Abstract
Background
Increasing prevalence of obesity requires the investigation of respective comorbidities, including tumor diseases like colorectal, renal, post-menopausal breast, prostate cancer, and leukemia. To date, molecular mechanisms of the malignant transformation of these peripheral tissues induced by obesity remain unclear. Adipose tissue secretes factors with hormone-like functions, the adipokines, and is therefore categorized as an endocrine organ. Current research demonstrates the ability of adipose tissue to alter DNA methylation and gene expression in peripheral tissues, probably affecting microRNA (miR) expression.
Methods
Literature was analyzed for adipokine-regulated miRs. Many of these adipokine upregulated or downregulated miRs exert either oncogenic or anti-tumoral potential.
Results
The three selected and analyzed adipokines, adiponectin, leptin, and resistin, induce more strongly oncogenic miRs and simultaneously reduce anti-tumoral miRs than vice versa. This effect is not only true for the pure number of regulated miRs, it is also the case by consideration of the abundance of the respective miR expression based on actual data sets derived from next-generation sequencing.
Conclusion
The link of obesity and cancer is analyzed under the aspect of adipokine-regulated miRs. At the same time the impact of miR abundance is considered as a regulatory variable. This context offers new strategies for tumor therapy and diagnostics.
Keywords: Obesity, MicroRNA, Cancer, Adipokines
Introduction
The increase of obesity (body mass index, BMI ≥30) and overweight (BMI ≥25) in the world population justifies the analysis of associated health issues [1]. The pure weight of the fat mass per se damages joints and increases the risk for an artificial hip and knee joint implantation [2, 3]. Obesity-associated lack of exercise and systemic metabolic dysfunctions promote inter alia type 2 diabetes, fatty liver disease, atherosclerosis, and cardiovascular disorders, and also contribute to a significantly reduced life expectancy [4, 5].
Interestingly, adipose tissue not only responds to hormonal signals, it also expresses and secretes factors with hormone-like functions, so-called adipokines, and is therefore categorized as an endocrine organ [6]. Meanwhile, a connection between obesity and certain tumor diseases, including colorectal cancer, renal cancer, post-menopausal breast cancer, esophageal adenocarcinoma, thyroid cancer, endometrial cancer, leukemia, and prostate cancer is demonstrated in numerous cohort studies [7, 8, 9]. Furthermore, with an increase of BMI of 5, a 10% increase in cancer mortality has been detected in both genders [10, 11].
Various immune cell functions are influenced by obesity. In particular, some of the approximately 600 proteins secreted by adipose tissues [9] exert anti-inflammatory or pro-inflammatory potential. Especially adiponectin, which is the most abundant adipokine secreted by adipocytes [7], exerts anti-inflammatory action by interfering with the functions of macrophages, T lymphocytes, and NK cells [12, 13, 14, 15]. Furthermore, the well-characterized adipokine leptin also exerts immunomodulatory functions on NK cells [16].
The detailed molecular mechanisms, which contribute to the immunomodulatory potential of adipose tissue, is the subject of ongoing research. It is a fact that adipose tissue is able to regulate the gene expression in other peripheral tissues of the organism. The gene expression per se can be regulated at different points – pretranscriptional and posttranscriptional, for example by altering the DNA methylation as a potential pretranscriptional regulatory control variable. Indeed, the gene expression patterns differ in human monozygotic twins with different BMIs [17]. Research has verified that obesity is associated with widespread changes in DNA methylation as a consequence of adiposity and not as a cause of it [18]. This important study could at least identify 187 gene loci, which were statistically significant and could be replicated differentially methylated at CpG sites. These gene loci belong to genes mostly involved in lipid and lipoprotein metabolism, substrate transport, and inflammatory pathways [18].
Many of these obesity-regulated genes contain intronic sequences harboring microRNA (miR) genes. Therefore, obesity also affects post-transcriptional gene regulation involving an altered miR transcriptome. MiRs are small, non-coding RNA molecules of about 21 nt in length. The miR genes are usually located within introns of coding genes or as separated genes. In both cases, the miR genes are transcribed by RNA polymerase II and the resulting primary miR transcripts are further processed into mature miRs [19, 20, 21, 22]. The sequence-specific binding of an miR to a target mRNA is determined by its “seed” region. miR binding to target mRNAs predominantly occurs at the 3′-untranslated region leading to translational inhibition and to mRNA decay [23]. Concerning the target specificity of miRs, it is noteworthy that one miR can regulate many different mRNAs [24]. Depending on the functions of these miR-regulated genes, several miRs can exert oncogenic or anti-tumoral potential [19].
This review analyzes and summarizes available PubMed database-listed literature of adipokine-regulated miRs and their functions regarding the association between obesity and tumor diseases. Focus was placed on the three well-characterized adipokines directly expressed by adipocytes: adiponectin, leptin, and resistin. The summarized adipokine-regulated miRs include human and murine datasets.
Adipokines and Their Impact on miR Expression
The adipose tissue interacts with the entire organism by secretion of hormone-like adipokines. Indeed, multiple factors are released or secreted by adipose tissue consisting of adipocytes, infiltrating immune cells, fibroblasts, endothelial cells, and others [25]. In 2004, Fain et al. [26] analyzed and compared the secretion of adipokines between isolated adipocytes and corresponding visceral adipose tissue. Many factors, including IL-6 and TNF-α, were expressed much higher by the adipose tissue than by adipocytes themselves. Other factors, like adiponectin and leptin, were almost equally expressed, reflecting that adiponectin and leptin are directly expressed by adipocytes. Furthermore, the adipokine resistin is also reported to be directly secreted by adipocytes [27].
These adipokines exert an impact on the gene expression in other peripheral tissues causing inter alia an altered miR expression pattern. Furthermore, many of these adipokine-regulated miRs have been characterized for their relevance on tumor biological functions, including proliferation, invasion, apoptosis inducibility, and angiogenesis. Subsequently, some of these adipokine-regulated miRs can be characterized as oncogenic or anti-tumoral miRs.
Nevertheless, next to the pure number of different adipokine-regulated miRs, also the abundance of each adipokine-regulated miR per se is an important variable, since it is known that cellular concentrations of miRs are critical for their regulatory potential [28]. For example, a duplication of the expression of a very high abundant miR upon adipokine stimulation could affect the translation of its target mRNAs in a stronger manner than the duplication of the expression of a very low abundant miR in regard to the absolute number of target mRNA molecules.
Meanwhile next-generation sequencing (NGS) is the state-of-the-art technology for miR analysis [29] and absolute NGS reads of miRs correlate with qPCR-based quantifications of these miRs. The absolute counts determined by NGS can be normalized into reads per million (rpm) using the formula: rpm = reads (miR)/(reads [all mapped reads] × 106) [30, 31]. Thus, in this review the rpm value of each adipokine-regulated miR was collected from the free online data base www.mirbase.org [32, 33]. For a better estimation of possible biological impacts of adipokine-regulated miRs with oncogenic or anti-tumoral functions, the respective rpms for adiponectin, leptin, and resistin stimulation were calculated and compared. At this point the human miR expression data and the human homologous miR expression data in the case of published murine miRs were applied and combined. The investigated three adipokines will be discussed separately and the respective regulated miRs, so far reported, are summarized.
The Role of Adiponectin
The gene of adiponectin is located on the long arm of chromosome 3 (3q27) and the protein has a molecular weight of about 30 kDa. In cells and in plasma, adiponectin protein forms homomultimers, trimers (LMW; approx. 67 kDa), hexamers (MMW; approx. 136 kDa), and high-molecular-weight (HMW; >300 kDa) multimers [34]. Adiponectin is also the most abundant adipokine within the human body [7]. The different adiponectin multimers exert different biological functions by binding to various adiponectin receptors. The adiponectin trimer binds to the adiponectin receptor 1 (AdipoR1), the adiponectin hexamer binds to the adiponectin receptor 2 (AdipoR2) and the adiponectin hexamers as well as the high-molecular-weight multimers, but not the adiponectin trimers that act as ligands for T-cadherin (CDH13) [35, 36]. Interestingly, these different adiponectin multimers do not interconvert during circulation [37].
