Abstract
The aim of this study was to reveal the biological activities and in vivo toxicity profiles of n-hexane, chloroform and methanol extracts of brown algae Halopteris scoparia L. Sauvageau. In this study, extracts were tested for their phytochemical contents and antioxidant activities. The cytotoxic activities of the extracts against cervical adenocarcinoma (HeLa), colon colorectal adenocarcinoma (CaCo-2) and breast adenocarcinoma (MCF7) cells were assessed by MTT assay and total RNAs derived from cell lines to analyze gene expression were analyzed by Real Time Ready Human Apoptosis Panel 96. Also, in vivo toxicity and irritation effects of extracts were evaluated by LD50 acute toxicity test and Hen’s egg test chorioallantoic membrane (HET-CAM) assay, respectively. Our results showed that the phenolic and flavonoid contents were determined only in methanol extract (33.20 ± 1.41 mg GAE/g and 1.26 ± 0.95 mg QE/g). Also, n-hexane has a broader spectrum of content than methanol and chloroform extracts. Furthermore, n-hexane extract in DPPH and methanol extract in ABTS+ exhibited the best antioxidant activity. In addition, MTT results revealed that each three extracts cause a significant reduction in cell viability, especially in HeLa cells. When the apoptotic gene expressions were examined after treatment of extracts, the expression of many pro-apoptotic genes in both caspase-independent and caspase-dependent intrinsic and extrinsic pathways increased. These findings suggest that, considering that it had not led to irritation and toxicity in vivo, edible H. scoparia is a natural antioxidant and its apoptotic/cytotoxic activities can potentially be used against human cancers.
Electronic supplementary material
The online version of this article (10.1007/s10616-019-00314-5) contains supplementary material, which is available to authorized users.
Keywords: Anticancer, Antioxidant, Apoptosis, Brown algae, Halopteris scoparia, HET-CAM assay
Introduction
Marine and terrestrial herbal resources have gained great attention to meet the nutritional and health needs of the growing human population. Especially, marine algae rich in active chemical compounds have been extensively consumed in many areas such as food, pharmacy, medicine and cosmetics industry since ancient times (Nigam and Pandey 2009).
Algae are mostly preferred for the elimination of oxidative stress-related damage, thanks to their antioxidant properties (Zubia et al. 2009). Blunt et al. (2010) revealed that polyunsaturated fatty acid, phenolic, and carotenoid ingredients of algae show antioxidant activity through radical scavenging and inhibiting lipid peroxidation. Similarly, there are numerous studies that reveal the antioxidant activity of algae (Cornish and Garbary 2010; Güner et al. 2015). However, biological activities of algae are not limited to alone their antioxidant properties. Algae compounds have also been extensively studied in anticancer applications and have shown promising results in cancer intervention process in vivo and in vitro studies (Kim et al. 2011; Martínez Andrade et al. 2018).
Cancer is a disease characterized by the malfunction of cellular homeostasis between cell differentiation and proliferation (Green and Reed 1998; Kaufmann and Hengartner 2001). Apoptosis or programmed cell death plays a key role in development and homeostasis throughout life. While the balance between apoptosis and cell survival is tightly controlled in the normal process, this control mechanism is lost in cancer. (Thompson 1995; Jubri et al. 2012). Activation of apoptosis or inhibition of cell proliferation by using different chemotherapeutic agents has become one of the most popular strategies in the fight against cancer. Synthetic drugs and other methods are widely used in cancer treatment, however, in many cases, they are inadequate and may cause serious side effects. Therefore, it is very important to discover new inhibitors with pro-apoptotic activity for cancer treatment. Compounds derived from natural sources such as plants, animals, and fungi have become important in the treatment of a variety of diseases including cancer (Mann et al. 2005; Gurib-Fakim 2006). The recent descriptions related to bioactive anti-cancer agents identified from algae are that it can offer a beneficial therapy at certain stages of cancer. Some edible brown algae and their extracts have been shown to reduce or prevent the development and progression of pre-cancerous cells. Fucoidans derived from the brown algae such as Laminaria spp., Eclonia cava, Sargassum hornery and Costaria costata induced apoptosis both via the activation of Fas, IGF-IR signaling and caspase-3 as well as the down-regulation of the extracellular signal-regulated kinase pathway in various cancer cells (Ermakova et al. 2011; Park et al. 2012; Senthilkumar et al. 2013).
Phaeophyceae, commonly called brown algae, is a large group of mostly marine multicellular algae rich in alginates and sulfated polysaccharides such as fucoidans and laminarins. The brown algae Halopteris scoparia (Linnaeus) Sauvageau (1904) belongs to Stypocaulacea family of Phaeophyceae class. It has an approximate height of 15 cm, and its thallus, which is attached to rocks, forms rigid and branched “broom-like” filaments (Cabioc’h et al. 1992; Cornish and Garbary 2010). H. scoparia is one of the algal species with high biological content, especially proven antimicrobial and antifungal efficacy. This promising medically powerful agent shows a wide range of distribution and is eaten with seafood salad or meals in Far Eastern countries (Holdt and Kraan 2011), on the other hand, it has not received enough attention in cancer chemoprevention area. The primary aim of this study was to determine the anticancer activity of H. scoparia extracts against cancer cells in cellular and sub-cellular levels. Thus, here we focused on alterations in apoptotic genes following changes in cell viability on cervix, colon, and breast cancer cells. In addition, other biological activities of extracts were evaluated by antioxidant activity (DPPH and ABTS+ assay), content determination (Folin-Ciocalteu assay, aluminum chloride colorimetric method, HPLC assay) and partially reliable consumption (acute toxicity and HET-CAM assay).
Materials and methods
Seaweed material
H. scoparia was collected at a depth of 1–2 m, in a region of high light intensity, from the coastline of Urla, Izmir, in April 2012 and was identified by one of the authors (A. Sukatar). Voucher specimens (number: 40796) were deposited in the Hydrobiology Laboratory of Ege University, Faculty of Science, Department of Biology. The samples were washed three times with tap water to remove salt, epiphytes, and sand attached to the surface, then carefully rinsed with fresh water, and maintained in a refrigerator at -20 °C.
Preparation of extracts
Algal samples were dried at 45 °C. Powdered material (108 g) was extracted subsequently with n-hexane (Hex) (purity ≥ 99.9%, Merck, Darmstadt, Germany), chloroform (Chl) (purity 99.0–99.4%, Merck, Darmstadt, Germany) and methanol (Met) (purity ≥ 99.9%, Merck, Darmstadt, Germany) at room temperature in an ultrasonic bath (3 × 1 L of each solvent, 40 °C, 24 h). The combined extracts were evaporated separately for each solvent under reduced pressure by using Rotary evaporator (Heidolph300 Labro Rota, Germany) to dryness and were obtained 166 mg, 228 mg, and 3902 mg, respectively, from the Hex, Chl and Met extracts (Table 1).
Table 1.
Extraction yields and content of flavonoid and total phenolic compounds of H. scoparia extracts expressed as quercetin (QEmg/g) and gallic acid (GA) equivalents (GAEs; mg of GA/g of extract) (Hex: n-Hexane, Chl: Chloroform, Met: Methanol)
| Sample | Extraction yield (%) | Flavonoid content (QE mg/g) | Phenolic content (GAE mg/g) |
|---|---|---|---|
| Hex | 0.15% | – | – |
| Chl | 0.21% | – | – |
| Met | 3.6% | 1.26 ± 0.95 | 33.20 ± 1.41 |
Each value is the average of three analyses ± standard deviation
Determination of total phenolic and flavonoid contents
Total phenolic content was determined by the Folin-Ciocalteu method (Meda et al. 2005). Briefly, 0.1 mL of extracts (0.5 mg/mL and 1 mg/mL) were mixed with 2.8 mL deionized water. This solution was mixed with 2 mL 2% sodium carbonate and 0.1 mL of 0.1 N Folin-Ciocalteu reagent. After incubation at room temperature for 30 min, the absorbance of the mixture was measured at 750 nm against a deionized water blank on a UNICAM 8625 UV/Vis spectrophotometer. Gallic acid was chosen as a standard. The data expressed as milligram gallic acid equivalents.
Total flavonoid content was determined by the aluminum chloride colorimetric method described by Chang et al. (2002). 0.5 mL of the extracts (0.5 mg/mL and 1 mg/mL) were mixed with 1.5 mL of ethanol, 0.1 mL of 10% aluminum chloride and 2.8 mL of distilled water. The mixture was kept at room temperature for 30 min and the absorbance was recorded at 415 nm with the help of UNICAM 8625 UV/Vis spectrophotometer. Quercetin equivalent (QE) was chosen as a standard. The amount of flavonoid was expressed as QE.
