Abstract
Background
Clinical assessment of skin stiffness is unreliable in many applications. The durometer, an industrial device to measure hardness, has previously been applied in scleroderma. The Myoton is a non-invasive handheld device for assessing soft tissue biomechanical parameters.
Materials and Methods
We evaluated the reproducibility of both devices in six healthy subjects in the volar forearm, extensor forearm, upper arm, shin, and calf bilaterally. The intraclass correlation coefficient (ICC) was used as a measure of reproducibility among three observers.
Results
The inter-observer intraclass correlation coefficient (ICC) of overall stiffness for the Myoton was 0.74 [95% confidence interval (CI) 0.45–1.00] and 0.71 [0.39–1.00] for the durometer. Coefficient of variation (CV) for Myoton was 6.4% [range 1.3–12.1] and 7.6% [range 4.4–13.8] for the durometer. Myoton and Durometer values had a Pearson correlation of 0.69. The intra-observer Myoton ICC was 0.89 [0.74–1.00] and CV 3.1% [range 1.6–5.0]. The 95% confidence minimal detectable change by the Myoton for a single observer is 32.4 N/m, which is 7.6% of the average subject’s overall stiffness.
Conclusion
The Myoton demonstrated high reproducibility, particularly in the overall stiffness parameter, and merits further investigation to assess disease progression and treatment efficacy.
Keywords: Myoton, durometer, sclerosis, stiffness, intraclass correlation coefficient
Introduction
There is no established reliable clinical method to evaluate patient skin biomechanics, which are important in many diseases and applications, such as monitoring disease status in scleroderma and chronic graft-versus-host disease. The standard of manual palpation lacks necessary sensitivity. Previously tested tools for stiffness measurements include the durometer, SkinFibroMeter, tonometer, and indurometer1–3. The durometer has been shown to have intraclass correlation coefficients (ICCs) ranging from 0.61–0.92 in patients with scleroderma4,5.
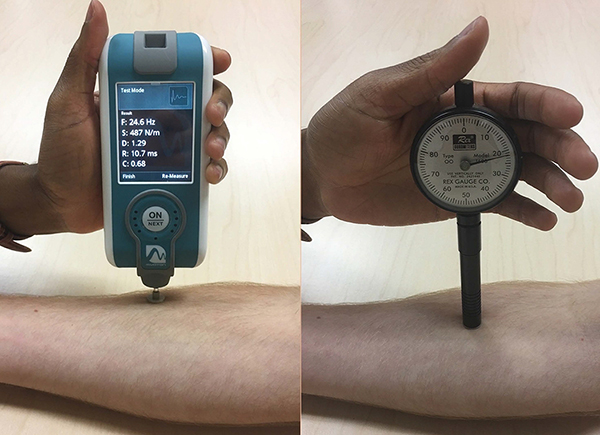
In healthy subjects, we evaluated the reliability to measure skin stiffness by two devices. The Myoton is a handheld device designed to measure muscle (Figure 1). It delivers a brief mechanical impulse and extracts soft tissue biomechanical parameters from the subsequent damped natural tissue oscillation curves6. Though used in diverse fields, it has not been validated for dermatologic disease. In contrast, the durometer is a commercial device designed for measuring non-biomaterial surface hardness. Though it is used clinically for assessing sclerosis, the amount of force applied is measurer-dependent, introducing variability. We hypothesized that the Myoton and durometer would each demonstrate high reproducibility for measurement of healthy skin.
Figure 1:
Myoton, a noninvasive handheld device modified to isolate cutaneous tissue, and the Rex gauge OO durometer
Materials and Methods
We evaluated the ICC of three observers’ Myoton skin stiffness measurement and the Rex gauge OO durometer measurement in six healthy subjects, which had 85% power to differentiate an ICC of 0.7 from 0.0 (α=0.05) for each device.
