Abstract
Background:
Opioid use disorder (OUD) is a public health crisis. Differences in opioid withdrawal severity that predict treatment outcome could facilitate the process of matching patients to treatments.
Methods:
This is a secondary analysis of a randomized controlled trial (RCT; Dunn et al., 2017) that enrolled treatment-seeking primary heroin users (N=89; males=78) into a residential study. Participants maintained on morphine (30mg, SC, QID) underwent a naloxone (0.4mg, IM) challenge session to precipitate withdrawal. Area-under-the-curve (AUC) values from self-reported withdrawal ratings during the challenge session were analyzed using K-means clustering, revealing 2 phenotype groups. Withdrawal and retention from the subsequent 14-day double-blind, double-dummy RCT comparing 3 study medications (clonidine, tramadol-ER, buprenorphine) were evaluated as a function of phenotype.
Results:
Cluster analyses suggested HIGH (N=37; mean[SD] SOWS-AUC 123.7[65.8]) and LOW (N=52; SOWS-AUC 68.0 [47.7]) withdrawal phenotype groups. HIGH participants were significantly more female and had lower body mass indices than LOW participants; no drug use variables were significant. Regarding RCT outcomes, HIGH phenotype participants were less likely to be retained in the study (p=0.02) and had higher mean self-reported withdrawal (p=0.05) than LOW phenotype participants. A significant interaction in RCT retention was observed between phenotype (p=0.02) and study medication (p<.01). Self-reported withdrawal was significant for phenotype (p=0.02); study medication trended towards significance (p=0.07).
Conclusions:
Results suggest patients have meaningfully different experiences of opioid withdrawal that may predict differential response to opioid pharmacotherapies during supervised withdrawal. Additional prospective research to replicate and more thoroughly evaluate withdrawal phenotype correlates and sex differences is warranted.
Keywords: opioid, personalized medicine, withdrawal, buprenorphine
1. Introduction
Nearly 12.5 million people in the United States misused an opioid pain reliever or heroin in 2016 and approximately 2.4 million were estimated to have opioid use disorder (OUD) (Substance Abuse and Mental Health Services Administration (SAMHSA). 2014); yet almost 80% of those believed to require OUD treatment were not engaged in treatment. Opioid use in the United States has been identified as a public health emergency, and unintentional drug poisonings, of which opioid overdose is a major contributor, are the leading cause of accidental death in adults aged 25-64 (S. L. Murphy et al. 2017). Several national organizations have called for increased efforts to expand and improve OUD treatment options and availability (K. Murphy et al. 2016; Blendon and Benson. 2018).
Chronic opioid use leads to the development of physical symptoms of opioid dependence, and the prominent and aversive withdrawal syndrome that manifests when opioid use is discontinued is a classic hallmark of OUD. Avoidance of the opioid withdrawal syndrome is a frequently cited reason for continued opioid use (Hutcheson et al. 2001) and OUD pharmacotherapies generally aim to minimize opioid withdrawal severity so patients can successfully achieve abstinence. Current medications used to treat opioid withdrawal include opioid agonists (e.g., methadone, buprenorphine; Gowing et al. 2017)) that directly suppress withdrawal, as well as non-opioids (e.g., clonidine; Gold et al. 1978)) that reduce individual symptom severity. While these medications can be used to transition patients off opioids, many patients with OUD are also maintained on an opioid agonist for extended periods (i.e., opioid maintenance treatment [OMT] with methadone or buprenorphine). Maintenance on the extended-release antagonist naltrexone (XR-NTX) is another treatment approach that targets relapse prevention. XR-NTX has been recently shown to be non-inferior to daily buprenorphine in maintenance treatment of OUD (Lee et al. 2017; Tanum et al. 2017), though its induction requires patients to successfully complete a supervised withdrawal protocol, which generally has a high rate of attrition. Of the OUD pharmacotherapies, buprenorphine has emerged as the most highly prescribed (IMS Institute for Healthcare Informatics. 2014; SAMHSA. 2014). This is likely due to its status as a Schedule III medication, which allows it to be prescribed by any physician, nurse pratictioner, or physician assistant who has received appropriate certification; this feature was also expected to increase patient access to OMT.
Yet the scale of the opioid epidemic has resulted in a profound discrepancy between OUD treatment need and availability. A recent large-scale survey reported 96% of states had OUD rates that exceeded their OMT availability and that the majority of OMT clinics were already operating at ≥80% capacity (Jones et al. 2015). Treatment options are particularly lacking in rural settings. Only 1.3% of authorized buprenorphine providers reside in rural areas and 82.5% of rural counties have no authorized prescribers (Rosenblatt et al. 2015). Evidence also indicates that buprenorphine-waivered physicians are treating far fewer patients than their legal capacity (Sigmon. 2015; Huhn and Dunn. 2017). Although barriers to agonist treatment infrastructure are being addressed, the severity of the opioid epidemic suggests it is equally critical that additional treatment options be explored. Of particular interest here is supervised withdrawal from opioids, which is the most commonly-accessed form of OUD treatment each year. For instance, among the 97% of residential detoxification beds filled in 2013, 85% were used for supervised opioid withdrawal (SAMHSA. 2014).
There is debate in the OUD treatment community regarding the value of supervised opioid withdrawal (Friedmann and Suzuki. 2017; Tetrault and Fiellin. 2018), because it is associated with a high rate of relapse relative to OMT (Weiss et al. 2011; Fiellin et al. 2014). Specifically, attrition is higher among patients undergoing supervised withdrawal relative to maintenance treatments (Fiellin et al. 2014), and relapse within 30 days of withdrawal is common in the absence of relapse prevention efforts (Ivers et al. 2017). This is particularly problematic because reductions in opioid tolerance can increase the risk of opioid-related overdose (Wines et al. 2007). Failure to effectively manage opioid withdrawal symptoms is a primary contributor to attrition from supervised withdrawal, with higher severity symptoms resulting in lower treatment retention (Strobbe et al. 2003; Northrup et al. 2015). Anecdotal evidence suggests patients may have clinically-meaningfully differences in their opioid withdrawal syndrome; however, there is a dearth of empirical evidence examining this phenomenon and its possible underlying mechanisms. Identifying phenotypical differences in withdrawal could eventually lead to a personalized medication approach to OUD treatment whereby patients who are at risk of more severe withdrawal could be prioritized to agonist treatments, while those with lower expected withdrawal could be managed with non-opioid agonist options. This is especially important in the current societal context of limited buprenorphine availability; a method for successfully matching patients to OUD pharmacotherapies and modalities could help providers maximize the impact that existing OUD pharmacotherapies would have on treatment, which could be a useful method for addressing current treatment capacity issues.
