Key Points
Question
What is the effect of omitting evaluation of prefeed gastric residuals on nutritional outcomes in extremely preterm infants?
Findings
In this randomized clinical trial of 143 premature, very low-birth-weight infants, those who did not undergo gastric residual evaluation advanced feeds more quickly and consumed more enteral nutrition at weeks 5 and 6 after birth.
Meaning
Among extremely preterm infants, the omission of gastric residual evaluation was safe and increased the delivery of enteral nutrition.
Abstract
Importance
Evaluating prefeed gastric residuals is considered routine care but has little supporting evidence.
Objective
To determine the effect of omitting prefeed gastric residual evaluation on nutritional outcomes in extremely preterm infants.
Design, Setting, and Participants
This single-center randomized clinical trial compared the omission of gastric residual evaluation with prefeed gastric residual evaluation. Infants were recruited from a level 4 neonatal intensive care unit and were enrolled from October 17, 2013, to October 8, 2016, and then followed up for 6 weeks after birth. Eligible participants were infants born at 32 or fewer weeks’ gestation with a birth weight of 1250 g or less; they were enrolled within 72 hours after birth and within 24 hours after feeding initiation. All participants (N = 143) were included in the modified intent-to-treat analysis, which was conducted from March to July 2018.
Interventions
The residual group underwent prefeed gastric residual evaluation; the no residual group did not. Feeding decisions were made according to nutritional guidelines, and infants received only human milk.
Main Outcomes and Measures
The primary outcome was weekly enteral nutrition intake in mL/kg for 6 weeks after birth.
Results
Of 143 infants, 74 (51.7%) were randomized to undergo gastric residual evaluation (residual group) and 69 (48.3%) to omitted gastric residual evaluation (no residual group). The residual group comprised an even number of male and female infants (37 [50.0%]) with a mean (SD) gestational age of 27.1 (2.4) weeks and a mean (SD) birth weight of 888.8 (206.6) grams, whereas the no residual group had more male infants (36 [52.17%]), a mean (SD) gestational age of 27 (1.2) weeks, and a mean (SD) birth weight of 915.2 (180) grams. The no residual group had feedings that advanced more quickly compared with the residual group (mean weekly increase, 20.7 mL/kg/d vs 17.9 mL/kg/d; P = .02) and consumed more feedings at weeks 5 (137.2 [95% CI, 128.6-145.8]; P = .03) and 6 (141.6 [95% CI, 133.2-150.0]; P = .03). Among the secondary outcomes, the no residual group had higher mean estimated log weights (7.01 [95% CI, 6.99-7.02] vs 6.98 [95% CI, 6.97-7.00]; P = .03), had fewer episodes of abdominal distention (0.59 [95% CI, 0.34-1.01] vs 1.79 [95% CI, 1.27-2.53]; P = .001), and were discharged 8 days earlier (4.21 [95% CI, 4.14-4.28] vs 4.28 [95% CI, 4.19-4.36]; P = .01). Odds for necrotizing enterocolitis (0.058 [95% CI, 0.018-0.190] vs 0.026 [95% CI, 0.006-0.109]), death (0.004 [95% CI, 0.0003-0.046] vs 0.012 [95% CI, 0.001-0.131]), late-onset sepsis (0.970 [95% CI, 0.67-1.40] vs 1.38 [95% CI, 0.97-1.94]), and ventilator-associated pneumonia (0.084 [95% CI, 0.033-0.214] vs 0.056 [95% CI, 0.019-0.168]) were similar between groups.
Conclusions and Relevance
Among extremely preterm infants, the omission of gastric residual evaluation increased the delivery of enteral nutrition as well as improved weight gain and led to earlier hospital discharge; these results may translate into evidence-based practice.
Trial Registration
ClinicalTrials.gov identifier: NCT01863043
This randomized clinical trial examines the effect of omitting prefeed gastric residual evaluation on enteral nutrition intake for 6 weeks after birth among very low-birth-weight infants.
Introduction
Providing sufficient enteral nutrition to critically ill patients reduces complications and improves health outcomes.1,2,3 Unfortunately, the feeding goals of patients who receive feedings through oro-nasogastric feeding tubes are rarely met, with the most important barrier being large gastric residuals.4,5 Evaluating gastric residuals has been considered standard care for decades without substantial supporting evidence. Large gastric residuals have traditionally been assumed to represent (1) feeding intolerance; (2) a risk for aspiration and ventilator-associated pneumonia (VAP); or (3) in extremely preterm infants, an early sign of necrotizing enterocolitis (NEC), a devastating disease affecting up to 7% to 14% of infants born at very low birth weight (VLBW).6,7,8,9 However, little evidence exists to indicate that evaluating gastric residuals improves patient outcomes and whether omitting this evaluation may cause harm.10,11 In addition, variation in the definition of large gastric residuals; lack of evidence-based management for abnormal gastric residuals; and potential variation in gastric residual volume owing to feeding tube type and size as well as patient position and aspiration technique further limit the clinical usefulness of evaluation.12,13,14
Prefeed gastric residuals are evaluated by more than 97% of neonatal intensive care unit (NICU) nurses,15 but evidence suggests this evaluation may be unnecessary.11,16 Omitting gastric residual evaluation has been shown to improve enteral nutrition delivery in adults and children without increasing feeding intolerance or VAP.10,16,17 Moreover, in several small studies, preterm infants who did not undergo gastric residual evaluation had improved nutritional outcomes.11,18,19 However, to our knowledge, this study is the first adequately powered randomized clinical trial to determine the risks and benefits of omitting gastric residual evaluation in VLBW infants, the population at greatest risk for adverse nutritional outcomes of gastric residual evaluation. Not only are these infants innately vulnerable but they also require frequent feedings (every 2-3 hours) and, because of their prematurity and level of acuity, require a feeding tube for weeks to months.20,21 Thus, the primary objective of this randomized clinical trial was to determine the effect of omitting prefeed gastric residual evaluation on the amount of enteral nutrition provided to premature VLBW infants for 6 weeks after birth. We hypothesized that infants who did not undergo gastric residual evaluation would have increased weekly enteral intake. Secondary objectives included determining the effect of omitting gastric residual evaluation on nutritional and infant outcomes as well as on risk of gastric content aspiration and VAP.
