Abstract
The cardinal motor symptoms of Parkinson’s disease (PD) are caused by the death of dopaminergic neurons in the substantia nigra pars compacta (SNc). Alpha-synuclein (aSYN) pathology and mitochondrial dysfunction have been implicated in PD pathogenesis, but until recently it was unclear why SNc dopaminergic neurons should be particularly vulnerable to these two types of insult. In this brief review, the evidence that SNc dopaminergic neurons have an anatomical, physiological and biochemical phenotype that predisposes them to mitochondrial dysfunction and synuclein pathology is summarized. The recognition that certain traits may predispose neurons to PD-linked pathology creates translational opportunities for slowing or stopping disease progression.
Keywords: neurodegeneration, pathogenesis, calcium, mitochondria, oxidant stress, synuclein, axon, dopamine, Lewy pathology, excitotoxicity, bioenergetics, autophagy
Graphical Abstract
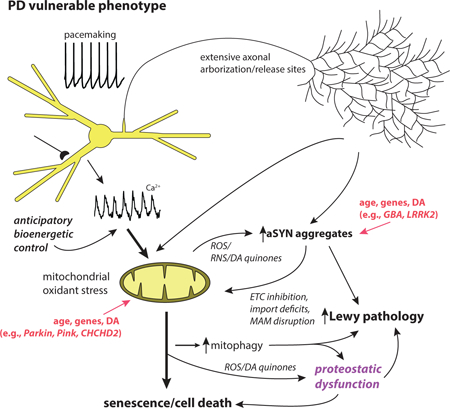
This review summarizes evidence that selective neuronal vulnerability in Parkinson’s disease results from several phenotypic traits: 1) calcium-dependent, feed-forward control of mitochondrial respiration leading to elevated reactive oxygen species and cytosolic calcium concentration; 2) an extensive axonal arbor; and 3) a reactive neurotransmitter. These traits increase vulnerability to genetic mutations associated with PD, age and environmental toxins.
Introduction
PD is the second most common neurodegenerative disease, afflicting 1% of the population above the age of 65 [1]. The prevalence of PD in the U.S. is projected to steadily increase, reaching 2 million by 2030 [2, 3]. A similar trend is expected in developed countries around the world. The disease is debilitating, being characterized by progressive bradykinesia, rigidity, resting tremor and gait impairment, as well as a spectrum of non-motor symptoms including autonomic and cognitive dysfunction. These disabilities underlie the enormous economic burden of PD, estimated to be over $23 billion annually in the U.S. alone [4, 5]. PD has no cure and nothing is known to modify the progression of the disease. The cardinal motor symptoms of PD– bradykinesia and rigidity – stem from the loss of SNc dopaminergic (DA) neurons [6, 7]. Although it is widely recognized that the pathology in PD is not limited to SNc DA neurons [8, 9], this brief review focuses on current thinking about the vulnerability of this particular group of neurons. The reader is referred to another recent review that address the broader questions associated with distributed vulnerability in PD and common features of at-risk neurons [9].
Two competing theories of PD pathogenesis
There are two widely held theories of why SNc DA neurons are lost in PD. One is built upon the observation that Lewy pathology (LP) — proteinaceous inclusions that are rich in fibrillary forms of aSYN —is commonly observed in the SNc of PD patients [10]. These inclusions or an earlier oligomeric form of aggregated aSYN are commonly thought to be toxic [11, 12], resulting in the death of SNc DA neurons. Point mutations in the SNCA gene, which encodes aSYN, or duplication or triplication of SNCA increase the risk of developing PD, solidifying the connection between PD and aSYN [13, 14].
A fundamental question is how LP (or oligomeric aSYN) arises in this small group of neurons in the mesencephalon. Comparison of patient brains taken at various times after a diagnosis has led to the hypothesis that in the preclinical stages of PD, LP first appears in either the olfactory bulb or the dorsal motor nucleus of vagus (DMV) in the caudal medulla and then propagates to the SNc through synaptically coupled networks [8, 15]. Indeed, there is compelling experimental evidence in support of the notion that some form of aggregated aSYN pathology can spread [16]. For example, histological analysis of fetal transplants into the striatum of patients with PD revealed that DA neurons exhibited proteinaceous inclusions that strongly resembled LP, suggesting that aSYN pathology has spread from the host into the graft [17, 18]. Moreover, when aSYN fibrils are directly injected into the brain, pathology can spread. In mice, synthetic, pre-formed aSYN fibrils propagate from the site of stererotaxic injection to neighboring structures, creating Lewy-like pathology [19, 20]. Similarly, in monkeys, proteins extracted from human brains with LP (that would contain aSYN fibrils and other LP proteins) can propagate [21]. Recent work has identified surface proteins that specifically interact with aSYN fibrils and promote spreading [22, 23]. Although there are methodological and biological issues surrounding these studies [24–26], they do demonstrate that extracellular aSYN aggregates can be taken up, spread and induce LP.
Despite of the unequivocal evidence for distributed, aSYN-laden LP in PD and the ability of aSYN aggregates to spread in animal models, the relationship between aSYN pathology, cell death and symptoms remains uncertain [9]. In particular, it is unclear why SNc DA neurons should be particularly vulnerable to propagated aSYN aggregates [16, 27]. Although aSYN fibrils inoculated into the brain can kill neurons [19], at lower, more biologically meaningful levels, LP does not appear to be particularly toxic. In many parts of the brain (particularly the brainstem), LP can be present for decades without causing any obvious degeneration or death. Why should DMV neurons tolerate LP and SNc DA neurons not? A related criticism of this hypothesis is that SNc DA neurons appear to be lost in sporadic PD cases before LP is present in the SNc and LP is not present in some familial cases despite loss of SNc DA neurons [9].
It is also possible that LP is a ‘red herring’ and that oligomeric aSYN (rather than fibrillar aSYN found in LP) is the real culprit in pathogenesis [28–33]. The problem with this hypothesis at present is that oligomeric species of aSYN are difficult to track in a cellular setting, making a rigorous test of the hypothesis problematic.
An alternative (but not mutually exclusive) hypothesis is that the loss of SNc DA neurons in PD is driven by mitochondrial dysfunction [34–36]. A major piece of evidence for this conclusion comes from studies of familial cases of PD. Loss of function mutations in DJ-1 (PARK 7), PINK1 (PARK 6) and parkin (PARK 2) cause recessive, early onset forms of PD and all three gene products are directly involved in mitochondrial biology, influencing a range of functions from oxidant defenses, to quality control and oxidative phosphorylation (OXPHOS) [37–39]. Mutations in genes associated with dominant forms of PD, including SNCA (PARK 1), LRRK2 (PARK 8), and CHCHD2, also have been linked to mitochondrial dysfunction [36, 40]. Another piece of evidence implicating mitochondria in PD comes from studies of environmental toxins. Toxins linked to PD, like rotenone, are invariably inhibitors of the mitochondrial electron transport chain (ETC), most commonly mitochondrial complex I [41, 42]. Post-mortem examination of the brains of PD patients also has implicated mitochondria in pathogenesis. The levels of functional complex I are diminished in the SNc of PD patients [43]. This is not just a consequence of neurodegeneration, as functional complex I levels are lower even in surviving SNc DA neurons [44]. Mitochondrial deoxyribonucleic acid (mtDNA) deletions, which can be caused by reactive oxygen species (ROS), are elevated in the SNc of PD patients [45–47]. These observations have led to the proposition that there is a ‘vicious cycle’ of oxidant stress and mitochondrial damage behind PD that ultimately leads to a bioenergetic crisis and the death of SNc DA neurons [36].