Chromatographic analysis
Sample solutions were prepared by dissolving the extracts in methanol at a concentration of 0.1 g/mL in a sonicator. The solution was passed through a 0.22 μm pore diameter filter before use. The gallic acid (purity 97%, CAS NO: 149-91-7), chlorogenic acid (purity ≥ 95%, CAS NO: 327-97-9), caffeic acid (purity ≥ 98%, CAS NO: 331-39-5), p-coumaric acid (purity ≥ 98%, CAS NO: 501-98-4) and quercetin (purity ≥ 95%, CAS NO: 117-39-5) which was purchased from Sigma content of the extracts was analyzed using the HPLC method (Agilent 1100 HPLC–DAD) (Spácil et al. 2008). An Ace C18 column (25 cm × 4.6 mm × 5 µm) was used. The mobile phase comprised water and formic acid (95:5, v/v). The detection wavelength, flow rate, and column temperature were set to 350 nm, 0.9 mL/min and 25 °C, respectively. For all solutions (samples and standards), a quantity of 10 µL was injected. The standards were identified by comparing the retention time and spectral characteristics of its peaks with that’s of standard and all measurements (standard and extracts) were made in triplicate.
A calibration curve was established by diluting with ethanol of gallic acid, chlorogenic acid, caffeic acid, p-coumaric acid, and quercetin stock solutions. Within the concentration range injections (0.25, 1 and 5 mg/L), the detector response for all standards was linear (R2 ≥ 0.9996).
DPPH radical scavenging activity
The capacity of the H. scoparia extract to scavenge the DPPH radical was measured according to Blois method with a slight modification (Turkoglu et al. 2006). One thousand µL of the extracts (0.5 mg/mL and 1 mg/mL) were added to a 4 mL of a 0.004% methanol solution of DPPH. After 30 min of incubation in dark at room temperature, the absorbance was read against a blank at 517 nm. Inhibition (I) of a free radical by DPPH in percent I (%) was calculated as follows:
where Ablank is the absorbance of the control reaction and Asample is the absorbance of the test compound. Tocopherol was used for comparison.
ABTS+ radical cation decolorization assay
The free radical scavenging activity was also determined by ABTS+ (2,2’-azino-bis 3-ethyl benzothiazoline-6-sulfuric acid) radical cation decolorization assay (Re et al. 1999). The mixture of ABTS+ (7 mM concentration) and potassium persulfate (2.45 mM) was left at room temperature in the dark for 16 h, then it was diluted with ethanol (70%) and its absorbance was adjusted to 0.70 ± 0.02 at 734 nm. ABTS+ cation solutions were added to the extract solution and the decrease of absorption was measured against 70% ethanol, 6 min later, at the end of the reaction at room temperature. All measurements were carried out in triplicate. The inhibition percentages were calculated as follows:
where A0 is the absorbance of control and A1 is the absorbance of the sample. Synthetic antioxidants were used as positive controls.
Cell culture and cytotoxicity assay
In this study, human cervix adenocarcinoma (HeLa) (ATCC-CCL-2), human colon colorectal adenocarcinoma (CaCo-2) (ATCC-HTB-37) and human breast adenocarcinoma (MCF7) (ATCC-HTB-22) were used as cancer cell lines while human embryonic kidney cells 293 (HEK293) (ATCC-CRL-1573) were used as a non-cancerous cell line. Cell lines were purchased from Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ, Braunschweig, Germany) and maintained in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, 100 U/mL penicillin and 100 µg/mL streptomycin (Biochrom AG, Berlin, Germany) at 37 °C in a humidified atmosphere of 5% CO2.
Cytotoxic effects of three extract were determined by a modified MTT [3-(4, 5- dimethyl-2-thiazolyl) -2, 5-diphenyl-2H-tetrazolium bromide)] cell viability assay (Mosmann 1983), which measures the reducing activity of viable cells colorimetrically. The formed formazan crystals were dissolved in dimethyl sulfoxide (DMSO), and the optical density (OD) of triplicate samples was measured at 570 nm. All cell lines were cultivated for 24 h in 96-well microplates with an initial concentration of 1 × 105 cells/mL. Then, cells were treated with different concentrations of the extracts (0.5, 5, 50 µg/mL) and incubated for 48 h at 37 °C. Each sample was tested in triplicate. The DMSO concentration in the assay mixtures was less than 5% which was not toxic to the cells. Parthenolide (Parth), a chemotherapeutic agent, was used as a positive control. Percentages of surviving cells in each culture were determined at the end of treatment, and % viability was determined according to the formula:
Also, the morphological changes were monitored at 48 h exposure. The concentrations of the extracts causing 50% inhibition of cell growth, compared to untreated controls, were calculated as described previously (Yalçin et al. 2014). Cytotoxicity was expressed as an increase of the mean percentage of cytotoxicity relative to the unexposed control ± standard deviation (SD). Control values were set at 0% cytotoxicity. IC50 was calculated by fitting the data to a sigmoidal curve and using a four parameters logistic model and presented as an average of three independent measurements. The IC50 values were reported at 95% confidence interval and calculation was performed using GraphPad Prism software (San Diego, USA). The values of the blank wells were subtracted from each well of treated and control cells and inhibition of growth 50% was calculated in comparison with untreated controls.
cDNA synthesis and panel assays
HeLa, MCF-7, CaCo-2 and HEK293 cells were plated at a density of 1 × 105 cells/mL. After 48 h of incubation, cells were treated with the IC50 concentration of the three solvent extracts for 24 h. Cells were harvested and total RNA was isolated using the TriPure isolation reagent (Roche, Basel, Switzerland, Cat. no. 11 667 157 001). The quality of the isolated RNA was controlled by NanoDrop (NanoDrop ND-2000c, Thermo Scientific, Waltham, MA, USA). First-strand cDNA was synthesized from total RNA with the Transcriptor First Strand cDNA Synthesis kit (Roche, Cat. no. 04 379 012 001), and cDNAs were stored at − 20 °C until gene expression analysis.
cDNA samples from treated and untreated cells were then used as probes in the analysis of the expression of 84 genes involved in apoptosis through the Real Time Ready Human Apoptosis Panel, 96 (Roche, Cat. No. 05 392 063 001) according to the manufacturer’s protocols. A master reaction mix was prepared using 1058.4 µl 2 × Roche LightCycler 480 Probes Master (10 µl per reaction), 348 µL cDNA sample (4 µl per reaction) and 518.4 µl water (5.4 µl per reaction). For amplification, 20 µl cDNA was interpolated to 980 µl of LightCycler 480 Probes Master kit. PCR cycling conditions were as follows: initially 95 °C for 10 min, amplification for 40 cycles of 15 s at 95 °C, 8 s at 62 °C and 13 s at 72 °C. The expression levels of the 84 apoptosis-related genes were standardized to those of seven housekeeping genes. The comparative Ct method (2−∆∆Ct method) was used to determine the expression level of analyzed genes (Livak and Schmittgen 2001). Genes with average a fold change values ≤ − 2 or ≥ 2 in their expression were considered as up- or downregulated.
HET-CAM (Hen’s egg test chorioallantoic membrane) irritation test
The irritation effects of algae extracts revealed by using a chorioallantoic membrane model on fertilized hen eggs. Irritation effects of samples were carried out on fertile Leghorn chicken eggs weighing 50–60 gr obtained from commercial sources (Lezita, İzmir, Turkey) by using HET-CAM method modified of Kishore et al. (2008) and Güner and Karabay Yavasoglu (2018). Fertilized hens’ eggs were placed into an incubator with conveyor rotation system at 37 ± 1 °C and 80 ± 2% humidity for 7 days. On day 7, the eggs were opened on the snub side sucked off through a hole on the pointed side and then a round piece of shell (3–4 cm diameter) was removed carefully with forceps. Then, the inner membrane carefully removed with forceps, without injury to the blood vessel. After that, 300 μl of the freshly prepared sample at 0.5 and 1 mg/mL concentration that dissolved in DMSO (0.05%) (0.5 to 1 mg/mL) was applied to the CAM. The irritation severity (IS) for a period of up to 5 min was scored as:
where h is the time of vascular hemorrhage occurred; l is the time of first vascular lysis occurred; and c is the time of first vascular coagulation occurred. Irritation classification based on IS: 0.0–0.9, non-irritation; 1.0–4.9, slight irritation; 5.0–8.9, moderate irritation; and 9.0–21.0, severe irritation. Also, 0.9% NaCl as a negative control and 0.1 N NaOH as a positive control at the concentration of 300 μL were also tested. For every test compound, 5 eggs were utilized. All samples were tested in triplicate at different times.
Acute toxicity test
In this study, the up and down technique was concluded to determine the lethal dose (LD50) of a substance that will kill 50% of test animals (Derelanko and Hollinger 1995; Barile 2008). Male and female albino mice weighing 15–20 g each were used for this test (n = 2 for each group). The protocol was approved by the Ege University, Local Ethical Committee of Animal Experiment (18.12.2013, no. 2013–052). Animals were kept in a room maintained at 22 ± 1 °C with an alternating 12 h light–dark cycle. Food and water were available ad libitum. The animals were transported to a quiet laboratory at least 1 h before the experiment. All experiments conformed to ethical guidelines for the investigation of experimental pain in conscious animals (Zimmermann 1983). Each animal was used once only and was humanely sacrificed immediately after completion of testing. The limit dose (2000 mg/kg body weight) for acute oral toxicity was used according to the Environmental Protection Agency (EPA) and The Organization for Economic Cooperation and Development (OECD).
