Abstract
Background:
Women with a history of hypertensive disorders of pregnancy and preterm delivery have an increased risk of cardiovascular disease (CVD). Chronic inflammation, endothelial dysfunction, and dyslipidemia may link pregnancy outcomes with CVD.
Objective:
We evaluated whether women with a history of HDP or normotensive preterm delivery had adverse CVD biomarker profiles after pregnancy.
Study Design:
We identified parous women from the Nurses’ Health Study II with C-reactive protein (CRP; n=2,614), interleukin-6 (IL-6; n=2,490), glycated hemoglobin (n=885), intracellular adhesion molecule-1 (n=1,231), high density lipoprotein cholesterol (n=931), low density lipoprotein cholesterol (n=931), triglycerides (n=1,428), or total cholesterol (n=2,940) assessed in stored blood samples. Multivariable-adjusted robust linear regression models evaluated percent differences and 95% confidence intervals (CIs) in each biomarker associated with a history of HDP or preterm delivery.
Results:
Ten percent of women had a history of HDP, while 11% with normotensive pregnancies had at least one preterm delivery. Median time from first pregnancy to blood draw was 17 years (interquartile range: 12, 22). Plasma levels of CRP and IL-6 were 34.4% (95% CI: 17.2, 54.1), and 11.6% higher (95% CI: 2.1, 21.9) respectively, among women with a history of HDP compared to those with only normotensive pregnancies. Altered CVD biomarker levels were otherwise not present in women with a history of HDP or preterm delivery.
Conclusion:
CRP and IL-6, but not other CVD biomarkers, were elevated in women with a history of HDP in the years following pregnancy, suggesting inflammation may be a pathway linking HDP with future CVD risk.
Keywords: Cardiovascular diseases, Inflammation, Mass screening, Pre-eclampsia, Premature birth, Women’s health
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of mortality in women [1]. The American Heart Association’s guidelines for CVD prevention in women identify preeclampsia, gestational hypertension, and gestational diabetes as CVD risk factors [2]. Pregnancy may act as a “stress test” that unmasks subclinical cardiovascular risk, providing early insight into a woman’s cardiovascular health [3–5].
Pregnancy complications, including preeclampsia, preterm delivery, gestational diabetes, and low birth weight, will impact approximately 20% of women [5]. Women with a history of a hypertensive disorder of pregnancy (HDP; gestational hypertension and preeclampsia) or preterm delivery are at nearly two-fold higher risk of CVD than women without these complications [6–14]. However, research evaluating mechanisms that link these conditions is limited and primarily focused on postpartum development of clinical CVD risk factors (e.g. chronic hypertension) [13,15–17]. Preeclampsia and preterm delivery have been linked to elevated inflammatory biomarkers and alterations in lipid levels during pregnancy [18–24], providing biological plausibility for persistent changes after pregnancy. Therefore, elevated inflammatory, endothelial, and lipid biomarkers may provide insight into the development of CVD risk after complicated pregnancies. However, studies that have investigated the relationship between HDP, preterm delivery, and CVD biomarkers have produced inconsistent results, and suffer from small sample sizes or short follow-up after pregnancy [25–30].
We evaluated the associations between HDP, preterm delivery, and CVD biomarkers 1 to 34 years after pregnancy in the Nurses’ Health Study II (NHSII), a cohort in which we have demonstrated associations of these pregnancy complications with increased risk of chronic hypertension, type 2 diabetes, dyslipidemia, and CVD events [14,31,32]. We hypothesized that women with a history of HDP or preterm delivery would have adverse CVD biomarker profiles in the years following pregnancy.
MATERIALS AND METHODS
Study Population
The study population consisted of participants in the NHSII, a longitudinal cohort of 116,429 female U.S. registered nurses between aged 25 and 42 years at baseline in 1989. Participants completed biennial questionnaires that assessed health-related behaviors, medication use, reproductive history, and incident disease. Women who responded to the 1995 questionnaire and had not previously reported a cancer diagnosis were invited to provide blood samples (n=92,888). Between 1996 and 2001, 29,611 women provided a blood sample. Our analysis was restricted to parous women whose blood sample had been analyzed for a previous sub-study (nested case-control studies of chronic diseases or cohort studies of lifestyle exposures). The NHSII was approved by the Partners Human Research Committee (Institutional Review Board) of Brigham and Women’s Hospital. Questionnaire return was considered informed consent.
Exposure Assessment
Hypertensive Disorders of Pregnancy
On the 2009 questionnaire, women self-reported whether each pregnancy lasting at least 20 weeks was complicated by ‘preeclampsia/toxemia’ or ‘high blood pressure’ (i.e. gestational hypertension). If a woman reported either of these conditions in any pregnancy prior to her blood draw, she was considered to have a history of HDP.
Gestational Length
In 2009, women self-reported gestation length for all pregnancies in the following categories: <8, 8-11, 12-19, 20-27, 28-31, 32-36, 37-39, 40-42, and 43+ weeks. For this analysis, these were collapsed into term (≥37 weeks), moderate preterm (≥32 to <37 weeks), and very preterm (≥20 to <32 weeks). Pregnancies lasting <20 weeks were not included. For women who had more than one birth, gestation length category was determined by the shortest length of all reported births prior to blood draw. Since HDP is an indication for preterm delivery [33], and because we hypothesized HDP to be associated with the CVD biomarkers of interest, primary analyses of gestation length focused on women with no history of HDP to isolate the preterm-CVD biomarker relationship.
Exposure Validity
We assessed the validity of self-reported preeclampsia and gestation length in a subset of participants who reported preeclampsia between 1991 and 2001. Among 462 women with complete medical records, the positive predictive value for preeclampsia was 89%. Gestation length validity was evaluated using a 3-category exposure (term, moderate preterm, very preterm) for 403 participants, yielding a Kappa statistic of 0.74.