Selective vulnerability – a convergence of traits?
But why should SNc DA neurons be particularly vulnerable to mitochondrial dysfunction, any more than aSYN pathology? There are three characteristics of SNc DA neurons that have been hypothesized to make them preferentially vulnerable to these insults.
One distinguishing feature of SNc DA neurons is a long and highly branched, unmyelinated axon with an extraordinary number of transmitter release sites. SNc DA neurons in the rodent have axons that branch profusely in the striatum and possess as many as 200,000 vesicular release sites [48]. Why might a long and highly branched axon increase vulnerability? There are several theories that have been proposed [49, 50]. Mitochondrial oxidant stress – one of the potential drivers of neurodegeneration – is elevated in the axons of SNc DA neurons and this stress is reduced by diminishing the size of the arbor [51]. The extraordinary large axonal arbor of SNc DA neurons is very likely to increase the expression of aSYN (which is largely a synaptic protein), adding to the potential for aSYN pathology. [52]. That said, not all neurons with long, branched axons are vulnerable in PD (e.g., striatal cholinergic interneurons [53]), suggesting that some other factor(s) is in play.
Another key feature of SNc DA neurons is their distinctive physiology. The action potential of these neurons is slow and broad, which maximizes calcium entry and promotes slow rhythmic activity [54]. The slow, rhythmic activity (2–10 Hz) in these neurons is autonomously generated and accompanied by slow oscillations in intracellular calcium concentration that are triggered by the opening of plasma membrane Cav1 calcium channels and release of calcium from intracellular, endoplasmic reticulum (ER) stores [55–58]. Once in the cytoplasm, calcium is relatively free to interact with other proteins as the abundance of calcium buffering proteins, like calbindin, is low [59]. This combination of features – broad spikes, pacemaking, low intrinsic calcium buffering and cytosolic calcium oscillations, distinguishes SNc DA neurons from the vast majority of neurons in the brain. For example, VTA DA neurons, which are significantly less vulnerable than SNc DA neurons (see above), are autonomous pacemakers with broad spikes, but have smaller Cav1 channel currents and strong intrinsic calcium buffering (by calbindin) [60–63].
The slow calcium oscillations in SNc DA neurons sub-serve two complementary functions. First, they help maintain the slow tonic spiking by creating a membrane potential oscillation [56, 57, 64]. Second, they promote calcium entry into mitochondria at specialized junctions with the ER [65]; mitochondrial calcium entry stimulates OXPHOS and the production of adenosine triphosphate (ATP) [55](Zampese et al., unpublished observations). In principle, this anticipatory control of OXPHOS helps to ensure that bioenergetic needs are met even in conditions of sustained stress [66, 67] and that intracellular ATP levels do not fall into a range that would trigger protective activation of K-ATP channels and cessation of on-going activity [68]. Even temporary cessation of SNc activity would disrupt basal ganglia function, slow movement and lessen the chances of survival in a threatening environment. As a consequence, there should have been strong evolutionary pressure to maintain this kind of ‘anticipatory’ (feed-forward) control mechanism.
Although anticipatory bioenergetic control clearly has an upside, but what are its downsides? There are two that are apparent. First, stimulating OXPHOS in the absence of strong ATP demand (which is most of the time) leads to mitochondrial hyperpolarization, slowed electron flux through the electron transport chain and increased production of ROS [69]. Both ROS and reactive nitrogen species (RNS) can damage proteins, lipids and DNA, particularly in mitochondria. This could be a major factor underlying declining mitochondrial function in at-risk neurons with age [70]. ROS and RNS also exacerbate the impact of genetic mutations and environmental toxins affecting mitochondria [71], as well as increase the propensity of aSYN to aggregate [72]. Moreover, mitochondrial damage stemming from oxidant stress should increase mitophagy, diminishing the ‘reserve’ autophagic capacity of neurons and their ability to deal with misfolded proteins, like aSYN fibrils [73]. Recent work by our group has demonstrated that mitophagy is in fact elevated in healthy SNc DA neurons [74]. The second downside associated with the anticipatory control of OXPHOS is that it results in high cytosolic calcium concentrations, which can have a variety of deleterious effects. Recent work has revealed that calcium concentrations in the dendrites of SNc DA neurons may rise into the low micromolar range with every spike during pacemaking, which is happening 2–10 times a second [74]. Elevated calcium directly promotes aSYN aggregation [75–77], activates the protease calpain (which increases aggregation) [78–80], activates the protein phosphatase calcineurin (which increases aSYN toxicity [81]); and impairs lysosomal motility and turnover of misfolded proteins, like aggregated aSYN [82]. aSYN oligomers may in turn elevate intracellular calcium, creating a damaging feedback loop [83].
Perhaps the most compelling piece of evidence that physiological phenotype is a determinant of pathology in PD is the observation that dihydropyridine inhibition of Cav1 channels in SNc DA neurons – which lowers cytosolic calcium levels, lowers mitochondrial oxidant stress and turnover, increases mitochondrial mass and decreases the sensitivity to toxins [55, 62, 74, 84–86] – has consistently been linked by epidemiological studies to reduced risk of developing PD [87–92]. The combination of pre-clinical and clinical data implicating Cav1 channels in PD pathogenesis motivated the National Institutes of Health in the U.S. to mount a 5 year, Phase III, disease modification clinical trial in early stage PD patients with the dihydropyridine isradipine; this trial will be completed later this year.
Thus, by design, SNc DA neurons appear to reside close to bioenergetic and protein degradation ‘tipping points’. Flagging mitochondrial and proteasomal/autophagic function with age [70, 93] – the biggest risk factor for PD – should undoubtedly push them closer to this tipping point, elevating the probability of de novo LP or an inability to handle the burden created by taking up pathological aSYN species from the extracellular space. Against this backdrop, it makes perfect sense that the genetic mutations and toxins associated with PD are ones that target mitochondria, protein degradation and aSYN expression, either directly or indirectly [94–96].
Is DA an accomplice?
Another trait that may contribute to selective vulnerability of SNc DA neurons is the reliance upon DA as a neurotransmitter. Cytosolic DA has long been known to be potentially toxic because of its oxidation to reactive quinones, but its role in pathogenesis has been contested for several reasons [97–99]. However, recent work has identified a new mechanism that might re-open the debate by tying DA toxicity to mitochondrial function, particularly in axons. First, mitochondrial oxidant stress in human (but not mouse) DA neurons promotes the generation of DA quinones that disrupt the function of glucocerebrosidase (GC) and lysosomes [100]. This species difference was linked to the relatively higher level of DA in human neurons and the accumulation of neuromelanin. GC was modified in its catalytic site by quinones, leading to lower activity. While it is widely accepted that lysosomal dysfunction can lead to the accumulation of damaged mitochondria [101], this work provides the first strong evidence that mitochondrial oxidant stress can cause lysosomal dysfunction. This observation complements earlier work linking DA, Cav1 channels, aSYN and lysosomes [102, 103]. Another potential linkage between DA and vulnerability could involve mitochondrially anchored monoamine oxidase (MAO). MAO degrades cytosolic DA, and is so doing, is widely thought to increase cytosolic oxidant stress by generating hydrogen peroxide [97]. Although appealing, this hypothesis has never been rigorously tested in situ. Moreover, this hypothesis doesn’t explain why MAO is anchored to the outer membrane of mitochondria. It is tempting to speculate that the electrons generated by DA metabolism are in fact shuttled to mitochondria to help with ATP production, rather than simply being ‘discarded’. If this were the case, there could be a summation in axonal mitochondrial oxidant stress arising from MAO metabolism of DA and that driven by calcium entry through Cav1 calcium channels [104].
