Abstract
The emergence of the bilateral embryonic body axis from a symmetrical egg has been a long-standing question in developmental biology. Historical and modern experiments point to an initial symmetry-breaking event leading to localized Wnt and Nodal growth factor signaling and subsequent induction and formation of a self-regulating dorsal “organizer.” This organizer forms at the site of notochord cell internalization and expresses primarily Bone Morphogenetic Protein (BMP) growth factor antagonists that establish a spatiotemporal gradient of BMP signaling across the embryo, directing initial cell differentiation and morphogenesis. Although the basics of this model have been known for some time, many of the molecular and cellular details have only recently been elucidated and the extent that these events remain conserved throughout vertebrate evolution remains unclear. This chapter summarizes historical perspectives as well as recent molecular and genetic advances regarding: (1) the mechanisms that regulate symmetry-breaking in the vertebrate egg and early embryo, (2) the pathways that are activated by these events, in particular the Wnt pathway, and the role of these pathways in the formation and function of the organizer, and (3) how these pathways also mediate anteroposterior patterning and axial morphogenesis. Emphasis is placed on comparative aspects of the egg-to-embryo transition across vertebrates and their evolution. The future prospects for work regarding self-organization and gene regulatory networks in the context of early axis formation are also discussed.
Keywords: Vertebrate embryology, Axis formation, Cortical rotation, Spemann organizer, Dorsoventral patterning, Anteroposterior patterning, Embryonic induction, Nieuwkoop center, Anterior visceral endoderm, Gastrulation
6.1. Introduction
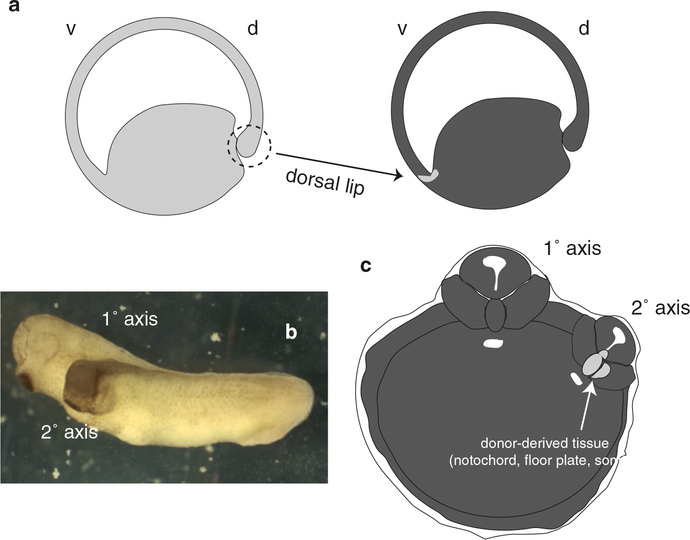
Bilaterality is a central feature of animal body organization. In certain invertebrates, such as some insects and cephalopods, this feature is determined by the structure of the egg itself (Wilson 1928), but vertebrates and many other animals define the plane of bilateral symmetry de novo in each embryo. In vertebrates, this plane is ultimately defined by the formation of the generalized vertebrate tissues, the dorsal neural tube, notochord and somites. The vertebrate body axis fully forms during gastrulation, following the internalization of axial mesendoderm at the future dorsal midline of the embryo (Fig. 6.1). This event initiates at the dorsal (upper) lip of the forming blastopore, the importance of which was first clearly recognized by Spemann in amphibians, and was defined as the “organizer” of axis formation (Spemann 1918; Spemann and Mangold 1924). Spemann performed transplantation experiments in salamanders, demonstrating that the dorsal lip could induce and organize a normally patterned second body axis when grafted to the opposite (ventral) side of a host gastrula. In this “secondary embryo,” the organizer cells contributed mostly to notochord themselves, whereas host cells populated the bulk of the induced axis, which included neural tube, somites, intermediate mesoderm and gut endoderm. Additional experiments showed that organizer might also contribute to anteroposterior patterning of the embryo, demonstrating a central role for the organizer tissue in controlling cell interactions during development.
Fig. 1.
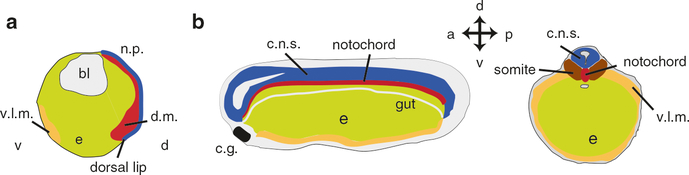
Vertebrate axial organization. (a) Diagram of a sagittal section through a Xenopus gastrula, showing the involution of the dorsal mesoderm (d.m., dark red) at the dorsal lip. The neural plate (n.p., blue) overlies the dorsal mesoderm. bl blastocoel, v.l.m. ventrolateral mesoderm (orange), e endoderm (yellow). (b) Sagittal (left panel) and coronal (right panel) diagrams of a tailbud-stage Xenopus embryo showing the elongated anterior-to-posterior axis and organization of tissues within. The neural tube is located dorsally and will form the entire central nervous system (c.n.s.). The dorsal mesoderm gives rise to the notochord and somites, ventrolateral mesoderm (v.l.m.) will form the kidneys, body wall muscles and vascular system. The endoderm forms the gut and its derivative organs. The cement gland (c.g.), a larval amphibian anchoring structure, is shown at the anterior end. After Hausen and Riebesell (1991)
Although these main findings were firmly established by the 1930s, it was not until the 1990s that the cellular and molecular mechanisms underlying the action of the organizer were revisited, resulting in the identification of conserved growth factor antagonists and transcription factors. The background and history of this work has been written about exhaustively by Spemann and his contemporaries and later by modern authors (Spemann 1938; Waddington 1940; Hamburger 1988; Grunz 2004). As outlined later in this chapter, the conservation of the organizer extends to the cellular and genetic levels and largely defines the core mechanisms of early vertebrate body plan formation.
In contrast to the conservation of the organizer and its components, the ultimate origins of axial bilateral symmetry in vertebrates are seemingly more diverse. Axis formation was first extensively studied using amphibians and was linked to cytoplasmic localizations in the egg. This was evident in the formation of a natural marker of the future dorsal side, what came to be called the “gray crescent” (Roux 1888). Early mechanistic studies suggested the crescent formed by rotation of the outer cortex over the yolky inner cytoplasm (reviewed in Clavert 1962; Ancel and Vintemberger 1948). This “cortical rotation” was verified by later authors and found to involve the organization and polarization of microtubules dorsally and the transport of dorsalizing determinants (Gerhart et al. 1989). Similar overall patterns are seen in primitive fish (Clavert 1962), suggesting that axis specification through cortical rotation in the fertilized egg is an ancestral condition in vertebrates.
By contrast, sauropsids (birds and reptiles) and more derived fish (teleost and selachiians/dogfish) lack an obvious physical marker of dorsoventral polarity. These eggs contain abundant yolk and undergo discoidal cleavage, and axis formation occurs after significant cleavage in the blastoderm. In birds and reptiles, evidence suggests that rotation of the egg during passage through the oviduct affects axis formation in the blastoderm. Similar gravitational mechanisms were originally thought to exist in dogfish and teleosts (Clavert 1962), although recently, mechanisms involving cytoskeletal polarization in the cortex, analogous to the amphibian cortical rotation have been found in teleosts (zebrafish and medaka).
With the exception of the egg-laying monotremes, which undergo discoidal cleavage and are likely similar to reptiles with regard to axial patterning, mammals represent a significant divergence from this broad trend. The eggs of therian mammals have lost yolk, reverted to holoblastic cleavage (secondary holoblastic cleavage) and evolved the blastocyst structure to facilitate implantation. Consequently, the first cell fate decisions are centered on distinguishing the embryo proper from extraembryonic lineages rather than on establishing bilateral symmetry. Axial patterning is thus rather late, only becoming apparent after implantation, about a week into development. Early blastomeres retain pluripotency for an extended time and axis formation requires multiple reciprocal interactions with extraembryonic tissues.
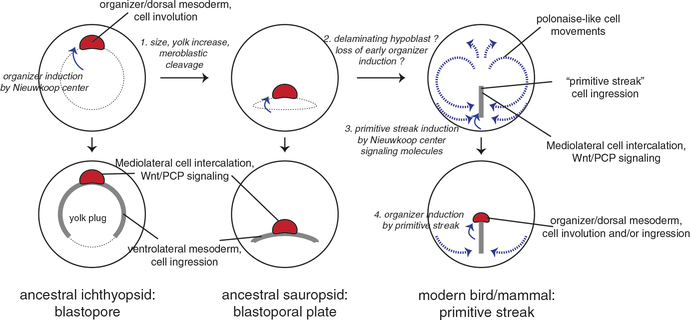
Although there was evidence that formation of the organizer depended on polarization of the egg, the mechanisms connecting the two were totally unknown to early embryologists. Studies in amphibians unexpectedly found that the organizer was itself formed through induction, rather than by inheriting gray crescent material. This organizer-inducing activity was predominantly found in dorsovegetal cells of the blastula, later termed the “Nieuwkoop center” after its discoverer, and its formation depended on cortical rotation (Gerhart et al. 1989). These experiments were a critical link in the chain of causality from egg to organizer and were represented in various three- and four-signal models familiar to developmental biologists (Slack 1991). The cortical rotation → Nieuwkoop center → organizer model has been a useful conceptual tool and has directed much of the research into the molecular basis for these processes and their conservation across vertebrates. It is now appreciated that cortical rotation results in dorsal Wnt/beta-catenin signaling, activating Transforming growth factor beta (Tgfb)/Nodal signaling in the vegetal cells, which induce and pattern the organizer in the overlying equatorial cells. Analogous mechanisms have been found acting in the teleost dorsal yolk syncytial layer (dYSL) of the egg and in the avian posterior marginal zone (PMZ) epiblast, based on molecular and functional data, suggesting deep conservation of these processes in the early vertebrate lineage.
Recent cellular and molecular characterization of axis formation and patterning has produced a wealth of examples of such deeply conserved vertebrate developmental mechanisms. Vertebrate embryology has historically been a comparative science, with investigations encompassing a wide range of diverse organisms. More recently, the use of specialized molecular and genetic approaches has largely limited the study of axis formation to a few tractable vertebrates, notably the mouse, chicken, Xenopus, and zebrafish. However, these species are all fairly evolutionarily derived representatives of their respective groups, making inference of primitive vertebrate characters problematic. With the growing ease of high-throughput genome analyses, stem cell technology and programmable genome editing, the barriers to performing comparative molecular and genetic studies are becoming increasingly reduced, potentially heralding a return to a broad comparative approach to vertebrate development.
In the context of the egg-to-embryo transition, the formation of the body axis is perhaps a defining process, since early developmental processes become organized into a unit comprising an individual. Indeed the idea of individuality in twinned embryos was an inspiration for Spemann to begin studying the embryological mechanisms of axis formation (Hamburger 1988), and remains relevant in current bioethics arguments regarding human embryos. This chapter shall review the core concepts relating to the origins and patterning of the axis, focusing on recent advances in understanding intracellular reorganizations, intercellular signaling events and cellular migrations. Emphasis has been given to recent molecular advances in the context of first discoveries and initial functional studies. Many of the ideas in this chapter have been extensively reviewed separately in the context of certain organisms, molecules or individual processes, but this chapter will attempt to tie these together to generate a more unified picture of axial development throughout the vertebrates.
6.2. Origins of Axial Polarity in the Egg and Early Embryo
The process of determining the initial plane of bilaterality and axis formation was first examined closely in the amphibians, where the gray crescent served as marker of the future axis (Fig. 6.2). Like most vertebrate eggs, amphibian eggs are initially symmetrical about the animal–vegetal axis (axisymmetrical), with the animal pole being the site of polar body extrusion, by definition, and more darkly pigmented. The vegetal pole is less pigmented and more concentrated with yolk. Using localized fertilization of frog eggs (Rana (Lithobates) spp.), Newport and Roux showed that the meridian of sperm entry coincided with the embryonic midline (and often the first cleavage plane), with the dorsoanterior axis forming opposite the sperm entry side (Newport 1851, 1854; Roux 1885, 1887). Importantly, Roux noted an apparent shifting of the animal–vegetal axis toward the sperm entry point, along with the appearance of a lightened area in the pigment on the opposing side. This feature formed before first cleavage and indicated the axial plane of the embryo, irrespective of the cleavage plane, which could be highly variable relative to sperm entry, depending on species (Roux 1887, 1888). Later studies confirmed these observations, further showing that the position of this “gray crescent”1 strongly predicts the area in which the future dorsal lip of the blastopore would form (Morgan and Tsuda 1894; Schultze 1899; Roux 1903; Morgan and Boring 1903; Brachet 1904). These classical embryological observations established that the dorsal axis and bilateral symmetry were determined upon fertilization of the egg and likely occurred prior to the first division, thus disputing the long-held idea that the cleavage itself was determinative.
Fig. 2.
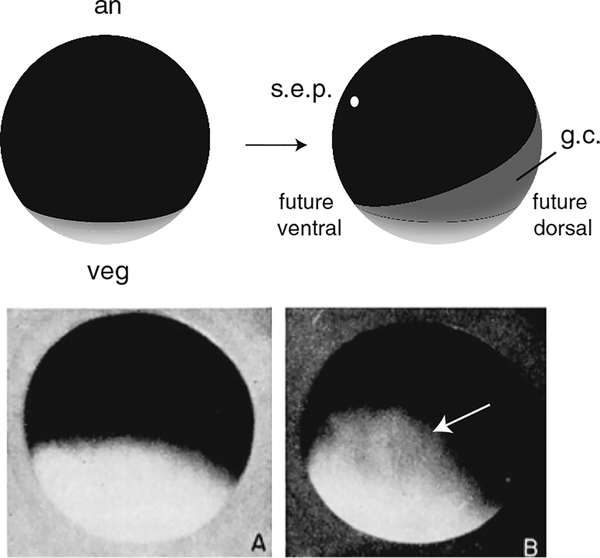
Gray crescent formation in amphibians. Top panel, diagram of an amphibian egg (e.g., Rana) before (left) and after fertilization (right). The heavily pigmented animal pole (an) and the paler vegetal pole (veg) are indicated. After fertilization, corticocytoplasmic movements opposite to the sperm entry point (s.e.p.) result in the appearance of the gray crescent (g.c.) on the prospective dorsal side. Bottom panel, images of a Rana egg at fertilization (a), and at 20 min post-fertilization, showing the gray crescent (b; dorsal view, arrow). Bottom panel reproduced from Rugh (1951)
Despite the important nature of the connection between the gray crescent and the dorsal axis, the mechanisms of gray crescent formation and function remained elusive for many years. In the first comprehensive effort to understand the cellular changes underlying gray crescent formation, Ancel and Vintemberger (1948; reviewed in Clavert 1962) examined the movement of electrocautery wounds that were made either in the deep yolk or on the surface of frog eggs. The motion of these markers revealed that outer egg cortex moves dorsally relative to the stationary deeper yolky cytoplasm. Although other models were also considered, such as asymmetric cortical contraction, a later series of marking and egg manipulation studies in Xenopus largely substantiated and clearly documented the cortical rotation model (Vincent et al. 1986; Vincent and Gerhart 1987).
Measurements of relative cortical displacement using superficially or deeply placed fluorescent dyes found that cortical rotation begins about halfway though the first cell cycle, eventually covering an average 30° of arc over the dense yolky cytoplasm. Also, cortical movement progressed along an animal–vegetal meridian, and toward the future dorsal side of the egg (generally away from the sperm entry point), with the region of greatest movement correlating with the position of the axial midline (Vincent et al. 1986; Gerhart et al. 1989). It is thought that changes in fluid dynamics in the fertilized egg result in a low viscosity/high elasticity shear zone in the subcortical region as well as an increase in firmness in the deep cytoplasm, allowing this differential movement between two parts of the egg (Elinson 1983; Gerhart et al. 1989).
In an extensive comparison of axis forming mechanisms, Clavert (1962) indicates that, in addition to amphibians, primitive fish including lampreys, lungfish, and chondrostean fish (sturgeons and paddlefish) likely form gray crescents, suggesting that these organisms also likely undergo cortical rotation. With these older comparative studies and more recent molecular data taken together, it is apparent that the basics of the amphibian cortical rotation model are conserved throughout the anamniotes (icthyopsids). And vestiges may exist even in some amniotes. It is also now generally appreciated that cortical rotation establishes a self-organizing, transient microtubule polarity in the zygote that is critical for the transport of cortical cytoplasmic dorsal determinants and activation of Wnt/beta-catenin signaling (reviewed in Gerhart 2004; Weaver and Kimelman 2004; Houston 2012). Wnt/beta-catenin signaling (see Sect. 6.3.5) also plays a key role in bird and mammal axis formation, but this is likely controlled by mechanisms other than cytoplasmic localization.
6.2.1. Mechanisms of Amphibian Cortical Rotation
6.2.1.1. Cortical Rotation in Anurans
A number of studies have shown that cortical rotation is primarily controlled by the assembly of parallel microtubule arrays in the vegetal cortex (Fig. 6.3). Treatment of fertilized eggs during the middle of the first cell cycle with microtubule-depolymerizing agents such as ultraviolet irradiation (UV), exposure to anti-microtubule drugs, cold and high pressure, can inhibit gray crescent formation and/or block axis formation in both frog and salamander eggs (Malacinski et al. 1975; Manes et al. 1978; Manes and Elinson 1980; Scharf and Gerhart 1983; Vincent and Gerhart 1987). Correspondingly, impressive arrays of parallel microtubules are assembled in the vegetal cortical region during the period of cortical rotation in Xenopus and Rana (Lithobates) (Elinson and Rowning 1988).
Fig. 3.
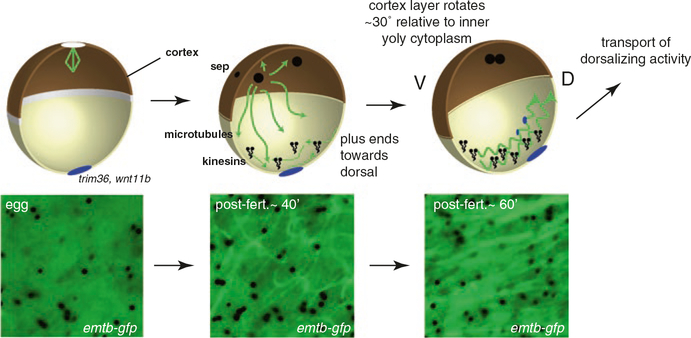
Events of cortical rotation in Xenopus. Microtubules are disassembled during oocyte maturation, and are absent from the egg cortex (left panels). Certain RNAs are localized to the vegetal cortex during oogenesis (blue) and encode proteins critical for cortical rotation and dorsalization (e.g., trim36, wnt11b). After fertilization, the incoming sperm pronucleus and associated centrosome initiate astral microtubule assembly. Cortical microtubule assembly also begins, forming a network by 40 min post-fertilization. A shear zone forms and microtubules associate with the yolky cytoplasmic core (not shown) and cortical rotation begins, under the action of kinesin-like proteins (kinesins). Relative cortical movement occurs dorsally, possibly the result of nudging by ventrally positioned astral microtubules, and rapidly orients microtubule plus ends dorsally (Olson et al. 2015) (middle panel). Microtubule assembly and organization becomes robust by 60 min post-fertilization and full cortical rotation commences, continuing until first cleavage. Rapid transport of dorsalizing activity occurs along parallel microtubule arrays using kinesin-like motors (right panel). The corresponding bottom panels show live images of microtubules labeled with Enconsin microtubule-binding domain tagged GFP (EMTB-GFP), showing progressive assembly and alignment during cortical rotation (Olson et al. 2015)
Microtubules are completely disassembled in the egg at fertilization, but progressively repolymerize over the first 35 min, approximately when relative cortical movement begins. Microtubules in the cortical region initially form a disorganized network that gradually becomes organized as cortical rotation progresses. By mid-cortical rotation, microtubules are predominantly oriented with the plus ends towards the dorsal side (Houliston and Elinson 1991; Olson et al. 2015). Live imaging studies indicate that the arrays associate and move with the deeper cytoplasm (Houliston 1994; Larabell et al. 1996; Olson et al. 2015). These microtubule arrays are transient and are progressively depolymerized upon first cleavage under the control of MPF activation (Marrari et al. 2003), thus terminating cortical rotation.
Surprisingly little is known about the regulation of microtubule activity during cortical rotation. Generalized kinesin-related protein activity in the cortex proper is thought to tether the microtubule array to the cortex and facilitate movement (Marrari et al. 2003). This has been assessed using function-blocking antibodies but specific roles for individual kinesins have not been identified. Kinesin1/Kif5b appears dispensable in Xenopus (Marrari et al. 2000, 2003), and Dynein has been shown to act early in rotation, as shown by injection of the antagonistic Dctn2 (Dynamitin/p50; Marrari et al. 2004). Recently, a suite of mRNAs localized to the vegetal pole in oocytes has also been implicated in regulating microtubule assembly. Maternal mRNA depletion experiments show that reductions in perilipin2 (plin2; Chan et al. 2007), tripartite motif containing 36 (trim36; Cuykendall and Houston 2009), and dead end homolog 1 (dnd1; Mei et al. 2013) lead to abnormal microtubule array formation and failure of cortical rotation. Trim36 can function as a single RING-finger-type ubiquitin ligase, and this activity is essential for its role in microtubule assembly (Cuykendall and Houston 2009). Dnd1 is an RNA-binding protein required to tether trim36 mRNA to the cortex, facilitating locally enriched Trim36 protein levels (Mei et al. 2013). Dnd1 is typically associated with germline specification (Weidinger et al. 2003), and it is not known whether these functions are related. The role of Plin2 is unclear. The protein is associated with lipid droplets (Chan et al. 2007), but a structural role for the plin2 RNA has also been suggested (Kloc 2009). A different set of localized mRNAs are involved in vegetal microtubule organization and transport in the zebrafish zygote (Nojima et al. 2010; Ge et al. 2014), although with slightly different functions (see Sect. 6.2.2). It remains to be determined how these localized molecules interact with microtubule regulatory proteins and motor proteins to control microtubule assembly in cortical rotation.
The initial cue for the direction of cortical rotation in normal development is thought to be sperm entry, as this site is generally opposite the direction of movement. The central model for orientation of the array is a reciprocal positive feedback loop, during which random asymmetry in microtubule growth is refined and amplified by rotation of the cortex (Gerhart et al. 1989; Gerhart 2004). Microtubules growing into the cortex, originating from the sperm aster and within the cortex may provide the initial movement cue (Houliston and Elinson 1991; Schroeder and Gard 1992). Cortical movement then serves to progressively stabilize microtubule growth and formation in the same direction. High-resolution live imaging of microtubule assembly and orientation has verified that cortical rotation begins before there is visible bias in plus end directionality or microtubule alignment (Olson et al. 2015), an observation that was suggested from earlier studies but never directly shown (Larabell et al. 1996). Additionally, plus end orientation occurs almost as soon as cortical rotation begins, indicating that directionality is determined in a punctuated manner rather than progressively (Olson et al. 2015).
In vivo, sperm entry or slight asymmetry with respect to gravity could be sufficient to initiate cortical movement, although a “vector summation” of microtubule polymerization forces, as initially proposed (Gerhart et al. 1989) cannot be ruled out. The shear-induced alignment of organelles (endoplasmic reticulum) may also play a role in perpetuating alignment, since ER and microtubules are often interdependent (Terasaki et al. 1986). Because cortical movement can have a role in determining the orientation of microtubules, the overall role of cortical rotation may be thought of as twofold; first to generate relative displacement of the cortex, and second to align the microtubule array facilitating the faster and longer range transport of determinants.
Evidence for these determinants came from 90° egg tipping experiments, which cause the axis to form in the uppermost part of a tipped egg (Ancel and Vintemberger 1948; Kirschner et al. 1980; Gerhart et al. 1981). Also, tipping can rescue axial development following UV-irradiation (Scharf and Gerhart 1980; Chung and Malacinski 1980). In amphibian eggs, denser yolk accumulates in the vegetal pole, which when tipped off axis, results in a tendency to “fall” downward against the cortex, which is immobilized in these experiments, creating relative displacement. Tipping does not restore microtubules (Zisckind and Elinson 1990), further suggesting that the relative displacement of cortical dorsal determinants is essential, whether achieved normally by microtubule motive force or experimentally by gravitational force.
Other studies indicated the existence of an essential, transplantable dorsalizing activity associated with the cortex/subcortical cytoplasm (Yuge et al. 1990; Hainski and Moody 1992; Holowacz and Elinson 1993; Kikkawa et al. 1996; Kageura 1997). And, live imaging studies have shown various substances moving dorsally within the shear zone during cortical rotation. These include a subset of pigment granules and organelles, fluorescent beads, and certain GFP fusion proteins (Miller et al. 1999; Weaver et al. 2003). Their movement is rapid (~50 μm/min) and saltatory, consistent with generalized kinesin-based transport along microtubules. Transport can be measured from 30°–120° of arc from the vegetal pole, equal to and greater than the overall relative cortical displacement (Rowning et al. 1997; Miller et al. 1999; Weaver et al. 2003). Interestingly, this distribution matches that of dorsalizing cytoplasm taken from the egg (Fujisue et al. 1993; Holowacz and Elinson 1993). Additionally, stimulation of microtubule assembly with deuterium can hyperdorsalize embryos, potentially through wide-spread distribution of this dorsalizing material along many egg meridians (Scharf et al. 1989; Miller et al. 1999). The identity of the molecules responsible for the activity of this cytoplasm in vivo is unclear but are likely related to Wnt/beta-catenin signaling (see Sect. 6.3.2).
Cortical rotation can thus be considered a robust self-organizing symmetry-breaking process that integrates cytoskeletal and physical forces to generate a single direction for the short-range relative displacement of the cortex and for the long-range distribution of molecules and putative determinants towards the presumptive dorsal side.
6.2.1.2. Cortical Rotation in Urodeles
Although much of the recent cell and molecular characterization of cortical rotation has been done in anurans (Xenopus and Rana (Lithobates)), urodeles Triturus and Ambystoma are known to form gray crescents (Bánki 1927; Clavert 1962). However, urodele eggs are normally polyspermic and the relationships between the site of sperm entry or male pronucleus formation and the site of the gray crescent are unclear. Recently, relative cortical displacements analogous to those in Xenopus have been observed in Cynops (Fujisue et al. 1991), which also exhibits vegetal microtubule array assembly during the period of cortical rotation (Iwao et al. 1997). Curiously, although some species are ventralized by UV-irradiation (see above), irradiation of Cynops eggs dorsalizes embryos (Doi et al. 2000), suggesting that putative dorsal determinants are more widely dispersed in these eggs and would remain so in the absence of microtubule assembly and cortical rotation. This situation may possibly mimic the random dispersion of determinants occurring in deuterium-treated Xenopus eggs. Thus, the basic mechanisms of microtubule-dependent cortical rotation and dorsal determinant transport are conserved in amphibians.
Urodeles are thought to lack vegetal cortical localization of RNAs (Elinson and del Pino 2011; Houston 2013), which is interesting given the connection between localized RNAs and cortical rotation in Xenopus and zebrafish. However, it has not been specifically demonstrated whether the exact RNAs implicated, including trim36 and syntabulin (sybu), are in fact unlocalized in urodeles. Since these RNAs are partially associated with the germ plasm, which is not found in urodeles (Nieuwkoop and Sutasurya 1979), one might expect an absence of localization. Urodeles may however localize important components posttranslationally. Nevertheless the basic mechanisms of polarizing the egg and distributing dorsal determinants appear conserved, but are not well understood in either case.
6.2.2. Cortical Rotation and Dorsal Determinant Transport in Zebrafish
Axis formation in zebrafish similarly relies on asymmetric localization of dorsal determinants and activation of Wnt/beta-catenin signaling. The polarizing mechanisms and the similarity of these to classical amphibian cortical rotation are only now becoming apparent, however. It has been traditionally thought that typical cortical rotation does not occur in teleost fish. The origin of this assumption is mysterious, but likely can be attributed to initial observations on the importance of a polarized dYSL in teleost axial patterning rather than formation of a gray crescent-like clear crescent, which does occur in non-teleosts (Long 1983; Ho 1992). There do not appear to have been any classical embryological studies directly addressing either relative displacement of cytoplasm and cortex or the existence of transplantable vegetal cortical cytoplasm.
However, parallel microtubule arrays have been noted at the vegetal pole cortex of the early post-fertilization (~20 min) medaka and zebrafish egg (Jesuthasan and Stähle 1997). By 30 min post-fertilization, this array is offset from the vegetal pole, giving bilateral symmetry to the egg. During cleavage of the blastoderm, a second set of microtubule arrays forms along the animal–vegetal axis (Strähle and Jesuthasan 1993; Solnica-Krezel and Driever 1994), and polarized transport of fluorescent beads has been observed to move animally into the YSL and marginal blastomeres (Jesuthasan and Stähle 1997). Disruption of either set of microtubules with UV, cold, or nocadazole disrupts axis formation as well as epiboly (Strähle and Jesuthasan 1993; Solnica-Krezel and Driever 1994; Jesuthasan and Stähle 1997).
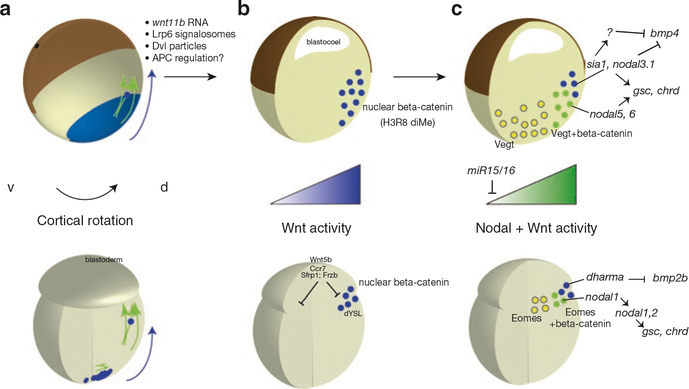
Thus a two-step transport pathway is proposed (Fig. 6.4). Asymmetry is initially established by the localization of determinants to the future dorsal vegetal side of the egg, followed by generalized upward movement of material into the YSL and marginal blastoderm cells (the dorsal determinants being carried along only on the dorsal side). In support of this idea, a recent live imaging study has demonstrated for the first time, as was tacitly assumed, that the plus ends of zebrafish vegetal cortical microtubules are oriented dorsally as in frogs (Tran et al. 2012). Given the relationship between movement of the cytoplasmic core and microtubule orientation in Xenopus eggs (Olson et al. 2015), it is likely that relative cortical movement, at least locally, is involved in orienting these microtubules in teleost eggs as well.
Fig. 4.
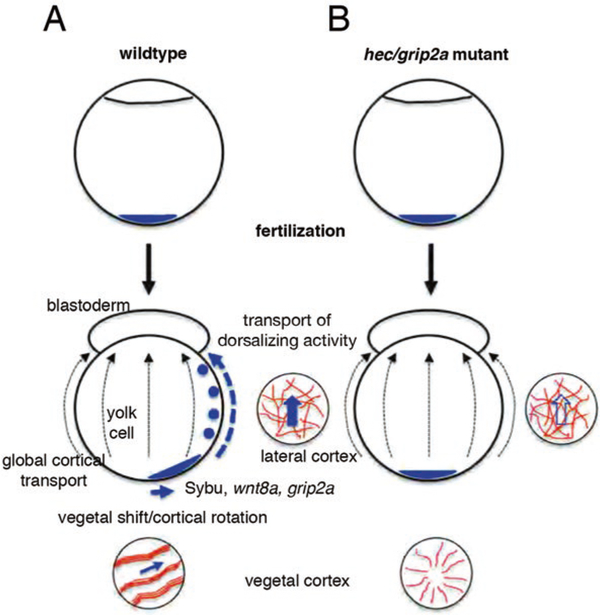
Dorsal determinant transport in zebrafish. (a) Sequence of events in wildtype embryos. RNAs and other dorsal determinants are localized vegetally during oogenesis (blue). After fertilization, cytoplasm streams to the animal pole, forming the future blastoderm. Microtubule assembly initiates ~20 min post-fertilization at the vegetal pole of the yolk cell; localized RNAs and Syntabulin protein (Sybu) are shifted toward the future dorsal side. Microtubule networks in the lateral cortex facilitate global transport animal-ward, which on the dorsal side would contain axis determinants. (b) In hecate (hec) mutants lacking Grip2a, maternal vegetal localization occurs, but cortical rotation and microtubule assembly are deficient post-fertilization. This image is reproduced and modified from, Ge X, Grotjahn D, Welch E, Lyman-Gingerich J, Holguin C, Dimitrova E, et al. (2014) Hecate/Grip2a acts to reorganize the cytoskeleton in the symmetry-breaking event of embryonic axis induction. PLoS Genet 10(6): e1004422. doi:10.1371/journal.pgen.1004422, under the terms of the Creative Commons Attribution License (CC BY 4.0)
Additional insight into microtubule assembly in zebrafish and further support for the dual-range model of transport in the yolk cell has recently come from analysis of maternal-effect mutants in zebrafish (Nojima et al. 2004, 2010; Lyman Gingerich et al. 2005; Ge et al. 2014). The mutants hecate (hec; Ge et al. 2014) and tokkaebi (tkk; Nojima et al. 2010) are ventralized with near complete penetrance and harbor disruptions in syntabulin (sybu) and glutamate receptor interacting protein 2a (grip2a) loci, respectively. Parallel microtubule assembly at the vegetal pole is normal in tkk mutants but disrupted in hec mutants. Upward movement to the YSL/blastoderm margin is normal in hec eggs, underscoring the independence of these two transport systems. Sybu encodes a potential cargo linking protein for Kif5b, suggesting a role in microtubule transport of dorsal determinants vegetally. Grip2a encodes a scaffolding protein important for subcellular localization in mammalian neurons.
Both sybu and grip2a mRNAs localize to the vegetal cortex, along with wnt8a mRNA (see below), and these RNAs all undergo an off-center “shift,” mirroring that of the microtubule array (Nojima et al. 2010; Lu et al. 2011; Ge et al. 2014). Sybu protein fails to localize to the prospective dorsal side in nocodazole-treated eggs, suggesting it is trafficked by microtubules. The exact role of Grip2a is not known but it may recruit protein complexes to vesicles or organelles that attach to and help align microtubules. Interestingly, grip2 mRNA is localized in Xenopus, but follows a germ plasm-like pattern and is not thought to play a role in axis formation (Tarbashevich et al. 2007). Similarly, sybu is localized to the germ plasm in Xenopus and may play an undefined role in axis formation, possibly in transport or in Wnt activation (Colozza and De Robertis 2014).
In zebrafish, maternal loss-of-function mutants have implicated kif5ba in vegetal microtubule formation and axis formation (Campbell et al. 2015), although its role is complex. Organized vegetal microtubules fail to form and wnt8a does not shift dorsally. However, grip2a asymmetric translocation still occurs and sybu RNA is not maintained vegetally (Campbell et al. 2015). It is unclear to what extent these phenotypes reflect roles for Kif5ba in localizing components vegetally during oogenesis or more acute roles during microtubule organization and transport.
6.2.3. Asymmetry in Early Amniote Embryos
Initial axis formation in fish and frogs occurs in the fertilized egg; the dorsal determinants are either inherited directly by dorsal cells (frogs, primitive fish) or transmitted from the uncleaved yolk cell to peripheral dYSL and overlying dorsal marginal blastomeres (teleost fish). The axis in amniotes (birds, reptiles and mammals) relies on mainly on reciprocal interactions between upper embryonic and lower extraembryonic tissue layers (epiblast and hypoblast, respectively) and asymmetric cell movements, with only hints that early asymmetry in the egg or early embryo are involved. Additionally, the links to localized activation of growth factor signaling pathways are much less clear.
6.2.3.1. The Role of Gravity in Axis Formation in Sauropsids
Classic experiments in the chick have suggested that axis specification occurs in response to gravity as the egg rotates as it passes through the oviduct (Fig. 6.5). Axis formation in reptile embryos is thought to occur in a similar fashion, although is less thoroughly studied in this regard. Bird and reptile embryos are both highly polyspermic, making it unlikely that sperm entry plays a role in axis formation (Waddington 1956). The long-noted von Baer’s rule of thumb suggested a relationship between the axis of the egg and that of the embryonic axis (von Baer 1828). This axis is most often (~70 %) situated perpendicular to the long axis of the egg, with the left side of the embryo oriented towards the blunt end. Eggs rotate clock-wise about the long axis as they travel through the oviduct (~0.2 rpm) and continue rotating in the uterus, where they acquire the shell membranes and shell. As the egg rotates, the blastoderm is maintained at an angle of ~45°, balancing the inertia of the rotation with the tendency of the buoyant blastoderm to float on the dense yolk (Clavert 1962). In this arrangement, the anterior of the embryo forms at the lower end. Most eggs enter the uterus and are laid sharp-end first and thus end up following von Baer’s rule. The minority of cases where the blunt end enters first are thus truly exceptions that prove the rule, since it is only the embryo’s orientation with respect to the external egg shape that is changed; the posterior of the embryo still forms at the upper end of the blastodisc.
Fig. 5.
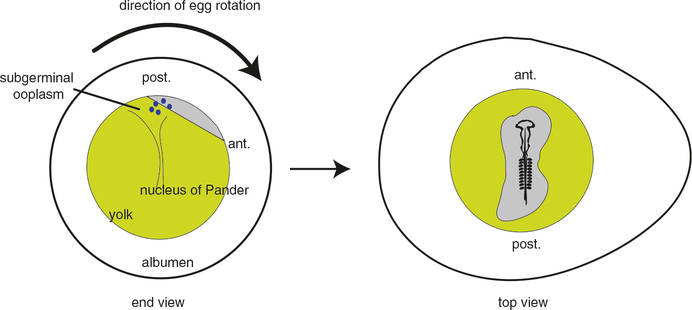
Model for establishment of asymmetry in bird eggs. Left, sectional view of a uterine chicken egg viewed from the sharp end. The direction of rotation is indicated; because of this rotation, the lighter blastoderm cytoplasm is maintained off angle as it continually floats to the highest point. The blastoderm is exposed to the subgerminal cytoplasm, which is hypothesized to contain axis determinants (blue). At this stage, the blastoderm is several thousand cells and has not formed the area pellucida epiblast. Right, top view of 2–3 day embryo showing anterior-to-posterior axial polarity. This embryo would conform to von Baer’s rule, with head oriented away with the blunt end positioned left. ant. anterior, post. posterior
Both in utero and in vitro experiments have defined a critical period for axis establishment in the uterus (Vintemberger and Clavert 1959; Clavert 1961; reviewed in Clavert 1962). Egg orientation was manipulated at different times prior to egg-laying and the orientation of the embryonic axis was altered if the presentation of the egg was changed at least six hours prior to laying. Additionally, eggs could be removed and incubated in vitro in a rotating cylinder and the orientation of the embryo was determined by the relative direction of rotation. Similar to the experiments in frogs, the effects of earlier rotations could be overridden by later manipulations up to the critical period of axis formation. This period correlates with the time when the chick blastoderm thins to a single layer, forming the area pellucida epiblast. Another set of studies showed that uterine eggs could be incubated in a variety of orientations without rotation, or even hung from a chalaza without shells, and the axis always formed along the gravitational axis with the posterior end uppermost (Kochav and Eyal-Giladi 1971; Eyal-Giladi and Fabian 1980). Thus, it is the response to gravity that is critical, not the stress of movement or effect of rotation per se.
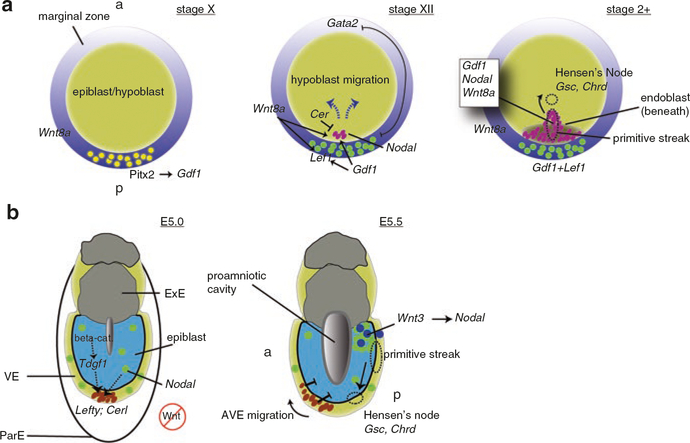
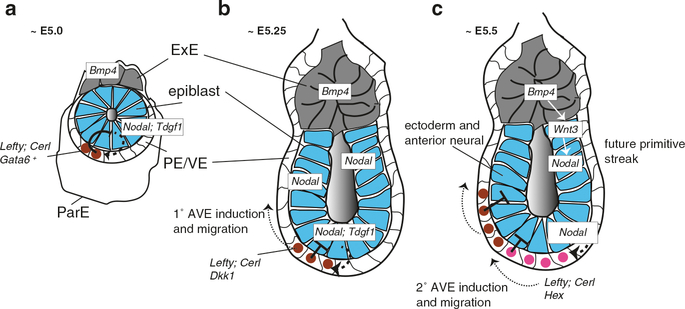
Axial polarity and bilateral symmetry in the blastoderm is evident both morphologically and molecularly prior to primitive streak formation. The future posterior half of the embryo, in which the primitive streak forms, can first be distinguished by the formation of a ridge of cells in the deep layer of the posterior area opaca, Koller’s/Rauber’s sickle (Callebaut and Van Nueten 1994). Additionally, the hypoblast layer (analogous to the anterior visceral endoderm, see below) begins to form in this region, coalescing from isolated hypoblast islands delaminating from the epiblast in a posterior-to-anterior progression. The hypoblast is then replaced by the endoblast (posterior visceral endoderm), derived from Koller’s sickle, in the same progression (Stern 1990; Stern and Downs 2012). Molecular analyses have also identified early differential gene expression in this region, including Gdf1 (alias cVg1; Seleiro et al. 1996; Shah et al. 1997) in the PMZ of the epiblast and Goosecoid (Gsc) in Koller’s sickle (Izpisúa Belmonte et al. 1993). Transplantation experiments showed non-cell/tissue autonomous induction of ectopic axes, sparking parallels between the PMZ and the amphibian Nieuwkoop center (see Sect. 6.3.5).
The mechanisms leading to these developmental events in the posterior are unknown. The prevailing hypothesis for the initiation of this posterior polarity is the differential exposure of presumptive areas to maternal cytoplasm during cleavage. In the chicken egg, a particular cytoplasmic layer, the subgerminal ooplasm (gamma- and delta-ooplasm) is contained in a central region below the blastodisc and overlying the latebra and Nucleus of Pander (Callebaut 2005). With the blastoderm offset from the animal pole by the inertia of the rotating egg (see above), this cytoplasm would have more prolonged contact with cells arising in the upper (future posterior end) of the embryo. The subgerminal cytoplasm may also be differentially inherited by primordial germ cells, which form in response to cytoplasmic determinants (germ plasm) in birds (Tsunekawa et al. 2000). It is thus possible that unknown axial determinants might also be localized to this region, as in amphibians.
6.2.3.2. Early Polarization of the Mammalian Embryo
The evolution of implantation in therian mammals resulted in many changes in the structure of the egg and early embryo, including a loss of yolk, the reemergence of holoblastic cleavage and the early segregation of embryonic and extra embryonic tissues, forming a preimplantation blastocyst (see Chap. 4). In light of these major alterations in life history, it has long been of interest to determine the extent that axes in the mammalian embryo are determined by cytoplasmic asymmetries as in other vertebrates.
