Key Points
Question
What is the association of an adverse environment, including low socioeconomic status and traumatic stressful events, with psychopathology, neurocognition, and brain parameters in puberty among children and young adults?
Findings
In this community-based cohort study of 9498 participants, low socioeconomic status was associated with reduced neurocognitive performance, and experiencing a higher number of traumatic stressful events was associated with greater psychopathology. Both factors were associated with multiple brain structural and functional parameters as well as earlier maturation.
Meaning
Low socioeconomic status and the experience of traumatic stressful events are environmental aspects that appear to have common and unique associations with the brain and behavior, and both are associated with accelerated maturation.
This community-based cohort study compares the association of low socioeconomic status and traumatic stressful events with psychopathology, puberty, neurocognition, and multimodal neuroimaging parameters in brain maturation among children and young adults.
Abstract
Importance
Low socioeconomic status (L-SES) and the experience of traumatic stressful events (TSEs) are environmental factors implicated in behavioral deficits, abnormalities in brain development, and accelerated maturation. However, the relative contribution of these environmental factors is understudied.
Objective
To compare the association of L-SES and TSEs with psychopathology, puberty, neurocognition, and multimodal neuroimaging parameters in brain maturation.
Design, Setting, and Participants
The Philadelphia Neurodevelopmental Cohort is a community-based study examining psychopathology, neurocognition, and neuroimaging among participants recruited through the Children’s Hospital of Philadelphia pediatric network. Participants are youths aged 8 to 21 years at enrollment with stable health and fluency in English. The sample of 9498 participants was racially (5298 European ancestry [55.8%], 3124 African ancestry [32.9%], and 1076 other [11.4%]) and economically diverse. A randomly selected subsample (n = 1601) underwent multimodal neuroimaging. Data were collected from November 5, 2009, through December 30, 2011, and analyzed from February 1 through November 7, 2018.
Main Outcomes and Measures
The following domains were examined: (1) clinical, including psychopathology, assessed with a structured interview based on the Schedule for Affective Disorders and Schizophrenia for School-Age Children, and puberty, assessed with the Tanner scale; (2) neurocognition, assessed by the Penn Computerized Neurocognitive Battery; and (3) multimodal magnetic resonance imaging parameters of brain structure and function.
Results
A total of 9498 participants were included in the analysis (4906 [51.7%] female; mean [SD] age, 14.2 [3.7] years). Clinically, L-SES and TSEs were associated with greater severity of psychiatric symptoms across the psychopathology domains of anxiety/depression, fear, externalizing behavior, and the psychosis spectrum. Low SES showed small effect sizes (highest for externalizing behavior, 0.306 SD; 95% CI, 0.269 to 0.342), whereas TSEs had large effect sizes, with the highest in females for anxiety/depression (1.228 SD; 95% CI, 1.156 to 1.300) and in males for the psychosis spectrum (1.099 SD; 95% CI, 1.032 to 1.166). Both were associated with early puberty. Cognitively, L-SES had moderate effect sizes on poorer performance, the greatest being on complex cognition (−0.500 SD 95% CI, −0.536 to −0.464), whereas TSEs were associated with slightly better memory (0.129 SD; 95% CI, 0.084 to 0.174) and poorer complex reasoning (−0.109 SD; 95% CI, −0.154 to −0.064). Environmental factors had common and distinct associations with brain structure and function. Structurally, both were associated with lower volume, but L-SES had correspondingly lower gray matter density, whereas TSEs were associated with higher gray matter density. Functionally, both were associated with lower regional cerebral blood flow and coherence and with accelerated brain maturation.
Conclusions and Relevance
Low SES and TSEs are associated with common and unique differences in symptoms, neurocognition, and structural and functional brain parameters. Both environmental factors are associated with earlier completion of puberty by physical features and brain parameters. These findings appear to underscore the need for identifying and preventing adverse environmental conditions associated with neurodevelopment.
Introduction
Associations of environmental stress with psychopathology, cognition, and brain structure and function are well described.1,2,3 However, studies typically assess low socioeconomic status (L-SES) or traumatic stressful events (TSEs) separately. These factors are often correlated4,5 but may show individual differences, as suggested by evidence on deprivation and threat.6,7
A growing body of literature reports associations between childhood adversity and psychopathology, focusing on TSEs with depression,8,9 posttraumatic stress disorder,10,11 and, more recently, psychosis.12,13,14 Cognition studies have examined SES disparities, initially with single measures of IQ and/or educational attainment15,16,17 and increasingly with parameters linked to brain systems. Implicating frontoparietal dysfunction, deficits in language18,19,20 and executive functioning21,22,23 have been associated with L-SES. A meta-analysis of children’s SES and executive function noted heterogeneity among studies, with small to medium effect sizes.24 For neuroimaging, structural magnetic resonance imaging (MRI) studies in small samples were inconclusive regarding associations of neuroanatomical measures and SES.18 The large-scale Pediatric Imaging, Neurocognition, and Genetics study reported that family income and parental educational attainment were associated with the cortical area in regions implicated in language, executive functions, and spatial skills.25 Furthermore, SES has been found to moderate age-related cortical thinning, especially in language regions,26 and lower volume and fractional anisotropy (FA) of white matter tracts.27
Traumatic stressful events are associated not only with symptom severity across psychiatric disorders5,28 but also with neurocognitive deficits29,30 and structural and functional MRI abnormalities.31,32,33 Stress-sensitive brain structures, such as the hippocampus, have lower volume,34,35 and the circuitry underlying emotion processing36 and executive function23 indicates abnormal activation. The stress acceleration hypothesis6 was recently linked to early puberty and DNA methylation age, relative to chronological age, specifically associated with life stressors but not with deprivation.7 Satterthwaite et al37 reported puberty associations with brain behavior and Barzilay et al38 reported associations with psychopathology, but these studies did not systematically assess associations with environment. Notably, the same neural aberrations associated with cognitive deficits are implicated in psychopathology and undergo protracted maturation, motivating joint examination of psychopathology, cognition, and brain maturation.
