Abstract
Prolonged exposure to socioeconomic hardship (SH) is associated with greater delayed reward discounting (DRD), a form of impulsive decision-making that reflects a reduced capacity to delay gratification and a significant correlate of diverse risk behaviors, but the neurobehavioral mechanisms linking SH and DRD are unknown. An emerging hypothesis suggests that cognitive and affective stress associated with poverty may tax neurocognitive functions, such as working memory (WM), and lead to impulsive DRD. Furthermore, research suggests that emotional reactivity (ER) is an important dispositional factor to consider in the link between executive functions and DRD. Thus, we longitudinally examined the indirect effect of SH on impulsive DRD via a network of brain regions associated with WM function in a sample of young adults, and whether that link was moderated by ER. Participants were 119 rural African Americans (aged 19–24 years) assessed behaviorally on four occasions, with fMRI at the last time point. Results showed that, among emerging adults with higher ER, SH severity was predictive of increased DRD via reduced response in brain regions activated during an n-back WM task. These findings reveal both the cognitive and affective mechanisms that underlie the relationship between SH and DRD.
Keywords: Socioeconomic hardship, Decision-making, Delayed reward discounting, Working memory, Emotional reactivity, Poverty, Early life stress
1. Introduction
Cortical brain maturation processes that are linked to cognitive control functions continue through the mid-20 s (Luna et al., 2004). These executive functions (EF), which include working memory (WM), complex attention, inhibitory control, and cognitive flexibility, represent a domain of higher-order cognitive processes that serve as a foundation for decision-making (Alloway and Alloway, 2010; Lezak et al., 2012). Accordingly, emerging adults demonstrate improved decision-making abilities, such as a decline in impulsivity, compared to adolescents (Mills et al., 2014). Despite expected improvements in EF and subsequent decision-making in emerging adulthood, individuals who experienced chronic life stress, including poverty, show reduced performance across these measures of cognitive control (Eamon, 2002; Holz et al., 2015; Johnson et al., 2016). Further, humans and animal subjects who have experienced stress related to significant resource scarcity (poverty in humans) make maladaptive choices that are correlated with reduced executive functions (Kishiyama et al., 2009; Sale et al., 2009). These findings provided support for the allostatic load theory (Juster et al., 2010), which suggests that chronic stress induces neurobiological risk and cognitive process that are particularly associated with WM (G. W. Evans and Schamberg, 2009; Kim et al., 2013). Specifically, the allostatic load theory suggests that exposure to prolonged chronic stress associated with poverty can result in neurocognitive vulnerabilities that are driven by physiological stress response systems (Juster et al., 2010). In turn, these vulnerabilities are linked to maladaptive decision-making (Lovallo, 2013). Hence, converging evidence suggests that socioeconomic hardship (SH) conditions are associated with compromised decision making and increased health risk behaviors among emerging adults and adults (Bickel et al., 2014; Braveman et al., 2010).
1.1. Socioeconomic hardship and impulsive delayed reward discounting
Some emerging adults who were raised under prolonged conditions of socioeconomic hardship are vulnerable to stress associated with poverty, including a lack of financial and food security and unstable housing (Farah et al., 2006). Early life stress associated with SH is correlated with neurobiological and cognitive vulnerabilities that are also associated with more impulsive decision-making (Hair et al., 2015; Luby et al., 2013). Youths’ ability to make less impulsive decisions and be more future-oriented is an important asset for their successful transition into responsible adult roles (Oshri et al., 2018a,b; Romer et al., 2010). More recent research evidence shows a link between stress and delay-reward discounting (DRD; Malesza, 2019), a person’s orientation toward smaller immediate rewards rather than larger delayed rewards (Shamosh et al., 2008; Wesley and Bickel, 2014). Emerging research and theory suggest that individuals exposed to SH may be more cognitively and emotionally taxed than those in less SH (Deck and Jahedi, 2015; Mani et al., 2013). Moreover, within scarce and less stable environments, impoverished individuals may resort to more immediate and available, as opposed to delayed, resources, even if waiting is expected to yield greater rewards (Frankenhuis et al., 2016; Lipina and Posner, 2012b). Although the associations between early life stress secondary to SH and impulsive decision-making are well established by behavioral research (Lovallo et al., 2018, 2013; Oshri et al., 2017), there have been no multi-method investigations (i.e., survey, behavioral, and neuroimaging methods) to specify the etiological mechanisms that link SH and DRD.
1.2. Influences of working memory and affective reactivity in the link between socioeconomic hardship and impulsive discounting
A related body of research suggests that early life stress associated with growing up and living in poverty is inversely related to executive functions, such as WM, in youth and emerging adults (G. W. Evans and Schamberg, 2009), independent of genetic ancestry (Noble et al., 2015). SH is linked to brain function and morphology in regions, such as the prefrontal cortex, that are active during WM tasks- supporting the hypothesis that exposure to chronic SH may result in disruption in working memory (Ganzel et al., 2010; Juster et al., 2011; McEwen and Gianaros, 2011; Seeman et al., 2010). When these limited WM resources are overextended, the dynamic process of decision-making is negatively affected, which may result in difficulty weighing one’s choices and considering consequences, thus leading to a tendency towards impulsive behaviors (Finn, 2002). Some studies have suggested that coping with the demands of poverty may change the allocation of some individuals’ cognitive resources in ways that can particularly undermine WM (Evans and Schamberg, 2009; Farah et al., 2006; Lipina et al., 2005). Evidence suggests that SH exerts a negative influence on general cognitive and WM functions, as evident in both alterations in behavioral performance and neural network response to WM challenges (Farah et al., 2006; Leonard et al., 2015; Noble et al., 2015). Accordingly, compensatory neural processes are often evident by overactivation in brain areas taxed by WM tasks across the community and clinical populations (Langenecker and Nielson, 2003; Philip et al., 2016; Sweet et al., 2006). Among impoverished individuals, WM or brain regions involved in learning and memory (e.g., the hippocampus) may be compromised possibly via cognitive burden (Evans and Schamberg, 2009; Hanson et al., 2011).
Neuroimaging studies aiming to predict the development of risk behaviors that precede addiction have also implicated the connection between WM and DRD. These studies suggest that the cognitive ability to concurrently process the costs and benefits of immediate versus delayed rewards is limited, as it requires the parallel engagement of the brain’s cognitive control and WM networks and decision-making (Wesley and Bickel, 2014). Increased neural response to WM challenges in fronto-parietal nodes of the cognitive control network is linked specifically to reduced top-down modulation of impulsivity (Hallowell et al., 2019) and decision-making (D’esposito and Postle, 2015; Jensen and Tesche, 2002; Olesen et al., 2004). Moreover, a recent experimental study demonstrated that neurocognitive training on working memory decreases delay discounting, providing further evidence of a functional relationship between delay discounting and working memory (Bickel et al., 2011).
1.3. Emotion reactivity and impulsive decision-making
Emotional reactivity, defined here as a self-reported emotional response to an emotionally valenced situation, serves an important intra-individual context that affects working memory, decision-making and youth risk behaviors (Blakemore and Robbins, 2012; Garfinkel et al., 2015; Quinn and Harden, 2013; Schreiber et al., 2012). For example, children’s performance on working memory task that involved emotional facial expressions was impaired when exposed to negatively valenced expressions but not to neutral and positively valenced expressions (Augusti et al., 2014). Similarly, and in line with research and theory on the role of emotions in decision-making (Bechara, 2004; Cyders and Smith, 2008), compromised cognitive control systems are thought to give rise to elevated emotional reactivity resulting in decision-making characterized by a preference for immediate rewards. This hypothesis is based on neuroimaging findings that activity in emotional processing networks moderated top-down regulation of decision-making (Ernst and Paulus, 2005; Miu et al., 2008). Emotionally reactive individuals have been shown to be more prone to use substances because of altered salience of rewards (Oshri et al., 2018a,b) and a reduced potential for executive systems, such as WM, to effectively evaluate and weigh long-term versus short-term behavioral outcomes to yield less impulsive choices (Bechara, 2005; Cohen et al., 2016; D’Argembeau et al., 2008).
1.4. Current study
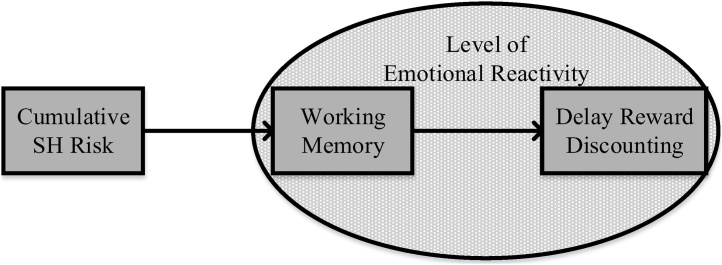
The present study concurrently examined cognitive, neural, and affective components in the pathway linking SH exposure to decision-making vulnerability in a longitudinal cohort of rural African American emerging adults. The focus on rural African Americans is important because data suggest that this group is particularly vulnerable to socioeconomic adversity and its consequences (Brody et al., 2014). However, this population is significantly understudied, particularly using neuroimaging methods (Falk et al., 2013), limiting empirical understanding of individual differences in neurocognitive and affective antecedents of reward discounting among African American youth. Lastly, a novel contribution is the use of covariance structure modeling analyses (Kircanski et al., 2018; Lahey et al., 2012) to examine brain response during WM performance, with validation of the correspondence between the behavioral and brain imaging data. Specifically, associations were modeled between neural responses during the 2-back WM task to formulate a latent index of common variance of neural activation in brain regions associated with the 2-back. To further validate the connection between neural response and behavioral performance, the association between the brain region activation and behavioral performance on the 2-back was then confirmed in separate regression analyses. Thus, per Fig. 1, we predicted that WM would play a central role in the indirect association between SH and increase in impulsive DRD. For poorer young people, we hypothesized that decrements in WM function would undermine the top-down regulation of decision-making processes when evaluating the salience of immediate versus delayed rewards. Specifically, we hypothesized that the neural systems associated with WM would be less effective among emerging adults who experienced chronic SH (Hypothesis 1), which would be associated with increased DRD (i.e., making decisions that prioritize immediate rewards: Hypothesis 2). Incorporating emotional reactivity in the hypothesized associations, we expected that SH would be associated with steeper discounting rate via decrements in WM as a function of emotional reactivity level (Hypothesis 3). Specifically, per Fig. 1, we predicted that emotional reactivity would be a positive moderator of the association between WM and riskier DRD (i.e., higher emotional reactivity would be associated with a stronger association between the two).
