Abstract
Introduction
Recent studies have suggested a higher recurrence rate of hepatocellular carcinoma (HCC) in patients with a history of HCC and hepatitis C virus (HCV)-associated cirrhosis treated with direct-acting antiviral (DAA) agents.
Material and methods
We conducted a prospective analysis of 24 patients with HCV-associated cirrhosis and treated HCC who received ombitasvir/paritaprevir/ritonavir+dasabuvir+ribavirin for 12 weeks. Prior therapies for HCC included resection (9/24 patients), radiofrequency ablation (RFA) (7/24) and trans-arterial chemoembolization (TACE) (8/24). All patients were eligible for treatment if they had no HCC recurrence 6 months after their last procedure. A control group was defined. All patients were followed every 6 months, with dynamic computed tomography and/or magnetic resonance imaging.
Results
The sustained virological response rate per protocol was 21/24 (87.5%). The study group included 14 (59%) males, median age 64 years (51–77), 50% with associated non-alcoholic steatohepatitis and 24% with Child–Pugh A6 points. HCC recurrence rate/100 patient-years was lower in the DAA-HCC group versus control: 5.5 versus 24.6% patient-years for the resection+RFA group (p = 0.044), respectively, and 18.6 versus 72.7% patient-years for TACE group (p = 0.002). Survival without recurrence was higher in the resection+RFA group (45 compared to 18 months (p < 0.001)) and also in the TACE group (44 compared to 11.5 months (p = 0.002)).
Conclusions
DAA therapy significantly reduced the recurrence rate of HCC and improved survival without recurrence in patients with treated HCV-associated HCC.
Keywords: Hepatitis C, direct antiviral therapy, hepatocellular carcinoma, ombitasvir/paritaprevir/r+dasabuvir+ribavirin, liver cirrhosis
Key summary
We conducted a prospective analysis including 24 patients diagnosed with hepatitis C virus (HCV)-associated cirrhosis and previously treated HCC (resection, radiofrequency ablation (RFA) or trans-arterial chemoembolization (TACE)) who received reimbursed ombitasvir/paritaprevir/ritonavir+dasabuvir+ribavirin for 12 weeks from the Romanian National Health Agency.
All patients were eligible for reimbursed treatment if they had no HCC recurrence 6 months after their last procedure. A control group was defined. All patients were followed for tumour recurrence every 6 months, with dynamic computer tomography and/or magnetic resonance imaging.
The sustained virological response rate per protocol was 21/24 (87.5%).
A reduced recurrence rate of HCC was observed in patients receiving direct-acting antiviral therapy compared to the control group: 5.5 versus 24.6% patient-years in patients treated by resection or RFA (p = 0.044), respectively, and 18.6 versus 72.7% patient-years in patients treated by TACE (p = 0.002).
Introduction
Hepatitis C virus (HCV) is a worldwide health problem that affects approximately 71 million people.1,2 Most of the affected people are viraemic, meaning that they will develop chronic hepatitis C that could potentially lead to end-stage liver disease, hepatocellular carcinoma (HCC) and liver-related death in a significant percentage of cases.3 The most important risk factor for HCC, the second leading cause of cancer mortality worldwide, regardless of the aetiology, is cirrhosis and it is responsible for 90% of HCC cases.4,5 The European prevalence of HCV infection ranges from 1.5% in Western Europe to 1.7% in Eastern Europe.6 In Romania, the prevalence of the HCV chronic infection has reached ∼4% with no available vaccine against infection.6,7 Previous treatment for HCV was limited to interferon (IFN)-based therapy, but nowadays the availability of oral direct-acting antivirals (DAAs) has changed HCV management.8 It is believed that HCV can promote carcinogenesis and its eradication directly decreases HCC risk.9 The DAAs are considered a revolutionary therapy with sustained virological response (SVR) rates consistently >90% for genotype 1 despite the presence of cirrhosis.10–12 There are inconsistent data regarding a higher recurrence rate of HCC in patients who previously had complete response to locoregional treatment and were subsequently treated with DAAs.13–17 Although IFN has been reported to have a suppressive activity in carcinogenesis and tumour recurrence, similar properties were not proven for the IFN-free DAAs.18–21 Despite the available literature, the indications of antiviral therapy in virus-related HCC patients remain incomplete in most clinical guidelines.22,23 There are some studies that suggest a lower rate of HCC recurrence and others that indicate a higher rate of HCC recurrence after DAA therapy.24–29
Possible explanations for the increased rate of HCC recurrence after DAA therapy include the rapid decrease in natural killer cell cytotoxicity, non-reversible mucosal-associated invariant T cell dysfunction and the ‘normalized’ liver microenvironment, all supporting HCC progression by disrupting immunological balances.3
The aim of our study was to assess the rate of HCC recurrence in patients with a history of treated HCC that received ombitasvir, paritaprevir, ritonavir, dasabuvir and ribavirin (OBV/PTV/r+DSV+RBV), since no consistent data from Eastern Europe have been published to date.
Material and methods
Patients
We selected patients with treated HCV-associated HCC from our national cohort, which currently has 5861 patients enrolled with HCV-associated cirrhosis. All had received reimbursed DAAs with OBV/PTV/r+DSV+RBV for 12 weeks between December 2015 and October 2016. According to current national inclusion criteria, patients were treated if they had absence of recurrence of hepatic cancer 6 months after their procedure (surgery, radiofrequency ablation (RFA) or trans-arterial chemoembolization (TACE)). Absence of recurrence of HCC was defined according to imaging criteria (via contrast-enhanced computed tomography (CT) or contrast-enhanced magnetic resonance imaging (MRI)) 6 months after the last therapeutic procedure.
