Abstract
The NF-κB transcription factor family influences breast cancer outcomes by regulating genes involved in tumor progression, angiogenesis, and metastasis. Dithiolethiones, a class of naturally occurring compounds with cancer chemoprevention effects that have become clinically available, have been found to inhibit NF-κB activity. However, the mechanism of this inhibition has not been identified, and the influence of dithiolethines on NF-κB pathway in breast cancer cells has not been examined. Here, we investigated the chemical and biochemical effects of dithiolethione on NF-κB and downstream effector molecules in estrogen receptor–negative breast cancer cells and murine tumor xenografts. The dithiolethiones ACS-1 and ACS-2 inhibited NF-κB transcriptional activity. Interestingly, this inhibition was not due to H2S release or protein phosphatase 2A activation, which are key properties of dithiolethiones, but occurred via a covalent reaction with the NF-κB p50 and p65 subunits to inhibit DNA binding. Dithiolethione-mediated inhibition of NF-κB–regulated genes resulted in the inhibition of interleukin (IL)-6, IL-8, urokinase-type plasminogen activator, and VEGF production. ACS-1 also inhibited matrix metalloproteinase-9 activity, cellular migration, and invasion, and ACS-2 reduced tumor burden and resulted in increased tumor host interactions. Together, our findings suggest that dithiolethiones show potential clinical use for estrogen negative breast cancer as a chemotherapeutic or adjuvant therapy.
Introduction
The NF-κB transcription factor family drives tumor progression and metastasis in many cancers by regulating genes involved in inflammation, cellular survival, and proliferation (1–3). In particular, NF-κB signaling is essential for estrogen receptor–negative (ER−) breast cancer tumorigenesis, progression, and metastasis (4–6). Furthermore, the role of NF-κB in expressing proinflammatory cytokines and enzymes contribute to the strong correlation between inflammation and breast cancer. Among the proinflammatory enzymes that are transcriptionally controlled by NF-κB are cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (NOS2; ref. 2). COX-2 and NOS2 expression in ER− breast cancer is highly correlated with poor patient prognosis (7, 8). The predictive value of NOS2 was tumor-node-metastasis stage independent suggesting that NF-κB activation of NOS2 may have a role in the initiation and progression of the disease state (8). These findings, in addition to many other reports, imply that pharmacologic targeting of NF-κB activity may provide a unique mechanism to limit cancer morbidity and mortality in patients with ER− disease (9–11).
Dithiolethione compounds, which are found in dietary vegetables and are clinically available, have been reported to inhibit NF-κB activity in multiple cell types and have been shown to ameliorate the effects of lipopolysaccharide toxicity in mice (12). This class of compounds has well-established chemopreventive effects in humans. For example, 4-methyl-5-(N-2-pyrazinyl)-1,2-dithiole-3-thione (oltipraz) increases aflatoxin metabolism and decreases the risk for aflatoxin-induced hepatocellular carcinoma, while 5-[p-methoxyphenyl]-1,2-dithiole-3-thione (anethole dithiolethione; ADT) is efficacious in limiting bronchial dysplasia in smokers (13, 14). Dithiolethiones exert their chemopreventive effects, in part, by activating the Nrf2 transcription factor that binds to the antioxidant response element on the promoter region of target genes (15, 16), in addition to inhibiting the xenobiotic response element, which is responsible for carcinogen activation (17). Beyond activation of Nrf2, dithiolethiones increase the activity of the tumor suppressor protein phosphatase 2A (PP2A) that inhibits oncogenic Akt and c-myc signaling (18). Modified nonsteroidal anti-inflammatory drugs (NSAID) such as diclofenac and valproic acid (VPA) with an ADT-like moiety and have shown that these agents reduce lung cancer xenograft volume and exhibit antiangiogenic properties (19, 20).
Because dithiolethione compounds exhibit multiple anti-cancer properties, the effects of ADT-like molecules (ACS-1, ACS-2, and ACS-92) were investigated in ER− breast cancer cell lines and focused on NF-κB activity and target gene products involved in tumor proliferation and metastasis. Previous reports show opposite effects of dithiolethiones (ADT and oltipraz) on NF-κB activation and DNA binding (21, 22) and here a series of ADT-based ditholethiones (ACS compounds) is examined in ER− breast cancer cells. ADT-based dithiolethiones inhibited NF-κB activity by forming a covalent modification with NF-κB subunits to inhibit NF-κB binding to its DNA consensus sequence. Furthermore, ACS-1 inhibited the production of NF-κB–regulated molecules involved in tumor progression and metastasis, such as interleukin-6 (IL-6), IL-8, VEGF and urokinase-type plasminogen activator (uPA). ACS-1 also inhibited the activity of matrix metalloproteinase-9 (MMP-9), which is involved in tumor metastasis. Using a mouse xenograft model, ACS-2 drastically reduced tumor burden and reduced tumor IL-6, IL-8, uPA, and VEGF. These data suggest that dithiolethione compounds, which are clinically available, may have beneficial effects in ER− breast cancer chemotherapy.
Materials and Methods
Cell culture
Human breast adenocarcinoma cell lines MDA-MB-231 and MDA-MB-468 (American Type Culture Collection, ATCC) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Invitrogen) containing 10% FBS (Atlanta Biologics) and 100 IU penicillin and 100 μg/mL streptomycin (Invitrogen). Cells were cultured at 37°C in 5% CO2 and passaged 2 to 3 times per week. Drs. Aldona Karaczyn and Joseph Verdi (Center for Molecular Medicine, Maine Medical Center Research Institute) kindly provided the MDA-MB-231 cells stably transfected with GFP, which were cultured as described above. The cell lines have been authenticated by short tandem repeat profiling (ATCC).
Statistical analyses
Data analyses were conducted with Prism 4 software (GraphPad Software, Inc.). Statistical significance was achieved with P < 0.05. One-way ANOVA analyses with the Dunnett posttest were compared with control values as indicated in figure legends. Tumor IL-6, IL-8, VEGF, uPA, and GFP values were analyzed by unpaired t test with a 1-tailed P value.
Detailed Materials and Methods are available in Supplementary Data.
