Abstract
Vaccination is a safe and effective way to prevent Human Papillomavirus (HPV) infection and related cancers; however, HPV vaccine uptake remains low in the US. After the 2011 Advisory Committee on Immunization Practices (ACIP) recommendation for routine HPV vaccination of adolescent males, several studies have examined predictors for initiating the vaccine series in this population of interest, particularly with regard to provider recommendations. This study examined racial and ethnic differences for HPV vaccine initiation and provider recommendation in male adolescents. Based on prior HPV vaccine uptake estimates and healthcare utilization data, we hypothesized that minority adolescents would be more likely to initiate HPV vaccines, but less likely to receive a provider recommendation compared to white counterparts. We analyzed the 2014 National Immunization Survey-Teen (NIS-Teen), which included 10,753 male adolescents with provider-verified vaccination data in 50 US states, using multivariate logistic regression models to evaluate racial/ethnic differences in HPV vaccine initiation and provider recommendation. The odds of HPV vaccine initiation were 76 percent higher for Hispanic adolescents and 43 percent higher for non-Hispanic Other or Multiple race adolescents compared to white adolescents. Approximately half of parents reported receiving a provider recommendation for vaccination, with no significant difference in the odds of receiving a provider recommendation across racial/ethnic groups. Despite similar frequency of recommendations across racial and ethnic groups, male adolescents who are racial/ethnic minorities are more likely to initiate vaccination. Future research should focus on developing tailored interventions to increase HPV vaccine receipt among males of all racial/ethnic groups.
Keywords: HPV vaccines, Human papillomavirus, Adolescent, Provider recommendation, Race, Ethnicity
1. Introduction
Over the past decade, vaccination against Human Papillomavirus (HPV) has emerged as a safe, effective way to prevent HPV infection and subsequent related cancers [1]. Because racial/ethnic minorities are disproportionately affected by several HPV-related cancers, vaccination can also help reduce HPV-related cancer disparities in minority populations [2].
However, HPV vaccine uptake remains low in the US, with current national coverage levels still significantly below the Healthy People 2020 goal of 80% coverage [3]. Unique barriers for parents and adolescents, such as awareness, acceptance, and lack of provider recommendation, all pose challenges to vaccine uptake [4]. Additionally, the majority of the HPV vaccine literature focuses on barriers to vaccine uptake for female adolescents. However, uptake for males has been considerably slower. Following FDA licensure for male vaccination in 2009, the Advisory Committee on Immunization Practices (ACIP) initially recommended permissive use of the vaccine for males in 2010, but did not recommend routine use until 2011 [5]. Vaccinating males can help reduce HPV transmission to female sexual partners [6,7] and prevent approximately 70% of HPV-attributable oropharyngeal cancers, which are expected to surpass the number of HPV-attributable cervical cancers in the US by 2020 [8]. Vaccinating males offers direct benefits for cancer prevention, as oropharyngeal cancers are often only detected at advanced stages [9]. Studies have also shown that emphasizing oropharyngeal cancer prevention is a more persuasive argument compared to altruism (i.e., cancer prevention in females) in increasing male vaccine uptake [10–12]. Vaccinating males also protects groups like men who have sex with men, who do not benefit from female-only vaccination approaches to decrease the spread of HPV [6].
More recently, researchers have also sought to better understand individual parent and adolescent socio-demographic characteristics that predict vaccine uptake, with hopes of tailoring patient care and interventions to improve protection from HPV. These predictors and the parental decision making process for adolescent males are still not fully understood [13].
We used the 2014 National Immunization Survey – Teen [14] to examine racial/ethnic differences in HPV vaccine initiation (first of three doses) and provider recommendation for adolescent males. Based on existing literature, we hypothesized that racial/ethnic minority adolescents would be more likely to initiate, but less likely to receive a provider recommendation for HPV vaccines compared to their white counterparts [15–18]. Using multiple logistic regression, we identified key socio-demographic predictors of HPV vaccine initiation in adolescent males.
Study results can help identify missed educational opportunities for adolescent males and their providers by identifying key predictors of initiation for males and exploring racial/ethnic differences. Findings can also help guide future interventions targeting male adolescents to increase patient-provider communication and reduce HPV-related cancer disparities.
