Abstract
BACKGROUND
Telomeres cap and protect DNA but shorten with each somatic cell division. Aging, environmental, and lifestyle factors contribute to the speed of telomere attrition. Current evidence suggests a link between relative telomere length (RTL) and depression, but directionality of the relationship remains unclear. We prospectively examined associations between RTL and subsequent depressive symptom trajectories.
METHODS
Among 8,801 women of the Nurses’ Health Study depressive symptoms were measured every four years from 1992–2012; group-based trajectories of symptoms were identified using latent class growth-curve analysis. Multinomial logistic models were used to relate mid-life RTLs to the probabilities of group assignment to subsequent depressive symptom trajectories.
RESULTS
We identified four depressive symptom trajectory groups: minimal depressive symptoms (62%), worsening depressive symptoms (14%), improving depressive symptoms (19%), and persistent severe depressive symptoms (5%). Longer mid-life RTLs were related to significantly lower odds of being in the worsening symptoms trajectory versus minimal trajectory but not to other trajectories. Compared to being in the minimal symptoms group, the multivariable-adjusted odds ratio of being in the worsening depressive symptoms group was 0.78 (95% CI (confidence interval): 0.62–0.97, p=0.02), for every standard deviation increase in baseline RTL.
CONCLUSIONS
In this large prospective study of generally healthy women, longer telomeres at mid-life were associated with significantly lower risk of a subsequent trajectory of worsening mood symptoms over 20 years. The results raise the possibility of telomere shortening as a novel contributing factor to late-life depression.
Keywords: Telomeres, late-life, depression, depressive symptoms, trajectories
INTRODUCTION
Telomere length (TL) is thought to be a marker of biological aging. Telomere attrition occurs with each somatic cell division and may be accelerated by oxidative stress and inflammation, both often elevated among individuals with depression.(Black, Bot, Scheffer, Cuijpers, & Penninx, 2015; Correia-Melo, Hewitt, & Passos, 2014; Setiawan et al., 2015) Prior studies have noted that persons with a history of depression have shorter TL than those without such history(Ridout, Ridout, Price, Sen, & Tyrka, 2016; Schutte & Malouff, 2015; Solomon et al., 2017; Starnino, Busque, Tardif, & D’Antono, 2016). Once telomeres reach critical length, apoptosis, mitochondrial damage and genomic-instability may occur.(Blackburn, 2005; Sahin et al., 2011)
While oxidative stress may result in telomere attrition and dysfunction, emerging data suggest that telomere shortening could itself trigger cascades of cell dysfunction and death.(Hovatta, 2015; Sahin & Depinho, 2010; Verhoeven, Revesz, Wolkowitz, & Penninx, 2014) Specifically, telomere shortening and dysfunction may further increase production of reactive oxygen species (ROS) – contributing to mitochondrial and metabolic dysfunction.(Sahin & Depinho, 2010) High energy-demand organs such as the heart and brain are particularly sensitive to damage from ROS(Magistretti & Allaman, 2015). Thus, associations between depression and TL may be bi-directional: depression may adversely affect telomeres, and telomere dysfunction may predispose to future depressive symptomatology – particularly in later-life when accumulated damage from ROS may be most prominent. As brain regions (e.g., hippocampus), central to mood health are damaged over time by ROS insults, depressive symptoms may emerge or worsen.(MacQueen et al., 2003)
Yet, associations of TL with future depressive symptoms have been inadequately explored. Therefore, we examined how mid-life TL related to subsequent 20-year trajectories of mood symptoms among 8,801 women in the Nurses’ Health Study (NHS).
METHODS AND MATERIALS
Study Sample
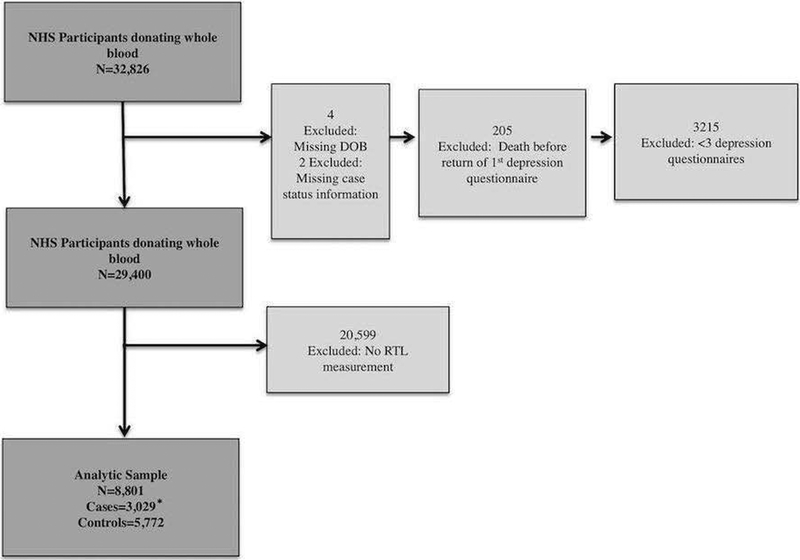
The NHS enrolled 121,700 female nurses, aged 30–55 years, in 1976 from 11 U.S. states. Participants have since completed biannual mailed questionnaires. Study retention has remained at ~90%. In 1989–1990, a subset of 32,826 NHS participants supplied blood samples; the sample for analysis was derived from these blood sub-cohort participants.(S. E. Hankinson et al., 1995) Out of n=32,826, 4 were excluded for missing date-of-birth, 2 for missing disease case status information (later used to select participants for telomere assays); 205 died before returning any depression questionnaire data (depressive symptoms were assessed using validated surveys beginning in 1992). An additional 3,215 were excluded for having <3 depression measures over follow-up. Those excluded for insufficient depression measures had: older age; higher depression scores; lower physical activity; higher medical co-morbidity; heavier smoking.
A total of 29,400 women remained after the above exclusions. Additional exclusions were then made, as our analytic sample consisted of NHS blood sub-cohort participants with relative TL (RTL) data. RTL was measured in over a dozen NHS nested case-control studies of different diseases.(De Vivo et al., 2009; Devore, Prescott, De Vivo, & Grodstein, 2011; Han et al., 2009; Page et al., 2008; Prescott, McGrath, Lee, Buring, & De Vivo, 2010) After exclusion of 20,599 women not included in these prior case-control studies and thus lacking RTL measures, the final sample included 8,801 (Figure 1). There were 3,029 cases (ie, from nested case-control studies) and 5,772 controls (matching healthy participants in the case-control studies).
Figure 1:
Study Population Exclusions
*Cases and controls from prior Nurses’ Health Study nested case-control studies of various diseases that measured RTL (i.e. not depression cases)
The study was approved by the institutional review board of Brigham and Women’s Hospital.