Patients with hepatic nodular lesions showing an atypical imaging pattern that did not fulfil HCC criteria did not receive DAA treatment and were excluded from the analysis.
The study was approved by the National Ethics Committee of Medicines and Medical Devices (number 27SNI/10 October 2016). The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected by this approval.
A control group was defined retrospectively, and included an equal number of HCV-related HCC patients and patients with HCV-associated cirrhosis treated by surgery, RFA or TACE who did not receive DAA therapy.
The patients were treated between 2009 and 2013 in the Clinic Fundeni Institute referral centre. They were chosen by adjusting age, gender and Barcelona Clinic Liver Cancer (BCLC) staging to match the patients in the DAA group, and included only if they had absence of recurrence of cancer 6 months after their last session of therapy (surgery, RFA or TACE).
All patients signed a written informed consent form before entering the study.
Patients with HBV coinfection, HIV infection or other causes of chronic liver disease (alcoholic liver injury, primary biliary cholangitis, autoimmune hepatitis or hepatotoxic drug abuse) were excluded.
All patients were followed for tumour recurrence every 6 months with contrast-enhanced CT and/or MRI. All subjects were divided into four groups: DAA-resection+RFA (patients with HCC treated by resection or RFA who received DAA therapy), DAA-TACE (patients with HCC treated by TACE who received DAA therapy), control- resection+RFA and control-TACE. Data were obtained from the Romanian National Health Agency.
The observation period for both cohorts (DAA-treated and controls) started from the last HCC intervention.
Recurrence of hepatic cancer was defined as the appearance of new HCC nodules, reappearance of HCC in the same segment or both. Recurrence rates were calculated from the initiation of DAA therapy to the time of the above-mentioned recurrence events.
Recorded data included in the analysis were: age, sex, body mass index, fibromax parameters (fibrosis stage, steatosis score and necroinflammatory activity), aspartate aminotransferase (AST) platelet ratio index score, presence of comorbidities and use of concomitant medications. Biological parameters were recorded every 6 months starting from HCC diagnosis, and included platelet count, international normalized ratio, total bilirubin, AST, alanine aminotransferase, glucose level, alpha-fetoprotein, HCV RNA viral load, creatinine and estimated clearance of creatinine.
Diagnosis of HCC and follow-up
The diagnosis of HCC was made based on imaging criteria, and it was based on typical vascular patterns revealed by contrast-enhanced CT or MRI.
Statistical analysis
Data analysis was performed with SPSS 16.0 (SPSS, Inc., Chicago, IL, USA). Categorical variables were reported as frequency and analysed by Fisher's exact test. Continuous variables that were not normally distributed were reported as median (minimum to maximum) and analysed with the Mann–Whitney U-test. Survival was compared by log rank test. Significance was regarded to be p < 0.05 (two-sided).
Results
The median observation period of the patients was 44 months (range = 24–96). The date of the last follow-up for this study was 28 February 2018.
Most of the 24 patients were treated by resection (9/24) followed by RFA (7/24), with TACE performed in 8/24.
The SVR rate per protocol was 21/24 (87.5%). Two female patients from the DAA-resection + RFA group decompensated and died because of acute liver insufficiency (9.5%), one immediately after finishing the 12 weeks of therapy and the other during the 7th week of DAA therapy. In the DAA-TACE group, one male had virological relapse 12 weeks after his DAA therapy finished.
For a more homogenous approach, we excluded the deceased patients from the follow-up and analysed the remaining 22 patients (the two patients were included in the overall survival rate, but not in survival without recurrence).
Recurrence of HCC occurred in six patients (27%), 4 males and 2 females. It was more frequent in the TACE group (38%) than in those with hepatic resection (25%), while the lowest risk of recurrence was in the RFA (17%); however, these data did not reach statistical significance.
An increased risk of recurrence in the TACE group was probably related to more advanced tumour stage (BCLC stage B in 100% of patients compared with patients treated by resection+RFA, which were mostly BCLC stage 0).
Demographic, clinical and laboratory parameters in the two groups (DAA-resection+RFA and control- resection+RFA) are shown in Table 1. The two groups have statistically comparable parameters, except for follow-up duration (longer in the DAA group (42 versus 28.5 months, p < 0.05) as depicted in Table 1). Statistically significant differences regarding recurrence rate (21 versus 86%, p = 0.002) and survival without recurrence (39.5 versus 16 months, p < 0.001), demonstrated a benefit for the OBV/PTV/r+DSV+RBV-treated patients. However, overall survival was not significantly different (44 versus 38.5 months). Instead, a trend of better survival was noticed in patients that received DAA therapy.
Table 1.