Results
Clinical association between NOS2 expression and NF-κB activity
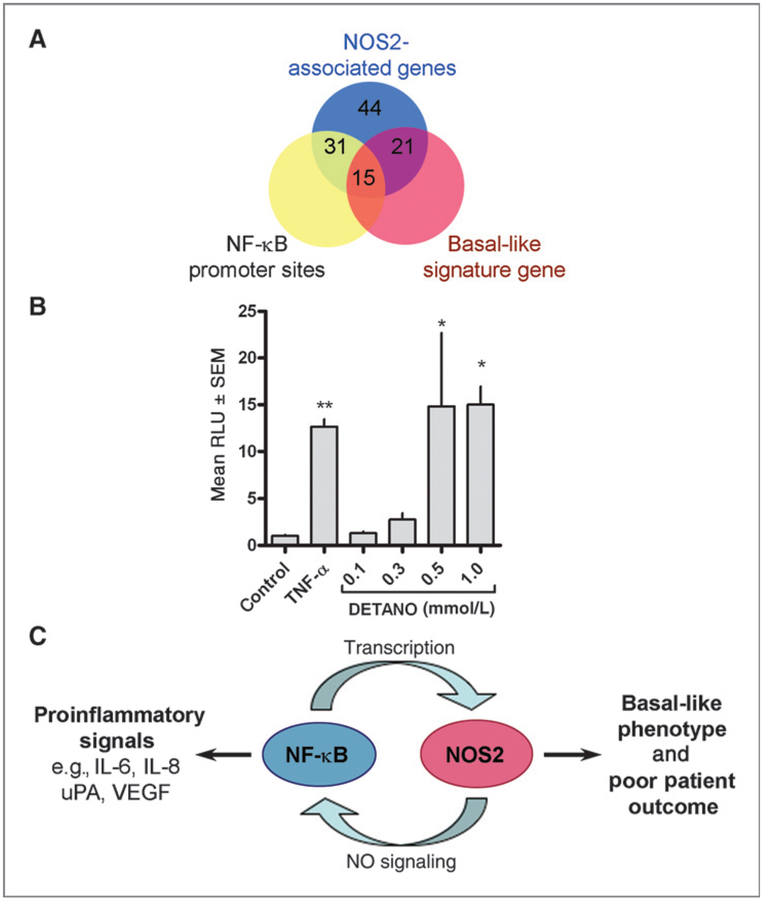
High tumor NOS2 expression is associated with a poor outcome in ER− breast cancer patients; more than 90% of the deceased patient within our cohort had elevated expression of NOS2 (8). In addition, high NOS2 expression results in the upregulation of 44 genes in the tumors of these patients (8). Genomic analyses reveal that most (31 of 44; 70%) of these genes contain at least 1 NF-κB consensus sequences in their respective promoter regions (Supplementary Table S1). Also, NF-κB contributes to the expression of the basal-like phenotype by nitric oxide (NO) as 48% (15 of 31) of genes containing an NF-κB promoter sequence are known basal-like signature genes (Supplementary Table S1 and Fig. 1A). Overall 34% (15 of 44) of NOS2 associated genes have both NF-κB promoter sequences and are basal-like markers (Fig. 1A), suggesting that NO signaling, NF-κB activation, and poor patient outcomes may be mechanistically linked. To investigate the role of NO on NF-κB activation, ER− MDA-MB-468 breast cancer cells were transfected with an NF-κB luciferase reporter construct and treated with the NO donor DETANO for 24 hours. A chemical NO donor was used because these cell lines do not express NOS2, and the use of DETANO allows for the generation of consistent and controlled NO concentrations. DETANO caused a concentration-dependent increase in luciferase activity compared with untreated control cells and similar to TNF-α–treated positive control cells (Fig. 1B). Statistical significance was achieved at 0.5 and 1 mmol/L concentrations of the donor, which are the same concentrations that significantly increased IL-8 production and cell migration in our previous report (8). These observations establish a mechanistic link between NF-κB signaling, NO-mediated inflammation, the basal-like phenotype, and ER− patient survival (Fig. 1C). Therefore NO is a physiologically relevant stimulator of NF-κB activity in ER− breast cancer patients and targeting NF-κB activity may have beneficial effects in limiting this disease state.
Figure 1.
Nitric oxide stimulates NF-κB activity in ER− breast cancer cells. A, Venn diagram depicting the relationship between genes overexpressed in high NOS2 tumors, promoter NF-κB sites, and the expression of basal-like signature genes. B, MDA-MB-468 cells were transiently transfected with an NF-κB luciferase reporter construct and exposed to TNF-α (20 ng/mL) or DETANO for 24 hours. Luciferase activity shown as mean RLU ± SD was normalized to untreated control and the fold increase relative to control is presented. Statistical significance (*, P < 0.05) compared with control was determined by one-way ANOVA. C, scheme representing the positive feedback loop between NOS2 and NF-κB activity and the products of this reciprocal signaling on tumor inflammation and patient outcomes.
Dithiolethiones inhibit NF-κB transcriptional activity in ER− breast cancer cells
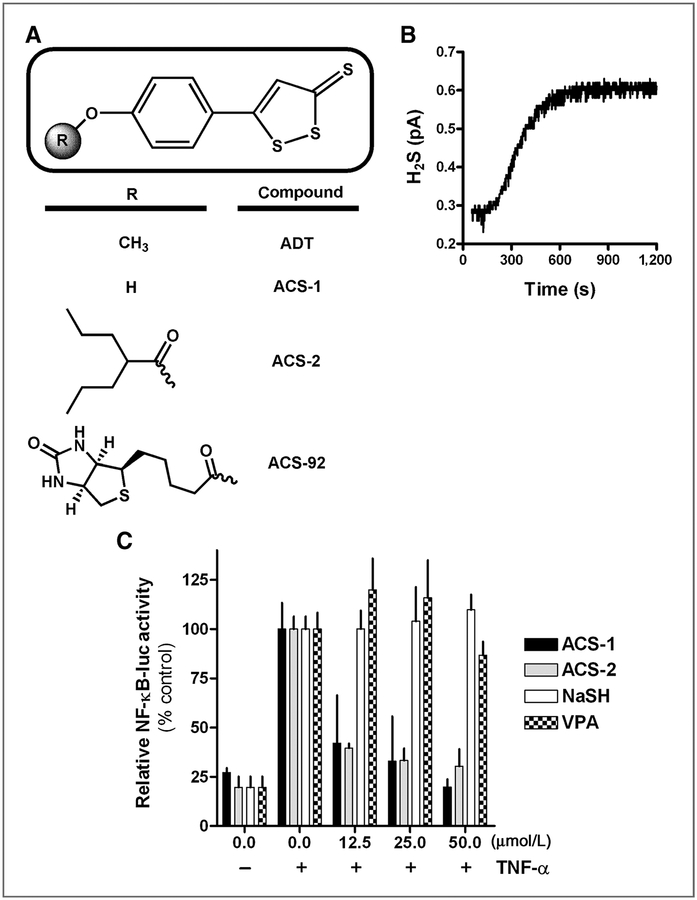
The dithiolethiones used in this study are shown in Fig. 2A. ACS-1 is the paraphenol analog of ADT. ACS-2 and ACS-92 are formed from the conjugation of VPA and biotin, respectively, with ACS-1 (12). As previously reported, these compounds hydrolyze to release hydrogen sulfide (H2S; ref. 12). ACS-1 in media containing 10% FBS rapidly released H2S as measured by a selective electrode (Fig. 2B). To measure the ability for ACS-1 and ACS-2 to inhibit NF-κB transcriptional activity, MDA-MB-231 cells were transiently transfected with an NF-κB luciferase reporter plasmid and incubated for 18 hours with the compounds and TNF-α. In addition to the dithiolethiones, the metabolites VPA and the conjugate base of H2S, sodium hydrosulfide (NaSH), were also examined in this assay. ACS-1 and ACS-2 inhibited NF-κB–dependent transcription of luciferase similar to untreated controls with an IC50 < 12.5 μmol/L, while VPA and NaSH did not alter luciferase production at the concentrations examined compared with TNF-α controls (Fig. 2C). These data indicate that the dithiolethione moiety of these compounds, not metabolites (i.e., H2S and VPA), are responsible for inhibiting NF-κB transcriptional activity.
