This randomized noninferiority trial compares the effect of laparoscopic distal gastrectomy with open distal gastrectomy for locally advanced gastric cancer.
Key Points
Question
Does laparoscopic distal gastrectomy yield inferior oncological outcomes to open distal gastrectomy for patients with locally advanced gastric cancer?
Findings
In this randomized clinical trial that included 1056 patients with clinically staged locally advanced gastric cancer, laparoscopic vs open distal gastrectomy resulted in a 3-year disease-free survival rate of 76.5% vs 77.8%, respectively, a difference that did not exceed the noninferiority margin of −10%.
Meaning
These findings support the use of laparoscopic gastrectomy for patients assessed as having locally advanced cancer preoperatively.
Abstract
Importance
Laparoscopic distal gastrectomy is accepted as a more effective approach to conventional open distal gastrectomy for early-stage gastric cancer. However, efficacy for locally advanced gastric cancer remains uncertain.
Objective
To compare 3-year disease-free survival for patients with locally advanced gastric cancer after laparoscopic distal gastrectomy or open distal gastrectomy.
Design, Setting, and Patients
The study was a noninferiority, open-label, randomized clinical trial at 14 centers in China. A total of 1056 eligible patients with clinical stage T2, T3, or T4a gastric cancer without bulky nodes or distant metastases were enrolled from September 2012 to December 2014. Final follow-up was on December 31, 2017.
Interventions
Participants were randomized in a 1:1 ratio after stratification by site, age, cancer stage, and histology to undergo either laparoscopic distal gastrectomy (n = 528) or open distal gastrectomy (n = 528) with D2 lymphadenectomy.
Main Outcomes and Measures
The primary end point was 3-year disease-free survival with a noninferiority margin of −10% to compare laparoscopic distal gastrectomy with open distal gastrectomy. Secondary end points of 3-year overall survival and recurrence patterns were tested for superiority.
Results
Among 1056 patients, 1039 (98.4%; mean age, 56.2 years; 313 [30.1%] women) had surgery (laparoscopic distal gastrectomy [n=519] vs open distal gastrectomy [n=520]), and 999 (94.6%) completed the study. Three-year disease-free survival rate was 76.5% in the laparoscopic distal gastrectomy group and 77.8% in the open distal gastrectomy group, absolute difference of −1.3% and a 1-sided 97.5% CI of −6.5% to ∞, not crossing the prespecified noninferiority margin. Three-year overall survival rate (laparoscopic distal gastrectomy vs open distal gastrectomy: 83.1% vs 85.2%; adjusted hazard ratio, 1.19; 95% CI, 0.87 to 1.64; P = .28) and cumulative incidence of recurrence over the 3-year period (laparoscopic distal gastrectomy vs open distal gastrectomy: 18.8% vs 16.5%; subhazard ratio, 1.15; 95% CI, 0.86 to 1.54; P = .35) did not significantly differ between laparoscopic distal gastrectomy and open distal gastrectomy groups.
Conclusions and Relevance
Among patients with a preoperative clinical stage indicating locally advanced gastric cancer, laparoscopic distal gastrectomy, compared with open distal gastrectomy, did not result in inferior disease-free survival at 3 years.
Trial Registration
ClinicalTrials.gov Identifier: NCT01609309
Introduction
Gastric cancer is a common cancer and a leading cause of cancer-related deaths worldwide.1,2,3 More than 90% of early gastric cancer is curative with surgical resection alone, and laparoscopic distal gastrectomy with limited lymphadenectomy is recommended for patients with early gastric cancer located in the middle or lower third of the stomach.4,5,6 In contrast to early-stage gastric cancer, surgery for locally advanced gastric cancer (defined as T2-4aN0-3M0, corresponding to stages Ib to IIIc excluding T1 or T4b tumors; American Joint Committee on Cancer’s AJCC Cancer Staging Manual)7 is technically more challenging because dissection of D2 lymph nodes is required.4 The ability to perform adequate D2 lymphadenectomy using laparoscopic approaches is uncertain.
Therefore, the Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) group conducted a multicenter randomized trial (CLASS-01 trial) to determine the noninferiority of laparoscopic distal gastrectomy (experimental group) compared with open distal gastrectomy (control group) for 3-year disease-free survival among patients whose preoperative clinical stage indicated locally advanced gastric cancer defined as having a stage T2, T3, or T4a tumor but lacking metastatic disease or bulky lymph nodes. Safety results demonstrated shorter hospital stays and faster postoperative recovery for laparoscopic distal gastrectomy than did open distal gastrectomy.8 The present study reports the trial’s primary outcome of 3-year disease-free survival and secondary outcomes of 3-year overall survival and recurrence patterns.
Methods
Study Design
The current study was a noninferiority, open-label, randomized clinical trial conducted at 14 hospitals in China from September 2012 to December 2017. Approval was obtained from the institutional review board of each participating hospital, and all patients provided written informed consent. An independent contract research organization was responsible for oversight of the study. The approved study protocol and statistical analysis plan are available in Supplement 1.
Cancer Staging
According to the Japanese classification of gastric carcinoma: 3rd English edition,9 early-stage gastric cancer is invasive gastric cancer that reaches no farther than the submucosa, irrespective of lymph node metastasis (ie, T1N0-3M0). The current study therefore defined locally advanced gastric cancer as clinical T2-4aN0-3M0, corresponding to AJCC clinical stages Ib to IIIc excluding T1 or T4b tumors (stage Ib: T2N0M0, stage II: T2N1-2M0, T3N0-1M0, T4aN0M0, stage III: T2N3M0, T3N2-3M0, T4aN1-3M0). The Japanese Classification was used to define tumors as localized vs locally advanced vs metastatic. Further categorization into staging subgroups was described on the basis of the AJCC Cancer Staging Manual, 7th edition (AJCC 7).7
Patients
Patients were included if they were aged 18 to 75 years; had an Eastern Cooperative Oncology Group (ECOG) score of 0 (asymptomatic) or 1 (symptomatic but completely ambulatory); had histologically confirmed gastric adenocarcinoma diagnosed at clinical locally advanced stage according to the Japanese Classification9 and to T2-4aN0-3M0, corresponding to stages Ib to IIIc excluding T1 or T4b tumors according to the AJCC 77; had tumors located in the lower or middle third of the stomach by preoperative evaluation; and were expected to undergo distal gastrectomy with D2 lymphadenectomy for curative intent. Patients were excluded if they had enlarged or bulky regional lymph nodes, larger than 3 cm at the long diameter according to preoperative imaging. Detailed eligibility criteria are shown in eTable 1 in Supplement 2.
