Abstract
The coevolution of parental supply and offspring demand has long been thought to involve offspring need driving begging and parental care, leaving other hypotheses underexplored. In a population of wild birds, we tested experimentally whether begging serves as a negatively condition-dependent signal of need or a positively condition-dependent signal of quality. Across multiple years, we food-supplemented nestling house wrens shortly after hatching, and simultaneously manipulated corticosterone levels to simulate the hunger-induced increase in glucocorticoids thought to mediate begging. This allowed us also to test whether begging is simply a proximate signal of hunger. Days after supplementation ended, food-supplemented nestlings were in better condition than non-supplemented nestlings and begged for food at an increased rate; their parents, in turn, increased provisioning to a greater extent than parents of non-supplemented young, as begging positively predicted provisioning. Food-supplemented nestlings, therefore, attained above-average condition, which predicted their recruitment as breeding adults in the local population. Glucocorticoids increased begging in the short-term, but this transient effect depended on satiety. Thus, glucocorticoids promoted begging as a proximate response to hunger, whereas the longer-term changes in nestling condition, begging, and food provisioning suggest that begging ultimately signals offspring quality to elicit increased investment, thereby enhancing offspring survival.
Keywords: corticosterone, glucocorticoid, house wren, life history, parental care, parent-offspring conflict
The evolutionary interests of parents and their offspring may not always be in perfect harmony, as maximizing the fitness of offspring requires that parents invest more resources in their offspring, which are limiting in time and space, than would maximize the parents’ own fitness (Trivers 1974; Mock and Parker 1997; Kölliker et al. 2005, 2012; Meunier and Kölliker 2012). Consequently, parents are generally thought to have been selected to invest less in any individual offspring than what would maximize a given offspring’s survival (Trivers 1974; Smith and Fretwell 1974; but see also Clutton-Brock 1984; Bowers et al. 2015a for examples of exceptions). Thus, it follows that selection should favor conspicuous behaviors in offspring that attract the attention of parents and increase the amount of food they receive. Indeed, altricial young solicit food in a variety of ways across taxa, including auditory and visual stimuli, to attract the attention of parents, and numerous hypotheses have been proposed to explain the diversity observed in these solicitations (Mock and Parker 1997; Drummond 2002; Roulin 2002; Kölliker et al. 2006; Trumbo 2012). The relationship between offspring signaling and parental provisioning has long been thought to involve decision rules governing parental responses to offspring begging, with parents responding to these signals through changes in provisioning (Leonard and Horn 1996, 1998; Ottoson et al. 1997; Burford et al. 1998; Saino et al. 2000; Kölliker and Richner 2001; Grodzinski and Lotem 2007).
For parents actively choosing which offspring to feed, or how much to invest in a brood as a whole, there are two main hypotheses explaining the information encoded in offspring begging solicitations in an ultimate, evolutionary framework (Mock 2011; Koykka and Wild 2018). The hypothesis that offspring begging is a signal of need (Godfray 1991, 1995a,b) has received the most attention from both theoreticians and empiricists (Price and Ydenberg 1995; Price et al. 1996; Kilner and Johnstone 1997; Cotton et al. 1999; Godfray and Johnstone 2000; Villaseñor and Drummond 2007; Koykka and Wild 2018). According to this model, parents should respond to the degree to which feeding a particular offspring will benefit that individual offspring’s fitness, and, thus, parents’ fitness. If this hypothesis is true, begging should be negatively condition dependent, whereby nestlings in poor condition (i.e., low reproductive value) should beg louder or more intensely than offspring in better condition as their fitness stands to increase the most from a unit increase in parental investment. As outlined by Mock et al. (2011), this hypothesis makes four key assumptions: (i) parents favor an equal investment in all of their offspring such that all offspring survive to leave the nest; (ii) siblings cooperate over which of them should get fed at any point in time (i.e., subordinate siblings receiving food when they need it likely depends on older, competitively dominant siblings not begging even if it might improve their personal fitness); (iii) individual nestlings are able to assess their own reproductive value (or level of need) and, thus, the marginal increase in their reproductive value accrued by a unit increase in food; and (iv) begging is ‘honest’ and reliably conveys information regarding a nestling’s need to parents and to nestmates. Some of these assumptions are not always satisfied in natural families, raising the possibility that begging signals offer different kinds of information to parents (Mock and Parker 1997; Cotton et al. 1999; Mock et al. 2011). For example, Lotem (1998) experimentally manipulated brood size to create enlarged broods and smaller broods, and found that the putatively neediest nestlings – small nestlings from large broods with many siblings – begged less than larger nestlings in a similar environment, a result that cannot be explained by the signal-of-need hypothesis. Indeed, with per-nestling food availability usually lower within larger broods than within smaller broods (Bowers et al. 2014c), the findings of Lotem (1998) suggest offspring may be signaling information other than need to their parents (Royle et al. 2002).
In contrast to the signal-of-need hypothesis, the signal-of-quality hypothesis postulates that feeding the offspring with the greatest chance of survival is likely a better investment than feeding offspring in poor condition and low probability of survival. In other words, the degree to which feeding a particular offspring benefits parental fitness is not driven by selection to increase the fitness of offspring in poor condition, but because feeding offspring in good condition will likely provide parents a greater return on their investment (Grafen 1990). In this view, begging is positively condition dependent (i.e., begging is a signal of quality). This model is somewhat simpler than the signal of need because it does not require kin-selected cooperation among family members – i.e., in most situations the larger, competitively dominant siblings usually command the lion’s share of food (Parker et al. 1989; Mock and Parker 1997; Cotton et al. 1999). Begging as a signal of quality also does not require that parents spread their investment equally among all offspring. Indeed, it is well established that females of many species produce more eggs and hatchlings than the number of fledglings surviving to leave the nest. This is commonly thought of as a form of bet hedging to protect against unpredictable food availability (Wiebe and Bortolotti 1994; Amundsen and Slagsvold 1998; Mock and Parker 1997), as a decline in food availability when rearing nestlings will likely lead to the youngest hatchling(s) being the first to die, early in life, before they reduce the amount of resources that would better serve parental fitness by being delivered to the older siblings. Thus, the distinction made by parents between a good investment and a wasted one may be facilitated if offspring can communicate who among them is the most likely to survive harsh conditions (Lotem 1998). Thus, for begging to serve as a signal of quality, the highest-quality offspring should be able to afford the energetic costs of begging (e.g., Kilner 2001) to a greater extent than their lower-quality, subordinate siblings (Royle et al. 2002), allowing parents to make clear associations between begging intensity and the probability of a return on their investment.
What mediates the expression of offspring solicitations? What prompts offspring to express those behaviors that enhance survival? In addition to the adaptive significance of condition-dependent offspring signaling, a proximate perspective may also shed light on what is perhaps a more parsimonious explanation for offspring begging, namely hunger (Mock et al. 2011). Detecting one’s own satiety at a physiological level, and expending energy to obtain food and regain homeostasis, suggests a role for the hypothalamus in regulating begging solicitations. Part of the limbic system (i.e., the emotional center of the brain), the hypothalamus has long been known to drive the physiological manifestation of hunger and thirst across vertebrate taxa, in addition to regulating growth, aggression, and sexual behavior. Moreover, acute stress, such as that which occurs when an individual is deprived of food, is often associated with emotional distress and increases in glucocorticoid production, in addition to affecting circulating growth hormone levels and thyroid hormones, also driven by the hypothalamus (e.g., McEwen 2007; Chrousos 2009). In short, corticotropin-releasing factor from the hypothalamus stimulates the release of adrenocorticotropic hormone from the anterior pituitary, which, in turn, stimulates glucocorticoid (e.g., corticosterone and cortisol) secretion from the adrenal gland. In the context of nestling begging and growth, hypothalamic function may drive these processes through its influence on a variety of mechanisms, including the production of glucocorticoids, which can enhance learning through long-term potentiation (Kerr et al. 1994; see also Kedar et al. 2000 for an example of cognitive changes in begging by altricial nestlings in response to parental feeding frequency). Increases in glucocorticoid secretion are often induced by hunger (Sapolsky et al. 2000; Kitaysky et al. 2001; Schwabl and Lipar 2002; but see also Kitaysky et al. 2005), and there is evidence that corticosterone (the major avian glucocorticoid) either in the egg or that which is endogenously produced by nestlings can affect their begging solicitations (Kitaysky et al. 2001; Schwabl and Lipar 2002; Love and Williams 2008; Smiseth et al. 2011; Bowers et al. 2016a). Thus, a nestling’s interest in being fed constitutes a more proximate hypothesis of begging as a signal of hunger that should be thought of as being distinct from need in an adaptive sense (Clark 2002; Grodzinski and Lotem 2007; Mock et al. 2011; Mock 2016; Fresneau et al. 2018). This signal of hunger may indeed be more parsimonious than the signal-of-need and signal-of-quality hypotheses (Mock et al. 2011). However, empirical studies have revealed that effects of corticosterone on offspring development are often context-dependent (Love et al. 2005; Chin et al. 2009; Sheriff and Love 2013; Weber et al. 2018); thus, effects of glucocorticoids on begging solicitations may depend on the level of satiety.