Data analysis
Values are presented as mean ± standard error of the mean (SEM). IC50 calculation and variance analysis (standard deviation calculation) were performed with Graph Pad Prism (San Diego USA). All statistical analyses were performed using SPSS for Windows 15.0. Furthermore, The Ct (cycle threshold) is defined as the number of cycles required for the fluorescent signal to cross the threshold (exceeds background level, etc.) and Ct values are inversely proportional to the amount of target nucleic acid in the sample. At the same time, this definition was evaluated taking into consideration of Cts < 29 are strong positive reactions, Cts of 30–37 are moderate positive reactions and Cts of 38–40 are weak reactions.
Results
Total phenol, flavonoid and HPLC contents
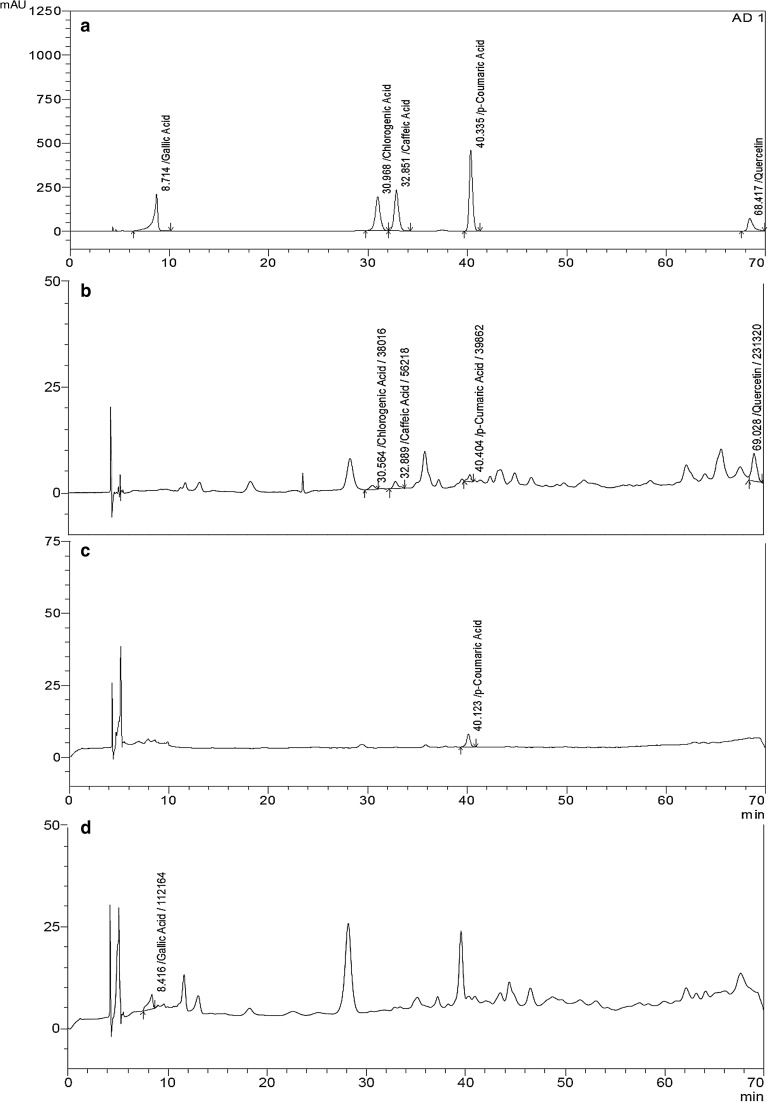
The results showed that Met extract had the highest total flavonoid and polyphenol contents of 33.20 ± 1.41 GAE/g extract and 1.26 ± 0.95 mg QE/g extract respectively while a computable value of Chl and Hex could not be established (Table 1). The different content of the extract was determined by HPLC and chromatograms of standard (A) and Met, Chl and Hex extract (respectively, B, C, and D) were shown in Fig. 1. These results revealed that caffeic acid (tR 32.889), p-coumaric acid (tR 40.404) and quercetin (tR 69.028) were the predominant content (mg/100 g extract) in Hex extract and 0.036 ± 0.0004, 0.017 ± 0.0002 and 0.41 ± 0.0003, respectively. However, gallic acid (tR 8.416) that eluted first among other phenolic only determined in Met extract as 0.11 ± 0.0002 mg/100 g extract, while p-coumaric acid was detected in Chl (tR 40.123) as 0.13 ± 0.0004 mg/100 g extract.
Fig. 1.
HPLC profiles and retention times (tR) of H. scoparia extracts with 0.1 g/mL concentration and standards at 350 nm analyzed with the improved method. Peak identification; a, b, c and d were determined for standards (gallic acid, chlorogenic acid, caffeic acid, p-coumaric acid, and quercetin), Hex, Chl and Met extracts, respectively. Hex: n-Hexane, Chl: Chloroform, Met: Methanol
Antioxidant activity
The DPPH antioxidant activities of the H. scoparia extracts are presented in Table 2. The results showed that Hex (ED50 = 14.0963%) extract exerted higher antioxidant activity than Chl (ED50 = 15.1228%) and Met (ED50 = 400.7910%). According to ABTS results, Met extract in 1 mg/mLconcentration showed the highest activity (Table 2). Additionally, Hex extract with 63.93 ± 0.31 values is remarkably higher than 57.45 ± 0. 12 value of Chl extract.
Table 2.
DPPH and ABTS+ antioxidant activities of H. scoparia extracts (Hex: n-Hexane, Chl: Chloroform, Met: Methanol)
| Sample | Concentration (mg/mL) | DPPH inhibition (%) | α-tocopherol equivalent antioxidant activity values (µg/mL) | ED50 (mg/mL) | ABTS+ inhibition (%) (1 mg/mL) |
|---|---|---|---|---|---|
| Hex | 0.25 | 4.11 ± 0.04 | 3.72 ± 0.01 | 14.0963 | 63.93 ± 0.31 |
| 0.5 | 4.67 ± 0.02 | 4.22 ± 0.02 | |||
| 1 | 7.89 ± 0.01 | 7.13 ± 0.01 | |||
| Chl | 0.25 | 3.27 ± 0.02 | 4.54 ± 0.01 | 15.1228 | 57.45 ± 0.12 |
| 0.5 | 4.13 ± 0.06 | 3.96 ± 0.02 | |||
| 1 | 8.96 ± 0.04 | 9.08 ± 0.02 | |||
| Met | 0.25 | 5.11 ± 0.02 | 5.72 ± 0.03 | 400.332 | 51.01 ± 0.9 |
| 0.5 | 6.43 ± 0.06 | 7.62 ± 0.02 | |||
| 1 | 11.28 ± 0.01 | 10.29 ± 0.4 |
Each value is the average of three analyses ± standard deviation
Cytotoxic effects of algal extracts on cancer cells lines
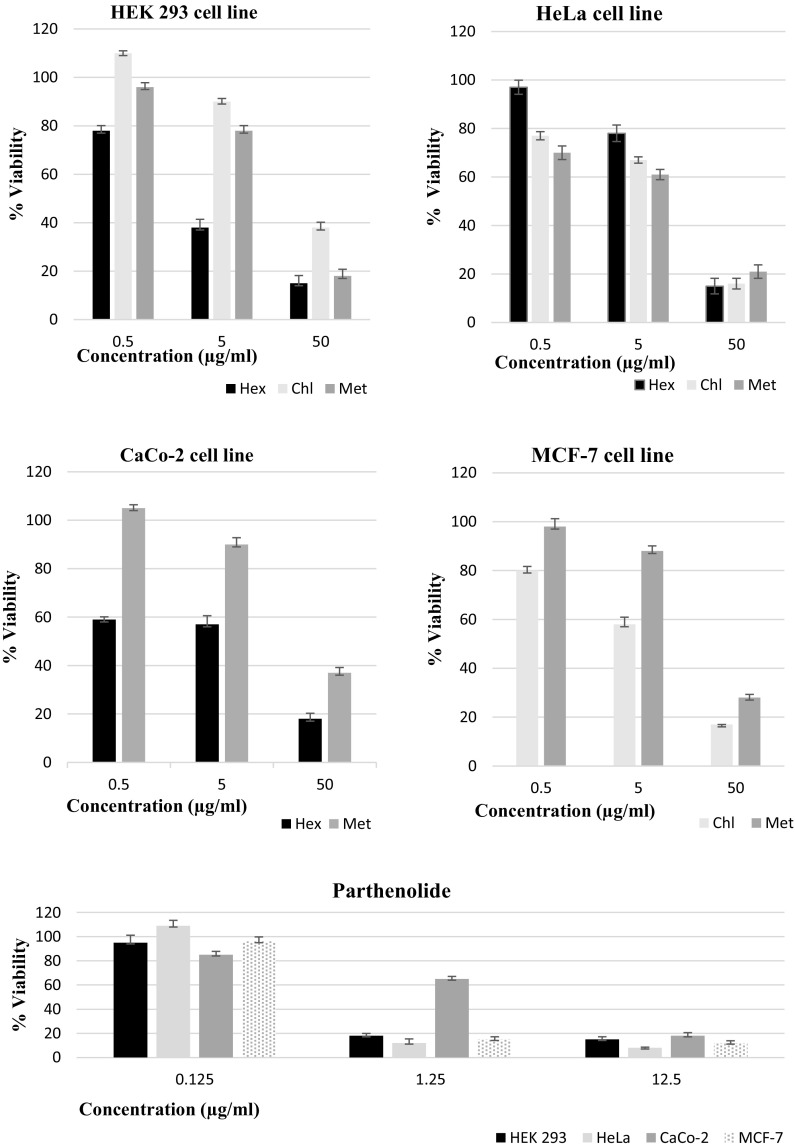
The cytotoxic effect and IC50 value of algae extracts on cell lines were determined by using different concentrations of the extracts. The MTT assay revealed that all extracts inhibit cell proliferation in a dose-dependent manner (Fig. 2). As shown in Table 3, Met-extract exhibited a more potent cytotoxic effect on HeLa cells as compared to Hex and Chl extracts. At the same time, Chl and Met extracts inhibited cell proliferation in MCF-7 while CaCo-2 cell proliferation was inhibited with Hex and Met extracts were treated. However, Hex, Chl, and Met extracts also inhibited the growth of HEK293 normal cells. As supporting these results, cells without Hex, Chl, and Met extracts treatment had a polygonal shape and a healthy appearance, maintaining normal cell growth. Increasing algae extracts concentrations resulted in cells lost their adhesion to the culture plates and an increased number of rounded cells in appearance simultaneously growth inhibition (see Online Resources 1).