The vast number of DA release sites of nigrostriatal axons also connects vulnerability to alterations in synaptic transmission per se. aSYN is strongly associated with vesicular trafficking at transmitter release sites [105, 106]. Other aspects of vesicular trafficking may also be disrupted in PD. Recent studies have implicated the synaptic proteins auxilin and synaptojanin-1, which regulate clathrin-mediated synaptic vesicle endocytosis, in PD [107–111]. But, how synaptic dysfunction contributes to the molecular mechanisms mediating dysfunction and the degeneration of SNc DA neurons remain unclear.
Does network dysfunction accelerate progression?
In addition to phenotypic traits that predispose SNc DA neurons to mitochondrial pathology, network dysfunction could contribute to their mitochondrial stress and disease progression, particularly in the symptomatic stages of the disease [112–117]. With symptom onset, rhythmic, synchronous bursting activity emerges in subthalamic nucleus (STN) glutamatergic neurons; because these STN neurons innervate SNc DA neurons, this pathological activity has been hypothesized to increase glutamate release in the SNc, initially compensating for DA release deficits but ultimately driving excitotoxicity [112, 118, 119]. Moreover, lesions of the STN or regularizing the output of the STN with deep brain stimulation has been reported to protect SNc DA neurons [112, 113, 115–117]. Another potential player in network-driven pathology is the pedunculopontine nucleus (PPN). PPN provides a potent glutamatergic innervation of vulnerable ventral tier SNc DA neurons [120–122]. Activity in the PPN, like that of the STN, rises in PD models [119, 123, 124] and in PD patients[125], suggesting that it could provide an additional excitotoxic drive.
Neuronal degeneration induced by glutamate is thought to be mediated by N-methyl-d-aspartate receptors that flux calcium [114]. Certainly, in SNc DA neurons adding to the calcium burden created by Cav1 calcium channels during pacemaking could prove problematic. However, there is another potential glutamatergic mechanism, particularly in SNc DA neurons with impaired mitochondrial function. Group I metabotropic glutamate receptors potently harness ER calcium stores to elevate cytosolic calcium levels and increase mitochondrial calcium loading in SNc DA neurons (Zampese et al. unpublished observations). This mechanism complements that engaged by Cav1 calcium channels during pacemaking, allowing excitatory glutamatergic synaptic input to stimulate mitochondrial ATP production in anticipation of the need created by synaptically evoked depolarization and spiking. Normally, this mechanism should be adaptive, helping mitochondrial ATP production meet demand. But, when mitochondrial function is impaired in PD or when glutamate rises in the absence of synaptic demand [118], mitochondrial calcium loading could drive pathology. Indeed, several lines of study suggest that antagonism of Group I mGluRs protects SNc DA neurons against toxins that compromise mitochondrial function [126–128].
Not an either-or proposition
Thus, in SNc DA neurons there may be a ‘perfect storm’ created by the convergence of their peculiar phenotype, aSYN pathology and mitochondrial dysfunction (Fig. 1). The available evidence clearly suggests that elevated mitochrondrial oxidant stress and cytosolic calcium will promote aSYN aggregation, increase aSYN toxicity and impair clearance, making SNc DA neurons not only more prone to de novo pathology, but to propagated aSYN pathology as well. Conversely, aSYN aggregates disrupt mitochondrial function [95, 129–133] and impair autophagy (see above), potentially leading to the accumulation of damaged and mis-regulated mitochondria. Recent work in SNc DA neurons over-expressing aSYN has provided new evidence that this interaction with mitochondria could create a ‘death spiral’ [134].
Figure 1:
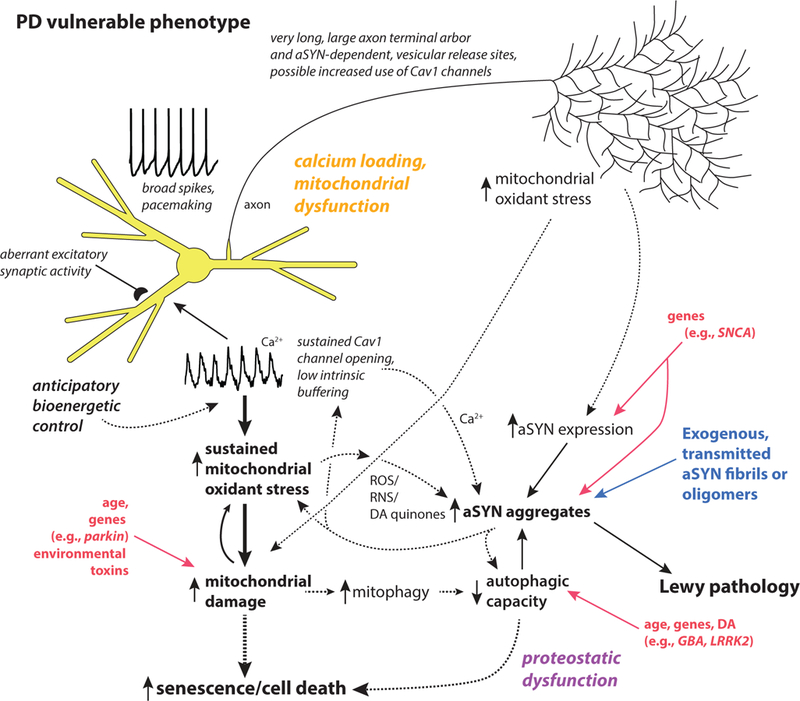
Schematic summary of the key traits of neurons that are vulnerable in PD. Two major drivers of pathogenesis are mitochondrial and proteostatic dysfunction. Mitochondrial dysfunction is proposed to be a consequence of anticipatory (feed-forward) control of mitochondrial respiration by calcium, and yet undefined axonal bioenergetic factors working in combination with genetic and environmental factors (e.g., toxins). Proteostatic dysfunction is proposed to arise from aSYN aggregation promoted by oxidant stress, elevated cytosolic calcium and DA quinones, in addition to lysosomal dysfunction promoted by increased mitophagy and oxidant damage to lysosomal proteins like glucocerebrosidase. Solid lines make connections between events that are well-established in mammalian models; dashed lines connect mechanisms for which there is good but not unequivocal support.
Unanswered questions and tools to build
Given all we know about aSYN pathology, mitochondrial dysfunction and SNc DA neurons, why is aging the most important risk factor for cPD? There are a number of recent reviews that have focused on the potential role of aging in the selective vulnerability of SNc DA neurons [70, 135, 136]. It is unclear to what extent aging diminishes the capacity of SNc DA neurons to successfully cope with stress arising from their phenotype and to what extent their phenotype actually accelerates the aging process. Many if not all of the causes of aging – genetic mutations, mitochondrial dysfunction, proteostatic dysfunction and telomere shortening [137] – could be promoted by the conditions found in SNc DA neurons. Telomere shortening, which is a new addition to this list, has recently been found to be driven by oxidant stress [138], making it relevant to aging of non-dividing neurons in the brain. In non-human primates, neurons in the ventral tier of the SNc, which is the among the first regions to degenerate in patients with PD, manifest signs of senescence (e.g., down-regulate tyrosine hydroxylase) sooner than do neurons in the dorsal tier or VTA [136]. ‘Premature’ cellular aging should increase vulnerability to challenges posed by protein aggregation, genetic mutations, environmental toxins, or infection, just as aging increases our vulnerability at the organismal level. Interestingly, rodent models do not recapitulate telomere shortening with aging seen in humans. This provides a potential explanation for why mouse genetic models of PD have consistently failed to reproduce the pattern of pathology observed in patients with PD.