In this study, we test the signal-of-need and signal-of-quality hypotheses in an ultimate, adaptive context. Under both hypotheses, offspring signals communicate condition and the signaler’s potential fitness, but in opposing directions. To discriminate between these hypotheses, we augmented nestling condition by experimentally supplementing their diets for four days posthatching by pipetting food into their mouths and observing their swallowing of the food, ensuring that nestlings received the intended supplement (Mock et al. 2005). We assessed nestling begging and parental provisioning twice, once during the period of supplementation and two days after food supplementation ended (fig. 1); thus, our design also allows us to test the hypothesis that begging serves as a more proximate signal of hunger during our first observation period. We predicted that, when experimental nestlings were fed immediately before our observations of begging and provisioning, these nestlings should be less hungry and beg for food at a reduced rate; thus, these observations should be reflective of short-term variation in satiety. As part of this proximate perspective, we also assessed a role for glucocorticoids by combining with the food-supplement treatment a corticosterone treatment in which experimental nestlings were fed corticosterone, creating a factorial design allowing us to test for a context-dependent effect of glucocorticoids on nestling begging. We predicted that increases in glucocorticoids within unfed nestlings would prompt an increase in begging, but expected that this orally provided corticosterone would be rapidly cleared from nestling circulation (e.g., within minutes; Kahn and Robert 2013). Thus, begging during this observation should be reflective of short-term, neuroendocrine responses to hunger in a proximate sense. For our second observation period (fig. 1), we predicted that if the condition (i.e., probability of survival) of offspring is augmented by supplemental food, this should affect offspring begging in different directions depending on whether the signal-of-need or signal-of-quality hypothesis is more likely to be true. The signal-of-need hypothesis predicts that experimentally fed young should beg less than non-experimental nestlings (i.e., begging is negatively condition-dependent), but the signal-of-quality hypothesis predicts that the experimental nestlings with enhanced condition should beg more than non-experimental nestlings (i.e., begging is positively condition-dependent). In other words, differences in nestling condition between groups should predict differences in their begging vocalizations, and parents should respond to this variation through food provisioning to enhance postfledging survival and recruitment.
Figure 1.
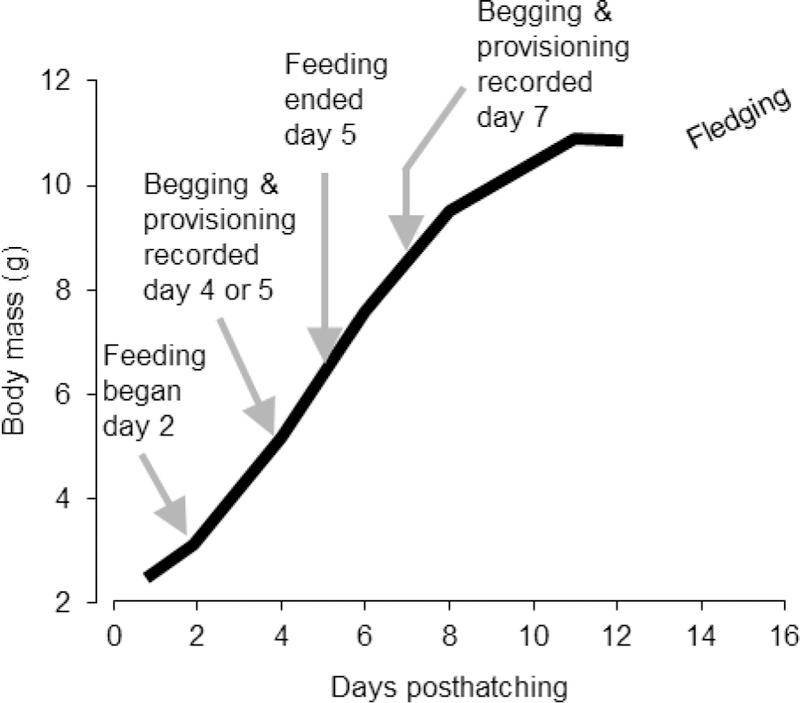
Schematic depiction of the timing of our treatments (feeding of food and corticosterone) and observations of nestling begging and adult provisioning (growth data from Bowers et al. 2015c).
Methods
Study area and species
House wrens (Troglodytes aedon) are secondary-cavity-nesting songbirds with a widespread distribution across North and South America (Johnson 2014). We studied a migratory population breeding in secondary deciduous forest in McLean County, Illinois, USA (40.665°N, 88.89°W) from 2014–2018. Females select a mate that is defending a nesting site and produce one egg per day until their clutch is completed. House wrens are multibrooded, with a modal clutch size of 7 eggs for nests produced during the first half of the breeding season, and 6 eggs for those produced later within seasons (Johnson 2014). Only females incubate eggs and brood nestlings, but both parents provision offspring with food after hatching, and the length of the nestling period is typically ca. 15 days (Bowers et al. 2016b). Over the 10 years preceding this study (2004–2013), brood reduction occurred in 49.0 ± 0.1 % (mean ± SD) of unmanipulated nests that fledged at least one young, typically biased against late- or last-hatching runts (Johnson 2014), and average brood size was 5.9 ± 0.1 young (mean ± SD) after hatching and 5.1 ± 0.3 at fledging. House wrens readily accept nestboxes for nesting, and the nestboxes in this study were spaced 30 m apart along north-south transects separated by 60 m and mounted atop 48.3-cm diameter aluminum predator baffles on 1.5-m poles. Lambrechts et al. (2010) provide further details on nestboxes.
Procedures
During incubation or early in the nestling-rearing stage, we captured adults inside nestboxes or by using mist nets near the box throughout the 2014–2015 breeding seasons (May-August). We measured their body mass (± 0.1 g) and tarsus length (± 0.1 mm), and banded them all with a numbered U. S. Geological Survey leg band. Males received three additional colored bands in a unique combination so they could be identified visually without needing to be recaptured (males are more difficult to capture at the nest than females).
We located nests in the egg-laying stage and, upon hatching, assigned whole broods at random to (i) one of three food treatments (experimental, control, natural/unmanipulated) and (ii) one of three corticosterone treatments (experimental, control, natural/unmanipulated), such that all nine possible treatment combinations were represented in a fully factorial design (N = 146 broods; table A1). New treatments were alternated by the order in which clutches hatched. Treatments were applied to all nestlings within a given brood once each day on days 2–5 posthatching (fig. 1) by pipetting the supplemental food and oil (the corticosterone vehicle) into the nestlings’ mouths, which they then swallowed. Because experimental changes in the begging of individual nestlings can often induce changes in begging by their non-experimental siblings within broods (Elderbrock et al. 2018), we treated all nestlings within broods in the same manner, such that the brood was the unit of replication, thereby removing the issues of sibling-induced changes in begging and non-independence among experimental units (Forbes et al. 2002). For the food treatment, we supplemented the diet of experimental broods with commercially available baby bird food (Kaytee “exact” hand-feeding formula for baby birds; Kaytee Products, Inc.) dissolved in water following the manufacturer’s instructions. This formulation is commonly used in food-supplementation experiments (e.g., Mock et al. 2005) and is a complete source of nutrition for hand-fed nestling passerines (e.g., Soha et al. 2009). The amount of food and water received varied with nestling age, and resulted in day 2 nestlings (i.e., 2 d after hatching began within a given nest) receiving 200 μL, day 3 nestlings 300 μL, day 4 nestlings 400 μL, and day 5 nestlings 500 μL. Control broods were fed with the water vehicle only in equivalent volumes as those in the experimental group. Natural, unmanipulated broods were not supplemented, but had a pipette tip inserted briefly into their mouths, as in the other treatments.