Three observers (AV, LD, FC) independently performed durometer and Myoton measurements on five unmarked sites bilaterally - shin, dorsal forearm, volar forearm, upper arm, and calf. The observers agreed on a general area of measurement at each site, but each chose a unique exact measurement spot. When one measurer completed all measurements (~10 min), he or she exited the room and the next measurer entered. Subjects were first measured with the durometer, which was held perpendicular to the skin, allowing gravity to dictate the force applied. When the measurement stabilized (typically 3 seconds), it was recorded by a fourth person (MP). Six repetitions were averaged per site, in contrast to four repetitions in previous studies4. Each observer practiced this technique for 1 hour, compared to 15 minutes in other studies5.
For Myoton measurements, two device modifications were selected for increased skin signal. First, a 12 mm diameter disk was attached to the standard 3 mm testing end. The larger surface area decreases surface power density. Second, impulse delivery time was reduced from the default 15 ms to 7 ms, resulting in a smaller effective mass of natural oscillation, selecting again for superficial tissue. Subjects laid supine on the exam table and relaxed without talking to minimize skeletal muscle contributions to variation. The disk rested flat against the skin during the device’s mechanical impulse. Skin stiffness (Newtons/meter) was averaged over 10 repetitions. Intra-observer reproducibility was also assessed with observer LD measuring six additional healthy controls in three full-body measurement sets. Myoton training was conducted by the inventor and observer 1, AV, for 6–8 hours. Before this study, observers LD and FC had 1 and 6 months supervised experience, respectively.
Coefficient of variation (CV) across three observers was used to test device repeatability, while ICC, calculated using a linear mixed model, assessed clinical reproducibility. Minimal detectable change (MDC95) was calculated for each site and device7. In addition to individual sites, we studied overall stiffness, which is the average stiffness across all measured sites for a single observer in a single measurement session.
Results
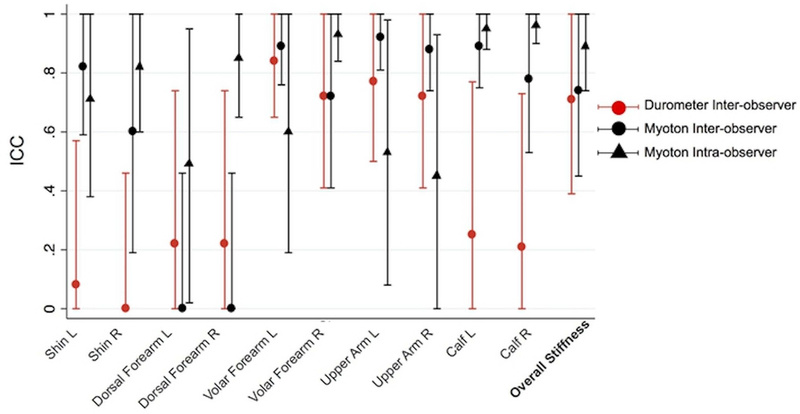
Inter-observer Myoton measurements yielded an overall stiffness CV of 6.4% [range 1.3–12.1] and ICC of 0.74 [95% confidence interval (CI) 0.45–1.00], whereas durometer measurements yielded an overall stiffness CV of 7.6% [range 4.4–13.8] and ICC of 0.71 [CI 0.39–1.00] (Table 1, Fig 2). Intra-observer Myoton measurements for six different healthy controls yielded an overall stiffness CV of 3.1% [range 1.6–5.0] and overall stiffness ICC of 0.89 [CI 0.74–1.00] (Table 1). The Pearson coefficient of correlation R between Myoton and durometer readings was 0.69 (Supplemental Figure S1).
Table 1.
