Abstract
The recommended protocols to prevent ventilator-associated pneumonia include keeping ventilated patients’ head and upper body elevated to an angle between 30 and 45 degrees. These recommendations are largely based on a study that has been difficult to replicate, because studies that have attempted to replicate the original conditions have failed to achieve the necessary bed angles consistently. This work suggests the possibility that two specific types of human error, slips and lapses, contribute to non-compliant bed angles. A novel device provided 83,655 samples of bed angles over a period of 1579 hours. The bed angle was out of compliance 64.2% of the time analyzed. Slips, the accident of raising the bed to an angle slightly less than the desired angle, accounted for most of the out-of-compliance measurements, or 55.9% of the time analyzed. It appears that stochastic variation in the bed adjustments results in the bed being out of compliance. Interventions should be investigated such as increasing the target angle and providing feedback at the moment the bed is raised to close to, but less than, the target angle.
Keywords: Protocol adherence, monitoring, head-of-bed, ventilator-associated pneumonia, wireless sensors
1. Introduction
Ventilator-associated pneumonia (VAP) is a common infection acquired in intensive care units (ICUs) (Tablan et al. 1994). In a study of 1658 mechanically-ventilated patients in 27 European ICUs, 23.7% of the patients developed VAP (Blot et al. 2011). A review of 429 research papers shows crude VAP mortality rates of 24–50%, reaching 76% for specific settings and infection by high-risk pathogens (Chastre and Fagon, 2002). Ventilated ICU patients with pneumonia have a 2- to 10-fold higher risk of death over patients without pneumonia (Eagye, Nicolau, and Kuti, 2009).
The financial costs of VAP are also striking. When Rello et al. (2002) compared patients who developed VAP to control subjects without VAP, they found that the VAP patients stayed on ventilation longer, stayed in the ICU longer, and had longer hospital stays. The VAP patients incurred average hospital charges of $104,983 compared to $63,689 for non-VAP patients (Rello et al., 2002). Another study in Canada found that VAP accounts for approximately 17,000 ICU days per year or around 2% of all ICU days (Muscedere, Martin, and Heyland, 2008).
2. Background
2.1. Ventilator associated pneumonia and compliance to HOBA
The lungs are typically sterile, but an invasion of bacteria via aspiration can lead to pneumonia (Efrati et al., 2010; Tablan et al., 1994). All patients supported by mechanical ventilation (without tracheotomy tubes) are intubated with endotracheal tubes. These endotracheal tubes have the potential to serve as a conduit for transferring secretions into the lungs.
Drakulovic et al. (1999) observed that intubated patients who were completely flat (0°) had significantly higher VAP and mortality rates than those patients with a head-of-bed angle (HOBA) elevated to 45°. Largely motivated by this study, the American Thoracic Society, the Infectious Disease Society of America (American Thoracic Society 2006) and the Canadian Association of Medical Microbiology and Infectious Diseases (Rotstein et al., 2008) all issued recommendations to position ventilated patients in a semi-recumbent position with bed backrest elevation between 30° and 45°.
Despite these guidelines, previous studies have found that patients’ beds are rarely elevated to 30°, let alone 45° (van Nieuwenhoven et al., 2006). A PubMed literature search with the MESH keyword “Pneumonia, Ventilator-Associated” and “bed” yielded 133 articles. A review of these and related articles either citing or cited by these revealed just 19 English reports in which bed angles were directly monitored as part of the primary study. These studies are summarized in Table 1 below. In this table, compliance is indicated by the percentage of measurements with the head-of-bed angle above 30 degrees, unless otherwise noted.
Table 1.
Published experiments specifically monitoring head-of-bed angles ordered by maximum compliance range
| Study | Sample Size | Sampling Technique | Results |
|---|---|---|---|
| Sedwick et al., 2012 | 4709 ventilator days | Reviewed patient chart | 100% compliance after education bundle implemented |
| Teixeira et al., 2013 | 2472 patients over 2 years | Checklist filled out during rounds | 97% compliance |
| Bird et al., 2010 | Intubated patients in large hospital over 31 months | Twice daily observation by respiratory care specialist | 65–99% compliance |
| Croce et al., 2013 | 630 patients | Evaluated once per day by member of the research team | 83.3–91.5% compliance |
| Lawrence and Fulbrook, 2012 | 315 observations | Once-per-week observation | 80–88% compliance |
| DuBose et al., 2008 | 570 patients | ICU fellow filled out checklist daily | 35.2%–84.5%, after intervention |
| Bingham et al., 2010 | 100 patients | Direct observation of VAP procedures during 2-hour interval | 70–72% compliance |
| Williams et al., 2008 | 268 measurements made over the course of 2 weeks | Daily observations by researcher using angle indicator | 23% compliance before intervention, 71.5% after |
| Rose et al., 2010 | 141 patients | Measured 3 times per day by research team member | 32–70% compliance |
| Lyerla et al., 2010 | 315 observations on 43 patients | Direct observation of angle indicator by researcher 1–3 times daily | 44% compliance before intervention and 67% compliance after intervention |
| Wolken et al., 2012 | 7720 hours of ventilated patient observations | Continuously monitored electronically. | 61% compliance (hrs >30 degrees) without feedback, 76% compliance with feedback |
| Bouadma et al., 2010 | 1649 ventilator-days over 4 weeks | 6 observations/day of a bicolored plastic ribbon attached at the head of the bed to indicate appropriate elevation | 5–58% compliance |
| Sasabuchi et al., 2012 | 12 patients, 265 intubated hours | Hourly for 24 hours by electronic bed monitor | 24% compliance before intervention; 45% compliance after |
| Grap et al., 2005 | 276 ventilator days | Continuous | 28% compliance |
| Liu et al., 2013 | 2842 ventilator days | 4 times daily at 5–7 hour intervals Measurement made by physician, corroborated by attending nurse | 27.8% compliant |
| van Nieuwenhoven et al., 2006 | 109 patients | Continuous | 15% compliance (defined as >45 degrees) |
| Markewitz et al., 2005 | 30 patients | Continuous | 3% compliance |
| Balonov et al., 2007 | 29 patients | Automatically every 20 minutes | Effectively 0% |
| Laux et al., 2010 | 24-bed trauma unit | Electronically monitored | Novel definition of compliance (more than 16 hours/day above 30 degrees). Compliance between 3 hours – 16 hours/day |
Although Drakulovic’s work is the basis of a standard protocol, published attempts to validate and extend his work (e.g., Von Nieuwenhoven et al., 2006; Banalov et al., 2007; Grap et al., 2005) have been unsuccessful because of the difficulty of maintaining bed-angle protocol. Von Nieuwenhoven and colleagues were unable to establish a difference in outcomes between supine and elevated positions, at least partly due to the failure of the intervention group to consistently reach the target 45°. In fact, the average performance for this group was less than 30° and, perhaps more strikingly, the intervention of 45° was achieved a mere 15% of the time, despite an aggressive push for compliance (van Nieuwenhoven et al., 2006). Balanov et al. devised a hydraulic pressure transducer to confirm that the hospital-wide VAP prevention initiative had led to average bed angles above 30°, but found instead that all patients had average HOBAs less than 30° (Balonov et al., 2007). Markewitz et al. used an inclinometer to measure the bed angle of 30 patients over a two-month period and found that the median amount of time spent above 30° was just 3% (Markewitz et al., 2005). Grap et al. (2005) measured the HOBA for 276 ventilator days and found that patients spent 73–78% of their time with bed angles less than 30°, with an average angle between 17° and 24°. The failure of these studies to maintain HOBAs makes it difficult to learn more about Drakulovic’s findings and casts doubt on whether the protocol is being followed in regular hospital settings.