A comparison between patients with hepatocellular carcinoma-treated trough resection or radiofrequency ablation + virus C-compensated liver cirrhosis that received direct-acting antiviral therapy, and the control group (hepatocellular carcinoma-treated trough resection or radiofrequency ablation + virus C-compensated liver cirrhosis without antiviral therapy).
| Parameter | DAA-resection+RFA (14) | Control-resection+RFA (14) | p-value |
|---|---|---|---|
| Sex (male)a | 7 (50%) | 6 (42.9%) | 1 |
| Mean age (years)** | 63 (51, 77) | 66 (50, 77) | 0.427 |
| Follow-up (months) ** | 42 (24, 90) | 28.5 (12, 105) | 0.039 |
| Platelets ** (×103/mm3) | 156.5 (65, 300) | 110.5 (68, 309) | 0.246 |
| AST ** (IU/ml) | 65.5 (22, 196) | 76 (26, 388) | 0.482 |
| ALT ** (IU/ml) | 61.5 (16, 183) | 70 (19, 353) | 0.401 |
| Child–Pugh score (points) ** | 5 (5, 6) | 5 (5, 7) | 0.804 |
| Blood glucose ** (mg/dl) | 95.5 (81, 118) | 103 (74, 188) | 0.056 |
| INR ** | 1.14 (0.94, 1.27) | 1.05 (0.94, 1.58) | 0.306 |
| Creatinine **(mg/dl) | 0.84 (0.56, 1.25) | 0.85 (0.5, 2.5) | 0.946 |
| Total bilirubin ** (mg/dl) | 0.87 (0.51, 1.7) | 0.95 (0.4, 4.3) | 0.910 |
| APRI | 1.91 (0.23, 5.37) | 2.03 (0.31, 15.42) | 0.571 |
| Alpha-fetoprotein | 11.1 (3.02, 123.33) | 31 (4.84, 410) | 0.252 |
| RNA viral load (×103)** | 1249 (43, 11,400) | 1680 × 103 (72.2, 5078) | 0.769 |
| BCLC stage 0a | 8/14 (57%) | 7/14 (50%) | 1 |
| Diabetes mellitusa | 2/14 (14.3%) | 2/14 (14.3%) | 1 |
| SVR 12 (per protocol)a | 12/14 (85.7%) | NA | NA |
| Time from last intervention to DAA therapy (months)** | 23 (7, 72) | NA | NA |
| Time from last intervention to recurrence (months)** | 24 (24, 60) | 14.5 (7, 36) | 0.101 |
| Recurrence ratea | 3 (21.4%) | 12 (85.7%) | 0.002 |
| Survival without recurrence ** | 39.5 (20, 80) | 16 (8, 36) | < 0.001 |
| Overall survival ** | 44 (20, 90) | 38.5 (12, 105) | 0.376 |
| HCC recurrence rate/100 patient-years*** | 5.5% | 24.6% | 0.044 |
ALT: alanine aminotransferase; APRI: aspartate aminotransferase platelet ratio index score; AST: aspartate aminotransferase; BCLC: Barcelona Clinic Liver Cancer; DAA: direct-acting antiviral; HCC: hepatocellular carcinoma; INR: international normalized ratio; NA: not applicable; RFA: radiofrequency ablation; SVR: sustained virological response.
Number (%), compared by Fisher's exact test.
bMedian (minimum, maximum), compared by Mann–Whitney U-test.
cRecurrence rate compared by log rank test.
Regarding the HCC recurrence rate/100 patient-years, this rate was significantly lower in the DAA-resection+RFA group compared to the control-resection+RFA group: 5.5 versus 24.6% (p = 0.044).
Table 2 describes the most important characteristics of the DAA-TACE and control-TACE groups. DAA therapy positively impacted the recurrence rate, which decreased from 100% in the control group to 37.5% in patients that received OBV/PTV/r+DSV+RBV. Also, the HCC recurrence rate/100 patient-years was considerably diminished in patients that received antiviral therapy: 18.6 versus 72.7% (p-value = 0.002). Survival without recurrence and overall survival were significantly higher in those treated with antivirals: 44 versus 11.5 months and 44 versus 17 months, respectively, for this group.
Table 2.
A comparison between patients with hepatocellular carcinoma-treated trough trans-arterial chemoembolization + virus C-compensated liver cirrhosis that received direct-acting antiviral therapy and the control group (hepatocellular carcinoma-treated trough trans-arterial chemoembolization + virus C-compensated liver cirrhosis without antiviral therapy).