Depressive symptoms
Depressive symptoms were assessed on NHS questionnaires at four-year intervals from 1992–2012. During this period, three different measures were utilized: the five-item Mental Health Inventory (MHI-5) from the Medical Outcomes Study Short Form-36 (1992, 1996, 2000)(Ware & Sherbourne, 1992); the 10-item Center for Epidemiologic Studies Depression Scale (CES-D-10) (2004); and the 15-item Geriatric Depression Scale (GDS-15) (2008, 2012). To model depressive symptom trajectories, we first harmonized and placed these three different measures on the same numerical scale, using an equipercentile equating method, described in detail elsewhere.(S.-C. Chang et al., 2016) The harmonized scale expressed the CES-D-10 and GDS-15 on the MHI-5 scale; original (1992/1996/2000) and estimated (2004/2008/2012) MHI-5 scores were then used to model trajectories. Prior work indicates excellent performance for identifying major depression and high concordance between estimated (using the equating method) and actual scores within a validation subset.(S.-C. Chang et al., 2016)
Relative telomere length
RTL was obtained as the ratio of telomere repeat copy number to single gene copy number (T/S) and determined by real-time quantitative PCR assay. Assays were conducted with genomic DNA extracted from peripheral blood leukocytes from the 1989–1990 blood samples. Details regarding these procedures have been described elsewhere.(Crous-Bou et al., 2014; De Vivo et al., 2009; Han et al., 2009; S E Hankinson et al., 1995) Because the RTL measures were pooled across various case-control studies, we adjusted for batch variation within the T/S ratio estimates using methods described by Rosner et al.(Rosner, Cook, Portman, Daniels, & Falkner, 2008) We derived z-scores of log-transformed batch-adjusted T/S ratios; this was the RTL exposure of interest. The average coefficient of variation for the exponentiated T/S ratio was 9.6%.
Statistical Analysis
Our statistical analyses proceeded in two stages of group-based trajectory modeling, as implemented using the TRAJ procedure (Proc Traj) for SAS 9.3.(Jones, Nagin, & Roeder, 2001) First, we estimated the number of latent groups; then we explored the optimal polynomial shape for each group-based trajectory using standard approaches.(Byers, Vittinghoff, Lui, & et al., 2012; S.-C. Chang et al., 2016; Jones et al., 2001) We considered models of 2–7 groups; scaled age ([age at measurement – age at baseline]/ 10) was the time variable. We used scaled aged rather than raw age, as other work indicates that scaling may yield better approximation of group trajectories.(S.-C. Chang et al., 2016; Jones et al., 2001) We compared performance of both forms of age, and confirmed that scaled-age better approximated actual group trajectories. To select the number of groups, we used standard practices of examining the Bayesian Information Criteria (BIC), average posterior probability of group membership, parsimony, and clinical relevance of the groups. After selecting the number of groups, optimal shape of each group’s mean trajectory was examined iteratively. For each group, we considered polynomial shapes of linear through quintic. All eligible participants donating blood (n=29,400) were included in the sample for the first stage.
In the second stage, we examined the association between baseline RTL and depressive symptom trajectories in the final analytic sample of n=8,801. We modeled odds of group-based trajectory membership associated with each standard deviation (SD) increase in RTL using multinomial logit models implemented in Proc Traj.(Jones et al., 2001) Basic models were unadjusted for covariates, but age was controlled for as it served as the method for relating depression symptoms to time. Multivariable-adjusted models included: smoking (pack-years); body mass index (BMI) (kg/m2); alcohol consumption (grams/day); physical activity (MET [Metabolic Equivalent] hours/week); Charlson co-morbidity index(Charlson, Pompei, Ales, & MacKenzie, 1987); paternal age-at-birth (years), antidepressant use (yes/no); menopausal/hormone therapy status (pre-menopausal, post-menopausal and currently on hormones, post-menopausal and not currently on hormones); case/control status. Covariates were selected based on prior literature regarding likely confounders of TL and depression. Covariates were ascertained from questionnaires returned just prior to or at time-of-blood draw.
Secondary Analyses
Secondarily, we examined RTL and depression incidence using Cox proportional hazards models. Individuals were classified as having incident depression based on first occurrence of: (1) symptom score beyond validated cut-off for major depressive disorder (MDD) (ie, MHI-5 ≤52; CES-D-10 ≥10; GDS-15 ≥6), (2) self-reported regular antidepressant use, or (3) self-reported physician/clinician-diagnosed depression. We also examined models where depression cases were classified by symptom scores only. Person-time was contributed from return of the baseline questionnaire until occurrence of depression, death, loss to follow-up, or end of follow-up period, whichever came first.
Finally, we explored how 10-year change in RTL was prospectively associated with incident depression between 2000 and 2012. This analysis included a limited sub-set (n=1039) of women who were part of a second NHS blood collection in 2000/2001, had available RTL data at both blood collections and were alive as of the 2000 questionnaire cycle, as described elsewhere.(Shun‐Chiao et al., 2018)
RESULTS
Participant Characteristics
Table 1 displays baseline characteristics of the analytic sample (n=8,801) by RTL z-score quartile. Those in the lowest quartile were older and had higher pack-years of smoking; other characteristics did not meaningfully differ across quartiles.
Table 1:
Baseline participant characteristics by relative telomere length quartile at blood draw in 1989/1990
| 1st Quartile (n=2200) | 2nd Quartile (n=2200) | 3rd Quartile (n=2201) | 4th Quartile (n=2200) | |
|---|---|---|---|---|
| Age*†, (SD) | 59.1 (6.2) | 58.6 (6.5) | 58.0 (6.4) | 57.5 (6.6) |
| MHI-5 score‡ (SD) | 78.0 (13.8) | 78.9 (13.4) | 77.7 (14.4) | 78.4 (13.4) |
| Case status§, % | 35 | 35 | 34 | 35 |
| Pack-years of smoking, (SD) | 12.5 (18.1) | 12.1 (17.7) | 12.0 (17.9) | 11.8 (18.0) |
| Body mass index, (SD) | 25.2 (4.8) | 25.4 (4.8) | 25.2 (4.7) | 25.2 (4.6) |
| Antidepressant use, % | 5 | 4 | 4 | 4 |
| Alcohol intake, gm/wk (SD) | 5.6 (9.7) | 5.5 (9.6) | 5.6 (9.9) | 5.2 (8.8) |
| Physical activity, MET hrs/wk (SD) | 16.9 (23.6) | 16.9 (21.8) | 17.1 (25.8) | 16.1 (17.7) |
| Education: BA, % | 20 | 21 | 19 | 22 |
| Education: MA or DR, % | 11 | 10 | 10 | 10 |
| Pre-menopausal, % | 14 | 14 | 14 | 13 |
| Post-menopausal & currently on HRT, % | 35 | 35 | 36 | 35 |
| Paternal age-at-birth, (SD) | 31.0 (6.9) | 31.7 (7.2) | 31.6 (7.1) | 31.7 (6.9) |
| Hypertension, % | 29 | 30 | 29 | 30 |
| High cholesterol, % | 42 | 43 | 44 | 44 |
| CVD (MI, stroke, CABG) , % | 7 | 5 | 6 | 5 |
| Diabetes, % | 4 | 5 | 4 | 4 |
| Respiratory disease , % | 9 | 9 | 8 | 7 |
| Charlson Index¶, (SD) | 0.2 (0.6) | 0.2 (0.6) | 0.2 (0.5) | 0.2 (0.5) |
Values are means (SD) or percentages and are standardized to the age distribution of the study population.