Biomarker Assessment
Participants returned blood samples to the laboratory by overnight courier for processing and storage at ≤130° Celsius, as described elsewhere [34,35]. Our analysis consists of women who had at least one of the following established CVD biomarkers [36–38] assayed: C-reactive protein (CRP), interleukin-6 (IL-6), glycated hemoglobin (HbA1C), intracellular adhesion molecule-1 (ICAM-1), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), total cholesterol, or triglycerides; 78% of these samples were provided after fasting for ≥8 hours. These biomarkers have been evaluated and shown to be stable within 36 hours of transport [39]. Assays used to measure each biomarker are presented in Supplemental Table 1. Most assays were conducted at the laboratory of Dr. Nader Rifai at Boston Children’s Hospital and Harvard Medical School. Intra-assay coefficients of variation (CVs) from replicate, blinded, quality-control samples ranged from 0.3% to 15.3%, with 92% of laboratory batches yielding CVs <10% (Supplemental Table 1).
Covariates
Covariates were chosen a priori either because they were matching factors used to select controls in the nested case-control studies or were hypothesized as potential confounders of the pregnancy history-CVD risk association. The following matching factors were included as covariates: menopausal status (pre, post, or unknown/missing), current smoking, alcohol intake (none, moderate (<1 drink/day), or heavy consumption (≥1 drink/day) in the month before blood draw), current post-menopausal hormone (PMH) use, and history of infertility at blood draw. We considered a woman to have a history of infertility if she reported trying to become pregnant for more than one year without success. Age and parity at blood draw, race/ethnicity, and pre-pregnancy body mass index (BMI, kilograms per meter2; <18.5, 18.5 to <25, 25 to <30, ≥30), diet (Alternative Healthy Eating Index-2010 (AHEI) [40] in quintiles), and strenuous physical activity (never, 1-3, 4-6, 7-9, 10-12 months per year) were considered potential confounders.
Exclusions
Our analytic sample included women who provided a blood sample, completed the 2009 pregnancy history questions, were parous before blood draw, and were ≥18 years old at first birth (Figure 1). For each biomarker analysis, we excluded women who did not have the biomarker of interest measured or who self-reported use of cholesterol-lowering or diabetes medication before blood draw. We also excluded women whose blood sample had been chosen for analysis because they were cases in CVD-related nested case-control studies (i.e. hypertension, diabetes, stroke, and myocardial infarction cases) due to known relationships between our pregnancy exposures, CVD biomarkers, and these diseases. While we did not exclude non-CVD related cases (e.g. Barrett’s esophagus cases), as a sensitivity analysis, we excluded all samples selected as cases for biomarker case-control studies. In the CRP analysis, we additionally excluded women with CRP values >10 mg/L, a clinical cutoff used to denote acute infection. Sample sizes differ for each biomarker analysis, ranging from 885 to 2,940 women, since they are composed of different sub-studies (based on which biomarkers were assayed).
Figure 1.
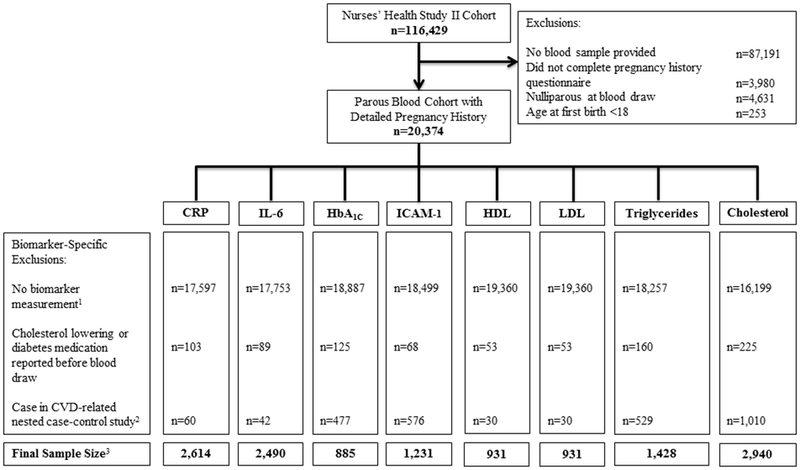
Exclusions by cardiovascular biomarker in the Nurses’ Health Study II
Abbreviations: CRP: C-reactive protein; IL-6: Interleukin-6, HbA1C: Glycated hemoglobin; ICAM-1: Intracellular adhesion molecule-1; HDL: High-density lipoprotein cholesterol; LDL: Low-density lipoprotein cholesterol
1N=109 excluded from CRP analysis due to level >10mg/L; N =1 excluded from HbA1C analysis due level < 0.5%; N=3 excluded from triglyceride analysis due to level >1000 mg/dL
2Number of CVD-related nested case-control studies differ by biomarker yielding a large variation in number of women excluded due to this criterion
3For the preterm analyses: N=2 additionally excluded from CRP, IL-6, and HbA1C, N=1 additionally excluded from ICAM-1, and N=7 additionally excluded from total cholesterol due to missing gestation length
Statistical Analysis
Biomarkers were log-transformed to improve normality. To reduce the potential for laboratory drift, we adjusted for batch using a method described by Rosner [41]. Multivariable adjusted robust regression models were used to estimate the percent differences in post-pregnancy CVD biomarker levels and 95% confidence intervals (CI) by history of HDP and normotensive preterm delivery. HDP was assessed as a binary exposure, while preterm delivery was evaluated as both dichotomous and categorical (3 categories: term, moderate preterm, very preterm) using indicator variables. Analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Table 1 shows age-standardized characteristics of study participants separately by history of HDP and normotensive preterm delivery: 9.9% of women had a history of HDP, while 11.3% of women with only normotensive pregnancies delivered an infant preterm. On average, women were 26.7 years old (standard deviation (SD)=4.3) at first birth and 44.0 years old (SD=4.5) at blood draw. Median time between first birth and blood draw was 17 years (interquartile range (IQR): 12, 22). Women were generally similar across exposure groups, although women with a history of HDP were more likely to be overweight or obese before first pregnancy, and more likely to smoke or use PMH at blood draw, compared to women with normotensive pregnancies. Women who delivered an infant preterm were more likely to be smokers at blood draw than women who delivered at term.
Table 1.