| Parameter | DAA-TACE (8) | Control-TACE(8) | p-value |
|---|---|---|---|
| Sex (male)a | 6 (75%) | 6 (75%) | 1 |
| Mean age (years)b | 68 (53, 75) | 63.5 (55, 76) | 0.574 |
| Platelets (×103/mm3)b | 81 (64, 95) | 120 (96, 151) | 0.959 |
| AST (IU/ml)b | 105 (90, 107) | 129 (42, 242) | 0.645 |
| ALT (IU/ml)b | 100 (77, 126) | 100 (39, 242) | 0.279 |
| Child score (points)b | 5 (5, 6) | 5 (5, 8) | 0.645 |
| Blood glucose (mg/dl)b | 98 (98, 140) | 100 (79, 171) | 0.505 |
| INRb | 1.02 (0.95, 1.23) | 1.14 (1.04, 1.63) | 0.021 |
| Creatinine (mg/dl)b | 0.88 (0.70, 1.20) | 0.91 (0.82, 1.00) | 1 |
| Total bilirubin (mg/dl)b | 0.80 (0.71, 1.20) | 0.80 (0.50, 0.90) | 0.959 |
| APRI | 3.14 (0.91, 7.62) | 1.82 (1.18, 4.33) | 0.574 |
| Alpha-fetoprotein | 9.6 (2.16, 170.97) | 269 (3, 400) | 0.083 |
| RNA viral load (×103)b | 911.479 (277.971, 2640) | 1004.6 × 103 (101.3, 2046) | 0.852 |
| BCLC stage Ba | 8/8 (100%) | 8/8 (100%) | 1 |
| Diabetes mellitusa | 3/8 (37.5%) | 1/8 (12.5%) | 0.442 |
| SVR 12 (per protocol)a | 7/8 (87.5%) | NA | NA |
| Time from last intervention to DAA therapy (months)b | 24 (16, 30) | NA | NA |
| Time from last intervention to recurrence (months)b | 24 (16, 30) | 24 (16, 30) | 1 |
| Recurrence ratea | 3 (37.5%) | 8 (100%) | 0.026 |
| Survival without recurrenceb | 44 (16, 51) | 11.5 (7, 31) | 0.002 |
| Overall survivalb | 44 (24, 51) | 17 (12, 46) | 0.048 |
| HCC recurrence rate/100 patient-yearsc | 18.6% | 72.7% | 0.002 |
ALT: alanine aminotransferase; APRI: aspartate aminotransferase platelet ratio index score; AST: aspartate aminotransferase; BCLC: Barcelona Clinic Liver Cancer; DAA: direct-acting antiviral; HCC: hepatocellular carcinoma; INR: international normalized ratio; NA: not applicable; SVR: sustained virological response; TACE: trans-arterial chemoembolization.
Number (%), compared by Fisher's exact test.
Median (minimum, maximum), compared by Mann–Whitney U-test.
Recurrence rate compared by log rank test.
If we take into account the entire group of patients treated with DAAs and compare it to the control group, the HCC recurrence rate/100 patient-years was considerably reduced in patients that received OBV/PTV/r+DSV+RBV: 7.37 versus 33.44% (p-value < 0.001).
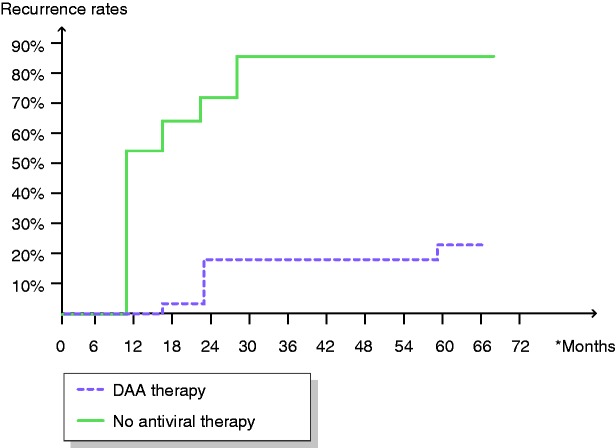
Figure 1 illustrates the time trend for recurrence, comparing all patients treated with OBV/PTV/r+DSV+RBV that were followed-up prospectively and the control group (patients with HCC and HCV-associated cirrhosis that did not receive antiviral therapy). In the control group, HCC reoccurred mostly during the first 12–36 months of follow-up, reaching a 91% recurrence rate. The time trend for the patients that received OBV/PTV/r+DSV+RBV was similar: indeed, in this situation, no recurrence was documented in the first 6 months after the last session of therapy for HCC (otherwise they would not have received DAA therapy), and liver tumour reappearance was recorded at 1.5–2.5 years.
Figure 1.
Recurrence rates in 22 patients with ombitasvir/paritaprevir/r+dasabuvir+ribavirin therapy after hepatocellular carcinoma therapy and in the control group without antiviral therapy. Hepatocellular carcinoma recurrence rates were significantly lower in those who received antiviral therapy.
DAA: direct-acting antiviral.
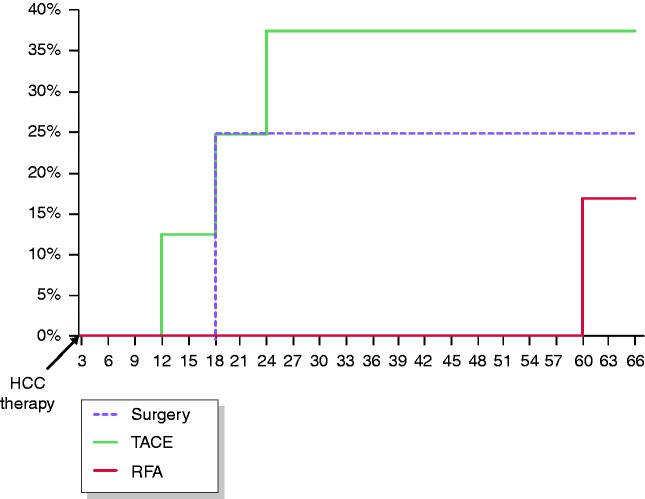
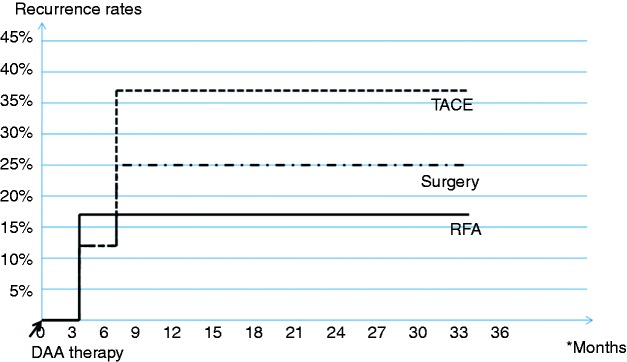
Recurrence rates over time according to type of cancer therapy in 22 patients who received OBV/PTV/r+DSV+RBV after curative treatment for HCC are depicted in Figure 2. Recurrence rates were not statistically different among the therapeutic approaches, but the subgroups were very small. We also studied the reappearance of HCC over time after patients started the DAA therapy. The results are depicted in Figure 3. All recurrences were recorded very early: 3 to 6 months after the patients received OBV/PTV/r+DSV+RBV.
