Abstract
The surgical treatment of patients with advanced lung cancer remains controversial. The current study aimed to identify the factors affecting the prognosis of patients with stage IV non-small cell lung cancer (NSCLC) and to clarify the surgery guidelines. A total of 27,725 patients diagnosed with stage IV NSCLC were selected from the Surveillance, Epidemiology, and End Results program between 2010 and 2013. The sex, age, ethnicity, marital status, Tumor-Node-Metastasis stage, radiation therapy received and surgical status of each patient were recorded. Patients were followed up to November 2015. Survival rates were estimated by the Kaplan-Meier method. Single- and multi-factor analyses were performed using the log-rank test and multivariate Cox regression analysis respectively. In the isolated organ metastasis cohort, patients with liver metastasis alone had the worst prognosis, with a median overall survival (OS) of 4 months (liver metastasis vs. other organ metastases; P<0.001). Patients with lung metastasis only had the best prognosis, with a median OS of 8 months (lung metastasis vs. other organ metastases; P<0.001). Furthermore, patients with only one metastasis had the best prognosis, with a median OS of 6 months (single metastasis vs. multiple-organ metastases; P<0.001). The multivariate Cox regression analysis of the isolated-organ metastasis cohort and the multiple-organ metastases cohort revealed that patients who were ≤60 years, female, married, Asian, with N0 stage, had only bone metastasis, accepted wedge resection or lobectomy of the primary tumor, had surgical procedure to distant lymph node(s), and received beam radiation had an improved prognosis compared with the other patients. Age, sex, tumor type, ethnicity, N stage, number and type of metastatic lesions, surgical treatment of primary and metastatic lesions and radiation therapy are factors which influence the prognosis of patients with stage IV NSCLC. Furthermore, surgery may still benefit these patients.
Keywords: non-small cell lung cancer, prognosis, surgery, prognosis, Surveillance, Epidemiology, and End Results program
Introduction
Lung cancer is among the most common malignant tumors and has increasing rates of morbidity and mortality worldwide (1). It is estimated that ~234,000 new lung cancer cases were diagnosed in 2018 in the United States, and that ~154,000 people will succumb to lung cancer in this year (2). The majority of patients with lung cancer (85%) are diagnosed with non-small cell lung cancer (NSCLC), and >80% of these patients have different degrees of metastasis (3).
The most common sites for lung cancer metastasis are the nervous system, bone, liver, respiratory system and adrenal glands. Bone metastasis is the most common in patients with lung adenocarcinoma (39%) (4). The prognosis and survival rate of patients with advanced lung cancer are very poor, and the survival rate is not satisfactory. The median survival time of patients with stage IV NSCLC is 5 months (5). Patients with stage IV NSCLC with liver metastasis have the worst prognosis, <3 months (5).
With advances in cancer treatment, molecular targeted therapy and immunotherapy may provide alternatives to the conventional surgery, radiotherapy and chemotherapy. However, targeted therapy is not effective in people without epidermal growth factor receptor (EGFR) mutations (6). The emergence of drug resistance in tumor cells may lead to treatment failure in a patient population that is suitable for targeted therapy (7). In addition, both targeted therapy and immunotherapy may be economically unfeasible for patients with lung cancer (8,9). Accordingly, cost-effective treatment alternatives for patients with lung cancer are required.
The gold standard treatment for patients with NSCLC with distant metastasis is a multidisciplinary comprehensive treatment including chemoradiotherapy, immunotherapy, targeted therapy and immunotherapy rather than surgery. Furák et al (10) reported that the 5-year survival rate of patients with NSCLC that did not undergo surgical treatment was 5.8%. However, improvements in surgical techniques have improved the 5-year survival rate and median survival of patients with stage IV NSCLC (11–13). Therefore, surgical treatment in patients with stage IV NSCLC may be beneficial.
To date, there have been few large clinical retrospective studies on the surgical treatment of patients with stage IV NSCLC (11,12). Accordingly, the current study aimed to investigate whether surgical treatment may improve the outcome of patients with stage IV NSCLC, as well as to identify the factors which influence the prognosis of patients. Relevant cases were selected from the Surveillance, Epidemiology, and End Results (SEER) (https://seer.cancer.gov/) program for further analysis.
Materials and methods
Data collection
A total of 27,725 patients with stage IV NSCLC in the United States diagnosed between January 1, 2010 and December 31, 2013 with distinct metastatic sites in bone, brain, lung and liver and multiple metastases, that had received chemotherapy at least once, were selected from the SEER program. Patients included in this study were followed up between January 1, 2010 and November 31, 2016. Patients with incomplete or missing information were excluded. The distant metastatic lesions included only bone, brain, lung and liver. Other common sites, such as the pleura, adrenal gland and gastrointestinal tract were not included. The inclusion codes and criteria from the SEER database were are as follows: The primary tumor type was coded as lung (063), the coding of tumor pathological tissue classification was squamous cell neoplasms (02), and adenomas and adenocarcinomas (05). The following patient data were collected: i) Marital status; ii) ethnicity; iii) sex; iv) age at diagnosis; v) survival time (months); vi) overall survival (OS) and cancer-specific survival (CSS); vii) T stage; viii) N stage; ix) surgery of the primary site; x) surgery of the metastatic sites; xi) radiation therapy received; and xii) whether there was bone (not including the bone marrow), brain (not including the spinal cord or other parts of the central nervous system), lung (not including the pleura or pleural fluid) or liver metastasis. According to the SEER program definition, survival time means the time between diagnosis and death or the last follow-up time. OS is the time from the date of diagnosis to the death of any cause. CSS is the time from the date of diagnosis to the date of cancer-associated mortality. Surgery of the primary site describes a surgical procedure that removes and/or destroys tissue of the primary site performed as part of the therapy. Surgery of the metastatic sites describes the surgical removal of distant lymph node(s) or other tissue(s) or organ(s) beyond the primary site. According to the definition of the 7th Edition of the American Joint Committee on Cancer (AJCC) staging system (14), all the included patients with NSCLC were stage IV patients (T0-4N0-3M1), and the histopathological types included adenocarcinoma and squamous cell carcinoma. Patients with adenomas did not meet the above criteria and therefore were excluded from this study.
Statistical analysis
The χ2 test was used to compare the clinicopathological features of the patients included in the study and determine whether there were differences between different metastatic lesions. The Kaplan-Meier method was used to estimate the survival function, and the differences were evaluated with the log-rank test by pair comparison. Multivariate Cox regression analysis was conducted to assess the association of specific factors that impact overall survival (OS) and CSS. Additionally, the 95% confidence interval (CI) for all hazard ratio (HRs) estimates across all strata were calculated. P<0.05 was considered to indicate a statistically significant difference. All statistical operations were performed using SPSS software (version 22.0; IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
A total of 27,725 patients with NSCLC in the United States diagnosed from January 1, 2010 to December 31, 2013 were included in the current study. A total of 17,603 patients had one metastatic lesion while 10,122 patients had ≥2 metastatic lesions. The number of patients with only bone, brain, lung and liver metastases was 5,989, 4,255, 5,717 and 1,642, respectively. The number of patients with two, three and four metastatic lesions was 7,275, 2,389 and 458 respectively. The mean age of the patients was 67.51 years, with a median of 68 years (range, 13–102 years). A total of 737, 1,761 and 146 patients received surgical intervention for their primary lesion only, metastatic lesion only and both primary and secondary lesions, respectively. However, the specific surgical intervention for each patient was not recorded. The basic information of the patients is presented in Table I.