Figure 2.
Dithiolethiones inhibit NF-κB transcriptional activity. A, chemical structures of the dithiolethiones used in this study compared with ADT. B, H2S release from ACS-1 over 1200 seconds as measured by chemical potential electrode. C, MDA-MB-231 cells transiently transfected with an NF-κB luciferase reporter construct exposed to TNF-α (20 ng/mL) alone or in combination with ACS-1, ACS-2, NaSH, or VPA. Luciferase activity shown as mean RLU (± SD) was normalized to percent of TNF-α control activity.
ACS-1 does not alter IKK signaling or NF-κB nuclear translocation
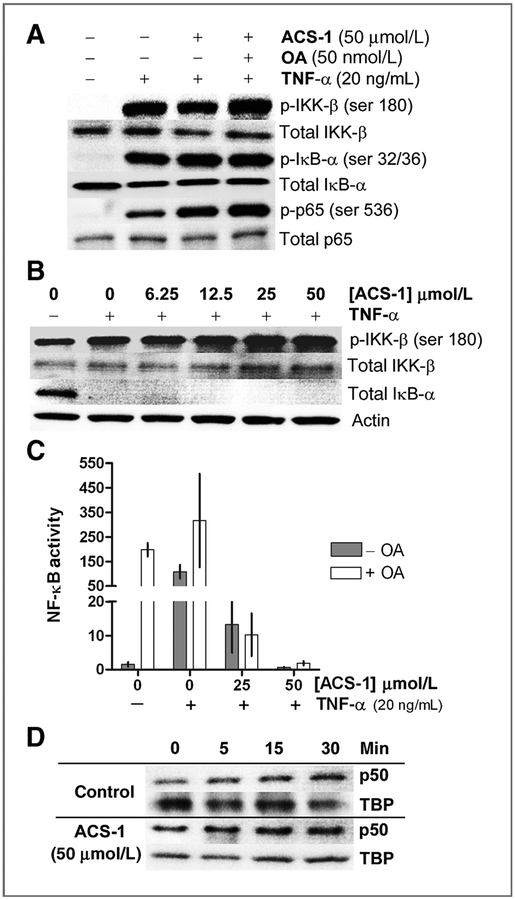
Dithiolethiones activate PP2A phosphatase activity (18). PP2A negatively regulates NF-κB activity by dephosphorylating IKK-β (23). To examine the role of PP2A activity in the ACS-1 inhibition of NF-κB, MDA-MB-231 cells were treated with ACS-1 alone or with the PP2A inhibitor okadaic acid (OA) for 2 hours before stimulation with TNF-α for 5 minutes. TNF-α stimulation resulted in marked phosphorylation of IKK-β, IkBα, and p65 (Fig. 3A). TNF-α–stimulated cells pretreated with either ACS-1 alone or ACS-1 and OA resulted in hyperphosphorylation of IKK-β, IkBα, and p65 indicating that ACS-1 does not inhibit NF-κB via increased PP2A activity (Fig. 3A). The hyperphosphorylation of IKK-β was also observed in cells treated with a range of ACS-1 concentrations while ACS-1 did not prevent the degradation of IkBα in response to TNF-α after 30 minutes (Fig. 3B). Using the NF-κB luciferase assay, ACS-1 inhibited NF-κB transcriptional activity in the presence of OA, indicating that PP2A activity is not involved in the inhibition of NF-κB by ACS-1 (Fig. 3C). To examine whether ACS-1 altered the kinetics of the NF-κB p50 subunit translocation to the nucleus, MDA-MB-468 cells were treated with or without 50 μmol/L ACS-1 for 2 hours and then stimulated with TNF-α. Cells were harvested at the indicated times and the nuclear p50 content was analyzed by Western blot (Fig. 3D). ACS-1 did not alter the rate of p50 nuclear translocation in response to TNF-α suggesting that ACS-1 does not interfere with the signaling cascade or nuclear localization of NF-κB subunits involved in NF-κB activation.
Figure 3.
NF-κB inhibition by dithiolethiones is not mediated by PP2A. A, serum-starved MDA-MB-231 cells were treated with ACS-1 or ACS-1 and OA for 2 hours before TNF-α stimulation for 5 minutes. Representative Western blot of IKK-β (ser 180), IκBα (ser 32/36), and p65 (ser 536) phosphorylation compared with total respective protein are shown. B, MDA-MB-231 cells were treated with ACS-1 for 2 hours before 30 minutes of TNF-α stimulation. IKK-β (ser 180) phosphorylation and total IkBα protein levels were measured by Western blot analysis and compared with actin. C, serum-starved MDA-MB-468 cells transiently transfected with NF-κB luciferase reporter construct were incubated 24 hours with TNF-α, ACS-1, and OA as shown. Luciferase activity was measured and normalized to fold increase over untreated controls. Mean RLU (± SD) are shown from 3 independent experiments. D, NF-κB p50 subunit nuclear accumulation was measured by Western analysis of MDA-MB-468 cells pretreated with ACS-1 or vehicle (DMSO) before TNF-α stimulation.
