Abstract
Objective
To test effects on care quality of Chronic Care Model-based Parkinson disease (PD) management.
Methods
This 2-group stratified randomized trial involved 328 veterans with PD in southwestern United States. Guided care management, led by PD nurses, was compared to usual care. Primary outcomes were adherence to 18 PD care quality indicators. Secondary outcomes were patient-centered outcome measures. Data sources were telephone survey and electronic medical record (EMR). Outcomes were analyzed as intent-to-treat comparing initial and final survey and repeated-measures mixed-effects models.
Results
Average age was 71 years; 97% of participants were male. Mean proportion of participants receiving recommended PD care indicators was significantly higher for the intervention than for usual care (0.77 vs 0.58) (mean difference 0.19, 95% confidence interval [CI] 0.16, 0.22). Of 8 secondary outcomes, the only significant difference of the changes over time was in the positive Patient Health Questionnaire–2 depression screen for intervention minus usual care (−11.52 [95% CI −20.42, −2.62]).
Conclusion
A nurse-led chronic care management intervention, Care Coordination for Health Promotion and Activities in Parkinson's Disease (CHAPS), substantially increased adherence to PD quality of care indicators among veterans with PD, as documented in the EMR. Of 8 secondary outcomes assessed, a screening measure for depressive symptomatology was the only measure that was better in the intervention compared to usual care. More telephone calls in CHAPS were the only utilization difference over usual care. While CHAPS appears promising for improving PD care, additional iterative research is needed to refine the CHAPS model in routine clinical care so that it measurably improves patient-centered outcomes (NCT01532986).
Classification of evidence
This study provides Class I evidence that for patients with PD, CHAPS increased adherence to PD quality of care indicators.
Parkinson disease (PD), the second most common neurodegenerative disorder, leads to progressive loss of motor function with secondary symptoms affecting quality of life,1 underscoring the need for integrated patient/person-centered care.2–4 In the Department of Veterans Affairs (VA) in 2016, about 50,000 of 110,000 veterans with PD received VA care (Tanner and Goldman, unpublished data, 2017). To improve VA quality of care, as measured by PD quality indicators,5 and address gaps in PD care,6 we created a guided care management intervention, Care Coordination for Health Promotion and Activities in Parkinson's Disease (CHAPS), based on the Chronic Care Model7 and adapted from our earlier research with patients with Alzheimer disease.8,9 Using a randomized trial design, we implemented the CHAPS intervention and evaluated its efficacy. We hypothesized that the care of veterans receiving CHAPS would (1) meet more PD quality care indicators as compared to usual VA care, (2) yield better secondary outcomes: health-related quality of life, self-efficacy, and perceptions of care quality, and (3) be feasible to implement and sustain relative to resource utilization.
Methods
Setting, eligible participants, data collection
Full details of the study protocol and intervention design have been published.9 In brief, this study was a randomized trial, to obtain Class I level of evidence, of a nurse-led care management intervention occurring at 5 VA medical centers (Greater Los Angeles, Loma Linda, Long Beach, and San Diego, California; and Las Vegas, Nevada). Potential participants with at least 2 ICD-9 diagnostic codes for PD over a 12-month period, from these 5 sites, were offered study participation on a rolling basis.
Using the statistical software program Stata, the study programmer created randomization tables with block size of 4 and stratified by site that assigned participants in a 1:1 ratio to either the intervention or control (usual care) arm. Patients were invited, by letter, to participate in the study. Research staff members obtained verbal consent and then patients were enrolled as participants.
After a research staff member consented and enrolled a participant and prior to randomization, a research interviewer administered the baseline survey via phone. After the baseline survey was completed, a research interviewer contacted the project manager (PM) by telephone to inform that individual of the new enrollment; the communication between the research interviewer and the PM ended at that point after the patient was enrolled. Then, the PM pulled up the randomization table that had been generated by the study programmer prior to initiation of the study and, working from the randomization list in sequence, gave that patient enrollee the next sequential randomization assignment number from the randomization table. If the enrollee's allocation assignment was to the intervention arm, the PM notified the appropriate nurse care manager (NCM). This randomization allocation sequence table was inaccessible to the research interviewers, who were the individuals who enrolled patients and collected study outcome data, as it was located on a password-protected file folder on a computer to which the research interviewers did not have access.
The research interviewer, blinded to the participant's study arm assignment, administered the follow-up 6-, 12-, and 18-month surveys by telephone, to assess 6 of the 18 PD quality of care indicators (primary outcomes) and all of the secondary outcome measures. Within the trial's first year, we experienced a 6-month period of NCM understaffing, so we temporarily halted recruitment and added an additional survey at 24 months for the first 59% (193 of 328) of enrolled participants.