The biological functions of adiponectin are pleiotropic and range from its role in energy homeostasis from anti-inflammatory and anti-apoptotic to pro-angiogenic activities [38, 39, 40]. Adiponectin reduces the phagocytotic activity of macrophages and inhibits the proliferation of myelomonocytic progenitor cells [12]. In primary human monocytes, in monocyte-derived macrophages, and in dendritic cells, adiponectin induces the secretion of the anti-inflammatory cytokines IL-10 and IL-1RA [41]. In addition, adiponectin extends its anti-inflammatory potential also by suppression of the IL-2-mediated activation of NK cells and T cells [15, 42].
Meanwhile, many pro-inflammatory adipokines have been described in the literature. However, only a few of the adipokines exert anti-inflammatory potential; among these are adiponectin, adipolin, and omentin [43]. Due to the fact that obesity is described as chronic low-grade inflammation, such anti-inflammatory adipokines are usually downregulated in obesity [43, 44]. This critical point will be discussed in more detail below.
Differential Adiponectin Expression in Hyperplasia and Hypertrophy as Two Different Patterns of Adipose Tissue
Two different patterns of obese tissue emerge regarding the cellular characteristics. A hyperplastic pattern with increased adipocyte cell number and a hypertrophic one with increased cell size and unchanged number of adipocytes [45]. The occurrence of hyperplasia or hypertrophy is independent of sex and body weight [46], but strongly relates to the total adipocyte number in adults [46]. Interestingly, in most studies investigating adiponectin expression and obesity this aspect is not considered.
The secretion of total adiponectin is higher in subcutaneous adipose tissue when compared to visceral adipose tissue. Indeed, adipocyte size is larger in subcutaneous adipose tissue. Additionally, adipocyte size is negatively correlated to adiponectin expression and secretion [47]. These important results could explain why adiponectin serum levels decrease with increasing BMI in certain individuals, while every adipocyte per se expresses adiponectin. This observed contradictory effect of decreased adiponectin levels with increased BMI is of course not statistically significant in all published studies, because this effect is obviously linked to the pattern of adipose tissue (hyperplasia or hypertrophy) as an individual parameter for each tested patient. That parameter is not considered in almost all studies, potentially leading to such contradictory results.
Furthermore, a significant sex difference in adiponectin expression is also reported. Women exert a higher percentage of body fat and higher adiponectin levels when compared to men of the same BMI [48]. Black women have significantly lower adiponectin levels than white subjects and show no clear decreasing trend with increasing severity of obesity [49]. Therefore, this review also focusses on adiponectin and its impact on miR expression in the dependency of obesity.
Adiponectin Strongly Increases Tumoral miRs and Downregulates Anti-Tumoral miRs
All so far identified adiponectin-regulated miRs are summarized and divided into miRs that are either induced or reduced in their expression upon adiponectin stimulation in Table 1. In the case of available information about tumor biology-relevant parameters, including proliferation, invasion, apoptosis inducibility, and angiogenesis, these adiponectin-regulated miRs were either grouped as oncogenic or as anti-tumoral. In Table 2 the adiponectin-induced miRs and in Table 3 the adiponectin downregulated miRs are listed and characterized. So far, 6 miRs have been described as induced and 3 miRs are reported to be reduced upon adiponectin stimulation. To estimate whether adiponectin stimulation alters the miR expression pattern into an oncogenic or anti-tumoral fashion, the adiponectin-regulated miRs were divided into “induced oncogenic miRs and reduced anti-tumoral miRs” as a first group, and into “reduced oncogenic miRs and induced anti-tumoral miRs” as a second group.
Table 1.
Summary of adipokine-regulated miRs
| Adipokine | miR induced | Originating tissue | Ref. | miR reduced | Originating tissue | Ref. |
|---|---|---|---|---|---|---|
| Adiponectin | miR-883b-5p | Murine adipose tissue | 50 | miR-27b | Human chondrosarcoma cells | 51 |
| miR-1934 | Murine adipose tissue | 50 | miR-532-5p | Murine adipose tissue | 50 | |
| miR-133a | Neonatal rat ventricular myocytes | 52 | miR-1983 | Murine adipose tissue | 50 | |
| miR-378 | Human adipocytes | 53 | ||||
| miR-155 | Murine RAW 264.7 cells | 54 | ||||
| miR-21 | Murine RAW 264.7 cells | 55 | ||||
| | ||||||
| Leptin | miR-21 | Human mature adipocytes, transgenic mice | 56, 57 | miR-27b | Human chondrosarcoma cells | 58 |
| miR-4443 | Human colon cancer cells | 59 | miR-93 | Human osteoblasts | 60 | |
| miR-199a-3p | Human adipocytes | 61 | miR-1908 | Human adipocytes | 62 | |
| miR-378 | Human adipocytes, human adipocytes | 53, 63 | miR-143 | Human adipocytes | 64 | |
| miR-335 | Human adipocytes | 65 | miR-221 | Human subcutaneous abdominal adipose tissue biopsies | 66 | |
| miR-182 | Human ovary carcinoma cell lines (SKOV3 and A2780) | 67 | miR-26b | Human adipocytes | 68 | |
| miR-96 | Human ovary carcinoma cell lines (SKOV3 and A2780) |
67 | miR-489 | Murine skeletal muscle | 69 | |
| miR-31 | Murine skeletal muscle | 69 | miR-103 | Murine skeletal muscle | 69 | |
| miR-223 | Murine skeletal muscle | 69 | miR-101b | Murine skeletal muscle | 69 | |
| miR-491 | Murine skeletal muscle | 69 | miR-let 7g | Murine skeletal muscle | 69 | |
| miR-142 | Murine skeletal muscle | 69 | miR-155 | Murine skeletal muscle | 69 | |
| miR-685 | Murine skeletal muscle | 69 | ||||
| | ||||||
| Resistin | miR-21 | Human mature adipocytes | 56 | miR-1908 | Human adipocytes | 62 |
| miR-335 | Human adipocytes | 65 | miR-143 | Human adipocytes | 64 | |
| miR-34a | Human liver cell line (Hep G2) | 70 | miR-26b | Human adipocytes | 68 | |
| miR-696 | Murine muscle cell line (C2C12) 71 | miR-206 | Human endothelial progenitor | 72 | ||
| cells | ||||||
| miR-145 | Human liver cell line (Hep G2) | 73 | miR-519d | Human chondrosarcoma cells | 74 | |
Table 2.
Induced miRs upon adiponectin, leptin, or resistin stimulation and their tumor biological impact
| miR | Induced by adipokine | Proliferation | Invasion | Apoptosis inducibility | Angiogenesis | Ongogenic or anti-tumoral potential |
|---|---|---|---|---|---|---|
| miR-133a | Adiponectin | Down | Down | Up | n.d. | Anti-tumoral |
| miR-142 | Leptin | Down | Down | Up | Up | Anti-tumoral |
| miR-145 | Resistin | Down | Down | Up | Down | Anti-tumoral |
| miR-223 | Leptin | Down | Down | Up | Down | Anti-tumoral |
| miR-34a | Resistin | Down | Down | Up | Down | Anti-tumoral |
| miR-378 | Adiponectin, leptin | Down | Down | Up | Down | Anti-tumoral |
| miR-491 | Leptin | Down | Down | Up | Up | Anti-tumoral |
| miR-155 | Adiponectin | Up | Up | Down | Up | Oncogenic |
| miR-182 | Leptin | Up | Up | Down | Up | Oncogenic |
| miR-21 | Adiponectin, leptin, resistin | Up | Up | Down | Up | Oncogenic |
| miR-31 | Leptin | Up | Up | Down | Up | Oncogenic |
| miR-96 | Leptin | Up | Up | Down | n.d. | Oncogenic |
| miR-1934 | Adiponectin | n.d. | n.d. | n.d. | n.d. | n.d. |
| miR-685 | Leptin | n.d. | n.d. | n.d. | n.d. | n.d. |
| miR-696 | Resistin | Up | n.d. | n.d. | n.d. | n.d. |
| miR-883b-5p | Adiponectin | n.d. | n.d. | n.d. | n.d. | n.d. |
| miR-199a-3p | Leptin | Controversially discussed | ||||
| miR-4443 | Leptin | Controversially discussed | ||||
| miR-335 | Leptin, resistin | Controversially discussed |
Table 3.