Value is not age- adjusted
Mental Health Inventory-5
Classified as a “case” in the nested case-control studies of various diseases used to form analytic sample
All comorbidities are from self-reports of diagnosis by a healthcare professional Charlson Comorbidity Index(Charlson et al., 1987)
Depression Trajectories
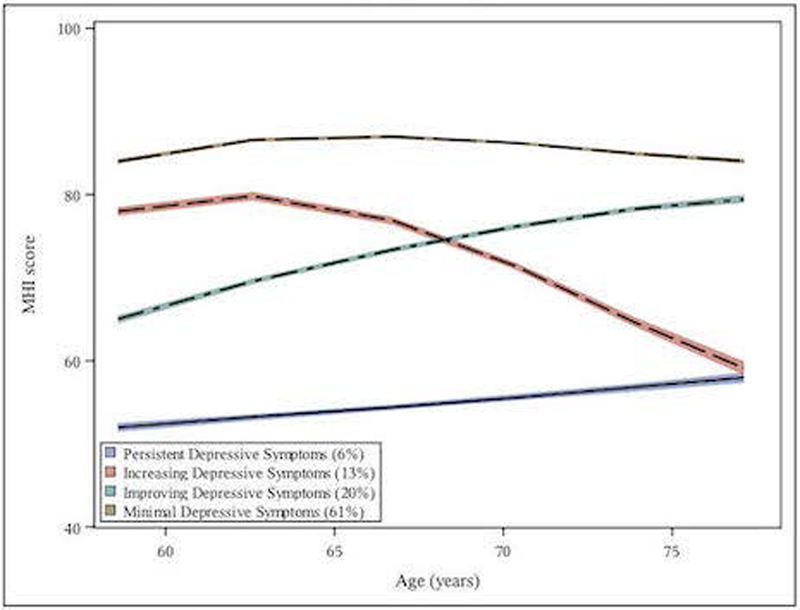
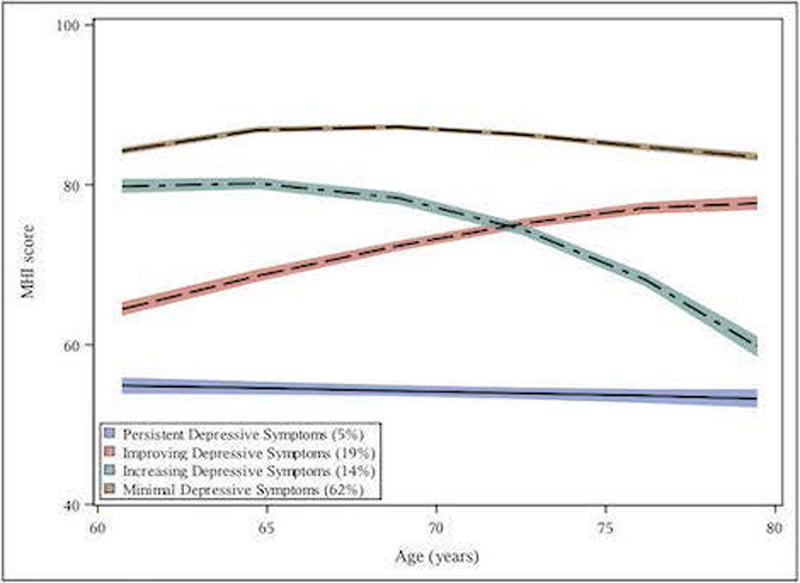
We examined models of between 2–7 groups and selected a 4-group model (Figures 2a and 2b) based on optimal combination of BIC, average posterior probabilities and clinical interpretability. There were four depressive symptom trajectory types (label hereafter, prevalence): Minimal Symptoms (“minimal”, 61%), Improving Symptoms (“improving”, 20%), Worsening Symptoms (“worsening”, 13%), and Persistent Severe Symptoms (“persistent-severe”, 6%). The worsening group initially showed similar levels of symptoms to that of the minimal group; however, over follow-up symptoms increased from a euthymic range to that consistent with clinical depression. The improving and persistent-severe group trajectories both began with clinical-range symptoms, although initial symptom levels were slightly less severe for the improving group. Over 20-year follow-up, however, the improving group showed symptom reduction – ultimately approaching symptom levels similar to those of the minimal group. Baseline characteristics by depressive symptom trajectory group are provided in Supplementary Table 1.
Figure 2a:
Twenty-year mean unadjusted MHI* depressive symptom trajectories** (lower scores indicate more depressive symptoms) by age among women in the Nurses Heath Study (N=29,400 in full blood cohort)
Figure 2b:
Twenty-year Multivariable-adjusted*** MHI* depressive symptom trajectories** (lower scores indicate more depressive symptoms) (N=8,801 in analytic sample)
* Mental Health inventory 5
** The figures illustrate group-based trajectories and their 95% confidence bands (shaded). Trajectories are modeled as a function of age. Group membership for identified trajectories was assigned using the group to which each participant had the highest posterior probability of membership.
***Adjusted for case/control status, pack years of smoking, BMI, antidepressant use, alcohol consumption, physical activity (MET hours/week), menopausal & hormone replacement therapy status, Charlson Index score, and paternal age at birth
As a sensitivity analysis, we derived the trajectories within only the subset of 8,801 participants with RTL measures. The numbers of groups and shapes of trajectories were identical to those derived using data from the larger sample of blood study participants (data not shown).
Multinomial Model Results
Table 2a displays results from the main analyses relating mid-life RTL to group membership in subsequent 20-year trajectories of depressive symptoms. The largest group was the reference category (ie, minimal group). In both age- and multivariable-adjusted models, the was no evidence of higher odds of membership in the improving or persistent-severe groups, compared to the minimal group, by RTL. However, mid-life RTL was significantly associated with odds of being in the worsening vs. minimal group, including after adjustment for multiple lifestyle and health confounders. Specifically, each SD-increase in RTLs was related to 22% lower odds of being in the worsening depressive symptoms group. Since we found a significant association among the groups with similar levels of low symptoms at baseline, we subsequently examined the association with RTL among groups with similar levels of high symptoms at baseline (persistent–severe vs. improving). We noted no significant association for this comparison, but groups were less similar in symptom levels than in our main analysis. The odds of being in the improving group vs. persistent-severe group decreased by a non-significant 0.94 times for each RTL increase, p=0.72 (data not shown in tables).
