Abstract
β-Apocarotenoids are eccentric cleavage products of carotenoids formed by chemical and enzymatic oxidations. They occur in foods containing carotenoids and thus might be directly absorbed from the diet. However, there is limited information about their intestinal absorption. The present research examined the kinetics of uptake and metabolism of β-apocarotenoids. Caco-2 cells were grown on 6-well plastic plates until a differentiated cell monolayer was achieved. β-Apocarotenoids were prepared in Tween 40 micelles, delivered to differentiated cells in serum-free medium, and incubated at 37°C for up to 8 h. There was rapid uptake of β-apo-8′-carotenal into cells, and β-apo-8′-carotenal was largely converted to β-apo-8′-carotenoic acid and a minor metabolite that we identified as 5,6-epoxy-β-apo-8′-carotenol. There was also rapid uptake of β-apo-10′-carotenal into cells, and β-apo-10′-carotenal was converted into a major metabolite identified as 5,6-epoxy-β-apo-10′-carotenol and a minor metabolite that is likely a dihydro-β-apo-10′-carotenol. Finally, there was rapid cellular uptake of β-apo-13-carotenone, and this compound was extensively degraded. These results suggest that dietary β-apocarotenals are extensively metabolized in intestinal cells via pathways similar to the metabolism of retinal. Thus, they are likely not absorbed directly from the diet.
Keywords: β-carotene, absorption, cell uptake, carotenoid metabolism, dietary lipids, intestine, nutrition/lipids, vitamin A
Vitamin A is a general term for structurally related compounds that have the functional activity of retinol (1). De novo synthesis of provitamin A carotenoids occurs almost exclusively in plants and some microorganisms, such as algae and fungi (2, 3). Dietary vitamin A can be obtained in two major forms: preformed vitamin A (retinol and retinyl esters) and provitamin A carotenoids (β-carotene, α-carotene, and β-cryptoxanthin) (1, 2). Preformed vitamin A is present in animal-derived products, such as liver, eggs, and dairy products. In contrast, plant food sources (mostly fruits and vegetables) account for significant intake of dietary provitamin A carotenoids (2, 3). Sweet potatoes and pumpkin, for example, provide high amounts of α-carotene; oranges, papaya, and peppers are excellent sources of β-cryptoxanthin; and carrots, squash, and dark-leafy vegetables are important sources of β-carotene (3, 4).
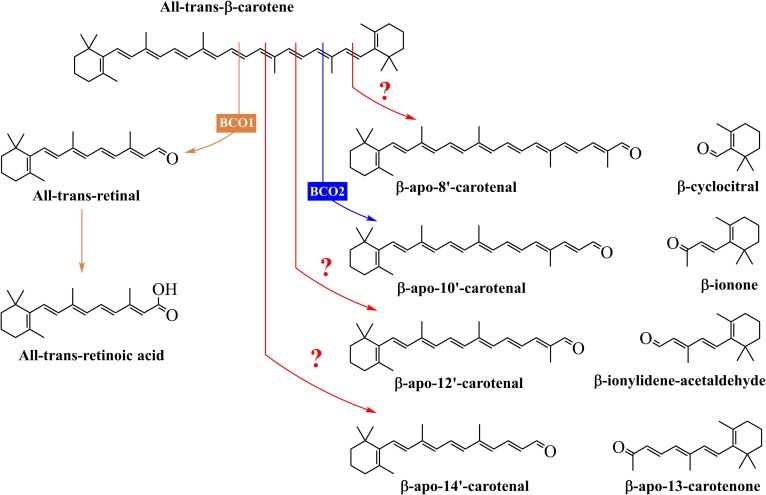
β-Carotene is an excellent precursor of vitamin A and exhibits this provitamin A activity because it has two unsubstituted β-ionone rings as well as the correct number and position of methyl groups in its long hydrocarbon chain (2). Two important enzymes, β-carotene 15,15′-oxygenase (BCO1) and β-carotene 9′,10′-oxygenase (BCO2), catalyze the oxidative cleavage of β-carotene to produce biologically active metabolites (Fig. 1) (5). BCO1, localized in the cytosol (6), cleaves β-carotene to give two molecules of retinal, which can be converted to retinoic acid (RA) or retinol (2, 7). On the other hand, BCO2, localized in the mitochondria (6, 8), cleaves β-carotene to yield β-ionone and β-apo-10′-carotenal, which can be converted to retinal by BCO1 (2, 9). Peroxidases and lipoxygenases have been suggested as enzymes that could catalyze the formation of β-apocarotenoids other than β-apo-10′-carotenal (10). Apocarotenoids are also formed nonenzymatically via chemical oxidation during food processing and cooking or in tissues under conditions of oxidative stress.
Fig. 1.
Structures of the products of the central and eccentric cleavage of β-carotene. All-trans-β-carotene can be cleaved by BCO1 to yield two molecules of retinal, which can be converted to retinol or RA. On the other hand, the main product of the eccentric cleavage pathway of β-carotene is β-apo-10′-carotenal, and this reaction is catalyzed by BCO2. Also, β-carotene can undergo chemical and enzymatic oxidations in vivo and in foods to yield other long-chain and short-chain β-apocarotenoids. The exact mechanisms and enzymes responsible for these products are unknown.
β-Apocarotenoids have biological activity and previous studies suggest that they may elicit their functions through gene regulation (5, 7). RA receptors (RARs) are a family of transcription factors that activate genes with RA response elements as part of their promoter sequence (7, 11). A number of studies have investigated the binding affinity and effects of β-apocarotenoids on these transcription factors. Work by Eroglu et al. (7) demonstrated that some β-apocarotenoids are potent antagonists of α, β, and γ isoforms of RARs. β-Apo-14′-carotenoic acid, β-apo-14′-carotenal, and β-apo-13-carotenone were high affinity ligands for RARs α, β, and γ and significantly antagonized the transactivation of all three RAR isoforms by RA. Also, these β-apocarotenoids effectively decreased the RA-induced upregulation of RARβ and cytochrome P450-26A1 in HepG2 cells. In addition, Eroglu et al. (12) examined the effects of β-apocarotenoids on the activation and signaling of retinoid X receptor α (RXRα). β-Apo-13-carotenone significantly inhibited the 9-cis-RA-induced activation of RXRα and its transcriptional response. Molecular modeling and ligand binding studies showed that β-apo-13-carotenone directly binds to the ligand binding domain of RXRα.
The exact mechanisms of formation of β-apocarotenoids, other than β-apo-10′-carotenal and retinal, from β-carotene and other carotenoids consumed in the diet by humans are not fully understood (10, 13). However, these eccentric cleavage products are present in a carotenoid-rich diet. Harrison and colleagues identified several β-apocarotenoids in cantaloupe and orange-fleshed honeydew melons (14). The quantities of β-apo-8′-carotenal, β-apo-10′-carotenal, β-apo-12′-carotenal, β-apo-14′-carotenal, and β-apo-13-carotenone detected using HPLC-MS were approximately 1.5% of β-carotene in both melons. In addition, our laboratory has evidence that these β-apocarotenoids are present in pumpkin, sweet potato, and yellow squash at about 2.5, 0.4, and 3% of β-carotene, respectively (M. K. Fleshman and E. H. Harrison, unpublished observations). Thus, it is possible that β-apocarotenoids might be directly absorbed from the diet (13). However, there is limited knowledge about their bioavailability and intestinal absorption.
The Caco-2 intestinal cell line is a well-established in vitro model that has been used to study the absorption of the C40 carotenes and xanthophylls (15, 16). Differentiated cells have morphological and functional characteristics comparable to enterocytes (17, 18) and these include very well developed brush border membranes and tight junctions that decrease the probability of transport of molecules between cells (19). Thus, Caco-2 cells are a good model to study the apical uptake and metabolism of β-apocarotenoids. In the present study, we investigated the uptake and metabolism of β-apo-8′-carotenal, β-apo-10′-carotenal, and β-apo-13-carotenone using Caco-2 cells as a model. We focused specifically on these β-apocarotenoids because β-apo-8′-carotenal is commercially available and is routinely utilized as a food colorant; β-apo-10′-carotenal is the major product of BCO2 cleavage of β-carotene; and β-apo-13-carotenone, with a similar structure to retinol, has been shown to be a potent antagonist of vitamin A signaling.
MATERIALS AND METHODS
Chemicals and supplies
Unless otherwise stated, all chemicals and supplies were purchased from Sigma-Aldrich (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA). Cell culture reagents were obtained from Gibco® Life Technologies Corporation (Grand Island, NY). TLC plates were purchased from Analtech (Newark, DE). Plates were 20 × 20 cm glass backed with a 1,000 micron layer of silica gel containing a fluorescent indicator. The synthesis of β-apo-8′-carotenoic acid, β-apo-10′-carotenal, β-apo-10′-carotenoic acid, β-apo-12′-carotenal, and β-apo-13-carotenone have been described previously (7). In addition, β-apo-10′-carotenol was synthesized as described elsewhere (20). Finally, β-apo-8′-carotenol and 5,6-epoxy-β-apo-8′-carotenol were synthesized according to the methods described below.
Synthesis of β-apocarotenoids
Synthesis of β-apo-8′-carotenol.
Five milligrams of β-apo-8′-carotenal, 15 mg (3.3 equivalents) of sodium borohydride, 5 ml of absolute ethanol, and 2 ml of toluene were mixed in a dry round bottom flask under argon with magnetic stirring. After 30 min, the color of the mixture had not changed. After 1 h, the mixture became a little lighter colored. More sodium borohydride was added, the reaction mixture stirred for 30 min, and 1 ml of 4°C saturated ammonium chloride was added. The mixture was now still lighter in color and was partitioned between saturated sodium chloride and ethyl acetate. The ethyl acetate layer was dried over sodium sulfate, filtered, and concentrated under reduced pressure. The dried residue was resolubilized in ethyl acetate, spread onto a preparative TLC plate, and allowed to develop for 40 min using 20% ethyl acetate/hexanes as mobile phase. The lower band, tentatively identified as β-apo-8′-carotenol was suspended and stirred in acetone for 20 min, filtered, and concentrated under reduced pressure. The newly synthesized compound was then characterized by NMR and HPLC (see HPLC analysis, Method 1).
Synthesis of 5,6-epoxy-β-apo-8′-carotenol.
Previously synthesized β-apo-8′-carotenol (13.6 mg) was dissolved in approximately 10 ml of dichloromethane and placed in an oven-dried round bottom flask with a magnetic stirrer and an argon inlet. After cooling to 4°C, 8.9 mg (1.2 equivalents) of meta-chloroperoxybenzoic acid were added. The mixture lost its color immediately. The ice bath was removed and stirring continued for 4 h. The solution was washed twice with saturated sodium bicarbonate, then with distilled water followed by saturated sodium chloride, and dried over sodium sulfate. Filtration and concentration under reduced pressure afforded crude product, which was purified by preparative TLC and characterized as for β-apo-8′-carotenol.