Figure 2.
Tumour recurrence rates according to methods of cancer therapy in 22 patients who received ombitasvir/paritaprevir/r+dasabuvir+ribavirin after treatment for hepatocellular carcinoma. Recurrence rates were not significantly different among resection, radiofrequency ablation or trans-arterial chemoembolization.
HCC, hepatocellular carcinoma; RFA: radiofrequency ablation; TACE: trans-arterial chemoembolization.
Figure 3.
Tumour recurrence rates according to methods of cancer therapy in 22 patients who received ombitasvir/paritaprevir/r+dasabuvir+ribavirin after curative treatment for hepatocellular carcinoma: evolution in time after starting direct-acting antiviral therapy.
DAA: direct-acting antiviral; RFA: radiofrequency ablation; TACE: trans-arterial chemoembolization.
Table 3 shows all the patients included in the statistical analysis and their previously treated HCC characteristics (size, location, number, time of last HCC procedure and recurrence). There were three recurrences in the DAA-resection+RFA group, two of which had multiple HCC nodules at initial diagnosis. Due to the small sample size, predictive factors of recurrence such as size, number and location could not be isolated.
Table 3.
Patients treated for hepatocellular carcinoma that received three-dimensional therapy: their hepatocellular carcinoma characteristics (size, location, number, time of last hepatocellular carcinoma procedure and recurrence).
| Patient initials, sex and age (years) | Size of HCC nodule (the largest diameter) | Location (hepatic segment) | Type of HCC therapy | Number of HCC nodules | Time of last HCC procedure (month and year) | Recurrence Y/N |
|---|---|---|---|---|---|---|
| 1. GM, F, 66 | 14 mm | VI | RFA | 1 | November 2012 | N |
| 2. IS, F, 55 | 15 mm | VI | Resection | 1 | February 2016 | N |
| 3. MM, M, 77 | 35 mm | VI | RFA | 1 | October 2013 | N |
| 4. TE, F, 69 | 35 mm | III | Resection | 1 | February 2015 | N |
| 5. UG, F, 64 | 22 mm | V–VI | Resection | 1 | November 2013 | N |
| 6. BM, M, 64 | 44 mm | III | RFA | 1 | March 2015 | N |
| 7. DA, M, 65 | 23 mm | III | Resection | 1 | December 2011 | N |
| 8. DV, M, 51 | 27 mm | III | RFA | 1 | October 2014 | N |
| 9. DN, F, 63 | 29 mm | VI | RFA | 1 | February 2015 | N |
| 10. CM, F, 63 | 32, 24, 29 mm | VI | Resection | 3 | January 2015 | Y |
| 11. LG, M, 59 | 25, 40, 40 mm | VI, VIII | Resection | 3 | October 2015 | Y |
| 12. PI, M, 69 | 30 mm | VIII | RFA | 1 | December 2010 | Y |
| 13. HI, M, 52 | 51 mm | VII | Resection | 1 | March 2015 | N |
| 14. PN, F, 62 | 18 mm | VII | Resection | 1 | February 2014 | N |
| 15. CF, M, 53y | 35 mm | VIII | TACE | 1 | September 2013 | N |
| 16. CC, M, 67 | 28 mm | VIII | TACE | 1 | August 2014 | Y |
| 17. RM, F, 75 | 64 mm | VI | TACE | 1 | November 2014 | N |
| 18. TM, F, 74 | 46 mm | IVa | TACE | 1 | December 2014 | N |
| 19. TT, M, 64 | 35 mm | VIII | TACE | 1 | July 2014 | N |
| 20. PG, M, 67 | 63, 17 mm | VIII | TACE | 2 | March 2015 | Y |
| 21. SI, M, 69 | 21, 28, 54 mm | V | TACE | 3 | October 2014 | N |
| 22. CG, M, 74 | 30 mm | VII | TACE | 1 | April 2015 | Y |
F: female; HCC: hepatocellular carcinoma; M: male; N: no; RFA: radiofrequency ablation; TACE: trans-arterial chemoembolization; Y: yes.
The pattern of recurrence was: intrahepatic growth (two patients), new intrahepatic lesion (up to three nodules ≤ 3 cm in one patient), and infiltrative ill-defined hepatocellular carcinoma and/or extrahepatic lesions in two patients.
Discussion
Our study addresses important issues in patients with previously treated HCC in HCV-associated cirrhosis: if, when and how to treat them using DAA, and what impact OBV/PTV/r+DSV+RBV has on the overall survival of these patients.