Reduced miRs upon adiponectin, leptin, or resistin stimulation and their tumor biological impact
| miR | Reduced by adipokine | Proliferation | Invasion | Apoptosis inducibility | Angiogenesis | Ongogenic or anti-tumoral potential |
|---|---|---|---|---|---|---|
| miR-143 | Leptin, resistin | Down | Down | Up | Down | Anti-tumoral |
| miR-206 | Resistin | Down | Down | Up | Down | Anti-tumoral |
| miR-26b | Leptin, resistin | Down | Down | Up | Down | Anti-tumoral |
| miR-27b | Adiponectin, leptin | Down | Down | Up | Up | Anti-tumoral |
| miR-489 | Leptin | Down | Down | Up | Down | Anti-tumoral |
| miR-519d | Resistin | Down | Down | Up | Down | Anti-tumoral |
| miR-532-5p | Adiponectin | Down | Down | Up | n.d. | Anti-tumoral |
| miR-let 7g | Leptin | Down | n.d. | Up | n.d. | Anti-tumoral |
| miR-155 | Leptin | Up | Up | Down | Up | Oncogenic |
| miR-221 | Leptin | Up | Up | Down | Up | Oncogenic |
| miR-101b | Leptin | n.d. | n.d. | n.d. | n.d. | n.d. |
| miR-1983 | Adiponectin | n.d. | n.d. | n.d. | n.d. | n.d. |
| miR-103 | Leptin | Controversially discussed | ||||
| miR-93 | Leptin | Controversially discussed | ||||
| miR-1908 | Leptin, resistin | Controversially discussed |
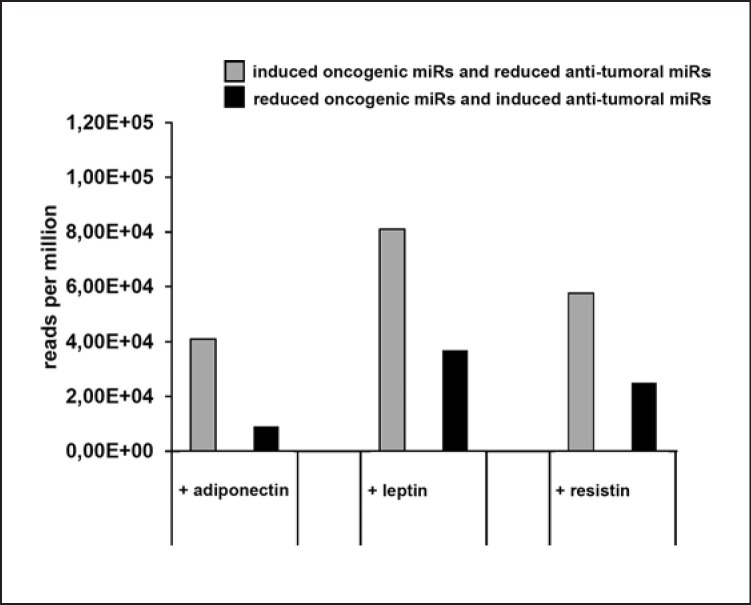
As previously discussed, the abundance of each miR is an important regulatory variable. Therefore, the rpm values reported at the free online database www.mirbase.org [32, 33] for each oncogenic and anti-tumoral miR are listed in Table 4, and a respective total amount for both adiponectin-regulated miR groups was calculated (Table 4; Fig. 1).
Table 4.
Consideration of the expression levels of “induced oncogenic miRs and reduced anti-tumoral miRs” compared to “reduced oncogenic miRs and induced anti-tumoral miRs” upon stimulation with adiponectin, leptin, or resistin
| Adipokine | Induced oncogenic miR and reduced anti-tumoral miR, rpm | Reduced oncogenic miR and induced anti-tumoral miR, rpm |
|---|---|---|
| Adiponectin | 2.56e+04 (miR-21), 2.94e+03 (miR-155), 1.02e+04 (miR-27b), 2.28e+03 (miR-532) =41,020 |
7.03e+03 (miR-378), 2.05e+03 (miR-133a) = <bold> 9,080 </bold> |
| | ||
| Leptin | 2.56e+04 (miR-21), 1.58e+03 (miR-182), 685 (miR-96), 3.89e+03 (miR-31), 1.02e+04 (miR-27b), 2.47e+04 (miR-143), 5.2e+03 (miR-26b), 72.4 (miR-489), 9e+03 (let 7g) = <bold> 80,927.4 </bold> |
8.06e+03 (miR-221), 2.94e+03 (miR-155), 7.03e+03 (miR-378), 5.81e+03 (miR-223), 60.6 (miR-491), 1.3e+04 (miR-142) =36,900.6 |
| | ||
| Resistin | 2.56e+04 (miR-21), 2.47e+04 (miR-143), 5.2e+03 (miR-26b), 1.86e+03 (miR-206), 199 (miR-519d) =57,559 |
1.13e+03 (miR-34a), 2.39e+04 (miR-145) =25,030 |
| Adiponectin, leptin, resistin (every miR just 1-fold) | = 98,406.4 | = 63,980.6 |
Instead of the murine miRs, the human homologous miR expression data were applied.
Fig. 1.
Impact of adipokines on tumor-associated miRs. The results presented in Table 4 are visualized as a bar diagram. Under consideration of the expression level in rpm, the group of “induced oncogenic miR and reduced anti-tumoral miR” contains much more dysregulated tumor-relevant miRs than the group of “reduced oncogenic miR and induced anti-tumoral miR.” This effect was observed for adiponectin, leptin, and resistin.
Interestingly, adiponectin stimulation is shifting the miR transcriptome towards an oncogenic direction. The absolute number of induced oncogenic miRs and reduced anti-tumoral miRs is about 4 times higher than the absolute number of reduced oncogenic miRs and induced anti-tumoral miRs (Table 4; Fig. 1).
The Role of Leptin
The adipocyte-derived leptin protein has a molecular weight of 16 kDa and is encoded on the long arm of chromosome 7 (7q32.1). Leptin is secreted into the bloodstream and involved in the regulation of the energy and physiological body weight homeostasis [75]. Leptin secretion is higher in subcutaneous fat cells which are also of bigger size than visceral fat cells, and its amount is correlated to the size and number of adipocytes [76]. Thus, leptin expression correlates to obesity. Leptin-deficient mice (ob/ob mice) or mice lacking the functional leptin receptor (db/db mice) suffer morbid obesity and type 2 diabetes [77, 78]. These ob/ob mice are sensitive to a leptin treatment, responding by fat mass reduction and regaining normal body weights. Furthermore, leptin treatment also lowers food intake and increases metabolic rates in lean mice [79].
The leptin receptor (LEPR) mRNA underlays alternative splicing, which leads to the generation of six LEPR isoforms (LEPRa, b, c, d, e, and f). All of these isoforms are able to bind leptin as ligand. Nevertheless, these different splicing variants differ in the intracellular domain affecting appropriate downstream signaling. Actually, the isoform LEPRb is the only isoform mediating functional leptin downstream signaling [80]. The functions of the other LEPR isoforms are still under investigation.