Cell culture conditions
Caco-2 cells were obtained from the American Type Culture Collection (Rockville, MD). Cells were grown and maintained as described elsewhere (21). Briefly, 75 cm2 flasks were seeded with 0.4 × 106 cells and grown in DMEM containing 25 mM glucose, 15% heat-inactivated FBS, 1% penicillin-streptomycin, 1% l-glutamine (200 mM), 1% nonessential amino acids, and 1% fungizone. Cells were incubated at 37°C in an atmosphere of air/CO2 (95:5 v/v) and cells were passaged every 7 days or when cells were at 75–80% confluency.
Preparation of β-apocarotenoids for uptake studies
We prepared β-apocarotenoids in Tween 40 as previously described (15) with some changes and delivered them in serum-free medium to cells (see description below). Briefly, β-apocarotenoids were solubilized in either hexane or ethanol to prepare a stock solution of the pure compounds. The required volume of each β-apocarotenoid dissolved in appropriate solvent, 22.5 μl of 10% v/v Tween 40 (in ethanol), and 400 μl of acetone were placed into a 4 ml glass vial. The resulting mixture was dried under a stream of nitrogen gas at 30–40°C, resolubilized in 200 μl of acetone, and dried again. Dried residues were solubilized in 1 ml of serum-free medium, vortexed briefly, and sonicated for 1 min. The resulting mixture was dissolved in additional serum-free medium to yield the desired test concentrations of the clear micellar solutions.
Stability of β-apocarotenoids during incubation in cell culture conditions
Previous studies have shown that carotenoids are susceptible to spontaneous chemical oxidation and degradation during incubation in cell culture environment (22, 23). Thus, we investigated the stability of the β-apocarotenoids in the same cell culture conditions as were used for the cell uptake experiments. The method for preparing β-apocarotenoids in Tween 40 has been described above. For the stability experiments, 2 ml of serum-free medium, containing the individual β-apocarotenoids (β-apo-8′-carotenal, β-apo-10′-carotenal, and β-apo-13-carotenone), were added to 6-well plates without Caco-2 cells and incubated at 37°C for time points up to 8 h. Aliquots of medium at 0, 1, 4, and 8 h were collected and stored at −80°C before extraction and HPLC analysis.
Uptake of β-apocarotenoids into Caco-2 cells
For experiments, 6-well plates were seeded with 0.15 × 106 cells and maintained in the cell culture conditions described above until a confluent monolayer was achieved. Once confluent, the amount of FBS used in the medium was decreased to 7.5% and the medium was regularly replaced after 2 days. Cell cultures used for experiments were between passages 22 and 40 when monolayers were 10–14 days postconfluent (i.e., fully differentiated). At the start of each experiment, cell monolayers were washed once with basal DMEM. Two milliliters of serum-free medium, containing a known concentration of each β-apocarotenoid (β-apo-8′-carotenal, β-apo-10′-carotenal, and β-apo-13-carotenone), were added to each well and incubated at 37°C.
After incubation, the integrity of the monolayer was examined by phase contrast microscopy and medium containing the test compound was collected into a 15 ml centrifuge tube. There were no observed changes in the morphology of cell monolayers after treatment with any β-apocarotenoids. Cell monolayers were washed once with 2 ml of ice-cold PBS containing 2 g/l of BSA and twice with 2 ml of ice-cold PBS to remove extracellular β-apocarotenoids. Two milliliters of ice-cold PBS were added to each well and cells were harvested into 15 ml centrifuge tubes using a plastic scraper. Tubes were then centrifuged at 1,000 g for 10 min at 4°C. The supernatant was discarded and the cell pellets were stored under nitrogen gas at −80°C before extraction and HPLC analysis (24).
Extraction of β-apocarotenoids
Extraction from medium.
Cell culture medium samples before and after incubation were extracted according to the method of Kopec et al. (25) with slight modifications. Briefly, 1 ml of medium and 1 ml of acetone were placed in a 15 ml centrifuge tube and vortexed for 1 min. Four milliliters of hexanes were added and the mixture was vortexed again for 1 min. The resulting mixture was then centrifuged at 3,000 g for 10 min at 4°C. The upper layer was transferred into an 11 ml screw cap glass vial and solvent was dried under a stream of nitrogen gas at 30–40°C. Extracts were then stored at −80°C before HPLC analysis.
Extraction from cell monolayer.
Cell pellets were resuspended in 1 ml of PBS and probe sonicated for 20 s. One milliliter of acetone was added and the tube was vortexed for 1 min. Four milliliters of hexanes were added and the mixture was vortexed again for 1 min. The resulting solution was then centrifuged at 3,000 g for 10 min at 4°C. The upper layer was transferred into an 11 ml screw cap glass vial and solvent was dried under a stream of nitrogen gas at 30–40°C. Extracts were stored at −80°C before HPLC analysis.
HPLC analysis
Method 1: analysis of β-apo-8′-carotenal and β-apo-10′-carotenal.
Sample extracts were solubilized in 200 μl of 1:1 v/v methanol/methyl tert-butyl ether (MTBE), syringe filtered (0.22 μm pores; Millipore), and 20 μl of the extract were injected for analysis. Separation of β-apocarotenoids was achieved using a YMC C30 column (4.6 × 150 mm, 5 μm; Waters, Ireland) on an Agilent 1200 series HPLC system coupled to a diode array detector (DAD) (Agilent Technologies, Santa Clara, CA) with a mobile phase of 98:2 v/v methanol/1 M ammonium acetate (pH 4.6) (solvent A) and MTBE (solvent B). The flow rate was set at 0.8 ml/min and a column temperature of 35°C was maintained. Analytes were separated with a linear gradient from 15% B to 40% B over 10 min, followed by elution at 40% B for 10 min before reequilibrating to the initial conditions for 4.1 min. Elution was monitored at 380 nm, 450 nm, and 460 nm for β-apo-8′-carotenal and at 380 nm and 438 nm for β-apo-10′-carotenal. β-Apocarotenoids in samples were quantified by comparing the peak area against a standard curve prepared with pure standard compounds.
Method 2: analysis of β-apo-13-carotenone.
Sample extracts were dissolved in 200 μl of 1:1 v/v methanol/HPLC-MS grade water, syringe filtered (0.22 μm pores; Millipore), and 20 μl of the extract were injected for analysis. The HPLC system and column used for this analysis were described in Method 1. The composition of the mobile phase was methanol (solvent A) and HPLC-MS grade water (solvent B). The flow rate was set at 0.8 ml/min and a column temperature of 35°C was maintained. Analytes were separated with 15% B for 15 min and elution was monitored at 345 nm. β-Apo-13-carotenone in samples was quantified by comparing the peak area against a standard curve prepared with known concentrations of pure β-apo-13-carotenone.
UHPLC-MS analysis of cellular metabolites
Method 1: analysis of cellular metabolites of β-apo-8′-carotenal.
Cellular metabolites of β-apo-8′-carotenal were separated by an Agilent 1290 Infinity I UHPLC (Agilent Technologies) using a YMC C30 column (2.0 × 150 mm, S3; Waters Corporation, Milford, MA) with a mobile phase of 80% methanol/20% water (solvent A) and 78% MTBE/20% methanol/2% water (solvent B) where both mobile phases contained 2% (w/v) ammonium acetate. The flow rate was set at 0.4 ml/min and ramped up linearly from 0% B to 60% B over 7 min and reequilibrated over the subsequent 5 min. The column temperature was maintained at 45°C. The entire eluent flow was introduced to an Agilent 6550 quadrupole TOF (QTOF) mass spectrometer (Agilent Technologies) with positive ion electrospray source parameters of 400°C sheath gas at 12 l/min, 150°C drying gas at 18 l/min, 30 psi nebulizer, 3 kV Vcap, and 1.5 kV nozzle. Ion funnel settings were 90 V low pressure funnel, 200 V high pressure funnel. Also, there was a DAD inline to collect UV-vis spectra from 230 nm through 640 nm.
Method 2: analysis of cellular metabolites of β-apo-10′-carotenal.
Cellular metabolites of β-apo-10′-carotenal were analyzed by an Agilent 1290 Infinity I UHPLC (Agilent Technologies) using an ACE super C18 column (2.1 × 150 mm, 2 μm; Advanced Chromatography Technologies, Scotland) with a mobile phase of 80% methanol/20% water (solvent A) and 78% MTBE/20% methanol/2% water (solvent B) where both mobile phases contained 2% (w/v) ammonium acetate. The flow rate was set at 0.5 ml/min and a column temperature of 45°C was maintained. Analytes were separated with 10% B for 1.5 min, followed by a linear gradient over 7 min to 65% B and subsequently to 100% B over 2 min before reequilibrating to the initial conditions for 2 min. The entire eluent flow was introduced to an Agilent 6550 QTOF mass spectrometer (Agilent Technologies) with positive ion electrospray source parameters of 400°C sheath gas at 12 l/min, 150°C drying gas at 18 l/min, 30 psi nebulizer, 3 kV Vcap, and 1.5 kV nozzle. Ion funnel settings were 90 V low pressure funnel, 200 V high pressure funnel. Also, there was a DAD inline to collect UV-vis spectra from 230 nm through 640 nm.
Statistical analyses
All data are presented as means and standard deviations. Means and standard deviations were calculated using Microsoft Excel 2013 (Microsoft Corporation, Redmond, WA). Graphs were produced using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA).