Our group recently compared clonidine, tramadol extended-release (tramadol-ER), and buprenorphine for supervised withdrawal from opioids in a double-blind, double-dummy, randomized controlled clinical trial (Dunn et al. 2017). Results indicated both buprenorphine and tramadol-ER produced significantly better outcomes than clonidine. The following exploratory analyses evaluated whether participants in that study expressed different levels of opioid withdrawal in a manner that could be used to predict efficacy of the study medications. This was assessed using two sets of withdrawal data from that trial. First, withdrawal ratings were taken from a non-randomized portion of the study, when all participants were maintained on the same dose of morphine and received an injection of the opioid antagonist naloxone to precipitate withdrawal (“naloxone challenge”). Since previous research has not yet analyzed opioid withdrawal scales in a manner necessary to identify rating score thresholds indicative of clinically-meaningful level of withdrawal or predictive of specific clinical outcomes, we utilized cluster analysis to determine whether latent groups that experienced phenotypically-different levels of naloxone-precipitated withdrawal existed. Phenotype group assignment was then evaluated as a potential predictor of clinical outcomes (e.g., retention, withdrawal severity) in the subsequent randomized clinical trial (RCT).
2. Methods and Materials
2.1. Participants:
Participants (N=103) were enrollees in a parent trial that evaluated clonidine, tramadol-ER, and buprenorphine for opioid tapering (NCT01188421). Only methods relevant to the present analyses are described here, a full description of the parent study and its outcomes is available elsewhere (Dunn et al. 2017). Eligible participants were aged 18-60, met DSM-IV (American Psychiatric Association. 2000) criteria for opioid dependence, provided a urine sample that tested positive for recent opioid use and/or had evidence of opioid withdrawal by the end of the screening session, and had no other significant medical and/or psychiatric illness. Participants were excluded for pregnancy, physical dependence on benzodiazepines or alcohol that required medical intervention, hypotension, history of seizures, known allergy to study medications, or current enrollment in opioid agonist treatment. The Johns Hopkins Institutional Review Board approved the study protocol and all participants provided informed consent to participate.
2.2. RCT Study Design:
Participants resided in a closed residential unit for the entire 26-28 day study. Participants were stabilized onto 30 mg of subcutaneous (SC) morphine that was injected four times daily for a 7-10 day period before assignment to study medication group. All participants were maintained on the same morphine dose and schedule to prevent differences in baseline opioid use potency and exposure from confounding outcomes of the experimental medications during the RCT. Participants were stratified on sex, race, CYP2D6 genotype, and peak Clinical Opiate Withdrawal Scale (COWS) scores from the naloxone challenge test to undergo a double-blind, double-dummy taper using oral clonidine, tramadol-ER, or sublingual buprenorphine/naloxone. All participants were transitioned from morphine to their assigned study medication on Study Day 1; medication doses were then tapered down in a step-wise fashion for 7 days and participants within each experimental group received an identical taper. On Study Day 8 all participants were transitioned to placebo for an additional 7-day post-taper period; though participants were informed they would be transitioned to placebo by the end of the study, neither staff nor participants were informed when the transition occurred. Withdrawal ratings were collected 7 times daily and peak withdrawal ratings for each day were analyzed as the primary outcome for the parent trial. Retention was defined as remaining enrolled on the final day of the 7-day taper.
2.3. Naloxone Challenge:
Following ≥4 days of morphine stabilization, participants underwent a naloxone challenge session. The naloxone challenge was used to support stratification into experimental group for the parent study and was scheduled for ≥4 days to allow particpants to acclimate to the same dose of morphine before evaluating the severity of physical dependence as assessed by the peak COWS withdrawal ratings. On the challenge day, participants received 30 mg SC morphine at 07:00 and a 0.4 mg intramuscular dose of the opioid antagonist naloxone at 11:00. Withdrawal ratings were collected 15 minutes prior to the naloxone injection and at 15-minute intervals following naloxone injection for 120 minutes. A 30 mg SC injection of morphine was administered at 13:00 to eliminate remaining withdrawal symptoms.
2.4. Study Measures:
The primary withdrawal measure for the present analyses is the Subjective Opiate Withdrawal Scale (SOWS; Handelsman et al. 1987), a 16-item self-report measure on which participants rate the severity of their opioid withdrawal symptoms on a scale of 0 (not at all) to 4 (extremely). Values are summed for a total score (0-64). The SOWS was selected as a primary outcome because all symptoms are listed independently (e.g., nausea and vomiting are separate items), which confers strong analytic sensitivity. Each item is also rated on the same 0-4 Likert scale, which enables refined evaluation of relative changes. Finally, self-reported withdrawal is a better predictor of treatment outcome than observer ratings (Kosten et al. 1985), making this an ecologically-valid outcome. Additional withdrawal ratings included the Clinical Opiate Withdrawal Scale (COWS; Wesson and Ling. 2003; Tompkins et al. 2009); an 11-item observer-rated measure that uses different ordinal scales for each item and sums to a total score (range 0-48), and physiological indices of withdrawal (e.g., respiration, oxygen saturation, blood pressure, pulse, temperature, and pupil diameter measured via pupilometer [Neuroptics, Inc]).
Demographics (sex, race, age, education), past 30-day and number years of drug use, history of injection drug use, and Body Mass Index (BMI) were also collected as part of the study eligibility screening process and evaluated as potential correlates of phenotype.
2.5. Data Analysis:
This secondary analysis hypothesized that participants would express opioid withdrawal differently and that differences would be evident when withdrawal was precipitated by naloxone in a challenge session. The study further hypothesized that withdrawal phenotypes defined by the challenge session would be associated with clinical outcomes from the RCT. The primary outcomes for the RCT analyses were treatment retention and withdrawal suppression.