This leads to another major shortcoming in the field of PD pathogenesis: the near absence of progressive models of pathogenesis that have construct validity. By construct validity, I mean a model that starts with an intervention that mimics a key event in human pathogenesis. Models predicated upon doses of toxin that kill SNc DA neurons virtually overnight do not fall into this category. For unknown reasons, mice harboring genetic mutations mimicking those found in human PD patients have failed to provide robust models of PD. Mice with intrastriatal injections of pre-formed fibrils of aSYN do manifest SNc degeneration and parkinsonism and, since LP is a feature of many forms of PD, these models do have construct validity [19, 139]. Moreover, our work and that of others suggests that aSYN pathology increases cytosolic and mitochondrial oxidant stress in SNc DA neurons [9, 132, 140]. However, the mitochondrial challenge in these mice is secondary to broader cellular stress, making it difficult to use them to assess the specific role of mitochondrial dysfunction and aSYN pathology in the evolution of PD – a question that is of fundamental importance for the development of therapies that slow SNc DA neuron loss in the early stages of PD. Moreover, these aSYN models do not manifest the progressive features of idiopathic PD; in particular, the early loss of DA axons innervating the striatum followed by loss of somatodendritic integrity [141]. The lack of progressive models not only makes hypothesis testing about the mechanisms underlying pathogenesis problematic, it makes it difficult to connect the motor symptoms of PD to stages in the degeneration of SNc DA neurons. Developing such a model would be a great leap forward.
Acknowledgements:
This work was supported by awards to DJS by the JPB Foundation, the IDP Foundation and the NIH (NS047085)
Abbreviations:
- (ATP)
adenosine triphosphate
- (aSYN)
alpha-synuclein
- (DA)
dopaminergic
- (DMV)
dorsal motor nucleus of vagus
- (ETC)
electron transport chain
- (ER)
endoplasmic reticulum
- (GC)
glucocerebrosidase
- (LP)
Lewy pathology
- (mtDNA)
mitochondrial deoxyribonucleic acid
- (MAO)
monoamine oxidase
- (PD)
Parkinson’s disease
- (PPN)
pedunculopontine nucleus
- (OXPHOS)
oxidative phosphorylation
- (RNS)
reactive nitrogen species
- (ROS)
reactive oxygen species
- (SNc)
substantia nigra pars compacta
- (STN)
subthalamic nucleus
References:
- 1.de Lau LM & Breteler MM (2006) Epidemiology of Parkinson’s disease, Lancet Neurol 5, 525–35. [DOI] [PubMed] [Google Scholar]
- 2.Bach JP, Riedel O, Klotsche J, Spottke A, Dodel R & Wittchen HU (2012) Impact of complications and comorbidities on treatment costs and health-related quality of life of patients with Parkinson’s disease, J Neurol Sci 314, 41–7. [DOI] [PubMed] [Google Scholar]
- 3.Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, Marshall FJ, Ravina BM, Schifitto G, Siderowf A & Tanner CM (2007) Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030, Neurology 68, 384–6. [DOI] [PubMed] [Google Scholar]
- 4.Huse DM, Schulman K, Orsini L, Castelli-Haley J, Kennedy S & Lenhart G (2005) Burden of illness in Parkinson’s disease, Mov Disord 20, 1449–54. [DOI] [PubMed] [Google Scholar]
- 5.Dorsey ER, George BP, Leff B & Willis AW (2013) The coming crisis: obtaining care for the growing burden of neurodegenerative conditions, Neurology 80, 1989–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal HK, Zhai S, Surmeier DJ & Ellis-Davies GCR (2017) Intracellular Uncaging of cGMP with Blue Light, ACS Chem Neurosci 8, 2139–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag AE & Lang AE (2017) Parkinson disease, Nat Rev Dis Primers 3, 17013. [DOI] [PubMed] [Google Scholar]
- 8.Braak H, Ghebremedhin E, Rub U, Bratzke H & Del Tredici K (2004) Stages in the development of Parkinson’s disease-related pathology, Cell Tissue Res 318, 121–34. [DOI] [PubMed] [Google Scholar]
- 9.Surmeier DJ, Obeso JA & Halliday GM (2017) Selective neuronal vulnerability in Parkinson disease, Nat Rev Neurosci 18, 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goedert M, Spillantini MG, Del Tredici K & Braak H (2013) 100 years of Lewy pathology, Nat Rev Neurol 9, 13–24. [DOI] [PubMed] [Google Scholar]
- 11.Iljina M, Garcia GA, Horrocks MH, Tosatto L, Choi ML, Ganzinger KA, Abramov AY, Gandhi S, Wood NW, Cremades N, Dobson CM, Knowles TP & Klenerman D (2016) Kinetic model of the aggregation of alpha-synuclein provides insights into prion-like spreading, Proc Natl Acad Sci U S A 113, E1206–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts HL & Brown DR (2015) Seeking a Mechanism for the Toxicity of Oligomeric α-Synuclein., Biomolecules 5, 282–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poulopoulos M, Levy OA & Alcalay RN (2012) The neuropathology of genetic Parkinson's disease., Movement disorders : official journal of the Movement Disorder Society 27, 831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konno T, Ross OA, Puschmann A, Dickson DW & Wszolek ZK (2016) Autosomal dominant Parkinson's disease caused by SNCA duplications., Parkinsonism & related disorders 22 Suppl 1, S1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawkes CH, Del Tredici K & Braak H (2007) Parkinson’s disease: a dual-hit hypothesis, Neuropathol Appl Neurobiol 33, 599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brundin P & Melki R (2017) Prying into the Prion Hypothesis for Parkinson’s Disease, J Neurosci 37, 9808–9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Bjorklund A, Widner H, Revesz T, Lindvall O & Brundin P (2008) Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation, Nat Med 14, 501–3. [DOI] [PubMed] [Google Scholar]
- 18.Kordower JH, Chu Y, Hauser RA, Freeman TB & Olanow CW (2008) Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease, Nat Med 14, 504–6. [DOI] [PubMed] [Google Scholar]
- 19.Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ & Lee VM (2012) Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice, Science 338, 949–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masuda-Suzukake M, Nonaka T, Hosokawa M, Oikawa T, Arai T, Akiyama H, Mann DMA & Hasegawa M (2013) Prion-like spreading of pathological α-synuclein in brain., Brain : a journal of neurology 136, 1128–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Recasens A, Dehay B, Bové J, Carballo Carbajal I, Dovero S, Pérez Villalba A, Fernagut PO, Blesa J, Parent A, Perier C, Fariñas I, Obeso JA, Bezard E & Vila M (2014) Lewy body extracts from Parkinson disease brains trigger α‐synuclein pathology and neurodegeneration in mice and monkeys, Annals of neurology 75, 351–362. [DOI] [PubMed] [Google Scholar]