Methods
Study Participants
From October 17, 2013, to October 8, 2016, infants were recruited from a level 4 NICU and enrolled in the study if eligible. They were eligible for inclusion if they were born at 32 or fewer weeks’ gestation, had a birth weight of 1250 g or less, were younger than 72 hours, and were receiving some feedings by 72 hours after birth. Infants were ineligible if they had congenital or chromosomal abnormalities, including complex congenital heart disease or a gastrointestinal condition. Infants were withdrawn from the study if stage II or greater NEC or spontaneous intestinal perforation occurred. This study was approved by the University of Florida Institutional Review Board, and the research protocol is available in Supplement 1. Parental written informed consent was obtained by the research team within 72 hours of life and within 24 hours of initiating feeds. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
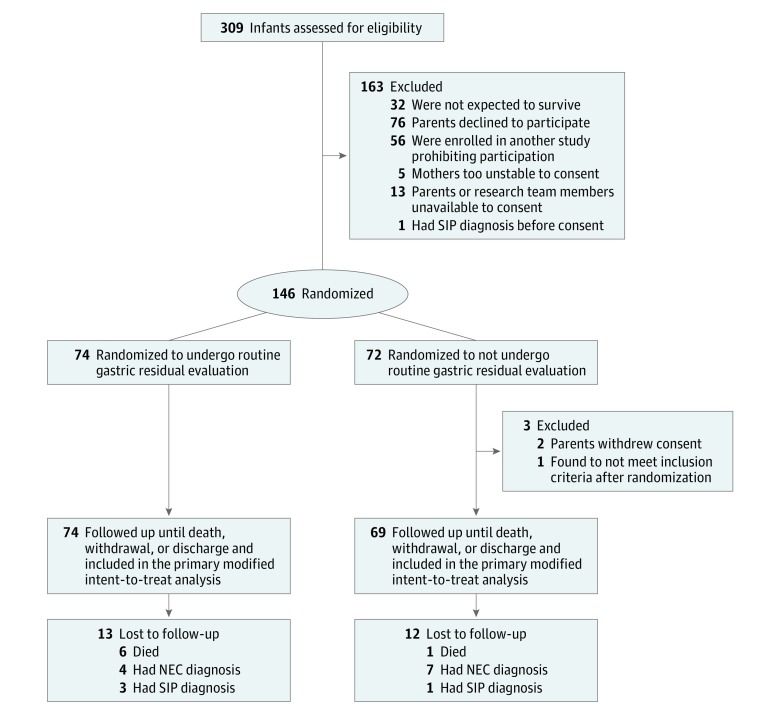
After parents or guardians signed the informed consent forms, infants were randomized to 1 of 2 groups by a computer-generated sequence with random-length permuted blocks of sizes (4, 6, or 8) to maintain an approximate balance of assignment to each treatment group. Infants were randomized to either gastric residual evaluation (residual group) or omitted gastric residual evaluation (no residual group) (Figure). Randomization was concealed until the intervention was assigned.
Figure. Consort Diagram.
NEC indicates necrotizing enterocolitis; SIP, spontaneous intestinal perforation.
Study Intervention
All infants were enrolled by the research team. The residual group underwent prefeed gastric residual evaluation, and the no residual group did not. Gastric content aspiration was not used to verify feeding tube placement for infants in the no residual group. Instead, a combination of 2 insertion depth measurement strategies and verification of the calculated depth prior to every feeding was used.22,23 Because nurses recorded information regarding gastric residual volume, neither the clinical team nor the research staff were blinded to the intervention. However, all feeding decisions, including time of initiation, rate of advancement, and human milk fortification, were made according to the NICU nutritional guidelines. Other clinical decisions, including initiation and duration of parenteral nutrition (PN), length of central line access, withholding of feedings, and the need for abdominal radiographs and laboratory analyses, were also based on the NICU nutritional guidelines. The clinical team made all of the clinical decisions except those regarding gastric residual evaluation. Infants received only human milk (mother’s own milk or pasteurized donor human milk).
Study Outcomes
The primary outcome for this study was weekly enteral nutrition measured in mL/kg for 6 weeks after birth. Prespecified secondary outcomes included days to full feeds (120 mL/kg/d), hours of PN, hours with a central line, evidence of PN–associated liver disease (PNALD) (ie, level of direct bilirubin [DB] and level of alkaline phosphatase; DB and alkaline phosphate levels were assessed with clinically ordered laboratory tests), growth indices (weekly weight, head circumference, and length), days to discharge, evidence of feeding intolerance (episodes of emesis and episodes of increased abdominal circumference by >2 cm during the 6-week trial), episodes of presumed (treated with antibiotics for ≥5 days) or culture-proven late-onset sepsis (occurring ≥3 days of life), and evidence of stage II or greater NEC. Other health outcomes, including incidence of intraventricular hemorrhage, death, bronchopulmonary dysplasia (BPD), and duration of respiratory support, were compared between groups. Secondary outcomes also included occult fecal blood, fecal calprotectin and S100A12 levels, as well as motilin and gastrin levels but these were not included in this study.
Evidence of gastric content aspiration and incidence of VAP were assessed through (1) evidence of VAP on a chest radiograph as read by a pediatric radiologist, (2) positive tracheal cultures when done in accordance with clinical care, and (3) if the infant was ventilated, tracheal aspirate samples collected weekly during routine endotracheal tube suctioning and sent to the hospital laboratory for analysis of pepsin level.
Sample Size and Statistical Analysis
In a preliminary study, 40 VLBW infants had a mean (SD) enteral intake at 14 days of 72 (65) mL/kg/d. To detect a 50% improvement (Cohen d = 0.55), 104 infants were required to achieve 80% power at 2-sided P = .05 for the primary outcome of weekly enteral intake. Given the 143 infants available for analysis, an effect size of 0.31 (Cohen f) provided 80% power to detect the treatment-by-week interaction within the 2 (treatment) by 6 (time) generalized linear mixed model (GLMM) used.24,25 The sample size was planned to accommodate dropouts, including deaths (estimated at 15%) and development of NEC (estimated at 7%).
