Abstract
Background:
Prostate cancer is characterized by T-cell exclusion, which is consistent with their poor responses to immunotherapy. In addition, T-cells restricted to the adjacent stroma and benign areas are characterized by anergic and immunosuppressive phenotypes. In order for immunotherapies to produce robust anti-tumor responses in prostate cancer, this exclusion barrier and immunosuppressive microenvironment must first be overcome. We have previously identified mesenchymal stem cells (MSCs) in primary and metastatic human prostate cancer tissue.
Methods:
An Opal Multiplex immunofluorescence assay based on CD73, CD90, and CD105 staining was used to identify triple-labeled MSCs in human prostate cancer tissue. T-cell suppression assays and flow cytometry were used to demonstrate the immunosuppressive potential of primary MSCs expanded from human bone marrow and prostate cancer tissue from independent donors.
Results:
Endogenous MSCs were confirmed to be present at sites of human prostate cancer. These prostate cancer-infiltrating MSCs suppress T-cell proliferation in a dose-dependent manner similar to their bone marrow-derived counterparts. Also similar to bone marrow-derived MSCs, prostate cancer-infiltrating MSCs upregulate expression of PD-L1 and PD-L2 on their cell surface in the presence of IFNγ and TNFα.
Conclusion:
Prostate cancer-infiltrating MSCs suppress T-cell proliferation similar to canonical bone marrow-derived MSCs, which have well-documented immunosuppressive properties with numerous effects on both innate and adaptive immune system function. Thus, we hypothesize that selective depletion of MSCs infiltrating sites of prostate cancer should restore immunologic recognition and elimination of malignant cells via broad re-activation of cytotoxic pro-inflammatory pathways.
Keywords: immunotherapy, mesenchymal stem cell, MSC, prostate cancer, T-cell exclusion
1 |. INTRODUCTION
The advent of cancer immunotherapy, in which the host immune system is augmented to generate a personalized anti-tumor response, has transformed care for lung, melanoma, bladder, and kidney cancer patients. This has been accomplished using a variety of diverse but complementary strategies including: adoptive transfer of tumorinfiltrating lymphocytes (TILs), allogeneic cell-based vaccines, genetically-engineered autologous T-cells with chimeric antigen receptors (CARs), and immune checkpoint inhibition in which antibodies are used to overcome negative regulators of the adaptive immune response (eg, PD-1/PD-L1). These immune-based approaches have led to remarkable and durable remissions in many cancer patients with advanced disease who previously responded poorly to conventional treatments.1,2
Unfortunately, immune checkpoint inhibitors as single agents or in combination have shown limited activity against prostate cancer in clinical trials thus far.3–7 Despite initial optimism, the lack of significant responses in the context of prostate cancer begs the question-why is prostate cancer different from other solid tumors, including many previously thought to be immunologically silent?
1.1 |. Prostate cancer is characterized by t-cell exclusion
For a robust anti-tumor response to immunotherapy, at least three things are required: 1) generation of tumor-reactive T-cells, 2) a physical interaction between target and effector cells, and 3) a microenvironment permissive to immune effector functions. Therefore, a possible explanation for the lack of anti-tumor immune responses is that prostate cancer lacks immunologically recognized tumor antigens. Indeed, prostate cancer is typically on the low end of the mutational burden spectrum, estimated to have ~20–40 non-synonymous mutations per tumor compared to ~100–200 for melanoma.8,9 However, accumulating clinical evidence indicates prostate cancer tumor-associated antigens are recognized by the adaptive immune system as demonstrated by the presence of tumor-reactive cytotoxic T-cells and auto-antibodies to prostate-specific proteins in the peripheral blood of patients.10–21 Unfortunately, despite this recognition, these adaptive responses are rendered ineffective in clinically relevant disease. Thus, low immunogenicity is not the primary reason that prostate cancer is unresponsive to immune checkpoint inhibition.
A second possibility is that immune effector cells never come into direct contact with cancer cells. Prostate cancer is characterized by T-cell exclusion (ie, poor infiltration of effector cells into malignant foci).5,17,21–23 Instead, T-cells are restricted to the adjacent stroma and benign areas of the gland, which prevents direct contact between effector and cancer (ie, target) cells. Furthermore, the immune cells that are present are frequently characterized by anergic and immunosuppressive phenotypes, including regulatory T-cells (Tregs), M2-polarized tumor-associated macrophages (TAMs), and myeloid-derived suppressor cells (MDSCs) that serve to reinforce this barrier.3,15,17,18,24 These phenotypic changes are largely driven via characteristics of the prostate cancer microenvironment that make it highly immunosuppressive. These include elevated levels of indoleamine 2, 3-dioxygenase (IDO), nitric oxide (NO), interleukin 10 (IL10), prostaglandin E2 (PGE2), hepatocyte growth factor (HGF), transforming growth factor-beta (TGF-β), arginase, adenosine, and others.22,25,26 These findings indicate that immune recognition of prostate cancer is restrained through orchestrated immune-dampening by the surrounding stroma. Consequently, in order for immunotherapy to produce robust anti-tumor responses in prostate cancer, this exclusion barrier and the immunosuppressive microenvironment must first be overcome.
1.2 |. Prostate cancer and chronic inflammation
Despite the presence of this exclusion barrier and immunosuppressive microenvironment in later stages of the disease, the positive association between chronic inflammation and prostate cancer initiation is well known.27,28 Proliferative inflammatory atrophy (PIA), a putative precursor lesion, is typified by highly proliferative atrophic foci in close proximity to a dense immune infiltrate.27,29 This infiltrate is thought to play a causal role in prostate cancer initiation through repeated cycles of tissue damage and regeneration resulting from oxidative stress produced during chronic inflammation. Autopsy studies have demonstrated prostate cancer often begins in the 3rd and 4th decades of life, but only becomes clinically detectable decades later due to its low proliferative index.30–33 This provides ample time for the immune system to develop adaptive immune responses to tumor-associated antigens.10–21 Unfortunately, this same extended time frame for clinical manifestation ultimately leads to a tolerized microenvironment with decreased numbers of cytotoxic T-cells and increased numbers of immunosuppressive Tregs, TAMs, and MDSCs as described above.3,15,18,24–26 This long, slow progression within the context of a chronic inflammatory microenvironment may also explain why these immunosuppressive and anergic cells are so entrenched and refractory to checkpoint inhibition. However, it should also be noted that current targets of checkpoint blockade such as PD-L1 are poorly expressed in localized disease; though evidence indicates expression may be higher in metastatic disease.34 This suggests that either different checkpoints are more relevant in this disease setting or that immune checkpoints in general are not the primary driver of immunosuppression in prostate cancer.