Early authors concluded that this must be the case, although these conclusions were admittedly based on a few cases of poorly characterized abnormal embryos generated following blastomere perturbations (Waddington 1956). However, in contrast to amphibians, the separation of early mammalian (rodent and rabbit) blastomeres does not result in complementary embryos either having or lacking dorsal structures (Seidel 1956; Tarkowski 1959; Tarkowski and Wróblewska 1967). Additionally, early mammalian blastomeres demonstrate a high degree of developmental plasticity, with each of the four-to-eight cell blastomeres contributing to all cell lineages in chimeric embryos (Tarkowski 1961; Mintz 1964; Kelly 1977). Furthermore, cell fate specification with respect to epiblast/primitive endoderm/trophectoderm is largely dependent on cell polarity related to inside or outside cell position within the morula, as well as on the timing of asymmetric cell division in generating inside cells (Hillman et al. 1972; Ziomek and Johnson 1980; Pedersen et al. 1986; Morris et al. 2010) (see also Chap. 4). Ablation experiments have found that removal of the animal or vegetal poles from fertilized eggs and early blastomeres is fully compatible with normal development (Zernicka-Goetz 1998; Ciemerych et al. 2000), unlike the case in amphibians. It is therefore generally concluded that segregation of maternal determinants in the egg is unlikely to direct axis formation or cell fate patterning in mammals, or that if such a bias exists it can be readily overridden by other cellular interactions.
Nevertheless, axis specification requires that symmetry breaking occur at some point prior to gastrulation. When this asymmetry is established and to what extent it depends on earlier developmental bias or is more or less random has been a recurring debate. There have been various attempts to correlate cleavage patterns in the early embryo with asymmetries in the blastocyst and conceptus and with the eventual anteroposterior axis of the embryo. A preponderance of the evidence however suggests that much of this observed “bilateral symmetry” likely results from physical constraints imposed by the zona pellucida (vitelline membrane) or other external constraints and is not connected to the orientation of the anteroposterior axis (for detailed reviews of this literature, please see Takaoka and Hamada 2012; Zernicka-Goetz 2013; Bedzhov et al. 2014 and references therein).
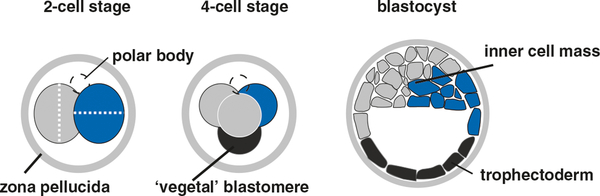
The most compelling evidence for an early cell fate bias is the observation that the order and orientation of rotational cleavages in the mouse embryo can influence blastomere fate in the blastocyst (Fig. 6.6). In particular, the vegetal blastomere (distal to the polar body) that arises from a particular tetrahedral cleavage pattern (which occurs in a subset of cases), will disproportionately contribute to the trophectoderm in normal embryos (Piotrowska-Nitsche et al. 2005; Torres-Padilla et al. 2007a). Furthermore, chimeras composed exclusively of vegetal blastomeres fail to survive (Piotrowska-Nitsche et al. 2005), likely because these cells cannot generate sufficient numbers of pluripotent epiblast cells to support development (Morris et al. 2012b).
Fig. 6.
Early bias of mouse blastomeres towards lineage fate but not axial polarity. Two-cell blastomeres undergo rotational cleavage (dotted white lines indicate cleavage planes), generating a fraction of embryos with a tetrahedral cell arrangement. In this formation, vegetal blastomeres are biased towards contributing to the trophectoderm (dark gray) in the blastocyst. The corresponding animal blastomeres are biased towards contributing to the inner cell mass (blue). After Zernicka-Goetz et al. (2009)
Lineage labeling studies of individual or all four cells have found a similarly biased contribution of four cell-stage blastomeres to either inner cell mass (ICM) or trophectoderm (TE) fates in a subset of embryos (Fujimori et al. 2003; Tabansky et al. 2013). Importantly, this developmental bias was reflected in cell lineage but not in relative cell positioning toward the embryonic or abembryonic poles of the blastocyst. For technical reasons however, the specific contribution of vegetal blastomeres could not be assessed in these studies. Mechanisms underlying this bias may include epigenetic regulation of cell polarity (Parfitt and Zernicka-Goetz 2010), decreased pluripotency transcription factor occupancy at target genes (Plachta et al. 2011) or a combination of factors. How this differential regulation is initiated is unknown, but the lack of maternal influences and a lack of differential gene expression in two- and three-cell blastomeres (VerMilyea et al. 2011) suggest that this bias is either an emergent property or a positioning effect in the four-cell stage embryo.
It is unclear at present whether any bias in early blastomere fate can be connected to axis specification in the mammalian embryo. Although this will be discussed further in Sect. 6.5.1 and similar to the bird embryo, the proximal events in mammalian axis formation involve the asymmetric migration of cells in the extraembryonic anterior visceral endoderm/hypoblast.
6.3. Initiation of Axis Induction by Dorsal Determinant Signaling
Numerous models have been suggested for how early asymmetries in the egg and embryo can lead to the specification of the organizer and ultimately to axis formation. Classical views, perhaps influenced by the importance of cytoplasmic localizations in invertebrates, suggested that the amphibian gray crescent contained precursors or determinants of the organizer (Wilson 1928). Another influential idea was that of a dorsal “cortical field” intersecting with a vegetal yolk gradient to determine the position of the organizer (Dalcq and Pasteels 1937). Later experiments showed that mesoderm in general, and the organizer in particular, required inductive cell–cell signaling by the vegetal prospective endoderm (see Chap. 7; Boterenbrood and Nieuwkoop 1973; Gimlich and Gerhart 1984; Dale et al. 1985), suggesting that cell-autonomous inheritance of organizer determinants was subordinate to mesoderm induction. Importantly with respect to axis formation, Nieuwkoop and colleagues showed that the blastula vegetal mass is dorsoventrally patterned, with only the dorsovegetal cells being able to induce dorsal mesoderm/organizer. This dorsal signaling center, or “Nieuwkoop center” as it became known (Gerhart et al. 1989), was also demonstrated by transplantation of dorsal vegetal cells into UV-ventralized hosts (or ventrally into normal hosts), resulting in largely non-cell-autonomous organizer and axis induction (Gimlich and Gerhart 1984; Gimlich 1986; Kageura 1990).
Cortical rotation emerged as the candidate upstream event leading to Nieuwkoop center formation in dorsovegetal cells, as embryos ventralized by UV-irradiation lack both Nieuwkoop center and organizer activity (Smith et al. 1985; Gerhart et al. 1989). Also, because the extent of mesoderm induction is unchanged in ventralized embryos (Cooke and Smith 1987), a hypothesis was formed that the Nieuwkoop center generates a distinct dorsalizing signal or a competence modifying signal, which acts along with a general mesoderm inducer to induce the organizer. This idea became enshrined in the influential three-signal models of axis formation (Smith et al. 1985; Smith 1989; Heasman 1997). It is now recognized, owing to the work of many labs over many years, that this “dorsal signal” is not a unique signal at all, but represents an early and elevated wave of Nodal-related Tgfb signaling that is regulated by dorsally enriched Wnt/beta-catenin signaling and other maternal factors (see below).
Although many of these studies were conducted using Xenopus embryos, transplantation experiments have shown that localized regions in the blastula-equivalent stages of the zebrafish and chicken embryo can induce axes non-cell autonomously (dYSL, Mizuno et al. 1999; PMZ epiblast, Bachvarova et al. 1998). These regions also ultimately act through elevated Nodal signaling, either downstream of or in concert with Wnt/beta-catenin signaling, suggesting that the mechanisms of axis induction are widely conserved vertebrate development. In mammals however, Nodal signaling likely precedes obvious Wnt asymmetry and is the main determinant of axis formation, albeit in conjunction with Wnt signaling. In this section, the roles of early Wnt signaling in establishing dorsal fates in amphibians and fish are reviewed, along with the conserved but divergent roles of Wnt and Nodal signaling in regulating organizer formation across vertebrates.
6.3.1. Basic Wnt Signaling Mechanisms
Since its initial discovery as a mammalian oncogene (Nusse and Varmus 1982), signaling by the deeply conserved Wnt1 (int-1/wingless (wg)) family of growth factors has emerged as a central feature of many aspects of animal development and disease. The reception of Wnt signals and intracellular signal transduction mechanisms has been extensively studied in vivo in both vertebrate and invertebrate organisms as well as in tissue culture cells. Although there are many variations that are important in specific tissues and disease states, three main arms of the pathway are widely implicated in vertebrate axis formation. These are: (1) the regulation of Ctnnb1 protein stability (beta-catenin protein hereafter), nuclear localization and transcriptional activity, (2) the regulation of cytoskeletal organization and cell polarity, and (3) the release of calcium from intracellular stores. With the caveats in mind that much of Wnt signaling entails complex, context-dependent and networked interactions, it remains useful to understand the basic features of Wnt pathways involved in early axis development. There are numerous comprehensive reviews on different aspects of Wnt signaling (MacDonald et al. 2009; Hikasa and Sokol 2013); here the key evidence of Wnt signaling in axis formation and the core signal transduction mechanisms most involved in axis formation and patterning will be briefly reviewed.
6.3.1.1. Wnt/Beta-Catenin Signal Transduction Mechanisms
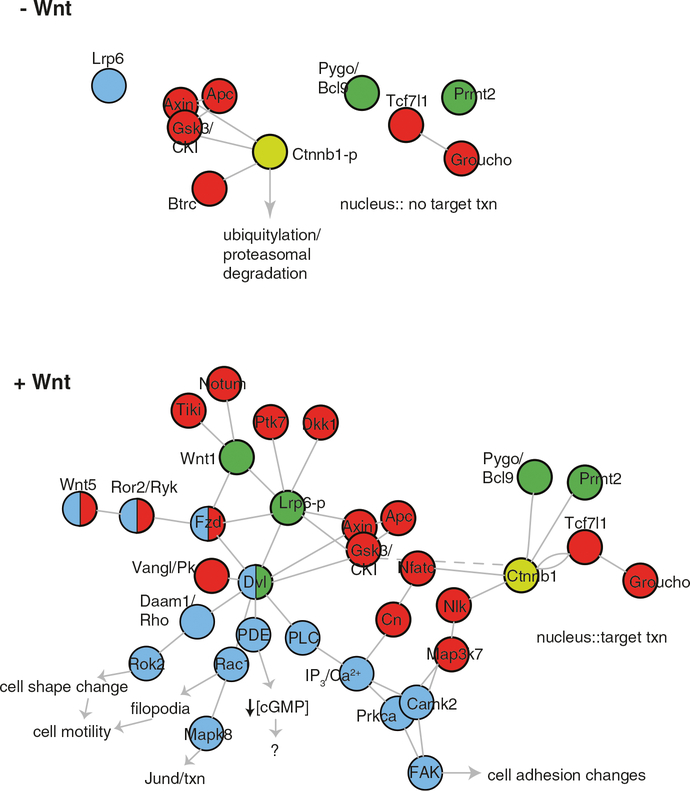
In Wnt-unstimulated cells, beta-catenin protein is constitutively turned over by the activity of a multiprotein “destruction” complex (Fig. 6.7). This complex contains Axin1, Adenomatosis polyopsis coli (Apc), Glycogen synthase kinase 3 beta (Gsk3b), and Casein kinase 1 alpha (Ck1a/Csnk1a1), and serves to regulate the phosphorylation of cytoplasmic beta-catenin by Gsk3b and Ck1a (reviewed in MacDonald et al. 2009; Clevers and Nusse 2012; Hikasa and Sokol 2013). These phosphorylations in the beta-catenin N-terminus allow recognition by members of the beta-transducin repeat containing E3 ubiquitin protein ligase family (Btrc, Jiang and Struhl 1998; Liu et al. 1999a) and target phospho-beta-catenin for ubiquitylation, resulting in degradation by proteasomes (Aberle et al. 1997). Axin is thought to be a key limiting component of this complex, regulating the assembly of the destruction complex (Lee et al. 2003) and recruiting the beta-catenin kinases (Ck1a and Gsk3b) for the priming and processive phosphorylation, respectively, of beta-catenin (Liu et al. 2002; Amit et al. 2002).
Fig. 7.
Generalized Wnt signaling networks. In the absence of activating Wnt ligands (top panel, -Wnt), beta-catenin protein (Ctnnb1) is phosphorylated by destruction complex components and tagged for proteasomal degradation. In the nucleus, Tcf7l1/Tcf3 represses Wnt target promoter activity through recruitment of Groucho. Upon stimulation with Wnt ligand, a variety of pathways are activated (see text for details). Predominantly positive-acting components with respect to beta-catenin regulation are shown in green, negative components in red, beta-catenin-independent components are light blue. Beta-catenin is shown in yellow. Circles indicate component nodes, lines indicate edges, or interacting components. This arrangement is not meant to convey specific exact binding relationships or stoichiometry. Wnt1 is shown as a beta-catenin-activating ligand, whereas Wnt5 is shown as a Wnt/PCP and Wnt/Calcium-stimulating ligand. Plot was generated with iGraph in R (Csardi and Nepusz 2014). txn transcription
Wnt stimulation inactivates the destruction complex through a little understood mechanism involving recruitment of the cytoskeletal adaptor protein Dishevelled (Dvl homologs 1–3). This blocks the activity of Gsk3b (Siegfried et al. 1992, 1994; Cook et al. 1996) and prevents beta-catenin phosphorylation, thereby allowing cytoplasmic accumulation and subsequent nuclear localization of beta-catenin (MacDonald et al. 2009; Clevers and Nusse 2012; Hikasa and Sokol 2013). Nuclear beta-catenin interacts with the LEF/TCF family of High Mobility Group (HMG) domain transcription factors (Brunner et al. 1997, see below) either to activate or to “derepress” transcription of Wnt-responsive genes (Fig. 6.7).
Signaling is initiated by binding of Wnts to one of seven-transmembrane domain Frizzled (Fzd) receptors plus a coreceptor, lipoprotein receptor-related protein Lrp5 or Lrp6 (Lrp5/6; reviewed in He et al. 2004). Fzds are heterotrimeric G protein-coupled receptors that are activated in response to Wnts (Slusarski et al. 1997b; Katanaev and Buestorf 2009). Wnts bind to Fzd through the receptor’s extracellular cysteine-rich domain (CRD), with key contacts being made between the Wnt lipid moiety and a separate hydrophobic “index finger” interacting with grooves in the CRD (Janda et al. 2012). Wnts also interact with the Lrp6 extracellular domain, leading to clustering of the receptors and coreceptors (Tamai et al. 2000; Mao et al. 2001; Kato et al. 2002; Liu et al. 2003; Itasaki et al. 2003). The induced proximity of the intracellular domains of Fzd and Lrp6 is necessary and sufficient to initiate downstream signaling and inhibition of beta-catenin phosphorylation (Tolwinski et al. 2003).
The inhibition of beta-catenin degradation following Wnt receptor activation remains incompletely understood. Recent observations together with extensive data on biochemical interactions have suggested that Wnt-Fzd binding activates Dvl, possibly through GPCR activation, to recruit Axin/Gsk3b/Ck1a complexes to the Lrp6 intracellular domain, resulting in phosphorylation by Gsk3b and Ck1a (Mao et al. 2001; Tolwinski et al. 2003; Cliffe et al. 2003; Tamai et al. 2004; Davidson et al. 2005; Zeng et al. 2005, 2008; Egger-Adam and Katanaev 2010; Jernigan et al. 2010). Lrp6 phosphorylation occurs at PPPSPxS motifs, which serve as sites for additional Axin complex recruitment and are thought to directly inhibit destruction complex Gsk3b activity (Piao et al. 2008; Cselenyi et al. 2008; Wu et al. 2009). Furthermore, Axin itself is a Gsk3b substrate (Yamamoto 1999) and Gsk3b inhibition results in Axin dephosphorylation and its dissociation from phospho-Lrp6 and beta-catenin (Kim et al. 2013). Axin is then free to be either phosphorylated again to reconstitute beta-catenin destruction complexes or degraded. These data are consistent with a kinetic analysis of beta-catenin regulation, which suggests that Wnt signaling results in partial inhibition of both Gsk3b and Ck1a activities (Hernández et al. 2012). It is possible that this effect could be explained by the inactivation of a subset of limiting and finite destruction complexes through Axin dephosphorylation, which would then depress overall beta-catenin phosphorylation at the population level in a distributed manner.
In a separate but not necessarily mutually exclusive model, Dvl recruitment leads to multimerization of phospho-Lrp6-Fzd complexes, followed by accumulation of Dvl aggregates, leading to positive feedback recruitment and inactivation of destruction complexes (Metcalfe and Bienz 2011; Dobrowolski and De Robertis 2012). There is also evidence that these receptor complexes are incorporated into signaling endosomes (Lrp6 signalosomes) to stabilize and amplify signaling (Bilic et al. 2007). Other data suggest that these signalosomes are eventually sequestered into multivesicular bodies, leading to the longer-term removal of Gsk3b activity and the inability to phosphorylate newly synthesized beta-catenin (Taelman et al. 2010).
6.3.1.2. Transcriptional Regulation by Wnt/Beta-Catenin Signaling
Transcriptional responses in response to beta-catenin are mediated by binding to Lymphoid Enhancer-binding Factor 1 (Lef1)/Transcription factor 7 (T-cell specific, HMG-box; Tcf7) proteins. These proteins are constitutively nuclear and typically repress target genes by recruiting Groucho family repressors (Roose et al. 1998; Barker et al. 2000). Beta-catenin accumulation can lead to displacement of Groucho and activation of target genes, through a combination of derepression and transcriptional activation, mediated by distinct Lef1/Tcf7 proteins. Tcf7l1 (Tcf3 hereafter) likely exclusively acts as a transcriptional repressor during early development, with Lef1 and Tcf1 proteins serving as activators, and Tcf7l2 (Tcf4) exhibiting spliceform-dependent activator and repressive functions (Pukrop et al. 2001; Gradl et al. 2002; Wöhrle et al. 2007; Weise et al. 2010).
This protein family has diverged in function, with Tcf3 primarily performing a repressive role during early development and others acting as beta-catenin-dependent co-activators later in development (see Sect. 6.5.3). Tcf3 constructs lacking the ability to interact with beta-catenin, by deletion of the N-terminal beta-catenin-binding domain (deltaNTcf3), have been used to inhibit Wnt/beta-catenin-regulated transcription, as these cannot be derepressed or activated by beta-catenin. Expression of deltaNTcf3 during the cleavage stages efficiently ventralizes embryos (Molenaar et al. 1996; Pelegri and Maischein 1998), but fails to inhibit ventrolateral development or to block a late Wnt overexpression effect in Xenopus (i.e., anterior truncations; Hamilton et al. 2001). Additionally, experimental depletion of Tcf3 is sufficient to activate Wnt target gene expression during vertebrate axis development (Kim et al. 2000; Houston et al. 2002; Dorsky et al. 2003; Merrill et al. 2004) and in embryonic stem cells (Yi et al. 2008). Recent data from mouse studies in which the mutant deltaNTcf3 was knocked into the endogenous Tcf7l1 locus have substantiated the idea that Tcf3-mediated repression is critical for its role in early development (Wu et al. 2012a). Gastrulation proceeded normally in these mice, suggesting that the proper amount of transcriptional derepression of Tcf3 targets can occur in the absence of beta-catenin-Tcf3 interactions during axis formation. However, beta-catenin interactions with Tcf3 and with other Lef1/Tcf7 proteins are required later in development and in cancer cells (Wu et al. 2012a; Shy et al. 2013).
Derepression of Tcf3 is sufficient for Wnt target gene activation, although co-activators are also required for normal development, suggesting both likely operate in vivo. Beta-catenin recruits a number of co-activators including p300 and the conserved nuclear complex containing Pygopus and Bcl9 proteins (Kramps et al. 2002; Parker et al. 2002; Belenkaya et al. 2002). Pygo/Bcl9 are thought to be dedicated to Wnt signaling and may regulate the extent that Tcfs and beta-catenin associate with chromatin (Hoffmans et al. 2005; Fiedler et al. 2008; Mieszczanek et al. 2008). Also, beta-catenin has also been implicated in establishing poised chromatin architecture prior to major zygotic gene activation. Evidence in Xenopus suggests that beta-catenin recruits Histone H3 Arginine 8 Methyltransferase (Prmt2; Blythe et al. 2010) to modify chromatin at target loci prior to the onset of target gene expression. Thus, Wnt target genes are regulated both by direct transcriptional activation following beta-catenin recruitment and by beta-catenin-regulated changes to chromatin, modes of regulation that may be temporally uncoupled. It is unclear, however to what extent the Pygo-regulated mechanisms and Prmt2 chromatin modifications are interrelated or instead exhibit overlapping or redundant regulation of Wnt targets genes.
6.3.1.3. Wnt/Planar Cell Polarity (PCP) Signaling
The first studies on Wnt signaling focused on the regulation of beta-catenin in development and cancer. Subsequent work found additional roles for a subset of Wnt ligands other components in controlling cell movements during axis organization and/or antagonism of the Wnt/beta-catenin pathway (Rauch et al. 1997; Rothbächer et al. 2000; Wallingford et al. 2000; Veeman et al. 2003a) (Fig. 6.7). In vertebrates, these beta-catenin-independent Wnt pathways (often referred to, malapropos, as the “noncanonical” Wnt pathways) were shown to act through conserved Drosophila planar cell polarity (PCP) homologs and/or through release of intracellular calcium (Wnt/PCP and Wnt/Calcium pathways). Although these different pathway designations are convenient conventions, there is likely a large degree of overlap and interaction among them in vivo, particularly in the case of the Wnt/PCP and Wnt/Calcium pathways, and the ultimate outcome of signaling is likely dependent on the complement of Fzd receptors and coreceptors present on a given cell. This important point was exemplified early on by experiments showing that Wnt5a, traditionally considered a beta-catenin-independent Wnt ligand, could induce second axes in Xenopus when co-expressed with its cognate receptor Frizzled 5 (He et al. 1997), and recently by studies demonstrating Wnt5a-mediated regulation of beta-catenin-dependent and -independent Wnt signaling in mammals (Mikels and Nusse 2006; van Amerongen et al. 2012).
PCP signaling in vertebrates involves a set of components largely homologous to those mediating planar cell polarity signaling during imaginal disc development in insects (Vinson and Adler 1987; Krasnow and Adler 1994). This core set of proteins controls asymmetric Fzd1 localization (Strutt 2001) independently of Wnt ligands (Lawrence et al. 2002) and have been characterized genetically and biochemically in Drosophila (reviewed in Maung and Jenny 2011; Jose Maria Carvajal-Gonzalez 2014). These proteins include Fzd, Dishevelled, Flamingo (Fmi, a seven transmembrane pass cadherin), Prickle (Pk, a LIM and PET domain protein), strabismus/Van Gogh (Stbm/Vang, a four transmembrane protein with a PDZ motif), and Diego (Dgo, an ankyrin repeat protein). Homologous proteins also control epithelial cell and tissue polarity in vertebrates, notably in the inner ear (reviewed in Veeman et al. 2003a; Bayly and Axelrod 2011). Additionally, vertebrate PCP proteins are critical for controlling cell shape and cell migration in mesenchymal-type cells. Cell intercalation and cell migration during vertebrate gastrulation and neurulation in particular are dependent on Wnt/PCP signaling (reviewed in Solnica-Krezel and Sepich 2012).
The mechanisms of signal transduction during Wnt/PCP signaling in vertebrates are more varied and less well characterized than those of the beta-catenin-dependent pathway. Activation of the Wnt/PCP pathway in vertebrates is dependent on certain Wnt-Fzd combinations and a different set of coreceptors instead of Lrp5/6, including Ryk (Kim et al. 2008; Yoshikawa et al. 2003), Ror2 (Schambony and Wedlich 2007; Gao et al. 2011), and various Glypican proteoglycans (Topczewski et al. 2001; Ohkawara et al. 2003). Additionally, the transmembrane protein encoding Protein tyrosine kinase 7 (Ptk7) has been characterized as a novel regulator of PCP signaling (Lu et al. 2004; Yen et al. 2009). The role of Ptk7 is unclear, but it may represent an additional Wnt coreceptor modulating beta-catenin inhibition and activation with PCP signaling (Peradziryi et al. 2011; Hayes et al. 2013; Bin-Nun et al. 2014; Linnemannstöns et al. 2014). Dvl involvement is also critical for beta-catenin-independent Wnt signaling, although different domains are important for each function by controlling protein complex assembly and subcellular localization (Axelrod et al. 1998; Boutros and Mlodzik 1999; Rothbächer et al. 2000). The N-terminal DIX domain (Dishevelled, Axin) is critical for beta-catenin regulation whereas the C-terminal DEP (Dvl, Egl-10, Pleckstrin) domain regulates PCP and calcium signaling. In Drosophila, Dvl associates with Fzd1 and localizes to the distal cell margin. This complex inhibits the distal accumulation of Vang/Pk complexes, which are restricted proximally.
In vertebrates, similar complexes are implicated but the assembly and asymmetry of these is less understood. Fzd/Dvl association likely occurs following GPCR activation, and Dvl and Fzds accumulate in asymmetric puncta in cells in various vertebrate tissues undergoing PCP. In the zebrafish gastrula, Dvl-GFP is localized to the posterior membrane of cells whereas injected Drosophila Prickle-GFP localizes to the opposite, anterior edge (Ciruna et al. 2006; Yin et al. 2008). Additionally, in the mouse posterior notochord/node, Prickle2 and Vangl1 colocalize at the anterior edge of cells (Antic et al. 2010) and Dvl-GFP localizes posteriorly (Hashimoto et al. 2010). However, in other tissues such as the cochlea, Vangl2 and Fzd3 colocalize (Wang and Nathans 2007), but Prickle2 and Fzd6 localize to opposite sides (Deans et al. 2007). Celsr1 (a vertebrate Fmi homolog; cadherin, EGF LAG seven-pass G-type receptor 1) may also play a role in recruiting Dvl/Fz complexes to adherens junctions in the neural plate and mediating subsequent signaling (Nishimura et al. 2012). Thus in vertebrates, the roles of the different core PCP components may have diverged following gene duplication and the acquisition of Wnt ligand dependence and may have taken on tissue- or cell type-specific roles.
Dvl recruitment in the context of Wnt/PCP signaling is implicated in the control of cytoskeletal dynamics through the activation of small GTPases. Dvl can recruit the Formin-related Daam1 protein to activate Rho in an Wnt-dependent manner and regulate actin dynamics (Habas et al. 2001). Additionally, Rho activation can lead to Rho kinase (Rok2) activation to control cell shape (Marlow et al. 2002; Tahinci and Symes 2003). In a separate and parallel pathway, Dvl can directly activate Rac downstream of Wnt, leading the stimulation of filopodial extensions and Mapk8 (Jun N-terminal kinase, JNK) activation (Habas et al. 2003; Tahinci and Symes 2003). The coordinate activity of Rho and Rac, and potentially other small GTPases, is required for cell intercalation and convergent extension morphogenesis in many developing tissues.
6.3.1.4. Wnt/Calcium Release Signaling
Certain Wnt-Fzd combinations can stimulate the release of intracellular calcium stores (reviewed in Veeman et al. 2003a; Kohn and Moon 2005) and signal independently of beta-catenin. The regulation of this pathway also begins with Fzd-mediated heterotrimeric G protein activation and involves well-characterized GPCR responses, namely phosphoinositide turnover (Slusarski et al. 1997b), activation of cGMP-phosphodiesterase (Ahumada et al. 2002), as well as Calmodulin-dependent protein kinase 2 (Camk2) and Protein kinase C (Prkca) activation (Sheldahl et al. 1999; Kuhl et al. 2000). Many of the same coreceptors involved in Wnt/PCP signaling are also critical for the Wnt/calcium pathway, suggesting that these pathways overlap considerably (Fig. 6.7). In line with this idea, overexpression of Dvl can initiate calcium flux and activate Camk2 and Prkca in fish and frog embryos (Sheldahl et al. 2003). Similarly, overexpression of Prickle1 indirectly regulates calcium dynamics (Veeman et al. 2003b). Also, recruitment of Dvl to the membrane during PCP signaling requires a calcium-regulated PKC isoform, Prkcd (Kinoshita et al. 2003). Wnt/PCP and Wnt/Calcium are likely to be tightly integrated, owing to shared components and shared roles in regulating morphogenesis during gastrulation and beta-catenin antagonism.
Evidence suggests that Wnt/Calcium signaling is essential for inhibiting beta-catenin activation during axis formation. Loss of maternal Wnt5b in zebrafish eliminates calcium flux in the blastula and triggers ectopic beta-catenin activity, resulting in dorsalized embryos (Westfall et al. 2003). This effect was partially rescued by Camk2, suggesting that calcium-mediated activation of this pathway is sufficient to suppress beta-catenin activity. Wnt/Calcium is also implicated in activating Nemo-like kinase (Nlk) (Ishitani et al. 1999, 2003; Meneghini et al. 1999) and Nfatc nuclear translocation (Saneyoshi et al. 2002) to antagonize beta-catenin activity.
6.3.1.5. Wnt Secretion and Extracellular Regulation
Wnts are secreted and are modified by glycosylation (Brown et al. 1987; Papkoff et al. 1987) and lipidation (Willert et al. 2003). Efficient secretion of Wnts requires glycosylation and palmitoleoylation, the latter of which is mediated by the Porcupine (Porcn) family of acyl transferases (van den Heuvel et al. 1993; Kadowaki et al. 1996; Hofmann 2000; Tanaka et al. 2000). Tyrosine sulfation has also been observed and may be necessary for activity in some cases (Cha et al. 2009). Wnt secretion also requires trafficking of Wnt from the Golgi apparatus to the plasma membrane by the Wntless Wnt ligand secretion mediator (Wls; alias Evi/Gpr177/Wingful) as well as efficient recycling of Wls through the endosome-retromer system (Bartscherer et al. 2006; Coudreuse 2006). Interestingly, Wls is a direct Wnt/beta-catenin target gene in mouse and is required for extracellular Wnt signaling during mouse axis formation (Fu et al. 2009), indicating that Wnt activity potentiates its own signaling. Additional evidence suggests Wnt proteins may also be packaged into lipoprotein particles and/or exosome vesicles (Panáková et al. 2005; Gross et al. 2012). Wnts can act as both long-and short-range signaling molecules in the extracellular space, acting as developmental morphogens (Zecca et al. 1996). Wnt signaling gradients can also interact with those of a Wnt antagonist, Dkk1, to establish hair follicle spacing through a Turing-like reaction-diffusion mechanism (Sick et al. 2006), illustrating one of the complex ways this pathway can used to establish tissue patterns in development.
Wnt signaling can be tightly regulated in the extracellular space by a host of different Wnt antagonists. Many of these proteins belong to large protein families and have redundant and tissue-specific functions throughout development (Cruciat and Niehrs 2013). The main secreted Wnt antagonists involved in axial patterning are the Secreted Frizzled-related proteins (Sfrps), which bind directly to Wnts and antagonize different Wnt ligands, and Dickkopf1 (Dkk1), which acts at the level of the Wnt/Lrp6 receptor complex. In addition, the secreted Notum pectinacetylesterase homolog was identified as a Wnt antagonist in Drosophila (Giraldez et al. 2002; Gerlitz and Basler 2002) and is thought to act by promoting membrane shedding of Glypican Wnt coreceptors (Kreuger et al. 2004). Recent data from flies and vertebrates also suggests that Notum acts as a Wnt deacylase, cleaving the Wnt palmitoleate moiety, resulting in Wnt ligand oxidation and inactivation (Kakugawa et al. 2015; Zhang et al. 2015). Notum is conserved and is involved in feedback regulation of Wnt signaling body axis patterning in Planaria (Petersen and Reddien 2011), and recent data suggest a role in dorsoventral neural tube pattering in zebrafish (Flowers et al. 2012).
Transmembrane antagonists have recently been identified as well. Those with roles in axis formation include the leucine-rich repeat protein Trophoblast glycoprotein (Tbgp/Waif1) and Tiki1 (Trabd2a). Tbgp is thought to act as a feedback Wnt inhibitor, acting in Wnt-receiving cells to alter Lrp6 subcellular localization (Kagermeier-Schenk et al. 2011). Trabd2a/Tiki1 is a transmembrane metalloproteinase enriched in the organizer that can cleave a subset of Wnt ligands, causing their abnormal oxidation and oligomerization and reduced receptor binding (Zhang et al. 2012).
6.3.2. Wnt/Beta-Catenin Signaling in Early Axis Formation
The central role of Wnt/beta-catenin signaling in axis formation was initially demonstrated largely through simple overexpression experiments in Xenopus and zebrafish embryos. The first of these was the induction of axis duplications in Xenopus by injected mouse Wnt1 mRNA (McMahon and Moon 1989). Xenopus wnt8a (Xwnt-8; Christian et al. 1991; Sokol et al. 1991, Smith and Harland 1991), and several other Wnt ligands (Wolda et al. 1993; Du et al. 1995; Kelly et al. 1995a) can also induce secondary axes and rescue UV ventralization. Importantly, beta-catenin also exhibits axis inducing activity (Funayama et al. 1995; Guger and Gumbiner 1995), and both Wnt and beta-catenin can induce axial structures non-cell autonomously when expressed in vegetal blastomeres, suggesting that this activity acts analogously to a Nieuwkoop center (Smith and Harland 1991; Guger and Gumbiner 1995).
Interestingly, later overexpression of wnt8a during gastrulation (from injected plasmid DNA as opposed to mRNA) causes a loss of anterior structures, indicating roles for Wnts in patterning of the axis as well as its induction (Christian and Moon 1993). Other Wnts, including Wnts 4, 5a and 11b, do not elicit axis duplications but disrupt gastrulation movements and cell adhesion when overexpressed (Moon et al. 1993; Ku and Melton 1993; Du et al. 1995). Additionally, these Wnts can be antagonistic to the axis-inducing Wnts in some cases (Torres et al. 1996). The same ligands were also shown to trigger intracellular calcium release when expressed in the early zebrafish embryo (Slusarski et al. 1997a, b).
Loss-of-function experiments have established that Wnt/beta-catenin signaling is essential for axis formation in vertebrates. The first evidence for this came from antisense oligonucleotide mRNA depletion of maternal ctnnb1 mRNA in Xenopus oocytes, leading to embryos lacking axial structures and dorsal-specific gene expression (Heasman et al. 1994). Additionally, in Nieuwkoop conjugate experiments, late blastula vegetal masses from ctnnb1-depleted embryos fail to induce dorsal mesoderm in animal caps, suggesting that maternal Wnt/beta-catenin is essential for the generation of the Nieuwkoop signal and acts upstream of other axis-inducing molecules (Wylie et al. 1996). Analysis of heterochronic Nieuwkoop conjugates pre-and post-midblastula transition also showed that dorsal and general mesoderm induction are primarily zygotic events (Wylie et al. 1996). Beta-catenin is present in dorsal nuclei prior to major zygotic genome activation in the Xenopus morula and blastula as well as in the zebrafish dYSL and dorsal marginal blastomeres (Schneider et al. 1996; Jesuthasan and Stähle 1997; Kelly et al. 2000; Dougan et al. 2003) demonstrating that Wnt/beta-catenin signaling is active in the relevant region of the embryo.
In addition to theses data, genetic studies in zebrafish identified a requirement for a maternally expressed beta-catenin in normal axis formation (ctnnb2/ichabod; Kelly et al. 2000). Furthermore, in the mouse, genetic deletion of Ctnnb1 results in embryos lacking axial structures and anteroposterior polarity, resulting from lack of all mesoderm and failure to form the anterior visceral endoderm (AVE) (Haegel et al. 1995; Huelsken et al. 2000; Morkel et al. 2003). Mouse beta-catenin is pre-dominantly required in the epiblast, as shown in chimeric embryo experiments (Huelsken et al. 2000) and is not required maternally (De Vries et al. 2004), reflecting a different mode of activation in mammals. The main Wnt ligand expressed at this time, Wnt3, is primarily expressed in the posterior epiblast and is required for embryonic axis and mesoderm formation (Liu et al. 1999b). Interestingly, the formation of the AVE is normal in Wnt3 null mice, indicating a differential role for beta-catenin in the development of this tissue. A Wnt ligand-independent role for beta-catenin in anteroposterior patterning has been proposed (Morkel et al. 2003), possibly through regulation of Tdgf1 (teratocarcinoma-derived growth factor 1, alias Cripto/frl1) expression and subsequent effects on Nodal activity (see Sect. 6.5). Although genetic manipulations are less tractable in the chicken, studies using extracellular Wnt inhibitors suggested that Wnt signaling is required for experimental axis induction (Skromne and Stern 2001).
6.3.3. Asymmetric Activation of Wnt/Beta-Catenin Signaling in Early Amphibian and Fish Embryos
6.3.3.1. Xenopus
Despite the well-documented roles for beta-catenin in axis formation in Xenopus, and more recently in zebrafish, it remains relatively unclear how Wnt/beta-catenin signaling is initiated in early embryos, as well as the extent that these activating mechanisms are conserved. Cytoplasmic transplantation studies in Xenopus identified the presence of a cytoplasmic, transplantable dorsalizing activity in the vegetal cortical region (Darras et al. 1997; Marikawa et al. 1997; Marikawa and Elinson 1999). By correlating its activity with various axis-inducing molecules, this vegetal cortical cytoplasm was found to mimic intracellular activation of the Wnt/beta-catenin signaling pathway (Marikawa and Elinson 1999). Curiously, UV-irradiation experiments in Xenopus oocytes indicated that this cytoplasm showed cell cycle-dependent sensitivity to UV Irradiation of the egg disrupts microtubule assembly and cortical rotation, although the activity of the vegetal cortical cytoplasm itself is not affected. By contrast, UV-irradiation of full-grown oocytes effectively does eliminate the dorsalizing ability of vegetal cytoplasm and ventralizes embryos (Holwill et al. 1987; Elinson and Pasceri 1989). Eggs irradiated as oocytes undergo normal cortical rotation and are not rescued by tipping (Elinson and Pasceri 1989), suggesting that a critical component of axis induction is absent.
The target of UV irradiation in the oocyte is not known, but either its action is completed by oocyte maturation or it is subsequently sequestered and no longer susceptible to irradiation. These features may be useful in identifying potential candidate molecules. In apparent support of a direct cytoplasmic beta-catenin activation model, particles of exogenous Wnt-activating proteins, Dvl2-GFP (Miller et al. 1999) and Frat1-GFP (Weaver et al. 2003) were shown to undergo dorsal translocation during cortical rotation, suggesting that the dorsalizing activity might be composed of beta-catenin stabilizing agents. These molecules might then directly stabilize beta-catenin, or act by sensitizing dorsal cells to Wnt signals. Further indications of potential Wnt ligand-independent dorsalizing mechanisms came from observations that overexpression of secreted Wnt antagonists were unable to suppress endogenous axis formation (Hoppler et al. 1996; Leyns et al. 1997; Wang et al. 1997).
More recent studies in Xenopus suggest a more typical Wnt signaling model, with maternal Wnt11b acting to induce beta-catenin activity. Maternal wnt11b is localized to the vegetal cortex during oogenesis via a mitochondrial cloud-dependent pathway (see Chap. 8) and was initially considered a prime candidate for the vegetal dorsally activity, based on its activity in UV-rescue experiments. In light of the evidence favoring the direct activation model (see above), and other experiments showing that Wnt11b can regulate beta-catenin-independent signaling, a role for wnt11b in axis formation was later discounted. However, a reinvestigation using antisense oligo mediated maternal mRNA depletion showed that maternal wnt11b is indeed required for axis formation (Tao et al. 2005).
Wnt11b is though to act in concert with uniformly expressed Wnt5a (Cha et al. 2008), forming extracellular complexes with each other and with other proteins, including heparin sulfate proteoglycans and the Nodal coreceptor Tdgf1 (Tao et al. 2005; Cha et al. 2008, 2009). The activity of this Wnt complex can be antagonized by maternal Dkk1, suggesting a model in which cortical rotation tips the balance of Wnt activity to overcome generalized Wnt antagonism (Cha et al. 2008). The mechanism of Wnt11b enrichment dorsally following cortical rotation is unclear, as both enrichment of total wnt11b RNA (Tao et al. 2005) or enhanced polyadenylation (Schroeder et al. 1999) have been proposed. A similar mechanism, albeit with different Wnts and antagonists, has been proposed in zebrafish (see below). However, Wnt11b can also regulate beta-catenin levels in an autocrine fashion in fully grown oocytes (Kofron et al. 2007), providing the possibility that Wnt activation may wholly or partially occur before fertilization. Possible mechanisms for Wnt activation in Xenopus are shown Fig. 6.8 (top panels).
Fig. 8.
Models for Wnt/beta-catenin activation in Xenopus and zebrafish. (a) During cortical rotation in Xenopus (top) and zebrafish (bottom), beta-catenin stabilizing dorsalizing activity is transported into the equatorial region of the embryo by microtubule-mediated rotation of the cortex and through transport along microtubule arrays. Candidates for this activity include wnt11b and Lrp6/Dvl particles in Xenopus and wnt8a in zebrafish. (b) By the cleavage stages (16–128-cell stage), beta-catenin be-comes activated and enriched in dorsal vegetal and marginal nuclei until MBT. In Xenopus, priming of Wnt target genes occurs through dimethylation of Histone3 at arginine 8 (H3R8). In zebrafish, beta-catenin accumulates in dorsal marginal and dYSL nuclei, and is antagonized by multiple antagonists and calcium signaling mediators. (c) During the peri-MBT stages, beta-catenin activates direct Wnt targets and cooperates with maternal T-domain proteins (Vegt, Eomes) to activate nodal initially on the dorsal side. The combination of nodal and BMP antagonism induced by beta-catenin induce the formation of the organizer (gsc, chrd)
Although there is good evidence for secreted Wnt11b activity, it is unclear when Wnt11b is required. Also, this role for Wnt11b has not been reconciled with the cytoplasmic activation model. One potential unifying model has been proposed; that ongoing Wnt signaling in the oocyte generates activated Lrp6 signaling endosomes that are transported dorsally (Dobrowolski and De Robertis 2012). However, it is not known to what extent these Lrp6-containing endosomes are formed in the oocyte. Lrp6 is phosphorylated in eggs but becomes dephosphorylated following egg activation (Davidson et al. 2009), suggesting that stable signaling complexes may actually be inactivated prior to cortical rotation. Furthermore, analyses of the relative activity of vegetal cortical cytoplasm suggests it acts at the level of the destruction complex and does not mimic the activity of activated receptors (Marikawa and Elinson 1999).
6.3.3.2. Zebrafish
The regulation of Wnt signaling in zebrafish also has intriguing parallels and differences to the situation in Xenopus (Fig. 6.8, bottom panels). Maternal/zygotic mutants of wnt11 form the axis normally in zebrafish (Heisenberg et al. 2000), suggesting that fish use other mechanisms or other Wnts. Maternal wnt8a has been proposed to act as a dorsal determinant in zebrafish (Kelly et al. 1995a; Lu et al. 2011). Wnt8a is the only vegetally localized wnt transcript in the yolk cell, and is shifted asymmetrically following cortical rotation. Vegetal localization also occurs through a mitochondrial cloud dependent mechanism, similar to frog wnt11b (Lu et al. 2011). In the fish however, injection of a dominant-negative Wnt8a construct was able to reduce the expression of chrd and dharma, the latter being a direct Wnt target gene. Full-length Wnt8a also rescues to some extent embryos ventralized by nocodazole (Lu et al. 2011). Also, depletion of Sfrp1 or Frzb hyperdorsalizes embryos, indicating dorsal enrichment of a maternal Wnt, Wnt8a in the case of fish, may be a trigger to overcome generalized Wnt antagonism in the embryo. While this and the similar mechanism proposed to act in frog are intriguing (Cha et al. 2008), it remains to seen whether relatively small changes in Wnt levels involved are responsible for dorsalization.