2. Methods
2.1. Data source: National Immunization Survey- Teen (NIS-Teen) 2014
In 2008, the National Center for Immunizations and Respiratory Diseases and the Center for Health Statistics at the CDC expanded the original NIS survey to sample parents or caregivers of adolescents 13–17 years old in all 50 states, District of Columbia, and Puerto Rico or Virgin Islands [19]. The NIS-Teen contacts participants by using a list-assisted random-digit-dialing telephone survey. In 2012, the CDC also started randomly sampling cell-phone only households. In 2014, response rates of 60.3% and 31.2% were achieved from those contacted via landline and cell phone, respectively [19]. This yielded a total sample of 38,703 adolescents (20,030 landline and 18,673 cell-phone only) [19]. Parents/caregivers were asked to self-report demographic characteristics and vaccination history of adolescents. Interviewers also requested permission to contact immunization providers to obtain provider-verified vaccination data. In 2014, 64.4% of landline respondents and 61.2% of cell-phone respondents gave oral consent for NIS to follow up with providers [19]. Ultimately, 94.9% of landline sample providers and 94.8% of cell-phone providers returned vaccine questionnaires [19]. Based on these responses, 11,243 (57.1%) of landline-sample teens were considered to have adequate provider vaccination data. For the cell-phone sample teens, 9,584 (52.3%) had adequate provider data. In 2014, adequate provider vaccination data weights were provided for adolescents in all 50 US states, excluding Puerto Rico [19].
2.2. Study sample
The analytic sample included the 2014 NIS-Teen used with a cross-sectional study design. Only male adolescents with provider-verified vaccination data and survey weights for all 50 US states, excluding teens sampled in Puerto Rico, were analyzed. The final sample included 10,743 adolescent males with provider-verified data in 50 US states.
2.3. Theoretical framework
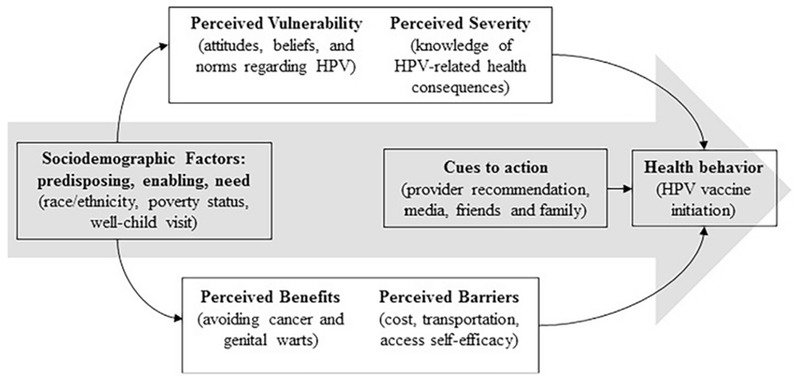
This study was guided by the Andersen and Aday Behavioral Model of Healthcare Utilization [20] and the Health Belief Model [21]. The Andersen and Aday model provides a well-developed structure for individual-level characteristics that motivate health behaviors, such as socioeconomic status [20]. The Health Belief Model is used to understand the decision-making process of using preventive services, and how “cues to action” influence health behaviors like vaccination [21]. We also examined provider recommendation as a potential moderator for the association between race/ethnicity and initiation as a method of investigating if minority adolescents and parents are more likely to adhere to the recommendation. The Health Belief Model has previously been used to map the predictors of HPV vaccination [22]. The theoretical framework is detailed in Fig. 1.
Fig. 1.
Conceptual framework: HPV vaccine initiation process for male racial/ethnic minority adolescents. Adapted from Thomas et al. [22].
2.4. Study outcomes
The primary outcome of interest was receipt of the first of three HPV vaccine doses (initiation). Initiation was measured using the number of provider verified HPV shots received by each adolescent. If the adolescent received one or more shots then he was considered to have initiated the vaccine. If the adolescent received 0 shots then he was considered to not have initiated the vaccine sequence.
2.5. Independent variables
Adolescent race/ethnicity was reported by caregivers and categorized as Hispanic, non-Hispanic White (reference), non-Hispanic Black, and non-Hispanic Other or Multiple Race. The second primary independent variable was provider recommendation. Caregivers were asked “Had or has doctor or other health care professional ever recommended that [Teen] receive HPV shots?” Recommendation was categorized as a dichotomous variable. Socio-demographic factors included age of adolescent, age of mother, health insurance, receipt of 11–12 year well-child visit, poverty status, mother’s education level, receipt of other adolescent vaccines (Tdap or Meningococcal), mother’s marital status and census region.