1.3 |. Mesenchymal stem cells infiltrate human prostate cancer
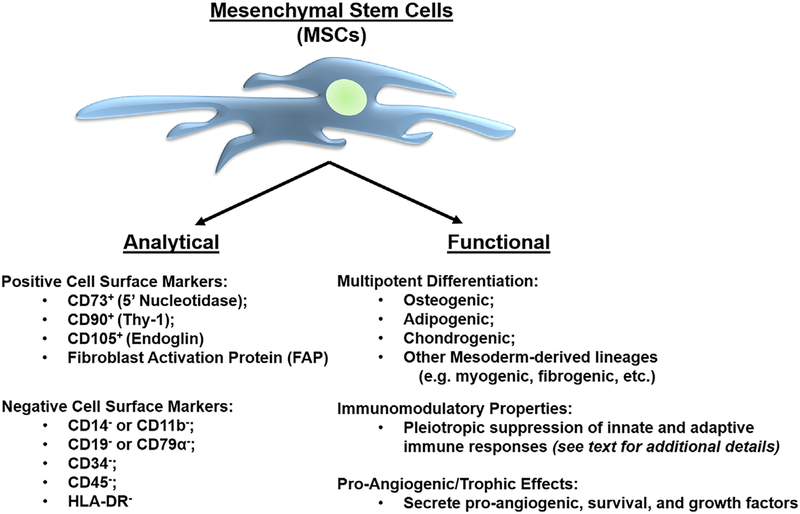
Another functional consequence of this chronic inflammation in the prostate is the recruitment of mesenchymal stem cells (MSCs) from the bone marrow.35–38 MSCs are multipotent cells functionally characterized by the ability to differentiate into cells of the mesoderm-lineage, including osteoblasts, chondrocytes, adipocytes, smooth muscle cells, and fibroblasts, among others. MSCs are defined analytically by the co-expression of CD73, CD90, and CD105 in the absence of hematopoietic lineage markers, such as CD14, CD20, CD34, CD45, and HLA-DR.39 In the adult, MSCs represent a rare population of cells in the bone marrow,40 but can be mobilized from these niches in response to inflammatory stimuli, such as CXCL12 (SDF-1), CCL5 (RANTES), CCL2 (MCP-1), IGF-1, and TGF-β; all of which are upregulated in the prostate cancer microenvironment.35,36,41–44 MSCs have also been identified at low frequency in tissues throughout the body,45–47 where they are thought to contribute to tissue homeostasis and repair via regenerative, trophic, and immunomodulatory properties.48–50
Based upon these analytical and functional characteristics (Figure 1), MSCs have been identified in radical prostatectomy tissue from men with prostate cancer in a systematic analysis of benign and malignant prostate tissue obtained from multiple donors representing different disease states and a wide range of age groups, from fetal development through adult death.37,38,51 Our group and others have demonstrated the in vivo recruitment of MSCs to prostate tissue from systemic sources using a variety of independent techniques, including transgenic chimeras, in vivo cell tracking methodologies, and tissue recombination models.37,38,44,52–54 This raises the question of whether MSCs are contributing to prostate cancer progression.
FIGURE 1.
Analytical and functional characterization of human mesenchymal stem cells. MSCs are defined by a series of positive and negative cell surface markers, in addition to functional properties, including multipotent differentiation potential, immunomodulatory properties, and pro-angiogenic/trophic effects
1.4 |. Role of mesenchymal stem cells in cancer progression
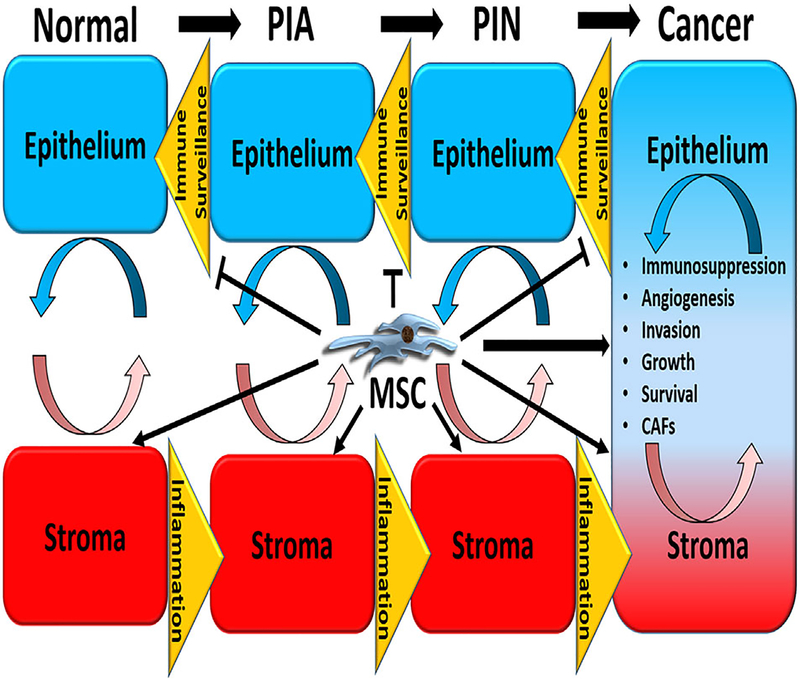
Reciprocal interactions between the epithelium and stroma have long been appreciated to play important roles in such diverse physiological processes as embryonic patterning, differentiation, and normal tissue homeostasis, in addition to pathophysiological processes, including cancer initiation and progression. The latter is at least partially facilitated via inflammatory inducers and the suppression of immune surveillance, which occurs as a negative feedback response to chronic inflammation. Though the exact role of tumor-infiltrating MSCs in cancer progression is unclear and may be disease-specific,36,55 there is supporting evidence that they promote disease progression through multiple mechanisms, including stimulating angiogenesis, invasion, growth, survival, and the generation of carcinoma-associated fibroblasts (CAFs), in addition to suppression of the innate and adaptive anti-tumor immune response (Figure 2).36,55–58 CAFs are derived from MSCs and are also associated with significant pro-tumorigenic properties.59–62 This suggests that MSCs sit at a critical node regulating multiple pro-tumorigenic pathways and that selectively targeting this tumor-infiltrating population may have significant anti-tumor effects.
FIGURE 2.
Potential roles of tumor-infiltrating mesenchymal stem cells in prostate cancer progression. Reciprocal interactions between the epithelium and stroma play important roles in diverse physiological and pathophysiological processes, including cancer initiation and progression. The latter is facilitated in part via inflammatory inducers and the suppression of immune surveillance. Tumor-infiltrating MSCs may play a role in cancer progression via multiple mechanisms, including stimulating angiogenesis, invasion, growth, survival, and the generation of carcinoma-associated fibroblasts (CAFs), in addition to suppression of innate and adaptive anti-tumor immune responses
1.5 |. Mesenchymal stem cells as mediators of immunosuppression
MSCs have well-documented immunomodulatory properties with numerous effects on the innate and adaptive immune system.36,63–67 The effects on the innate immune system include inhibition of NK cell proliferation and cytotoxicity, suppression of mast cell degranulation, promoting M2 polarization in macrophages (ie, TAMs), and blocking dendritic cell (DC) maturation and antigen presentation.65,68–70 Of note, recent evidence also indicates that MSCs are key regulators of MDSC expansion and function;71–74 another heterogeneous population of cells with pleiotropic immunosuppressive properties thought to contribute to tumor progression.57,75,76 In addition to the indirect effects on B- and T-cells that result from impaired DC maturation, MSCs have direct effects on the adaptive immune system as well. These include suppression of B-cell proliferation and immuglobulin (Ig) production, inhibition of T-cell proliferation and effector functions, and promoting Treg differentiation.77–79 These immunosuppressive effects are mediated primarily via the expression of soluble paracrine factors, such as IDO, IL-6, IL-10, HGF, PGE2, leukocyte inhibitor factor (LIF), TNF-stimulated gene 6 (TSG-6), and TGF-β, among others.36,63,67,80 These factors can be produced locally in the tumor microenvironment by tumor-infiltrating MSCs or act at distant sites via MSC-derived exosome trafficking.50
Cell-cell contact has also been implicated in a subset of these immunosuppressive properties, particularly T-cell suppression via engaging inhibitory immune checkpoint receptors such as PD-1 and other cell-cell interactions.67,81–83 Though the immunosuppressive properties of MSCs are consistent across species, tissue sources, and culture methods; there are potential differences in the pathways producing these effects that need to be considered when designing experiments and/or interpreting results. For example, human MSCs activated or “licensed” with interferon gamma (IFNγ) to mimic an inflammatory microenvironment, suppress T-cell proliferation primarily via production of IDO, whereas mouse and rat MSCs rely on the inducible nitric oxide synthase (iNOS) pathway to achieve this same effect.84–86 Collectively, these properties are thought to represent a normal physiologic feedback loop to prevent uncontrolled inflammation leading to autoimmune disorders and further tissue damage.