Inter- and Intra-Observer Repeatability of Myoton and Inter-Observer Repeatability of Durometer Measurements in Healthy Subjects#
| Myoton Inter-observer | |||||
| Site | Average Values (N/m) | ICC* | CV | MDC95^(N/m) | Normalized MDC (%)$ |
| Shin L | 657.1 [466.3–864.8] | 0.82 [0.59–1.00] | 10.3 [1.3–17.0] | 192.7 | 29.3 |
| Shin R | 729.2 [572.2–950.5] | 0.60 [0.19– 1.00] | 13.5 [3.1–27.5] | 267.0 | 36.6 |
| Dorsal Forearm L | 454.5 [402.4–494.9] | 0.00 [0.00– 0.46] | 20.6 [5.4–46.1] | 98.9 | 21.8 |
| Dorsal Forearm R | 450.3 [406.4–525.3] | 0.00 [0.00– 0.46] | 24.4 [11.4–37.9] | 126.9 | 28.2 |
| Volar Forearm L | 456.5 [318.5–653.9] | 0.89 [0.76– 1.00] | 7.5 [2.0–15.4] | 107.1 | 23.5 |
| Volar Forearm R | 435.1 [344.2–545.8] | 0.72 [0.41– 1.00] | 10.1 [3.9–16.5] | 124.5 | 28.6 |
| Upper Arm L | 310.6 [249.8–512.8] | 0.92 [0.81–1.00] | 7.5 [2.5–12.8] | 81.4 | 26.2 |
| Upper Arm R | 306.2 [250.3–428.5] | 0.88 [0.74– 1.00] | 6.5 [2.9–11.4] | 63.1 | 20.6 |
| Calf L | 380.3 [286.6–482.8] | 0.89 [0.75– 1.00] | 6.3 [1.9–10.2] | 72.1 | 19.0 |
| Calf R | 378.3 [303.5–516.5] | 0.78 [0.53– 1.00] | 8.7 [3.1–12.8] | 99.6 | 26.3 |
| Overall Stiffness! | 455.8 [379.5–539.6] | 0.74 [0.45– 1.00]! | 6.4 [1.3–12.1]! | 91.5! | 20.1! |
| Durometer Inter-observer | |||||
| Site | Average Values (durometer units) | ICC | CV | MDC95 (durometer units) | Normalized MDC (%) |
| Shin L | 29.4 [23.2–37.1] | 0.08 [0.00– 0.57] | 26.0 [18.7–32.8] | 14.0 | 47.5 |
| Shin R | 30.4 [25.7–34] | 0.00 [0.00– 0.46] | 25.8 [18.7–35.0] | 8.8 | 29.1 |
| Dorsal Forearm L | 23.3 [15.9–26.6] | 0.22 [0.00– 0.74] | 20.1 [9.2–49.4] | 9.4 | 40.4 |
| Dorsal Forearm R | 23.9 [19.6–26.8] | 0.22 [0.00– 0.74] | 16.4 [8.7–23.7] | 7.7 | 32.1 |
| Volar Forearm L | 24.1 [19.4–27.6] | 0.84 [0.65– 1.00] | 6.4 [4.2–11.1] | 4.0 | 16.8 |
| Volar Forearm R | 23.6 [17.3–29.2] | 0.72 [0.41– 1.00] | 10.0 [5.1–19.3] | 6.4 | 27.1 |
| Upper Arm L | 17.3 [11.6–26.2] | 0.77 [0.50– 1.00] | 12.0 [2.8–25.8] | 6.9 | 39.7 |
| Upper Arm R | 16.7 [12.6–24.3] | 0.72 [0.41– 1.00] | 14.7 [3.2–24.5] | 7.2 | 43.3 |
| Calf L | 25.1 [21.7–30] | 0.25 [0.00– 0.77] | 14.4 [10.9–16.6] | 7.4 | 29.3 |
| Calf R | 27.2 [23.9–30.8] | 0.21 [0.00– 0.73] | 14.8 [9.2–27.6] | 8.0 | 29.3 |
| Overall Stiffness! | 24.1 [20.0–28.2]! | 0.71 [0.39– 1.00]! | 7.6 [4.4–13.8]! | 5.0! | 20.7! |
| Myoton Intra-observer | |||||
| Site | Average Values (N/m) | ICC | CV | MDC95 (N/m) | Normalized MDC (%) |
| Shin L | 617.1 [487.0–749.0] | 0.71 [0.38– 1.00] | 8.4 [5.4–17.1] | 125.9 | 20.4 |
| Shin R | 656.8 [475.8–814.4] | 0.82 [0.60–1.00] | 9.2 [6.4–11.8] | 140.9 | 21.5 |
| Dorsal Forearm L | 449.6 [378.6–506.0] | 0.49 [0.02– 0.95] | 8.8 [4.4–15.8] | 90.4 | 20.1 |
| Dorsal Forearm R | 464.1 [357.8–552.0] | 0.85 [0.65– 1.00] | 7.2 [2.1–12.2] | 84.0 | 18.1 |
| Volar Forearm L | 415.7 [390.7–510.9] | 0.60 [0.19– 1.00] | 5.9 [1.4–13.7] | 75.0 | 18.1 |
| Volar Forearm R | 414.1 [307.6–587.9] | 0.93 [0.84– 1.00] | 5.4 [2.8–8.1] | 62.7 | 15.1 |
| Upper Arm L | 280.7 [256.2–318.4] | 0.53 [0.08– 0.98] | 6.5 [1.5–11.5] | 44.4 | 15.8 |
| Upper Arm R | 265.1 [208.6–288.3] | 0.45 [0.00– 0.93] | 8.6 [1.6–19.5] | 55.1 | 20.8 |