Evidence in fish is also suggests that other mechanisms of Wnt antagonism are critical in suppressing beta-catenin activation. Notably, maternal/zygotic mutants for wnt5b are hyperdorsalized, owing to defective Wnt/calcium signaling and failure to repress Wnt/beta-catenin signaling (Westfall et al. 2003). A similar but not well-characterized pathway may exist in frogs (Saneyoshi et al. 2002). In Xenopus, the maternal role of Wnt5b has not been assessed, however Wnt5a is proposed to activate instead of repress Wnt/beta-catenin signaling in frogs (Cha et al. 2008). Axis formation is largely normal however in wnt5a, wnt5b double mutant mice (Agalliu et al. 2009; spinal cord formation), suggesting that the roles of Wnt5 paralogues may be species-specific. Recent data suggest that calcium transients downstream of the chemokine receptor Ccr7 GPCR signaling are also involved in suppressing beta-catenin activity (Wu et al. 2012b). The prominence of calcium regulation mechanisms suggests tight control over beta-catenin stabilization in the zebrafish blastula.
6.3.4. Beta-Catenin Activity Dorsalizes the Primary Germ Layers
Beta-catenin stabilization in the blastula following cortical rotation is the central mechanism for establishing early dorsal fates across all three primary germ layers (Fig. 6.8). In the vegetal prospective endodermal cells, beta-catenin is critical for the dynamic regulation of Nodal expression and signaling, which is directly involved in mesendoderm induction and patterning and likely constitutes what is referred to as the Nieuwkoop center (see Chap. 7). In Xenopus, the typical nodal homolog genes (e.g., nodal homologs 1, 2, 4, 5, and 6) are expressed in a temporally and spatially graded fashion in the late blastula/early gastrula. These genes depend on vegetally localized maternal vegt activity, showing high dorsovegetal expression at the onset of gastrulation, followed by a shift ventrolaterally by late gastrulation, mirrored by Smad2 activity (Agius et al. 2000; Faure et al. 2000; Lee et al. 2001). Maternal beta-catenin directly patterns this activity first by contributing to early nodal paralogue expression (nodal5, nodal6) in dorsovegetal blastomeres prior to and immediately after the onset of zygotic transcription at the mid-blastula transition (MBT; see Chap. 9) (Takahashi et al. 2000; Rex et al. 2002; Xanthos et al. 2002; Hilton et al. 2003; Blythe et al. 2010). This role of beta-catenin may be related to its function in recruiting Prmt2 to prime gene expression during the cleavage stages (Blythe et al. 2010). Beta-catenin also synergizes with Nodal activity to generate higher nodal1 expression dorsally in the early gastrula, although mid-late gastrula expression becomes uniform and is independent of beta-catenin (Agius et al. 2000; Lee et al. 2001).
In addition to overlapping with Vegt, beta-catenin might also functionally interact with Gdf1 (alias Vg1), which is encoded by another maternally localized mRNA (Rebagliati et al. 1985; Melton 1987; Weeks and Melton 1987). Gdf1 is a Nodal-related Tgfb family ligand and activates Smad2 signaling through Nodal receptors/coreceptors. Wnt/beta-catenin activity synergizes with gdf1 overexpression to induce axial structures in Xenopus (Cui et al. 1996). Maternally depleted gdf1 embryos lack anterior structures and are deficient in the expression of certain organizer markers, including secreted antagonists nog, chrd, dkk1, and cer (Birsoy et al. 2006). Early Smad2 activation is also compromised, indicating that Gdf1 contributes to overall Nodal signaling on the dorsal side of the late blastula (Birsoy et al. 2006). Thus, beta-catenin activity can enrich Nodal expression and activity dorsally at multiple regulatory levels.
This temporal control of Nodal activity is functionally significant. In Nieuwkoop conjugate experiments, late blastula vegetal explants from ctnnb1-depleted Xenopus embryos fail induce dorsal fates in early equatorial or animal cap tissue (Xanthos et al. 2002; Wylie et al. 1996). However, if ctnnb1-depleted vegetal explants from late gastrula embryos are used, which now express nodal genes, dorsal mesoderm is induced (Xanthos et al. 2002). Additionally, pre-MBT Nodal activity regulated by beta-catenin is essential for normal axis formation, possibly for perpetuating nodal expression through autoinduction (Skirkanich et al. 2011). Beta-catenin also likely contributes to Nodal ongoing autoregulation, both directly and indirectly. Dorsal beta-catenin activity is required to repress microRNAs 15 and 16 (miR15/16) through an unknown mechanism (Martello et al. 2007). These miRs target and downregulate an essential component of the Nodal receptor complex, activin A receptor, type IIA (acrv2a) (Martello et al. 2007), which contributes to the early dorsal bias in Nodal activity.
In zebrafish, maternal beta-catenin signaling similarly regulates early nodal homolog gene expression. Two paralogues are expressed in the early dYSL (nodal1/squint and nodal2/cyclops); nodal1 is likely a direct beta-catenin target (Kelly et al. 2000) and nodal2 is autoregulated by Nodal signaling itself. Genetic and other loss-of-function experiments support a role for the early Nieuwkoop center nodal genes in axis formation. Single and double mutants for nodal1, nodal2 (squint, cyclops) exhibit axial patterning and mesendoderm defects (Rebagliati et al. 1998; Feldman et al. 1998; Sampath et al. 1998; Erter et al. 1998), and these abnormalities can be rescued by YSL-specific expression of Nodal ligands. Additionally, nodal1 is maternally expressed in zebrafish and its mRNA localized to the presumptive dorsal side at the 4-cell stage, through an uncharacterized mechanism involving microtubules (Gore et al. 2005). The role of this maternal transcript is not fully clear; it may lead to enhanced Nodal activity dorsally, although there are hints that the 3′UTR may help promote dorsal nuclear beta-catenin accumulation by acting as a noncoding RNA (Lim et al. 2012).
In addition to stimulating higher levels of Nodal activity, dorsal beta-catenin directly initiates a set of gene regulatory interactions that integrate with Nodal signaling in the prospective mesendoderm (Fig. 6.8). In Xenopus, Wnt/beta-catenin signaling directly activates the paired homeobox paralogues siamois1 (sia1) and siamois2 (twin/sia2) in dorsovegetal cells in the blastula (Lemaire et al. 1995; Brannon and Kimelman 1996; Brannon et al. 1997). Sia1/2 integrate with Nodal/Tgfb signaling in activating additional genes in the anterior endoderm and organizer, including cerberus, hhex, and goosecoid, whose promoters are bound by Sia1 (Ishibashi et al. 2008; Rankin et al. 2011; Sudou et al. 2012). Maternal beta-catenin signaling is also required to repress early bmp4 expression dorsally in Xenopus (Baker et al. 1999), and this is also mediated through Sia1/2. These proteins are required to repress Bone Morphogenetic Protein (BMP) expression and activity dorsally, which is essential for dorsal mesoderm formation (see Sect. 6.4.1). Sia1/2 activate the expression of several BMP antagonists, including noggin (nog) and chordin (chrd) (Ishibashi et al. 2008), and also indirectly repress bmp4 expression, through an unknown intermediate. Nog and chrd expression are maintained by Nodal signaling, but initial expression depends only on maternal beta-catenin (Wessely et al. 2001). Although Sia1/2 are predominantly expressed in mesendodermal precursors, their expression can extend into some equatorial precursors that escape mesoderm induction (Kuroda et al. 2004), where they specify early neural fate and the prospective anterior neuroectoderm (Ishibashi et al. 2008; Klein and Moody 2015).
Similarly, in zebrafish maternal beta-catenin activates expression of dharma in dorsal marginal blastomeres and dYSL. Dharma is a paired homoebox gene some- what functionally analogous to sia1, but unrelated by descent (Fekany et al. 1999; Kelly et al. 2000). Dharma has numerous roles in dorsoventral patterning, including direct repression of bmp2b (Koos and Ho 1999; Leung et al. 2003a). Interestingly, both sia1 and dharma are expressed prior to major zygotic gene activation in frogs and fish (Yang et al. 2002; Leung et al. 2003b; Blythe et al. 2010) suggesting very early and direct roles for these proteins in dividing the embryo into prospective BMP-expressing or -absent territories. Additionally in fish, the accumulation of nuclear beta-catenin occurs concomitantly with a change in cell division patterns relative to the body axis as well as slower cell division in the presumptive dorsal shield region, but a causal relationship between these events has not been established (Keller et al. 2008).
Although the activation of Nodal and Sia1/2 appears to mediate many of the functions of maternal beta-catenin signaling, other less characterized pathways are likely required as well. One other beta-catenin target gene, nodal homolog 3.1 (nodal3.1; Smith et al. 1995), likely functions in controlling morphogenesis during organizer formation. Nodal 3.1 is an atypical member of the Nodal protein family present only in anuran amphibians (Smith et al. 1995). This protein has BMP antagonist activity (Hansen et al. 1997) and, during gastrulation, is restricted to the superficial epithelial layer of the organizer (Glinka et al. 1996), a region with discrete morphogenetic regulatory ability (Shih and Keller 1992a). Loss-of-function experiments suggest that Nodal3.1 regulates convergent extension, acting as a Fibroblast growth factor (FGF) receptor ligand (Yokota et al. 2003), although this mechanism is not well understood.
In summary, beta-catenin function is critical at many different regulatory levels to specify dorsal fate across all prospective germ layers in the early blastula in Xenopus and zebrafish. These general functions would include generating dorsal-inducing signals, mediating competence to respond to those signals, reducing the ability of dorsal cells to respond to ventralizing BMP signals, and regulating dorsal morphogenesis. In this context, the strong expression of nodals in the dorsovegetal blastomeres is likely responsible for the Nieuwkoop center phenomenon, although beta-catenin activity is required in prospective organizer mesoderm and ectoderm as well. In amniotes, however, axis formation is also governed by the dynamic regulation of Nodal signaling in concert with Wnt/beta-catenin activity. In these cases, the initial establishment of restricted Nodal signaling occurs without early maternal Wnt signaling and the role of a Nieuwkoop center analog in inducing the organizer is less clear.
6.3.5. Wnt/Beta-Catenin and Nodal Signaling During Axis Formation in Amniotes
6.3.5.1. Chick
The role of concerted Wnt and Tgfb signaling in the dynamic regulation of Nodal expression and activity is conserved during amniote axis formation. In contrast however to the case in fish and frogs, axis formation in birds and mammals is driven by spatially restricted Nodal activity overlapping with more generalized Wnt/beta-catenin signaling in the marginal zone of the epiblast (Fig. 6.9a). In the chicken, there is no evidence for localized Wnt expression in the egg or early cleavage stages. Wnt8a (alias, CWnt8C) is the predominant Wnt expressed prior to gastrulation and is enriched in the PMZ epiblast, but is also found in a decreasing gradient around the marginal zone from posterior-to-anterior (Hume and Dodd 1993; Skromne and Stern 2001). By contrast, the Nodal-related gene Gdf1 is specific to the PMZ at the same stage (Shah et al. 1997; Skromne and Stern 2001) and represents one of the earliest asymmetrically expressed genes in the chick blastoderm (and the first to be discovered).
Fig. 9.
Models for axis induction signaling in chick and mouse. (a) In the chick blastoderm (left panel, top/dorsal view, ~stage X, Eyal-Giladi and Kochav 1976) the outer marginal zone of the epiblast expresses Wnt8a in a posterior-to-anterior gradient (purple shading). In the PMZ, Pitx2 (yellow) activates Gdf1 expression (green). Subsequently (middle panel), the newly formed hypoblast (below the plane of the page) begins anterior migration and Gdf1 + Wnt8a cooperate to induce Lef1 in the PMZ and Nodal in the adjacent epiblast. Gata2 is expressed in the anterior marginal zone and antagonizes Gdf1 long-range. Nodal (magenta) is antagonized by Cerberus (Cer), which is expressed in the hypoblast. By the initial primitive streak stage (right panel, stage 2+, Hamburger and Hamilton 1951), the anterior migration of the hypoblast and migration of the endoblast beneath the posterior epiblast removes the inhibition of Nodal and allows feed-forward signaling leading to primitive streak formation. The same signaling molecules are ex-pressed in the primitive streak and induce organizer genes in Hensen’s node (dotted circle, Gsc, Chrd) at the anterior tip of the streak. (b) In the mouse, the earliest asymmetries are the expression of Lefty1 and Cerl (red-brown) at the tip of the postimplantation AVE (left panel, ~E5.0). These genes are regulated by Nodal (green) and Tdgf1, and Tdgf1 is regulated by beta-catenin in the absence of secreted Wnt ligand activity (stop symbol). Lefty1 and Cerl antagonize Nodal and feedback regulation drives AVE migration towards the proximal egg cylinder on one side (right panel, ~E5.5). Nodal activity is restricted to the posterior epiblast and is responsible for Wnt3 expression (blue), which in turn maintains Nodal. These signals cooperate to induce the primitive streak, which induces Hensen’s node toward the distal tip later in gastrulation. a anterior, p posterior, ExE extraembryonic ectoderm, VE visceral endoderm, ParE parietal endoderm
Grafting of Gdf1-expressing cells induces an ectopic axis anywhere in the marginal zone (Shah et al. 1997; Skromne and Stern 2001). Interestingly, ectopic expression of Wnt alone is not a robust axis inducer in chicken (Joubin and Stern 1999). Gdf1 and Wnt8a can synergize in axis induction, and the axis-inducing ability of Gdf1 is blocked by co-expression with Wnt antagonists (Skromne and Stern 2001). These Gdf1 and Wnt8a signals cooperate to induce Lef1, a Wnt signaling transcriptional activator, in the PMZ (Skromne and Stern 2001) coincident with the activation of Nodal in the adjacent area pellucida epiblast (Skromne and Stern 2002). Nodal itself is required for primitive streak and axis formation (Bertocchini and Stern 2002). Based on shared embryological and molecular characteristics, it has been speculated that the PMZ epiblast in chick represents the equivalent of the Nieuwkoop center (Bachvarova et al. 1998). However, in birds and likely in mammals as well (see below), the middle regions of the primitive streak also express Gdf1, Wnt8a, and Nodal and may be more relevant for inducing the anterior streak and Hensen’s node/organizer (Joubin and Stern 1999; Bertocchini and Stern 2002). The Nieuwkoop center in chicks may thus be considered a primitive streak-inducer, as opposed to its more traditional organizer-inducing amphibian role (also see Sect. 6.7). As compared to mammalian axis formation, which depends initially on Nodal asymmetry (see below), the chick may represent an intermediate condition, where Wnt signaling is widely active prior to gastrulation but the role of Nodal-related proteins in inducing the axis is becoming predominant.
The mechanisms controlling localized Gdf1 expression in the PMZ remain largely unknown. In contrast to the strict requirement for maternal factors in fish and amphibians, there is only a presumptive early influence of maternal determinants in birds, and axis formation is highly self-organizing (i.e., regulative). Classic embryological studies showed that partitioning of the blastoderm results in axis initiation in each individual fragment (Lutz 1949; Spratt and Haas 1960; Callebaut and Van Nueten 1995), suggesting that axis determinants are not uniquely restricted to one region. In recent reinvestigations of this phenomenon, Gdf1 expression reinitiates stochastically at the “new posterior” pole of these blastoderm explants and regulates formation of the new primitive streak (Bertocchini et al. 2004). The Wnt8a gradient presumably reforms around the new site of Gdf1, suggesting that Wnt8a is likely downstream or independent of Gdf1. There is no evidence to suggest whether this regulation is direct or indirect. Data also show that the transcription factor Gata2 is expressed earlier than Gdf1, and in a roughly complementary anteriorly biased gradient (Bertocchini and Stern 2012). Both Gata2 and Gdf1 control each other’s expression indirectly through a global signaling gradient, likely mediated by BMP signaling downstream of Gata2. Additionally, recent microarray screening and functional analyses identified the Pitx2 homeodomain protein as an essential upstream activator of Gdf1 expression in the PMZ (Torlopp et al. 2014). This Pitx/Gdf1/Nodal regulatory relationship is deeply conserved in several well-known developmental processes, including sea urchin axis patterning, amphibian mesendoderm induction and left-right pattering (Torlopp et al. 2014 and references therein), indicating these genes form a robust module can be readily redeployed for novel functions. However, there are as yet no clues to any mechanisms that would potentially activate Pitx2 or connect this pathway to any maternal asymmetries. Interestingly, recent data suggest that chick embryos form a yolk syncytial layer (Nagai et al. 2015), similar to that of teleosts. This is likely the result of independent convergent evolution and it will be exciting to learn to what extent this structure might function similarly in axis induction.
6.3.5.2. Mouse
In contrast to the case in chick, in which the graded expression of Wnt8a in the marginal zone still plays some role in normal axis development, several lines of evidence in the mouse suggest that active Wnt/beta-catenin signaling is not part of the early axial polarization mechanism (Fig. 6.9b). Many Wnt genes are expressed in the preimplantation blastocyst (Kemp et al. 2005), although studies in Wnt reporter mice initially suggested that Wnt signaling is not active in the blastocyst prior to implantation (Mohamed et al. 2004; Na et al. 2007). Recent studies with more sensitive reporter constructs have detected some beta-catenin-dependent activity in the ICM/epiblast of peri-implantation blastocysts (E3.0–4.5; Granier et al. 2011; ten Berge et al. 2011). Similarly, transient nuclear beta-catenin has been observed by immuno-localization at the same stage (Chazaud and Rossant 2006). However, the extent of activity is unclear, since overexpression of stabilized beta-catenin does not ectopically induce Wnt target genes before gastrulation (Kemler et al. 2004). Thus, although there are indications of early but non-polarized beta-catenin activity in the preimplantation mammalian embryo, the role of this signaling appears to be dispensable or easily compensated for, at least with regard to axis formation.
Correspondingly, mutants for the major Wnts, Wnt/beta-catenin coreceptors, as well as regulators of Wnt secretion and activity all undergo normal preimplantation development, including anteroposterior patterning in the AVE. These mutants also fail to form mesoderm and do not gastrulate. These Wnt/beta-catenin pathway mutants include (but are not limited to) Wnt3 (Liu et al. 1999b), Lrp5/6 (Kelly et al. 2004), Mesdc1 (Hsieh et al. 2003), Wntless (Fu et al. 2009), and Porcn (Biechele et al. 2011, 2013; Barrott et al. 2011). Since beta-catenin mutants exhibit these same mesoderm defects but additionally fail to form the AVE, a Wnt ligand-independent role for beta-catenin in anteroposterior patterning has been proposed (Morkel et al. 2003), possibly mediated by activation of Tdgf1 expression and subsequent effects on Nodal activity (see Sect. 6.5.2). These results suggest possible similarities to the potential role of Wnt ligand independent signaling in Xenopus axis formation (see Sect. 6.3.3), but too little is known about either mechanism to draw meaningful comparisons. There are other agonist ligands of Wnt/beta-catenin signaling, such as Norrin and R-spondin (Cruciat and Niehrs 2013), but these also rely on Lrp5/6. Other potential mechanisms of regulating beta-catenin activity may exist, such as regulation through Hippo signaling (Varelas et al. 2010) or through Seven in abstentia homologs (Siah) (Topol et al. 2003), although these have not been extensively investigated in the context of axis formation.
Rather than relying on polarized Wnt activity, the earliest asymmetries in the mouse blastocyst are the expression of Nodal antagonists Cerl and Lefty1 in the primitive endoderm of the peri-implantation blastocyst (~E4.0; Torres-Padilla et al. 2007b). Expression of these genes likely depends on FoxH1-mediated Nodal signaling (Takaoka et al. 2006; Torres-Padilla et al. 2007b), initiating a cascade of both positive and negative feedback regulation of Nodal signaling in the embryo. Cerl/Lefty1 asymmetry is likely to arise stochastically at this early stage, as the blastocyst contains a rather small number of cells and it is unlikely that the few Cerl/Lefty1 expressing cells would arise precisely in the center. These cells are then thought to lead and propagate AVE migration toward the future anterior side, i.e., towards the side of initial asymmetry (Takaoka et al. 2011; Morris et al. 2012a), restricting Nodal activity to the prospective posterior. This aspect will be discussed further in Sect. 6.5. Additionally, Nodal is expressed at low levels in the ICM prior to implantation, but there is no evidence that Wnt/beta-catenin signaling activates this expression. Later in development, prior to and during gastrulation, Nodal expression is maintained by Wnt3 signals in the posterior epiblast and in the forming primitive streak (Brennan et al. 2001; Ben-Haim et al. 2006), indicating that Wnt regulation of Nodal remains conserved, but is deployed modularly in development.
Interestingly, although Wnt may not activate early Nodal expression in the blastocyst, data suggest that Tcf3 repression of Nodal is a conserved feature of vertebrate development. Deletion of mouse Tcf3 (Merrill et al. 2004) results in the formation of secondary axes (Hensen’s nodes and notochord) and in upregulation of Nodal target genes (e.g., Foxa2; Hoodless et al. 2001; Yamamoto et al. 2001) in the pregastrula embryo. And, Nodal is upregulated in Tcf3 null embryonic stem (ES) cells (Cole et al. 2008). In fish and frogs, early-localized Wnt signals provide an initial signal to relieve repression by Tcf3 (Kim et al. 2000; Houston et al. 2002; Dorsky et al. 2003). Mammals must have evolved different mechanisms to overcome this repression in the absence of pervasive early Wnt activity. Recent data suggest that Nodal initiation and potentiation may be controlled by a distinct enhancer bound by pluripotency transcription factors Oct4 and Klf4, in addition to ongoing Smad2-dependent signaling (Papanayotou et al. 2014). Interestingly, Tcf3 null ES cells also exhibit derepression of other genes, in addition to Nodal, that are co-regulated by pro-pluripotency factors (Cole et al. 2008), suggesting that in normal ES cells, these proteins may help overcome Tcf3-mediated repression in the absence of strong activation of Wnt/beta-catenin signaling.
Is there a mammalian Nieuwkoop center? In line with a lack of asymmetry in the mammalian egg, clonal analyses and transplant experiments performed in the pregastrula mouse embryo have not identified a region with the requisite Nieuwkoop properties; indeed transplanted epiblast cells typically change fate according to their new position (Lawson et al. 1991; Lawson and Pedersen 1992; Parameswaran and Tam 1995). Also, there is a high degree of cell mixing in the epiblast during normal development (Gardner and Cockroft 1998), which would be inconsistent with a lineage-restricted Nieuwkoop center. However, there is overlap of Nodal and Wnt3 in the posterior proximal epiblast and subsequently throughout the primitive streak (Tam et al. 2006) and high levels of Nodal signaling are required for organizer formation (Vincent et al. 2003). The early proximal epiblast may have some hallmarks of a Nieuwkoop center, although the region of highest and most complete axis-inducing activity is the anterior primitive streak itself, prior to Hensen’s node formation (Kinder et al. 2001), similar to the case in chicken. Interestingly, these cells do not significantly contribute to the organizer/Hensen’s node or notochord, instead making contributions to the anterior mesendoderm (Kinder et al. 2001). Thus, organizer induction in mammals may still require non-cell-autonomous induction by Wnt and Nodal signals, with the signals being generated from the nascent mesendoderm, as opposed to strictly extra-mesodermal Nieuwkoop center-like mechanisms.
6.4. The Organizer and Dorsoventral Patterning
Axis formation and patterning are largely determined by a discrete zone of midline mesoderm in the prospective dorsal region of the gastrula. The collective cell signaling and morphogenetic properties of these cells and their descendants during normal development and under experimental conditions led to their designation as the “organization center” or “organizer” (Organisationszentrum/Organisator) (Spemann 1921; Spemann and Mangold 1924). During experiments to test the extent of cell fate determination in the gastrula, Spemann and Mangold showed that the dorsal (upper) lip of the newt gastrula could retain its fate when transplanted to the ventral side, typically forming a normally patterned “secondary embryo” that matched the axial organization of the host. By grafting dorsal lips from albino embryos into pigmented hosts (Fig. 6.10), they assessed the contribution of donor cells, and strikingly demonstrated that the secondary dorsal tissues including the neural tube were formed from induced host cells, with donor cells forming notochord and somites (Spemann 1921; Spemann and Mangold 1924). The term organizer was thus coined by Spemann to reflect the ability of the dorsal (upper) blastopore lip to direct the development of the characteristic embryonic structures in vertebrates (notochord, somites, dorsal neural tube).
Fig. 10.
The organizer experiment of Spemann and Mangold. (a) Diagrammatic model of Spemann and Mangold’s dorsal lip transplantation from a lightly pigmented species (light gray) to the ventral region of a darker species (dark gray). (b) Image of a Xenopus tadpole following the successful grafting of an early gastrula dorsal lip, showing the endogenous axis (1° axis) and the induced partial axis (2° axis). The dark pigment in the head of the 2° axis is related to abnormal head development in the induced axis. (c) Diagram of a cross section through an embryo resulting from a dorsal lip transplant as in (a). The typical lineage contribution of the donor lip is lightly shaded reflecting the species origin, indicating contributions to the notochord, floor plate and medial somite (c is after Spemann and Mangold 1924). v ventral, d dorsal
Spemann’s characterization of the organizer was highly influential and rapidly led to the identification of homologous regions in other vertebrates: birds (chick and duck, Hunt 1929; Waddington 1930), mammals (chick into rabbit, Waddington 1934; rabbit into rabbit/chick, Waddington 1936, 1937; mouse into frog, Blum et al. 1992; and mouse into mouse, Beddington 1994), lamprey (Bytinski-Salz 1937; Yamada 1938), and teleost fish (minnow, perch, and trout, Oppenheimer 1934a, b; Luther 1935). Similar dorsalizing and neuralizing activities have also been found for the prospective notochordal cells of invertebrate cephalochordates (Tung et al. 1962), suggesting the organizer is a basal feature of the chordates.
In bird and mammalian embryos, organizer activity is limited to the anterior primitive streak, including the discrete anterior tip (Hensen’s node, “Knoten,” Viebahn 2001; Blum et al. 2007).2 In teleosts, the organizer corresponded to a thickened area of the blastoderm on the dorsal side, termed the embryonic shield. The initial characterization of the organizer identified several embryological properties of the organizer that are central to its functions and are still studied in the context of axial development. These are: the tissue-autonomous involution and convergent extension of the dorsal lip and the differentiation into axial mesoderm (notochord), the induction and regionalization of the neural plate, and self-organization/developmental plasticity (i.e., regulation) (Spemann 1938).
As intriguing as these observations were, the organizer remained largely a phenomenon until the application of molecular biological methods to developmental biology in the 1980s–1990s. These were first used to characterize the neural inducing activity of the organizer. This aspect was the historical readout of organizer function prior to the molecular era, owing to ease of identifying induced neural plates and nervous system tissue in sectioned material. Beginning with the observations that dissociation and delayed reaggregation of amphibian animal cap ectoderm could cause neural differentiation (Grunz and Tacke 1989, 1990; Saint-Jeannet et al. 1990; Godsave and Slack 1991), and the cloning of organizer-specific transcription factors (Cho et al. 1991; Dirksen and Jamrich 1992; Taira et al. 1992), a series of experiments from many labs identified antagonism of Bone Morphogenetic Protein (BMP) signaling activity as a key activity of the organizer in vertebrate axial development (Smith and Harland 1992; Sasai et al. 1994, 1995; Wilson and Hemmati-Brivanlou 1995; Zimmerman et al. 1996). The early history of the molecular characterization of the organizer and its impact on the fields of developmental biology and the evolution of development has been extensively reviewed over the years by many of the key participants (Harland 1994, 2008; Elinson and Holowacz 1995; De Robertis and Sasai 1996; Harland and Gerhart 1997; Chang and Hemmati-Brivanlou 1998; De Robertis et al. 2000; De Robertis 2006, 2009; Harland and Grainger 2011).
6.4.1. The Differentiation of Axial Mesoderm and the Role of BMP Antagonism
It is clear from numerous studies that the formation of a ventral-to-dorsal BMP activity gradient through extracellular antagonism underlies the main functions of the organizer in dorsoventral axial patterning (Fig. 6.11a). BMPs are members of the Tgfb superfamily of secreted growth factors. These function as dimers and activate heteromeric cell surface serine/threonine kinase receptors, resulting in the phosphorylation of BMP-specific Smad transcription factors (Smad 1/5/8) (Chap. 7; reviewed in Little and Mullins 2006; Ozair et al. 2012). BMPs are expressed in non-organizer tissue, namely the ventrolateral regions in amphibians and posterior primitive streak of the epiblast in chick and mouse. These molecules function in a dose-dependent fashion to promote ventrolateral mesendoderm and epidermis, and can prevent neural development of dissociated animal caps and inhibit dorsal mesendoderm formation in explant assays (Jones et al. 1992; Dale et al. 1992; Fainsod et al. 1994; Suzuki et al. 1994; Graff et al. 1994; Wilson and Hemmati-Brivanlou 1995; Hammerschmidt et al. 1996b; Streit et al. 1998).
Fig. 11.
Dorsoventral patterning of the gastrula. (a) Image of an early gastrula Xenopus embryo (left image) showing gsc mRNA expression in the organizer region (purple). This area expresses BMP antagonists Chrd, Fst, and Nog in a dorsoventral gradient (blue shading), and is complementary to a gradient of BMP signaling (red shading), resulting in a graded pattern of phospho-Smad1/5 (p-Smad1/5). On the right is a late gastrula showing continued ventroposterior pattering by BMP signaling, specifying in progressively later fashion (differing line thicknesses) the anterior neural crest (a.n.c), posterior neural crest (p.n.c.) and epidermis (ep.), in addition to the underlying germ layers (not shown). (b) Simplified network model of secreted protein interactions acting in dorsoventral patterning. Chrd secreted by the organizer antagonizes Bmp activity, mediated by Bmp4/7 ventrally and Admp within the organizer. Bmp activity inhibits chrd expression. Tld/Bmp1 acts as a Chrd inhibitor via proteolysis; Szl is a Tld inhibitor, indirectly promoting Chrd activity in the extreme ventral region (dotted arrow). The critical reciprocal control interactions responsible for self-organization are indicated with blue lines; Bmp4/7 positively controls its own expression but inhibits admp expression. Bmps also upregulate cv2/bmper, a ventral Bmp antagonist, and inhibit chrd dorsally. Model after De Robertis (2009)
The first insights into the molecular biology of the organizer came from the isolation of conserved homeobox and forkhead transcription factors expressed in the organizer: goosecoid (gsc), lim homeobox 1 (lhx1), and forkhead box a4 (foxa4) (Cho et al. 1991; Taira et al. 1992; Dirksen and Jamrich 1992). Importantly, ectopic expression of gsc in ventral cells caused second axes with notochordal tissue, indicating that gsc was not merely a marker of, but a critical functional component of the organizer (Cho et al. 1991). Studies in other organisms subsequently found a deeply conserved role for these proteins in regulating axial development in both protostomes and deuterostomes and even to some extent in diploblasts (Martindale 2005).
Although it was clear that overexpression of these proteins, notably Gsc, could induce most aspects of organizer function and regulate cell–cell signaling during axis formation (Niehrs et al. 1993), the nature of these signals was not immediately apparent. In a series of seminal experiments, expression cloning and differential cDNA screening in Xenopus identified secreted molecules encoded by noggin (nog; Smith and Harland 1992; Lamb et al. 1993), chordin (chrd; Sasai et al. 1994), and follistatin (fst; Hemmati-Brivanlou et al. 1994) as potent dorsalizing and neuralizing molecules specifically expressed in the organizer. These genes turned out to encode extracellular BMP antagonists that bind to secreted BMPs and inhibit the activation of BMP receptors (Zimmerman et al. 1996; Piccolo et al. 1996). Importantly, overexpression of these BMP antagonists mimicked the action of the organizer in nearly every respect.
Functional interference and genetic manipulations confirmed the requirements for BMP signaling and BMP antagonism in dorsoventral patterning. Of particular importance, genetic mutations in zebrafish bmp7 and bmp2b, BMP receptor acvr1 and smad5 (snailhouse, swirl, lost-a-fin, and somitabun mutants, respectively) result in dorsalized embryos (Kishimoto et al. 1997; Nguyen et al. 1998; Hild et al. 1999; Dick et al. 2000; Schmid et al. 2000; Mintzer et al. 2001), whereas embryos lacking chrd function (chordino; Hammerschmidt et al. 1996a, b) are ventralized. Results using antisense oligo-based loss-of-function in Xenopus similarly showed dorsalization upon reduction of BMPs and ventralization following inhibition of Nog, Chrd, Fst (Oelgeschläger et al. 2003; Khokha et al. 2005; Reversade et al. 2005). In the mouse and the chick, the roles of BMPs are more complex owing to their functions in extra embryonic tissues and in controlling cell proliferation (Hogan 1996). Nonetheless, Nog and Chrd are expressed in the anterior primitive streak and Hensen’s node (Connolly et al. 1997; Streit et al. 1998; Streit and Stern 1999; Bachiller et al. 2000), and Nog, Chrd double mouse mutants exhibit dorsoanterior truncations (Bachiller et al. 2000). Additionally, genetic deletion of Bmp4 in mouse results in ventral mesoderm defects (Winnier et al. 1995).
A host of other BMP antagonists as well as antagonists of other ligands were also discovered and a commonly accepted model emerged; namely that dorsoventral patterning across germ layers is mediated by a gradient of BMP activity originating ventrally and antagonized dorsally by organizer molecules. Additional support for this model came from characterization of patterns of BMP activity, finding that levels of phosphorylated active Smad1 were enriched ventrally and absent from the organizer (Faure et al. 2000; Schohl and Fagotto 2002).
BMP antagonism derived from the organizer also patterns the convergent extension movements of the axial mesoderm (also see Sect. 6.6). High levels of BMP inhibit convergent extension behavior in Xenopus (Graff et al. 1994), and in fish there is evidence that BMP signaling inhibits expression of convergent extension-promoting Wnt/PCP ligands, wnt11 and wnt5b (Myers et al. 2002). BMP signaling may also inhibit dorsal mesoderm involution but this has not been extensively studied (Nakayama et al. 1998). And, BMPs can promote epithelial-mesenchymal transitions and ingression behavior, which occurs in more ventrolateral (non-organizer) mesoderm. Thus, the production of secreted BMP antagonists is essential and largely sufficient for the self-differentiation and morphogenesis of the organizer into notochord mesoderm and for the patterning of the surrounding germ layers.
6.4.2. The Role of the Organizer and BMP Antagonism in Neural Induction
Because of the historical link to early organizer studies and a general interest in the development of the central nervous system, the role of the organizer in neural induction has received considerable attention. Also, conceptually, neural induction is thought of differently from axis and mesendoderm induction. Mesoderm and endoderm are induced from ectoderm, whereas ectoderm develops if inducers are absent, and can thus be considered the “default” germ layer. Surprisingly and perhaps counter-intuitively, experiments in Xenopus suggested that neural, rather than epidermal, was the default state of the ectoderm (Hemmati-Brivanlou and Melton 1997; Chang and Hemmati-Brivanlou 1998; Stern 2006; Ozair et al. 2012). Inhibition of mesendoderm-inducing Tgfb signaling using dominant-negative receptors or endogenous antagonists (Hemmati-Brivanlou and Melton 1994; Chang and Harland 2007) or depletion of maternal vegt mRNA (Zhang et al. 1998) results in neuroectoderm formation in all presumptive germ layers. Critical evidence for the neural default model includes experiments indicating that BMPs induce epidermis in dissociated cells that would otherwise become neuralized, and that Nog, Chrd and Fst act as direct neural inducers in ectoderm tissue by blocking BMP signaling. Interestingly, these antagonists elicited only anterior neural fates, which hinted at possible mechanisms of anteroposterior patterning by the organizer (discussion of this aspect will be deferred to the following section).
Whereas the central requirement for BMP antagonism is clear, the extent that BMP inhibition alone is sufficient for neural induction is unclear. Studies in chick and Xenopus have indicated that neural induction also requires Wnt antagonism (Wilson et al. 2001; Pera et al. 2003; Fuentealba et al. 2007) as well as ongoing FGF signaling (Linker and Stern 2004; Delaune et al. 2005; Marchal et al. 2009). Subsequent experiments using different methodologies have suggested that FGF signaling is particularly involved in establishing “pre-neural” ectodermal fate in the chick and is not sufficient for neural induction, whereas in Xenopus, BMP antagonism is largely sufficient in vivo (Wills et al. 2010; Pinho et al. 2011). Experiments in mammalian embryonic stem cells indicate that culture under low density or Tgfb-inhibited conditions can lead to neural development (Tropepe et al. 2001; Chambers et al. 2009), largely supporting the default model in mammalian cells.
The relative roles of FGF signaling and/or Wnt antagonism may reflect species level differences in the function of the organizer. The Xenopus organizer expresses shisa2, an antagonist of Fzds and FGF receptors (Yamamoto et al. 2005). Shisa homologues are not expressed in the organizer in chicken and mouse, although they are expressed in anterior regions (Furushima et al. 2007; Hedge and Mason 2008). Thus, FGF signaling may be less critical in Xenopus neural induction because it is normally inhibited in the presumptive neural region. FGF signaling may contribute to neural induction by inhibiting Smad1, through MAPK-mediated linker domain phosphorylation (Pera et al. 2003), or by inducing the expression of various pre-neural genes (Sheng et al. 2003; Pinho et al. 2011). In chick, non-organizer Wnt signaling can promote BMP activity, both indirectly by inhibiting the ability of FGF activity to repress Bmp expression and by acting more directly to promote BMP activity (Wilson et al. 2001). This latter activity may occur at the level of C-terminally phosphorylated active Smad1. FGF/MAPK and Gsk3b activity (i.e., absence of Wnt signaling) can phosphorylate the linker region of active Smad1 leading to its turnover, whereas limiting MAPK activity of Wnt treatment can prolong active Smad1 signaling (Fuentealba et al. 2007).
In general, FGF may be more critical in cases where neural development occurs over a longer time course and many rounds of cell divisions, as in the chick and mouse. Additionally, FGFs may have different roles in the context of an epiblast epithelial architecture (i.e., pseudostratified, interdigitating columnar epithelia). A similar idea has come from experiments in embryonic stem cells, where FGF signaling and low Wnt activity are thought to promote a pluripotent stem cell state resembling the postimplantation epiblast (EpiSC) (reviewed in Ozair et al. 2012). Thus FGFs may not just promote a “pre-neural” state, but a “pre-germ layer differentiation” state, which would encompass neural fate.
6.4.3. Self-Organization and Developmental Plasticity in the Organizer
In addition to the neural inducing and mesoderm patterning functions, the developmental plasticity of the organizer can be largely explained by BMP regulation. Early experiments by Spemann showed that partial constriction of the gastrula along the midline led to double-headed tadpoles, each with a normally formed anterior axis (Spemann 1903). Thus, half an organizer can restore normal bilateral symmetry locally and scale its effects accordingly. Similarly, rather normal embryos develop from dorsal half explants as opposed to hyperdorsalized embryos, which would be the expected result in the absence of self-organization (Reversade and De Robertis 2005).
Recent experiments in Xenopus have shown that this self-organization ability relies on the feedback regulation of Chrd flux in the embryo (De Robertis 2009) (Fig. 6.11b). The stability and activity of Chrd is regulated in the extracellular space by proteolytic degradation, mediated by Bmp1, a member of the Tolloid family of metallopeptidases. The activity of Bmp1 can be inhibited by the Sizzled (Szl) protein (Lee et al. 2006), a secreted Frizzled related protein. Szl is expressed ventrally in Xenopus as well as zebrafish, and becomes restricted to an extreme ventral domain in the ventral mesoderm (Salic et al. 1997; Yabe et al. 2003; Collavin and Kirschner 2003; Martyn and Schulte-Merker 2003). Szl therefore promotes Chrd activity, creating a relatively shallow gradient of dorsalizing activity, which can explain the apparent paradox that szl loss-of-function embryos are ventralized despite its expression ventrally (Yabe et al. 2003; Collavin and Kirschner 2003; Martyn and Schulte-Merker 2003; Lee et al. 2006). Chrd and BMPs proteins can form long-range gradients within Brachet’s cleft, the narrow intraembryonic space in the gastrula separating the ectoderm from the mesendoderm, possibly using the fibronectin-rich extracellular matrix to facilitate distribution (Plouhinec et al. 2013).
Self-organization becomes possible because key dorsal and ventral genes encode proteins with opposing molecular activities, but are under differential transcriptional control. Although the organizer expresses many growth factor antagonists, this region also contains several BMP receptor agonists, admp (anti-dorsalizing morphogenetic protein) and bmp2 (Moos et al. 1995; Joubin and Stern 1999; Lele et al. 2001; Inomata et al. 2008). A subset of BMP antagonists are expressed in a ventral domain, including bmper/crossveinless2 (Ambrosio et al. 2008) and bambi (Onichtchouk et al. 1999). A central element of this regulatory network is the positive feedback control of the dorsal BMP agonists (De Robertis 2009): The prominent ventrally expressed BMPs (bmp4/7) are positively regulated by BMP signaling itself, whereas admp/bmp2 agonists are inhibited by BMP activity. Similarly, the ventrally expressed BMP antagonists and szl are induced by BMPs, whereas chrd is repressed. Additionally, all BMPs are inhibited by binding to Chrd and can be released for action by Bmp1-mediated cleavage of Chrd.
Thus, in a dorsal half explant, loss of BMPs and Szl would lead to enhanced Chrd cleavage and inhibition resulting in higher admp/bmp2 expression. These ligands would then accumulate in the area of highest Bmp1/lowest Chrd activity, activating bmp4/7 expression. Increasing levels of these “new” ventral BMPs would limit the extent of chrd and admp expression as well as establish a new szl domain, resetting the Chrd gradient to the size of the new “embryo” (Reversade and De Robertis 2005). Analogous regulatory networks have been uncovered in zebrafish (Lele et al. 2001; Xue et al. 2014) and are likely to exist in chick, as orthologues of the main genes are present. Mammals appear to lack Admp and Szl (although Bmp2 is present), making it unclear whether similar axial self-organization occurs or to what extent mechanistically similar processes have evolved. As the appreciation of network effects in biology is growing, it will be interesting to discover whether these principles of embedded antagonistic proteins under complementary transcriptional control are general features of self-organization in development.
6.4.4. Molecular Interpretation of BMP Gradient Signaling
Accumulated data has provided insight into the mechanisms through which cells interpret different BMP signaling levels to achieve different dorsoventral cell fates. Typical models invoke a positional information mechanism, in which the graded concentration of a morphogen determines cell fate. However, it is becoming clear that there are spatiotemporal aspects to growth factor signaling gradients in general, and to BMP gradients in particular. Recent experiments have shown that dorsalized maternal-zygotic BMP receptor (acvr1) mutant fish (lost-a-fin) are rescued by inducible acvr1 expression only if expression is initiated prior to the midgastrula stage (Tucker et al. 2008). Similarly, inducible expression of chrd after the middle of gastrulation fails to dorsalize wildtype embryos. Analogous results were seen in Xenopus and in fish using timed application of an anti-BMPR drug (Wawersik et al. 2005; Kwon et al. 2010), further supporting the idea that early but not late exposure to BMP signals is critical for inhibiting dorsal fates and promoting ventral fates.
Temporal regulation was particularly evident in timed Chrd induction experiments. Dorsoanterior markers become progressively refractory to BMP inhibition, whereas more lateral-posterior tissues (neural crest and pronephros) became progressively more sensitive (Tucker et al. 2008). These studies thus suggest that BMP signaling is active later and longer in ventroposterior tissues and never active in prospective organizer. Additionally, dorsoventral and anteroposterior patterning occurred along a similar time course, indicating these processes are coordinated (see also Sect. 6.5.2). Interestingly in these studies, embryos do not seem to sense cumulative BMP exposure, as ventroposterior fates could still be induced with a late burst of BMP signals (Tucker et al. 2008). As discussed above, the BMP gradient is self-organizing, therefore a small pulse of BMP activity could potentially regenerate the activity gradient to regulate different cell fates. Threshold dose and temporal aspects of exposure to BMP signals thus cooperate to specify axial patterning.