RESULTS
Stability of β-apo-8′-carotenal, β-apo-10′-carotenal, and β-apo-13-carotenone
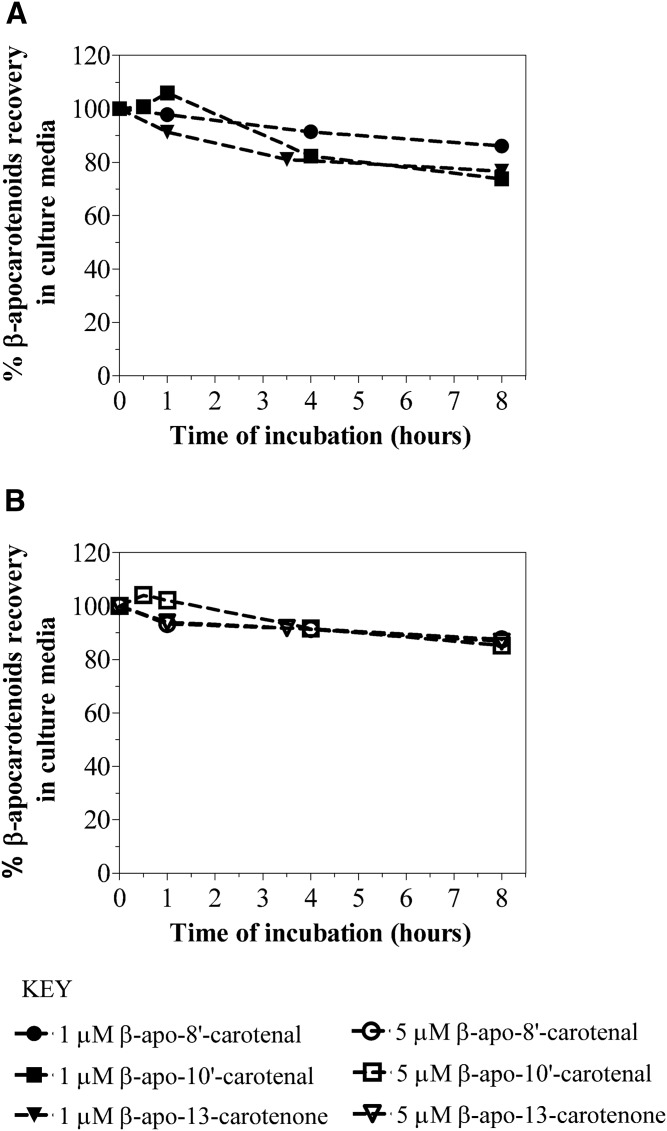
Given that β-apocarotenoids can undergo chemical oxidation and degradation during incubation in cell culture, we investigated the stability of these compounds in the same conditions as were used for the cell uptake experiments. Serum-free medium containing 1 μM or 5 μM of individual β-apocarotenoids (β-apo-8′-carotenal, β-apo-10′-carotenal, and β-apo-13-carotenone) was incubated in 6-well plates without cells for up to 8 h. The percentage recovery of β-apocarotenoids was calculated relative to the concentration of β-apocarotenoids in serum-free medium at the start of the experiment. Figure 2 summarizes the results for the stability of β-apocarotenoids in cell culture conditions. After 1 h of incubation, the recovery of 1 μM of β-apo-8′-carotenal in serum-free medium was 97.9%, whereas the recovery of 5 μM of β-apo-8′-carotenal was 93.3% at the same incubation time. There was a slight degradation of β-apo-8′-carotenal as incubation time increased, but our recovery of this β-apocarotenal was still good. Specifically, the recovery of 1 μM of β-apo-8′-carotenal in serum-free medium was 86.1% after 8 h of incubation. We obtained similar results for the stability of 5 μM of β-apo-8′-carotenal after 8 h of incubation.
Fig. 2.
Stability of β-apocarotenoids in cell culture conditions. Serum-free medium containing Tween 40 micelles of 1 μM (A) or 5 μM (B) of individual β-apocarotenoids (β-apo-8′-carotenal, β-apo-10′-carotenal, and β-apo-13-carotenone) were incubated at 37°C for time points up to 8 h. Following incubation, β-apocarotenoids were extracted from the medium and analyzed by HPLC-DAD. Percent recovery at each time point was calculated relative to the initial concentration of β-apocarotenoids in cell culture medium at the start of the stability experiment.
Similarly, the recovery of β-apo-10′-carotenal at early time points was very good. The recovery of 1 μM of β-apo-10′-carotenal in serum-free medium was 106.0% after 1 h of incubation, while the recovery of 5 μM of β-apo-10′-carotenal was 102.2% after 1 h of incubation. As with β-apo-8′-carotenal, there was also minimal degradation of β-apo-10′-carotenal as incubation time increased. The recovery of 1 μM of β-apo-10′-carotenal in serum-free medium was 73.7% after 8 h of incubation. On the other hand, we obtained a higher recovery (85.2%) after 8 h of incubation for 5 μM of β-apo-10′-carotenal. Furthermore, after 1 h of incubation, the recovery of 1 μM of β-apo-13-carotenone in serum-free medium was 91.3%, while the recovery of 5 μM of β-apo-13-carotenone was 93.9% at the same incubation time. There was some degradation of β-apo-13-carotenone in serum-free medium as incubation time increased. Specifically, the recovery of 1 μM of β-apo-13-carotenone in medium was 76.6% after 8 h of incubation. In contrast, the recovery of 5 μM of β-apo-13-carotenone was 86.8%. The results of these stability experiments are important because they established that these compounds are very stable under cell culture conditions and do not rapidly degrade before we incubate them with cells.
Kinetics of uptake of β-apo-8′-carotenal: effect of incubation time and initial β-apo-8′-carotenal concentration
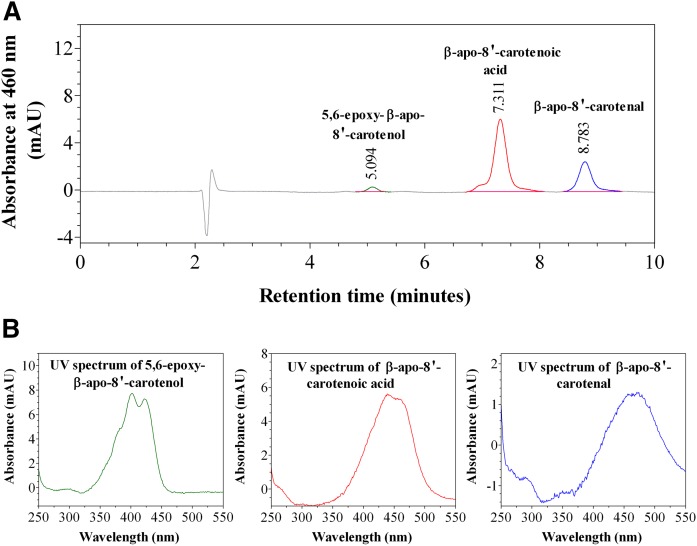
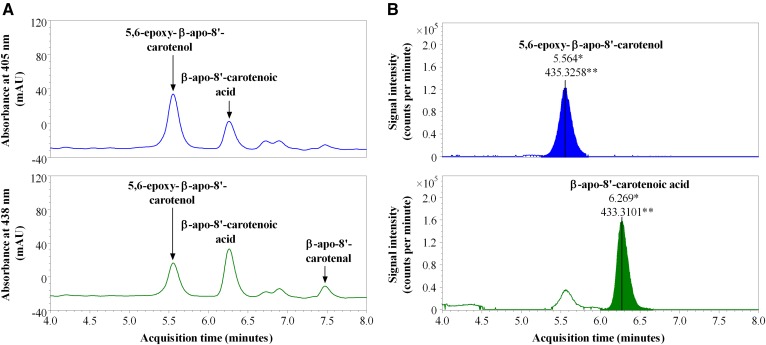
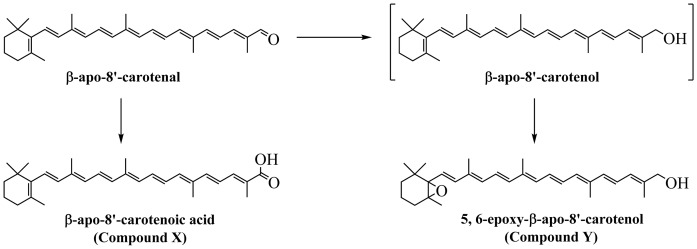
We investigated the kinetics of uptake of β-apo-8′-carotenal as a function of incubation time and concentration. Figures 3–6 summarize the results. Figure 3A shows a representative chromatogram of the uptake of β-apo-8′-carotenal into Caco-2 cells. The UV absorption spectra of the indicated peaks are also given (Fig. 3B). When cells were incubated with β-apo-8′-carotenal for 1 h, we detected two peaks (initially named compounds X and Y) in addition to β-apo-8′-carotenal. Compound X was the major peak detected, while compound Y and β-apo-8′-carotenal were present in small amounts. Our HPLC conditions enabled us to resolve both cellular metabolites from the parent apocarotenal. We also observed that compounds X and Y do not get released into the medium after they are formed within cells. We then determined the identities of the cellular metabolites detected. The components of cell extracts obtained after incubation with β-apo-8′-carotenal were separated by UHPLC (Fig. 4A), and the accurate masses of the cellular metabolites were determined using a high resolution mass spectrometer. The major peak, compound X, differed from β-apo-8′-carotenal by a mass of +16, while the minor peak, compound Y, differed from β-apo-8′-carotenal by a mass of +18 (Fig. 4B, Table 1).
Fig. 3.
HPLC-DAD analysis of cell extract after incubation with β-apo-8′-carotenal. A: Representative HPLC-DAD chromatogram of the uptake of 1 μM of β-apo-8′-carotenal into fully differentiated Caco-2 cells after incubation at 37°C for 1 h. B: UV-vis spectra of β-apo-8′-carotenal and its cellular metabolites, the major one of which was confirmed as β-apo-8′-carotenoic acid (compound X) and the other we identified as 5,6-epoxy-β-apo-8′-carotenol (compound Y).
Fig. 6.
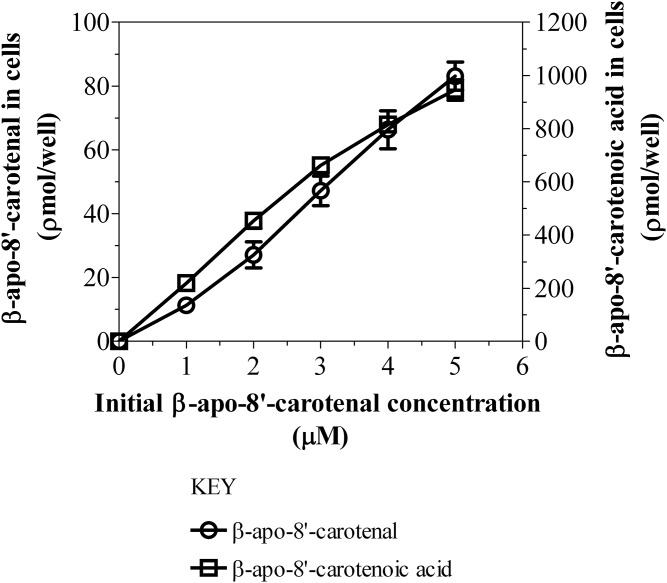
Dose-dependent uptake and metabolism of β-apo-8′-carotenal in Caco-2 cells. Differentiated Caco-2 cells were incubated with serum-free medium containing 1, 2, 3, 4, or 5 μM of β-apo-8′-carotenal at 37°C for 4 h. After incubation, the extracts from cells were analyzed using HPLC-DAD. The results shown represent an individual experiment and the points are the mean ± standard deviation of three or more wells. Furthermore, the data shown above have been confirmed with three independent experiments.