Table 2a:
Odds of being in each trajectory group, compared to the odds of being in the minimal symptoms group (reference category), per standard deviation increase in relative telomere length (cases and controls, n=8801).†
| Depressive Symptom Trajectory Group | Age-only adjusted Odds Ratio | 95% CI | P-Value | Adjusted Odds Ratio* | 95% CI | P-Value |
|---|---|---|---|---|---|---|
| Minimal Symptoms (n=5456) | Ref | Ref | Ref | Ref | Ref | Ref |
| Improving Symptoms (1672) | 1.10 | (0.91, 1.34) | 0.32 | 1.10 | (0.90,1.34) | 0.36 |
| Worsening Symptoms (n=1232) | 0.69 | (0.55, 0.86) | 0.001 | 0.78 | (0.62, 0.97) | 0.02 |
| Persistent Symptoms (n=441) | 0.95 | (0.71, 1.27) | 0.72 | 1.02 | (0.76, 1.37) | 0.89 |
Adjusted for case/control status, pack years of smoking, BMI, antidepressant use, alcohol consumption, physical activity (MET hours/week), menopausal & hormone replacement therapy status, Charlson Index score, and paternal age at birth. Cases and controls were from nested case-control studies of various diseases in the Nurses’ Health Study blood cohort.
Additional Analyses
To address potential bias by including disease cases from the nested case-control studies, we repeated models with only the 5,772 healthy controls. Results from these analyses (Table 2b) were comparable to those observed in the main analyses.
Table 2b:
Odds of being in each trajectory group, compared to the odds of being in the minimal symptoms group (reference category), per standard deviation increase in relative telomere length (controls only, n=5772)‡
| Depressive Symptom Trajectory Group | Age-only adjusted Odds Ratio | 95% CI | P-Value | Adjusted Odds Ratio** | 95% CI | P-Value |
|---|---|---|---|---|---|---|
| Minimal Symptoms (n=3536) | Ref | Ref | Ref | Ref | Ref | Ref |
| Improving Symptoms (n=993) | 1.22 | (0.95, 1.57) | 0.13 | 1.19 | (0.92, 1.54) | 0.19 |
| Worsening Symptoms (n=916) | 0.68 | (0.52, 0.89) | 0.005 | 0.76 | (0.58, 1.00) | 0.05 |
| Persistent Symptoms (n=327) | 0.94 | (0.66, 1.34) | 0.72 | 1.07 | (0.74,1.53) | 0.73 |
Adjusted for pack years of smoking, BMI, antidepressant use, alcohol consumption, physical activity (MET hours/week), menopausal & hormone replacement therapy status, Charlson Index score, and paternal age at birth. Controls were from nested case-control studies of various diseases in the Nurses’ Health Study blood cohort.
We examined the association of RTL with depression incidence in Cox proportional hazards models. Whether telomere length was measured at the 1st (Supplemental Table 2a) or 2nd blood draw (Supplemental Table 2b), there were no associations between RTL and depression incidence. Further, there was no significant relation of 10-year change in RTL to subsequent risk of depression among the subset of participants (n=1039) with RTL measures at both timepoints (Supplemental Table 3). Finally, results were similar regardless of whether incident depression cases were classified by symptoms only or by self-reported diagnosis or antidepressant use.
DISCUSSION
Among nearly 9,000 women followed over 20 years, we found significant relations of mid-life telomere length to subsequent trajectory of depressive symptoms into later-life. For each SD increment in RTL, the odds of being in the worsening symptoms group vs. minimal symptoms group was 0.78 (95% CI: 0.62–0.97, p=0.02) – or 22% lower. RTL was not associated with likelihood of being in other trajectory groups. Our results suggest a novel potential link between telomeres lengths and the evolution of depressive symptoms between mid- and later-life.
Although RTL was associated with worsening depression among those with comparable symptom levels at baseline, it was not associated with being in the persistently high vs. persistently low symptom group over time, and mean RTL did not differ at baseline between these groups. One potential explanation for this is the relatively small number of participants in the persistent-severe category (n=441, or 5% of sample); there may not have been adequate power to detect a significant contrast between the persistently high and persistently low symptom groups, and thus caution is warranted in the interpretation. Alternatively, it is possible that the group that has persistently severe symptoms – where persistent depression is associated with a variety of adverse health consequences, including earlier mortality(Murphy et al., 2016) – and yet remains living and available for 20-year follow-up, may include older adults who are particularly resilient. For example, a speculative possibility is that genetic or other factors may be associated with both RTL and resilience to ill effects of depression; thus, observed estimates could have been influenced by a survivor effect among participants in that group. The members of these two groups were similar in RTL at baseline but may have been dissimilar in levels of resilience to oxidative stress or other factors that promote telomere attrition – as impacts of oxidative stress would be expected to be more pronounced in late-life when telomeres have shortened with age and with cumulative exposure to damaging environmental factors. Differences in survival may also explain some of the inconsistencies in findings noted between both cross-sectional and longitudinal studies of TL and depression.
Previous work has frequently, but not uniformly, indicated relations of depression to TLs in cross-sectional and longitudinal studies, where depression was examined as an exposure.(Garcia-Rizo et al., 2013; Georgin-Lavialle et al., 2014; Hartmann, Boehner, Groenen, & Kalb, 2010; Hassett et al., 2012; Hoen et al., 2011; Hoen et al., 2013; Huzen et al., 2010; Karabatsiakis, Kolassa, Kolassa, Rudolph, & Dietrich, 2014; Ladwig et al., 2013; Liu, Zhang, Yan, Wang, & Li, 2014; Lung, Chen, & Shu, 2007; Needham et al., 2015; Phillips et al., 2013; Ridout et al., 2016; Rius-Ottenheim et al., 2012; Schaakxs, Verhoeven, Oude Voshaar, Comijs, & Penninx, 2015; Schutte & Malouff, 2015; Shaffer et al., 2012; Shalev et al., 2014; Simon et al., 2015; Solomon et al., 2017; Starnino et al., 2016; Surtees et al., 2011; Teyssier, Chauvet-Gelinier, Ragot, & Bonin, 2012; Verhoeven et al., 2018; Verhoeven, van Oppen, Revesz, Wolkowitz, & Penninx, 2016; Wikgren et al., 2012) Most studies, thus far, have been cross-sectional. Some cross-sectional studies among adults reported significant associations between depression and shorter TL(Garcia-Rizo et al., 2013; Hartmann et al., 2010; Hassett et al., 2012; Hoen et al., 2011; Karabatsiakis et al., 2014; Needham et al., 2015; Starnino et al., 2016; Teyssier et al., 2012; Verhoeven et al., 2016; Wikgren et al., 2012); others showed null associations(Georgin-Lavialle et al., 2014; Hoen et al., 2011; Hoen et al., 2013; Huzen et al., 2010; Ladwig et al., 2013; Schaakxs et al., 2015; Shaffer et al., 2012; Simon et al., 2015; Surtees et al., 2011). Relatively fewer studies have examined how depression related to TL over time; these longitudinal studies show mixed findings.(Liu et al., 2014; Lung et al., 2007; Phillips et al., 2013; Rius-Ottenheim et al., 2012; Schutte & Malouff, 2015; Shalev et al., 2014; Vance et al., 2018; Verhoeven et al., 2018; Verhoeven et al., 2016) A recent meta-analysis of longitudinal and cross-sectional studies examining the association of depression with TL, found no association for the pooled longitudinal result (R=−0.001, 95% CI: −0.08, 0.08; p=0.98);(Schutte & Malouff, 2015) a significant association was indicated for the pooled cross-sectional studies.(Schutte & Malouff, 2015) In the last year, our group also examined relations of depression to prospective change in RTL in a subset of n=1,250 NHS participants; Chang et al. similarly observed no significant association between baseline depression and 11-year telomere attrition, although point estimates and statistical trends were consistently in the direction of worse telomere attrition among those with vs. without depression(S. C. Chang et al., 2018). Overall, longitudinal evidence remains relatively sparse. Indeed, the meta-analysis by Schutte and Malouff was more limited for the pooled longitudinal studies: only 5 studies met inclusion criteria, compared to the 25 studies included in the cross-sectional analysis.(Schutte & Malouff, 2015) Also, it is possible that the inconsistencies in findings may relate to the extent of survivor effects present in the different cohorts under examination, where greater influences of survival would be expected with older cohorts.