These combined results raise the question of whether prostate cancer-infiltrating MSCs are immunosuppressive. Thus, this hypothesis was tested experimentally.
2 |. METHODS
2.1 |. Human tissue and cell culture
LNCaP and PC3 were obtained from ATCC (Manassas, VA) and cultured in RPMI-1650 medium (Life Technologies-Invitrogen; Carlsbad, CA) supplemented with 10% fetal bovine serum (Gemini Bioproducts; West Sacramento, CA) and 2% L-glutamine (Invitrogen). All cells are routinely checked for mycoplasma using the MycoSensor PCR assay kit (Agilent; Santa Clara, CA) and authenticated via STR analysis in the Johns Hopkins Genetic Resources Core Facility. All cells are incubated in a 5% CO2, 95% air humidified incubator at 37°C with regular media changes every 3–4 days.
Radical prostatectomy tissue was obtained from patients undergoing radical prostatectomy at the Brady Urological Institute at Johns Hopkins via the Prostate Biospecimen Repository in accordance with IRB-approved protocols as previously described.38 Tissue was either formalin-fixed, paraffin-embedded, and sectioned for immunofluorescence labeling as described below, or digested into a single cell suspension to generate primary prostate stromal cell cultures (ie, PrCSCs), as previously described.38,62 Bone marrow-derived MSCs (BM-MSCs) were purchased from RoosterBio, Inc. (Frederick, MD). hMSC high performance media was also purchased from RoosterBio, Inc. BM-MSCs and PrCSCs expanded from two independent donors each and cultured according to previously optimized methods38,62 were used for the studies reported herein.
2.2 |. Triple-labeling of MSCs in formalin-fixed paraffin-embedded (FFPE) tissue
FFPE tissue was deparaffinized and underwent heat-mediated antigen retrieval in citrate buffer according to standard protocols in the SKCCC Tissue Services Core prior to triple labeling with the Opal Multiplex Immunostaining kit (PerkinElmer; Waltham, MA) according to the manufacturer’s instructions. Briefly, slides were blocked with Dako Endogenous Dual block (Agilent) for 10 min at room temperature, rinsed with TBST, and incubated with the anti-CD73 primary antibody [1:100, Clone D7F9A, (Cell Signaling Technology; Danvers, MA)] for 45 min at room temperature followed by washing with TBST (3 × 1 min). Slides were incubated with secondary antibody (PVR) diluted 1:125 in DPBS for 30 min at room temperature, washed with TBST (3 × 1 min), incubated with the Opal520 fluorophore working solution for 10 min at room temperature, and washed with TBST (3 × 1 min). Slides then underwent heat-mediated antigen retrieval and antibody removal in citrate buffer. After cooling, the process was repeated two additional rounds for labeling with anti-CD90 [1:100, Clone EPR3132, (Abcam; Cambridge, MA)] and anti-CD105 [1:100, #HPA067440, (Sigma Aldrich; St. Louis, MO)] followed by secondary labeling with the respective fluorophore working solution (ie, Opal570 and Opal690, respectively) as described above. Next, nuclei were labeled with DAPI for 10 min in a humid chamber, washed with dH2O, and coverslips mounted with ProLong Gold (ThermoFisher Scientific; Waltham, MA). Antibody specificity was previously documented using shRNA controls to the respective antibody targets. Slide images were captured using a Nikon 50i epifluorescence microscope equipped with X-Cite series 120 illuminator using a 40X/0.95 NA PlanApo lens with correction collar.
2.3 |. T-cell suppression assay
PBMCs were labeled with CellTrace Violet (CTV) according to the manufacturer’s instructions (BD Biosciences; San Jose, CA). CTV-labeled PBMCs (0.4e6) alone or with increasing ratios of “unlicensed” BM- or PCa-infiltrating MSCs (ie, PrCSCs) were used in a direct co-culture assay. Cells were incubated with anti-CD3/-CD28 beads [Dynabeads Human T-Activator; (ThermoFisher Scientific)] for 4 days at 37°C, then collected for analysis by flow cytometry using a Beckman Coulter Gallios instrument (Indianapolis, IN). T-cell proliferation was defined as the number of CD3+ cells in the CTV-low population and was calculated as a percentage of the stimulated PBMCs only control (ie, 0:1).
2.4 |. Constitutive and inducible expression of PD-L1 and PD-L2
The respective cultures were incubated in the presence or absence of IFNγ [10–100 ng/mL, (ThermoFisher Scientific)] and/or TNFα [1–10 ng/mL, (ThermoFisher Scientific)] for 24 h prior to staining for PD-L1 [clone MIH1, (BD Pharmingen)], PD-L2 [clone MIH18, (BD Pharmingen)], or the appropriate isotype control according to standard protocols as previously described.38 Analysis was performed by flow cytometry.