Fig. 4.
UHPLC-MS analysis of cellular metabolites of β-apo-8′-carotenal. A: UHPLC separation of molecules in Caco-2 cells incubated with 1 μM of β-apo-8′-carotenal for 1 h prior to accurate mass analysis. B: Determination of the accurate masses for the cellular metabolites of β-apo-8′-carotenal by QTOF MS. The major peak, compound X, differed from β-apo-8′-carotenal by a mass of +16, while the minor peak, compound Y, differed from β-apo-8′-carotenal by a mass of +18. Based on accurate mass, retention time, and UV absorption spectra, compound X was identified as β-apo-8′-carotenoic acid, while compound Y is 5,6-epoxy- β-apo-8′-carotenol. An * indicates acquisition time, while ** indicates accurate mass for each peak shown.
TABLE 1.
Mass for β-apo-8′-carotenal and the cellular metabolites of β-apo-8′-carotenal
Value indicates nominal mass.
Value indicates accurate mass as determined by UHPLC-MS.
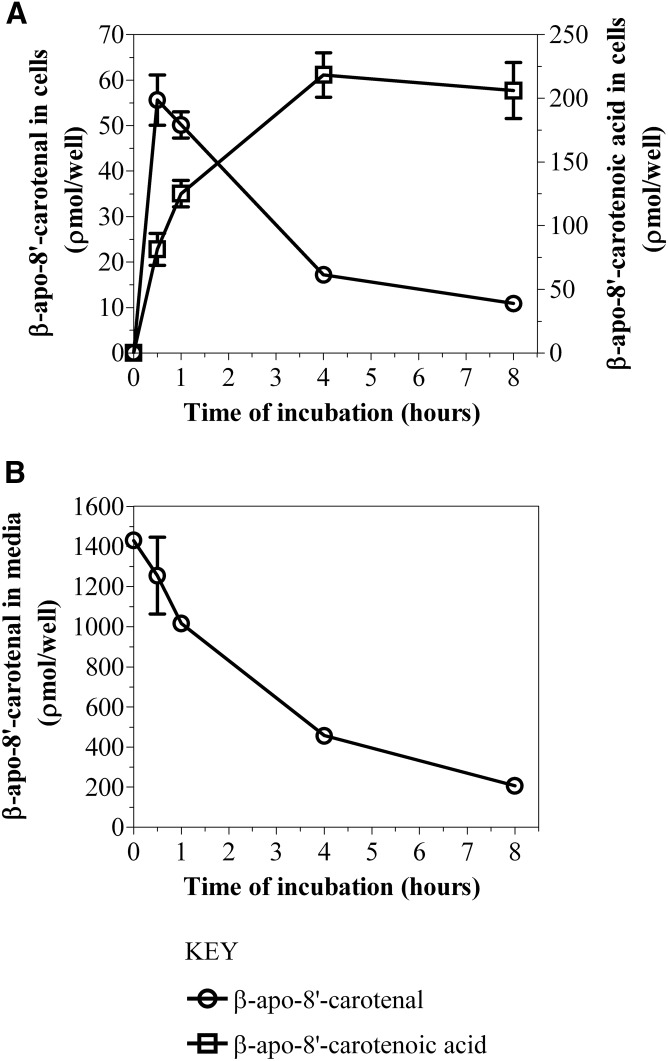
Based on this UHPLC-MS analysis, we proposed putative compounds that could be metabolites X and Y. The proposed compounds were synthesized, purified by TLC, and characterized by NMR spectroscopy. Subsequently, we analyzed the synthetic compounds by HPLC-DAD (see HPLC analysis, Method 1) and compared their retention times and UV absorption spectra to those of the cellular metabolites of β-apo-8′-carotenal. Based on accurate mass as well as identical retention time and UV absorption spectrum of an authentic standard, compound X was confirmed as β-apo-8′-carotenoic acid. In addition, we identified the minor peak (Y) as 5,6-epoxy-β-apo-8′-carotenol based on its accurate mass, retention time, and UV absorption spectrum showing fine structure consistent with an alcohol and a hypsochromic shift in absorption maximum consistent with the loss of a double bond (Fig. 3B, Table 1). Figure 5A shows the kinetics of uptake of β-apo-8′-carotenal for an individual experiment. Fully differentiated Caco-2 cells were incubated with 1 μM of β-apo-8′-carotenal for 30 min to 8 h. As mentioned previously, we observed the rapid accumulation of β-apo-8′-carotenal in cells and detected two metabolites, the major one of which was confirmed as β-apo-8′-carotenoic acid, and the other we identified as 5,6-epoxy-β-apo-8′-carotenol. We observed that the formation of β-apo-8′-carotenoic acid in cells increased as a function of incubation time and this corresponds with a decrease in the concentration of β-apo-8′-carotenal that accumulated within cells. In addition, we observed the disappearance of β-apo-8′-carotenal from medium as a function of incubation time (Fig. 5B).
Fig. 5.
A: Time course of uptake and metabolism of β-apo-8′-carotenal in Caco-2 cells. B: Effect of incubation time on the disappearance of β-apo-8′-carotenal from medium. Differentiated Caco-2 cells were incubated with serum-free medium containing 1 μM of β-apo-8′-carotenal at 37°C for 30 min, 1 h, 4 h, and 8 h. After incubation, the extracts from medium and cells were analyzed using HPLC-DAD. The results shown represent an individual experiment and the points are the mean ± standard deviation of three or more wells. Furthermore, the data shown in A have been confirmed with two independent experiments.
Taken together with the cell uptake data, we calculated the percent recovery of β-apo-8′-carotenal at each incubation time point. In preliminary experiments, we observed the rapid uptake of β-apo-8′-carotenal into cells, but we could only account for about 10% of this apocarotenal after 24 h of incubation (data not shown). Thus, we reduced our maximum incubation time to 8 h. β-Apo-8′-carotenal and β-apo-8′-carotenoic acid accounted for 97.3% of the initial β-apo-8′-carotenal concentration after 30 min of incubation, and both of those compounds accounted for 83.2% of the initial β-apo-8′-carotenal concentration after 1 h of incubation. These recovery values were expected because there is some degradation of β-apo-8′-carotenal with increasing incubation time. Also, the amount of 5,6-epoxy-β-apo-8′-carotenol that accumulated in Caco-2 cells was much lower than that of β-apo-8′-carotenoic acid based on peak height comparisons (Fig. 3A). We also studied the uptake of β-apo-8′-carotenal as a function of concentration. Figure 6 describes the dose-dependent accumulation of β-apo-8′-carotenal into Caco-2 cells. When differentiated monolayers were incubated with β-apo-8′-carotenal (1–5 μM) for 4 h, we observed that the amount of β-apo-8′-carotenal in cells increased with increasing initial concentration of β-apo-8′-carotenal. Also, we observed that the formation of β-apo-8′-carotenoic acid in cells increased linearly. Similarly to the time course experiments, the amount of 5,6-epoxy-β-apo-8′-carotenol formed within cells was much lower than that of β-apo-8′-carotenoic acid based on peak height comparisons for the different levels of concentration we studied (data not shown).
Kinetics of uptake of β-apo-10′-carotenal: effects of incubation time
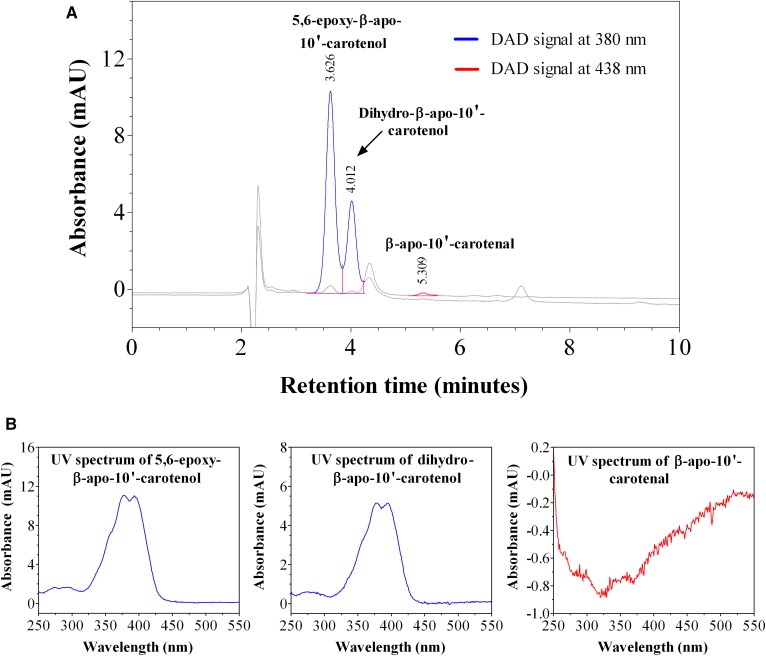
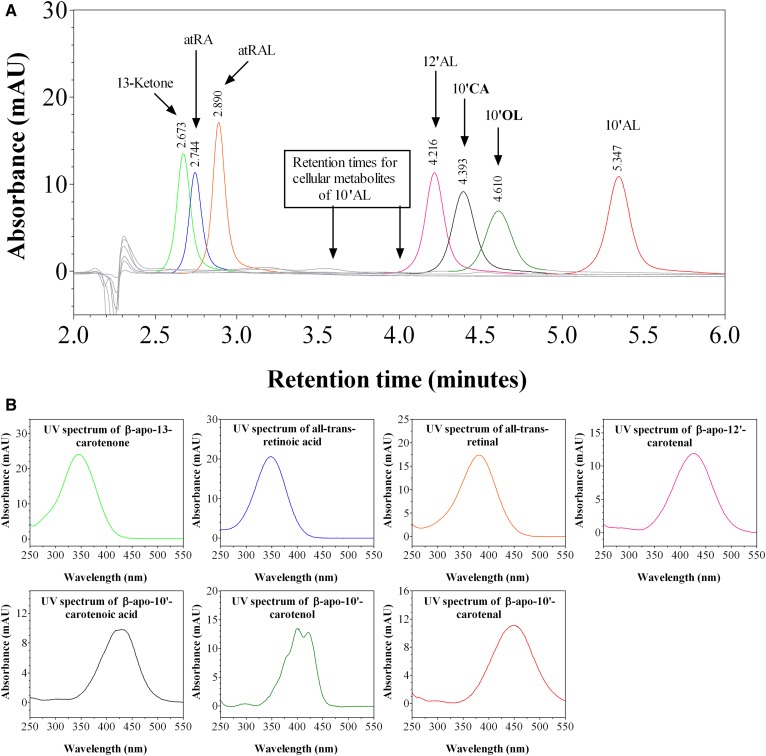
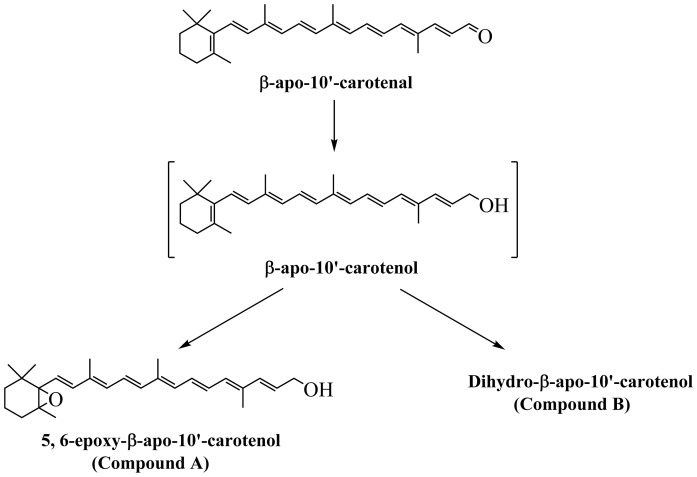
We investigated the kinetics of uptake of β-apo-10′-carotenal into Caco-2 cells as a function of incubation time. Figure 7A is a representative chromatogram of the uptake and metabolism of β-apo-10′-carotenal in Caco-2 cells. The UV absorption spectra of the indicated peaks are also shown (Fig. 7B). When cells were incubated with β-apo-10′-carotenal for 4 h, two peaks (initially named compounds A and B) were detected along with β-apo-10′-carotenal. Compound A was the major peak detected, whereas compound B and β-apo-10′-carotenal were present in minor amounts. We also observed that compounds A and B do not get released into the medium after they are formed within cells. We next established the identities of cellular metabolites A and B. Compound A elutes at 3.63 min and its UV absorption spectrum has a fine structure with absorption maxima at 378 nm and 394 nm. On the other hand, compound B elutes at 4.01 min and its UV absorption spectrum has fine structure with absorption maxima at 379 nm and 395 nm. This indicates that both compounds are more hydrophilic than β-apo-10′-carotenal. We initially thought that the cellular metabolites of β-apo-10′-carotenal would be similar to those observed with β-apo-8′-carotenal. Hence, we analyzed several short-chain β-apocarotenoids in the same HPLC-DAD conditions used to separate β-apo-10′-carotenal and its metabolites. The standard compounds examined were β-apo-13-carotenone, retinal, RA, β-apo-12′-carotenal, β-apo-10′-carotenoic acid, and β-apo-10′-carotenol. However, none of these compounds eluted at the same retention time as compound A or B (Fig. 8).