All infants were included in the modified intent-to-treat analysis. Data analysis was performed from March to July 2018. Descriptive statistics were generated to characterize groups. Given the range of measurement levels (eg, continuous and censored), several statistical methods appropriate for outcome variable distribution were used. Statistical assumption tenability and model fit were evaluated for each model; when appropriate, remediation, such as Box-Cox family of transformation, non-normal link function, or inclusion of a scale measurement, was used. Adjacent categories were merged for categorical variables with small cell sizes. Variables clinically relevant to outcomes were evaluated for inclusion as covariates within models. Covariates were retained if they were statistically significant or if their removal reduced model fit as evaluated by the Bayes Information Criterion. Inclusion of relevant covariates within a randomized design reduces error variance, thereby increasing the power to detect treatment differences, and enables exploring the heterogeneity of response to treatment through evaluating interaction with treatment. The trial was not powered to detect those interactions, but they provide information to better understand the effect of omitting gastric residual evaluation. A description of the statistical methods and associated outcome variables appears below.
Generalized linear mixed model analysis was used for outcomes consisting of repeated measures. With the exception of DB, unstructured-type (when each variance and each covariance is estimated uniquely from the data) within-participant covariance matrix forms best fit the data as assessed using Bayes Information Criterion. Effects included in models consisted of main effects for treatment (gastric residual: no or yes) and week (1-6), the treatment-by-week interaction, and selected covariates relevant to specific outcomes. Treatment-by-covariate interactions with P > .05 were removed from the model. Simple main effects, which deconstruct interacting effects, were examined for statistically significant interaction effects involving treatment.
Generalized linear model (GLM) analysis was used for outcomes consisting of single noncensored values compared between groups. Effects tested consisted of main effects for treatment, selected covariates relevant to the specific outcome modeled, and treatment-by-covariate interactions. Interactions with P > .05 were removed from the model.
Days to full feeds (120 mL/kg/d), hours of PN, hours with a central line, days to discharge, and respiratory support were compared using techniques suitable for censored data. By default, those accelerated failure time regression models transform the response with the natural logarithm before fitting specified models for γ, log-logistic, and log-normal distributions.26,27 Data were censored at the end of the 6-week trial.
Differences in maximum alkaline phosphatase level and mean pepsin value were tested using a Wilcoxon 2-sample test, with exact P value estimates owing to residual-value non-normal distribution.26
An interim analysis, executed after half the planned accrual had been followed for 6 weeks, was performed to assess whether continued randomization was ethical. After evaluating the results, the data monitoring committee recommended the completion of planned accrual.
Results
In total, 143 infants were included in the study; 309 infants were eligible for inclusion, and 163 were excluded or parents refused consent. Of the 143 infants, 74 (51.7%) were randomized to undergo gastric residual evaluation (residual group) and 69 (48.3%) were randomized to omitted gastric residual evaluation (no residual group) (Figure). The residual group comprised an even number of male and female infants (37 [50.0%]) with a mean (SD) gestational age of 27.1 (2.4) weeks and a mean (SD) birth weight of 888.8 (206.6) grams, whereas the no residual group had more male infants (36 [52.17%]), a mean (SD) gestational age of 27 (1.2) weeks, and a mean (SD) birth weight of 915.2 (180) grams.
Eighteen infants (26.1%) in the no residual group had 1 or more gastric residuals evaluated, either inadvertently or when ordered, for symptoms of gastrointestinal dysfunction. Four infants (5.8%) were withdrawn for NEC, and no infant had gastric residuals evaluated for more than 1 day. The 2 groups were similar in sex, delivery mode, multiple birth, exposure to antenatal steroids, Apgar or SNAP (Score for Neonatal Acute Physiology) II score, and feeding type (mother’s own milk vs donor human milk). However, infants in the no residual group compared with the residual group were nearly twice as likely to be African American (39 of 69 infants [56.5%] vs 22 of 74 infants [29.7%]; Table 1).
Table 1. Baseline Characteristics of Infants.
| Variable | Frequency, No. (%) | |
|---|---|---|
| Residual Group (n = 74) | No Residual Group (n = 69) | |
| Gestational age, mean (SD), wk | 27.1 (2.4) | 27.0 (1.2) |
| Birth weight, mean (SD), g | 888.8 (206.6) | 915.2 (180.0) |
| Race | ||
| White | 49 (66.2) | 28 (40.58) |
| African American | 22 (29.7) | 39 (56.5) |
| Asian | 0 | 1 (1.45) |
| Other | 3 (4.1) | 1 (1.45) |
| Ethnicity | ||
| Hispanic | 10 (13.51) | 6 (8.70) |
| Sex | ||
| Male | 37 (50.0) | 36 (52.17) |
| Female | 37 (50.0) | 33 (44.14) |
| Mode of delivery | ||
| Cesarean | 55 (74.32) | 54 (78.26) |
| Vaginal | 19 (25.68) | 23 (33.33) |
| Multiple births | 18 (24.32) | 15 (21.75) |
| Received antenatal steroids | 68 (91.89) | 56 (81.16) |
| 5-m Apgar score, mean (SD) | 6.58 (2.26) | 6.39 (2.39) |
| SNAP-II score, mean (SD) | 20.58 (12.58) | 21.25 (12.51) |
| Type of feeding | ||
| % MOM, mean (SD) | 55.04 (39.90) | 49.89 (38.52) |
Abbreviations: MOM, mother’s own milk; SNAP, Score for Neonatal Acute Physiology.
Primary Outcome
Results of the GLMM comparing enteral nutrition between groups over the 6-week study are presented in Table 2. The change in enteral nutrition over time differed between groups. Both groups increased over time, but the no residual group exhibited a steeper increase compared with the residual group (mean weekly increase, 20.7 mL/kg/d vs 17.9 mL/kg/d; P = .02). As seen in the simple main effects results, least square means for the groups were similar in weeks 1 through 4 but higher for the no residual group at weeks 5 (137.2 [95% CI, 128.6-145.8] vs 123.9 [95% CI, 115.2-132.6]; P = .03) and 6 (141.6 [95% CI, 133.2-150.0] vs 128.4 [95% CI, 119.9-136.9]; P = .03).