3 |. RESULTS
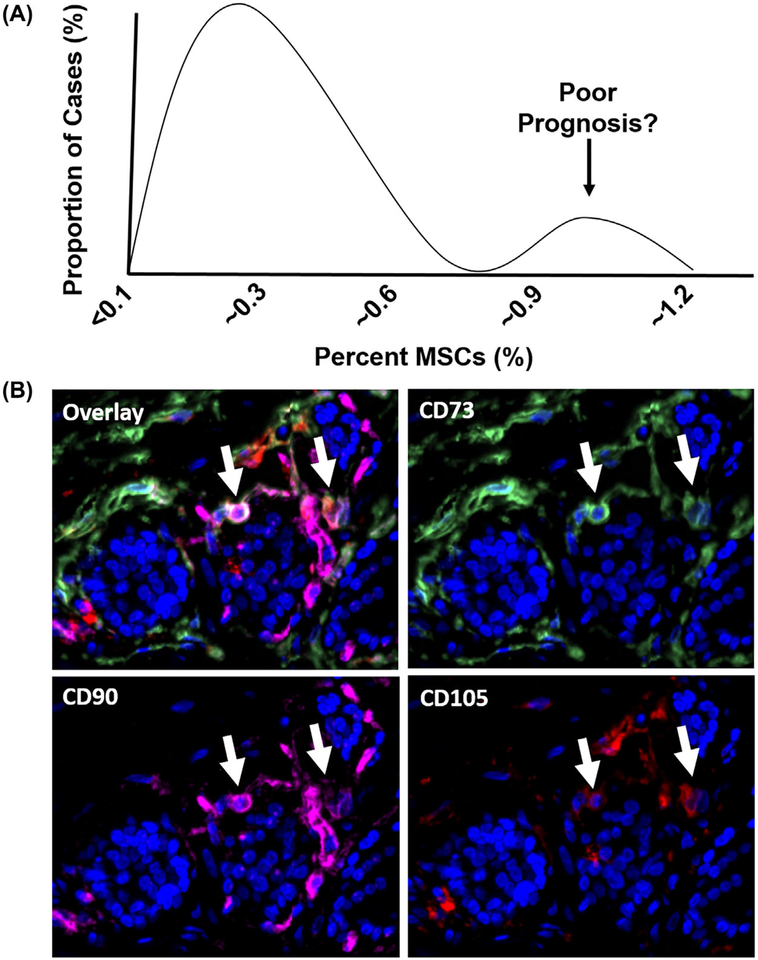
MSCs have previously been identified in radical prostatectomy tissue based upon the analytical and functional characteristics described above.37,38,51 Of note, a subset of these patients had elevated numbers of MSCs in their prostates (~1% of all cells in the tumor) based on multiparameter flow cytometry; an observation that does not correlate with Gleason score.38 Furthermore, rather than the expected Gaussian distribution, a bimodal pattern was observed with ~10–15% of cases in this MSC-enriched fraction (Figure 3A), suggesting that quantification of tumor-infiltrating MSCs may have prognostic value independent of Gleason score. We have recently confirmed that MSCs are present in human prostate cancer tissue using independent methodology—that is, a triple-label immunofluorescence assay for canonical MSC markers (Figure 3B). Additionally, the identification of MSCs in human mCRPC lesions from multiple organ sites at the time of death demonstrates this recruitment occurs throughout disease progression and may contribute to the lethal phenotype.38 This hypothesis is supported by preclinical prostate cancer models documenting that MSC recruitment promotes metastasis via a CXCL12- and CXCL16-dependent mechanism87 and that MSCs are critical components regulating the metastatic niche.88–90 The ongoing recruitment of MSCs to tumor tissue also raises the possibility of exploiting this inherent tumor tropism to develop a cell-based drug delivery vector.36,91,92
FIGURE 3.
Mesenchymal Stem Cells are present in human primary prostate cancer. A, Bimodal distribution pattern of MSCs in primary human prostate cancer based on multiparameter flow cytometry as previously described.39 B, Endogenous MSCs (white arrows) identified in human prostate cancer tissue based on positive staining for CD73 (green), CD90 (pink), and CD105 (red) using a multiplex immunofluorescence assay. Nuclei stained with DAPI (blue). 200× magnification
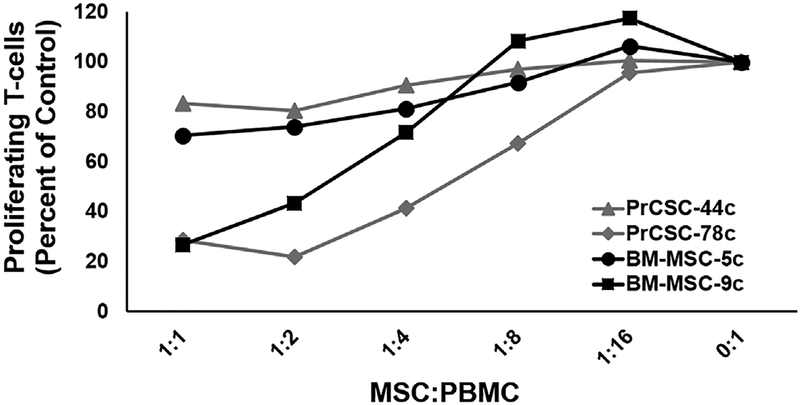
MSCs are rapidly selected for in primary prostate stromal cultures derived from benign and malignant human tissue.62 Using such primary cultures, human prostate cancer-infiltrating MSCs (ie, PrCSCs) were confirmed to suppress T-cell proliferation in a dose-dependent manner similar to their bone marrow-derived counterparts in a direct co-culture assay (Figure 4). Depending on the donor, T-cell proliferation ranged from ~20–75% of that observed in the controls (ie, PBMCs stimulated with anti-CD3/-CD28 in the absence of MSC co-culture) at the lowest ratio tested [ie, 1:1; (Figure 4)]. It should be noted that these studies were performed with “unlicensed” MSCs (ie, not pre-treated with IFNγ), which has previously been shown to significantly enhance MSC-mediated suppression of T-cell proliferation and effector functions.81
FIGURE 4.
Human bone marrow-derived and tumor-infiltrating mesenchymal stem cells suppress T-cell proliferation. CellTrace Violet (CTV)-labeled PBMCs alone or with increasing ratios of “unlicensed” BM- or PCa-infiltrating MSCs (ie, PrCSCs) expanded from two independent donors each were used in a direct co-culture assay. Cultures incubated for 4 days in the presence of anti-CD3/-CD28 beads at 37°C, then collected for analysis by flow cytometry. T-cell proliferation defined as the number of CD3+ cells in the CTV-low population and calculated as a percentage of the stimulated PBMCs alone control (ie, 0:1)
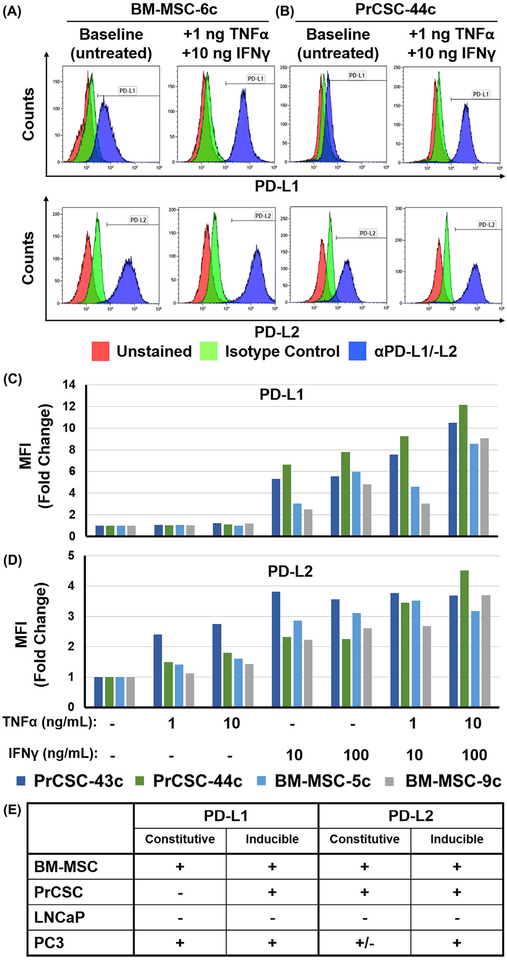
Cell-cell contact was previously implicated in MSC-mediated T-cell suppression and bone marrow-derived MSCs have previously been shown upregulate expression of immune checkpoint ligands, particularly in an inflammatory microenvironment.67,81–83 This led to the question of whether these factors also have a role in the functional effect of human PrCSCs on T-cell proliferation. Bone marrow-derived MSCs constitutively express PD-L1 and PD-L2, but can upregulate both in response to IFNγ signaling with additive effects seen in combination with TNFα (Figure 5A and C). Intriguingly, PrCSCs only robustly express PD-L2 at baseline, but can also upregulate both PD-L1 and PD-L2 under the same pro-inflammatory conditions (Figures 5B and D). In general, expression of these ligands by PrCSCs is comparable to or higher than that observed on bone marrow-derived MSCs. LNCaP and PC3 prostate cancer cells were used as additional controls, which were consistent with previously documented baseline and inducible expression patterns for these checkpoints (Figure 5E).93 These studies do not delineate the relative contributions of cell-cell interactions as opposed to soluble factors in mediating PrCSC-dependent suppression of T-cell proliferation, but this relationship along with detailed mechanistic analyses are being explored in ongoing studies.
