Abstract
The two thrombopoietin receptor agonists (TPO-RA), eltrombopag and romiplostim, were licensed in the US for treatment of immune thrombocytopenia (ITP) in 2008 and, since then, their use has progressively increased around the world; they are currently used in more than 100 countries. The six largest randomized controlled trials conducted in ITP have used one of these two agents. All studies have demonstrated a platelet response rate between 50-90%, depending on the criteria used, with good safety and tolerability. TPO-RA were shown to be effective in reducing bleeding and the need for concomitant or rescue medication. Many other investigations of their mechanism of effect, prospective and retrospective trials, and studies focusing on toxicity have been performed widening our knowledge of these two agents. Initial concerns on issues such as myelofibrosis have not been confirmed. Only a small number of patients develop moderate-severe reticulin fibrosis and/or collagen fibrosis; however, these are usually reversed after discontinuation of TPO-RA. Studies indicate, however, that TPO-RA may increase the risk of venous thromboembolism. Both TPO-RA are currently approved in patients with chronic ITP aged >1-year who are refractory to at least one other treatment. Eltrombopag has acquired two additional indications: severe aplastic anemia refractory to first-line treatment and hepatitis C patients undergoing treatment with interferon-ribavirin. Despite these wide-ranging studies, important questions still need to be answered. This summary review on TPO-RA will summarize what is known regarding efficacy in ITP, evaluate safety concerns in more depth, and focus on the questions that remain.
Introduction
Over the last 20 years, and before the regular availability of thrombopoietin receptor agonists (TPO-RA), the most commonly used second-line treatments for patients with immune thrombocytopenia (ITP) were splenectomy and rituximab. Both options have the potential to provide a cure. However, long-term responses are not completely satisfactory (60% after splenectomy and only 20% 2-5 year long-term responses after rituximab).1,2 Adverse events following these interventions are also significant, if uncommon: post-operative morbidity and increased risk of infections and thromboembolism (TE) after splenectomy, and very rare cases of progressive multifocal leukoencephalopathy (PML) and slight increased infectious rates after rituximab.3
The two TPO-RA, romiplostim and eltrombopag, represent a completely different approach to ITP; they both have a very good chance of supporting the platelet count with undemanding daily or weekly treatment. Their goal is to support the patient’s platelet count until adequate levels are achieved and treatment is no longer required. The TPO-RA were licensed in the US for the treatment of ITP in 2008, and, since then, their use has progressively increased around the world; they are currently used in more than 100 countries. Their introduction heralded a paradigm shift in the treatment of ITP. They are now widely used and many hematologists are well-acquainted with them. This is the 10-year anniversary of their licensure in the US for ITP and it seems appropriate to review the state of the art of these agents: what is known about their mechanism of effect, efficacy, and toxicity, and what remains to be learned, including an exploration of other clinical situations in which they might be useful.
Mechanism of action
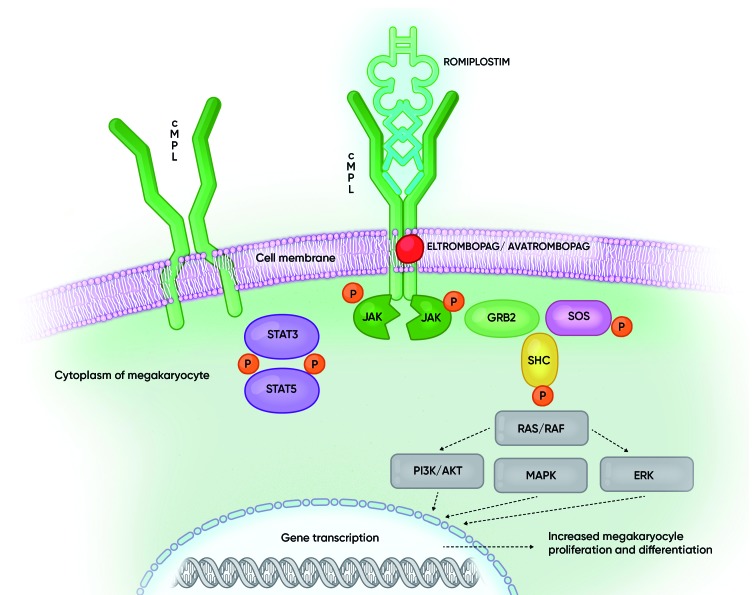
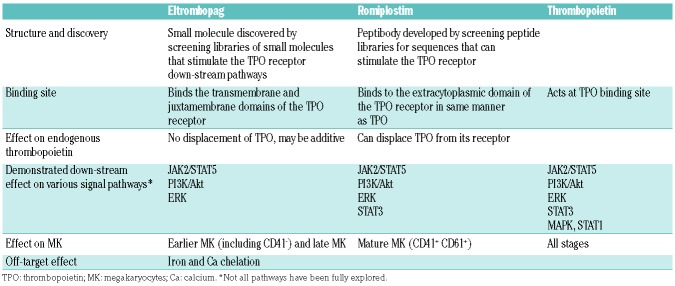
Romiplostim and eltrombopag both bind to the thrombopoietin (TPO) receptor, causing conformational change in the TPO receptor, activation of the JAK2/STAT5 pathway, and a resulting increased megakaryocyte progenitor proliferation and increased platelet production.4,5 However, there are some differences between the two agents (Figure 1). Romiplostim is a peptibody that binds directly and competitively at the TPO binding site, whereas eltrombopag is a small molecule which binds at a trans-membrane site. There are also differences in the activation of other signaling pathways in megakaryocytes (MK) such as STAT3, ERK and AKT (Table 1).6–8 Furthermore, romiplostim mostly stimulates mature precursors, while eltrombopag appears to act earlier in the pathway, stimulating MK precursor cells and MK differentiation.4,6
Figure 1.
Cellular mechanisms of action of thrombopoietin (TPO) and of thrombopoietin receptor agonists (TPO-RA). Binding of the ligand (TPO/TPO-RA) to the c-MPL receptor on the megakaryocyte causes conformational change in the receptor, resulting in downstream activation of the various signaling pathways including JAK2/STAT5, PI3K/AKT, ERK, ultimately resulting in increased platelet production. Various pathways can be activated by the different substances (see also Table 1). GRB2: growth factor receptor-binding protein 2; JAK: Janus kinase; MAPK: mitogen-activated protein kinase; P: phosphorylation; RAF: rapidly accelerated fibrosarcoma kinase; RAS: rat sarcoma GTPase; SHC: Src homology collagen protein; STAT: signal transducer and activator of transcription; PI3K: phosphatidylinositol 3-kinases; ERK: extracellular-signal-regulated kinase.
Table 1.
Characteristics and down-stream effect of eltrombopag, romiplostim and endogenous thrombopoietin.
In addition to differences in TPO-receptor activation, eltrombopag also has off-target effects. For example, eltrombopag chelates both extra- and intra-cellular calcium and iron and can shuttle iron out of cells.9 The iron-chelating action of eltrombopag causes anti-proliferative effects on leukemic cells lines,10 and a TPO-independent effect on stimulating stem cells and MK precursors in vivo.
These differences may explain why some patients respond to one agent and not the other,11,12 and why treatment with both agents can be useful in very refractory patients.
Although the prime mechanism of action of the TPO-RA is thought to be due to increased platelet production, both TPO-RA have also been described to have immunomodulatory effects, with increased regulatory T-and B-cell effects in patients on TPO-RA.13 This effect has been suggested to be mediated by TGF-B, a major cytokine involved in T-regulatory (Treg) cell development, and found in abundance in MK and platelets.14,15 Alternatively, TPO-RA may also affect antigen processing and presentation by MK.16 Whether these potential immunomodulatory effects result in the treatment-free durable responses reported with both TPO-RA has not yet been understood.