Table 2. Repeated Measures Outcomes Analyzed Using Generalized Linear Mixed Model Analysis.
| Variable | P Value | Estimate (95% CI)a | |
|---|---|---|---|
| Residual Group | No Residual Group | ||
| Weekly feedings, mL/kg/d | |||
| Birth weight | .03 | NA | NA |
| GA | <.001 | NA | NA |
| GA × week | <.001 | NA | NA |
| Week | .006 | NA | NA |
| Treatment | .048 | NA | NA |
| Treatment × week | .02 | NA | NA |
| Simple main effects | |||
| Week 1 | .27 | 25.7 (21.8 to 29.5) | 22.6 (18.6 to 26.5) |
| Week 2 | .12 | 83.2 (72.9 to 93.4) | 94.7 (84.3 to 105.2) |
| Week 3 | .28 | 109.2 (98.5 to 120.0) | 117.7 (106.9 to 128.5) |
| Week 4 | .15 | 119.4 (109.5 to 129.4) | 129.6 (119.7 to 139.6) |
| Week 5 | .03 | 123.9 (115.2 to 132.6) | 137.2 (128.6 to 145.8) |
| Week 6 | .03 | 128.4 (119.9 to 136.9) | 141.6 (133.2 to 150.0) |
| Estimated log direct bilirubin | |||
| Week | <.001 | NA | NA |
| GA | <.001 | NA | NA |
| Birth weight | <.001 | NA | NA |
| Treatment | |||
| Transformed values | .98 | −0.777 (−0.892 to −0.661) | −0.778 (−0.895 to −0.661) |
| Untransformed valuesb | NA | 0.690 (0.563 to 0.819)b | 0.671 (0.542 to 0.799)b |
| Occurrence of elevated (>2 mg/dL) direct bilirubin | |||
| GA | .07 | NA | NA |
| Birth weight | .008 | NA | NA |
| Treatment | .53 | 0.040 (0.017 to 0.092) | 0.057 (0.025 to 0.131) |
| Estimated log transformed weight | |||
| Birth weight | <.001 | NA | NA |
| GA | <.001 | NA | NA |
| Week | <.001 | NA | NA |
| GA × treatment | .047 | NA | NA |
| Treatment | |||
| Transformed values | .03 | 6.98 (6.97 to 7.00) | 7.01 (6.99 to 7.02) |
| Untransformed valuesb | NA | 1129.7 (1111 to 1148)b | 1145.3 (1126 to 1164)b |
| Head circumference | |||
| Week | .02 | NA | NA |
| Birth HC | <.001 | NA | NA |
| GA | <.001 | NA | NA |
| Birth HC × week | <.001 | NA | NA |
| GA × week | <.001 | NA | NA |
| Treatment | .56 | 25.94 (25.71 to 26.17) | 25.85 (25.62 to 26.08) |
| Length | |||
| Birth length | <.001 | NA | NA |
| GA | <.001 | NA | NA |
| Week | <.001 | NA | NA |
| Birth length × treatment | .008 | NA | NA |
| GA × treatment | .005 | NA | NA |
| Treatment | .59 | 36.41 (36.08 to 36.74) | 36.78 (36.44 to 37.11) |
Abbreviations: GA, gestational age; HC, head circumference; NA, not applicable.
All estimates are least square means except for occurrence of elevated bilirubin, which are odds.
Untransformed values provided for clinical reference only.
Secondary Outcomes
Results of the groups’ days to full feeds, hours on PN, and hours with a central line appear in Table 3. Product-limit curves are similar between groups, with P values for homogeneity of strata ranging from 0.3 to 0.8 (eFigure in Supplement 2). The accelerated failure time regression models comparing mean time to outcome demonstrated no statistically significant differences, ranging from P = .59 for days to 120 mL/kg/d to P = .97 for days requiring invasive ventilation. After natural log transformation to normalize the distribution of residual values, mean log-transformed DB and odds for occurrence of an elevated DB (>2 mg/dL) were similar between groups (Table 3). Maximum alkaline phosphate levels were similar between the residual group and no residual group (median interquartile range [IQR], 481 [371-664] vs 495.5 [393-650.5]; P = .95).
Table 3. Outcomes Analyzed Using Survival Analysis.
| Variable | P Value | LSM (95% CI) | |
|---|---|---|---|
| Residual Group | No Residual Group | ||
| Days to full feeds, 120 mL/kg/da | |||
| GA | .006 | NA | NA |
| Treatment | |||
| Transformed values | .59 | 2.47 (2.29-2.65) | 2.51 (2.38-2.63) |
| Untransformed valuesb | NA | 18.1 (16.3-20.0)b | 15.9 (14.1-17.8)b |
| Hours of parenteral nutritionc | |||
| Birth weight | <.001 | NA | NA |
| Weekly median % of MOM | .003 | NA | NA |
| Treatment | |||
| Transformed values | .64 | 5.67 (5.55-5.78) | 5.71 (5.59-5.82) |
| Untransformed valuesb | NA | 358.8 (312.2-405.4)b | 356.8 (310.6-403.1)b |
| Hours with a central accessc | |||
| Birth weight | <.001 | NA | NA |
| Weekly median % of MOM | .001 | ||
| Treatment | .95 | 5.81 (5.69-5.93)/402.6 (352.4-452.8)b | 5.81 (5.70-5.93)/398.6 (350.2-447.0)b |
| Transformed values | .95 | 5.81 (5.69-5.93) | 5.81 (5.70-5.93) |
| Untransformed valuesb | NA | 402.6 (352.4-452.8)b | 398.6 (350.2-447.0)b |
| Days requiring invasive ventilationd | |||
| GA | <.001 | NA | NA |
| Treatment | .97 | 1.96 (1.70-2.23)/14.3 (11.2-17.4)b | 1.97 (1.69-2.25)/13.3 (10.1-16.5)b |
| Days to dischargea | |||
| GA | <.001 | NA | NA |
| GA × treatment | .03 | 4.28 (4.19-4.36) | 4.21 (4.14-4.28) |
| Treatment | |||
| Transformed values | .01 | 4.28 (4.19-4.36) | 4.21 (4.14-4.28) |
| Untransformed valuesb | NA | 86.4 (80.7-92.1)b | 79.1 (73.17-84.5)b |
Abbreviations: GA, gestational age; LSM, least square means; MOM, mother’s own milk; NA, not applicable.
Gamma distribution modeled.
Untransformed values provided for clinical reference only.
Log-logistic distribution modeled.
Log-normal distribution modeled.