While prior studies have examined prospective relations of depression to TL, similar attention has not focused on the association of TL with subsequent depression. The possibility of this temporal direction has been raised by earlier studies among children and adolescents.(Gotlib et al., 2015; Rackley et al., 2012; Wojcicki et al., 2015) For example, individuals born with dyskeratosis congenita, a disorder impeding normal telomerase activity and rapidly accelerating telomere attrition, show an increased risk of psychiatric disorders when compared to individuals suffering from other chronic illnesses.(Rackley et al., 2012) However, the relevance of these findings to older adults is not clear: the biological relationships between depression and RTL may differ among these groups, and other confounders and factors associated with disorders of aging may play a role. Similarly, a temporal association between TL and subsequent depression outcomes was also suggested by data from a more recent preliminary study. Hough et al.(Hough et al., 2016) examined antidepressant response in relation to baseline TL among adults 20–65 years (n=27; mean age=38 years) diagnosed with MDD; shorter TL was associated with worse antidepressant response and with more negative affect over an eight-week period.(Hough et al., 2016)
When results from the current study are considered alongside the above-mentioned reports, an intriguing possibility is raised that having shorter telomeres may predispose to development of worse psychiatric symptoms among adults. Indeed, in a separate NHS cohort analysis using a latent class (finite mixture modeling) approach to identify phobic anxiety symptom groups, Ramin et al.(Ramin et al., 2015) reported a non-statistically significant trend (multivariable-adjusted p=0.09) for mid-life RTLs and risk of late-life anxiety. Among n=3,194 women with no or minimal phobic anxiety symptoms at baseline, those with shorter compared to the longest telomeres at mid-life had nearly 2-fold higher odds of severe phobic anxiety 16 years later. Nonetheless, an alternative explanation for those preliminary findings is that short TLs may be peripheral biomarkers for other biological processes that impact subsequent affective outcomes more directly.
The potential for a bi-directional association between telomeres and psychopathology has a plausible biologic basis. While depression and oxidative stress have been associated with shorter telomeres, it is also possible that telomere shortening may itself potentiate oxidative stress.(Liu et al., 2014) One mechanism may involve the release of ROS and subsequent cellular stress cascades.(Cai et al., 2015; Karabatsiakis et al., 2014; Sahin & Depinho, 2010; Savitz & Drevets, 2009; Szebeni et al., 2014) Depression and other forms of psychological stress may increase ROS, resulting in damage to DNA and other cellular components.(Karabatsiakis et al., 2014; Teyssier et al., 2012; Tyrka et al., 2015; Verhoeven et al., 2014; Wei, Backlund, Wegener, Mathe, & Lavebratt, 2015) This damage may impede normal cell and antioxidant defenses and result in apoptosis or accelerated telomere attrition. Yet, as noted by Sahin and DePinho(Sahin & Depinho, 2010), dysfunctional telomeres can activate their own cascade of DNA damage, oxidative stress and mitochondrial dysfunction via a p53-mediated process; this may lead to further downstream mitochondrial and metabolic dysfunction, including among post-mitotic cells that are energy demand-sensitive.(Hovatta, 2015; Sahin et al., 2011; Verhoeven et al., 2014; Wolkowitz et al., 2015) Indeed, as the brain has the highest demand for mitochondrial energy of any organ, neurons are particularly vulnerable to deficiencies in energy production.(Pei & Wallace, 2018; Wallace, 2017) Indeed, such processes may result in loss of susceptible neurons in key regions for mood maintenance and neurogenesis, such as the hippocampus (which has the highest telomerase activity of any brain region). For example, mice with critically short telomeres show reduced neurogenesis and neural differentiation – suggesting that telomere dysfunction could reduce neuroplasticity with age.(Ferron et al., 2009) Thus, a cascade of telomere attrition and downstream consequences may be particularly relevant for older adults with respect to potential for disruptions of homeostatic mechanisms within the brain.(Duman, Malberg, Nakagawa, & D’Sa, 2000; Szebeni et al., 2014) Nonetheless, while these potential mechanisms relating telomere shortening and dysfunction to brain outcomes are compelling, we were not able to observe such processes directly in the present study.
This study features important strengths, including a large cohort of participants followed over decades for depressive symptoms and potential confounders that were measured repeatedly using validated questionnaires. Additionally, this study makes a novel contribution by applying group-based trajectory modeling to explore the relationship between TL and subsequent depressive symptoms. The ability to examine mood trajectories over 20 years in a large, well-characterized sample afforded finer discrimination of depression outcomes and their relations to antecedent TL. When we explored, as a sensitivity analysis, this association using a simple case classification of depression in survival models, TL was not significantly related to incident depression. These differences may reflect that a more nuanced understanding of the data might be achieved in this context when depression is treated as a continuous, dimensional outcome in group-based trajectory models vs. as a dichotomous, case endpoint in traditional survival models. Specifically, survival analyses are based on reaching a threshold of caseness; once classified as a case, an individual contributes no further information. Participants may also experience the competing risk of death before ever being counted as a case. By contrast, the group-based trajectory modeling approach allowed information at all available timepoints to be included in analyses.
Limitations should also be considered. First, depressive symptoms were only assessed at four-year intervals; thus, interim fluctuations in symptoms could be missed. Shorter intervals between evaluations would have provided finer granularity of trajectories. However, we employed data from six time points over 20-years and provided sufficient information regarding symptoms over time such that latent groups could be identified. Second, the study cohort consisted of women who were mostly non-Latina white (95%), had access to healthcare resources, and had relatively high education levels (associate degree and above). Although there is not presently a strong biological rationale to suggest that the association between TL and depressive trajectories would differ by sex, education or race/ethnicity, the limited generalizability of the sample indicate that results should be interpreted with caution. Third, RTL is a biological measure with potential sources of error in both the telomere and single gene assays. Random measurement error would likely be non-differential and could result in bias in estimates toward the null.