Efficacy of romiplostim and eltrombopag
Platelet response
The response rate of these agents depends on the definition of “response.” If a consistent “durable” platelet count response is required, then the response rate may be 40-60%. This type of response, with platelet counts consistently higher than 50×109/L without bleeding and/or need for rescue therapy, is a realistic goal for patients with ITP. If, however, a “response” is a single platelet count over 50×109/L during a finite period of time, then the response rate is closer to 60-90%.17–23
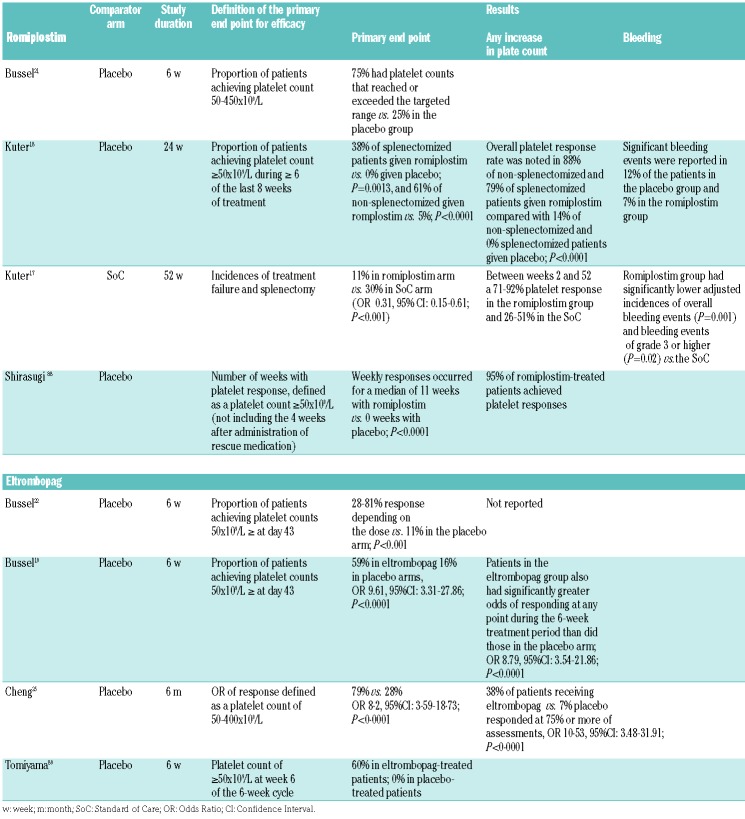
Table 2 summarizes the effect of the two agents in randomized controlled trials (RCT) performed in adult ITP patients. The rate for a durable response in the pivotal trials for romiplostim was around 60% in non-splenectomized patients but lower in previously splenectomized patients. Splenectomized patients treated with eltrombopag in the Randomized Placebo-Controlled Idiopathic Thrombocytopenic Purpura (RAISE) and Eltrombopag Extended Dosing (EXTEND) studies also had lower response rates than non-splenectomized patients. While splenectomized patients and those with platelet counts <15×109/L respond less well to both romiplostim18 and eltrombopag,24,25 there is still good evidence of the effect of these agents in these patients.
Table 2.
Summary of the randomized controlled trials performed in adult immune thrombocytopenia patients with romiplostim or eltrombopag.
A recent meta-analysis, which included 1126 patients from 13 RCT performed in eight adult and five pediatric ITP populations, showed that TPO-RA significantly increased platelet response by 3-fold and durable response rates by almost 8-fold as compared to placebo or Standard of Care (SoC).26 In adult studies, a 3-fold increase in response [Risk Ratio (RR): 3.1, 95% Confidence Interval (CI): 2.0-5.0] and 7.5-fold increase in durable response (RR: 7.4, 95%CI: 3.2-17.1) was seen.26 Therefore, while this is a very effective approach to treatment of ITP, not all patients will have a clinically meaningful response to TPO-RA, whereas some have to discontinue TPO-RA because of the lack of response.20,24,27 In the EXTEND study, of the 302 patients enrolled, 55% withdrew from the study due to adverse events (14%), patient decision (13%), lack of efficacy (11%), or other reasons,20,24,27 whereas, in the long-term romiplostim study, 31% of the 292 enrolled patients discontinued because of patient decision (27%), adverse events (12%), alternative therapy (12%), or for other reasons.20,24,27
Reduction in bleeding and concomitant medications
The meta-analysis showed that TPO-RA significantly reduced incidences of any or severe bleeding events (RR: 0.8, 95%CI: 0.7-0.9; RR: 0.5, 95%CI: 0.3-0.99, respectively).26 Especially with eltrombopag, there were substantial reductions in any or severe bleeding events in treated patients compared with controls (RR: 0.7, 95%CI: 0.5-0.9; and RR: 0.3, 95%CI: 0.1-1.0, respectively). In parallel with reduced bleeding episodes, pooled results of eight studies indicated a significant reduction in the need for rescue medications in the TPO-RA groups compared with control groups (RR: 0.5, 95%CI: 0.4-0.6).26 Treatment studies with both agents have also demonstrated an ability to reduce or stop concomitant medications (RR: 1.8, 95%CI: 1.1-3.0).
Health-related quality of life and thrombopoietin treatment
Health-related quality of life (HRQoL) was studied in many of the RCT and extension studies conducted with TPO-RA using different generic and disease-specific questionnaires. Unquestionably, ITP has a major negative impact on HRQoL.28,29
In general, short-term treatment with TPO-RA does not seem to affect HRQoL,19,22 while long-term studies with both agents show improvements in HRQoL.17,25 In the open-label RCT comparing romiplostim to SoC, clinically significant improvement in seven scales of the Immune Thrombocytopenic Purpura Patient Assessment Questionnaire (ITP-PAQ) was observed in both treatment arms at 52 weeks compared with baseline.17 However, the romiplostim group, and in particular the responders, had significantly greater improvements, although the magnitude of the effect was of uncertain clinical benefit.30 In the RAISE study, HRQoL was significantly improved in the eltrombopag arm only, in five of the eight SF-36 domains at week 26 compared to baseline.25 In the EXTEND trial, all the HRQoL instruments used had positive mean changes from baseline over time. The improvements from baseline persisted through five years of treatment.31 This study found positive and clinically-meaningful mean changes from baseline in all HRQoL scores.
Practical issues related to use of thrombopoietin receptor agonists
Indication and dosage
The current label for both TPO-RA in Europe and in the US is patients aged ≥1 year with chronic ITP who are refractory to at least one other treatment (e.g. corticosteroids, immunoglobulins).
Initial dosing with eltrombopag starts at 50 mg daily, unless the patient is East Asian in whom a lower dose should initially be used. If a response is not seen in two weeks, the dose is increased to 75 mg daily, the maximum dose licensed for ITP. In the RAISE study of 197 adults, approximately equal numbers of patients were on 50 and 75 mg daily after six months of treatment. With romiplostim, the package insert recommends 1 μg/kg/week and increasing by 1 μg/kg/week until a response is achieved; however, this approach would take nine weeks to achieve the maximum dose of 10 μg/kg/week. A more practical schema would be to start at 3 μg/kg/week, particularly if a rapid response is needed, or one full vial of 250 μg, and increasing weekly to 5, 7, and then 10 μg/kg/week until a response is achieved. Median dose of romiplostim in adults is 3-5 μg/kg/week.27,32 In Europe, approximately one-third of the patients self-administer romiplostim subcutaneously.32 In the US, self-administration is still not licensed; however, this appears likely to be allowed in the near future.