FIGURE 5.
Human bone marrow-derived and tumor-infiltrating mesenchymal stem cells upregulate PD-L1 and PD-L2 in response to IFNγ signaling. Representative flow plots documenting that MSCs expanded from human (A) bone marrow or (B) primary prostate cancer tissue (BM-MSC or PrCSC, respectively) upregulate PD-L1 and PD-L2 in response to IFNγ and TNFα. Median fluorescence intensity (MFI) of (C) PD-L1 and (D) PD-L2 expression on BM-MSCs and PrCSCs incubated in the presence or absence of IFNγ (10–100 ng/mL) and/or TNFα (1–10 ng/mL) for 24 h prior to staining for the respective marker or isotype control. PC3 and LNCaP used as controls to document baseline constitutive or inducible expression as previously reported.92 E, Summary of constitutive and inducible PD-L1 and PD-L2 expression
4 |. DISCUSSION
The physiological relevance of the immunosuppressive activity of MSCs is strongly supported by the clinical development of cell-based immunotherapy using MSCs for a variety of pro-inflammatory immune-related indications, including Crohn’s disease, heart failure, and graft versus host disease (GvHD). Approval of an allogeneic MSC infusion product for acute GvHD in Canada and New Zealand first occurred in 2012, and a few years later in Japan as well; in addition to an independent product recently approved in the European Union for the treatment of enterocutaneous fistulas associated with Crohn’s Disease.94 Additionally, the full results from an open-label multi-center phase 3 trial in the U.S. evaluating remestemcel-L, a proprietary allogeneic MSC infusion product by Mesoblast Limited that has received fast track status by the Food and Drug Administration (FDA) for pediatric patients with steroid-refractory acute GvHD, are anticipated soon; though early reports via press releases and professional society presentations indicate the primary endpoint was successfully met.95,96 The use of allogeneic (ie, non-HLA-matched) MSCs in hundreds of clinical trials worldwide provides further evidence of their strong immunosuppressive properties, which lead to immune evasion following adoptive transfer into an unmatched host.97
4.1 |. Pro-angiogenic properties of mesenchymal stem cells
Tumor-infiltrating MSCs likely contribute to disease progression via their pro-angiogenic properties as well.51 Bone marrow-derived MSCs promote angiogenesis by expressing proteolytic enzymes that degrade the extracellular matrix, including multiple matrix metalloproteases (MMPs), and secreting soluble factors that promote angiogenesis, such as VEGF and bFGF.56,98–101 Additionally, MSCs derived from human prostate cancer (ie, PrCSCs) induce a robust angiogenic response as demonstrated using an in vitro 3D fibrin matrix assay.51 This assay is particularly relevant because it accurately recapitulates each of the major physiologic stages necessary for new vessel formation102; ultimately generating a complex, multicellular capillary network of branched and interconnected lumens with a collagen IV-rich basement membrane surrounding each vessel. Furthermore, this assay demonstrated that MSCs are essential for angiogenesis as conditioned media from multiple prostate cancer cell lines (eg, LNCaP, PC3, CWR22Rv1) were unable to induce vessel sprouting in this assay.51 In addition to restoring immune recognition, these observations suggest that selective depletion of tumor-infiltrating MSCs will also limit the production of required pro-angiogenic factors within the tumor microenvironment to suppress tumor growth.
4.2 |. Potential for therapeutic targeting
Recent lines of evidence have demonstrated that there may be subsets of prostate cancer patients who are well suited for conventional immunomonotherapy. These include the early evidence of anti-PD-1 activity using pembrolizumab in a subset of enzalutamide-resistant mCRPC patients,103 rare exceptional responders to the anti-CTLA4 agent ipilimumab,104 and a small proportion of prostate cancer patients harboring mutations in DNA repair genes and/or microsatellite instability (MSI) that are characterized by a hyper-mutated phenotype who may respond to single agent checkpoint inhibition.105,106 Inactivation of cyclin-dependent kinase (CDK)-12 was also recently documented to identify a distinct subset of advanced prostate cancer patients characterized by increased neo-antigen burden and T-cell infiltration who may benefit from immune checkpoint inhibition.107
For the majority of patients however, combination approaches to take the brakes off immune-dampening at both the checkpoint (eg, PD-L1/PD-1) and the cellular level (eg, MSCs) may be required. Though MSCs express membrane-bound PD-L1 and PD-L2, in addition to secreting soluble forms of these ligands, and inhibition of the PD-1 axis can attenuate MSC-dependent suppression of T-cell proliferation in vitro81,82,108; the functional significance of this inhibitory axis in vivo is less clear. However, the poor clinical responses to PD1-axis targeted therapies indicate that either this is not the dominant suppressive mechanism utilized by MSCs in vivo, at least in the context of prostate cancer and other tumor types refractory to these agents, or that functional redundancies exist as a result of the pleiotropic suppressive signaling pathways attributed to MSCs as described above. Regardless of the mechanism, the therapeutic potential of eliminating this immunosuppressive population has been documented using genetically-engineered mouse models, which have demonstrated that conditional depletion of MSCs promotes immunologic control of tumor growth via increased accumulation and infiltration of tumorspecific cytotoxic T-cells109,110; essentially, taking the “brakes” off of pre-existing tumor-reactive effector cells.
Collectively, these observations strongly suggest MSCs are key regulators of the immunosuppressive microenvironment, which ultimately allows the tumor to escape immunosurveillance by preventing cytotoxic immune effector cells from infiltrating malignant foci and promoting tolerance to tumor-associated antigens. This further suggests that depletion of tumor-infiltrating MSCs can restore immunologic recognition of tumors, which is predicted to result in a compensatory upregulation of immune checkpoints as a consequence of chronic antigen exposure leading to exhaustion. Thus, there is significant potential for synergistic activity with therapeutic strategies targeting tumor-infiltrating MSCs in combination with immune checkpoint inhibition. However, it should be noted there is potential for “on-target, off-tumor” toxicity resulting from the localization of MSCs at low levels throughout the body, including the bone marrow.111 In order to generate a therapeutic index and prevent toxicity to peripheral tissues, care must be taken to selectively target either the tumor-infiltrating population over those in other tissues or the MSC-dependent signaling pathways co-opted by the tumor to promote immunologic escape and tumor progression.