- 22.Mao X, Ou MT, Karuppagounder SS, Kam TI, Yin X, Xiong Y, Ge P, Umanah GE, Brahmachari S, Shin JH, Kang HC, Zhang J, Xu J, Chen R, Park H, Andrabi SA, Kang SU, Goncalves RA, Liang Y, Zhang S, Qi C, Lam S, Keiler JA, Tyson J, Kim D, Panicker N, Yun SP, Workman CJ, Vignali DA, Dawson VL, Ko HS & Dawson TM (2016) Pathological alpha-synuclein transmission initiated by binding lymphocyte-activation gene 3, Science 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shrivastava AN, Redeker V, Fritz N, Pieri L, Almeida LG, Spolidoro M, Liebmann T, Bousset L, Renner M, Lena C, Aperia A, Melki R & Triller A (2015) alpha-synuclein assemblies sequester neuronal alpha3-Na+/K+-ATPase and impair Na+ gradient, EMBO J 34, 2408–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uchihara T & Giasson BI (2016) Propagation of alpha-synuclein pathology: hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies., Acta neuropathologica 131, 49–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh DM & Selkoe DJ (2016) A critical appraisal of the pathogenic protein spread hypothesis of neurodegeneration, Nature reviews Neuroscience 17, 251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sacino AN, Brooks MM, Chakrabarty P, Saha K, Khoshbouei H, Golde TE & Giasson BI (2017) Proteolysis of alpha-synuclein fibrils in the lysosomal pathway limits induction of inclusion pathology, J Neurochem 140, 662–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braak H & Del Tredici K (2017) Neuropathological Staging of Brain Pathology in Sporadic Parkinson’s disease: Separating the Wheat from the Chaff, J Parkinsons Dis 7, S73–S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts HL & Brown DR (2015) Seeking a mechanism for the toxicity of oligomeric alpha-synuclein, Biomolecules 5, 282–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rockenstein E, Nuber S, Overk CR, Ubhi K, Mante M, Patrick C, Adame A, Trejo-Morales M, Gerez J, Picotti P, Jensen PH, Campioni S, Riek R, Winkler J, Gage FH, Winner B & Masliah E (2014) Accumulation of oligomer-prone alpha-synuclein exacerbates synaptic and neuronal degeneration in vivo, Brain 137, 1496–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colla E, Jensen PH, Pletnikova O, Troncoso JC, Glabe C & Lee MK (2012) Accumulation of toxic alpha-synuclein oligomer within endoplasmic reticulum occurs in alpha-synucleinopathy in vivo, J Neurosci 32, 3301–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winner B, Jappelli R, Maji SK, Desplats PA, Boyer L, Aigner S, Hetzer C, Loher T, Vilar M, Campioni S, Tzitzilonis C, Soragni A, Jessberger S, Mira H, Consiglio A, Pham E, Masliah E, Gage FH & Riek R (2011) In vivo demonstration that alpha-synuclein oligomers are toxic, Proc Natl Acad Sci U S A 108, 4194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helwig M, Klinkenberg M, Rusconi R, Musgrove RE, Majbour NK, El-Agnaf OM, Ulusoy A & Di Monte DA (2016) Brain propagation of transduced alpha-synuclein involves non-fibrillar protein species and is enhanced in alpha-synuclein null mice, Brain 139, 856–70. [DOI] [PubMed] [Google Scholar]
- 33.Ulusoy A, Musgrove RE, Rusconi R, Klinkenberg M, Helwig M, Schneider A & Di Monte DA (2015) Neuron-to-neuron alpha-synuclein propagation in vivo is independent of neuronal injury, Acta Neuropathol Commun 3, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dauer W & Przedborski S (2003) Parkinson’s disease: mechanisms and models, Neuron 39, 889–909. [DOI] [PubMed] [Google Scholar]
- 35.Schapira AH (2011) Mitochondrial pathology in Parkinson’s disease, Mt Sinai J Med 78, 872–81. [DOI] [PubMed] [Google Scholar]
- 36.Haelterman NA, Yoon WH, Sandoval H, Jaiswal M, Shulman JM & Bellen HJ (2014) A mitocentric view of Parkinson’s disease, Annu Rev Neurosci 37, 137–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beilina A & Cookson MR (2016) Genes associated with Parkinson’s disease: regulation of autophagy and beyond, J Neurochem 139 Suppl 1, 91–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cookson MR (2010) DJ-1, PINK1, and their effects on mitochondrial pathways, Mov Disord 25 Suppl 1, S44–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heo JY, Park JH, Kim SJ, Seo KS, Han JS, Lee SH, Kim JM, Park JI, Park SK, Lim K, Hwang BD, Shong M & Kweon GR (2012) DJ-1 null dopaminergic neuronal cells exhibit defects in mitochondrial function and structure: involvement of mitochondrial complex I assembly, PLoS One 7, e32629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Funayama M & Hattori N (2015) CHCHD2 and Parkinson’s disease--authors’ reply, Lancet Neurol 14, 682–3. [DOI] [PubMed] [Google Scholar]
- 41.Greenamyre JT, Cannon JR, Drolet R & Mastroberardino PG (2010) Lessons from the rotenone model of Parkinson’s disease, Trends Pharmacol Sci 31, 141–2; author reply 142–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Przedborski S, Tieu K, Perier C & Vila M (2004) MPTP as a mitochondrial neurotoxic model of Parkinson’s disease, J Bioenerg Biomembr 36, 375–9. [DOI] [PubMed] [Google Scholar]
- 43.Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB & Marsden CD (1989) Mitochondrial complex I deficiency in Parkinson’s disease, Lancet 1, 1269. [DOI] [PubMed] [Google Scholar]
- 44.Grunewald A, Rygiel KA, Hepplewhite PD, Morris CM, Picard M & Turnbull DM (2016) Mitochondrial DNA Depletion in Respiratory Chain-Deficient Parkinson Disease Neurons, Ann Neurol 79, 366–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, Taylor RW & Turnbull DM (2006) High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease, Nat Genet 38, 515–7. [DOI] [PubMed] [Google Scholar]
- 46.Bender A, Schwarzkopf RM, McMillan A, Krishnan KJ, Rieder G, Neumann M, Elstner M, Turnbull DM & Klopstock T (2008) Dopaminergic midbrain neurons are the prime target for mitochondrial DNA deletions, J Neurol 255, 1231–5. [DOI] [PubMed] [Google Scholar]
- 47.Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, Kowall NW & Khrapko K (2006) Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons, Nat Genet 38, 518–20. [DOI] [PubMed] [Google Scholar]
- 48.Matsuda W, Furuta T, Nakamura KC, Hioki H, Fujiyama F, Arai R & Kaneko T (2009) Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum, J Neurosci 29, 444–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bolam JP & Pissadaki EK (2012) Living on the edge with too many mouths to feed: why dopamine neurons die, Mov Disord 27, 1478–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hunn BHM, Cragg SJ, Bolam JP, Spillantini MG & Wade-Martins R (2015) Impaired intracellular trafficking defines early Parkinson's disease., Trends in neurosciences 38, 178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pacelli C, Giguère N, Bourque M-J, Lévesque M, Slack RS & Trudeau L-E (2015) Elevated Mitochondrial Bioenergetics and Axonal Arborization Size Are Key Contributors to the Vulnerability of Dopamine Neurons., Current biology : CB 25, 2349–2360. [DOI] [PubMed] [Google Scholar]