Fig. 1.
Theoretical Model: The indirect effect of SH and delayed reward discounting via working memory in emotional reactivity context.
2. Materials and methods
2.1. Participants
The present study included a sample of 119 right-handed young African American men and women (53.78% women) from rural Georgia, who were randomly selected from a larger longitudinal parent study. Individuals were screened to exclude those with a history of neurological or psychiatric disorder, left-handedness, and contraindications for MRI (e.g., ferrous implants, claustrophobia). Participants in the parent study were selected randomly from lists of students that schools provided (see Brody et al., 2014 for a full description of recruitment procedures). Among the 119 participants, data were deemed inadequate (and were estimated as missing data) among 17 (14.3%) participants due to inadequate performance on the 2-back task and 7 (5.9%) were missing due to excessive movement (4; 3.4%) and other artifacts (3; 2.5%).
2.2. Procedures
Emerging adults and their former caregivers provided data during four assessments (T1-T4) beginning at 19 years of age. There were 6 months between T1 (Mage = 19.31) and T2 (Mage = 19.98), 12 months between T2 and T3 (Mage = 21.03), and 24 months between T3 and T4 (Mage = 24.62). These assessments were collected in participants’ homes with a standardized protocol using an audio computer-assisted self-interview (ACASI); details of these assessments are provided in prior publications (Brody et al., 2014). Neuroimaging was completed within two weeks of the T4 in-home visit. During this visit, participants completed an MRI protocol followed by a behavioral assessment in the same facility. Prior to the neuroimaging assessment, participants were trained to perform the n-back WM task and given an opportunity to practice. Practice consisted of a 15 consonant 2-back task, which was administered a minimum of twice: once with feedback from the administrator, followed by as many subsequent attempts as necessary to achieve a criterion score of 77% correct responses. During the fMRI procedure, participants wore earplugs and lay supine on the scanner table. Visual stimuli were presented through goggles, and participants responded using a two-button box held in the right hand. Responses were recorded using E-prime Software, which also presented the n-back paradigm. The MRI session lasted approximately 30 min. At each wave, including the neuroimaging assessment, individuals consented to participate. All study protocols were approved by The University of Georgia’s Institutional Review Board.
2.3. Measures
2.3.1. Cumulative SH risk index
A cumulative SH risk index, derived from data at T1, T2, and T3 (ages 19–21 years), was created based on a composite of six caregiver-reported items: poverty status, parental education, perceived adequacy of income, parent employment, single-parent status, and government assistance receipt. The SH risk scores across the first three waves were summed to represent a cumulative risk index, with a possible range from 0 to 18. This index has been used in several previous studies (Brody et al., 2017).
2.3.2. Delayed reward discounting
DRD was assessed with the Monetary Choice Questionnaire (MCQ; Kirby et al., 1999) at T3 and T4. The MCQ consists of 27 items that pair a relatively smaller immediate reward with a larger delayed reward (e.g., ‘Would you rather have $54 today or $75 in 117 days?’). Participants were instructed to choose their preferred rewards. The MCQ provides estimates of an individual’s temporal discounting of rewards at three magnitudes (small: $25-35; medium: $50-60; large: $75-85). Responses were analyzed using the standard method (Gray et al., 2016), and the hyperbolic discounting functions (i.e., k) were estimated within each magnitude, with a higher k representing a higher hyperbolic discounting function. In the primary analyses, a latent difference score (Hamaker et al., 2015; McArdle and Hamagami, 2001; Selig and Preacher, 2009; Steyer et al., 1997) was used to assess the change in DRD from T3 to T4, consisting of the small, medium, and large magnitude log transformed k values. In addition, previous research suggests that smaller delayed rewards present the largest effect size (Amlung and MacKillop, 2014, 2011; Oshri et al., 2018a,b) possibly because the small magnitudes are of greatest relevance to a sample of low SES. Thus, to examine the ecological validity of the survey task, we tested the model using small, medium, and large magnitude k values separately.
2.3.3. Emotional reactivity
Participants’ emotional reactivity was assessed at T4 using a six-item subscale from the MacArthur Reactive Responding Scale (Taylor and Seeman, 1999). Items include, “I operate on a short fuse when my emotions are involved”, “I often respond quickly and emotionally when something happens.”, “Sometimes I overreact to situations”, “I let my emotions cool before I act”, “I keep a cool head when I am angry or frightened”, and “I stop and think before I act, even if I am angry”. Responses ranged from strongly disagree to strongly agree using a five-point scale. The latter three items were reverse-coded, and then a sum score of the six items was used in the analysis, with a possible range from 6 to 30. The internal consistency across the six items was α = .73.
2.3.4. Demographics
At T4, participants reported their gender, age, and household income level. Gender was coded as 0 = male and 1 = female. Household income was coded as 1 = Less than $ 15,000, 2 = $15,001-$30,000, 3 = $30,001-$45,000, 4 = $45,001- $60,000, 5 = larger than $60,000.
2.4. Brain region activation assessment
2.4.1. Paradigm
WM performance and associated brain response were measured during the n-back task, a widely employed and reliable WM fMRI paradigm (Sweet et al., 2008, 2006). The task requires participants to buffer, update, match, encode, and respond to patterns of consonants. The present study included two components: task-free baseline and 2-back. Stimulus presentation parameters were based on those used in previous studies (Braver et al., 1997; Smith and Jonides, 1997; Sweet et al., 2008, 2006). Participants completed two imaging runs of the n-back paradigm. Each run included three 2-back blocks alternating with two 27-second rest periods (i.e., 2 per imaging run). During the 2-back condition, participants were asked to respond “yes” if a consonant was the same as the consonant presented two earlier, and “no” if it was not. The task consisted of six series of 15 consonants of random case, 33% of which were targets. In total, six series of individual consonants were presented visually; a consonant was presented every 3 s (500 ms with a 2500 ms inter-stimulus interval). Consonants were arranged in a pseudo-random order from a list of all consonants except “L” (excluded due to ambiguity in lower-case). The n-back task was presented using E-Prime 2.0 software (Psychology Software Tools, PA) installed on a desktop computer. The computer video signal was projected through MR-compatible goggles. The MR-compatible button response box was connected to transmit responses to the computer.
2.4.2. MRI acquisition
Whole-brain fMRI was conducted using the s General Electric 16-channel fixed-site Signa HDx 3.0 T MRI scanner in the Bio-Imaging Research Center of the University of Georgia. The scanner was equipped with a three-axis local gradient head coil and elliptical endcapped quadrature radio frequency coil. Structural images were acquired for anatomical reference using a high-resolution T1-weighted, fast-spoiled gradient echo scan to cover the whole brain (TR = 7.8 ms; TE = 3.1 ms; FOV = 256 × 256 mm; matrix = 256 × 256; 160 contiguous 1 mm axial slices; voxel size, 1 mm3). Functional images were collected using a single-shot, gradient-echo echoplanar pulse sequence (TR = 2500; TE = 40 ms; FOV = 224 x 224 mm; matrix = 64 × 64). Contiguous 3.5-mm thick axial slices were selected to provide coverage of the entire brain in isometric 3.5 mm voxels.
2.4.3. Imaging processing
Data processing and statistical analyses were conducted using Analysis of NeuroImages software (AFNI; Cox, 1996). Standard preprocessing of the raw data was used to strip the skull, ensure alignment to T1 anatomical data sets, and censor any volumes that exhibited outlying values or excessive movement (> 0.3 mm per repetition). Data sets were transformed into Talairach space (Talairach and Tournoux, 1988), and spatial registration of each time series was set to the 3rd volume of the first imaging run using an iterative linear least-squares method. The AFNI 3D registration program yielded a root-mean-square difference for each image, which was used to screen for uncorrected movement. Participants that showed excessive movement (greater than 3.5 mm in any direction or greater than 25% censored TRs) were estimated in the analyses. Spatial blurring over a 6-mm radius was applied using a Gaussian kernel to compensate for typical variations in functional neuroanatomy across participants.
A voxelwise General Linear Model (GLM) procedure was conducted to quantify the relationship between observed brain activity and the 2-back paradigm, using four blocks of 27 s. For each voxel of every individual, a GLM of the temporal pattern of 2-back presentation (including hemodynamic transitions modeled as a gamma function) and covariates (observed movement, linear drift) was performed using the BOLD signal over time as the dependent variable. The GLM included a general linear contrast of the 2-back versus resting baseline. Therefore, resulting individual activation maps included beta coefficients for each voxel that represented the 2-back effects relative to baseline. Individual activation maps were then used in subsequent group-level analyses.
To create group summary maps of 2-back effects, 2-back parameter estimates were compared to a hypothetical mean of zero for each voxel using pooled-variance one-sample Student’s t-tests. The group summary activation maps were examined qualitatively for comparison to prior literature and quantitatively to generate functionally defined regions of interest (ROIs) for quantification of 2-back effects for orthogonal hypothesis testing. Also, group summary maps were used for a data-driven functional ROI strategy for further hypothesis testing. Task-related ROIs were clusters that were significantly recruited during the 2-back task compared to rest in our sample. Functional ROIs were defined using a family-wise error rate of p < .005 with a minimum cluster size of five adjacent voxels, to allow for separation of distinct clusters into individual ROIs. Thus, in the present study, mean 2-back-associated fMRI responses within each of these functionally defined ROIs were extracted to examine the relationship between 2-back brain response, DRD, and emotional reactivity in the current sample.