2.2. Reasons for non-compliance to HOBA
Reviewing the studies in Table 1, it would appear that the less structured and controlled the observations, the higher the perceived compliance rate. For example, Sedwick et al.’s 2010 analysis of patient charts suggested a 100% compliance rate after an educational intervention. In a study that measured HOBA compliance twice daily over three years, compliance rates increased from 57–82% in 2007 to 77–100% in 2009 (Bird et al., 2010). A large-scale implementation of the VAP bundle of 112 ICUs with 550,800 ventilator days published self-reported HOBA compliance, with lapses cited as the primary reason for non-compliance (Bingham et al., 2010). The studies near the bottom of the table that include automatic monitoring tend to have much lower compliance rates than those reported in studies in which human observations are used.
A clear visual presentation of the angle seems to improve compliance. Rose et al. (2010) found that an inclinometer mounted on the bed improved HOBAs so that they were in compliance 70% of the time rather than 32% without the inclinometer. Another study found similar compliance differences and attributed this benefit to the increased visibility of the angle (Williams, Chan, and Kelly, 2008).
The failure to achieve full compliance with bed-angle recommendations, particularly compliance with the 30–45° position, may be caused by: (i) the contraindications to raised elevations; (ii) nursing concerns; and/or (iii) human error. Rose et al. (Rose, Baldwin, and Crawford 2010) noted a contraindication rate of 14% in their 1154 patient study. The contraindications include hemodynamic instability, undergoing a medical procedure in the bed, intracranial hypertension, and intra-aortic balloon pumping. An-other reason for not achieving the desired angles may be nursing concerns. A survey of nurses found that the most common reasons for not raising the bed were concerns that: (i) the patient would slide down in bed; (ii) it would be too difficult to rotate the patient laterally; (iii) the patient would not be comfortable; (iv) skin breakdown would occur; or (v) hemodynamic stability would be compromised (Helman et al., 2003). Human error relates to differences between intent and resulting action. For example, the challenge of achieving the desired angle may have to do with the difficulty of visually estimating bed angles. In a study of 160 nurses and trainees, 61.6% of the bed-rest angles were overestimated, compared to just 14.9% that were estimated accurately (Peterlini et al., 2006).
2.3. Human error and non-compliance
Though it has not yet been carefully studied in this context, human error likely plays an important role in HOBA non-compliance. Several studies noted the need for more investigation of precisely why the desired bed angles were not obtained, pointing to factors related to the knowledge, motivation and behavior of the professionals who set the bed angle. Van Nieuwenhoven et al., for example, suggest that units performed differently because of “healthcare-worker related factors such as motivation and commitment to adhering to scientific protocols” (van Nieuwenhoven et al., 2006). Bingham et al. (2010) suggest that forgetfulness or miscommunication may be at the root of the problem:
It is challenging to explain why compliance with elevating the head-of-bed was poor and showed little change after the intervention. When asked about this failure, staff noted occasions where patients were lowered for a valid reason and then not returned to the correct position because of interruptions or other demands. Sometimes other members of the healthcare team changed the patient’s position for an intervention and failed to return the patient to the correct position.
There are a number of theoretical approaches to the study of human error and how systems may be designed to protect against these errors. For example, Rasmussen’s drift theory suggests that deviations from safe operations occur dynamically over time as the accumulation of gradually accepted deviations (Rasmussen, 1997), resulting from value and cost tradeoffs. At an even higher systems-level perspective, safety experts are shifting their focus on how to make systems resilient in the face of uncertainty by emphasizing normative processes and deemphasizing outlying errors (e.g., Hollnagel, 2014). Although these large-scale perspectives are important to consider in designing a robust work system, identifying specific behaviors that can lead to errors, and tracking factors that affect performance are key steps prior to comprehensive design. The traditional approach to human error helps identify and describe specific error-prone behaviors, and may be divided into two main categories: (i) mistakes; and (ii) lapses and slips (Leape, 1994; Reason, 2000; Zapf and Reason, 1994)
Mistakes occur when a plan of action is insufficient to achieve the desired goal. Slips and lapses occur when a plan is sufficient to achieve the goal, but the plan is not correctly implemented. Slips occur when an action is begun, but improperly executed. This might happen if a nurse intends to set the bed to 30°, but inadvertently sets it to only 25°.A lapse occurs when an action is intended to be undertaken, but is forgotten. This might occur, for example, if a nurse is interrupted after lowering the bed to perform a patient care activity and leaves the room before returning the bed to the recommended angle. Bingham et al.’s (2010) attribution of non-compliance to forgetfulness suggests that lapses may be an important source of error.
Previous researchers have addressed bed-angle compliance from the viewpoints of knowledge and adherence (Cason et al., 2007; El-Khatib et al., 2010; Kaynar et al., 2007; Labeau et al., 2007), visibility (Rose, Baldwin, and Crawford, 2010), checklists (Dubose et al., 2010) and training (Bloos et al., 2009; Hawe et al., 2009; Marra et al., 2009). To our knowledge, none have addressed the problem from the viewpoint of human error. The human error perspective requires the analysis of individual moments at which the desired behavior does not occur, which often leads to important insights into how to avoid such behaviors (Bion, Abrusci, and Hibbert, 2010; Kohn et al., 2000; Leape, 1994; Reason, 2005; Sexton, Thomas, and Helmreich, 2000). Immediate feedback may be effective in addressing some categories of human error.
2.4. Monitoring and providing feedback about HOBA
Auditing and feedback have a long history in medicine and has been used successfully to modify behavior (Grimshaw et al., 2001; Hysong, 2009). A meta-analysis on feedback effectiveness suggests that the most effective feedback: (i) provides a correct solution; (ii) delivers the information in writing; (iii) provides feedback to both the group and the individual; and (iv) delivers the feedback privately (Hysong, 2009). Hysong agrees with Kluger and DeNisi’s finding (Kluger and DeNisi, 1998) that appropriate graphical feed-back can be more effective than text, although this attribute was not fairly tested in her meta-analysis. Other studies have suggested that feedback must be timely, individualized and meaningful (Hyson, Best, and Pugh, 2006). Finally, feedback is more effective when paired with goal-setting (Kluger and DeNisi, 1998). Effective feedback requires accurate measuring.
Several researchers have instrumented beds to monitor HOBAs (Balonov et al., 2007; Markewitz et al., 2005; Rose, Baldwin, and Crawford, 2010; Williams, Chan, and Kelly, 2008). Several new hospital bed models even have electronic monitoring of bed angles built in. Unfortunately, such devices are not easy to introduce into an existing medical environment, because of the complexity of the instrumentation and the prohibitive cost of replacing beds. Hospitals that already have beds with the necessary instrumentation may experience difficulties in finding the correct equipment with which to store the data or in connecting the bed infrastructure with their existing electronic medical record infrastructure.
A portable sensing system can be deployed in a variety of environments to quickly provide a continuous report of bed angles over a sustained period of time lasting days or weeks. This allows for the performance in maintaining head-of-bed angles to be assessed. Such monitoring systems have proven to be essential in industrial engineering settings, because they provide a stream of data that can then be processed and analyzed, typically within the context of other available data, in order to more clearly understand and diagnose the root causes of a process issue. Such a system would allow the bed angles to be studied in greater detail in order to determine which behavior patterns are most likely the causes for the lack of compliance.
In summary, the role of human error in HOBA non-compliance is not well understood. The relatively few studies employing mechanical bed-angle measurements suggest that bed angles are lower than recommended. They do not suggest what types of errors account for this. They also do not determine whether electronic reporting yields more accurate bed-angle estimates than previous recording techniques. The purpose of this study is to measure bed angles for intubated patients to determine: (i) whether the measured bed angles agreed with the electronically reported bed angles; (ii) the frequency and magnitude of lapses and slips; and (iii) the pattern of bed-angle adjustments. If the types of behaviors associated with bed angles can be better understood, it seems likely that a more effective intervention can be defined.