For the corticosterone treatment, we fed experimental broods with corticosterone (product number 27840–500MG, Sigma-Aldrich Corp. St. Louis, MO, USA) at mass-specific dosage concentrations following Strange et al. (2016), each dissolved in 15 μL of peanut oil. Peanut oil is a widely used vehicle containing high amounts of unsaturated fatty acids, namely linoleic and oleic acids. Passerines prefer diets with unsaturated fats (specifically linoleic and oleic acids), and these fatty acids are commonly found in insects (see summary in Ríos et al. 2014). Control broods were fed only the peanut oil vehicle in equivalent volumes as those in the experimental group, whereas natural, unmanipulated broods were not supplemented, but had a pipette tip inserted briefly into their mouths, as in the other treatments. This oral administration causes a 4- to 5-fold increase in circulating corticosterone within minutes of dosing (Strange et al. 2016), comparable to the increase in corticosterone observed following 30 minutes of restraint stress (Weber et al. 2018). Thus, our oral dosing causes increases in corticosterone within a physiologically relevant range, consistent with what is produced in response to putatively stressful events (see also Pakkala et al. 2016). Similarly, Kitaysky et al. (2001) experimentally increased offspring corticosterone by a similar magnitude above baseline as our 4–5-fold increase, and they observed a resulting corticosterone level that was identical to what is observed in food-deprived young (Kitaysky et al. 2001).
In an earlier study (Bowers et al. 2016), we found that nestlings exposed to maternally derived corticosterone within the egg begged at a higher rate than control nestlings after hatching, as some yolk of maternal origin is retained within hatchlings, and has activational effects on behavior (in addition to organizational effects during embryonic development; Schwabl & Lipar 2002). It is important to note, however, that hunger may not be the only motivation for increasing begging, and corticosterone may not mediate all changes in begging. Nonetheless, there is no evidence that nestlings produce begging calls in situations that cause them discomfort, such as being exposed to an unpleasant taste. A previous study of nestling begging vocalizations in another population of house wrens (Sawhney et al. 2006) reported that these calls were given only when nestlings were attempting to attract the attention of a parent; they did not report any other contexts in which begging calls were expressed. Given the poor taste acuity of birds and our feeding procedure, the effect on nestlings also does not appear to be a response to an unpleasant taste. Birds are generally thought to have a poor sense of taste, as taste buds in birds are universally low in number. Blue tits, for example, have only 24 compared with 9000 in humans, and in passerines they are confined to the palate and the posterior of the tongue (Klasing 1998), both of which are anterior to the region where we inserted the pipet tip.
Immediately following supplementation on day 4 posthatching (or occasionally on day 5 if rain on day 4 precluded an observation of sufficient duration), we recorded nestling begging vocalizations using a small microphone within nests attached to a digital voice recorder outside the nest (Barnett et al. 2011; Bowers et al. 2016a). We then returned to the nest on day 7 posthatching to record begging vocalizations again, this time without having supplemented the nestlings beforehand. We also observed parental food provisioning during both begging recordings by filming parental provisioning to nests using pocket-sized digital video cameras (Kodak Sport Zx5 or Zx1, Eastman Kodak, Rochester, NY) positioned ca. 1.5 m from the nestbox and secured at the top of a 1.5-m length of metal conduit using a cell-phone holster (Barnett et al. 2011; Bowers et al. 2016a). Adults were habituated to the presence of the metal pole and camera by setting up the pole and a dummy camera 24 h before the actual recording began. We video-recorded nests for approximately 100 min or more between 0630 and 1100 h Central Daylight Time, and observed the 60 min following supplementation, which provides a representative sample of parental behavior (Murphy et al. 2015). Parental provisioning assessed using this approach is especially relevant, as the ages at which we observed begging and provisioning are periods of rapid nestling growth, and the amount of food delivered by parents is positively correlated with nestling growth, fledging success, and the recruitment of offspring into the breeding population (Bowers et al. 2014a,c, 2015a). Following the observation of begging and provisioning on day 7, we subsequently monitored nests and banded nestlings on day 11 posthatching, 3–5 days prior to normal fledging age. At the time of banding, we weighed nestlings and measured their tarsus length to assess size-adjusted body mass (García-Berthou 2001). When measured prior to fledging, this size-adjusted mass positively predicts offspring recruitment and their reproductive success in the population as adults (Bowers et al. 2014a, 2015c), and, when measured in adulthood during breeding, maternal lifetime allelic fitness (Sakaluk et al. 2018). We checked nests daily beginning on day 13 to check for fledging. We also attempted to capture all adults breeding in subsequent years through the 2018 breeding season to assess the recruitment of offspring into the population as breeding adults.
In the laboratory, we counted begging vocalizations using Raven Pro 1.5 (Cornell Lab of Ornithology). By targeting properties of the begging calls (e.g., frequency/pitch, duration), we were able to count the number of vocalizations efficiently and accurately in an automated fashion, with analysis of each file optimized according to properties of individual nests. For example, adult male house wrens sing near the nests and can be heard in the recordings, but their song is at a slightly lower pitch than the begging signals, and our method therefore excludes components of the resident male’s song as false positives. We assessed the accuracy of our approach by manually comparing the actual frequency of begging signals in a randomly selected subset of files (see fig. A1 in the appendix, available online) with the values obtained using Raven. Although false positives and false negatives did occasionally occur, this approach was able to count many signals with a high degree of accuracy (R2 > 0.97; fig. A1). Begging recordings and videos of parental provisioning were scored blind with respect to treatment.
Figure A1.
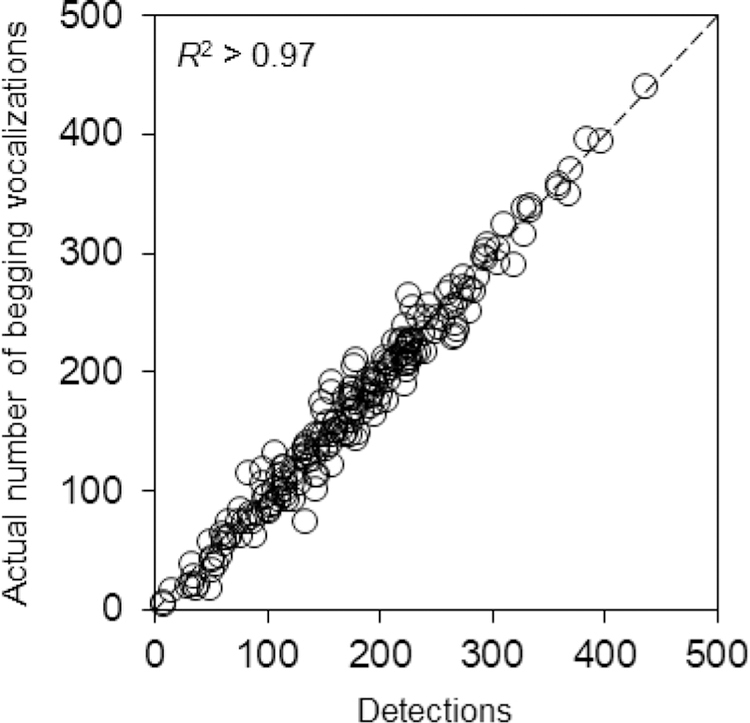
Accuracy of Raven software. Accuracy was assessed using ten randomly selected clips (10 sec each) from each begging file (total of 100 sec per file). The sum total of detections is compared with the actual number of begging signals produced by nestlings. The dashed diagonal indicates perfect correspondence. Each point represents a single file (N = 195).
Data and analyses
We used SAS (version 9.4) for all analyses, all tests are two-tailed (α = 0.05), and we used Satterthwaite’s degrees-of-freedom approximation, which can result in non-integer denominator degrees of freedom. Whenever appropriate, we also included nest as a random effect to account for the non-independence of multiple observations within the same nest. We first assessed effects of the food and corticosterone supplementation on nestling begging immediately following the treatment application using linear models with food and corticosterone treatments as crossed main effects, and included brood size as a covariate. We anticipated that the effect of corticosterone would be short-lived, so we subdivided the observation period into 5-min increments to test for effects on nestling begging in the first 5 min following supplementation, during min 6–10 post-treatment, etc., but did not analyze effects of corticosterone beyond 10 min because these effects disappeared by min 6–10 (see Results). We then assessed effects of food supplementation on nestling begging during the entirety of the hour-long observations using a repeated-measures ANOVA, with the hour subdivided into six 10-min segments to assess changes occurring over the course of the hour. Here we included the food treatment, time since the treatment (i.e., time since the beginning of the observation), and the treatment × time interaction, in addition to brood size, hatching date, and an interaction between brood size and time as we expected that broods with more siblings would increase begging over time more rapidly than broods with fewer siblings.
We then assessed nestling growth from day 2 posthatching (i.e., the beginning of the supplementations) to day 5 posthatching (i.e., the last day of the supplementations) using a repeated-measures ANOVA with the food and corticosterone treatments, time since the beginning of the observation, and the food × time and corticosterone × time interactions in addition to hatching date and brood size as covariates. We analyzed brood means for body mass, as nestlings within broods were not uniquely identifiable until being banded on day 11. We then conducted a repeated-measures ANOVA of nestling begging vocalizations per nestling per hr at the two different ages with the food treatment and nestling age as crossed fixed effects, with hatching date as a covariate. We assessed total parental food deliveries to the nest using a similar approach, but we included brood size as a covariate in this analysis (we did not include brood size in the analysis of begging vocalizations per hr because brood size was included as part of the calculation of the dependent variable). We then analyzed nestling prefledging mass on day 11 posthatching with food treatment, and included hatching date, brood size, and tarsus length as covariates. The analysis is, thus, reflective of size-adjusted body mass or condition (García-Berthou 2001; but see also Barnett et al. 2015). Finally, we assessed whether increased nestling mass prior to fledging affected the recruitment of these offspring as adult breeders within the population in subsequent years. Data underlying this article are deposited in the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.53885k7 (Bowers et al. 2019).