LEPRe encodes for a soluble protein due to the missing transmembrane domain. Furthermore, LEPRe is analogical to secreted leptin circulating in the bloodstream [81]. Especially in this case, it is indicated that the downstream signaling-incompetent leptin receptors compete for leptin binding as a kind of negative regulator [82].
Leptin stimulation is reported to act in a pro-inflammatory way by the induction of certain cytokines, like TNFα, IL-1, and IL-6 [83, 84]. Nevertheless, there also exist studies reporting impaired NK cell functions and proliferation upon long-term leptin stimulation [16].
Leptin Increases Tumoral miRs and Downregulates Anti-Tumoral miRs
Twelve miRs have been reported to be upregulated, and 11 miRs have been reported to be downregulated upon leptin stimulation (Table 1). The characterization of these leptin-regulated miRs in regard to their impact on tumor biology leads to 4 induced oncogenic miRs and 5 reduced anti-tumoral miRs. On the other hand, 4 anti-tumoral miRs were induced and 2 oncogenic miRs were reduced (Table 2, 3). By addressing the respective abundance of these regulated miRs, the group of “induced oncogenic miRs and reduced anti-tumoral miRs” was about two times higher than the group of “reduced oncogenic miRs and induced anti-tumoral miRs” upon leptin stimulation (Table 4; Fig. 1).
Resistin
The resistin gene is located at the short arm of chromosome 19 (19p13). Human resistin is a 10-kDa cysteine-rich polypeptide. Resistin is synthesized as a 108-amino acid (aa) precursor containing an 18-aa signal sequence and a 90-aa mature region. It is predominantly secreted by adipocytes, whereby the resistin expression is higher in murine adipocytes as compared to human ones for so far unknown reasons [85, 86, 87]. The secreted resistin circulates either as homotrimers, homohexamers, or even as higher molecular weight oligomers [88, 89, 90]. In addition to that, Lee et al. [91] reported that the applied purified recombinant resistin protein forms a multimeric quaternary structure at 55 kDa. Adenylyl cyclase-associated protein 1 (CAP1) has been identified as a receptor for human resistin.
In humans, conflicting results regarding the resistin secretion and obesity are reported, which might be contributed to by the fact that the respective studies did not distinguish between hyperplasia or hypertrophy of adipose tissue as two different mechanisms for increasing fat mass in obese humans. This point is not a factor in studies investigating obesity only in a single monogenetic mouse strain.
Furthermore, human resistin stimulates the secretion of pro-inflammatory cytokines and chemokines, including TNF-α and IL-12 [92, 93]. Resistin is also involved in mediating insulin resistance and type 2 diabetes mellitus in obese humans [94].
Resistin Also Induces Tumoral miRs and Reduces Anti-Tumoral miRs
Until now, 10 different miRs have been reported to be affected in their expression level upon resistin stimulation. Among them, 5 miRs are upregulated and 5 miRs are downregulated (Table 1). One of these regulated miRs exerts oncogenic functions, whereas 6 exert anti-tumoral functions. The other miRs were either not yet investigated or controversially discussed in the literature. Interestingly, resistin induces the oncogenic miRs and reduces four anti-tumoral miRs (Table 2, 3).
Opposing that, only two anti-tumoral miRs are induced and no oncogenic miRs are decreased by resistin. The resistin-regulated group of “induced oncogenic miRs and reduced anti-tumoral miRs” is two times higher expressed than the group of “induced anti-tumoral miRs and reduced oncogenic miRs” (Table 4; Fig. 1).
Conclusion
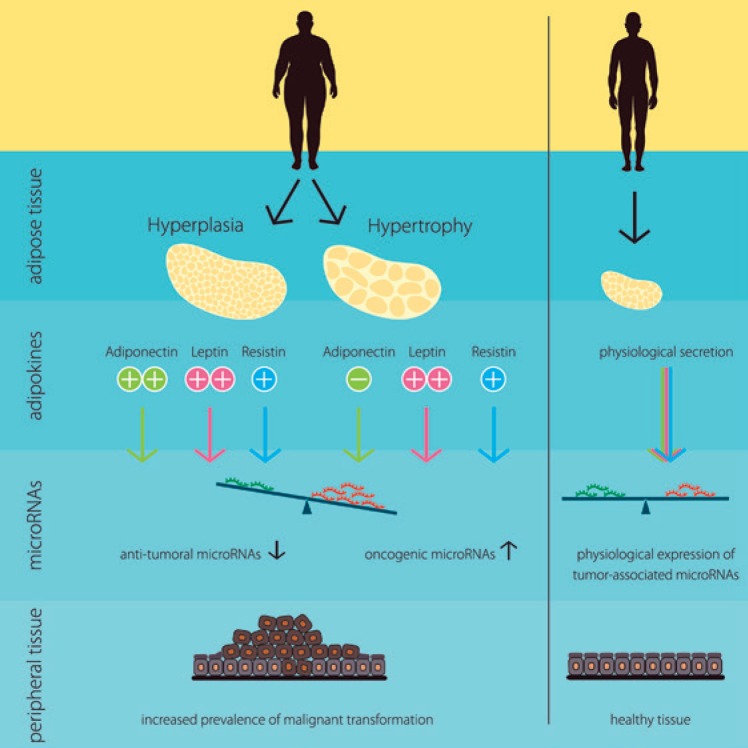
Obesity is linked to certain tumor diseases, including colorectal cancer, renal cancer, post-menopausal breast cancer, leukemia, and prostate cancer [7, 8]. However, the connection between adipose tissue and malignant transformation in peripheral tissues is still under investigation. The authors hypothesized in this review that in addition to already known carcinogenic factors like mutations, pathogens, external stimuli, etc., also hormone-like factors secreted by the adipose tissue itself are a putative factor involved in that malignant transformation by disrupting the balance of anti-tumoral and oncogenic miRs. This hypothesis is summarized in Figure 2.
Fig. 2.
Schematic summary of the hypothesized molecular link between obesity and certain tumor diseases. In dependency of the individual composition of adipose tissue (hyperplasia or hypertrophy) obese individuals exert an altered secretion of the adipokines adiponectin, leptin, and resistin when compared to normal-weight individuals. As a consequence, the expression of tumorassociated miRs in peripheral tissues is out of balance with induced oncogenic miRs and reduced anti-tumoral miRs, probably enhancing the prevalence of malignant transformation.
In various studies the expression of adiponectin, leptin, and resistin was analyzed for a potential correlation to the risk of development and/or grading and staging of certain tumor diseases. Unfortunately, the results of these numerous studies are partially controversial (Table 5). Nevertheless, in the case of post-menopausal breast cancer, different investigators could demonstrate that increased leptin and resistin levels were statistically significantly associated with enhanced tumor risk.
Table 5.
Summary of adiponectin, leptin, and resistin expression analyses in selected obesity-linked tumor diseases
| Tumor disease | Adiponectin | Leptin | Resistin |
|---|---|---|---|
| Colorectal cancer | Controversial | Controversial | Controversial |
| | |||
| Renal cancer | Controversial | No effect | No effect |
| | |||
| Post-menopausal breast cancer | Increased adiponectin levels associated with lowered tumor risk | Increased leptin levels are statistically significantly associated with enhanced tumor risk | Increased resistin levels are statistically significantly associated with enhanced tumor risk |
| | |||
| Prostate cancer | Statistically significant decreased adiponectin levels in tumor patients | Controversial | No effect |
Adiposity influences gene expression, for example by altering the DNA methylation pattern [18], an important mechanism, which affects inter alia also the miR transcriptome. One of the adipokine-regulated miRs that was induced upon stimulation of adiponectin, leptin, and resistin is miR-21. Interestingly, miR-21 was rapidly characterized as oncogenic by murine knock-in experiments [95]. Indeed, miR-21 negatively regulates certain tumor suppressors, including PTEN [96], and therefore acts as an anti-apoptotic and pro-survival factor [97]. The gene is located on the long arm of chromosome 17.
miRs act dose dependently. In fact, the miR-21-mediated oncogenic effects occur especially at high levels of miR-21 expression. In tumor diseases high levels of miR-21 are also correlated to poor survival and to a worse prognosis [98]. Studies are currently investigating the applicability of different miR-21 inhibitors as anti-cancer drugs [99]. Interestingly, the connection of obesity and miR-21 also works vice versa, whereby long-term inhibition of miR-21 leads to a reduction of obesity [100]. Additionally, the circumstance in which adiponectin induces the oncogenic miR-21 should be highlighted and considered, while investigating its eligibility as a cardioprotective therapeutic.