The nature of interpretation of the BMP gradient at the transcriptional level may also partly underlie this temporally progressive dorsoventral patterning. In Xenopus ectoderm cells, competence to respond to BMP is maintained throughout gastrulation (Simeoni and Gurdon 2007). And transcription can be triggered rapidly and requires the continuous receptor activation to maintain appropriate steady-state levels of Smad1 in the nucleus (Simeoni and Gurdon 2007). These responses are distinct from those elicited during Activin/Tgfb-mediated mesoderm induction (Chap. 7), which are characterized by a more limited period of competence, extending signaling from active endocytic complexes, delayed onset of transcription, and cumulative sensing of ligand levels (Dyson and Gurdon 1998; Shimizu and Gurdon 1999; Bourillot et al. 2002; Piepenburg et al. 2004; Jullien and Gurdon 2005). The molecular bases for the differences between Tgfb and BMP signaling, despite similar pathway architecture, is not known but may underlie the general tendency of ventral signals to limit dorsal ones. These differences may also help explain the temporal aspects of BMP responses, as discussed above, as well as the importance of Smad1 as a center of signaling integration.
BMP-mediated transcriptional regulation is controlled by the formation of phosphorylated Smad1/Smad4 complexes and subsequent nuclear translocation and association with target promoters. In general, Smad-responsive cis-regulatory elements are thought to mediate low affinity interactions and Smad-associated cofactors are therefore required for specific target promoter interactions. Smad1-binding transcriptional cofactors Znf423 (Oaz; Seoane et al. 2000 and Hivep1 (Human immunodeficiency virus type I enhancer binding protein 1/Schnurri1; Yao et al. 2006) have been characterized and facilitate binding of BMP-activated Smads to BMP-responsive enhancer elements. These cofactors likely recruit general transcriptional activators or repressors depending on the cellular or epigenetic context (Blitz and Cho 2009).
6.4.5. Complexity of Cross-Regulation Between BMP Signaling and the Organizer
BMP signaling activates a conserved cascade of gene regulation involving several immediate response genes, including the homeobox ventx genes, as well as msx1 and wnt8a (Gawantka et al. 1995; Schmidt et al. 1996; Ault et al. 1996; Ladher et al. 1996; Onichtchouk et al. 1996; Suzuki et al. 1997; Hoppler and Moon 1998), and a secondary target, even-skipped homolog 1 (evx1/xhox3) (Ruiz i Altaba et al. 1991). Additionally, Ventx and Gsc proteins cross-repress each other’s expression, mediating part of the negative feedback regulation of BMP and organizer gene expression (Fainsod et al. 1994; Gawantka et al. 1995; Onichtchouk et al. 1996; Trindade et al. 1999; Sander et al. 2007). In the mesoderm, Brachyury homolog (T) provides an essential input to ventx expression (and hence gsc repression) through its interaction with Smad1 (Messenger et al. 2005). Interestingly, double inhibition of Ventx and Gsc results in normal embryos (Sander et al. 2007) indicating that these proteins are only strictly required to regulate each other, and that redundant mechanisms pattern the axis in their absence. Multiple such independent and redundant cross-regulatory interactions between Gsc-Ventx and other protein pairs likely would provide robustness to dorsoventral patterning mechanisms.
Similar cross-regulatory loops exist in zebrafish (Nikaido et al. 1997; Melby et al. 2000; Kawahara et al. 2000; Imai et al. 2001), although the Ventx genes have undergone a unique evolutionary trajectory in other organisms (Scerbo et al. 2014). These genes appear to have been lost in rodents and are only expressed in a limited set of hematopoietic cells in humans (Rawat et al. 2010). The Ventxs bear ancient homology to Nanog, a gene implicated in pluripotency and primitive endoderm formation (Kozmik et al. 2001). Nanog may have been co-opted for some of the functions of Ventx in mammals, including possibly Gsc repression (Vallier et al. 2009), whereas Ventx may have substituted for Nanog function in fish and amphibians (Camp et al. 2009; Scerbo et al. 2012). This case is particularly likely in the anuran lineage, which appears to have secondarily lost Nanog (Scerbo et al. 2014). In spite of this reduced dependency on Ventx in mammals, cross-antagonism between BMP signaling and Gsc in patterning the axis/primitive streak has been evolutionarily maintained, as this relationship exists as reciprocal repression of EVX1 (activated by BMP signaling) and GSC (Kalisz et al. 2012) in human ES cells. Thus, although the involvement of the well-characterized Ventx proteins is variable throughout evolution, possibly related to the timing and requirement for ventral mesoderm in hematopoiesis in early development (Kozmik et al. 2001), there remains an ancient and conserved network of reciprocal repression between BMPs and organizer genes.
6.5. Anteroposterior Axis Patterning
In general, within the dorsal blastopore/anterior primitive streak, the sequence of internalizing mesoderm determines anteroposterior character, with anterior mesendoderm (anterior definitive endoderm and prechordal plate) being followed by the chordamesoderm (notochord). Signals from the organizer have long been implicated in establishing anteroposterior fates along the body axis. This effect was seen in Spemann and Mangold’s early experiments in which second axes induced by organizer transplantation often showed varying degrees of completeness at the anterior end (Spemann and Mangold 1924). These results were roughly correlated with the stage of gastrula from which the donor dorsal lip was taken; earlier lips induced more complete axes with well-formed heads and later lips induced truncated axes or second tails (Spemann 1931). These experiments were interpreted to suggest that the organizer comprised a spatially distinct “head organizer” that would induce forebrain in the early gastrula, and a “trunk organizer” that would induce hindbrain and spinal cord in more posterior ectoderm during later gastrulation. The remaining region, the midbrain, is formed largely by interactions between forebrain and hindbrain.
Work by Nieuwkoop and others later suggested a different model. Implantation of folded strips of competent animal cap ectoderm at various anteroposterior levels in the prospective neuroectoderm generated a graded pattern of neural fates in the implant, with anterior neural/neural plate border tissue forming distally in the implant and more posterior neural forming proximally (Nieuwkoop 1952, 1999) (Fig. 6.12a). These observations were interpreted to suggest a neural “activation” step that initiates a tendency toward forebrain differentiation, followed by a “transforming” event that converts activated anterior neural tissue to more posterior fates (Nieuwkoop 1952, 1999). Earlier experiments had shown that artificial neural induction in urodele animal caps using nonspecific chemical and physical methods invariably generated forebrain (Holtfreter 1944). Also, explants taken from early prospective neuroectoderm showed progressive waves of activation followed by transformation, occurring from posterior to anterior and coincident with mesoderm internalization (Eyal-Giladi 1954). These different activities were found to depend on the nature of the underlying axial mesoderm, with activation predominating in the prechordal mesoderm and anterior notochord and transformation predominating in the posterior notochord (Sala 1955). Experimental data have at various times favored one model or the other, but accumulating molecular evidence supports a modified version of the Nieuwkoop model (Fig. 6.12b and c). This support is based on three major findings:
that BMP antagonists, Noggin, Chordin and Follistatin act as endogenous neural inducers, and induce exclusively anterior neural fate in competent ectoderm (see Sect. 6.4, Smith and Harland 1992; Hemmati-Brivanlou et al. 1994; Sasai et al. 1994),
the identification of “transforming” molecules, such as FGFs, Wnts, Nodals, and retinoids, which posteriorize anterior tissue (reviewed in Stern 2005) but do not directly neuralize ectoderm per se, and,
the elucidation of proposed “anterior stabilizing” signals (Fraser and Stern 2004). These are mediated by continuing BMP antagonism (Hartley et al. 2001), Wnt antagonism by inhibitors such as Frzb1 and Dickkopf1 (Leyns et al. 1997; Wang et al. 1997; Glinka et al. 1998), multifunctional antagonism by members of the Cerberus/DAN family of proteins (Bouwmeester et al. 1996) and the feedback Nodal antagonism by Lefty1 (Thisse et al. 2000; Cheng et al. 2000). These molecules are expressed prior to gastrulation in the extraembryonic endoderm (anterior visceral endoderm (AVE)/hypoblast/foregut endoderm), and during gastrulation in the anterior definitive endoderm and prechordal plate, providing signals that initiate and stabilize anterior pattern, respectively.
Fig. 12.
Models for anteroposterior axis patterning in vertebrates. (a) Depiction of Nieuwkoop’s ectodermal fold implantation experiments (Nieuwkoop 1952; Nieuwkoop and Nigtevecht 1954). Dorsal posterior view of a neurula stage amphibian embryo; neural fate is represented as a gradient from light-to-dark with darker color indicating more posterior fates; the epidermis is yellow. The implanted folds are shown as boxes, divided to show the approximately position of induced neural fates. Each fold is characterized by a distal epidermal portion (ep.) bounded by general neural/neural plate border (activated tissue); this is followed proximally by graded neural fates, reflecting the hypothesized influence of a transforming gradient (as opposed to a distinct inducer at each AP level). (b) Molecular interpretation of Nieuwkoop’s model. In the gastrula, neural induction is accomplished by BMP antagonism, which induces neural tissue with forebrain character. (c) During later gastrulation, the expression of Wnts directly induces posterior fates in anterior neural-fated tissue in a dose-dependent fashion. FGF signaling is required in a permissive role. Wnt antagonists expressed in the anterior mesendoderm limit the extent of Wnt signaling and the anterior remains forebrain
6.5.1. Cellular and Tissue Basis of Anteroposterior Patterning
6.5.1.1. The Hypoblast/Anterior Visceral Endoderm in Anterior Patterning
Comparative studies have suggested that the role of the hypoblast/AVE in anterior patterning is widely conserved, based on analogous gene expression patterns in the AVE and later anterior definitive endoderm, cell migration and anterior signaling activities (rabbit hypoblast, Knoetgen et al. 1999; chicken hypoblast, Foley et al. 2000; teleost YSL, Ho et al. 1999; amphibian foregut/anterior endoderm, Bouwmeester et al. 1996; Jones et al. 1999). Interestingly, long before it was molecularly characterized, the hypoblast received considerable attention as a potential regulator of anteroposterior patterning through the control of cell movements. Classical cell tracking experiments in the chicken showed that the pregastrula epiblast undergoes bilaterally symmetrical “polonaise-like” or “double vortex” cell movements, which head anteriorly along the midline before circling back and converging at the posterior, where the primitive streak will form (Wetzel 1929; Gräper 1929). This pattern of cell movements was shown to be controlled by the hypoblast (Waddington 1930, 1933). In these studies, which were repeated and extended by later authors (e.g., Azar and Eyal-Giladi 1981; Foley et al. 2000), changing the orientation of the underlying hypoblast changed the orientation and placement of the forming primitive streak. Although this effect of the hypoblast on cell movements was clearly recognized by Waddington at the time (Waddington 1933), later experiments focused mainly on presumed signaling/inductive properties of the hypoblast in head and mesoderm induction (Azar and Eyal-Giladi 1981).
In mammals, the AVE has been extensively studied at the cellular and genetic level. The AVE forms prior to gastrulation and is characterized by cells with a distinct columnar morphology (rabbit, Viebahn et al. 1995; mouse, Kimura et al. 2000; Rivera-Pérez et al. 2003) as well as specific gene expression patterns (VE-1 antigen, Rosenquist and Martin 1995; Hesx1, Thomas and Beddington 1996; Otx2, Acampora et al. 1995; Hhex, Thomas et al. 1998; Lhx1, Gsc, Foxa2, Cerl, Belo et al. 1997). In mouse, these genes are expressed in visceral endoderm cells at the distal tip of the forming egg cylinder (Thomas et al. 1998). Cell labeling with DiI (Thomas et al. 1998) or with transgenic methods (Kimura et al. 2000; Rivera-Pérez et al. 2003; Srinivas et al. 2004), showed that these Hhex-expressing AVE cells unilaterally migrate towards the future anterior region of the embryo. Physical ablation of patches of AVE compromised development of the underlying forebrain (Thomas and Beddington 1996), demonstrating a requirement for the AVE in anterior patterning. Also, gene targeting analysis of chimeric embryos showed that a number of transcription factors are required in the AVE for its proper formation and migration and for anterior specification (Otx2, Acampora et al. 1995; Rhinn et al. 1998; Perea-Gomez et al. 2001; Foxa2, Dufort et al. 1998; Lhx1, Shawlot et al. 1999 and Hhex, Martinez Barbera et al. 2000 among others).
Despite this requirement for anterior development, several experiments have revealed that the hypoblast and related tissues largely lack head organizing properties. Einsteck implantation of the prospective foregut endoderm in Xenopus fails to result in ectopic head induction (Bouwmeester et al. 1996). Similar results were seen in chick and mouse regarding the function of the AVE/hypoblast, which like-wise fail to induce anterior structures in transplant assays (Tam and Steiner 1999; Foley et al. 2000). Additionally, removal of the hypoblast/AVE results in ectopic primitive streak and mesoderm formation and anteriorization of the embryo, demonstrating that these tissues are repressors rather than inducers of mesoderm (Bertocchini and Stern 2002) as well as permissive regulators of anterior development (Yamamoto et al. 2004). Thus a more complex picture of anteroposterior patterning is emerging in which the hypoblast/AVE/anterior endoderm self-organizes its own morphogenesis and engages in reciprocal signaling with the epiblast/prospective definitive mesendoderm to control cell fate and morphogenesis in the embryo.
6.5.1.2. Formation and Migration of the Hypoblast/AVE
The initial formation of the hypoblast/AVE has been studied with considerable detail in the mouse but is little understood in the chicken. In the latter case, hypoblast cells delaminate from the early epiblast, forming isolated clusters, which merge together from posterior-to-anterior (Stern 2004). While it has never been studied as such, it is likely that this process is analogous to the cell sorting of primitive endoderm from epiblast in the mouse blastocyst. In the mouse, the primitive endoderm in contact with the epiblast (embryonic visceral endoderm) differentiates into the visceral endoderm after implantation, under the control of BMP signaling (Yamamoto et al. 2009). Continuing BMP signals from the epiblast and extraembryonic ectoderm likely inhibit the formation of the AVE at the proximal end of the mouse egg cylinder (Rodriguez 2005; Mesnard et al. 2006). The AVE is ultimately specified through the activation of Nodal/Smad2 signals from the epiblast (Waldrip et al. 1998; Brennan et al. 2001) and the inhibition of BMP/Smad1 signals (Yamamoto et al. 2009).
In the presumptive AVE, Nodal indirectly and directly induces a number of AVE transcription factors as well as the expression of Nodal antagonists Lefty1 and Cerberus-like (Cerl) and the Wnt antagonists Dickkopf1 and Sfrp5 (Brennan et al. 2001, see below). The expression of Lefty1 and Cerl in the AVE inhibits the autoregulatory maintenance of Nodal expression in the distal epiblast (Norris et al. 2002). As AVE migration commences and is replaced by posterior visceral endoderm, Nodal and Nodal target gene expression are gradually restricted to the posterior epiblast, mediating mesoderm induction and primitive steak formation (Ding et al. 1998; Brennan et al. 2001; Perea-Gomez et al. 2002; Yamamoto et al. 2004). Thus, anterior migration of the AVE has several consequences for anteroposterior patterning: the maintenance of ectoderm germ layer and anterior neural in the anterior epiblast, and the removal of inhibitory signals for primitive streak/mesendoderm formation (Fig. 6.13).
Fig. 13.
Model for origin and role of the AVE in anteroposterior pattering in the mouse. (a) In the peri-implantation blastocyst (E5.0), a subpopulation of primitive endoderm (PE) expressing Lefty1 and Cerl arise stochastically positioned asymmetrically in the distal egg cylinder. This population requires Nodal and Tdgf1 in the epiblast and is inhibited by Bmp4 in the extraembryonic ectoderm. (b) As the conceptus grows after implantation (E5.25), the AVE begins to also express Wnt antagonists and is repelled by Nodal and Wnt signals; the action of BMP is limited to the proximal epiblast and PE, allowing migration of AVE in the distal region. (c) As AVE migration proceeds, a second set of Lefty1; Cerl-expressing cells is induced in the distal VE (2°AVE) by Tdgf1-independent Nodal signaling. These cells and the 1°AVE migrate in a coherent stream toward the presumptive anterior, inhibiting Nodal and Wnt signaling in the epiblast and specifying the anterior neuroectoderm. Progressive loss of Nodal from the anterior limits activity to the posterior, where Nodal and Wnt maintain and amplify each other’s expression through BMP4, inducing the primitive steak (PS) in the prospective posterior. ParE parietal endoderm, PE/VE primitive/visceral endoderm, ExE extra-embryonic ectoderm. Images were modified and adapted from: Bedzhov I, Graham SJL, Leung CY, Zernicka-Goetz M (2014) Developmental plasticity, cell fate specification and morphogenesis in the early mouse embryo. Philosophical Transactions of the Royal Society B: Biological Sciences 369:20130538. doi:10.1098/rstb.2013.0538 under the terms of the Creative Commons Attribution License CC BY 3.0
Recent experiments have identified considerable complexity in the control of AVE specification and migration. Gain-of-function studies in mouse embryos showed that Cerl/Lefty1 and Dkk1 can act as attractants for AVE migration, whereas Nodal and Wnts can act as repellants (Yamamoto et al. 2004; Kimura-Yoshida et al. 2005). Additionally, it is also now better appreciated that the AVE is heterogeneous, arising from an early sub-population of primitive endoderm in the preimplantation blastocyst, in addition to a later population established from naïve distal visceral endoderm (Takaoka et al. 2006). Importantly, the primary AVE cells (alias DVE, in some literature) originate asymmetrically in the visceral endoderm and are necessary to “lead” the migration of the secondary AVE in unilateral anterior migration (Takaoka et al. 2011; Morris et al. 2012a). This complexity of the AVE was first hinted at in mice mutant for the Nodal coreceptor Tdgf1, which fail to express early AVE markers (Ding et al. 1998). Although these do become expressed later, the AVE nevertheless fails to migrate. Reporter gene analyses showed that the Cerl and Lefty1-expressing primary AVE arises asymmetrically within the primitive endoderm (E4.0–5.0; Takaoka et al. 2006; Torres-Padilla et al. 2007b), with live imaging of cultured embryos revealing a second population gaining Cerl-expression at E5.0 (Torres-Padilla et al. 2007b). Genetic lineage labeling of Lefty1-expressing cells confirmed that the secondary AVE arises from progenitors that lack early Lefty1-expression (Takaoka et al. 2011).
The leading role of the primary AVE was suggested through microsurgical ablation of the primary AVE, which resulted in a wider defect in remaining VE migration (Miura and Mishina 2007). Importantly, inducible genetic lineage ablation of Lefty1-expressing cells clearly showed that the secondary AVE does not migrate anteriorly if early Lefty1-expressing primitive endoderm is eliminated (Takaoka et al. 2011). Similar results were seen in live imaging of embryos cultured on a collagen-coated polyacrylamide matrix to mimic implantation (Morris et al. 2012a). In this case, laser ablation of leading “pioneer” cells expressing the highest levels of Cerl prevented the migration of the AVE. Interestingly, ablation of an immediate “follower” cell also inhibited migration, suggesting that a coherent stream of interacting cells is necessary for anterior migration (Morris et al. 2012a).
This behavior conceptually resembles “follow-the-leader” collective cell migration strategies evident in epithelial wound healing, lateral line and neural crest cell migration, cancer metastasis and other paradigms (Friedl and Gilmour 2009; Wynn et al. 2012; Cheung et al. 2013; Dalle Nogare et al. 2014; Yamaguchi et al. 2015). However, many of the well-characterized cases involve mesenchymal cells or epithelial-to-mesenchymal transitions, whereas AVE migration occurs by directional intercalation and neighbor exchange within the simple epithelium of the visceral endoderm (Srinivas et al. 2004). Trinkaus performed seminal work on coordinated migration in epithelial sheets and coupled protrusive behavior over 40 years ago (Vaughan and Trinkaus 1966), but the mechanisms controlling follow-the-leader behavior are only beginning to be studied. Recent data in Xenopus suggest that cell–cell adhesion between leaders and followers results in mechanical stimulation of cadherins and formation of a cadherin-keratin-plakoglobin complex, which is critical for stimulating protrusive behavior opposite the site of cell–cell contact (Weber et al. 2012). This maintenance of cellular tension as well as chemoattraction by the leading cells is likely to play key roles in this coordinated chain migration.
The initiation of AVE migration is similarly not well understood. Nodal-dependent cell proliferation in the epiblast is required (Stuckey et al. 2011), suggesting that the egg cylinder may need to reach a critical size, possibly to extend the AVE outside the reach of inhibitory signals. Thus, an initial asymmetry in the AVE, established when the embryo is small, would be amplified during growth and would determine the direction of subsequent migration, which is maintained by coordinated cell movements.
6.5.2. Molecular Regulation of Anteroposterior Patterning
6.5.2.1. Nodal and Wnt Antagonists as Regulators of Anterior Patterning
Clues to the molecular regulation of anteroposterior patterning first came from the characterization of conserved Nodal and Wnt growth factor antagonists expressed in the amphibian organizer endoderm region and in the amniote hypoblast/AVE (see below). Xenopus cerberus (cer) was identified through differential screening of dorsal lip-specific molecules (Bouwmeester et al. 1996). Cer overexpression on the ventral side could induce multiple second axes consisting only of heads (Kerberos was the multi-headed guard dog of the underworld in Greek mythology; Hesiod and Evelyn-White 1914). This head-inducing activity was attributed to the ability of Cer to antagonize multiple developmental signaling molecules extracellularly, including BMP, Wnt and Nodal proteins (Piccolo et al. 1999), although Nodal antagonism is likely paramount. The activity of Cer could largely be recapitulated by co-expression of the Cerberus Nodal antagonist domain (Cerberus-short, CerS) with Chrd. Additionally, overexpression of nodal1 (nodal homolog 1) after the mid-blastula transition (i.e., from a plasmid vector) resulted in anterior truncations (Piccolo et al. 1999). Additional studies with amniote Cerl suggested the anti-Wnt activity is minimal in mouse and chick and that Cerl represents mainly a Nodal and BMP antagonist (Marvin et al. 2001). These and studies in zebrafish using Lefty, another Nodal antagonist, established that inhibition of Nodal activity is critical for proper patterning of anterior fate in the axial mesendoderm (Thisse et al. 2000).
In addition to anti-Nodal signals, Wnt inhibitors are expressed within the organizer and in the hypoblast/AVE and Wnt signaling is antagonistic to anterior development in a stage-dependent manner. Although Wnt signaling is critical for axis induction, other experiments show that activating Wnt/beta-catenin signaling during gastrulation causes anterior truncations (Christian and Moon 1993; Kelly et al. 1995b; Hoppler et al. 1996) whereas inhibiting late Wnt signaling results in hyperanteriorization (Hoppler et al. 1996; Leyns et al. 1997; Wang et al. 1997; Glinka et al. 1997, 1998). Additionally, inhibition of BMP and Wnt signaling together is needed to induce secondary axes with head structures, whereas induction with BMP antagonists alone generates only trunk structures (i.e., notochord; Glinka et al. 1997). Genetic studies in zebrafish tcf3 (headless, hdl) mutants show a reduction in anterior structures and expanded ventrolateral derivatives (Kim et al. 2000), whereas the opposite occurs in wnt8a mutants (Kim et al. 2000; Lekven et al. 2001). Wnt antagonists are also required in vivo for anterior patterning. Interference with Dkk1 function in fish and frogs leads to anterior truncations (Hashimoto et al. 2000; Shinya et al. 2000), mimicking Wnt overexpression. Similarly, genetic deletion of Dkk1 in the mouse epiblast led to loss of forebrain neural markers (Mukhopadhyay et al. 2001). Additionally, Dkk1 is a direct target of Otx2, a key anterior specifying gene, in both the anterior mesendoderm (Ip et al. 2014) and anterior visceral endoderm (Kimura-Yoshida et al. 2005). And, either overexpression of Dkk1 or additional deletion of one Ctnnb1 allele is sufficient to partially rescue loss of Otx2 (Kimura-Yoshida et al. 2005), suggesting that controlling Wnt levels is a critical role of Otx2.
6.5.2.2. Self-Regulation of Nodal and Wnt Signaling During Anterior Patterning
As is the case for BMP signaling/antagonism and the organizer, Nodal and Wnt signaling are also precisely regulated through auto-regulation and negative feedback loops controlled by secreted antagonists. Nodal can directly auto-regulate its own expression in the epiblast and visceral endoderm through a Smad2/Foxh1-dependent enhancer in the first intron of the Nodal locus (Osada et al. 2000; Norris et al. 2002). Also, Wnt3 is expressed in the proximal egg cylinder ectoderm and can regulate Nodal expression through a Wnt/beta-catenin-responsive proximal ectoderm enhancer (Brennan et al. 2001; Ben-Haim et al. 2006). Wnt3 is in turn regulated by secreted Bmp4 signals emanating from a population of extra-embryonic ectoderm adjacent to the epiblast. This population is maintained by epiblast Nodal signaling, forming the second feedback loop (Ben-Haim et al. 2006). Nodal signals emanating primarily from the epiblast also induce the AVE at the distal end of the egg cylinder in a Smad2-dependent manner, regulating (both directly and indirectly) the expression of Nodal antagonists Cerl and Lefty1. These in turn feedback and inhibit Nodal signaling in the underlying epiblast.
Wnt3 also activates its own expression in the posterior epiblast and visceral endoderm directly through beta-catenin-dependent Wnt signaling (Tortelote et al. 2013) and Wnt signals (likely including Wnt3) directly activate Dkk1 expression in a number of cell types (Chamorro et al. 2005). Interestingly, the autoregulation of Wnt3 is evolutionarily ancient, occurring in sponges and in cnidarians (Holstein 2012). Both Wnt/Dkk1 and Nodal/Lefty1 interactions have been suggested to constitute reaction-diffusion systems in vivo, with regard to hair follicle spacing in the case of Wnt (Sick et al. 2006) and to left-right patterning in the case of Nodal (Nakamura et al. 2006). Whether or not these match the strict mathematical assumptions of Turing reaction-diffusion systems (the case for Wnt has been disputed, Meinhardt 2012), it is clear that interacting networks of self-propagating and self-limiting loops of Nodal, Wnt and BMP signaling underlie much of axis formation in vertebrates and indeed likely all animals.
In addition to reciprocal regulation of Nodal and Wnt, there is extensive interaction between Wnt/BMP and Nodal/BMP at the level of signaling integration during anteroposterior patterning. Wnt signaling can potentiate BMP signaling through several possible mechanisms. In the frog, BMP-activated Smad1 can be inhibited by Gsk3b phosphorylation of the linker region, and this in turn can be inhibited by Wnt leading to the perdurance of active Smad1 (Fuentealba et al. 2007). In zebrafish embryos, dorsoventral and anteroposterior patterning occur along a similar time course (Tucker et al. 2008). Correspondingly, manipulation of Wnt signaling can alter the anteroposterior character of Chrd-induced tissues at any time during gastrulation (Hashiguchi and Mullins 2013), suggesting that BMP and Wnt signaling mechanisms are active together, along with FGF and retinoid signaling (Hashiguchi and Mullins 2013). Additionally, in the chick epidermis Wnt signaling can block FGF inhibition of Bmp4 transcription during neural plate induction (Wilson et al. 2001), although it is unclear whether this interaction is also critical for anteroposterior neural plate patterning.
Nodal and BMP signaling also interact at several different levels. Nodal and certain BMPs can form inactive heterodimeric ligands that fail to stimulate either signaling pathway and exhibit mutual antagonism (Yeo and Whitman 2001). Also, in the frog organizer Nodal and Admp (a BMP ligand) form a self-regulating network to control anteroposterior patterning (Inui et al. 2012). Nodal and Admp can compete for a shared receptor, Acvr2a, to oppositely regulate Nodal and Wnt antagonists in the anterior endoderm. High Nodal levels activate high expression of Nodal antagonists, which subsequently block Nodal-Acvr2a interaction and permit the occupancy by Admp. This alternate pathway limits the expression of Nodal antagonists, allowing Nodal signaling to reengage (Inui et al. 2012). Interestingly, Admp depletion results in the anterior endoderm gaining head-inducing activity in Einsteck transplantation assays, owing to overproduction of Nodal and Wnt antagonists (Inui et al. 2012). The high degree of interrelatedness of these signaling pathways thus can underlie many of the self-organizing properties of early axis formation.
6.5.3. Regulation of Posterior Development by Wnt Signaling
6.5.3.1. Default Specification of Anterior Neural Fate
In line with the activation-transformation model, experimental neural induction in Xenopus animal caps (ectoderm), either by Noggin treatment (Lamb et al. 1993) or by disaggregation (Grunz and Tacke 1989, 1990), results exclusively in anterior neural induction. Additionally, anteroposterior patterning is maintained in frog embryos lacking mesoderm and endoderm, generated by vegt depletion or CerS overexpression (Zhang et al. 1998; Wessely et al. 2001), and in those lacking both organizer and ventral BMP signals (Reversade and De Robertis 2005). Similarly in zebrafish, embryos lacking Nodal signaling and thus mesendoderm also retain anterior identity in the ectoderm (Gritsman et al. 2000). In the mouse, loss of Nodal signaling results in anterior neural specification in the epiblast in the absence of mesendoderm and the AVE (Conlon et al. 1994; Brennan et al. 2001; Camus et al. 2006). The molecules mediating this anterior default state are not known but are hypothesized to be present maternally in fish and amphibians and in the early epiblast of amniotes. Because embryonic stem cells can also acquire anterior neural fates by default (Tropepe et al. 2001; Chambers et al. 2009), it is possible that core pluripotency factors or proteins activated at the onset of epiblast differentiation would be good candidates for anterior specifiers.
6.5.3.2. Graded Wnt Signals and Posterior Specification
Although many different signaling molecules have been implicated in posterior neural fate specification, current evidence suggests that the graded activity of Wnt signaling is the critical proximate determinant of early posterior identity. Wnt activity can be seen in Xenopus embryos as a gradient of nuclear beta-catenin in the prospective neural plate of the gastrula, with the highest levels posteriorly (Kiecker and Niehrs 2001). Similarly, in the mouse gastrula, posterior gradients of Wnt reporter construct activity have been observed (Maretto et al. 2003; Lewis et al. 2007, 2008; Fossat et al. 2011). Experiments in neuralized Xenopus animal caps showed that inducible expression of activated beta-catenin during gastrulation directly induces posterior neural markers (McGrew et al. 1997; Domingos et al. 2001). Additionally, cell–cell contact and FGF signaling are required in this case, suggesting the involvement of a “community-effect” response, as described for muscle formation (Standley and Gurdon 2004). Similar requirements for Wnt and FGF signaling were seen in chicken, where Wnt treatment directly and dose-dependently posteriorizes competent anterior neural tissue explants (Nordstrom et al. 2002). Correspondingly, Wnt inhibition in posterior neural explants causes anteriorization. FGF signaling is also required for posterior specification, but likely in a permissive role, acting in a non-dose-dependent fashion. And loss of FGF signaling does not anteriorize posterior neural fates (Nordstrom et al. 2002). Wnt signaling also has subsequent roles in specifying the posterior portion of major brain subdivisions later in development, in particular distinguishing diencephalon versus telencephalon (Heisenberg et al. 2001; Houart et al. 2002). It is therefore of great interest to determine how this signaling system is compartmentalized and reiterated during multiple aspects of individual tissue and organ development.
At the transcriptional level, Wnt/beta-catenin signaling during posterior specification is thought to proceed through the use of Tcf/Lef proteins as coactivators. This mechanism of gene activation is likely different from early, axis-inducing Wnt signals, which involve mainly derepression of Tcf3 (see Sect. 6.3.1). In mouse development, double homozygous genetic deletion of Lef1 and Tcf7(Tcf1) results in posterior defects in addition to abnormalities in mesoderm specification (Galceran et al. 1999). Similarly in Xenopus, Lef1 and Tcf1 are required for the late/ventral response to Wnt (Roël et al. 2002; Liu et al. 2005), whereas Tcf3 is dispensable in this regard (Hamilton et al. 2001). Recall that Tcf3 is necessary for the repression of early Wnt target genes in vertebrates. Experiments in mouse and frog have suggested a “Tcf exchange” model for the changes in target gene responses to Wnt signaling prior to and following axis formation. Lef1 can be transcriptionally activated by Wnt signaling (chick, Skromne and Stern 2001; mouse, Wu et al. 2012a), suggesting that cells experiencing Wnt signals undergo a qualitative change in their potential underlying responses to subsequent Wnt exposure during development. Similarly, early Tcf3 repressor function is inactivated by Wnt-activated kinases and replaced by activating Tcf1 function in both Xenopus and in mouse embryonic stem cells (Hikasa et al. 2010; Yi et al. 2011). Thus, the activation of genes during posterior specification is likely to involve both beta-catenin-dependent derepression of Tcf3, followed by replacement and activation by Lef1/Tcf1. In support of this idea, mouse knock-ins in which Tcf3 has been replaced by the deltaNTcf3 construct (non-derepressible) lack Lef1 expression and show defective late Wnt responses (Wu et al. 2012a).
6.6. Axial Morphogenesis
The physical form of the vertebrate axis is brought about during gastrulation. During this process, the presumptive mesoderm and endoderm germ layers are internalized and undergo a set of morphogenetic events that elongate the axis in the anteroposterior direction and establish dorsoventral (mediolateral) organization in the mesoderm. Vertebrate embryos undergo gastrulation in one of two basic ways, depending on the architecture of the embryo. In spherical anamniote embryos, internalization occurs through the blastopore, which circumscribes the marginal zone, and forms the archenteron (primitive gut tube). In the flat blastoderm of amniote embryos, internalization generally occurs through the primitive streak in the posterior of the embryo, forming a trilaminar embryo with the endoderm remaining open to the yolk and forming a tube secondarily.
In recent years, the cellular mechanics of vertebrate gastrulation have been studied in great depth using live imaging approaches. Surprisingly, the underlying cell and tissue mechanics and their molecular regulation are extensively conserved, despite differing patterns and timing of gastrulation movements. Largely based on the seminal work of Trinkaus, Keller, Stern and others, a common set of vertebrate gastrulation movements and cell behaviors have been identified. These have been extensively reviewed in recent years (Keller 1986; Keller and Shook 2004; Stern 2004; Solnica-Krezel 2005; Tam et al. 2006; Shook and Keller 2008; Solnica-Krezel and Sepich 2012) and fall into three main categories, internalization, convergent extension and epiboly. Together, these processes form a “mosaic of regional processes” (Keller 1986) that are exquisitely integrated to accomplish the feat of gastrulation.
Mesendoderm internalization typically initiates by invagination (in-pocketing) in a tissue layer (the epiblast), followed either by involution as an in-rolling sheet of cells over a lip (Einrollung; Vogt 1929), or by ingression of individual cells. Invagination and ingression proceed by apical constriction of epiblast cells, and in the case of ingression, epithelial-to-mesenchymal transition and subsequent cell migration. Convergent extension is a thinning and narrowing of a tissue and occurs by mediolateral intercalation of closely packed cells or by migration of cells toward a midline. Cells in the mesendoderm undergoing convergent extension typically elongate along the mediolateral axis and exhibit bipolar protrusive behavior using lateral lamellopodia. The traction generated by this bipolar activity is thought to facilitate cell intercalation. Epiboly is a thinning and spreading/extension of a tissue and involves radial intercalation to form a thinner cell layer, as well as cell shape changes. In spherical embryos, epiboly is essential for the ectoderm to spread and cover the yolk mass during gastrulation. Uniquely in teleost embryos, epiboly encompasses all germ layers and the entire embryonic blastoderm covers the yolk cell.
6.6.1. Amphibian Gastrulation
Gastrulation in amphibians initiates at a latitudinal slit at the boundary of equatorial and vegetal regions, forming the dorsal lip of the incipient blastopore. Cell internalization begins with apical constriction of the so-called “bottle cells,” creating the initial invagination at the blastopore (Keller and Shook 2004). Extension of this cavity during gastrulation creates the archenteron. Internal vegetal rotation cell movements in the presumptive endoderm pull the marginal zone vegetally and initiate internalization by involution at the dorsal lip. Internalization spreads laterally from the dorsal lip eventually forming a circular blastopore, with axial mesendoderm entering at the early dorsal lip and more paraxial and lateral mesendoderm internalizing from the lateral and ventral blastopore (Keller and Shook 2004).
On the dorsal side, the anterior leading edge of the mesoderm (prechordal/head mesendoderm) migrates as a cellular stream along the blastocoel roof (Winklbauer and Nagel 1991; Winklbauer et al. 1992). This migration requires fibronectin fibrils deposited by the blastocoel roof cells (Boucaut et al. 1985; Winklbauer and Nagel 1991; Nagel and Winklbauer 1999). Internalizing mesendoderm cells maintain what is termed “tissue separation” with the ectoderm, and cannot intercalate back into the ectoderm (Wacker et al. 2000). This segregation is evident by the presence of Brachet’s Cleft between the mesendoderm and ectoderm/neuroectoderm (Nieuwkoop and Florschütz 1950). An analogous cleft separating these tissue layers is found throughout the vertebrates.
In Xenopus and other anurans, the majority of the mesoderm arises from deep rather than superficial cells (Nieuwkoop and Florschütz 1950; Keller 1975). Involution of the mesendoderm is thus internal in these species and provides the main mechanical forces driving gastrulation. The mesendoderm and deep neural ectoderm undergo radial intercalation just prior to involution, occurring first in more anterior cells and later in posterior ones (Keller et al. 1985). Additionally, tissue extension is biased in the anterior–posterior direction, likely because some initial mediolateral intercalation occurs. After involution, cells become elongated perpendicular to the axis of extension and exhibit bipolar protrusive behavior and undergo mediolateral intercalation (Keller and Tibbetts 1989; Shih and Keller 1992b). This behavior drives convergent extension of the marginal zone and is the major biomechanical force behind internalization and elongation of the axis. Mediolateral intercalation behavior begins in more anterior tissue as it involutes and spreads to more posterior axial tissue as well as lateral mesoderm as the blastopore closes.
Closure of the blastopore at the ventral marginal zone is accomplished by convergent thickening, a little understood behavior in which prospective posterior somitic mesoderm converges into a pile of cells that do not extend, although they do so later in neurulation (Keller and Danilchik 1988). In general, the process of convergence can lead either to extension or thickening, or both, with the degree of each outcome depending on the constraints applied by the surrounding tissue. In either case, the progressive expansion of mediolateral intercalation behavior around the blastopore and the linking of forces around the marginal zone is thought to create “hoop stress” that pulls the blastopore lip over the yolk plug and internalizes the endoderm (Keller et al. 2000; Keller and Shook 2004).
Urodele and anuran amphibians undergo significant differences in development that have been long noted but perhaps not well appreciated. The process of gastrulation in salamanders and newts is outwardly similar to the situation described in Xenopus, but has several important distinctions. Mesoderm is derived from the surface layer in urodeles (Vogt 1929). Axial mesoderm undergoes similar involution at the dorsal lip in urodeles, but internalization in the ventral and lateral regions occurs mostly by ingression of individual cells (Shook et al. 2002). This pattern of ingression has been referred to as a “bilateral primitive streak.” In urodeles, lateral and ventral convergent forces are generated by the ingressing cells in these regions, as these cells undergo apical constriction and bring additional cells into the ingression zone (Keller and Shook 2004).
Interestingly, the urodele pattern of dorsal lip involution and lateral ingression is conserved in more primitive organisms including cyclostomes and lungfish (Shook and Keller 2008). It is likely that both the anuran and teleost mechanisms of primary internalization by involution evolved independently within those lineages. These changes in gastrulation may be correlated with the cellular organization of the ectodermal moiety, occurring as a multilayered ectodermal “deep layer” of non-polarized cells, covered by a superficial polarized epithelial layer in the anurans and teleosts, as opposed to the typical “epiblast” of pseudostratified interdigitating epithelial cells (Shook and Keller 2008).
6.6.2. Teleost Gastrulation
Similar morphogenetic events occur in the zebrafish/teleost gastrula, albeit in the context of a different embryonic architecture. One of the main features of fish gastrulation is prominent epiboly, in which all three germ layers (as opposed to just ectoderm) undergo extensive radial intercalation as the blastoderm expands to cover the yolk cell. Epiboly in teleost fish is somewhat unusual, and involves interconnections between the yolk cell and the superficial epithelial layer of the blastoderm. Epiboly begins just prior to gastrulation with YSL nuclei migrating vegetally in advance of the blastoderm margin. Cytoskeletal perturbations and in vivo observations suggest that the YSL “tows” the blastoderm margin vegetally (Betchaku and Trinkaus 1978; Solnica-Krezel and Driever 1994).
Internalization initiates by an involution-like “synchronized ingression” around the blastoderm margin, forming a bilayered structure, the germ ring (Adams and Kimmel 2004). Typical ingression is not thought to occur, as teleost gastrulation lacks a classical epithelial-to-mesenchymal transition (EMT) and cell migration characteristic of typical ingression movements (Shook and Keller 2008). Internalizing cells migrate beneath the outer layer toward the animal pole, forming an inner hypoblast (this is mesendoderm, and not homologous to the amniote hypoblast) and outer epiblast (ectoderm). Both layers are covered by a flattened superficial epithelium, the enveloping layer (EVL), which remains extraembryonic and may provide structural support for morphogenetic movements (Kimmel et al. 1990). On the dorsal side of the margin, involuting and single “delaminating” cells form a thicker layer, constituting the embryonic shield (Montero 2005). Also dorsally, a teleost-specific population of epiblast cells, the dorsal forerunner cells (DFCs) detach and migrate vegetally ahead of the dorsal lip, in association with the margin of the EVL (Cooper and D’Amico 1996). Their function at this stage is unknown, but later these cells will form the Kuppfer’s vesicle, which functions in left-right asymmetry. Also, similar to the process in anurans, convergent extension elongates the axial mesendoderm as a result of bipolar protrusive behavior and well as dorsally directed migration of more lateral cells (Solnica-Krezel 2005). Teleost thus appear to have independently evolved an amphibian-style method of gastrulation as a solution to concentrated yolk and discoidal cleavage in the egg.
6.6.3. Amniote Gastrulation
In the mammals and birds, gastrulation initiates at the primitive streak, a groove formed by internalizing cells along the posterior axial midline. Anterior–posterior patterning, and thus the positioning of the primitive streak towards the posterior, is controlled by the anterior migration of the hypoblast/AVE (Sect. 6.5.1). Mesoderm precursors are initially found throughout the posterior epiblast but migrate toward the posterior end prior to primitive streak formation. In the chick, this occurs through the double-vortex polonaise-like cell movements (see Sect. 6.5.1) and similar mechanisms have typically been thought to occur in mammals. However, mouse embryos appear to lack these large-scale cell movements in the epiblast (Williams et al. 2011), possibly owing to a smaller number of cells at the equivalent stage or to constraints of the cup-shaped architecture. Alternatively, the cell movements positioning the streak may occur in the visceral endoderm layer in mouse (Weber et al. 1999). Polonaise-like movements appear to occur in rabbit embryos, which have a flat morphology similar to chick embryos (Halacheva et al. 2011). Interestingly though, in this case, a more localized extreme intercalation cell movement event (“processional cell movements”), predominates (Halacheva et al. 2011). These observations indicate that overall primitive streak positioning in amniotes can result from many different patterns of cell movements depending on the species.