Age-standardized characteristics of participants by history of pregnancy complications
| Only Normotensive (n=4,442) | Ever Hypertensive Disorder of Pregnancy (n=491) | Normotensive |
||
|---|---|---|---|---|
| Only Term (n=3,931) | Ever Preterm (n=502) | |||
| Age at first birth in years,1 mean (SD) | 26.6 (4.3) | 26.9 (4.5) | 26.7 (4.2) | 26.4 (4.6) |
| Age at blood draw1 | ||||
| <40 years | 17.3 | 19.8 | 16.8 | 21.1 |
| 40 to <45 years | 35.6 | 39.7 | 36.4 | 29.5 |
| ≥45 years | 47.2 | 40.5 | 46.9 | 49.4 |
| White | 97.4 | 97.5 | 97.6 | 96.0 |
| Years between first birth and blood draw, mean (SD) | 17.3 (6.6) | 17.2 (6.7) | 17.3 (6.6) | 17.4 (7.0) |
| Characteristics at Blood Draw: | ||||
| Smoking at blood draw | ||||
| Past or never smoker | 93.1 | 90.0 | 93.5 | 90.1 |
| Current smoker | 6.9 | 10.0 | 6.5 | 9.9 |
| Alcohol consumption in month prior to blood draw | ||||
| No drinks in past month | 32.3 | 40.3 | 31.8 | 36.2 |
| Moderate drinker in past month | 59.2 | 52.1 | 59.4 | 57.5 |
| Heavy drinker in past month | 8.6 | 7.6 | 8.8 | 6.3 |
| Menopausal status at blood draw | ||||
| Pre-menopausal | 82.2 | 78.7 | 82.3 | 81.8 |
| Post-menopausal | 10.5 | 11.4 | 10.7 | 9.7 |
| Unknown | 7.2 | 9.8 | 7.1 | 8.5 |
| Post-menopausal hormone use at blood draw | 16.8 | 22.0 | 16.7 | 17.2 |
| History of infertility at blood draw | 7.7 | 10.0 | 7.2 | 11.5 |
| Parity at blood draw | ||||
| 1 birth | 15.5 | 17.3 | 15.9 | 11.9 |
| ≥2 births | 84.5 | 82.7 | 84.1 | 88.1 |
| Potential Confounders: | ||||
| Pre-pregnancy BMI | ||||
| Underweight (<18.5 kg/m2) | 2.2 | 2.1 | 2.2 | 2.6 |
| Normal weight (18.5-24.9 kg/m2) | 88.5 | 79.4 | 88.5 | 88.4 |
| Overweight (25-29.9 kg/m2) | 7.9 | 14.0 | 7.9 | 8.2 |
| Obese (≥30 kg/m2) | 1.3 | 4.6 | 1.4 | 0.8 |
| Pre-pregnancy Alternative Healthy Eating Index | ||||
| First quintile (unhealthiest) | 21.1 | 23.5 | 21.1 | 20.8 |
| Fifth quintile (healthiest) | 18.1 | 16.0 | 17.8 | 20.7 |
| Strenuous physical activity, age 18-22 years | ||||
| Never | 30.6 | 30.5 | 30.8 | 29.9 |
| 10-12 months per year | 10.3 | 10.1 | 10.1 | 12.2 |
Values are percentages unless otherwise specified and are standardized to the age distribution of the study population. Values of polytomous variables may not sum to 100% due to rounding. Characteristics at blood draw were matching factors in sub-studies included in the analysis
Value is not age adjusted
HDP and CVD Biomarkers
Crude post-pregnancy lipid and HbA1C levels were similar between women with a history of HDP and those with only normotensive pregnancies, while levels of CRP, IL-6, and ICAM-1 were higher among women with HDP (Table 2). For example, median CRP was 1.25 mg/L in women with HDP compared to 0.78 mg/L in women with normotensive pregnancies.
Table 2.
Percent difference in post-pregnancy biomarkers and 95% confidence intervals comparing women with only normotensive pregnancies to women with a history of a hypertensive disorder of pregnancy (HDP)
| Only Normotensive | Ever HDP | |
|---|---|---|
| CRP | n=2,356 | n=258 |
| CRP level, mg/L, median (IQR) | 0.78 (0.36, 1.93) | 1.25 (0.51, 2.58) |
| Model 1 | ref | 40.5 (22.2, 61.6) |
| Model 2 | ref | 34.4 (17.2, 54.1) |
| IL-6 | n=2,241 | n=249 |
| IL-6 level, pg/mL, median (IQR) | 0.89 (0.63, 137) | 1.01 (0.72, 1.72) |
| Model 1 | ref | 15.1 (5.3, 25.8) |
| Model 2 | ref | 11.6 (2.1, 21.9) |
| HbA1C | n=792 | n=93 |
| HbA1C, %, median (IQR) | 5.38 (5.21, 5.53) | 5.38 (5.21, 5.48) |
| Model 1 | ref | −0.1 (−1.0, 0.8) |
| Model 2 | ref | −0.3 (−1.2, 0.6) |
| ICAM-1 | n=1,117 | n=114 |
| ICAM-1, ng/mL, median (IQR) | 231.8 (207.5, 261.1) | 244.7 (213.5, 275.1) |
| Model 1 | ref | 4.3 (−0.4, 9.2) |
| Model 2 | ref | 3.1 (−1.5, 7.9) |
| HDL-C | n=833 | n=98 |
| HDL-C level, mg/dL, median (IQR) | 58.8 (51.0, 69.0) | 55.7 (47.2, 66.3) |
| Model 1 | ref | −3.2 (−8.3, 2.2) |
| Model 2 | ref | −2.3 (−7.3, 3.0) |
| LDL-C | n=833 | n=98 |
| LDL-C level, mg/dL, median (IQR) | 111.5 (92.6, 130.0) | 109.3 (91.4, 128.7) |
| Model 1 | ref | −1.2 (−6.8, 4.8) |
| Model 2 | ref | −2.0 (−7.6, 3.9) |
| Triglycerides | n=1,275 | n=153 |
| Triglyceride level, mg/dL, median (IQR) | 85.3 (65.0, 121.3) | 86.8 (67.4, 132.2) |
| Model 1 | ref | 4.1 (−3.9, 12.7) |
| Model 2 | ref | 0.4 (−7.0, 8.5) |
| Total Cholesterol | n=2,658 | n=282 |
| Total cholesterol, mg/dL, median (IQR) | 190.6 (170.9, 214.2) | 194.9 (172.6, 214.8) |
| Model 1 | ref | 0.7 (−1.3, 2.8) |
| Model 2 | ref | 0.2 (−1.8, 2.3) |
Model 1 is adjusted for the following criteria for sample selection for laboratory analysis: age at blood draw, race, menopausal status at blood draw, smoking at blood draw, alcohol intake at blood draw, post-menopausal hormone use at blood draw, and history of infertility at blood draw.