In the pediatric studies, many children needed the maximum dose of 10 μg/kg/week of romiplostim and 75 mg of eltrombopag, which corresponds to 3-6 mg/kg as compared to 0.5-1 mg/kg for adults.23,33–35
Choice of agent
The two TPO-RA have comparable overall efficacy. Eltrombopag is given orally while romiplostim is dosed as a weekly subcutaneous injection. However, eltrombopag must be given on an empty stomach; in particular, it should be taken four hours after and two hours before food containing cations, e.g. iron, calcium, milk or other dairy products. In the US, different criteria for medical insurance are used for the two agents, which may impact on the decision to adopt one treatment or the other depending on which is likely to be approved first. If patients have absorption problems or transaminitis, it may be prudent to use romiplostim. If patients do not have stable platelet counts, or if they do not want to come to the clinic every week for injections, then eltrombopag may be better.
Dealing with non-responders: switching or combination
Approximately one-third of the patients discontinue TPO-RA because of lack of response.36 If one TPO-RA does not work, switching to the other TPO-RA has been seen to be surprisingly effective. In a study of 46 patients who switched from one agent to another, 80% of the patients who failed to respond to eltrombopag eventually responded to romiplostim, and 46% of patients who did not respond to romiplostim responded to eltrombopag.37 These results were confirmed in a more recent retrospective study in which 106 patients underwent switching with 60% achieving response with either agent after switching.38 Switching can also be an effective policy in case of severe platelet-count fluctuations or side-effects.37–39 Finally, stopping one agent before starting the other is not essential, unless adverse effects are the indication to switch (W Ghanima et al., personal observation, 2019). The addition of a small dose of steroid (2.5-5 mg prednisolone) to a TPO-RA may have a good effect in some patients and can be tried in non-responding patients (W Ghanima et al., personal observation, 2019).
Treatment-free durable responses after discontinuation of thrombopoietin receptor agonists
Approximately 10-30% of patients taking a TPO-RA will be able to discontinue their TPO-RA and maintain response after discontinuation.40 In one study of 75 adults with ITP of <6 months duration treated with romiplostim for ≤12 months, 32% were able to discontinue the medication and to obtain treatment-free durable responses (platelet counts >50×109/L) lasting at least six months.41 Higher mean platelet count (138×109/L) for the first two months was associated with remission. However, lasting treatment-free response has also been reported in chronic patients. In a retrospective study, 10% of 260 patients treated with eltrombopag maintained acceptable platelet counts after discontinuation of the drug.42 In another small retrospective study of 54 patients who were treated with TPO-RA for at least five years, TPO-RA were discontinued in 20 out of 28 patients who achieved a complete response. Of these, eight patients showed a sustained response for a median of 13 months (range 5-27 months).40 However, it is still not known how TPO-RA induce long-lasting off-treatment responses, although it is unlikely that this is simply due to a selection of patients who would eventually remit.41 Durable responses have even been observed in patients with long-lasting disease. Potential mechanisms include: restored immune tolerance by increased exposure to platelet autoantigens, thereby reducing platelet antibodies through increased presence of MK and platelets,41,43 or through improvement of Treg function, which in turn could restore immune tolerance to platelets.13
Predicting who will achieve a durable response and how to discontinue TPO-RA is challenging. We recommend tapering in a patient who achieves and maintains a stable platelet count over 50-100×109/L for at least 3-6 months, particularly if using low doses of a TPO-RA and achieving a normal, stable platelet count for some months. One way to taper treatment would involve gradually decreasing and/or increasing the interval between doses until the platelet count remains <30×109/L or it is possible to discontinue treatment.
Safety and tolerability of thrombopoietin receptor agonists
Ten years after their availability, TPO-RA have been proven to be well-tolerated. The long follow up of the patients included in the pivotal studies and “real-life” reports generally give reassuring data. Many of the initial theoretical concerns, such as uncontrolled stem cell proliferations and myelofibrosis with TPO-RA, have not materialized. However, there are emerging reports of adverse events, such as an increased incidence of thrombosis, which remains unexplained.
In the EXTEND study, evaluating long-term safety and efficacy of eltrombopag in 302 adults ITP patients treated with eltrombopag for a mean duration of >2 years, important adverse events were rare and did not increase with treatment duration over one year.24 Fourteen percent of patients on eltrombopag stopped treatment because of adverse events. Although plasma levels and exposure have been shown to be much higher in Asian populations,44 tolerability of eltrombopag in the Chinese population appears similar to that observed in Caucasian populations. The good tolerance of eltrombopag observed in pivotal studies has been confirmed in the Spanish eltrombopag registry including 220 ITP adults.45
In a pooled analysis from 13 completed studies of romiplostim including 1111 patients, exposure-adjusted rates of adverse events were lower in the romiplostim group than in the placebo/SoC group.46 These data were confirmed in another registry study.36
Bone marrow reticulin deposition and TE events are associated with the TPO-RA drug class. However, the safety profiles of TPO-RA do not fully overlap and specific adverse events, i.e. cataract and transaminitis, are more frequently seen with eltrombopag. Others, such as development of neutralizing antibodies, are mainly observed with romiplostim, as is pain after administration. This absence of overlapping toxicity encourages switching when a TPO-RA is stopped because of an adverse event that is not due to class effect.
Bone marrow fibrosis
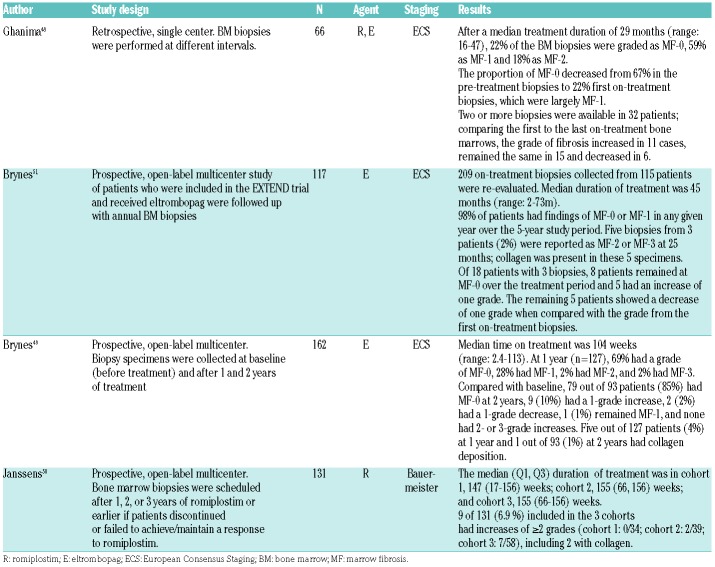
Early concerns were raised regarding the possible induction of bone marrow fibrosis because of sustained stimulation of megakaryopoiesis by TPO-RA, as seen in animal studies.47 Table 3 summarizes results of the published trials showing that, in most patients, grade of fibrosis did not change during treatment with TPO-RA, while a slight, non-progressive reticulin fibrosis (MF-1 or Baumeister <2) was observed in 10-50% of patients.48,49 In one study, a moderate increase in reticulin fibrosis (MF-2) was observed in 18% at median time of treatment of 2.5 years,48 whereas in three other studies, reticulin fibrosis progressed by >2 grades or developed ≥MF-2 during the study periods in less than 10%.49–51 Severe grades of reticulin fibrosis (MF-3 and/or collagen fibrosis) were extremely rare in all studies.48–51 In general, it does not seem that TPO-RA induce substantial fibrosis or changes in number or morphology of peripheral blood cells. Both reticulin and collagen fibrosis regressed in most patients after discontinuation of TPO-RA; in a few patients, fibrosis regressed despite continuing therapy.48,49
Table 3.
Summary of studies determining the grade of bone marrow fibrosis in patients treated with thrombopoietin receptor agonists.