Of 38 PD indicators, we chose a subset of 18 indicators that represented the range of categories of all 38 indicators, so that all indicator domains were covered except for a single diagnosis confirmation-related indicator (which would not have been applicable to most of our sample, who had diagnosed PD for more than a year). In addition, we selected these specific 18 indicators based on (1) their feasibility of measurement from medical records or from survey and (2) the extent to which they were a focus of the intervention protocol. Six of these indicators were measured through the participant survey and 12 from the electronic medical record (EMR) review.
A research nurse reviewed outpatient EMR notes with PD-related information through 18 months after a participant's enrollment date. Baseline adherence to PD quality indicators was not collected. To assess the reliability of data abstraction, 2 raters independently reviewed a 25-patient random sample. Interrater agreement was assessed using the Cohen kappa statistic.
Intervention fidelity measures were (1) NCM coverage, defined as the percent of days a veteran was in the study and NCM was available; (2) number of initial CHAPS intervention assessments; (3) receipt of health care notebooks; and (4) number of follow-up encounters, including yearly structured reassessments, all measured through EMR abstraction and research logs for the same 18 months as above.
Intervention
The guided care management intervention, CHAPS, consisted of NCMs collaborating with site PD specialists to provide comprehensive care. The NCMs used (1) a telephone-administered structured and comprehensive assessment using Microsoft Access with embedded algorithms for identifying 28 problem areas, (2) evidence-based practice in the CHAPS model containing 3 components: care protocols as derived from practice guidelines (where they exist) and expert consensus where they do not,5 and veteran priorities and preferences,10 (3) communication tools (VA's patient portal MyHealtheVet and personalized health care notebook), and (4) documentation templates to provide coordinated, patient-centered care.9 NCMs were registered nurses with education ranging from associate degree through nurse practitioner. Initial assessments were done after study enrollment and identified PD-related problems and topics for each veteran. NCMs then developed action plans with patients that included problem-specific interventions such as information, problem-solving collaboratively, and clinical referrals. NCMs had problem-specific care plans to refer to for management options. The first follow-up call was within approximately 1 month, and then every 6 months or sooner, as needed. The follow-up calls monitored how patients were doing and their follow-through of interventions, including self-management activities.