In the current literature, a lot of studies have addressed HCC recurrence, but only two included a control group of age-, gender- and BCLC staging-matched patients without antiviral therapy after initial HCC therapy, similar to our study.14,18 The major drawback of our study is the small number of patients (our included only 24 patients who were followed-up prospectively; because two patients decompensated and died during the DAA therapy, only 22 could be analysed regarding HCC recurrence). Most of the available studies were performed on 18–189 patients with complete response to prior treatment of HCC who received anti-HCV DAA therapy.13,18–21,23,24,29–32
The first retrospective cohort study that addressed this issue came from Italy, and enrolled 59 chronic HCV-infected cirrhotic patients with a history of HCC, followed-up for ≤ 24 weeks after DAA therapy. While de novo occurrence of HCC was detected in 26 of 285 (7.6%) patients, an alarmingly high rate of recurrence of previous successfully treated HCC was reported in 17 (29%) of 59 patients.29 We report a similar recurrence rate (27%), in concordance with other data from the literature.14,19,28,29
Regarding HCC recurrence rate/100 patient-years, some studies on HCC relapse in patients after HCC treatment with curative intent report quite variable percentages, ranging from 2.2 to 47%. Our study found a recurrence rate of 5.5% in this subgroup of patients, which is a smaller than in previous reports.13,19,29 However, several other publications have reported no increase in the HCC relapse rate.
Zavaglia et al. did not confirm the findings of Reig et al. and Conti et al., since they declared only one case of HCC recurrence in their series of 31 consecutive patients who were followed-up for a median of 8 months. The authors suggested that their longer interval between complete tumour eradication and antiviral therapy (median 19 months in their series versus 11 months in the study by Reig et al.) could explain, at least in part, the contrasting results. In fact, the longer the interval, the lower the risk that residual tumour tissue is present at the start of DAA therapy.30
In our study, this interval was longer (median 24 months; minimum 7 months and maximum 72 months), but a high recurrence rate was still noticed (27%). However, considering the fact that recurrence rate in the control group reached 92%, this ultimately translated in improved survival without recurrence (40 versus 14 months) and improved overall survival (44 versus 38.5 months), favouring the DAA-treated group.
Besides the control group, our study has other important strengths: a long follow-up median of 44 months (minimum 24 and maximum 96 months) and a homogenous study population, all patients presenting with genotype 1 b, all with compensated cirrhosis and all treated with the same drug combination. Other publications reported a median follow-up between 5.7 and 26.1 months, and patients had different genotypes and different DAA therapy (mostly treated with sofosbuvir and ledipasvir).13,18–24,29–32
In the ANRS CO12 CirVir substudy, 13 of 79 patients received DAA therapy 3 months after complete response to HCC treatment with curative intent.13 The rate of HCC recurrence was 7.7% in the DAA group (1.11/100 person-years) and 47.0% in the untreated group (1.73/100 person-years; p = 0.748).
In the ANRS CO22 HEPATHER substudy, 189 of 267 HCC patients received DAA therapy after HCC treatment with curative intent (median time between HCC treatment and onset of DAA therapy/study inclusion: 22.8/19.2 months).14 The median follow-up times were 20.2 and 26.1 months, and the relapse rates were 12.7% (0.73/100 person-years) and 20.5% (0.66/100 person-years; p = 0.8756), for the DAA-treated and untreated groups, respectively. However, contrary to both previous studies from Italy and Spain, and our data, the ANRS register also included patients who received orthotopic liver transplantation. Still, these data suggest that the rates of recurrence would be lowest if the median time between HCC treatment and the onset of DAA therapy is 3 months.23,29
Another important issue is the SVR rate, as this patient group was difficult to treat. We report a good SVR rate per protocol of 87.5%, similar to other studies that found SVR rates ranging between 90 and 100%.10,23,27,29,32–35 According to a recent meta-analysis (Ji et al., in press), the two-/three-dimensional (2D/3D) regimen was used in only 101 HCC patients from non-Asian studies, limiting comparability among the different DAA regimens. That is because the 2D/3D regimen may have been avoided in HCC patients with more severe liver disease, which is demonstrated by the fact that 2 out of our 24 patients decompensated and died during therapy.
Another important finding of our study was the benefit of DAA therapy with OBV/PTV/r+DSV+RBV, which was significant in patients successfully treated by TACE. A decreased recurrence rate was observed (from 100 to 37.5%) as well as improved survival without recurrence (from a median of 11.5 to 44 months). Moreover, we noticed increased overall survival from 17 to 44 months. Very few previous studies have included this category of patients.23 Reig et al. reported no recurrence in patients treated by TACE, but the median follow-up duration was shorter than in our study (5.7 months). These recurrences were diagnosed radiologically very early after the start of DAA therapy, with a median time of 4 months (3–6 months), similar to the data from the cohort of Reig et al.23 The pattern of recurrence was mainly intrahepatic growth (two patients) and infiltrative, ill-defined HCC and/or extrahepatic lesions (two patients), similar to the data of Reig et al. In addition, Abdelaziz et al. observed significantly worse response to ablation in patients with recurrent HCC compared with de novo lesions.25
Because of the low sample size, it was impossible to identify predictive factors for HCC recurrence in our cohort, but other authors have found that the main tumour size and a history of prior HCC recurrence are independent risk factors.28
Another limitation of our study is that protease inhibitor regimens (such as OBV/PTV/r+DSV+RBV) are contraindicated in patients with cirrhosis and previous liver decompensation (a parameter that was not evaluated at inclusion) due to severe side effects. However, in 2016 this was the only reimbursed therapy available in Romania. Better treatment options are now available.