3. Methods
Our approach (Fig. 1) differs from past attempts (e.g., Grap et al. 2005 and Sasabuchi, et al. 2012) in that it employs a device that is portable, battery-operated and wireless. These technical adaptations allow the monitor to be easily placed, observed and retrieved without disrupting the workflow in the ICU.
Fig. 1.

On the top, a prototype of the bed angle monitor with: a) bubble level indicator, b) USB port, and c) LED indicators. On the bottom, the inside of the device revealing: d) two circuit boards, e) a rechargeable battery, and f) the magnet on the back cover.
3.1. Apparatus
A magnet fixes the device to the bed frame where it periodically senses the angle of inclination at the head of the bed with its inclinometer. The inclinometer is connected to a microprocessor that can either save the readings and/or silently emit radio broadcasts that can be recorded and/or displayed remotely (outside a patient room). The device (i) is easy to use and installs in seconds for each bed; (ii) weighs about 200 grams; (iii) can operate for one week without servicing; and (iv) has data that can be collected in an efficient manner as each device is equipped with a radio.
This device is similar to several developed previously by our team over the past four years (Fries et al., 2009; Herman et al., 2009; Hornbeck et al., 2011; Polgreen et al., 2010). These designs were used to track hand hygiene compliance among health care workers (Fries et al., 2009; Polgreen et al., 2010); improve procedures for human observations of hand hygiene compliance (Fries et al., 2011); better understand the distribution of individual movement within the ICU (Hornbeck et al., 2011); and identify peripatetic health care workers who are especially at risk to be super spreaders (Naylor et al., 2011). These projects each relied on some combination of our wearable, battery-operated devices to track user position, monitor the use of hand hygiene dispensers, and detect passages through doorways.
The bed angle monitor design is based on a dual axis, high precision accelerometer chip (Analog Devices’ adxl203ce) and a TelosB, a microprocessor/radio circuit that has been the core component in many of our past hardware designs (Moteiv Corporation, 2004). The monitor also includes a custom-designed circuit board and case. The case has an embedded bubble level to ensure that the sensor is properly aligned when initially installing the device on the bed.
The magnetic strip allows the device to be mounted on the bed frame, parallel to either the bed’s long or short axis. In a week-long pilot experiment with 20 beds experiencing normal patient activity, we confirmed that the sensor placement was stable and consistent. The sensor’s output is linearly related to the sine of the head-of-bed angle. To test the precision of the sensor output, a sensor was oriented at −50 to 50 degrees in increments of ten degrees in two rotational dimensions. At each position, 20 reports from the on-chip analog-to-digital were collected and averaged, in a manner typical of the sensor’s normal use. The experiment was repeated three times. The sensor values were then fit to the sine of the inclination angle. As the calibration data illustrated in Figure 2 shows, the R2 values for both the x and y regressions are very close to 1, indicating that more than 99.95% of the sensor variation is attributable to predictable changes in angle. The sensor’s sensitivity is better than 1° in the range of 0° to 45° elevation. The same procedure was used to determine the slope and intercept between each sensor and the inclination angle.
Fig. 2.
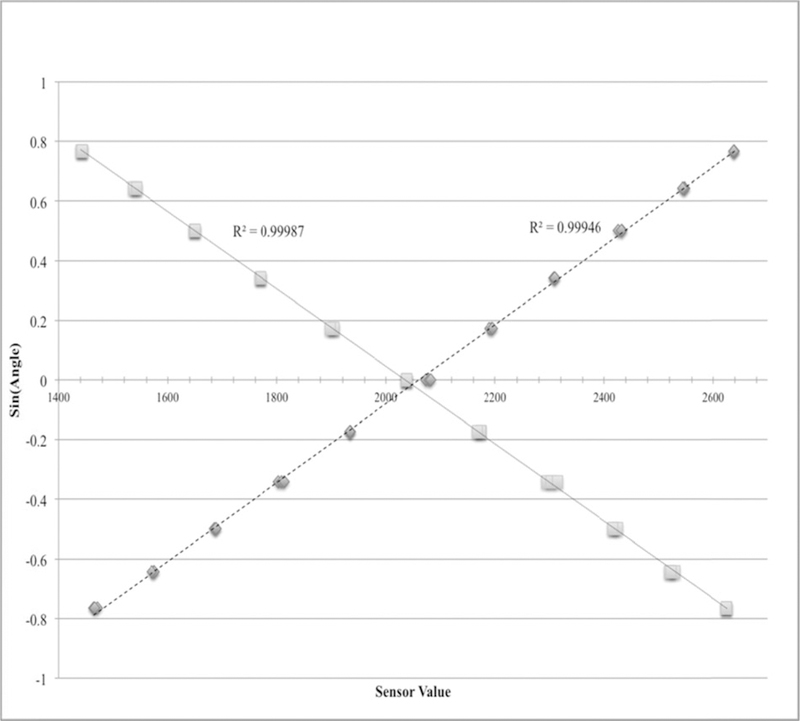
Calibration curves for the two rotation axes of the tilt sensor. The horizontal axis is the digital-to-analog converter output. The vertical axis is the sine of the inclination angle. Diamonds are for x-axis orientation (left and right rotation), squares for the y-axis orientation (forward and backward rotation).
A rechargeable cell phone battery powers the device and the software is designed to use the battery power efficiently. The sensor’s sampling frequency may be easily altered, but is currently programmed to sample every 0.5 seconds and broadcast and store the measurements when either: (i) five minutes have elapsed since the last recording, or (ii) the bed has moved since the last recording. In normal use, the device will operate at least one week between recharging. The data may then either be read wirelessly or downloaded directly from the device. A status light blinks every seven seconds to indicate the sensor’s battery and memory health; green for all is well, red when the memory is low or the battery is running out. The device is a core and novel component for achieving our specific aims.
3.2. Procedure
In September 2013 we deployed these devices on twenty patient beds in a medical intensive care unit at a large, Mid-western hospital for a continuous, 21-day interval. Twice each day a doctor on the unit recorded which patients were intubated, what angles had been recorded for these patients’ bed angle in the patient record by the nurses, and the ordered bed angle. The broadcasts from the tilt sensors were received by several tablet PCs, placed near the nurse stations associated with the rooms under study. The nurses in the unit were aware of the experiment and the fact that the head-of-bed angle was being monitored, but they received no special instructions regarding the bed angle or emphasis, as this was primarily a test of the data collection procedure and instrument rather than an intervention. The protocol was IRB-approved.
3.3. Data processing
To define intervals of approximately consistent bed angle, the bed-angle reports for each bed containing an intubated patient were resampled to produce a time series with a consistent, one-second interval spacing. For seconds with more than one bed angle, available values were averaged. For seconds without bed-angle measurements, values were interpolated from the closest readings. Significant changes in bed angles were detected by convolving the time series with the vector [−1, −1, −1, −1, 1, 1, 1, 1] and dividing the sequence whenever the absolute convolved product was greater than 20, representing an event in which the average angle changed by greater than 20 degrees over a 4-second interval. Sub-intervals with lengths less than 300 seconds were discarded. The first and last 10 seconds were trimmed from the remaining readings.
The intervals are first divided into three groups: compliant, slips and low-angle. Compliant intervals have an average angle greater than or equal to 30 degrees. Slips have an angle less than 30 degrees and greater than or equal to 15 degrees. The remaining, low-angle, intervals are again split. Patient care intervals are low-angle intervals with durations less than 1000 seconds. Lapses are low-angle intervals with durations longer than 1000 seconds.