However, the analyzed adipokines adiponectin, leptin, and resistin not only induce several oncogenic miRs, they also downregulate several anti-tumoral miRs, including miR-27b. Indeed, miR-27b has been characterized as a suppressor for several genes associated with cancer, including PPARγ [101]. Furthermore, miR-27b synergizes with anticancer drugs by p53 activation [102].
The adipokine-induced oncogenic miRs (miR-21, miR-31, miR-96, miR-182, and miR-155) were further analyzed for their expression profiles in obesity-linked tumor diseases, like esophageal adenocarcinoma, renal carcinoma, and colon cancer. While the expressions of miR-21, miR-96, and miR-155 are reported to be increased in these tumor diseases in comparison to adjacent normal tissues, the information about miR-31 and miR-182 is either not yet available or contradictory in different tumor entities. BMI-related expression levels of such oncogenic miRs should be considered in further expression studies investigating the suitability of these oncogenic miRs as predictive biomarkers in several tumor diseases.
Actual research focuses on the development of miR-based therapeutics for the treatment of diseases including HCV infections, metabolic diseases, atherosclerosis, and tumor diseases. Mimics of the anti-tumoral miR-34a are being tested in phase I studies to reduce the expression of oncogenes in liver cancer. In contrast to that, the blockade of the oncogenic miR-21 and miR-221 by use of anti-miR constructs is being tested in preclinical trials [103]. Besides these direct approaches, indirect approaches affecting miR expression should also be discussed, for instance the block of circulating oncogenic adipokines.
This analysis is limited due to the small number of available studies investigating the effects of the adipokines on the miR transcriptome. Often just single miRs were analyzed instead of a complete miR transcriptome screening upon adipokine stimulation. In addition, it was also necessary to include murine studies. However, from such murine miRs the respective human homologues were representatively observed, which is also rather an estimate. Therefore, the calculated data need to be nuanced and only demonstrate the potential of these three adipokines to induce or reduce oncogenic/anti-tumoral miRs in peripheral tissues.
Indeed, the reported serum levels of these three adipokines differ between humans and mice. Human fasting leptin serum concentration is about 6.9 ± 0.3 ng/mL in males (n = 333) and 15.2 ± 1.3 ng/mL in females (n = 63) [104], while in healthy C57BL/6 mice the serum leptin concentration is about 0.1 ng/mL [105]. In the case of human fasting resistin serum levels the overall median is 8.93 ng/mL in males and 10.42 ng/mL in females [106], while in healthy C57BL/6 mice the serum resistin concentration is about 19 ng/mL [105]. Furthermore, the mean cohort human adiponectin level is 9.41 + 5.30 μg/mL (range 3.1–45.8) [107], while in healthy C57BL/6 mice the adiponectin serum concentration is about 44 ng/mL [105]. However, one cannot necessarily assume that the pure abundance of the three investigated adipokines determines their regulatory impact to affect miR expression in certain peripheral tissues. Also, many other factors need to be considered, like the expression of respective receptors on target cells, methylation status of miR genes harboring promotors in the target cells, or the expression of the miR-processing factors in the peripheral target cells, and even many more factors.
Nevertheless, direct studies with animal models should investigate the connection of adipokines and peripheral malignant transformation upon dysregulated miR expression patterns in dependence of obesity. Also, clinical studies in humans should verify the results of the published in vitro studies of the adipokine-regulated miRs under consideration of hyperplasia and hypertrophy as two types of adipose tissue with different endocrinological impact. The link between obesity and cancer is discussed upon the aspect of adipokine-regulated miRs, offering new strategies for tumor therapy and diagnostics.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors declare that no conflicts of interest exist.
Funding Sources
This work was performed with a grant of the Dr. Werner Jackstädt Foundation.
References
- 1.Jia H, Lubetkin EI. Trends in quality-adjusted life-years lost contributed by smoking and obesity. Am J Prev Med. 2010 Feb;38((2)):138–44. doi: 10.1016/j.amepre.2009.09.043. [DOI] [PubMed] [Google Scholar]
- 2.Jasinski-Bergner S, Radetzki AL, Jahn J, Wohlrab D, Kielstein H. Impact of the body mass index on perioperative immunological disturbances in patients with hip and knee arthroplasty. J Orthop Surg Res. 2017 Apr;12((1)):58. doi: 10.1186/s13018-017-0557-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guenther D, Schmidl S, Klatte TO, Widhalm HK, Omar M, Krettek C, et al. Overweight and obesity in hip and knee arthroplasty: evaluation of 6078 cases. World J Orthop. 2015 Jan;6((1)):137–44. doi: 10.5312/wjo.v6.i1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011 Feb;11((2)):85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006 Dec;444((7121)):875–80. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 6.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004 Jun;89((6)):2548–56. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 7.Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med. 2010;61((1)):301–16. doi: 10.1146/annurev.med.080708.082713. [DOI] [PubMed] [Google Scholar]
- 8.Larsson SC, Wolk A. Overweight and obesity and incidence of leukemia: a meta-analysis of cohort studies. Int J Cancer. 2008 Mar;122((6)):1418–21. doi: 10.1002/ijc.23176. [DOI] [PubMed] [Google Scholar]
- 9.Lehr S, Hartwig S, Sell H. Adipokines: a treasure trove for the discovery of biomarkers for metabolic disorders. Proteomics Clin Appl. 2012 Jan;6((1-2)):91–101. doi: 10.1002/prca.201100052. [DOI] [PubMed] [Google Scholar]
- 10.Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al. Prospective Studies Collaboration Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009 Mar;373((9669)):1083–96. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basen-Engquist K, Chang M. Obesity and cancer risk: recent review and evidence. Curr Oncol Rep. 2011 Feb;13((1)):71–6. doi: 10.1007/s11912-010-0139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000 Sep;96((5)):1723–32. [PubMed] [Google Scholar]
- 13.Yamaguchi N, Argueta JG, Masuhiro Y, Kagishita M, Nonaka K, Saito T, et al. Adiponectin inhibits Toll-like receptor family-induced signaling. FEBS Lett. 2005 Dec;579((30)):6821–6. doi: 10.1016/j.febslet.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Okamoto Y, Folco EJ, Minami M, Wara AK, Feinberg MW, Sukhova GK, et al. Adiponectin inhibits the production of CXC receptor 3 chemokine ligands in macrophages and reduces T-lymphocyte recruitment in atherogenesis. Circ Res. 2008 Feb;102((2)):218–25. doi: 10.1161/CIRCRESAHA.107.164988. [DOI] [PubMed] [Google Scholar]
- 15.Kim KY, Kim JK, Han SH, Lim JS, Kim KI, Cho DH, et al. Adiponectin is a negative regulator of NK cell cytotoxicity. J Immunol. 2006 May;176((10)):5958–64. doi: 10.4049/jimmunol.176.10.5958. [DOI] [PubMed] [Google Scholar]