2.6. Statistical analysis
We generated descriptive statistics for key variables of interest and socio-demographic characteristics of adolescents and caregivers (Table 1). Chi-square tests were used to compare individual characteristics of adolescent HPV vaccine initiators and non-initiators. To evaluate the effect of race/ethnicity on initiation, we used two logistic regression models and controlled for confounding variables. A third logistic regression model examined racial/ethnic differences in rates of provider recommendation, controlling for the same set of confounding variables (Table 2). Statistics were performed using SAS 9.4 (SAS Institute, Inc, Cary, NC), using appropriate complex survey procedures to account for the NIS-Teen’s dual-frame sampling and weighting techniques.[19] Significance levels were set as p < .05 and logistic regression results were expressed as odds ratios. The Emory University Institutional Review Board exempted this study from review.
Table 1.
Weighted adolescent male and parent characteristics by HPV vaccine initiation status, NIS Teen 2014.
| Total, % (N = 10,743) | Initiated, % (N = 4436) | Did not initiate, % (N = 6307) | p Value | |
|---|---|---|---|---|
| Adolescent characteristics | ||||
| US weighted sample size | 10,659,765 | 4,450,370 | 6,209,395 | |
| Proportion of sample | 100 | 41.3 | 58.7 | |
| Mean age of teen at survey (years) | 15.0 | 15.0 | 14.9 | |
| Race/ethnicity | <.0001 | |||
| Hispanic | 22.0 | 54.2 | 45.8 | |
| Non-Hispanic white | 55.9 | 36.4 | 63.6 | |
| Non-Hispanic black | 13.2 | 42.1 | 57.9 | |
| Non-Hispanic other/multiple race | 8.9 | 44.0 | 56.0 | |
| Had 11–12 year well-child visit | .3051 | |||
| Yes | 90.4 | 44.4 | 55.6 | |
| No | 5.6 | 36.0 | 64.0 | |
| Don’t know | 4.0 | 43.6 | 56.4 | |
| Health insurance | .0008 | |||
| Employer/Union | 57.1 | 38.5 | 61.5 | |
| Any Medicaid/SCHIP | 31.4 | 47.3 | 52.7 | |
| Indian/Military/Other | 4.8 | 40.1 | 59.9 | |
| Uninsured | 6.7 | 40.5 | 59.5 | |
| Region of country | <.0001 | |||
| Northeast | 16.7 | 48.0 | 52.0 | |
| Midwest | 21.7 | 37.8 | 62.2 | |
| South | 37.8 | 37.9 | 62.1 | |
| West | 23.9 | 47.0 | 53.0 | |
| UTD Tdap or Meningococcal | <.0001 | |||
| Yes | 91.3 | 45.4 | 54.6 | |
| No | 8.7 | 3.4 | 96.6 | |
| Parent characteristics | ||||
| Received provider recommendation for HPV vaccine | <.0001 | |||
| Yes | 53.7 | 63.4 | 36.6 | |
| No | 46.3 | 19.2 | 80.8 | |
| Maternal education level | <.0001 | |||
| Less than 12 years | 13.4 | 55.8 | 44.2 | |
| 12 years | 23.8 | 42.4 | 57.6 | |
| 12 + years, non college grad | 25.7 | 36.4 | 63.6 | |
| College graduate | 37.1 | 40.0 | 60.0 | |
| Maternal age group | .0672 | |||
| ≤34 years | 8.3 | 48.9 | 51.1 | |
| 35–44 years | 45.6 | 41.2 | 58.8 | |
| ≥45 years | 46.1 | 41.0 | 59.0 | |
| Marital status | .0207 | |||
| Married | 67.5 | 40.2 | 59.8 | |
| Not currently married | 32.5 | 45.0 | 55.0 | |
| Poverty status | <.0001 | |||
| Below poverty | 22.4 | 51.6 | 48.4 | |
| Above poverty ≤ $75,000 | 35.8 | 39.4 | 60.6 | |
| Above poverty > $75,000 | 36.5 | 39.6 | 60.4 | |
| Unknown | 5.3 | 30.7 | 69.3 |
Source: CDC, NCRID and NCHS (2015), 2014 National Immunization Survey – Teen.
Initiation = 1 or more shots; UTD = Up-to-date; Tdap = Tetanus Diphtheria Pertussis; Note: Row percents shown for Initiated and Did not initiate categories.
Table 2.