3.4. Verbal interviews
After initial analysis of bed-angle data, members of the research team not associated with the hardware development and deployment conducted semi-structured interviews with nurses and residents in the medical intensive care unit. The interview questions gathered information regarding: (1) providers’ understanding and practice of setting and adjusting bed angles; (2) their initial impression on the bed-angle data presented to them; and (3) their reasoning on why the error-associated patterns in data occur. After gathering their comprehension/awareness about the bed-angle setting and adjustment process, the interviewers explained what the different error types such as slips, lapses and mistakes mean, using plots of specific instances from the data set as examples. Participants were asked why those error-associated patterns might occur and what procedural and behavioral aspects might cause those errors. They were also asked for their perceptions on the frequency of such events. The interviews were audio-recorded and transcribed. The participant comments were qualitatively coded with categories developed to describe reasons for error-associated patterns.
4. Results
The experiment included 1579 hours of monitored bed angles of intubated patients. The doctor-ordered head-of-bed angle for all these patient hours was 30 degrees. The medical records for these patients included 526 angles recorded by nurses and 83,655 angles produced by the bed-angle monitor.
The average bed angles reported by the nurses was 30.7 degrees, with a standard deviation of 7.6 degrees. Ninety-five percent of the reports were for angles 30 degrees or above. The most common reported angle was 30 degrees, which accounted for 85.9% of all the reported angles. Except for four values, all the reported angles were multiples of 5 degrees.
The data contained 668 intervals, which accounted for 5,130,000 s, or 90.3% of the time during which intubated patients were studied. Figure 3 illustrates the results of reducing the raw data into intervals for a typical dataset. Across all the data, the average interval length was 7,685 s and the average interval angle was 24.76 degrees. The time-weighted average interval head-of-bed angle was 27.34 degrees. The 432 intervals with an average angle less than 30 degrees accounted for 64.2% of the total interval lengths.
Fig. 3.
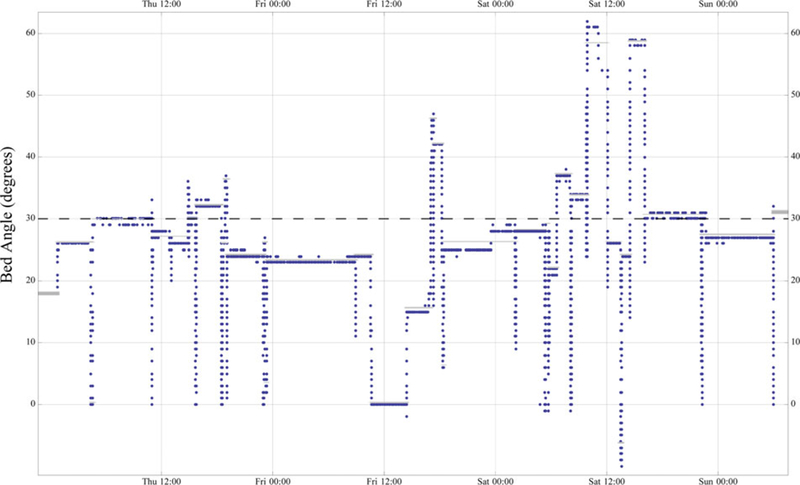
Reported head-of-bed angles in degrees for an intubated patient over a 3-day period. The horizontal dashed line indicates the doctor-ordered head-of-bed angle. The grey bars indicate the average angle for intervals, using the algorithm defined in the data processing section.
Figure 4 plots each interval according to its duration and average angle, with the markers indicating the interval’s category. There were 423 compliant intervals, 272 slips, 47 lapses and 104 patient care intervals. Compliant intervals, lapses, slips and patient care intervals accounted for 1,838,000 s (35.8%), 367,000 s (7.1%), 2,883,729 s (56.2%) and 45,254 s (0.9%), respectively.
Fig. 4.
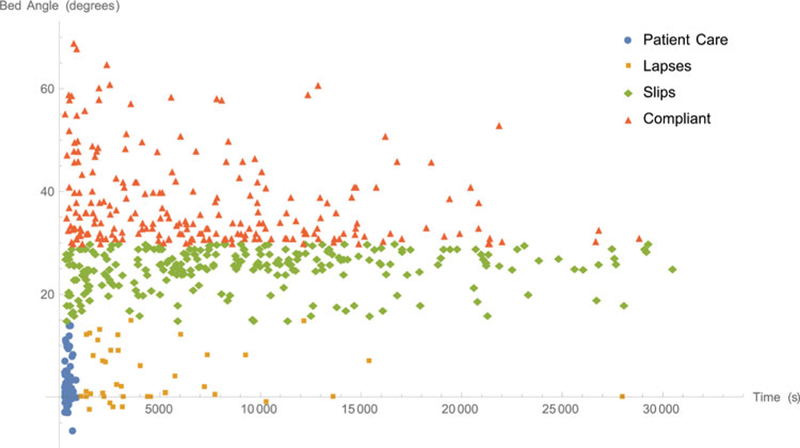
Plot of interval angles versus interval lengths, separated by interval category.
A naïve, one-sided sign test of all interval angles indicates that the median is significantly less than 30 degrees at the 5% level (W(n = 668) = 243, p ≤ 2*10−12). A more generous definition of compliant intervals that account for potential rounding to 5 degrees, as indicated in the nursing charts, reduces the compliance threshold angle to 27.5 degrees. With this threshold, and eliminating the nursing care intervals, a one-sided sign test rejects the hypothesis that the median interval angles is less than or equal to 25.7 degrees at the 5% level (W(n = 564) = 243, p < 5*10−6).
Figure 5 displays the detail of the interval angle versus interval length plot in the region near the patient care intervals. This graph reveals how modifying the patient care interval criteria would reclassify lapses and slips.
Fig. 5.
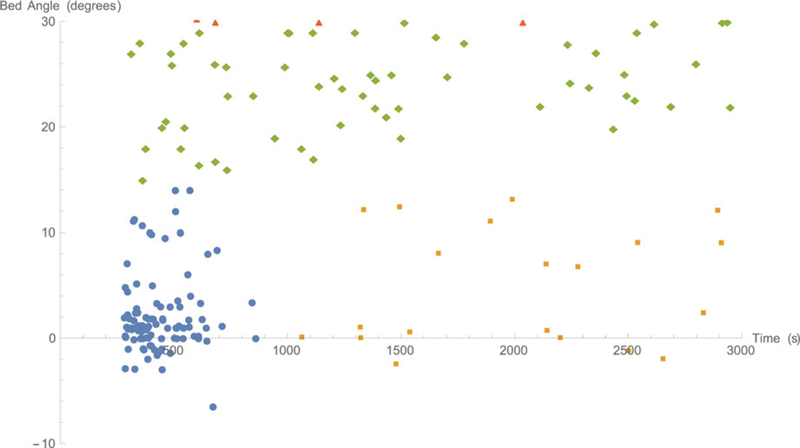
Plot of interval angles versus interval lengths near the region defined as patient care intervals, with the marker coding used in Figure 5.
Figure 6 presents the histograms for the intervals, coded by interval category. The histogram suggests a bimodal distribution with one peak near 30 degrees and another peak near zero degrees.
Fig. 6.