Table I.
Clinicopathological characteristics of patients with metastatic non-small cell lung cancer.
| Variable | Bone metastasis | Brain metastasis | Liver metastasis | Lung metastasis | Multiple metastasis | χ2 value | P-value |
|---|---|---|---|---|---|---|---|
| Age at diagnosis | |||||||
| ≤60 | 1,483 | 1,493 | 335 | 1,101 | 3,087 | 5,842.255 | <0.001 |
| >60 | 4,506 | 2,762 | 1,307 | 4,616 | 7,035 | ||
| Sex | |||||||
| Female | 2,417 | 2,021 | 692 | 2,684 | 4,517 | 3,38.394 | <0.001 |
| Male | 3,572 | 2,234 | 950 | 3,033 | 5,605 | ||
| Tumor type | |||||||
| Squamous cell | 1,569 | 838 | 603 | 1,958 | 1,934 | 6,989.873 | <0.001 |
| carcinomas | |||||||
| Adenocarcinoma | 4,420 | 3,417 | 1,039 | 3,759 | 8,188 | ||
| Marital status | |||||||
| Unmarried | 2,643 | 2,042 | 783 | 2,820 | 4,462 | 178.562 | <0.001 |
| Married | 3,346 | 2,231 | 859 | 2,897 | 5,660 | ||
| Ethnicity | |||||||
| Caucasian | 4,879 | 3,391 | 1,338 | 4,513 | 7,860 | 44,453.648 | <0.001 |
| African-American | 716 | 573 | 214 | 759 | 1,284 | ||
| Asian | 387 | 282 | 83 | 432 | 948 | ||
| Australoid | 7 | 9 | 7 | 13 | 30 | ||
| T stage | |||||||
| 0 | 72 | 66 | 28 | 9 | 50 | 11,588.136 | <0.001 |
| 1 | 1,068 | 780 | 245 | 241 | 782 | ||
| 2 | 1,871 | 1,452 | 490 | 665 | 1,808 | ||
| 3 | 1,441 | 996 | 420 | 1,687 | 2,962 | ||
| 4 | 1,537 | 991 | 459 | 3,115 | 4,520 | ||
| N stage | |||||||
| 0 | 1,637 | 1,253 | 475 | 1,646 | 1,827 | 8,358.724 | <0.001 |
| 1 | 616 | 424 | 145 | 349 | 764 | ||
| 2 | 2,755 | 1,931 | 778 | 2,351 | 5,036 | ||
| 3 | 984 | 647 | 244 | 1,371 | 2,495 | ||
| Surgery of the primary site | |||||||
| No | 5,847 | 4,006 | 1,596 | 5,403 | 9,990 | 76,277.022 | <0.001 |
| Wedge resection | 75 | 84 | 27 | 200 | 105 | ||
| Lobectomy | 59 | 156 | 18 | 102 | 24 | ||
| Pneumonectomy | 8 | 9 | 1 | 12 | 3 | ||
| Surgery of the metastases | |||||||
| No | 5,695 | 3,385 | 1,612 | 5,610 | 9,516 | 92,989.834 | P<0.001 |
| Surgical procedure to other regional sites | 25 | 16 | 6 | 27 | 26 | ||
| Surgical procedure to distant lymph node(s) | 24 | 26 | 3 | 20 | 51 | ||
| Surgical procedure to distant site | 241 | 827 | 21 | 56 | 519 | ||
| Combination of all the above | 4 | 1 | 0 | 4 | 10 | ||
| Radiation therapy | |||||||
| No | 2,755 | 775 | 1,351 | 4,346 | 4,303 | 41,417.869 | P<0.001 |
| Beam radiation | 3,226 | 3,476 | 289 | 1,362 | 5,801 | ||
| Radioactive implants | 3 | 3 | 0 | 4 | 3 | ||
| Radioisotopes | 2 | 0 | 1 | 1 | 6 | ||
| Combination of 2 or | 3 | 1 | 1 | 4 | 9 | ||
3 above
Survival outcomes
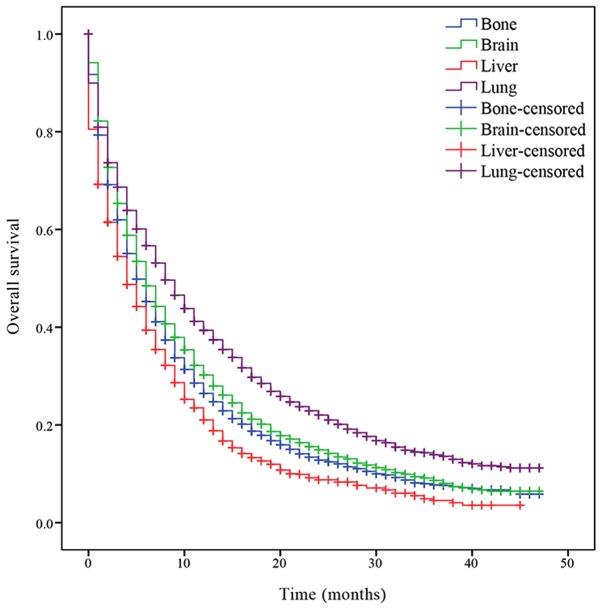
Survival analysis was performed to determine the OS of patients with the different isolated metastatic lesions. The median OS of patients with NSCLC with bone, brain, liver and lung metastases was 5, 6, 4 and 8 months, respectively. Patients with lung metastasis had an increased prognosis compared with the other patients (P<0.001; Fig. 1), while patients with liver metastasis had a decreased prognosis (P<0.001; Fig. 1). Significant differences of median OS time between patients with different organ metastasis were indicated.
Figure 1.
Kaplan-Meier curve of overall survival based on the site of isolated organ metastases. P<0.001 bone metastasis vs. brain metastasis; bone metastasis vs. lung metastasis; bone metastasis vs. liver metastasis; brain metastasis vs. lung metastasis; brain metastasis vs. liver metastasis and lung metastasis vs. liver metastasis.
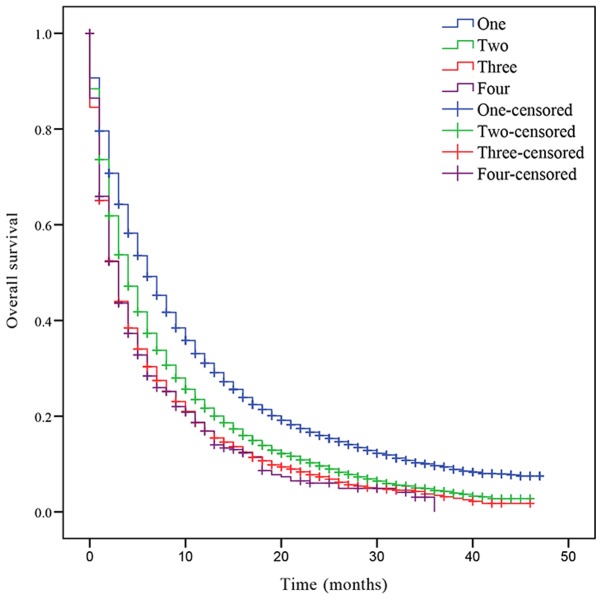
In addition, the OS was also assessed based on the number of metastatic lesions. The median OS of patients with NSCLC with one, two, three or four metastatic sites was 6, 4, 3 and 3 months, respectively. Patients with one metastatic lesion had a significant increased prognosis compared with patients with >1 metastatic lesion (Fig. 2). Patients with two metastases had a significantly improved prognosis compared with patients with three and four metastases (two sites vs. three sites, P<0.001; two sites vs. four sites, P<0.001; Fig. 2). However, there was no statistically significant difference in OS between patients with three and four metastatic lesions (P=0.721; Fig. 2).