Model 2 is additionally adjusted for pre-pregnancy BMI, diet, and physical activity, and parity at blood draw.
The null value comparing ever HDP to only normotensive is 0.0 percent difference.
After multivariable adjustment, mean CRP levels were 40.5% (95% CI: 22.2, 61.6) higher among women with HDP than women with normotensive pregnancies (Table 2, Model 1). After further adjustment for parity at blood draw and pre-pregnancy BMI, diet, and physical activity, HDP remained associated with higher levels of CRP following pregnancy (Table 2, Model 2). Similarly, in the fully adjusted model, women with a history of HDP had 11.6% (95% CI: 2.1, 21.9) higher IL-6 levels compared to women with only normotensive pregnancies (Table 2, Model 2). There were no significant associations between HDP and HbA1C, ICAM-1, HDL-C, LDL-C, triglycerides, or total cholesterol (Table 2).
Preterm Delivery and CVD Biomarkers
In contrast to HDP, we found no associations between preterm delivery and any of the CVD biomarkers, adjusting for age, race/ethnicity, sub-study matching factors, pre-pregnancy lifestyle factors, and parity (Table 3).
Table 3.
Percent difference in post-pregnancy biomarkers and 95% confidence intervals among women with no history of HDP, comparing women with only term deliveries to women with a history of preterm deliveries
| Only Term (≥37 weeks) | Ever Preterm (<37 weeks) | Ever Moderate Preterm (≥32 to <37 weeks) | Ever Very Preterm (<32 weeks) | |
|---|---|---|---|---|
| CRP | n=2,096 | n=258 | n=200 | n=58 |
| CRP level, mg/L, median (IQR) | 0.78 (0.35, 1.94) | 0.77 (0.39, 1.63) | 0.76 (0.38, 1.70) | 0.86 (0.40, 1.56) |
| Model 1 | ref | −3.5 (−15.5, 10.2) | −1.5 (−14.8, 13.9) | −10.2 (−32.5, 19.5) |
| Model 2 | ref | −2.9 (−14.9, 10.8) | −0.9 (−14.3, 14.5) | −9.4 (−31.7, 20.3) |
| IL-6 | n=1,986 | n=253 | n=197 | n=56 |
| IL-6 level, pg/mL, median (IQR) | 0.89 (0.63, 1.37) | 0.89 (0.61, 1.39) | 0.87 (0.59, 1.33) | 0.96 (0.76, 1.52) |
| Model 1 | ref | 0.0 (−8.2, 9.0) | −2.9 (−11.6, 6.7) | 11.0 (−7.8, 33.6) |
| Model 2 | ref | 0.3 (−8.1, 9.3) | −2.6 (−11.5, 7.2) | 11.1 (−7.5, 33.4) |
| HbA1C | n=706 | n=84 | n=65 | n=19 |
| HbA1C, %, median (IQR) | 5.38 (5.21, 5.53) | 5.41 (5.24, 5.53) | 5.42 (5.21, 5.53) | 5.38 (5.26, 5.53) |
| Model 1 | ref | 0.2 (−0.8, 1.2) | 0.2 (−0.9, 1.4) | 0.2 (−1.2, 1.6) |
| Model 2 | ref | 0.2 (−0.8, 1.1) | 0.2 (−1.0, 1.3) | 0.1 (−1.3, 1.5) |
| ICAM-1 | n=990 | n=126 | n=93 | n=33 |
| ICAM-1, ng/mL, median (IQR) | 231.9 (208.3, 260.1) | 231.2 (203.6, 265.5) | 225.8 (201.3, 262.9) | 246.1 (220.7, 278.6) |
| Model 1 | ref | 1.7 (−2.2, 5.7) | 0.3 (−4.0, 4.8) | 5.5 (−2.2, 13.8) |
| Model 2 | ref | 1.6 (−2.3, 5.6) | 0.6 (−3.8, 5.1) | 4.4 (−3.2, 12.6) |
| HDL-C | n=743 | n=90 | n=70 | n=20 |
| HDL-C level, mg/dL, median (IQR) | 58.7 (50.6, 68.7) | 59.5 (51.7, 70.8) | 59.7 (51.4, 71.0) | 57.1 (52.1, 70.5) |
| Model 1 | ref | 2.5 (−2.9, 8.1) | 1.8 (−4.3, 8.2) | 4.9 (−5.0, 15.7) |
| Model 2 | ref | 2.7 (−2.7, 8.4) | 2.1 (−4.0, 8.5) | 5.1 (−5.1, 16.3) |
| LDL-C | n=743 | n=90 | n=70 | n=20 |
| LDL-C level, mg/dL, median (IQR) | 111.3 (92.0, 130.0) | 112.8 (96.3, 131.2) | 111.3 (94.6, 126.1) | 117.3 (106.9, 135.4) |
| Model 1 | ref | 2.9 (−2.2, 8.3) | 2.1 (−3.7, 8.3) | 5.9 (−2.9, 15.5) |
| Model 2 | ref | 2.5 (−2.6, 7.8) | 1.7 (−4.0, 7.9) | 5.1 (−3.5, 14.4) |
| Triglycerides | n=1,133 | n=141 | n=108 | n=33 |
| Triglycerides, mg/dL, median (IQR) | 85.3 (65.0, 122.1) | 85.0 (61.6, 117.3) | 83.8 (61.3, 114.8) | 100.7 (70.2, 134.7) |
| Model 1 | ref | −4.4 (−12.0, 3.8) | −6.7 (−15.2, 2.7) | 3.6 (−10.1, 19.4) |
| Model 2 | ref | −4.2 (−11.7, 3.9) | −6.3 (−14.8, 3.0) | 3.1 (−10.3, 18.5) |
| Total Cholesterol | n=2,360 | n=292 | n=218 | n=74 |
| Total Cholesterol, mg/dL, median (IQR) | 190.2 (170.2, 214.2) | 193.0 (175.8, 213.8) | 192.3 (173.5, 213.6) | 199.4 (182.9, 217.7) |
| Model 1 | ref | 0.8 (−1.1, 2.7) | 0.2 (−2.0, 2.4) | 2.5 (−1.1, 6.2) |
| Model 2 | ref | 0.7 (−1.2, 2.6) | 0.2 (−2.0, 2.4) | 2.1 (−1.4, 5.8) |
Model 1 is adjusted for the following criteria for sample selection for laboratory analysis: age at blood draw, race, menopausal status at blood draw, smoking at blood draw, alcohol intake at blood draw, post-menopausal hormone use at blood draw, and history of infertility at blood draw.