Due to the known association between BMI and drug response and the fact that doses in this study were not adjusted for body weight, analyses were restricted to participants who had a BMI in the normal range (e.g., 18-30), resulting in a final sample of N=89. All withdrawal data were analyzed as area-under-the-curve (AUC), and the pre-naloxone withdrawal time point from the Challenge session was included in those AUC calculations to control for baseline values (i.e., AUC data were effectively areas-under-the-change-from-baseline curves). Cluster analyses were used to examine whether different latent withdrawal groups existed, based upon the self-reported withdrawal ratings from the SOWS total score AUC results from the Challenge session and participant BMI. Since SOWS and BMI were assessed using different measurement scales, these variables were standardized prior to cluster analysis. Hierarchical agglomerative clustering with a Euclidean distance matrix and a dendrogram was used to visualize the data and a model-based approach was used to determine the number of clusters. The Bayesian Information Criteria (BIC) was calculated for 2 to 9 cluster models and the optimal model was defined as the one that maximized BIC. Analyses identified an optimal two-cluster solution, which was then evaluated using K-means clustering to identify group membership.
For ease of interpretation, the two clusters were conceptualized as representing HIGH (N=37) and LOW (N=52) withdrawal phenotype groups, and participant group assignment into the HIGH or LOW phenotype group was hereafter defined as the group to which this cluster analysis had assigned them. Demographic and drug use characteristics were compared between the two phenotype groups using chi-squares for dichotomous and independent groups t-tests for continuous variables. To examine whether elevated reporting on a subset of symptoms was driving cluster membership, AUC values for each of the 16 individual self-reported symptoms were compared between phenotype groups using independent groups t-tests. Challenge test values from the observer-rated COWS total and individual scores, as well as physiological indices, were also compared across groups using independent groups t-test to examine their convergence with the SOWS self-reported challenge outcomes. Analysis of Variance (ANOVAs) were used to explore the degree to which sex drove effects on the AUCS for SOWS, COWS, and systolic blood pressure.
To assess whether the HIGH or LOW withdrawal phenotype was associated with RCT outcomes, AUC values for the self-reported SOWS total score, the observer-rated COWS total score, and a representative physiological index (pupil diameter) were derived for each participant using his or her daily peak ratings from the randomized taper and post-taper RCT phases. Missing values were treated as missing and not interpolated. Total AUC for each outcome was then compared between phenotype groups using independent-groups t-tests. Logistic regression and Analyses of Variance (ANOVA) with Cohen’s d measures of effect size for significant results were used to examine potential interactions between phenotype and study medication group (e.g., whether participants were tapered using clonidine, tramadol-ER, or buprenorphine) on the RCT outcomes of retention and withdrawal, respectively. HIGH and LOW phenotype groups differed significantly with regard to BMI and sex; because these items were significantly correlated (r=.29, p<.01), only BMI was included as a covariate for the regression and ANOVA. Alpha levels were set at .05 and analyses were conducted using R version 3.3.2 and SPSS version 24.
3. Results
3.1. Participants:
Participants were 86% male, 45% African American, an average (SD) 40.8 (10.6) years old, and had an average BMI of 23.6 (2.0). Participants were predominately heroin users and reported using heroin a mean (SD) of 25.3 (8.1) days in the past 30 and for 12.1 (10.0) years in their lifetime. Approximately half (56.5%) reported lifetime injection drug use (Table 1).
Table 1.
Demographic and Drug Use Characteristics
| LOW (N=52) | HIGH (N=37) | p-value | |
|---|---|---|---|
| Demographic Characteristics | |||
| Age (mean yrs ± SD) | 41.9 ± 9.2 | 39.6± 11.9 | 0.34 |
| Female (%) | 2 | 27 | <.001 |
| African American (%) | 39 | 51 | 0.28 |
| Body Mass Index (mean, SD) | 25.7 ± 2.3 | 21.5 ± 1.6 | <.001 |
| Education (mean yrs ± SD) | 12.1 ± 1.9 | 12.4 ± 1.8 | 0.48 |
| Drug Use Characteristics | |||
| Past 30-day Use (mean days ± SD ) | |||
| Alcohol | 2.0± 3.1 | 1.5± 2.2 | 0.38 |
| Heroin | 23.8 ± 8.7 | 26.8 ± 7.4 | 0.09 |
| Other Opioids | 2.8± 7.5 | 2.0± 6.9 | 0.61 |
| Cocaine | 3.8± 8.0 | 5.7± 9.0 | 0.28 |
| Cannabis | 2.4± 5.5 | 3.9± 8.9 | 0.37 |
| Years Used (mean ± SD) | |||
| Alcohol | 2.2 ± 3.4 | 2.8± 7.1 | 0.63 |
| Heroin | 13.3± 10.0 | 10.9± 10.0 | 0.27 |
| Other Opioids | 1.3± 3.5 | 1.8± 4.4 | 0.58 |
| Cocaine | 2.7± 5.1 | 3.1± 5.6 | 0.73 |
| Cannabis | 3.8± 5.9 | 3.5± 5.3 | 0.79 |
| Injection Drug User (%) | 52 | 61 | 0.26 |
| RCT Group Assignment (%) | 0.44 | ||
| Clonidine | 32 | 37 | |
| Tramadol-ER | 42 | 29 | |
| Buprenorphine | 26 | 34 |
Results based upon independent t-tests for continuous and chi-square for dichotomous variables. SD=standard deviation, yrs=years, RCT=Randomized Controlled Trial, ER=extended release
3.2. Phenotype Determination:
K-means clustering based upon SOWS self-reported withdrawal ratings from the challenge session revealed two distinct subgroups, categorized here as HIGH and LOW withdrawal phenotypes. The time courses of withdrawal ratings from the self-reported SOWS total score collected during the challenge session are shown in Figure 1 (top left panel). Ratings for each of the 16 individual SOWS self-reported items from the challenge session were then compared between the phenotype groups. There was no evidence that elevated reporting on a single symptom drove phenotype differentiation; rather, HIGH phenotype participants rated all 16 symptoms as being more severe than LOW phenotype participants (Table 2). The same pattern of effects, with the HIGH phenotype group evidencing greater withdrawal during the challenge session than the LOW phenotype group, was observed for the observer-rated COWS total score (Figure 1, left middle panel) and 10 of the 11 individual COWS items, as well as systolic (Figure 1, left bottom panel) and diastolic blood pressure (Table 2). The convergence in these challenge session results and the fact they reveal the same time course and pattern as the self-reported SOWS values (the measure upon which the cluster analyses was based) supports the notion of meaningfully different withdrawal phenotypes.
Figure 1. Naloxone Challenge Session Results.