Figure 2.
Kaplan-Meier curve of overall survival based on the number of metastatic organs. P<0.001 one site vs. two sites; one site vs. three sites; one site vs. four sites; two sites vs. three sites and two sites vs. four sites. P=0.721 three sites vs. four sites.
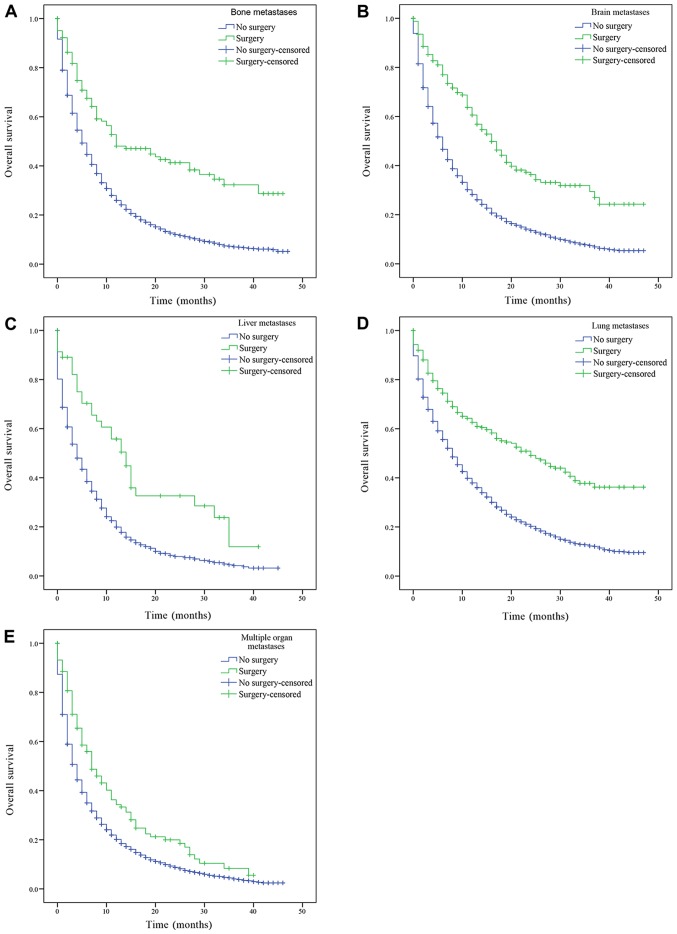
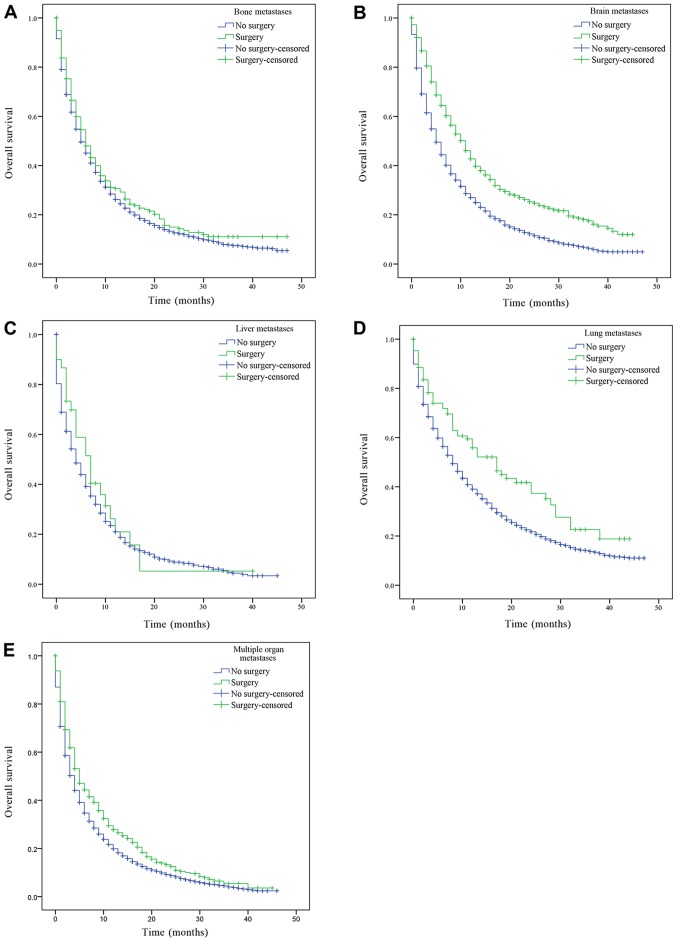
The patients with only bone, brain, liver, lung metastasis and patients with multiple metastases were divided into groups according to whether the primary or metastatic lesions were treated by surgery, and their OS was subsequently estimated. Patients with bone, brain, liver, lung metastasis and multiple metastatic lesions had a significantly increased prognosis following surgery on the primary lesions compared with patients who had not received surgery (bone, brain, liver, lung metastasis and multiple organ metastases, P<0.001; Fig. 3). Similarly, patients with only bone, brain, liver, lung metastasis and multiple metastatic lesions who received surgery on distant lesions had an improved OS compared with patients who had not received surgery (bone metastasis, P=0.043; lung metastasis, P=0.001; brain and multiple organ metastases, P<0.001; Fig. 4). There was a statistically significant difference in OS between patients who had underwent surgery and those who had not received surgery. However, there was no statistically significant difference in the median OS of patients with liver metastasis who had received surgery compared with patients who had not (P=0.388; Fig. 4C).
Figure 3.
Kaplan-Meier curve of overall survival based on whether surgery of the primary tumor was performed. (A) Patients with isolated bone metastasis. (B) Patients with isolated brain metastasis. (C) Patients with isolated liver metastasis. (D) Patients with isolated lung metastasis. (E) Patients with multiple organ metastases. P<0.001 surgery vs. no surgery.
Figure 4.
Kaplan-Meier curve of overall survival based on whether surgery of the metastatic lesions was performed. (A) Patients with isolated bone metastasis. P=0.043 surgery vs. no surgery. (B) Patients with isolated brain metastasis. P<0.001 surgery vs. no surgery. (C) Patients with isolated liver metastasis P=0.388 surgery vs. no surgery. (D) Patients with isolated lung metastasis. P=0.001 surgery vs. no surgery. (E) Patients with multiple organ metastases. P<0.001 surgery vs. no surgery.