Randomization
For randomization, a central dynamic, stratified strategy was adopted.10 The randomization sequence was generated using the Pocock-Simon minimization method in SAS version 9.3 (SAS Institute Inc) and stratified by participating site (14 hospitals), patient age (≤60 or >60 years), clinical TNM stage (I or II or III, but excluding T1 or T4b tumors),7 and histological type (signet ring cell carcinoma or not). Participating sites submitted the above information to the data center at the Department of Biological Statistics, Southern Medical University, Guangzhou, China, where central randomization was performed. Information on treatment allocation was subsequently sent to each participating site.
Procedures
Both laparoscopic distal gastrectomy and open distal gastrectomy complied with the principles of the extent of distal gastrectomy and D2 lymph node dissection in accordance with the Japanese guidelines.11 Laparoscopic distal gastrectomy was performed using 5 trocars for diagnostic tumor staging and lymph node dissection and a minilaparotomy for specimen retraction and anastomosis. In laparoscopic distal gastrectomy, the ultrasonic scalpel was adopted for mobilization and dissection, whereas in open distal gastrectomy an electronic or ultrasonic scalpel was used. The methods of anastomosis were determined by the surgeon’s preference and the patient’s anatomy. Once the length of the minilaparotomy exceeded 10 cm, laparoscopic distal gastrectomy was considered an open surgery conversion according to the study protocol.
For patients with pathologic stage II or higher tumors,7 adjuvant chemotherapy with 6 months of a fluorouracil–based chemotherapy was mandated, with the choice of regimen and treatment duration at the discretion of the treating oncologist.
Outcomes
The study’s primary end point was 3-year disease-free survival, and the secondary end points were 3-year overall survival and recurrence patterns. Secondary outcomes pertaining to safety, including intraoperative and postoperative (≤30 days) morbidities and mortalities, have been previously reported.8
A minimum follow-up of 36 months was required and achieved for each patient after surgery. Follow-up care included (1) medical history every 3 months for the first 2 years and every 6 months thereafter; (2) physical examination and blood testing with carcinoembryonic antigen and cancer antigen 19-9 every 3 months for the first 2 years, and every 6 months thereafter; (3) chest x-ray and abdominal computed tomographic scans every 6 months for 3 years; and (4) upper gastrointestinal endoscopy annually for 3 years. Positron emission tomography–computed tomography was performed if recurrence was suspected. Recurrence was identified by medical history and physical examination in combination with imaging evaluation, cytology, or tissue biopsy (preferred when feasible).
Statistical Considerations
Noninferiority Margin and Sample Size
Trials involving early gastric cancer comparing laparoscopic with open surgery have used a noninferiority margin of 5% for disease-free survival (corresponding to a hazard ratio [HR] of 1.54).5,6 Given that the absolute difference in survival rate corresponding to an equal length of survival time tended to increase with the increasing tumor stage, a noninferiority margin of −10% for 3-year disease-free survival corresponding to a similar HR of 1.46 was selected for the current study. The relatively wide noninferiority margin was deemed acceptable to clinicians and patients, considering that (1) it was smaller than the survival differences between stage IIa and IIb and between stage IIIa and IIIc gastric cancer based on data reported in the AJCC 77 and that (2) the expectation that laparoscopic gastrectomy might be superior to open gastrectomy with less surgical trauma, fewer overall complications, and quicker recovery.12
Based on a previous randomized clinical trial of locally advanced gastric cancer in which 3-year disease-free survival was 72.2% for patients undergoing open gastrectomy,13 a sample size of 422 patients per group was calculated as necessary for 90% power to detect a noninferiority margin of −10% with a 1-sided α of .025. Assuming a dropout rate of 20%, sample size was increased to 528 patients for each group. The sample size calculation was conducted using nQuery Advisor 7.0 (Statistical Solutions Ltd).
Analytic Populations
The primary analysis was based on the primary analysis set, defined as the cohort of participants randomized, excluding those who withdrew consent preoperatively or who had unresectable gastric cancer detected intraoperatively. A prespecified secondary analysis of the per-protocol population further excluded patients who received total rather than distal gastrectomy, had inadequate D2 lymphadenectomy, or were switched to the other surgical approach preoperatively or intraoperatively. A third analysis was performed on the as-treated population, which included all participants in the per-protocol population as well as those who received a different surgical treatment than the one assigned at random. In this post hoc analysis, patients were assigned to a treatment group based on the treatment they actually received.
The primary analysis set population was used for all analyses; the secondary per-protocol analysis and the post hoc as-treated analysis were conducted for the primary end point only.
Statistical Analyses
A noninferiority analysis was used as the primary analysis for 3-year disease-free survival. Noninferiority was established if the lower bound of the 1-sided 97.5% CI derived by the Newcombe method was greater than the prespecified margin of −10% (laparoscopic distal gastrectomy minus open distal gastrectomy). All other analyses of disease-free survival and secondary outcomes were performed using conventional 2-tailed superiority hypothesis tests with α = .05 and with 2-sided 95% CIs. All analyses of secondary outcomes were post hoc except for the log-rank tests and Cox regressions.
The 3-year disease-free and overall survival rates were calculated using the Kaplan-Meier method. The HRs comparing laparoscopic distal gastrectomy with open distal gastrectomy were estimated by mixed-effects Cox regression which accounted for center effects. Multivariable mixed effects Cox regressions were performed to evaluate the effect of surgery type on disease-free and overall survival, after adjustment for clinicopathologic covariates that were significantly associated with the outcome in bivariable analyses, including age, sex, body mass index, ECOG score, comorbidities, tumor size, histology, pathologic T and N stages, and adjuvant chemotherapy status. The proportional hazard assumption was checked for each variable by testing the independence of Schoenfeld residuals with time, and a time-dependent Cox regression was used if the assumption was violated.
For recurrence, all-cause mortality was treated as the competing event. Cumulative incidence in the presence of competing risks was calculated and competing-risks survival regression was used as an alternative to Cox regression.
Subgroup analyses, using log-rank tests, were conducted for disease-free and overall survival stratified by pathologic stage (ie, stage I, II, III, IV; )7 and by the lymph node status of the pathologic T4a stage (ie, T4aN0 vs T4aN+).7 Mixed-effects Cox regression with an interaction term was used to test if the differences in effect size among subgroups were statistically significant. In addition, 2 post hoc sensitivity analyses were performed: considering the high proportion of overstaged patients who did not have pathologic T stage of at least T2, the noninferiority analysis for the primary end point was repeated after the exclusion of patients with pathologic T1N0-3M0 or with pathologic stage I defined as T1N0-1M0 or T2N0M0.7
The level of missingness was determined for each variable, and multiple imputations were performed if the missing data accounted for more than 5%. Because all superiority tests were considered exploratory, no correction for type I error was made. Analyses were performed with SAS software version 9.3 (SAS Institute Inc), Stata software version 14 (StataCorp LP), and RStudio version 1.1.419 (RStudio Inc).