Infants in the no residual group had higher least square means values for estimated mean log weight (7.01 [95% CI, 6.99-7.02] vs 6.98 [95% CI, 6.97-7.00]; P = .03); however, head circumference (25.85 [95% CI, 25.62-26.08] vs 25.94 [95% CI, 25.71-26.17]; P = .56) and length (36.78 [95% CI, 36.44-37.11] vs 36.41 [05% CI, 36.08-36.74; P = .59) were similar between groups (Table 2). The no residual group had fewer episodes of abdominal distention (0.59 [95% CI, 0.34-1.12] vs 1.79 ]95% CI, 1.27-2.53]; P = .001) but more emesis episodes (5.01 [95% CI, 3.87-6.50] vs 2.46 [95% CI, 1.70-3.56]; P = .002), and the number of abdominal radiographs were similar between groups (3.81 [95% CI, 3.24-4.64] vs 4.34 [95% CI, 3.66-5.34]; Table 4).
Table 4. Outcomes Analyzed Using Generalized Linear Model Analysis.
| Variable | P Value | Estimate (95% CI)a | |
|---|---|---|---|
| Residual Group | No Residual Group | ||
| No. of emesis episodes | |||
| Race | .26 | NA | NA |
| Treatment × race | .008 | NA | NA |
| GA | .002 | NA | NA |
| Weekly median % of MOM | <.001 | NA | NA |
| Treatment | .002 | 2.46 (1.70-3.56) | 5.01 (3.87-6.50) |
| Simple main effect | |||
| African American | .006 | 2.62 (1.97-3.49) | 4.16 (3.54-4.89) |
| Non–African American | <.001 | 2.22 (1.83-2.68) | 6.18 (5.33-7.16) |
| No. of episodes of abdominal distension | |||
| Race | <.001 | NA | NA |
| Weekly median % of MOM | .03 | NA | NA |
| Treatment | .001 | 1.79 (1.27-2.53) | 0.590 (0.344-1.012) |
| No. of abdominal radiographs | |||
| GA | <.001 | NA | NA |
| Weekly median % of MOM | .08 | NA | NA |
| Treatment | .25 | 4.34 (3.66-5.34) | 3.81 (3.24-4.64) |
| Positive tracheal aspirate occurrence | |||
| GA | <.001 | NA | NA |
| Weekly mean % of MOM | .03 | NA | NA |
| Treatment | .48 | 0.056 (0.019-0.168) | 0.084 (0.033-0.214) |
| No. of positive tracheal aspirate samples (modeling probability of higher number of positives) | |||
| GA | <.001 | NA | NA |
| Weekly mean % of MOM | <.001 | NA | NA |
| Treatment | NA | NA | |
| 1 | .17 | 0.055 (0.029-0.107) | 0.088 (0.051-0.152) |
| ≥2 | 0.015 (0.007-0.033) | 0.024 (0.012-0.047) | |
| Brochopulmonary dysplasia | |||
| Overall | .001 | NA | NA |
| GA | <.001 | NA | NA |
| Treatment | .43 | 0.512 (0.305-0.858) | 0.680 (0.414-01.12) |
| Respiratory supportb | |||
| GA | <.001 | NA | NA |
| Treatment | .053 | 6.02 (2.79-12.96) | 2.45 (1.34-4.47) |
| IVH stage (modeling probability of higher stage) | |||
| Overall | .06 | NA | NA |
| GA | .03 | NA | NA |
| Treatment | NA | NA | |
| Stage 2 | .62 | 3.95 (1.74-8.96) | 3.07 (1.39-6.78) |
| Stage 3 or 4 | 0.365 (0.167-0.796) | 0.284 (0.126-0.638) | |
| No. of late-onset sepsis episodesc | |||
| Poisson model | |||
| Length of stay | <.001 | NA | NA |
| Treatment | .08 | 1.38 (0.97-1.94) | 0.97 (0.67-1.40) |
| Zero-inflation model | |||
| GA | .002 | NA | NA |
| Occurrence of NECc | |||
| GA | .02 | NA | NA |
| Estimated log weekly mean % of MOM | .08 | NA | NA |
| Treatment | .25 | 0.026 (0.006-0.109) | 0.058 (0.018-0.190) |
| Death within 6 weeksc | |||
| GA | <.001d | NA | NA |
| Treatment | .60d | 0.012 (0.001-0.131) | 0.004 (0.0003-0.046) |
Abbreviations: GA, gestational age; IVH, intraventricular hemorrhage; MOM, mother’s own milk; NA, not applicable; NEC, necrotizing enterocolitis.
All estimates are odds except for IVH stage and number of positive tracheal aspirate samples (which are odds ratios) and number of late-onset sepsis episodes, number of emesis episodes, number of abdominal distensions, and number of abdominal radiographs (which are least square means).
Whether or not infant required respiratory support.
Death, late-onset sepsis, and NEC were mutually exclusive.
Exact conditional P value.
Variables indicative of VAP were similar between groups, including odds for a positive tracheal aspirate and number of positive aspirates (0.084 [95% CI, 0.033-0.214] vs 0.056 [95% CI, 0.019-0.168]) (Table 4). Pepsin levels were similar between the residual and no residual groups (median [IQR], 1.00 [0.2-5.7] vs 0.90 [0-2.0]; P = .27). No instances of VAP were found on chest radiograph. Odds for BPD (0.512 [95% CI, 0.305-.858] vs 0.680 [95% CI, 0.414-1.120]; P = .43) and use of respiratory support (6.02 [95% CI, 2.79-12.96] vs 2.45 [95% CI, 1.34-4.47]; P = .53; Table 4) were similar between the residual and no residual groups. Least square means for estimated log days requiring invasive ventilation were also similar (1.96 [95% CI, 1.70-2.23] vs 1.97 [95% CI, 1.69-2.25]; P = .97; Table 3).
The product-limit survival curves for days to discharge were similar (eFigure in Supplement 2), but when gestational age entered into the model, infants in the no residual group were discharged 8 days earlier than those in the residual group (4.21 [95% CI, 4.14-4.28] vs 4.28 [95% CI, 4.19-4.36]; P = .01; Table 3). Odds for NEC (0.058 [95% CI, 0.018-0.190] vs 0.026 [95% CI, 0.006-0.109]), death (0.004 [95% CI, 0.0003-0.046] vs 0.012 [95% CI, 0.001-0.131]), and late-onset sepsis (0.970 [95% CI, 0.67-1.40] vs 1.38 [95% CI, 0.97-1.94]), and VAP (0.084 [95% CI, 0.033-0.214] vs 0.056 [95% CI, 0.019-0.168]) were similar between groups (Table 4).