Fig. 7.
HPLC-DAD analysis of cell extract after incubation with β-apo-10′-carotenal. A: Representative HPLC-DAD chromatogram of the uptake of 1 μM of β-apo-10′-carotenal into fully differentiated Caco-2 cells after incubation at 37°C for 4 h. B: UV-vis spectra of β-apo-10′-carotenal and its cellular metabolites, the major one of which was identified as 5,6-epoxy-β-apo-10′-carotenol (compound A) and the other is likely a dihydro-β-apo-10′-carotenol (compound B).
Fig. 8.
HPLC-DAD analysis of possible cellular metabolites of β-apo-10ʹ-carotenal. (A): Overlap of HPLC-DAD chromatograms for reference compounds of known retinoids and β-apocarotenoids. (B): UV-vis spectra for the retinoids and β-apocarotenoids analyzed by HPLC-DAD. 13-ketone, β-apo-13-carotenone; atRA, all-trans-RA; atRAL, all-trans-retinal; 12′AL, β-apo-12′-carotenal; 10′CA, β-apo-10′-carotenoic acid; 10′OL, β-apo-10′-carotenol; 10′AL, β-apo-10′-carotenal.
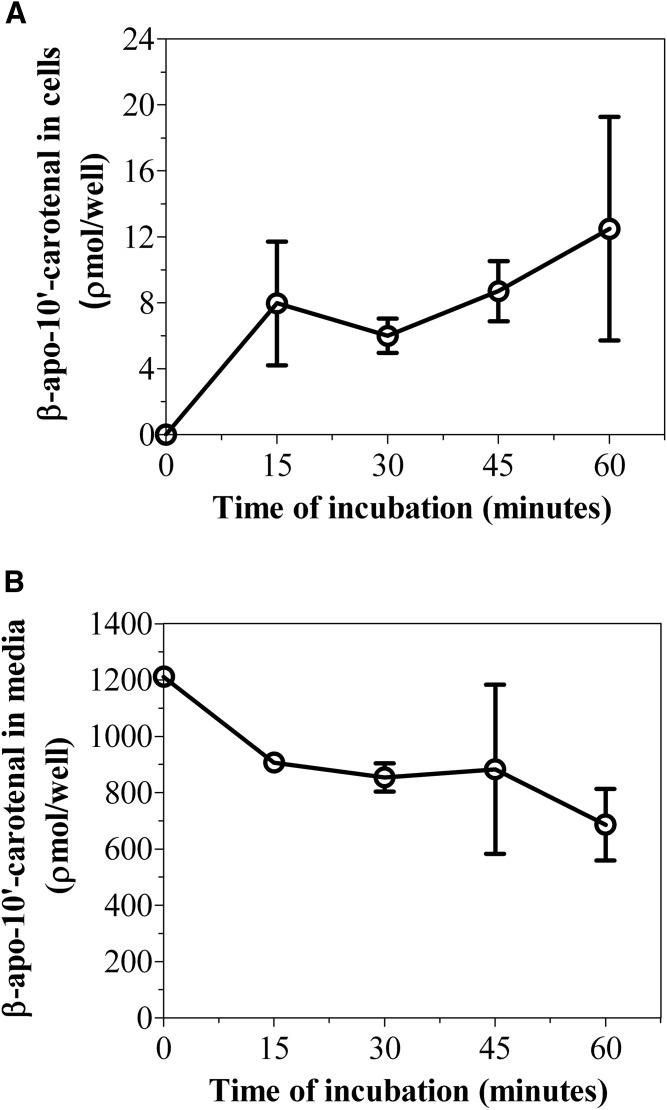
We then determined the accurate masses of both metabolites using a high resolution mass spectrometer. The major peak, compound A, differed from β-apo-10′-carotenal by a mass of +18, whereas the minor peak, compound B, differed from β-apo-10′-carotenal by a mass of +4 (Table 2). Based on accurate mass, retention time, and UV absorption spectra showing fine structure consistent with an alcohol and a hypsochromic shift in absorption maximum consistent with the loss of a double bond, compound A was identified as 5,6-epoxy-β-apo-10ʹ-carotenol, while compound B is likely a dihydro-β-apo-10ʹ-carotenol (Fig. 7B, Table 2). Figure 9A shows the kinetics of uptake of β-apo-10′-carotenal for an individual experiment. We observed the rapid uptake of β-apo-10′-carotenal into cells up to 1 h. There was a subsequent decrease in cellular accumulation of β-apo-10′-carotenal with increasing incubation time (data not shown). Also, we observed the disappearance of β-apo-10′-carotenal from medium as a function of time (Fig. 9B). We then determined the recovery of β-apo-10′-carotenal in cells and medium after incubation. The percent recovery of β-apo-10′-carotenal after incubation was about 75.5% at 15 min, 71.0% at 30 min, 73.6% at 45 min, and 57.6% at 1 h.
TABLE 2.
Mass for β-apo-10′-carotenal and the cellular metabolites of β-apo-10′-carotenal
| Compound | Mass (g/mol) |
| β-Apo-10′-carotenal | 376.5840a |
| Compound A | 394.2866b |
| 395.2935b | |
| Compound B | 380.3072b |
Value indicates nominal mass.
Value indicates accurate mass as determined by UHPLC-MS.
Fig. 9.
A: Time course of uptake and metabolism of β-apo-10′-carotenal in Caco-2 cells. B: Effect of incubation time on the disappearance of β-apo-10′-carotenal from medium. Differentiated Caco-2 cells were incubated with serum-free medium containing 1 μM of β-apo-10′-carotenal at 37°C for 15, 30, 45, and 60 min. After incubation, the extracts from medium and cells were analyzed by HPLC-DAD. The results shown represent an individual experiment and the points are the mean ± standard deviation of three or more wells. Furthermore, the data shown in A have been confirmed with two independent experiments.