2.5. Analyses
Study aims were tested using structural equation modeling (SEM) in Mplus Version 7.4 (Muthén and Muthén, 1998-2012) with maximum likelihood estimation with robust standard errors (Yuan and Bentler, 2000). Missing data ranged from 0% to 22.8%. Little’s Missing Completely at Random test suggested that the missing data were completely random (χ2 (47) = 62.08, p > .05) and therefore qualified for estimation with full-informative maximum likelihood (FIML) algorithm (Rubin and Little, 2002). FIML yields more efficient and less biased parameter estimates than do traditional methods of handling missing data, including non-normally distributed missing data (Enders and Bandalos, 2001). Per Hu and Bentler’s recommendations (Hu and Bentler, 1999), model fit was assessed using the chi-square test of model fit, the root mean square error of approximation (RMSEA < .06), the comparative fit index (CFI > .90), the Tucker-Lewis index (TLI > .90), the McDonald and Marsh’s relative non-centrality index (RNI > .90), and the standardized root mean square residual (SRMR < .06).
A confirmatory factor analysis (CFA) was used to model a factor that reflects the common variance associated with activation of specific brain regions during the n-back. A sensitivity analysis was conducted to confirm the prior measurement hypothesis. Then, a latent difference score was calculated to assess the change in DRD from T3 to T4, consisting of the small, medium, and large magnitude log transformed k values. Next, SEM was used to model the indirect link between cumulative SH risk and DRD latent change score via the n-back activation factor. Emotional reactivity was tested as a moderator of the association between the brain activation factor and DRD change. In the SEM model, gender, age, and income were controlled. To examine the conditional indirect effects, a mediation procedure (i.e. R-Mediation) was used. This mediation strategy produces a confidence interval for the product of two normal random variables using three methods: the distribution of the product of coefficients, Monte Carlo, and asymptotic normal theory with the multivariate-delta standard error (Asymptotic-Delta) method (MacKinnon et al., 2007; Tofighi and MacKinnon, 2011). Johnson-Neyman’s techniques (Johnson and Neyman, 1936) were used to interpret the moderation effect.
Previous research suggests that smaller delayed rewards present the largest effect size (Amlung and MacKillop, 2014, 2011; Oshri et al., 2018a,b) possibly because the small magnitudes are of greatest relevance to a sample of low SES. To examine the ecological validity of the survey task, we tested the SEM models with different magnitudes of DRD separately. Specifically, the indirect links between cumulative SH risk and DRD (small, medium, and large magnitude k values assessed at T4) via the n-back activation factor conditional on emotional reactivity were examined, after controlling for gender, age, income, and corresponding DRD magnitude assessed at T3. The results of other magnitudes than small are presented in the supplemental materials.
3. Results
3.1. Descriptive and correlation analyses
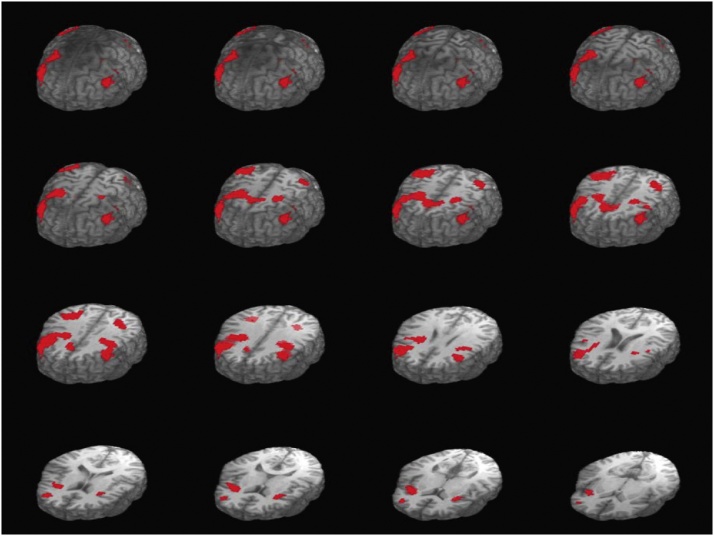
Descriptive statistics and bivariate correlations are presented in Table 1. Significant activation responses were observed in six clusters comprising four ROIs: the bilateral inferior parietal lobule (IPL) and middle frontal gyrus (MFG), and the right insula, and right anterior cingulate/medial frontal gyrus (see Fig. 2 and Table 2), which are consistent with prior 2-back fMRI literature (Braver et al., 1997; Sweet et al., 2008, 2006). These 4 key ROIs were used in hypothesis testing. Variables were correlated in the predicted directions. 2-back performance accuracy was positively and significantly associated with 2-back associated activation magnitude in these ROIs. Generally, cumulative SH risk was negatively associated with activation across ROIs, which was also negatively associated with DRD at both T3 and T4.
Table 1.
Descriptive Statistics and Correlations of Study Variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Cumulative SH Risk -T1, 2, 3 | -- | |||||||||||||||
| 2. MFG -T4 | −.19 | -- | ||||||||||||||
| 3. IPL – T4 | −.12 | .71** | -- | |||||||||||||
| 4. RCingulate – T4 | −.18 | .66** | .71** | -- | ||||||||||||
| 5. RInsula -T4 | −.19 | .65** | .60** | .62** | -- | |||||||||||
| 6. 2-back performance | −.24* | .20 | .26* | .21* | .07 | -- | ||||||||||
| 7. Emotional Reactivity – T4 | −.08 | .02 | −.14 | −.02 | −.05 | .07 | -- | |||||||||
| 8. DRD Small – T4 | .02 | −.11 | −.01 | −.07 | −.13 | −.06 | .18* | -- | ||||||||
| 9. DRD Medium – T4 | .04 | −.14 | −.03 | −.11 | −.07 | −.16 | .10 | .80** | -- | |||||||
| 10. DRD Large – T4 | .05 | −.18 | −.07 | −.18 | −.11 | −.17 | .00 | .77** | .92** | -- | ||||||
| 11. DRD Small – T3 | .07 | .03 | −.08 | −.19 | −.07 | −.08 | .10 | .24** | .30** | .31** | -- | |||||
| 12. DRD Medium – T3 | .03 | −.04 | −.14 | −.21* | −.05 | −.13 | .11 | .40** | .51** | .51** | .82** | -- | ||||
| 13. DRD Large – T3 | .06 | −.09 | −.18 | −.27* | −.10 | −.18 | .07 | .35** | .49** | .49** | .80** | .89** | -- | |||
| 14. Gender | −.08 | −.07 | −.10 | .02 | −.07 | −.15 | .22* | −.15 | −.23* | −.23* | −.26** | −.25** | −.19* | -- | ||
| 15. Age | .14 | −.13 | −.20 | −.11 | −.10 | −.10 | .08 | −.05 | .03 | .01 | .00 | .02 | .02 | .01 | -- | |
| 16. Income | −.26* | −.01 | .10 | −.01 | .03 | .22* | −.05 | −.17 | −.10 | −.04 | −.03 | −.03 | −.09 | −.28** | −.08 | -- |
| Mean | 8.44 | .51 | .37 | .18 | .19 | .73 | 15.90 | −1.30 | −1.48 | −1.68 | −1.19 | −1.43 | −1.58 | .52 | 24.45 | 2.29 |
| SD | 4.10 | .41 | .35 | .18 | .17 | .13 | 5.16 | .88 | .80 | .89 | .83 | .76 | .83 | .50 | .66 | 1.40 |
| Minimum | .00 | −.22 | −.57 | −.25 | −.19 | .38 | 6.00 | −3.80 | −3.80 | −3.80 | −3.80 | −3.80 | −3.80 | .00 | 23.00 | 1.00 |
| Maximum | 6.00 | 1.45 | 1.45 | .84 | .75 | .96 | 26.40 | −.61 | −.61 | −.61 | −.61 | −.61 | −.61 | 1.00 | 26.00 | 8.00 |
Note. T1, 2, 3, 4 = Time 1, 2, 3, 4, respectively; SH = Socioeconomic hardship; IPL = Inferior parietal lobule; MFG = Middle frontal gyrus; RInsula = Right insula; RCaudate = Right cingulate/ right medial frontal; DRD = Delayed reward discounting, small, medium, and large indicate the DRD reward magnitudes; SD = Standard deviation. Gender was coded as 0 = male and 1 = female. *p < .05, **p < .01.
Fig. 2.
Neural activity associated with 2-back vs. rest, regions that exhibited significant activity during the 2-back task compared to baseline.
Note. Talaraich Z-plane: +75 to 0in 5 mm slices, t ≥ 4.318, p < .005. This figure presents the group-level N-back activation: 2-back versus the baseline condition. Functionally defined regions of interest (ROIs) used in the present analyses. Axial slices show three-dimensional brain regions that exhibited significant activity during the 2-back task above and beyond that exhibited during rest. Underlying anatomical image of a single representative participant was used for this figure. Signal outside of the brain was masked.
Table 2.
Functionally Defined Regions Associated with Working Memory.
| ROI | Voxels | Center of Mass Coordinates |
|||
|---|---|---|---|---|---|
| X | Y | Z | |||
| ROI 1 | R. Middle Frontal Gyrus | 174 | −39.2 | −23.2 | 35.5 |
| L. Middle Frontal Gyrus | 42 | 41.9 | −24.4 | 30.4 | |
| ROI 2 | R. Inferior Parietal Lobule | 113 | −43.8 | 47.4 | 43.4 |
| L. Inferior Parietal Lobule | 30 | 34.1 | 46.8 | 39.1 | |
| ROI 3 | R. Cingulate/Medial Frontal | 68 | −4.5 | −19.1 | 41.7 |
| ROI 4 | R. Insula | 54 | −34.1 | −16.9 | 6.4 |
Note. L = left, R = right. Coordinates are in Talairach space (RAI). Functional ROIs were defined using a family-wise error rate of p < .005 with a minimum cluster size of five adjacent voxels, to allow for separation of distinct clusters into individual ROIs. : Talaraich-Tournoux Atlas was used for anatomical labels. Information in this table corresponds to regions in Fig. 2.
Additionally, we conducted t-tests and chi-square tests to examine the differences of participants whose fMRI data were available (n = 95) and missing (n = 24) in the current study (Supplemental Table 1). Results indicated that these two groups were not significantly different in demographics and major study variables except for cumulative SH risk (t (117) = 2.25, p < .05). Participants whose fMRI data were missing had significantly higher cumulative SH risk compared to participants whose fMRI data were available. Overall, analyses were conducted with FIML to estimate for missing values under RAM using all 119 participants.