ACS-1 inhibits NF-κB–DNA binding
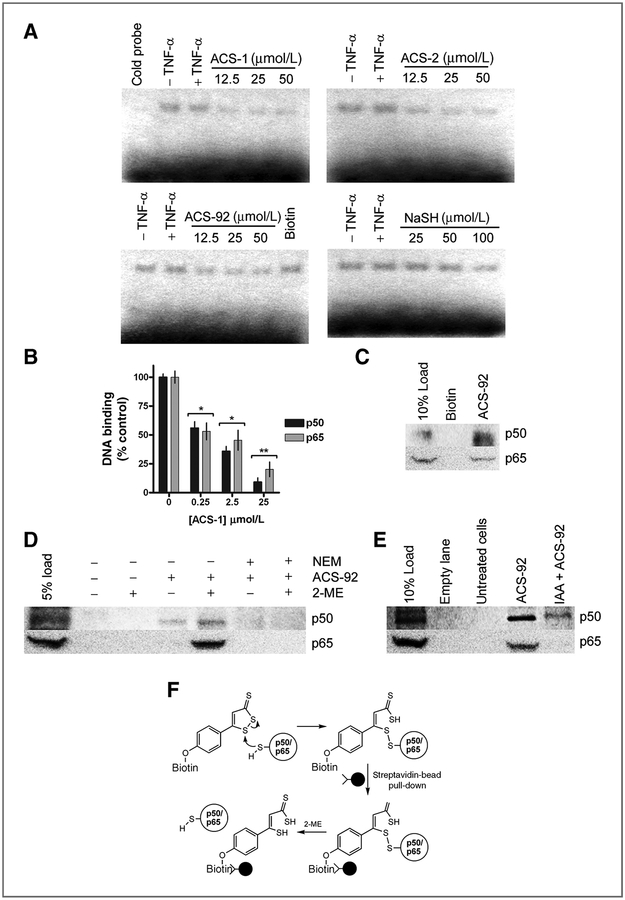
Because ACS-1 did not alter NF-κB signal transduction or nuclear translocation upon TNF-α stimulation, the effect of dithiolethiones on NF-κB binding to DNA was investigated. MDA-MB-468 cells were treated with ACS-1, ACS-2, ACS-92, or NaSH for 2 hours before TNF-α stimulation and compared with untreated cells. Nuclear fractions were isolated after 30 minutes of TNF-α stimulation and NF-κB binding to its consensus DNA sequence was measured by gel shift assay (Fig. 4A). TNF-α stimulation resulted in modest increases in DNA oligonucleotide retardation compared with untreated controls, which was eliminated upon incubation with unlabelled DNA. ACS-1, ACS-2, and ACS-92 inhibited NF-κB binding in a concentration-dependent manner, however up to 50 μmol/L NaSH/H2S did not reduce NF-κB binding, however modest decrease in binding was observed at 100 μmol/L NaSH. These data indicate that dithiolethiones inhibit NF-κB binding to DNA independent of H2S release, consistent with NF-κB luciferase results in Fig. 2C. Furthermore, 50 μmol/L biotin did not reduce NF-κB binding to DNA indicating that the dithiolethione moiety of ACS-92 is responsible for the inhibition of NF-κB binding.
Figure 4.
Dithiolethiones covalently react with NF-kB subunits to inhibit DNA binding. A, images from electrophoresis mobility shift assay experiments from MDA-MB-468 cells treated for 2 hours with ACS-1 (with cold probe competition), ACS-2, ACS-92 (with biotin control), and NaSH before TNF-α stimulation for 3 hours. B, recombinant human p50 and p65 were incubated with ACS-1 and DNA binding was measured as described. Statistical differences versus control samples were calculated by one-way ANOVA with the Dunnett posttest (*, P < 0.05; **, P < 0.01). C, p50 and p65 protein pull down from MDA-MB-231 whole-cell lysate by biotin or ACS-92 compared with lysate load control. D, Western blot of p50 and p65 eluted from ACS-92 pull down of MDA-MB-231 whole-cell lysate in the presence or absence of 2-ME. E, serum-starved MDA-MB-231 treated with or without IAA (100 μmol/L) before ACS-92 (50 μmol/L). Whole-cell lysates were incubated with streptavidin beads, and p50 and p65 were eluted in the presence of 2-ME and measured by Western blot. F, schematic representing ACS-92 covalently reacting with p50 and p65, which are pulled down from cell lysate via streptavidin-conjugated beads. Protein is eluted from the beads only in the presence of thiol-reducing agent (2-ME), indicating a disulfide bond formed between ACS-92 and the NF-κB subunits.
Because dithiolethiones inhibited NF-κB binding to its DNA consensus sequence, the effect of ACS-1 to alter the DNA-binding ability of recombinant NF-κB subunits was further examined. Recombinant human p50 or p65 (1 μg/100 μL) was incubated with ACS-1 and DNA binding was measured using an ELISA-based assay with immobilized NF-κB consensus sequences. ACS-1 inhibited both p50 and p65 DNA binding in a concentration-dependent manner as shown in Fig. 4B with an apparent IC50 of approximately 250 nmol/L in this recombinant system.
Dithiolethiones form disulfide bonds with NF-κB subunits
Due to the inhibition of NF-κB–DNA binding by dithiolethiones, the direct chemical modification of NF-κB subunit by dithiolethiones was investigated. Biotin-labeled ACS-1 (ACS-92; Fig. 1A) was used to determine whether dithiolethiones covalently modified NF-κB subunits, as the biotin-streptavidin bond is commonly used for protein pull-down experiments. MDA-MB-231 whole-cell lysate was treated with ACS-92 or biotin for 10 minutes and bound proteins were pulled down using streptavidin-conjugated beads. Western blotting revealed that p50 was present in the cell lysate (load lane) and was pulled down by ACS-92 but not by biotin alone, indicating that the dithiolethione moiety physically interacts with the NF-κB subunit (Fig. 4C). Similarly, p65 was also pulled down by ACS-92, but not biotin (Fig. 4C). To explore the nature of this interaction, a similar pull-down assay was conducted with MDA-MB-231 whole-cell lysate and the bound protein was eluted off the beads in loading buffer containing 5% 2-mercaptoethanol (2-ME) or loading buffer alone. Both p50 and p65 subunits were released from the ACS-92–conjugated bead only in the presence of 2-ME (Fig. 4D), indicating that a disulfide bond had been formed between the NF-κB subunit and ACS-92, as summarized in Fig. 4F. In addition, ACS-92 did not pull down p50 or p65 from cell lysates pretreated with N-ethyl maleimide (Fig. 4D). Furthermore, MDA-MB-231 cells were pretreated with the thiol-blocking agent iodoacetic acid (IAA) followed by ACS-92 exposure and compared with untreated cells. Whole-cell lysates were then incubated with streptavidin beads and bound protein was eluted in the presence of 2-ME. ACS-92 pull down of p50 and p65 was measured by Western blot analyses. p50 and p65 were not detected in untreated cells, indicating that the association between NF-κB subunits and the streptavidin beads was not nonspecific. Both p50 and p65 were pulled down from ACS-92–treated cells, however, IAA pretreatment markedly decreased the ability of ACS-92 to associate with and pull down p50 and p65 subunits (Fig. 4E), indicating that protein thiols are required for interaction with dithiolethiones as shown in Fig. 4F. These data indicate that dithiolethione compounds covalently interact with p50 and p65 via disulfide formation.