Effects of incubation time and initial β-apo-13-carotenone concentration on the uptake and metabolism of β-apo-13-carotenone
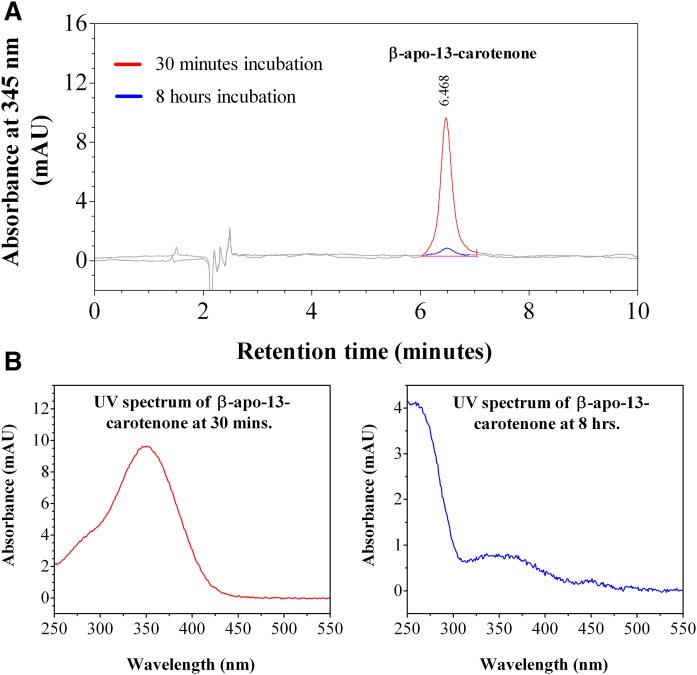
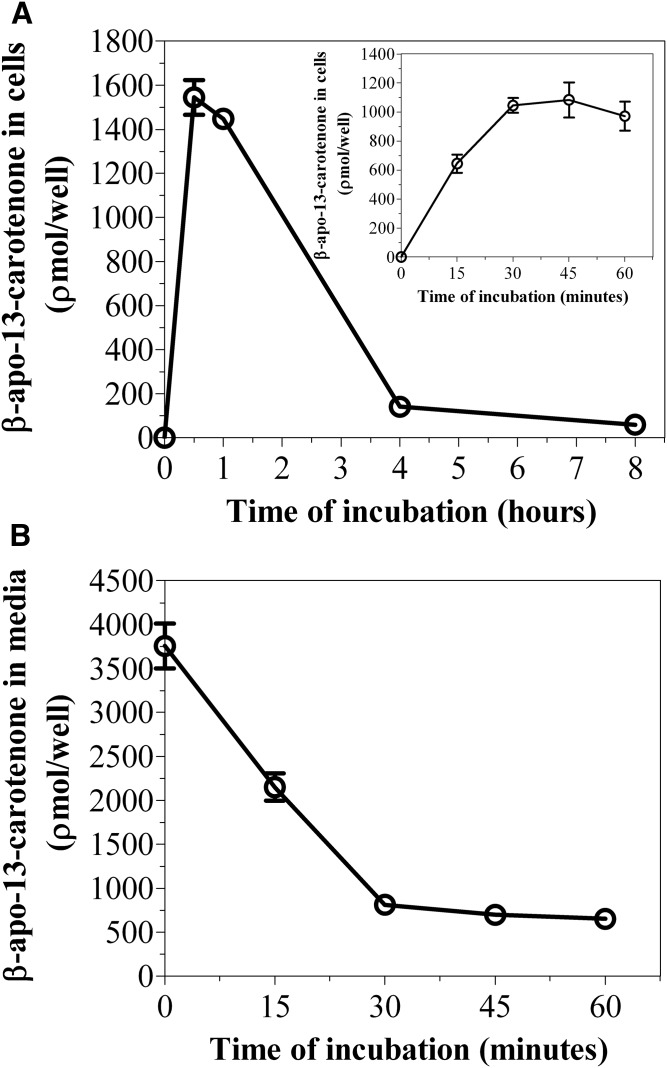
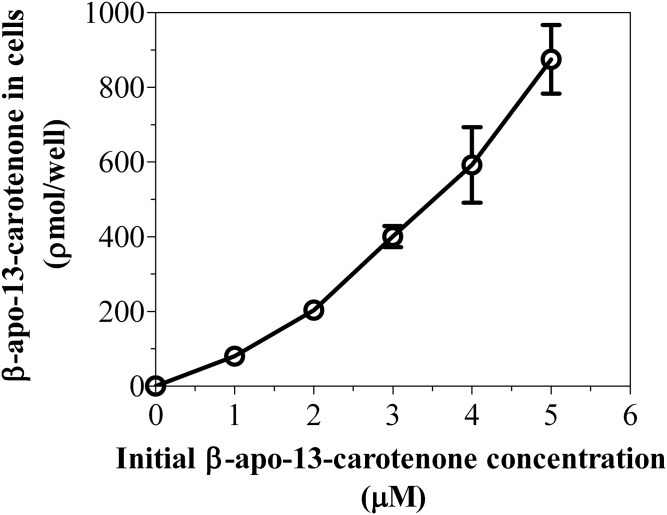
We investigated the kinetics of uptake of β-apo-13-carotenone into Caco-2 cells as a function of incubation time and concentration. Figure 10A is a representative chromatogram of the uptake of β-apo-13-carotenone into Caco-2 cells. The UV absorption spectra of the indicated peaks are also given (Fig. 10B). In contrast to the β-apocarotenals, we did not detect any UV-absorbing metabolites when cells were incubated with 5 μM of β-apo-13-carotenone for 30 min or 8 h. Figure 11A summarizes the kinetics of uptake of β-apo-13-carotenone as a function of incubation time. We observed the rapid uptake of β-apo-13-carotenone into cells but subsequent extensive degradation with increasing incubation time. Also, we observed the disappearance of β-apo-13-carotenone from the medium as a function of time (Fig. 11B). In addition, we determined the recovery of β-apo-13-carotenone in cells after incubation. Initial experiments had shown that β-apo-13-carotenone recovery in cells after 4 h of incubation was less than 5% (data not shown). Thus, we reduced our maximum incubation time to 1 h. The percent recovery of β-apo-13-carotenone after incubation was about 62.1% at 15 min, 59.2% at 30 min, 53.1% at 45 min, and 49.6% at 1 h. These recovery values were expected because there is some degradation of β-apo-13-carotenone with increasing incubation time. Figure 12 summarizes the dose dependent accumulation of β-apo-13-carotenone into Caco-2 cells. Fully differentiated monolayers of Caco-2 cells were incubated for 45 min with β-apo-13-carotenone (1–5 μM). We observed that the cellular accumulation of β-apo-13-carotenone increased with increasing initial concentration of β-apo-13-carotenone.
Fig. 10.
HPLC-DAD analysis of cell extract after incubation with β-apo-13-carotenone. A: Representative HPLC-DAD chromatogram of the uptake of 5 μM of β-apo-13-carotenone into fully differentiated Caco-2 cells after incubation at 37°C for either 30 min or 8 h. B: UV-vis spectra of β-apo-13-carotenone at both incubation time points. No cellular metabolites were detected.
Fig. 11.
A: Time course of uptake of β-apo-13-carotenone into Caco-2 cells. B: Effect of incubation time on the disappearance of β-apo-13-carotenone from medium. Differentiated Caco-2 cells were incubated with serum-free medium containing 5 μM of β-apo-13-carotenone at 37°C for time points up to 8 h. After incubation, the extracts from medium and cells were analyzed by HPLC-DAD. The inset graph in A shows the uptake of β-apo-13-carotenone into cells at time points up to 1 h. No peaks were detected in medium after 4 or 8 h of incubation. The results shown represent an individual experiment and the points are the mean ± standard deviation of three or more wells. Furthermore, the data shown in A have been confirmed with two independent experiments.
Fig. 12.
Dose-dependent uptake of β-apo-13-carotenone into Caco-2 cells. Differentiated Caco-2 cells were incubated with serum-free medium containing 1, 2, 3, 4, or 5 μM of β-apo-13-carotenone at 37°C for 45 min. After incubation, the extracts from cells were analyzed using HPLC-DAD. The results shown represent an individual experiment and the points are the mean ± standard deviation of three or more wells. Furthermore, the data shown above have been confirmed with two independent experiments.
DISCUSSION
β-Apocarotenoids are eccentric cleavage products of carotenoids formed by chemical and enzymatic oxidations. These cleavage products demonstrate potent bioactivity in different experimental models. β-Apocarotenoids inhibit the cell proliferation of breast tumor cells via a RAR-independent mechanism (26) and human leukemia cell line, HL-60 (27). These compounds have also been shown to upregulate the gene expression of adipocyte markers during the differentiation of 3T3-L1 cells (28). In addition, β-apocarotenoids can bind to retinoid- and/or lipid-responsive transcription factors and may modulate these proteins’ abilities to stimulate transcription of target genes (7, 11, 12). The presence of β-apocarotenoids in carotenoid-rich fruits, vegetables, and other plant food items has been reported by our laboratory (14) and others (29–31). Furthermore, numerous lines of evidence demonstrate that these eccentric cleavage products are detected in the serum or tissues of WT (32–34) and BCO knockout animals (34, 35) as well as the plasma of humans consuming diets containing carotenoids or given a bolus of β-carotene (7, 36). Collectively, these data suggest that β-apocarotenoids might be directly absorbed from the diet. Here, we have examined the uptake of β-apo-8′-carotenal, β-apo-10′-carotenal, and β-apo-13-carotenone into Caco-2 cells. Also, we have demonstrated the metabolism of these eccentric cleavage products after cellular uptake.
When cells were incubated with β-apo-8′-carotenal for 1 h, we detected two peaks (initially named compounds X and Y) in addition to β-apo-8′-carotenal. Compound X was the major peak detected, while compound Y and β-apo-8′-carotenal were present in minor amounts (Fig. 3A). Based on accurate mass as well as identical retention time and UV absorption spectrum of an authentic standard, compound X was confirmed as β-apo-8ʹ-carotenoic acid. Compound Y was identified as 5,6-epoxy-β-apo-8ʹ-carotenol based on its accurate mass, retention time, and UV absorption spectrum showing fine structure consistent with an alcohol and a hypsochromic shift in absorption maximum consistent with the loss of a double bond (Fig. 3B, Table 1). The results presented here are in agreement with previous studies that examined the absorption of β-apo-8′-carotenal in different species. Early work detected β-apo-8ʹ-carotenoic acid as a urinary metabolite in dogs given a daily dose of β-apo-8ʹ-carotenal (37). Subsequent work by multiple research groups also corroborates that this apocarotenoic acid is a major metabolite present in the serum and tissues of animals fed β-apo-8ʹ-carotenal (38, 39). In one of those studies, the intact aldehyde and β-apo-8ʹ-carotenol were also present in minor amounts (38). This is similar to what is observed in the present study, the difference being that we detected 5,6-epoxy-β-apo-8ʹ-carotenol.
Although there is limited evidence, it is not too far-fetched to suggest that β-apo-8ʹ-carotenal is similarly converted to β-apo-8ʹ-carotenoic acid in humans. Olson and colleagues examined the uptake and metabolism of a single dose of β-apo-8ʹ-carotenal in healthy male volunteers with adequate vitamin A status (40). β-Apo-8ʹ-carotenal was rapidly absorbed and was extensively metabolized in all subjects. Serum metabolites observed were β-apo-8ʹ-carotenoic acid, β-apo-8ʹ-carotenol, β-apo-8ʹ-carotenyl palmitate, β-apo-10ʹ-carotenol, and β-apo-12ʹ-carotenal. Unsurprisingly, the unchanged aldehyde was only detected in minor amounts. Taken together, these data suggest that the major proportion of β-apo-8ʹ-carotenal is metabolized to the corresponding β-apocarotenoic acid (38). This metabolic conversion is similarly observed with retinal, which can be converted to retinol or RA. Previous studies also demonstrate the formation of epoxy metabolites of vitamin A in vivo (41, 42). Thus, we propose that β-apo-8ʹ-carotenal taken up from carotenoid-rich foods is metabolized by intestinal cells according to the pathway shown in Fig. 13.
Fig. 13.
Structures of the metabolites in the metabolic pathway of β-apo-8′-carotenal in Caco-2 cells. There was rapid uptake of β-apo-8′-carotenal into cells, and β-apo-8′-carotenal was largely converted to β-apo-8′-carotenoic acid. Several studies show that this apocarotenoic acid is a major metabolite present in the serum and tissues of animals fed β-apo-8ʹ-carotenal (38, 39). This metabolic conversion is similar to what is observed with retinal, which can be converted to retinol or RA. β-Apo-8′-carotenal was also converted to a minor metabolite that was identified as 5,6-epoxy-β-apo-8′-carotenol, which is analogous to the formation of epoxy metabolites of vitamin A in vivo (41, 42).