- 16.Wrann CD, Laue T, Hübner L, Kuhlmann S, Jacobs R, Goudeva L, et al. Short-term and long-term leptin exposure differentially affect human natural killer cell immune functions. Am J Physiol Endocrinol Metab. 2012 Jan;302((1)):E108–16. doi: 10.1152/ajpendo.00057.2011. [DOI] [PubMed] [Google Scholar]
- 17.Pietiläinen KH, Ismail K, Järvinen E, Heinonen S, Tummers M, Bollepalli S, et al. DNA methylation and gene expression patterns in adipose tissue differ significantly within young adult monozygotic BMI-discordant twin pairs. Int J Obes. 2016 Apr;40((4)):654–61. doi: 10.1038/ijo.2015.221. [DOI] [PubMed] [Google Scholar]
- 18.Wahl S, Drong A, Lehne B, Loh M, Scott WR, Kunze S, et al. Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature. 2017 Jan;541((7635)):81–6. doi: 10.1038/nature20784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jasinski-Bergner S, Mandelboim O, Seliger B. The role of microRNAs in the control of innate immune response in cancer. J Natl Cancer Inst. 2014 Sep;106((10)):dju257. doi: 10.1093/jnci/dju257. [DOI] [PubMed] [Google Scholar]
- 20.Jasinski-Bergner S, Stehle F, Gonschorek E, Kalich J, Schulz K, Huettelmaier S, et al. Identification of 14-3-3β gene as a novel miR-152 target using a proteome-based approach. J Biol Chem. 2014 Nov;289((45)):31121–35. doi: 10.1074/jbc.M114.556290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003 Sep;425((6956)):415–9. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 22.Yi R, Pasolli HA, Landthaler M, Hafner M, Ojo T, Sheridan R, et al. DGCR8-dependent microRNA biogenesis is essential for skin development. Proc Natl Acad Sci USA. 2009 Jan;106((2)):498–502. doi: 10.1073/pnas.0810766105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007 Jul;27((1)):91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009 Jan;136((2)):215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fasshauer M, Blüher M. Adipokines in health and disease. Trends Pharmacol Sci. 2015 Jul;36((7)):461–70. doi: 10.1016/j.tips.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 26.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004 May;145((5)):2273–82. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 27.Kim KH, Lee K, Moon YS, Sul HS. A cysteine-rich adipose tissue-specific secretory factor inhibits adipocyte differentiation. J Biol Chem. 2001 Apr;276((14)):11252–6. doi: 10.1074/jbc.C100028200. [DOI] [PubMed] [Google Scholar]
- 28.Kuchen S, Resch W, Yamane A, Kuo N, Li Z, Chakraborty T, et al. Regulation of microRNA expression and abundance during lymphopoiesis. Immunity. 2010 Jun;32((6)):828–39. doi: 10.1016/j.immuni.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee IH, Hong X, Mathur SC, Sharma M, Rastogi A, Sharma P, et al. A detailed analysis of next generation sequencing reads of microRNA expression in Barrett's esophagus: absolute versus relative quantification. BMC Res Notes. 2014 Apr;7((1)):212. doi: 10.1186/1756-0500-7-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stokowy T, Eszlinger M, Świerniak M, Fujarewicz K, Jarząb B, Paschke R, et al. Analysis options for high-throughput sequencing in miRNA expression profiling. BMC Res Notes. 2014 Mar;7((1)):144. doi: 10.1186/1756-0500-7-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008 Jul;5((7)):621–8. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 32.Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014 Jan;42((Database issue)):D68–73. doi: 10.1093/nar/gkt1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozomara A, Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011 Jan;39((Database issue)):D152–7. doi: 10.1093/nar/gkq1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005 May;26((3)):439–51. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 35.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003 Jun;423((6941)):762–9. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 36.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci USA. 2004 Jul;101((28)):10308–13. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schraw T, Wang ZV, Halberg N, Hawkins M, Scherer PE. Plasma adiponectin complexes have distinct biochemical characteristics. Endocrinology. 2008 May;149((5)):2270–82. doi: 10.1210/en.2007-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng Y, Scherer PE. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann N Y Acad Sci. 2010 Nov;1212((1)):E1–19. doi: 10.1111/j.1749-6632.2010.05875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajala MW, Scherer PE. Minireview: the adipocyte—at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003 Sep;144((9)):3765–73. doi: 10.1210/en.2003-0580. [DOI] [PubMed] [Google Scholar]
- 40.Landskroner-Eiger S, Qian B, Muise ES, Nawrocki AR, Berger JP, Fine EJ, et al. Proangiogenic contribution of adiponectin toward mammary tumor growth in vivo. Clin Cancer Res. 2009 May;15((10)):3265–76. doi: 10.1158/1078-0432.CCR-08-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004 Oct;323((2)):630–5. doi: 10.1016/j.bbrc.2004.08.145. [DOI] [PubMed] [Google Scholar]
- 42.Wilk S, Scheibenbogen C, Bauer S, Jenke A, Rother M, Guerreiro M, et al. Adiponectin is a negative regulator of antigen-activated T cells. Eur J Immunol. 2011 Aug;41((8)):2323–32. doi: 10.1002/eji.201041349. [DOI] [PubMed] [Google Scholar]
- 43.Ohashi K, Shibata R, Murohara T, Ouchi N. Role of anti-inflammatory adipokines in obesity-related diseases. Trends Endocrinol Metab. 2014 Jul;25((7)):348–55. doi: 10.1016/j.tem.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 44.Pietrzyk L, Torres A, Maciejewski R, Torres K. Obesity and Obese-related Chronic Low-grade Inflammation in Promotion of Colorectal Cancer Development. Asian Pac J Cancer Prev. 2015;16((10)):4161–8. doi: 10.7314/apjcp.2015.16.10.4161. [DOI] [PubMed] [Google Scholar]
- 45.Salans LB, Cushman SW, Weismann RE. Studies of human adipose tissue. Adipose cell size and number in nonobese and obese patients. J Clin Invest. 1973 Apr;52((4)):929–41. doi: 10.1172/JCI107258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arner E, Westermark PO, Spalding KL, Britton T, Rydén M, Frisén J, et al. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes. 2010 Jan;59((1)):105–9. doi: 10.2337/db09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meyer LK, Ciaraldi TP, Henry RR, Wittgrove AC, Phillips SA. Adipose tissue depot and cell size dependency of adiponectin synthesis and secretion in human obesity. Adipocyte. 2013 Oct;2((4)):217–26. doi: 10.4161/adip.24953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003 Jul;52((7)):1779–85. doi: 10.2337/diabetes.52.7.1779. [DOI] [PubMed] [Google Scholar]
- 49.Cohen SS, Gammon MD, Signorello LB, North KE, Lange EM, Fowke JH, et al. Serum adiponectin in relation to body mass index and other correlates in black and white women. Ann Epidemiol. 2011 Feb;21((2)):86–94. doi: 10.1016/j.annepidem.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ge Q, Gérard J, Noël L, Scroyen I, Brichard SM. MicroRNAs regulated by adiponectin as novel targets for controlling adipose tissue inflammation. Endocrinology. 2012 Nov;153((11)):5285–96. doi: 10.1210/en.2012-1623. [DOI] [PubMed] [Google Scholar]