3.2. Measurement model
CFAs were conducted to test the factor structure of 2-back-associated ROIs (Brown, 2015). Table 3 presents a measurement model of ROIs brain activation. The factor of brain activation consisted of four indicators: inferior parietal lobule (bilaterally), middle frontal gyrus (bilaterally), right insula, and right cingulate/right medial frontal gyrus. All factor loadings were moderate to high (λ > .50) (Brown, 2015) and significant (p < .01). The measurement model fit was excellent: χ2 (2) = 2.16 (p = .34). CFI = 1.00, TLI = 1.00, RNI = 1.00, RMSEA = .03, SRMR = .01 (Hu and Bentler, 1999). Furthermore, the ROI activation factor was significantly and positively associated with participants’ 2-back performance accuracy (r = .21, p < .05). To confirm a prior measurement hypothesis, a sensitivity analysis was conducted to test the loadings of prior working memory ROIs on the WM brain activation factor during the 2-back task constructed through CFA in the current study. Five WM ROIs (bilateral rostral and caudal medial frontal gyrus, right medial frontal gyrus, inferior parietal lobule, and superior parietal lobule) were tested separately and all yielded significant (p < .001) and moderate to high (λ > .30) loading coefficients. The results of sensitivity analysis support the expected covariances between ROIs in the present and the apriori ROIs from the literature (See supplemental materials Table 3).
Table 3.
Measurement Model of Working Memory Associated Brain Regions Activation during the 2-back Task.
| Factors and Indicators | B (SE) | λ (SE) | R2 | 95%CI of λ |
|---|---|---|---|---|
| WM Brain Regions Activation | ||||
| WM Brain Activation → IPL | 1.00 (.00) | .85 (.04) | .72 | [.77, .93]*** |
| WM Brain Activation → MFG | 1.16 (.14) | .83 (.04) | .69 | [.75, .91]*** |
| WM Brain Activation → RInsula | .99 (.11) | .82 (.03) | .56 | [.75, .89]*** |
| WM Brain Activation → RCingulate | .84 (.12) | .75 (.06) | .67 | [.63, .87]*** |
Note. WM Brain Activation = Working memory associated brain regions activation during the 2-back task latent factor. IPL = Inferior parietal lobule. MFG = Middle frontal gyrus. RInsula = Right insula. RCaudate = Right cingulate/ right medial frontal. Model fit is excellent: χ2 (2) = 2.16 (p = .34). CFI = 1.00, TLI = 1.00, RNI = 1.00, RMSEA = 0.03, SRMR = .01.
p < .001.
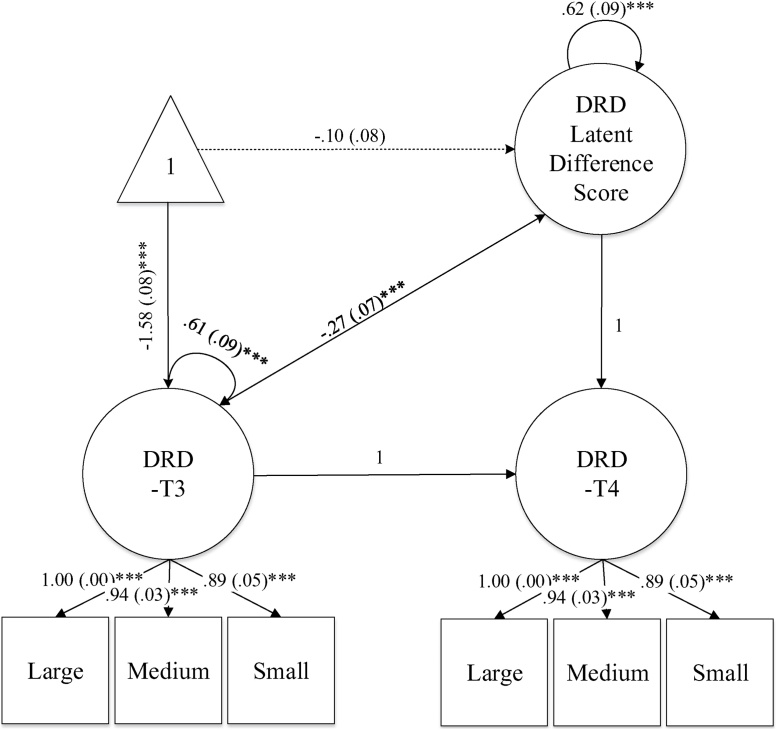
A latent change model (McArdle and Hamagami, 2001; Selig and Preacher, 2009; Steyer et al., 1997) was used to examine the inter-individual differences in DRD from T3 to T4 (Table 4 and Fig. 3). At each time-point, DRD consisted of three indicators: small, medium, and large magnitude k values. The regression coefficients of DRD at T3 and the latent difference score on the follow-up DRD were fixed to one, and the residual of T4 was fixed to zero. The mean of the DRD latent difference score marginally significantly different from zero (M = -.21, p = .05) and the estimated variance was significantly larger than zero (Σ2 = 1.72, p < .001), suggesting individual differences in the latent difference score of DRD from T3 to T4. The overall measurement model fit was good: χ2 (10) = 14.75 (p = .14). CFI = .99, TLI = .99, RNI = .99, RMSEA = .06, SRMR = .05 (Hu and Bentler, 1999).
Table 4.
Latent Change Model of DRD.
| Factors and Indicators | Λ (SE) | λ (SE) | 95%CI of Λ |
|---|---|---|---|
| DRD – T3 → DRD Large – T3 | 1.00 (.00) | .93 (.02) | [1.00, 1.00]*** |
| DRD – T3 → DRD Medium – T3 | .94 (.03) | .97 (.02) | [.85, 1.04]*** |
| DRD – T3 → DRD Small – T3 | .89 (.05) | .84 (.03) | [.79, .98]*** |
| DRD – T4 → DRD Large – T4 | 1.00 (.00) | .94 (.02) | [1.00, 1.00]*** |
| DRD – T4 → DRD Medium – T4 | .94 (.03) | .98 (.01) | [.85, 1.02]*** |
| DRD – T4 → DRD Small – T4 | .89 (.05) | .82 (.03) | [.79, .98]*** |
| Paths & Covariance | B (SE) | β(SE) | 95%CI of B |
| DRD – T3 → DRD – T4 | 1.00 (.00) | .94 (.09) | [1.00, 1.00]*** |
| ΔDRD → DRD – T4 | 1.00 (.00) | .95 (.08) | [1.00, 1.00]*** |
| ΔDRD & DRD – T3 | −.27 (.07) | −.44 (.09) | [-.40, -.12]*** |
| Means | M (SE) | μ (SE) | 95%CI of M |
| DRD – T3 | −1.58 (.08) | −2.04 (.18) | [-1.73, -1.43]*** |
| ΔDRD | −.10 (.08) | −.13 (.10) | [-.26, .06] |
| Variances | Σ2(SE) | σ2(SE) | 95%CI of Σ2 |
| DRD – T3 | .61 (.09) | 1.00 (.00) | [.42, .78]*** |
| ΔDRD | .62 (.09) | 1.00 (.00) | [.43, .79]*** |
Note. T3 = Time 3; T4 = Time 4; SE = Standard error; CI = Confidence interval; DRD = Delayed reward discounting; Small, medium, and large indicate the DRD magnitude; ΔDRD = DRD latent difference score. Model fit is excellent: χ2 (10) = 14.75 (p = .14). CFI = .99, TLI = .99, RNI = .99, RMSEA = 0.06, SRMR = 0.05, *p < .05.
p < .001.
Fig. 3.
Latent Change Model of the Delayed Reward Discounting.
Note: Unstandardized coefficients are presented in the figure. DRD = Delayed reward discounting. Large, medium, and small indicate the DRD reward magnitudes. ***p < .001.
3.3. Primary analyses
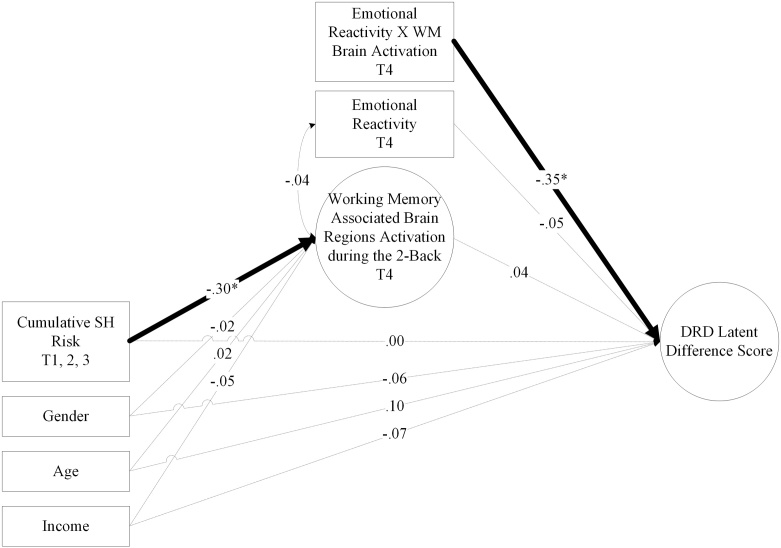
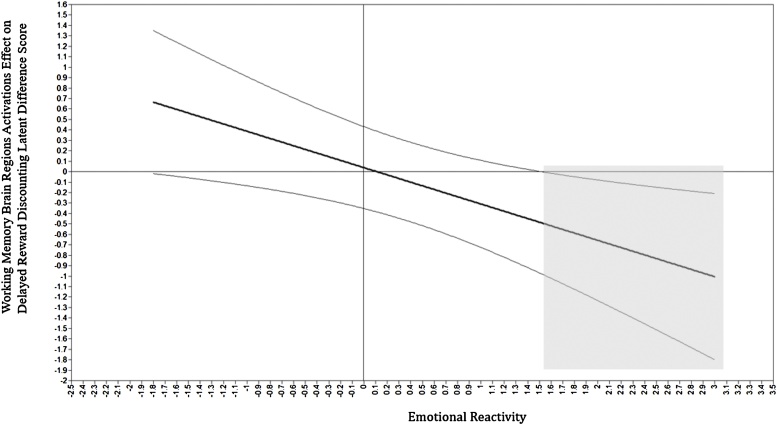
An SEM was used to test the associations among cumulative risk, subsequent brain region activation during the 2-back task, DRD latent change score, and emotional reactivity. Gender, age, and income were entered as covariates. Table 5 and Fig. 4 present the SEM model of the associations among cumulative risk and subsequent activation during the 2-back task, DRD latent change score, and emotional reactivity. This model exhibited an acceptable fit: χ2 (77) = 104.78 (p < .05). CFI = .96 TLI = .95, RNI = .96, RMSEA = .06. Cumulative SH risk predicted reduced brain activation during the 2-back task at T4 (β = -.30, p < .05). In addition, the interaction between WM brain activation and emotional reactivity was negatively associated with DRD latent change score (β = -.36, p < .05), after controlling cumulative risk (β = .00, p = .88). Based on the Johnson-Neyman’s plot (P. O. Johnson and Neyman, 1936), as shown in Fig. 5, among participants with elevated levels of emotional reactivity, lower levels of WM brain activation were significantly associated with higher levels of DRD. In testing mechanistic conditional indirect effects, R-Mediation (Tofighi and MacKinnon, 2011) showed that the indirect effect of cumulative SH risk on DRD through WM brain region activation during the 2-back task was significant, conditional level of emotional reactivity (α*β = .11, p < .05). For participants high in emotional reactivity, the indirect effect was positive and significant.