Model 2 is additionally adjusted for pre-pregnancy BMI, diet, and physical activity, and parity at blood draw.
The null value comparing ever preterm groups to only term is 0.0 percent difference.
Sensitivity Analyses
Supplemental Table 2 shows age-standardized characteristics of controls separately by history of HDP and normotensive preterm delivery. The distributions of these characteristics are similar to those in Table 1, which includes controls and non-CVD related cases. Additionally, results from multivariable-adjusted models in only controls were similar to our primary analyses (Supplemental Tables 3 and 4).
Further adjustment for history of miscarriage and gestational diabetes mellitus before blood draw in primary analyses did not change the results. We additionally investigated associations between HDP and preterm delivery and each CVD biomarker among women who had their blood drawn before age 45, since NHSII data suggest stronger relative risks of chronic hypertension, type 2 diabetes, and dyslipidemia closer to the complicated pregnancy [31,32]. In this analysis, sample size was reduced by 47% and mean time from first birth to blood draw was 13.2 years (SD=4.7, range: 1-26). Results were similar to the primary analysis for HDP. However, we found slightly higher HDL-C (9.8%, 95% CI: 1.9, 18.3), LDL-C (8.3%, 95% CI: 1.5, 15.6), and total cholesterol (3.3%, 95% CI: 0.5, 6.1) levels in women with a history of preterm delivery compared to women with term deliveries.
DISCUSSION
We found that CRP and IL-6 levels measured 17 years after first birth, on average, were elevated among women with a history of HDP compared to women with normotensive pregnancies. Other CVD biomarkers did not vary by history of HDP or preterm delivery.
HDP and CVD Biomarkers
The literature on HDP and post-pregnancy CVD biomarker profiles is inconsistent [25]. Although most studies report higher IL-6 levels after HDP, as we observed [22,42–44], studies of CRP levels after HDP are less consistent. Four studies found higher CRP levels after HDP [28,42,45,46], while another 4 found no difference [22,47–49]. Two null studies had sample sizes under 80 women, while the remaining null studies evaluated CRP closer to pregnancy – at 1 and 10 years postpartum [22,48,49]. In contrast, the Avon Longitudinal Study of Parents and Children (ALSPAC), the most comparable to ours with respect to when the biomarkers were measured post-pregnancy (mean=18 years) and sample size (n=3,187), suggests higher CRP levels among women with a history of HDP [28]. Our results, with those from ALSPAC, provide support that CRP levels remain elevated years after a pregnancy complicated by HDP.
Previous research is inconsistent on HbA1C and ICAM-1 levels following a pregnancy complicated by HDP. For HbA1C, some studies suggest higher levels in women with a history of HDP, while others found no differences [43,45,49,50]. Similarly, for ICAM-1, studies suggest either higher levels in women with a history of HDP or no difference [22,43,50,51]. Our results are consistent with studies reporting no association with HDP and HbA1C or ICAM-1 after pregnancy. However, HDP does predict type 2 diabetes in the NHSII [31], indicating that our single measurement of HbA1C may not adequately capture the window of increased risk for type 2 diabetes.
Studies with LDL-C and total cholesterol measured closer to pregnancy (mean <8 years) found higher LDL-C and total cholesterol levels in women with a history of HDP [42,45,48], while most studies with blood measurements taken further after pregnancy (mean >15 years) showed no differences [28,46,49,50,52]. The latter is analogous to our study where blood draw occurred at a median of 17 years after pregnancy and revealed no differences in LDL-C or total cholesterol levels between women with HDP and those with normotensive pregnancies. Our sensitivity analysis restricted to women with blood drawn before age 45, with a mean follow-up of 13.2 years, also found no differences in lipid levels. This suggests that an adverse lipid profile may be present in the short term following pregnancies complicated by HDP, but does not persist throughout adulthood, consistent with NHSII data showing higher relative risks of clinical CVD risk factor development in the years soon after HDP, which attenuate as risk factors increase with age in the general population [31].
Preterm Delivery and CVD Biomarkers
The literature regarding preterm delivery and CVD biomarkers is more limited. We found no difference in CRP levels in women with a history of preterm delivery (but no history of HDP), consistent with previous publications [28,30,53]. Another study found higher CRP levels among women with indicated preterm deliveries, but no difference between women with spontaneous preterm deliveries and those with term deliveries [29]. Because HDP is one of the primary indications for preterm delivery [33], their association may reflect the presence of HDP in women with indicated preterm delivery. We did not have sufficient data to evaluate spontaneous and indicated preterm delivery, but, because we evaluated only normotensive preterm deliveries, many, if not most, preterm deliveries in our women were likely spontaneous.
Intrauterine infection, which yields an inflammatory response in pregnant women, underlies approximately 30% of preterm deliveries [54,55]. Elevated CRP levels in early pregnancy are associated with spontaneous preterm delivery [19,56,57], and both preterm delivery and CRP have been shown to be associated with future CVD in women [6–14,37]. Further investigation of the changes in CRP levels after spontaneous and indicated preterm deliveries is warranted.
Consistent with the literature, we also found no association between preterm delivery and IL-6 levels [30,53]. To our knowledge, this study is the first to evaluate the associations between preterm delivery and HbA1C and ICAM-1 levels following pregnancy. Consequently, our null findings merit replication.
Results from previous studies generally vary with respect to duration of time between preterm delivery and evaluation of lipids. Studies which measure lipids more proximal to pregnancy (≤12 years) report an adverse lipid profile in women who delivered an infant preterm, while a study measuring lipids further from pregnancy (mean=18 years) found no association with LDL-C, triglycerides, or total cholesterol [26,27,28,53]. Our primary results, which are based on a median of 17 years of follow-up, are consistent with no association. Taken together, this literature suggests that an adverse lipid profile may be present shortly after a preterm pregnancy, but does not persist in the longer term. This is similar to NHSII data, which show that the relative risk of self-reported hypercholesterolemia is highest in the first 10 years after preterm delivery and weakens over time [32].