- 51.Huang CY, Chang AC, Chen HT, Wang SW, Lo YS, Tang CH. Adiponectin promotes VEGF-C-dependent lymphangiogenesis by inhibiting miR-27b through a CaMKII/AMPK/p38 signaling pathway in human chondrosarcoma cells. Clin Sci (Lond) 2016 Sep;130((17)):1523–33. doi: 10.1042/CS20160117. [DOI] [PubMed] [Google Scholar]
- 52.Li Y, Cai X, Guan Y, Wang L, Wang S, Li Y, et al. Adiponectin Upregulates MiR-133a in Cardiac Hypertrophy through AMPK Activation and Reduced ERK1/2 Phosphorylation. PLoS One. 2016 Feb;11((2)):e0148482. doi: 10.1371/journal.pone.0148482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang X, Xue M, Fu Z, Ji C, Guo X, Zhu L, et al. Insight into the effects of adipose tissue inflammation factors on miR-378 expression and the underlying mechanism. Cell Physiol Biochem. 2014;33((6)):1778–88. doi: 10.1159/000362957. [DOI] [PubMed] [Google Scholar]
- 54.Subedi A, Park PH. Autocrine and paracrine modulation of microRNA-155 expression by globular adiponectin in RAW 264.7 macrophages: involvement of MAPK/NF-κB pathway. Cytokine. 2013 Dec;64((3)):638–41. doi: 10.1016/j.cyto.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 55.Subedi A, Kim MJ, Nepal S, Lee ES, Kim JA, Sohn DH, et al. Globular adiponectin modulates expression of programmed cell death 4 and miR-21 in RAW 264.7 macrophages through the MAPK/NF-κB pathway. FEBS Lett. 2013 May;587((10)):1556–61. doi: 10.1016/j.febslet.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 56.Zhang N, Zhang N, Song L, Xie H, Zhao C, Li S, et al. Adipokines and free fatty acids regulate insulin sensitivity by increasing microRNA-21 expression in human mature adipocytes. Mol Med Rep. 2017 Aug;16((2)):2254–8. doi: 10.3892/mmr.2017.6769. [DOI] [PubMed] [Google Scholar]
- 57.Pourhoseini S, Seth RK, Das S, Dattaroy D, Kadiiska MB, Xie G, et al. Upregulation of miR21 and repression of Grhl3 by leptin mediates sinusoidal endothelial injury in experimental nonalcoholic steatohepatitis. PLoS One. 2015 Feb;10((2)):e0116780. doi: 10.1371/journal.pone.0116780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang WH, Chang AC, Wang SW, Wang SJ, Chang YS, Chang TM, et al. Leptin promotes VEGF-C production and induces lymphangiogenesis by suppressing miR-27b in human chondrosarcoma cells. Sci Rep. 2016 Jun;6((1)):28647. doi: 10.1038/srep28647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meerson A, Yehuda H. Leptin and insulin up-regulate miR-4443 to suppress NCOA1 and TRAF4, and decrease the invasiveness of human colon cancer cells. BMC Cancer. 2016 Nov;16((1)):882. doi: 10.1186/s12885-016-2938-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang WH, Tsai CH, Fong YC, Huang YL, Wang SJ, Chang YS, et al. Leptin induces oncostatin M production in osteoblasts by downregulating miR-93 through the Akt signaling pathway. Int J Mol Sci. 2014 Sep;15((9)):15778–90. doi: 10.3390/ijms150915778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gu N, You L, Shi C, Yang L, Pang L, Cui X, et al. Expression of miR-199a-3p in human adipocytes is regulated by free fatty acids and adipokines. Mol Med Rep. 2016 Aug;14((2)):1180–6. doi: 10.3892/mmr.2016.5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang X, Yang L, Pang L, Chen L, Guo X, Ji C, et al. Expression of obesity‑related miR‑1908 in human adipocytes is regulated by adipokines, free fatty acids and hormones. Mol Med Rep. 2014 Aug;10((2)):1164–9. doi: 10.3892/mmr.2014.2297. [DOI] [PubMed] [Google Scholar]
- 63.Xu LL, Shi CM, Xu GF, Chen L, Zhu LL, Zhu L, et al. TNF-α, IL-6, and leptin increase the expression of miR-378, an adipogenesis-related microRNA in human adipocytes. Cell Biochem Biophys. 2014 Nov;70((2)):771–6. doi: 10.1007/s12013-014-9980-x. [DOI] [PubMed] [Google Scholar]
- 64.Zhu L, Shi C, Ji C, Xu G, Chen L, Yang L, et al. FFAs and adipokine-mediated regulation of hsa-miR-143 expression in human adipocytes. Mol Biol Rep. 2013 Oct;40((10)):5669–75. doi: 10.1007/s11033-013-2668-2. [DOI] [PubMed] [Google Scholar]
- 65.Zhu L, Chen L, Shi CM, Xu GF, Xu LL, Zhu LL, et al. MiR-335, an adipogenesis-related microRNA, is involved in adipose tissue inflammation. Cell Biochem Biophys. 2014 Mar;68((2)):283–90. doi: 10.1007/s12013-013-9708-3. [DOI] [PubMed] [Google Scholar]
- 66.Meerson A, Traurig M, Ossowski V, Fleming JM, Mullins M, Baier LJ. Human adipose microRNA-221 is upregulated in obesity and affects fat metabolism downstream of leptin and TNF-α. Diabetologia. 2013 Sep;56((9)):1971–9. doi: 10.1007/s00125-013-2950-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu X, Dong Z, Li Y, Yang Y, Yuan Z, Qu X, et al. The upregulation of signal transducer and activator of transcription 5-dependent microRNA-182 and microRNA-96 promotes ovarian cancer cell proliferation by targeting forkhead box O3 upon leptin stimulation. Int J Biochem Cell Biol. 2013 Mar;45((3)):536–45. doi: 10.1016/j.biocel.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 68.Xu G, Ji C, Shi C, Fu H, Zhu L, Zhu L, et al. Modulation of hsa-miR-26b levels following adipokine stimulation. Mol Biol Rep. 2013 May;40((5)):3577–82. doi: 10.1007/s11033-012-2431-0. [DOI] [PubMed] [Google Scholar]
- 69.Hamrick MW, Herberg S, Arounleut P, He HZ, Shiver A, Qi RQ, et al. The adipokine leptin increases skeletal muscle mass and significantly alters skeletal muscle miRNA expression profile in aged mice. Biochem Biophys Res Commun. 2010 Sep;400((3)):379–83. doi: 10.1016/j.bbrc.2010.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wen F, Li B, Huang C, Wei Z, Zhou Y, Liu J, et al. MiR-34a is Involved in the Decrease of ATP Contents Induced by Resistin Through Target on ATP5S in HepG2 Cells. Biochem Genet. 2015 Dec;53((11-12)):301–9. doi: 10.1007/s10528-015-9693-x. [DOI] [PubMed] [Google Scholar]
- 71.Wen F, Zhang H, Bao C, Yang M, Wang N, Zhang J, et al. Resistin Increases Ectopic Deposition of Lipids Through miR-696 in C2C12 Cells. Biochem Genet. 2015 Jun;53((4-6)):63–71. doi: 10.1007/s10528-015-9672-2. [DOI] [PubMed] [Google Scholar]
- 72.Su CM, Hsu CJ, Tsai CH, Huang CY, Wang SW, Tang CH. Resistin Promotes Angiogenesis in Endothelial Progenitor Cells Through Inhibition of MicroRNA206: Potential Implications for Rheumatoid Arthritis. Stem Cells. 2015 Jul;33((7)):2243–55. doi: 10.1002/stem.2024. [DOI] [PubMed] [Google Scholar]
- 73.Wen F, Yang Y, Jin D, Sun J, Yu X, Yang Z. MiRNA-145 is involved in the development of resistin-induced insulin resistance in HepG2 cells. Biochem Biophys Res Commun. 2014 Mar;445((2)):517–23. doi: 10.1016/j.bbrc.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 74.Tsai CH, Tsai HC, Huang HN, Hung CH, Hsu CJ, Fong YC, et al. Resistin promotes tumor metastasis by down-regulation of miR-519d through the AMPK/p38 signaling pathway in human chondrosarcoma cells. Oncotarget. 2015 Jan;6((1)):258–70. doi: 10.18632/oncotarget.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000 Apr;404((6778)):661–71. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 76.Van Harmelen V, Reynisdottir S, Eriksson P, Thörne A, Hoffstedt J, Lönnqvist F, et al. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes. 1998 Jun;47((6)):913–7. doi: 10.2337/diabetes.47.6.913. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994 Dec;372((6505)):425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 78.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995 Dec;83((7)):1263–71. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 79.Mistry AM, Swick AG, Romsos DR. Leptin rapidly lowers food intake and elevates metabolic rates in lean and ob/ob mice. J Nutr. 1997 Oct;127((10)):2065–72. doi: 10.1093/jn/127.10.2065. [DOI] [PubMed] [Google Scholar]