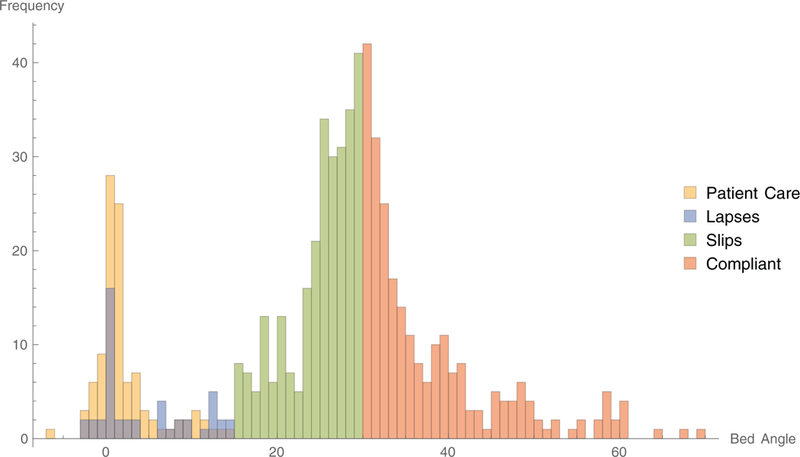
A histogram of the bed angles and frequency of occurrence for each interval.
The qualitative analysis yielded a consistent explanation for the process of setting the head-of-bed angle requirements. Typically, physicians initiate the patient head-of-bed orders and route it to the nurses as part of a ventilator bundle. Nurses control the head-of-bed angle and chart the angle values every four hours. Nurses typically do not communicate with physicians when they lower the bed below ordered values. Nurses provided several reasons why the bed will be angled less than or greater than 30 degrees. Some of these are determined by patient condition and procedures being performed on patients, in concordance with or against physician orders. Multiple nurses and residents reported that angles 30 degrees and above prevent aspirations and ventilator associated pneumonia, trading off from setting the angle less than 30 degrees which prevents pressure ulcer formation.
Nurses reported that they may set the angle to less than 30 degrees to accommodate various patient medical conditions, such as patients who are hemodynamically unstable or who have suffered spinal trauma. The participants reported that such patients are unable to tolerate a high head-of-bed angle. Nurses also suggested that the head-of-bed angle is lowered for certain procedures such as bathing patients, cleaning beds, and inserting catheters. During these procedures, frequent interruptions occur and may cause personnel to leave bed angles for longer durations than intended when they are called away and forget to return. Participants suggested that the repetition of the reported angles may be, at least in part, a result of copying chart values from previous patient records, particularly in intervals between staff changes and handoffs. Additionally, nurses mentioned that the visual indicator for angles is inaccurate and imprecise, along with a delay in feedback, from the indicator in showing the correct angle when the bed has been moved. Two discrepancies were encountered among nurses regarding when the bed should be less than or greater than 30 degrees. Participants had contrasting responses that angles should be above or less than 30 degrees to prevent aspirations as well as if and when the patient is intubated.
5. Discussion
The purpose of this study is to measure bed angles for intubated patients to determine: (i) whether the measured bed angles agreed with the electronically reported bed angles; the frequency and magnitude of lapses and slips; and the pattern of bed-angle adjustments.
The average angle reported in the electronic medical record was 30.7 degrees and the time-weighted measured angle was 27.34 degrees. Given that the annotations in the electronic medical record appear to be rounded to the closest 5 degrees, some clinicians may not perceive this difference as practically significant. Nevertheless, the median value of the interval bed angles was significantly lower than 30 degrees. In fact, two-thirds of the time when the bed is in a stable position, it is at an angle less than 30 degrees. Viewed another way, 95% of the reports in the electronic medical record indicate protocol compliance, whereas the continuous measurements indicate compliance only 35.8% of the time. To complicate the story, if the interval angles are analyzed in a manner that accounts for patient care intervals and allows for rounding decimal places, there is no evidence to suggest that the median bed angles is significantly less than 30 degrees.
There are at least two analysis perspectives to under-stand the bed-angle data. From the first perspective, each time a health care worker adjusts the bed, he or she makes a decision about the appropriate angle at which to set the bed. This decision is either in compliance or out of compliance. From this perspective, the record of the average interval angles yields a tally of 423 compliant and 245 non-compliant decisions. From a second perspective, the health care worker distinguishes between short-term and long-term decisions, seeking to optimize the bed position over the course of the day, permitting short deviations for patient care activities, while emphasizing long-term compliance. This perspective yields the conclusion that, although the bed angle was compliant only 35.8% of the time, the time-weighted average angle was fairly close to the desired bed angle. The first perspective emphasizes the moment of decision making, while the second perspective recognizes the need to occasionally reduce the bed angle for patient care, a perspective that forgives small, short duration adjustments that occur over the course of the day. Thus, the second measurement approach is more appropriate for the clinical realities of patient care. Still, such a low compliance rate begs an explanation.
The values in the electronic medical record and the comments made in the interviews support the conjecture that the health care workers are aware of the desired bed angle. The primary reasons offered for consistently differing from the prescribed values are medical contraindications, which were excluded from the study. The other reasons for lowering the head-of-bed angle include patient care activities and concern for ulcer formation. Our analysis accounts for patient care activities, which have a relatively small effect on the time-averaged bed angle and compliance rate. The remaining concern is for ulcer formation. If this was a principal concern, however, it was not well represented in the interviews or in annotations found in patient records. Were slips or lapses the cause for the non-compliant angles?
Of the 668 intervals, 272 were classified as slips and 47 as lapses. Most of the time (56.2%), the beds were set at an angle categorized as a slip. Lapses accounted for only 7.1% of the bed setting time, but had a disproportionately large impact on the time-weighted bed angle.
The interviews suggested that the slips might be caused, at least in part, by difficulties with the bed indicators. The rough symmetry of the graph reinforces the conjecture that the nurses’ intention is to place the head-of-bed at or near 30 degrees, but this intention is acted upon imprecisely. Viewed as a stochastic distribution with a mean of 30 degrees, natural variance would cause the angle to be out of compliance as often as it is in compliance. However, the measurement variance of the central peak in Figure 6 seems larger than one would expect if the angle indicator was observed for each measurement. The large variance is more consistent with health care workers visually estimating the bed angle. Whatever the cause, the nominal compliance rate is generally dominated by slips. The specific degree to which this is true, however, depends on the manner in which slips, lapses and patient care are defined.
The categories were defined by a series of demarcations. The first demarcates compliant angles from non-compliant angles. We considered both 30 degree and 27.5 degree thresholds. This choice has an important effect on whether the beds are found to be in compliance or not. The second demarcation is between low angles and slips. Here, we arbitrarily chose 15 degrees. Figure 5 illustrates the relative dearth of long intervals in this mid-range, between 12 and 18 degrees, particularly for intervals longer than 1000 seconds. Although the specific location of the angle is arbitrary, the existence of a division is indicated both by language in the interviews relating to “lowering the patient” and to a pattern in the observed data. The third demarcation is within the low-angle intervals. Again, Figure 5 suggests a clear cluster among short intervals and a sporadic sampling for longer intervals. This is consistent with the statement in the interviews that the beds are generally lowered briefly for patient-care activities, but occasionally a nurse is distracted before he or she can raise the bed again. Although the cutoff of 1000s was arbitrary, the data suggest that any value between 800 s and 1500 s would have yielded similar results.
The third objective of the study was to determine the pattern of bed-angle adjustments. Figure 6 suggests a bi-modal pattern with a central peak located at 30 degrees. A second peak near the origin tends to be populated by shorter intervals, as indicated in Figure 5. If the adjustments of bed angle were consistent in that the bed was lowered to close to zero and then raised up again, the size of the two peaks would be similar. The fact that the central peak is larger suggests that health care workers often change the bed position among raised angles. This pattern may be observed in several places in the sample data in Figure 3. However, Figure 3 reveals that the bed is most frequently lowered for a period shorter than the 300-second interval threshold, then raised to a higher level. These short deviations to lower angles would have increased the number of patient-care intervals, but would not have contributed substantially to the time-weighted average angles.