Table 5.
Model of the Associations Between Cumulative Socioeconomic Stress Risk, WM Associated Brain Regions Activation, Delayed Reward Discounting Latent Change Score, and Emotional Reactivity.
| Direct Paths | B (SE) | β | 95%CI of B |
|---|---|---|---|
| Direct effects | |||
| CSH (T1, 2, 3) → WM Brain (T4) | −.059 (.024) | −.298 | [-.105, -.012]* |
| WM Brain (T4) → ΔDRD | .039 (.200) | .040 | [-.353, .431] |
| CSH (T1, 2, 3) → ΔDRD | −.003 (.020) | −.016 | [-.043, .037] |
| ER (T4) → ΔDRD | −.038 (1.529) | −.049 | [-3.035, 2.960] |
| Interaction Effect | |||
| WM Brain ER (T4) → ΔDRD (T4) | −.348 (.135) | −.355 | [-.612, -.084]* |
| Conditional Indirect Effect | |||
| CSH (T1, 2, 3) → WM Brain ER (T4) → ΔDRD | .021 (.012) | .106 | [.002, .048]* |
| Control | |||
| Gender → WM Brain (T4) | −.029 (.213) | −.018 | [-.447, .388] |
| Age → WM Brain (T4) | .021 (.175) | .018 | [-.322, .364] |
| Income → WM Brain (T4) | −.026 (.060) | −.046 | [-.143, .091] |
| Gender → ΔDRD | −.098 (.178) | −.064 | [-.448, .251] |
| Age → ΔDRD | .115 (.097) | .101 | [-.075, .305] |
| Income → ΔDRD | −.039 (.061) | −.070 | [-.159, .081] |
Note. T1, 2, 3 = Combination of Times 1, 2, and 3. T3 = Time 3. T4 = Time 4. SE = Standard error. CI = Confidence interval. CSH = Cumulative socioeconomic hardship Risk. WM Brain = Working memory associated brain regions activation during the 2-back task. ER = Emotional Reactivity. ΔDRD = Delayed reward discounting latent difference score. Gender was coded as 0 for male and 1 for female. Model fit is acceptable: χ2 (77) = 104.78 (p < .05). CFI = .96 TLI = .95, RNI = 0.96, RMSEA = 0.06, SRMR = .14.
p < .05.
Fig. 4.
Moderated Mediation Model of the Associations between Cumulative SH Risk, Working Memory Associated Brain Regions Activation, Delayed Reward Discounting, and Emotional Reactivity.
Note: Standardized coefficients are presented in the figure. SH = Socioeconomic hardship. DRD = Delayed reward discounting. WM = Working memory. Gender and DRD at T3 were controlled. *p < .05, **p < .01, ***p < .001.
Fig. 5.
Probing and Interpretation of the Moderating Role of Emotional Reactivity on the Associations between Working Memory Associated Brain Activation and DRD Latent Change Score.
Note. Shadowed area indicates regions of significance in which the working memory brain regions activation was significantly negatively associated with delayed reward discounting. This figure suggested that only among participants with higher emotional reactivity, lower levels of brain activation was associated with higher levels of delayed reward discounting significantly. Emotional reactivity was standardized.
To examine the effects on different DRD reward magnitudes, we tested the SEM models with small, medium, and magnitudes of DRD separately. The results are presented in the supplemental Table 2. The three models with different reward magnitudes all fit well and are consistent with the primary findings using the DRD latent difference score. However, despite that the indirect effects of cumulative SH risk on DRD through WM brain activation conditional on emotional reactivity are significant for the small and medium magnitudes of DRD, this conditional indirect effect was not significant for the large DRD magnitude. In addition, income is only significantly and negatively associated with small, but not medium or large DRD magnitudes at T4 (supplemental Table 2).
4. Discussion
Previous studies have documented associations between socioeconomic adversity early in life, WM and impulsive decision-making, and a range of risk behaviors (Barrett and Turner, 2006;Evans and Kim, 2010;Galvan and Rahdar, 2013; Lipina and Posner, 2012a; Mueller et al., 2010). Missing are multidisciplinary multimethod investigations of the associations between poverty and decision-making via the underlying neuro-circuitry of cognitive control functions that contribute to decision-making (Haushofer and Fehr, 2014). Findings from the present study expand on current knowledge regarding this risk pathway in several ways. First, the study documented a mechanistic pathway linking SH and increased DRD via neural activation during a WM task. Our findings confirmed that cumulative SH risk was significantly and negatively predictive of regional brain response to the 2-back WM task. In addition, reduced activation in these regions (as reflected by the latent factor of the WM effects across ROIs) was associated with less accurate performance on the 2-back, providing behavioral confirmation of these neuroimaging findings. Subsequently, the connection between reduced activation in these brain regions and impulsive decision-making (i.e., higher DRD) was found to be significant in the context of elevated emotional reactivity. The connection between 2-back-associated network activation and attendant DRD behavioral performance represents an implementation of interdisciplinary research that aims to better our understanding for how DRD may vary by emotional reactivity context (Casey et al., 2016). These findings corroborate in part, the dual systems hypothesis that compromised decision-making in adolescence is associated with a mismatch between socioemotional reward and cognitive control regulatory brain circuitry (Mills et al., 2014; Shulman et al., 2016).
Expanding on previous behavioral research, the current study used neuroimaging techniques to identify activation patterns in four key ROIs associated with 2-back performance that underlies the associations between SH and DRD. We identified a latent factor that was composed of a network of neural activity elicited during the 2-back task, which is consistent with prior literature (Owen et al., 2005; Owens et al., 2018; Stoodley et al., 2012). The bilateral-IPL and MFG, the right insula, and the right cingulate/right medial frontal gyrus are four nodes of the fronto-parietal cognitive control network, which is routinely activated during goal-directed task performance (Leung and Alain, 2011; Philip et al., 2016; Ragland et al., 2002). Activity in this network was associated with WM performance and significantly mediated the associations between cumulative SH risk and DRD among emerging adults with higher emotional reactivity, implicating these regions in the neural substrate in DRD.
The present findings have several implications for basic research on the impact of SH on decision-making bias. The findings are consistent with theories that suggest that poverty may be associated with decision-making preferences for immediate rewards because SH consumes mental resources that result in cognitive overload (Mani et al., 2013). Given that WM capacity is known to be correlated with decision-making process, these hypotheses are corroborated by theoretical connection between WM engagement and the process of evaluating delayed rewards (Evans and Stanovich, 2013). WM is one of several related executive functions necessary for decision-making (D'esposito & Postle, 2015; Hinson et al., 2003; McCabe et al., 2010). Evans and colleagues further suggest that working memory processing is a critical capacity for the mental operations necessary during decision-making operations (Evans and Stanovich, 2013). Moreover, our results are also supportive of recent research in suggesting that impulsive decision-making may be construed as a rational cognitive strategy in unreliable and impoverished contexts (Kidd et al., 2013). Specifically, youth experience harsh and unreliable environment are objectively correct not to delay gratification but to resort to more immediate rewards even if they are smaller compared to delayed options. This research is also concordant with a more nuanced neuro-economics hypothesis and experimental research suggesting that poor individuals exhibit attentional bias linked to their financial scarcity (Shah et al., 2015). Nonetheless, impulsive decisions that could be objectively regarded as irrational may also reflect cognitive adaptations that promote survival for individuals who grew up in enduring scarcity (Pepper and Nettle, 2017). These cognitive adaptations to scarcity may be functional for youth in the short-term, but could become maladaptive as they prepare for more autonomous living independent from their parents (Willoughby et al., 2014).
Our findings elucidate a mechanism in which emotionally reactive emerging adults are particularly inclined to choose immediate reward when WM is impaired. Theory and research on why adolescents and emerging adults are prone to impulsive decision-making have suggested the existence of two (dual) systems that coordinate the process of decision-making (Steinberg, 2010). In particular, dual systems theories suggest that during adolescence proclivity for impulsive decisions are attributed to the gap in developmental maturation of subcortical brain regions linked to incentive-reward systems (also referred to as socioemotional system (Shulman et al., 2016), and prefrontal regions that undergird cognitive control systems (Shulman et al., 2016). Specifically, adolescents have accelerated sub-cortical brain maturation but protracted development of regions associated with cognitive control, creating a gap between these two systems that impedes effective regulation of the socioemotional system by the cognitive control system (Harden and Tucker-Drob, 2011). This maturation gap leads to the dual system hypothesis that in the absence of mature prefrontal brain areas and attendant executive control functions, youth are expected to experience greater anticipation to rewarding stimuli and heightened responses for the receipt of rewards (Shulman et al., 2016). The findings from the present study further suggest that exposure to SH may extend this decision-making risk among youth who are more vulnerable emotionally. Thus, in line with the dual system perspective, lack of emotional control moderates risk for delayed reward discounting.