Multivariate Cox proportional hazard models were used to determine the prognostic factors of patients with NSCLC with single and multiple organ metastases. The analysis in patients with single organ metastases revealed that patients with the following characteristics: i) Age (≤60); ii) sex (female); iii) tumor type (adenocarcinoma); iv) marital status (married); v) ethnicity (Asian); vi) N0 stage; vii) received surgery of the primary tumor (wedge resection and lobectomy) and metastatic lesion [distant tissue(s) or organ(s)]; and viii) received beam radiation therapy had improved OS and CSS compared with other patients (Table II). Using bone metastasis as a reference, patients with brain and liver metastases had a decreased OS (brain, HR, 1.162, 95% CI, 1.106–1.220; liver, HR, 1.081, 95% CI, 1.015–1.151), while patients with lung metastases had an improved OS (HR, 0.636; 95% CI, 0.607–0.667). The analysis in overall metastatic patient cohort revealed that patients with the following characteristics: i) Age (≤60); ii) sex (female); iii) tumor type (adenocarcinoma); iv) marital status (married); v) ethnicity (Asian); vi) N0 stage; vii) one metastatic lesion; viii) received surgery of the primary (wedge resection and lobectomy) and metastatic lesion (distant tissue(s) or organ(s)); and ix) received beam radiation therapy had improved OS and CSS (Table III). Using single metastatic organ as a reference, patients with two, three and four metastases had a decreased OS (two metastases, HR, 1.336, 95% CI, 1.294–1.379; three metastases, HR, 1.649, 95% CI, 1.571–1.732; four metastases, HR, 1.787, 95% CI, 1.613–1.980) and CSS (two metastases, HR, 1.322, 95% CI, 1.275–1.372; three metastases, HR, 1.628, 95% CI, 1.541–1.721; four metastases, HR, 1.805, 95% CI, 1.613–2.019).
Table II.
Multivariate Cox regression analysis for OS and CSS in patients with a single metastatic site.
| OS | CSS | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Age at diagnosis | ||||
| ≤60 | 1.00 (reference) | 1.00 (reference) | ||
| >60 | 1.017 (1.015–1.019) | <0.001 | 1.017 (1.015–1.019) | <0.001 |
| Sex | ||||
| Female | 1.00 (reference) | 1.00 (reference) | ||
| Male | 1.291 (1.244–1.338) | <0.001 | 1.275 (1.222–1.330) | <0.001 |
| Tumor type | ||||
| Squamous cell | 1.00 (reference) | 1.00 (reference) | ||
| carcinomas | ||||
| Adenocarcinoma | 0.930 (0.918–0.942) | <0.001 | 0.921 (0.907–0.935) | <0.001 |
| Marital status | ||||
| Unmarried | 1.00 (reference) | 1.00 (reference) | ||
| Married | 0.841 (0.812–0.872) | <0.001 | 0.831 (0.797–0.866) | <0.001 |
| Ethnicity | ||||
| Caucasian | 1.00 (reference) | 1.00 (reference) | ||
| African-American | 1.000 (0.949–1.053) | 0.991 | 1.022 (0.963–1.085) | 0.471 |
| Asian | 0.674 (0.625–0.728) | <0.001 | 0.669 (0.614–0.730) | <0.001 |
| Australoid | 0.717 (0.457–1.125) | 0.148 | 0.709 (0.427–1.178) | 0.185 |
| T stage | ||||
| 0 | 1.00 (reference) | 1.00 (reference) | ||
| 1 | 0.781 (0.654–0.932) | 0.006 | 0.845 (0.689–1.035) | 0.104 |
| 2 | 0.888 (0.746–1.057) | 0.181 | 0.954 (0.781–1.165) | 0.642 |
| 3 | 1.008 (0.847–1.200) | 0.931 | 1.090 (0.893–1.332) | 0.396 |
| 4 | 1.042 (0.861–1.219) | 0.787 | 1.119 (0.917–1.366) | 0.268 |
| N stage | ||||
| 0 | 1.00 (reference) | 1.00 (reference) | ||
| 1 | 1.134 (1.059–1.214) | <0.001 | 1.114 (1.029–1.207) | 0.008 |
| 2 | 1.276 (1.222–1.332) | <0.001 | 1.237 (1.176–1.302) | <0.001 |
| 3 | 1.340 (1.271–1.413) | <0.001 | 1.314 (1.235–1.398) | <0.001 |
| Surgery of the primary | ||||
| No | 1.00 (reference) | 1.00 (reference) | ||
| Wedge resection | 0.718 (0.630–0.819) | <0.001 | 0.687 (0.586–0.805) | <0.001 |
| Lobectomy | 0.361 (0.305–0.428) | <0.001 | 0.346 (0.281–0.426) | <0.001 |
| Pneumonectomy | 0.791 (0.525–1.191) | 0.262 | 0.840 (0.535–1.319) | 0.449 |
| Surgery of the metastases | ||||
| No | 1.00 (reference) | 1.00 (reference) | ||
| Surgical procedure to other regional sites | 0.843 (0.634–1.120) | 0.237 | 0.786 (0.572–1.082) | 0.140 |
| Surgical procedure to distant lymph node(s) | 0.806 (0.605–1.075) | 0.142 | 0.796 (0.571–1.112) | 0.181 |
| Surgical procedure to distant site | 0.778 (0.720–0.841) | <0.001 | 0.768 (0.704–0.838) | <0.001 |
| Combination of all the above | 1.402 (0.629–3.126) | 0.409 | 1.419 (0.636–3.166) | 0.393 |
| Radiation therapy | ||||
| No | 1.00 (reference) | 1.00 (reference) | ||
| Beam radiation | 0.808 (0.776–0.841) | <0.001 | 0.823 (0.786–0.863) | <0.001 |
| Radioactive implants | 1.616 (0.769–3.396) | 0.205 | 1.315 (0.423–4.803) | 0.636 |
| Radioisotopes | 1.317 (0.494–3.512) | 0.582 | 1.264 (0.407–3.923) | 0.685 |
| Combination of 2 or 3 above | 0.958 (0.430–2.135) | 0.916 | 0.987 (0.410–2.377) | 0.976 |
| Metastatic site | ||||
| Bone only | 1.00 (reference) | 1.00 (reference) | ||
| Brain only | 1.162 (1.106–1.220) | <0.001 | 1.131 (1.070–1.197) | <0.001 |
| Liver only | 1.081 (1.015–1.151) | 0.016 | 1.075 (0.999–1.157) | 0.053 |
| Lung only | 0.636 (0.607–0.667) | <0.001 | 0.635 (0.600–0.671) | <0.001 |
OS, overall survival; CSS, cancer-specific survival; HR, hazard ratio; CI, confidence interval.
Table III.