Dithiolethiones inhibit NF-κB–regulated target genes involved in tumor inflammation and metastasis
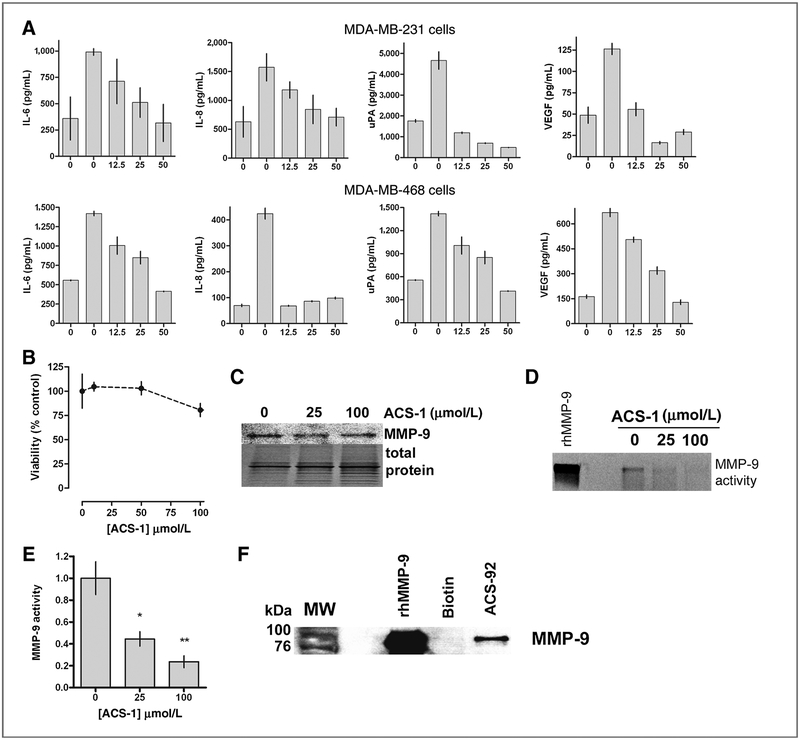
Inhibition of NF-κB by dithiolethiones suggests that the production of downstream target genes would also be decreased. NF-κB regulates the transcription of genes that have roles in tumor progression and metastasis such as IL-6, IL-8, VEGF, and uPA. Serum-starved MDA-MB-231 and MDA-MB-468 cells were treated with TNF-α and ACS-1 for 24 hours. TNF-α treatment alone resulted in increased expression of IL-6, IL-8, uPA, and VEGF compared with untreated serum-starved controls. ACS-1 resulted in the marked decrease in IL-6, IL-8, uPA, and VEGF secreted by the TNF-α–stimulated cells (Fig. 5A). Cell viability after 24 hours was determined in ACS-1–treated cells by measuring total cellular ATP levels. ACS-1 did not cause a significant decrease in cellular viability up to 100 μmol/L (Fig. 5B), indicating that the decrease in IL-6, IL-8, uPA, and VEGF are due to NF-κB inhibition and not due to cellular toxicity.
Figure 5.
Dithiolethiones inhibits NF-κB–regulated molecules involved in cancer progression and metastasis. A, serum-starved MDA-MB-231 (top panels) and MDA-MB-468 (bottom panels) cells were incubated with TNF-α alone or with ACS-1 for 24 hours and IL-6, IL-8, uPA, and VEGF were measured in the conditioned media by ELISA. Data represent the mean of triplicate experiments (± SD). B, MDA-MB-231 cells were treated with ACS-1 for 24 hours and cellular viability was determined by total ATP levels measured by a luminescent assay. Data represent the mean (± SD, n = 6) and are shown as percent of untreated controls. C, Western blot of MMP-9 from MDA-MB-231 cells treated with ACS-1. MMP-9 enzymatic activity from cells treated with ACS-1 was measured by gel zymography (D) and quantified by densitometry (E). Statistical significance from untreated controls was calculated by one-way ANOVA with the Dunnett posttest (*, P < 0.05;**, P < 0.01). F, recombinant human pro-MMP-9 was incubated with biotin or ACS-92, and bound protein was pulled down by streptavidin-conjugated beads. Bound protein was eluted in loading buffer containing 5% 2-ME and analyzed by Western blot.
An NF-κB consensus sequence is present in the promoter region of the MMP-9 gene, which is essential for tumor cell metastasis. ACS-1 did not alter the secretion of MMP-9 by MDA-MB-231 cells over a wide concentration range as shown in Fig. 5C, suggesting that MMP-9 transcription is not dependent on NF-κB. However, MMP-9 proteinase activity of conditioned media was significantly inhibited by ACS-1 as determined by zymography and quantified by densitometry (Fig. 5D and E). To examine a potential chemical mechanism of this inhibition of MMP-9 by dithiolethiones, human recombinant pro–MMP-9 was incubated with ACS-92 or biotin and pulled down as described above. ACS-92 was able to pull down the latent form of MMP-9 while biotin did not, indicating that the dithiolethione moiety covalently reacts with MMP-9 to inhibit its proteinase activity (Fig. 5F).
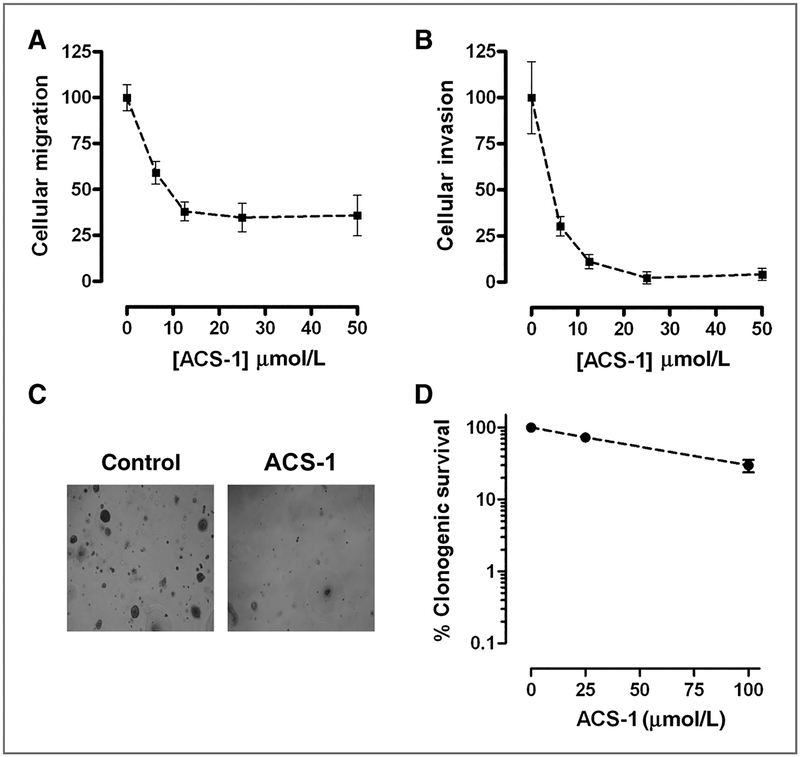
MDA-MB-231 cell motility is regulated by NF-κB–mediated production of uPA (24). To examine the effects of ACS-1 on cell motility, MDA-MB-231 cells were seeded onto Trans-well plates in serum-free DMEM containing ACS-1 and cells were allowed to migrate toward 5% FBS chemotractant for 24 hours. ACS-1 caused a concentration-dependent decrease in cell migration consistent with NF-κB and uPA inhibition (Fig. 6A). ACS-1 also inhibited MDA-MB-231 invasion through Matrigel basement membrane matrix with an IC50 < 6.25 μmol/L, consistent with NF-κB and MMP-9 inhibition (Fig. 6B). Furthermore, ACS-1 inhibited MDA-MB-231 Matrigel outgrowth (Fig. 6C) indicating that cellular proliferation, migratory and invasive potential are reduced by this dithiolethione (25). To determine whether the effect of ACS-1 was due to cell death or cytostasis, MDA-MB-231 cells were treated for 24 hours with ACS-1 and colony formation was assessed after 7 days (Fig. 6D). ACS-1 did not result in a logarithmic decrease in cell survival, and minimal toxicity was observed below 25 μmol/L indicating that dithiolethiones exert a cytostatic effect on ER− breast cancer cells and that the inhibition of Matrigel outgrowth was not due to cell viability.