Results
Study Population
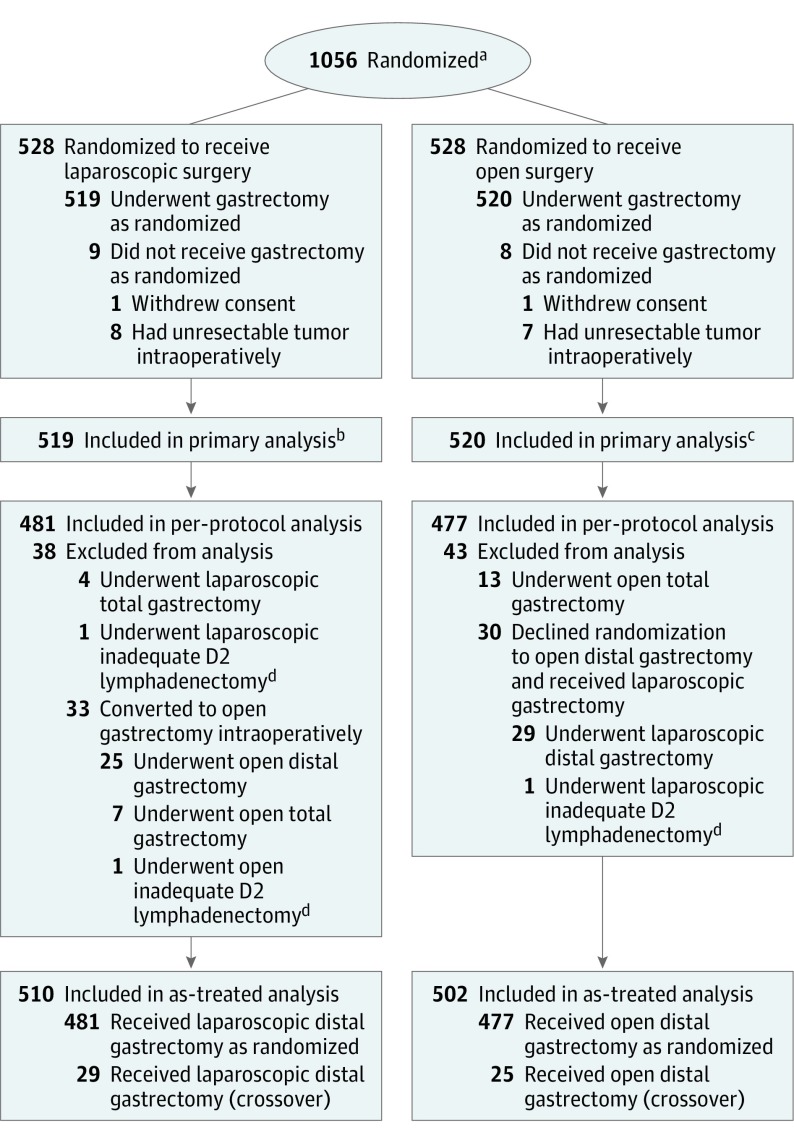
From September 12, 2012, to December 3, 2014, 1056 patients were randomly assigned to the laparoscopic distal gastrectomy group or the open distal gastrectomy group (n = 528 per group) (Figure 1). In the laparoscopic group, 1 patient withdrew informed consent and 8 had unresectable gastric cancer detected intraoperatively. In the open group, 1 patient withdrew informed consent and 7 had unresectable gastric cancer detected intraoperatively. The primary analysis set consisted of 519 patients in the laparoscopic group and 520 in the open group. The per-protocol population consisted of 958 patients, with 481 in the laparoscopic group (519 patients in the primary analysis set minus 38 patients who did not adhere to their treatment plans) and 477 in the open group (520 patients in the primary analysis set minus 43 patients who did not adhere to their treatment plans). The as-treated population consisted of 510 patients in the laparoscopic group (481 per-protocol patients plus 29 patients with protocol crossovers), and 502 patients in the open group (477 per-protocol patients plus 25 patients with protocol crossovers). The median follow-up period was 37.9 months (interquartile range, 35.9-42.3 months), with a total of 40 patients (3.8%) lost to follow-up (26 in the laparoscopic group and 14 in the open group).
Figure 1. Flow of Patient Enrollment and Randomization.
aData for number screened for eligibility and reasons for exclusion were not available.
bIncludes 26 patients who were lost to follow-up and 2 patients who died within 30 days after the surgery (1 due to respiratory failure as a result of pneumonia and the other due to a cerebrovascular accident).
cIncludes 14 patients who were lost to follow-up.
dIndicates cases with more than 1 missing lymph node station according to the guidelines of The Japanese Research Society for Gastric Cancer (JRSGC) lymph node grouping.
The baseline and postoperative characteristics of the patients are shown in Table 1 and Table 2, respectively. Most patients in both groups were men (380 [73.2%] in the laparoscopic group and 346 [66.5%] in the open group). Both groups were moderately skewed to a younger age, with a mean age of 56.5 years (SD, 10.4 years) in the laparoscopic group and 55.8 years (SD, 11.1 years) in the open group. Although all patients were diagnosed at clinical T2 stage or higher, 248 patients (23.9%) were found to have pathologic T1 tumors (116 [22.4%] in the laparoscopic group and 132 [25.4%] in the open group). The amount of missing data was low (no variables with missingness >5%; Table 1 and Table 2), and therefore, no multiple imputations were conducted.
Table 1. Baseline Demographic and Clinical Characteristics of Patients Who Underwent Laparoscopic or Open Surgery.
| Characteristics | Surgery, No. (%) | |
|---|---|---|
| Laparoscopic (n = 519)a | Open (n = 520)a | |
| Sex | ||
| Men | 380 (73.2) | 346 (66.5) |
| Women | 139 (26.8) | 174 (33.5) |
| Age, y | (n = 518) | |
| Mean (SD) | 56.5 (10.4) | 55.8 (11.1) |
| Median (IQR) | 57 (51-64) | 57 (50-64) |
| BMI | (n = 504) | (n = 511) |
| Mean (SD) | 22.7 (3.2) | 22.7 (3.2) |
| Eastern Cooperative Oncology Group scoreb | (n = 517) | (n = 518) |
| 0 (asymptomatic) | 375 (72.5) | 391 (75.5) |
| 1 (symptomatic but completely ambulatory) | 142 (27.5) | 127 (24.5) |
| Comorbidities present | (n = 518) | (n = 517) |
| No. (%) | 159 (30.7) | 139 (26.9) |
| Tumor size, cm | (n = 499) | (n = 503) |
| Mean (SD) | 4.0 (2.0) | 4.0 (2.1) |
| Histology | ||
| Signet-ring cell | 79 (15.2) | 99 (19.0) |
| Otherc | 440 (84.8) | 421 (81.0) |
| Clinical T stage | ||
| T2 | 115 (22.2) | 146 (28.1) |
| T3 | 177 (34.1) | 173 (33.3) |
| T4a | 227 (43.7) | 201 (38.7) |
| Clinical N stage | ||
| N0 | 252 (48.6) | 258 (49.6) |
| N+ | 267 (51.5) | 262 (50.4) |
| Clinical M stage (M0) | 519 (100) | 520 (100) |
| Clinical TNM staged | ||
| I | 64 (12.3) | 88 (16.9) |
| II | 248 (47.8) | 247 (47.5) |
| III | 207 (39.9) | 185 (35.6) |
Abbreviations: BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; IQR, interquartile range.