Participants maintained on morphine received 0.4mg IM injection of naloxone and provided withdrawal ratings at 15-minute intervals for 120 minutes. Results show challenge session time course for representative self-reported withdrawal ratings (Subjective Opiate Withdrawal Scale [SOWS]; Top), clinician-observed (Clinical Opiate Withdrawal Scale [COWS]; middle), and physiological rating (systolic blood pressure; bottom). Left column presents results collapsed across sex for HIGH (open circles; N=37) and LOW (filled circles; N=52) phenotype groups, as defined by K-means clustering of SOWS area-underthe-curve (AUC) total score values derived from naloxone-precipitated withdrawal. Phenotype groups differed significantly on all three measures. Right column presents results as a function of sex and phenotype; phenotype group (but not sex) was significant for SOWS and COWS outcomes. Y-axes represent mean scores (maximum range) or ratings, X-axis presents minutes post-dose, and error bars represent SEM.
Table 2.
Naloxone Challenge Session AUC Values
| LOW (N=52) | HIGH (N=37) | p-value | |
|---|---|---|---|
| SOWS Individual Items | |||
| Anxious | 5.1 (4.8) | 8.7 (5.9) | 0.002 |
| Yawning | 6.9 (5.1) | 10.6 (5.8) | 0.002 |
| Perspiring | 3.7 (3.5) | 6.7 (5.0) | 0.002 |
| Tears Running | 4.3 (4.0) | 8.6 (5.7) | 0.001 |
| Nose Running | 4.8 (4.8) | 8.3 (5.8) | <.001 |
| Gooseflesh | 3.6 (4.0) | 7.3 (6.4) | 0.001 |
| Shaking | 2.6 (3.6) | 6.7 (6.5) | <.001 |
| Hot Flashes | 4.3 (3.9) | 8.3 (6.2) | <.001 |
| Cold Flashes | 5.1 (4.3) | 8.5 (6.3) | 0.003 |
| Bone Aches | 3.6 (4.5) | 7.1 (6.5) | 0.005 |
| Restlessness | 5.2 (4.8) | 10.2 (6.7) | <.001 |
| Nausous | 2.7 (3.3) | 6.4 (6.0) | <.001 |
| Vomiting | 1.3 (2.5) | 4.0 (5.7) | 0.003 |
| Muscle Twitching | 2.8 (4.1) | 5.1 (5.6) | 0.02 |
| Stomach Cramping | 3.8 (3.9) | 7.3 (6.0) | 0.001 |
| Feel Like Using | 7.0 (8.3) | 15.6 (9.6) | <.001 |
| COWS | |||
| Pulse | 2.5 (2.7) | 2.8 (3.2) | 0.63 |
| GI Upset | 5.4 (5.3) | 9.6 (68) | 0.001 |
| Sweating | 4.6 (2.8) | 6.2 (2.5) | 0.004 |
| Tremors | 1.6 (3.6) | 5.0 (7.1) | 0.004 |
| Restlessness | 4.4 (3.7) | 8.4 (6.6) | <.001 |
| Yawning | 4.1 (3.9) | 3.6 (6.7) | 0.02 |
| Pupil | 9.5 (5.0) | 9.6 (4.7) | 0.97 |
| Anxious | 4.1 (3.6) | 7.2 (5.0) | 0.001 |
| Bones Ache | 2.6 (3.1) | 4.0 (3.4) | 0.05 |
| Nose and Eyes Tearing | 6.0 (4.4) | 8.9 (5.8) | 0.008 |
| Gooseflesh | 3.7 (7.4) | 9.9 (13.0) | 0.006 |
| Total Score | 48.3 (24.9) | 78.1 (40.0) | <.001 |
| Physiological Measures | |||
| Systolic Blood Pressure | 1017.0 (117.6) | 901.6 (177.3) | <.001 |
| Diastolic Blood Pressure | 606.6 (73.2) | 563.1 (115.9) | 0.03 |
| Heart Rate | 590.5 (83.4) | 574.1 (132.4) | 0.48 |
| Temperature | 256.9 (38.6) | 247.2 (63.9) | 0.38 |
| Oxygen Saturation | 777.7 (32.6) | 740.6 (143.5) | 0.08 |
| Respiration | 119.0 (23.0) | 105.1 (30.6) | 0.02 |
| Pupil Diameter | 39.1 (11.2) | 39.6 (12.5) | 0.82 |
Values represent mean (SD) area-under-the-curve (AUC) values derived from ratings collected at baseline and every 15-minutes for the 120-minute naloxone challenge session. Results compared between the LOW and HIGH phenotype groups using independent groups t-tests. Phenotype designation based upon K-means clustering of SOWS Total Score AUC. SOWS=Subjective Opiate Withdrawal Scale, COWS=Clinical Opiate Withdrawal Scale.
3.3. Phenotype Group Comparisons:
Examination of demographic characteristics revealed HIGH phenotype participants were significantly more likely to be female (27% vs. 2% p<.001) and have lower BMIs (21.5 vs. 25.7, p<.001) than LOW phenotype participants, respectively. Withdrawal phenotype was not significantly associated with any of the demographic or drug use characteristics evaluated or assignment into the RCT study medication group (Table 1). ANOVAs to assess the relative contribution of phenotype and sex on outcomes suggested significant effects of phenotype on SOWS AUC (F(1,89)=9.53, p=.003) and COWS AUC (F(1,89)=6.41, p=.01) but not systolic blood pressure (F(1,89)=0.78, p=.40). No significant sex or sex-phenotype interactions were observed. The right column of Figure 1 presents results as a function of sex and phenotype for the SOWS, COWS, and systolic blood pressure.
3.4. Association Between Phenotype and RCT Outcomes:
The HIGH and LOW withdrawal phenotypes defined by the SOWS self-reported withdrawal during the challenge session were significantly associated with some RCT outcomes. Participants with a HIGH withdrawal phenotype were significantly less likely to be retained at the end of the 7-day taper (60% vs. 82%, χ2=5.05, p=0.02) and had a higher mean AUC (SD) on their self-reported SOWS total score during the RCT (16.8 [13.1] vs. 10.2 [8.9]; t(56)=2.04, p=0.05) than the LOW phenotype group, respectively. No significant group differences in the observer-rated COWS or pupil diameter from the RCT were identified.