A conceptual framework for understanding how life stressors derail development by affecting brain structure and function, leading to enhanced maturation and consequent symptoms and cognitive deficits, requires information on all pertinent parameters within a developmental context. Such data are a prerequisite for elucidating mechanisms through which adversity during development leads to the evolutionary adaptive response of accelerated maturation. To our knowledge, the associations of L-SES have not been previously compared with TSE load in the same sample across domains. The Philadelphia Neurodevelopmental Cohort provides this opportunity with data on environment,39 psychopathology,40 neurocognition,41 and, in a subsample, multimodal neuroimaging.42 Herein we test the hypothesis that L-SES and TSEs are associated with increased psychopathology, neurocognitive deficits, and abnormalities in parameters of brain structure and function, as well as earlier puberty6,7 and brain maturation.43
Methods
Recruitment and clinical, neurocognitive, and neuroimaging procedures for data acquisition, processing, and analysis in the Philadelphia Neurodevelopmental Cohort were published previously (eMethods 1 and 2 in the Supplement).40,41,42 The Philadelphia Neurodevelopmental Cohort (n = 9498), aged 8 to 21 years, is a racially and economically diverse population ascertained from nonpsychiatric pediatric services (Table). Data were acquired from November 5, 2009, through December 30, 2011, concomitantly applying standard protocols. Procedures were approved by the institutional review boards of the University of Pennsylvania and Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania. Written informed consent was obtained from legal guardians; children provided written assent. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Table. Participant Characteristics.
| Sample Variable | No. of TSEs Stratified by Sex | |||||
|---|---|---|---|---|---|---|
| Male | Female | |||||
| 0 | 1 | ≥2 | 0 | 1 | ≥2 | |
| Total Sample | ||||||
| No. of participants | 2654 | 988 | 950 | 2735 | 1195 | 976 |
| Age, mean (SD), y | 13.01 (3.49) | 14.18 (3.54) | 15.66 (3.44) | 13.79 (3.66) | 14.87 (3.4) | 16.25 (3.19) |
| No. (%) EA | 1690 (63.7) | 588 (59.5) | 405 (42.6) | 1626 (59.5) | 634 (53.1) | 355 (36.4) |
| No. (%) AA | 701 (26.4) | 280 (28.3) | 444 (46.7) | 795 (29.1) | 408 (34.1) | 496 (50.8) |
| No. (%) other | 263 (9.9) | 120 (12.1) | 101 (10.6) | 314 (11.5) | 153 (12.8) | 125 (12.8) |
| Parent educational attainment, mean (SD), y | 14.65 (2.36) | 14.30 (2.27) | 13.71 (2.09) | 14.52 (2.34) | 14.11 (2.27) | 13.47 (2.07) |
| SES, mean (SD)a | 0.16 (0.94) | 0.07 (0.97) | −0.35 (1.05) | 0.12 (0.97) | −0.07 (0.99) | −0.44 (1.03) |
| Imaging Sample | ||||||
| No. of participants | 345 | 155 | 165 | 378 | 189 | 163 |
| Age, mean (SD), y | 13.52 (3.45) | 14.22 (3.31) | 16 (3.02) | 13.94 (3.71) | 15.14 (3.1) | 16.09 (2.81) |
| No. (%) EA | 181 (52.5) | 91 (58.7) | 50 (30.3) | 176 (46.6) | 71 (37.6) | 50 (30.7) |
| No. (%) AA | 112 (32.5) | 51 (32.9) | 97 (58.8) | 151 (39.9) | 93 (49.2) | 102 (62.6) |
| No. (%) other | 52 (15.1) | 13 (8.4) | 18 (10.9) | 51 (13.5) | 25 (13.2) | 11 (6.7) |
| Parent educational attainment, mean (SD), y | 14.28 (2.47) | 14.12 (2.13) | 13.69 (2.18) | 14.23 (2.31) | 13.69 (2.19) | 13.33 (2.04) |
| SES, mean (SD)a | −0.05 (1.00) | −0.04 (0.95) | −0.49 (1.06) | −0.13 (1.02) | −0.41 (1.06) | −0.64 (1.02) |
| Mean RMS | 0.07 (0.04) | 0.07 (0.04) | 0.07 (0.04) | 0.07 (0.04) | 0.06 (0.04) | 0.06 (0.04) |
Abbreviations: AA, African ancestry; EA, European ancestry; RMS, root mean square motion estimate; SES, socioeconomic status index in SD units; TSE, traumatic stressful event.
Indicates factor score calculated on data ascertained using census-based geocoding.
Clinical Assessment
Psychiatric
The in-person interview based on the structured Schedule for Affective Disorders and Schizophrenia for School-Age Children44 (GOASSESS)40 evaluated lifetime history of clinical symptoms across major psychopathology domains. Participants were assessed dimensionally and categorically and had to have significant symptoms associated with distress and affecting functioning to score high on specific domains of anxiety/misery, fear, psychosis, and externalizing behavior.45
Pubertal Stage
Self-reported Tanner stage,46,47which correlates with physical examination results,48 was quantified on a scale of 1 to 5 using schematic drawings of secondary sex characteristics. The score (stage 5) used was based on the pubic hair item, which is common to boys and girls.
Environmental Risk Parameters
The first environmental factor was SES, ascertained using census-based geocoding variables obtained with the participants’ addresses. Census-based variables, public in 2010 and proximal to the enrollment period (2009-2011), included neighborhood characteristics that correlate with race and parental educational attainment39 (eMethods 1 and eTable 1 in the Supplement). Neighborhoods were census block groups, which vary in size, population, and population density. Generally, block groups contain 600 to 3000 persons, and the mean number of persons sampled per block group in the Philadelphia Neurodevelopmental Cohort was 2.4. Second, TSEs were ascertained using GOASSESS to probe lifetime exposure to natural disasters or bad accidents; concern that someone close was hurt badly or killed; witnessing someone getting killed, badly beaten, or die; seeing a dead body; and/or ever experiencing assault, including being attacked or badly beaten, threatened with a weapon, and/or sexually assaulted. Traumatic stress load was quantified by the cumulative number of endorsed TSEs (range, 0-8).28
Neurocognitive Assessment
The Penn Computerized Neurocognitive Battery provided measures of accuracy and response time for executive function, episodic memory, complex cognition, and social cognition.41,49,50 To examine efficiency, accuracy and median response time were given as z scores. The response time z score was multiplied by −1 (so that higher numbers indicate faster response times), and the mean of these 2 z scores was calculated for efficiency.
Neuroimaging
All MRIs were acquired on the same 3-T scanner (Total Imaging Matrix Trio; Siemens).51 Image quality procedures were applied across modalities,52,53,54 and an accurate multiatlas labeling procedure, with joint label fusion as implemented in Advanced Normalization Tools, provided whole-brain anatomical parcellation.55 Parameters examined were associated with normative and pathological behavior. Brain structure was quantified by brain volume, gray matter density (GMD), diffusion tenor imaging–based mean diffusivity for white and gray matter regions, and FA for white matter tracts. Brain function was quantified by cerebral blood flow (CBF) measured with arterial spin–labeled MRI and resting-state functional MRI measures of regional homogeneity (ReHo) and amplitude of low-frequency fluctuations (ALFF), the main functional MRI parameters linked to development and performance.