Multivariate Cox regression analysis for OS and CSS in overall metastatic patient cohort.
| OS | CSS | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Age at diagnosis | ||||
| ≤60 | 1.00 (reference) | 1.00 (reference) | ||
| >60 | 1.269 (1.229–1.310) | <0.001 | 1.275 (1.231–1.321) | <0.001 |
| Sex | ||||
| Female | 1.00 (reference) | 1.00 (reference) | ||
| Male | 1.287 (1.251–1.324) | <0.001 | 1.281 (1.240–1.323) | <0.001 |
| Tumor types | ||||
| Squamous cell carcinomas | 1.00 (reference) | 1.00 (reference) | ||
| Adenocarcinoma | 0.930 (0.920–0.940) | <0.001 | 0.920 (0.909–0.931) | <0.001 |
| Marital status | ||||
| Unmarried | 1.00 (reference) | 1.00 (reference) | ||
| Married | 0.834 (0.811–0.857) | <0.001 | 0.823 (0.797–0.849) | <0.001 |
| Ethnicity | ||||
| Caucasian | 1.00 (reference) | 1.00 (reference) | ||
| African-American | 0.964 (0.825–1.004) | 0.074 | 0.981 (0.937–1.028) | 0.421 |
| Asian | 0.658 (0.622–0.696) | <0.001 | 0.655 (0.616–0.697) | <0.001 |
| Australoid | 0.745 (0.544–1.021) | 0.067 | 0.721 (0.507–1.026) | 0.069 |
| T stage | ||||
| 0 | 1.00 (reference) | 1.00 (reference) | ||
| 1 | 0.756 (0.649–0.882) | <0.001 | 0.831 (0.697–0.990) | 0.039 |
| 2 | 0.850 (0.732–0.988) | 0.034 | 0.928 (0.781–1.102) | 0.393 |
| 3 | 0.874 (0.753–1.016) | 0.079 | 0.964 (0.812–1.145) | 0.675 |
| 4 | 0.842 (0.725–0.978) | 0.024 | 0.933 (0.787–1.107) | 0.430 |
| N stage | ||||
| 0 | 1.00 (reference) | 1.00 (reference) | ||
| 1 | 1.129 (1.069–1.193) | <0.001 | 1.122 (1.052–1.196) | <0.001 |
| 2 | 1.231 (1.188–1.274) | <0.001 | 1.190 (1.143–1.240) | <0.001 |
| 3 | 1.194 (1.145–1.245) | <0.001 | 1.175 (1.120–1.234) | <0.001 |
| Surgery of the primary | ||||
| No | 1.00 (reference) | 1.00 (reference) | ||
| Wedge resection | 0.670 (0.598–0.750) | <0.001 | 0.670 (0.587–0.765) | <0.001 |
| Lobectomy | 0.362 (0.308–0.424) | <0.001 | 0.349 (0.287–0.425) | <0.001 |
| Pneumonectomy | 0.720 (0.486–1.066) | 0.101 | 0.774 (0.504–1.188) | 0.242 |
| Surgery of the metastases | ||||
| No | 1.00 (reference) | 1.00 (reference) | ||
| Surgical procedure to other regional sites | 0.857 (0.676–1.086) | 0.202 | 0.836 (0.638–1.096) | 0.194 |
| Surgical procedure to distant lymph node(s) | 0.883 (0.713–1.093) | 0.252 | 0.875 (0.686–1.118) | 0.286 |
| Surgical procedure to distant site | 0.852 (0.802–0.906) | <0.001 | 0.829 (0.775–0.888) | <0.001 |
| Combination of all the above | 1.021 (0.604–1.726) | 0.938 | 0.984 (0.570–1.697) | 0.952 |
| Radiation therapy | ||||
| No | 1.00 (reference) | 1.00 (reference) | ||
| Beam radiation | 0.881 (0.856–0.906) | <0.001 | 0.892 (0.864–0.921) | <0.001 |
| Radioactive implants | 1.498 (0.805–2.786) | 0.202 | 1.302 (0.585–2.901) | 0.518 |
| Radioisotopes | 0.646 (0.308–1.335) | 0.248 | 0.613 (0.255–1.475) | 0.275 |
| Combination of 2 or 3 above | 0.821 (0.454–1.484) | 0.513 | 1.051 (0.565–1.958) | 0.874 |
| Number of metastatic sites | ||||
| Single | 1.00 (reference) | 1.00 (reference) | ||
| Double | 1.336 (1.294–1.379) | <0.001 | 1.322 (1.275–1.372) | <0.001 |
| Triple | 1.649 (1.571–1.732) | <0.001 | 1.628 (1.541–1.721) | <0.001 |
| Four | 1.787 (1.613–1.980) | <0.001 | 1.805 (1.613–2.019) | <0.001 |
OS, overall survival; CSS, cancer-specific survival; HR, hazard ratio; CI, confidence interval.
Discussion
Improvements in lung cancer diagnosis and treatment have increased the 5-year survival rate and median survival time of patients with stage IV NSCLC (15). In recent years, an increased understanding of the genetic changes involved in lung cancer has led to molecular targeted therapy (6). Additionally, surgical techniques are also rapidly evolving (16). The emergence of radiofrequency ablation (17) and endoscopy (18) has contributed to the improved safety of surgical procedures. Individualized treatment plans may reduce the occurrence of adverse events and improve the quality of life of the patients (19).
The current study involved a retrospective analysis of a large population of patients with stage IV NSCLC selected from the SEER program. Compared with patients with other organ metastases, patients with lung metastasis had the longest OS, and patients with single organ metastasis had an increased OS compared with patients with multiple organ metastases. This suggested that the type and number of metastatic organs may affect the prognosis of patients with stage IV NSCLC. This is similar to previously published studies investigating bladder and colorectal cancer (20,21). Furthermore, the current study established that surgical resection of the primary and metastatic organs may significantly improve the prognosis of patients with stage IV NSCLC.
The current study demonstrated that patients with only lung metastases had the best prognosis, patients with only brain metastases had a slightly improved prognosis compared with patients with only bone metastases, while those with liver metastases had the worst prognosis. Patients with only one metastasis had an improved prognosis compared with patients with multiple metastases. Previous studies have revealed similar results; patients with NSCLC and SCLC with liver metastasis and multiple metastases have the worst prognosis (22–25). Similar results were obtained using the AJCC staging system, where the number of metastatic organs had an effect on the prognosis of patients (26). Notably, the effect of the number of metastases is not same for different types of cancer, the OS of patients with pancreatic cancer is not affected by either single or multiple organ metastases (27). Pancreatic cancer is characterized by rapid growth, abundant pancreatic blood and lymphatic vessels, and incomplete pancreatic capsule. Therefore, the time of distant metastasis is relatively early, so whether there is distant metastasis or not, has little impact on OS (28).
Multivariate Cox regression analysis revealed that the prognosis of patients who underwent surgery for primary and metastatic lesions was better compared with patients who did not undergo the above. Surgical treatment remains the main approach used for the treatment of the majority of malignant tumors (16). Previously published studies revealed that certain patients with advanced NSCLC with unilateral contralateral lung metastasis, single brain, bone or adrenal metastasis may be treated surgically (29–31). For patients with NSCLC with isolated metastases and resectable pulmonary lesions, resection of the metastatic organs may also be considered. However, how isolated liver metastases should be removed remains unclear (32–34). Previous studies demonstrated that surgery serves an important role in the treatment of liver metastasis of neuroendocrine carcinoma and colorectal cancer, but not in lung cancer (32,35). With advances in liver resection and the continuous improvement of surgical safety, previous case reports described surgical resection of liver metastatic carcinoma with satisfactory results (33,34).