5 |. CONCLUSION
In conclusion, tumor-infiltrating MSCs in prostate cancer represent a common denominator between chronic inflammation, the immunosuppressive microenvironment, T-cell exclusion, and the suppression of immune effector functions that are ultimately permissive to immunologic escape and tumor progression. Furthermore, many of these immunosuppressive properties are independent of checkpoint expression, which explains the poor responses to checkpoint inhibition and suggests a logical strategy to overcome this limitation. Based on these observations, we hypothesize that selective depletion of tumor-infiltrating MSCs can restore immunologic recognition and elimination of malignant cells via broad re-activation of cytotoxic pro-inflammatory pathways that will enhance responses to checkpoint inhibition and other forms of immunotherapy. Furthermore, this suggests that tumor-infiltrating MSCs are directly correlated with immunologic escape and disease progression and may thus provide prognostic information to identify men with aggressive lethal disease.
ACKNOWLEDGMENTS
The authors would like to acknowledge the expert assistance of the Sidney Kimmel Comprehensive Cancer Center (SKCCC) Tissue Services and Immunohistochemistry Core supported by the SKCCC Cancer Center Support Grant [CCSG, (P30 CA006973)], the Prostate Cancer Biorepository Network (PCBN) funded by the Department of Defense Prostate Cancer Research Program (W81XWH-10-2-0056 and W81XWH-10-2-0046), in addition to the following sources of financial support: Prostate Cancer Foundation Young Investigator Award (WNB and DLJT), the Patrick C. Walsh Prostate Cancer Research Fund (WNB and DLJT), the Hopkins-Allegheny Health Network Cancer Research Fund (DLJT, WNB), NIH-Prostate SPORE [P50 CA058236, (SRD, JTI)], Department of Defense Prostate Cancer Research Program [W81XWH-16-1-0410, (JTI) and W81XWH-17-1-0528, (WNB)], and the NCI [R01CA201035, (DLJT)].
Funding information
NIH-Prostate SPORE, Grant number: P30 CA058236; Patrick C. Walsh Prostate Cancer Research Fund; U.S. Department of Defense, Grant numbers: W81XWH-10-2-0046, W81XWH-10-2-0056, W81XWH-16-1-0410, W81XWH-17-1-0528; National Cancer Institute, Grant number: R01CA201035; Hopkins-Allegheny Health Network Cancer Research Fund; Prostate Cancer Foundation; NIH, Grant number: P30 CA006973
Footnotes
CONFLICT OF INTEREST
The authors have declared there are no relevant conflicts of interest to disclose.
REFERENCES
- 1.Helmink BA, Gaudreau PO, Wargo JA. Immune Checkpoint Blockade across the Cancer Care Continuum. Immunity. 2018; 48:1077–1080. [DOI] [PubMed] [Google Scholar]
- 2.June CH, Sadelain M. Chimeric Antigen Receptor Therapy. New Engl J Med. 2018; 379:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schweizer MT, Drake CG. Immunotherapy for prostate cancer: recent developments and future challenges. Cancer Metastasis Rev. 2014; 33:641–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slovin SF. Immunotherapy for prostate cancer: is prostate an immune responsive tumor? Curr Opin Urol. 2016;26:529–534. [DOI] [PubMed] [Google Scholar]
- 5.Bilusic M, Madan RA, Gulley JL. Immunotherapy of Prostate Cancer: Facts and Hopes. Clin Cancer Res. 2017;23:6764–6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laccetti AL, Subudhi SK. Immunotherapy for metastatic prostate cancer: immuno-cold or the tip of the iceberg? Curr Opin Urol. 2017;27:566–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nuhn P, De Bono JS, Fizazi K, et al. Update on Systemic Prostate Cancer Therapies: Management of Metastatic Castration-resistant Prostate Cancer in the Era of Precision Oncology. Eur Urol. 2018. [Epub ahead of print]. 10.1016/j.eururo.2018.03.028. [DOI] [PubMed] [Google Scholar]
- 8.Berger MF, Lawrence MS, Demichelis F, et al. The genomic complexity of primary human prostate cancer. Nature. 2011;470:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grasso CS, Wu YM, Robinson DR, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487: 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bander NH, Yao D, Liu H, et al. MHC class I and II expression in prostate carcinoma and modulation by interferon-alpha and −gamma. Prostate. 1997;33:233–239. [DOI] [PubMed] [Google Scholar]
- 11.Mercader M, Bodner BK, Moser MT, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci USA. 2001;98:14565–14570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNeel DG, Nguyen LD, Ellis WJ, Higano CS, Lange PH, Disis ML. Naturally occurring prostate cancer antigen-specific T cell responses of a Th1 phenotype can be detected in patients with prostate cancer. Prostate. 2001;47:222–229. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Yu J, Sreekumar A, et al. Autoantibody signatures in prostate cancer. N Engl J Med. 2005;353:1224–1235. [DOI] [PubMed] [Google Scholar]
- 14.Olson BM, McNeel DG. Antibody and T-cell responses specific for the androgen receptor in patients with prostate cancer. Prostate. 2007;67:1729–1739. [DOI] [PubMed] [Google Scholar]
- 15.Sfanos KS, Bruno TC, Maris CH, et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gannon PO, Poisson AO, Delvoye N, Lapointe R, Mes-Masson AM, Saad F. Characterization of the intra-prostatic immune cell infiltration in androgen-deprived prostate cancer patients. J Immunol Methods. 2009;348:9–17. [DOI] [PubMed] [Google Scholar]
- 17.Ebelt K, Babaryka G, Frankenberger B, et al. Prostate cancer lesions are surrounded by FOXP3+, PD-1+ and B7-H1+ lymphocyte clusters. Eur J Cancer. 2009;45:1664–1672. [DOI] [PubMed] [Google Scholar]
- 18.Sfanos KS, Bruno TC, Meeker AK, De Marzo AM, Isaacs WB, Drake CG. Human prostate-infiltrating CD8+ T lymphocytes are oligoclonal and PD-1+. Prostate. 2009;69:1694–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drake CG. Prostate cancer as a model for tumour immunotherapy. Nat Rev Immunol. 2010;10:580–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiessling A, Wehner R, Fussel S, Bachmann M, Wirth MP, Schmitz M. Tumor-associated antigens for specific immunotherapy of prostate cancer. Cancers (Basel). 2012;4:193–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molon B, Ugel S, Del Pozzo F, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011; 208:1949–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348:74–80. [DOI] [PubMed] [Google Scholar]
- 23.Yuan H, Hsiao YH, Zhang Y, et al. Destructive impact of T-lymphocytes, NK and Mast cells on basal cell layers: implications for tumor invasion. BMC Cancer. 2013;13:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez-Bujanda Z, Drake CG. Myeloid-derived cells in prostate cancer progression: phenotype and prospective therapies. J Leukoc Biol. 2017;102:393–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu AA, Drake V, Huang HS, Chiu S, Zheng L. Reprogramming the tumor microenvironment: tumor-induced immunosuppressive factors paralyze T cells. Oncoimmunology. 2015;4:e1016700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munn DH, Bronte V. Immune suppressive mechanisms in the tumor microenvironment. Curr Opin Immunol. 2016;39:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Marzo AM, Platz EA, Sutcliffe S, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7:256–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sfanos KS, De Marzo AM. Prostate cancer and inflammation: the evidence. Histopathology. 2012;60:199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am J Pathol. 1999;155:1985–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.NCI. SEER Stat Fact Sheets: Prostate. 2015. Accessed January 19, 2016.