Weighted logistic regressions for racial/ethnic differences in adolescent male HPV vaccine initiation and provider recommendation, NIS Teen 2014.
| Model 1: initiation OR (95% CI) | Model 2: initiation (with recommendation) OR (95% CI) | Model 3: provider recommendation OR (95% CI) | |
|---|---|---|---|
| Race/ethnicity of adolescent | |||
| Non-Hispanic white | Ref | Ref | Ref |
| Hispanic | 1.76 (1.32–2.34) | 1.91 (1.41–2.57) | 1.16(0.87–1.55) |
| Non-Hispanic black | 1.27 (0.96–1.68) | 1.20 (0.86–1.68) | 1.22 (0.93–1.61) |
| Non-Hispanic other/multiple race | 1.43 (1.05–1.96) | 1.59 (1.11–2.29) | 0.98 (0.71–1.34) |
Note. All models control for age of teen, well-child visit, health insurance, region, Tdap or Meningococcal, maternal age group, maternal education level, marital status, and poverty status;
Model 1 N = 9099; Model 2 N = 8268; Model 3 N = 8268.
3. Results
Table 1 illustrates weighted individual characteristics for adolescent male initiators and non-initiators of HPV vaccines. Out of 10,743 male adolescents with adequate provider data, 4436 adolescents (41.3%) initiated the HPV vaccine. The majority of male adolescents were non-Hispanic white (55.9%), had an 11–12 year-old well-child visit (90.4%), had insurance coverage through an employer/union (57.1%), lived in the South (37.8%), and were up-to-date on either Tdap or Meningococcal vaccines (75.3%). Overall, 53.7% of parents self-reported receiving a recommendation for HPV vaccines from a healthcare provider. The largest percentage of adolescents came from families with reported incomes above poverty and making >$75 K (36.4%). The largest number of their mothers were college graduates (37.1%), age 45 years or older (46.1%), and were currently married (67.5%). The sample significantly differed on several adolescent and parental characteristics by initiation status, including race/ethnicity, health insurance, region, being up-to-date on Tdap or Meningococcal, HPV recommendation, maternal education level, maternal marital status and poverty status (Chi-squared tests, p < .05) (Table 1). For example, 54.2% of Hispanic adolescents initiated the HPV vaccine series, compared to only 36.4% of non-Hispanic white adolescents. The majority of adolescents with employer/union insurance (61.5%) and adolescents in the South (62.1%) and Midwest (62.2%) did not initiate, respectively. While those with public insurance were more likely to initiate (47.3%), rates for male adolescents remained low overall.
After controlling for various individual adolescent and parental characteristics (excluding provider recommendation), we found that adolescent race/ethnicity significantly predicted likelihood of HPV vaccine initiation (Table 2, Model 1). More specifically, Hispanic adolescents had 76 percent higher odds of initiating HPV vaccines compared to non-Hispanic whites (OR: 1.76; 95% CI: 1.32–2.34). Non-Hispanic other and multiple race adolescents had 43 percent higher odds of initiation (OR: 1.43; 95% CI: 1.05–1.96). Finally, non-Hispanic black adolescents were no more likely to initiate than their non-Hispanic white counterparts (OR: 1.27; 95% CI: 0.96–1.68).
Model 3 examined the relationship between race/ethnicity and provider recommendation (Table 2). After controlling for individual-level covariates, we found no statistically significant racial/ethnic differences for provider recommendation in male adolescents. Hispanic and Non-Hispanic black adolescents had 16 percent (95% CI: 0.87–1.55) and 22 percent (95% CI: 0.93–1.61) higher odds of receiving a recommendation compared to white counterparts, respectively. Non-Hispanic other and multiple race adolescents had 2 percent lower odds of receiving a recommendation in comparison to whites (95% CI: 0.71–1.34).
To assess the effect of provider recommendation on the relationship between race/ethnicity and vaccine initiation, we included an interaction term between race/ethnicity and recommendation in a separate model; overall, the interaction term was significant (p = .0318) when controlling for the same set of individual adolescent and parent characteristics. Figs. 2 and 3 illustrate how provider recommendation affected the association between race/ethnicity and initiation. Fig. 2 was stratified by recommendation status (yes/no), and compared minority racial/ethnic groups to non-Hispanic whites (reference group) for each recommendation status. For those who received a recommendation, Hispanic adolescents had by far the highest odds of initiating HPV vaccines compared to whites. For those who did not receive a recommendation, Hispanics, non-Hispanic blacks, and Non-Hispanic other/multiple race adolescents had odds of initiation twice as high compared to whites. We then stratified Fig. 3 by race/ethnicity, and compared adolescents who received recommendations to those who did not for each racial/ethnic group. Using no recommendation as the reference group, we found that non-Hispanic white adolescents had nearly 11 times higher odds of initiation when they had received a recommendation. The odds of Hispanic adolescents initiating after receiving a recommendation were nearly 9 times higher, while the odds of non-Hispanic black and other/multiple race adolescents were nearly 5 times higher and 7 times higher, respectively. Overall, the relationship between receiving a provider recommendation and initiation was strong for all racial/ethnic groups.