Results
Nestling begging
Corticosterone supplementation had an immediate, but transient, effect on nestling begging solicitations within 5 min of supplementation, but this effect only occurred within the unmanipulated food treatment (fig. 2A), as there was an interaction between the corticosterone- and food-supplementation treatments in their effect on nestling begging (table 1). Follow-up tests revealed that, among the natural nestlings not supplemented with food, experimental increases in corticosterone led to a significant increase in begging vocalizations relative to control and unmanipulated nestlings within 5 min of treatment (F1, 26 = 17.03, P < .001; fig. 2A), but, at the same time, there was no demonstrable effect of corticosterone on begging within either the experimental food treatment (F1, 30 = 2.98, P = .095) or the control food treatment (F1, 30 = 1.96, P = .172). The effect of corticosterone on begging was short-lived, however, as the interaction and main effect of corticosterone supplementation disappeared after the first 5 min post-treatment (table 1; fig. 2A). Consequently, there were no effects of corticosterone supplementation overall on begging vocalizations or parental provisioning per hour, nor on nestling body mass at any point during development (see below). Thus, when assessing begging and provisioning at the level of the brood overall (e.g., food deliveries or begging vocalizations per hour), we pooled all corticosterone treatments within each food treatment to test for effects of supplemental feeding.
Figure 2.
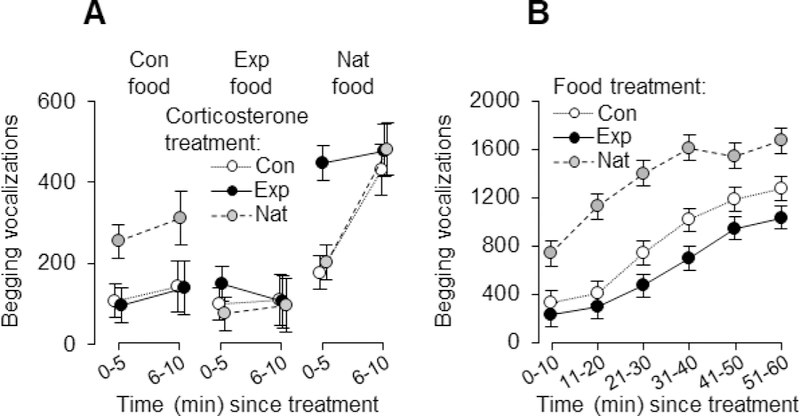
Interactive effects of food and corticosterone supplementation were apparent within the first 5 min of treatment application on day 4/5, but disappeared in min 6–10 (A). Food supplementation affected nestling begging during the entirety of our hour-long observation on this day (B), with unfed nestlings begging at a higher rate than nestlings fed either food and water or water only. Plotted are least-squares means ± SE. Filled black symbols depict experimental (Exp) nests for either the corticosterone (A) or food (B) supplement, open symbols depict control (Con) nests in which nestlings were fed the vehicle only, and filled grey symbols depict unmanipulated, natural (Nat) nests that were not supplemented.
Table 1:
Effects on nestling begging on day 4/5 posthatching, immediately following application of the food and corticosterone treatments
| F | df | P | |
|---|---|---|---|
| 0−5 min post-treatment: | |||
| Food treatment | 11.07 | 2, 87.1 | < .001 |
| Corticosterone treatment | 4.17 | 2, 87.9 | .019 |
| Food × corticosterone | 6.30 | 4, 87.9 | < .001 |
| Brood size | .93 | 1, 87.9 | .338 |
| 5–10 min post-treatment: | |||
| Food treatment | 21.79 | 2, 88.0 | < .001 |
| Corticosterone treatment | .93 | 2, 88.0 | .398 |
| Food × corticosterone | .78 | 2, 88.0 | .544 |
| Brood size | 3.21 | 2, 88.0 | .076 |
Food supplementation affected nestling begging during the entire hour-long recording session on day 4/5 (table 2; fig. 2B), with unfed nestlings begging at a higher rate than experimental nestlings (supplemented with food and water) and control nestlings (supplemented with water only). Nestling begging generally increased over the course of the hour since supplementation, but to a lesser extent for food-supplemented nestlings (fig. 2B). The rate of this increase also varied with brood size, as indicated by an interaction between brood size and time since the supplementation and beginning of our observation, with larger broods associated with a more rapid increase in begging (table 2; fig. 3).
Table 2:
Effects on nestling begging on day 4/5 posthatching, immediately following application of the food treatment. Time is assessed here as 10-min intervals since the treatment was applied for 60 min
| F | df | P | |
|---|---|---|---|
| Food treatment | 20.81 | 2, 110 | < .001 |
| Time since treatment | .54 | 5, 442 | .744 |
| Food × time | 2.59 | 10, 442 | .005 |
| Brood size | 17.45 | 1, 110 | < .001 |
| Hatching date | 5.56 | 1, 109 | .020 |
| Brood size × time | 2.55 | 5, 442 | .027 |
Figure 3.
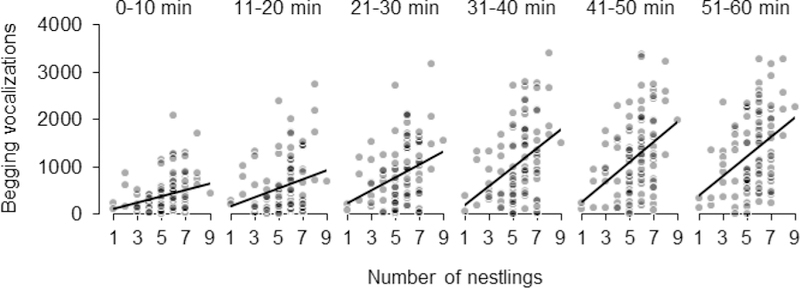
Effect of brood size on begging vocalizations on day 4/5 in the time since application of treatments (i.e., 0–10 min is the first 10 min since feeding nestlings and the beginning of the observation).
Condition-dependent begging
We then assessed nestling growth from day 2–5 posthatching (i.e., the respective beginning and end of our supplementation treatments) using a repeated-measures analysis of nestling mass, and detected a significant effect of our food supplementation over time, as indicated by an interaction between food supplementation and nestling age (table 3; fig. 4A). By day 5 posthatching, the end of food-supplementation, experimental nestlings were heavier than control and unmanipulated nestlings (F1, 135 = 9.07, P = .003; fig. 4A), even though they were weighed before receiving their food supplement that day.
Table 3:
Effects on growth, begging, and body mass of nestlings, and on parental provisioning
| Nestling growth, days 2−5 | F | df | P |
|---|---|---|---|
| Food treatment | 2.48 | 2, 198 | .086 |
| Corticosterone treatment | .01 | 2, 154 | .985 |
| Nestling age | 3123.94 | 1, 153 | < .001 |
| Food × age | 5.71 | 2, 153 | .004 |
| Corticosterone × age | .14 | 2, 153 | .874 |
| Hatching date | 7.83 | 1, 222 | .006 |
| Brood size | .07 | 1, 209 | .790 |
| Nestling begginga, days 4/5 and 7 | |||
| Food treatment | .13 | 2, 171 | .875 |
| Nestling age | 1.0 | 1, 146 | .289 |
| Food × age | 18.95 | 2, 147 | < .001 |
| Hatching date | 14.41 | 1, 196 | < .001 |
| Parental food deliveries, days 4/5 and 7 | |||
| Food treatment | .22 | 2, 159 | .800 |
| Nestling age | 13.8 | 1, 158 | < .001 |
| Food × age | 7.69 | 2, 158 | < .001 |
| Hatching date | 24.64 | 1, 177 | < .001 |
| Brood size | 11.8 | 1, 162 | < .001 |
| Nestling prefledging mass, day 11 | |||
| Food treatment | 5.77 | 2, 137 | .004 |
| Hatching date | 5.45 | 1, 137 | .021 |
| Brood size | .05 | 1, 137 | .828 |
| Nestling tarsus length | 14.55 | 1, 137 | < .001 |
Begging assessed as the number of vocalizations per nestling per hr
Figure 4.