The benefit of surgical treatment on the prognosis of patients with advanced lung cancer remains controversial (29–32). A previous study based on SEER program analysis suggested that no further surgical treatment is recommended for patients with advanced lung cancer (36). However, additional studies do not concur with this recommendation (37). Patients with stage IV NSCLC who received pneumonectomy and thoracic wall enlargement resection had an improved quality of life and 5-year survival (38). However, this is contrary to what was observed in the current study. Results from a previous study suggested that the long-term survival rate of patients is related to the degree of tumor infiltration into the chest wall, and thus the scope of resection should be determined according to the degree of infiltration (39). Using the SEER program, previous studies have revealed that the size of the lung cancer lesions should guide the choice of surgical intervention and expanding the scope of surgical resection will not improve prognosis (40). A previous study revealed that lymph node dissection for distant metastatic lesions is necessary to improve the prognosis (41). Taken together, the results from the aforementioned studies suggest that it is important to identify specific patients who may benefit from surgical procedures.
Radiotherapy is widely used for patients with advanced lung cancer (42). A previous study revealed that surgery following radiotherapy may be beneficial to patients (43). The most commonly employed method of radiotherapy is beam radiation (44), which was consistent with the results obtained in the current study. However, previous studies reported that radioactive implants and radioisotopes may offer promising results for patients with advanced NSCLC (45,46).
Multivariate Cox regression analysis revealed that age, sex, marital status, ethnicity, N stage and tumor type affected the prognosis of patients with NSCLC in the current study. Patients >60 years had an improved prognosis compared with other patients. Toffart et al (47) revealed that patients with NSCLC >63 years had significantly decreased OS compared with other younger patients using a multivariate cox analysis (HR=1.63; 95% CI: 1.013–2.63; P=0.04). The current study demonstrated that the prognosis of female patients was improved compared with male patient. This may be attributed to different hormone and corresponding receptor expression levels (48). In terms of marital status, previous large epidemiological studies revealed that marriage benefits patients with less aggressive cancer (49,50), which is consistent with the results obtained in the current study. The effect of ethnicity on the prognosis of patients with NSCLC patients remains controversial (51–54). A previous study revealed that African-Americans with lung cancer had a decreased 5-year survival rate compared with Caucasians (51). Similar survival rates for African-Americans and Caucasians have been reported for patients with lung cancer (52,53). Tannenbaum et al (54) reported that Asian patients with NSCLC had significantly increased survival rates compared with Caucasian patients, which is consistent with the results obtained in the current study.
The results obtained in the current study suggested that there was no statistical difference in the prognosis of patients with different T stages. This is not in accordance with the AJCC staging system. However, the patients selected in the current study all had stage IV NSCLC, according to the 7 Edition of the AJCC staging system with only four sites of metastases identified, and may not conform with the principles of the staging system, due to a limited representative sample. The current study revealed that the N stage influenced the prognosis of patients with stage IV NSCLC. This was consistent with a previous study which suggested that lymph node metastasis is an adverse prognostic factor for the surgical treatment of patients with advanced NSCLC (55). The aforementioned study recommended that patients with N0 stage should be eligible for surgical treatment and that surgery for patients with extensive lymph node metastases may not be beneficial (55). There are few studies investigating the prognosis of patients with stage IV NSCLC with adenocarcinoma and squamous carcinoma (56,57). A retrospective study of 148 Chinese patients with NSCLC revealed that non-lung adenocarcinoma was a prognostic risk factor in patients with NSCLC (57), consistent with the results obtained in the current study.
The present study had certain limitations. Firstly, due to the retrospective nature of the study, confounding factors, such as smoking history and age, were not easily excluded. Secondly, the specific chemotherapy regimens and radiation doses were not detailed in the SEER program, and these may have had an impact on the prognosis of the patients (58). Thirdly, the SEER program did not include data on whether the patients were treated with tyrosine kinase inhibitors, due to EGFR mutations being more prevalent in non-smoking, female, Asian patients (59). The OS would be affected if they were treated with tyrosine kinase inhibitors (6), influencing our conclusions. Finally, more distal metastases, such as in the adrenal gland and gastrointestinal tract, cannot be included without relevant data, and at the same time, the sequence of metastatic lesions cannot be determined. The results obtained in the current study require further examination by future well-designed studies to validate this study's results.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in the Surveillance, Epidemiology, and End Results (SEER) repository (https://seer.cancer.gov/).
Authors' contributions
YL designed the study, work that led to the submission, acquired data and played an important role in interpreting the results. XF and XW analyzed the data and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The SEER database does not include any human or demographic identifying information, and the data used for analysis were de-identified. Therefore, ethics approval and formal informed consent to participate was not required.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 3.Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, et al. Bethesda, MD: 2018. [Sep 10;2018 ]. SEER Cancer Statistics Review, 1975–2015, National Cancer Institute. [Google Scholar]
- 4.Duan L, Pang HL, Chen WJ, Shen WW, Cao PP, Wang SM, Liu LL, Zhang HL. The role of GDF15 in bone metastasis of lung adenocarcinoma cells. Oncol Rep. 2019;41:2379–2388. doi: 10.3892/or.2019.7024. [DOI] [PubMed] [Google Scholar]
- 5.Riihimäki M, Hemminki A, Fallah M, Thomsen H, Sundquist K, Sundquist J, Hemminki K. Metastatic sites and survival in lung cancer. Lung Cancer. 2014;86:78–84. doi: 10.1016/j.lungcan.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Xie S, He B. Effect of EGFR gene polymorphism on efficacy of chemotherapy combined with targeted therapy for non-small cell lung cancer in Chinese patients. Am J Cancer Res. 2019;9:619–627. [PMC free article] [PubMed] [Google Scholar]