Finally, the control group was chosen from a single hospital cohort, which was from a tertiary referral centre for hepatic diseases and cancer (Fundeni Clinical Institute).
Conclusions
SVR rate per protocol in patients with treated HCC and HCV-associated cirrhosis that received OBV/PTV/r+DSV+RBV was very high, reaching 87.5%. Recurrence rates in patients with HCC treated by resection and RFA or TACE, and who subsequently received DAA therapy, were 21 and 38%, respectively, significantly lower compared to those of the control groups (86 and 100%, respectively). The decision to treat patients with compensated HCV-associated cirrhosis and HCV-associated HCC with an absence of recurrence 6 months after their last procedure (surgery or RFA or TACE) lead to very good results in terms of lower recurrence rates of HCC, improved survival without recurrence and overall survival.
Declaration of conflicting interests
Carmen Monica Preda, Cristian Baicus, Irina Sandra, Alexandru Oproiu, Teodora Manuc, Ileana Constantinescu, Daniel Gavrila, Radu Dumitru, Catalin Vasilescu, Cristian Tieranu, Doina Istratescu, Theodor Voiosu, Mircea Manuc have nothing to disclose. Mircea Diculescu has served as a speaker has received research funding from: Abbvie.
Funding
None to disclose.
Ethics approval
The study was approved by the Romanian National Ethics Committee of Medicines and Medical Devices (No.27SNI/October 10, 2016).
Informed consent
All patients have signed a written informed consent before entering in the study.
References
- 1.World Health Organization. Hepatitis C: Fact sheet, http://www.who.int/mediacentre/factsheets/fs164/en/. (2017, accessed 23 January 2018).
- 2.Preda CM, Popescu CP, Baicus C, et al. Real-world efficacy and safety of ombitasvir, paritaprevir/r+dasabuvir+ribavirin in genotype 1b patients with hepatitis C virus cirrhosis. Liver Int 2018; 38: 602–610. [DOI] [PubMed] [Google Scholar]
- 3.Werner JM, Adenugba A, Protzer U. Immune reconstitution after HCV clearance with direct antiviral agents: Potential consequences for patients with HCC?. Transplantation 2017; 101: 904–909. [DOI] [PubMed] [Google Scholar]
- 4.Baumert TF, Jühling F, Ono A, et al. Hepatitis C-related hepatocellular carcinoma in the era of new generation antivirals. BMC Med 2017; 15: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wörns MA, Galle PR, Zeuzem S, et al. Drug treatment for chronic hepatitis C infection and cancer risk. Dtsch Arztebl Int 2017; 114: 597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gheorghe L, Csiki IE, Iacob S, et al. The prevalence and risk factors of hepatitis C virus infection in adult population in Romania: A nationwide survey 2006 - 2008. J Gastrointestin Liver Dis 2010; 19: 373–379. [PubMed] [Google Scholar]
- 7.Pascu O, Gheorghe L, Voiculescu M, et al. How severe is chronic hepatitis with HCV genotype 1b? A study of 1,220 cases on the waiting list for antiviral therapy in Romania. J Gastrointestin Liver Dis 2011; 20: 51–55. [PubMed] [Google Scholar]
- 8.Saadi T, Khoury J. Is there a relationship between treatment with direct antiviral agents for HCV infection and the development of malignancies?. J Clin Gastroenterol 2018; 52: 353–359. [DOI] [PubMed] [Google Scholar]
- 9.Manthravadi S, Paleti S, Pandya P. Impact of sustained viral response postcurative therapy of hepatitis C-related hepatocellular carcinoma: A systematic review and meta-analysis. Int J Cancer 2017; 140: 1042–1049. [DOI] [PubMed] [Google Scholar]
- 10.Prenner SB, VanWagner LB, Flamm SL, et al. Hepatocellular carcinoma decreases the chance of successful hepatitis C virus therapy with direct-acting antivirals. J Hepatol 2017; 66: 1173–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gheorghe L, Iacob S, Curescu M, et al. Real-life use of 3 direct-acting antiviral regimen in a large cohort of patients with genotype-1b HCV compensated cirrhosis. J Gastrointestin Liver Dis 2017; 26: 275–281. [DOI] [PubMed] [Google Scholar]
- 12.Ogata F, Kobayashi M, Akuta N, et al. Outcome of all-oral direct-acting antiviral regimens on the rate of development of hepatocellular carcinoma in patients with hepatitis C virus genotype 1-related chronic liver disease. Oncology 2017; 93: 92–98. [DOI] [PubMed] [Google Scholar]
- 13.ANRS collaborative study group on hepatocellular carcinoma (ANRS CO22 HEPATHER, CO12 CirVir and CO23 CUPILT cohorts). Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: Data from three ANRS cohorts. J Hepatol 2016; 65: 734–740. [DOI] [PubMed] [Google Scholar]
- 14.Waziry R, Hajarizadeh B, Grebely J, et al. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: A systematic review, meta-analyses, and meta-regression. J Hepatol 2017; 67: 1204–1212. [DOI] [PubMed] [Google Scholar]