- 52.Zharikov AD, Cannon JR, Tapias V, Bai Q, Horowitz MP, Shah V, El Ayadi A, Hastings TG, Greenamyre JT & Burton EA (2015) shRNA targeting alpha-synuclein prevents neurodegeneration in a Parkinson’s disease model, J Clin Invest 125, 2721–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou F-M, Wilson CJ & Dani JA (2002) Cholinergic interneuron characteristics and nicotinic properties in the striatum, J Neurobiol 53, 590–605. [DOI] [PubMed] [Google Scholar]
- 54.Bean BP (2007) The action potential in mammalian central neurons, Nat Rev Neurosci 8, 451–65. [DOI] [PubMed] [Google Scholar]
- 55.Guzman JN, Sanchez-Padilla J, Wokosin D, Kondapalli J, Ilijic E, Schumacker PT & Surmeier DJ (2010) Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1, Nature 468, 696–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nedergaard S, Flatman JA & Engberg I (1993) Nifedipine- and omega-conotoxin-sensitive Ca2+ conductances in guinea-pig substantia nigra pars compacta neurones, J Physiol 466, 727–47. [PMC free article] [PubMed] [Google Scholar]
- 57.Puopolo M, Raviola E & Bean BP (2007) Roles of subthreshold calcium current and sodium current in spontaneous firing of mouse midbrain dopamine neurons, J Neurosci 27, 645–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morikawa H & Paladini CA (2011) Dynamic regulation of midbrain dopamine neuron activity: intrinsic, synaptic, and plasticity mechanisms, Neuroscience 198, 95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Foehring RC, Zhang XF, Lee JC & Callaway JC (2009) Endogenous calcium buffering capacity of substantia nigral dopamine neurons, J Neurophysiol 102, 2326–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khaliq ZM & Bean BP (2010) Pacemaking in dopaminergic ventral tegmental area neurons: depolarizing drive from background and voltage-dependent sodium conductances, J Neurosci 30, 7401–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guzman JN, Sanchez-Padilla J, Chan CS & Surmeier DJ (2009) Robust pacemaking in substantia nigra dopaminergic neurons, J Neurosci 29, 11011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, Meredith GE & Surmeier DJ (2007) ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease, Nature 447, 1081–6. [DOI] [PubMed] [Google Scholar]
- 63.Philippart F, Destreel G, Merino-Sepulveda P, Henny P, Engel D & Seutin V (2016) Differential Somatic Ca2+ Channel Profile in Midbrain Dopaminergic Neurons, J Neurosci 36, 7234–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Putzier I, Kullmann PH, Horn JP & Levitan ES (2009) Cav1.3 channel voltage dependence, not Ca2+ selectivity, drives pacemaker activity and amplifies bursts in nigral dopamine neurons, J Neurosci 29, 15414–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hayashi T, Rizzuto R, Hajnoczky G & Su TP (2009) MAM: more than just a housekeeper, Trends Cell Biol 19, 81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Balaban RS (2009) The role of Ca(2+) signaling in the coordination of mitochondrial ATP production with cardiac work, Biochim Biophys Acta 1787, 1334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nicholls DG & Budd SL (1998) Mitochondria and neuronal glutamate excitotoxicity, Biochim Biophys Acta 1366, 97–112. [DOI] [PubMed] [Google Scholar]
- 68.Dragicevic E, Schiemann J & Liss B (2015) Dopamine midbrain neurons in health and Parkinson’s disease: emerging roles of voltage-gated calcium channels and ATP-sensitive potassium channels, Neuroscience 284, 798–814. [DOI] [PubMed] [Google Scholar]
- 69.Votyakova TV & Reynolds IJ (2001) DeltaPsi(m)-Dependent and -independent production of reactive oxygen species by rat brain mitochondria, J Neurochem 79, 266–77. [DOI] [PubMed] [Google Scholar]
- 70.Reeve A, Simcox E & Turnbull D (2014) Ageing and Parkinson’s disease: why is advancing age the biggest risk factor?, Ageing Res Rev 14, 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gegg ME & Schapira AH (2016) Mitochondrial dysfunction associated with glucocerebrosidase deficiency, Neurobiol Dis 90, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gupta A, Dawson VL & Dawson TM (2008) What causes cell death in Parkinson’s disease?, Ann Neurol 64 Suppl 2, S3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wong E & Cuervo AM (2010) Autophagy gone awry in neurodegenerative diseases, Nat Neurosci 13, 805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guzman JN, Ilijic E, Yang B, Sanchez-Padilla J, Wokosin D, Galtieri D, Kondapalli J, Schumacker PT & Surmeier DJ (2018) Systemic isradipine treatment diminishes calcium-dependent mitochondrial oxidant stress, J Clin Invest 128, 2266–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rcom-H’cheo-Gauthier A, Goodwin J & Pountney DL (2014) Interactions between calcium and alpha-synuclein in neurodegeneration, Biomolecules 4, 795–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nath S, Goodwin J, Engelborghs Y & Pountney DL (2011) Raised calcium promotes α-synuclein aggregate formation, Molecular and Cellular Neuroscience 46, 516–526. [DOI] [PubMed] [Google Scholar]
- 77.Lautenschlager J, Stephens AD, Fusco G, Strohl F, Curry N, Zacharopoulou M, Michel CH, Laine R, Nespovitaya N, Fantham M, Pinotsi D, Zago W, Fraser P, Tandon A, St George-Hyslop P, Rees E, Phillips JJ, De Simone A, Kaminski CF & Schierle GSK (2018) C-terminal calcium binding of alpha-synuclein modulates synaptic vesicle interaction, Nat Commun 9, 712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Diepenbroek M, Casadei N, Esmer H, Saido TC, Takano J, Kahle PJ, Nixon RA, Rao MV, Melki R, Pieri L, Helling S, Marcus K, Krueger R, Masliah E, Riess O & Nuber S (2014) Overexpression of the calpain-specific inhibitor calpastatin reduces human alpha-Synuclein processing, aggregation and synaptic impairment in [A30P]alphaSyn transgenic mice, Hum Mol Genet 23, 3975–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dufty BM, Warner LR, Hou ST, Jiang SX, Gomez-Isla T, Leenhouts KM, Oxford JT, Feany MB, Masliah E & Rohn TT (2007) Calpain-cleavage of alpha-synuclein: connecting proteolytic processing to disease-linked aggregation, Am J Pathol 170, 1725–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Melachroinou K, Xilouri M, Emmanouilidou E, Masgrau R, Papazafiri P, Stefanis L & Vekrellis K (2013) Deregulation of calcium homeostasis mediates secreted α-synuclein-induced neurotoxicity., Neurobiology of aging 34, 2853–2865. [DOI] [PubMed] [Google Scholar]
- 81.Caraveo G, Auluck PK, Whitesell L, Chung CY, Baru V, Mosharov EV, Yan X, Ben-Johny M, Soste M, Picotti P, Kim H, Caldwell KA, Caldwell GA, Sulzer D, Yue DT & Lindquist S (2014) Calcineurin determines toxic versus beneficial responses to alpha-synuclein, Proc Natl Acad Sci U S A 111, E3544–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gómez-Sintes R, Ledesma MD & Boya P (2016) Lysosomal cell death mechanisms in aging., Ageing research reviews [DOI] [PubMed]