In summary, results from this study indicated that mid-life women with longer telomeres at baseline were less likely to experience worsening depressive symptoms over the subsequent 20 years. Findings suggest that mechanisms involved in telomere shortening could be relevant to the evolution of depressive symptoms during aging. As data are minimal regarding the potential for a bi-directional relationship between depressive symptoms and telomere attrition, novel findings from this study make a notable contribution to the evidence base on telomeres and psychiatry.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank the NHS staff and participants without whom none of this research would be possible. Dr. Gillis was supported by a National Research Service Award, Training Program in Psychiatric Epidemiology T32 MH1711928 and by a National Institute for Occupational Safety and Health (NIOSH) training grant T42 OH008416. This work was supported by US National Institutes of Health (NIH) grants [R01 MH096776, R01 CA49449, P01 CA087969, UM1 CA186107]. The funding sources were not involved in the data collection, data analysis, manuscript writing or publication.
Footnotes
FINANCIAL DISCLOSURES
The authors have no financial conflicts of interest to disclose.
DATA SHARING
Data used in this study are property of the Nurses’ Health Study. For information on data sharing opportunities, please contact the Nurses’ Health Study staff.
REFERENCES
- Black CN, Bot M, Scheffer PG, Cuijpers P, & Penninx BW (2015). Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology, 51, 164–175. doi: 10.1016/j.psyneuen.2014.09.025 [DOI] [PubMed] [Google Scholar]
- Blackburn EH (2005). Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett, 579(4), 859–862. doi: 10.1016/j.febslet.2004.11.036 [DOI] [PubMed] [Google Scholar]
- Byers AL, Vittinghoff E, Lui L, & et al. (2012). Twenty-year depressive trajectories among older women. Archives of General Psychiatry, 69(10), 1073–1079. doi: 10.1001/archgenpsychiatry.2012.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai N, Chang S, Li Y, Li Q, Hu J, Liang J, … Flint J (2015). Molecular Signatures of Major Depression. Current Biology, 25(9), 1146–1156. doi: 10.1016/j.cub.2015.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S-C, Wang W, Pan A, Jones RN, Kawachi I, & Okereke OI (2016). Racial Variation in Depression Risk Factors and Symptom Trajectories among Older Women. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry, 24(11), 1051–1062. doi: 10.1016/j.jagp.2016.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SC, Crous-Bou M, Prescott J, Rosner B, Simon NM, Wang W, … Okereke OI (2018). Prospective association of depression and phobic anxiety with changes in telomere lengths over 11 years. Depress Anxiety, 35(5), 431–439. doi: 10.1002/da.22732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, & MacKenzie CR (1987). A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases, 40(5), 373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- Correia-Melo C, Hewitt G, & Passos JF (2014). Telomeres, oxidative stress and inflammatory factors: partners in cellular senescence? Longev Healthspan, 3(1), 1. doi: 10.1186/2046-2395-3-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous-Bou M, Fung TT, Prescott J, Julin B, Du M, Sun Q, … De Vivo I (2014). Mediterranean diet and telomere length in Nurses’ Health Study: population based cohort study. Bmj, 349, g6674. doi: 10.1136/bmj.g6674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vivo I, Prescott J, Wong JY, Kraft P, Hankinson SE, & Hunter DJ (2009). A prospective study of relative telomere length and postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev, 18(4), 1152–1156. doi: 10.1158/1055-9965.epi-08-0998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devore EE, Prescott J, De Vivo I, & Grodstein F (2011). Relative telomere length and cognitive decline in the Nurses’ Health Study. Neurosci Lett, 492(1), 15–18. doi: 10.1016/j.neulet.2011.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Nakagawa S, & D’Sa C (2000). Neuronal plasticity and survival in mood disorders. Biol Psychiatry, 48(8), 732–739. [DOI] [PubMed] [Google Scholar]
- Ferron SR, Marques-Torrejon MA, Mira H, Flores I, Taylor K, Blasco M, & Farinas I (2009). Telomere shortening in neural stem cells disrupts neuronal differentiation and neuritogenesis. Journal of Neuroscience, 29(46), 14394–14407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rizo C, Fernandez-Egea E, Miller BJ, Oliveira C, Justicia A, Griffith JK, … Kirkpatrick B (2013). Abnormal glucose tolerance, white blood cell count, and telomere length in newly diagnosed, antidepressant-naive patients with depression. Brain Behav Immun, 28, 49–53. doi: 10.1016/j.bbi.2012.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgin-Lavialle S, Moura DS, Bruneau J, Chauvet-Gelinier JC, Damaj G, Soucie E, … Hermine O (2014). Leukocyte telomere length in mastocytosis: correlations with depression and perceived stress. Brain Behav Immun, 35, 51–57. doi: 10.1016/j.bbi.2013.07.009 [DOI] [PubMed] [Google Scholar]
- Gotlib IH, LeMoult J, Colich NL, Foland-Ross LC, Hallmayer J, Joormann J, … Wolkowitz OM (2015). Telomere length and cortisol reactivity in children of depressed mothers. Mol Psychiatry, 20(5), 615–620. doi: 10.1038/mp.2014.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Qureshi AA, Prescott J, Guo Q, Ye L, Hunter DJ, & De Vivo I (2009). A Prospective Study of Telomere Length and the Risk of Skin Cancer. The Journal of investigative dermatology, 129(2), 415–421. doi: 10.1038/jid.2008.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankinson SE, Manson JE, Spiegelman D, Willett WC, Longcope C, & Speizer FE (1995). Reproducibility of plasma hormone levels in postmenopausal women over a 2–3-year period. Cancer Epidemiology Biomarkers & Prevention, 4(6), 649–654. [PubMed] [Google Scholar]
- Hankinson SE, Willett WC, Manson JE, Hunter DJ, Colditz GA, Stampfer MJ, … Speizer FE (1995). Alcohol, height, and adiposity in relation to estrogen and prolactin levels in postmenopausal women. J Natl Cancer Inst, 87(17), 1297–1302. [DOI] [PubMed] [Google Scholar]
- Hartmann N, Boehner M, Groenen F, & Kalb R (2010). Telomere length of patients with major depression is shortened but independent from therapy and severity of the disease. Depress Anxiety, 27(12), 1111–1116. doi: 10.1002/da.20749 [DOI] [PubMed] [Google Scholar]
- Hassett AL, Epel E, Clauw DJ, Harris RE, Harte SE, Kairys A, … Williams DA (2012). Pain is associated with short leukocyte telomere length in women with fibromyalgia. J Pain, 13(10), 959–969. doi: 10.1016/j.jpain.2012.07.003 [DOI] [PubMed] [Google Scholar]