CHAPS assessment, problems/topics, follow-up notes, and patient notebooks were organized by the Siebens Domain Management Model (SDMM), a comprehensive 4-domain clinical framework (I. Medical/Surgical Issues; II. Mental Status/Emotions/Coping; III. Physical Function; IV. Living Environment).11 The health care notebook contained the CHAPS assessment, education sheets, a patient-specific “My Action Plan,” and text explaining notebook purpose and options for how to use it.9,12
Outcome measures
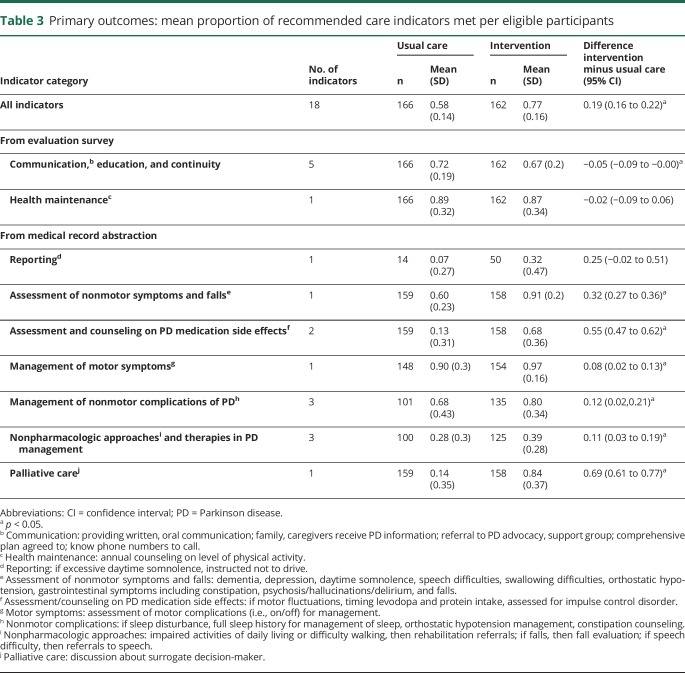
The study's primary goal was adherence to PD quality indicators as measured across 9 categories: (1) communication, education, and continuity; (2) health maintenance; (3) regulatory reporting; (4) assessment of nonmotor symptoms and falls; (5) assessment and counseling about PD medication side effects; (6) management of motor symptoms; (7) management of nonmotor complications of PD; (8) use of nonpharmacologic approaches and therapies; and (9) palliative care.13
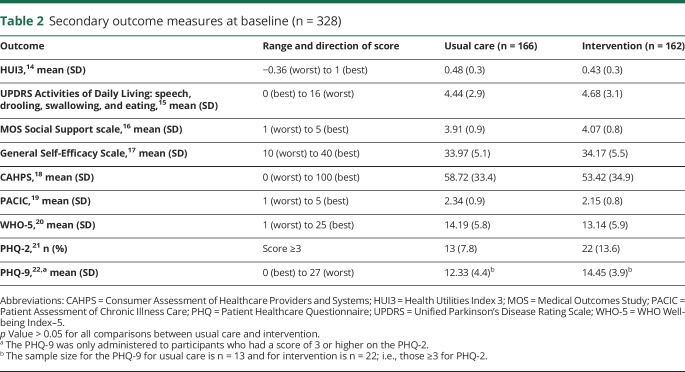
Secondary outcomes covered health-related quality of life (Health Utilities Index 3 [HUI3]),14 speech, drooling, swallowing and eating (Unified Parkinson's Disease Rating Scale Activities of Daily Living),15 social support (Medical Outcomes Study [MOS] Social Support Scale),16 self-efficacy (General Self-Efficacy Scale),17 perceptions of care quality (Consumer Assessment of Healthcare Providers and Systems [CAHPS]), and the Patient Assessment of Chronic Illness Care (PACIC),18,19 and self-reported depression screeners (WHO Well-being Index–5 [WHO-5], Patient Healthcare Questionnaire [PHQ]–2 , and PHQ-9 administered to participants who had a score of 3 or higher on the PHQ-2).10,20–22
For CHAPS process evaluation, a convenience sample of intervention veterans, in the last several months of the study, answered questions about their CHAPS experience after completing their final survey. NCMs and providers completed an anonymous survey about their impressions of CHAPS.
VA utilization data were abstracted during EMR review. This included outpatient visits (by provider type), emergency department visits, telephone calls, other non-face-to-face contacts, and VA hospitalizations.
Analytic sample size, statistical power, and enrollment sample size
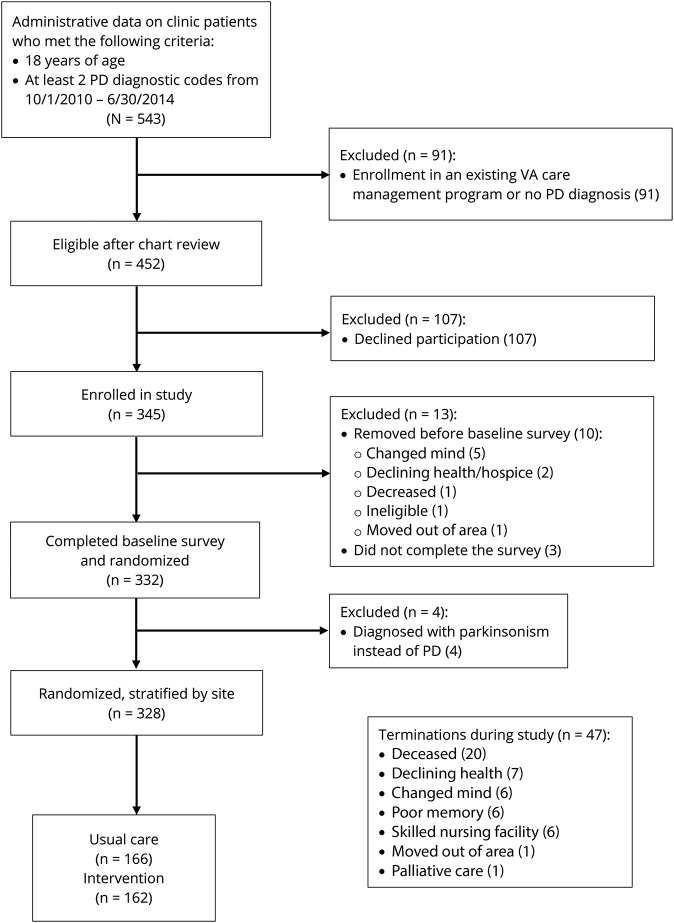
The sample size calculation was based on the primary outcome of guideline adherence to 38 PD indicators, expressed as the mean across the study participant group of the per-patient proportion of applicable guideline measures for which there was adherence.9 Using a medium effect size of 0.4 (Cohen d), similar to other chronic disease studies, significance level of 0.05 and power of 90%, we calculated an analytic sample size of 266 (133 in each arm). Using an estimate of 80% study sample retention rate, the target enrollment was 167 in each arm for a total of 334. The figure shows flow of enrollment, allocations, follow-up, and analytic sample.
Figure. Flow diagram of enrollment and randomization and terminations.
PD = Parkinson disease; VA = Veterans Affairs.