There is no consensus for patients on TPO-RA as to whether or how to monitor bone marrow (BM) fibrosis. At the moment, hardly any centers perform routine BM biopsy in TPO-RA treated patients. However, if a biopsy is performed and severe reticulin (MF3) or collagen is discovered, then it is recommended that TPO-RA be discontinued. With moderately increased fibrosis, e.g. MF 2, a patient may continue TPO-RA but may need a repeat biopsy in six months. Older age and splenectomy could be associated with higher grades of BM fibrosis; fibrosis was not associated with type, dose or duration of treatment.20,27,48
Risk of clonal evolution and malignancy
The TPO-receptor is expressed in many hematopoietic cells, including early stem cells.52 Sustained stimulation of the hematopoietic cells raised concerns regarding potential clonal evolution associated with prolonged use of TPO-RA. Based on clinical trials, safety databases and ten years of clinical experience, there are no indications that TPO-RA induce neoplastic changes in ITP patients. A safety analysis of more than 1000 patients treated with romiplostim showed that rates of hematologic and non-hematologic malignancies were comparable between the romiplostim group and the placebo/SoC.53 In the EXTEND study, ten (3%) patients reported malignancies diagnosed during the 6-year study.24 In one ITP study, routine BM flow cytometry and cytogenetic studies were performed and no karyotypic or immunophenotypic changes indicative of monoclonality were detected.48
In myelodysplastic syndromes (MDS), the risk of leukemogenesis had been a matter of concern.54–56 In aplastic anemia, comparison of natural history between eltrombopag-treated patients and those receiving immunosuppression alone showed no difference in incidence of malignancy, although those treated with eltrombopag tended to develop malignancy sooner.57
Thromboembolism
In early trials with TPO-RA, sporadic thromboembolic events (TEE) gave impetus to extensive epidemiological studies exploring the association between thrombosis and ITP and the role of TPO-RA. The incidence of TEE in patients with chronic ITP not exposed to TPO-RA was compared with age- and sex-matched non-ITP control populations.58–62 The annualized incidence was 0.41-0.67 for venous thromboembolism (VTE) and 0.96-1.15 for arterial thrombosis (AT), whereas the control populations had 0.28-0.42 and 0.67-0.91, respectively, showing a slightly but statistically significantly higher risk of VTE and possibly AT in ITP patients.63
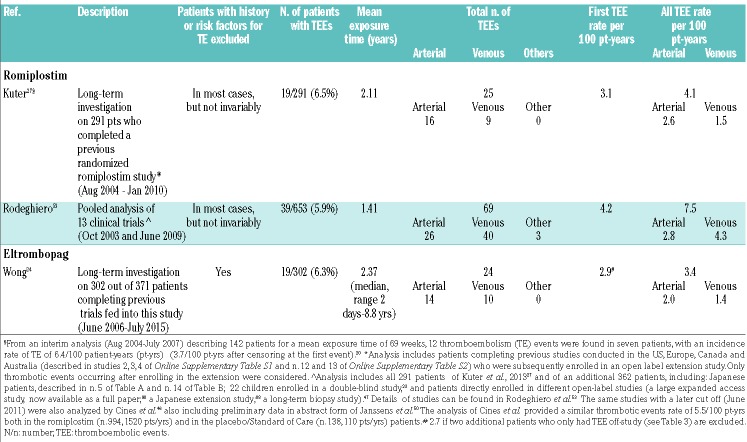
Thromboembolic events in the long-term studies and pooled analyses are summarized in Table 4. Online Supplementary Table S1 describes thromboembolic events in phase I-II and in randomized, placebo-controlled studies, while Online Supplementary Table S2 refers to single arm trials. As shown in Online Supplementary Table S1, the exposure time to TPO-RA was generally short and ranged from a few weeks to ≤6 months (or 1 year in a single study17). Overall, there have been 15 events out of 415 patients exposed to romiplostim (3.6%) versus 4 events in 202 controls (2%), and 5 events in the 391 patients exposed to eltrombopag (1.3%) versus none out of 155 controls. Conversely, more consistent and significant data could be derived from the large long-term studies or pooled analyses mainly based on the long-term extension studies, fed by patients who had completed previous trials and in a single-arm study investigating a large number of patients. These studies included greater numbers of patients and report on longer treatment exposure.24,27,50,53 Notably, patients with history of or important risk factors for thrombosis were excluded upfront from the RCT, and patients experiencing TEE during their previous study were excluded from long-term extension studies, resulting in a generally smaller thrombosis risk population in the long-term studies. Despite that, a relatively large number of TEE occurred in the long term-studies.
Table 4.
Incidence of thromboembolism with romiplostim and eltrombopag in long-term studies or in pooled analyses in adults.
The incidence per 100 patient-years (censoring after first TEE) ranged from 3.1 to 4.2 with romiplostim and was 2.9 in the single eltrombopag study. Without censoring after first event, the incidence ranged from 4.1 to 7.5 with romiplostim and 3.4 with eltrombopag.63 In a pooled analysis of romiplostim studies, an incidence rate per 100 patient-years of 5.5 was reported for both patients exposed to romiplostim or to placebo/SoC.46 Unfortunately, the low number and short exposure of placebo/SoC make the figures in non-exposed patients unreliable.
Thrombotic events have also been reported in pediatric trials. In a multicenter retrospective study on 79 children with ITP treated with eltrombopag, romiplostim or both, two cases of pulmonary embolism were reported.64 The randomized controlled trials did not identify any TEE, and overall TEE incidence is clearly lower than in adults.23,33–35,65
The TEE events were neither associated with thrombocytosis nor with a higher dose of TPO-RA. At least 30-50% of cases occurred in patients with lower than normal platelet counts. In general, TEE events tended to happen in the first year of treatment, creating a trend towards lower incidence figures with more prolonged exposure time. Among arterial events, cerebrovascular (stroke) and myocardial (infarction) were predominant and seen more in patients >70 years of age. However, <20% of TEE resulted in permanent disability and only three deaths could be attributed to thrombosis. In a pooled analysis, the annualized risk of thromboembolism in splenectomized patients (6.3) was not significantly higher than nonsplenectomized patients (4.3).66
The pathogenic mechanisms responsible for the increased thrombotic risk linked to TPO-RA have not yet been identified.67 The expected findings that TPO-RA lower the threshold of platelet activation have not been demonstrated.68,69 In general, ITP per se seems to be a pro-coagulant condition, as indicated by an increase in the various coagulation activation markers, including D-dimer, prothrombin fragment F1+2 and thrombin generation, and in the antifibrinolytic marker plasminogen activator inhibitor-1 (PAI-1) compared to controls.70,71 No further increase in the coagulation activation markers has been observed after the initiation of TPO-RA.70,71 However, a recent study reported increased PAI-1 levels in patients treated with TPO-RA, possibly leading to the formation of a more fibrinolysis-resistant clot; the study also showed increased microparticle-associated phosphatidylserine procoagulant activity.72 Moreover, levels of soluble P-selectin and basal exposure of P-selectin in quiescent platelets were significantly increased in TPO-RA treated patients compared to pretreatment levels or to untreated patients; however, the significance of these findings is still not known.70,72
In summary, although they have not been substantiated in properly designed trials, the annualized thrombosis rates in adults appear to be 2-3 times higher (annualized incidence rate of TEE of 4-7%) with TPO-RA treatment than in an ITP population not treated with TPO-RA, and even higher if compared to non-ITP control populations.63 On the other hand, most available data on the risk of thrombosis are based on retrospective and registry studies, which probably underestimate the risk of thrombosis in the ITP population. The patient’s individual risk profile should be considered when initiating treatment with a TPO-RA to evaluate if the expected reduction in bleeding risk outweighs the risk of thrombotisis. Comorbidities more prevalent in ITP should be considered and/or investigated; these include previous thromboembolism, splenectomy, presence of antiphospholipid antibodies, and concomitant medications like estroprogestinic preparations. Efforts should be made to correct modifiable risk factors, and thrombo-prophylaxis is recommended for surgery, provided the patient has a safe platelet count.67 Furthermore, antiplatelet agents or even anticoagulation could be considered in patients at high risk of thrombosis once platelet counts reach >50×109/L after initiation of TPO-RA.