- Hoen PW, de Jonge P, Na BY, Farzaneh-Far R, Epel E, Lin J, … Whooley MA (2011). Depression and leukocyte telomere length in patients with coronary heart disease: data from the Heart and Soul Study. Psychosom Med, 73(7), 541–547. doi: 10.1097/PSY.0b013e31821b1f6e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoen PW, Rosmalen JG, Schoevers RA, Huzen J, van der Harst P, & de Jonge P (2013). Association between anxiety but not depressive disorders and leukocyte telomere length after 2 years of follow-up in a population-based sample. Psychol Med, 43(4), 689–697. doi: 10.1017/s0033291712001766 [DOI] [PubMed] [Google Scholar]
- Hough CM, Bersani FS, Mellon SH, Epel ES, Reus VI, Lindqvist D, … Wolkowitz OM (2016). Leukocyte Telomere Length Predicts SSRI Response in Major Depressive Disorder: A Preliminary Report. Molecular Neuropsychiatry, 2(2), 88–96. doi: 10.1159/000446500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovatta I (2015). Genetics: Dynamic Cellular Aging Markers Associated with Major Depression. Current Biology, 25(10), R409–R411. doi: 10.1016/j.cub.2015.03.036 [DOI] [PubMed] [Google Scholar]
- Huzen J, van der Harst P, de Boer RA, Lesman-Leegte I, Voors AA, van Gilst WH, … van Veldhuisen DJ (2010). Telomere length and psychological well-being in patients with chronic heart failure. Age Ageing, 39(2), 223–227. doi: 10.1093/ageing/afp256 [DOI] [PubMed] [Google Scholar]
- Jones BL, Nagin DS, & Roeder K (2001). A SAS Procedure Based on Mixture Models for Estimating Developmental Trajectories. Sociological Methods & Research, 29(3), 374–393. doi: 10.1177/0049124101029003005 [DOI] [Google Scholar]
- Karabatsiakis A, Kolassa IT, Kolassa S, Rudolph KL, & Dietrich DE (2014). Telomere shortening in leukocyte subpopulations in depression. BMC Psychiatry, 14, 192. doi: 10.1186/1471-244x-14-192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladwig KH, Brockhaus AC, Baumert J, Lukaschek K, Emeny RT, Kruse J, … Peters A (2013). Posttraumatic stress disorder and not depression is associated with shorter leukocyte telomere length: findings from 3,000 participants in the population-based KORA F4 study. PLoS One, 8(7), e64762. doi: 10.1371/journal.pone.0064762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhang J, Yan J, Wang Y, & Li Y (2014). Leucocyte telomere shortening in relation to newly diagnosed type 2 diabetic patients with depression. Oxid Med Cell Longev, 2014, 673959. doi: 10.1155/2014/673959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung FW, Chen NC, & Shu BC (2007). Genetic pathway of major depressive disorder in shortening telomeric length. Psychiatr Genet, 17(3), 195–199. doi: 10.1097/YPG.0b013e32808374f6 [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Campbell S, McEwen B, Macdonald K, Amano S, Joffe R, … Young LT (2003). Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci U S A, 100(3), 1387–1392. doi: 10.1073/pnas.0337481100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti Pierre J. Allaman I (2015). A Cellular Perspective on Brain Energy Metabolism and Functional Imaging. Neuron, 86(4), 883–901. doi: 10.1016/j.neuron.2015.03.035 [DOI] [PubMed] [Google Scholar]
- Murphy RA, Hagaman AK, Reinders I, Steeves JA, Newman AB, Rubin SM, … Harris TB (2016). Depressive Trajectories and Risk of Disability and Mortality in Older Adults: Longitudinal Findings From the Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci, 71(2), 228–235. doi: 10.1093/gerona/glv139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham BL, Mezuk B, Bareis N, Lin J, Blackburn EH, & Epel ES (2015). Depression, anxiety and telomere length in young adults: evidence from the National Health and Nutrition Examination Survey. Mol Psychiatry, 20(4), 520–528. doi: 10.1038/mp.2014.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page JH, Ma J, Rexrode KM, Rifai N, Manson JE, & Hankinson SE (2008). Plasma Dehydroepiandrosterone and Risk of Myocardial Infarction in Women. Clinical Chemistry, 54(7), 1190–1196. doi: 10.1373/clinchem.2007.099291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei L, & Wallace DC (2018). Mitochondrial Etiology of Neuropsychiatric Disorders. Biol Psychiatry, 83(9), 722–730. doi: 10.1016/j.biopsych.2017.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AC, Robertson T, Carroll D, Der G, Shiels PG, McGlynn L, & Benzeval M (2013). Do symptoms of depression predict telomere length? Evidence from the west of Scotland twenty-07 study. Psychosom Med, 75(3), 288–296. doi: 10.1097/PSY.0b013e318289e6b5 [DOI] [PubMed] [Google Scholar]
- Prescott J, McGrath M, Lee IM, Buring JE, & De Vivo I (2010). Telomere length and genetic analyses in population-based studies of endometrial cancer risk. Cancer, 116(18), 4275–4282. doi: 10.1002/cncr.25328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackley S, Pao M, Seratti GF, Giri N, Rasimas JJ, Alter BP, & Savage SA (2012). Neuropsychiatric conditions among patients with dyskeratosis congenita: a link with telomere biology? Psychosomatics, 53(3), 230–235. doi: 10.1016/j.psym.2011.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramin C, Wang W, Prescott J, Rosner B, Simon NM, De Vivo I, & Okereke OI (2015). A prospective study of leukocyte telomere length and risk of phobic anxiety among women. Psychiatry Res, 230(2), 545–552. doi: 10.1016/j.psychres.2015.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridout KK, Ridout SJ, Price LH, Sen S, & Tyrka AR (2016). Depression and telomere length: A meta-analysis. J Affect Disord, 191, 237–247. doi: 10.1016/j.jad.2015.11.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rius-Ottenheim N, Houben JM, Kromhout D, Kafatos A, van der Mast RC, Zitman FG, … Giltay EJ (2012). Telomere length and mental well-being in elderly men from the Netherlands and Greece. Behav Genet, 42(2), 278–286. doi: 10.1007/s10519-011-9498-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner B, Cook N, Portman R, Daniels S, & Falkner B (2008). Determination of blood pressure percentiles in normal-weight children: some methodological issues. Am J Epidemiol, 167(6), 653–666. doi: 10.1093/aje/kwm348 [DOI] [PubMed] [Google Scholar]
- Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, … DePinho RA (2011). Telomere dysfunction induces metabolic and mitochondrial compromise. Nature, 470(7334), 359–365. doi: 10.1038/nature09787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin E, & Depinho RA (2010). Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature, 464(7288), 520–528. doi: 10.1038/nature08982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz JB, & Drevets WC (2009). Imaging phenotypes of major depressive disorder: genetic correlates. Neuroscience, 164(1), 300–330. doi: 10.1016/j.neuroscience.2009.03.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaakxs R, Verhoeven JE, Oude Voshaar RC, Comijs HC, & Penninx BW (2015). Leukocyte telomere length and late-life depression. The American Journal of Geriatric Psychiatry, 23(4), 423–432. [DOI] [PubMed] [Google Scholar]