- 31.Sakr WA, Partin AW. Histological markers of risk and the role of high-grade prostatic intraepithelial neoplasia. Urology. 2001;57:115–120. [DOI] [PubMed] [Google Scholar]
- 32.Zlotta AR, Egawa S, Pushkar D, et al. Prevalence of prostate cancer on autopsy: cross-sectional study on unscreened Caucasian and Asian men. J Natl Cancer Inst. 2013;105:1050–1058. [DOI] [PubMed] [Google Scholar]
- 33.Berges RR, Vukanovic J, Epstein JI, et al. Implication of cell kinetic changes during the progression of human prostatic cancer. Clin Cancer Res. 1995;1:473–480. [PMC free article] [PubMed] [Google Scholar]
- 34.Haffner MC, Guner G, Taheri D, et al. Comprehensive evaluation of programmed death-ligand 1 expression in primary and metastatic prostate cancer. Am J Pathol. 2018;188:1478–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spaeth E, Klopp A, Dembinski J, Andreeff M, Marini F. Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 2008;15:730–738. [DOI] [PubMed] [Google Scholar]
- 36.Brennen WN, Denmeade SR, Isaacs JT. Mesenchymal stem cells as a vector for the inflammatory prostate microenvironment. Endocr Relat Cancer. 2013;20:R269–R290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brennen WN, Chen S, Denmeade SR, Isaacs JT. Quantification of Mesenchymal Stem Cells (MSCs) at sites of human prostate cancer. Oncotarget 2013;4:106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brennen WN, Zhang B, Kulac I, et al. Mesenchymal stem cell infiltration during neoplastic transformation of the human prostate. Oncotarget. 2017;8:46710–46727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- 40.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284: 143–147. [DOI] [PubMed] [Google Scholar]
- 41.Wan M, Li C, Zhen G, et al. Injury-activated transforming growth factor beta controls mobilization of mesenchymal stem cells for tissue remodeling. Stem Cells. 2012;30:2498–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y, Yu X, Lin S, Li X, Zhang S, Song YH. Insulin-like growth factor 1 enhances the migratory capacity of mesenchymal stem cells. Biochem Biophys Res Commun. 2007;356:780–784. [DOI] [PubMed] [Google Scholar]
- 43.Ponte AL, Marais E, Gallay N, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737–1745. [DOI] [PubMed] [Google Scholar]
- 44.Wang L, Xie L, Tintani F, et al. Aberrant transforming growth factorbeta activation recruits mesenchymal stem cells during prostatic hyperplasia. Stem Cells Transl Med. 2017;6:394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. [DOI] [PubMed] [Google Scholar]
- 47.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. [DOI] [PubMed] [Google Scholar]
- 48.Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dimarino AM, Caplan AI, Bonfield TL. Mesenchymal stem cells in tissue repair. Front Immunol. 2013;4:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Phinney DG, Pittenger MF. Concise review: MSC-Derived exosomes for cell-free therapy. Stem Cells. 2017;35:851–858. [DOI] [PubMed] [Google Scholar]
- 51.Brennen WN, Nguyen H, Dalrymple SL, et al. Assessing angiogenic responses induced by primary human prostate stromal cells in a three-dimensional fibrin matrix assay. Oncotarget. 2016;7: 71298–71308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Placencio VR, Li X, Sherrill TP, Fritz G, Bhowmick NA. Bone marrow derived mesenchymal stem cells incorporate into the prostate during regrowth. PLoS ONE. 2010;5:e12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakata W, Nakai Y, Yoshida T, et al. Bone marrow-derived cells contribute to regeneration of injured prostate epithelium and stroma. Prostate. 2015;75:806–814. [DOI] [PubMed] [Google Scholar]
- 54.Kim W, Barron DA, Martin RS, et al. RUNX1 is essential for mesenchymal stem cell proliferation and myofibroblast differentiation. P Natl Acad Sci USA. 2014;111:16389–16394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klopp AH, Gupta A, Spaeth E, Andreeff M, Marini F 3rd. Concise review: dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells. 2011; 29:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bergfeld SA, DeClerck YA. Bone marrow-derived mesenchymal stem cells and the tumor microenvironment. Cancer Metastasis Rev. 2010;29:249–261. [DOI] [PubMed] [Google Scholar]
- 57.Bianchi G, Borgonovo G, Pistoia V, Raffaghello L. Immunosuppressive cells and tumour microenvironment: focus on mesenchymal stem cells and myeloid derived suppressor cells. Histol Histopathol. 2011;26:941–951. [DOI] [PubMed] [Google Scholar]
- 58.Shi Y, Du L, Lin L, Wang Y. Tumour-associated mesenchymal stem/stromal cells: emerging therapeutic targets. Nat Rev Drug Discov. 2017;16:35–52. [DOI] [PubMed] [Google Scholar]
- 59.Mishra PJ, Mishra PJ, Humeniuk R, et al. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008;68:4331–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paunescu V, Bojin FM, Tatu CA, et al. Tumour-associated fibroblasts and mesenchymal stem cells: more similarities than differences. J Cell Mol Med. 2011;15:635–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brennen WN, Isaacs JT, Denmeade SR. Rationale behind targeting fibroblast activation protein-expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy. Mol Cancer Ther. 2012;11:257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brennen WN, Kisteman LN, Isaacs JT. Rapid selection of mesenchymal stem and progenitor cells in primary prostate stromal cultures. Prostate. 2016;76:552–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.English K, Mahon BP. Allogeneic mesenchymal stem cells: agents of immune modulation. J Cell Biochem. 2011;112:1963–1968. [DOI] [PubMed] [Google Scholar]
- 64.Caplan AI, Sorrell JM. The MSC curtain that stops the immune system. Immunol Lett. 2015;168:136–139. [DOI] [PubMed] [Google Scholar]
- 65.Prockop DJ. Concise review: two negative feedback loops place mesenchymal stem/stromal cells at the center of early regulators of inflammation. Stem Cells. 2013;31:2042–2046. [DOI] [PubMed] [Google Scholar]
- 66.Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haddad R, Saldanha-Araujo F. Mechanisms of T-cell immunosuppression by mesenchymal stromal cells: what do we know so far? Biomed Res Int. 2014;2014:216806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2, 3-dioxygenase and prostaglandin E2. Blood. 2008;111: 1327–1333. [DOI] [PubMed] [Google Scholar]
- 69.Brown JM, Nemeth K, Kushnir-Sukhov NM, Metcalfe DD, Mezey E. Bone marrow stromal cells inhibit mast cell function via a COX2-dependent mechanism. Clin Exp Allergy. 2011;41:526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Djouad F, Charbonnier LM, Bouffi C, et al. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. 2007;25:2025–2032. [DOI] [PubMed] [Google Scholar]
- 71.Ren G, Zhao X, Wang Y, et al. CCR2-dependent recruitment of macrophages by tumor-educated mesenchymal stromal cells promotes tumor development and is mimicked by TNFalpha. Cell Stem Cell. 2012;11:812–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen HW, Chen HY, Wang LT, et al. Mesenchymal stem cells tune the development of monocyte-derived dendritic cells toward a myeloid-derived suppressive phenotype through growth-regulated oncogene chemokines. J Immunol. 2013;190:5065–5077. [DOI] [PubMed] [Google Scholar]