| Calf L | 338.3 [218.4–471.9] | 0.95 [0.88– 1.00] | 6.2 [1.8–10.1] | 54.5 | 16.1 |
| Calf R | 372.7 [237.7–513.9] | 0.96 [0.90– 1.00] | 5.5 [3.0–7.5] | 50.3 | 13.5 |
| Overall Stiffness! | 427.4 [371.4–465.2]! | 0.89 [0.74–1.00]! | 3.1 [1.6–5.0]! | 32.4! | 7.6! |
Values are shown as ICC [95% confidence interval] and Coefficient of Variation [Range]
ICC: intraclass correlation coefficient
MDC95: minimal detectable change based on a 95% confidence interval calculated as:
MDC95 = population standard deviation × sqrt(1-ICC value) × 1.96 × sqrt(2)
Normalized MDC95 = MDC95 / average site measurement × 100
The overall stiffness row is not the average of the other rows. Instead, overall stiffness reflects the corresponding outcomes for the average skin stiffness across all measured sites for a single observer in a single measurement session.
Figure 2. Inter- and Intra-Observer Repeatability of Myoton and Inter-Observer Repeatability of Durometer Measurements in Healthy Subjects.
Scatter plot with error bars showing the inter- and intra-observer Myoton and inter-observer durometer ICCs per site across six healthy subjects. Error bars represent 95% confidence interval.
Discussion
In this study, the Myoton exhibited higher reproducibility than the durometer. Subjects with high Myoton stiffness readings reach a maximum durometer reading of approximately 35 durometer units, suggesting that the durometer may be unable to differentiate varying degrees of elevated stiffness (Supplemental Fig 1). Interestingly, observed durometer reproducibility (ICC 0.71) in healthy subjects is lower than in studies measuring patients with scleroderma. Merkel et al. reported an overall ICC of 0.92 over six sites (forearms, thighs, calves) in 43 scleroderma patients5. Notably, these patients can have a wide range of skin biomechanical properties at a given measurement site4. For mathematical reasons, high inter-subject variability can falsely elevate ICC estimates8.
Our data suggest that the Myoton may provide a reliable assessment of skin stiffness. The observed inter-observer ICC of 0.74 and intra-observer ICC of 0.89 for overall stiffness in healthy subjects indicate substantial agreement by Landis and Koch criteria9. In healthy subjects, the CI of the Myoton ICC [0.45–1.00] compared favorably to the NIH Skin Score for non-moveable sclerosis [0.21–0.60]10. The CVs for overall stiffness were under 10%, suggesting high device repeatability as well. With the Myoton, the dorsal forearm ICC had lower inter-observer [CI 0.00–0.46] than intra-observer values [CI 0.02–1.00], which may reflect the variable contribution of fascial plains of extensor carpi muscles for small deviations in position selection by different observers. In contrast, the calf ICC exhibited inter- [CI 0.53–1.00] and intra-observer values [CI 0.88–1.00] near perfect agreement by Landis and Koch criteria9. Importantly, for a single observer, the Myoton should report true changes in overall stiffness with high accuracy, as evidenced by the MDC95 of 32.4 N/m, which is only 7.6% of the average subject’s overall stiffness.