Fig. 2.
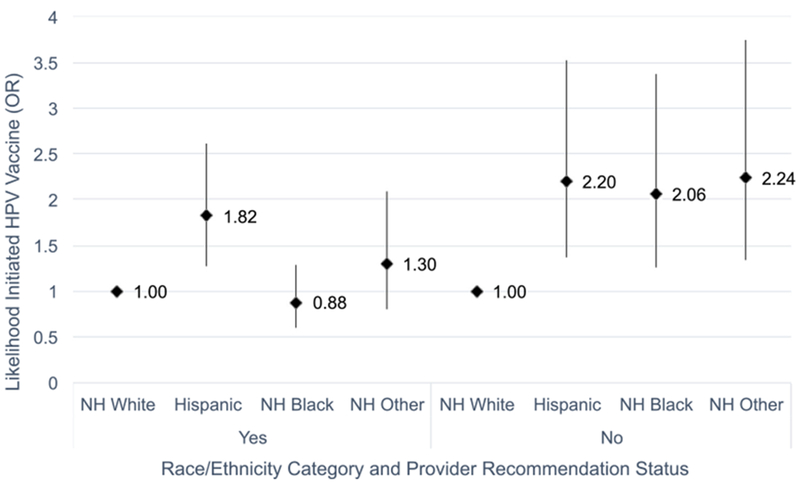
Interaction between race/ethnicity and provider recommendation on male HPV vaccine initiation (stratified by provider recommendation status), NIS-Teen 2014. Source: CDC, NCRID and NCHS (2015), 2014 National Immunization Survey – Teen.
Fig. 3.
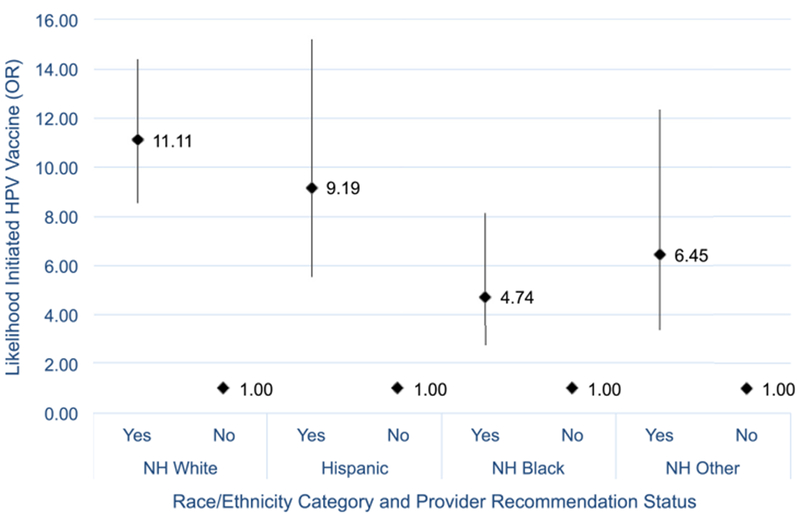
Interaction between race/ethnicity and provider recommendation on male HPV vaccine initiation (stratified by race/ethnicity), NIS-Teen 2014. Source: CDC, NCRID and NCHS (2015), 2014 National Immunization Survey – Teen.
4. Discussion
Our analysis found that male HPV vaccination remains low (41.7%), although it had increased from the 2013 data (34.6%). [18] Similar to previous studies, Hispanic male adolescents had 76% higher odds of initiating HPV vaccines compared to whites [15,16,23]. It is possible that Hispanic parents are more likely to adhere to provider recommendations, or have a more positive view of vaccines [24]. Additional research should aim to explain these racial/ethnic differences by examining sociocultural views on HPV vaccination.
Contrary to our hypothesis, the odds of male adolescents receiving a provider recommendation for HPV vaccines did not differ by race/ethnicity. For all racial/ethnic groups, approximately 53% of parents reported receiving provider recommendations. This suggests that minority male adolescents are receiving similar opportunities for patient-provider discussion on HPV vaccines compared to their white counterparts, but recommendations rates should be improved for all adolescents to reduce HPV-related cancer outcomes.