The results are both supported by and help to explain the patterns seen earlier in the literature review. Studies that emphasize processes similar to those used in the electronic medical record, such as filling out a check list (Teixeira, et al., 2013; DuBose et al., 2008), reviewing a chart (Sedwick, et al., 2012), periodic observations of bed angle as part of a VAP bundle intervention (Lawrence and Fulbrook, 2012; Croce et al., 2013), or observing VAP procedures over a 2-hour period (Bingham et al., 2010) tend to have compliance rates that reach into the range above 75%. This may be because the observers are recording the intent of the health care workers in setting the bed angle, rather than measuring the bed angle independently. These results are similar to the results we found in the electronic medical record of reported bed angles. When research studies include periodic measurements with an angle indicator (Williams, et al., 2008; Lyerla et al., 2010; Liu, et al., 2013; Bouadma et al., 2010), the compliance rates are in the 20– 70% range. This might be explained by the omission of the short-term bed angles, if the observers thought it “unfair” to record the low bed angle while the nurse was in the middle of patient care activities and simply waited until the care activities were complete.
Reports involving continuous measurements of bed angle have the lowest levels of compliance. Wolken et al., 2012 found compliance ranges in the 61%−76% range, which is higher than the 35.8% range that we found. Our findings are more consistent with those of Sasabuchi et al. (2013) (24%−45%) and Grap et al. (2005) (28%) and certainly better than Balonov et al. (2007) and Markewitz et al. (2005) who effectively never observed compliant bed angles. This may be due to the difference in the processes in varying hospitals. The hospital we studied has been engaged in VAP prevention studies for many years. The awareness of desired head-of-bed angles indicated in both the charting and the interviews supports the idea that the health care workers are well aware of the goal. Other institutions may not enjoy this awareness, which would likely lead to lower compliance rates.
The efforts of van Niewenhoven et al. to maintain the bed angles at 45 degrees were even less effective, despite very intense efforts to achieve bed angles of 45 degrees. This is unlikely to be a factor of awareness, and may be a tradeoff between the concerns for skin ulcers against the goal to prevent VAP. This tradeoff has not been sufficiently explored in the literature.
It is interesting to note that the primary support for the head-of-bed angle comes from Drakulovic’s original experiment that has not been replicated. Standard practice seeks to achieve 30 degrees rather than Drakulovic’s 45-degree, 24-hour protocol, most likely for the practical difficulties of patient care. Moreover, his zero-degree control condition is not standard practice. Although clinical benefits have been reported for VAP bundles, the benefit of head-of-bed angle has not been specifically measured. In light of these deviations from the original parameters of Drakulovic’s 45-degree protocol, it seems reasonable to consider other bed criteria, such as Laux et al. 2010 novel compliance definition requiring that the head-of-bed be inclined by more than 30 degrees at least 16 hours per day.
Assuming that stochastic variation around the target value is the primary source of error, at least two strategies would help to correct the problem. The first strategy would be to increase the target angle. The official recommendations are for bed angles between 30 and 45 degrees. Rather than interpreting the target as 30 degrees, the nurses might target the middle of that range. For example, consider the effect of changing the target angle to 37.5 degrees. Assuming that the patient care activities were to remain consistent, increasing the remaining angles by 7.5 degrees would increase the frequency of adjustments to compliant angles from 40.1% to 78.2%. Thus, simply changing the target angle to the middle of the target range could have an important influence on the compliance rate.
A second strategy would be to use feedback, bed-angle indicators, or other technologies to remind the nursing staff to continue to raise the bed until the target angle was achieved. This would probably not affect the 47 lapses and 104 adjustments associated with patient care activities, but would reduce the variance around the 30-degree peak or bias the distribution above the 30-degree mark. If that technology were perfectly consistent in eliminating slip errors, extrapolating our results suggests that the bed angle would have been compliant 80% of the time, or even 93% of the time, if exceptions were allowed for patient care activities.
There are several important limitations to this study. First, it does not measure the impact of head-of-bed angle on VAP outcomes. This is due to the relatively low incidence of VAP at the hospital studied. A much larger study would be required to find any significant effect. Second, the definition of slips and lapses is based on the analysis of bed angles and may not reflect the intention or actions of the health care workers. Third, this study does not account for concerns over skin ulcers, which may have affected the setting of head-of-bed angles in some instances.
6. Conclusion
This investigation represents the most detailed study of continuous head-of-bed angles yet undertaken. The results suggest that the values entered in the electronic medical record do not accurately represent the head-of-bed angle experienced by ventilated patients during the period studied. The actual bed angles were significantly less than 30 degrees.
The angles were analyzed to determine whether the non-compliant bed angles were caused by lapses and slips. There were relatively few lapses, meaning periods in which the bed was lowered and then not raised for a long period. The most likely explanation for non-compliance is that stochastic variation occurs around the target angle. Consequently, since the target is set at the threshold for compliance, even accounting for rounding and periods in which the bed is intentionally lowered for patient care activities, the bed is raised to a non-compliant angle approximately half the time.
We propose two solutions to bring the bed angles into compliance. The first is to change the target angle communicated to the staff to 37.5 degrees, in the middle of the desired range. The second solution is to introduce technology that either reminds staff to bring head-of-bed angles into compliance or that automatically brings the bed to the correct position when staff indicate a desire to raise the head-of-bed angle.
The effect of head-of-bed angle on the incidence of VAP has not been widely studied, largely because of the difficulty of achieving sufficient compliance with bed-angle protocols. This work suggests that the cause behind lack of compliance is neither negligence nor concern for patient safety, but natural variation. The findings are consistent with and explain previous results showing that making the bed angle easier to read and interpret has improved compliance rates.
Once the compliance rates have been addressed, it will become possible to study the benefit of head-of-bed angle on patient outcomes and ultimately help to reduce the burden of this major hospital-acquired disease on the healthcare system and the patients it serves.
Acknowledgments
Funding
This investigation was supported by the Agency for Health-care Research and Quality (AHRQ) under Grant Award (R03 HS021558). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the AHRQ.
Footnotes
Color versions of one or more of the figures in the article can be found online at www.tandfonline.com/uhse.