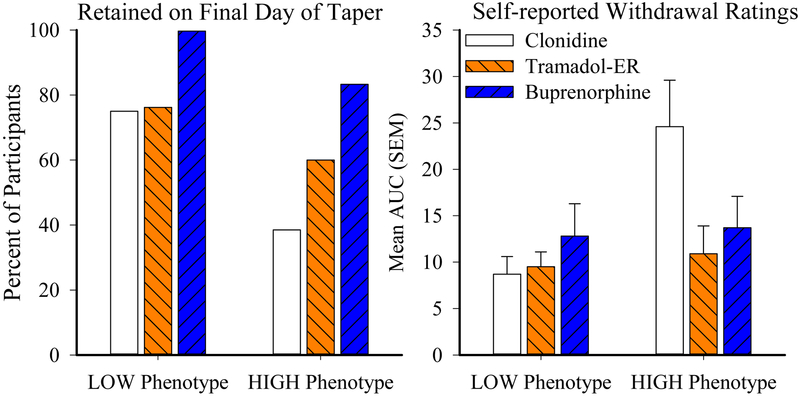
Interactions between withdrawal phenotype and medication group on RCT retention and withdrawal ratings were also examined. A logistic regression revealed that both phenotype (χ2 (1)=5.31, p=.02, OR=0.29, 95%CI [0.10 – 0.83]) and study medication group (χ2 (1)=7.38, p<.01, OR=2.64, 95%CI [1.31 – 5.31]) were significantly associated with treatment retention (Figure 2, left panel). Specifically, retention among LOW phenotype participants who received clonidine, tramadol-ER, and buprenorphine was 75%, 76%, and 100%, respectively, whereas retention among HIGH phenotype participants was 38%, 60%, and 83%, respectively. BMI was not a significant covariate in this model.
Figure 2. Phenotype Associations with Randomized Controlled Trial Outcomes.
Figure presents percent participants retained on the final day of the 7-day taper (left panel) and the area-underthe-curve (AUC) values from daily self-report ratings of peak withdrawal severity, as measured by the Subjective Opiate Withdrawal Scale (SOWS; right panel), collapsed across taper and posttaper periods. Results are shown as a function of experimental group, representing participants assigned to receive clonidine (white), tramadol-ER (orange), or buprenorphine (blue). Y-axes represent percent of participants (left) and mean AUC value (right), X-axes present phenotype group, and error bars represent SEM. Sample sizes for clonidine, tramadol-ER, and buprenorphine participants in the LOW group are N=16, N=18, N=12 and in the HIGH group are N=13, N=10, N=11, respectively.
Analysis of variance (ANOVA) of the AUC values from the daily peak rating of the self- reported SOWS total score collected during the RCT (Figure 2, right panel) also revealed a significant main effect of phenotype (F(1,56)=6.1, p=.02) and a trend towards a phenotype x study medication group interaction (F(2,56)=2.7, p=.07); the main effect of study medication group approached but did not reach significance (F(1,56)=2.1, p=0.12). The significant main effect of phenotype (F(1,56)=7.10, p=.01) was retained when BMI was included as a covariate, and pairwise comparisons revealed the effect was driven by differences between the clonidine and tramadol-ER groups (p=.04). Cohen’s d values for clonidine vs. tramadol-ER, clonidine vs. buprenorphine, and tramadol-ER vs. buprenorphine groups were .01, .44, and .43 for the LOW group and 1.0, .76, and .42 for the HIGH group, respectively. The time course data from the SOWS total score from the RCT for the LOW (top panel) and HIGH (bottom panel) phenotype groups are presented for descriptive purposes in Figure 3. Analyses of the AUC values from the observer-rated COWS total score from the RCT did not reveal a significant main effect of phenotype or study medication group, or a phenotype x study medication group interaction. Evaluation of the AUC from pupil diameter ratings during the RCT revealed a trend towards a significant main effect of phenotype (F(1, 57)=3.11, p=.08) and phenotype x study medication group interaction (F(2,57)=2.68, p=.08); the main effect of study medication group did not approach significance in this model (F(2,57)=0.16, p=0.85).
Figure 3. Time Course of Self-reported Withdrawal from Randomized Controlled Trial.
Figure shows results of daily peak self-report ratings of withdrawal severity as measured by the Subjective Opiate Withdrawal Scale (SOWS) over the nonrandomized morphine stabilization period (days -7 through -1), double-blind taper (days 1 through 7), and double-blind post-taper placebo-dosing period (days 8 through 14). Results presented as function of experimental group, representing participants assigned to receive clonidine (white), tramadol-ER (orange), or buprenorphine (blue) within the LOW (Top Panel) or HIGH (Bottom Panel) withdrawal phenotype groups. Y-axes represent mean (range) rating, X-axes represent study day, and error bars represent SEM. RCT=Randomized Controlled Trial
4. Discussion
These analyses revealed three main findings. First, they provide initial evidence that different opioid withdrawal phenotypes may exist, insofar that quantitative differences in ratings of withdrawal instensity represent qualitatively different expressions of withdrawal. Specifically, evaluation of the self-reported SOWS ratings following naloxone-precipitated withdrawal suggested participants in this study expressed either HIGH or LOW levels of opioid withdrawal. Further evaluation refuted the notion that phenotype designations were due to reporting on a specific symptom by demonstrating that HIGH phenotype participants reported more severe withdrawal than LOW phenotype participants on every SOWS symptom during the challenge session. Observers also rated HIGH phenotype participants as having significantly more severe withdrawal than LOW phenotype participants on the COWS, and several additional physiological indices of withdrawal collected during the challenge session differed between the groups. The convergence of these results suggests that elevated withdrawal during the challenge session was not an artifact of participant self-report.
Second, this preliminary evidence suggests withdrawal phenotype may be associated with differential outcomes following random assignment to clonidine, tramadol-ER, and buprenorphine for opioid tapering. Both the withdrawal phenotype that was defined during the naloxone challenge and the study medication group to which participants were assigned in the RCT were significantly associated with important clinical outcomes. For instance, retention rates were drug-dependent among HIGH phenotype participants, with clonidine and buprenorphine producing stark differences in retention (38% vs. 83%, respectively; Figure 2). In contrast, LOW phenotype participants had relatively high rates of retention overall (82%) and showed only minor improvement following treatment with buprenorphine relative to clonidine and tramadol- ER. In addition, LOW phenotype participants reported relatively uniform and mild levels of withdrawal during the clinical trial, regardless of the medication to which they had been assigned, whereas HIGH phenotype participants reported substantial variability in withdrawal levels among the study medication groups. Figure 3 illustrates the time course of self-reported withdrawal ratings during the 14-day, double-blind, double-dummy RCT. This figure suggests medication assignment was not differentially related to withdrawal experience among LOW phenotype participants but that HIGH phenotype participants who received clonidine reported significantly greater withdrawal during the clinical trial than HIGH phenotype participants who received tramadol-ER or buprenorphine. Together these data provide initial evidence that choice of OUD pharmacotherapy could have been more critical among participants expressing a HIGH versus LOW withdrawal phenotype.