Internalization through the primitive streak involves massive epithelial-to-mesenchymal transition (EMT) and ingression of individual cells to form definitive mesoderm and endoderm (Stern 2004). Early ingressing mesoderm contributes mainly to pharyngeal and cardiac mesoderm anteriorly, as well as extraembryonic mesoderm posteriorly. Definitive endoderm ingresses by mid-streak stages and intercalates into and displaces the hypoblast/visceral endoderm. The primitive streak elongates and progresses anteriorly (distally in the mouse egg cylinder) until mid-gastrulation (late streak stage), approximately to the level of the future hindbrain, and begins to form the anatomically distinct Hensen’s node at the anterior tip. Internalization at Hensen’s node in many birds and mammals, including humans, resembles that of the amphibian dorsal lip, with invaginating bottle cells and involution of notochord and somitic mesoderm (Shook and Keller 2008). Chick and mouse embryos however appear to have separately evolved ingression as the main mode of internalization, although dorsal lip involution may be evolutionarily ancestral.
Reptiles (non-avians) represent a useful but little-studied intermediate case. These embryos typically form a dorsal lip perpendicular to the axial midline in the posterior of the blastoderm, through which chordamesoderm involutes. Lateral and ventral cells ingress through a reptilian blastoporal plate, which resembles both the urodele blastopore and amniote primitive streak (Bertocchini et al. 2013).
After its formation, the Hensen’s node begins to regress posteriorly and the primitive streak closes in an anterior-to-posterior progression. Notochord and somites form in the mesoderm behind the regressing node. At this point Hensen’s node and surrounding cells likely comprise a pluripotent stem cell population that persists throughout regression and into tailbud formation (Wilson et al. 2009). It is often not discussed as such, but amniote primitive streak regression is likely an analogous process to blastopore closure, with mediolateral intercalation behavior driving convergent extension in the axial midline tissues and convergent behavior following ingression at the primitive streak. For the mouse, mediolateral intercalation of mesoderm has been established as a mechanism of axial elongation, and convergent-extension behavior has been demonstrated to initiate in pre-somitic mesoderm cells immediately after they exit the primitive streak (Yen et al. 2009).
In all vertebrates, the embryo lengthens and narrows along the AP axis at the culmination of gastrulation. The neural plate is visible in the dorsal ectoderm and the closed blastopore represents the posterior pole of the embryo. Patterning of the AP and DV axes is essentially accomplished during gastrulation. Although the left-right axis is thus specified topologically, the cellular and molecular mechanisms that regulate left-right asymmetry of the internal organs do not come into play until after gastrulation. These are primarily mediated by a distinct ciliated structure that forms during late gastrulation at the posterior end of the notochord along the gastrocoel roof plate, a part of the archenteron lining (Blum et al. 2014). This structure is derived from surface mesoderm in the late involuting dorsal lip and is homologous in many respects across vertebrates. Monocilia in the posterior notochord region are thought to create a leftward fluid flow, generating asymmetry in Nodal signaling in the left lateral plate mesoderm, leading to morphological left-right asymmetry and the generation of internal bilateral asymmetry in organs such as the heart, gut and lungs (also, see Blum et al. (2014) for review of caveats in chick and pig, which lack nodal cilia (Gros et al. 2009)).
6.6.4. Molecular Regulation of Axial Morphogenesis
The control of convergent extension has been extensively studied at the molecular level in Xenopus and zebrafish embryos. A host of cellular and genetic experiments have implicated components of the Wnt/PCP pathway as critical regulators of cell shape, cell polarity and activity during convergent extension. In Xenopus, overexpression of certain beta-catenin-independent Wnts and dominant-negative Dishevelled 2 (Xdd1; Sokol 1996) were sufficient to block axis elongation without blocking mesoderm formation. Additionally, mutation or inhibition of wnt11 function in fish and frogs indicated that Wnt/PCP signaling was indeed required for convergent extension behavior in vivo (Tada and Smith 2000; Heisenberg et al. 2000). Importantly, Wallingford et al. (2000) showed in detailed live imaging of explants that Dvl activity was needed for bipolar cell shape changes and for stabilizing mediolaterally oriented bilateral protrusive activity of lamellopodia in converging and extending mesodermal cells. And similar regions of the protein were required for controlling CE in Xenopus as for Drosophila PCP, namely the C-terminal DEP domain (Wallingford et al. 2000). Analogous results were found for zebrafish wnt11 and wnt5a mutants, which showed unstable monopolar protrusive activity in dorsally migrating cells (Kilian et al. 2003; Ulrich et al. 2003). Other PCP proteins have also been shown to control cell intercalation by regulating mediolateral lamellopodia in fish and frogs, including Frizzleds, Vangl2, and Prickle (Djiane et al. 2000; Darken et al. 2002; Goto and Keller 2002; Jessen et al. 2002; Wallingford et al. 2002; Veeman et al. 2003b; Takeuchi et al. 2003), as well an extra-cellular modulator of Wnt, Glypican 4 (knypek, Topczewski et al. 2001).
More recent data from amniotes has shown that the regulation of cell shape and the formation of mediolateral lamellopodia are conserved in mouse (Ybot-Gonzalez et al. 2007; Yen et al. 2009; Williams et al. 2014) and chicken (Voiculescu et al. 2007). Additionally, mutations in core Wnt/PCP proteins in mice and humans display axis elongation and neural tube defects, both of which depend on convergent extension (Vangl1/2, Kibar et al. 2001; Murdoch et al. 2001; Montcouquiol et al. 2003; Kibar et al. 2007; Torban et al. 2008; Celsr1, Curtin et al. 2003; and Dvl1/2, Wang et al. 2006). Also, noncore vertebrate Wnt/PCP regulators Ptk7 and Scrib are also required for axis elongation and convergent extension, with Ptk7 likely directly controlling bipolar protrusive activity (Montcouquiol et al. 2003; Lu et al. 2004; Yen et al. 2009). The exact role of Ptk7 is unclear, although genetic experiments in zebrafish have suggested a role in antagonizing Wnt/beta-catenin signaling to allow Wnt/PCP signaling (Hayes et al. 2013). However, beta-catenin-activating roles have also been described suggesting temporal or context-dependent roles.
In addition to controlling cell intercalation during convergent extension, Wnt/PCP signaling is also implicated in cell intercalation prior to primitive streak formation. In the chicken, local cell intercalation in the presumptive primitive streak region prior to gastrulation is though to result from, and may facilitate the polonaise movements in the epiblast. A number of Wnt/PCP components are expressed in the midline prior to primitive streak formation and inhibition by electroporation of anti-PCP morpholino oligos can inhibit streak morphogenesis (Voiculescu et al. 2007). Additionally, and consistent with the role of the hypoblast in controlling polonaise movements, rotation of the hypoblast can alter Wnt/PCP gene expression, possibly through Fgf8 signaling (Voiculescu et al. 2007). Interestingly, it is likely that ingression itself can function locally to organize polonaise-like movements and pull additional cells into the streak, thus driving ingression in a feed-forward manner (Voiculescu et al. 2014).
The situation is less clear in mammals, as Wnt/PCP components have not been widely implicated in primitive streak formation in the mouse. However, ingression at the primitive streak in the mouse does require the down-regulation of Vangl2 protein, mediated by Dact1 (Dishevelled-binding antagonist of beta-catenin 1; Suriben et al. 2009). Thus, whereas mice may exhibit a reduced role for cell movement-driven mediolateral intercalation during primitive streak formation (see above), a role for Wnt/PCP components may have been retained at the level of EMT in the forming primitive streak.
Wnt/PCP signaling exerts its effects on intercalating cells by coordinately regulating bipolar cell shape and polarization of actin dynamics to generate tensile forces along the cell’s mediolateral axis. The mechanisms that orient this bilateral protrusive activity perpendicular to the elongating anteroposterior axis are not well understood. Experiments using dissociated Xenopus animal cap cells treated with graded Activin doses or the recombination of anterior versus posterior notochord explants showed that apposition of tissue from different axial levels was necessary for convergent extension along a perpendicular axis, whereas recombination of equivalent levels produced no elongation (Ninomiya et al. 2004). It is possible that cells could respond to this gradient of Tgfb signaling through changes in cadherin-and protocadherin-mediated cell adhesion during intercalation (Brieher and Gumbiner 1994; Kraft et al. 2012). Similar results have been obtained by opposing gradients of Nodal and BMP activity in zebrafish blastoderm explants (Xu et al. 2014), suggesting an important role for integration of the these pathways in controlling elongation. Temporal changes in Nodal signaling are also correlated with the onset of mediolateral intercalation behavior, further suggesting that the local activity of Wnt/PCP may be cued by differences in Tgfb activity.
Tgfb signaling, most likely through Nodal and Nodal-related proteins, is also involved in inducing ingression behavior and bottle cell formation by activating EMT. This can be shown in Xenopus by overexpression of Nodal in animal caps, which results in ectopic bottle cell formation (Lustig et al. 1996; Agius et al. 2000; Kurth and Hausen 2000). Also, loss of Nodal signaling dramatically reduces EMT-like cell ingression in zebrafish embryos (Keller et al. 2008). Movement of the hypoblast in amniotes, which expresses Nodal antagonists, is also highly correlated with primitive streak formation in the epiblast formed by those cells left behind. This Nodal-controlled ingression may also be self-regulating, as ingression may facilitate recruitment of additional cells to the streak (see above), which would expose more cells to Nodal signals, further triggering more EMT and ingression (Voiculescu et al. 2014).
Signaling by FGFs also plays a key role in regulating convergent extension and mediolateral intercalation. FGF signaling can regulate brachyury (t) expression, which in turn can activate wnt11 (Tada and Smith 2000). In addition to its role in morphogenesis, FGF signaling also has a well-known role in mesoderm formation (Chap. 7). Experiments with cytoplasmic antagonists of FGF signaling, Sprouty/Spry and Spred, indicate that maternal Sprys antagonize convergent extension behavior by blocking PKC and calcium outputs of FGF signaling (Sivak et al. 2005). Later during gastrulation, zygotic Spred proteins accumulate and antagonize Mapk signaling, allowing the morphogenetic signals to predominate after medoderm specification has occurred (Sivak et al. 2005). Additionally, FGF regulation of convergent extension is controlled by the Nodal related 3.1 protein through an atypical and little characterized mechanism (Yokota et al. 2003).
6.7. Concluding Remarks
The overall conservation of axial patterning mechanisms in vertebrates has long been recognized. However, detailed investigations at the cellular, molecular and genetic levels are only now revealing the true extent of this conservation and are also uncovering important differences. The broadest pattern of axis specification involves the generation of animal–vegetal polarity in the oocyte followed by the dorsal localization of determinants in the egg. This ab ovo specification mechanism operates throughout the primitive vertebrates, reptiles and possibly the egg-laying mammals. Based on various cell biological, genetic, and morphological observations, the basic pattern of axis generation is the involution of notochordal precursors at the early dorsal lip/organizer and the later ingression of ventrolateral precursors around the lateral blastopore, or “bilateral primitive streaks.”
This pattern exists throughout the anamniotes (icthyopsids) and the reptiles and is initially established by cortical rotation in the egg, leading to Wnt/beta-catenin asymmetry, elevated dorsal Nodal signaling and organizer induction and dorsal mesoderm involution (Fig. 6.14). Interestingly, much of this sequence of events is likely specific to the vertebrates. In basal invertebrate chordates, such as Branchiostoma, cortical rotation and early dorsal enrichment of beta-catenin do not occur and gastrulation occurs symmetrically via cell ingression, with minimal involution (Zhang et al. 1997; Holland and Onai 2012). Early Nodal asymmetry is present and likely leads to the induction of organizer/BMP antagonist gene expression on the dorsal side of the blastopore (Yu et al. 2007; Holland and Onai 2012). Thus, a key series of events in early vertebrate evolution was likely the appearance of cortical rotation, leading to the restriction of gastrulation initiation dorsally, coupled with a transition to cell involution behavior in the organizer.
Fig. 14.
Model for the evolution of axis formation in vertebrates. Highly generalized schematic diagrams of different vertebrate embryos during early (upper panels) and middle (lower panels) gastrulation. In the basal vertebrates (left panels; vegetal views), gastrulation initiates at the organizer with induction by the Nieuwkoop center (high/early Nodal signaling). The initial internalization movements are through involution. By mid-gastrulation, ingression of mesendoderm progresses around the vegetal cells (yolk plug) and forms the nascent blastopore. Involuted dorsal mesendoderm undergoes convergent extension via mediolateral cell intercalation under the control of Wnt/PCP signaling. During the evolution of amniotes (middle panels; top/dorsal views), eggs increased in size and yolk content and began to undergo meroblastic cleavage. Organizer induction by Nieuwkoop center molecules is retained in early gastrulation. Ingression proceeds through the horizontal slit of the blastoporal plate/blastopore and does not circumferentially envelop the non-cleaving yolk (not shown). In the evolution of modern birds and mammals, gastrulation initiates with ingression at the primitive streak, which would be homologous to the later ventrolateral blastopore in ancestral forms. The organizer (Hensen’s node) is induced later by Nodal signaling from the middle primitive streak. The heterochrony in the pattern of gastrulation morphogenesis and organizer formation could result from several main events; the hypoblast/anterior endoderm segregating from the epiblast as opposed to forming from cleaving vegetal cells, the loss of early organizer induction, the apparent emergent behavior of polonaise-like movements leading to primitive streak formation and the relatively later induction of Hensen’s node by the primitive steak
In contrast to this early asymmetry mechanism, therian mammals undergo apparent ab embryo axis specification, with the early phase of Wnt asymmetry being lost and evolving into new mechanisms of symmetry breaking in the blastocyst, based on cell position and migration in the visceral endoderm. In both ab ovo and ab embryo cases, the egg and the blastocyst exhibit marked metastability, rendering development with radial symmetry improbable under normal conditions. The evolutionary transitions between these mechanisms are not well understood. Increases in yolk and egg size and the shift to meroblastic cleavage have been suggested to drive the evolution towards primitive streak-based gastrulation (Arendt and Nübler-Jung 1999), although these cannot be the sole factors involved. Reptiles have large eggs and undergo meroblastic cleavage but still form the organizer first and develop a horizontal slit blastopore/blastoporal plate, maintaining the ancestral dorsal involution/lateral ingression pattern. Additionally, teleosts also evolved meroblastic cleavage but this change resulted in additional adaptations to keep a blastoporal gastrulation pattern, rather than evolving primitive streak-like movements. Furthermore, the “primitive steak” evolved independently in birds and in mammals, possibly multiple times, which could explain the differences in cellular mechanisms underlying formation of the primitive streak, as well as observations such as the presence of left-right asymmetries around Hensen’s node and lack of nodal cilia in both chick and pig (Gros et al. 2009; Blum et al. 2014).
This convergent evolution toward the primitive streak could be correlated with changes in the development of the hypoblast/anterior endoderm. In amphibians and reptiles, these cells arise during cleavage from yolky vegetal cells, whereas in birds and mammals the hypoblast develops from cells delaminating or sorting out from the epiblast. Formation of the hypoblast in this manner could be a prerequisite for the emergent behavior of coordinated polonaise-like cell movements in the epiblast, which themselves are likely sufficient to position and initiate the primitive streak (Voiculescu et al. 2007). Additionally, owing to the more prominent role of hypoblast migration in birds and mammals, cell internalization initiates with the primitive streak, not with the organizer. Full organizer formation, in terms of gene expression and inducing activity, does not occur until mid-gastrulation. Thus, there is significant heterochrony in the main pattern of axis formation in birds and mammals, with the appearance of the organizer and cell involution lagging behind that of ventrolateral cell ingression (Fig. 6.14). It is possible that the lessened importance of cortical rotation-like mechanisms led to subtle reorganization of the organizer gene regulatory networks, causing organizer genes such as Noggin to be expressed later relative to other dorsal genes.
Mammals exhibit the greatest changes in axis-forming mechanisms within the vertebrates. There is also wide variation in these mechanisms within the mammals themselves. Mammalian eggs have evolved to lose size and yolk content, as well as any notable animal–vegetal asymmetry and cortical rotation-related events in the process. The evolution of implantation also necessitated the early segregation of embryonic and extra-embryonic lineages and the maintenance of embryonic pluripotency. Furthermore, gastrulation in mammals initiates with far fewer cells than in other groups of organisms, ~250 cells in the mouse (compared with >10,000 in the frog and chicken), and is accompanied by rapid cell division. This rapid cell cycle of 2–6 h (Snow 1977) coincides with germ layer and axis patterning in the mammalian embryo, and has sparked evolutionary comparisons to the cleavage stage cell cycle in more primitive animals (O’Farrell et al. 2004). Whether this rapid division is indeed a conserved but temporally deferred event, as has been proposed, is unclear. Alternatively, there may exist a general correlation between pluripotency and shorter G1/G2 phases, with limited cell cycle checkpoints, as is seen in cultured stem cells (Becker et al. 2006).
What are some future prospects for the study of axis formation? After many decades of research, the ideas of symmetry breaking and self-organization in development remain compelling yet incompletely understood. Indeed, the ideas of self-organization and biological “regulation” predate the discovery of the organizer and have been developed into the concepts of the self-regulating embryonic “field” and pattern formation by positional information (Child 1915; Weiss 1926; Nieuwkoop 1967; Wolpert 1969, 1971; Green and Sharpe 2015). The ability of the organizer to undergo self-organization was noted by Spemann and others and clearly implies a complex network of interactions. Recent work showing inverse transcriptional regulation within the BMP regulatory circuitry has shed some light onto these processes. This observation is only the first step toward understanding self-organization at the gene regulatory network level, and additional general principles are likely to be identified. Although the conserved transcriptional control of genes like Goosecoid has long been noted, these mechanisms have not necessarily been fully characterized or compared. Remarkably, large and robust organizer gene regulatory networks have not yet been elucidated for any vertebrate organism. It should eventually be possible to generate comparative organizer gene regulatory networks across vertebrates and quantitatively compare their properties. Such analyses might shed light on possible changes in relative onset of organizer activity and also provide insight into the control of cell behaviors during gastrulation, as there have been several evolutionary transitions between involution and ingression in similar tissues.
Additionally, with regard to the complex interactions underlying symmetry breaking events in early vertebrate development, the molecular basis of microtubule organization during cortical rotation, the prime example of a symmetry-breaking process, remains largely unknown. The cell movements underlying asymmetry in the mammalian blastocyst are similarly mysterious. Both of these areas would likely benefit from advances in live imaging technology that are rendering the molecular activities of cells and tissues more visible and quantifiable. In particular, new methods for culturing and imaging different vertebrate embryos (e.g., Bedzhov and Zernicka-Goetz 2014; Keller et al. 2008) will likely provide novel insights into morphogenesis.
There have recently been rapid advances in whole genome analysis and genome editing technology that will likely transform the study of developmental biology and biology in general. Large-scale gene expression, chromatin analysis and proteomics are becoming commonplace and should yield abundant material for data science analysis. It is probably not a stretch to imagine that real-time whole transcriptome analyses will also become technologically feasible in living cells in the near future. TALEN and Cas9/CRISPR-mediated genome editing are also on the verge of becoming routine, extending mutational analysis to many areas previously intractable. Thus, in the foreseeable future, a variety of “-omics” data should be readily available for any organism, and investigators will have the capability to interrogate the function of any genomic region and immediately read out responses in gene expression and cell behavior.
The lack of detailed genomic and genetic information and methodologies has often been a barrier to work in many nontraditional model organisms. However, newer technologies should soon allow the genetic analysis of axis formation and other processes in organisms rationally chosen based on phylogenetic position or other criteria based on the biological question. Indeed multiple related species could be analyzed in parallel to capture extant variation in developmental mechanisms. Such ideas are not new (Tzika and Milinkovitch 2008) but are much closer to realization. Some interesting candidates for developmental analysis could include tree shrews, owing to their similarity to primates, and echidnas, as an egg-laying mammal model (Tzika and Milinkovitch 2008). The increased availability of human embryonic stem cells is also leading to a better understanding of human development. Gene regulatory networks can be analyzed in embryonic stem cells and induced-pluripotent stem cells and tissue “organoids” are increasingly being used to understand human organ development (e.g., McCracken et al. 2014). Certain ethical considerations would have to navigated however to study human axis formation in this way. Comparative studies of early development across all vertebrates could be facilitated by reviving the idea of a “standardized vertebrate normal table” (Witschi 1956; Hopwood 2007). Such a standard series could be based on conserved gene expression data, gene regulatory network organization and morphogenetic patterns. Potential in-roads to such a project have already been made with the construction of comparative drawings based on gene expression patterns, the “Molecular Haeckels” (Elinson and Kezmoh 2010).
It is perhaps ironic that just as long-awaited sequenced genomes and targeted genome editing technologies are becoming routinely available in organisms like fish and amphibians, interest in the basic science of development in these organisms is perceived to be waning in favor of human-centered translational research. It remains to be seen whether this perception is borne out in the long term. An optimist might argue that the processes of asymmetry, axis formation, and self-organization have fascinated scientists and have generated fundamental biological insight for well over 100 years, and should continue to do so long into the future.
Acknowledgements
The author would like to thank B. Fritzsch for assistance with German translations. This work was supported by the University of Iowa.
Abbreviations
- AP
Anteroposterior, Anterior-to-posterior
- AVE
Anterior visceral endoderm
- BMP
Bone morphogenetic protein
- CRD
Cysteine-rich domain
- DEP
domain Dishevelled, Egl10, Pleckstrin
- DFC
Dorsal forerunner cell
- DIX
domain Dishevelled, Axin
- DV
Dorsoventral, Dorsal-to-ventral
- dYSL
Dorsal yolk syncytial layer
- EMT
Epithelial-to-mesenchymal transition
- EpiSC
Epiblast stem cells
- ES
Embryonic stem
- EVL
Enveloping layer
- FGF
Fibroblast growth factor
- GPCR
G protein-coupled receptor
- HMG
High mobility group
- ICM
Inner cell mass
- MAPK
Mitogen-activated protein kinase
- MBT
Mid-blastula transition
- MPF
Maturation promoting factor
- PCP
Planar cell polarity
- PDZ
domain Postsynaptic density protein (PSD95), Disc large tumor suppressor (Dlg1), and zonula occludens1 protein (ZO-1)
- PMZ
Posterior marginal zone
- TALEN
TAL-effector nuclease
- TE
Trophectoderm
- Tgfb
Transforming growth factor beta
- UV
Ultraviolet irradiation
Footnotes
The nomenclature of the gray crescent has been quite variable. Roux (1888) referred to this feature as a “crescent-shaped gray seam” (halbmondförmigen grauen Saumes). Morgan and Tsuda (1894) used the term “white crescent,” whereas Morgan and Boring (1903) used “grey crescent” [sic], translated as “graue Feld” (gray field). Later this became universally referred to as the gray crescent/grauer Halbmond/croissant gris.
In this chapter, Hensen’s node is used to refer to the anterior tip of the primitive streak in all birds and mammals, and is considered equivalent to the dorsal lip/organizer. Often the mouse organizer is referred to as the “node” without the eponym. However, the node can also refer to the posterior notochord “node” involved in left-right patterning, which lacks organizer activity. This terminology can cause confusion since the latter structure is embryologically distinct from Hensen’s node. In human embryology, Hensen’s node is referred to as the primitive node/knot or Hensens’ knot (Gray 1918; Larsen et al. 2009).
References
- Aberle H, Bauer A, Stappert J et al. (1997) Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J 16:3797–3804. doi: 10.1093/emboj/16.13.3797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acampora D, Mazan S, Lallemand Y et al. (1995) Forebrain and midbrain regions are deleted in Otx2−/− mutants due to a defective anterior neuroectoderm specification during gastrulation. Development 121:3279–3290 [DOI] [PubMed] [Google Scholar]
- Adams RJ, Kimmel CB (2004) Morphogenetic cellular flows during zebrafish gastrulation In: Stern CD (ed) Gastrulation: from cells to embryo. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 305–316 [Google Scholar]
- Agalliu D, Takada S, Agalliu I et al. (2009) Motor neurons with axial muscle projections specified by Wnt4/5 signaling. Neuron 61:708–720. doi: 10.1016/j.neuron.2008.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agius E, Oelgeschläger M, Wessely O et al. (2000) Endodermal Nodal-related signals and mesoderm induction in Xenopus. Development 127:1173–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahumada A, Slusarski DC, Liu X et al. (2002) Signaling of rat Frizzled-2 through phosphodiesterase and cyclic GMP. Science 298:2006–2010. doi: 10.1126/science.1073776 [DOI] [PubMed] [Google Scholar]
- Ambrosio AL, Taelman VF, Lee HX et al. (2008) Crossveinless-2 is a BMP feedback inhibitor that binds Chordin/BMP to regulate Xenopus embryonic patterning. Dev Cell 15:248–260. doi: 10.1016/j.devcel.2008.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amit S, Hatzubai A, Birman Y et al. (2002) Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev 16:1066–1076. doi: 10.1101/gad.230302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancel P, Vintemberger P (1948) Recherches sur le déterminisme de la symétrie bilatérale dans l’oeuf des Amphibiens. Bull Biol Fr Belg (Suppl) 31:1–182 [Google Scholar]
- Antic D, Stubbs JL, Suyama K, Kintner C, Scott MP and Axelrod JD (2010) Planar Cell Polarity Enables Posterior Localization of Nodal Cilia and Left-Right Axis Determination during Mouse and Xenopus Embryogenesis. PLoS ONE 5, e8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt D, Nübler-Jung K (1999) Rearranging gastrulation in the name of yolk: evolution of gastrulation in yolk-rich amniote eggs. Mech Dev 81:3–22 [DOI] [PubMed] [Google Scholar]
- Ault KT, Dirksen ML, Jamrich M (1996) A novel homeobox gene PV.1 mediates induction of ventral mesoderm in Xenopus embryos. Proc Natl Acad Sci U S A 93:6415–6420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod JD, Miller JR, Shulman JM et al. (1998) Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev 12:2610–2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azar Y, Eyal-Giladi H (1981) Interaction of epiblast and hypoblast in the formation of the primitive streak and the embryonic axis in chick, as revealed by hypoblast-rotation experiments. J Embryol Exp Morphol 61:133–144 [PubMed] [Google Scholar]
- Bachiller D, Klingensmith J, Kemp C et al. (2000) The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature 403:658–661. doi: 10.1038/35001072 [DOI] [PubMed] [Google Scholar]
- Bachvarova RF, Skromne I, Stern CD (1998) Induction of primitive streak and Hensen’s node by the posterior marginal zone in the early chick embryo. Development 125:3521–3534 [DOI] [PubMed] [Google Scholar]
- von Baer KE (1828) Über Entwicklungsgeschichte der Thiere Beobachtung und Reflexion. Gebrüder Borntrager, Konigsberg [Google Scholar]
- Baker J, Beddington R, Harland R (1999) Wnt signaling in Xenopus embryos inhibits bmp4 expression and activates neural development. Genes Dev 13:3149–3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, Morin P, Clevers H (2000) The Yin-Yang of TCF/beta-catenin signaling. Adv Cancer Res 77:1–24 [DOI] [PubMed] [Google Scholar]
- Barrott JJ, Cash GM, Smith AP et al. (2011) Deletion of mouse Porcn blocks Wnt ligand secretion and reveals an ectodermal etiology of human focal dermal hypoplasia/Goltz syndrome. Proc Natl Acad Sci U S A 108:12752–12757. doi: 10.1073/pnas.1006437108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartscherer K, Pelte N, Ingelfinger D, Boutros M (2006) Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell 125:523–533. doi: 10.1016/j.cell.2006.04.009 [DOI] [PubMed] [Google Scholar]
- Bayly R, Axelrod JD (2011) Pointing in the right direction: new developments in the field of planar cell polarity. Nat Rev Genet 12:385–391. doi: 10.1038/nrg2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bánki Ö (1927) Die Langebeziehung der Spermiumeintritts stelle zur Medianeben und zur ersten Furche nach Versuchen mit örtlicher Vitälfarbung am Axolotl Ei. Verh. Anat. Ges., Jena, 36: 198–208 [Google Scholar]
- Becker KA, Ghule PN, Therrien JA et al. (2006) Self-renewal of human embryonic stem cells is supported by a shortened G1 cell cycle phase. J Cell Physiol 209:883–893. doi: 10.1002/jcp.20776 [DOI] [PubMed] [Google Scholar]
- Beddington RS (1994) Induction of a second neural axis by the mouse node. Development 120:613–620 [DOI] [PubMed] [Google Scholar]
- Bedzhov I, Graham SJL, Leung CY, Zernicka-Goetz M (2014) Developmental plasticity, cell fate specification and morphogenesis in the early mouse embryo. Philos Trans R Soc B Biol Sci 369:20130538. doi: 10.1098/rstb.2013.0538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedzhov I, Zernicka-Goetz M (2014) Self-organizing properties of mouse pluripotent cells initiate morphogenesis upon implantation. Cell 156:1032–1044. doi: 10.1016/j.cell.2014.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenkaya TY, Han C, Standley HJ et al. (2002) pygopus Encodes a nuclear protein essential for wingless/Wnt signaling. Development 129:4089–4101 [DOI] [PubMed] [Google Scholar]
- Belo JA, Bouwmeester T, Leyns L et al. (1997) Cerberus-like is a secreted factor with neutralizing activity expressed in the anterior primitive endoderm of the mouse gastrula. Mech Dev 68:45–57 [DOI] [PubMed] [Google Scholar]
- Ben-Haim N, Lu C, Guzman-Ayala M et al. (2006) The nodal precursor acting via activin receptors induces mesoderm by maintaining a source of its convertases and BMP4. Dev Cell 11:313–323. doi: 10.1016/j.devcel.2006.07.005 [DOI] [PubMed] [Google Scholar]
- ten Berge D, Kurek D, Blauwkamp T et al. (2011) Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nat Cell Biol 13:1070–1075. doi: 10.1038/ncb2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertocchini F, Alev C, Nakaya Y, Sheng G (2013) A little winning streak: the reptilian-eye view of gastrulation in birds. Dev Growth Differ 55:52–59. doi: 10.1111/dgd.12014 [DOI] [PubMed] [Google Scholar]
- Bertocchini F, Skromne I, Wolpert L, Stern CD (2004) Determination of embryonic polarity in a regulative system: evidence for endogenous inhibitors acting sequentially during primitive streak formation in the chick embryo. Development 131:3381–3390. doi: 10.1242/dev.01178 [DOI] [PubMed] [Google Scholar]
- Bertocchini F, Stern CD (2002) The hypoblast of the chick embryo positions the primitive streak by antagonizing nodal signaling. Dev Cell 3:735–744 [DOI] [PubMed] [Google Scholar]
- Bertocchini F, Stern CD (2012) Gata2 provides an early anterior bias and uncovers a global positioning system for polarity in the amniote embryo. Development 139:4232–4238. doi: 10.1242/dev.081901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betchaku T, Trinkaus JP (1978) Contact relations, surface activity, and cortical microfilaments of marginal cells of the enveloping layer and of the yolk syncytial and yolk cytoplasmic layers of fundulus before and during epiboly. J Exp Zool 206:381–426. doi: 10.1002/jez.1402060310 [DOI] [PubMed] [Google Scholar]
- Biechele S, Cockburn K, Lanner F et al. (2013) Porcn-dependent Wnt signaling is not required prior to mouse gastrulation. Development 140:2961–2971. doi: 10.1242/dev.094458 [DOI] [PubMed] [Google Scholar]
- Biechele S, Cox BJ, Rossant J (2011) Porcupine homolog is required for canonical Wnt signaling and gastrulation in mouse embryos. Dev Biol 355:275–285. doi: 10.1016/j.ydbio.2011.04.029 [DOI] [PubMed] [Google Scholar]
- Bilic J, Huang Y-L, Davidson G et al. (2007) Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science 316:1619–1622. doi: 10.1126/science.1137065 [DOI] [PubMed] [Google Scholar]
- Bin-Nun N, Lichtig H, Malyarova A et al. (2014) PTK7 modulates Wnt signaling activity via LRP6. Development 141:410–421. doi: 10.1242/dev.095984 [DOI] [PubMed] [Google Scholar]
- Birsoy B, Kofron M, Schaible K et al. (2006) Vg 1 is an essential signaling molecule in Xenopus development. Development 133:15–20. doi: 10.1242/dev.02144 [DOI] [PubMed] [Google Scholar]
- Blitz IL, Cho KWY (2009) Finding partners: how BMPs select their targets. Dev Dyn 238:1321–1331. doi: 10.1002/dvdy.21984 [DOI] [PubMed] [Google Scholar]
- Blum M, Andre P, Muders K, et al. (2007) Ciliation and gene expression distinguish between node and posterior notochord in the mammalian embryo. Differentiation [DOI] [PubMed] [Google Scholar]
- Blum M, Feistel K, Thumberger T, Schweickert A (2014) The evolution and conservation of left-right patterning mechanisms. Development 141:1603–1613. doi: 10.1242/dev.100560 [DOI] [PubMed] [Google Scholar]
- Blum M, Gaunt SJ, Cho KW et al. (1992) Gastrulation in the mouse: the role of the homeobox gene goosecoid. Cell 69:1097–1106 [DOI] [PubMed] [Google Scholar]
- Blythe SA, Cha S-W, Tadjuidje E et al. (2010) Beta-catenin primes organizer gene expression by recruiting a histone H3 arginine 8 methyltransferase, Prmt2. Dev Cell 19:220–231. doi: 10.1016/j.devcel.2010.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boterenbrood EC, Nieuwkoop PD (1973) The formation of the mesoderm in urodelean amphibians. Wilhelm Roux’ Arch Entwicklungsmech 173:319–332. doi: 10.1007/BF00575837 [DOI] [PubMed] [Google Scholar]
- Boucaut JC, Darribere T, De Li S, Boulekbache H, Yamada KM, Thiery JP (1985) Evidence for the role of fibronectin in amphibian gastrulation. Development 89:211–227 [PubMed] [Google Scholar]
- Bourillot PY, Garrett N, Gurdon JB (2002) A changing morphogen gradient is interpreted by continuous transduction flow. Development 129:2167–2180 [DOI] [PubMed] [Google Scholar]
- Boutros M, Mlodzik M (1999) Dishevelled: at the crossroads of divergent intracellular signaling pathways. Mech Dev 83:27–37 [DOI] [PubMed] [Google Scholar]
- Bouwmeester T, Kim S-H, Sasai Y et al. (1996) Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann’s organizer. Nature 382:595–601. doi: 10.1038/382595a0 [DOI] [PubMed] [Google Scholar]
- Brachet A (1904) Recherches expérimentales sur l’oeuf de Rana fusca. Arch Biol 21:103–160 [Google Scholar]
- Brannon M, Gomperts M, Sumoy L et al. (1997) A beta-catenin/XTcf-3 complex binds to the siamois promoter to regulate dorsal axis specification in Xenopus. Genes Dev 11:2359–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannon M, Kimelman D (1996) Activation of Siamois by the Wnt pathway. Dev Biol 180:344–347 [DOI] [PubMed] [Google Scholar]
- Brennan J, Lu CC, Norris DP et al. (2001) Nodal signalling in the epiblast patterns the early mouse embryo. Nature 411:965–969. doi: 10.1038/35082103 [DOI] [PubMed] [Google Scholar]
- Brieher WM, Gumbiner BM (1994) Regulation of C-cadherin function during activin induced morphogenesis of Xenopus animal caps. J Cell Biol 126:519–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A, Papkoff J, Fung Y et al. (1987) Identification of protein products encoded by the proto-oncogene int-1. Mol Cell Biol 7:3971–3977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner E, Peter O, Schweizer L, Basler K (1997) pangolin encodes a Lef-1 homologue that acts downstream of Armadillo to transduce the Wingless signal in Drosophila. Nature 385:829–833 [DOI] [PubMed] [Google Scholar]
- Bytinski-Salz H (1937) Trapianti di organizzatore nelle uova di Lampreda. Tip Luigi Niccolai [Google Scholar]
- Callebaut M (2005) Origin, fate, and function of the components of the avian germ disc region and early blastoderm: role of ooplasmic determinants. Dev Dyn 233:1194–1216. doi: 10.1002/dvdy.20493 [DOI] [PubMed] [Google Scholar]
- Callebaut M, Van Nueten E (1994) Rauber’s (Koller’s) sickle: the early gastrulation organizer of the avian blastoderm. Eur J Morphol 32:35–48 [PubMed] [Google Scholar]
- Callebaut M, Van Nueten E (1995) Gastrulation inducing potencies of endophyll and Rauber’s sickle in isolated caudocranially oriented prestreak avian blastoderm quadrants (or fragments) in vitro. Eur J Morphol 33:221–235 [PubMed] [Google Scholar]
- Camp E, Sánchez-Sánchez AV, García-España A et al. (2009) Nanog regulates proliferation during early fish development. Stem Cells 27:2081–2091. doi: 10.1002/stem.133 [DOI] [PubMed] [Google Scholar]
- Campbell PD, Heim AE, Smith MZ, Marlow FL (2015) Kinesin-1 interacts with Bucky ball to form germ cells and is required to pattern the zebrafish body axis. Development 142:2996–3008. doi: 10.1242/dev.124586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camus A, Perea-Gomez A, Moreau A, Collignon J (2006) Absence of Nodal signaling promotes precocious neural differentiation in the mouse embryo. Dev Biol 295:743–755. doi: 10.1016/j.ydbio.2006.03.047 [DOI] [PubMed] [Google Scholar]
- Cha S-W, Tadjuidje E, Tao Q et al. (2008) Wnt5a and Wnt11 interact in a maternal Dkk1-regulated fashion to activate both canonical and non-canonical signaling in Xenopus axis formation. Development 135:3719–3729. doi: 10.1242/dev.029025 [DOI] [PubMed] [Google Scholar]
- Cha S-W, Tadjuidje E, White J et al. (2009) Wnt11/5a complex formation caused by tyrosine sulfation increases canonical signaling activity. Curr Biol 19:1573–1580. doi: 10.1016/j.cub.2009.07.062 [DOI] [PubMed] [Google Scholar]
- Chambers SM, Fasano CA, Papapetrou EP et al. (2009) Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol 27:275–280. doi: 10.1038/nbt.1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro MN, Schwartz DR, Vonica A et al. (2005) FGF-20 and DKK1 are transcriptional targets of beta-catenin and FGF-20 is implicated in cancer and development. EMBO J 24:73–84. doi: 10.1038/sj.emboj.7600460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AP, Kloc M, Larabell CA et al. (2007) The maternally localized RNA fatvg is required for cortical rotation and germ cell formation. Mech Dev 124:350–363. doi: 10.1016/j.mod.2007.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Harland RM (2007) Neural induction requires continued suppression of both Smad1 and Smad2 signals during gastrulation. Development 134:3861–3872. doi: 10.1242/dev.007179 [DOI] [PubMed] [Google Scholar]
- Chang C, Hemmati-Brivanlou A (1998) Cell fate determination in embryonic ectoderm. J Neurobiol 36:128–151 [PubMed] [Google Scholar]
- Chazaud C, Rossant J (2006) Disruption of early proximodistal patterning and AVE formation in Apc mutants. Development 133:3379–3387. doi: 10.1242/dev.02523 [DOI] [PubMed] [Google Scholar]
- Cheng AM, Thisse B, Thisse C, Wright CV (2000) The lefty-related factor Xatv acts as a feedback inhibitor of nodal signaling in mesoderm induction and L-R axis development in Xenopus. Development 127:1049–1061 [DOI] [PubMed] [Google Scholar]