We also found that adolescents up-to-date on Tdap or Meningococcal vaccines had significantly higher odds of both initiation (OR: 18.59; 95% CI: 11.17–30.94) and provider recommendation (OR: 3.20; 95% CI: 2.35–4.37) compared to those who were not up-to-date or did not report receiving a provider recommendation. This suggests that receipt of other adolescent vaccines plays an important role in the HPV vaccination process, and supports previous research on the benefit of “bundling” HPV, Tdap, and Meningococcal vaccines together during provider visits [25]. It is also possible that adolescents who were up-to-date on one or both 11–12 year vaccines may be more likely to have a usual source of healthcare and, therefore, have more consistent opportunities to discuss HPV vaccination with providers.
Provider recommendation was a strong predictor of HPV vaccination, with adolescents who reported receiving a recommendation having odds of initiation more than eight times greater than those who did not report receiving a recommendation (OR: 8.92; 95% CI: 7.22–11.02). The importance of provider recommendations for HPV vaccines has been established in the literature [26–28]. One study that examined initiation and provider recommendation in adolescent females found that minorities were less likely to report receiving a provider recommendation for HPV vaccines compared to whites [17]. However, we found no significant racial/ethnic differences in provider recommendation for this male sample. This could be the result of provider hesitancy to discuss HPV vaccination with female minorities compared to male minorities, possibly due to perceived cultural norms about female adolescent sexual education or activity.
We also found regional differences in provider recommendations, which may reflect differing social norms surrounding discussion of preventing sexually transmitted diseases. Parents in the Midwest (OR: 0.65; 95% CI: 0.52–0.82) and South (OR: 0.59; 95% CI: 0.47–0.74) were significantly less likely to report receiving provider recommendations for HPV vaccines compared to their counterparts in the Northeast. Improving uptake in these regions is important, particularly in the South, due to an established lack of preventive HPV screenings and HPV-related cancer disparities (e.g. anal cancer) [29].
One positive finding was that provider recommendation rates were consistent across all racial/ethnic groups. However, recommendation rates should be improved for all male adolescents, as the literature shows that recommendations for males still substantially lag behind their female counterparts [30,31]. Policymakers and clinical decision makers should support continued education for healthcare providers in order to increase recommendation rates and enable providers to send effective messages to their patients. States can also promote policies to fund research and implementation of evidence-based communication strategies to improve uptake for all adolescent males. Additionally, passage of the Affordable Care Act (ACA) in 2010 helped eliminate a significant financial barrier for HPV vaccines, which previously cost up to $500 for all three doses. Researchers have attributed the passage of the ACA with an uptake of HPV vaccination for women [32]. Therefore, it is likely that male HPV vaccination will also benefit.
The interaction results also have interesting implications for male HPV vaccination. We observed how the relationship between race/ethnicity and HPV vaccine initiation might be moderated by provider recommendation status. Figs. 2 and 3 illustrated how receiving a provider recommendation may be more important for parents of non-Hispanic white adolescents. For adolescents who did not receive recommendations, all three minority groups had significantly higher odds of HPV initiation compared to whites (Fig. 2). In Fig. 3, we observed how the magnitude of increased likelihood of initiation was smallest for non-Hispanic blacks. This suggests that recommendation may not be as important in the decision-making process, and other educational tools may improve uptake for non-Hispanic black adolescents. Additionally, we focused on predictors for initiation of the three-dose series, but more recently, a two-dose schedule was recommended for younger adolescents [33]. Several studies have demonstrated that while minority adolescents have higher rates of vaccine initiation, they have lower rates of completion [34]. A shift from three doses to two doses may continue to help improve uptake for all adolescents. Overall, we found that provider recommendation is a strong “cue to action” for all racial/ethnic groups, but that recommendations are not the only driving force behind minority adolescent vaccination.
This study has several important limitations. Both landline and cellphone response rates for NIS-Teen are traditionally low (68% and 23%, respectively) [19]. While this is similar to other national telephone-based surveys, it may bias results because parents who are active in health-seeking behaviors may be more likely to participate. The self-reported nature of provider recommendation also makes the study vulnerable to parent recall bias. Parents may have been confused as to what constitutes a recommendation, or may have reported a recommendation regardless of whether or not it was received before the time of initiation. Notably, NIS-Teen does not survey 11–12-year-olds, who are the primary recommended population for HPV vaccination. In addition, the cumulative nature of NIS-Teen means that some adolescents have had more time to receive HPV vaccines and be categorized as having initiated or completed the series. These adolescents and their parents may have had more exposure to the 2011 ACIP routine use recommendation for males. This may affect parent recommendation recall, especially if parents are more able to remember recommendations immediately following the ACIP endorsement. Finally, because the NIS-Teen recently changed the definition of adequate provider data, the 2014 estimates will not be directly comparable to estimates published using previous years of NIS-Teen data. Despite these limitations, the findings are generalizable to US males aged 13–17, and using provider-verified data strengthens the internal validity of the study.