References
- American Thoracic Society. (2005) Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. American Journal of Respiratory and Critical Care Medicine, 171, 388–416. [DOI] [PubMed] [Google Scholar]
- Balonov K, Miller AD, Lisbon A, and Kaynar AM (2007) A novel method of continuous measurement of head of bed elevation in ventilated patients. Intensive Care Medicine, 33(6), 1050–1054. [DOI] [PubMed] [Google Scholar]
- Berenholtz SM, Pham JC, Thompson DA, Needham DM, Lubomski LH, Hyzy RC, Welsh R, et al. (2011) Collaborative cohort study of an intervention to reduce ventilator-associated pneumonia in the intensive care unit. Infection Control and Hospital Epidemiology, 32(4), 305–314. [DOI] [PubMed] [Google Scholar]
- Bingham M, Ashley J, De Jong M, and Swift C (2010) Implementing a unit-level intervention to reduce the probability of ventilator-associated pneumonia. Nursing Research, 59(1 Suppl), S40–S47. [DOI] [PubMed] [Google Scholar]
- Bion JF, Abrusci T and Hibbert P (2010) Human factors in the management of the critically ill patient. British Journal of Anaesthesia, 105(1), 26–33. [DOI] [PubMed] [Google Scholar]
- Bird D, Zambuto A, O’Donnell C, Silva J, Korn C, Burke R, et al. (2010) Adherence to ventilator-associated pneumonia bundle and incidence of ventilator-associated pneumonia in the surgical intensive care unit. Archives of Surgery, 145(5), 465–470. [DOI] [PubMed] [Google Scholar]
- Bloos F, Müller S, Harz A, Gugel M, Geil D, Egerland K, et al. (2009) Effects of staff training on the care of mechanically ventilated patients: A prospective cohort study. British Journal of Anaesthesia, 103(2), 232–237. [DOI] [PubMed] [Google Scholar]
- Blot SI, Serra ML, Koulenti D, Lisboa T, Deja M, Myrianthefs P, et al. (2011) Patient to nurse ratio and risk of ventilator-associated pneumonia in critically ill patients. American Journal of Critical Care, 20(1), e1–e9. [DOI] [PubMed] [Google Scholar]
- Bouadma L, Mourvillier B, Deiler V, Le Corre B, Lolom I, Régnier B, Wolff M, and Lucet JC (2010) A multifaceted program to prevent ventilator-associated pneumonia: Impact on compliance with preventive measures*. Critical Care Medicine, 38(3), 789–796. [DOI] [PubMed] [Google Scholar]
- Cason CL, Tyner T, Saunders S, and Broome L (2007) Nurses’ implementation of guidelines for ventilator-associated pneumonia from the Centers for Disease Control and Prevention. American Journal of Critical Care, 16(1), 28–37. [PubMed] [Google Scholar]
- Chastre J, and Fagon JY (2002) Ventilator-associated pneumonia. American Journal of Respiratory and Critical Care Medicine, 165(7), 867–903. [DOI] [PubMed] [Google Scholar]
- Croce MA, Brasel KJ, Coimbra R, Adams CA Jr., Miller PR, Pasquale MD, McDonald CS, Vuthipadadon S, Fabian TC, and Tolley EA (2013) National Trauma Institute prospective evaluation of the ventilator bundle in trauma patients: Does it really work? Journal of Trauma and Acute Care Surgery, 74(2), 354–362. [DOI] [PubMed] [Google Scholar]
- Drakulovic MB, Torres A, Bauer TT, Nicolas JM, Nogué S, and Ferrer M (1999) Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: A randomised trial. The Lancet, 354(9193), 1851–1858. [DOI] [PubMed] [Google Scholar]
- DuBose JJ, Inaba K, Shiflett A, Trankiem C, Teixeira PG, Salim A, Rhee P, Demetriades D, and Belzberg H (2008) Measurable outcomes of quality improvement in the trauma intensive care unit: the impact of a daily quality rounding checklist. Journal of TraumaInjury, Infection, and Critical Care, 64(1), 22–29. [DOI] [PubMed] [Google Scholar]
- Dubose J, Teixeira PGR, Inaba K, Lam L, Talving P, Putty B, Plurad D, et al. (2010) Measurable outcomes of quality improvement using a daily quality rounds checklist: one-year analysis in a trauma intensive care unit with sustained ventilator-associated pneumonia reduction. Journal of Trauma and Acute Care Surgery, 69(4), 855–860. [DOI] [PubMed] [Google Scholar]
- Eagye KJ, Nicolau DP, and Kuti JL (2009) Impact of superinfection on hospital length of stay and costs in patients with ventilator-associated pneumonia. Seminars in Respiratory and Critical Care Medicine, 30(1), 116–123. [DOI] [PubMed] [Google Scholar]
- Efrati S, Deutsch I, Antonelli M, Hockey PM, Rozenblum R, and Gurman GM (2010) Ventilator-associated pneumonia: Current status and future recommendations. Journal of Clinical Monitoring and Computing, 24(2), 161–168. [DOI] [PubMed] [Google Scholar]
- El-Khatib MF, Zeineldine S, Ayoub C, Husari A, and Bou-Khalil PK (2010) Critical care clinicians’ knowledge of evidence-based guidelines for preventing ventilator-associated pneumonia. American Journal of Critical Care, 19(3), 272–276. [DOI] [PubMed] [Google Scholar]
- Fries J, Hlady C, Herman T, Polgreen PM, and Segre AM (2012) A low-cost non-RFID based method for automated monitoring of hand hygiene compliance. Program and abstracts of the 19th Annual Scientific Meeting of the Society for Healthcare Epidemiology of America (SHEA). San Diego, CA Abstract 123. [Google Scholar]
- Fries J, Segre AM, Thomas GW, Herman T, Ellingson K, and Polgreen PM (2012) Monitoring hand hygiene via human observers: How should we be sampling? Infection Control and Hospital Epidemiology, 33(7), 689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grap MJ, Munro CL, Hummel RS, Elswick RK, McKinney JL, and Sessler CN (2005) Effect of backrest elevation on the development of ventilator-associated pneumonia. American Journal of Critical Care, 14(4), 325–332. [PubMed] [Google Scholar]
- Grimshaw JM, Shirran L, Thomas R, Mowatt G, Fraser C, Bero L, Grilli R, Harvey E, Oxman A, and O’Brien MA (2001) Changing provider behavior: An overview of systematic reviews of interventions. Medical Care, 39 (8 Suppl 2), II2–II45. [PubMed] [Google Scholar]
- Hawe CS, Ellis KS, Cairns CJ, and Longmate A (2009) Reduction of ventilator-associated pneumonia: Active versus passive guideline implementation. Intensive Care Medicine, 35(7), 1180–1186. [DOI] [PubMed] [Google Scholar]
- Helman DL Jr, Sherner JH III, Fitzpatrick TM, Callender ME, and Shorr AF (2003) Effect of standardized orders and provider education on head-of-bed positioning in mechanically ventilated patients. Critical Care Medicine, 31(9), 2285–2290. [DOI] [PubMed] [Google Scholar]
- Herman T, Pemmaraju SV, Segre AM, Polgreen PM, Curtis DE, Fries J, et al. (2009) Wireless applications for hospital epidemiology. Proceedings of the 1st ACM International Workshop on Medical-Grade Wireless Networks. New Orleans, LA, 45–50. [Google Scholar]
- Hornbeck T, Curtis DE, Herman T, Thomas G, Segre AM, and Polgreen PM (2011) Contact patterns for HCWs: Not everyone is the ‘average.’ 21st Annual Scientific Meeting of the Society for Healthcare Epidemiology of America Dallas, TX Abstract 423. [Google Scholar]
- Hysong SJ, Best RG, and Pugh JA (2006) Audit and feedback and clinical practice guideline adherence: Making feedback actionable. Implementation Science, 1(1): 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysong SJ (2009) Meta-analysis: Audit and feedback features impact effectiveness on care quality. Medical Care, 47(3), 356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaynar AM, Mathew JJ, Hudlin MM, Gingras DJ, Ritz RH, Jackson MR, et al. (2007) Attitudes of respiratory therapists and nurses about measures to prevent ventilator-associated pneumonia: A multicenter, cross-sectional survey study. Respiratory Care, 52(12), 1687–1694. [PubMed] [Google Scholar]
- Kluger AN, and DeNisi A (1998) Feedback interventions: Toward the understanding of a double-edged sword. Current Directions in Psychological Science, 7(3), 67–72. [Google Scholar]
- Kohn LT, Corrigan JM, and Donaldson MS (2000) To err is human: Building a safer health system. A report of the Committee on Quality of Health Care in America, Institute of Medicine The National Academies Press. [PubMed] [Google Scholar]
- Labeau S, Vandijck DM, Claes B, Van Aken P, Blot SI, and on behalf of the executive board of the Flemish Society for Critical Care Nurses. (2007) Critical care nurses’ knowledge of evidence-based guidelines for preventing ventilator-associated pneumonia: An evaluation questionnaire. American Journal of Critical Care, 16(4), 371–377. [PubMed] [Google Scholar]
- Laux L, Dysert K, Kiely S, and Weimerskirch J (2010) Trauma VAP SWAT team: a rapid response to infection prevention. Critical care nursing quarterly, 33(2), 126–131. [DOI] [PubMed] [Google Scholar]
- Lawrence P, and Fulbrook P (2012) Effect of feedback on ventilator care bundle compliance: before and after study. Nursing in critical care, 17(6), 293–301. [DOI] [PubMed] [Google Scholar]
- Leape LL (1994) Error in medicine. JAMA: The Journal of the American Medical Association, 272(23), 1851–1857. [PubMed] [Google Scholar]
- Leape LL, and Berwick DM (2005) Five years after to err is human. JAMA: The Journal of the American Medical Association, 293(19), 2384–2390. [DOI] [PubMed] [Google Scholar]
- Liu JT, Song HJ, Wang Y, Kang Y, Jiang L, Lin SH, Bin D, and Ma PL (2013) Factors associated with low adherence to head-of-bed elevation during mechanical ventilation in Chinese intensive care units. Chin Med J, 126, 834–838. [PubMed] [Google Scholar]
- Lyerla F, LeRouge C, Cooke DA, Turpin D, and Wilson L (2010) A nursing clinical decision support system and potential predictors of head-of-bed position for patients receiving mechanical ventilation. American Journal of Critical Care, 19(1), 39–47. [DOI] [PubMed] [Google Scholar]
- Markewitz BA, Mayer J, Westenskow D, and Richardson S (2005) Use of an inclinometer-data logger tool for continuous recording of head of bed position in patients undergoing mechanical ventilation. CHEST Journal, 128(4 MeetingAbstracts), 303S-b. [Google Scholar]
- Marra AR, Cal RGR, Silva CV, Caserta RA, Paes ÂT, Moura DF Jr, et al. (2009) Successful prevention of ventilator-associated pneumonia in an intensive care setting. American Journal of Infection Control, 37(8), 619–625. [DOI] [PubMed] [Google Scholar]
- Moteiv Corporation. (2004) Telos Rev B (Low Power Wireless Sensor Module) Preliminary Datasheet Accessed at: http://www2.ece.ohio-state.edu/bibyk/ee582/telosMote.pdf.