- 73.Yen BL, Yen ML, Hsu PJ, et al. Multipotent human mesenchymal stromal cells mediate expansion of myeloid-derived suppressor cells via hepatocyte growth factor/c-met and STAT3. Stem Cell Reports. 2013;1:139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Giallongo C, Tibullo D, Parrinello NL, et al. Granulocyte-like myeloid derived suppressor cells (G-MDSC) are increased in multiple myeloma and are driven by dysfunctional mesenchymal stem cells (MSC). Oncotarget. 2016;7:85764–85775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gabrilovich DI. Myeloid-derived suppressor cells. Cancer Immunol Res. 2017;5:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Calcinotto A, Spataro C, Zagato E, et al. IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature. 2018;559: 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. [DOI] [PubMed] [Google Scholar]
- 78.Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76:1208–1213. [DOI] [PubMed] [Google Scholar]
- 79.English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4 + CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009;156:149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fontaine MJ, Shih H, Schafer R, Pittenger MF. Unraveling the mesenchymal stromal cells’ paracrine immunomodulatory effects. Transfus Med Rev. 2016;30:37–43. [DOI] [PubMed] [Google Scholar]
- 81.Chinnadurai R, Copland IB, Patel SR, Galipeau J. IDO-independent suppression of T cell effector function by IFN-gamma-licensed human mesenchymal stromal cells. J Immunol. 2014;192: 1491–1501. [DOI] [PubMed] [Google Scholar]
- 82.Augello A, Tasso R, Negrini SM, et al. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35:1482–1490. [DOI] [PubMed] [Google Scholar]
- 83.Suva D, Passweg J, Arnaudeau S, Hoffmeyer P, Kindler V. In vitro activated human T lymphocytes very efficiently attach to allogenic multipotent mesenchymal stromal cells and transmigrate under them. J Cell Physiol. 2008;214:588–594. [DOI] [PubMed] [Google Scholar]
- 84.Ren G, Su J, Zhang L, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27:1954–1962. [DOI] [PubMed] [Google Scholar]
- 85.Meisel R, Brockers S, Heseler K, et al. Human but not murine multipotent mesenchymal stromal cells exhibit broad-spectrum antimicrobial effector function mediated by indoleamine 2,3-dioxygenase. Leukemia. 2011;25:648–654. [DOI] [PubMed] [Google Scholar]
- 86.Su J, Chen X, Huang Y, et al. Phylogenetic distinction of iNOS and IDO function in mesenchymal stem cell-mediated immunosuppression in mammalian species. Cell Death Differ. 2014;21:388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jung Y, Kim JK, Shiozawa Y, et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat Commun. 2013;4:1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Corcoran KE, Trzaska KA, Fernandes H, et al. Mesenchymal stem cells in early entry of breast cancer into bone marrow. PLoS ONE. 2008;3:e2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shiozawa Y, Pedersen EA, Havens AM, et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011;121:1298–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Correa D, Somoza RA, Lin P, Schiemann WP, Caplan AI. Mesenchymal stem cells regulate melanoma cancer cells extravasation to bone and liver at their perivascular niche. Int J Cancer. 2016;138:417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Levy O, Brennen WN, Han E, et al. A prodrug-doped cellular Trojan Horse for the potential treatment of prostate cancer. Biomaterials. 2016;91:140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Krueger TEG, Thorek DLJ, Denmeade SR, Isaacs JT, Brennen WN. Concise review: mesenchymal stem cell-based drug delivery: the good, the bad, the ugly, and the promise. Stem Cells Transl Med. 2018;9:651–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martin AM, Nirschl TR, Nirschl CJ, et al. Paucity of PD-L1 expression in prostate cancer: innate and adaptive immune resistance. Prostate Cancer Prostatic Dis. 2015;18:325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Galipeau J, Sensebe L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22:824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.MesoblastLimited. Primary Endpoint Successfully Achieved in Mesoblast’s Phase 3 Cell Therapy Trial for Acute Graft Versus Host Disease. GlobeNewswire; 2018. [Google Scholar]
- 96.MesoblastLimited. Key Day 100 Survival Outcomes of Phase 3 Trial for Acute Graft Versus Host Disease Presented at 2018 ISSCR Annual Meeting. GlobeNewswire; 2018. [Google Scholar]
- 97.Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32:252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ghajar CM, Blevins KS, Hughes CC, George SC, Putnam AJ. Mesenchymal stem cells enhance angiogenesis in mechanically viable prevascularized tissues via early matrix metalloproteinase upregulation. Tissue Eng. 2006;12:2875–2888. [DOI] [PubMed] [Google Scholar]
- 99.Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tao H, Han Z, Han ZC, Li Z. Proangiogenic features of mesenchymal stem cells and their therapeutic applications. Stem Cells Int. 2016;2016:1314709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Merino-Gonzalez C, Zuniga FA, Escudero C, et al. Mesenchymal stem cell-derived extracellular vesicles promote angiogenesis: potencial clinical application. Front Physiol. 2016;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nakatsu MN, Sainson RC, Aoto JN, et al. Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: the role of fibroblasts and Angiopoietin-1. Microvasc Res. 2003;66:102–112. [DOI] [PubMed] [Google Scholar]
- 103.Graff JN, Alumkal JJ, Drake CG, et al. Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget. 2016;7:52810–52817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cabel L, Loir E, Gravis G, et al. Long-term complete remission with Ipilimumab in metastatic castrate-resistant prostate cancer: case report of two patients. J Immunother Cancer. 2017;5:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pritchard CC, Morrissey C, Kumar A, et al. Complex MSH2 and MSH6 mutations in hypermutated microsatellite unstable advanced prostate cancer. Nat Commun. 2014;5:4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Isaacsson Velho P, Antonarakis ES. PD-1/PD-L1 pathway inhibitors in advanced prostate cancer. Expert Rev Clin Pharmacol. 2018;11: 475–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wu YM, Cieslik M, Lonigro RJ, et al. Inactivation of CDK12 delineates a distinct immunogenic class of advanced prostate cancer. Cell. 2018;173:1770–1782. e1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Davies LC, Heldring N, Kadri N, Le Blanc K. Mesenchymal stromal cell secretion of programmed death-1 ligands regulates t cell mediated immunosuppression. Stem Cells. 2017;35: 766–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kraman M, Bambrough PJ, Arnold JN, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330:827–830. [DOI] [PubMed] [Google Scholar]
- 110.Feig C, Jones JO, Kraman M, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci USA. 2013;110:20212–20217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Roberts EW, Deonarine A, Jones JO, et al. Depletion of stromal cells expressing fibroblast activation protein-alpha from skeletal muscle and bone marrow results in cachexia and anemia. J Exp Med. 2013;210:1137–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]