Unless otherwise indicated.
The Eastern Cooperative Oncology Group score ranges from 0 to 5, with 0 denoting asymptomatic, 1 denoting “symptomatic but completely ambulatory,” 2 denoting “symptomatic, <50% in bed during the day,” 3 denoting “symptomatic, >50% in bed, but not bedbound,” 4 denoting bedbound, and 5 denoting death.
Includes papillary, tubular, mucous, and poorly differentiated adenocarcinoma.
According to the Cancer Staging Manual, 7th edition,7 a T2 tumor invades muscularis propria; a T3 tumor penetrates subserosal connective tissue without invasion of visceral peritoneum or adjacent structures; a T4a tumor invades serosa (visceral peritoneum); and a T4b tumor invades adjacent structures. N0 indicates no regional lymph node metastasis and N+, metastasis in lymph nodes. M0 indicates no distant metastasis and M1, distant metastasis. In the current study, clinical stage I includes T2N0M0; stage II, T2N1M0, T3N0M0, T2N2M0, T3N1M0, and T4aN0M0; and stage III, T2N3M0, T3N2M0, T4aN1M0, T4aN2M0, T4aN3M0, and T3N3M0.
Table 2. Postoperative Pathologic and Clinical Characteristics of Patients Who Underwent Laparoscopic or Open Surgery.
| Characteristics | Surgery, No. (%) | |
|---|---|---|
| Laparoscopic (n = 519)a | Open (n = 520)a | |
| No. of retrieved lymph nodes | (n = 509) | (n = 507) |
| Mean (SD) | 36.1 (16.7) | 36.9 (16.1) |
| No. of metastatic lymph nodes | (n = 516) | (n = 516) |
| Mean (SD) | 4.9 (8.0) | 4.5 (6.9) |
| Received chemotherapy | 192 (37.0) | 217 (41.7) |
| Pathologic T stage | (n = 518) | (n = 519) |
| T1 | 116 (22.4) | 132 (25.4) |
| T2-T4a | 394 (76.1) | 383 (73.8) |
| T4b | 8 (1.5) | 4 (0.8) |
| Pathologic N stage | (n = 518) | (n = 519) |
| N0 | 214 (41.3) | 216 (41.6) |
| N1 | 87 (16.8) | 79 (15.2) |
| N2 | 88 (17.0) | 98 (18.9) |
| N3 | 129 (24.9) | 126 (24.3) |
| Pathologic M stage | (n = 518) | (n = 519) |
| M0 | 510 (98.5) | 511 (98.5) |
| M1 | 8 (1.5) | 8 (1.5) |
| Pathologic TNM stageb | (n = 518) | (n = 519) |
| Ia | 87 (16.8) | 99 (19.1) |
| Ib | 64 (12.4) | 53 (10.2) |
| IIa | 66 (12.7) | 59 (11.4) |
| IIb | 71 (13.7) | 79 (15.2) |
| IIIa | 69 (13.3) | 77 (14.8) |
| IIIb | 73 (14.1) | 78 (15.0) |
| IIIc | 77 (14.9) | 66 (12.7) |
| IV | 11 (2.1) | 8 (1.5) |
| Received adjuvant chemotherapy | 192 (37.0) | 217 (41.7) |
Unless otherwise indicated.
According the Cancer Staging Manual, 7th edition,7 a T1 tumor invades lamina propria, muscularis mucosae, or submucosa; a T2 tumor invades muscularis propria; a T3 tumor penetrates subserosal connective tissue without invasion of visceral peritoneum or adjacent structures; a T4a tumor invades serosa (visceral peritoneum); and a T4b tumor invades adjacent structures. N0 denotes no regional lymph node metastasis; N1, metastasis in 1 to 2 regional lymph nodes; N2, metastasis in 3 to 6 regional lymph nodes; N3, metastasis in 7 or more regional lymph nodes. M0, denotes no distant metastasis and M1, distant metastasis. In the current study, pathologic stage Ia includes T1N0M0; stage Ib, T2N0M0 and T1N1M0; stage IIa, T2N1M0, T3N0M0, and T1N2M0; stage IIb, T1N3M0, T2N2M0, T3N1M0, and T4aN0M0; stage IIIa, T2N3M0, T3N2M0, and T4aN1M0; stage IIIb, T4aN2M0, T3N3M0, T4bN0M0, and T4bN1M0; stage IIIc, T4bN2M0, T4aN3M0, and T4bN3M0; stage IV, TanyNanyM1.
Primary Outcome: Disease-Free Survival
Primary Analysis Set
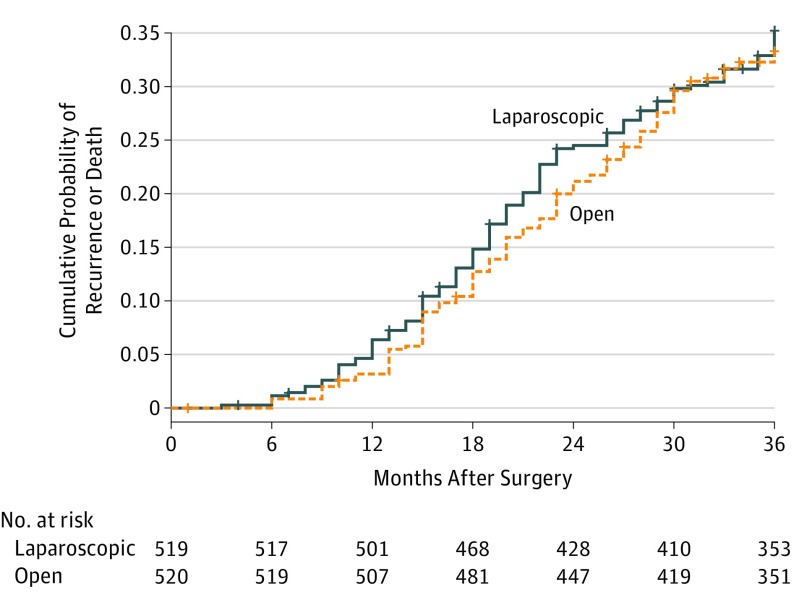
The 3-year disease-free survival rates were 76.5% (119) in the laparoscopic group and 77.8% (114) in the open group, with an absolute difference of −1.3% and a 1-sided 97.5% CI (−6.5% to ∞) that did not cross the prespecified noninferiority margin of −10% (Figure 2). In the post hoc sensitivity analysis comparing laparoscopic with open distal gastrectomy, the exclusion of the 248 patients with pathologic T1N0-3M0 tumors resulted in an absolute difference of −2.4% (1-sided 97.5% CI, −8.7% to ∞). Exclusion of the 303 patients with pathologic stage I tumors (corresponding to T1N0-1M0 and T2N0M0) resulted in an absolute difference of −3.9% with a 1-sided 97.5% CI (−10.6% to ∞) that crossed the prespecified noninferiority margin.