In addition, the fact that results following a precipitated naloxone challenge were associated with clinical treatment outcomes also provides initial evidence that a precipitated naloxone challenge session could have value for OUD medication development. These data support additional research into the value of using a naloxone challenge as a laboratory model for screening potential OUD medications prior to an RCT. Validated human laboratory models have already been developed and employed to screen potential medications for nicotine (McKee et al. 2012) and alcohol (Plebani et al. 2012) prior to formal RCT evaluations, but such a model has yet to be established for opioids. The ability to expeditiously screen medications to determine the degree to which they may suppress opioid withdrawal symptoms before commencing a large-scale RCT could help to more quickly advance the science of OUD treatment, particularly with regard to concomitant medications for the treatment of specific OUD withdrawal symptoms, if additional research supported this approach.
It is interesting that pupil diameter, which is often used in clinical pharmacology studies as a sensitive index of opioid effects and withdrawal, approached but was ultimately not significantly associated with withdrawal phenotypes. Although this may be due to limited statistical power to detect an effect on this outcome, it is also the case that associations between pupil diameter and opioid withdrawal severity are not clearly delineated. One previous study that examined how pupil diameter correlated with other observer-rated withdrawal symptoms following a similar naloxone challenge test reported only minor correlations (Tompkins et al. 2009), whereas a factor analysis of observer-rated withdrawal symptoms collected in the context of a RCT observed strong correlations between pupil and other withdrawal symptoms among men but not women (Barbosa-Leiker et al. 2015). It is possible that differences in the type of withdrawal (naloxone-precipitated vs. clinical) and the sensitivity of pupil measurement contributed to these outcomes. Specifically, while this and the previous study that conducted a naloxone challenge test used a pupilometer to precisely quantify pupil changes at the level of millimeters, the clinical trial rated pupil changes on a 4-item Likert scale. A second interpretation is that a core construct that differentiated the present withdrawal-based phenotypes is emotional distress, as opposed to opioid agonist or antagonist activity. Pupil diameter is a sensitive physiological index of opioid agonist and antagonist activity, but it does not reflect the critical emotional distress aspect that is so prominent in opioid withdrawal and the clinical presentations that differentiate opioid-dependent and withdrawing subjects from one another. Notably, the physiological indices that did contribute to phenotype differentiation here are also commonly associated with emotional arousal (heart rate, blood pressure). Finally, it is possible that nonopioid transmitter systems may underlie the manifestation of opioid withdrawal symptoms, including pupil changes. Preclinical literature has demonstrated serotonergic, adrenergic, and dopaminergic contributions to opioid-induced effects on behavior, yet the role of non-opioid transmitter systems in human opioid withdrawal has not yet been well-established (Maldonado et al. 1997; Sastre-Coll et al. 2002; Cecchi et al. 2007). More research in this area is warranted and could lead to innovative strategies for managing human opioid withdrawal.
Detailed information related to prior opioid use and several other factors that may have impacted withdrawal ratings were not collected because the parent study did not necessitate this information for eligibility determinations. Though we compared all available demographic and drug use data (see Table 1) these analyses do not thoroughly evaluate the contribution that differences in degree of opioid tolerance, opioid use history, and primary route of administration had on opioid withdrawal expression. Perhaps for that reason, none of the drug use characteristics sampled were observed to be significantly different between the groups. In contrast, comparison of demographic characteristics revealed the HIGH and LOW phenotype groups differed significantly with regards to sex and BMI. Specifically, while the parent trial had a low representation of women overall (N=11), 91% (10/11) of enrolled women were categorized into the HIGH phenotype group. Although comparisons of SOWS, COWS, and systolic blood pressure AUC values revealed no significant effect of sex or sex-phenotype interactions, Figure 1 clearly suggests that HIGH phenotype women experienced greater withdrawal than all other groups. It should be noted that this study was not powered to appropriately examine the contribution of sex on outcomes and that results should be considered preliminary due to the small and imbalanced samples. Nevertheless, the divergence between male and female subjects is consistent with known sex-based differences in opioid receptor expression and response to opioids (Bodnar and Kest. 2010). These results support additional research on this topic, particularly since only a limited number of studies examining sex-based differences in opioid withdrawal exist (Papaleo and Contarino. 2006; Allahverdiyev et al. 2015). The results from this study also provide support for more focused evaluation of opioid withdrawal phenotypes to tailor OUD treatment. Such personalized medicine approaches have been widely embraced by the field of medicine and the alcohol treatment field, and several studies have highlighted its value for customizing opioid analgesic doses (Branford et al. 2012; Linares et al. 2014; Stauble et al. 2014; Bruehl et al. 2015; Senagore et al. 2017). Despite growing evidence that metabolic and genetic differences may produce variable opioid responses, we know of no studies that have applied personalized medicine to improve OUD treatment outcomes.
This study was not prospectively designed to evaluate opioid withdrawal phenotypes and their association with clinical outcomes. However, the convergence of withdrawal phenotype results across several diverse measures from the challenge session, and their highly significant association with phenotype groups and clinical outcomes from a rigorous RCT (despite small cell sizes), provide compelling support for the continuation of this research. It will be important to replicate these results using a prospective design and for a more exhaustive array of demographic, drug use, and physiological (e.g., metabolic and genetic status) correlates of withdrawal phenotype groups to be examined, as well as different clinical outcomes such as opioid abstinence. It will also be important for equal numbers of men and women to be enrolled to examine whether the sex-based differences observed here are replicable, and to more thoroughly examine the role of BMI in phenotype outcomes. Further, though maintaining all participants on the same dose of morphine prior to study procedures was an empirical strength that helped to produce a more standardized level of physical dependence in the sample, this procedure may reduce the generalizability of these results for clinical treatment populations as it may not reflect each participant’s natural level of physical dependence based upon their typical opioid use. It will be important to investigate this phenomenon in clinical samples of patients who are using different types and quantities of opioids. Finally, all participants in this study received the same dose of morphine and naloxone independent of body weight. Thus, there was some variance in physiological dose quantities of the study drugs based upon BMI. BMI was included in our cluster analysis and as a covariate in other analyses to adjust for such differences, but the degree to which differences in body weight may have contributed to outcome are not known and warrant further evaluation.