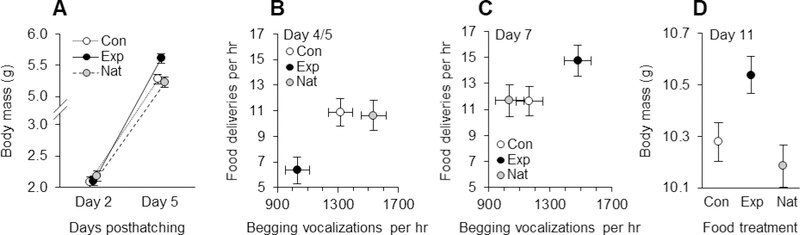
Effects of food supplementation. (A) Nestling growth from the onset of the manipulation (day 2 posthatching) to the end of supplemental feeding (day 5 posthatching). On both days, nestlings were weighed before the treatments were applied. (B) Total food provisioning by both parents (deliveries per hr) in relation to begging vocalizations 4/5 d posthatching (vocalizations per nestling per hr), immediately following the feeding treatment. (C) Total food provisioning in relation to begging vocalizations (as in B) 7 d posthatching, two days after the supplemental feeding had ended. (D) Nestling prefledging mass. All panels depict least-squares means ± SE. Black symbols depict experimental (Exp) nests (nestlings fed food dissolved in water), open symbols depict control (Con) nests (nestlings were fed water only), and grey symbols depict unmanipulated, natural (Nat) nests that were not supplemented.
We then conducted a repeated-measures analysis of nestling begging across different ages (i.e., days 4/5 and 7) in relation to the food-supplementation treatment, which revealed an interaction between nestling age and food supplementation in their effect on begging vocalizations (table 3; fig. 4B). Follow-up tests revealed that experimental nestlings begged for food at a reduced rate on day 4/5 after having been supplemented with food relative to control and unmanipulated nestlings (F1, 127 = 14.65, P < .001; fig. 4B). However, on day 7, two days after food-supplementation ended and experimental nestlings were heavier than controls, these experimental nestlings also begged for food at an increased rate (F1, 105 = 16.31, P < .001; fig. 4C). Thus, there was a positive correlation overall between begging frequency and nestling mass (standardized estimate ± SE = .234 ± .098, F1, 21 = 5.73, P = .026).
Parental provisioning
Parental provisioning mirrored the treatment effects on nestling begging observed on days 4/5 and 7 posthatching. Consistent with the effects on nestling begging, we saw a similar interaction between food treatment and nestling age in their effect on parental provisioning (table 3; fig. 4B,C). Indeed, total parental food deliveries per hour were lower at experimental nests than at control and unmanipulated nests on day 4/5 (F1, 123 = 9.03, P = .003; fig. 4B), but increased on day 7 (F1, 104 = 5.04, P = .027; fig. 4C). Parental provisioning per hour also increased over the course of the breeding season (effect of hatching date: estimate ± SE = .126 ± .025; table 3), and increased with the number of nestlings within the nest (effect of brood size: estimate ± SE = 1.222 ± .356; table 3).
Nestling condition and return rates of offspring and adults
The food supplementation augmented nestling mass earlier in development, and persisted to affect prefledging, asymptotic body mass (table 3; fig. 4D), while controlling for hatching date (estimate ± SE = –.005 ± .002), brood size (estimate ± SE = .007 ± .033), and skeletal size (i.e., tarsus length; estimate ± SE = .325 ± .085). There was also a positive effect of prefledging body mass on the probability that a given brood would produce an adult recruit in the breeding population in subsequent years (estimate ± SE = .952 ± .461, F1, 125 = 4.27, P = .041; fig. 5), and this was the case while controlling for breeding date, which indicated a decline in recruitment over the course of the breeding season (estimate ± SE = –.065 ± .022, F1, 125 = 8.63, P = .004). There was no effect of corticosterone supplementation on offspring recruitment (F2, 125 = 0.03, P = .966). Of the parents in the current study, 32 out of 118 unique females (27%) of known identity returned to breed in the following year, and 50 of 100 unique males (50%) returned. Among them, there were no effects of either their provisioning rates or either manipulative treatment (food and corticosterone treatments) on their probability of returning to breed (all P > 0.2).
Figure 5.
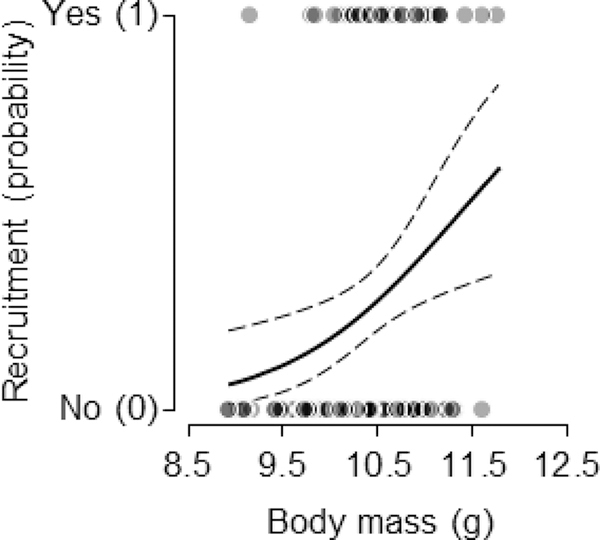
The probability that a given nesting attempt would produce at least one recruit to the breeding population in relation to the average prefledging mass of nestlings within broods. The solid curve represents the predicted value and dashed curves the 95% CI.
Discussion
Food supplementation had a strong effect on begging, both immediately after having been fed and later after supplemental feeding had ended. Recently fed experimental and control nestlings begged at a lower rate than non-food-supplemented nestlings throughout our observations on day 4/5, and increases in brood size were associated with a more rapid increase in begging. The control offspring that were fed water but not food showed a slightly steeper rise in begging rate than did the experimentally fed nestlings, suggesting that nestlings have stretch and tension receptors that induce a feeling of satiety even in the absence of any caloric content (Paintal 1954), which is known to alter hypothalamic activity (see also Berthoud 2008; Mayer 2011). Indeed, experimentally elevated glucocorticoids did not induce begging when satiated, but did so when unsatiated. Although this effect was ephemeral, these results suggest that intermittent fasting between feedings over the course of a day is associated with a dynamic balance between proximate manifestations of hunger, signaled from gut to brain, and increases in glucocorticoids that mediate behavioral changes to elicit increases in parental care (Smiseth et al. 2011; Mock 2016). Thus, the results from these early observations on day 4/5 immediately after feeding are consistent with the proximate signal-of-hunger hypothesis.
Two days after supplemental feeding had ended, however, at a time when experimentally food-supplemented nestlings were heavier than unfed control and natural nestlings, these nestlings with enhanced body condition begged for food at a higher rate than unfed nestlings. These effects were not trivial, as the experimental nestlings subsequently attained increased asymptotic, prefledging body mass, a trait that positively predicted their recruitment as breeding adults in the local population, a common pattern in passerine birds (Tinbergen and Boerlijst 1990; Young 1996; Both et al. 1999; Naef-Daenzer et al. 2001). Thus, results from our observations on day 7, two days after supplementation ended, suggest that parents also differentially allocate resources to their offspring based on positively condition-dependent signals (Smith and Montgomerie 1991; Dugas 2009; Mattey et al. 2018), and, at an ultimate level, support the signal-of-quality hypothesis while contravening the signal-of-need hypothesis.
The relationship between parental supply and offspring demand is likely subject to a number of opposing selective forces. Although parents may be selected to respond to begging solicitations (Godfray 1991, 1995b; Kilner and Johnstone 1997; Ottosson et al. 1997; Dor and Lotem 2003; Caro et al. 2016), it seems just as plausible that parents should be selected not to respond to begging, lest they be forced to provide a greater-than-optimal level of care. Indeed, because parental investment is costly, parents should be selected to avoid (i) investing in a lost cause (i.e., offspring that are unlikely to survive), and to avoid (ii) being exploited (e.g., by greedy offspring that want a greater share of resources than the parental optimum). Notwithstanding the effects observed on begging and parental provisioning in the current study, a recent study conducted on the same population induced treatment effects on nestling begging, but found no corresponding difference between treatments in parental provisioning (Barnett et al, 2011). Parents were responsive to differences in begging in the current study. Thus, our findings suggest that parents might not always respond to increases in begging (e.g., Barnett et al. 2011), but may do so when nestlings demonstrate they are worth the increased investment by virtue of their superior condition (Cotton et al. 1999; Whittingham et al. 2003; Mock et al. 2005, 2011). Thus, signals sent by offspring to parents when soliciting food may be better thought of as boasting than as begging if high-quality offspring beg more intensely than poor-quality offspring as a means of signaling the increased returns on parental investment when biased toward those of the highest reproductive value (Mock et al. 2011). The results of this study suggest a need for further experimental field studies, including those comparing differences between siblings within nests. Indeed, the factors contributing to parent-offspring communication from both proximate and ultimate perspectives warrant further study (Müller et al. 2007).