Statistical Analysis
Data were analyzed from February 1 through November 7, 2018. To reduce data dimensionality, the dependent measures selected were (1) clinical factor scores, including mood/anxiety, fear, externalizing behavior, and psychosis spectrum45; (2) neurocognitive factor scores, including executive function, episodic memory, complex cognition, and social cognition49,50; and (3) sectional neuroimaging values, including white matter, cerebellum, basal ganglia/striatum, and limbic, frontal, parietal, temporal, occipital, and FA for white matter tracts.56,57 These regions were quantified for volume, GMD, mean diffusivity, FA, CBF, ReHo, and ALFF. Notably, GMD is not available for white matter, nor is FA available for gray matter or CBF available for cerebellum (due to inadequate coverage of the arterial spin labeling sequence). Each dependent measure was entered into mixed-model repeated-measures analysis with continuous SES and TSEs as the main risk factors of interest; age, sex, and race as fixed factors; and the within-modality values (clinical factor scores, neurocognitive domains, and brain section) as within-individual factors. Significant interactions were followed by appropriate contrasts in which individuals at the low SES tertile (L-SES) were compared with the remaining sample (not L-SES; ie, middle and high SES tertiles combined), and individuals with at least 2 TSEs were compared with those with 1 or 0 TSEs (eTable 2 in the Supplement provides demographic characteristics of these groups). The proportion reaching stage 5, which is the dependent measure for puberty, was analyzed with logistic regression. To examine whether SES and TSEs are associated with accelerated brain maturation, we first trained a random forest regression58 to estimate adulthood (dichotomous age, 8-17 vs ≥18 years) in the benign sample (no TSEs and not L-SES), using all sectional brain parameters. This model was then applied to the whole sample (all environment types) to obtain an estimated age category (adult vs not adult). These values (adult brain vs not) were then entered into a logistic regression using age, sex, race, TSEs, and SES (all 2-way interactions). A significant age × L-SES or age × TSEs interaction (in the appropriate direction) would indicate premature brain aging in the TSE or L-SES group. The differences between groups in significance of proportions was determined using the Fisher z test (https://www.socscistatistics.com/tests/ztest/Default2.aspx). For all statistical tests, a 2-sided P < .05 was used, unless correction for multiple comparison is indicated.
Results
Environment and Clinical Measures
Symptom Dimensions
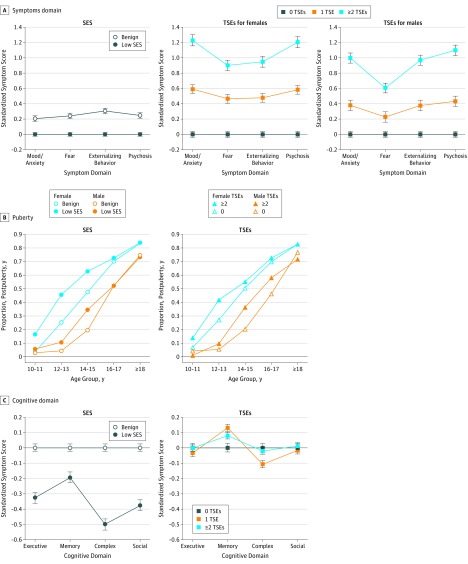
A total of 9498 participants were included in the analysis (4906 [51.7%] females and 4592 [48.3%] males; mean [SD] age, 14.2 [3.7] years). All main variables had significant associations with symptom severity across domains (eTable 3 in the Supplement). Notably, TSEs had greater effect sizes than SES. Low SES had small and uniform effect sizes (approximately 0.200 SD) across psychopathology domains and were highest for externalizing (0.306 SD; 95% CI, 0.269-0.342), whereas even 1 TSE was associated with moderate effect sizes (approximately 0.400 SD), and at least 2 TSEs had large effect sizes (>0.800 SD), with mood/anxiety having the highest score in females (1.228 SD; 95% CI, 1.156-1.300) and psychosis spectrum having the highest score in males (1.099 SD; 95% CI, 1.032-1.166) (Figure 1A). The significant SES × TSEs interaction indicated that the associations for TSEs were stronger than those for L-SES, especially mood/anxiety and psychosis spectrum relative to fear. A sex × TSEs × symptom domain interaction indicated that in females, higher symptom severity with TSE load was pronounced for mood/anxiety and psychosis spectrum, whereas in males, externalizing behavior was more pronounced (Figure 1A).
Figure 1. Association of Socioeconomic Status (SES) and Traumatic Stressful Events (TSEs) With Clinical, Puberty, and Cognitive Domains.
Whiskers indicate 95% CIs.
Puberty Stage
Age interacted with SES and TSEs (eTable 4 in the Supplement). As illustrated in Figure 1B, the proportion of youth completing puberty at an earlier chronological age was higher for participants with L-SES and those who experienced at least 2 TSEs. Main effects for SES and TSEs were −1.23 and 0.57, respectively.
Environment and Neurocognitive Domains
eFigure 1 in the Supplement shows the scatterplot of SES and TSEs. Only SES had a significant association with neurocognitive performance across domains (eTable 5 in the Supplement). Significant interactions of SES × domain (P < .001) and TSEs × domain (P < .001) were found, and the 3-way SES × TSEs × domain interaction (P = .01) indicated that each environmental factor was differentially associated with neurocognitive domains (Figure 1C). Low SES was associated with lower overall efficiency, but this association was diminished for memory relative to other domains. The strongest association with L-SES was with complex cognition (−0.500 SD; 95% CI, −0.536 to −0.464). Traumatic stressful events were not associated with overall performance, but the group with at least 2 TSEs had poorer complex cognition (−0.109 SD; 95% CI, −0.154 to −0.064) and better memory (0.129 SD; 95% CI, 0.084 to 0.174). eTable 6 in the Supplement shows results for accuracy and speed separately, and eFigure 2 in the Supplement illustrates associations (z scale) by TSE categories. A significant 5-way interaction (sex × SES × TSE × domain × race) indicated that z scores for trauma were similar across groups, with relative sparing of memory compared with executive function and complex cognition and more severe decline for L-SES. However, for males with African ancestry and benign SES, TSEs were even associated with enhanced performance in these functions (eFigure 3 in the Supplement).