Fig. 2.
Viability of cancer cells line after treatment of H. scoparia extracts and parthenolide which is used as a control for 48 h by MTT assay. The viability of untreated cells was exposed to vehicle only which was taken as 100%. Data are expressed as mean ± SD. Each value is the average of three analyses ± standard deviation. Hex: n-Hexane, Chl: Chloroform, Met: Methanol, Parth: Parthenolide
Table 3.
IC50 values of HEK 293, CaCo-2, MCF-7, and A549 cell lines after treatments of H. scoparia extracts and parthenolide which is used as a control for 48 h
| Cell lines | IC50 of samples (µg/mL) with SD | |||
|---|---|---|---|---|
| Hex | Chl | Met | Parth | |
| HEK293 | 26.50 ± 0.14 | 12 ± 0.13 | 2.50 ± 0.11 | 0.5583 ± 0.10 |
| HeLa | 12.2 ± 0.12 | 7.6 ± 0.08 | 6.5 ± 0.10 | 0.9865 ± 0.06 |
| CaCo-2 | 4.53 ± 0.12 | > 50 | 22 ± 0.11 | 0.4762 ± 0.06 |
| MCF-7 | > 50 | 13 ± 0.11 | 26.8 ± 0.13 | 0.527 ± 0.06 |
(Hex: n-Hexane, Chl: Chloroform, Met: Methanol, Parth: Parthenolide)
SD Standard deviation
Change of Ct values and expression of pro/anti-apoptotic genes in cell lines
Most of the control and extract treated groups gave Ct values between 21 and 29, and it revealed that the lower the Ct value, the better the PCR performance (see Online Resources 2). As seen in Table 4 related to the analysis of gene expression associated with algae extract-treatment in a cancer cell line, 59 gene-profiles (39 pro-apoptotic and 20 anti-apoptotic gene expression) in a total of 84 genes have given meaning. These genes; 14 members of the death receptors and their ligands, 12 members of the Bcl-2 family, 11 members of caspase family, 5 members of the pro-survival NF-κB family, 3 members of the inhibitors of apoptosis (IAP) family as well as 10 members of the other proteins related to apoptosis and cell survival.
Table 4.
The fold changes in gene expression levels in cell lines exposed to H. scoparia extracts using RT2 Profiler PCR Array System
| Gene symbol/alias | Gene name | HeLa cells | CaCo-2 cells | MCF-7 cells | ||||
|---|---|---|---|---|---|---|---|---|
| Hex | Chl | Met | Hex | Met | Chl | Met | ||
| TNF receptor | ||||||||
| TNF/TNF-α | Tumor necrosis factors | 3.45 | − 2.8 | 2.01 | − 1.6 | − 3.6 | 11.2 | 9.19 |
|
FAS/ TNFRSF6 |
Tumor necrosis factor receptor superfamily member 6 precursor | 4.5 | − 3.7 | − 3.2 | 5.09 | − 1.1 | − 3.2 | − 1.2 |
| FASLG | Fas ligand | 3.34 | − 8.3 | 4.57 | – | 2.54 | – | 2.03 |
| FADD | Fas-associated via death domain | 2.32 | − 4.6 | − 2.3 | − 1.3 | − 4.4 | − 3.3 | − 2.3 |
| CRADD | Death domain-containing protein | − 2.2 | 4.57 | 1.6 | − 1.9 | − 1.2 | 2.42 | 4.2 |
| NGFR | Tumor necrosis factor receptor superfamily member 16 | 2.01 | 3.59 | 2.59 | – | − 3.2 | 4.07 | − 1.2 |
| TNFRSF10A | Tumor necrosis factor receptor superfamily. member 10A | 2.8 | − 3.9 | − 5.4 | − 1.4 | 1.2 | 1.25 | − 1.5 |
| TNFRSF10C | Tumor necrosis factor receptor superfamily. member 10C | 5.2 | – | – | − 3.2 | − 2.3 | – | − 1.3 |
| TNFRSF10D | Tumor necrosis factor receptor superfamily. member 10D | 3.12 | − 4.3 | 2.87 | − 1.4 | 2.54 | – | − 1.3 |
| TNFRSF1A | Tumor necrosis factor receptor superfamily. member 1A | 6.8 | 3.9 | 2.06 | – | – | 3.42 | – |
| TNFRSF11B | Tumor necrosis factor receptor superfamily member 11B | – | – | – | –1.78 | –1.40 | –1.02 | –2.54 |
| TNFSF8 | TNF superfamily member 8 | – | – | – | – | − 3.1 | 1.25 | 1.28 |
| TNFSF10 | TNF superfamily member 10 | – | – | – | 11.9 | 1.21 | − 1.4 | 4.45 |
| TNFSF11 | TNF superfamily member 11 | – | – | – | − 1.4 | – | 2.41 | − 1.6 |
| TRAF family | ||||||||
| TRAF1 | TNF receptor-associated factor1 | – | – | 1.23 | 5.68 | 10.1 | − 1.3 | − 3.7 |
| TRAF2 | TNF receptor-associated factor2 | 2.03 | 7.2 | 4.19 | 1.42 | – | − 1.1 | − 1.4 |
| TRAF3 | TNF receptor-associated factor3 | − 5.2 | 4.1 | 2.58 | − 1.3 | – | − 2.9 | − 1.7 |
| TRAF5 | TNF receptor-associated factor5 | – | – | – | − 1.5 | − 1.7 | − 1.3 | – |
| TRAF6 | TNF receptor-associated factor6 | – | – | – | − 1.2 | − 5.4 | − 1.2 | 2.47 |
| BCL2 family | ||||||||
| Anti-apoptotic members | ||||||||
| BCL2 | B cell CLL/lymphoma 2 | − 5.2 | 5.27 | − 7.1 | – | − 2.2 | − 3.6 | − 1.1 |
| BCL2L1 | B-cell lymphoma like X (BCLX) | 2.38 | – | − 3.2 | − 1.4 | − 4.2 | − 2.1 | – |
| MCL1 | Myeloid cell leukemia sequence 1 (BCL2-related) | − 2.4 | 3.78 | − 5.3 | 2.69 | – | − 1.1 | 2.00 |
| BCL2L10 | Bcl-2-like protein 10 | – | – | – | – | – | 1.28 | 2.34 |
| BCL2L2 | Bcl-2-like protein 2 | – | – | – | – | – | 18.5 | 1.90 |
| Pro-apoptotic members | ||||||||
| BH multidomain members | ||||||||
| BAX | Bcl-2-associated X protein | – | – | – | – | − 1.5 | 2.15 | − 1.5 |
| BAK1 | BCL2-antagonist killer 1 | 5.2 | − 7.3 | 6.78 | − 1.3 | − 2.6 | − 2.5 | 1.93 |
| BOK | BCL2-related ovarian killer | 3.5 | − 3.2 | 2.4 | – | − 1.7 | 5.28 | 3.31 |
| BH3-only members | ||||||||
| BBC3 | BCL2 binding component 3 | 4.84 | − 2.7 | 3.4 | – | – | 7.90 | 1.20 |
| BAD | Bcl2 antagonist Of Cell Death | 5.3 | − 4.6 | 4.42 | – | − 1.3 | 1.58 | − 1.5 |
| BID | BH3 interacting domain death agonist | 2.87 | − 4.2 | − 2.3 | − 1.4 | – | − 2.2 | 3.94 |
| BIK | BCL2-interacting killer (apoptosis-inducing) | 4.12 | − 2.3 | − 3.4 | – | – | 2.34 | 3.58 |
| NF-kB family | ||||||||
| NF-kB 1 | Nuclear factor of kappa light polypeptide gene enhancer in B-cells 2 | – | – | – | − 1.3 | − 1.2 | − 2.9 | − 3.1 |
| NF-kB 2 | Nuclear factor of kappa light polypeptide gene enhancer in B-cells 2 | 2.1 | 4.6 | − 2.2 | − 2.5 | − 1.7 | − 1.4 | 2.31 |
| REL | v-rel reticuloendotheliosis viral oncogene homolog (avian) | − 2.3 | − 2.6 | 2.12 | − 1.9 | − 1.2 | 3.98 | − 2.4 |
| RELA | v-rel reticuloendotheliosis viral oncogene homolog B | – | – | – | – | − 1.2 | − 3.5 | − 3.4 |
| RELB | v-rel reticuloendotheliosis viral oncogene homolog B | 2.54 | 3.16 | 2.01 | 4.07 | – | 2.96 | 2.00 |
| Caspases and regulators | ||||||||
| CASP2 | Caspase 2. apoptosis-related cysteine peptidase | – | 2.45 | 3.56 | − 1.3 | 3.21 | − 1.5 | 9.25 |
| CASP3 | Caspase 3. apoptosis-related cysteine peptidase | 4.23 | 7.34 | 6.8 | − 3.2 | − 1.9 | − 4.2 | − 2.4 |
| CASP4 | Caspase 4. apoptosis-related cysteine peptidase | – | – | – | – | – | – | 2.3 |
| CASP5 | Caspase 5. apoptosis-related cysteine peptidase | – | – | – | 2.40 | – | – | 2.02 |