Figure 2. Kaplan-Meier Curves of Cumulative Probability of Recurrence or Death for Laparoscopic Distal Gastrectomy vs Open Distal Gastrectomy Within 3 Years After Surgery.
For both curves the median follow-up was 38 months (interquartile range, 36-42 months).
Accounting for center effects, the mixed-effects Cox regression model yielded a nonsignificant HR of 1.07 (95% CI, 0.83-1.39; P = .59) comparing laparoscopic with open distal gastrectomy (eTable 2 in Supplement 2). A similar HR was observed after adjusting for age, tumor size, pathologic T stage, pathologic N stage, and adjuvant chemotherapy (laparoscopic vs open distal gastrectomy HR, 1.10; 95% CI, 0.84-1.43; P = .49) (eTable 2 in Supplement 2).
Per-Protocol and As-Treated Populations
In the per-protocol analysis, the 3-year disease-free survival rates were 77.6% (105 of 481) patients who died or had a recurrence calculated by time to event in the laparoscopic group and 78.5% (101 of 477) in the open group, with an absolute difference of −0.9% (1-sided 97.5% CI, −6.1% to ∞). In the as-treated analysis, the 3-year disease-free survival rates were 77.7% (107 of 510) in the laparoscopic group and 78.4% (111 of 502) in the open group, with an absolute difference of −0.7% (1-sided 97.5% CI, −6.0% to ∞).
Secondary Outcomes
Overall Survival
The deaths of 160 patients resulted in 3-year overall survival rates of 83.1% (85 of 519) in the laparoscopic group and 85.2% (75 of 520) in the open group (Table 3). The HR for all-cause mortality in the laparoscopic group compared with that in the open group was 1.17 (95% CI, 0.86-1.59; P = .33) in the univariate mixed-effects Cox model (eTable 2 in Supplement 2). This estimate remained similar after controlling for age, tumor size, pathologic T stage, and pathologic N stage (laparoscopic vs open groups HR, 1.19; 95% CI, 0.87-1.64; P = .28) (eTable 2 in Supplement 2). The cumulative incidence for cause-specific death did not significantly differ between the 2 groups (all P > .05; Table 3).
Table 3. Frequencies of Causes of First Recurrence and Death Within 3 Years After Surgery in Patients Who Underwent Laparoscopic or Open Surgery.
| Events | Surgery, No. (%) | Risk Differencea | Hazard Ratio (95% CI)b | P Valuec | |
|---|---|---|---|---|---|
| Laparoscopic (n = 519) | Open (n = 520) | ||||
| Any recurrenced | 95 (18.3) | 85 (16.3) | 0.022 | 1.15 (0.86-1.54) | .35 |
| Local | 15 (15.8) | 22 (25.9) | −0.013 | 0.68 (0.35-1.31) | .25 |
| Peritoneum | 18 (18.9) | 19 (22.4) | −0.002 | 0.96 (0.51-1.83) | .91 |
| Liver | 18 (18.9) | 11 (12.9) | 0.014 | 1.67 (0.78-3.51) | .19 |
| Multiple sitese | 18 (18.9) | 10 (11.8) | 0.017 | 1.82 (0.84-3.95) | .13 |
| Other or uncertain sitesf | 26 (27.4) | 23 (27.1) | 0.006 | 1.16 (0.66-2.02) | .61 |
| Cause of deathg | 85 (16.4) | 75 (14.4) | −0.021 | 1.17 (0.86-1.59) | .33 |
| Gastric cancer | 70 (82.4) | 57 (76.0) | 0.027 | 1.27 (0.89-1.79) | .19 |
| Other causesh | 15 (17.6) | 18 (24.0) | −0.006 | 0.84 (0.42-1.67) | .62 |
Except for all-cause death, the risk difference was calculated by subtracting the cumulative incidence in the first 3 years of the open group from that of the laparoscopic group, in presence of competing events; for all-cause death, the risk difference was calculated by subtracting the 3-year overall survival rate of the open group from that of the laparoscopic group.
Except for all-cause death, competing-risks survival regression was used to derive the hazard ratio, 95% CI, and P value. For total recurrence, all-cause death was the competing event; for the specific types of recurrence, other types of recurrence and death were the competing events; for gastric cancer cause of death, other causes of death were the competing events, and vice versa. Mixed-effects Cox regression was used for all-cause death.
P value for the hazard ratios.
Refers only to first-time recurrence, even though patients can have recurrence at multiple times.
Includes patients who have recurrence simultaneously in 2 or more metastatic sites, including peritoneum, liver, lung, bone, brain, distant lymph node, or other hematogenous metastatic sites.
Includes hematogenous recurrence at sites other than liver (ie, lung, bone, brain), recurrence at distant lymph node, and recurrence at uncertain sites.
Post hoc exploratory outcomes.
Includes other cancers, diseases other than cancer, unintentional injuries, and unknown causes.
Recurrence
Within the first 3 years of follow-up, recurrence was found in 95 (cumulative incidence, 18.8%) and 85 (cumulative incidence, 16.5%) patients in the laparoscopic and open groups, respectively (Table 3). The cumulative incidence of recurrence is shown in eFigure 1 in Supplement 2. Treating death as the competing risk, no significant difference in the recurrence cumulative incidence was found comparing laparoscopic with open distal gastrectomy (subhazard ratio, 1.15; 95% CI, 0.86-1.54; P = .35). The cumulative incidence of recurrence for all other specific types of recurrence did not significantly differ between the 2 groups (all P > .05; Table 3). The sites of recurrence were similar between the 2 groups (χ2 P = .29).