Environment and Neuroimaging Parameters
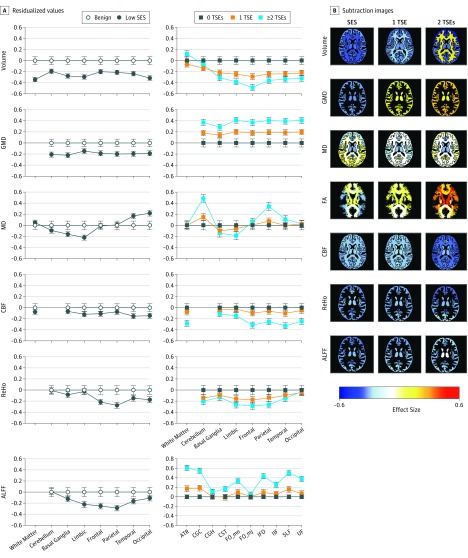
Neuroimaging parameters (eTable 7 in the Supplement) showed main associations or region interactions for nearly all parameters for SES and TSEs. For volume, there was a main interaction of SES and TSEs by section. Low SES was associated with lower volume across regions, including white matter and all gray matter regions, with small effect sizes (0.200-0.400 SD) (Figure 2). Traumatic stressful events had no association with white matter or cerebellar volumes, but were associated with lower volumes in specific regions, which scaled with TSE load to reach moderate effect sizes (>0.400 SD) in limbic and frontal regions. For GMD, findings were opposite for SES and TSEs: L-SES was associated with lower GMD (small effect sizes of approximately 0.200 SD), whereas TSE load was associated with higher GMD (moderate effect sizes of approximately 0.400 SD for ≥2 TSEs). For mean diffusivity, L-SES and TSEs were associated with lower values in basal ganglia and limbic regions; however, L-SES was associated with higher values in temporal and occipital cortex (small effect sizes of approximately 0.200 SD), whereas TSE load was associated with higher values in cerebellum and parietal cortex (effect sizes of approximately 0.400 SD). For CBF, L-SES was associated with mildly reduced values across regions, whereas TSEs showed reduced white matter and cortical perfusion that scaled with load (small effect sizes of ≤0.400 SD). For resting-state functional MRI, reduced ReHo and ALFF were associated with L-SES and most pronounced in frontoparietal regions, whereas TSEs were associated with lower cortical ReHo that scaled with the TSEs load and was most pronounced for frontoparietal regions. Finally, for FA, there was no association with SES, but TSEs were associated with elevated values in several tracts, which scaled with TSE load and reached moderate effect sizes (≥0.400 SD) for anterior thalamic radiation, cingulate gyrus–cingulum bundle, inferior fronto-occipital fasciculus, superior longitudinal fasciculus, and uncinate fasciculus (Figure 2A).
Figure 2. Association of Socioeconomic Status (SES) and Traumatic Stress Events (TSEs) With Multimodal Regional Brain Parameters.
A, Mean (SEM) residualized values compare groups with and without adversity and controlling for age, sex, and race for each region by modality for SES and TSEs. Whiskers indicate 95% CIs in the benign (control) group and SEM in the adverse (low SES and all TSA) groups. B, Subtraction images based on magnetic resonance imaging show the effect sizes of differences between groups with low SES compared with benign SES, with 1 compared with 0 TSEs, and with at least 2 compared with 0 TSEs. Images show z = 86, but each frame can be activated in a movie mode to scroll up and down the z scale in axial, sagittal, and coronal orientations (https://pennbbl.github.io/ers/index.html). ALFF indicates amplitude of low-frequency fluctuations; ATR, anterior thalamic radiation; CBF, cerebral blood flow; CGC, cingulate gyrus–cingulum bundle; CGH, cingulate gyrus proximal to hippocampus; CST, corticospinal tract; FA, fractional anisotropy; FO_mn, forceps minor; FO_mj, forceps major; GMD, gray matter density; IFO, inferior fronto-occipital fasciculus; ILF, inferior longitudinal fasciculus; MD, mean diffusivity; ReHo, resting state functional magnetic resonance imaging measures of regional homogeneity; SLF, superior longitudinal fasciculus; and UF, uncinate fasciculus. There is no graph for FA and SES because this effect size was not significant.
Environment and Brain Maturation
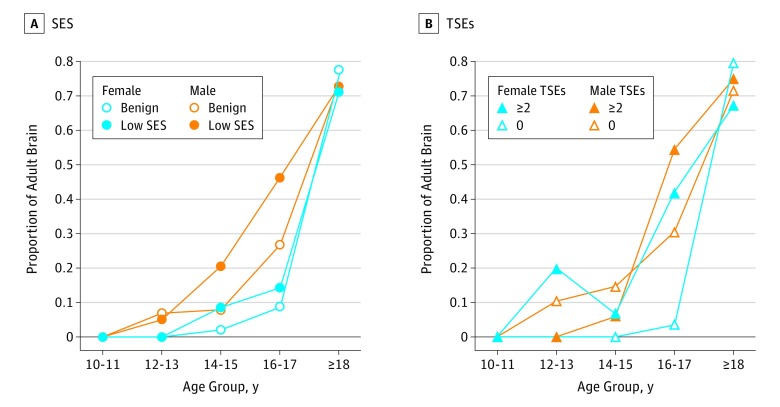
Low SES and TSEs were associated with accelerated brain maturation as indicated by higher proportions of individuals misclassified as adults at age bins younger than 18 years (eTable 8 in the Supplement). The association with SES was more pronounced in males (Figure 3). A structural equation model was specified, with all outcomes of interest regressed on all independent variables of interest (eResults and eTable 9 in the Supplement), and the correlation matrix among the dependent measures is shown in eTable 10 in the Supplement.
Figure 3. Estimated Brain Age Relative to Chronological Age.
Participants were stratified into groups with low socioeconomic status (SES) compared with benign SES and groups with at least 2 traumatic stressful events (TSEs) compared with 0 TSEs.
Discussion
This study compared SES and TSE associations across behavioral and multimodal brain parameters in a sufficiently powered community youth sample, enabling examination of individual differences. We found evidence for common and unique associations with accelerated puberty and brain maturation.
Associations With Clinical Features
Psychopathology Domains
Adverse SES and TSEs were associated with elevated psychopathology, more pronounced for TSEs. Low SES had a small effect size, highest for externalizing behavior, yet even 1 TSE had moderate effect sizes for increased symptoms, and at least 2 TSEs had large effect sizes, especially for mood/anxiety and psychosis spectrum. The association of TSEs with greater mood/anxiety psychopathology aligns with reports in adults.59,60,61 Furthermore, although L-SES had similar associations in males and females, females showed larger effect sizes of trauma, especially mood/anxiety relative to externalizing behavior. These results confirm earlier reports8,9 but show the relative association of L-SES compared with high TSEs.
Puberty Stage
The associations of chronological age with proportion of puberty was observed in L-SES and TSEs. As expected, given earlier physical maturation of girls,62 the association appeared earlier for girls (age range, 10-13 years) than boys (age range, 14-17 years). These findings support a prior report7 on advanced maturation associated with threat events. We found similar associations with SES, and the discrepancy could be due to measures used; although our measure of TSEs aligns with the previous threat exposure measure, our SES parameters differ from the previous deprivation measure in that ours are geocoding based, whereas Sumner et al7 relied on self-reports with partial overlap of items.