Rebound thrombocytopenia
In most patients receiving TPO-RA, platelet counts return to pre-therapy baseline values on discontinuation of therapy; however, in up to 10% of patients, platelet counts temporarily drop below pretreatment levels after discontinuation of TPO-RA.18 Endogenous TPO activity, which is regulated by platelet mass, may be suppressed while platelet and MK levels are elevated on TPO-RA and may not rapidly re-equilibrate when TPO-RA are abruptly discontinued. However, the REPEAT study, which involved intermittent administration of eltrombopag, provided reassuring data.73 Nonetheless, when TPO-RA treatment is discontinued, tapering is preferred to immediate withdrawal.
Fluctuating platelet counts
Substantial fluctuations in platelet count on stable treatment doses may occur and can be difficult to manage. They are more common with romiplostim than eltrombopag, possibly due to the longer dosing intervals and inconsistent delivery with subcutaneous administration.36–38 Some patients experiencing such platelet fluctuations with romiplostim can be stabilized by switching to eltrombopag.74
Adverse events mainly associated with eltrombopag
Cataract
Treatment-related cataracts were observed in juvenile rodents on eltrombopag and were dose and time dependent. Cataracts have been reported with both eltrombopag and romiplostim. Given multiple confounding risk factors, e.g. steroid use, older age, smoking, no clinical study has unequivocally demonstrated this suspected risk with TPO-RA. In a 6-month study, the incidence of cataract in patients treated with eltrombopag was similar to placebo.25 In the open-label EXTEND study, cataracts developed in 28 patients (9%) in up to eight years of treatment. In 16 (5%), it was considered a severe adverse event, which led to withdrawal of eltrombopag in four (1.3%) patients.24 In the Pediatric Patients with Thrombocytopenia from Idiopathic Thrombocytopenic Purpura (PETIT2) study, two children developed cataracts, raising serious concern.33 The analysis of up to 1000 patients treated with romiplostim for ITP reported 37 events of cataracts, but only one case in patients with placebo or SoC, suggesting cataracts, given the big difference in exposure, might also be associated with romiplostim.46
An alternative option to routine ophthalmological evaluation for all patients on eltrombopag is to reserve ophthalmic examination for patients with one or more risk factors.
Transaminitis
Hepatocyte degeneration, associated with increased serum liver enzymes, was observed in animals at doses that were associated with morbidity and mortality. In humans, development of transaminitis occurs in up to 10%, especially on eltrombopag.22,25 Bilirubin elevations are also possible but involve mainly non-conjugated bilirubin (not indicative of serious liver injury). Transaminitis is mostly asymptomatic and reversible with dose interruption, reduction or discontinuation; only 3% of children and adults were unable to tolerate eltrombopag in large studies.24,25 Transaminitis occurs more in the first year of treatment, which justifies regular monitoring of liver enzymes at more frequent intervals particularly during the first years.
Adverse events mainly associated with romiplostim
Risk of antibody development
Romiplostim is a chimeric fusion protein produced by genetic engineering. Neutralizing antibodies directed against romiplostim have been reported. In contrast, because small molecules do not typically elicit an immune response, development of neutralizing antibodies is not considered to be a risk for eltrombopag. In an analysis of up to 1000 patients treated with romiplostim, neutralizing antibodies to romiplostim were reported in only six patients; importantly, none cross-reacted with endogenous TPO. All had a platelet response, and detection of neutralizing antibodies did not automatically result in loss of response.66 However, neutralizing antibodies were detected in a group of four patients treated with romiplostim who lost response.75 Current estimates, based on very limited data, suggest that these occur at a rate of up to 1%, more frequently in children than adults. Monitoring could be yearly and when response is lost.
Other indications for thrombopoietin receptor agonists
Treatment of severe aplastic anemia (SAA) with eltrombopag yielded multilineage clinical responses in certain patients with refractory severe aplastic anemia.11 Consequently, eltrombopag has been approved for use in patients with SAA failing immunosuppression who are not eligible for transplantation. A recent study has shown benefit when eltrombopag was used upfront together with immunosuppression, with more than one-third of the patients achieving a complete response by six months.57
Thrombocytopenia is a common complication of liver disease, and eltrombopag was licensed to support the platelet count in patients with hepatitis C undergoing treatment with interferon and ribavirin. However, improvements in current hepatitis C therapy have meant that interferon is no longer used.76
Studies into the use of TPO-RA in MDS are no longer actively pursued, perhaps not so much because of the risk of induction of leukemia, as because of failure to provide evidence of survival benefit.54,55
The major indication under study is in solid tumor chemotherapy. Studies are now available that demonstrate promising results. Schedule and dosing in solid tumor chemotherapy can be better maintained, but translating this into a survival advantage still has to be clearly demonstrated.77
Treatment of inherited thrombocytopenias with eltrombopag has been studied in MYH9-related disorders and Wiskott-Aldrich syndrome, showing platelet response in both conditions.78–80
Other thrombopoietic agents
Two current studies with avatrombopag and lusutrombopag, both of which were recently approved for procedures in thrombocytopenic patients with liver disease in the US, were careful to verify adequate pre-procedure portal flow and use only several days of TPO-RA prior to and during the procedure to avoid risks of thrombosis, especially that of the portal vein. Avatrombopag, an oral small molecule, apparently binds to the TPO-R similarly to eltrombopag, but does not have any dietary limitations.81 It was shown to be effective in a phase II study of ITP,81 and also in a recently published phase III trial, which confirmed the superiority of avatrombopag (5-40 mg daily) over placebo with regard to acute and durable platelet response in patients with chronic ITP. Headache was the most frequent side effect.82 In view of the two RCT in ITP and the approved indication in liver disease, we expect that avatrombopag will be licensed for ITP.
A recombinant human thrombopoietin (rhTPO) has been licensed in China for many years in adults and children with ITP. Studies have also recently been performed in pregnant women, all with good results.83,84 One concern is the uncertainty regarding development of antibodies to the TPO agent which, unlike those seen with romiplostim, might cross-react with endogenous TPO and create lasting and substantial thrombocytopenia in affected recipients. Importantly, neither romiplostim nor eltrombopag are recommended to be used in pregnancy; however, there are limited case reports in which the use of these agents in pregnant women with difficult ITP appeared to be safe.
Future treatments for immune thrombocytopenia and the role of thrombopoietin receptor agonists
The two TPO-RA licensed for use in ITP are now both licensed for use after one year from diagnosis after failure of corticosteroids, further consolidating their position as the mainstay for second-line therapy in ITP. However, many other agents are currently under development, at various stages of clinical testing, or are being considered for registration. Among these, fostamatinib, an inhibitor of spleen tyrosine kinase (syk) has an overall response rate of almost 50% with continued treatment, and an 18% rate of stable responses in heavily pretreated ITP patients.85 This agent was licensed in the US in 2018 for treatment of chronic ITP in adults. Several blockers of FcRn have entered trials in adults with persistent and chronic ITP; at least two (rozanolixizumab and ARGX-117) have completed phase II studies.86 The mechanism is a dramatic increase in IgG turnover as a result of inhibition of IgG recycling; not only “normal” but also IgG autoantibody levels decrease markedly.87 Preliminary results are encouraging but efficacy and toxicity need to be better defined in phase III studies. It remains to be seen how these agents will influence the future role of TPO-RA.