In the present study, the role of WM performance and associated brain responses are also consistent with the executive information processing hypothesis (Bickel et al., 2007; McClure et al., 2004), which posits that decision-making is partially supported by effective WM and processing of information, as evident in maintenance and manipulation of information to evaluate long-term versus short-term consequences of a decision. The moderation of these associations by emotional reactivity is consistent with the functioning of the salience and motivational system, suggesting that emotional reactivity is a critical component in the process of DRD (Haushofer and Fehr, 2014). This supports the hypothesis that decision-making process is rooted in one’s ability to process emotions (Bechara and Damasio, 2005). Accordingly, the finding suggests that DRD is a process that is emotionally regulated by driven marker signals that possibly arise through bio-regulatory processes. Haushofer and Fehr (2014) also suggest that decision making in the context of emotional reactivity and stress induced by poverty might be related to attention, specifically, in the context of scarcity individuals tend to induce a focus on more immediate and safe payoffs. Similarly, these findings may provide limited but preliminary support to models that emphasize the important of emotional reactivity in decision-making. For example, the triadic model (Ernst, 2014) suggests that in addition to the dual systems perspective, it is important to view emotional reactivity as an additional component in processing risk assessment.
The findings bear important implications for programs designed to attenuate the risk associated with socioeconomic adversity and risk behaviors such as substance use among emerging adults in general, and African American emerging adults in particular. Epidemiological data suggesting that young African American adults are particularly susceptible to a delayed onset yet rapid escalation of substance use compared to members of other racial and ethnic groups (French et al., 2002;Galvan and Caetano, 2003; Watt, 2008). The present study goes beyond behavioral research that has documented correlations between WM performance and DRD by integrating behavioral, neuropsychological, and neuroscience data to delineate brain activation in a network that has been previously implicated in WM performance. Specifically, we corroborated research on the link between SH and WM (Evans and Schamberg, 2009; Philip et al., 2016), as well as other neuroimaging studies indicating an overlap of brain regions (such as the dorsolateral prefrontal cortex) that are recruited during WM and DRD tasks (McClure et al., 2007, 2004; Owen et al., 2005). Clinically, the findings further support the use of intervention strategies that focus on improving WM performance, especially in the context of emotional reactivity, to decrease DRD, a strong behavioral marker for addiction (Bickel et al., 2012; MacKillop et al., 2011). This conclusion is further corroborated by recent experimental evidence on the effect of WM training on DRD performance (Bickel et al., 2011). The findings provide support for studies that have demonstrated DRD to be behaviorally malleable through several types of interventions (Daniel et al., 2013). Specifically, the current study concurs with research demonstrating that WM training might mitigate devaluation of delayed rewards (Bickel et al., 2011.)
This study has several limitations. First, this study focused on rural African American emerging adults and may have limited generalization to other more urban youth. However, rural African American youth are an understudied population, particularly in neuroimaging studies, and therefore these findings serve an important contribution to the literature. Second, although findings document associations between brain activation during WM performance and DRD, neuroimaging data during DRD would substantially improve the capacity for future research to further determine underlying neural mechanisms. Future research would benefit from the use of repeated neuroimaging measures to longitudinally examine the link between SH and impulsive decision-making among impoverished emerging adults. Third, because missing data reached 20 percent on some variables, interpretation of the results from the present study should be done with caution. Yet, missing data we determined to be missing in random and were analyzed with FIML, a data analyses estimation method that introduces very little estimation bias (Enders and Bandalos, 2001). Lastly, emotional reactivity was found to exacerbate the association between WM activation and DRD. Although negative affect is being shown to influence delay discounting tasks in the context of emotion regulation, positive emotional states have been shown to affect DRD in an opposite way (Ifcher and Zarghamee, 2011). In the present study we assessed emotional reactivity, that mostly reflect negative affective states, in relation to DRD.
Acknowledging these considerations, the current findings nonetheless shed new light on the mechanisms linking adversity with steep discounting of delayed rewards. Notably, these mechanisms are both cognitive and affective, further revealing how adversity appears to shape both information processing and emotional processing in relation to decision making in emerging adults.
Financial disclosure and conflict of interest
JM is a principal in BEAM Diagnostics, Inc. No other authors report financial interests or potential conflicts of interest.
Funding
This work was supported by The National Institute on Drug Abuse (NIDA) Grants K01 DA045219-02 (PI Oshri), R01 DA029488 and P30 DA027827 (PI Brody). NIDA had no role in the study design; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the paper for publication. James MacKillop’s contributions were partially supported by the Peter Boris Chair in Addictions Research, Macmaster University, Canada.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2019.100642.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Alloway T.P., Alloway R.G. Investigating the predictive roles of working memory and IQ in academic attainment. J. Exp. Child Psychol. 2010;106(1):20–29. doi: 10.1016/j.jecp.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Amlung M., MacKillop J. Delayed reward discounting and alcohol misuse: the roles of response consistency and reward magnitude. J. Exp. Psychopathol. 2011;2(3) jep. 017311. [PMC free article] [PubMed] [Google Scholar]
- Amlung M., MacKillop J. Understanding the effects of stress and alcohol cues on motivation for alcohol via behavioral economics. Alcohol. Clin. Exp. Res. 2014;38(6):1780–1789. doi: 10.1111/acer.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augusti E.-M., Torheim H.K., Melinder A. The effect of emotional facial expressions on children’s working memory: associations with age and behavior. Child Neuropsychol. 2014;20(1):86–105. doi: 10.1080/09297049.2012.749225. [DOI] [PubMed] [Google Scholar]
- Barrett A.E., Turner R.J. Family structure and substance use problems in adolescence and early adulthood: examining explanations for the relationship. Addiction. 2006;101(1):109–120. doi: 10.1111/j.1360-0443.2005.01296.x. [DOI] [PubMed] [Google Scholar]
- Bechara A. The role of emotion in decision-making: evidence from neurological patients with orbitofrontal damage. Brain Cogn. 2004;55(1):30–40. doi: 10.1016/j.bandc.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat. Neurosci. 2005;8(11):1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bechara A., Damasio A.R. The somatic marker hypothesis: a neural theory of economic decision. Games Econ. Behav. 2005;52(2):336–372. [Google Scholar]
- Bickel W.K., Miller M.L., Yi R., Kowal B.P., Lindquist D.M., Pitcock J.A. Behavioral and neuroeconomics of drug addiction: competing neural systems and temporal discounting processes. Drug Alcohol Depend. 2007;90:S85–S91. doi: 10.1016/j.drugalcdep.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel W.K., Yi R., Landes R.D., Hill P.F., Baxter C. Remember the future: working memory training decreases delay discounting among stimulant addicts. Biol. Psychiatry. 2011;69(3):260–265. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel W.K., Jarmolowicz D.P., Mueller E.T., Koffarnus M.N., Gatchalian K.M. Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: emerging evidence. Pharmacol. Ther. 2012;134(3):287–297. doi: 10.1016/j.pharmthera.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel W.K., Moody L., Quisenberry A., Ramey C., Sheffer C. A competing neurobehavioral decision systems model of SES-related health and behavioral disparities. Prev. Med. 2014;68:37–43. doi: 10.1016/j.ypmed.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.-J., Robbins T.W. Decision-making in the adolescent brain. Nat. Neurosci. 2012;15(9):1184. doi: 10.1038/nn.3177. [DOI] [PubMed] [Google Scholar]
- Braveman P.A., Cubbin C., Egerter S., Williams D.R., Pamuk E. Socioeconomic disparities in health in the United States: what the patterns tell us. Am. J. Public Health. 2010;100(S1):S186–S196. doi: 10.2105/AJPH.2009.166082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver T.S., Cohen J.D., Nystrom L.E., Jonides J., Smith E.E., Noll D.C. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5(1):49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Brody G.H., Lei M.K., Chae D.H., Yu T., Kogan S.M., Beach S.R. Perceived discrimination among African American adolescents and allostatic load: a longitudinal analysis with buffering effects. Child Dev. 2014;85(3):989–1002. doi: 10.1111/cdev.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody G.H., Gray J.C., Yu T., Barton A.W., Beach S.R., Galván A. Protective prevention effects on the association of poverty with brain development. JAMA Pediatr. 2017;171(1):46–52. doi: 10.1001/jamapediatrics.2016.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T.A. Guilford Publications; 2015. Confirmatory Factor Analysis for Applied Research. [Google Scholar]