This study found that for males, Hispanic adolescents and non-Hispanic other or Multiple Race adolescents had higher odds of HPV vaccine initiation than their white counterparts. However, there were no significant racial/ethnic differences in the likelihood of receiving a provider recommendation, suggesting that recommendations serve as a strong “cue to action” for male adolescents across racial/ethnic groups. Future studies should expand upon these findings with a focus on both vaccination initiation and completion. Future studies can also examine important differences in HPV vaccination outcomes by measuring race and ethnicity with two separate constructs. Further, qualitative analysis may be useful for identifying predictors of vaccination, the vaccination decision-making process for adolescent males and their parents, and whether or not cultural norms play an important role.
There is still much room for improvement in male HPV vaccination uptake, and more research is needed to determine reasons behind racial/ethnic differences in facilitators of vaccination. Given the relative newness of HPV vaccination for males, there is a prime opportunity to expand vaccine updates and promote policy action such as school-mandated education and social marketing targeted to male adolescents.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The National Immunization Survey 2014 is available from the Centers for Disease Control and Prevention. [US Department of Health and Human Services (DHHS). National Center for Health Statistics. The 2014 National Immunization Survey. Hyattsville, MD: Centers for Disease Control and Prevention, 2014.http://www.cdc.gov/NCHS/his/data_files.htm]. All analyses, interpretations or conclusions reached are attributable to the authors and not to the National Center for Health Statistics (NCHS), which is responsible only for the initial data.
Disclosure statement
Ms. Landis and Drs. Bednarczyk and Gaydos report no conflicts of interest. Dr. Bednarczyk receives funding from National Institutes of Health grant 5K01AI106961-04.
Abbreviations:
- HPV
human papillomavirus
- NIS-Teen
National Immunization Survey – Teen
- ACIP
Advisory Committee on Immunization Practices
- SCHIP
State Children’s Health Insurance Program
- UTD
Up-to-Date
- Tdap
Diphtheria Tetanus Pertussis
- CDC
Centers for Disease Control and Prevention
- NCRID
National Center for Immunization and Respiratory Diseases
- NCHS
National Center for Health Statistics
Footnotes
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.vaccine.2018.04.075.
References
- [1].Plotkin SA, Orenstein WA, Offit PA. Vaccines. 6th ed Philadelphia, Pa: Elsevier Saunders; 2013. xix, 1550 pages. [Google Scholar]
- [2].Accelerating HPV Vaccine Uptake: Urgency for Action to Prevent Cancer A Report to the President of the United States from the President’s Cancer Panel, in Bethesda, MD: National Cancer Institute; 2014. [Google Scholar]
- [3].Healthy People 2020. Washington, DC: US Department of Health and Human Services 2012; October 24,2015]; Available from: <http://www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicId=23>. [Google Scholar]
- [4].Berenson AB. An update on barriers to adolescent human papillomavirus vaccination in the USA. Expert Rev Vaccines 2015:1–8. [DOI] [PubMed] [Google Scholar]
- [5].FDA Licensure of Quadrivalent Human Papillomavirus Vaccine (HPV4, Gardasil) for Use in Males and Guidance From the Advisory Committee on Immunization Practices (ACIP) (Reprinted from MMWR, vol 59, pg 630-632, 2010). Jama-J Am Med Assoc 2010; 304(5): 518–9. [PubMed] [Google Scholar]
- [6].Sadlier C et al. Prevalence of human papillomavirus in men who have sex with men in the era of an effective vaccine; a call to act. HIV Med 2014;15 (8):499–504. [DOI] [PubMed] [Google Scholar]
- [7].Luyten J, Engelen B, Beutels P. The sexual ethics of HPV vaccination for boys. HEC Forum 2014;26(1):27–42. [DOI] [PubMed] [Google Scholar]
- [8].Chaturvedi AK et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011;29(32):4294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gillison ML et al. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol 2015;33(29):3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Liddon N et al. Acceptability of human papillomavirus vaccine for males: a review of the literature. J Adolesc Health 2010;46(2):113–23. [DOI] [PubMed] [Google Scholar]
- [11].McRee AL et al. Does framing human papillomavirus vaccine as preventing cancer in men increase vaccine acceptability? Cancer Epidemiol Biomarkers Prevention 2010;19(8):1937–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Newman PA et al. HPV vaccine acceptability among men: a systematic review and meta-analysis. Sex Trans Infect 2013;89(7):568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dunne EF et al. Recommendations on the use of quadrivalent human papillomavirus vaccine in males-advisory committee on immunization practices (ACIP), 2011 (Reprinted from MMWR, vol 60, pg 1705, 2011). Jama-J Am Med Assoc 2012;307(6):557–9. [PubMed] [Google Scholar]