Conclusion
This study directly calculated the reproducibility of two devices within the same subjects, but the small sample of healthy individuals limits generalizability. Though Myoton alterations presumably isolated dermal tissue, the signal may be affected by positioning relative to underlying muscles, tendons, or bones. Overall stiffness demonstrated high reproducibility and merits further investigation to assess disease progression and treatment efficacy.
Future directions include testing the Myoton’s reproducibility in sclerotic patients, and its ability to track disease longitudinally.
Supplementary Material
Supplemental Figure 1: Correlation of Average Myoton and Durometer Measurements Across All Measured Sites for All Observers Scatter plot with error bars showing the inter-observer Myoton versus inter-observer durometer average measurements per site across six healthy subjects. Error bars represent the range of the average Myoton (horizontal error bars) and durometer (vertical error bars) measurements for the three observers.
Acknowledgements
We would like to acknowledge and thank the participating volunteers. Funding and support for this study came from Vanderbilt University School of Medicine Research Immersion (LD), NIH K12 CA090625 (ET), Baltic-American Freedom Foundation (AV), the Archimedes Foundation Kristjan Jaak Scholarship (MP), and the Vanderbilt Medical Scholars Program (JG). This work was also supported in part by Career Development Award Number IK2 CX001785 from the United States (U.S.) Department of Veterans Affairs Clinical Sciences R&D (CSRD) Service.
References
- 1.Seyger MM, van den Hoogen FH, de Boo T & de Jong EM Reliability of two methods to assess morphea: skin scoring and the use of a durometer. J. Am. Acad. Dermatol 37, 793–6 (1997). [DOI] [PubMed] [Google Scholar]
- 2.Douglass J, Graves P & Gordon S Intrarater Reliability of Tonometry and Bioimpedance Spectroscopy to Measure Tissue Compressibility and Extracellular Fluid in the Legs of Healthy Young People in Australia and Myanmar. Lymphat. Res. Biol 15, 57–63 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Sun D et al. The Value of Using a SkinFibroMeter for Diagnosis and Assessment of Secondary Lymphedema and Associated Fibrosis of Lower Limb Skin. Lymphat. Res. Biol 15, 70–76 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Kissin EY et al. Durometry for the assessment of skin disease in systemic sclerosis. Arthritis Rheum. 55, 603–609 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Merkel PA et al. Validity, reliability, and feasibility of durometer measurements of scleroderma skin disease in a multicenter treatment trial. Arthritis Rheum. 59, 699–705 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vain A, inventor; Myoton AS, assignee. Device and method for real-time measurement of parameters of mechanical stress state and biomechanical properties of soft biological tissue. United States Patent: 9808183 (2011). [Google Scholar]
- 7.Beaton DE Understanding the relevance of measured change through studies of responsiveness. Spine (Phila. Pa. 1976). 25, 3192–9 (2000). [DOI] [PubMed] [Google Scholar]
- 8.Russek L Factors Affecting Interpretation of Reliability Coefficients. J. Orthop. Sport. Phys. Ther 34, 341–349 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Landis JR & Koch GG The measurement of observer agreement for categorical data. Biometrics 33, 159–74 (1977). [PubMed] [Google Scholar]
- 10.Mitchell SA et al. A Multicenter Pilot Evaluation of the National Institutes of Health Chronic Graft-versus-Host Disease (cGVHD) Therapeutic Response Measures: Feasibility, Interrater Reliability, and Minimum Detectable Change. Biol. Blood Marrow Transplant. 17, 1619–1629 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Correlation of Average Myoton and Durometer Measurements Across All Measured Sites for All Observers Scatter plot with error bars showing the inter-observer Myoton versus inter-observer durometer average measurements per site across six healthy subjects. Error bars represent the range of the average Myoton (horizontal error bars) and durometer (vertical error bars) measurements for the three observers.