| CASP6 | Caspase 6. apoptosis-related cysteine peptidase | 3.87 | − 2.4 | 2.68 | 3.58 | 4.58 | 9.65 | 8.41 |
| CASP7 | Caspase 7. apoptosis-related cysteine peptidase | − 4.21 | 2.98 | − 3.59 | 1.09 | − 2.1 | 5.46 | − 2.1 |
| CASP8 | Caspase 8. apoptosis-related cysteine peptidase | 8.19 | 2.54 | 4.93 | − 2.4 | − 1.2 | − 2.3 | − 3.6 |
| CASP9 | Caspase 9. apoptosis-related cysteine peptidase | 5.62 | 3.65 | 2.78 | 1.86 | − 2.6 | − 3.4 | − 2.4 |
| CASP1 | Caspase 1. apoptosis-related cysteine peptidase | – | – | 1.50 | − 1.4 | − 1.6 | 1.45 | − 3.2 |
| CASP10 | Caspase 10. apoptosis-related cysteine peptidase | 4.52 | 2.04 | 5.21 | − 4.2 | 6.25 | − 1.5 | – |
| CASP14 | Caspase 14. apoptosis-related cysteine peptidase | – | – | – | – | – | − 1.9 | 2.00 |
| IAP family | ||||||||
| BIRC2 | Baculoviral IAP repeat-containing protein 2 (Inhibitor of apoptosis protein 2) | − 4.58 | − 2.2 | – | − 1.9 | − 1.4 | 3.12 | 2.83 |
| BIRC3 | Baculoviral IAP repeat-containing protein 3 (Inhibitor of apoptosis protein 3) | − 3.05 | 2.04 | − 3.5 | − 1.5 | – | 2.42 | 4.3 |
| BIRC5 | Baculoviral IAP repeat-containing protein 5 (Apoptosis inhibitor survivin) | 3.98 | 2. 2 | 2. 1 | – | – | − 1.9 | − 1.5 |
| Other proteins | ||||||||
| APAF1 | Apoptotic protease-activating factor 1 (Apaf-1) | 6.05 | 3.12 | 3.25 | 11.8 | 2.03 | 2.10 | 2.45 |
| HTRA2 | Serine protease HTRA2. mitochondrial precursor | 2.86 | 4.71 | 2.34 | − 2.1 | − 1.4 | − 2 | − 3.1 |
| endoG | Endonuclease G. mitochondrial precursor | 3.48 | 2.02 | 2.36 | − 1.59 | – | 5.46 | − 2.00 |
| DFFA | DNA fragmentation factor subunit alpha | − 5.6 | − 2.4 | − 2.5 | 5.46 | – | 4.4 | − 1.2 |
| CAD | CAD protein | 4.36 | 5.9 | 3.63 | − 2.3 | − 3.4 | 4.78 | 6.09 |
| HMGB1 | High mobility group box 1 | 6.65 | 5.2 | 4.7 | − 1.8 | − 3.2 | 2.50 | 2.14 |
| PTEN | Phosphatase and tensin homolog | 2.13 | 2.45 | − 3.1 | 1.54 | 2.14 | 4.10 | 2.49 |
| TP53 | Cellular tumor antigen P53(Tumor suppressor P53) | 12.4 | 8.05 | 7.2 | 3.88 | 2.04 | 2.98 | 2.40 |
| AKT1 | AKT serine/threonine kinase 1 | 2.4 | – | 2.8 | − 3.1 | − 2.7 | − 4.3 | – |
(Hex: n-Hexane, Chl: Chloroform, Met: Methanol)
– No activity
When gene expressions in Hela cells were examined after treatment of extracts, Hex and Met treatments led to upregulation (with a fold change value in between 2.32 to 12.4 and 2.01 to 7.2, respectively) of many pro-apoptotic genes in death receptor pathway, intrinsic pathway, caspase family (Casp − 3, − 6, − 8, − 9 and − 10) and caspase-independent pathway (TP53, PTEN, endoG). Chl treatment caused positive induction in only the TNFRSF1A gene (with a fold change value of 3.9) of the extrinsic pathway, in − 2, − 3, − 7, − 8, − 9, and − 10 genes of caspases family (with a fold change value in between 2.04 and 7.34) and in HTRA2, PTEN, TP53, endoG genes of caspase-independent pathway (with a fold change value in between 2.02 and 8.05).
Gen expressions changes in CaCo-2 cells after treatments of Hex and Met extracts indicated that Hex treatment provoked positively stimulation in FAS and TNFSF10 genes (with a fold change value of 5.09 and 11.9, respectively) of extrinsic pathway and in -5 and -6, genes (with a fold change value of 2.4 and 3.58, respectively) of caspase family. Met treatment stimulated upregulation in FASLG and TNFRSF10D genes (with a fold change value of 2.6) of extrinsic pathway and in -2, -6 and -10 genes (with a fold change value of 3.2, 4.58, and 6.25, respectively) of caspase family.
When analyzed the gene expressions in MCF-7 cells after treatments of Chl and Met, Chl treatment caused upregulation in TNF, CRADD, TNFRSF1A, and TNFSF11 genes (with a fold change value of 11.2, 2.42, 3.42, and 2.41, respectively) of the death receptor pathway, in BOX, BOK, BBC3 and BIK genes (with a fold change value of 2.5, 5.28, 7.9, and 2.34, respectively) of intrinsic pathway and in Casp -6, and -7 genes (with a fold change value of 9.65 and 5.46, respectively) of caspase family. Met treatment led to positive increases in TNF, FASLG, CRADD, TNFRSF11B, and TNFSF10 genes (with a fold change value of 9.02, 2.03, 4.2, 2.54, and 4.45, respectively) of the extrinsic pathway, in BOK, BID, and BIK genes (with a fold change value of 3.31, 3.9, and 3.6, respectively) of intrinsic pathway and in − 2, − 4, − 5, − 6, and − 14 genes of caspases family (with a fold change value of 9.25, 2.3, 2.02, 8.41, and 2, respectively). Also, all extracts led to positive increases in other apoptosis-activated genes such as APAF1, PTEN, and TP53 with a fold change value in between 11.8 and 2.04 in CaCo-2 and MCF-7 cells.
Anti-apoptotic gene expression results after extract treatments showed that TRAF 3 gene after Hex extract in HeLa cells and TRAF 6 gene after Met extract in MCF-7 and CaCo-2 cells were downregulated with a fold change value of − 5.4, − 5.2, and − 3.7, respectively. When Bcl-2 family gene expressions were evaluated, Bcl-2 and Mcl-1 genes after Hex treatment and Bcl-2, Bcl2 l-1, and Mcl-1 genes after Met treatment in HeLa cells were negatively regulated with a fold change value of − 5.2, − 2.4, − 7.1, − 3.2, and − 5.3, respectively. In CaCo-2 cells, Met treatments led to a downregulation in Bcl-2 and Bcl2 l-1 genes in HeLa cells with a fold change value of − 3.6 and − 2.1, respectively. NF-κB and IAP family gene expression result showed that the REL gene after Hex and Chl treatments and NF-κB 2 gene after Met treatment in HeLa cells were negatively induced. NF-κB 1 and RELA gene after Chl and NFkB1, REL and RELA genes after Met treatment in MCF-7 cells were negatively regulated while only NF-κB 2 gene downregulated by Hex treatment in CaCo-2 cells.
In vivo acute toxicity and irritation test
According to the Fig. 3, while n-hexane extract had no lead to any irritation even at the highest concentrations with IS value of 0.03 ± 0.01, chloroform and methanol extracts caused a slight irritation with weak lysis on the CAM after 5 min, respectively 1.2 ± 0.03 and 1.4 ± 0.02 values for IS. However, while in the negative control and DMSO groups, no irritation was found, the positive control immediately interacting with the CAM resulted in hemorrhage, lysis, and coagulation, respectively, and cause severe irritation with the IS score of 18.4 ± 0.2 for a period of up to 5 min.
Fig. 3.