Associations With Neurocognitive Domains
Adverse SES and TSEs were associated with altered cognitive performance, but SES showed larger effect sizes than TSEs. The association of L-SES with poorer cognitive performance is well established for general and specific domains,18,19,20,21,22 and our study indicated overall decreased performance across neurocognitive domains, with relative sparing of memory. In contrast, TSEs were associated with a small reduction in efficiency on complex cognition tests and a small elevation in episodic memory performance. The improved memory is consistent with studies indicating that traumatic experiences can accelerate learning and memory in rodents,63 as well as with a meta-analysis of human research64 suggesting that stress disrupts some episodic memory processes while enhancing others. Notably, no SES × TSE interaction indicates that the association of TSEs is similar across social strata.
Associations With Brain Parameters
Socioeconomic status and TSEs were associated with effects in multiple brain parameters. For volume, both were associated with lower values, although the associations for SES were more widespread, including in white matter and cerebellum, whereas TSE-associated differences were limited to frontolimbic regions. This pattern supports earlier studies.65,66 For GMD, L-SES and TSEs showed uniform associations across regions but in opposite directions; L-SES was associated with lower GMD whereas TSEs were associated with higher GMD. Such a dissociation could reflect the more chronic nature of SES adversity relative to the acute effects of specific trauma. Reduced volume combined with reduced GMD characterizes brain changes associated with senescence-related neurodegenerative processes,67 whereas reduced volume and increased GMD characterizes developmental brain changes in childhood and adolescence.68 Thus, the TSE associations are consistent with accelerated biological maturation.7 Examining volume and GMD before and after exposure to stress could illuminate this issue. For example, we found reduced volume and increased GMD in preliminary data from winter-over expeditions to Antarctica.69 Such changes in brain anatomical parameters may reflect adaptation to TSEs.6,70
Socioeconomic status and TSEs showed regionally specific association with mean diffusivity, measuring constraints of water movement in the brain. Low SES and TSEs were associated with lower mean diffusivity in basal ganglia and limbic regions, but L-SES was associated with higher mean diffusivity in temporo-occipital cortex, whereas TSEs were associated with higher mean diffusivity in cerebellum and parietal cortex. Fractional anisotropy, measuring white matter microstructural organization, was not associated with SES, but a significant interaction of TSEs × white matter tract revealed higher FA values in anterior thalamic radiation, cingulate gyrus–cingulum bundle, inferior fronto-occipital fasciculus, superior longitudinal fasciculus, and uncinate fasciculus. Lifespan trajectories of mean diffusivity and FA are nonlinear.71 Late adolescence is associated with a decline in mean diffusivity and rise in FA, followed by a relative stability into senescence, when mean diffusivity rises and FA declines. Our results are consistent with reports that TSEs and SES appear to accelerate this overall pattern of biological aging in a regionally dependent manner.72,73 The accelerated decline of mean diffusivity associated with significant TSEs is most evident in brain structures undergoing protracted white matter development, including deep brain structures (eg, anterior thalamic radiation, cingulate gyrus–cingulum bundle). However, developmentally stable brain regions,71 such as those within the occipital, temporal, and parietal cortices, show a pattern consistent with accelerated biological aging. The timing of adverse SES or TSE burden likely interacts with critical neurodevelopmental processes that accelerate biological alterations of brain connectivity.
Socioeconomic status had significant associations with all functional parameters, with lower CBF, ReHo, and ALFF. The CBF associations were more pronounced for temporoparietal cortex, whereas those for ReHo and ALFF were more pronounced in frontoparietal cortex. Traumatic stressful events were associated with markedly lower CBF compared with L-SES, with greatest associations in basal ganglia and temporal cortex. Lower ReHo was seen in the high-trauma group and was especially pronounced in limbic and frontoparietal cortex. The results suggest that L-SES and TSEs are associated with reduced cerebral perfusion and associated activation coherence. The finding of altered resting state coherence is consistent with reports on associations with adversity.70,74
Brain Maturation
We examined whether SES and TSE associations with early puberty, evident in physical maturation, are also seen in brain parameters. Results supported the accelerated development hypothesis for SES and TSEs in that a higher proportion of the L-SES and high-TSE groups were misclassified as having adult brain parameters in the younger chronological age bins. Thus, our study provides evidence that accelerated maturation, considered an adaptive response to environmental deprivation,6,70 may be reflected in altered brain structure, function, and connectivity that could mediate behavioral deficits.
Limitations
We report cross-sectional data with a restricted range of measures in each domain. Although it is parsimonious to posit that adverse SES and TSEs cause the differences in behavior and brain parameters, other chains of causality cannot be ruled out. Our community sample was not ascertained for stress exposure or the seeking of psychiatric help. Therefore, we have limited information on timing and duration of stressors. To control type I error, we restricted granularity of measures analyzed, preventing the discovery of more specific associations. This choice was necessitated by the aim to examine SES and TSE associations across domains and modalities, each consisting of multiple measures. Our findings could motivate additional analyses of measures showing the most pronounced associations. Another limitation of the study is that physical maturity was established by a single item from a self-report measure rather than by physical examination. It was not feasible in this large-scale study to conduct physical examination, but self-report correlates moderately with physical examination results,48 and pubic hair is common to boys and girls. Finally, our sample, while diverse, included mostly urban and suburban participants and deviates from the US racial composition, which may limit generalizability.