Subgroup Analysis
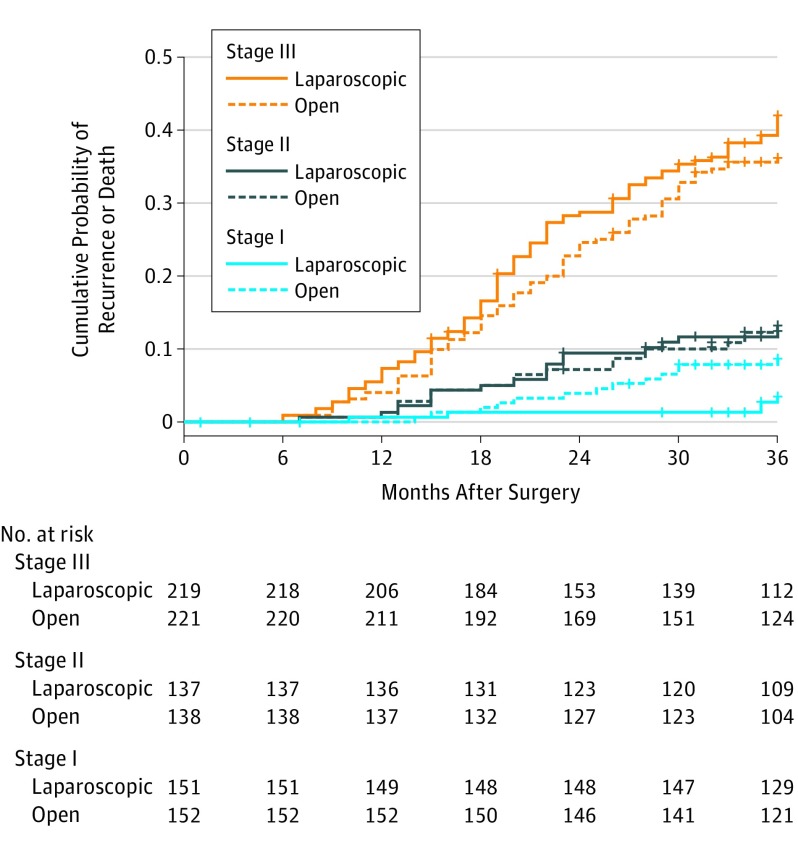
The 3-year disease-free survival rates for the laparoscopic and open groups, for patients with pathologic stage I were 96.5% vs 91.3% (log-rank P = .05); for stage II, 87.5% vs 86.8% (log-rank P = .89); for stage III, 58.0% vs 63.8% (log-rank P = .23), and for stage IV, 20.8% vs 58.3% (log-rank P = .13). Interaction tests showed that the difference in disease-free survival between the 2 groups was significantly larger among patients with pathologic stages III and IV than among patients with pathologic stage I (interaction P = .04 and 0.02, respectively). The between-group differences in 3-year disease-free survival rates did not differ by lymph node status among patients with pathologic T4a tumors (pT4aN0: 81.4% in the laparoscopic group vs 87.6% in the open group, log-rank P = .39; pT4aN+: 55.1% in the laparoscopic group vs 61.8% in the open group, log-rank P = .26; interaction P = .64). The cumulative probability of event by treatment for the subgroups of stages and the subgroups of T4a tumors are shown in Figure 3; eFigure 2 in Supplement 2, respectively.
Figure 3. Kaplan-Meier Curves of Cumulative Probability of Recurrence or Death for Laparoscopic Distal Gastrectomy vs Open Distal Gastrectomy Within 3 Years After Surgery by Pathologic Stage.
The median follow-up for patients with stage III in the laparoscopic group was 37 months (interquartile range [IQR], 31-41 months) and in the open group, 38 months (IQR, 34-41 months; with stage II in the laparoscopic group, 38 months (IQR, 36-44 months) and in the open group, 39 months (IQR, 37-45 months); and with stage I in the laparoscopic, 39 months (IQR, 37-45 months) and in the open group, 39 months (IQR, 37-45 months).
The 3-year overall survival rates for the laparoscopic and open groups, among patients with pathologic stage I was 97.9% vs 97.3% (P = .72); stage II, 92.5% vs 92.6% (P = .96); stage III, 69.5% vs 73.2% (P = .42); and stage IV, 20.0% vs 66.7% (P = .06). Interaction tests showed that the differences in overall survival between the 2 groups did not significantly differ across the stages (all interaction, P > .05). The between-group differences in 3-year overall survival rates did not differ by lymph node status among patients with pathologic T4a tumors (pT4aN0: 86.1% in the laparoscopic group vs 92.5% in the open group, log-rank P = .33; pT4aN+: 63.9% in the laparoscopic group vs 69.0% in the open, log-rank P = .37; interaction P = .53). The cumulative mortality rates by treatment for the subgroups of pathologic stages and the subgroups of T4a tumors are shown in eFigure 3 and eFigure 4 in Supplement 2, respectively.
Discussion
This multicenter randomized clinical trial conducted at 14 centers in China among patients with locally advanced gastric cancer (clinical stage T2-4aN0-3M0), who had distal gastrectomy with D2 lymphadenectomy performed by 1 of 15 experienced surgeons, found that the 3-year disease-free survival of patients assigned to the laparoscopic distal gastrectomy group was not inferior to that of patients assigned to the open distal gastrectomy group. Additionally, no significant differences were found between the groups in the overall survival rate and cumulative incidence of recurrence over the 3-year period.
Surgical safety of laparoscopic gastrectomy for treatment of locally advanced gastric cancer has previously been confirmed by 2 large randomized clinical trials.8,14 Compared with open gastrectomy, laparoscopic gastrectomy has been shown to have lower rates of intraoperative and postoperative morbidity as well as faster recovery.8,14 The safety and efficacy of laparoscopic distal gastrectomy for stage I gastric cancer has recently been confirmed by a Korean randomized trial, which showed decreased morbidity5 and noninferior 5-year overall survival15 of the laparoscopic approach compared with the open approach. However, in the context of early-stage gastric cancer, a D2 lymphadenectomy is not necessarily required and there has been uncertainty about the balance of risks and benefits for laparoscopic approaches when D2 resection is indicated to remove all potentially malignant lymph nodes.15 The safety of laparoscopic approaches with D2 lymphadenectomy have been demonstrated previously by a number of clinical trials.14,16,17 In addition, for the present study, short-term surgical outcomes including postoperative morbidity, mortality, and complication rates were similar for laparoscopic vs open distal gastrectomy.8
From the technical point of view, laparoscopic approaches have the benefits over open surgeries through visual magnification, better exposure, and more delicate maneuvers of organs, vessels, and nerves. Although the safety is now established, the oncological efficacy of laparoscopic approaches with D2 lymphadenectomy has continued to be the subject of debate. Compared with open gastrectomy, a laparoscopic approach can compromise the ability to perform an adequate D2 lymphadenectomy and a complete resection (R0). The laparoscopic manipulation and pneumoperitoneum effects could increase the risk of cancer cell dissemination to nearby organs, particularly for tumors with serosal invasion (ie, T4a) and positive lymph node metastasis; therefore potentially elevating the risk of recurrence.18
The current multicenter trial found that the 3-year disease-free survival rate in the laparoscopic distal gastrectomy group (76.5%) was not inferior to that in the open distal gastrectomy group (77.8%), using −10% as the noninferiority margin. Previously published observational studies19,20 and smaller-scale randomized trials21,22 have similarly reported that neither 3-year nor 5-year disease-free survival were significantly different between laparoscopic gastrectomy and open gastrectomy groups. This noninferiority of efficacy, along with the superiority of safety over open gastrectomy,12 suggest that the indication for laparoscopic distal gastrectomy could be extended to include locally advanced gastric cancer.