- 7.Sosa Iglesias V, Giuranno L, Dubois LJ, Theys J, Vooijs M. Drug resistance in non-small cell lung cancer: A potential for NOTCH Targeting? Front Oncol. 2018;8:267. doi: 10.3389/fonc.2018.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kogure Y. Cost effectiveness and health economics of immune checkpoint inhibitors for non-small cell lung cancer. Gan To Kagaku Ryoho. 2018;45:781–784. (In Japanese) [PubMed] [Google Scholar]
- 9.Skinner KE, Fernandes AW, Walker MS Pavilack M, VanderWalde A. Healthcare costs in patients with advanced non-small cell lung cancer and disease progression during targeted therapy: A real-world observational study. J Med Econ. 2018;21:192–200. doi: 10.1080/13696998.2017.1389744. [DOI] [PubMed] [Google Scholar]
- 10.Furák J, Troján I, Szöke T, Agócs L, Csekeö A, Kas J, Svastics E, Eller J, Tiszlavicz L. Lung cancer and its operable brain metastasis: Survival rate and staging problems. Ann Thorac Surg. 2005;79:241–247. doi: 10.1016/j.athoracsur.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 11.Hanagiri T, Takenaka M, Oka S, Shigematsu Y, Nagata Y, Shimokawa H, Uramoto H, Tanaka F. Results of a surgical resection for patients with stage IV non-small-cell lung cancer. Clin Lung Cancer. 2012;13:220–224. doi: 10.1016/j.cllc.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Congedo MT, Cesario A, Lococo F, De Waure C, Apolone G, Meacci E, Cavuto S, Granone P. Surgery for oligometastatic non-small cell lung cancer: Long-term results from a single center experience. J Thorac Cardiovasc Surg. 2012;144:444–452. doi: 10.1016/j.jtcvs.2012.05.051. [DOI] [PubMed] [Google Scholar]
- 13.Tieqin L, Hongxu L, Yu L, Shun X, Chunlu Y. Influencing factors of survival rate on 44 cases of postoperative stage IV non-small-celllung cancer. Chin J Ciinicians (Eiectronic Ed) 2013;7:605–608. [Google Scholar]
- 14.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. 7th. Springer; New York, NY: 2009. AJCC Cancer Staging Manual. [Google Scholar]
- 15.Delea T, Langer C, McKiernan J, Liss M, Edelsberg J, Brandman J, Sung J, Raut M, Oster G. The cost of treatment of skeletal-related events in patients with bone metastases from lung cancer. Oncology. 2004;67:390–396. doi: 10.1159/000082923. [DOI] [PubMed] [Google Scholar]
- 16.Hofferberth SC, Grinstaff MW, Colson YL. Nanotechnology applications in thoracic surgery. Eur J Cardiothorac Surg. 2016;50:6–16. doi: 10.1093/ejcts/ezw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaughn C, Mychaskiw G, II, Sewell P. Massive hemorrhage during radiofrequency ablation of a pulmonary neoplasm. Anesth Analg. 2002;94:1149–1151. doi: 10.1097/00000539-200205000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Tutar N, Yurci A, Güneş I, Gülmez İ, Gürsoy Ş, Önal Ö, Canöz Ö. The role of endobronchial and endoscopic ultrasound guided fine needle aspiration for mediastinal nodal staging of non-small-cell lung cancer. Tuberk Toraks. 2018;66:85–92. doi: 10.5578/tt.66866. [DOI] [PubMed] [Google Scholar]
- 19.Reck M, Rabe KF. Precision diagnosis and treatment for advanced non-small-cell lung cancer. N Engl J Med. 2017;377:849–861. doi: 10.1056/NEJMra1703413. [DOI] [PubMed] [Google Scholar]
- 20.Dong F, Shen Y, Gao F, Xu T, Wang X, Zhang X, Zhong S, Zhang M, Chen S, Shen Z. Prognostic value of site-specific metastases and therapeutic roles of surgery for patients with metastatic bladder cancer: A population-based study. Cancer Manag Res. 2017;9:611–626. doi: 10.2147/CMAR.S148856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo D, Liu Q, Yu W, Ma Y, Zhu J, Lian P, Cai S, Li Q, Li X. Prognostic value of distant metastasis sites and surgery in stage IV colorectal cancer: A population-based study. Int J Colorectal Dis. 2018;33:1241–1249. doi: 10.1007/s00384-018-3091-x. [DOI] [PubMed] [Google Scholar]
- 22.Tamura T, Kurishima K, Nakazawa K, Kagohashi K, Ishikawa H, Satoh H, Hizawa N. Specific organ metastases and survival in metastatic non-small-cell lung cancer. Mol Clin Oncol. 2015;3:217–221. doi: 10.3892/mco.2014.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakazawa K, Kurishima K, Tamura T, Kagohashi K, Ishikawa H, Satoh H, Hizawa N. Specific organ metastases and survival in small cell lung cancer. Oncol Lett. 2012;4:617–620. doi: 10.3892/ol.2012.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang J, Zhang Y, Sun X, Gusdon AM, Song N, Chen L, Jiang G, Huang Y. The prognostic value of multiorgan metastases in patients with non-small cell lung cancer and its variants: A SEER-based study. J Cancer Res Clin Oncol. 2018;144:1835–1842. doi: 10.1007/s00432-018-2702-9. [DOI] [PubMed] [Google Scholar]
- 25.Gibson AJW, Li H, D'Silva A, Tudor RA, Elegbede AA, Otsuka SM, Bebb DG, Cheung WY. Impact of number versus location of metastases on survival in stage IV M1b non-small cell lung cancer. Med Oncol. 2018;35:117. doi: 10.1007/s12032-018-1182-8. [DOI] [PubMed] [Google Scholar]
- 26.Rami-Porta R, Bolejack V, Crowley J, Ball D, Kim J, Lyons G, Rice T, Suzuki K, Thomas CF, Jr, Travis WD, et al. The IASLC lung cancer staging project: Proposals for the revisions of the T descriptors in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol. 2015;10:990–1003. doi: 10.1097/JTO.0000000000000559. [DOI] [PubMed] [Google Scholar]
- 27.Oweira H, Petrausch U, Helbling D, Schmidt J, Mannhart M, Mehrabi A, Schöb O, Giryes A, Decker M, Abdel-Rahman O. Prognostic value of site-specific metastases in pancreatic adenocarcinoma: A Surveillance Epidemiology and End Results database analysis. World J Gastroenterol. 2017;23:1872–1880. doi: 10.3748/wjg.v23.i10.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 29.Shi Y, Sun Y, Yu J, Ding C, Wang Z, Wang C, Wang D, Wang C, Wang Z, Wang M, et al. China experts consensus on the diagnosis and treatment of advanced stage primary lung cancer (2016 version) Asia Pac J Clin Oncol. 2017;13:87–103. doi: 10.1111/ajco.12608. [DOI] [PubMed] [Google Scholar]
- 30.Shi Y, Sun Y, Yu J, Ding C, Ma Z, Wang Z, Wang D, Wang Z, Wang M, Wang Y, et al. China experts consensus on the diagnosis and treatment of brain metastases of lung cancer (2017 version) Zhongguo Fei Ai Za Zhi. 2017;20:1–13. doi: 10.3779/j.issn.1009-3419.2017.01.01. (In Chinese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun Y, Guan Z, Liao M, Yu X, Wang C, Wang J, Niu X, Shi Y, Zhi X, Liu Y, et al. Expert consensus on the diagnosis and treatment of bone metastasis in lung cancer (2014 version) Zhongguo Fei Ai Za Zhi. 2014;17:57–72. doi: 10.3779/j.issn.1009-3419.2014.02.01. (In Chinese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmelzle M, Eisenberger CF, Am Esch JS, II, Matthaei H, Krausch M, Knoefel WT. Non-colorectal, non-neuroendocrine, and non-sarcoma metastases of the liver: Resection as a promising tool in the palliative management. Langenbecks Arch Surg. 2010;395:227–234. doi: 10.1007/s00423-009-0580-y. [DOI] [PubMed] [Google Scholar]
- 33.Ileana E, Greillier L, Moutardier V, Barlesi F. Surgical resection of liver non-small cell lung cancer metastasis: A dual weapon? Lung Cancer. 2010;70:221–222. doi: 10.1016/j.lungcan.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 34.Di Carlo I, Grasso G, Patane' D, Russello D, Latteri F. Liver metastases from lung cancer: Is surgical resection justified? Ann Thorac Surg. 2003;76:291–293. doi: 10.1016/S0003-4975(03)00149-8. [DOI] [PubMed] [Google Scholar]