- Cheung KJ, Gabrielson E, Werb Z, Ewald AJ (2013) Collective invasion in breast cancer requires a conserved basal epithelial program. Cell 155:1639–1651. doi: 10.1016/j.cell.2013.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child CM (1915) Individuality in organisms (Google Books). The University of Chicago Press, Chicago, IL [Google Scholar]
- Cho KW, Blumberg B, Steinbeisser H, De Robertis EM (1991) Molecular nature of Spemann’s organizer: the role of the Xenopus homeobox gene goosecoid. Cell 67:1111–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian J, Moon R (1993) Interactions between Xwnt-8 and Spemann organizer signaling pathways generate dorsoventral pattern in the embryonic mesoderm of Xenopus. Genes Dev 7:13–28 [DOI] [PubMed] [Google Scholar]
- Christian JL, McMahon JA, McMahon AP, Moon RT (1991) Xwnt-8, a Xenopus Wnt-1/int-1-related gene responsive to mesoderm-inducing growth factors, may play a role in ventral mesodermal patterning during embryogenesis. Development 111:1045–1055 [DOI] [PubMed] [Google Scholar]
- Chung HM, Malacinski GM (1980) Establishment of the dorsal/ventral polarity of the amphibian embryo: use of ultraviolet irradiation and egg rotation as probes. Dev Biol 80:120–133 [DOI] [PubMed] [Google Scholar]
- Ciemerych MA, Mesnard D, Zernicka-Goetz M (2000) Animal and vegetal poles of the mouse egg predict the polarity of the embryonic axis, yet are nonessential for development. Development 127:3467–3474 [DOI] [PubMed] [Google Scholar]
- Ciruna B, Jenny A, Lee D et al. (2006) Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nat Cell Biol 439:220–224. doi: 10.1038/nature04375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavert J (1962) Symmetrization of the egg of vertebrates. Adv Morphol 2:27–60 [Google Scholar]
- Clavert J (1961) Développement de la symétrie chez les Vertébrés. Bull Soc Zool Fr 86:381–401 [Google Scholar]
- Clevers H, Nusse R (2012) Wnt/β-catenin signaling and disease. Cell 149:1192–1205. doi: 10.1016/j.cell.2012.05.012 [DOI] [PubMed] [Google Scholar]
- Cliffe A, Hamada F, Bienz M (2003) A role of Dishevelled in relocating Axin to the plasma membrane during wingless signaling. Curr Biol 13:960–966 [DOI] [PubMed] [Google Scholar]
- Cole MF, Johnstone SE, Newman JJ et al. (2008) Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes Dev 22:746–755. doi: 10.1101/gad.1642408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collavin L, Kirschner MW (2003) The secreted Frizzled-related protein Sizzled functions as a negative feedback regulator of extreme ventral mesoderm. Development 130:805–816 [DOI] [PubMed] [Google Scholar]
- Colozza G, De Robertis EM (2014) Maternal syntabulin is required for dorsal axis formation and is a germ plasm component in Xenopus. Differentiation 88:17–26. doi: 10.1016/j.diff.2014.03.002 [DOI] [PubMed] [Google Scholar]
- Conlon FL, Lyons KM, Takaesu N et al. (1994) A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development 120:1919–1928 [DOI] [PubMed] [Google Scholar]
- Connolly DJ, Patel K, Cooke J (1997) Chick noggin is expressed in the organizer and neural plate during axial development, but offers no evidence of involvement in primary axis formation. Int J Dev Biol 41:389–396 [PubMed] [Google Scholar]
- Cook D, Fry MJ, Hughes K et al. (1996) Wingless inactivates glycogen synthase kinase-3 via an intracellular signalling pathway which involves a protein kinase C. EMBO J 15:4526–4536 [PMC free article] [PubMed] [Google Scholar]
- Cooke J, Smith JC (1987) The midblastula cell cycle transition and the character of mesoderm in UV-induced nonaxial Xenopus development. Development 99:197–210 [DOI] [PubMed] [Google Scholar]
- Cooper MS, D’Amico LA (1996) A cluster of noninvoluting endocytic cells at the margin of the zebrafish blastoderm marks the site of embryonic shield formation. Dev Biol 180:184–198. doi: 10.1006/dbio.1996.0294 [DOI] [PubMed] [Google Scholar]
- Coudreuse DYM (2006) Wnt gradient formation requires retromer function in Wnt-producing cells. Science 312:921–924. doi: 10.1126/science.1124856 [DOI] [PubMed] [Google Scholar]
- Cruciat C-M, Niehrs C (2013) Secreted and transmembrane wnt inhibitors and activators. Cold Spring Harb Perspect Biol 5:a015081. doi: 10.1101/cshperspect.a015081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csardi G, Nepusz T (2014) igraph: Network analysis and visualization. R package version 0.7 [Google Scholar]
- Cselenyi CS, Jernigan KK, Tahinci E et al. (2008) LRP6 transduces a canonical Wnt signal independently of Axin degradation by inhibiting GSK3’s phosphorylation of beta-catenin. Proc Natl Acad Sci U S A 105:8032–8037. doi: 10.1073/pnas.0803025105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Tian Q, Christian JL (1996) Synergistic effects of Vg1 and Wnt signals in the specification of dorsal mesoderm and endoderm. Dev Biol 180:22–34. doi: 10.1006/dbio.1996.0281 [DOI] [PubMed] [Google Scholar]
- Curtin JA, Quint E, Tsipouri V et al. (2003) Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol 13:1129–1133 [DOI] [PubMed] [Google Scholar]
- Cuykendall TN, Houston DW (2009) Vegetally localized Xenopus trim36 regulates cortical rotation and dorsal axis formation. Development 136:3057–3065. doi: 10.1242/dev.036855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalcq A, Pasteels J (1937) Une conception nouvelle des bases physiologiques de la morphogénèse. Arch Biol (Liege) 48:699–710 [Google Scholar]
- Dale L, Howes G, Price B, Smith J (1992) Bone morphogenetic protein 4: a ventralizing factor in early Xenopus development. Development 115:573–585 [DOI] [PubMed] [Google Scholar]
- Dale L, Smith JC, Slack JM (1985) Mesoderm induction in Xenopus laevis: a quantitative study using a cell lineage label and tissue-specific antibodies. J Embryol Exp Morphol 89:289–312 [PubMed] [Google Scholar]
- Dalle Nogare D, Somers K, Rao S et al. (2014) Leading and trailing cells cooperate in collective migration of the zebrafish posterior lateral line primordium. Development 141:3188–3196. doi: 10.1242/dev.106690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darken R, Scola A, Rakeman A et al. (2002) The planar polarity gene strabismus regulates convergent extension movements in Xenopus. EMBO J 21:976–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darras S, Marikawa Y, Elinson RP, Lemaire P (1997) Animal and vegetal pole cells of early Xenopus embryos respond differently to maternal dorsal determinants: implications for the patterning of the organiser. Development 124:4275–4286 [DOI] [PubMed] [Google Scholar]
- Davidson G, Shen J, Huang Y-L et al. (2009) Cell cycle control of wnt receptor activation. Dev Cell 17:788–799. doi: 10.1016/j.devcel.2009.11.006 [DOI] [PubMed] [Google Scholar]
- Davidson G, Wu W, Shen J et al. (2005) Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature 438:867–872. doi: 10.1038/nature04170 [DOI] [PubMed] [Google Scholar]
- De Robertis E, Larrain J, Oelgeschlager M, Wessely O (2000) The establishment of Spemann’s organizer and patterning of the vertebrate embryo. Nat Rev Genet 1:171–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EM (2009) Spemann’s organizer and the self-regulation of embryonic fields. Mech Dev 126:925–941. doi: 10.1016/j.mod.2009.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EM (2006) Spemann’s organizer and self-regulation in amphibian embryos. Nat Rev Mol Cell Biol 7:296–302. doi: 10.1038/nrm1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EM, Sasai Y (1996) A common plan for dorsoventral patterning in Bilateria. Nature 380:37–40. doi: 10.1038/380037a0 [DOI] [PubMed] [Google Scholar]
- De Vries WN, Evsikov AV, Haac BE et al. (2004) Maternal beta-catenin and E-cadherin in mouse development. Development 131:4435–4445. doi: 10.1242/dev.01316 [DOI] [PubMed] [Google Scholar]
- Deans MR, Antic D, Suyama K et al. (2007) Asymmetric distribution of Prickle-like 2 reveals an early underlying polarization of vestibular sensory epithelia in the inner ear. J Neurosci 27:3139–3147. doi: 10.1523/JNEUROSCI.5151-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaune E, Lemaire P, Kodjabachian L (2005) Neural induction in Xenopus requires early FGF signalling in addition to BMP inhibition. Development 132:299–310. doi: 10.1242/dev.01582 [DOI] [PubMed] [Google Scholar]
- Dick A, Hild M, Bauer H, Imai Y, Maifeld H, Schier AF, Talbot WS, Bouwmeester T, Hammerschmidt M (2000) Essential role of Bmp7 (snailhouse) and its prodomain in dorsoventral patterning of the zebrafish embryo. Development 127:343–354 [DOI] [PubMed] [Google Scholar]
- Ding J, Yang L, Yan YT et al. (1998) Cripto is required for correct orientation of the anterior-posterior axis in the mouse embryo. Nature 395:702–707. doi: 10.1038/27215 [DOI] [PubMed] [Google Scholar]
- Dirksen ML, Jamrich M (1992) A novel, activin-inducible, blastopore lip-specific gene of Xenopus laevis contains a fork head DNA-binding domain. Genes Dev 6:599–608 [DOI] [PubMed] [Google Scholar]
- Djiane A, Riou J, Umbhauer M et al. (2000) Role of frizzled 7 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development 127:3091–3100 [DOI] [PubMed] [Google Scholar]
- Dobrowolski R, De Robertis EM (2012) Endocytic control of growth factor signalling: multivesicular bodies as signalling organelles. Nat Rev Mol Cell Biol 13:53–60. doi: 10.1038/nrm3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi JY, Niigaki H, Sone K et al. (2000) Distribution of dorsal-forming activity in precleavage embryos of the Japanese newt, Cynops pyrrhogaster: effects of deletion of vegetal cytoplasm, UV irradiation, and lithium treatment. Dev Biol 223:154–168. doi: 10.1006/dbio.2000.9735 [DOI] [PubMed] [Google Scholar]
- Domingos PM, Itasaki N, Jones CM et al. (2001) The Wnt/beta-catenin pathway posteriorizes neural tissue in Xenopus by an indirect mechanism requiring FGF signalling. Dev Biol 239:148–160. doi: 10.1006/dbio.2001.0431 [DOI] [PubMed] [Google Scholar]
- Dorsky RI, Itoh M, Moon RT, Chitnis A (2003) Two tcf3 genes cooperate to pattern the zebrafish brain. Development 130:1937–1947 [DOI] [PubMed] [Google Scholar]
- Dougan ST, Warga RM, Kane DA et al. (2003) The role of the zebrafish nodal-related genes squint and cyclops in patterning of mesendoderm. Development 130:1837–1851 [DOI] [PubMed] [Google Scholar]
- Du SJ, Purcell SM, Christian JL et al. (1995) Identification of distinct classes and functional domains of Wnts through expression of wild-type and chimeric proteins in Xenopus embryos. Mol Cell Biol 15:2625–2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufort D, Schwartz L, Harpal K, Rossant J (1998) The transcription factor HNF3beta is required in visceral endoderm for normal primitive streak morphogenesis. Development 125:3015–3025 [DOI] [PubMed] [Google Scholar]
- Dyson S, Gurdon JB (1998) The interpretation of position in a morphogen gradient as revealed by occupancy of activin receptors. Cell 93:557–568 [DOI] [PubMed] [Google Scholar]
- Egger-Adam D, Katanaev VL (2010) The trimeric G protein Go inflicts a double impact on axin in the Wnt/frizzled signaling pathway. Dev Dyn 239:168–183. doi: 10.1002/dvdy.22060 [DOI] [PubMed] [Google Scholar]
- Elinson R, Pasceri P (1989) Two UV-sensitive targets in dorsoanterior specification of frog embryos. Development 106:511–518 [DOI] [PubMed] [Google Scholar]
- Elinson RP (1983) Cytoplasmic phases in the first cell cycle of the activated frog egg. Dev Biol 100:440–451 [DOI] [PubMed] [Google Scholar]
- Elinson RP, del Pino EM (2011) Developmental diversity of amphibians. WIREs Dev Biol 1:345–369. doi: 10.1002/wdev.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinson RP, Holowacz T (1995) Specifying the dorsoanterior axis in frogs: 70 years since Spemann and Mangold. Curr Top Dev Biol 30:253–285 [DOI] [PubMed] [Google Scholar]
- Elinson RP, Kezmoh L (2010) Molecular Haeckel. Dev Dyn 239:1905–1918. doi: 10.1002/dvdy.22337 [DOI] [PubMed] [Google Scholar]
- Elinson RP, Rowning B (1988) A transient array of parallel microtubules in frog eggs: potential tracks for a cytoplasmic rotation that specifies the dorso-ventral axis. Dev Biol 128:185–197 [DOI] [PubMed] [Google Scholar]
- Erter CE, Solnica-Krezel L, Wright CV (1998) Zebrafish nodal-related 2 encodes an early mesendodermal inducer signaling from the extraembryonic yolk syncytial layer. Dev Biol 204:361–372. doi: 10.1006/dbio.1998.9097 [DOI] [PubMed] [Google Scholar]
- Eyal-Giladi H (1954) Dynamic aspects of neural induction in amphibia. Arch Biol (Liege) 65:179–259 [PubMed] [Google Scholar]
- Eyal-Giladi H, Fabian BC (1980) Axis determination in uterine chick blastodiscs under changing spatial positions during the sensitive period for polarity. Dev Biol 77:228–232 [DOI] [PubMed] [Google Scholar]
- Eyal-Giladi H, Kochav S (1976) From cleavage to primitive streak formation: a complementary normal table and a new look at the first stages of the development of the chick: I. General morphology. Dev Biol 49:321–337 [DOI] [PubMed] [Google Scholar]
- Fainsod A, Steinbeisser H, De Robertis E (1994) On the function of BMP-4 in patterning the marginal zone of the Xenopus embryo. EMBO J 13:5015–5025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure S, Lee MA, Keller T et al. (2000) Endogenous patterns of TGFbeta superfamily signaling during early Xenopus development. Development 127:2917–2931 [DOI] [PubMed] [Google Scholar]
- Fekany K, Yamanaka Y, Leung T, Sirotkin HI, Topczewski J, Gates MA, Hibi M, Renucci A, Stemple D, Radbill A et al. (1999) The zebrafish bozozok locus encodes Dharma, a homeodomain protein essential for induction of gastrula organizer and dorsoanterior embryonic structures. Development 126:1427–1438 [DOI] [PubMed] [Google Scholar]
- Feldman B, Gates MA, Egan ES et al. (1998) Zebrafish organizer development and germ-layer formation require nodal-related signals. Nature 395:181–185. doi: 10.1038/26013 [DOI] [PubMed] [Google Scholar]
- Fiedler M, Sánchez-Barrena MJ, Nekrasov M et al. (2008) Decoding of methylated histone H3 tail by the Pygo-BCL9 Wnt signaling complex. Mol Cell 30:507–518. doi: 10.1016/j.molcel.2008.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers GP, Topczewska JM, Topczewski J (2012) A zebrafish Notum homolog specifically blocks the Wnt/β-catenin signaling pathway. Development 139:2416–2425. doi: 10.1242/dev.063206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley AC, Skromne I, Stern CD (2000) Reconciling different models of forebrain induction and patterning: a dual role for the hypoblast. Development 127:3839–3854 [DOI] [PubMed] [Google Scholar]
- Fossat N, Jones V, Khoo P-L et al. (2011) Stringent requirement of a proper level of canonical WNT signalling activity for head formation in mouse embryo. Development 138:667–676. doi: 10.1242/dev.052803 [DOI] [PubMed] [Google Scholar]
- Fraser SE, Stern CD (2004) Early rostrocaudal patterning of the mesoderm and neural plate In: Stern CD (ed) Gastrulation. CSHL Press, New York, pp 389–401 [Google Scholar]
- Friedl P, Gilmour D (2009) Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol 10:445–457. doi: 10.1038/nrm2720 [DOI] [PubMed] [Google Scholar]
- Fu J, Jiang M, Mirando AJ et al. (2009) Reciprocal regulation of Wnt and Gpr177/mouse Wntless is required for embryonic axis formation. Proc Natl Acad Sci U S A 106:18598–18603. doi: 10.1073/pnas.0904894106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba LC, Eivers E, Ikeda A et al. (2007) Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell 131:980–993. doi: 10.1016/j.cell.2007.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimori T, Kurotaki Y, Miyazaki J-I, Nabeshima Y-I (2003) Analysis of cell lineage in two- and four-cell mouse embryos. Development 130:5113–5122. doi: 10.1242/dev.00725 [DOI] [PubMed] [Google Scholar]
- Fujisue M, Kobayakawa Y, Yamana K (1993) Occurrence of dorsal axis-inducing activity around the vegetal pole of an uncleaved Xenopus egg and displacement to the equatorial region by cortical rotation. Development 118:163–170 [DOI] [PubMed] [Google Scholar]
- Fujisue M, Sakai M, Yamana K (1991) Subcortical rotation and specification of the dorsoventral axis in newt eggs. Dev Growth Differ 33:34–51 [DOI] [PubMed] [Google Scholar]
- Funayama N, Fagotto F, McCrea P, Gumbiner BM (1995) Embryonic axis induction by the armadillo repeat domain of beta-catenin: evidence for intracellular signaling. J Cell Biol 128:959–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furushima K, Yamamoto A, Nagano T et al. (2007) Mouse homologues of Shisa antagonistic to Wnt and Fgf signalings. Dev Biol 306:480–492. doi: 10.1016/j.ydbio.2007.03.028 [DOI] [PubMed] [Google Scholar]
- Galceran J, Fariñas I, Depew MJ et al. (1999) Wnt3a−/−-like phenotype and limb deficiency in Lef1(−/−)Tcf1(−/−) mice. Genes Dev 13:709–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Song H, Bishop K et al. (2011) Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev Cell 20:163–176. doi: 10.1016/j.devcel.2011.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RL, Cockroft DL (1998) Complete dissipation of coherent clonal growth occurs before gastrulation in mouse epiblast. Development 125:2397–2402 [DOI] [PubMed] [Google Scholar]
- Gawantka V, Delius H, Hirschfeld K et al. (1995) Antagonizing the Spemann organizer: role of the homeobox gene Xvent-1. EMBO J 14:6268–6279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Grotjahn D, Welch E et al. (2014) Hecate/Grip2a acts to reorganize the cytoskeleton in the symmetry-breaking event of embryonic axis induction. PLoS Genet 10:e1004422. doi: 10.1371/journal.pgen.1004422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J (2004) Symmetry breaking in the egg of Xenopus laevis. 341–351. Cold Spring Harbor, New York: CSHL Press [Google Scholar]
- Gerhart J, Danilchik M, Doniach T et al. (1989) Cortical rotation of the Xenopus egg: consequences for the anteroposterior pattern of embryonic dorsal development. Development 107(Suppl):37–51 [DOI] [PubMed] [Google Scholar]
- Gerhart J, Ubbels G, Black S et al. (1981) A reinvestigation of the role of the grey crescent in axis formation in Xenopus laevis. Nature 292:511–516 [DOI] [PubMed] [Google Scholar]
- Gerlitz O, Basler K (2002) Wingful, an extracellular feedback inhibitor of Wingless. Genes Dev 16:1055–1059. doi: 10.1101/gad.991802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimlich RL (1986) Acquisition of developmental autonomy in the equatorial region of the Xenopus embryo. Dev Biol 115:340–352 [DOI] [PubMed] [Google Scholar]
- Gimlich RL, Gerhart JC (1984) Early cellular interactions promote embryonic axis formation in Xenopus laevis. Dev Biol 104:117–130 [DOI] [PubMed] [Google Scholar]
- Giraldez AJ, Copley RR, Cohen SM (2002) HSPG modification by the secreted enzyme Notum shapes the Wingless morphogen gradient. Dev Cell 2:667–676 [DOI] [PubMed] [Google Scholar]
- Glinka A, Delius H, Blumenstock C, Niehrs C (1996) Combinatorial signalling by Xwnt-11 and Xnr3 in the organizer epithelium. Mech Dev 60:221–231 [DOI] [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H et al. (1998) Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391:357–362. doi: 10.1038/34848 [DOI] [PubMed] [Google Scholar]
- Glinka A, Wu W, Onichtchouk D et al. (1997) Head induction by simultaneous repression of Bmp and Wnt signalling in Xenopus. Nature 389:517–519. doi: 10.1038/39092 [DOI] [PubMed] [Google Scholar]
- Godsave SF, Slack JM (1991) Single cell analysis of mesoderm formation in the Xenopus embryo. Development 111:523–530 [DOI] [PubMed] [Google Scholar]
- Gore AV, Maegawa S, Cheong A et al. (2005) The zebrafish dorsal axis is apparent at the four-cell stage. Nature 438:1030–1035. doi: 10.1038/nature04184 [DOI] [PubMed] [Google Scholar]
- Goto T, Keller R (2002) The planar cell polarity gene strabismus regulates convergence and extension and neural fold closure in Xenopus. Dev Biol 247:165–181 [DOI] [PubMed] [Google Scholar]
- Gradl D, König A, Wedlich D (2002) Functional diversity of Xenopus lymphoid enhancer factor/T- - cell factor transcription factors relies on combinations of activating and repressing elements. J Biol Chem 277:14159–14171. doi: 10.1074/jbc.M107055200 [DOI] [PubMed] [Google Scholar]
- Graff JM, Thies RS, Song JJ et al. (1994) Studies with a Xenopus BMP receptor suggest that ventral mesoderm-inducing signals override dorsal signals in vivo. Cell 79:169–179 [DOI] [PubMed] [Google Scholar]
- Granier C, Gurchenkov V, Perea-Gomez A et al. (2011) Nodal cis-regulatory elements reveal epiblast and primitive endoderm heterogeneity in the peri-implantation mouse embryo. Dev Biol 349:350–362. doi: 10.1016/j.ydbio.2010.10.036 [DOI] [PubMed] [Google Scholar]
- Gray H (1918) Anatomy of the human body, 20th edn. Lea and Febiger, Philadelphia, PA [Google Scholar]
- Gräper L (1929) Die Primitiventwicklung des Hühnchens nach stereokinematographischen Untersuchungen, kontrolliert durch vitale Farbmarkierung und verglichen mit der Entwicklung anderer Wirbeltiere. Wilhelm Roux’ Arch Entwicklungsmech 116:382–429. doi: 10.1007/BF02145235 [DOI] [PubMed] [Google Scholar]
- Green JBA, Sharpe J (2015) Positional information and reaction-diffusion: two big ideas in developmental biology combine. Development 142:1203–1211. doi: 10.1242/dev.114991 [DOI] [PubMed] [Google Scholar]
- Gritsman K, Talbot WS, Schier AF (2000) Nodal signaling patterns the organizer. Development 127:921–932 [DOI] [PubMed] [Google Scholar]
- Gros J, Feistel K, Viebahn C et al. (2009) Cell movements at Hensen’s node establish left/right asymmetric gene expression in the chick. Science 324:941–944. doi: 10.1126/science.1172478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JC, Chaudhary V, Bartscherer K, Boutros M (2012) Active Wnt proteins are secreted on exosomes. Nat Cell Biol 14:1036–1045. doi: 10.1038/ncb2574 [DOI] [PubMed] [Google Scholar]
- Grunz H (2004) The vertebrate organizer. Springer Science & Business Media, Berlin [Google Scholar]
- Grunz H, Tacke L (1989) Neural differentiation of Xenopus laevis ectoderm takes place after disaggregation and delayed reaggregation without inducer. Cell Differ Dev 28:211–217 [DOI] [PubMed] [Google Scholar]
- Grunz H, Tacke L (1990) Extracellular matrix components prevent neural differentiation of disaggregated Xenopus ectoderm cells. Cell Differ Dev 32:117–123 [DOI] [PubMed] [Google Scholar]
- Guger KA, Gumbiner BM (1995) beta-Catenin has Wnt-like activity and mimics the Nieuwkoop signaling center in Xenopus dorsal-ventral patterning. Dev Biol 172:115–125. doi: 10.1006/dbio.1995.0009 [DOI] [PubMed] [Google Scholar]
- Habas R, Dawid IB, He X (2003) Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev 17:295–309. doi: 10.1101/gad.1022203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R, Kato Y, He X (2001) Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell 107:843–854 [DOI] [PubMed] [Google Scholar]
- Haegel H, Larue L, Ohsugi M et al. (1995) Lack of beta-catenin affects mouse development at gastrulation. Development 121:3529–3537 [DOI] [PubMed] [Google Scholar]
- Hainski AM, Moody SA (1992) Xenopus maternal RNAs from a dorsal animal blastomere induce a secondary axis in host embryos. Development 116:347–355 [DOI] [PubMed] [Google Scholar]
- Halacheva V, Fuchs M, Dönitz J et al. (2011) Planar cell movements and oriented cell division during early primitive streak formation in the mammalian embryo. Dev Dyn 240:1905–1916. doi: 10.1002/dvdy.22687 [DOI] [PubMed] [Google Scholar]
- Hamburger V (1988) The heritage of experimental embryology: Hans Spemann and the organizer. Oxford University Press, USA [Google Scholar]
- Hamburger V, Hamilton HL (1951) A series of normal stages in the development of the chick embryo. J Morphol 88:231–272. doi: 10.1002/aja.1001950404 [DOI] [PubMed] [Google Scholar]
- Hamilton FS, Wheeler GN, Hoppler S (2001) Difference in XTcf-3 dependency accounts for change in response to beta-catenin-mediated Wnt signalling in Xenopus blastula. Development 128:2063–2073 [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Pelegri F, Mullins MC et al. (1996a) Mutations affecting morphogenesis during gastrulation and tail formation in the zebrafish, Danio rerio. Development 123:143–151 [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Serbedzija GN, McMahon AP (1996b) Genetic analysis of dorsoventral pattern formation in the zebrafish: requirement of a BMP-like ventralizing activity and its dorsal repressor. Genes Dev 10:2452–2461. doi: 10.1101/gad.10.19.2452 [DOI] [PubMed] [Google Scholar]
- Hansen C, Marion C, Steele K et al. (1997) Direct neural induction and selective inhibition of mesoderm and epidermis inducers by Xnr3. Development 124:483–492 [DOI] [PubMed] [Google Scholar]
- Harland R (2008) Induction into the Hall of Fame: tracing the lineage of Spemann’s organizer. Development 135:3321–3323 [DOI] [PubMed] [Google Scholar]
- Harland R, Gerhart J (1997) Formation and function of Spemann’s organizer. Annu Rev Cell Dev Biol 13:611–667 [DOI] [PubMed] [Google Scholar]
- Harland RM (1994) Neural induction in Xenopus. Curr Opin Genet Dev 4:543–549 [DOI] [PubMed] [Google Scholar]
- Harland RM, Grainger RM (2011) Xenopus research: metamorphosed by genetics and genomics. Trends Genet 27:507–515. doi: 10.1016/j.tig.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley KO, Hardcastle Z, Friday RV et al. (2001) Transgenic Xenopus embryos reveal that anterior neural development requires continued suppression of BMP signaling after gastrulation. Dev Biol 238:168–184. doi: 10.1006/dbio.2001.0398 [DOI] [PubMed] [Google Scholar]
- Hashiguchi M, Mullins MC (2013) Anteroposterior and dorsoventral patterning are coordinated by an identical patterning clock. Development 140:1970–1980. doi: 10.1242/dev.088104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H, Itoh M, Yamanaka Y, Yamashita S, Shimizu T, Solnica-Krezel L, Hibi M, Hirano T (2000) Zebrafish Dkk1 functions in forebrain specification and axial mesendoderm formation. Dev Biol 217:138–152 [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Shinohara K, Wang J et al. (2010) Planar polarization of node cells determines the rotational axis of node cilia. Nat Cell Biol 12:170–176. doi: 10.1038/ncb2020 [DOI] [PubMed] [Google Scholar]
- Hausen P, Riebesell M (1991) The early development of Xenopus laevis: an atlas of the histology. Springer, New York [Google Scholar]
- Hayes M, Naito M, Daulat A et al. (2013) Ptk7 promotes non-canonical Wnt/PCP-mediated morphogenesis and inhibits Wnt/β-catenin-dependent cell fate decisions during vertebrate development. Development 140(8):1807–1818 [DOI] [PubMed] [Google Scholar]
- He X, Saint-Jeannet JP, Wang Y et al. (1997) A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science 275:1652–1654 [DOI] [PubMed] [Google Scholar]
- He X, Semenov M, Tamai K, Zeng X (2004) LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development 131:1663–1677. doi: 10.1242/dev.01117 [DOI] [PubMed] [Google Scholar]
- Heasman J (1997) Patterning the Xenopus blastula. Development 124:4179–4191 [DOI] [PubMed] [Google Scholar]
- Heasman J, Crawford A, Goldstone K et al. (1994) Overexpression of cadherins and underexpression of beta-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell 79:791–803 [DOI] [PubMed] [Google Scholar]
- Hedge TA, Mason I (2008) Expression of Shisa2, a modulator of both Wnt and Fgf signaling, in the chick embryo. Int J Dev Biol 52:81–85. doi: 10.1387/ijdb.072355th [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Houart C, Take-Uchi M et al. (2001) A mutation in the Gsk3-binding domain of zebrafish Masterblind/Axin1 leads to a fate transformation of telencephalon and eyes to diencephalon. Genes Dev 15:1427–1434. doi: 10.1101/gad.194301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ et al. (2000) Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature 405:76–81. doi: 10.1038/35011068 [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Kelly O, Melton DA (1994) Follistatin, an antagonist of activin, is expressed in the Spemann organizer and displays direct neuralizing activity. Cell 77:283–295 [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Melton D (1997) Vertebrate embryonic cells will become nerve cells unless told otherwise. Cell 88(1):13–17 [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Melton DA (1994) Inhibition of activin receptor signaling promotes neuralization in Xenopus. Cell 77:273–281 [DOI] [PubMed] [Google Scholar]
- Hernández AR, Klein AM, Kirschner MW (2012) Kinetic responses of β-catenin specify the sites of Wnt control. Science 338:1337–1340. doi: 10.1126/science.1228734 [DOI] [PubMed] [Google Scholar]
- Hesiod, Evelyn-White HG (1914) Works and days. The Homeric Hymns and Homerica 174–ff [Google Scholar]
- Hikasa H, Ezan J, Itoh K et al. (2010) Regulation of TCF3 by Wnt-dependent phosphorylation during vertebrate axis specification. Dev Cell 19:521–532. doi: 10.1016/j.devcel.2010.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikasa H, Sokol SY (2013) Wnt signaling in vertebrate axis specification. Cold Spring Harb Perspect Biol 5:a007955. doi: 10.1101/cshperspect.a007955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hild M, Dick A, Rauch GJ et al. (1999) The smad5 mutation somitabun blocks Bmp2b signaling during early dorsoventral patterning of the zebrafish embryo. Development 126:2149–2159 [DOI] [PubMed] [Google Scholar]
- Hillman N, Sherman MI, Graham C (1972) The effect of spatial arrangement on cell determination during mouse development. J Embryol Exp Morphol 28:263–278 [PubMed] [Google Scholar]
- Hilton E, Rex M, Old R (2003) VegT activation of the early zygotic gene Xnr5 requires lifting of Tcf-mediated repression in the Xenopus blastula. Mech Dev 120:1127–1138 [DOI] [PubMed] [Google Scholar]
- Ho C-Y, Houart C, Wilson SW, Stainier DYR (1999) A role for the extraembryonic yolk syncytial layer in patterning the zebrafish embryo suggested by properties of the hex gene. Curr Biol 9:1131–1134. doi: 10.1016/S0960-9822(99)80485-0 [DOI] [PubMed] [Google Scholar]
- Ho RK (1992) Cell movements and cell fate during zebrafish gastrulation. Dev Suppl 65–73 [PubMed] [Google Scholar]
- Hoffmans R, Städeli R, Basler K (2005) Pygopus and legless provide essential transcriptional coactivator functions to armadillo/beta-catenin. Curr Biol 15:1207–1211. doi: 10.1016/j.cub.2005.05.054 [DOI] [PubMed] [Google Scholar]
- Hofmann K (2000) A superfamily of membrane-bound O-acyltransferases with implications for wnt signaling. Trends Biochem Sci 25:111–112 [DOI] [PubMed] [Google Scholar]
- Hogan BL (1996) Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev 10:1580–1594 [DOI] [PubMed] [Google Scholar]
- Holland LZ, Onai T (2012) Early development of cephalochordates (amphioxus). WIREs Dev Biol 1:167–183. doi: 10.1002/wdev.11 [DOI] [PubMed] [Google Scholar]
- Holowacz T, Elinson RP (1993) Cortical cytoplasm, which induces dorsal axis formation in Xenopus, is inactivated by UV irradiation of the oocyte. Development 119:277–285 [DOI] [PubMed] [Google Scholar]
- Holstein TW (2012) The evolution of the Wnt pathway. Cold Spring Harb Perspect Biol 4:a007922. doi: 10.1101/cshperspect.a007922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtfreter J (1944) Neural differentiation of ectoderm through exposure to saline solution. J Exp Zool 95:307–343. doi: 10.1002/jez.1400950303 [DOI] [Google Scholar]
- Holwill S, Heasman J, Crawley C, Wylie CC (1987) Axis and germ line deficiencies caused by UV irradiation of Xenopus oocytes cultured in vitro. Development 100:735–743 [Google Scholar]
- Hoodless PA, Pye M, Chazaud C et al. (2001) FoxH1 (Fast) functions to specify the anterior primitive streak in the mouse. Genes Dev 15:1257–1271. doi: 10.1101/gad.881501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppler S, Brown JD, Moon RT (1996) Expression of a dominant-negative Wnt blocks induction of MyoD in Xenopus embryos. Genes Dev 10:2805–2817 [DOI] [PubMed] [Google Scholar]
- Hoppler S, Moon RT (1998) BMP-2/−4 and Wnt-8 cooperatively pattern the Xenopus mesoderm. Mech Dev 71:119–129 [DOI] [PubMed] [Google Scholar]
- Hopwood N (2007) A history of normal plates, tables and stages in vertebrate embryology. Int J Dev Biol 51:1–26. doi: 10.1387/ijdb.062189nh [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houart C, Caneparo L, Heisenberg C et al. (2002) Establishment of the telencephalon during gastrulation by local antagonism of Wnt signaling. Neuron 35:255–265 [DOI] [PubMed] [Google Scholar]
- Houliston E (1994) Microtubule translocation and polymerisation during cortical rotation in Xenopus eggs. Development 120:1213–1220 [Google Scholar]
- Houliston E, Elinson RP (1991) Evidence for the involvement of microtubules, ER, and kinesin in the cortical rotation of fertilized frog eggs. J Cell Biol 114:1017–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston DW (2012) Cortical rotation and messenger RNA localization in Xenopus axis formation. WIREs Dev Biol 1:371–388. doi: 10.1002/wdev.29 [DOI] [PubMed] [Google Scholar]
- Houston DW (2013) Regulation of cell polarity and RNA localization in vertebrate oocytes. Int Rev Cell Mol Biol 306:127–185. doi: 10.1016/B978-0-12-407694-5.00004-3 [DOI] [PubMed] [Google Scholar]
- Houston DW, Kofron M, Resnik E et al. (2002) Repression of organizer genes in dorsal and ventral Xenopus cells mediated by maternal XTcf3. Development 129:4015–4025 [DOI] [PubMed] [Google Scholar]
- Hsieh J-C, Lee L, Zhang L et al. (2003) Mesd encodes an LRP5/6 chaperone essential for specification of mouse embryonic polarity. Cell 112:355–367 [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Brinkmann V et al. (2000) Requirement for beta-catenin in anterior-posterior axis formation in mice. J Cell Biol 148:567–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume CR, Dodd J (1993) Cwnt-8C: a novel Wnt gene with a potential role in primitive streak formation and hindbrain organization. Development 119:1147–1160 [DOI] [PubMed] [Google Scholar]
- Hunt TE (1929) Hensen’s node as an organiser in the formation of the chick embryo. Anat Rec 42:22 [Google Scholar]
- Imai Y, Gates MA, Melby AE et al. (2001) The homeobox genes vox and vent are redundant repressors of dorsal fates in zebrafish. Development 128:2407–2420 [DOI] [PubMed] [Google Scholar]
- Inomata H, Haraguchi T, Sasai Y (2008) Robust stability of the embryonic axial pattern requires a secreted scaffold for chordin degradation. Cell 134:854–865. doi: 10.1016/j.cell.2008.07.008 [DOI] [PubMed] [Google Scholar]
- Inui M, Montagner M, Ben-Zvi D et al. (2012) Self-regulation of the head-inducing properties of the Spemann organizer. Proc Natl Acad Sci U S A 109:15354–15359. doi: 10.1073/pnas.1203000109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip CK, Fossat N, Jones V et al. (2014) Head formation: OTX2 regulates Dkk1 and Lhx1 activity in the anterior mesendoderm. Development 141:3859–3867. doi: 10.1242/dev.114900 [DOI] [PubMed] [Google Scholar]
- Ishibashi H, Matsumura N, Hanafusa H et al. (2008) Expression of Siamois and Twin in the blastula Chordin/Noggin signaling center is required for brain formation in Xenopus laevis embryos. Mech Dev 125:58–66. doi: 10.1016/j.mod.2007.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani T, Kishida S, Hyodo-Miura J et al. (2003) The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/beta-catenin signaling. Mol Cell Biol 23:131–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani T, Ninomiya-Tsuji J, Nagai S et al. (1999) The TAK1-NLK-MAPK-related pathway antagonizes signalling between beta-catenin and transcription factor TCF. Nature 399:798–802 [DOI] [PubMed] [Google Scholar]
- Itasaki N, Jones CM, Mercurio S et al. (2003) Wise, a context-dependent activator and inhibitor of Wnt signalling. Development 130:4295–4305 [DOI] [PubMed] [Google Scholar]
- Iwao Y, Yasumitsu K, Narihira M et al. (1997) Changes in microtubule structures during the first cell cycle of physiologically polyspermic newt eggs. Mol Reprod Dev 47:210–221. [DOI] [PubMed] [Google Scholar]
- Izpisúa Belmonte JC, De Robertis EM, Storey KG, Stern CD (1993) The homeobox gene goosecoid and the origin of organizer cells in the early chick blastoderm. Cell 74:645–659 [DOI] [PubMed] [Google Scholar]
- Janda CY, Waghray D, Levin AM et al. (2012) Structural basis of Wnt recognition by frizzled. Science 337:59–64. doi: 10.1126/science.1222879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan KK, Cselenyi CS, Thorne CA et al. (2010) Gbetagamma activates GSK3 to promote LRP6-mediated beta-catenin transcriptional activity. Sci Signal 3:ra37. doi: 10.1126/scisignal.2000647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen JR, Topczewski J, Bingham S et al. (2002) Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat Cell Biol 4:610–615. doi: 10.1038/ncb828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesuthasan S, Stähle U (1997) Dynamic microtubules and specification of the zebrafish embryonic axis. Curr Biol 7:31–42 [DOI] [PubMed] [Google Scholar]
- Jiang J, Struhl G (1998) Regulation of the Hedgehog and Wingless signalling pathways by the F-box/WD40-repeat protein Slimb. Nature 391:493–496 [DOI] [PubMed] [Google Scholar]
- Jones C, Lyons K, Lapan P et al. (1992) DVR-4 (bone morphogenetic protein-4) as a posterior-ventralizing factor in Xenopus mesoderm induction. Development 115:639–647 [DOI] [PubMed] [Google Scholar]
- Jones CM, Broadbent J, Thomas PQ et al. (1999) An anterior signalling centre in Xenopus revealed by the homeobox gene XHex. Curr Biol 9:946–954 [DOI] [PubMed] [Google Scholar]
- Jose Maria Carvajal-Gonzalez MM (2014) Mechanisms of planar cell polarity establishment in Drosophila. F1000Prime Reports. doi: 10.12703/P6-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubin K, Stern CD (1999) Molecular interactions continuously define the organizer during the cell movements of gastrulation. Cell 98:559–571 [DOI] [PubMed] [Google Scholar]
- Jullien J, Gurdon JB (2005) Morphogen gradient interpretation by a regulated trafficking step during ligand-receptor transduction. Genes Dev 19:2682–2694. doi: 10.1101/gad.341605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T, Wilder E, Klingensmith J et al. (1996) The segment polarity gene porcupine encodes a putative multitransmembrane protein involved in Wingless processing. Genes Dev 10:3116–3128 [DOI] [PubMed] [Google Scholar]
- Kagermeier-Schenk B, Wehner D, Ozhan-Kizil G et al. (2011) Waif1/5 T4 inhibits Wnt/β-catenin signaling and activates noncanonical Wnt pathways by modifying LRP6 subcellular localization. Dev Cell 21:1129–1143. doi: 10.1016/j.devcel.2011.10.015 [DOI] [PubMed] [Google Scholar]
- Kageura H (1997) Activation of dorsal development by contact between the cortical dorsal determinant and the equatorial core cytoplasm in eggs of Xenopus laevis. Development 124:1543–1551 [DOI] [PubMed] [Google Scholar]
- Kageura H (1990) Spatial distribution of the capacity to initiate a secondary embryo in the 32-cell embryo of Xenopus laevis. Dev Biol 142:432–438 [DOI] [PubMed] [Google Scholar]
- Kakugawa S, Langton PF, Zebisch M et al. (2015) Notum deacylates Wnt proteins to suppress signalling activity. Nature 519:187–192. doi: 10.1038/nature14259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisz M, Winzi M, Bisgaard HC, Serup P (2012) EVEN-SKIPPED HOMEOBOX 1 controls human ES cell differentiation by directly repressing GOOSECOID expression. Dev Biol 362:94–103. doi: 10.1016/j.ydbio.2011.11.017 [DOI] [PubMed] [Google Scholar]
- Katanaev V, Buestorf S (2009) Frizzled proteins are bona fide G protein-coupled receptors. Nature Precedings 1–19 [Google Scholar]
- Kato M, Patel MS, Levasseur R et al. (2002) Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol 157:303–314. doi: 10.1083/jcb.200201089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara A, Wilm T, Solnica-Krezel L, Dawid IB (2000) Antagonistic role of vega1 and bozozok/dharma homeobox genes in organizer formation. Proc Natl Acad Sci U S A 97:12121–12126. doi: 10.1073/pnas.97.22.12121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller PJ, Schmidt AD, Wittbrodt J, Stelzer EHK (2008) Reconstruction of zebrafish early embryonic development by scanned light sheet microscopy. Science 322:1065–1069 [DOI] [PubMed] [Google Scholar]
- Keller R, Danilchik M (1988) Regional expression, pattern and timing of convergence and extension during gastrulation of Xenopus laevis. Development 103:193–209 [DOI] [PubMed] [Google Scholar]
- Keller R, Davidson L, Edlund A et al. (2000) Mechanisms of convergence and extension by cell intercalation. Philos Trans R Soc Lond B Biol Sci 355:897–922. doi: 10.1098/rstb.2000.0626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R, Shook D (2004) Gastrulation in amphibians In: Stern CD (ed) Gastrulation: from cells to embryo. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 171–203 [Google Scholar]
- Keller R, Tibbetts P (1989) Mediolateral cell intercalation in the dorsal, axial mesoderm of Xenopus laevis. Dev Biol 131:539–549 [DOI] [PubMed] [Google Scholar]
- Keller RE (1986) The cellular basis of amphibian gastrulation. Dev Biol (NY) 2:241–327 [DOI] [PubMed] [Google Scholar]
- Keller RE (1975) Vital dye mapping of the gastrula and neurula of Xenopus laevis: I. Prospective areas and morphogenetic movements of the superficial layer. Dev Biol 42(2):222–241 [DOI] [PubMed] [Google Scholar]
- Keller RE, Danilchik M, Gimlich R, Shih J (1985) The function and mechanism of convergent extension during gastrulation of Xenopus laevis. J Embryol Exp Morphol 89(Suppl):185–209 [PubMed] [Google Scholar]
- Kelly C, Chin AJ, Leatherman JL et al. (2000) Maternally controlled (beta)-catenin-mediated signaling is required for organizer formation in the zebrafish. Development 127:3899–3911 [DOI] [PubMed] [Google Scholar]
- Kelly G, Greenstein P, Erezyilmaz D, Moon R (1995a) Zebrafish wnt8 and wnt8b share a common activity but are involved in distinct developmental pathways. Development 121:1787–1799 [DOI] [PubMed] [Google Scholar]
- Kelly GM, Erezyilmaz DF, Moon RT (1995b) Induction of a secondary embryonic axis in zebrafish occurs following the overexpression of beta-catenin. Mech Dev 53:261–273 [DOI] [PubMed] [Google Scholar]
- Kelly OG, Pinson KI, Skarnes WC (2004) The Wnt co-receptors Lrp5 and Lrp6 are essential for gastrulation in mice. Development 131:2803–2815. doi: 10.1242/dev.01137 [DOI] [PubMed] [Google Scholar]
- Kelly SJ (1977) Studies of the developmental potential of 4- and 8-cell stage mouse blastomeres. J Exp Zool 200:365–376. doi: 10.1002/jez.1402000307 [DOI] [PubMed] [Google Scholar]