We next investigated the uptake and metabolism of β-apo-10′-carotenal in cells. In vitro enzymatic assays show that β-apo-10′-carotenal is the major product of eccentric cleavage of β-carotene by BCO2 (43, 44). The unchanged aldehyde has been detected in the plasma and tissues of mice on a β-carotene-containing diet (45). The corresponding alcohol and acid are also generated in vivo (35). WT and BCO1−/− but not BCO2−/− and BCO1−/−/BCO2−/− mice accumulate β-apo-10′-carotenol, mainly in its esterified form, and β-apo-10′-carotenoic acid in their livers when given a diet with β-carotene as the only vitamin A source (35, 44). There is renewed interest in β-apo-10′-carotenoids because of emerging reports about the role these compounds play in embryogenesis. Costabile et al. (20) showed that β-apo-10′-carotenal and β-apo-10′-carotenol are biologically active metabolites that regulate placental lipoprotein biosynthesis and allow for effective transport of β-carotene from the maternal circulation to the embryo independent of changes to the mRNA expression of RA target genes. Instead, these β-apocarotenoids activate hepatic nuclear factor 4α, which in turn upregulates the gene expression and activity of the placental microsomal triglyceride transfer protein (20), which is important for the assembly and secretion of apoB-containing lipoproteins (46, 47). Recently, Quadro and colleagues showed that β-apo-10′-carotenal can support normal embryonic development during periods of vitamin A deficiency (48).
Caco-2 cells incubated with β-apo-10′-carotenal for 4 h produced two peaks detected by HPLC-DAD (Fig. 7A). The two metabolites, appearing at retention time 3.63 min (compound A) and 4.01 min (compound B) both have UV absorption spectra with fine structure. Compound A has absorption maxima at 378 nm and 394 nm, whereas compound B has absorption maxima at 379 nm and 395 nm. We initially thought that the cellular metabolites of β-apo-10′-carotenal would be similar to those observed with β-apo-8′-carotenal. Thus, we analyzed β-apo-13-carotenone, retinal, RA, β-apo-12′-carotenal, β-apo-10′-carotenoic acid, and β-apo-10′-carotenol by HPLC-DAD. However, none of the compounds tested had the retention time or absorption maxima of the metabolites (Fig. 8). We then determined the accurate masses of both metabolites using a high resolution mass spectrometer. Based on accurate mass, retention time, and UV absorption spectrum showing fine structure consistent with an alcohol and a hypsochromic shift in absorption maximum consistent with the loss of a double bond, compound A was identified as 5,6-epoxy-β-apo-10ʹ-carotenol, whereas compound B is likely a dihydro-β-apo-10ʹ-carotenol (Fig. 7B, Table 2).
Several studies suggest that β-oxidation, involving the stepwise cleavage of two carbons, is a possible pathway for the metabolism of β-apocarotenoids to shorter chain β-apocarotenoids, retinal, and RA. Wang et al. (49) showed that β-apo-10′-carotenoic acid, β-apo-12′-carotenoic acid, and β-apo-14′-carotenoic acid are produced following in vitro incubation of β-apo-8′-carotenoic acid with mitochondrial fractions isolated from rabbit livers. Similarly, al-Hasani et al. (39) reported the formation of β-apo-10′-carotenoic acid and β-apo-12′-carotenoic acid in the serum and tissues of vitamin A-depleted quails maintained on a diet with β-apo-10′-carotenal as the only source of provitamin A activity. We have now proposed two additional metabolites (5,6-epoxy-β-apo-10ʹ-carotenol and a dihydro-β-apo-10ʹ-carotenol) that may be formed when β-apo-10ʹ-carotenal taken up from carotenoid-rich foods is metabolized by intestinal cells. The chemical structures for the newly proposed metabolites are shown in Fig. 14.
Fig. 14.
Structures of the metabolites in the metabolic pathway of β-apo-10′-carotenal in Caco-2 cells. There was rapid uptake of β-apo-10′-carotenal into cells, and β-apo-10′-carotenal was converted to a major metabolite that we identified as 5,6-epoxy-β-apo-10′-carotenol and a minor metabolite that is likely a dihydro-β-apo-10′-carotenol. Previous studies have suggested that β-apo-10′-carotenol, β-apo-10′-carotenoic acid, and β-apo-12′-carotenoic acid are detected in serum and tissues of animals given β-apo-10′-carotenal as the only source of provitamin A activity (39). However, in the present study, we did not detect any of these metabolites.
We also examined the uptake and metabolism of β-apo-13-carotenone. Caco-2 cells incubated with β-apo-13-carotenone for 30 min produced an individual peak with retention time and absorption maximum equivalent to authentic β-apo-13-carotenone standard. On the other hand, when cells were incubated with β-apo-13-carotenone for 4 or 8 h, no peaks were detected (Fig. 10). One possible explanation is that the β-apo-13-carotenone might be metabolized to short chain metabolites with limited UV absorption. We also cannot rule out the possibility that the compound was converted into metabolites with UV absorption, but that once the metabolites were generated, they were rapidly degraded.
In summary, our results are consistent with a recent clinical trial showing a lack of direct absorption of β-apocarotenals in healthy volunteers consuming a daily dose of high β-carotene tomato juice, containing precisely quantitated amounts of β-apocarotenals, for 4 weeks (31). The findings from that study also support our conclusions that β-apocarotenals are rapidly metabolized in the intestine. In contrast to the β-apocarotenals, β-apo-13-carotenone was quantitatively detected in all subjects, including the control group consuming a carotenoid-free juice. Thus, the authors of that study concluded that β-apo-13-carotenone could possibly be formed from the oxidative cleavage of preformed vitamin A. Further research is required to understand the mechanisms of absorption and metabolism of β-apocarotenoids in animal models and humans.
Acknowledgments
The authors thank Dr. Mark L. Failla for his expert technical assistance and contributions to the successful completion of this research project. The authors also thank Dr. Chureeporn Chitchumroonchokchai for laboratory training in aseptic cell culture techniques and assistance with carotenoid extraction protocols.
Footnotes
Abbreviations:
- BCO1
- β-carotene 15, 15′-oxygenase
- BCO2
- β-carotene 9′, 10′-oxygenase
- DAD
- diode array detector
- MTBE
- methyl tert-butyl ether
- QTOF
- quadrupole time-of-flight
- RA
- retinoic acid
- RAR
- retinoic acid receptor
- RXRα
- retinoid X receptor alpha
This work was supported by funds from National Heart, Lung, and Blood Institute Grant R01-HL049879 and the Virginia M. Vivian Graduate Student Research Award (College of Education and Human Ecology, Ohio State University). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Ross C. A., and Harrison E. H.. 2007. Vitamin A: nutritional aspects of retinoids and carotenoids. In Handbook of Vitamins. J. Zempleni, R. B. Rucker, D. B. McCormick, et al., editors. CRC Press, Boca Raton, FL. 2–30. [Google Scholar]
- 2.Harrison E. H. 2012. Mechanisms involved in the intestinal absorption of dietary vitamin A and provitamin A carotenoids. Biochim. Biophys. Acta. 1821: 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.During A., and Harrison E. H.. 2004. Intestinal absorption and metabolism of carotenoids: insights from cell culture. Arch. Biochem. Biophys. 430: 77–88. [DOI] [PubMed] [Google Scholar]
- 4.Weber D., and Grune T.. 2012. The contribution of beta-carotene to vitamin A supply of humans. Mol. Nutr. Food Res. 56: 251–258. [DOI] [PubMed] [Google Scholar]
- 5.Sharoni Y., Linnewiel-Hermoni K., Khanin M., Salman H., Veprik A., Danilenko M., and Levy J.. 2012. Carotenoids and apocarotenoids in cellular signaling related to cancer: a review. Mol. Nutr. Food Res. 56: 259–269. [DOI] [PubMed] [Google Scholar]
- 6.Raghuvanshi S., Reed V., Blaner W. S., and Harrison E. H.. 2015. Cellular localization of beta-carotene 15,15′ oxygenase-1 (BCO1) and beta-carotene 9′,10′ oxygenase-2 (BCO2) in rat liver and intestine. Arch. Biochem. Biophys. 572: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eroglu A., Hruszkewycz D. P., dela Seña C., Narayanasamy S., Riedl K. M., Kopec R. E., Schwartz S. J., Curley R. W. Jr., and Harrison E. H.. 2012. Naturally occurring eccentric cleavage products of provitamin A beta-carotene function as antagonists of retinoic acid receptors. J. Biol. Chem. 287: 15886–15895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amengual J., Lobo G. P., Golczak M., Li H. N., Klimova T., Hoppel C. L., Wyss A., Palczewski K., and von Lintig J.. 2011. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J. 25: 948–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.dela Seña C., Narayanasamy S., Riedl K. M., Curley R. W. Jr., Schwartz S. J., and Harrison E. H.. 2013. Substrate specificity of purified recombinant human beta-carotene 15,15′-oxygenase (BCO1). J. Biol. Chem. 288: 37094–37103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrison E. H., dela Seña C., Eroglu A., and Fleshman M. K.. 2012. The formation, occurrence, and function of beta-apocarotenoids: beta-carotene metabolites that may modulate nuclear receptor signaling. Am. J. Clin. Nutr. 96: 1189S–1192S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziouzenkova O., Orasanu G., Sukhova G., Lau E., Berger J. P., Tang G., Krinsky N. I., Dolnikowski G. G., and Plutzky J.. 2007. Asymmetric cleavage of beta-carotene yields a transcriptional repressor of retinoid X receptor and peroxisome proliferator-activated receptor responses. Mol. Endocrinol. 21: 77–88. [DOI] [PubMed] [Google Scholar]