- [14].U.S. Department of Health and Human Services (DHHS). National Center for Health Statistics The 2014 National Immunization Survey – Teen. 2015, Centers for Disease Control and Prevention: Hyattsville, MD. [Google Scholar]
- [15].Stokley S et al. Human papillomavirus vaccination coverage among adolescents, 2007-2013, and post licensure vaccine safety monitoring, 2006–2014-United States. Mmwr-Morbidity and Mortality Weekly Report 2014;63(29):620–4. [PMC free article] [PubMed] [Google Scholar]
- [16].Taylor JL et al. Vaccinating sons against HPV: results from a US national survey of parents. Plos One 2014;9(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ylitalo KR, Lee H, Mehta NK. Health care provider recommendation, human papillomavirus vaccination, and race/ethnicity in the US national immunization survey. Am J Publ Health 2013;103(1):164–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Reagan-Steiner S et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years-United States, 2014. MMWR Morb Mortal Wkly Rep 2015;64(29):784–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Centers for Disease Control and Prevention. Teen Vaccination Coverage. A User’s Guide for the 2014 Public-Use Data File National Immunization Survey (NIS) - Teen. [cited 2014; Available from: <ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/NIS/NISTEENPUF14_DUG.pdf>.
- [20].Aday LA, Andersen R. A framework for the study of access to medical care. Health Serv Res 1974;9(3):208–20. [PMC free article] [PubMed] [Google Scholar]
- [21].Janz NK, Becker MH. The health belief model: a decade later. Health Educ Q 1984;11(1):1–47. [DOI] [PubMed] [Google Scholar]
- [22].Thomas TL et al. An opportunity for cancer prevention during preadolescence and adolescence: stopping human papillomavirus (HPV)-related cancer through HPV vaccination. J Adolesc Health 2013;52(5 Suppl):S60–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Curtis CR et al. National and state vaccination coverage among adolescents aged 13-17 years – United States, 2012. Mmwr-Morbidity Mortality Weekly Report 2013;62(34):685–93. [PMC free article] [PubMed] [Google Scholar]
- [24].Reiter PL et al. Early adoption of the human papillomavirus vaccine among Hispanic adolescent males in the United States. Cancer 2014;120(20):3200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ogunbajo A et al. “I think they’re all basically the same”: parents’ perceptions of human papilloma virus (HPV) vaccine compared with other adolescent vaccines. Child Care Health Dev 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gargano LM et al. Impact of a physician recommendation and parental immunization attitudes on receipt or intention to receive adolescent vaccines. Human Vacc Immunother 2013;9(12):2627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dorell C, Yankey D, Strasser S. Parent-reported reasons for nonreceipt of recommended adolescent vaccinations, national immunization survey: teen, 2009. Clin Pediatr (Phila) 2011;50(12):1116–24. [DOI] [PubMed] [Google Scholar]
- [28].Rosenthal SL et al. Predictors of HPV vaccine uptake among women aged 19-26: importance of a physician’s recommendation. Vaccine 2011;29(5):890–5. [DOI] [PubMed] [Google Scholar]
- [29].Joseph DA et al. Understanding the burden of human papillomavirus-associated anal cancers in the US. Cancer 2008;113(10 Suppl):2892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].McRee AL, Gilkey MB, Dempsey AF. HPV vaccine hesitancy: findings from a statewide survey of health care providers. J Pediatr Health Care 2014;28 (6):541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Luque JS et al. Recommendations and administration of the HPV vaccine to11-to 12-year-old girls and boys: a statewide survey of Georgia vaccines for children provider practices. J Low Genit Tract Dis 2014;18(4):298–303. [DOI] [PubMed] [Google Scholar]
- [32].Lipton BJ, Decker SL. ACA provisions associated with increase in percentage of young adult women initiating and completing the HPV vaccine. Health Aff (Millwood) 2015;34(5):757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Markowitz LE, Meites E, Unger ER. Two vs three doses of human papillomavirus vaccine: new policy for the second decade of the vaccination program. JAMA 2016;316(22):2370–2. [DOI] [PubMed] [Google Scholar]
- [34].Ackerson B et al. Human papillomavirus vaccine series completion in boys before and after recommendation for routine immunization. Vaccine 2017;35 (6):897–902. [DOI] [PubMed] [Google Scholar]