- Casey B., Galván A., Somerville L.H. Beyond simple models of adolescence to an integrated circuit-based account: a commentary. Dev. Cogn. Neurosci. 2016;17:128–130. doi: 10.1016/j.dcn.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A.O., Breiner K., Steinberg L., Bonnie R.J., Scott E.S., Taylor-Thompson K. When is an adolescent an adult? Assessing cognitive control in emotional and nonemotional contexts. Psychol. Sci. 2016;27(4):549–562. doi: 10.1177/0956797615627625. [DOI] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cyders M.A., Smith G.T.J.Pb. Emotion-based dispositions to rash action: positive and negative urgency. Psychol. Bull. 2008;134(6):807. doi: 10.1037/a0013341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Argembeau A., Xue G., Lu Z.-L., Van der Linden M., Bechara A. Neural correlates of envisioning emotional events in the near and far future. Neuroimage. 2008;40(1):398–407. doi: 10.1016/j.neuroimage.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’esposito M., Postle B.R. The cognitive neuroscience of working memory. Annu. Rev. Psychol. 2015;66 doi: 10.1146/annurev-psych-010814-015031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel T.O., Stanton C.M., Epstein L.H. The future is now: reducing impulsivity and energy intake using episodic future thinking. Psychol. Sci. 2013;24(11):2339–2342. doi: 10.1177/0956797613488780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deck C., Jahedi S. The effect of cognitive load on economic decision making: a survey and new experiments. Eur. Econ. Rev. 2015;78:97–119. [Google Scholar]
- Eamon M.K. Effects of poverty on mathematics and reading achievement of young adolescents. J. Early Adolesc. 2002;22(1):49–74. [Google Scholar]
- Enders C.K., Bandalos D.L. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Struct. Equ. Model. 2001;8(3):430–457. [Google Scholar]
- Ernst M. The triadic model perspective for the study of adolescent motivated behavior. Brain Cogn. 2014;89:104–111. doi: 10.1016/j.bandc.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M., Paulus M.P. Neurobiology of decision making: a selective review from a neurocognitive and clinical perspective. Biol. Psychiatry. 2005;58(8):597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Evans G.W., Kim P. Multiple risk exposure as a potential explanatory mechanism for the socioeconomic status–health gradient. Ann. N. Y. Acad. Sci. 2010;1186(1):174–189. doi: 10.1111/j.1749-6632.2009.05336.x. [DOI] [PubMed] [Google Scholar]
- Evans G.W., Schamberg M.A. Childhood poverty, chronic stress, and adult working memory. Proc. Natl. Acad. Sci. 2009;106(16):6545–6549. doi: 10.1073/pnas.0811910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J.S.B., Stanovich K.E. Dual-process theories of higher cognition: advancing the debate. Perspect. Psychol. Sci. 2013;8(3):223–241. doi: 10.1177/1745691612460685. [DOI] [PubMed] [Google Scholar]
- Falk E.B., Hyde L.W., Mitchell C., Faul J., Gonzalez R., Heitzeg M.M. What is a representative brain? Neuroscience meets population science. Proc. Natl. Acad. Sci. 2013;110(44):17615–17622. doi: 10.1073/pnas.1310134110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah M.J., Shera D.M., Savage J.H., Betancourt L., Giannetta J.M., Brodsky N.L. Childhood poverty: Specific associations with neurocognitive development. Brain Res. 2006;1110(1):166–174. doi: 10.1016/j.brainres.2006.06.072. [DOI] [PubMed] [Google Scholar]
- Finn P.R. Motivation, working memory, and decision making: a cognitive-motivational theory of personality vulnerability to alcoholism. Behav. Cogn. Neurosci. Rev. 2002;1(3):183–205. doi: 10.1177/1534582302001003001. [DOI] [PubMed] [Google Scholar]
- Frankenhuis W.E., Panchanathan K., Nettle D. Cognition in harsh and unpredictable environments. Curr. Opin. Psychol. 2016;7:76–80. [Google Scholar]
- French K., Finkbiner R., Duhamel L. Caliber Associates; Fairfax, VA: 2002. Patterns of Substance Use among Minority Youth and Adults in the United States: an Overview and Synthesis of National Survey Findings. [Google Scholar]
- Galvan F.H., Caetano R. Alcohol use and related problems among ethnic minorities in the United States. Alcohol Res. Health. 2003;27(1):87–95. [PMC free article] [PubMed] [Google Scholar]
- Galvan A., Rahdar A. The neurobiological effects of stress on adolescent decision making. Neuroscience. 2013;249:223–231. doi: 10.1016/j.neuroscience.2012.09.074. [DOI] [PubMed] [Google Scholar]
- Ganzel B.L., Morris P.A., Wethington E. Allostasis and the human brain: integrating models of stress from the social and life sciences. Psychol. Rev. 2010;117(1):134. doi: 10.1037/a0017773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel S.N., Zorab E., Navaratnam N., Engels M., Mallorquí-Bagué N., Minati L. Anger in brain and body: The neural and physiological perturbation of decision-making by emotion. Soc. Cogn. Affect. Neurosci. 2015;11(1):150–158. doi: 10.1093/scan/nsv099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J.C., Amlung M.T., Palmer A.A., MacKillop J. Syntax for calculation of discounting indices from the monetary choice questionnaire and probability discounting questionnaire. J. Exp. Anal. Behav. 2016;106(2):156–163. doi: 10.1002/jeab.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hair N.L., Hanson J.L., Wolfe B.L., Pollak S.D. Association of child poverty, brain development, and academic achievement. JAMA Pediatr. 2015;169(9):822–829. doi: 10.1001/jamapediatrics.2015.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaker E., Ceulemans E., Grasman R., Tuerlinckx F.J. Modeling affect dynamics: state of the art and future challenges. Emot. Rev. 2015;7(4):316–322. [Google Scholar]
- Hanson J.L., Chandra A., Wolfe B.L., Pollak S.D. Association between income and the hippocampus. PLoS One. 2011;6(5) doi: 10.1371/journal.pone.0018712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden K.P., Tucker-Drob E.M. Individual differences in the development of sensation seeking and impulsivity during adolescence: further evidence for a dual systems model. Dev. Psychol. 2011;47(3):739. doi: 10.1037/a0023279. [DOI] [PubMed] [Google Scholar]
- Haushofer J., Fehr E. On the psychology of poverty. Science. 2014;344(6186):862–867. doi: 10.1126/science.1232491. [DOI] [PubMed] [Google Scholar]
- Hinson J.M., Jameson T.L., Whitney P. Impulsive decision making and working memory. J. Exp. Psychol. Learn. Mem. Cogn. 2003;29(2):298. doi: 10.1037/0278-7393.29.2.298. [DOI] [PubMed] [Google Scholar]
- Holz N.E., Boecker R., Hohm E., Zohsel K., Buchmann A.F., Blomeyer D. The long-term impact of early life poverty on orbitofrontal cortex volume in adulthood: results from a prospective study over 25 years. Neuropsychopharmacology. 2015;40(4):996. doi: 10.1038/npp.2014.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Lt., Bentler P.M. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct. Equ. Model. A Multidiscip. J. 1999;6(1):1–55. [Google Scholar]
- Ifcher J., Zarghamee H. Happiness and time preference: the effect of positive affect in a random-assignment experiment. Am. Econ. Rev. 2011;101(7):3109–3129. [Google Scholar]
- Jensen O., Tesche C.D. Frontal theta activity in humans increases with memory load in a working memory task. Eur. J. Neurosci. 2002;15(8):1395–1399. doi: 10.1046/j.1460-9568.2002.01975.x. [DOI] [PubMed] [Google Scholar]
- Johnson P.O., Neyman J. Tests of certain linear hypotheses and their application to some educational problems. Stat. Res. Mem. 1936 [Google Scholar]
- Johnson S.B., Riis J.L., Noble K.G. State of the art review: poverty and the developing brain. Pediatrics. 2016 doi: 10.1542/peds.2015-3075. peds. 2015-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster R.-P., McEwen B.S., Lupien S.J. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci. Biobehav. Rev. 2010;35(1):2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Juster R.-P., Bizik G., Picard M., Arsenault-Lapierre G., Sindi S., Trepanier L. A transdisciplinary perspective of chronic stress in relation to psychopathology throughout life span development. Dev. Psychopathol. 2011;23(3):725–776. doi: 10.1017/S0954579411000289. [DOI] [PubMed] [Google Scholar]
- Kidd C., Palmeri H., Aslin R.N. Rational snacking: young children’s decision-making on the marshmallow task is moderated by beliefs about environmental reliability. Cognition. 2013;126(1):109–114. doi: 10.1016/j.cognition.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P., Evans G.W., Angstadt M., Ho S.S., Sripada C.S., Swain J.E. Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proc. Natl. Acad. Sci. 2013 doi: 10.1073/pnas.1308240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby K.N., Petry N.M., Bickel W.K. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J. Exp. Psychol. Gen. 1999;128(1):78. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Kircanski K., White L.K., Tseng W.-L., Wiggins J.L., Frank H.R., Sequeira S. A latent variable approach to differentiating neural mechanisms of irritability and anxiety in youth. JAMA Psychiatry. 2018 doi: 10.1001/jamapsychiatry.2018.0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishiyama M.M., Boyce W.T., Jimenez A.M., Perry L.M., Knight R.T. Socioeconomic disparities affect prefrontal function in children. J. Cogn. Neurosci. 2009;21(6):1106–1115. doi: 10.1162/jocn.2009.21101. [DOI] [PubMed] [Google Scholar]
- Lahey B.B., McNealy K., Knodt A., Zald D.H., Sporns O., Manuck S.B. Using confirmatory factor analysis to measure contemporaneous activation of defined neuronal networks in functional magnetic resonance imaging. Neuroimage. 2012;60(4):1982–1991. doi: 10.1016/j.neuroimage.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenecker S.A., Nielson K.A. Frontal recruitment during response inhibition in older adults replicated with fMRI. Neuroimage. 2003;20(2):1384–1392. doi: 10.1016/S1053-8119(03)00372-0. [DOI] [PubMed] [Google Scholar]
- Leonard J.A., Mackey A.P., Finn A.S., Gabrieli J.D. Differential effects of socioeconomic status on working and procedural memory systems. Front. Hum. Neurosci. 2015;9 doi: 10.3389/fnhum.2015.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung A.W., Alain C. Working memory load modulates the auditory “What” and “Where” neural networks. Neuroimage. 2011;55(3):1260–1269. doi: 10.1016/j.neuroimage.2010.12.055. [DOI] [PubMed] [Google Scholar]
- Lezak M., Howieson D., Bigler E., Tranel D. 2012. Neuropsychological assessment: OUP USA. [Google Scholar]
- Lipina S.J., Posner M.I. The impact of poverty on the development of brain networks. Front. Hum. Neurosci. 2012;6 doi: 10.3389/fnhum.2012.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipina S.J., Posner M.I. The impact of poverty on the development of brain networks. Front. Hum. Neurosci. 2012;6:238. doi: 10.3389/fnhum.2012.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipina S.J., Martelli M.I., Vuelta B., Colombo J.A. Performance on the A-not-B task of Argentinean infants from unsatisfied and satisfied basic needs homes. Int. Am. J. Psychol. 2005;39(1) [Google Scholar]