- Muscedere JG, Martin CM, and Heyland DK (2008) The impact of ventilator-associated pneumonia on the Canadian health care system. Journal of Critical Care, 23(1), 5–10. [DOI] [PubMed] [Google Scholar]
- Naylor D, Hornbeck T, Segre AM, and Polgreen PM (2011) Analyzing the Impact of Superspreading Using Hospital Contact Networks. International Meeting on Emerging Diseases and Surveillance (IMED)
- Peterlini MAS, Rocha PK, Kusahara DM, and Pedreira ML (2006) Subjective assessment of backrest elevation: Magnitude of error. Heart and Lung: The Journal of Acute and Critical Care, 35(6), 391–396. [DOI] [PubMed] [Google Scholar]
- Polgreen PM, Hlady CS, Severson MA, Segre AM, and Herman T (2010) Method for automated monitoring of hand hygiene adherence without radio-frequency identification. Infection Control and Hospital Epidemiology: The Official Journal of the Society of Hospital Epidemiologists of America, 31(12), 1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reason J (2000) Human error: Models and management. British Medical Journal, 320(7237), 768–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reason J (2005) Safety in the operating theatre - part 2: Human error and organisational failure. Quality and Safety in Health Care, 14(1), 56–60. [PMC free article] [PubMed] [Google Scholar]
- Reid PP, Compton WD, Grossman JH, and Fanjiang G (Eds.). (2005) Building a Better Delivery System: ANew Engineering/Health Care Partnership National Academies Press, Washington, D.C. [PubMed] [Google Scholar]
- Rello J, Ollendorf DA, Oster G, Vera-Llonch M, Bellm L, Red-man R, and Kollef MH (2002) Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. CHEST Journal, 122(6), 2115–2121. [DOI] [PubMed] [Google Scholar]
- Rose L, Baldwin I, and Crawford T (2010) The use of bed-dials to maintain recumbent positioning for critically ill mechanically ventilated patients (The RECUMBENT study): Multicentre before and after observational study. International Journal of Nursing Studies, 47(11), 1425–1431. [DOI] [PubMed] [Google Scholar]
- Rotstein C, Evans G, Born A, Grossman R, Light RB, Magder S, et al. (2008) Clinical practice guidelines for hospital-acquired pneumonia and ventilator-associated pneumonia in adults. The Canadian Journal of Infectious Diseases and Medical Microbiology, 19(1), 19–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasabuchi Y, Sanui M, Onuma T, Shimozono T, and Lefor AT (2012) A bedside placard significantly increases compliance with head of the bed elevation in the intensive care unit: a pilot study. Anaesthesia and Intensive Care, 40(4), 731. [PubMed] [Google Scholar]
- Sedwick MB, Lance-Smith M, Reeder SJ, and Nardi J (2012) Using evidence-based practice to prevent ventilator-associated pneumonia. Critical Care Nurse, 32(4), 41–51. [DOI] [PubMed] [Google Scholar]
- Sexton JB, Thomas EJ, and Helmreich RL (2000) Error, stress, and teamwork in medicine and aviation: Cross sectional surveys. British Medical Journal, 320(7237), 745–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tablan OC, Anderson LJ, Arden NH, Breiman RF, Butler JC, and McNeil MM (1994) Guideline for prevention of nosocomial pneumonia. Infection Control and Hospital Epidemiology, 15(9), 587–627. [DOI] [PubMed] [Google Scholar]
- Teixeira PG, Inaba K, DuBose J, Melo N, Bass M, Belzberg H, and Demetriades D (2013) Measurable outcomes of quality improvement using a daily quality rounds checklist: Two-year prospective analysis of sustainability in a surgical intensive care unit. Journal of Trauma and Acute Care Surgery, 75(4), 717–721. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services. (2012) Healthcare-Associated Infection (HAI) Accessed January 18 at http://www.hhs.gov/ash/initiatives/hai/index.html.
- Valdez RS, Ramly E, and Brennan PF (2010) Final report: Industrial and systems engineering and health care: Critical areas of research. AHRQ Publication 10–0079 Rockville, MD: Agency for Healthcare Research and Quality. [Google Scholar]
- van Nieuwenhoven CA, Vandenbroucke-Grauls C, van Tiel FH, Joore HC, van Schijndel RJS, van der Tweel I, et al. (2006) Feasibility and effects of the semirecumbent position to prevent ventilator-associated pneumonia: A randomized study. Critical Care Medicine, 34(2), 396–402. [DOI] [PubMed] [Google Scholar]
- Williams Z, Chan R, and Kelly E (2008) A simple device to increase rates of compliance in maintaining 30-degree head-of-bed elevation in ventilated patients. Critical Care Medicine, 36(4), 1155–1157. [DOI] [PubMed] [Google Scholar]
- Wolken RF, Woodruff RJ, Smith J, Albert RK, and Douglas IS (2012) Observational study of head of bed elevation adherence using a continuous monitoring system in a medical intensive care unit. Respiratory Care, 57(4), 537–543. [DOI] [PubMed] [Google Scholar]
- Zapf D, and Reason JT (1994) Introduction: Human errors and error handling. Applied Psychology, 43(4), 427–432. [Google Scholar]
- Zaydfudim V, Dossett LA, Starmer JM, Arbogast PG, Feurer ID, Ray WA, et al. (2009) Implementation of a real-time compliance dashboard to help reduce SICU ventilator-associated pneumonia with the ventilator bundle. Archives of Surgery, 144(7), 656–662. [DOI] [PubMed] [Google Scholar]