Conclusions
This study establishes associations of adverse SES and TSEs with psychiatric symptom severity, puberty, cognition, and brain structure and function parameters. Adverse SES and TSEs were associated with moderate to large differences in symptom severity, with TSEs showing large effect sizes, especially in females. Both were associated with early puberty and cognitive deficits in complex cognition. However, L-SES showed additional deficits in executive and social cognition performance, whereas TSEs were associated with better memory. Both showed some similar effects on regional brain parameters—reduced volume, CBF, and ReHo—but also some unique effects; in particular, L-SES was associated with lower GMD and TSEs with higher GMD. A multivariate brain-age measure showed accelerated neurodevelopment associated with L-SES and with TSEs. The relationship among these associations remains to be elucidated, along with how they combine to manifest in the evolutionarily adaptive accelerated maturation. We examined pertinent factors (sex, race, and age), providing the context and parameters for mechanistically elucidating common and unique associations of adverse SES and TSEs, and the sample has been genotyped so that such models can be probed. The results underscore the marked and pervasive associations of adverse SES and TSEs with symptoms, cognition, and brain structure and function and highlight the need to identify, ameliorate, and mitigate exposome conditions contributing to these associations.3,75
eMethods 1. Clinical Assessment and SES Score Creation
eMethods 2. Neurocognitive Assessment, Neuroimaging, and Neuroimaging Data Processing Details
eResults. Structural Equation Model
eTable 1. Variables included in Socioeconomic Status (SES) Score With Weights
eTable 2. Demographics for the 4 Groups Contrasted in Figures
eTable 3. Results of the MMRM on the Main Demographic and Trauma Factors With Symptom Domains as Dependent Measures
eTable 4. Results of Logistic Regression Analysis on the Main Demographic and Trauma Factors With Proportion of Individuals at Each Age Bracket That Has Reached Puberty According to the Pubic Hair Item of the Tanner Scale
eTable 5. Results of the MMRM on the Main Demographic and Trauma Factors With Cognitive Domain Scores as Dependent Measures
eTable 6. Mixed-Model Repeated Measures Results for Neurocognitive Performance Accuracy and Speed
eTable 7. Results of the Mixed-Model Repeated Measures on the Main Demographic and Trauma Factors With Brain Parameters as Dependent Measures
eTable 8. Results of Logistic Regression Analysis on the Main Demographic and Trauma Factors With Proportion of Individuals at Each Age Bracket That Were Classified as Having Adult Brain by the Machine-Learning Classifier
eTable 9. Structural Model Results Using All Variables
eTable 10. Correlation Matrix Among the Dependent Measures
eFigure 1. Scatterplot of Association Between Number of Traumatic Experiences and Socioeconomic Status
eFigure 2. The Relation of Trauma Category to Cognitive Performance on the 4 Efficiency Domains
eFigure 3. The 5-Way Interaction (Sex × SES × TSE × Domain × Race)
eReferences.
References
- 1.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434-445. doi: 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190-222. doi: 10.1111/j.1749-6632.2009.05331.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guloksuz S, van Os J, Rutten BPF. The exposome paradigm and the complexities of environmental research in psychiatry. JAMA Psychiatry. 2018;75(10):985-986. doi: 10.1001/jamapsychiatry.2018.1211 [DOI] [PubMed] [Google Scholar]
- 4.Dong M, Anda RF, Felitti VJ, et al. . The interrelatedness of multiple forms of childhood abuse, neglect, and household dysfunction. Child Abuse Negl. 2004;28(7):771-784. doi: 10.1016/j.chiabu.2004.01.008 [DOI] [PubMed] [Google Scholar]
- 5.McLaughlin KA, Greif Green J, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and first onset of psychiatric disorders in a national sample of US adolescents. Arch Gen Psychiatry. 2012;69(11):1151-1160. doi: 10.1001/archgenpsychiatry.2011.2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callaghan BL, Tottenham N. The stress acceleration hypothesis: effects of early-life adversity on emotion circuits and behavior. Curr Opin Behav Sci. 2016;7:76-81. doi: 10.1016/j.cobeha.2015.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sumner JA, Colich NL, Uddin M, Armstrong D, McLaughlin KA. Early experiences of threat, but not deprivation, are associated with accelerated biological aging in children and adolescents. Biol Psychiatry. 2019;85(3):268-278. doi: 10.1016/j.biopsych.2018.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heim C, Binder EB. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp Neurol. 2012;233(1):102-111. doi: 10.1016/j.expneurol.2011.10.032 [DOI] [PubMed] [Google Scholar]
- 9.Björkenstam E, Vinnerljung B, Hjern A. Impact of childhood adversities on depression in early adulthood: a longitudinal cohort study of 478,141 individuals in Sweden. J Affect Disord. 2017;223:95-100. doi: 10.1016/j.jad.2017.07.030 [DOI] [PubMed] [Google Scholar]
- 10.Liu H, Petukhova MV, Sampson NA, et al. ; World Health Organization World Mental Health Survey Collaborators . Association of DSM-IV posttraumatic stress disorder with traumatic experience type and history in the World Health Organization World Mental Health Surveys. JAMA Psychiatry. 2017;74(3):270-281. doi: 10.1001/jamapsychiatry.2016.3783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLaughlin KA, Koenen KC, Bromet EJ, et al. . Childhood adversities and post-traumatic stress disorder: evidence for stress sensitisation in the World Mental Health Surveys. Br J Psychiatry. 2017;211(5):280-288. doi: 10.1192/bjp.bp.116.197640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGrath JJ, McLaughlin KA, Saha S, et al. . The association between childhood adversities and subsequent first onset of psychotic experiences: a cross-national analysis of 23 998 respondents from 17 countries. Psychol Med. 2017;47(7):1230-1245. doi: 10.1017/S0033291716003263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trotta A, Murray RM, Fisher HL. The impact of childhood adversity on the persistence of psychotic symptoms: a systematic review and meta-analysis. Psychol Med. 2015;45(12):2481-2498. doi: 10.1017/S0033291715000574 [DOI] [PubMed] [Google Scholar]
- 14.van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468(7321):203-212. doi: 10.1038/nature09563 [DOI] [PubMed] [Google Scholar]
- 15.Bradley RH, Corwyn RF. Socioeconomic status and child development. Annu Rev Psychol. 2002;53:371-399. doi: 10.1146/annurev.psych.53.100901.135233 [DOI] [PubMed] [Google Scholar]
- 16.Hanscombe KB, Trzaskowski M, Haworth CM, Davis OS, Dale PS, Plomin R. Socioeconomic status (SES) and children’s intelligence (IQ): in a UK-representative sample SES moderates the environmental, not genetic, effect on IQ. PLoS One. 2012;7(2):e30320. doi: 10.1371/journal.pone.0030320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sirin SR. Socioeconomic status and academic achievement: a meta-analytic review of research. Rev Educ Res. 2005;75(3):417-453. doi: 10.3102/00346543075003417 [DOI] [Google Scholar]