Although the purpose of this study was to compare the effectiveness of laparoscopic distal gastrectomy and open distal gastrectomy for locally advanced gastric cancer, a large proportion of participants in the trial were overstaged and at pathology were found to have early stage gastric cancer. Specifically, 248 patients (23.9%) with clinical T2 and above had pathologic T1 tumors, and 303 (29.2%) had pathologic stage I tumors, corresponding to early-stage not locally advanced gastric cancer. Similar degree of inconsistency between clinical and pathologic T stages has recently been reported by 2 large validation studies conducted in Japan.23,24
To address the issue of overstaging, 2 post hoc sensitivity analyses were conducted and yielded contradictory results. After exclusion of patients with pathologic T1 tumors, noninferiority remained significant. However, after exclusion of patients with pathologic stage I tumors, noninferiority became nonsignificant, with the lower bound (−10.6%) just crossing the 10% noninferiority margin. Although the current study supports the noninferiority of laparoscopic distal gastrectomy compared with open distal gastrectomy for locally advanced gastric cancer—defined as clinical T2-4aN0-3M0 with the important caveat that about a quarter of patients’ tumors were down staged to T1 by pathologic review of the resected tumor specimen—generalizability of these study results to patients who undergo more intensive initial staging and/or have lower rates of pathologic downstaging is uncertain.
The previously reported safety results revealed that the postoperative complication rates including rate of positive margins, number of lymph nodes retrieved, and blood transfusions required were similar for both laparoscopic and open gastrectomy groups.8 The mean length of the surgical incision was 8 cm for laparoscopic vs 18 cm for the open approach and 6.4% of participants assigned to laparoscopic surgery were converted to an open distal gastrectomy intraoperatively. The median duration of hospital stay was 1 day shorter for laparoscopic operations (9 vs 10 days). Taken together, the short-term and long-term results of this study suggest that in the setting of locally advanced gastric cancer, laparoscopic distal gastrectomy is noninferior to open distal gastrectomy when performed by expert surgeons at high-volume referral centers in China. Generalizability of these findings to practice settings where staging, surgical training, and use of adjuvant systemic chemotherapy and radiation are different may be limited.
Limitations
This study has several limitations. First, although larger than prior studies in locally advanced gastric cancer, this study had limited power to detect small effect sizes that may nevertheless be clinically important. Specifically, the lower bound of the noninferiority margin point estimate of 3-year disease-free survival rate difference was 6.5%, corresponding to an HR of 1.38 such that the hazard rate could be 38% higher for patients who underwent laparoscopic distal gastrectomy. Second, the study did not measure patient-centered outcomes such as toxicity, quality of life, satisfaction, or return to normal role functioning. It is not clear whether shorter hospitalization (median and mean difference of 1 day) and shorter incisions (mean difference 10 cm) for laparoscopic gastrectomy provide sufficient benefit to offset residual concern about differences in long-term recurrence and survival, particularly for patients with gastric cancer involving lymph nodes or adherent to adjacent structures. Third, the differences in 3-year disease-free survival between the laparoscopic and open distal gastrectomy groups increased with tumor stage, although statistical significance was not reached in any of the stage subgroups. However, these null results should be interpreted with caution because statistical power may have been insufficient for subgroup analyses. Fourth, the study was performed in China and results may be less pertinent to western settings where proximal gastrectomy is a more common operation than distal gastrectomy. Fifth, no patients received neoadjuvant chemotherapy or radiation, which can influence surgical outcomes. Sixth, distal gastrectomies were performed at high-volume referral centers by Chinese surgeons who submitted videos establishing their technical proficiency in order to participate in the trial and may not extend to surgeons with less intensive training. Seventh, allowing minilaparotomy incisions of up to 10 cm in patients assigned to the laparoscopic group could potentially have attenuated differences in outcomes between the laparoscopic and open surgical techniques compared with more stringent thresholds.
Conclusions
Among patients with a preoperative clinical stage indicating locally advanced gastric cancer, laparoscopic distal gastrectomy, compared with open distal gastrectomy, did not result in inferior disease-free survival at 3 years.
Trial Protocol and Statistical Analysis Plan
eTable 1. Eligibility Criteria for the Enrollment of the Patients
eTable 2. Univariate and Multivariable Mixed Effects Cox Regression Analyses of Risk Factors for Survival
eFigure 1. Cumulative Incidence of Any Recurrence for Laparoscopic Distal Gastrectomy vs Open Distal Gastrectomy within 3 Years after Surgery
eFigure 2. Kaplan-Meier Curves of Cumulative Probability of Recurrence or Death for Laparoscopic Distal Gastrectomy vs Open Distal Gastrectomy within 3 Years after Surgery by Lymph Node Status of Pathologic T4a Stage
eFigure 3. Kaplan-Meier Curves of Cumulative Probability of Death for Laparoscopic Distal Gastrectomy vs Open Distal Gastrectomy within 3 Years after Surgery, Overall and by Pathologic Stage (I, II, III)
eFigure 4. Kaplan-Meier Curves of Cumulative Probability of Death for Laparoscopic Distal Gastrectomy vs Open Distal Gastrectomy within 3 Years after Surgery by Lymph Node Status of Pathologic T4a Stage
Data Sharing Statement
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87-108. doi: 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012: estimated cancer incidence, mortality, and prevalence worldwide in 2012. International Agency for Research on Cancer website. http://publications.iarc.fr/Databases/Iarc-Cancerbases/GLOBOCAN-2012-Estimated-Cancer-Incidence-Mortality-And-Prevalence-Worldwide-In-2012-V1.0-2012. Accessed August 25, 2018.