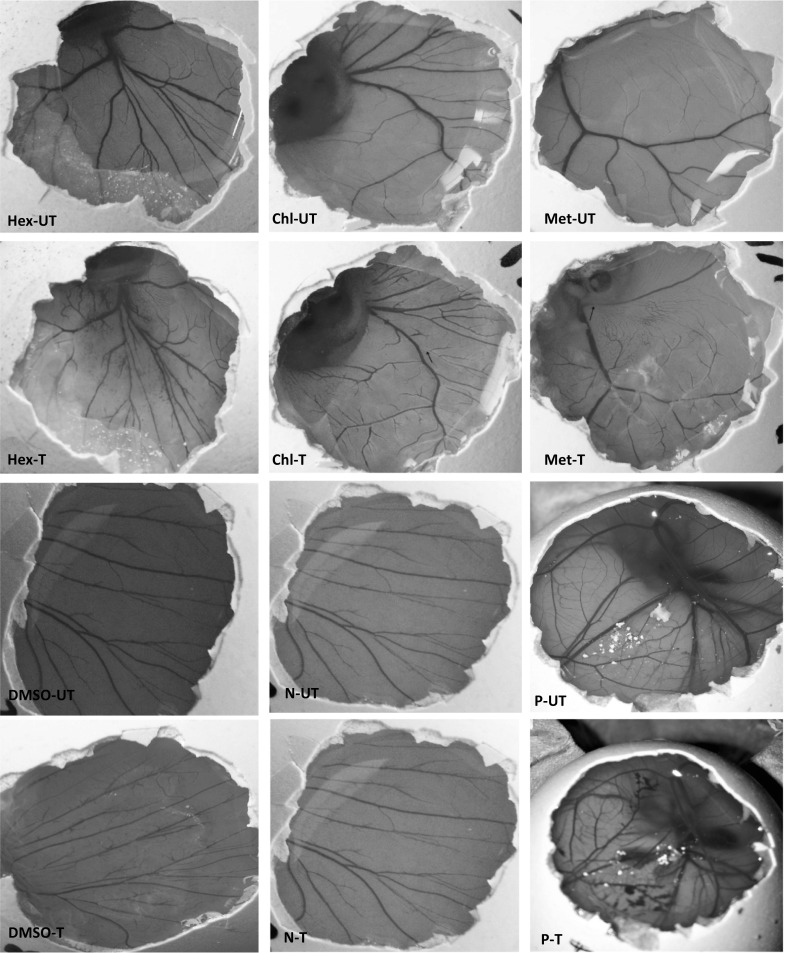
Photographs illustrating the potential irritation or toxicity on vascularization before exposure to the test samples (0 min) and after the exposure for up to 5 min by using HET-CAM assay. Hex: n-Hexane, Chl: Chloroform, Met: Methanol, UT: before treatment, T: after treatment, N: Negative control (0.9% NaCl), P: Positive control (0.1 N NaOH)
In vivo acute oral toxicity study revealed that no lethality was observed among mice treated with oral doses of H. scoparia extracts.
Discussion
Therapeutic activities of algae have been intensively studied in many fields and the novel biologically active compounds discovered were converted into medicinal herbs and functional foods. In particular, edible marine algae have great attention thanks to their mineral, protein, essential amino acids, dietary fiber, and vitamin contents that enhance the bioavailability of body (Pereira 2011). H. scoparia which contains rich in anti-oxidative cytokines, polyphenols, and betaines are widely harvested for nutritional and pharmaceutical purposes (Pereira 2016). In the present study, we observed that solvent extracts of H. scoparia had antioxidant activity and caused changes in expressions of the apoptotic pathway genes of different cancer cells.
The potential activities of active metabolites are associated with the extraction methods and different polarities of solvents used. ABTS+ and DPPH assays are the main methods to determine the antioxidant potential of active compounds in natural sources. While DPPH reacts in the organic phase (especially in ethanol), ABTS+ can only be soluble in the both aqueous and organic phase (Thaipong et al. 2006). For this reason, whether there is a correlation between ABTS+ and DPPH is still a matter of debate. According to the present study, the highest activity of both Hex apolar extracts in the DPPH assay and Met polar extracts in ABTS+ indicated no correlation between these assays tend to support the findings of the Wang et al. (2007) and recent investigations have documented that these antioxidant activities of solvents may result from many phenolic compounds (Yusnawan 2013). However, our results showed that there is a correlation between total phenolic content with ABTS+ activity for polar Met extract, but similar results have not been observed for DPPH. This phenomenon may be explained that antioxidant activity of Hex in DPPH test is thought to originate from synergistic or single effects contents such as caffeic acid, p-coumaric acid, and quercetin compared to Met and Chl extracts. This supporting information may not mean to be that compounds specified in the standard are absolutely present in the solvent extracts, but it can give a general idea about phytochemical groups to which it belongs (Chakraborty and Paulraj 2010). For this reason, the possible antioxidant activity of Hex is revealed to be closely related to the number and hydroxyl groups of phenolic compounds as well as lipophilic content such as carotenoid and unsaturated fatty acid (Cheng et al. 2006). Similarly, study related to the antioxidant activities of the different solvent of red algae Laurencia obtusa conducted by Demirel et al. (2011) declared that methanol extracts exhibit too low an activity compared to the other solvent in DPPH assays.
Exogenous and endogenous originated stimulants can cause an imbalance in the antioxidant mechanism following the increase of free radicals in cells and this phenomenon is considered the basis of many diseases such as cancer. This situation can be reduced or eliminated by the consumption of terrestrial or marine plants that is rich in antioxidant compounds. Today, approximately 50% of chemotherapeutic drugs is originated from either natural resources or their derivative products (Simmons et al. 2005; Newman and Cragg 2012). In our study, H. scoparia extracts showed different cytotoxic effects in cancer cell lines. The results obtained from HeLa cells indicated that H. scoparia Met fraction showed a more potent cytotoxic effect compared to Chl and Hex. Supporting our data, Khanavi et al. (2010) declared that polar extracts of algae show cytotoxic effects against different cancer cells. When assayed more aggressive cell lines, Hex extract in MCF-7 cells and Chl extract in CaCo-2 cells showed more cytotoxic effects than Met extract. Some studies showed that synergistic formulations of plant extracts can be more effective than synthetic alone or pure natural compound alone (de Kok et al. 2008). In accordance with these findings, present cytotoxic effects may be related that the efficacy of phytochemicals such as lipid and carotenoid dissolved in nonpolar solvents as well as originated from caffeic acid, p-coumaric acid and quercetin contents for Hex extracts and p-coumaric acid contents for Chl extracts, and as a result of this it can also be expressed by interacting with different receptors on the cell surface suitable for the structure of the active compounds. Furthermore, all extracts exhibited cytotoxicity against non-tumor HEK-293 cells indicating that the activity was not specific to cancer cells.
Elimination of any defect or abnormality throughout the apoptotic pathways is a useful approach in cancer treatment. Numerous studies reported that diet-derived compounds have provided effective protection by with intervention to any stage of cancer, especially induce apoptotic genes (Khan and Mlungwana 1999; Ramos 2008). The correct interpretation of expression changes in apoptosis genes is essential in determining the future of the cell. To date, numerous analyzes have been developed to verify and demonstrate these changes. The RT2 Profiler PCR Array System that is one of the latest trends is the most reliable and accurate tool to express the changes of a focused panel of genes using TaqMan probe-based real-time PCR. The present study is the first to reveal the mechanisms of anticancer/anti-proliferative action of Hex, Chl and Met extracts of H. scoparia through the different apoptotic pathways in HeLa, MCF-7 and CaCo-2 cancer cell lines. In this context, the extract treatments contributed to the regulation of 59 apoptotic genes in association with tumor suppressor p53 (TP 53) pathway, the extrinsic/intrinsic receptor pathway and transcription factor NF-κB pathway.
In this study, extrinsic (death receptor pathway) and intrinsic (mitochondrial) pathways had a critical role in extract-induced apoptosis. TNF family and their receptors, especially TNF receptor-associated death domain (TRADD) and Fas-associated death domain (FADD), have an important role in death and survival, proliferation, and homeostasis of cells (Ashkenazi and Dixit 1998; Locksley et al. 2001). Our results showed that each extract treatment led to different gene expression on the same pathway.
When HeLa cells were examined after H. scoparia applications, Hex and Met extracts were upregulated different pro-apoptotic genes such as TNF, FASLG, TRAIL and adaptor protein FADD in the cell membrane while Chl extract was only promoted TNFRSF1A and adaptor protein CRADD. The caspase-cascade system plays a central role in the regulation of apoptosis and changes in these pathway genes are determinative for the fate of the extrinsic pathway. Caspases − 3, − 8 and − 9 have a vital role in controlling traffic of apoptosis signal pathway. Especially, caspase-8 can lead to the caspase activation cascade signal via the extrinsic pathway (Salvesen and Dixit 1997). In this study, all extract treatments caused upregulation of Casp − 3, − 8 and − 10 and this situation revealed to continue of stimulations in the extrinsic pathway. In another saying, extracts tend to drag to the apoptosis the cancer cells via the extrinsic pathway. However, upregulations of CRADD and Casp-2 gene expression for Chl extract suggested that apoptosis is active through a different extrinsic pathway.