- Kemler R, Hierholzer A, Kanzler B et al. (2004) Stabilization of beta-catenin in the mouse zygote leads to premature epithelial-mesenchymal transition in the epiblast. Development 131:5817–5824. doi: 10.1242/dev.01458 [DOI] [PubMed] [Google Scholar]
- Kemp C, Willems E, Abdo S et al. (2005) Expression of all Wnt genes and their secreted antagonists during mouse blastocyst and postimplantation development. Dev Dyn 233:1064–1075. doi: 10.1002/dvdy.20408 [DOI] [PubMed] [Google Scholar]
- Khokha M, Yeh J, Grammer T, Harland R (2005) Depletion of three BMP antagonists from Spemann’s organizer leads to a catastrophic loss of dorsal structures. Dev Cell 8:401–411 [DOI] [PubMed] [Google Scholar]
- Kibar Z, Torban E, McDearmid JR et al. (2007) Mutations in VANGL1 associated with neural-tube defects. N Engl J Med 356:1432–1437. doi: 10.1056/NEJMoa060651 [DOI] [PubMed] [Google Scholar]
- Kibar Z, Vogan KJ, Groulx N et al. (2001) Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat Genet 28:251–255. doi: 10.1038/90081 [DOI] [PubMed] [Google Scholar]
- Kiecker C, Niehrs C (2001) A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development 128:4189–4201 [DOI] [PubMed] [Google Scholar]
- Kikkawa M, Takano K, Shinagawa A (1996) Location and behavior of dorsal determinants during first cell cycle in Xenopus eggs. Development 122:3687–3696 [DOI] [PubMed] [Google Scholar]
- Kilian B, Mansukoski H, Barbosa F et al. (2003) The role of Ppt/Wnt5 in regulating cell shape and movement during zebrafish gastrulation. Mech Dev 120:467–476 [DOI] [PubMed] [Google Scholar]
- Kim CH, Oda T, Itoh M et al. (2000) Repressor activity of Headless/Tcf3 is essential for vertebrate head formation. Nature 407:913–916. doi: 10.1038/35038097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GH, Her JH, Han JK (2008) Ryk cooperates with Frizzled 7 to promote Wnt11-mediated endocytosis and is essential for Xenopus laevis convergent extension movements. J Cell Biol 182:1073–1082. doi: 10.1083/jcb.200710188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SE, Huang H, Zhao M et al. (2013) Wnt stabilization of beta-catenin reveals principles for morphogen receptor-scaffold assemblies. Science 340:867–870. doi: 10.1126/science.1232389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Warga RM, Schilling TF (1990) Origin and organization of the zebrafish fate map. Development 108:581–594 [DOI] [PubMed] [Google Scholar]
- Kimura C, Yoshinaga K, Tian E et al. (2000) Visceral endoderm mediates forebrain development by suppressing posteriorizing signals. Dev Biol 225:304–321. doi: 10.1006/dbio.2000.9835 [DOI] [PubMed] [Google Scholar]
- Kimura-Yoshida C, Nakano H, Okamura D et al. (2005) Canonical Wnt signaling and its antagonist regulate anterior-posterior axis polarization by guiding cell migration in mouse visceral endoderm. Dev Cell 9:639–650. doi: 10.1016/j.devcel.2005.09.011 [DOI] [PubMed] [Google Scholar]
- Kinder SJ, Tsang TE, Wakamiya M et al. (2001) The organizer of the mouse gastrula is composed of a dynamic population of progenitor cells for the axial mesoderm. Development 128:3623–3634 [DOI] [PubMed] [Google Scholar]
- Kinoshita N, Iioka H, Miyakoshi A, Ueno N (2003) PKC delta is essential for Dishevelled function in a noncanonical Wnt pathway that regulates Xenopus convergent extension movements. Genes Dev 17:1663–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner M, Gerhart JC, Hara K, Ubbels GA (1980) Initiation of the cell cycle and establishment of bilateral symmetry in Xenopus eggs. Symp Soc Dev Biol 38:187–215 [Google Scholar]
- Kishimoto Y, Lee KH, Zon L, Hammerschmidt M, Schulte-Merker S (1997) The molecular nature of zebrafish swirl: BMP2 function is essential during early dorsoventral patterning. Development 124:4457–4466 [DOI] [PubMed] [Google Scholar]
- Klein SL, Moody SA (2015) Early neural ectodermal genes are activated by siamois and twin during blastula stages. Genesis 53:308–320. doi: 10.1002/dvg.22854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloc M (2009) Teachings from the egg: new and unexpected functions of RNAs. Mol Reprod Dev 76:922–932. doi: 10.1002/mrd.21043 [DOI] [PubMed] [Google Scholar]
- Knoetgen H, Viebahn C, Kessel M (1999) Head induction in the chick by primitive endoderm of mammalian, but not avian origin. Development 126:815–825 [DOI] [PubMed] [Google Scholar]
- Kochav S, Eyal-Giladi H (1971) Bilateral symmetry in chick embryo determination by gravity. Science 171:1027–1029 [DOI] [PubMed] [Google Scholar]
- Kofron M, Birsoy B, Houston D et al. (2007) Wnt11/beta-catenin signaling in both oocytes and early embryos acts through LRP6-mediated regulation of axin. Development 134:503–513. doi: 10.1242/dev.02739 [DOI] [PubMed] [Google Scholar]
- Kohn AD, Moon RT (2005) Wnt and calcium signaling: β-catenin-independent pathways. Cell Calcium 38:439–446. doi: 10.1016/j.ceca.2005.06.022 [DOI] [PubMed] [Google Scholar]
- Koos DS, Ho RK (1999) The nieuwkoid/dharma homeobox gene is essential for bmp2b repression in the zebrafish pregastrula. Dev Biol 215(2):190–207 [DOI] [PubMed] [Google Scholar]
- Kozmik Z, Holland LZ, Schubert M et al. (2001) Characterization of amphioxusamphivent, an evolutionarily conserved marker for chordate ventral mesoderm. Genesis 29:172–179. doi: 10.1002/gene.1021 [DOI] [PubMed] [Google Scholar]
- Kraft B, Berger CD, Wallkamm V et al. (2012) Wnt-11 and Fz7 reduce cell adhesion in convergent extension by sequestration of PAPC and C-cadherin. J Cell Biol 198:695–709. doi: 10.1083/jcb.201110076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramps T, Peter O, Brunner E et al. (2002) Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell 109:47–60 [DOI] [PubMed] [Google Scholar]
- Krasnow R, Adler P (1994) A single frizzled protein has a dual function in tissue polarity. Development 120:1883–1893 [DOI] [PubMed] [Google Scholar]
- Kreuger J, Perez L, Giraldez AJ, Cohen SM (2004) Opposing activities of Dally-like glypican at high and low levels of Wingless morphogen activity. Dev Cell 7:503–512. doi: 10.1016/j.devcel.2004.08.005 [DOI] [PubMed] [Google Scholar]
- Ku M, Melton DA (1993) Xwnt-11: a maternally expressed Xenopus wnt gene. Development 119:1161–1173 [DOI] [PubMed] [Google Scholar]
- Kuhl M, Sheldahl LC, Malbon CC, Moon RT (2000) Ca(2+)/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J Biol Chem 275:12701–12711 [DOI] [PubMed] [Google Scholar]
- Kuroda H, Wessely O, De Robertis EM (2004) Neural induction in Xenopus: requirement for ectodermal and endomesodermal signals via Chordin, Noggin, beta-Catenin, and Cerberus. PLoS Biol 2:E92. doi: 10.1371/journal.pbio.0020092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth T, Hausen P (2000) Bottle cell formation in relation to mesodermal patterning in the Xenopus embryo. Mech Dev 97:117–131. doi: 10.1016/S0925-4773(00)00428-7 [DOI] [PubMed] [Google Scholar]
- Kwon H-J, Bhat N, Sweet EM et al. (2010) Identification of early requirements for preplacodal ectoderm and sensory organ development. PLoS Genet 6:e1001133. doi: 10.1371/journal.pgen.1001133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladher R, Mohun TJ, Smith JC, Snape AM (1996) Xom: a Xenopus homeobox gene that mediates the early effects of BMP-4. Development 122:2385–2394 [DOI] [PubMed] [Google Scholar]
- Lamb TM, Knecht AK, Smith WC et al. (1993) Neural induction by the secreted polypeptide noggin. Science 262:713–718 [DOI] [PubMed] [Google Scholar]
- Larabell C, Rowning B, Wells J et al. (1996) Confocal microscopy analysis of living Xenopus eggs and the mechanism of cortical rotation. Development 122:1281–1289 [DOI] [PubMed] [Google Scholar]
- Larsen WJ, Schoenwolf GC, Bleyl SB et al. (2009) Larsen’s human embryology. Churchill Livingstone, Philadelphia, PA [Google Scholar]
- Lawrence P, Casal J, Struhl G (2002) Towards a model of the organisation of planar polarity and pattern in the Drosophila abdomen. Development 129:2749–2760 [DOI] [PubMed] [Google Scholar]
- Lawson KA, Meneses JJ, Pedersen RA (1991) Clonal analysis of epiblast fate during germ layer formation in the mouse embryo. Development 113:891–911 [DOI] [PubMed] [Google Scholar]
- Lawson KA, Pedersen RA (1992) Clonal analysis of cell fate during gastrulation and early neurulation in the mouse. Ciba Found Symp 165:3–21, discussion 21–26 [DOI] [PubMed] [Google Scholar]
- Lee E, Salic A, Kruger R et al. (2003) The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol 1:E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HX, Ambrosio AL, Reversade B, De Robertis EM (2006) Embryonic dorsal-ventral signaling: secreted frizzled-related proteins as inhibitors of tolloid proteinases. Cell 124:147–159. doi: 10.1016/j.cell.2005.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MA, Heasman J, Whitman M (2001) Timing of endogenous activin-like signals and regional specification of the Xenopus embryo. Development 128:2939–2952 [DOI] [PubMed] [Google Scholar]
- Lekven A, Thorpe C, Waxman J, Moon R (2001) Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev Cell 1:103–114 [DOI] [PubMed] [Google Scholar]
- Lele Z, Nowak M, Hammerschmidt M (2001) Zebrafish admp is required to restrict the size of the organizer and to promote posterior and ventral development. Dev Dyn 222:681–687. doi: 10.1002/dvdy.1222 [DOI] [PubMed] [Google Scholar]
- Lemaire P, Garrett N, Gurdon JB (1995) Expression cloning of Siamois, a Xenopus homeobox gene expressed in dorsal-vegetal cells of blastulae and able to induce a complete secondary axis. Cell 81:85–94 [DOI] [PubMed] [Google Scholar]
- Leung T, Bischof J, Söll I et al. (2003a) bozozok directly represses bmp2b transcription and mediates the earliest dorsoventral asymmetry of bmp2b expression in zebrafish. Development 130:3639–3649 [DOI] [PubMed] [Google Scholar]
- Leung T, Söll I, Arnold SJ et al. (2003b) Direct binding of Lef1 to sites in the boz promoter may mediate pre-midblastula-transition activation of boz expression. Dev Dyn 228:424–432. doi: 10.1002/dvdy.10408 [DOI] [PubMed] [Google Scholar]
- Lewis SL, Khoo P-L, Andrea De Young R et al. (2007) Genetic interaction of Gsc and Dkk1 in head morphogenesis of the mouse. Mech Dev 124:157–165. doi: 10.1016/j.mod.2006.10.001 [DOI] [PubMed] [Google Scholar]
- Lewis SL, Khoo PL, De Young RA et al. (2008) Dkk1 and Wnt3 interact to control head morphogenesis in the mouse. Development 135:1791–1801. doi: 10.1242/dev.018853 [DOI] [PubMed] [Google Scholar]
- Leyns L, Bouwmeester T, Kim SH et al. (1997) Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell 88:747–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S, Kumari P, Gilligan P et al. (2012) Dorsal activity of maternal squint is mediated by a non-coding function of the RNA. Development 139:2903–2915. doi: 10.1242/dev.077081 [DOI] [PubMed] [Google Scholar]
- Linker C, Stern CD (2004) Neural induction requires BMP inhibition only as a late step, and involves signals other than FGF and Wnt antagonists. Development 131:5671–5681. doi: 10.1242/dev.01445 [DOI] [PubMed] [Google Scholar]
- Linnemannstöns K, Ripp C, Honemann-Capito M et al. (2014) The PTK7-related transmembrane proteins off-track and off-track 2 are co-receptors for Drosophila Wnt2 required for male fertility. PLoS Genet 10:e1004443. doi: 10.1371/journal.pgen.1004443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little SC, Mullins MC (2006) Extracellular modulation of BMP activity in patterning the dorsoventral axis. Birth Defects Res C Embryo Today 78:224–242. doi: 10.1002/bdrc.20079 [DOI] [PubMed] [Google Scholar]
- Liu C, Kato Y, Zhang Z et al. (1999a) beta-Trcp couples beta-catenin phosphorylation-degradation and regulates Xenopus axis formation. Proc Natl Acad Sci U S A 96:6273–6278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Li Y, Semenov M et al. (2002) Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108:837–847 [DOI] [PubMed] [Google Scholar]
- Liu F, van den Broek O, Destrée O, Hoppler S (2005) Distinct roles for Xenopus Tcf/Lef genes in mediating specific responses to Wnt/beta-catenin signalling in mesoderm development. Development 132:5375–5385. doi: 10.1242/dev.02152 [DOI] [PubMed] [Google Scholar]
- Liu G, Bafico A, Harris VK, Aaronson SA (2003) A novel mechanism for Wnt activation of canonical signaling through the LRP6 receptor. Mol Cell Biol 23:5825–5835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ et al. (1999b) Requirement for Wnt3 in vertebrate axis formation. Nat Genet 22:361–365. doi: 10.1038/11932 [DOI] [PubMed] [Google Scholar]
- Long WL (1983) The role of the yolk syncytial layer in determination of the plane of bilateral symmetry in the rainbow trout, Salmo gairdneri Richardson. J Exp Zool 228:91–97 [Google Scholar]
- Lu F-I, Thisse C, Thisse B (2011) Identification and mechanism of regulation of the zebrafish dorsal determinant. Proc Natl Acad Sci U S A 108:15876–15880. doi: 10.1073/pnas.1106801108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Borchers AGM, Jolicoeur C et al. (2004) PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature 430:93–98. doi: 10.1038/nature02677 [DOI] [PubMed] [Google Scholar]
- Lustig KD, Kroll KL, Sun EE, Kirschner MW (1996) Expression cloning of a Xenopus T-related gene (Xombi) involved in mesodermal patterning and blastopore lip formation. Development 122:4001–4012 [DOI] [PubMed] [Google Scholar]
- Luther WH (1935) Entwicklungsphysiologische Untersuchungen am Forellenkeim: die Rolle des Organisationszentrums bei der Entstehung der Embryonalanlage. Biol Zentralbl 55:114–137 [Google Scholar]
- Lutz H (1949) Sur la production expérimentale de la polyembryone et de la monstruosité double chez les oiseaux. Arch Anat Microsc Morphol Exp 39:79–144 [Google Scholar]
- Lyman Gingerich J, Westfall TA, Slusarski DC, Pelegri F (2005) hecate, a zebrafish maternal effect gene, affects dorsal organizer induction and intracellular calcium transient frequency. Dev Biol 286:427–439. doi: 10.1016/j.ydbio.2005.07.031 [DOI] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X (2009) Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 17:9–26. doi: 10.1016/j.devcel.2009.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malacinski GM, Benford H, Chung HM (1975) Association of an ultraviolet irradiation sensitive cytoplasmic localization with the future dorsal side of the amphibian egg. J Exp Zool 191:97–110. doi: 10.1002/jez.1401910110 [DOI] [PubMed] [Google Scholar]
- Manes ME, Elinson RP (1980) Ultraviolet light inhibits grey crescent formation on the frog egg. Wilhelm Roux’s Arch Dev Biol 189:73–76 [DOI] [PubMed] [Google Scholar]
- Manes ME, Elinson RP, Barbieri FD (1978) Formation of the amphibian grey crescent: effects of colchicine and cytochalasin B. Wilhelm Roux’s Arch Dev Biol 185:99–104 [DOI] [PubMed] [Google Scholar]
- Mao J, Wang J, Liu B et al. (2001) Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol Cell 7:801–809 [DOI] [PubMed] [Google Scholar]
- Marchal L, Luxardi G, Thomé V, Kodjabachian L (2009) BMP inhibition initiates neural induction via FGF signaling and Zic genes. Proc Natl Acad Sci U S A 106:17437–17442. doi: 10.1073/pnas.0906352106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S et al. (2003) Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A 100:3299–3304. doi: 10.1073/pnas.0434590100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marikawa Y, Elinson RP (1999) Relationship of vegetal cortical dorsal factors in the Xenopus egg with the Wnt/beta-catenin signaling pathway. Mech Dev 89:93–102 [DOI] [PubMed] [Google Scholar]
- Marikawa Y, Li Y, Elinson RP (1997) Dorsal determinants in the Xenopus egg are firmly associated with the vegetal cortex and behave like activators of the Wnt pathway. Dev Biol 191:69–79. doi: 10.1006/dbio.1997.8710 [DOI] [PubMed] [Google Scholar]
- Marlow F, Topczewski J, Sepich D, Solnica-Krezel L (2002) Zebrafish Rho kinase 2 acts downstream of Wnt11 to mediate cell polarity and effective convergence and extension movements. Curr Biol 12:876–884 [DOI] [PubMed] [Google Scholar]
- Marrari Y, Clarke EJ, Rouvière C, Houliston E (2003) Analysis of microtubule movement on isolated Xenopus egg cortices provides evidence that the cortical rotation involves dynein as well as Kinesin Related Proteins and is regulated by local microtubule polymerisation. Dev Biol 257:55–70 [DOI] [PubMed] [Google Scholar]
- Marrari Y, Rouvière C, Houliston E (2004) Complementary roles for dynein and kinesins in the Xenopus egg cortical rotation. Dev Biol 271:38–48. doi: 10.1016/j.ydbio.2004.03.018 [DOI] [PubMed] [Google Scholar]
- Marrari Y, Terasaki M, Arrowsmith V, Houliston E (2000) Local inhibition of cortical rotation in Xenopus eggs by an anti-KRP antibody. Dev Biol 224:250–262. doi: 10.1006/dbio.2000.9773 [DOI] [PubMed] [Google Scholar]
- Martello G, Zacchigna L, Inui M et al. (2007) MicroRNA control of Nodal signalling. Nature 449:183–188. doi: 10.1038/nature06100 [DOI] [PubMed] [Google Scholar]
- Martindale MQ (2005) The evolution of metazoan axial properties. Nat Rev Genet 6:917–927. doi: 10.1038/nrg1725 [DOI] [PubMed] [Google Scholar]
- Martinez Barbera JP, Clements M, Thomas P et al. (2000) The homeobox gene Hex is required in definitive endodermal tissues for normal forebrain, liver and thyroid formation. Development 127:2433–2445 [DOI] [PubMed] [Google Scholar]
- Martyn U, Schulte-Merker S (2003) The ventralized ogon mutant phenotype is caused by a mutation in the zebrafish homologue of Sizzled, a secreted Frizzled-related protein. Dev Biol 260:58–67 [DOI] [PubMed] [Google Scholar]
- Marvin MJ, Di Rocco G, Gardiner A et al. (2001) Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev 15:316–327. doi: 10.1101/gad.855501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maung S, Jenny A (2011) Planar cell polarity in Drosophila. Organogenesis 7(3):165–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken KW, Catá EM, Crawford CM et al. (2014) Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 516:400–404. doi: 10.1038/nature13863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrew LL, Hoppler S, Moon RT (1997) Wnt and FGF pathways cooperatively pattern anteroposterior neural ectoderm in Xenopus. Mech Dev 69:105–114 [DOI] [PubMed] [Google Scholar]
- McMahon AP, Moon RT (1989) Ectopic expression of the proto-oncogene int-1 in Xenopus embryos leads to duplication of the embryonic axis. Cell 58:1075–1084 [DOI] [PubMed] [Google Scholar]
- Mei W, Jin Z, Lai F et al. (2013) Maternal Dead-End1 is required for vegetal cortical microtubule assembly during Xenopus axis specification. Development 140:2334–2344. doi: 10.1242/dev.094748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt H (2012) Turing’s theory of morphogenesis of 1952 and the subsequent discovery of the crucial role of local self-enhancement and long-range inhibition. Interface Focus 2:407–416. doi: 10.1098/rsfs.2011.0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melby AE, Beach C, Mullins M, Kimelman D (2000) Patterning the early zebrafish by the opposing actions of bozozok and vox/vent. Dev Biol 224(2):275–285 [DOI] [PubMed] [Google Scholar]
- Melton DA (1987) Translocation of a localized maternal mRNA to the vegetal pole of Xenopus oocytes. Nature 328:80–82. doi: 10.1038/328080a0 [DOI] [PubMed] [Google Scholar]
- Meneghini M, Ishitani T, Carter J et al. (1999) MAP kinase and Wnt pathways converge to downregulate an HMG-domain repressor in Caenorhabditis elegans. Nature 399:793–797 [DOI] [PubMed] [Google Scholar]
- Merrill BJ, Pasolli HA, Polak L et al. (2004) Tcf3: a transcriptional regulator of axis induction in the early embryo. Development 131:263–274. doi: 10.1242/dev.00935 [DOI] [PubMed] [Google Scholar]
- Mesnard D, Guzman-Ayala M, Constam DB (2006) Nodal specifies embryonic visceral endoderm and sustains pluripotent cells in the epiblast before overt axial patterning. Development 133:2497–2505. doi: 10.1242/dev.02413 [DOI] [PubMed] [Google Scholar]
- Messenger NJ, Kabitschke C, Andrews R et al. (2005) Functional specificity of the Xenopus T-domain protein Brachyury is conferred by its ability to interact with Smad1. Dev Cell 8:599–610. doi: 10.1016/j.devcel.2005.03.001 [DOI] [PubMed] [Google Scholar]
- Metcalfe C, Bienz M (2011) Inhibition of GSK3 by Wnt signalling—two contrasting models. J Cell Sci 124:3537–3544. doi: 10.1242/jcs.091991 [DOI] [PubMed] [Google Scholar]
- Mieszczanek J, de la Roche M, Bienz M (2008) A role of Pygopus as an anti-repressor in facilitating Wnt-dependent transcription. Proc Natl Acad Sci U S A 105:19324–19329. doi: 10.1073/pnas.0806098105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R (2006) Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol 4:e115. doi: 10.1371/journal.pbio.0040115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J, Rowning B, Larabell C et al. (1999) Establishment of the dorsal-ventral axis in Xenopus embryos coincides with the dorsal enrichment of dishevelled that is dependent on cortical rotation. J Cell Biol 146:427–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz B (1964) Gene expression in the morula stage of mouse embryos, as observed during development of t12/t12 lethal mutants in vitro. J Exp Zool 157:267–272 [DOI] [PubMed] [Google Scholar]
- Mintzer KA, Lee MA, Runke G, Trout J, Whitman M, Mullins MC (2001) Lost-a-fin encodes a type I BMP receptor, Alk8, acting maternally and zygotically in dorsoventral pattern formation. Development 128:859–869 [DOI] [PubMed] [Google Scholar]
- Miura S, Mishina Y (2007) The DVE changes distal epiblast fate from definitive endoderm to neurectoderm by antagonizing nodal signaling. Dev Dyn 236:1602–1610. doi: 10.1002/dvdy.21166 [DOI] [PubMed] [Google Scholar]
- Mizuno T, Yamaha E, Kuroiwa A, Takeda H (1999) Removal of vegetal yolk causes dorsal deficencies and impairs dorsal-inducing ability of the yolk cell in zebrafish. Mech Dev 81:51–63 [DOI] [PubMed] [Google Scholar]
- Mohamed OA, Clarke HJ, Dufort D (2004) Beta-catenin signaling marks the prospective site of primitive streak formation in the mouse embryo. Dev Dyn 231:416–424. doi: 10.1002/dvdy.20135 [DOI] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M et al. (1996) XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell 86:391–399 [DOI] [PubMed] [Google Scholar]
- Montcouquiol M, Rachel RA, Lanford PJ et al. (2003) Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature 423:173–177. doi: 10.1038/nature01618 [DOI] [PubMed] [Google Scholar]
- Montero JA (2005) Shield formation at the onset of zebrafish gastrulation. Development 132:1187–1198. doi: 10.1242/dev.01667 [DOI] [PubMed] [Google Scholar]
- Moon RT, Campbell RM, Christian JL et al. (1993) Xwnt-5A: a maternal Wnt that affects morphogenetic movements after overexpression in embryos of Xenopus laevis. Development 119:97–111 [DOI] [PubMed] [Google Scholar]
- Moos M, Wang S, Krinks M (1995) Anti-dorsalizing morphogenetic protein is a novel TGF-beta homolog expressed in the Spemann organizer. Development 121:4293–4301 [DOI] [PubMed] [Google Scholar]
- Morgan TH, Boring AM (1903) The relation of the first plane of cleavage and the grey crescent to the median plane of the embryo of the frog. Wilhelm Roux’ Arch Entwicklungsmech 16:680–690. doi: 10.1007/BF02301271 [DOI] [Google Scholar]
- Morgan TH, Tsuda U (1894) The orientation of the frog’s egg. Q J Microsc Sci 35:373–405 [Google Scholar]
- Morkel M, Huelsken J, Wakamiya M et al. (2003) Beta-catenin regulates Cripto- and Wnt3-dependent gene expression programs in mouse axis and mesoderm formation. Development 130:6283–6294. doi: 10.1242/dev.00859 [DOI] [PubMed] [Google Scholar]
- Morris SA, Grewal S, Barrios F et al. (2012a) Dynamics of anterior-posterior axis formation in the developing mouse embryo. Nat Commun 3:673. doi: 10.1038/ncomms1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SA, Guo Y, Zernicka-Goetz M (2012b) Developmental plasticity is bound by pluripotency and the Fgf and Wnt signaling pathways. Cell Rep 2:756–765. doi: 10.1016/j.celrep.2012.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SA, Teo RTY, Li H et al. (2010) Origin and formation of the first two distinct cell types of the inner cell mass in the mouse embryo. Proc Natl Acad Sci U S A 107:6364–6369. doi: 10.1073/pnas.0915063107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C et al. (2001) Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell 1:423–434 [DOI] [PubMed] [Google Scholar]
- Murdoch JN, Doudney K, Paternotte C et al. (2001) Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification. Hum Mol Genet 10:2593–2601 [DOI] [PubMed] [Google Scholar]
- Myers DC, Sepich DS, Solnica-Krezel L (2002) Bmp activity gradient regulates convergent extension during zebrafish gastrulation. Dev Biol 243:81–98. doi: 10.1006/dbio.2001.0523 [DOI] [PubMed] [Google Scholar]
- Na J, Lykke-Andersen K, Torres-Padilla M-E, Zernicka-Goetz M (2007) Dishevelled proteins regulate cell adhesion in mouse blastocyst and serve to monitor changes in Wnt signaling. Dev Biol 302:40–49. doi: 10.1016/j.ydbio.2006.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai H, Sezaki M, Kakiguchi K, Nakaya Y, Lee HC, Ladher R, Sasanami T, Han JY, Yonemura S and Sheng G (2015) Cellular analysis of cleavage-stage chick embryos reveals hidden conservation in vertebrate early development. Development 142, 1279–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel M, Winklbauer R (1999) Establishment of substratum polarity in the blastocoel roof of the Xenopus embryo. Development 126:1975–1984 [DOI] [PubMed] [Google Scholar]
- Nakamura T, Mine N, Nakaguchi E et al. (2006) Generation of robust left-right asymmetry in the mouse embryo requires a self-enhancement and lateral-inhibition system. Dev Cell 11:495–504. doi: 10.1016/j.devcel.2006.08.002 [DOI] [PubMed] [Google Scholar]
- Nakayama T, Snyder MA, Grewal SS et al. (1998) Xenopus Smad8 acts downstream of BMP-4 to modulate its activity during vertebrate embryonic patterning. Development 125:857–867 [DOI] [PubMed] [Google Scholar]
- Newport G (1851) On the impregnation of the Ovum in the Amphibia. (First Series). Philos Trans R Soc Lond B Biol Sci 141:169–242 [Google Scholar]
- Newport G (1854) Researches on the impregnation of the Ovum in the Amphibia; and on the early stages of development of the Embryo. (Third Series). Philos Trans R Soc Lond B Biol Sci 144:229–244 [Google Scholar]
- Nguyen VH, Schmid B, Trout J et al. (1998) Ventral and lateral regions of the zebrafish gastrula, including the neural crest progenitors, are established by a bmp2b/swirl pathway of genes. Dev Biol 199:93–110. doi: 10.1006/dbio.1998.8927 [DOI] [PubMed] [Google Scholar]
- Niehrs C, Keller R, Cho KW, De Robertis EM (1993) The homeobox gene goosecoid controls cell migration in Xenopus embryos. Cell 72:491–503 [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P, Nigtevecht G (1954) Neural activation and transformation in explants of competent ectoderm under the influence of fragments of anterior notochord in Urodeles. J Embryol Exp Morphol 2:175–193 [Google Scholar]
- Nieuwkoop PD (1999) The neural induction process; its morphogenetic aspects. Int J Dev Biol 43:615–623 [PubMed] [Google Scholar]
- Nieuwkoop PD (1952) Activation and organization of the central nervous system in amphibians. Part III. Synthesis of a new working hypothesis. J Exp Zool 120:83–108 [Google Scholar]
- Nieuwkoop PD (1967) The “organization centre”: II. Field phenomena, their origin and significance. Acta Biotheor 17:151–177 [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Florschütz PA (1950) Quelques caractères spéciaux de la gastrulation et de la neurulation de l’oeuf de Xenopus laevis, Daud, et de quelques autres Anoures. Arch Biol 61:113–150 [Google Scholar]
- Nieuwkoop PD, Sutasurya LA (1979) Primordial germ cells in the chordates: embryogenesis and phylogenesis
- Nikaido M, Tada M, Saji T, Ueno N (1997) Conservation of BMP signaling in zebrafish mesoderm patterning. Mech Dev 61:75–88 [DOI] [PubMed] [Google Scholar]
- Ninomiya H, Elinson RP, Winklbauer R (2004) Antero-posterior tissue polarity links mesoderm convergent extension to axial patterning. Nature 430:364–367. doi: 10.1038/nature02620 [DOI] [PubMed] [Google Scholar]
- Nishimura T, Honda H, Takeichi M (2012) Planar cell polarity links axes of spatial dynamics in neural-tube closure. Cell 149:1084–1097. doi: 10.1016/j.cell.2012.04.021 [DOI] [PubMed] [Google Scholar]
- Nojima H, Rothhamel S, Shimizu T et al. (2010) Syntabulin, a motor protein linker, controls dorsal determination. Development 137:923–933. doi: 10.1242/dev.046425 [DOI] [PubMed] [Google Scholar]
- Nojima H, Shimizu T, Kim C-H et al. (2004) Genetic evidence for involvement of maternally derived Wnt canonical signaling in dorsal determination in zebrafish. Mech Dev 121:371–386. doi: 10.1016/j.mod.2004.02.003 [DOI] [PubMed] [Google Scholar]
- Nordstrom U, Jessell T, Edlund T (2002) Progressive induction of caudal neural character by graded Wnt signaling. Nat Neurosci 5:525–532 [DOI] [PubMed] [Google Scholar]
- Norris DP, Brennan J, Bikoff EK, Robertson EJ (2002) The Foxh1-dependent autoregulatory enhancer controls the level of Nodal signals in the mouse embryo. Development 129:3455–3468 [DOI] [PubMed] [Google Scholar]
- Nusse R, Varmus H (1982) Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 31:99–109 [DOI] [PubMed] [Google Scholar]
- O’Farrell PH, Stumpff J, Su TT (2004) Embryonic cleavage cycles: how is a mouse like a fly? Curr Biol 14:R35–R45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelgeschläger M, Kuroda H, Reversade B, De Robertis EM (2003) Chordin is required for the Spemann organizer transplantation phenomenon in Xenopus embryos. Dev Cell 4:219–230 [DOI] [PubMed] [Google Scholar]
- Ohkawara B, Yamamoto T, Tada M, Ueno N (2003) Role of glypican 4 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development 130:2129–2138 [DOI] [PubMed] [Google Scholar]
- Olson DJ, Oh D, Houston DW (2015) The dynamics of plus end polarization and microtubule assembly during Xenopus cortical rotation. Dev Biol 401:249–263. doi: 10.1016/j.ydbio.2015.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onichtchouk D, Chen YG, Dosch R et al. (1999) Silencing of TGF-beta signalling by the pseudoreceptor BAMBI. Nature 401:480–485. doi: 10.1038/46794 [DOI] [PubMed] [Google Scholar]
- Onichtchouk D, Gawantka V, Dosch R et al. (1996) The Xvent-2 homeobox gene is part of the BMP-4 signalling pathway controlling [correction of controling] dorsoventral patterning of Xenopus mesoderm. Development 122:3045–3053 [DOI] [PubMed] [Google Scholar]
- Oppenheimer JM (1934a) Experimental studies on the developing perch (Perca flavescens Mitchill). Exp Biol Med 31:1123–1124. doi: 10.3181/00379727-31-7465P [DOI] [Google Scholar]
- Oppenheimer JM (1934b) Experiments on early developing stages of fundulus. Proc Natl Acad Sci U S A 20:536–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada SI, Saijoh Y, Frisch A et al. (2000) Activin/nodal responsiveness and asymmetric expression of a Xenopus nodal-related gene converge on a FAST-regulated module in intron 1. Development 127:2503–2514 [DOI] [PubMed] [Google Scholar]
- Ozair MZ, Kintner C, Brivanlou AH (2012) Neural induction and early patterning in vertebrates. WIREs Dev Biol 2:479–498. doi: 10.1002/wdev.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panáková D, Sprong H, Marois E et al. (2005) Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature 435:58–65. doi: 10.1038/nature03504 [DOI] [PubMed] [Google Scholar]
- Papanayotou C, Benhaddou A, Camus A et al. (2014) A novel nodal enhancer dependent on pluri-potency factors and smad2/3 signaling conditions a regulatory switch during epiblast maturation. PLoS Biol 12:e1001890. doi: 10.1371/journal.pbio.1001890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkoff J, Brown A, Varmus H (1987) The int-1 proto-oncogene products are glycoproteins that appear to enter the secretory pathway. Mol Cell Biol 7:3978–3984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran M, Tam P (1995) Regionalisation of cell fate and morphogenetic movement of the mesoderm during mouse gastrulation. Dev Genet 17:16–28 [DOI] [PubMed] [Google Scholar]
- Parfitt D-E, Zernicka-Goetz M (2010) Epigenetic modification affecting expression of cell polarity and cell fate genes to regulate lineage specification in the early mouse embryo. Mol Biol Cell 21:2649–2660. doi: 10.1091/mbc.E10-01-0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker D, Jemison J, Cadigan K (2002) Pygopus, a nuclear PHD-finger protein required for Wingless signaling in Drosophila. Development 129:2565–2576 [DOI] [PubMed] [Google Scholar]
- Pedersen RA, Wu K, Bałakier H (1986) Origin of the inner cell mass in mouse embryos: cell lineage analysis by microinjection. Dev Biol 117:581–595 [DOI] [PubMed] [Google Scholar]
- Pelegri F, Maischein HM (1998) Function of zebrafish beta-catenin and TCF-3 in dorsoventral patterning. Mech Dev 77:63–74 [DOI] [PubMed] [Google Scholar]
- Pera EM, Ikeda A, Eivers E, De Robertis EM (2003) Integration of IGF, FGF, and anti-BMP signals via Smad1 phosphorylation in neural induction. Genes Dev 17:3023–3028. doi: 10.1101/gad.1153603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peradziryi H, Kaplan NA, Podleschny M et al. (2011) PTK7/Otk interacts with Wnts and inhibits canonical Wnt signalling. EMBO J 30:3729–3740. doi: 10.1038/emboj.2011.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea-Gomez A, Lawson KA, Rhinn M et al. (2001) Otx2 is required for visceral endoderm movement and for the restriction of posterior signals in the epiblast of the mouse embryo. Development 128:753–765 [DOI] [PubMed] [Google Scholar]
- Perea-Gomez A, Vella FDJ, Shawlot W et al. (2002) Nodal antagonists in the anterior visceral endoderm prevent the formation of multiple primitive streaks. Dev Cell 3:745–756 [DOI] [PubMed] [Google Scholar]
- Petersen CP, Reddien PW (2011) Polarized notum activation at wounds inhibits Wnt function to promote planarian head regeneration. Science 332:852–855. doi: 10.1126/science.1202143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao S, Lee S-H, Kim H et al. (2008) Direct inhibition of GSK3beta by the phosphorylated cytoplasmic domain of LRP6 in Wnt/beta-catenin signaling. PLoS One 3:e4046. doi: 10.1371/journal.pone.0004046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Leyns L et al. (1999) The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature 397:707–710. doi: 10.1038/17820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Sasai Y, Lu B, De Robertis EM (1996) Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell 86:589–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepenburg O, Grimmer D, Williams PH, Smith JC (2004) Activin redux: specification of mesodermal pattern in Xenopus by graded concentrations of endogenous activin B. Development 131:4977–4986. doi: 10.1242/dev.01323 [DOI] [PubMed] [Google Scholar]
- Pinho S, Simonsson PR, Trevers KE et al. (2011) Distinct steps of neural induction revealed by Asterix, Obelix and TrkC, genes induced by different signals from the organizer. PLoS One 6:e19157. doi: 10.1371/journal.pone.0019157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowska-Nitsche K, Perea-Gomez A, Haraguchi S, Zernicka-Goetz M (2005) Four-cell stage mouse blastomeres have different developmental properties. Development 132:479–490. doi: 10.1242/dev.01602 [DOI] [PubMed] [Google Scholar]
- Plachta N, Bollenbach T, Pease S, Fraser SE (2011) Oct4 kinetics predict cell lineage patterning in the early mammalian embryo. Nat Cell Biol 13:117–123 [DOI] [PubMed] [Google Scholar]
- Plouhinec J-L, Zakin L, Moriyama Y, De Robertis EM (2013) Chordin forms a self-organizing morphogen gradient in the extracellular space between ectoderm and mesoderm in the Xenopus embryo. Proc Natl Acad Sci U S A 110:20372–20379. doi: 10.1073/pnas.1319745110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukrop T, Gradl D, Henningfeld KA et al. (2001) Identification of two regulatory elements within the high mobility group box transcription factor XTCF-4. J Biol Chem 276:8968–8978. doi: 10.1074/jbc.M007533200 [DOI] [PubMed] [Google Scholar]
- Rankin SA, Kormish J, Kofron M et al. (2011) A gene regulatory network controlling hhex transcription in the anterior endoderm of the organizer. Dev Biol 351:297–310. doi: 10.1016/j.ydbio.2010.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch G, Hammerschmidt M, Blader P et al. (1997) Wnt5 is required for tail formation in the zebrafish embryo. Cold Spring Harb Symp Quant Biol 62:227–234 [PubMed] [Google Scholar]
- Rawat VPS, Arseni N, Ahmed F et al. (2010) The vent-like homeobox gene VENTX promotes human myeloid differentiation and is highly expressed in acute myeloid leukemia. Proc Natl Acad Sci U S A 107:16946–16951. doi: 10.1073/pnas.1001878107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebagliati MR, Toyama R, Haffter P, Dawid IB (1998) cyclops encodes a nodal-related factor involved in midline signaling. Proc Natl Acad Sci U S A 95:9932–9937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebagliati MR, Weeks DL, Harvey RP, Melton DA (1985) Identification and cloning of localized maternal RNAs from Xenopus eggs. Cell 42:769–777 [DOI] [PubMed] [Google Scholar]
- Reversade B, De Robertis EM (2005) Regulation of ADMP and BMP2/4/7 at opposite embryonic poles generates a self-regulating morphogenetic field. Cell 123:1147–1160. doi: 10.1016/j.cell.2005.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reversade B, Kuroda H, Lee H et al. (2005) Depletion of Bmp2, Bmp4, Bmp7 and Spemann organizer signals induces massive brain formation in Xenopus embryos. Development 132:3381–3392. doi: 10.1242/dev.01901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex M, Hilton E, Old R (2002) Multiple interactions between maternally-activated signalling pathways control Xenopus nodal-related genes. Int J Dev Biol 46:217–226 [PubMed] [Google Scholar]
- Rhinn M, Dierich A, Shawlot W et al. (1998) Sequential roles for Otx2 in visceral endoderm and neuroectoderm for forebrain and midbrain induction and specification. Development 125:845–856 [DOI] [PubMed] [Google Scholar]
- Rivera-Pérez JA, Mager J, Magnuson T (2003) Dynamic morphogenetic events characterize the mouse visceral endoderm. Dev Biol 261:470–487 [DOI] [PubMed] [Google Scholar]
- Rodriguez TA (2005) Induction and migration of the anterior visceral endoderm is regulated by the extra-embryonic ectoderm. Development 132:2513–2520. doi: 10.1242/dev.01847 [DOI] [PubMed] [Google Scholar]
- Roël G, Hamilton FS, Gent Y et al. (2002) Lef-1 and Tcf-3 transcription factors mediate tissue-specific Wnt signaling during Xenopus development. Curr Biol 12:1941–1945 [DOI] [PubMed] [Google Scholar]
- Roose J, Molenaar M, Peterson J et al. (1998) The Xenopus Wnt effector XTcf-3 interacts with Groucho-related transcriptional repressors. Nature 395:608–612 [DOI] [PubMed] [Google Scholar]
- Rosenquist TA, Martin GR (1995) Visceral endoderm-1 (VE-1): an antigen marker that distinguishes anterior from posterior embryonic visceral endoderm in the early post-implantation mouse embryo. Mech Dev 49:117–121 [DOI] [PubMed] [Google Scholar]
- Rothbächer U, Laurent MN, Deardorff MA et al. (2000) Dishevelled phosphorylation, subcellular localization and multimerization regulate its role in early embryogenesis. EMBO J 19:1010–1022. doi: 10.1093/emboj/19.5.1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux W (1888) Beiträge zur Entwickelungsmechanik des Embryo. V. Virchows Arch Pathol Anat 114:113–153 [Google Scholar]
- Roux W (1885) Ueber die bestimmung der hauptrichtungen des froschembryo im ei und über die erste theilung des froscheies. Zeitschrift 20:1–54 [Google Scholar]
- Roux W (1887) Beiträge zur Entwickelungsmechanik des Embryo. Arch Mikrosk Anat 29:157–211 [Google Scholar]
- Roux W (1903) Über die Ursachen der Bestimmung der Hauptrichtungen des Embryo im Froschei. Anat Anz 23:65–183 [Google Scholar]
- Rowning BA, Wells J, Wu M et al. (1997) Microtubule-mediated transport of organelles and localization of beta-catenin to the future dorsal side of Xenopus eggs. Proc Natl Acad Sci U S A 94:1224–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugh R (1951) The frog, its reproduction and development. The Blakiston Co., Philadelphia, PA [Google Scholar]
- Ruiz i Altaba A, Choi T, Melton DA (1991) Expression of the Xhox3 homeobox protein in Xenopus embryos: blocking its early function suggests the requirement of Xhox3 for normal posterior development (axial pattern/central nervous system/embryonic mesoderm/homeobox gene/Xenopus laevis). Dev Growth Differ 33:651–669. doi: 10.1111/j.1440-169X.1991.00651.x [DOI] [PubMed] [Google Scholar]
- Saint-Jeannet JP, Huang S, Duprat AM (1990) Modulation of neural commitment by changes in target cell contacts in Pleurodeles waltl. Dev Biol 141:93–103 [DOI] [PubMed] [Google Scholar]
- Sala M (1955) Distribution of activating and transforming influences in the archenteron roof during the induction of the nervous system in amphibians. PNAS 58:635–647 [Google Scholar]
- Salic AN, Kroll KL, Evans LM, Kirschner MW (1997) Sizzled: a secreted Xwnt8 antagonist expressed in the ventral marginal zone of Xenopus embryos. Development 124:4739–4748 [DOI] [PubMed] [Google Scholar]
- Sampath K, Rubinstein AL, Cheng AM et al. (1998) Induction of the zebrafish ventral brain and floorplate requires cyclops/nodal signalling. Nature 395:185–189. doi: 10.1038/26020 [DOI] [PubMed] [Google Scholar]
- Sander V, Reversade B, De Robertis EM (2007) The opposing homeobox genes Goosecoid and Vent1/2 self-regulate Xenopus patterning. EMBO J 26:2955–2965. doi: 10.1038/sj.emboj.7601705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saneyoshi T, Kume S, Amasaki Y, Mikoshiba K (2002) The Wnt/calcium pathway activates NF-AT and promotes ventral cell fate in Xenopus embryos. Nature 417:295–299. doi: 10.1038/417295a [DOI] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, De Robertis EM (1995) Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature 376:333–336. doi: 10.1038/376333a0 [DOI] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H et al. (1994) Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell 79:779–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerbo P, Girardot F, Vivien C et al. (2012) Ventx factors function as nanog-like guardians of developmental potential in Xenopus. PLoS One 7:e36855. doi: 10.1371/journal.pone.0036855.s008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerbo P, Markov GV, Vivien C et al. (2014) On the origin and evolutionary history of NANOG. PLoS One 9:e85104. doi: 10.1371/journal.pone.0085104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schambony A, Wedlich D (2007) Wnt-5A/Ror2 regulate expression of XPAPC through an alternative noncanonical signaling pathway. Dev Cell 12:779–792. doi: 10.1016/j.devcel.2007.02.016 [DOI] [PubMed] [Google Scholar]