- 80.Zhou Y, Rui L. Leptin signaling and leptin resistance. Front Med. 2013 Jun;7((2)):207–22. doi: 10.1007/s11684-013-0263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gorska E, Popko K, Stelmaszczyk-Emmel A, Ciepiela O, Kucharska A, Wasik M. Leptin receptors. Eur J Med Res. 2010 Nov;15(Suppl 2):50–4. doi: 10.1186/2047-783X-15-S2-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Münzberg H, Morrison CD. Structure, production and signaling of leptin. Metabolism. 2015 Jan;64((1)):13–23. doi: 10.1016/j.metabol.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shen J, Sakaida I, Uchida K, Terai S, Okita K. Leptin enhances TNF-alpha production via p38 and JNK MAPK in LPS-stimulated Kupffer cells. Life Sci. 2005 Aug;77((13)):1502–15. doi: 10.1016/j.lfs.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 84.Faggioni R, Jones-Carson J, Reed DA, Dinarello CA, Feingold KR, Grunfeld C, et al. Leptin-deficient (ob/ob) mice are protected from T cell-mediated hepatotoxicity: role of tumor necrosis factor alpha and IL-18. Proc Natl Acad Sci USA. 2000 Feb;97((5)):2367–72. doi: 10.1073/pnas.040561297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001 Jan;409((6818)):307–12. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 86.Nagaev I, Smith U. Insulin resistance and type 2 diabetes are not related to resistin expression in human fat cells or skeletal muscle. Biochem Biophys Res Commun. 2001 Jul;285((2)):561–4. doi: 10.1006/bbrc.2001.5173. [DOI] [PubMed] [Google Scholar]
- 87.McTernan PG, McTernan CL, Chetty R, Jenner K, Fisher FM, Lauer MN, et al. Increased resistin gene and protein expression in human abdominal adipose tissue. J Clin Endocrinol Metab. 2002 May;87((5)):2407. doi: 10.1210/jcem.87.5.8627. [DOI] [PubMed] [Google Scholar]
- 88.Patel SD, Rajala MW, Rossetti L, Scherer PE, Shapiro L. Disulfide-dependent multimeric assembly of resistin family hormones. Science (80-) 2004;304:1154–1158. doi: 10.1126/science.1093466. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 89.Gerber M, Boettner A, Seidel B, Lammert A, Bär J, Schuster E, et al. Serum resistin levels of obese and lean children and adolescents: biochemical analysis and clinical relevance. J Clin Endocrinol Metab. 2005 Aug;90((8)):4503–9. doi: 10.1210/jc.2005-0437. [DOI] [PubMed] [Google Scholar]
- 90.Juan CC, Kan LS, Huang CC, Chen SS, Ho LT, Au LC. Production and characterization of bioactive recombinant resistin in Escherichia coli. J Biotechnol. 2003 Jun;103((2)):113–7. doi: 10.1016/s0168-1656(03)00099-3. [DOI] [PubMed] [Google Scholar]
- 91.Lee S, Lee HC, Kwon YW, Lee SE, Cho Y, Kim J, et al. Adenylyl cyclase-associated protein 1 is a receptor for human resistin and mediates inflammatory actions of human monocytes. Cell Metab. 2014 Mar;19((3)):484–97. doi: 10.1016/j.cmet.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Silswal N, Singh AK, Aruna B, Mukhopadhyay S, Ghosh S, Ehtesham NZ. Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB-dependent pathway. Biochem Biophys Res Commun. 2005 Sep;334((4)):1092–101. doi: 10.1016/j.bbrc.2005.06.202. [DOI] [PubMed] [Google Scholar]
- 93.Zhang Z, Xing X, Hensley G, Chang LW, Liao W, Abu-Amer Y, et al. Resistin induces expression of proinflammatory cytokines and chemokines in human articular chondrocytes via transcription and messenger RNA stabilization. Arthritis Rheum. 2010 Jul;62((7)):1993–2003. doi: 10.1002/art.27473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sadashiv TS, Tiwari S, Paul BN, Kumar S, Chandra A, Dhananjai S, et al. Over expression of resistin in adipose tissue of the obese induces insulin resistance. World J Diabetes. 2012 Jul;3((7)):135–41. doi: 10.4239/wjd.v3.i7.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Medina PP, Nolde M, Slack FJ. OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma. Nature. 2010 Sep;467((7311)):86–90. doi: 10.1038/nature09284. [DOI] [PubMed] [Google Scholar]
- 96.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007 Aug;133((2)):647–58. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005 Jul;65((14)):6029–33. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 98.Huang CS, Yu W, Cui H, Wang YJ, Zhang L, Han F, et al. Increased expression of miR-21 predicts poor prognosis in patients with hepatocellular carcinoma. Int J Clin Exp Pathol. 2015 Jun;8((6)):7234–8. [PMC free article] [PubMed] [Google Scholar]
- 99.Aravalli RN. Development of MicroRNA Therapeutics for Hepatocellular Carcinoma. Diagnostics (Basel) 2013 Mar;3((1)):170–91. doi: 10.3390/diagnostics3010170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Seeger T, Fischer A, Muhly-Reinholz M, Zeiher AM, Dimmeler S. Long-term inhibition of miR-21 leads to reduction of obesity in db/db mice. Obes (Silver Spring) 2014;22:2352–2360. doi: 10.1002/oby.20852. [DOI] [PubMed] [Google Scholar]
- 101.Lee JJ, Drakaki A, Iliopoulos D, Struhl K. MiR-27b targets PPARγ to inhibit growth, tumor progression and the inflammatory response in neuroblastoma cells. Oncogene. 2012 Aug;31((33)):3818–25. doi: 10.1038/onc.2011.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mu W, Hu C, Zhang H, Qu Z, Cen J, Qiu Z, et al. miR-27b synergizes with anticancer drugs via p53 activation and CYP1B1 suppression. Cell Res. 2015 Apr;25((4)):477–95. doi: 10.1038/cr.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Christopher AF, Kaur RP, Kaur G, Kaur A, Gupta V, Bansal P. MicroRNA therapeutics: discovering novel targets and developing specific therapy. Perspect Clin Res. 2016 Apr-Jun;7((2)):68–74. doi: 10.4103/2229-3485.179431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hickey MS, Israel RG, Gardiner SN, Considine RV, McCammon MR, Tyndall GL, et al. Gender differences in serum leptin levels in humans. Biochem Mol Med. 1996 Oct;59((1)):1–6. doi: 10.1006/bmme.1996.0056. [DOI] [PubMed] [Google Scholar]
- 105.Teixeira LG, Leonel AJ, Aguilar EC, Batista NV, Alves AC, Coimbra CC, et al. The combination of high-fat diet-induced obesity and chronic ulcerative colitis reciprocally exacerbates adipose tissue and colon inflammation. Lipids Health Dis. 2011 Nov;10((1)):204. doi: 10.1186/1476-511X-10-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lausten-Thomsen U, Christiansen M, Hedley PL, Nielsen TR, Fonvig CE, Pedersen O, et al. Reference values for fasting serum resistin in healthy children and adolescents. Clin Chim Acta. 2017 Jun;469:161–5. doi: 10.1016/j.cca.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 107.Jiang Y, Owei I, Wan J, Ebenibo S, Dagogo-Jack S. Adiponectin levels predict prediabetes risk: the Pathobiology of Prediabetes in A Biracial Cohort (POP-ABC) study. BMJ Open Diabetes Res Care. 2016 Mar;4((1)):e000194. doi: 10.1136/bmjdrc-2016-000194. [DOI] [PMC free article] [PubMed] [Google Scholar]