- 35.Reddy SK, Barbas AS, Marroquin CE, Morse MA, Kuo PC, Clary BM. Resection of noncolorectal nonneuroendocrine liver metastases: A comparative analysis. J Am Coll Surg. 2007;204:372–382. doi: 10.1016/j.jamcollsurg.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 36.Abdel-Rahman O. Outcomes of surgery as part of the management of metastatic Non-small-cell lung cancer: A Surveillance, epidemiology and End results database analysis. Cancer Invest. 2018;36:238–245. doi: 10.1080/07357907.2018.1466895. [DOI] [PubMed] [Google Scholar]
- 37.Liu K, Zheng D, Xu G, Du Z, Wu S. Local thoracic therapy improve prognosis for stage IV non-small cell lung cancer patients combined with chemotherapy: A Surveillance, Epidemiology, and End results database analysis. PLoS One. 2017;12:e0187350. doi: 10.1371/journal.pone.0187350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Setzer M, Robinson LA, Vrionis FD. Management of locally advanced pancoast (superior sulcus) tumors with spine involvement. Cancer Control. 2014;21:158–167. doi: 10.1177/107327481402100209. [DOI] [PubMed] [Google Scholar]
- 39.Rui M, Pengqing Y, Chang C. Progress in resection and reconstruction of chest wall in non-small lung cancer. Chin J Clin Thorac Cardiovasc Surg. 2015;22:685–690. [Google Scholar]
- 40.Dai C, Shen J, Ren Y, Zhong S, Zheng H, He J, Xie D, Fei K, Liang W, Jiang G, et al. Choice of surgical procedure for patients with Non-small-cell lung cancer ≤1 cm or >1 to 2 cm among lobectomy, segmentectomy, and wedge resection: A population-based study. J Clin Oncol. 2016;34:3175–3182. doi: 10.1200/JCO.2015.64.6729. [DOI] [PubMed] [Google Scholar]
- 41.Ludwig MS, Goodman M, Miller DL, Johnstone PA. Postoperative survival and the number of lymph nodes sampled during resection of node-negative non-small cell lung cancer. Chest. 2005;128:1545–1550. doi: 10.1378/chest.128.3.1545. [DOI] [PubMed] [Google Scholar]
- 42.Zhang R, Li P, Li Q, Qiao Y, Xu T, Ruan P, Song Q, Fu Z. Radiotherapy improves the survival of patients with stage IV NSCLC: A propensity score matched analysis of the SEER database. Cancer Med. 2018;7:5015–5026. doi: 10.1002/cam4.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herskovic A, Chitti B, Christos P, Wernicke AG, Parashar B. Addition of surgery after radiation significantly improves survival in stage IIIB Non-small cell lung cancer: A population-based analysis. World J Surg. 2017;41:758–762. doi: 10.1007/s00268-016-3764-y. [DOI] [PubMed] [Google Scholar]
- 44.Verma V, Rwigema JM, Adeberg S, Simone CB., II Enrollment of elderly patients with locally advanced non-small cell lung cancer in multi-institutional trials of proton beam radiation therapy. Clin Lung Cancer. 2017;18:441–443. doi: 10.1016/j.cllc.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 45.He M, Li S, Chen Y, Ouyang M, Chen P, Zhang J. 131I-chTNT injection to relieve tracheal obstruction in advanced NSCLC patient. Technol Health Care. 2016;24(Suppl 2):S513–S519. doi: 10.3233/THC-161176. [DOI] [PubMed] [Google Scholar]
- 46.Hendriks LE, Hermans BC, van den Beuken-van Everdingen MH, Hochstenbag MM, Dingemans AM. Effect of Bisphosphonates, Denosumab, and radioisotopes on bone pain and quality of life in patients with non-small cell lung cancer and bone metastases: A systematic review. J Thorac Oncol. 2016;11:155–173. doi: 10.1016/j.jtho.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 47.Toffart AC, Duruisseaux M, Brichon PY, Pirvu A, Villa J, Selek L, Guillem P, Dumas I, Ferrer L, Levra MG, Moro-Sibilot D. Operation and chemotherapy: Prognostic factors for lung cancer with one synchronous metastasis. Ann Thorac Surg. 2018;105:957–965. doi: 10.1016/j.athoracsur.2017.10.040. [DOI] [PubMed] [Google Scholar]
- 48.Cheng TD, Darke AK, Redman MW, Zirpoli GR, Davis W, Payne Ondracek R, Bshara W, Omilian AR, Kratzke R, Reid ME, et al. Smoking, Sex, and Non-small cell lung cancer: Steroid hormone receptors in tumor tissue (S0424) J Natl Cancer Inst. 2018;110:734–742. doi: 10.1093/jnci/djx260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Merrill RM, Johnson E. Benefits of marriage on relative and conditional relative cancer survival differ between males and females in the USA. J Cancer Surviv. 2017;11:578–589. doi: 10.1007/s11764-017-0627-y. [DOI] [PubMed] [Google Scholar]
- 50.Wu Y, Ai Z, Xu G. Marital status and survival in patients with non-small cell lung cancer: An analysis of 70006 patients in the SEER database. Oncotarget. 2017;8:103518–103534. doi: 10.18632/oncotarget.21568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richards TB, Henley SJ, Puckett MC, Weir HK, Huang B, Tucker TC, Allemani C. Lung cancer survival in the United States by race and stage (2001–2009): Findings from the CONCORD-2 study. Cancer. 2017;123:5079–5099. doi: 10.1002/cncr.31029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams CD, Salama JK, Moghanaki D, Karas TZ, Kelley MJ. Impact of race on treatment and survival among U.S. Veterans with early-stage lung cancer. J Thorac Oncol. 2016;11:1672–1681. doi: 10.1016/j.jtho.2016.05.030. [DOI] [PubMed] [Google Scholar]
- 53.Videtic GM, Reddy CA, Chao ST, Rice TW, Adelstein DJ, Barnett GH, Mekhail TM, Vogelbaum MA, Suh JH. Gender, race, and survival: A study in non-small-cell lung cancer brain metastases patients utilizing the radiation therapy oncology group recursive partitioning analysis classification. Int J Radiat Oncol Biol Phys. 2009;75:1141–1147. doi: 10.1016/j.ijrobp.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 54.Tannenbaum SL, Koru-Sengul T, Zhao W, Miao F, Byrne MM. Survival disparities in non-small cell lung cancer by race, ethnicity, and socioeconomic status. Cancer J. 2014;20:237–245. doi: 10.1097/PPO.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 55.Yoshinaga Y, Enatsu S, Iwasaki A, Shirakusa T. Surgical treatment for primary non-small cell lung cancer with synchronous brain metastases. Kyobu Geka. 2016;59:41–45. (In Japanese) [PubMed] [Google Scholar]
- 56.Ramalingam S, Dinan MA, Crawford J. Survival comparison in patients with Stage IV lung cancer in Academic versus Community centers in the United States. J Thorac Oncol. 2018;13:1842–1850. doi: 10.1016/j.jtho.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 57.Xiaolan J, Jia W. Prognostic factors for bone metastasis of patients with non-small-cell lung cancer. Shandong Med. 2016;56:88–90. [Google Scholar]
- 58.Masters GA, Temin S, Azzoli CG, Giaccone G, Baker S, Jr, Brahmer JR, Ellis PM, Gajra A, Rackear N, Schiller JH, et al. Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2015;33:3488–3515. doi: 10.1200/JCO.2015.62.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang YL, Yuan JQ, Wang KF, Fu XH, Han XR, Threapleton D, Yang ZY, Mao C, Tang JL. The prevalence of EGFR mutation in patients with non-small cell lung cancer: A systematic review and meta-analysis. Oncotarget. 2016;7:78985–78993. doi: 10.18632/oncotarget.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the Surveillance, Epidemiology, and End Results (SEER) repository (https://seer.cancer.gov/).