In conclusion, these analyses add to a large preclinical and growing human literature regarding qualitative differences in the expression of opioid withdrawal (Chopra et al. 2008) and provide additional evidence that OUD patients may express different clinically-relevant opioid withdrawal phenotypes. These efforts are consistent with other studies that have attempted to identify meaningful subgroups of OUD patients (Chan et al. 2011; Sun et al. 2012) and the use of cluster analysis to evaluate this concept extends this body of research to measures of withdrawal in a manner that has not yet been previously utilized. Studies that replicate these results and more thoroughly examine their associated underlying mechanisms are necessary, and research to establish clinically-relevant withdrawal thresholds will be of significant value to the field. Understanding the breadth of individual differences in opioid withdrawal, as well as underlying mechanisms, could contribute to advancements in OUD treatment (including both supervised withdrawal and maintenance), as well as the use of opioids for pain management. Efforts to facilitate treatment matching would be of particular value, and could ultimately help maximize patient success and the impact that OUD pharmacotherapies may have in environments where access to buprenorphine or OMT is not already ubiquitous.
Acknowledgements
The parent study was funded by National Institute on Drug Abuse grant R01DA-01815 (Strain) with additional salary support provided by grants R01DA035246, R01DA040644 (Dunn), K23DA029609 (Tompkins), T32DA007209 (Bigelow). The authors acknowledge Jessica Sides and Hye Jeong Han for their collection of parent trial data. Salary support for the current analyses was provided by R01DA035246, R01DA040644 (Dunn) and T32DA007209 (Bigelow). Analyses were conceptualized by authors KD, EW, AH, JS, and EC. KD and JS conducted statistical analyses. KD drafted the manuscript, and all authors provided important intellectual contributions to the manuscript revision. All authors approved the manuscript for content and approved the final version for publication. The authors have no conflicts of interest that are relevant to the analyses in this manuscript.
References
- Allahverdiyev O, TüRKMEN AZ, Nurten A, ŞEHİRLİ İ, Enginar N (2015) Spontaneous withdrawal in intermittent morphine administration in rats and mice: effect of clonidine coadministration and sex-related differences. Turkish journal of medical sciences 45:1380–1389. [PubMed] [Google Scholar]
- American Psychiatric Association, American Psychiatric Association (2000) DSM-IV-TR: Diagnostic and statistical manual of mental disorders, text revision. Washington, DC: American Psychiatric Association; 75:78–85. [Google Scholar]
- Barbosa-Leiker C, McPherson S, Mamey MR, Burns GL, Layton ME, Roll J, Ling W (2015) Examining the factor structure of the Clinical Opiate Withdrawal Scale: A secondary data analysis from the National Drug Abuse Treatment Clinical Trials Network (CTN) 0003. Drug Alcohol Depend 152:218–223. DOI: 10.1016/j.drugalcdep.2015.03.036 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blendon RJ, Benson JM (2018) The Public and the Opioid-Abuse Epidemic. N Engl J Med. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, Kest B (2010) Sex differences in opioid analgesia, hyperalgesia, tolerance and withdrawal: central mechanisms of action and roles of gonadal hormones. Horm Behav 58:72–81. [DOI] [PubMed] [Google Scholar]
- Branford R, Droney J, Ross J (2012) Opioid genetics: the key to personalized pain control? Clin Genet 82:301–310. [DOI] [PubMed] [Google Scholar]
- Bruehl S, Burns JW, Passik SD, Gupta R, Buvanendran A, Chont M, Schuster E, Orlowska D, France CR (2015) The contribution of differential opioid responsiveness to identification of opioid risk in chronic pain patients. The Journal of Pain 16:666–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi M, Capriles N, Watson SJ, Akil H (2007) β 1 adrenergic receptors in the bed nucleus of stria terminalis mediate differential responses to opiate withdrawal. Neuropsychopharmacology 32:589. [DOI] [PubMed] [Google Scholar]
- Chan G, Gelernter J, Oslin D, Farrer L, Kranzler HR (2011) Empirically derived subtypes of opioid use and related behaviors. Addiction 106:1146–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra MP, Feldman Z, Mancino MJ, Oliveto A (2008) Sex and opioid maintenance dose influence response to naloxone in opioid-dependent humans: a retrospective analysis. Pharmacology Biochemistry and Behavior 90:787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Tompkins DA, Bigelow GE, Strain EC (2017) Efficacy of tramadol extended-release for opioid withdrawal: a randomized clinical trial. JAMA psychiatry 74:885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiellin DA, Schottenfeld RS, Cutter CJ, Moore BA, Barry DT, O'Connor PG (2014) Primary Care-Based Buprenorphine Taper vs Maintenance Therapy for Prescription Opioid Dependence: A Randomized Clinical Trial. JAMA Intern Med 174:1947–1954. DOI: 10.1001/jamainternmed.2014.5302 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann PD, Suzuki J (2017) More beds are not the answer: transforming detoxification units into medication induction centers to address the opioid epidemic. Addiction science & clinical practice 12:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M, Redmond DE Jr, Kleber H (1978) Clonidine blocks acute opiate-withdrawal symptoms. The lancet 312:599–602. [DOI] [PubMed] [Google Scholar]
- Gowing L, Ali R, White JM, Mbewe D (2017) Buprenorphine for managing opioid withdrawal. The Cochrane Library. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD (1987) Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse 13:293–308. DOI: 10.3109/00952998709001515. [DOI] [PubMed] [Google Scholar]
- Huhn AS, Dunn KE (2017) Why aren’t physicians prescribing more buprenorphine? J Subst Abuse Treat 78:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson D, Everitt B, Robbins T, Dickinson A (2001) The role of withdrawal in heroin addiction: enhances reward or promotes avoidance? Nat Neurosci 4:943. [DOI] [PubMed] [Google Scholar]