The primary limitation of our study is that, to be included in this analysis, women had to provide a blood sample and be selected previously into a relevant sub-study. However, adjustment for the sub-study selection criteria yielded similar results to models adjusted only for age, suggesting limited bias due to case-control selection. Additionally, women included in this analysis were similar to all parous NHSII women who provided a blood sample (data not shown). Our study population is primarily white, impacting generalizability to other races, as the rates of HDP and preterm delivery are higher among African-Americans and may result from a different mix of causes [54,58]. This study may also suffer from exposure misclassification; however, a validation study completed in the NHSII suggests the participants recalled their pregnancy histories with good accuracy. Furthermore, in this cohort, self-reported history of both preterm delivery and HDP predicts CVD events with similar hazard ratios to those from studies based on pregnancy exposures documented in vital statistics registries [14,59].
As with all observational studies, this investigation is susceptible to unmeasured confounding. However, we adjusted for multiple pre-pregnancy lifestyle factors, including BMI, diet, and physical activity as well as matching factors. Only 78% of the blood samples were collected after fasting; however, we expect this to be random regarding our exposures. For highly variable biomarkers, a single blood measurement may not adequately represent that biomarker over time. Lastly, it is possible that women who developed HDP had elevated CRP levels before pregnancy [60, 61] and that the higher levels of CRP continue to persist after pregnancy. We were unable to evaluate this in our cohort as we have only 27 women with CRP measured before first pregnancy. However, we did adjust for pre-pregnancy diet and lifestyle factors associated with CRP levels [34,62–65]. Regardless, HDP could be useful as an early marker of chronic inflammation in these women. Future studies would benefit from serial blood sample collections before and after pregnancy to characterize biomarker trajectories over time.
The main strengths are our large sample sizes and long follow-up after pregnancy. Our sample sizes ranged from 885 to 2,940 women. In contrast, most published studies on these pregnancy complications and CVD biomarkers had sample sizes less than 800, and just over half contained fewer than 200 women. While our blood samples were collected at a median of 17 years, but up to 34 years after pregnancy, approximately half of previous studies had mean follow-up of less than 10 years. Furthermore, we investigated a wide range of established CVD biomarkers, providing insight into the specific mechanisms that link pregnancy complications and CVD.
CONCLUSIONS
Nearly two decades after first birth, on average, CRP and IL-6 levels were elevated among women with a history of HDP compared to women with normotensive pregnancies. Altered CVD biomarker profiles were otherwise not present among women with HDP or preterm delivery. Previous findings consistently identify HDP and CRP as predictors of maternal CVD [15,16,66,67]. Our findings suggest the possibility that women with a history of HDP have higher levels of inflammation in the years after pregnancy, which may ultimately contribute to their elevated risk of CVD.
Supplementary Material
Acknowledgements
We thank the participants of the Nurses’ Health Study II blood cohort for providing valuable blood samples and information. We also thank Eileen L. Hibert for her programming assistance.
Sources of Funding:
This work was supported by grants from the National Institutes of Health (UM1 CA176726, R01 CA67262, F31 HL131222, T32 HL098048, K01 HL130650); and the American Heart Association (AHA 13GRNT17070022).
Footnotes
Publisher's Disclaimer: Disclaimer: This is the prepublication, author-produced version of a manuscript accepted for publication in Pregnancy Hypertension. This version does not include post-acceptance editing and formatting. The publisher of Pregnancy Hypertension is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to https://www.journals.elsevier.com/pregnancy-hypertension or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.
Conflicts of Interest: The authors report no conflicts of interest
REFERENCES
- [1].Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135(10):e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the American Heart Association. Journal of the American College of Cardiology. 2011;57(12):1404–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sattar N, Greer IA. Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening? BMJ. 2002;325(7356):157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rich-Edwards JW. Reproductive health as a sentinel of chronic disease in women. Women’s Health. 2009;5(2):101–105. [DOI] [PubMed] [Google Scholar]
- [5].Rich-Edwards JW, McElrath TF, Karumanchi SA, Seely EW. Breathing life into the lifecourse approach: pregnancy history and cardiovascular disease in women. Hypertension. 2010;56(3):331–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Smith GCS, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129,290 births. The Lancet. 2001;357(9273):2002–2006. [DOI] [PubMed] [Google Scholar]
- [7].Pell JP, Smith GC, Walsh D. Pregnancy complications and subsequent maternal cerebrovascular events: a retrospective cohort study of 119,668 births. American Journal of Epidemiology. 2004;159(4):336–342. [DOI] [PubMed] [Google Scholar]
- [8].Catov JM, Wu CS, Olsen J, Sutton-Tyrrell K, Li J, Nohr EA. Early or recurrent preterm birth and maternal cardiovascular disease risk. Annals of Epidemiology. 2010;20(8):604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bonamy AK, Parikh NI, Cnattingius S, Ludvigsson JF, Ingelsson E. Birth characteristics and subsequent risks of maternal cardiovascular disease: effects of gestational age and fetal growth. Circulation. 2011;124(25):2839–2846. [DOI] [PubMed] [Google Scholar]
- [10].Hastie CE, Smith GC, Mackay DF, Pell JP. Maternal risk of ischaemic heart disease following elective and spontaneous pre-term delivery: retrospective cohort study of 750 350 singleton pregnancies. International Journal of Epidemiology. 2011;40(4):914–919. [DOI] [PubMed] [Google Scholar]