Conclusion
Romiplostim and eltrombopag are well tolerated and effective therapies for ITP with acceptable toxicity. Both agents increase the platelet count in up to three-quarters of patients. Ten years after their introduction, available evidence from short- and long-term and registry-based studies confirm the general safety of chronic long-term use of these medications, as well as persistent efficacy in most patients.
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/104/6/1112
References
- 1.Rodeghiero F. A critical appraisal of the evidence for the role of splenectomy in adults and children with ITP. Br J Haematol. 2018;181(2):183–195. [DOI] [PubMed] [Google Scholar]
- 2.Patel VL, Mahevas M, Lee SY, et al. Outcomes 5 years after response to rituximab therapy in children and adults with immune thrombocytopenia. Blood. 2012;119(25):5989–5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghanima W, Godeau B, Cines DB, Bussel JB. How I treat immune thrombocytopenia: the choice between splenectomy or a medical therapy as a second-line treatment. Blood. 2012;120(5):960–969. [DOI] [PubMed] [Google Scholar]
- 4.Erickson-Miller CL, Delorme E, Tian SS, et al. Preclinical activity of eltrombopag (SB-497115), an oral, nonpeptide thrombopoietin receptor agonist. Stem Cells. 2009;27(2):424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broudy VC, Lin NL. AMG531 stimulates megakaryopoiesis in vitro by binding to Mpl. Cytokine. 2004;25(2):52–60. [DOI] [PubMed] [Google Scholar]
- 6.Will B, Kawahara M, Luciano JP, et al. Effect of the nonpeptide thrombopoietin receptor agonist Eltrombopag on bone marrow cells from patients with acute myeloid leukemia and myelodysplastic syndrome. Blood. 2009;114(18):3899–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun H, Tsai Y, Nowak I, Liesveld J, Chen Y. Eltrombopag, a thrombopoietin receptor agonist, enhances human umbilical cord blood hematopoietic stem/primitive progenitor cell expansion and promotes multi-lineage hematopoiesis. Stem Cell Res. 2012;9(2):77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Buduo CA, Currao M, Pecci A, Kaplan DL, Balduini CL, Balduini A. Revealing eltrombopag’s promotion of human megakaryopoiesis through AKT/ERK-dependent pathway activation. Haematologica. 2016;101(12):1479–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vlachodimitropoulou E, Chen YL, Garbowski M, et al. Eltrombopag: a powerful chelator of cellular or extracellular iron(III) alone or combined with a second chelator. Blood. 2017;130(17):1923–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roth M, Will B, Simkin G, et al. Eltrombopag inhibits the proliferation of leukemia cells via reduction of intracellular iron and induction of differentiation. Blood. 2012;120(2):386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olnes MJ, Scheinberg P, Calvo KR, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367(1):11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Arena G, Guariglia R, Mansueto G, et al. No cross-resistance after sequential use of romiplostim and eltrombopag in chronic immune thrombocytopenic purpura. Blood. 2013;121(7):1240–1242. [DOI] [PubMed] [Google Scholar]
- 13.Bao W, Bussel JB, Heck S, et al. Improved regulatory T-cell activity in patients with chronic immune thrombocytopenia treated with thrombopoietic agents. Blood. 2010;116(22):4639–4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wan YY, Flavell RA. ‘Yin-Yang’ functions of transforming growth factor-beta and T regulatory cells in immune regulation. Immunol Rev. 2007;220:199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schifferli A, Kuhne T. Thrombopoietin receptor agonists: a new immune modulatory strategy in immune thrombocytopenia? Semin Hematol. 2016;53 Suppl 1:S31–4. [DOI] [PubMed] [Google Scholar]
- 16.Zufferey A, Kapur R, Semple JW. Pathogenesis and Therapeutic Mechanisms in Immune Thrombocytopenia (ITP). J Clin Med. 2017;6(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuter DJ, Rummel M, Boccia R, et al. Romiplostim or standard of care in patients with immune thrombocytopenia. N Engl J Med. 2010;363(20):1889–1899. [DOI] [PubMed] [Google Scholar]
- 18.Kuter DJ, Bussel JB, Lyons RM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008;371(9610):395–403. [DOI] [PubMed] [Google Scholar]
- 19.Bussel JB, Provan D, Shamsi T, et al. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373(9664):641–648. [DOI] [PubMed] [Google Scholar]
- 20.Bussel JB, Kuter DJ, Pullarkat V, Lyons RM, Guo M, Nichol JL. Safety and efficacy of long-term treatment with romiplostim in thrombocytopenic patients with chronic ITP. Blood. 2009;113(10):2161–2171. [DOI] [PubMed] [Google Scholar]
- 21.Bussel JB, Kuter DJ, George JN, et al. AMG 531, a thrombopoiesis-stimulating protein, for chronic ITP. N Engl J Med. 2006;355(16):1672–1681. [DOI] [PubMed] [Google Scholar]
- 22.Bussel JB, Cheng G, Saleh MN, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med. 2007;357(22):2237–2247. [DOI] [PubMed] [Google Scholar]
- 23.Bussel JB, Buchanan GR, Nugent DJ, et al. A randomized, double-blind study of romiplostim to determine its safety and efficacy in children with immune thrombocytopenia. Blood. 2011;118(1):28–36. [DOI] [PubMed] [Google Scholar]
- 24.Wong RSM, Saleh MN, Khelif A, et al. Safety and efficacy of long-term treatment of chronic/persistent ITP with eltrombopag: final results of the EXTEND study. Blood. 2017;130(23):2527–2536. [DOI] [PubMed] [Google Scholar]
- 25.Cheng G, Saleh MN, Marcher C, et al. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. Lancet. 2011;377:393–402. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Gao Z, Chen XP, et al. Efficacy and safety of thrombopoietin receptor agonists in patients with primary immune thrombocytopenia: A systematic review and meta-analysis. Sci Rep. 2016;6:39003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuter DJ, Bussel JB, Newland A, et al. Long-term treatment with romiplostim in patients with chronic immune thrombocytopenia: safety and efficacy. Br J Haematol. 2013;161(3):411–423. [DOI] [PubMed] [Google Scholar]
- 28.McMillan R, Bussel JB, George JN, Lalla D, Nichol JL. Self-reported health-related quality of life in adults with chronic immune thrombocytopenic purpura. Am J Hematol. 2008;83(2):150–154. [DOI] [PubMed] [Google Scholar]
- 29.Mathias SD, Gao SK, Miller KL, et al. Impact of chronic Immune Thrombocytopenic Purpura (ITP) on health-related quality of life: a conceptual model starting with the patient perspective. Health Qual Life Outcomes. 2008;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuter DJ, Mathias SD, Rummel M, et al. Health-related quality of life in nonsplenectomized immune thrombocytopenia patients receiving romiplostim or medical standard of care. Am J Hematol. 2012;87(5): 558–561. [DOI] [PubMed] [Google Scholar]
- 31.Saleh MN, Bussel JB, Khelif A, et al. Improvements in Patient Health-Related Quality of Life (HRQoL) with Clinical Efficacy in Patients Treated with Eltrombopag: Final Results from the Long-Term, Open-Label Extend Study. Blood. 2016;128(22):3742. [Google Scholar]