- Scharf SR, Gerhart JC (1983) Axis determination in eggs of Xenopus laevis: a critical period before first cleavage, identified by the common effects of cold, pressure and ultraviolet irradiation. Dev Biol 99:75–87 [DOI] [PubMed] [Google Scholar]
- Scharf SR, Gerhart JC (1980) Determination of the dorsal-ventral axis in eggs of Xenopus laevis: complete rescue of uv-impaired eggs by oblique orientation before first cleavage. Dev Biol 79:181–198 [DOI] [PubMed] [Google Scholar]
- Scharf SR, Rowning B, Wu M, Gerhart JC (1989) Hyperdorsoanterior embryos from Xenopus eggs treated with D2O. Dev Biol 134:175–188 [DOI] [PubMed] [Google Scholar]
- Schmid B, Fürthauer M, Connors SA et al. (2000) Equivalent genetic roles for bmp7/snailhouse and bmp2b/swirl in dorsoventral pattern formation. Development 127:957–967 [DOI] [PubMed] [Google Scholar]
- Schmidt JE, von Dassow G, Kimelman D (1996) Regulation of dorsal-ventral patterning: the ventralizing effects of the novel Xenopus homeobox gene Vox. Development 122:1711–1721 [DOI] [PubMed] [Google Scholar]
- Schneider S, Steinbeisser H, Warga RM, Hausen P (1996) Beta-catenin translocation into nuclei demarcates the dorsalizing centers in frog and fish embryos. Mech Dev 57:191–198 [DOI] [PubMed] [Google Scholar]
- Schohl A, Fagotto F (2002) Beta-catenin, MAPK and Smad signaling during early Xenopus development. Development 129:37–52 [DOI] [PubMed] [Google Scholar]
- Schroeder KE, Condic ML, Eisenberg LM, Yost HJ (1999) Spatially regulated translation in embryos: asymmetric expression of maternal Wnt-11 along the dorsal-ventral axis in Xenopus. Dev Biol 214:288–297. doi: 10.1006/dbio.1999.9426 [DOI] [PubMed] [Google Scholar]
- Schroeder MM, Gard DL (1992) Organization and regulation of cortical microtubules during the first cell cycle of Xenopus eggs. Development 114:699–709 [DOI] [PubMed] [Google Scholar]
- Schultze O (1899) Ueber das erste auftreten der bilateralen symmetrie im verlauf der entwicklung. Arch Mikrosk Anat 55:171–201 [Google Scholar]
- Seidel F (1956) Nachweis eines Zentrums zur Bildung der Keimscheibe im Säugetierei. Naturwissenschaften 43:306–307 [Google Scholar]
- Seleiro EA, Connolly DJ, Cooke J (1996) Early developmental expression and experimental axis determination by the chicken Vg1 gene. Curr Biol 6:1476–1486 [DOI] [PubMed] [Google Scholar]
- Seoane J, Lagna G, Montalvo E, Hemmati-Brivanlou A (2000) OAZ uses distinct DNA- and protein-binding zinc fingers in separate BMP-Smad and Olf signaling pathways. Cell 100(2):229–240 [DOI] [PubMed] [Google Scholar]
- Shah SB, Skromne I, Hume CR, Kessler DS, Lee KJ, Stern CD, Dodd J (1997) Misexpression of chick Vg1 in the marginal zone induces primitive streak formation. Development 124:5127–5138 [DOI] [PubMed] [Google Scholar]
- Shawlot W, Wakamiya M, Kwan KM et al. (1999) Lim1 is required in both primitive streak-derived tissues and visceral endoderm for head formation in the mouse. Development 126:4925–4932 [DOI] [PubMed] [Google Scholar]
- Sheldahl L, Park M, Malbon C, Moon R (1999) Protein kinase C is differentially stimulated by Wnt and Frizzled homologs in a G-protein-dependent manner. Curr Biol 9:695–698 [DOI] [PubMed] [Google Scholar]
- Sheldahl L, Slusarski D, Pandur P et al. (2003) Dishevelled activates Ca2+ flux, PKC, and CamKII in vertebrate embryos. J Cell Biol 161:769–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng G, dos Reis M, Stern CD (2003) Churchill, a zinc finger transcriptional activator, regulates the transition between gastrulation and neurulation. Cell 115:603–613 [DOI] [PubMed] [Google Scholar]
- Shih J, Keller R (1992a) The epithelium of the dorsal marginal zone of Xenopus has organizer properties. Development 116:887–899 [DOI] [PubMed] [Google Scholar]
- Shih J, Keller R (1992b) Patterns of cell motility in the organizer and dorsal mesoderm of Xenopus laevis. Development 116:915–930 [DOI] [PubMed] [Google Scholar]
- Shimizu K, Gurdon JB (1999) A quantitative analysis of signal transduction from activin receptor to nucleus and its relevance to morphogen gradient interpretation. Proc Natl Acad Sci U S A 96:6791–6796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinya M, Eschbach C, Clark M et al. (2000) Zebrafish Dkk1, induced by the pre-MBT Wnt signaling, is secreted from the prechordal plate and patterns the anterior neural plate. Mech Dev 98:3–17 [DOI] [PubMed] [Google Scholar]
- Shook DR, Keller R (2008) Epithelial type, ingression, blastopore architecture and the evolution of chordate mesoderm morphogenesis. J Exp Zool B Mol Dev Evol 310:85–110. doi: 10.1002/jez.b.21198 [DOI] [PubMed] [Google Scholar]
- Shook DR, Majer C, Keller R (2002) Urodeles remove mesoderm from the superficial layer by subduction through a bilateral primitive streak. Dev Biol 248:220–239. doi: 10.1006/dbio.2002.0718 [DOI] [PubMed] [Google Scholar]
- Shy BR, Wu C-I, Khramtsova GF et al. (2013) Regulation of Tcf7l1 DNA binding and protein stability as principal mechanisms of Wnt/β-catenin signaling. Cell Rep 4:1–9. doi: 10.1016/j.celrep.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sick S, Reinker S, Timmer J, Schlake T (2006) WNT and DKK determine hair follicle spacing through a reaction-diffusion mechanism. Science 314:1447–1450. doi: 10.1126/science.1130088 [DOI] [PubMed] [Google Scholar]
- Siegfried E, Chou T, Perrimon N (1992) wingless signaling acts through zeste-white 3, the Drosophila homolog of glycogen synthase kinase-3, to regulate engrailed and establish cell fate. Cell 71:1167–1179 [DOI] [PubMed] [Google Scholar]
- Siegfried E, Wilder E, Perrimon N (1994) Components of wingless signalling in Drosophila. Nature 367:76–80 [DOI] [PubMed] [Google Scholar]
- Simeoni I, Gurdon JB (2007) Interpretation of BMP signaling in early Xenopus development. Dev Biol 308:82–92. doi: 10.1016/j.ydbio.2007.05.010 [DOI] [PubMed] [Google Scholar]
- Sivak J, Petersen L, Amaya E (2005) FGF signal interpretation is directed by sprouty and spred proteins during mesoderm formation. Dev Cell 8:689–701. doi: 10.1016/j.devcel.2005.02.011 [DOI] [PubMed] [Google Scholar]
- Skirkanich J, Luxardi G, Yang J et al. (2011) An essential role for transcription before the MBT in Xenopus laevis. Dev Biol 357:478–491. doi: 10.1016/j.ydbio.2011.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skromne I, Stern CD (2001) Interactions between Wnt and Vg1 signalling pathways initiate primitive streak formation in the chick embryo. Development 128:2915–2927 [DOI] [PubMed] [Google Scholar]
- Skromne I, Stern CD (2002) A hierarchy of gene expression accompanying induction of the primitive streak by Vg1 in the chick embryo. Mech Dev 114:115–118 [DOI] [PubMed] [Google Scholar]
- Slack JMW (1991) From egg to embryo: regional specification in early development. Cambridge University Press, Cambridge [Google Scholar]
- Slusarski D, Yang-Snyder JA, Busa W, Moon R (1997a) Modulation of embryonic intracellular Ca2+ signaling by Wnt-5A. Dev Biol 182:114–120 [DOI] [PubMed] [Google Scholar]
- Slusarski DC, Corces VG, Moon RT (1997b) Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature 390:410–413. doi: 10.1038/37138 [DOI] [PubMed] [Google Scholar]
- Smith JC (1989) Mesoderm induction and mesoderm-inducing factors in early amphibian development. Development 105:665–677 [DOI] [PubMed] [Google Scholar]
- Smith JC, Dale L, Slack JM (1985) Cell lineage labels and region-specific markers in the analysis of inductive interactions. J Embryol Exp Morphol 89(Suppl):317–331 [PubMed] [Google Scholar]
- Smith W, Harland R (1992) Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell 70:829–840 [DOI] [PubMed] [Google Scholar]
- Smith WC, Harland RM (1991) Injected Xwnt-8 RNA acts early in Xenopus embryos to promote formation of a vegetal dorsalizing center. Cell 67:753–765 [DOI] [PubMed] [Google Scholar]
- Smith WC, McKendry R, Ribisi S, Harland RM (1995) A nodal-related gene defines a physical and functional domain within the Spemann organizer. Cell 82:37–46 [DOI] [PubMed] [Google Scholar]
- Snow M (1977) Gastrulation in the mouse: growth and regionalization of the epiblast. Development 42:293–303 [Google Scholar]
- Sokol S (1996) Analysis of Dishevelled signalling pathways during Xenopus development. Curr Biol 6:1456–1467 [DOI] [PubMed] [Google Scholar]
- Sokol S, Christian JL, Moon RT, Melton DA (1991) Injected Wnt RNA induces a complete body axis in Xenopus embryos. Cell 67:741–752 [DOI] [PubMed] [Google Scholar]
- Solnica-Krezel L (2005) Conserved patterns of cell movements during vertebrate gastrulation. Curr Biol 15:R213–R228. doi: 10.1016/j.cub.2005.03.016 [DOI] [PubMed] [Google Scholar]
- Solnica-Krezel L, Driever W (1994) Microtubule arrays of the zebrafish yolk cell: organization and function during epiboly. Development 120:2443–2455 [DOI] [PubMed] [Google Scholar]
- Solnica-Krezel L, Sepich DS (2012) Gastrulation: making and shaping germ layers. Annu Rev Cell Dev Biol 28:687–717. doi: 10.1146/annurev-cellbio-092910-154043 [DOI] [PubMed] [Google Scholar]
- Spemann H (1938) Embryo development and induction Yale University Press, New Haven [Google Scholar]
- Spemann H (1931) Über den Anteil von Implantat und Wirtskeim an der Orientierung und Beschaffenheit der induzierten Embryonalanlage. Wilhelm Roux’ Arch Entwicklungsmech 123:389–517. doi: 10.1007/BF01380646 [DOI] [PubMed] [Google Scholar]
- Spemann H (1918) Über die Determination der ersten Organanlagen des Amphibienembryo I–VI. Wilhelm Roux’ Arch Entwicklungsmech 43:448–555. doi: 10.1007/BF02267308 [DOI] [Google Scholar]
- Spemann H (1921) Die Erzeugung tierischer Chimären durch heteroplastische embryonale Transplantation zwischen Triton cristatus und taeniatus. Wilhelm Roux’ Arch Entwicklungsmech 48:533–570. doi: 10.1007/BF02554578 [DOI] [Google Scholar]
- Spemann H (1903) Entwickelungsphysiologische Studien am Triton-Ei. Wilhelm Roux’ Arch Entwicklungsmech 16:551–631. doi: 10.1007/BF02301267 [DOI] [Google Scholar]
- Spemann H, Mangold (1924) Über Induktion von Embryonenanlagen durch Implantation artfremder Organisatoren. Wilhelm Roux’ Arch EntwMech Org 100:599–638 [Google Scholar]
- Spratt NT, Haas H (1960) Integrative mechanisms in development of the early chick blastoderm. I. Regulative potentiality of separated parts. J Exp Zool 145:97–137 [Google Scholar]
- Srinivas S, Rodriguez T, Clements M et al. (2004) Active cell migration drives the unilateral movements of the anterior visceral endoderm. Development 131:1157–1164. doi: 10.1242/dev.01005 [DOI] [PubMed] [Google Scholar]
- Standley HJ, Gurdon JB (2004) The community effect in Xenopus development In: Grunz H (Ed.) The vertebrate organizer. Springer, Berlin, Heidelberg, pp 73–91 [Google Scholar]
- Stern CD (1990) The marginal zone and its contribution to the hypoblast and primitive streak of the chick embryo. Development 109:667–682 [DOI] [PubMed] [Google Scholar]
- Stern CD (2004) Gastrulation in the chick. From Cells to Embryo, Gastrulation [Google Scholar]
- Stern CD (2006) Neural induction: 10 years on since the “default model”. Curr Opin Cell Biol 18:692–697. doi: 10.1016/j.ceb.2006.09.002 [DOI] [PubMed] [Google Scholar]
- Stern CD (2005) Neural induction: old problem, new findings, yet more questions. Development 132:2007–2021. doi: 10.1242/dev.01794 [DOI] [PubMed] [Google Scholar]
- Stern CD, Downs KM (2012) The hypoblast (visceral endoderm): an evo-devo perspective. Development 139:1059–1069. doi: 10.1242/dev.070730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strähle U, Jesuthasan S (1993) Ultraviolet irradiation impairs epiboly in zebrafish embryos: evidence for a microtubule-dependent mechanism of epiboly. Development 119:909–919 [DOI] [PubMed] [Google Scholar]
- Streit A, Lee KJ, Woo I et al. (1998) Chordin regulates primitive streak development and the stability of induced neural cells, but is not sufficient for neural induction in the chick embryo. Development 125:507–519 [DOI] [PubMed] [Google Scholar]
- Streit A, Stern CD (1999) Establishment and maintenance of the border of the neural plate in the chick: involvement of FGF and BMP activity. Mech Dev 82:51–66 [DOI] [PubMed] [Google Scholar]
- Strutt D (2001) Asymmetric localization of frizzled and the establishment of cell polarity in the Drosophila wing. Mol Cell 7:367–375 [DOI] [PubMed] [Google Scholar]
- Stuckey DW, Clements M, Di-Gregorio A et al. (2011) Coordination of cell proliferation and anterior-posterior axis establishment in the mouse embryo. Development 138:1521–1530. doi: 10.1242/dev.063537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudou N, Yamamoto S, Ogino H, Taira M (2012) Dynamic in vivo binding of transcription factors to cis-regulatory modules of cer and gsc in the stepwise formation of the Spemann-Mangold organizer. Development 139:1651–1661. doi: 10.1242/dev.068395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suriben R, Kivimäe S, Fisher DAC et al. (2009) Posterior malformations in Dact1 mutant mice arise through misregulated Vangl2 at the primitive streak. Nat Genet 41:977–985. doi: 10.1038/ng.435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Thies R, Yamaji N et al. (1994) A truncated bone morphogenetic protein receptor affects dorsal-ventral patterning in the early Xenopus embryo. Proc Natl Acad Sci U S A 91:10255–10259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Ueno N, Hemmati-Brivanlou A (1997) Xenopus msx1 mediates epidermal induction and neural inhibition by BMP4. Development 124:3037–3044 [DOI] [PubMed] [Google Scholar]
- Tabansky I, Lenarcic A, Draft RW et al. (2013) Developmental bias in cleavage-stage mouse blastomeres. Curr Biol 23:21–31. doi: 10.1016/j.cub.2012.10.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M, Smith J (2000) Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development 127:2227–2238 [DOI] [PubMed] [Google Scholar]
- Taelman VF, Dobrowolski R, Plouhinec J-L et al. (2010) Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell 143:1136–1148. doi: 10.1016/j.cell.2010.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahinci E, Symes K (2003) Distinct functions of Rho and Rac are required for convergent extension during Xenopus gastrulation. Dev Biol 259:318–335 [DOI] [PubMed] [Google Scholar]
- Taira M, Jamrich M, Good PJ, Dawid IB (1992) The LIM domain-containing homeo box gene Xlim-1 is expressed specifically in the organizer region of Xenopus gastrula embryos. Genes Dev 6:356–366 [DOI] [PubMed] [Google Scholar]
- Takahashi S, Yokota C, Takano K et al. (2000) Two novel nodal-related genes initiate early inductive events in Xenopus Nieuwkoop center. Development 127:5319–5329 [DOI] [PubMed] [Google Scholar]
- Takaoka K, Hamada H (2012) Cell fate decisions and axis determination in the early mouse embryo. Development 139:3–14. doi: 10.1242/dev.060095 [DOI] [PubMed] [Google Scholar]
- Takaoka K, Yamamoto M, Hamada H (2011) Origin and role of distal visceral endoderm, a group of cells that determines anterior-posterior polarity of the mouse embryo. Nat Cell Biol 13:743–752. doi: 10.1038/ncb2251 [DOI] [PubMed] [Google Scholar]
- Takaoka K, Yamamoto M, Shiratori H et al. (2006) The mouse embryo autonomously acquires anterior-posterior polarity at implantation. Dev Cell 10:451–459. doi: 10.1016/j.devcel.2006.02.017 [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Nakabayashi J, Sakaguchi T et al. (2003) The Prickle-related gene in vertebrates is essential for gastrulation cell movements. Curr Biol 13:674–679 [DOI] [PubMed] [Google Scholar]
- Tam PP, Loebel DA, Tanaka SS (2006) Building the mouse gastrula: signals, asymmetry and lineages. Curr Opin Genet Dev 16:419–425. doi: 10.1016/j.gde.2006.06.008 [DOI] [PubMed] [Google Scholar]
- Tam PP, Steiner KA (1999) Anterior patterning by synergistic activity of the early gastrula organizer and the anterior germ layer tissues of the mouse embryo. Development 126:5171–5179 [DOI] [PubMed] [Google Scholar]
- Tamai K, Semenov M, Kato Y et al. (2000) LDL-receptor-related proteins in Wnt signal transduction. Nature 407:530–535 [DOI] [PubMed] [Google Scholar]
- Tamai K, Zeng X, Liu C et al. (2004) A mechanism for Wnt coreceptor activation. Mol Cell 13:149–156 [DOI] [PubMed] [Google Scholar]
- Tanaka K, Okabayashi K, Asashima M et al. (2000) The evolutionarily conserved porcupine gene family is involved in the processing of the Wnt family. Eur J Biochem 267:4300–4311 [DOI] [PubMed] [Google Scholar]
- Tao Q, Yokota C, Puck H et al. (2005) Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell 120:857–871. doi: 10.1016/j.cell.2005.01.013 [DOI] [PubMed] [Google Scholar]
- Tarbashevich K, Koebernick K, Pieler T (2007) XGRIP2.1 is encoded by a vegetally localizing, maternal mRNA and functions in germ cell development and anteroposterior PGC positioning in Xenopus laevis. Dev Biol 311:554–565. doi: 10.1016/j.ydbio.2007.09.012 [DOI] [PubMed] [Google Scholar]
- Tarkowski AK (1959) Experiments on the development of isolated blastomeres of mouse eggs. Nature 184:1286–1287. doi: 10.1038/1841286a0 [DOI] [PubMed] [Google Scholar]
- Tarkowski AK (1961) Mouse chimaeras developed from fused eggs. Nature 190:857–860 [DOI] [PubMed] [Google Scholar]
- Tarkowski AK, Wróblewska J (1967) Development of blastomeres of mouse eggs isolated at the 4- and 8-cell stage. J Embryol Exp Morphol 18:155–180 [PubMed] [Google Scholar]
- Terasaki M, Chen LB, Fujiwara K (1986) Microtubules and the endoplasmic reticulum are highly interdependent structures. J Cell Biol 103:1557–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse B, Wright CV, Thisse C (2000) Activin- and Nodal-related factors control antero-posterior patterning of the zebrafish embryo. Nature 403:425–428. doi: 10.1038/35000200 [DOI] [PubMed] [Google Scholar]
- Thomas P, Beddington R (1996) Anterior primitive endoderm may be responsible for patterning the anterior neural plate in the mouse embryo. Curr Biol 6:1487–1496 [DOI] [PubMed] [Google Scholar]
- Thomas PQ, Brown A, Beddington RS (1998) Hex: a homeobox gene revealing peri-implantation asymmetry in the mouse embryo and an early transient marker of endothelial cell precursors. Development 125:85–94 [DOI] [PubMed] [Google Scholar]
- Tolwinski NS, Wehrli M, Rives A et al. (2003) Wg/Wnt signal can be transmitted through arrow/LRP5,6 and Axin independently of Zw3/Gsk3beta activity. Dev Cell 4:407–418 [DOI] [PubMed] [Google Scholar]
- Topczewski J, Sepich DS, Myers DC et al. (2001) The zebrafish glypican knypek controls cell polarity during gastrulation movements of convergent extension. Dev Cell 1:251–264 [DOI] [PubMed] [Google Scholar]
- Topol L, Jiang X, Choi H et al. (2003) Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol 162:899–908. doi: 10.1083/jcb.200303158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torban E, Patenaude A-M, Leclerc S et al. (2008) Genetic interaction between members of the Vangl family causes neural tube defects in mice. Proc Natl Acad Sci U S A 105:3449–3454. doi: 10.1073/pnas.0712126105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torlopp A, Khan MAF, Oliveira NMM et al. (2014) The transcription factor Pitx2 positions the embryonic axis and regulates twinning. eLife Sci 3:e03743. doi: 10.7554/eLife.03743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Yang-Snyder JA, Purcell SM et al. (1996) Activities of the Wnt-1 class of secreted signaling factors are antagonized by the Wnt-5A class and by a dominant negative cadherin in early Xenopus development. J Cell Biol 133:1123–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Padilla M-E, Parfitt D-E, Kouzarides T, Zernicka-Goetz M (2007a) Histone arginine methylation regulates pluripotency in the early mouse embryo. Nature 445:214–218. doi: 10.1038/nature05458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Padilla M-E, Richardson L, Kolasinska P et al. (2007b) The anterior visceral endoderm of the mouse embryo is established from both preimplantation precursor cells and by de novo gene expression after implantation. Dev Biol 309:97–112. doi: 10.1016/j.ydbio.2007.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortelote GG, Hernández-Hernández JM, Quaresma AJC et al. (2013) Wnt3 function in the epiblast is required for the maintenance but not the initiation of gastrulation in mice. Dev Biol 374:164–173. doi: 10.1016/j.ydbio.2012.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran LD, Hino H, Quach H et al. (2012) Dynamic microtubules at the vegetal cortex predict the embryonic axis in zebrafish. Development 139:3644–3652. doi: 10.1242/dev.082362 [DOI] [PubMed] [Google Scholar]
- Trindade M, Tada M, Smith JC (1999) DNA-binding specificity and embryological function of Xom (Xvent-2). Dev Biol 216:442–456. doi: 10.1006/dbio.1999.9507 [DOI] [PubMed] [Google Scholar]
- Tropepe V, Hitoshi S, Sirard C et al. (2001) Direct neural fate specification from embryonic stem cells: a primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron 30:65–78 [DOI] [PubMed] [Google Scholar]
- Tsunekawa N, Naito M, Sakai Y et al. (2000) Isolation of chicken vasa homolog gene and tracing the origin of primordial germ cells. Development 127:2741–2750 [DOI] [PubMed] [Google Scholar]
- Tucker JA, Mintzer KA, Mullins MC (2008) The BMP signaling gradient patterns dorsoventral tissues in a temporally progressive manner along the anteroposterior axis. Dev Cell 14:108. doi: 10.1016/j.devcel.2007.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung TC, Wu SC, Tung Y (1962) Experimental studies on neural induction in Amphioxus. Sci Sin 11:805 [Google Scholar]
- Tzika A, Milinkovitch MC (2008) A pragmatic approach for selecting evo-devomodel species in amniotes In: Minelli A, Fusco G (eds) Evolving pathways; key themes in evolutionary developmental biology. Cambridge University Press, Cambridge, pp 119–140 [Google Scholar]
- Ulrich F, Concha M, Heid P et al. (2003) Slb/Wnt11 controls hypoblast cell migration and morphogenesis at the onset of zebrafish gastrulation. Development 130:5375–5384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallier L, Mendjan S, Brown S et al. (2009) Activin/Nodal signalling maintains pluripotency by controlling Nanog expression. Development 136:1339–1349. doi: 10.1242/dev.033951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amerongen R, Fuerer C, Mizutani M, Nusse R (2012) Wnt5a can both activate and repress Wnt/β-catenin signaling during mouse embryonic development. Dev Biol 369:101–114. doi: 10.1016/j.ydbio.2012.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M, Harryman-Samos C, Klingensmith J et al. (1993) Mutations in the segment polarity genes wingless and porcupine impair secretion of the wingless protein. EMBO J 12:5293–5302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varelas X, Miller BW, Sopko R et al. (2010) The Hippo pathway regulates Wnt/beta-catenin signaling. Dev Cell 18:579–591. doi: 10.1016/j.devcel.2010.03.007 [DOI] [PubMed] [Google Scholar]
- Vaughan RB, Trinkaus JP (1966) Movements of epithelial cell sheets in vitro. J Cell Sci 1:407–413 [DOI] [PubMed] [Google Scholar]
- Veeman M, Axelrod JD, Moon R (2003a) A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell 5:367–377 [DOI] [PubMed] [Google Scholar]
- Veeman MT, Slusarski DC, Kaykas A et al. (2003b) Zebrafish Prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol 13:680–685. doi: 10.1016/S0960-9822(03)00240-9 [DOI] [PubMed] [Google Scholar]
- VerMilyea MD, Maneck M, Yoshida N et al. (2011) Transcriptome asymmetry within mouse zygotes but not between early embryonic sister blastomeres. EMBO J 30:1841–1851. doi: 10.1038/emboj.2011.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viebahn C (2001) Hensen’s node. Genesis 29:96–103 [DOI] [PubMed] [Google Scholar]
- Viebahn C, Mayer B, Hrabé de Angelis M (1995) Signs of the principle body axes prior to primitive streak formation in the rabbit embryo. Anat Embryol 192:159–169 [DOI] [PubMed] [Google Scholar]
- Vincent J, Oster G, Gerhart J (1986) Kinematics of gray crescent formation in Xenopus eggs: the displacement of subcortical cytoplasm relative to the egg surface. Dev Biol 113:484–500 [DOI] [PubMed] [Google Scholar]
- Vincent JP, Gerhart JC (1987) Subcortical rotation in Xenopus eggs: an early step in embryonic axis specification. Dev Biol 123:526–539 [DOI] [PubMed] [Google Scholar]
- Vincent SD, Dunn NR, Hayashi S et al. (2003) Cell fate decisions within the mouse organizer are governed by graded Nodal signals. Genes Dev 17:1646–1662. doi: 10.1101/gad.1100503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson CR, Adler PN (1987) Directional non-cell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. Nature 329:549–551. doi: 10.1038/329549a0 [DOI] [PubMed] [Google Scholar]
- Vintemberger P, Clavert J (1959) On the determinism of bilateral symmetry in birds: XI. The moment of embryonic axis determination, according to the results of our experiences in rotation of the chicken egg in the uterus. C R Seances Soc Biol Fil 153:661–665 [PubMed] [Google Scholar]
- Vogt W (1929) Gestaltungsanalyse am Amphibienkeim mit Örtlicher Vitalfärbung. Wilhelm Roux’ Arch Entwicklungsmech 120:384–706. doi: 10.1007/BF02109667 [DOI] [PubMed] [Google Scholar]
- Voiculescu O, Bertocchini F, Wolpert L et al. (2007) The amniote primitive streak is defined by epithelial cell intercalation before gastrulation. Nature 449:1049–1052. doi: 10.1038/nature06211 [DOI] [PubMed] [Google Scholar]
- Voiculescu O, Bodenstein L, Lau I-J, Stern CD (2014) Local cell interactions and self-amplifying individual cell ingression drive amniote gastrulation. eLife Sci 3:e01817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker S, Grimm K, Joos T, Winklbauer R (2000) Development and control of tissue separation at gastrulation in Xenopus. Dev Biol 224:428–439. doi: 10.1006/dbio.2000.9794 [DOI] [PubMed] [Google Scholar]
- Waddington CH (1940) Organisers and genes. Cambridge University Press, Cambridge UK [Google Scholar]
- Waddington CH (1956) Principles of embryology. The Macmillian Company, New York [Google Scholar]
- Waddington CH (1930) Developmental mechanics of chicken and duck embryos. Nature 125:924–925. doi: 10.1038/125924b0 [DOI] [Google Scholar]
- Waddington CH (1934) Experiments on Embryonic induction. III. A note on inductions by chick primitive streak transplanted to the rabbit Embryo. J Exp Biol 11:224–227 [Google Scholar]
- Waddington CH (1936) Organizers in mammalian development: Abstract. Nature [Google Scholar]
- Waddington CH (1937) Experiments on determination in the rabbit embryo. Arch Biol 48:273–290 [Google Scholar]
- Waddington CH (1933) Induction by the endoderm in birds. Wilhelm Roux’ Arch Entwicklungsmech 128:502–521. doi: 10.1007/BF00649862 [DOI] [PubMed] [Google Scholar]
- Waldrip WR, Bikoff EK, Hoodless PA et al. (1998) Smad2 signaling in extraembryonic tissues determines anterior-posterior polarity of the early mouse embryo. Cell 92:797–808 [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Goto T, Keller R, Harland RM (2002) Cloning and expression of Xenopus Prickle, an orthologue of a Drosophila planar cell polarity gene. Mech Dev 116:183–186 [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Rowning BA, Vogeli KM et al. (2000) Dishevelled controls cell polarity during Xenopus gastrulation. Nature 405:81–85. doi: 10.1038/35011077 [DOI] [PubMed] [Google Scholar]
- Wang J, Hamblet NS, Mark S et al. (2006) Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development 133:1767–1778. doi: 10.1242/dev.02347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Krinks M, Lin K et al. (1997) Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell 88:757–766 [DOI] [PubMed] [Google Scholar]
- Wang Y, Nathans J (2007) Tissue/planar cell polarity in vertebrates: new insights and new questions. Development 134:647–658. doi: 10.1242/dev.02772 [DOI] [PubMed] [Google Scholar]
- Wawersik S, Evola C, Whitman M (2005) Conditional BMP inhibition in Xenopus reveals stage-specific roles for BMPs in neural and neural crest induction. Dev Biol 277:425–442. doi: 10.1016/j.ydbio.2004.10.002 [DOI] [PubMed] [Google Scholar]
- Weaver C, Farr GH, Pan W et al. (2003) GBP binds kinesin light chain and translocates during cortical rotation in Xenopus eggs. Development 130:5425–5436. doi: 10.1242/dev.00737 [DOI] [PubMed] [Google Scholar]
- Weaver C, Kimelman D (2004) Move it or lose it: axis specification in Xenopus. Development 131:3491–3499. doi: 10.1242/dev.01284 [DOI] [PubMed] [Google Scholar]
- Weber RJ, Pedersen RA, Wianny F et al. (1999) Polarity of the mouse embryo is anticipated before implantation. Development 126:5591–5598 [DOI] [PubMed] [Google Scholar]
- Weber GF, Bjerke MA, DeSimone DW (2012) A mechanoresponsive cadherin-keratin complex directs polarized protrusive behavior and collective cell migration. Dev Cell 22:104–115. doi: 10.1016/j.devcel.2011.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks DL, Melton DA (1987) A maternal mRNA localized to the vegetal hemisphere in Xenopus eggs codes for a growth factor related to TGF-beta. Cell 51:861–867 [DOI] [PubMed] [Google Scholar]
- Weidinger G, Stebler J, Slanchev K et al. (2003) dead end, a novel vertebrate germ plasm component, is required for zebrafish primordial germ cell migration and survival. Curr Biol 13:1429–1434 [DOI] [PubMed] [Google Scholar]
- Weise A, Bruser K, Elfert S et al. (2010) Alternative splicing of Tcf7l2 transcripts generates protein variants with differential promoter-binding and transcriptional activation properties at Wnt/beta-catenin targets. Nucleic Acids Res 38:1964–1981. doi: 10.1093/nar/gkp1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss PA (1926) Morphodynamik: Ein Einblick in die Gesetzte der organischen Gestaltung an Hand von experimentellen Ergebnissen In: Schaxel J (ed) Abhandlungen zur theoretischen Biologie, vol 23 Gebrüder Borntraeger, Berlin [Google Scholar]
- Wessely O, Agius E, Oelgeschlager M et al. (2001) Neural induction in the absence of mesoderm: beta-catenin-dependent expression of secreted BMP antagonists at the blastula stage in Xenopus. Dev Biol 234:161–173. doi: 10.1006/dbio.2001.0258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall T, Brimeyer R, Twedt J et al. (2003) Wnt-5/pipetail functions in vertebrate axis formation as a negative regulator of Wnt/beta-catenin activity. J Cell Biol 162:889–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel R (1929) Untersuchungen am Hühnchen. Die Entwicklung des Keims Während der Ersten Beiden Bruttage In: Untersuchungen am Hühnchen. Springer, Berlin, Heidelberg, pp 188–321 [DOI] [PubMed] [Google Scholar]
- Willert K, Brown J, Danenberg E et al. (2003) Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423:448–452 [DOI] [PubMed] [Google Scholar]
- Williams M, Burdsal C, Periasamy A et al. (2011) Mouse primitive streak forms in situ by initiation of epithelial to mesenchymal transition without migration of a cell population. Dev Dyn 241:270–283. doi: 10.1002/dvdy.23711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Yen W, Lu X, Sutherland A (2014) Distinct apical and basolateral mechanisms drive planar cell polarity-dependent convergent extension of the mouse neural plate. Dev Cell 29:34–46. doi: 10.1016/j.devcel.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills AE, Choi VM, Bennett MJ et al. (2010) BMP antagonists and FGF signaling contribute to different domains of the neural plate in Xenopus. Dev Biol 337:335–350. doi: 10.1016/j.ydbio.2009.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson E (1928) The cell in development and heredity, 3rd edn. The Macmillian Company, New York [Google Scholar]
- Wilson PA, Hemmati-Brivanlou A (1995) Induction of epidermis and inhibition of neural fate by Bmp-4. Nature 376:331–333. doi: 10.1038/376331a0 [DOI] [PubMed] [Google Scholar]
- Wilson SI, Rydstrom A, Trimborn T et al. (2001) The status of Wnt signalling regulates neural and epidermal fates in the chick embryo. Nature 411:325–330. doi: 10.1038/35077115 [DOI] [PubMed] [Google Scholar]
- Wilson V, Olivera-Martinez I, Storey KG (2009) Stem cells, signals and vertebrate body axis extension. Development 136:1591–1604. doi: 10.1242/dev.021246 [DOI] [PubMed] [Google Scholar]
- Winklbauer R, Nagel M (1991) Directional mesoderm cell migration in the Xenopus gastrula. Dev Biol 148:573–589 [DOI] [PubMed] [Google Scholar]
- Winklbauer R, Selchow A, Nagel M, Angres B (1992) Cell interaction and its role in mesoderm cell migration during Xenopus gastrulation. Dev Dyn 195:290–302 [DOI] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BL (1995) Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev 9:2105–2116 [DOI] [PubMed] [Google Scholar]
- Witschi E (1956) Proposals for an international agreement on normal stages in vertebrate embryology In: XIV international congress of zoology, Copenhagen, pp 260–262 [Google Scholar]
- Wolda SL, Moody CJ, Moon RT (1993) Overlapping expression of Xwnt-3A and Xwnt-1 in neural tissue of Xenopus laevis embryos. Dev Biol 155:46–57. doi: 10.1006/dbio.1993.1005 [DOI] [PubMed] [Google Scholar]
- Wolpert L (1969) Positional information and the spatial pattern of cellular differentiation. J Theor Biol 25:1–47 [DOI] [PubMed] [Google Scholar]
- Wolpert L (1971) Positional information and pattern formation. Curr Top Dev Biol 6:183–224 [DOI] [PubMed] [Google Scholar]
- Wöhrle S, Wallmen B, Hecht A (2007) Differential control of Wnt target genes involves epigenetic mechanisms and selective promoter occupancy by T-cell factors. Mol Cell Biol 27:8164–8177. doi: 10.1128/MCB.00555-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C-I, Hoffman JA, Shy BR et al. (2012a) Function of Wnt/β-catenin in counteracting Tcf3 repression through the Tcf3-β-catenin interaction. Development 139:2118–2129. doi: 10.1242/dev.076067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Huang H, Garcia Abreu J, He X (2009) Inhibition of GSK3 phosphorylation of beta-catenin via phosphorylated PPPSPXS motifs of Wnt coreceptor LRP6. PLoS One 4:e4926. doi: 10.1371/journal.pone.0004926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S-Y, Shin J, Sepich DS, Solnica-Krezel L (2012b) Chemokine GPCR signaling inhibits β-catenin during zebrafish axis formation. PLoS Biol 10:e1001403. doi: 10.1371/journal.pbio.1001403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie CC, Kofron M, Payne C et al. (1996) Maternal beta-catenin establishes a “dorsal signal” in early Xenopus embryos. Development 122:2987–2996 [DOI] [PubMed] [Google Scholar]
- Wynn ML, Kulesa PM, Schnell S (2012) Computational modelling of cell chain migration reveals mechanisms that sustain follow-the-leader behaviour. J R Soc Interface 9:1576–1588. doi: 10.1002/dvdy.22612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xanthos JB, Kofron M, Tao Q et al. (2002) The roles of three signaling pathways in the formation and function of the Spemann Organizer. Development 129:4027–4043 [DOI] [PubMed] [Google Scholar]
- Xu PF, Houssin N, Ferri-Lagneau KF et al. (2014) Construction of a vertebrate embryo from two opposing morphogen gradients. Science 344:87–89. doi: 10.1126/science.1248252 [DOI] [PubMed] [Google Scholar]
- Xue Y, Zheng X, Huang L, et al. (2014) Organizer-derived Bmp2 is required for the formation of a correct Bmp activity gradient during embryonic development. Nat Commun. doi: 10.1038/ncomms4766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe T, Shimizu T, Muraoka O, Bae YK, Hirata T, Nojima H, Kawakami A, Hirano T, Hibi M (2003) Ogon/secreted frizzled functions as a negative feedback regulator of Bmp signaling. Development 130:2705–2716 [DOI] [PubMed] [Google Scholar]
- Yamada T (1938) Induktion der sekundaren Embryonalanlage im Neunaugenkeim. Okajimas Folia Anatomica Japonica [Google Scholar]
- Yamaguchi N, Mizutani T, Kawabata K, Haga H (2015) Leader cells regulate collective cell migration via Rac activation in the downstream signaling of integrin β1 and PI3K. Sci Rep 5:7656. doi: 10.1038/srep07656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A, Nagano T, Takehara S et al. (2005) Shisa promotes head formation through the inhibition of receptor protein maturation for the caudalizing factors, Wnt and FGF. Cell 120:223–235. doi: 10.1016/j.cell.2004.11.051 [DOI] [PubMed] [Google Scholar]
- Yamamoto H (1999) Phosphorylation of axin, a Wnt signal negative regulator, by glycogen synthase kinase-3beta regulates its stability. J Biol Chem 274:10681–10684. doi: 10.1074/jbc.274.16.10681 [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Beppu H, Takaoka K et al. (2009) Antagonism between Smad1 and Smad2 signaling determines the site of distal visceral endoderm formation in the mouse embryo. J Cell Biol 184:323–334. doi: 10.1038/361543a0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Meno C, Sakai Y et al. (2001) The transcription factor FoxH1 (FAST) mediates Nodal signaling during anterior-posterior patterning and node formation in the mouse. Genes Dev 15:1242–1256. doi: 10.1101/gad.883901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Saijoh Y, Perea-Gomez A et al. (2004) Nodal antagonists regulate formation of the anteroposterior axis of the mouse embryo. Nature 428:387–392. doi: 10.1038/nature02418 [DOI] [PubMed] [Google Scholar]
- Yang J, Tan C, Darken RS et al. (2002) Beta-catenin/Tcf-regulated transcription prior to the midblastula transition. Development 129:5743–5752 [DOI] [PubMed] [Google Scholar]
- Yao L-C, Blitz IL, Peiffer DA et al. (2006) Schnurri transcription factors from Drosophila and vertebrates can mediate Bmp signaling through a phylogenetically conserved mechanism. Development 133:4025–4034. doi: 10.1242/dev.02561 [DOI] [PubMed] [Google Scholar]
- Ybot-Gonzalez P, Savery D, Gerrelli D et al. (2007) Convergent extension, planar-cell-polarity signalling and initiation of mouse neural tube closure. Development 134:789–799. doi: 10.1242/dev.000380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen WW, Williams M, Periasamy A et al. (2009) PTK7 is essential for polarized cell motility and convergent extension during mouse gastrulation. Development 136:2039–2048. doi: 10.1242/dev.030601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo C, Whitman M (2001) Nodal signals to Smads through Cripto-dependent and Cripto-independent mechanisms. Mol Cell 7:949–957 [DOI] [PubMed] [Google Scholar]
- Yi F, Pereira L, Hoffman JA et al. (2011) Opposing effects of Tcf3 and Tcf1 control Wnt stimulation of embryonic stem cell self-renewal. Nat Cell Biol 13:762–770. doi: 10.1038/ncb2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi F, Pereira L, Merrill BJ (2008) Tcf3 functions as a steady-state limiter of transcriptional programs of mouse embryonic stem cell self-renewal. Stem Cells 26:1951–1960. doi: 10.1634/stemcells.2008-0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C, Kiskowski M, Pouille PA et al. (2008) Cooperation of polarized cell intercalations drives convergence and extension of presomitic mesoderm during zebrafish gastrulation. J Cell Biol 180:221–232. doi: 10.1016/S1534-5807(04)00060-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota C, Kofron M, Zuck M et al. (2003) A novel role for a nodal-related protein; Xnr3 regulates convergent extension movements via the FGF receptor. Development 130:2199–2212 [DOI] [PubMed] [Google Scholar]
- Yoshikawa S, McKinnon R, Kokel M, Thomas J (2003) Wnt-mediated axon guidance via the Drosophila Derailed receptor. Nature 422:583–588 [DOI] [PubMed] [Google Scholar]
- Yu J-K, Satou Y, Holland ND et al. (2007) Axial patterning in cephalochordates and the evolution of the organizer. Nature 445:613–617. doi: 10.1038/nature05472 [DOI] [PubMed] [Google Scholar]
- Yuge M, Kobayakawa Y, Fujisue M, Yamana K (1990) A cytoplasmic determinant for dorsal axis formation in an early embryo of Xenopus laevis. Development 110:1051–1056 [DOI] [PubMed] [Google Scholar]
- Zecca M, Basler K, Struhl G (1996) Direct and long-range action of a wingless morphogen gradient. Cell 87:833–844 [DOI] [PubMed] [Google Scholar]
- Zeng X, Huang H, Tamai K et al. (2008) Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development 135:367–375. doi: 10.1242/dev.013540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Tamai K, Doble B et al. (2005) A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature 438:873–877. doi: 10.1038/nature04185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernicka-Goetz M (1998) Fertile offspring derived from mammalian eggs lacking either animal or vegetal poles. Development 125:4803–4808 [DOI] [PubMed] [Google Scholar]
- Zernicka-Goetz M (2013) Development: do mouse embryos play dice? Curr Biol 23:R15–R17. doi: 10.1016/j.cub.2012.10.032 [DOI] [PubMed] [Google Scholar]
- Zernicka-Goetz M, Morris SA, Bruce AW (2009) Making a firm decision: multifaceted regulation of cell fate in the early mouse embryo. Nat Rev Genet 10:467–477. doi: 10.1038/nrg2564 [DOI] [PubMed] [Google Scholar]
- Zhang J, Houston DW, King ML et al. (1998) The role of maternal VegT in establishing the primary germ layers in Xenopus embryos. Cell 94:515–524 [DOI] [PubMed] [Google Scholar]
- Zhang S, Holland ND, Holland LZ (1997) Topographic changes in nascent and early mesoderm in amphioxus embryos studied by DiI labeling and by in situ hybridization for a Brachyury gene. Dev Genes Evol 206:532. doi: 10.1007/s004270050083 [DOI] [PubMed] [Google Scholar]
- Zhang X, Abreu JG, Yokota C et al. (2012) Tiki1 is required for head formation via Wnt cleavage-oxidation and inactivation. Cell 149:1565–1577. doi: 10.1016/j.cell.2012.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Cheong S-M, Amado NG et al. (2015) Notum is required for neural and head induction via wnt deacylation, oxidation, and inactivation. Dev Cell 32:719–730. doi: 10.1016/j.devcel.2015.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman LB, De Jesus-Escobar J, Harland R (1996) The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell 86:599–606 [DOI] [PubMed] [Google Scholar]
- Ziomek CA, Johnson MH (1980) Cell surface interaction induces polarization of mouse 8-cell blastomeres at compaction. Cell 21:935–942 [DOI] [PubMed] [Google Scholar]
- Zisckind N, Elinson R (1990) Gravity and microtubules in dorsoventral polarization of the Xenopus egg. Dev Growth Differ 32:575–581 [DOI] [PubMed] [Google Scholar]