The intrinsic pathway (via mitochondrial perturbation) plays a vital role in the regulation of apoptosis as a response to cellular stress and this pathway is controlled by anti and pro-apoptotic genes of Bcl-2 families. The change of the Bax/Bcl-2 ratio is one of the key factors that determine the destiny of cells. In the intrinsic pathway mechanism, once activated pro-caspase 8 can cleave Bid into tBID, tBID then binds to pro-apoptotic protein BAX and can activate other executioner caspases. These stimuli cause variation in the mitochondrial membrane permeabilization and trigger the release of both caspase-dependent (cytochrome c, Smac/Diablo, HtrA2) and caspase-independent (endoG, AIF) death pathways from the mitochondria into the cytosol. Cytochrome c to activate caspase-9 binds to APAF 1 and then leading to the formation of the so-called apoptosome which may induce the activation of caspase-3 (Adams and Cory 2007; Ola et al. 2011). We have also revealed the changes in Bcl-2 genes to make it more meaningful for the expression of apoptosis-related genes after treatments of H. scoparia extracts. Both increases in the pro-apoptotic Bcl-2 and HtrA2 genes and downregulations in the expression of anti-apoptotic genes in HeLa cells after Hex and Met-treatment suggested that occurs an induction through caspase dependent-mitochondrial pathway. In other words, extracts tend to induce apoptosis by activating pro-apoptotic genes in the intrinsic pathway following stimulation in the extrinsic pathway.
DNA fragmentation factors (DFF) which protect chromosome stability is another important indicator of apoptosis mechanism. DNA fragmentation factor (DFF) consists of two subunits: DFFA/DFF45/ICAD and DFFB/DFF40/CAD. DFFA is the substrate for caspase-3 and triggers DNA fragmentation during apoptosis. DFF becomes activated when DFFA is cleaved by caspase-3. After DFFA cleavage by caspase 3, DFFA dissociates from CAD and HMGB1 binds to CAD (High-mobility group protein) (Yan et al. 2006). When we examine the changes in genes after treatment of extracts in this pathway, decreases in ICAD gene along with increases in CAD and HMGB1 gene expression revealed that Hex and Met extracts in HeLa promoted apoptosis by increasing the expression of multiple intrinsic pathway genes after the extrinsic pathway. However, increases in both the Bcl-2/Bax ratio and CAD/HMGB1 genes after treatment may be associated with the tendency the escape from apoptosis via caspase depend-mitochondria of cells or the relevant for the survival of the cells. But increases in expressions of endoG released mitochondrial during apoptosis suggested that Chl extract could induce the apoptosis by the caspase-independent pathway. Additionally, Hex and Met extracts led to activation of TP53 and endoG genes. Briefly, Hex and Met extract provoked to the expression of pro-apoptotic genes via both caspase-dependent extrinsic/intrinsic pathways and caspase independent-pathways such as p53. This broad efficacy may be attributable to the phenolic content of Met (gallic acids) and Hex (caffeic acid, chlorogenic, p-coumaric acid, and quercetin) extract. Many studies on the inhibition mechanism of different cancer cell lines have revealed that gallic acid induces apoptosis via intrinsic pathway thoroughly Casp-3 (Ji et al. 2009) as well as NF-κB inhibition via TNF receptors (Choi et al. 2009). Furthermore, although the Hex and Chl extracts are similar in terms of their p-coumaric acid contents which has apoptotic effects (Shailasree et al. 2015), the triggering of apoptosis via different pathway suggested the synergistic effects of Hex extract contents and this inference supports the studies carried out in different cancer cells in the literature (Mertens-Talcott and Percival 2005).
When analyzed the effects of H. scoparia extracts in CaCo-2 and MCF-7 cancer cells compared to HeLa cells, pro-apoptotic genes in CaCo-2 cells had undergone minimal changes. Met and Hex extracts in CaCo-2 cells led to increases expression in a limited number of genes in extrinsic (Casp-6, FAS and TNFSF10 genes) and intrinsic pathway (Casp-9 and APAF1). But, upregulations in anti-apoptotic genes (TRAF1, DFFA, some BCL2, and BIRC2 members) and downregulations in pro-apoptotic genes (TNF family, FADD, mitochondrial genes, CAD, HMG1, and Casp-3) had been associated with the survival mechanism of the CaCo-2 cancer cells. H. scoparia extracts caused changes in the expression of apoptotic pathway genes of MCF-7 cells as well. In the extrinsic pathway, negative regulations in NF-kB (NF-kB1, REL, REL-A) genes and TRAF family genes, as well as an increase in the TNF gene, suggested that extracts were effective in sensitizing MCF-7 cells by TNFα-induced apoptosis via obstruction of NF-κB activation. At the same time, although Chl and Met extracts showed similar effects in the intrinsic pathway, Met extract-treated considered to make the MCF-7 cells more sensitive by affecting expressions of Bcl-2 family members in a wider spectrum. This phenomenon may be related to the structure of the phenolic content of Met. However, downregulations in Casp-3, Casp-9, and HtrA2 genes as well as upregulations in IAP family (BIRC2 and BIRC3) expression that is an inhibitor of apoptosis had been associated with the force-driven survival of MCF-7 cells after treatments of Chl and Met extracts. Lastly, Tumor-suppressor p53 (TP53) positively regulates the expression of the PTEN tumor-suppressor gene and it is thought to induce apoptosis via inactivation of the PI3 K/Akt cell survival pathway (Abraham and O’Neill 2014). In light of this information, Met and Hex extracts in CaCo-2 cells and Chl extract in MCF-7 cells caused inhibition of AKT signaling pathways by inducing TP53 and PTEN gene and consequently, they had triggered apoptosis.
In conclusion, results from the present study showed that all extracts of H. scoparia tended to positively regulate the apoptotic gene expression through different pathways following high inhibition effects on viability of cervix, breast, and colon cancer cells, especially on cervix cells. In particular, Met extracts rich in phenol/phenolic contents showed a significant cytotoxic/apoptotic activity on all cancer cells. However, differences in gene expressions after treatment of extracts were associated with changes in the post-translational modifications and the active contents soluble in the different solvent of extracts. In addition, the extracts showed an important radical scavenging activity in both antioxidant assays depending on the solvent used. In the context of these promising results, given that the extracts did not cause any irritation and toxicity in vivo, H. scoparia as a reliable natural compound has been shown to have potential to be used as a protective and preventive agent against human cancers via enhancing antioxidant mechanism or improving anticancer gene therapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Authors are appreciated to Assoc. Prof. Dr. Baykan, Ş., for her scientific contribution. The study was supported by TÜBİTAK-2211 Scholarship and Ege University BAP project (2013/FEN/015).
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Adem Güner, Phone: +90 454 310 4011, Email: ademeguner@gmail.com.
Ayşe Nalbantsoy, Email: ayse.nalbantsoy@ege.edu.tr.
Atakan Sukatar, Email: atakan.sukatar@ege.edu.tr.
Nefise Ülkü Karabay Yavaşoğlu, Email: ulku.karabay@ege.edu.tr.