Females and their mates often differentially allocate resources to their offspring according to a variety of traits, including offspring sex, paternity, and the order in which they are produced within clutches (Cotton et al. 1999; Smiseth 2007a,b; Maddox and Weatherhead 2008; Johnson et al. 2009a,b; Magrath et al. 2009; Krist and Munclinger 2011; Bowers et al. 2014b, 2015b). Brood reduction is also common in house wrens, with the youngest members of the brood most likely to starve (Johnson 2014), ideally before reducing the amount of resources that would better serve parental fitness by being delivered to the older siblings. Thus, parents may bias their investment toward the offspring most likely to survive harsh conditions (Lotem 1998; Cotton et al. 1999). Results from our experiment suggest that this is indeed the case. Our treatments were assigned to whole broods, although one could envision a similar experiment with a split-brood design in which different treatment groups are represented within a single brood. However, under this latter scenario, differences in mass gain and begging behavior might be caused by displacement or asymmetric sibling competition over food, and not necessarily a response by parents to condition-dependent signaling, a possibility eliminated by the whole-brood design. Similarly, one might infer that the increased provisioning on day 7 by experimental parents in our field study might have been attributable to their reduced provisioning on day 4/5 as part of a trade-off between current and future provisioning effort, and less a response to nestling begging. If this hypothesis were true, we would expect experimental parents to be most responsive to begging vocalizations on day 7, being even more responsive than parents of non-experimental young. However, this was not the case, as the slope of the relationship between nestling begging and parental provisioning was generally positive, but did not differ between experimental and non-experimental nests at either age (data not presented).
It must be acknowledged, however, that the patterns we have detected may be reflective of a combination of parental strategies, in which parents embark upon a breeding event with the ‘intention’ of distributing resources equally among their offspring, but as the conditions for rearing those offspring change, so must the feeding strategies employed by parents to maximize fitness. For example, the feeding behavior of wild, free-living zebra finches (Taeniopygia guttata) actually differs from that of domesticated and captive zebra finches, with captive birds providing twice the amount of food as free-living ones (Gilby et al. 2011). A potential explanation for this difference might involve differences in whether parents respond to offspring need or quality between contexts and study systems. When food is abundant or provided ad libitum, with trivial flight distances, parents may be able to divide investment equally among their young and respond to signals of need, whereas, when resources are limiting, parents must be strategic in how they partition their investment of limiting resources among offspring of varying reproductive value (see also Koykka and Wild 2018). In other words, parents might start out aiming for whole-brood survival (as with the signal-of-need hypothesis), before realizing they cannot afford to feed the entire brood well, then switching to a signal-of-quality strategy; but the opposite pattern may also operate in species such as ours, in which brood reduction is common (Johnson 2014), where parents might conform to the signal-of-quality hypothesis until the runts die, and then follow the signal-of-need model to get the rest of the brood through to fledging.
Environmental conditions early in life can often have a profound effect on fitness (Lindström 1999; but see also Drummond and Ancona 2015). In young altricial birds, a particularly critical period of neonatal development with long-term consequences appears to involve a narrow window of time shortly after hatching. In our study population, for example, we recently found that the rate at which parents provision food to offspring at ca. 4 or 5 d posthatching positively predicts whether their offspring will survive and reproduce as adults within the breeding population (Bowers et al. 2014c). This is not unexpected, given the critical window of time for offspring to maximize growth prior to encountering the energetic demands of endothermy and to obtain the nutritional resources they will need to survive outside the nest. Thus, it follows that selection might favor overt solicitations from these offspring for parental food resources to enhance offspring survival, and that parents should selectively invest in offspring that are most likely to increase their inclusive fitness.
In conclusion, we detected a rapid effect of increasing glucocorticoid levels on nestling begging, but this effect was transient and depended on the nestlings’ level of satiety. Our results suggest that glucocorticoids may promote begging as a short-term, immediate reflection of offspring hunger, whereas the increase in nestling condition, and corresponding changes in parental food provisioning, suggest that, in an adaptive sense, begging can positively signal quality to elicit increased parental allocation. Positively condition-dependent begging may, thus, allow parents to allocate limiting resources differentially into the offspring most likely to provide a return on their investment.
Acknowledgments
We thank the 2014–2017 Wren Crews for field assistance, and the ParkLands Foundation (Merwin Preserve), the Illinois Great Rivers Conference of the United Methodist Church, and the Sears and Butler families for the use of their properties. We also thank Doug Mock and an anonymous reviewer, in addition to Emilie Snell-Rood and Russel Bonduriansky, for helpful comments that improved the paper. All research activities complied with current laws of the United States of America and were in accordance with the Illinois State University Institutional Animal Care and Use Committee, United States Geological Survey banding permit 09211 and U.S. Fish and Wildlife Service collecting permit MB692148–0. Financial support was provided by NSF grants IBN-0316580, IOS-0718140, and IOS-1118160; NIH grant R15HD076308; the Department of Biological Sciences at the University of Memphis and School of Biological Sciences at Illinois State University; and a postdoctoral research grant from the American Ornithological Society.
Appendix
Table A1:
Sample sizes of nests in which offspring survived at least to the beginning of supplementation
| Food treatment | ||||
|---|---|---|---|---|
| Control | Experimental | Natural | ||
| Corticosterone treatment | Control | 17 | 19 | 13 |
| Experimental | 16 | 17 | 13 | |
| Natural | 17 | 17 | 17 | |
Literature Cited
- Amundsen T, and Slagsvold T. 1998. Hatching asynchrony in great tits: a bet-hedging strategy? Ecology 79:295–304. [Google Scholar]
- Barnett CA, Clairardin SG, Thompson CF, and Sakaluk SK. 2011. Turning a deaf ear: a test of the manipulating androgens hypothesis in house wrens. Animal Behaviour 81:113–120. [Google Scholar]
- Barnett CA, Suzuki TN, Sakaluk SK, and Thompson CF. 2015. Mass-based condition measures and their relationship with fitness: in what condition is condition? Journal of Zoology 296:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud H-R 2008. Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neurogastroenterology and Motility 20:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both C, Visser ME, and Verboven N. 1999. Density-dependent recruitment rates in great tits: the importance of being heavier. Proceedings of the Royal Society of London B 266:465–469. [Google Scholar]
- Bowers EK, Thompson CF, and Sakaluk SK. 2014b. Offspring sex ratio varies with clutch size for female house wrens induced to lay supernumerary eggs. Behavioral Ecology 25:165–171. [Google Scholar]
- Bowers EK, Thompson CF, and Sakaluk SK. 2015c. Persistent sex-by-environment effects on offspring fitness and sex-ratio adjustment in a wild bird population. Journal of Animal Ecology 84:473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Nietz D, Thompson CF, and Sakaluk SK. 2014c. Parental provisioning in house wrens: effects of varying brood size and consequences for offspring. Behavioral Ecology 25:1485–1493. [Google Scholar]
- Bowers EK, Bowden RM, Sakaluk SK, and Thompson CF. 2015a. Immune activation generates corticosterone-mediated terminal reproductive investment in a wild bird. American Naturalist 185:769–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Bowden RM, Thompson CF, and Sakaluk SK. 2016a. Elevated corticosterone during egg production elicits increased maternal investment and promotes nestling growth in a wild songbird. Hormones and Behavior 83:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Jenkins JB, Mueller AJ, Miller KD, Thompson CF, and Sakaluk SK. 2019. Data from: Condition-dependent begging elicits increased parental investment in a wild bird population. American Naturalist, Dryad Digital Repository, 10.5061/dryad.53885k7. [DOI] [PMC free article] [PubMed]