- Schutte NS, & Malouff JM (2015). The association between depression and leukocyte telomere length: a meta-analysis. Depress Anxiety, 32(4), 229–238. doi: 10.1002/da.22351 [DOI] [PubMed] [Google Scholar]
- Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, … Meyer JH (2015). Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry, 72(3), 268–275. doi: 10.1001/jamapsychiatry.2014.2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer JA, Epel E, Kang MS, Ye S, Schwartz JE, Davidson KW, … Shimbo D (2012). Depressive symptoms are not associated with leukocyte telomere length: findings from the Nova Scotia Health Survey (NSHS95), a population-based study. PLoS One, 7(10), e48318. doi: 10.1371/journal.pone.0048318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I, Moffitt TE, Braithwaite AW, Danese A, Fleming NI, Goldman-Mellor S, … Caspi A (2014). Internalizing disorders and leukocyte telomere erosion: a prospective study of depression, generalized anxiety disorder and post-traumatic stress disorder. Mol Psychiatry, 19(11), 1163–1170. doi: 10.1038/mp.2013.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shun-Chiao C, Marta CB, Jennifer P, Bernard R, M. SN, Wei W, … I. OO (2018). Prospective association of depression and phobic anxiety with changes in telomere lengths over 11 years. Depress Anxiety, 35(5), 431–439. doi:doi: 10.1002/da.22732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NM, Walton ZE, Bui E, Prescott J, Hoge E, Keshaviah A, … Wong KK (2015). Telomere length and telomerase in a well-characterized sample of individuals with major depressive disorder compared to controls. Psychoneuroendocrinology, 58, 9–22. doi: 10.1016/j.psyneuen.2015.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon Z, Tsur N, Levin Y, Uziel O, Lahav M, & Ohry A (2017). The implications of war captivity and long-term psychopathology trajectories for telomere length. Psychoneuroendocrinology, Jul;81, 122–128. doi: 10.1016/j.psyneuen.2017.04.004. [DOI] [PubMed] [Google Scholar]
- Starnino L, Busque L, Tardif JC, & D’Antono B (2016). Psychological Profiles in the Prediction of Leukocyte Telomere Length in Healthy Individuals. PLoS One, 11(10), e0165482. doi: 10.1371/journal.pone.0165482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surtees PG, Wainwright NW, Pooley KA, Luben RN, Khaw KT, Easton DF, & Dunning AM (2011). Life stress, emotional health, and mean telomere length in the European Prospective Investigation into Cancer (EPIC)-Norfolk population study. J Gerontol A Biol Sci Med Sci, 66(11), 1152–1162. doi: 10.1093/gerona/glr112 [DOI] [PubMed] [Google Scholar]
- Szebeni A, Szebeni K, DiPeri T, Chandley MJ, Crawford JD, Stockmeier CA, & Ordway GA (2014). Shortened telomere length in white matter oligodendrocytes in major depression: potential role of oxidative stress. Int J Neuropsychopharmacol, 17(10), 1579–1589. doi: 10.1017/s1461145714000698 [DOI] [PubMed] [Google Scholar]
- Teyssier JR, Chauvet-Gelinier JC, Ragot S, & Bonin B (2012). Up-regulation of leucocytes genes implicated in telomere dysfunction and cellular senescence correlates with depression and anxiety severity scores. PLoS One, 7(11), e49677. doi: 10.1371/journal.pone.0049677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Carpenter LL, Kao HT, Porton B, Philip NS, Ridout SJ, … Price LH (2015). Association of telomere length and mitochondrial DNA copy number in a community sample of healthy adults. Exp Gerontol, 66, 17–20. doi: 10.1016/j.exger.2015.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance MC, Bui E, Hoeppner SS, Kovachy B, Prescott J, Mischoulon D, … Simon NM (2018). Prospective association between major depressive disorder and leukocyte telomere length over two years. Psychoneuroendocrinology, 90, 157–164. doi: 10.1016/j.psyneuen.2018.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven JE, Revesz D, Picard M, Epel EE, Wolkowitz OM, Matthews KA, … Puterman E (2018). Depression, telomeres and mitochondrial DNA: between- and within-person associations from a 10-year longitudinal study. Mol Psychiatry, 23(4), 850–857. doi: 10.1038/mp.2017.48 [DOI] [PubMed] [Google Scholar]
- Verhoeven JE, Revesz D, Wolkowitz OM, & Penninx BW (2014). Cellular aging in depression: Permanent imprint or reversible process?: An overview of the current evidence, mechanistic pathways, and targets for interventions. Bioessays, 36(10), 968–978. doi: 10.1002/bies.201400068 [DOI] [PubMed] [Google Scholar]
- Verhoeven JE, van Oppen P, Revesz D, Wolkowitz OM, & Penninx BW (2016). Depressive and Anxiety Disorders Showing Robust, but Non-Dynamic, 6-Year Longitudinal Association With Short Leukocyte Telomere Length. Am J Psychiatry, 173(6), 617–624. doi: 10.1176/appi.ajp.2015.15070887 [DOI] [PubMed] [Google Scholar]
- Wallace DC (2017). A Mitochondrial Etiology of Neuropsychiatric Disorders. JAMA Psychiatry, 74(9), 863–864. doi: 10.1001/jamapsychiatry.2017.0397 [DOI] [PubMed] [Google Scholar]
- Ware JE Jr., & Sherbourne CD (1992). The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care, 30(6), 473–483. [PubMed] [Google Scholar]
- Wei YB, Backlund L, Wegener G, Mathe AA, & Lavebratt C (2015). Telomerase dysregulation in the hippocampus of a rat model of depression: normalization by lithium. Int J Neuropsychopharmacol, 18(7), pyv002. doi: 10.1093/ijnp/pyv002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikgren M, Maripuu M, Karlsson T, Nordfjall K, Bergdahl J, Hultdin J, … Norrback KF (2012). Short telomeres in depression and the general population are associated with a hypocortisolemic state. Biol Psychiatry, 71(4), 294–300. doi: 10.1016/j.biopsych.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Wojcicki JM, Heyman MB, Elwan D, Shiboski S, Lin J, Blackburn E, & Epel E (2015). Telomere length is associated with oppositional defiant behavior and maternal clinical depression in Latino preschool children. Transl Psychiatry, 5, e581. doi: 10.1038/tp.2015.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkowitz OM, Mellon SH, Lindqvist D, Epel ES, Blackburn EH, Lin J, … Mueller S (2015). PBMC telomerase activity, but not leukocyte telomere length, correlates with hippocampal volume in major depression. Psychiatry Res, 232(1), 58–64. doi: 10.1016/j.pscychresns.2015.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.