- 83.Angelova PR, Ludtmann MH, Horrocks MH, Negoda A, Cremades N, Klenerman D, Dobson CM, Wood NW, Pavlov EV, Gandhi S & Abramov AY (2016) Ca2+ is a key factor in alpha-synuclein-induced neurotoxicity, J Cell Sci 129, 1792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kupsch A, Sautter J, Schwarz J, Riederer P, Gerlach M & Oertel WH (1996) 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity in non-human primates is antagonized by pretreatment with nimodipine at the nigral, but not at the striatal level, Brain Res 741, 185–96. [DOI] [PubMed] [Google Scholar]
- 85.Ilijic E, Guzman JN & Surmeier DJ (2011) The L-type channel antagonist isradipine is neuroprotective in a mouse model of Parkinson’s disease, Neurobiol Dis 43, 364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singh A, Verma P, Balaji G, Samantaray S & Mohanakumar KP (2016) Nimodipine, an L-type calcium channel blocker attenuates mitochondrial dysfunctions to protect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinsonism in mice, Neurochem Int 99, 221–232. [DOI] [PubMed] [Google Scholar]
- 87.Marras C, Gruneir A, Rochon P, Wang X, Anderson G, Brotchie J, Bell CM, Fox S & Austin PC (2012) Dihydropyridine calcium channel blockers and the progression of parkinsonism, Ann Neurol 71, 362–9. [DOI] [PubMed] [Google Scholar]
- 88.Pasternak B, Svanstrom H, Nielsen NM, Fugger L, Melbye M & Hviid A (2012) Use of calcium channel blockers and Parkinson’s disease, Am J Epidemiol 175, 627–35. [DOI] [PubMed] [Google Scholar]
- 89.Ritz B, Rhodes SL, Qian L, Schernhammer E, Olsen JH & Friis S (2010) L-type calcium channel blockers and Parkinson disease in Denmark, Ann Neurol 67, 600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gudala K, Kanukula R & Bansal D (2015) Reduced Risk of Parkinson’s Disease in Users of Calcium Channel Blockers: A Meta-Analysis, Int J Chronic Dis 2015, 697404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee YC, Lin CH, Wu RM, Lin JW, Chang CH & Lai MS (2014) Antihypertensive agents and risk of Parkinson’s disease: a nationwide cohort study, PLoS One 9, e98961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Becker C, Jick SS & Meier CR (2008) Use of antihypertensives and the risk of Parkinson disease, Neurology 70, 1438–44. [DOI] [PubMed] [Google Scholar]
- 93.Rodriguez M, Rodriguez-Sabate C, Morales I, Sanchez A & Sabate M (2015) Parkinson’s disease as a result of aging, Aging Cell 14, 293–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Duda J, Potschke C & Liss B (2016) Converging roles of ion channels, calcium, metabolic stress, and activity pattern of Substantia nigra dopaminergic neurons in health and Parkinson’s disease, J Neurochem 139 Suppl 1, 156–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Calì T, Ottolini D & Brini M (2014) Calcium signaling in Parkinson's disease., Cell and tissue research 357, 439–454. [DOI] [PubMed] [Google Scholar]
- 96.Surmeier DJ, Guzman JN, Sanchez J & Schumacker PT (2012) Physiological phenotype and vulnerability in Parkinson’s disease, Cold Spring Harb Perspect Med 2, a009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sulzer D (2007) Multiple hit hypotheses for dopamine neuron loss in Parkinson’s disease, Trends Neurosci 30, 244–50. [DOI] [PubMed] [Google Scholar]
- 98.Burke WJ, Li SW, Chung HD, Ruggiero DA, Kristal BS, Johnson EM, Lampe P, Kumar VB, Franko M, Williams EA & Zahm DS (2004) Neurotoxicity of MAO metabolites of catecholamine neurotransmitters: role in neurodegenerative diseases, Neurotoxicology 25, 101–15. [DOI] [PubMed] [Google Scholar]
- 99.Hastings TG (2009) The role of dopamine oxidation in mitochondrial dysfunction: implications for Parkinson’s disease, J Bioenerg Biomembr 41, 469–72. [DOI] [PubMed] [Google Scholar]
- 100.Burbulla LF, Song P, Mazzulli JR, Zampese E, Wong YC, Jeon S, Santos DP, Blanz J, Obermaier CD, Strojny C, Savas JN, Kiskinis E, Zhuang X, Kruger R, Surmeier DJ & Krainc D (2017) Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease, Science 357, 1255–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Plotegher N & Duchen MR (2017) Crosstalk between Lysosomes and Mitochondria in Parkinson’s Disease, Front Cell Dev Biol 5, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mosharov EV, Larsen KE, Kanter E, Phillips KA, Wilson K, Schmitz Y, Krantz DE, Kobayashi K, Edwards RH & Sulzer D (2009) Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons, Neuron 62, 218–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jain MK & Bhat R (2014) Modulation of human alpha-synuclein aggregation by a combined effect of calcium and dopamine, Neurobiol Dis 63, 115–28. [DOI] [PubMed] [Google Scholar]
- 104.Brimblecombe KR, Gracie CJ, Platt NJ & Cragg SJ (2015) Gating of dopamine transmission by calcium and axonal N-, Q-, T- and L-type voltage-gated calcium channels differs between striatal domains, J Physiol 593, 929–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wong YC & Krainc D (2017) alpha-synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies, Nat Med 23, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Abeliovich A & Gitler AD (2016) Defects in trafficking bridge Parkinson’s disease pathology and genetics, Nature 539, 207–216. [DOI] [PubMed] [Google Scholar]
- 107.Gorenberg EL & Chandra SS (2017) The Role of Co-chaperones in Synaptic Proteostasis and Neurodegenerative Disease, Front Neurosci 11, 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kirola L, Behari M, Shishir C & Thelma BK (2016) Identification of a novel homozygous mutation Arg459Pro in SYNJ1 gene of an Indian family with autosomal recessive juvenile Parkinsonism, Parkinsonism Relat Disord 31, 124–128. [DOI] [PubMed] [Google Scholar]
- 109.Chen KH, Wu RM, Lin HI, Tai CH & Lin CH (2015) Mutational analysis of SYNJ1 gene (PARK20) in Parkinson’s disease in a Taiwanese population, Neurobiol Aging 36, 2905 e7–8. [DOI] [PubMed] [Google Scholar]
- 110.Drouet V & Lesage S (2014) Synaptojanin 1 mutation in Parkinson’s disease brings further insight into the neuropathological mechanisms, Biomed Res Int 2014, 289728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Elsayed LE, Drouet V, Usenko T, Mohammed IN, Hamed AA, Elseed MA, Salih MA, Koko ME, Mohamed AY, Siddig RA, Elbashir MI, Ibrahim ME, Durr A, Stevanin G, Lesage S, Ahmed AE & Brice A (2016) A Novel Nonsense Mutation in DNAJC6 Expands the Phenotype of Autosomal-Recessive Juvenile-Onset Parkinson’s Disease, Ann Neurol 79, 335–7. [DOI] [PubMed] [Google Scholar]
- 112.Benazzouz A, Piallat B, Ni ZG, Koudsie A, Pollak P & Benabid AL (2000) Implication of the subthalamic nucleus in the pathophysiology and pathogenesis of Parkinson’s disease, Cell Transplant 9, 215–21. [DOI] [PubMed] [Google Scholar]