- 15.Renzulli M, Buonfiglioli F, Conti F, et al. Imaging features of microvascular invasion in hepatocellular carcinoma developed after direct-acting antiviral therapy in HCV-related cirrhosis. Eur Radiol 2018; 28: 506–513. [DOI] [PubMed] [Google Scholar]
- 16.Villani R, Facciorusso A, Bellanti F, et al. DAAs rapidly reduce inflammation but increase serum VEGF level: A rationale for tumor risk during anti-HCV treatment. PLoS One 2016; 11: e0167934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chu PS, Nakamoto N, Taniki N, et al. On-treatment decrease of NKG2D correlates to early emergence of clinically evident hepatocellular carcinoma after interferon-free therapy for chronic hepatitis C. PLoS One 2017; 12: e0179096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikeda K, Kawamura Y, Kobayashi M, et al. Direct-acting antivirals decreased tumor recurrence after initial treatment of hepatitis C virus-related hepatocellular carcinoma. Dig Dis Sci 2017; 62: 2932–2942. [DOI] [PubMed] [Google Scholar]
- 19.Reig M, Boix L, Bruix J. The impact of direct antiviral agents on the development and recurrence of hepatocellular carcinoma. Liver Int 2017; 37: 136–139. [DOI] [PubMed] [Google Scholar]
- 20.Bielen R, Moreno C, Van Vlierberghe H, et al. The risk of early occurrence and recurrence of hepatocellular carcinoma in hepatitis C-infected patients treated with direct-acting antivirals with and without pegylated interferon: A Belgian experience. J Viral Hepat 2017; 24: 976–981. [DOI] [PubMed] [Google Scholar]
- 21.Nagata H, Nakagawa M, Asahina Y, et al. Effect of interferon-based and -free therapy on early occurrence and recurrence of hepatocellular carcinoma in chronic hepatitis C. J Hepatol 2017; 67: 933–939. [DOI] [PubMed] [Google Scholar]
- 22.European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol 2018; 69: 461–511. [DOI] [PubMed] [Google Scholar]
- 23.Reig M, Mariño Z, Perelló C, et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol 2016; 65: 719–726. [DOI] [PubMed] [Google Scholar]
- 24.Grandhe S, Frenette CT. Occurrence and recurrence of hepatocellular carcinoma after successful direct-acting antiviral therapy for patients with chronic hepatitis C virus infection. Gastroenterol Hepatol (NY) 2017; 13: 421–425. [PMC free article] [PubMed] [Google Scholar]
- 25.Abdelaziz AO, Nabil MM, Abdelmaksoud AH, et al. De-novo versus recurrent hepatocellular carcinoma following direct-acting antiviral therapy for hepatitis C virus. Eur J Gastroenterol Hepatol 2018; 30: 39–43. [DOI] [PubMed] [Google Scholar]
- 26.Alberti A, Piovesan S. Increased incidence of liver cancer after successful DAA treatment of chronic hepatitis C: Fact or fiction?. Liver Int 2017; 37: 802–808. [DOI] [PubMed] [Google Scholar]
- 27.Beste LA, Green PK, Berry K, et al. Effectiveness of hepatitis C antiviral treatment in a USA cohort of veteran patients with hepatocellular carcinoma. J Hepatol 2017; 67: 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cabibbo G, Petta S, Calvaruso V, et al. Is early recurrence of hepatocellular carcinoma in HCV cirrhotic patients affected by treatment with direct-acting antivirals? A prospective multicentre study. Aliment Pharmacol Ther 2017; 46: 688–695. [DOI] [PubMed] [Google Scholar]
- 29.Conti F, Buonfiglioli F, Scuteri A, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol 2016; 65: 727–733. [DOI] [PubMed] [Google Scholar]
- 30.Zavaglia C, Okolicsanyi S, Cesarini L, et al. Is the risk of neoplastic recurrence increased after prescribing direct-acting antivirals for HCV patients whose HCC was previously cured?. J Hepatol 2017; 66: 236–237. [DOI] [PubMed] [Google Scholar]
- 31.Minami T, Tateishi R, Nakagomi R, et al. The impact of direct-acting antivirals on early tumor recurrence after radiofrequency ablation in hepatitis C-related hepatocellular carcinoma. J Hepatol 2016; 65: 1272–1273. [DOI] [PubMed] [Google Scholar]
- 32.Virlogeux V, Pradat P, Hartig-Lavie K, et al. Direct-acting antiviral therapy decreases hepatocellular carcinoma recurrence rate in cirrhotic patients with chronic hepatitis C. Liver Int 2017; 37: 1122–1127. [DOI] [PubMed] [Google Scholar]
- 33.Barone M, Iannone A, Shahini E, et al. A different perspective on sofosbuvir-ledipasvir treatment of patients with HCV genotype 1b cirrhosis: The ITAL-C network study. J Viral Hepat 2017; 25: 56–62. [DOI] [PubMed] [Google Scholar]
- 34.Lubel J, Strasser S, Stuart KA, et al. Real-world efficacy and safety of ritonavir-boosted paritaprevir, ombitasvir, dasabuvir +/− ribavirin for hepatitis C genotype 1 - final results of the REV1TAL study. Antivir Ther 2017; 22: 699–710. [DOI] [PubMed] [Google Scholar]
- 35.Eletreby R, Elakel W, Said M, et al. Real life Egyptian experience of efficacy and safety of simeprevir/sofosbuvir therapy in 6211 chronic HCV genotype IV infected patients. Liver Int 2017; 37: 534–541. [DOI] [PubMed] [Google Scholar]