- 32.Steurer M, Quittet P, Papadaki HA, et al. A large observational study of patients with primary immune thrombocytopenia receiving romiplostim in European clinical practice. Eur J Haematol. 2017;98(2):112–120. [DOI] [PubMed] [Google Scholar]
- 33.Grainger JD, Locatelli F, Chotsam -pancharoen T, et al. Eltrombopag for children with chronic immune thrombocytopenia (PETIT2): a randomised, multicentre, placebo-controlled trial. Lancet. 2015;386(10004):1649–1658. [DOI] [PubMed] [Google Scholar]
- 34.Bussel JB, de Miguel PG, Despotovic JM, et al. Eltrombopag for the treatment of children with persistent and chronic immune thrombocytopenia (PETIT): a randomised, multicentre, placebo-controlled study. Lancet Haematol. 2015;2(8):e315–325. [DOI] [PubMed] [Google Scholar]
- 35.Tarantino MD, Bussel JB, Blanchette VS, et al. Romiplostim in children with immune thrombocytopenia: a phase 3, randomised, double-blind, placebo-controlled study. Lancet. 2016;388(10039):45–54. [DOI] [PubMed] [Google Scholar]
- 36.Khellaf M, Michel M, Quittet P, et al. Romiplostim safety and efficacy for immune thrombocytopenia in clinical practice: 2-year results of 72 adults in a romiplostim compassionate-use program. Blood. 2011;118(16):4338–4345. [DOI] [PubMed] [Google Scholar]
- 37.Khellaf M, Viallard JF, Hamidou M, et al. A retrospective pilot evaluation of switching thrombopoietic receptor-agonists in immune thrombocytopenia. Haematologica. 2013;98(6):881–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cantoni S, Carpenedo M, Mazzucconi MG, et al. Alternate use of thrombopoietin receptor agonists in adult primary immune thrombocytopenia patients: A retrospective collaborative survey from Italian hematology centers. Am J Hematol. 2018;93(1):58–64. [DOI] [PubMed] [Google Scholar]
- 39.Kuter DJ, Macahilig C, Grotzinger KM, et al. Treatment patterns and clinical outcomes in patients with chronic immune thrombocytopenia (ITP) switched to eltrombopag or romiplostim. Int J Hematol. 2015;101(3): 255–263. [DOI] [PubMed] [Google Scholar]
- 40.Mahevas M, Fain O, Ebbo M, et al. The temporary use of thrombopoietin-receptor agonists may induce a prolonged remission in adult chronic immune thrombocytopenia. Results of a French observational study. Br J Haematol. 2014;165(6):865–869. [DOI] [PubMed] [Google Scholar]
- 41.Newland A, Godeau B, Priego V, et al. Remission and platelet responses with romiplostim in primary immune thrombocytopenia: final results from a phase 2 study. Br J Haematol. 2016;172(2):262–273. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez-Lopez TJ, Pascual C, Alvarez-Roman MT, et al. Successful discontinuation of eltrombopag after complete remission in patients with primary immune thrombocytopenia. Am J Hematol. 2015;90(3):E40–43. [DOI] [PubMed] [Google Scholar]
- 43.Ghadaki B, Nazi I, Kelton JG, Arnold DM. Sustained remissions of immune thrombocytopenia associated with the use of thrombopoietin receptor agonists. Transfusion. 2013;53(11):2807–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gibiansky E, Zhang J, Williams D, Wang Z, Ouellet D. Population pharmacokinetics of eltrombopag in healthy subjects and patients with chronic idiopathic thrombocytopenic purpura. J Clin Pharmacol. 2011;51(6):842–856. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez-Lopez TJ, Alvarez-Roman MT, Pascual C, et al. Use of eltrombopag for secondary immune thrombocytopenia in clinical practice. Br J Haematol. 2017;178(6):959–970. [DOI] [PubMed] [Google Scholar]
- 46.Cines DB, Gernsheimer T, Wasser J, et al. Integrated analysis of long-term safety in patients with chronic immune thrombocytopaenia (ITP) treated with the thrombopoietin (TPO) receptor agonist romiplostim. Int J Hematol. 2015;102(3):259–270. [DOI] [PubMed] [Google Scholar]
- 47.Kuter DJ, Mufti GJ, Bain BJ, Hasserjian RP, Davis W, Rutstein M. Evaluation of bone marrow reticulin formation in chronic immune thrombocytopenia patients treated with romiplostim. Blood. 2009;114(18): 3748–3756. [DOI] [PubMed] [Google Scholar]
- 48.Ghanima W, Geyer JT, Lee CS, et al. Bone marrow fibrosis in 66 Immune Thrombocytopenia patients treated with thrombopoietin receptor agonists: a single center long-term follow-up. Haematologica. 2014;99(5):937–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brynes RK, Wong RS, Thein MM, et al. A 2-Year, Longitudinal, Prospective Study of the Effects of Eltrombopag on Bone Marrow in Patients with Chronic Immune Thrombocytopenia. Acta Haematol. 2017;137(2):66–72. [DOI] [PubMed] [Google Scholar]
- 50.Janssens A, Rodeghiero F, Anderson D, et al. Changes in bone marrow morphology in adults receiving romiplostim for the treatment of thrombocytopenia associated with primary immune thrombocytopenia. Ann Hematol. 2016;95(7):1077–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brynes RK, Orazi A, Theodore D, et al. Evaluation of bone marrow reticulin in patients with chronic immune thrombocytopenia treated with eltrombopag: Data from the EXTEND study. Am J Hematol. 2015;90(7):598–601. [DOI] [PubMed] [Google Scholar]
- 52.Imbach P, Crowther M. Thrombopoietin-receptor agonists for primary immune thrombocytopenia. N Engl J Med. 2011;365(8):734–741. [DOI] [PubMed] [Google Scholar]
- 53.Rodeghiero F, Stasi R, Giagounidis A, et al. Long-term safety and tolerability of romiplostim in patients with primary immune thrombocytopenia: a pooled analysis of 13 clinical trials. Eur J Haematol. 2013;91(5): 423–436. [DOI] [PubMed] [Google Scholar]
- 54.Mittelman M, Platzbecker U, Afanasyev B, et al. Eltrombopag for advanced myelodysplastic syndromes or acute myeloid leukaemia and severe thrombocytopenia (ASPIRE): a randomised, placebo-controlled, phase 2 trial. Lancet Haematol. 2018;5(1):e34–e43. [DOI] [PubMed] [Google Scholar]
- 55.Fenaux P, Muus P, Kantarjian H, et al. Romiplostim monotherapy in thrombocytopenic patients with myelodysplastic syndromes: long-term safety and efficacy. Br J Haematol. 2017;178(6):906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Platzbecker U, Wong RS, Verma A, et al. Safety and tolerability of eltrombopag versus placebo for treatment of thrombocytopenia in patients with advanced myelodysplastic syndromes or acute myeloid leukaemia: a multicentre, randomised, placebo-controlled, double-blind, phase 1/2 trial. Lancet Haematol. 2015;2(10):e417–426. [DOI] [PubMed] [Google Scholar]
- 57.Townsley DM, Scheinberg P, Winkler T, et al. Eltrombopag Added to Standard Immunosuppression for Aplastic Anemia. N Engl J Med. 2017;376(16):1540–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Severinsen MT, Engebjerg MC, Farkas DK, et al. Risk of venous thromboembolism in patients with primary chronic immune thrombocytopenia: a Danish population-based cohort study. Br J Haematol. 2011;152(3):360–362. [DOI] [PubMed] [Google Scholar]
- 59.Sarpatwari A, Bennett D, Logie JW, et al. Thromboembolic events among adult patients with primary immune thrombocytopenia in the United Kingdom General Practice Research Database. Haematologica. 2010;95(7):1167–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Norgaard M, Severinsen MT, Lund Maegbaek M, Jensen AO, Cha S, Sorensen HT. Risk of arterial thrombosis in patients with primary chronic immune thrombocytopenia: a Danish population-based cohort study. Br J Haematol. 2012;159(1):109–111. [DOI] [PubMed] [Google Scholar]