Discussion
We found that infants who did not undergo gastric residual evaluation received considerably more enteral nutrition without an increase in adverse health outcomes. When gastric residual evaluation was omitted, infants advanced feedings more quickly and consumed more enteral nutrition at weeks 5 and 6 after birth. These findings are consistent with those in other studies that found increased enteral intake when gastric residual evaluations were omitted. In their randomized clinical trial of 61 preterm VLBW infants, Torrazza et al11 found that, when gastric residual evaluations were omitted, infants reached full feedings (150 mL/kg/d) nearly 6 days earlier. Although not statistically significant, these findings may be clinically important in preterm infants at substantial risk for complications associated with suboptimal enteral nutrition.11 In another study after a practice change that omitted gastric residual evaluation, infants at 34 weeks’ gestation or younger reached full feedings (150 mL/kg/d) 1 day earlier.18 When prefeed abdominal circumferences were evaluated in place of gastric residuals in a study, VLBW infants experienced fewer feeding interruptions (0 vs 2; P < .001) and required less time to reach full feeds (10 vs 14 days; P < .001).19 Conversely, Singh et al,28 in a randomized clinical trial of infants with a birth weight between 1500 g and 2000 g, found no difference in days to reach full feeds (120 mL/kg/d).
Evidence is lacking that large gastric residuals indicate feeding intolerance16,29 and, in infants born extremely preterm, may be normal owing to gastrointestinal immaturity and reduced motility.30 We found that, when gastric residual evaluation was omitted, infants experienced fewer episodes of abdominal distension and, although not statistically significant, required fewer abdominal radiography. Similarly, Shulman et al,31 in a prospective study of 50 preterm infants, found no association between feeding intolerance (longer time to full feedings and/or decreased feedings) and large gastric residuals (>50% and/or >2 mL/kg), and gastric residuals up to 2 to 3 mL/kg have not been shown to indicate feeding intolerance (less feedings at 14 days of life) in preterm infants.32
Contrary to results in previous studies, we found no differences in length of time PN or a central line was required nor in the incidence of PNALD or metabolic bone disease. When feedings are interrupted or decreased, PN is required in greater amounts and/or for longer periods, thereby increasing the risk for PNALD, prolonged need for central lines, and metabolic bone disease.33,34,35 Torrazza et al11 found that infants who did not undergo gastric residual evaluation required nearly central line 6 days less and more than 1 day less on PN, although these findings were not statistically significant. Two additional small studies reported that, when gastric residual evaluation was omitted, infants required fewer days of PN, but differences in timing of feeding initiation and inclusion of infants older than 32 weeks’ gestation may have affected results. Neither study included the number of days that a central line was required or the incidence rate of PNALD.18,19
In addition, we found that infants who did not undergo gastric residual evaluation were discharged home 8 days earlier. These findings are consistent with research in adults and children indicating that improved enteral nutrition reduces both intensive care unit and hospital stay,4,36,37 but previous research in preterm infants has not indicated differences in length of NICU stay.19 Furthermore, when gastric residual evaluations were omitted, improved weight gain in infants was observed but no substantial differences in either head circumference or length. When feedings are interrupted or decreased owing to large gastric residuals, growth may be negatively affected.38 Because of the association between adequate growth and improved neurodevelopmental outcome in extremely preterm infants, these findings may be particularly important.39,40,41
Large gastric residuals have traditionally been considered an early symptom of NEC,6 but we found no differences in incidence of NEC between the residual and no residual groups. Two retrospective case-control studies have suggested gastric residual volume may be higher in infants who develop NEC,6,8 but our findings are consistent with other studies of preterm infants in which omission of gastric residual evaluation was not associated with an increased incidence of NEC.11,18,19,28
Large gastric residuals have traditionally been believed to increase the risk of emesis and aspiration, thus increasing the risk of VAP, but evidence to support this assumption is lacking.10,42 Preterm infants are at an increased risk for VAP owing to the use of uncuffed endotracheal tubes, reduced immune competency, decreased lower esophageal sphincter tone, and decreased gastrointestinal motility.43
Previous research suggests that evaluating gastric residuals does not protect against VAP in adults and children,4,16,44 but the present study, to our knowledge, is the first to address the effect of omitting gastric residual evaluation on the incidence of VAP in extremely preterm infants. Using objective determinants of VAP, including tracheal pepsin levels, positive endotracheal cultures, and radiographic evidence, we did not find increased incidence of VAP when gastric residual evaluations were omitted. Although VAP has been associated with morbidity, including increased risk for BPD in preterm infants,45,46 we found no difference in incidence of BPD, need for respiratory support, or days requiring invasive ventilation between the 2 groups in this study.
Strengths and Limitations
Strengths of this study include omitting the evaluation of aspiration of gastric residuals to verify feeding tube placement, thereby limiting any potential disruption in digestion. In addition, all infants were fed only human milk to minimize the differences in gastric emptying and feeding intolerance that may occur with formula feedings.47
This trial has several limitations. Because information on gastric residual evaluation is included in medical records and monitored by clinicians, blinding was impossible. Therefore, a change in clinician behavior resulting from group assignment could not be completely excluded. In addition, the study was not powered to address safety concerns, including the risk for NEC.
Conclusions
The results of this trial suggest that evaluating gastric residuals is unnecessary and may decrease the delivery of enteral nutrition to extremely preterm infants. Limiting gastric residual evaluation to infants with symptoms of gastrointestinal dysfunction (including abdominal distension or tenderness, emesis, and clinical decompensation or bloody stools) may increase enteral nutrition delivery in this vulnerable population. These findings can be translated into evidence-based practice in the care of VLBW infants.
Trial Protocol.
eFigure. Product-limit Curves Comparing Gastric Residual and No Gastric Residual Groups
Data Sharing Statement.