References
- Abraham AG, O’Neill E. PI3 K/Akt-mediated regulation of p53 in cancer. Biochem Soc Trans. 2014;42:798–803. doi: 10.1042/BST20140070. [DOI] [PubMed] [Google Scholar]
- Adams JM, Cory S. Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Curr Opin Immunol. 2007;19:488–496. doi: 10.1016/j.coi.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- Barile FA. Principles of toxicology testing. New York: CRC Press; 2008. [Google Scholar]
- Blunt JW, Copp BR, Munro MH, Northcote PT, Prinsep MR. Marine natural product. Nat Prod Rep. 2010;27:165–237. doi: 10.1039/b906091j. [DOI] [PubMed] [Google Scholar]
- Cabioc’h J, Floc’h J, Le Toquin A. Guide mes algues des mers d’Europe. Paris: Delachaux et Niestle; 1992. [Google Scholar]
- Chakraborty K, Paulraj R. Sesquiterpenoids with free radical scavenging properties from marine macroalga Ulva fasciata Delile. Food Chem. 2010;122:31–41. doi: 10.1016/j.foodchem.2010.02.012. [DOI] [Google Scholar]
- Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–182. [Google Scholar]
- Cheng Z, Moore J, Yu L. High-throughput relative DPPH radical scavenging capacity assay. J Agric Food Chem. 2006;54:7429–7436. doi: 10.1021/jf0611668. [DOI] [PubMed] [Google Scholar]
- Choi KC, Lee YH, Jung MG, Kwon SH, Kim MJ, Jun WJ, Lee J, Lee JM, Yoon HG. Gallic acid suppresses lipopolysaccharide-induced nuclear factor-kappaB signaling by preventing rel A acetylation in A549 lung cancer cells. Mol Cancer Res. 2009;7:2011–2021. doi: 10.1158/1541-7786.MCR-09-0239. [DOI] [PubMed] [Google Scholar]
- Cornish ML, Garbary DJ. Antioxidants from macroalgae: potential applications in human health and nutrition. Algae. 2010;25:155–171. doi: 10.4490/algae.2010.25.4.155. [DOI] [Google Scholar]
- De Kok TM, Van Breda SG, Manson MM. Mechanisms of combined action of different chemopreventive dietary compounds: a review. Eur J Nutr. 2008;47:51–59. doi: 10.1007/s00394-008-2006-y. [DOI] [PubMed] [Google Scholar]
- Demirel Z, Yılmaz-Koz FF, Karabay-Yavasoglu NU, Ozdemır G, Sukatar A. Antimicrobial and antioxidant activities of solvent extracts and the essential oil composition of Laurencia obtusa and Laurencia obtusa var. pyramidata. Rom Biotechnol Lett. 2011;16:5927–5936. [Google Scholar]
- Derelanko MJ, Hollinger MA, editors. CRC handbook of toxicology. 2. Boca Raton: CRC Press; 1995. [Google Scholar]
- Ermakova S, Sokolova R, Kim SM, Um BH, Isakov V, Zvyagintseva T. Fucoidans from brown seaweeds Sargassum hornery, Eclonia cava, Costaria costata: structural characteristics and anticancer activity. Appl Biochem Biotechnol. 2011;164:841–850. doi: 10.1007/s12010-011-9178-2. [DOI] [PubMed] [Google Scholar]
- Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Güner A, Karabay Yavasoğlu NU. Evaluation of antioxidant, antimicrobial and antimutagenic activity with irritation effects of Ceramium rubrum (Red Algae) extract. Int J Second Metab. 2018;5:279–287. doi: 10.21448/ijsm.432654. [DOI] [Google Scholar]
- Güner A, Köksal Ç, Erel ŞB, Kayalar H, Nalbantsoy A, Sukatar A, Karabay Yavaşoğlu NÜ. Antimicrobial and antioxidant activities with acute toxicity, cytotoxicity and mutagenicity of Cystoseira compressa (Esper) Gerloff & Nizamuddin from the coast of Urla (Izmir, Turkey) Cytotechnology. 2015;67:135–143. doi: 10.1007/s10616-013-9668-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurib-Fakim A. Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol Aspects Med. 2006;27:1–93. doi: 10.1016/j.mam.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Holdt SL, Kraan S. Bioactive compounds in seaweed: functional food applications and legislation. J Appl Phycol. 2011;23:543–597. doi: 10.1007/s10811-010-9632-5. [DOI] [Google Scholar]
- Ji BC, Hsu WH, Yang JS, Hsia TC, Lu CC, Chiang JH, Yang JL, Lin CH, Lin JJ, Suen LJ, Gibson Wood W, Chung JG. Gallic acid induces apoptosis via caspase-3 and mitochondrion-dependent pathways in vitro and suppresses lung xenograft tumor growth in vivo. J Agric Food Chem. 2009;57:7596–7604. doi: 10.1021/jf901308p. [DOI] [PubMed] [Google Scholar]
- Jubri Z, Najib Narayanan NN, Norwahidah AK, Wan Ngah WZ. Antiproliferative activity and apoptosis induction by Gelam honey on liver cancer cell line. Int J Appl Sci Technol. 2012;2:4. [Google Scholar]
- Kaufmann SH, Hengartner MO. Programmed cell death: alive and well in the new millennium. Trends Cell Biol. 2001;11:526–534. doi: 10.1016/S0962-8924(01)02173-0. [DOI] [PubMed] [Google Scholar]
- Khan MR, Mlungwana SM. C-sitosterol, a cytotoxic sterol from Markhamia zanzibarica and Kigelia Africana. Fitoterapia. 1999;70:96–97. doi: 10.1016/S0367-326X(99)00005-2. [DOI] [Google Scholar]
- Khanavi M, Nabavi M, Sadati N, Ardekani MS, Sohrabipour J, Nabavi BM, Ghaeli P, Ostad NS. Cytotoxic activity of some marine brown algae against cancer cell lines. Biol Res. 2010;43:31–37. doi: 10.4067/S0716-97602010000100005. [DOI] [PubMed] [Google Scholar]
- Kim SK, Thomas NV, Li X. Anticancer compounds from marine macroalgae and their application as medical foods. Adv Food Nutr Res. 2011;64:213–224. doi: 10.1016/B978-0-12-387669-0.00016-8. [DOI] [PubMed] [Google Scholar]
- Kishore AS, Surekha PA, Sekhar PV, Srinivas A, Murthy PB. Hen egg chorioallantoic membrane bioassay: an in vitro alternative to Draize eye irritation test for pesticide screening. Int J Toxicol. 2008;27:449–453. doi: 10.1080/10915810802656996. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor super families: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/S0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Mann JR, Backlund MG, DuBois RN. Mechanisms of disease: inflammatory mediators and cancer prevention. Nat Clin Pract Oncol. 2005;2:202–210. doi: 10.1038/ncponc0140. [DOI] [PubMed] [Google Scholar]
- Martínez Andrade K, Lauritano C, Romano G, Ianora A. Marine microalgae with anti-cancer properties. Marine Drugs. 2018;16:165. doi: 10.3390/md16050165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–577. doi: 10.1016/j.foodchem.2004.10.006. [DOI] [Google Scholar]
- Mertens-Talcott SU, Percival SS. Ellagic acid and quercetin interact synergistically with resveratrol in the induction of apoptosis and cause transient cell cycle arrest in human leukemia cells. Cancer Lett. 2005;218:141–151. doi: 10.1016/j.canlet.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigam PS, Pandey A. Production of bioactive secondary metabolites: biotechnology for agro-industrial residues utilization. 1. Netherlands: Springer; 2009. pp. 129–145. [Google Scholar]
- Ola MS, Nawaz M, Ahsan H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem. 2011;351:41–58. doi: 10.1007/s11010-010-0709-x. [DOI] [PubMed] [Google Scholar]
- Park HK, Kim IH, Kim J, Nam TJ. Induction of apoptosis by laminarin, regulating the insulin-like growth factor-IR signaling pathways in HT-29 human colon cells. Int J Mol Med. 2012;30:734–738. doi: 10.3892/ijmm.2012.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L. A revıew of the nutrıent composıtıon of selected edıble seaweeds. In: Pomin VH, editor. Seaweed: ecology, nutrient composition and medicinal uses. Nova lorque: Nova Science Publishers Inc; 2011. pp. 15–47. [Google Scholar]
- Pereira L. Edible seaweeds of the world. Boca Raton: CRC Press; 2016. p. 52. [Google Scholar]
- Ramos S. Cancer chemoprevention and chemo- therapy: dietary polyphenols and signalling pathways. Mol Nutr Food Res. 2008;52:507–526. doi: 10.1002/mnfr.200700326. [DOI] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Salvesen GS, Dixit VM. Caspase activation: the induced-proximity model. Cell. 1997;91:443–446. doi: 10.1016/S0092-8674(00)80430-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvageau C. Remarques sur les Sphacélariacées. Bordeaux: Féret et fils; 1904. pp. 321–480. [Google Scholar]
- Senthilkumar K, Manivasagan P, Venkatesan J, Kim SK. Brown seaweed fucoidan: biological activity and apoptosis, growth signaling mechanism in cancer. Int J Biol Macromol. 2013;60:366–374. doi: 10.1016/j.ijbiomac.2013.06.030. [DOI] [PubMed] [Google Scholar]
- Shailasree S, Venkataramana M, Niranjana SR, Prakash HS. Cytotoxic effect of p-coumaric acid on neuroblastoma, N2a cell via generation of reactive oxygen species leading to dysfunction of mitochondria inducing apoptosis and autophagy. Mol Neurobiol. 2015;51:119–130. doi: 10.1007/s12035-014-8700-2. [DOI] [PubMed] [Google Scholar]
- Simmons TL, Andrianasolo E, McPhail K, Flatt PM, Gerwick WH. Marine natural products as anticancer drugs. Mol Cancer Ther. 2005;4:333–342. [PubMed] [Google Scholar]
- Thaipong K, Boonprakob U, Crosby K, Cisneros-Zevallos L, Hawkins D. Comparison of ABTS, DPHH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Compost Anal. 2006;19:669–675. doi: 10.1016/j.jfca.2006.01.003. [DOI] [Google Scholar]
- Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- Turkoğlu A, Kıvrak I, Mercan N, Duru ME, Gezer K, Turkoğlu H. Antioxidant and antimicrobial activities of Morchella conica Pers. African J Biotechnol. 2006;5:1146–1150. [Google Scholar]
- Wang LJ, Li D, Zou XD, Yong-Qiang Chen C. Antioxidative activity of Douchi (A Chinese traditional salt fermented soybean food) extracts during processing. Int J Food Properties. 2007;10:385–396. doi: 10.1080/10942910601052715. [DOI] [Google Scholar]
- Yalçın HT, Ozen MO, Gocmen B, Nalbantsoy A. Effect of ottoman viper (Montivipera xanthina (Gray, 1849)) venom on various cancer cells and on microorganisms. Cytotechnology. 2014;66:87–94. doi: 10.1007/s10616-013-9540-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B, Wang H, Wang H, Zhuo D, Li F, Kon T, Dewhirst M, Li CY. Apoptotic DNA fragmentation factor maintains chromosome stability in a P53-independent manner. Oncogene. 2006;25:5370–5376. doi: 10.1038/sj.onc.1209535. [DOI] [PubMed] [Google Scholar]
- Yusnawan E. The effectiveness of polar and non polar fractions of Ageratum conyzoıdes L. to control peanut rust dısease and phytochemıcal screenıngs of secondary metabolıtes. J Hpt Tropika. 2013;13:159–166. [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Zubia M, Fabre MS, Kerjean V, Deslandes E. Antioxidant and cytotoxic activities of some red algae (Rhodophyta) from Brittany coasts (France) Bot Mar. 2009;52:268–277. doi: 10.1515/BOT.2009.037. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.