- 61.Norgaard M, Cetin K, Maegbaek ML, et al. Risk of arterial thrombotic and venous thromboembolic events in patients with primary chronic immune thrombocytopenia: a Scandinavian population-based cohort study. Br J Haematol. 2016;174(4):639–642. [DOI] [PubMed] [Google Scholar]
- 62.Enger C, Bennett D, Forssen U, Fogarty PF, McAfee AT. Comorbidities in patients with persistent or chronic immune thrombocytopenia. Int J Hematol. 2010;92(2):289–295. [DOI] [PubMed] [Google Scholar]
- 63.Rodeghiero F. Is ITP a thrombophilic disorder? Am J Hematol. 2016;91(1):39–45. [DOI] [PubMed] [Google Scholar]
- 64.Ramaswamy K, Hsieh L, Leven E, Thompson MV, Nugent D, Bussel JB. Thrombopoietic agents for the treatment of persistent and chronic immune thrombocytopenia in children. J Pediatr. 2014;165(3): 600–6055.e4. [DOI] [PubMed] [Google Scholar]
- 65.Elalfy MS, Abdelmaksoud AA, Eltonbary KY. Romiplostim in children with chronic refractory ITP: randomized placebo controlled study. Ann Hematol. 2011;90(11): 1341–1344. [DOI] [PubMed] [Google Scholar]
- 66.Cines DB, Wasser J, Rodeghiero F, et al. Safety and efficacy of romiplostim in splenectomized and nonsplenectomized patients with primary immune thrombocytopenia. Haematologica. 2017;102(8):1342–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rodeghiero F. ITP and thrombosis: an intriguing association. Blood Adv. 2017;1(24):2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Psaila B, Bussel JB, Linden MD, et al. In vivo effects of eltrombopag on platelet function in immune thrombocytopenia: no evidence of platelet activation. Blood. 2012;119(17): 4066–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haselboeck J, Kaider A, Pabinger I, Panzer S. Function of eltrombopag-induced platelets compared to platelets from control patients with immune thrombocytopenia. Thromb Haemost. 2013;109(4):676–683. [DOI] [PubMed] [Google Scholar]
- 70.Garabet L, Ghanima W, Monceyron Jonassen C, et al. Effect of thrombopoietin receptor agonists on markers of coagulation and P-selectin in patients with immune thrombocytopenia. Platelets. 2017:1–7. [DOI] [PubMed] [Google Scholar]
- 71.Alvarez Roman MT, Fernandez Bello I, Arias-Salgado EG, et al. Effects of thrombopoietin receptor agonists on procoagulant state in patients with immune thrombocytopenia. Thromb Haemost. 2014;112(1):65–72. [DOI] [PubMed] [Google Scholar]
- 72.Justo Sanz R, Monzon Manzano E, Fernandez Bello I, et al. Platelet Apoptosis and PAI-1 are Involved in the Pro-Coagulant State of Immune Thrombocytopaenia Patients Treated with Thrombopoietin Receptor Agonists. Thromb Haemost. 2019. February 11 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 73.Bussel JB, Saleh MN, Vasey SY, Mayer B, Arning M, Stone NL. Repeated short-term use of eltrombopag in patients with chronic immune thrombocytopenia (ITP). Br J Haematol. 2013;160(4):538–546. [DOI] [PubMed] [Google Scholar]
- 74.Gonzalez-Porras JR, Mingot-Castellano ME, Andrade MM, et al. Use of eltrombopag after romiplostim in primary immune thrombocytopenia. Br J Haematol. 2015;169(1):111–116. [DOI] [PubMed] [Google Scholar]
- 75.Carpenedo M, Cantoni S, Coccini V, Pogliani EM, Cairoli R. Response loss and development of neutralizing antibodies during long-term treatment with romiplostim in patients with immune thrombocytopenia: a case series. Eur J Haematol. 2016;97(1):101–103. [DOI] [PubMed] [Google Scholar]
- 76.Afdhal NH, Dusheiko GM, Giannini EG, et al. Eltrombopag increases platelet numbers in thrombocytopenic patients with HCV infection and cirrhosis, allowing for effective antiviral therapy. Gastroenterology. 2014;146(2):442–452.e1. [DOI] [PubMed] [Google Scholar]
- 77.Zhang X, Chuai Y, Nie W, Wang A, Dai G. Thrombopoietin receptor agonists for prevention and treatment of chemotherapy-induced thrombocytopenia in patients with solid tumours. Cochrane Database Syst Rev. 2017;11:CD012035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pecci A, Gresele P, Klersy C, et al. Eltrombopag for the treatment of the inherited thrombocytopenia deriving from MYH9 mutations. Blood. 2010;116(26): 5832–5837. [DOI] [PubMed] [Google Scholar]
- 79.Gerrits AJ, Leven EA, Frelinger AL, 3rd, et al. Effects of eltrombopag on platelet count and platelet activation in Wiskott-Aldrich syndrome/X-linked thrombocytopenia. Blood. 2015;126(11):1367–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rodeghiero F, Pecci A, Balduini CL. Thrombopoietin receptor agonists in hereditary thrombocytopenias. J Thromb Haemost. 2018;16(9):1700–1710. [DOI] [PubMed] [Google Scholar]
- 81.Bussel JB, Kuter DJ, Aledort LM, et al. A randomized trial of avatrombopag, an investigational thrombopoietin-receptor agonist, in persistent and chronic immune thrombocytopenia. Blood. 2014;123(25):3887–3894. [DOI] [PubMed] [Google Scholar]
- 82.Jurczak W, Chojnowski K, Mayer J, et al. Phase 3 randomised study of avatrombopag, a novel thrombopoietin receptor agonist for the treatment of chronic immune thrombocytopenia. Br J Haematol. 2018;183(3):479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kong Z, Qin P, Xiao S, et al. A novel recombinant human thrombopoietin therapy for the management of immune thrombocytopenia in pregnancy. Blood. 2017;130(9): 1097–1103. [DOI] [PubMed] [Google Scholar]
- 84.Huang Y, Liu X, Xue F, et al. [Efficacy and safety of rhTPO in the treatment of pediatric primary immune thrombocytopenia]. Zhonghua Xue Ye Xue Za Zhi. 2015;36(6): 511–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bussel J, Arnold DM, Grossbard E, et al. Fostamatinib for the treatment of adult persistent and chronic immune thrombocytopenia: Results of two phase 3, randomized, placebo-controlled trials. Am J Hematol. 2018;93(7):921–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Robak T, Jarque I, Musteata V, et al. Phase II, Multiple-Dose Study of Anti-FcRn Antibody, Rozanolixizumab (UCB7665), in Patients with Primary Immune Thrombocytopenia: Interim Analysis. Blood. 2017;130(Suppl 1):15. [Google Scholar]
- 87.Kiessling P, Lledo-Garcia R, Watanabe S, et al. The FcRn inhibitor rozanolixizumab reduces human serum IgG concentration: A randomized phase 1 study. Sci Transl Med. 2017;9(414). [DOI] [PubMed] [Google Scholar]
- 88.Shirasugi Y, Ando K, Miyazaki K, et al. Romiplostim for the treatment of chronic immune thrombocytopenia in adult Japanese patients: a double-blind, randomized Phase III clinical trial. Int J Hematol. 2011;94(1):71–80. [DOI] [PubMed] [Google Scholar]
- 89.Tomiyama Y, Miyakawa Y, Okamoto S, et al. A lower starting dose of eltrombopag is efficacious in Japanese patients with previously treated chronic immune thrombocytopenia. J Thromb Haemost. 2012;10(5):799–806. [DOI] [PubMed] [Google Scholar]
- 90.Janssens A, Tarantino M, Bird RJ, et al. Romiplostim Treatment in Adults with Immune Thrombocytopenia of Varying Duration and Severity. Acta Haematol. 2015;134(4):215–228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.