- 18.Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends Cogn Sci. 2009;13(2):65-73. doi: 10.1016/j.tics.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hackman DA, Farah MJ, Meaney MJ. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat Rev Neurosci. 2010;11(9):651-659. doi: 10.1038/nrn2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noble KG, Houston SM, Kan E, Sowell ER. Neural correlates of socioeconomic status in the developing human brain. Dev Sci. 2012;15(4):516-527. doi: 10.1111/j.1467-7687.2012.01147.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hackman DA, Gallop R, Evans GW, Farah MJ. Socioeconomic status and executive function: developmental trajectories and mediation. Dev Sci. 2015;18(5):686-702. doi: 10.1111/desc.12246 [DOI] [PubMed] [Google Scholar]
- 22.Lawson GM, Farah MJ. Executive function as a mediator between SES and academic achievement throughout childhood. Int J Behav Dev. 2017;41(1):94-104. doi: 10.1177/0165025415603489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheridan MA, Peverill M, Finn AS, McLaughlin KA. Dimensions of childhood adversity have distinct associations with neural systems underlying executive functioning. Dev Psychopathol. 2017;29(5):1777-1794. doi: 10.1017/S0954579417001390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawson GM, Hook CJ, Farah MJ. A meta-analysis of the relationship between socioeconomic status and executive function performance among children. Dev Sci. 2018;21(2). Published online May 30, 2017. doi: 10.1111/desc.12529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noble KG, Houston SM, Brito NH, et al. . Family income, parental education and brain structure in children and adolescents. Nat Neurosci. 2015;18(5):773-778. doi: 10.1038/nn.3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piccolo LR, Merz EC, He X, Sowell ER, Noble KG; Pediatric Imaging, Neurocognition, Genetics Study . Age-related differences in cortical thickness vary by socioeconomic status. PLoS One. 2016;11(9):e0162511. doi: 10.1371/journal.pone.0162511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ursache A, Noble KG; Pediatric Imaging, Neurocognition and Genetics Study . Socioeconomic status, white matter, and executive function in children. Brain Behav. 2016;6(10):e00531. doi: 10.1002/brb3.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barzilay R, Calkins ME, Moore TM, et al. . Association between traumatic stress load, psychopathology, and cognition in the Philadelphia Neurodevelopmental Cohort. Psychol Med. 2019;49(2):325-334. doi: 10.1017/S0033291718000880 [DOI] [PubMed] [Google Scholar]
- 29.Malarbi S, Abu-Rayya HM, Muscara F, Stargatt R. Neuropsychological functioning of childhood trauma and post-traumatic stress disorder: a meta-analysis. Neurosci Biobehav Rev. 2017;72:68-86. doi: 10.1016/j.neubiorev.2016.11.004 [DOI] [PubMed] [Google Scholar]
- 30.Barzilay R, White LK, Calkins ME, et al. . Sex-specific association between high traumatic stress exposure and social cognitive functioning in youths. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(10):860-867. doi: 10.1016/j.bpsc.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calem M, Bromis K, McGuire P, Morgan C, Kempton MJ. Meta-analysis of associations between childhood adversity and hippocampus and amygdala volume in non-clinical and general population samples. Neuroimage Clin. 2017;14:471-479. doi: 10.1016/j.nicl.2017.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hart H, Rubia K. Neuroimaging of child abuse: a critical review. Front Hum Neurosci. 2012;6:52. doi: 10.3389/fnhum.2012.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDermott TJ, Kirlic N, Aupperle RL. Roadmap for optimizing the clinical utility of emotional stress paradigms in human neuroimaging research. Neurobiol Stress. 2018;8:134-146. doi: 10.1016/j.ynstr.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luby J, Belden A, Botteron K, et al. . The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatr. 2013;167(12):1135-1142. doi: 10.1001/jamapediatrics.2013.3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Logue MW, van Rooij SJH, Dennis EL, et al. . Smaller hippocampal volume in posttraumatic stress disorder: a multisite ENIGMA-PGC study: subcortical volumetry results from posttraumatic stress disorder consortia. Biol Psychiatry. 2018;83(3):244-253. doi: 10.1016/j.biopsych.2017.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herringa RJ, Birn RM, Ruttle PL, et al. . Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc Natl Acad Sci U S A. 2013;110(47):19119-19124. doi: 10.1073/pnas.1310766110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Satterthwaite TD, Shinohara RT, Wolf DH, et al. . Impact of puberty on the evolution of cerebral perfusion during adolescence. Proc Natl Acad Sci U S A. 2014;111(23):8643-8648. doi: 10.1073/pnas.1400178111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barzilay R, Patrick A, Calkins ME, et al. . Obsessive compulsive symptomatology in community youth: typical development or a red flag for psychopathology? J Am Acad Child Adolesc Psychiatry. 2019;58(2):277-286.e4. doi: 10.1016/j.jaac.2018.06.038 [DOI] [PubMed] [Google Scholar]
- 39.Moore TM, Martin IK, Gur OM, et al. . Characterizing social environment’s association with neurocognition using census and crime data linked to the Philadelphia Neurodevelopmental Cohort. Psychol Med. 2016;46(3):599-610. doi: 10.1017/S0033291715002111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calkins ME, Merikangas KR, Moore TM, et al. . The Philadelphia Neurodevelopmental Cohort: constructing a deep phenotyping collaborative. J Child Psychol Psychiatry. 2015;56(12):1356-1369. doi: 10.1111/jcpp.12416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gur RC, Richard J, Calkins ME, et al. . Age group and sex differences in performance on a computerized neurocognitive battery in children age 8-21. Neuropsychology. 2012;26(2):251-265. doi: 10.1037/a0026712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Satterthwaite TD, Connolly JJ, Ruparel K, et al. . The Philadelphia Neurodevelopmental Cohort: A publicly available resource for the study of normal and abnormal brain development in youth. Neuroimage. 2016;124(Pt B):1115-1119. doi: 10.1016/j.neuroimage.2015.03.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erus G, Battapady H, Satterthwaite TD, et al. . Imaging patterns of brain development and their relationship to cognition. Cereb Cortex. 2015;25(6):1676-1684. doi: 10.1093/cercor/bht425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaufman J, Birmaher B, Brent D, et al. . Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980-988. doi: 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- 45.Shanmugan S, Wolf DH, Calkins ME, et al. . Common and dissociable mechanisms of executive system dysfunction across psychiatric disorders in youth. Am J Psychiatry. 2016;173(5):517-526. doi: 10.1176/appi.ajp.2015.15060725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291-303. doi: 10.1136/adc.44.235.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45(239):13-23. doi: 10.1136/adc.45.239.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980;9(3):271-280. doi: 10.1007/BF02088471 [DOI] [PubMed] [Google Scholar]