- Bowers EK, Forsman AM, Masters BS, Johnson BGP, Johnson LS, Sakaluk SK, et al. 2015b. Increased extra-pair paternity in broods of aging males and enhanced recruitment of extra-pair young in a migratory bird. Evolution 69:2533–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Grindstaff JL, Soukup SS, Drilling NE, Eckerle KP, Sakaluk SK, et al. 2016b. Spring temperatures influence selection on breeding date and the potential for phenological mismatch in a migratory bird. Ecology 97:2880–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Hodges CJ, Forsman AM, Vogel LA, Masters BS, Johnson BGP, et al. 2014a. Neonatal body condition, immune responsiveness, and hematocrit predict longevity in a wild bird population. Ecology 95:3027–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burford JE, Friedrich TJ, and Yasukawa K. 1998. Response to playback of nestling begging in the red-winged blackbird, Agelaius phoeniceus. Animal Behaviour 56:555–561. [DOI] [PubMed] [Google Scholar]
- Chin EH, Love OP, Verspoor JJ, Williams TD, Rowley K, and Burness G. 2009. Juveniles exposed to embryonic corticosterone have enhanced flight performance. Proceedings of the Royal Society B 276:499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP 2009. Stress and disorders of the stress system. Nature Reviews Endocrinology 5:374–381. [DOI] [PubMed] [Google Scholar]
- Caro SM, Griffin AS, Hinde CA, and West SA. 2016. Unpredictable environments lead to the evolution of parental neglect in birds. Nature Communications 7:10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AB 2002. Appetite and the subjectivity of nestling hunger. Pages 173–198 in Wright J and Leonard ML, eds. The evolution of begging: competition, cooperation, and communication Kluwer Academic Publishers, New York. [Google Scholar]
- Clutton-Brock TH 1984. Reproductive effort and terminal investment in iteroparous animals. American Naturalist 123:212–229. [Google Scholar]
- Cotton PA, Wright J, and Kacelnik A. 1999. Chick begging strategies in relation to brood hierarchies and hatching asynchrony. American Naturalist 153:412–420. [DOI] [PubMed] [Google Scholar]
- Dor R, and Lotem A. 2010. Parental effort and response to nestling begging in the house sparrow: repeatability, heritability and parent–offspring co-evolution. Journal of Evolutionary Biology 23:1605–1612. [DOI] [PubMed] [Google Scholar]
- Drummond H 2002. Begging versus aggression in avian broodmate competition. Pages 337–360 in Wright J and Leonard ML, eds. The evolution of begging: competition, cooperation, and communication Kluwer Academic Publishers, New York. [Google Scholar]
- Drummond H, and Ancona S. 2015. Observational field studies reveal wild birds responding to early-life stresses with resilience, plasticity, and intergenerational effects. Auk 132:563–576. [Google Scholar]
- Dugas MB 2009. House sparrow, Passer domesticus, parents preferentially feed nestlings with mouth colours that appear carotenoid-rich. Animal Behaviour 78:767–772. [Google Scholar]
- Elderbrock EK, Small TW, and Schoech SJ. 2018. Influence of corticosterone treatment on nestling begging in Florida scrub-jays (Aphelocoma coerulescens). General and Comparative Endocrinology 259:213–222. [DOI] [PubMed] [Google Scholar]
- Forbes S 2002. Statistical challenges in the study of nestling begging. Pages 473–491 in Wright J and Leonard ML, eds. The evolution of begging: competition, cooperation, and communication Kluwer Academic Publishers, New York. [Google Scholar]
- Fresneau N, Iserbyt A, Lucass C, and Müller W. 2018. Size matters but hunger prevails—begging and provisioning rules in blue tit families. PeerJ 6:e5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Berthou E 2001. On the misuse of residuals in ecology: testing regression residuals vs. the analysis of covariance. Journal of Animal Ecology 70:708–711. [Google Scholar]
- Gilby AJ, Mainwaring MC, Rollins LA, and Griffith SC. 2011. Parental care in wild and captive zebra finches: measuring food delivery to quantify parental effort. Animal Behaviour 81:289–295. [Google Scholar]
- Godfray HCJ 1991. Signalling of need by offspring to their parents. Nature 352:328–330. [Google Scholar]
- Godfray HCJ 1995a. Evolutionary theory of parent-offspring conflict. Nature 376:133–138. [DOI] [PubMed] [Google Scholar]
- Godfray HCJ 1995b. Signaling of need between parents and young: parent-offspring conflict and sibling rivalry. American Naturalist 146:1–24. [Google Scholar]
- Godfray HCJ, and Johnstone RA. 2000. Begging and bleating: the evolution of parent-offspring signalling. Philosophical Transactions of the Royal Society of London B 355:1581–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafen A 1990. Biological signals as handicaps. Journal of Theoretical Biology 144:517–546. [DOI] [PubMed] [Google Scholar]
- Grodzinski U, and Lotem A. 2007. The adaptive value of parental responsiveness to nestling begging. Proceedings of the Royal Society B 274:2449–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LS 2014. House wren (Troglodytes aedon). In Poole A, ed. The Birds of North America Online Cornell Lab of Ornithology, Ithaca, NY. doi: 10.2173/bna.380. [DOI] [Google Scholar]
- Johnson LS, Brubaker JL, Johnson BGP, and Masters BS. 2009a. Evidence for a maternal effect benefiting extra-pair offspring in a songbird, the house wren Troglodytes aedon. Journal of Avian Biology 40:248–253. [Google Scholar]
- Johnson LS, Thompson CF, Sakaluk SK, Neuhäuser M, Johnson BGP, Soukup SS, et al. 2009b. Extra-pair young in house wren broods are more likely to be male than female. Proceedings of the Royal Society B 276:2285–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn N, and Robert K. 2013. Does sex matter? Differential responses to corticosterone administration in the zebra finch. Zoology 116:293–299. [DOI] [PubMed] [Google Scholar]
- Kedar H, Rodríguez-Gironés MA, Yedvab S, Winkler DW, and Lotem A. 2000. Experimental evidence for offspring learning in parent-offspring communication. Proceedings of the Royal Society of London B 267:1723–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr DS, Huggett AM, and Abraham WC. 1994. Modulation of hippocampal long-term potentiation and long-term depression by corticosteroid receptor activation. Psychobiology 22:123–133. [Google Scholar]
- Kilner R, and Johnstone RA. 1997. Begging the question: are offspring solicitation behaviours signals of need? Trends in Ecology and Evolution 12:11–15. [DOI] [PubMed] [Google Scholar]
- Kilner RM 2001. A growth cost of begging in captive canary chicks. Proceedings of the National Academy of Sciences of the USA 98:11394–11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaysky AS, Wingfield JC, and Piatt JF. 2001. Corticosterone facilitates begging and affects resource allocation in the black-legged kittiwake. Behavioral Ecology 12:619–625. [Google Scholar]
- Kitaysky AS, Romano MD, Piatt JF, Wingfield JC, and Kikuchi M. 2005. The adrenocortical response of tufted puffin chicks to nutritional deficits. Hormones and Behavior 47:606–619. [DOI] [PubMed] [Google Scholar]
- Klasing KC 1998. Comparative avian nutrition CAB International, Wallingford, U.K. [Google Scholar]
- Kölliker M, and Richner H. 2001. Parent-offspring conflict and the genetics of offspring solicitation and parental response. Animal Behaviour 62:395–407. [Google Scholar]
- Kölliker M, Brodie ED III, and Moore AJ. 2005. The coadaptation of parental supply and offspring demand. American Naturalist 166:506–516. [DOI] [PubMed] [Google Scholar]
- Kölliker M, Chuckalovcak JP, Haynes KF, and Brodie ED III. 2006. Maternal food provisioning in relation to condition-dependent offspring odours in burrower bugs (Sehirus cinctus). Proceedings of the Royal Society B 273:1523–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölliker M, Royle NJ, and Smiseth PT. 2012. Parent-offspring co-adaptation. Pages. 285–303 in Royle NJ, Smiseth PT, and Kölliker M, eds. The evolution of parental care Oxford University Press, Oxford. [Google Scholar]
- Koykka C, and Wild G. 2018. The influence of environmental variance on the evolution of signaling behavior. Behavioral Ecology 29:814–820. [Google Scholar]
- Krist M, and Munclinger P. 2011. Superiority of extra-pair offspring: maternal but not genetic effects as revealed by a mixed cross-fostering design. Molecular Ecology 20:5074–5091. [DOI] [PubMed] [Google Scholar]
- Lambrechts MM. Adriaensen F, Ardia DR, Artemyev AV, Atiénzar F, Bańbura J, et al. 2010. The design of artificial nestboxes for the study of secondary hole-nesting birds: a review of methodological inconsistencies and potential biases. Acta Ornithologica 45:1–26. [Google Scholar]
- Leonard M, and Horn A. 1996. Provisioning rules in tree swallows. Behavioral Ecology and Sociobiology 38:341–347. [Google Scholar]