Figure 6.
Dithiolethiones inhibit breast cancer cell migration and invasion. MDA-MB-231 cells were incubated in serum-free media containing ACS-1 and allowed to migrate (A) and invade through matrigel (B) toward 5% FBS chemotractant for 24 hours. Data represent the mean (± SD, n = 6) and are shown as percent of untreated controls. C, MDA-MB-231 cells were seeded between Matrigel layers and incubated with or with out ACS-1 for 14 days and Matrigel outgrowth was photographed (magnification, ×10). D, MDA-MB-231 cells were exposed to ACS-1 for 24 hours and assessed for colony formation. The clonogenic survival is represented as the mean number of colonies formed (± SD, n = 3) and is shown as percent of untreated controls.
ACS-2 reduces tumor xenograft burden
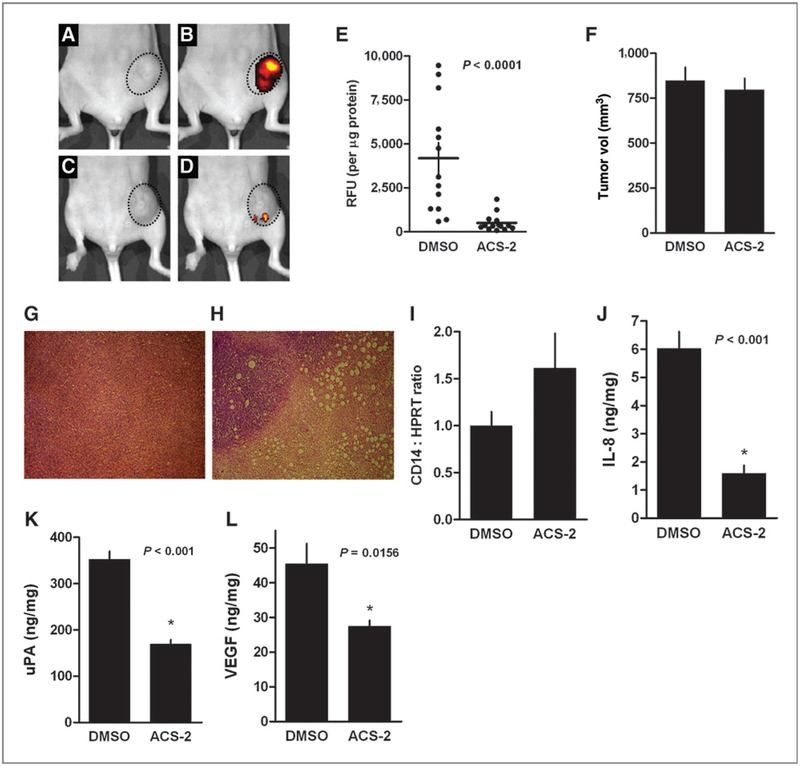
The antitumor effects of dithiolethiones were examined in a murine model of breast cancer. MDA-MB-231 cells stably expressing GFP were injected into the fourth inguinal mammary fat pad of 10-week-old female athymic nude mice. Tumors were allowed to grow for 10 days at which time ACS-2 (20 mg/kg) or DMSO (vehicle control) was administered by i.p. injection 3 times per week for 5 weeks. ACS-2 was used for in vivo studies due to the rapid metabolism and poor pharmacokinetics of ACS-1 in mice. After 5 weeks of drug administration, the mice were anesthetized and in vivo tumor GFP fluorescence was measured. The ACS-2–treated mice had a marked decrease in tumor GFP fluorescence compared with the DMSO-treated mice as shown in Fig. 7A–D. After in vivo GFP measurements, the mice were euthanized and the tumors were resected. Tumor sections were homogenized and GFP fluorescence was measured in the tumor homogenate and normalized to total protein content (Fig. 7E). Final tumor volumes were not significantly different between the 2 groups (Fig. 7F). Although the gross tumor volume was not significantly reduced by ACS-2, the ACS-2–treated tumors had significantly lower relative GFP fluorescence than control tumors (P = 0.0001), indicating that the tumor burden was reduced in the ACS-2–treated mice.
Figure 7.
Dithiolethione reduces MDA-MB-231-GFP tumor xenograft burden. Mammary fat pad MDA-MB-231–GFP tumor xenograft-bearing female athymic nude mice were treated with ACS-2 (20 mg/kg) or DMSO (vehicle control) for 5 weeks. Photograph of representative DMSO-treated tumor (encircled area; A) and in vivo GFP fluorescence of the same tumor (B). Photograph of representative ACS-2 treated tumor (encircled area; C) and in vivo GFP fluorescence of the same tumor (D). E, homogenized tumor GFP fluorescence normalized to total protein content. Horizontal line indicate mean fluorescence (± SEM). Statistical significance (P < 0. 0001) was calculated by unpaired t test with a one-tailed P value. F, the mean final tumor volumes are shown (± SEM) from DMSO and ACS-2–treated mice. Microphotographs of H&E stained xenograft tumor sections from DMSO (G) and ACS-2 (H) treated mice. I, CD14/HPRT ratios determined from densitometric analysis of Western blots normalized to mean DMSO levels. Data indicate normalized mean values (± SD). Human IL-8 (J), uPA (K), and VEGF (L) content of homogenized tumor xenografts are shown. Data represent the mean value (± SEM). Statistical significance was calculated by unpaired t test with a one-tailed P value.
Hematoxylin and eosin (H&E)-stained tumor sections reveal that ACS-2 (Fig. 7G) caused decreased mitotic index compared with DMSO-treated mice (Fig. 7H). ACS-2 also increased intracellular vacuoles consistent with the antiangiogenic properties of dithiolethiones including decreased VEGF production and MMP-9 activity. Histopathology examination of tumor sections revealed that ACS-2 induced necrosis throughout the tumor mass and the host was unable to clear the necrotic cells from the tumor. The potential loss of the GFP fluorescence in response to chronic ACS-2 treatment was examined by treating MDA-MB-231-GFP cells to ACS-2 for 30 days. Under these conditions, the cells retained GFP DNA vector and MDA-MB-231-GFP fluorescence was not quenched by ACS-2 (data not shown). These data indicate that ACS-2 does not eliminate the GFP gene, inhibit GFP protein production, or physically interfere with GFP fluorescence.
To measure the potential increase of host immune response in ACS-2–treated mice, whole-cell lysates were prepared from homogenized tumors and the relative amount of CD14 was analyzed by Western blotting and normalized to hypoxanthine phosphoribosyltransferase (HPRT). Densitometric analysis show that ACS-2-treated tumors had an increased mean content of the monocyte marker compared with DMSO controls, however the increase did not achieve statistical significance (Fig. 7I). However, tumor content of human IL-8, uPA, and VEGF were significantly reduced in the ACS-2–treated mice compared with DMSO control mice (Fig. 7J–L), indicating that tumor burden and NF-κB activity was reduced by dithiolethione treatment.