- 49.Gur RC, Richard J, Hughett P, et al. . A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J Neurosci Methods. 2010;187(2):254-262. doi: 10.1016/j.jneumeth.2009.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moore TM, Reise SP, Gur RE, Hakonarson H, Gur RC. Psychometric properties of the Penn Computerized Neurocognitive Battery. Neuropsychology. 2015;29(2):235-246. doi: 10.1037/neu0000093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Satterthwaite TD, Elliott MA, Ruparel K, et al. . Neuroimaging of the Philadelphia Neurodevelopmental Cohort. Neuroimage. 2014;86:544-553. doi: 10.1016/j.neuroimage.2013.07.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ciric R, Wolf DH, Power JD, et al. . Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. Neuroimage. 2017;154:174-187. doi: 10.1016/j.neuroimage.2017.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roalf DR, Quarmley M, Elliott MA, et al. . The impact of quality assurance assessment on diffusion tensor imaging outcomes in a large-scale population-based cohort. Neuroimage. 2016;125:903-919. doi: 10.1016/j.neuroimage.2015.10.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosen AFG, Roalf DR, Ruparel K, et al. . Quantitative assessment of structural image quality. Neuroimage. 2018;169:407-418. doi: 10.1016/j.neuroimage.2017.12.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang H, Suh JW, Das SR, Pluta JB, Craige C, Yushkevich PA. Multi-atlas segmentation with joint label fusion. IEEE Trans Pattern Anal Mach Intell. 2013;35(3):611-623. doi: 10.1109/TPAMI.2012.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51(5):527-539. doi: 10.1016/j.neuron.2006.08.012 [DOI] [PubMed] [Google Scholar]
- 57.Hua K, Zhang J, Wakana S, et al. . Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39(1):336-347. doi: 10.1016/j.neuroimage.2007.07.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Breiman M. Random forests. Mach Learn. 2001;45(1):5-32. doi: 10.1023/A:1010933404324 [DOI] [Google Scholar]
- 59.Lowe SR, Quinn JW, Richards CA, et al. . Childhood trauma and neighborhood-level crime interact in predicting adult posttraumatic stress and major depression symptoms. Child Abuse Negl. 2016;51:212-222. doi: 10.1016/j.chiabu.2015.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parent AS, Franssen D, Fudvoye J, Gérard A, Bourguignon JP. Developmental variations in environmental influences including endocrine disruptors on pubertal timing and neuroendocrine control: revision of human observations and mechanistic insight from rodents. Front Neuroendocrinol. 2015;38:12-36. doi: 10.1016/j.yfrne.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 61.Copeland WE, Shanahan L, Hinesley J, et al. . Association of childhood trauma exposure with adult psychiatric disorders and functional outcomes. JAMA Netw Open. 2018;1(7):e184493. doi: 10.1001/jamanetworkopen.2018.4493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cousminer DL, Widén E, Palmert MR. The genetics of pubertal timing in the general population: recent advances and evidence for sex-specificity. Curr Opin Endocrinol Diabetes Obes. 2016;23(1):57-65. doi: 10.1097/MED.0000000000000213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Callaghan BL, Richardson R. The effect of adverse rearing environments on persistent memories in young rats: removing the brakes on infant fear memories. Transl Psychiatry. 2012;2(7):e138. doi: 10.1038/tp.2012.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shields GS, Sazma MA, McCullough AM, Yonelinas AP. The effects of acute stress on episodic memory: a meta-analysis and integrative review. Psychol Bull. 2017;143(6):636-675. doi: 10.1037/bul0000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hanson JL, Chung MK, Avants BB, et al. . Structural variations in prefrontal cortex mediate the relationship between early childhood stress and spatial working memory. J Neurosci. 2012;32(23):7917-7925. doi: 10.1523/JNEUROSCI.0307-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gee D, Gabard-Durnam LJ, Flannery J, et al. . Early developmental emergence of mature human amygdala-prefrontal phenotype following maternal deprivation. Proc Natl Acad Sci U S A. 2013;110:15638-15643. doi: 10.1073/pnas.1307893110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62(1):42-52. doi: 10.1016/j.neuron.2009.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gennatas ED, Avants BB, Wolf DH, et al. . Age-related effects and sex differences in gray matter density, volume, mass, and cortical thickness from childhood to young adulthood. J Neurosci. 2017;37(20):5065-5073. doi: 10.1523/JNEUROSCI.3550-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gur RC, Dinges DF, Nasrini J, et al. Neurostructural, cognitive, and physiological changes during a 1-year Antarctic winter-over mission. Paper presented at: NASA Human Research Program Investigators’ Workshop; January 24, 2019; Houston, TX. [Google Scholar]
- 70.Teicher MH, Samson JA, Anderson CM, Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci. 2016;17(10):652-666. doi: 10.1038/nrn.2016.111 [DOI] [PubMed] [Google Scholar]
- 71.Westlye LT, Walhovd KB, Dale AM, et al. . Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cereb Cortex. 2010;20(9):2055-2068. doi: 10.1093/cercor/bhp280 [DOI] [PubMed] [Google Scholar]
- 72.Bath KG, Manzano-Nieves G, Goodwill H. Early life stress accelerates behavioral and neural maturation of the hippocampus in male mice. Horm Behav. 2016;82:64-71. doi: 10.1016/j.yhbeh.2016.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tanti A, Kim JJ, Wakid M, Davoli MA, Turecki G, Mechawar N. Child abuse associates with an imbalance of oligodendrocyte-lineage cells in ventromedial prefrontal white matter. Mol Psychiatry. 2018;23(10):2018-2028. doi: 10.1038/mp.2017.231 [DOI] [PubMed] [Google Scholar]
- 74.Barch DM, Belden AC, Tillman R, Whalen D, Luby JL. Early childhood adverse experiences, inferior frontal gyrus connectivity, and the trajectory of externalizing psychopathology. J Am Acad Child Adolesc Psychiatry. 2018;57(3):183-190. doi: 10.1016/j.jaac.2017.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blair C, Raver CC. Poverty, stress, and brain development: new directions for prevention and intervention. Acad Pediatr. 2016;16(3)(suppl):S30-S36. doi: 10.1016/j.acap.2016.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Clinical Assessment and SES Score Creation
eMethods 2. Neurocognitive Assessment, Neuroimaging, and Neuroimaging Data Processing Details
eResults. Structural Equation Model
eTable 1. Variables included in Socioeconomic Status (SES) Score With Weights
eTable 2. Demographics for the 4 Groups Contrasted in Figures
eTable 3. Results of the MMRM on the Main Demographic and Trauma Factors With Symptom Domains as Dependent Measures
eTable 4. Results of Logistic Regression Analysis on the Main Demographic and Trauma Factors With Proportion of Individuals at Each Age Bracket That Has Reached Puberty According to the Pubic Hair Item of the Tanner Scale
eTable 5. Results of the MMRM on the Main Demographic and Trauma Factors With Cognitive Domain Scores as Dependent Measures
eTable 6. Mixed-Model Repeated Measures Results for Neurocognitive Performance Accuracy and Speed
eTable 7. Results of the Mixed-Model Repeated Measures on the Main Demographic and Trauma Factors With Brain Parameters as Dependent Measures
eTable 8. Results of Logistic Regression Analysis on the Main Demographic and Trauma Factors With Proportion of Individuals at Each Age Bracket That Were Classified as Having Adult Brain by the Machine-Learning Classifier
eTable 9. Structural Model Results Using All Variables
eTable 10. Correlation Matrix Among the Dependent Measures
eFigure 1. Scatterplot of Association Between Number of Traumatic Experiences and Socioeconomic Status
eFigure 2. The Relation of Trauma Category to Cognitive Performance on the 4 Efficiency Domains
eFigure 3. The 5-Way Interaction (Sex × SES × TSE × Domain × Race)
eReferences.