References
- 1.McClave SA, Taylor BE, Martindale RG, et al. ; Society of Critical Care Medicine; American Society for Parenteral and Enteral Nutrition . Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2016;40(2):159-211. doi: 10.1177/0148607115621863 [DOI] [PubMed] [Google Scholar]
- 2.Mehta NM, Skillman HE, Irving SY, et al. . Guidelines for the provision and assessment of nutrition support therapy in the pediatric critically ill patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition. JPEN J Parenter Enteral Nutr. 2017;41(5):706-742. doi: 10.1177/0148607117711387 [DOI] [PubMed] [Google Scholar]
- 3.Reintam Blaser A, Starkopf J, Alhazzani W, et al. ; ESICM Working Group on Gastrointestinal Function . Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med. 2017;43(3):380-398. doi: 10.1007/s00134-016-4665-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tume LN, Latten L, Kenworthy L. Paediatric intensive care nurses’ decision-making around gastric residual volume measurement. Nurs Crit Care. 2017;22(5):293-297. doi: 10.1111/nicc.12304 [DOI] [PubMed] [Google Scholar]
- 5.Kuslapuu M, Jõgela K, Starkopf J, Reintam Blaser A. The reasons for insufficient enteral feeding in an intensive care unit: a prospective observational study. Intensive Crit Care Nurs. 2015;31(5):309-314. doi: 10.1016/j.iccn.2015.03.001 [DOI] [PubMed] [Google Scholar]
- 6.Cobb BA, Carlo WA, Ambalavanan N. Gastric residuals and their relationship to necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2004;113(1, pt 1):50-53. doi: 10.1542/peds.113.1.50 [DOI] [PubMed] [Google Scholar]
- 7.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364(3):255-264. doi: 10.1056/NEJMra1005408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertino E, Giuliani F, Prandi G, Coscia A, Martano C, Fabris C. Necrotizing enterocolitis: risk factor analysis and role of gastric residuals in very low birth weight infants. J Pediatr Gastroenterol Nutr. 2009;48(4):437-442. doi: 10.1097/MPG.0b013e31817b6dbe [DOI] [PubMed] [Google Scholar]
- 9.Montejo JC, Miñambres E, Bordejé L, et al. . Gastric residual volume during enteral nutrition in ICU patients: the REGANE study. Intensive Care Med. 2010;36(8):1386-1393. doi: 10.1007/s00134-010-1856-y [DOI] [PubMed] [Google Scholar]
- 10.Reignier J, Mercier E, Le Gouge A, et al. ; Clinical Research in Intensive Care and Sepsis (CRICS) Group . Effect of not monitoring residual gastric volume on risk of ventilator-associated pneumonia in adults receiving mechanical ventilation and early enteral feeding: a randomized controlled trial. JAMA. 2013;309(3):249-256. doi: 10.1001/jama.2012.196377 [DOI] [PubMed] [Google Scholar]
- 11.Torrazza RM, Parker LA, Li Y, Talaga E, Shuster J, Neu J. The value of routine evaluation of gastric residuals in very low birth weight infants. J Perinatol. 2015;35(1):57-60. doi: 10.1038/jp.2014.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen SS, Tzeng YL, Gau BS, Kuo PC, Chen JY. Effects of prone and supine positioning on gastric residuals in preterm infants: a time series with cross-over study. Int J Nurs Stud. 2013;50(11):1459-1467. doi: 10.1016/j.ijnurstu.2013.02.009 [DOI] [PubMed] [Google Scholar]
- 13.Metheny NA, Stewart J, Nuetzel G, Oliver D, Clouse RE. Effect of feeding-tube properties on residual volume measurements in tube-fed patients. JPEN J Parenter Enteral Nutr. 2005;29(3):192-197. doi: 10.1177/0148607105029003192 [DOI] [PubMed] [Google Scholar]
- 14.Sangers H, de Jong PM, Mulder SE, et al. . Outcomes of gastric residuals whilst feeding preterm infants in various body positions. J Neonatal Nurs. 2013;19(6):337-341. doi: 10.1016/j.jnn.2012.12.003 [DOI] [Google Scholar]
- 15.Metheny NA, Mills AC, Stewart BJ. Monitoring for intolerance to gastric tube feedings: a national survey. Am J Crit Care. 2012;21(2):e33-e40. doi: 10.4037/ajcc2012647 [DOI] [PubMed] [Google Scholar]
- 16.Poulard F, Dimet J, Martin-Lefevre L, et al. . Impact of not measuring residual gastric volume in mechanically ventilated patients receiving early enteral feeding: a prospective before-after study. JPEN J Parenter Enteral Nutr. 2010;34(2):125-130. doi: 10.1177/0148607109344745 [DOI] [PubMed] [Google Scholar]
- 17.Tume LN, Bickerdike A, Latten L, et al. . Routine gastric residual volume measurement and energy target achievement in the PICU: a comparison study. Eur J Pediatr. 2017;176(12):1637-1644. doi: 10.1007/s00431-017-3015-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riskin A, Cohen K, Kugelman A, Toropine A, Said W, Bader D. The impact of routine evaluation of gastric residual volumes on the time to achieve full enteral feeding in preterm infants. J Pediatr. 2017;189:128-134. doi: 10.1016/j.jpeds.2017.05.054 [DOI] [PubMed] [Google Scholar]
- 19.Kaur A, Kler N, Saluja S, et al. . Abdominal circumference or gastric residual volume as measure of feed intolerance in VLBW infants. J Pediatr Gastroenterol Nutr. 2015;60(2):259-263. doi: 10.1097/MPG.0000000000000576 [DOI] [PubMed] [Google Scholar]
- 20.Ibrahim NR, Kheng TH, Nasir A, et al. . Two-hourly versus 3-hourly feeding for very low birthweight infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2017;102(3):F225-F229. doi: 10.1136/archdischild-2015-310246 [DOI] [PubMed] [Google Scholar]
- 21.Lau C. Development of infant oral feeding skills: what do we know? Am J Clin Nutr. 2016;103(2):616S-621S. doi: 10.3945/ajcn.115.109603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallaher KJ, Cashwell S, Hall V, Lowe W, Ciszek T. Orogastric tube insertion length in very low birth weight infants. J Perinatol. 1993;13(2):128-131. [PubMed] [Google Scholar]
- 23.Cirgin Ellett ML, Cohen MD, Perkins SM, Smith CE, Lane KA, Austin JK. Predicting the insertion length for gastric tube placement in neonates. J Obstet Gynecol Neonatal Nurs. 2011;40(4):412-421. doi: 10.1111/j.1552-6909.2011.01255.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1988. [Google Scholar]
- 25.PASS 15 Power Analysis and Sample Size Software [computer program]. Kaysville, UT: NCSS Statistical Software; 2017.