Discussion
NF-κB has a central role in inflammation and cancer. The proinflammatory protein NOS2 is a strong prognostic marker in ER− breast cancer, and its expression is transcriptionally regulated by NF-κB activity (2, 8). Our data indicate that the product of NOS2 catalysis, NO, activates NF-κB signaling forming a positive feedback loop to enhance inflammatory signaling in ER− breast tumors. These results indicate that the aggressive basal-like phenotype is promoted in part by NOS2/NO/NF-κB pathway activation (Supplementary Table S1 and Fig. 1). These observations further highlight the clinical significance of developing agents that inhibit NF-κB activity for the treatment of ER− breast patients.
Dithiolethiones have chemopreventative effects in humans (13, 15, 26), however the data shown here indicate that this class of naturally occurring compounds may also have effects in cancer chemotherapy and/or adjuvant therapy. The results presented here establish dithiolethione compounds as inhibitors of the tumor-promoting NF-κB signaling pathway in estrogen-negative breast cancer cells. A prototypical dithiolethione ADT has been found to inhibit NF-κB activation in human T cells, however the mechanism of this inhibition has not been determined (22). A unique feature of dithiolethiones is their ability to release H2S. A ditholethione-modified NSAID has been shown to limit lipopolysaccharide-induced inflammation and NF-κB activity and H2S release was proposed to be responsible for the observed effects (12). H2S has been shown to reduce NF-κB activation in astrocytes and microglia cells (27). However, from the data presented here, it seems that H2S has no role in inhibiting NF-κB activity in breast cancer cells treated with dithiolethiones. Another report indicates that H2S increases proinflammatory cytokine production by activating NF-κB (28). These seemingly contradictory findings indicate that the effects of H2S on NF-κB signaling are dependent on context and cell type and that the physiology of H2S needs further elucidation.
The mechanism of NF-κB inhibition by dithiolethiones is via covalent thiol modification of NF-κB p50 and p65 subunits. This is consistent with other observations that NF-κB subunit cysteine oxidation or alkylation inhibits DNA-binding functions (29, 30). We conclude that the electrophilic nature of the dithiolethione moiety is responsible for the covalent modification of NF-κB subunits and are functionally similar to alkylating agents.
NF-κB is a molecular target for anticancer therapies because of the effects of NF-κB target genes on tumor proliferation and microenvironment (2, 9). NF-κB regulated genes involved in tumor progression, angiogenesis, and metastasis include IL-6, IL-8, uPA, VEGF, and MMP-9 (2, 3, 9, 24, 31, 32). The dithiolethiones studied here reduced IL-6, IL-8, uPA, and VEGF secreted by ER− breast cancer cells consistent with NF-κB inhibition. We predict that dithiolethiones may inhibit other cancer-related transcription factors and therefore we cannot rule out that the effects on cytokine production are solely due to NF-κB inhibition. We are in the process of examining and identifying other signaling pathways and transcription factors altered by dithiolethiones (such as AP-1 and β-catenin). Many protumor signaling pathways exhibit cross-talk and redundancy that has made targeting single pathways difficult. However, the inhibition of cytokine production is indicative of the overall antitumor effects of these compounds. Furthermore, ACS-1 treatment inhibited the activity but not the production of MMP-9, which is critical for tumor cell migration and invasion. The inhibition of MMP-9 activity is further characterized by the inhibition of both cellular migration and invasion by ACS-1 in a concentration-dependent manner. These results highlight the potential numerous benefits of NF-κB inhibition by dithiolethiones with respect to breast cancer chemotherapy or adjuvant therapy.
Despite the profound in vitro effects of dithiolethiones on NF-κB activity and downstream gene products, administration of ACS-2 (20 mg/kg) to mice with MDA-MB-231-GFP tumors implanted into the mammary fat pad did not alter total tumor volume compared with vehicle control mice upon gross measurements. However, the tumor burden was markedly decreased in mice treated with ACS-2 as inferred from in vivo and ex vivo tumor GFP measurements suggesting that ACS-2 may reduce the potential surgical or radiation fields and improve therapeutic margins. The potential loss of the GFP gene or GFP fluorescence quenching by ACS-2 was examined, however the dithiolethione did not alter the presence of the GFP gene or fluorescence of GFP expressed in the MDA-MB-231 cell line. Furthermore, histopathologic examination indicates that ACS-2 resulted in increased tumor cell necrosis in the absence of host clearance, reduced mitotic indices, and reduced tumor angiogenesis. From these observations, it seems that the dithiolethione altered tumor proliferation and/or host immune response to the tumor. Furthermore, tumor levels of NF-κB regulated IL-8, uPA, and VEGF were significantly reduced, indicating that dithiolethiones inhibit NF-κB activity in vivo and contributes to the reduced tumor burden observed. These results indicate that dithiolethione treatment induces an antitumor effect by inhibiting NF-κB.
In summary, dithiolethione compounds have potential use in ER− breast cancer as a chemotherapeutic or adjuvant therapy as these compounds inhibit NF-κB and downstream inflammatory molecules that promote tumor growth and metastasis. This novel feature of dithiolethiones, in addition to the antiangiogenic and tumor suppressor PP2A activation properties already reported (18, 20), indicate that this class of compounds have multiple anticancer properties and can be considered a potential multitargeted pharmaceutical. Furthermore, these compounds result in no observable toxic side effects in vivo, are naturally occurring in dietary cruciferous vegetables, and ADT is currently a clinically available drug.
Supplementary Material
Acknowledgments
The authors thank Drs. Aldona Karaczyn and Joseph Verdi of the Center for Molecular Medicine, Maine Medical Center Research Institute, Scarborough, ME, for generously supplying the MDA-MB-231-GFP cell line. Dithiolethione compounds, studied in this work, were synthesized in the lab of A. Sparatore under her supervision.
Grant Support
This work was supported by the Intramural Research Program of the NIH, National Cancer Institute (1ZIASC007281–16 and 1ZIABC010899–02).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
A. Sparatore and P. Del Soldato are stockholders in Sulfidris, which has patent rights on reagents used in this study. No potential conflicts of interest were disclosed by the other authors.