- [11].Kessous R, Shoham-Vardi I, Pariente G, Holcberg G, Sheiner E. An association between preterm delivery and long-term maternal cardiovascular morbidity. American Journal of Obstetrics and Gynecology. 2013;209(4):368.e361–368. [DOI] [PubMed] [Google Scholar]
- [12].Rich-Edwards JW, Klungsoyr K, Wilcox AJ, Skjaerven R. Duration of pregnancy, even at term, predicts long-term risk of coronary heart disease and stroke mortality in women: a population-based study. American Journal of Obstetrics and Gynecology. 2015;213(4):518.e511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lykke JA, Paidas MJ, Damm P, Triche EW, Kuczynski E, Langhoff-Roos J. Preterm delivery and risk of subsequent cardiovascular morbidity and type-II diabetes in the mother. BJOG. 2010;117(3):274–281. [DOI] [PubMed] [Google Scholar]
- [14].Tanz LJ, Stuart JJ, Williams PL, et al. Preterm Delivery and Maternal Cardiovascular Disease in Young and Middle-Aged Adult Women. Circulation. 2017;135(6):578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335(7627):974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. European Journal of Epidemiology. 2013;28(1):1–19. [DOI] [PubMed] [Google Scholar]
- [17].James-Todd TM, Karumanchi SA, Hibert EL, et al. Gestational age, infant birth weight, and subsequent risk of type 2 diabetes in mothers: Nurses’ Health Study II. Preventing Chronic Disease. 2013;10:E156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Catov JM, Bodnar LM, Kip KE, et al. Early pregnancy lipid concentrations and spontaneous preterm birth. American Journal of Obstetrics and Gynecology. 2007;197(6):610.e611–617. [DOI] [PubMed] [Google Scholar]
- [19].Catov JM, Bodnar LM, Ness RB, Barron SJ, Roberts JM. Inflammation and dyslipidemia related to risk of spontaneous preterm birth. American Journal of Epidemiology. 2007;166(11):1312–1319. [DOI] [PubMed] [Google Scholar]
- [20].Charlton F, Tooher J, Rye KA, Hennessy A. Cardiovascular risk, lipids and pregnancy: preeclampsia and the risk of later life cardiovascular disease. Heart, Lung & Circulation. 2014;23(3):203–212. [DOI] [PubMed] [Google Scholar]
- [21].Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. American Journal of Reproductive Immunology. 1998;40(2):102–111. [DOI] [PubMed] [Google Scholar]
- [22].Freeman DJ, McManus F, Brown EA, et al. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension. 2004;44(5):708–714. [DOI] [PubMed] [Google Scholar]
- [23].Greer IA, Lyall F, Perera T, Boswell F, Macara LM. Increased concentrations of cytokines interleukin-6 and interleukin-1 receptor antagonist in plasma of women with preeclampsia: a mechanism for endothelial dysfunction? Obstetrics and Gynecology. 1994;84(6):937–940. [PubMed] [Google Scholar]
- [24].Vince GS, Starkey PM, Austgulen R, Kwiatkowski D, Redman CW. Interleukin-6, tumour necrosis factor and soluble tumour necrosis factor receptors in women with pre-eclampsia. British Journal of Obstetrics and Gynaecology. 1995;102(1):20–25. [DOI] [PubMed] [Google Scholar]
- [25].Zoet GA, Koster MP, Velthuis BK, et al. Determinants of future cardiovascular health in women with a history of preeclampsia. Maturitas. 2015;82(2):153–161. [DOI] [PubMed] [Google Scholar]
- [26].Catov JM, Dodge R, Yamal JM, Roberts JM, Piller LB, Ness RB. Prior preterm or small-for-gestational-age birth related to maternal metabolic syndrome. Obstetrics and Gynecology. 2011;117(2 Pt 1):225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Catov JM, Dodge R, Barinas-Mitchell E, et al. Prior preterm birth and maternal subclinical cardiovascular disease 4 to 12 years after pregnancy. Journal of Women’s Health. 2013;22(10):835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fraser A, Nelson SM, Macdonald-Wallis C, et al. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the Avon Longitudinal Study of Parents and Children. Circulation. 2012;125(11):1367–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hastie CE, Smith GC, Mackay DF, Pell JP. Association between preterm delivery and subsequent C-reactive protein: a retrospective cohort study. American Journal of Obstetrics and Gynecology. 2011;205(6):556.e551–554. [DOI] [PubMed] [Google Scholar]
- [30].Catov JM, Lewis CE, Lee M, Wellons MF, Gunderson EP. Preterm birth and future maternal blood pressure, inflammation, and intimal-medial thickness: the CARDIA study. Hypertension. 2013;61(3):641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Stuart J, James-Todd T, Missmer S, Rimm E, Spiegelman D, Hibert EL, Rich-Edwards J. Hypertensive Disorders in Pregnancy and Cardiovascular Risk Factor Development [abstract]. In: Abstracts of the 47th Annual Meeting of the Society for Epidemiologic Research; June 24-27, 2014; Seattle, WA Abstract nr 496-S. Online URL: http://epiresearch.org/wp-content/uploads/2014/08/abstract-book-printed.pdf. [Google Scholar]
- [32].Tanz L, Stuart JJ, Rimm EB, Williams PL, Missmer SA, James-Todd TM, Rich-Edwards JW. Preterm Delivery and Maternal Cardiovascular Disease Risk Factors [abstract]. In: Society for Epidemiologic Research 50th Annual Meeting Abstract Book June 20–23, 2017; Seattle, WA: Abstract nr 0492. Online URL: https://epiresearch.org/annual-meeting/archives-2/50th-anniversary/. [Google Scholar]
- [33].Sibai BM. Preeclampsia as a cause of preterm and late preterm (near-term) births. Seminars in Perinatology. 2006;30(1):16–19. [DOI] [PubMed] [Google Scholar]
- [34].Pai JK, Hankinson SE, Thadhani R, Rifai N, Pischon T, Rimm EB. Moderate alcohol consumption and lower levels of inflammatory markers in US men and women. Atherosclerosis. 2006;186(1):113–120. [DOI] [PubMed] [Google Scholar]
- [35].Poole EM, Lee IM, Ridker PM, Buring JE, Hankinson SE, Tworoger SS. A prospective study of circulating C-reactive protein, interleukin-6, and tumor necrosis factor alpha receptor 2 levels and risk of ovarian cancer. American Journal of Epidemiology. 2013;178(8):1256–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473(7347):317–325. [DOI] [PubMed] [Google Scholar]
- [37].Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. The New England Journal of Medicine. 2000;342(12):836–843. [DOI] [PubMed] [Google Scholar]