- 26.SAS Institute Inc SAS/STAT 14.1 User’s Guide. Cary, NC: SAS Institute Inc; 2015. [Google Scholar]
- 27.Therneau T, Grambsch P. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer; 2000. doi: 10.1007/978-1-4757-3294-8 [DOI] [Google Scholar]
- 28.Singh B, Rochow N, Chessell L, et al. . Gastric residual volume in feeding advancement in preterm infants (GRIP Study): a randomized trial. J Pediatr. 2018;200(Jun):79-83.e1. doi: 10.1016/j.jpeds.2018.04.072 [DOI] [PubMed] [Google Scholar]
- 29.Elke G, Felbinger TW, Heyland DK. Gastric residual volume in critically ill patients: a dead marker or still alive? Nutr Clin Pract. 2015;30(1):59-71. doi: 10.1177/0884533614562841 [DOI] [PubMed] [Google Scholar]
- 30.Moore TA, Wilson ME. Feeding intolerance: a concept analysis. Adv Neonatal Care. 2011;11(3):149-154. doi: 10.1097/ANC.0b013e31821ba28e [DOI] [PubMed] [Google Scholar]
- 31.Shulman RJ, Ou CN, Smith EO. Evaluation of potential factors predicting attainment of full gavage feedings in preterm infants. Neonatology. 2011;99(1):38-44. doi: 10.1159/000302020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mihatsch WA, von Schoenaich P, Fahnenstich H, et al. . The significance of gastric residuals in the early enteral feeding advancement of extremely low birth weight infants. Pediatrics. 2002;109(3):457-459. doi: 10.1542/peds.109.3.457 [DOI] [PubMed] [Google Scholar]
- 33.Ukarapong S, Venkatarayappa SKB, Navarrete C, Berkovitz G. Risk factors of metabolic bone disease of prematurity. Early Hum Dev. 2017;112:29-34. doi: 10.1016/j.earlhumdev.2017.06.010 [DOI] [PubMed] [Google Scholar]
- 34.Hsieh CH, Kuo SW, Pei D, et al. . Thyrotoxic periodic paralysis: an overview. Ann Saudi Med. 2004;24(6):418-422. doi: 10.5144/0256-4947.2004.418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alcock G, Liley HG, Cooke L, Gray PH. Prevention of neonatal late-onset sepsis: a randomised controlled trial. BMC Pediatr. 2017;17(1):98-105. doi: 10.1186/s12887-017-0855-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeh DD, Fuentes E, Quraishi SA, et al. . Early protein inadequacy is associated with longer intensive care unit stay and fewer ventilator-free days: a retrospective analysis of patients with prolonged surgical intensive care unit stay. JPEN J Parenter Enteral Nutr. 2018;42(1):212-218. [DOI] [PubMed] [Google Scholar]
- 37.Villet S, Chiolero RL, Bollmann MD, et al. . Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin Nutr. 2005;24(4):502-509. doi: 10.1016/j.clnu.2005.03.006 [DOI] [PubMed] [Google Scholar]
- 38.McKenzie BL, Edmonds L, Thomson R, Haszard JJ, Houghton LA. Nutrition practices and predictors of postnatal growth in preterm infants during hospitalization: a longitudinal study. J Pediatr Gastroenterol Nutr. 2018;66(2):312-317. doi: 10.1097/MPG.0000000000001747 [DOI] [PubMed] [Google Scholar]
- 39.Schneider J, Fischer Fumeaux CJ, Duerden EG, et al. . Nutrient intake in the first two weeks of life and brain growth in preterm neonates. Pediatrics. 2018;141(3): e20172169. doi: 10.1542/peds.2017-2169 [DOI] [PubMed] [Google Scholar]
- 40.Sammallahti S, Kajantie E, Matinolli HM, et al. . Nutrition after preterm birth and adult neurocognitive outcomes. PLoS One. 2017;12(9):e0185632. doi: 10.1371/journal.pone.0185632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117(4):1253-1261. doi: 10.1542/peds.2005-1368 [DOI] [PubMed] [Google Scholar]
- 42.Kuppinger DD, Rittler P, Hartl WH, Rüttinger D. Use of gastric residual volume to guide enteral nutrition in critically ill patients: a brief systematic review of clinical studies. Nutrition. 2013;29(9):1075-1079. doi: 10.1016/j.nut.2013.01.025 [DOI] [PubMed] [Google Scholar]
- 43.Dhillon AS, Ewer AK. Diagnosis and management of gastro-oesophageal reflux in preterm infants in neonatal intensive care units. Acta Paediatr. 2004;93(1):88-93. doi: 10.1111/j.1651-2227.2004.tb00680.x [DOI] [PubMed] [Google Scholar]
- 44.McClave SA, Lukan JK, Stefater JA, et al. . Poor validity of residual volumes as a marker for risk of aspiration in critically ill patients. Crit Care Med. 2005;33(2):324-330. doi: 10.1097/01.CCM.0000153413.46627.3A [DOI] [PubMed] [Google Scholar]
- 45.Farhath S, Aghai ZH, Nakhla T, et al. . Pepsin, a reliable marker of gastric aspiration, is frequently detected in tracheal aspirates from premature ventilated neonates: relationship with feeding and methylxanthine therapy. J Pediatr Gastroenterol Nutr. 2006;43(3):336-341. doi: 10.1097/01.mpg.0000232015.56155.03 [DOI] [PubMed] [Google Scholar]
- 46.Pileggi C, Mascaro V, Bianco A, Nobile CGA, Pavia M. Ventilator bundle and its effects on mortality among ICU patients: a meta-analysis. Crit Care Med. 2018;46(7):1167-1174. doi: 10.1097/CCM.0000000000003136 [DOI] [PubMed] [Google Scholar]
- 47.Corvaglia L, Fantini MP, Aceti A, et al. ; “Emilia Romagna Perinatal Network” . Predictors of full enteral feeding achievement in very low birth weight infants. PLoS One. 2014;9(3):e92235. doi: 10.1371/journal.pone.0092235 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
eFigure. Product-limit Curves Comparing Gastric Residual and No Gastric Residual Groups
Data Sharing Statement.