- Leonard ML, and Horn AG. 1998. Need and nestmates affect begging in tree swallows. Behavioral Ecology and Sociobiology 42:431–436. [Google Scholar]
- Lindström J 1999. Early development and fitness in birds and mammals. Trends in Ecology and Evolution 14:343–348. [DOI] [PubMed] [Google Scholar]
- Lotem A 1998. Differences in begging behaviour between barn swallow, Hirundo rustica, nestlings. Animal Behaviour 55:809–818. [DOI] [PubMed] [Google Scholar]
- Love OP, and Williams TD. 2008. The adaptive value of stress-induced phenotypes: effects of maternally derived corticosterone on sex-biased investment, cost of reproduction, and maternal fitness. American Naturalist 172:E135–E149. [DOI] [PubMed] [Google Scholar]
- Love OP, Chin EH, Wynne-Edwards KE, and Williams TD. 2005. Stress hormones: a link between maternal condition and sex-biased reproductive investment. American Naturalist 166:751–766. [DOI] [PubMed] [Google Scholar]
- Maddox JD, and Weatherhead PJ. 2008. Egg size variation in birds with asynchronous hatching: is bigger really better? American Naturalist 171:358–365. [DOI] [PubMed] [Google Scholar]
- Magrath MJL, Vedder O, van der Velde M, and Komdeur J. 2009. Maternal effects contribute to the superior performance of extra-pair offspring. Current Biology 19:792–797. [DOI] [PubMed] [Google Scholar]
- Mattey SN, Richardson J, Ratz T, and Smiseth PT. 2018. Effects of offspring and parental inbreeding on parent-offspring communication. American Naturalist 191:716–725. [DOI] [PubMed] [Google Scholar]
- Mayer EA 2011. Gut feelings: the emerging biology of gut-brain communication. Nature Reviews Neuroscience 12:453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS 2007. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiological Reviews 87:873–904. [DOI] [PubMed] [Google Scholar]
- Meunier J, and Kölliker M. 2012. Parental antagonism and parent–offspring co-adaptation interact to shape family life. Proceedings of the Royal Society B 279:3981–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mock DW 2016. Animal behaviour: some begging is actually bragging. Nature 532:180–181. [DOI] [PubMed] [Google Scholar]
- Mock DW, and Parker GA. 1997. The evolution of sibling rivalry Oxford University Press, Oxford. [Google Scholar]
- Mock DW, Dugas MB, and Strickler SA. 2011. Honest begging: expanding from signal of need. Behavioral Ecology 22:909–917. [Google Scholar]
- Mock DW, Schwagmeyer PL, and Parker GA. 2005. Male house sparrows deliver more food to experimentally subsidized offspring. Animal Behaviour 70:225–236. [Google Scholar]
- Müller W, Lessells CM, Korsten P, and von Engelhardt N. 2007. Manipulative signals in family conflict? On the function of maternal yolk hormones in birds. American Naturalist 169:E84–E96. [DOI] [PubMed] [Google Scholar]
- Murphy MT, Chutter CM, and Redmond LJ. 2015. Quantification of avian parental behavior: what are the minimum necessary sample times? Journal of Field Ornithology 86:41–50. [Google Scholar]
- Naef-Daenzer B, Widmer F, and Nuber M. 2001. Differential post-fledging survival of great and coal tits in relation to their condition and fledging date. Journal of Animal Ecology 70:730–738. [Google Scholar]
- Ottosson U, Bäckman J, and Smith HG. 1997. Begging affects parental effort in the pied flycatcher, Ficedula hypoleuca. Behavioral Ecology and Sociobiology 41:381–384. [Google Scholar]
- Paintal AS 1954. A study of gastric stretch receptors. Their role in the peripheral mechanism of satiation of hunger and thirst. Journal of Physiology 126:255–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakkala JJ, Norris DR, Sedinger JS, and Newman AEM. 2016. Experimental effects of early-life corticosterone on the hypothalamic-pituitary-adrenal axis and pre-migratory behaviour in a wild songbird. Functional Ecology 30:1149–1160. [Google Scholar]
- Parker GA, Mock DW, and Lamey TC. 1989. How selfish should stronger sibs be? American Naturalist 133:846–868. [Google Scholar]
- Price K, and Ydenberg R. 1995. Begging and provisioning in broods of asynchronously-hatched yellow-headed blackbird nestlings. Behavioral Ecology and Sociobiology 37:201–208. [Google Scholar]
- Price K, Harvey H, and Ydenberg R. 1996. Begging tactics of nestling yellow-headed blackbirds, Xanthocephalus xanthocephalus. Animal Behaviour 51:421–435. [Google Scholar]
- Ríos JM, Barceló GF, Narváez C, Maldonado K, and Sabat P. 2014. Feeding and digestive responses to fatty acid intake in two South American passerines with different food habits. Journal of Comparative Physiology B 184:729–739. [DOI] [PubMed] [Google Scholar]
- Roulin A 2002. The sibling negotiation hypothesis. Pages 107–126 in Wright J and Leonard ML, eds. The evolution of begging: competition, cooperation, and communication Kluwer Academic Publishers, New York. [Google Scholar]
- Royle NJ, Hartley IR, and Parker GA. 2002. Begging for control: when are offspring solicitation behaviours honest? Trends in Ecology and Evolution 17:434–440. [Google Scholar]
- Sakaluk SK, Thompson CF, and Bowers EK. 2018. Experimental manipulation of incubation period reveals no apparent costs of incubation in house wrens. Animal Behaviour 137:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saino N, Ninni P, Incagli M, Calza S, Sacchi R, and Møller AP. 2000. Begging and parental care in relation to offspring need and condition in the barn swallow (Hirundo rustica). American Naturalist 156:637–649. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, and Munck AU. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews 21:55–89. [DOI] [PubMed] [Google Scholar]
- Sawhney MH, Baker MC, and Bisbee BR. 2006. Development of vocalisations in nestling and fledgling house wrens in natural populations. Bioacoustics 15:271–287. [Google Scholar]
- Schwabl H, and Lipar J. 2002. Hormonal regulation of begging behaviour. Pages 221–244 in Wright J and Leonard ML, eds. The evolution of begging: competition, cooperation, and communication Kluwer Academic Publishers, New York. [Google Scholar]
- Sheriff MJ, and Love OP 2013. Determining the adaptive potential of maternal stress. Ecology Letters 16:271–280. [DOI] [PubMed] [Google Scholar]
- Smiseth PT, Lennox L, and Moore AJ. 2007a. Interaction between parental care and sibling competition: parents enhance offspring growth and exacerbate sibling competition. Evolution 61:2331–2339. [DOI] [PubMed] [Google Scholar]
- Smiseth PT, Ward RJS, and Moore AJ. 2007b. Parents influence asymmetric sibling competition: experimental evidence with partially dependent young. Ecology 88:3174–3182. [DOI] [PubMed] [Google Scholar]
- Smiseth PT, Scott MP, and Andrews C. 2011. Hormonal regulation of offspring begging and mediation of parent-offspring conflict. Animal Behaviour 81:507–517. [Google Scholar]
- Smith CC, and Fretwell SD. 1974. The optimal balance between size and number of offspring. American Naturalist 108:499–506. [Google Scholar]
- Smith HG, and Montgomerie R. 1991. Nestling American robins compete with siblings by begging. Behavioral Ecology and Sociobiology 29:307–312. [Google Scholar]
- Soha JA, Lohr B, and Gill DE. 2009. Song development in the grasshopper sparrow, Ammodramus savannarum. Animal Behaviour 77:1479–1489. [Google Scholar]
- Strange MS, Bowden RM, Thompson CF, and Sakaluk SK. 2016. Pre- and postnatal effects of corticosterone on fitness-related traits and the timing of endogenous corticosterone production in a songbird. Journal of Experimental Zoology 325A:347–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinbergen JM, and Boerlijst MC. 1990. Nestling weight and survival in individual great tits (Parus major). Journal of Animal Ecology 59:1113–1127. [Google Scholar]
- Trivers RL 1974. Parent-offspring conflict. American Zoologist 14:249–264. [Google Scholar]
- Trumbo ST 2012. Patterns of parental care in invertebrates. Pages 81–100 in Royle NJ, Smiseth PT, and Kölliker M, eds. The evolution of parental care Oxford University Press, Oxford. [Google Scholar]
- Villaseñor E, and Drummond H. 2007. Honest begging in the blue-footed booby: signaling food deprivation and body condition. Behavioral Ecology and Sociobiology 61:1133–1142. [Google Scholar]
- Weber BM, Bowers EK, Terrell KA, Falcone JF, Thompson CF, and Sakaluk SK. 2018. Pre- and postnatal effects of experimentally manipulated maternal corticosterone on growth, stress reactivity and survival of nestling house wrens. Functional Ecology 32: 1995–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittingham LA, Dunn PO, and Clotfelter ED. 2003. Parental allocation of food to nestling tree swallows: the influence of nestling behaviour, sex and paternity. Animal Behaviour 65:1203–1210. [Google Scholar]
- Wiebe KL, and Bortolotti GR. 1994. Food supply and hatching spans of birds: energy constraints or facultative manipulation? Ecology 75:813–823. [Google Scholar]
- Young BE 1996. An experimental analysis of small clutch size in tropical house wrens. Ecology 77:472–488. [Google Scholar]