- IMS Institute for Healthcare Informatics (2014) Medicine use and shifting costs of healthcare. A review of the use of medicines in the United States in 2013. [Google Scholar]
- Ivers J, Zgaga L, Sweeney B, Keenan E, Darker C, Smyth BP, Barry J (2017) A naturalistic longitudinal analysis of post-detoxification outcomes in opioid-dependent patients. Drug Alcohol Rev. [DOI] [PubMed] [Google Scholar]
- Jones CM, Campopiano M, Baldwin G, McCance-Katz E (2015) National and state treatment need and capacity for opioid agonist medication-assisted treatment. Journal Information 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Rounsaville BJ, Kleber HD (1985) Comparison of clinician ratings to self reports of withdrawal during clonidine detoxification of opiate addicts. Am J Drug Alcohol Abuse 11:1–10. [DOI] [PubMed] [Google Scholar]
- Lee JD, Nunes EV Jr, Novo P, Bachrach K, Bailey GL, Bhatt S, Farkas S, Fishman M, Gauthier P, Hodgkins CC (2017) Comparative effectiveness of extended-release naltrexone versus buprenorphine- naloxone for opioid relapse prevention (X: BOT): a multicentre, open-label, randomised controlled trial. The Lancet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares OA, Daly D, Linares AD, Stefanovski D, Boston RC (2014) Personalized oxycodone dosing: Using pharmacogenetic testing and clinical pharmacokinetics to reduce toxicity risk and increase effectiveness. Pain Medicine 15:791–806. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Saiardi A, Valverde O, Samad TA, Roques BP, Borrelli E (1997) Absence of opiate rewarding effects in mice lacking dopamine D2 receptors. Nature 388:586. [DOI] [PubMed] [Google Scholar]
- McKee SA, Weinberger AH, Shi J, Tetrault J, Coppola S (2012) Developing and validating a human laboratory model to screen medications for smoking cessation. Nicotine Tobacco Res 14:1362–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Becker M, Locke J, Kelleher C, McLeod J, Isasi F (2016) Finding solutions to the prescription opioid and heroin crisis: A road map for states. National Governors Association Center for Best Practices, Washington, DC. [Google Scholar]
- Murphy SL, Xu J, Kochanek KD, Curtin SC, Arias E (2017) Deaths: Final data for 2015. [PubMed] [Google Scholar]
- Northrup TF, Stotts AL, Green C, Potter JS, Marino EN, Walker R, Weiss RD, Trivedi M (2015) Opioid withdrawal, craving, and use during and after outpatient buprenorphine stabilization and taper: A discrete survival and growth mixture model. Addict Behav 41:20–28. DOI: 10.1016/j.addbeh.2014.09.021 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F, Contarino A (2006) Gender-and morphine dose-linked expression of spontaneous somatic opiate withdrawal in mice. Behav Brain Res 170:110–118. [DOI] [PubMed] [Google Scholar]
- Plebani JG, Ray LA, Morean ME, Corbin WR, MacKillop J, Amlung M, King AC (2012) Human laboratory paradigms in alcohol research. Alcoholism: Clinical and Experimental Research 36:972–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt RA, Andrilla CH, Catlin M, Larson EH (2015) Geographic and specialty distribution of US physicians trained to treat opioid use disorder. Ann Fam Med 13:23–26. DOI: 10.1370/afm.1735 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre-Coll A, Esteban S, García-Sevilla JA (2002) Supersensitivity of 5-HT 1A-autoreceptors and α 2- adrenoceptors regulating monoamine synthesis in the brain of morphine-dependent rats. Naunyn Schmiedebergs Arch Pharmacol 365:210–219. [DOI] [PubMed] [Google Scholar]
- Senagore AJ, Champagne BJ, Dosokey E, Brady J, Steele SR, Reynolds HL, Stein SL, Delaney CP (2017) Pharmacogenetics-guided analgesics in major abdominal surgery: Further benefits within an enhanced recovery protocol. The American Journal of Surgery 213:467–472. [DOI] [PubMed] [Google Scholar]
- Sigmon SC (2015) The untapped potential of office-based buprenorphine treatment. JAMA psychiatry 72:395–396. [DOI] [PubMed] [Google Scholar]
- Stauble ME, Moore AW, Langman LJ, Boswell MV, Baumgartner R, McGee S, Metry J, Jortani SA (2014) Hydrocodone in postoperative personalized pain management: pro-drug or drug? Clinica Chimica Acta 429:26–29. [DOI] [PubMed] [Google Scholar]
- Strobbe S, Brower KJ, Galen LW (2003) Predicting completion of outpatient opioid detoxification with clonidine. Am J Addict 12:260–269. DOI: FLGJB56CAJ7FNXK1 [pii]. [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) (2014) National Survey of Substance Abuse Treatmetn Services (N-SSATS): 2013. Data on substance abuse treatment facilities. BHSIS Series S-73 HHS Publication No. (SMA) 14–489. [Google Scholar]
- Sun J, Bi J, Chan G, Oslin D, Farrer L, Gelernter J, Kranzler HR (2012) Improved methods to identify stable, highly heritable subtypes of opioid use and related behaviors. Addict Behav 37:1138–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanum L, Solli KK, Benth JŠ, Opheim A, Sharma-Haase K, Krajci P, Kunøe N (2017) Effectiveness of Injectable Extended-Release Naltrexone vs Daily Buprenorphine-Naloxone for Opioid Dependence: A Randomized Clinical Noninferiority Trial. JAMA psychiatry 74:1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetrault JM, Fiellin DA (2018) More Beds or More Chairs? Using a Science-Based Approach to Address the Opioid Epidemic. Ann Intern Med 168:73–74. [DOI] [PubMed] [Google Scholar]
- Tompkins DA, Bigelow GE, Harrison JA, Johnson RE, Fudala PJ, Strain EC (2009) Concurrent validation of the Clinical Opiate Withdrawal Scale (COWS) and single-item indices against the Clinical Institute Narcotic Assessment (CINA) opioid withdrawal instrument. Drug Alcohol Depend 105:154–159. DOI: 10.1016/j.drugalcdep.2009.07.001 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Fiellin DA, Byrne M, Connery HS, Dickinson W, Gardin J, Griffin ML, Gourevitch MN, Haller DL (2011) Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry 68:1238–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DR, Ling W (2003) The clinical opiate withdrawal scale (COWS). J Psychoactive Drugs 35:253–259. [DOI] [PubMed] [Google Scholar]
- Wines JD Jr, Saitz R, Horton NJ, Lloyd-Travaglini C, Samet JH (2007) Overdose after detoxification: a prospective study. Drug Alcohol Depend 89:161–169. DOI: S0376-8716(06)00471-6 [pii]. [DOI] [PubMed] [Google Scholar]