- 113.Rodriguez MC, Obeso JA & Olanow CW (1998) Subthalamic nucleus-mediated excitotoxicity in Parkinson’s disease: a target for neuroprotection, Ann Neurol 44, S175–88. [DOI] [PubMed] [Google Scholar]
- 114.Beal MF (1998) Excitotoxicity and nitric oxide in Parkinson’s disease pathogenesis, Ann Neurol 44, S110–4. [DOI] [PubMed] [Google Scholar]
- 115.Wallace BA, Ashkan K, Heise CE, Foote KD, Torres N, Mitrofanis J & Benabid AL (2007) Survival of midbrain dopaminergic cells after lesion or deep brain stimulation of the subthalamic nucleus in MPTP-treated monkeys, Brain 130, 2129–45. [DOI] [PubMed] [Google Scholar]
- 116.Temel Y, Visser-Vandewalle V, Kaplan S, Kozan R, Daemen MA, Blokland A, Schmitz C & Steinbusch HW (2006) Protection of nigral cell death by bilateral subthalamic nucleus stimulation, Brain Res 1120, 100–5. [DOI] [PubMed] [Google Scholar]
- 117.Takada M, Matsumura M, Kojima J, Yamaji Y, Inase M, Tokuno H, Nambu A & Imai H (2000) Protection against dopaminergic nigrostriatal cell death by excitatory input ablation, Eur J Neurosci 12, 1771–80. [DOI] [PubMed] [Google Scholar]
- 118.Meredith GE, Totterdell S, Beales M & Meshul CK (2009) Impaired glutamate homeostasis and programmed cell death in a chronic MPTP mouse model of Parkinson’s disease, Exp Neurol 219, 334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Breit S, Bouali-Benazzouz R, Benabid AL & Benazzouz A (2001) Unilateral lesion of the nigrostriatal pathway induces an increase of neuronal activity of the pedunculopontine nucleus, which is reversed by the lesion of the subthalamic nucleus in the rat, Eur J Neurosci 14, 1833–42. [DOI] [PubMed] [Google Scholar]
- 120.Scarnati E, Campana E & Pacitti C (1984) Pedunculopontine-evoked excitation of substantia nigra neurons in the rat, Brain Res 304, 351–61. [DOI] [PubMed] [Google Scholar]
- 121.Galtieri DJ, Estep CM, Wokosin DL, Traynelis S & Surmeier DJ (2017) Pedunculopontine glutamatergic neurons control spike patterning in substantia nigra dopaminergic neurons, Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Futami T, Takakusaki K & Kitai ST (1995) Glutamatergic and cholinergic inputs from the pedunculopontine tegmental nucleus to dopamine neurons in the substantia nigra pars compacta, Neurosci Res 21, 331–42. [DOI] [PubMed] [Google Scholar]
- 123.Geng X, Xie J, Wang X, Wang X, Zhang X, Hou Y, Lei C, Li M, Qu Q, He T, Han H, Yao X & Wang M (2016) Altered neuronal activity in the pedunculopontine nucleus: An electrophysiological study in a rat model of Parkinson’s disease, Behav Brain Res 305, 57–64. [DOI] [PubMed] [Google Scholar]
- 124.Zhang QJ, Liu J, Wang Y, Wang S, Wu ZH, Yan W, Hui YP & Ali U (2008) The firing activity of presumed cholinergic and non-cholinergic neurons of the pedunculopontine nucleus in 6-hydroxydopamine-lesioned rats: an in vivo electrophysiological study, Brain Res 1243, 152–60. [DOI] [PubMed] [Google Scholar]
- 125.Huang C, Tang C, Feigin A, Lesser M, Ma Y, Pourfar M, Dhawan V & Eidelberg D (2007) Changes in network activity with the progression of Parkinson’s disease, Brain 130, 1834–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vernon AC, Zbarsky V, Datla KP, Croucher MJ & Dexter DT (2007) Subtype selective antagonism of substantia nigra pars compacta Group I metabotropic glutamate receptors protects the nigrostriatal system against 6-hydroxydopamine toxicity in vivo, J Neurochem 103, 1075–91. [DOI] [PubMed] [Google Scholar]
- 127.Litim N, Morissette M & Di Paolo T (2017) Metabotropic glutamate receptors as therapeutic targets in Parkinson’s disease: An update from the last 5 years of research, Neuropharmacology 115, 166–179. [DOI] [PubMed] [Google Scholar]
- 128.Masilamoni GJ, Bogenpohl JW, Alagille D, Delevich K, Tamagnan G, Votaw JR, Wichmann T & Smith Y (2011) Metabotropic glutamate receptor 5 antagonist protects dopaminergic and noradrenergic neurons from degeneration in MPTP-treated monkeys, Brain 134, 2057–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Reeve AK, Ludtmann MH, Angelova PR, Simcox EM, Horrocks MH, Klenerman D, Gandhi S, Turnbull DM & Abramov AY (2015) Aggregated alpha-synuclein and complex I deficiency: exploration of their relationship in differentiated neurons, Cell Death Dis 6, e1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Luth ES, Stavrovskaya IG, Bartels T, Kristal BS & Selkoe DJ (2014) Soluble, prefibrillar alpha-synuclein oligomers promote complex I-dependent, Ca2+-induced mitochondrial dysfunction, J Biol Chem 289, 21490–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Abramov AY, Berezhnov AV, Fedotova EI, Zinchenko VP & Dolgacheva LP (2017) Interaction of misfolded proteins and mitochondria in neurodegenerative disorders, Biochem Soc Trans [DOI] [PubMed]
- 132.Dryanovski DI, Guzman JN, Xie Z, Galteri DJ, Volpicelli-Daley LA, Lee VM, Miller RJ, Schumacker PT & Surmeier DJ (2013) Calcium entry and alpha-synuclein inclusions elevate dendritic mitochondrial oxidant stress in dopaminergic neurons, J Neurosci 33, 10154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Guardia-Laguarta C, Area-Gomez E, Schon EA & Przedborski S (2015) A new role for alpha-synuclein in Parkinson’s disease: Alteration of ER-mitochondrial communication, Mov Disord 30, 1026–33. [DOI] [PubMed] [Google Scholar]
- 134.Subramaniam M, Althof D, Gispert S, Schwenk J, Auburger G, Kulik A, Fakler B & Roeper J (2014) Mutant alpha-synuclein enhances firing frequencies in dopamine substantia nigra neurons by oxidative impairment of A-type potassium channels, J Neurosci 34, 13586–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zucca FA, Segura-Aguilar J, Ferrari E, Munoz P, Paris I, Sulzer D, Sarna T, Casella L & Zecca L (2017) Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease, Prog Neurobiol 155, 96–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Collier TJ, Kanaan NM & Kordower JH (2017) Aging and Parkinson’s disease: Different sides of the same coin?, Mov Disord 32, 983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kirkwood TB (2010) Global aging and the brain, Nutr Rev 68 Suppl 2, S65–9. [DOI] [PubMed] [Google Scholar]
- 138.Sahin E & DePinho RA (2012) Axis of ageing: telomeres, p53 and mitochondria, Nat Rev Mol Cell Biol 13, 397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Thakur P, Breger LS, Lundblad M, Wan OW, Mattsson B, Luk KC, Lee VMY, Trojanowski JQ & Bjorklund A (2017) Modeling Parkinson’s disease pathology by combination of fibril seeds and alpha-synuclein overexpression in the rat brain, Proc Natl Acad Sci U S A 114, E8284–E8293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ & Lee VM (2000) Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions, Science 290, 985–9. [DOI] [PubMed] [Google Scholar]
- 141.Burke RE & O’Malley K (2013) Axon degeneration in Parkinson’s disease, Exp Neurol 246, 72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]