References
- 1.Rayet B, Gelinas C. Aberrant rel/nfkb genes and activity in human cancer. Oncogene 1999;18:6938–47. [DOI] [PubMed] [Google Scholar]
- 2.Karin M, Cao Y, Greten FR, Li Z-W. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer 2002;2:301–10. [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell 2004;6:203–8. [DOI] [PubMed] [Google Scholar]
- 4.Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ Jr, Sledge GW Jr. Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Mol Cell Biol 1997;17:3629–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huber MA, Azoitei N, Baumann B, Grunert S, Sommer A, Pehamberger H, et al. NF-κB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest 2004;114:569–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biswas DK, Shi Q, Baily S, Strickland I, Ghosh S, Pardee AB, et al. NF-kappa B activation in human breast cancer specimens and its role in cell proliferation and apoptosis. Proc Natl Acad Sci U S A 2004;101:10137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glynn S, Prueitt R, Ridnour L, Boersma B, Dorsey T, Wink D, et al. COX-2 activation is associated with Akt phosphorylation and poor survival in ER-negative, HER2-positive breast cancer. BMC Cancer 2010;10:626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glynn SA, Boersma BJ, Dorsey TH, Yi M, Yfantis HG, Ridnour LA, et al. Increased NOS2 predicts poor survival in estrogen receptor-negative breast cancer patients. J Clin Invest 2010;120:3843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orlowski RZ, Baldwin AS. NF-kappaB as a therapeutic target in cancer. Trends Mol Med 2002;8:385–9. [DOI] [PubMed] [Google Scholar]
- 10.Gupta SC, Sundaram C, Reuter S, Aggarwal BB. Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim Biophys Acta 2010;1799:775–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biswas DK, Dai S-C, Cruz A, Weiser B, Graner E, Pardee AB. The nuclear factor kappa B (NF-kappa B): a potential therapeutic target for estrogen receptor negative breast cancers. Proc Natl Acad Sci U S A 2001;98:10386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L, Rossoni G, Sparatore A, Lee LC, Del Soldato P, Moore PK. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free Radic Biol Med 2007;42:706–19. [DOI] [PubMed] [Google Scholar]
- 13.Lam S, MacAulay C, le Riche JC, Dyachkova Y, Coldman A, Guillaud M, et al. A randomized phase IIb trial of anethole dithiolethione in smokers with bronchial dysplasia. J Natl Cancer Inst 2002;94:1001–9. [DOI] [PubMed] [Google Scholar]
- 14.Wang J-S, Shen X, He X, Zhu Y-R, Zhang B-C, Wang J-B, et al. Protective alterations in phase 1 and 2 metabolism of aflatoxin B1 by oltipraz in residents of Qidong, People’s Republic of China. J Natl Cancer Inst 1999;91:347–54. [DOI] [PubMed] [Google Scholar]
- 15.Kwak M-K, Egner PA, Dolan PM, Ramos-Gomez M, Groopman JD, Itoh K, et al. Role of phase 2 enzyme induction in chemoprotection by dithiolethiones. Mutat Res 2001;480–481:305–15. [DOI] [PubMed] [Google Scholar]
- 16.Lee J-S, Surh Y-J. Nrf2 as a novel molecular target for chemoprevention. Cancer Lett 2005;224:171–84. [DOI] [PubMed] [Google Scholar]
- 17.Bass SE, Sienkiewicz P, MacDonald CJ, Cheng RYS, Sparatore A, Del Soldato P, et al. Novel dithiolethione-modified nonsteroidal anti-inflammatory drugs in human hepatoma HepG2 and colon LS180 cells. Clin Cancer Res 2009;15:1964–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Switzer CH, Ridnour LA, Cheng RYS, Sparatore A, Del Soldato P, Moody TW, et al. Dithiolethione compounds inhibit Akt signaling in human breast and lung cancer cells by increasing PP2A activity. Oncogene 2009;28:3837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isenberg JS, Jia Y, Field L, Ridnour LA, Sparatore A, Del Soldato P, et al. Modulation of angiogenesis by dithiolethione-modified NSAIDs and valproic acid. Br J Pharmacol 2007;151: 142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moody TW, Switzer C, Santana-Flores W, Ridnour LA, Berna M, Thill M, et al. Dithiolethione modified valproate and diclofenac increase E-cadherin expression and decrease proliferation of non-small cell lung cancer cells. Lung Cancer 2010;68:154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nho CW, O’Dwyer PJ. NF-κB activation by the chemopreventive dithiolethione oltipraz is exerted through stimulation of MEKK3 signaling. J Biol Chem 2004;279:26019–27. [DOI] [PubMed] [Google Scholar]
- 22.Sen CK, Traber KE, Packer L. Inhibition of NF-[kappa]B activation in human T-cell lines by anetholdithiolthione. Biochem Biophys Res Commun 1996;218:148–53. [DOI] [PubMed] [Google Scholar]
- 23.Fu DX, Kuo YL, Liu BY, Jeang KT, Giam CZ. Human T-lymphotropic virus type I tax activates I-kappa B kinase by inhibiting I-kappa B kinase-associated serine/threonine protein phosphatase 2A. J Biol Chem 2003;278:1487–93. [DOI] [PubMed] [Google Scholar]
- 24.Sliva D, Rizzo MT, English D. Phosphatidylinositol 3-kinase and NF-κB regulate motility of invasive MDA-MB-231 human breast cancer cells by the secretion of urokinase-type plasminogen activator. J Biol Chem 2002;277:3150–7. [DOI] [PubMed] [Google Scholar]
- 25.Zajchowski DA, Bartholdi MF, Gong Y, Webster L, Liu H-L, Munishkin A, et al. Identification of gene expression profiles that predict the aggressive behavior of breast cancer cells. Cancer Res 2001;61: 5168–78. [PubMed] [Google Scholar]
- 26.Zhang Y, Munday R. Dithiolethiones for cancer chemoprevention: where do we stand? Mol Cancer Ther 2008;7:3470–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee M, Schwab C, Yu S, McGeer E, McGeer PL. Astrocytes produce the antiinflammatory and neuroprotective agent hydrogen sulfide. Neurobiol Aging 2009;30:1523–34. [DOI] [PubMed] [Google Scholar]
- 28.Zhi L, Ang AD, Zhang H, Moore PK, Bhatia M. Hydrogen sulfide induces the synthesis of proinflammatory cytokines in human monocyte cell line U937 via the ERK-NF-κB pathway. J Leukoc Biol 2007;81:1322–32. [DOI] [PubMed] [Google Scholar]
- 29.Toledano MB, Leonard WJ. Modulation of transcription factor NF-kappa B binding activity by oxidation-reduction in vitro. Proc Natl Acad Sci U S A 1991;88:4328–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pande V, Sousa SF, Ramos MJ. Direct covalent modification as a strategy to inhibit nuclear factor-kappa B. Curr Med Chem 2009;16: 4261–73. [DOI] [PubMed] [Google Scholar]
- 31.Farina AR, Tacconelli A, Vacca A, Maroder M, Gulino A, Mackay AR. Transcriptional up-regulation of matrix metalloproteinase-9 expression during spontaneous epithelial to neuroblast phenotype conversion by SK-N-SH neuroblastoma cells, involved in enhanced invasivity, depends upon GT-box and nuclear factor kappaB elements. Cell Growth Differ 1999;10:353–67. [PubMed] [Google Scholar]
- 32.Bhat-Nakshatri P, Newton TR, Goulet R, Nakshatri H. NF-κB activation and interleukin 6 production in fibroblasts by estrogen receptor-negative breast cancer cell-derived interleukin 1b. Proc Natl Acad Sci U S A 1998;95:6971–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.