- 12.Eroglu A., Hruszkewycz D. P., Curley R. W. Jr., and Harrison E. H.. 2010. The eccentric cleavage product of beta-carotene, beta-apo-13-carotenone, functions as an antagonist of RXRalpha. Arch. Biochem. Biophys. 504: 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eroglu A., and Harrison E. H.. 2013. Carotenoid metabolism in mammals, including man: formation, occurrence, and function of apocarotenoids. J. Lipid Res. 54: 1719–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleshman M. K., Lester G. E., Riedl K. M., Kopec R. E., Narayanasamy S., Curley R. W. Jr., Schwartz S. J., and Harrison E. H.. 2011. Carotene and novel apocarotenoid concentrations in orange-fleshed Cucumis melo melons: determinations of beta-carotene bioaccessibility and bioavailability. J. Agric. Food Chem. 59: 4448–4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.During A., Hussain M. M., Morel D. W., and Harrison E. H.. 2002. Carotenoid uptake and secretion by CaCo-2 cells: beta-carotene isomer selectivity and carotenoid interactions. J. Lipid Res. 43: 1086–1095. [DOI] [PubMed] [Google Scholar]
- 16.During A., and Harrison E. H.. 2007. Mechanisms of provitamin A (carotenoid) and vitamin A (retinol) transport into and out of intestinal Caco-2 cells. J. Lipid Res. 48: 2283–2294. [DOI] [PubMed] [Google Scholar]
- 17.Quick T. C., and Ong D. E.. 1990. Vitamin A metabolism in the human intestinal Caco-2 cell line. Biochemistry. 29: 11116–11123. [DOI] [PubMed] [Google Scholar]
- 18.Delie F., and Rubas W.. 1997. A human colonic cell line sharing similarities with enterocytes as a model to examine oral absorption: advantages and limitations of the Caco-2 model. Crit. Rev. Ther. Drug Carrier Syst. 14: 221–286. [PubMed] [Google Scholar]
- 19.Garrett D. A., Failla M. L., Sarama R. J., and Craft N.. 1999. Accumulation and retention of micellar beta-carotene and lutein by Caco-2 human intestinal cells. J. Nutr. Biochem. 10: 573–581. [DOI] [PubMed] [Google Scholar]
- 20.Costabile B. K., Kim Y. K., Iqbal J., Zuccaro M. V., Wassef L., Narayanasamy S., Curley R. W. Jr., Harrison E. H., Hussain M. M., and Quadro L.. 2016. β-Apo-10′-carotenoids modulate placental microsomal triglyceride transfer protein expression and function to optimize transport of intact β-carotene to the embryo. J. Biol. Chem. 291: 18525–18535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Failla M. L., Chitchumroonchokchai C., Siritunga D., De Moura F. F., Fregene M., Manary M. J., and Sayre R. T.. 2012. Retention during processing and bioaccessibility of β-carotene in high β-carotene transgenic cassava root. J. Agric. Food Chem. 60: 3861–3866. [DOI] [PubMed] [Google Scholar]
- 22.Lornejad-Schäfer M. R., Lambert C., Breithaupt D. E., Biesalski H. K., and Frank J.. 2007. Solubility, uptake and biocompatibility of lutein and zeaxanthin delivered to cultured human retinal pigment epithelial cells in tween40 micelles. Eur. J. Nutr. 46: 79–86. [DOI] [PubMed] [Google Scholar]
- 23.O’Sullivan S. M., Woods J. A., and O’Brien N. M.. 2004. Use of Tween 40 and Tween 80 to deliver a mixture of phytochemicals to human colonic adenocarcinoma cell (CaCo-2) monolayers. Br. J. Nutr. 91: 757–764. [DOI] [PubMed] [Google Scholar]
- 24.Berni P., Chitchumroonchokchai C., Canniatti-Brazaca S. G., De Moura F. F., and Failla M. L.. 2014. Impact of genotype and cooking style on the content, retention, and bioacessibility of β-carotene in biofortified cassava (Manihot esculenta Crantz) conventionally bred in Brazil. J. Agric. Food Chem. 62: 6677–6686. [DOI] [PubMed] [Google Scholar]
- 25.Kopec R. E., Cooperstone J. L., Schweiggert R. M., Young G. S., Harrison E. H., Francis D. M., Clinton S. K., and Schwartz S. J.. 2014. Avocado consumption enhances human postprandial provitamin A absorption and conversion from a novel high-β-carotene tomato sauce and from carrots. J. Nutr. 144: 1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tibaduiza E. C., Fleet J. C., Russell R. M., and Krinsky N. I.. 2002. Excentric cleavage products of beta-carotene inhibit estrogen receptor positive and negative breast tumor cell growth in vitro and inhibit activator protein-1-mediated transcriptional activation. J. Nutr. 132: 1368–1375. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki T., Matsui M., and Murayama A.. 1995. Biological activity of (all-E)-beta-apo-12′-carotenoic acid and the geometrical isomers on human acute promyelocytic leukemia cell line HL-60. J. Nutr. Sci. Vitaminol. (Tokyo). 41: 575–585. [DOI] [PubMed] [Google Scholar]
- 28.Wang C. X., Jiang H., Yuen J. J., Lee S. A., Narayanasamy S., Curley R. W. Jr., Harrison E. H., and Blaner W. S.. 2015. Actions of β-apo-carotenoids in differentiating cells: differential effects in P19 cells and 3T3–L1 adipocytes. Arch. Biochem. Biophys. 572: 2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mínguez-Mosquera M. I., Hornero-Méndez D., and Garrido-Fernández J.. 1995. Detection of bixin, lycopene, canthaxanthin, and beta-apo-8′-carotenal in products derived from red pepper. J. AOAC Int. 78: 491–496. [PubMed] [Google Scholar]
- 30.Schaub P., Wust F., Koschmieder J., Yu Q., Virk P., Tohme J., and Beyer P.. 2017. Nonenzymatic β-carotene degradation in provitamin A-biofortified crop plants. J. Agric. Food Chem. 65: 6588–6598. [DOI] [PubMed] [Google Scholar]
- 31.Cooperstone J. L., Novotny J. A., Riedl K. M., Cichon M. J., Francis D. M., Curley R. W. Jr., Schwartz S. J., and Harrison E. H.. 2018. Limited appearance of apocarotenoids is observed in plasma after consumption of tomato juices: a randomized human clinical trial. Am. J. Clin. Nutr. 108: 784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma R. V., Mathur S. N., Dmitrovskii A. A., Das R. C., and Ganguly J.. 1976. Studies on the metabolism of beta-carotene and apo-beta-carotenoids in rats and chickens. Biochim. Biophys. Acta. 486: 183–194. [DOI] [PubMed] [Google Scholar]
- 33.Wang X. D., Liu C., Bronson R. T., Smith D. E., Krinsky N. I., and Russell M.. 1999. Retinoid signaling and activator protein-1 expression in ferrets given beta-carotene supplements and exposed to tobacco smoke. J. Natl. Cancer Inst. 91: 60–66. [DOI] [PubMed] [Google Scholar]
- 34.Shmarakov I., Fleshman M. K., D’Ambrosio D. N., Piantedosi R., Riedl K. M., Schwartz S. J., Curley R. W. Jr., von Lintig J., Rubin L. P., Harrison E. H., et al. 2010. Hepatic stellate cells are an important cellular site for beta-carotene conversion to retinoid. Arch. Biochem. Biophys. 504: 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amengual J., Widjaja-Adhi M. A., Rodriguez-Santiago S., Hessel S., Golczak M., Palczewski K., and von Lintig J.. 2013. Two carotenoid oxygenases contribute to mammalian provitamin A metabolism. J. Biol. Chem. 288: 34081–34096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho C. C., de Moura F. F., Kim S. H., and Clifford A. J.. 2007. Excentral cleavage of beta-carotene in vivo in a healthy man. Am. J. Clin. Nutr. 85: 770–777. [DOI] [PubMed] [Google Scholar]
- 37.Bagdon R. E., Impellizzeri C., and Osadca M.. 1962. Studies on the toxicity and metabolism of beta-apo-8′ -carotenal in dogs. Toxicol. Appl. Pharmacol. 4: 444–456. [DOI] [PubMed] [Google Scholar]
- 38.Sharma R. V., Mathur S. N., and Ganguly J.. 1976. Studies on the relative biopotencies and intestinal absorption of different apo-beta-carotenoids in rats and chickens. Biochem. J. 158: 377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.al-Hasani S. M., and Parrish D. B.. 1972. Forms of vitamin A and of carotenoids in tissues, blood serum and yolk of eggs from Cotournix coturnix japonica fed beta-apo-carotenals. J. Nutr. 102: 1437–1440. [DOI] [PubMed] [Google Scholar]
- 40.Zeng S., Furr H. C., and Olson J. A.. 1992. Metabolism of carotenoid analogs in humans. Am. J. Clin. Nutr. 56: 433–439. [DOI] [PubMed] [Google Scholar]
- 41.McCormick A. M., Napoli J. L., Schnoes H. K., and DeLuca H. F.. 1978. Isolation and identification of 5,6-epoxyretinoic acid: a biologically active metabolite of retinoic acid. Biochemistry. 17: 4085–4090. [DOI] [PubMed] [Google Scholar]
- 42.McCormick A. M., Napoli J. L., Yoshizawa S., and DeLuca H. F.. 1980. 5,6-Epoxyretinoic acid is a physiological metabolite of retinoic acid in the rat. Biochem. J. 186: 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mein J. R., Dolnikowski G. G., Ernst H., Russell R. M., and Wang X. D.. 2011. Enzymatic formation of apo-carotenoids from the xanthophyll carotenoids lutein, zeaxanthin and beta-cryptoxanthin by ferret carotene-9′,10′-monooxygenase. Arch. Biochem. Biophys. 506: 109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dela Seña C., Sun J., Narayanasamy S., Riedl K. M., Yuan Y., Curley R. W. Jr., Schwartz S. J., and Harrison E. H.. 2016. Substrate specificity of purified recombinant chicken beta-carotene 9′,10′-oxygenase (BCO2). J. Biol. Chem. 291: 14609–14619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee S. A., Jiang H., Trent C. M., Yuen J. J., Narayanasamy S., Curley R. W. Jr., Harrison E. H., Goldberg I. J., Maurer M. S., and Blaner W. S.. 2014. Cardiac dysfunction in beta-carotene-15,15′-dioxygenase-deficient mice is associated with altered retinoid and lipid metabolism. Am. J. Physiol. Heart Circ. Physiol. 307: H1675–H1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jamil H., Dickson J. K. Jr., Chu C. H., Lago M. W., Rinehart J. K., Biller S. A., Gregg R. E., and Wetterau J. R.. 1995. Microsomal triglyceride transfer protein. Specificity of lipid binding and transport. J. Biol. Chem. 270: 6549–6554. [DOI] [PubMed] [Google Scholar]
- 47.Hussain M. M., Shi J., and Dreizen P.. 2003. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J. Lipid Res. 44: 22–32. [DOI] [PubMed] [Google Scholar]
- 48.Spiegler E., Kim Y. K., Hoyos B., Narayanasamy S., Jiang H., Savio N., Curley R. W. Jr., Harrison E. H., Hammerling U., and Quadro L.. 2018. Beta-apo-10′-carotenoids support normal embryonic development during vitamin A deficiency. Sci. Rep. 8: 8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang X. D., Russell R. M., Liu C., Stickel F., Smith D. E., and Krinsky N. I.. 1996. Beta-oxidation in rabbit liver in vitro and in the perfused ferret liver contributes to retinoic acid biosynthesis from beta-apocarotenoic acids. J. Biol. Chem. 271: 26490–26498. [PubMed] [Google Scholar]