- [38].Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. The New England Journal of Medicine. 2010;362(9):800–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Pai JK, Curhan GC, Cannuscio CC, Rifai N, Ridker PM, Rimm EB. Stability of novel plasma markers associated with cardiovascular disease: processing within 36 hours of specimen collection. Clinical chemistry. 2002;48(10):1781–1784. [PubMed] [Google Scholar]
- [40].Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. The Journal of Nutrition. 2012;142(6):1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rosner B, Cook N, Portman R, Daniels S, Falkner B. Determination of blood pressure percentiles in normal-weight children: some methodological issues. American Journal of Epidemiology. 2008;167(6):653–666. [DOI] [PubMed] [Google Scholar]
- [42].Girouard J, Giguere Y, Moutquin JM, Forest JC. Previous hypertensive disease of pregnancy is associated with alterations of markers of insulin resistance. Hypertension. 2007;49(5):1056–1062. [DOI] [PubMed] [Google Scholar]
- [43].Visser S, Hermes W, Ket JC, et al. Systematic review and metaanalysis on nonclassic cardiovascular biomarkers after hypertensive pregnancy disorders. American Journal of Obstetrics and Gynecology. 2014;211(4):373.e371–379. [DOI] [PubMed] [Google Scholar]
- [44].Vitoratos N, Economou E, Iavazzo C, Panoulis K, Creatsas G. Maternal serum levels of TNF-alpha and IL-6 long after delivery in preeclamptic and normotensive pregnant women. Mediators of Inflammation. 2010;2010:908649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hermes W, Franx A, van Pampus MG, et al. Cardiovascular risk factors in women who had hypertensive disorders late in pregnancy: a cohort study. American Journal of Obstetrics and Gynecology. 2013;208(6):474.e471–478. [DOI] [PubMed] [Google Scholar]
- [46].Hubel CA, Powers RW, Snaedal S, et al. C-reactive protein is elevated 30 years after eclamptic pregnancy. Hypertension. 2008;51(6):1499–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Drost JT, Arpaci G, Ottervanger JP, et al. Cardiovascular risk factors in women 10 years post early preeclampsia: the Preeclampsia Risk EValuation in FEMales study (PREVFEM). European Journal of Preventive Cardiology. 2012;19(5):1138–1144. [DOI] [PubMed] [Google Scholar]
- [48].Smith GN, Walker MC, Liu A, et al. A history of preeclampsia identifies women who have underlying cardiovascular risk factors. American Journal of Obstetrics and Gynecology. 2009;200(1):58.e51–58. [DOI] [PubMed] [Google Scholar]
- [49].Spaan JJ, Houben AJ, Musella A, Ekhart T, Spaanderman ME, Peeters LL. Insulin resistance relates to microvascular reactivity 23 years after preeclampsia. Microvascular Research. 2010;80(3):417–421. [DOI] [PubMed] [Google Scholar]
- [50].Sattar N, Ramsay J, Crawford L, Cheyne H, Greer IA. Classic and novel risk factor parameters in women with a history of preeclampsia. Hypertension. 2003;42(1):39–42. [DOI] [PubMed] [Google Scholar]
- [51].Drost JT, Maas AH, Holewijn S, et al. Novel cardiovascular biomarkers in women with a history of early preeclampsia. Atherosclerosis. 2014;237(1):117–122. [DOI] [PubMed] [Google Scholar]
- [52].Magnussen EB, Vatten LJ, Smith GD, Romundstad PR. Hypertensive disorders in pregnancy and subsequently measured cardiovascular risk factors. Obstetrics and Gynecology. 2009;114(5):961–970. [DOI] [PubMed] [Google Scholar]
- [53].Perng W, Stuart J, Rifas-Shiman SL, Rich-Edwards JW, Stuebe A, Oken E. Preterm birth and long-term maternal cardiovascular health. Annals of Epidemiology. 2015;25(1):40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG. 2006;113 Suppl 3:17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Lohsoonthorn V, Qiu C, Williams MA. Maternal serum C-reactive protein concentrations in early pregnancy and subsequent risk of preterm delivery. Clinical Biochemistry. 2007;40(5–6):330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Moghaddam Banaem L, Mohamadi B, Asghari Jaafarabadi M, Aliyan Moghadam N. Maternal serum C-reactive protein in early pregnancy and occurrence of preterm premature rupture of membranes and preterm birth. The Journal of Obstetrics and Gynaecology. 2012;38(5):780–786. [DOI] [PubMed] [Google Scholar]
- [58].Breathett K, Muhlestein D, Foraker R, Gulati M. Differences in preeclampsia rates between African American and Caucasian women: trends from the National Hospital Discharge Survey. Journal of Women’s Health. 2014;23(11):886–893. [DOI] [PubMed] [Google Scholar]
- [59].Stuart JJTL, Rimm EB, Spiegelman D, Missmer SA, Rexrode KM, Mukamal KJ, Rich-Edwards JW. Hypertensive Disorders in Pregnancy and Maternal Cardiovascular Disease: Mediation by Pospartum Cardiovascular Risk Factors. Society for Epidemiologic Research; June 20-23, 2017, 2017; Seattle, WA. [Google Scholar]
- [60].Srinivas SK, Sammel MD, Bastek J, et al. Evaluating the association between all components of the metabolic syndrome and pre-eclampsia. The Journal of Maternal-Fetal & Neonatal Medicine. 2009;22(6):501–509. [DOI] [PubMed] [Google Scholar]
- [61].van Rijn BB, Veerbeek JH, Scholtens LC, et al. C-reactive protein and fibrinogen levels as determinants of recurrent preeclampsia: a prospective cohort study. Journal of Hypertension. 2014;32(2):408–414. [DOI] [PubMed] [Google Scholar]
- [62].Basu A, Devaraj S, Jialal I. Dietary factors that promote or retard inflammation. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26(5):995–1001. [DOI] [PubMed] [Google Scholar]
- [63].Bazzano LA, He J, Muntner P, Vupputuri S, Whelton PK. Relationship between cigarette smoking and novel risk factors for cardiovascular disease in the United States. Annals of Internal Medicine. 2003;138(11):891–897. [DOI] [PubMed] [Google Scholar]
- [64].Dietrich M, Jialal I. The effect of weight loss on a stable biomarker of inflammation, C-reactive protein. Nutrition Reviews. 2005;63(1):22–28. [DOI] [PubMed] [Google Scholar]
- [65].Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. Journal of the American College of Cardiology. 2005;45(10):1563–1569. [DOI] [PubMed] [Google Scholar]
- [66].McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. American Heart Journal. 2008;156(5):918–930. [DOI] [PubMed] [Google Scholar]
- [67].Wu P, Haththotuwa R, Kwok CS, et al. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circulation Cardiovascular Quality and Outcomes. 2017;10(2). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