- Lovallo W.R. Early life adversity reduces stress reactivity and enhances impulsive behavior: implications for health behaviors. Int. J. Psychophysiol. 2013;90(1):8–16. doi: 10.1016/j.ijpsycho.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo W.R., Farag N.H., Sorocco K.H., Acheson A., Cohoon A.J., Vincent A.S. Early life adversity contributes to impaired cognition and impulsive behavior: studies from the Oklahoma Family Health Patterns Project. Alcohol. Clin. Exp. Res. 2013;37(4):616–623. doi: 10.1111/acer.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo W.R., Cohoon A.J., Acheson A., Sorocco K.H., Vincent A.S. Blunted stress reactivity reveals vulnerability to early life adversity in young adults with a family history of alcoholism. Addiction. 2018 doi: 10.1111/add.14501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J., Belden A., Botteron K., Marrus N., Harms M.P., Babb C. The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatr. 2013;167(12):1135–1142. doi: 10.1001/jamapediatrics.2013.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B., Garver K.E., Urban T.A., Lazar N.A., Sweeney J.A. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75(5):1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- MacKillop J., Amlung M.T., Few L.R., Ray L.A., Sweet L.H., Munafò M.R. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology. 2011;216(3):305–321. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon D.P., Fritz M.S., Williams J., Lockwood C.M. Distribution of the product confidence limits for the indirect effect: program PRODCLIN. Behav. Res. Methods. 2007;39(3):384–389. doi: 10.3758/bf03193007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malesza M. Stress and delay discounting: the mediating role of difficulties in emotion regulation. Pers. Individ. Dif. 2019;144:56–60. [Google Scholar]
- Mani A., Mullainathan S., Shafir E., Zhao J. Poverty impedes cognitive function. Science. 2013;341(6149):976–980. doi: 10.1126/science.1238041. [DOI] [PubMed] [Google Scholar]
- McArdle J.J., Hamagami F. 2001. Latent Difference Score Structural Models for Linear Dynamic Analyses with Incomplete Longitudinal Data. [Google Scholar]
- McCabe D.P., Roediger H.L., III, McDaniel M.A., Balota D.A., Hambrick D.Z. The relationship between working memory capacity and executive functioning: evidence for a common executive attention construct. Neuropsychology. 2010;24(2):222. doi: 10.1037/a0017619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure S.M., Laibson D.I., Loewenstein G., Cohen J.D. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306(5695):503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- McClure S.M., Ericson K.M., Laibson D.I., Loewenstein G., Cohen J.D. Time discounting for primary rewards. J. Neurosci. 2007;27(21):5796–5804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Gianaros P.J.J.Ar. Stress-and allostasis-induced brain plasticity. Annu. Rev. Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills K.L., Goddings A.-L., Clasen L.S., Giedd J.N., Blakemore S.-J. The developmental mismatch in structural brain maturation during adolescence. Dev. Neurosci. 2014;36(3-4):147–160. doi: 10.1159/000362328. [DOI] [PubMed] [Google Scholar]
- Miu A.C., Heilman R.M., Houser D. Anxiety impairs decision-making: psychophysiological evidence from an Iowa Gambling Task. Biol. Psychol. 2008;77(3):353–358. doi: 10.1016/j.biopsycho.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Mueller S.C., Maheu F.S., Dozier M., Peloso E., Mandell D., Leibenluft E. Early-life stress is associated with impairment in cognitive control in adolescence: an fMRI study. Neuropsychologia. 2010;48(10):3037–3044. doi: 10.1016/j.neuropsychologia.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén L., Muthén B. 1998. Mplus user’s guide. 7. 2012. [Google Scholar]
- Noble K.G., Houston S.M., Brito N.H., Bartsch H., Kan E., Kuperman J.M. Family income, parental education and brain structure in children and adolescents. Nat. Neurosci. 2015;18(5):773. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen P.J., Westerberg H., Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nat. Neurosci. 2004;7(1):75–79. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- Oshri A., Kogan S.M., Kwon J.A., Wickrama K., Vanderbroek L., Palmer A.A., Mackillop J. Impulsivity as a mechanism linking child abuse and neglect with substance use in adolescence and adulthood. Dev. Psychopathol. 2017:1–19. doi: 10.1017/S0954579417000943. [DOI] [PubMed] [Google Scholar]
- Oshri A., Duprey E.B., Kogan S.M., Carlson M.W., Liu S. Growth patterns of future orientation among maltreated youth: a prospective examination of the emergence of resilience. Dev. Psychol. 2018;54(8):1456. doi: 10.1037/dev0000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshri A., Liu S., Duprey E.B., MacKillop J. Child maltreatment, delayed reward discounting, and alcohol and other drug use problems: the moderating role of heart rate variability. Alcohol. Clin. Exp. Res. 2018;42(10):2033–2046. doi: 10.1111/acer.13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen A.M., McMillan K.M., Laird A.R., Bullmore E. N‐back working memory paradigm: A meta‐analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005;25(1):46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens M.M., Duda B., Sweet L.H., MacKillop J. Distinct functional and structural neural underpinnings of working memory. NeuroImage. 2018 doi: 10.1016/j.neuroimage.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper G.V., Nettle D. The behavioural constellation of deprivation: causes and consequences. Behav. Brain Sci. 2017;40 doi: 10.1017/S0140525X1600234X. [DOI] [PubMed] [Google Scholar]
- Philip N.S., Sweet L.H., Tyrka A.R., Carpenter S.L., Albright S.E., Price L.H., Carpenter L.L. Exposure to childhood trauma is associated with altered n-back activation and performance in healthy adults: implications for a commonly used working memory task. Brain Imaging Behav. 2016;10(1):124–135. doi: 10.1007/s11682-015-9373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn P.D., Harden K.P. Differential changes in impulsivity and sensation seeking and the escalation of substance use from adolescence to early adulthood. Dev. Psychopathol. 2013;25(1):223–239. doi: 10.1017/S0954579412000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland J.D., Turetsky B.I., Gur R.C., Gunning-Dixon F., Turner T., Schroeder L. Working memory for complex figures: an fMRI comparison of letter and fractal n-back tasks. Neuropsychology. 2002;16(3):370. [PMC free article] [PubMed] [Google Scholar]
- Romer D., Duckworth A.L., Sznitman S., Park S. Can adolescents learn self-control? Delay of gratification in the development of control over risk taking. Prev. Sci. 2010;11(3):319–330. doi: 10.1007/s11121-010-0171-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin D.B., Little R.J. J Wiley & Sons; Hoboken, NJ: 2002. Statistical Analysis with Missing Data. [Google Scholar]
- Sale A., Berardi N., Maffei L. Enrich the environment to empower the brain. Trends Neurosci. 2009;32(4):233–239. doi: 10.1016/j.tins.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Schreiber L.R., Grant J.E., Odlaug B.L. Emotion regulation and impulsivity in young adults. J. Psychiatr. Res. 2012;46(5):651–658. doi: 10.1016/j.jpsychires.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman T., Epel E., Gruenewald T., Karlamangla A., McEwen B.S. Socio‐economic differentials in peripheral biology: Cumulative allostatic load. Ann. N. Y. Acad. Sci. 2010;1186(1):223–239. doi: 10.1111/j.1749-6632.2009.05341.x. [DOI] [PubMed] [Google Scholar]
- Selig J.P., Preacher K.J. Mediation models for longitudinal data in developmental research. Res. Hum. Dev. 2009;6(2-3):144–164. [Google Scholar]
- Shah A.K., Shafir E., Mullainathan S. Scarcity frames value. Psychol. Sci. 2015;26(4):402–412. doi: 10.1177/0956797614563958. [DOI] [PubMed] [Google Scholar]
- Shamosh N.A., DeYoung C.G., Green A.E., Reis D.L., Johnson M.R., Conway A.R. Individual differences in delay discounting: relation to intelligence, working memory, and anterior prefrontal cortex. Psychol. Sci. 2008;19(9):904–911. doi: 10.1111/j.1467-9280.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- Shulman E.P., Smith A.R., Silva K., Icenogle G., Duell N., Chein J., Steinberg L. The dual systems model: review, reappraisal, and reaffirmation. Dev. Cogn. Neurosci. 2016;17:103–117. doi: 10.1016/j.dcn.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E.E., Jonides J. Working memory: a view from neuroimaging. Cogn. Psychol. 1997;33(1):5–42. doi: 10.1006/cogp.1997.0658. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A dual systems model of adolescent risk‐taking. Dev. Psychobiol. 2010;52(3):216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- Steyer R., Eid M., Schwenkmezger P. Modeling true intraindividual change: true change as a latent variable. Methods Psychol. Res. Online. 1997;2(1):21–33. [Google Scholar]
- Stoodley C.J., Valera E.M., Schmahmann J.D. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage. 2012;59(2):1560–1570. doi: 10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet L.H., Rao S.M., Primeau M., Durgerian S., Cohen R.A. Functional magnetic resonance imaging response to increased verbal working memory demands among patients with multiple sclerosis. Hum. Brain Mapp. 2006;27(1):28–36. doi: 10.1002/hbm.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet L.H., Paskavitz J.F., Haley A.P., Gunstad J.J., Mulligan R.C., Nyalakanti P.K., Cohen R.A. Imaging phonological similarity effects on verbal working memory. Neuropsychologia. 2008;46(4):1114–1123. doi: 10.1016/j.neuropsychologia.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. 1988. Co-Planar Stereotaxic Atlas of the Human Brain. 3-Dimensional Proportional System: An Approach to Cerebral Imaging. [Google Scholar]
- Taylor S.E., Seeman T.E. Psychosocial resources and the SES‐health relationship. Ann. N. Y. Acad. Sci. 1999;896(1):210–225. doi: 10.1111/j.1749-6632.1999.tb08117.x. [DOI] [PubMed] [Google Scholar]
- Tofighi D., MacKinnon D.P. RMediation: an R package for mediation analysis confidence intervals. Behav. Res. Methods. 2011;43(3):692–700. doi: 10.3758/s13428-011-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt T.T. The race/ethnic age crossover effect in drug use and heavy drinking. J. Ethn. Subst. Abuse. 2008;7(1):93–114. doi: 10.1080/15332640802083303. [DOI] [PubMed] [Google Scholar]
- Wesley M.J., Bickel W.K. Remember the future II: meta-analyses and functional overlap of working memory and delay discounting. Biol. Psychiatry. 2014;75(6):435–448. doi: 10.1016/j.biopsych.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby T., Good M., Adachi P.J., Hamza C., Tavernier R. Examining the link between adolescent brain development and risk taking from a social–developmental perspective (reprinted) Brain Cogn. 2014;89:70–78. doi: 10.1016/j.bandc.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Yuan K.H., Bentler P.M. Three likelihood‐based methods for mean and covariance structure analysis with nonnormal missing data. Sociol. Methodol. 2000;30(1):165–200. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.