- 3.Allemani C, Matsuda T, Di Carlo V, et al. . Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023-1075. doi: 10.1016/S0140-6736(17)33326-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2014 (ver 4). Gastric Cancer. 2017;20(1):1-19. doi: 10.1007/s10120-016-0622-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim W, Kim HH, Han SU, et al. ; Korean Laparo-endoscopic Gastrointestinal Surgery Study (KLASS) Group . Decreased morbidity of laparoscopic distal gastrectomy compared with open distal gastrectomy for stage I gastric cancer: short-term outcomes from a multicenter randomized controlled trial (KLASS-01). Ann Surg. 2016;263(1):28-35. doi: 10.1097/SLA.0000000000001346 [DOI] [PubMed] [Google Scholar]
- 6.Katai H, Mizusawa J, Katayama H, et al. . Short-term surgical outcomes from a phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer: Japan Clinical Oncology Group Study JCOG0912. Gastric Cancer. 2017;20(4):699-708. doi: 10.1007/s10120-016-0646-9 [DOI] [PubMed] [Google Scholar]
- 7.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. AJCC Cancer Staging Manual. 7th ed New York, NY: Springer; 2010. [Google Scholar]
- 8.Hu Y, Huang C, Sun Y, et al. . Morbidity and mortality of laparoscopic versus open D2 distal gastrectomy for advanced gastric cancer: a randomized controlled trial. J Clin Oncol. 2016;34(12):1350-1357. doi: 10.1200/JCO.2015.63.7215 [DOI] [PubMed] [Google Scholar]
- 9.Japanese Gastric Cancer Association Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14(2):101-112. doi: 10.1007/s10120-011-0041-5 [DOI] [PubMed] [Google Scholar]
- 10.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103-115. doi: 10.2307/2529712 [DOI] [PubMed] [Google Scholar]
- 11.Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2010 (ver 3). Gastric Cancer. 2011;14(2):113-123. doi: 10.1007/s10120-011-0042-4 [DOI] [PubMed] [Google Scholar]
- 12.Viñuela EF, Gonen M, Brennan MF, Coit DG, Strong VE. Laparoscopic versus open distal gastrectomy for gastric cancer: a meta-analysis of randomized controlled trials and high-quality nonrandomized studies. Ann Surg. 2012;255(3):446-456. doi: 10.1097/SLA.0b013e31824682f4 [DOI] [PubMed] [Google Scholar]
- 13.Sakuramoto S, Sasako M, Yamaguchi T, et al. ; ACTS-GC Group . Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357(18):1810-1820. doi: 10.1056/NEJMoa072252 [DOI] [PubMed] [Google Scholar]
- 14.Lee HJ, Hyung WJ, Yang HK, et al. ; Korean Laparo-endoscopic Gastrointestinal Surgery Study (KLASS) Group . Short-term outcomes of a multicenter randomized controlled trial comparing laparoscopic distal gastrectomy with D2 lymphadenectomy to open distal gastrectomy for locally advanced gastric cancer (KLASS-02-RCT) [published online February 9, 2019]. Ann Surg. 2019. doi: 10.1097/SLA.0000000000003217 [DOI] [PubMed] [Google Scholar]
- 15.Kim HH, Han SU, Kim MC, et al. ; Korean Laparoendoscopic Gastrointestinal Surgery Study (KLASS) Group . Effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long-term survival among patients with stage I gastric cancer: the KLASS-01 randomized clinical trial [published online February 7, 2019]. JAMA Oncol. 2019. doi: 10.1001/jamaoncol.2018.6727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei HB, Wei B, Qi CL, et al. . Laparoscopic versus open gastrectomy with D2 lymph node dissection for gastric cancer: a meta-analysis. Surg Laparosc Endosc Percutan Tech. 2011;21(6):383-390. doi: 10.1097/SLE.0b013e31822d02dc [DOI] [PubMed] [Google Scholar]
- 17.Inaki N, Etoh T, Ohyama T, et al. . A multi-institutional, prospective, phase II feasibility study of laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for locally advanced gastric cancer (JLSSG0901). World J Surg. 2015;39(11):2734-2741. doi: 10.1007/s00268-015-3160-z [DOI] [PubMed] [Google Scholar]
- 18.Mathis KL, Nelson H. Controversies in laparoscopy for colon and rectal cancer. Surg Oncol Clin N Am. 2014;23(1):35-47. doi: 10.1016/j.soc.2013.09.006 [DOI] [PubMed] [Google Scholar]
- 19.Kinoshita T, Uyama I, Terashima M, et al. ; LOC-A Study Group . Long-term outcomes of laparoscopic versus open surgery for clinical stage II/III gastric cancer: a multicenter cohort study in Japan (LOC-A Study) [published online April 24, 2018]. Ann Surg. 2019;269(5):887-894. doi: 10.1097/SLA.0000000000002768 [DOI] [PubMed] [Google Scholar]
- 20.Kim HH, Han SU, Kim MC, et al. . Long-term results of laparoscopic gastrectomy for gastric cancer: a large-scale case-control and case-matched Korean multicenter study. J Clin Oncol. 2014;32(7):627-633. doi: 10.1200/JCO.2013.48.8551 [DOI] [PubMed] [Google Scholar]
- 21.Park YK, Yoon HM, Kim YW, et al. ; COACT group . Laparoscopy-assisted versus open D2 distal gastrectomy for advanced gastric cancer: results from a randomized phase II multicenter clinical trial (COACT 1001). Ann Surg. 2018;267(4):638-645. doi: 10.1097/SLA.0000000000002168 [DOI] [PubMed] [Google Scholar]
- 22.Huscher CG, Mingoli A, Sgarzini G, et al. . Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005;241(2):232-237. doi: 10.1097/01.sla.0000151892.35922.f2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bando E, Makuuchi R, Irino T, Tanizawa Y, Kawamura T, Terashima M. Validation of the prognostic impact of the new tumor-node-metastasis clinical staging in patients with gastric cancer. Gastric Cancer. 2019;22(1):123-129. doi: 10.1007/s10120-018-0799-9 [DOI] [PubMed] [Google Scholar]
- 24.Fukagawa T, Katai H, Mizusawa J, et al. ; Stomach Cancer Study Group of the Japan Clinical Oncology Group . A prospective multi-institutional validity study to evaluate the accuracy of clinical diagnosis of pathological stage III gastric cancer (JCOG1302A). Gastric Cancer. 2018;21(1):68-73. doi: 10.1007/s10120-017-0701-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eTable 1. Eligibility Criteria for the Enrollment of the Patients
eTable 2. Univariate and Multivariable Mixed Effects Cox Regression Analyses of Risk Factors for Survival
eFigure 1. Cumulative Incidence of Any Recurrence for Laparoscopic Distal Gastrectomy vs Open Distal Gastrectomy within 3 Years after Surgery
eFigure 2. Kaplan-Meier Curves of Cumulative Probability of Recurrence or Death for Laparoscopic Distal Gastrectomy vs Open Distal Gastrectomy within 3 Years after Surgery by Lymph Node Status of Pathologic T4a Stage
eFigure 3. Kaplan-Meier Curves of Cumulative Probability of Death for Laparoscopic Distal Gastrectomy vs Open Distal Gastrectomy within 3 Years after Surgery, Overall and by Pathologic Stage (I, II, III)
eFigure 4. Kaplan-Meier Curves of Cumulative Probability of Death for Laparoscopic Distal Gastrectomy vs Open Distal Gastrectomy within 3 Years after Surgery by Lymph Node Status of Pathologic T4a Stage
Data Sharing Statement