The actual number of indicators measured was 18 and not 38. This change has no effect on the sample size because the number is the denominator of the primary outcome of proportion/percent adherence.
Analysis
Distributions of baseline characteristics between usual care and intervention arms were compared using the 2 group t test for continuous measures and χ2 test or Fisher exact test for categorical data. For the primary outcome, the average proportion of 18 PD indicators met was compared between the 2 arms using a 2-group t test, in an intent-to-treat analysis. Changes in secondary outcomes were assessed from baseline to the veteran's final survey (24-month survey for the first 193 participants and 18-month survey for the remaining 135 participants).
For the secondary outcomes, we analyzed data in 2 ways. Outcomes were compared using 2 time points (the baseline survey and final survey) and using repeated-measures mixed-effects models (SAS PROC MIXED) that included all time points (baseline, and then 6, 12, 18, and 24 months) based on an intent-to-treat analysis. Missing values were imputed with the last-value-carried-forward method. Covariates of site and NCM noncoverage (% of days veteran was in the study when there was no NCM available) were included in all models. For each outcome, 2 covariance structures were modeled: autoregressive (assumes measurements for the same individual become less correlated as they were further apart in time) and compound symmetry (assumes the correlation between observations is constant, regardless of the time lapse between measurements). For each outcome, the model with the lowest Akaike Information Criterion23 was selected. The key p value was the interaction term of time by study arm.
Four sensitivity analyses were run on secondary outcomes. Two adjusted the repeated measures model for missing data due to terminations and incomplete survey collection. The model was adjusted by including predicted probabilities as a covariate, where the predicted probabilities were derived from logistic models with covariates of age, race, HUI3, and MOS Social Support scores at baseline. The third sensitivity analysis excluded participants who did not complete their final survey. The fourth sensitivity analysis replaced the categorical measure of time (survey) with a continuous measure of time defined as number of days the survey was conducted after the baseline survey. Post hoc analyses assessed for baseline differences between those who completed the study and those who did not. A 5% significance level was used throughout and 2-sided tests were used as applicable.
For the primary outcome (average of proportions) effect size, Cohen d was used to describe the standardized mean difference of the effect. The confidence interval (CI) was bootstrapped. All secondary outcomes' effect sizes, except PHQ-2, used partial η2 using the output from PROC MIXED. The CI was bootstrapped. For the PHQ-2, a repeated measures model with proportions, the odds ratio (OR) was converted to an effect size and the CI for the OR to a SE.24 Analyses were performed with SAS 9.4 statistical software (SAS Institute, Cary, NC).
Standard protocol approvals, registration, and patient consent
Institutional review board (IRB) approvals were obtained on November 9, 2011, for all 5 sites. The IRB waived the requirement for documentation of written consent and allowed verbal consent. This study is listed in ClinicalTrials.gov as NCT01532986, registered on January 13, 2012.
Data availability policy
We cannot share adequately de-identified data (i.e., by the time we de-identify data to the degree that would be acceptable, too many key covariates are taken out, given how one can re-identify veterans with enough social or personal demographic and area information).
Results
Of 452 eligible veterans invited to participate, 328 enrolled and were randomized and stratified by site from August 2012 through October 2015 (figure). Study enrollment retention rate was 83%. Survey response rates were 92%, 78%, 74%, and 69% at 6, 12, 18, and 24 months, respectively, collected through March 2017. There were no serious adverse events related to the study.
Surveys took, on average, 34 minutes to administer. Response rates and terminations from the study did not differ between the intervention and usual care arms (p value > 0.05 for each). EMR notes were abstracted for 97% (317 of 328) of the participants. Eleven participants had no relevant EMR notes. On average, 6.7 notes were abstracted per participant with an average of 5.1 in usual care and 8.3 in the intervention. NCM notes comprised 36% of the intervention notes. The mean kappa for agreement between the 2 nurses was 0.86 (range 0.57–1.00).
At baseline, there were no significant differences in sociodemographic characteristics (p > 0.05; table 1). In addition, we compared the 2 study arms in baseline secondary outcome measures and found no differences (p > 0.05; table 2). Final surveys were completed by 71% of usual care participants and 67% of intervention participants. There were no differences in race/ethnicity, primary language spoken, education, or employment between participants who completed their final survey and those who did not, but those who did not complete the final survey had a higher mean age (72.9 [SD 9.5] vs 69.3 [SD 9.6] years) and represented a higher proportion of men compared to the group who did complete the final survey (103 [100%] vs 216 [96.0%]). Also, participants who did not complete their final survey had significantly worse HUI3 (0.38 [SD 0.3] vs 0.49 [0.3]) and PACIC scores at baseline than those who did complete the final survey (2.08 [SD 0.7] vs 2.33 [SD 0.9]).
Table 1.
Sociodemographic characteristics (n = 328)
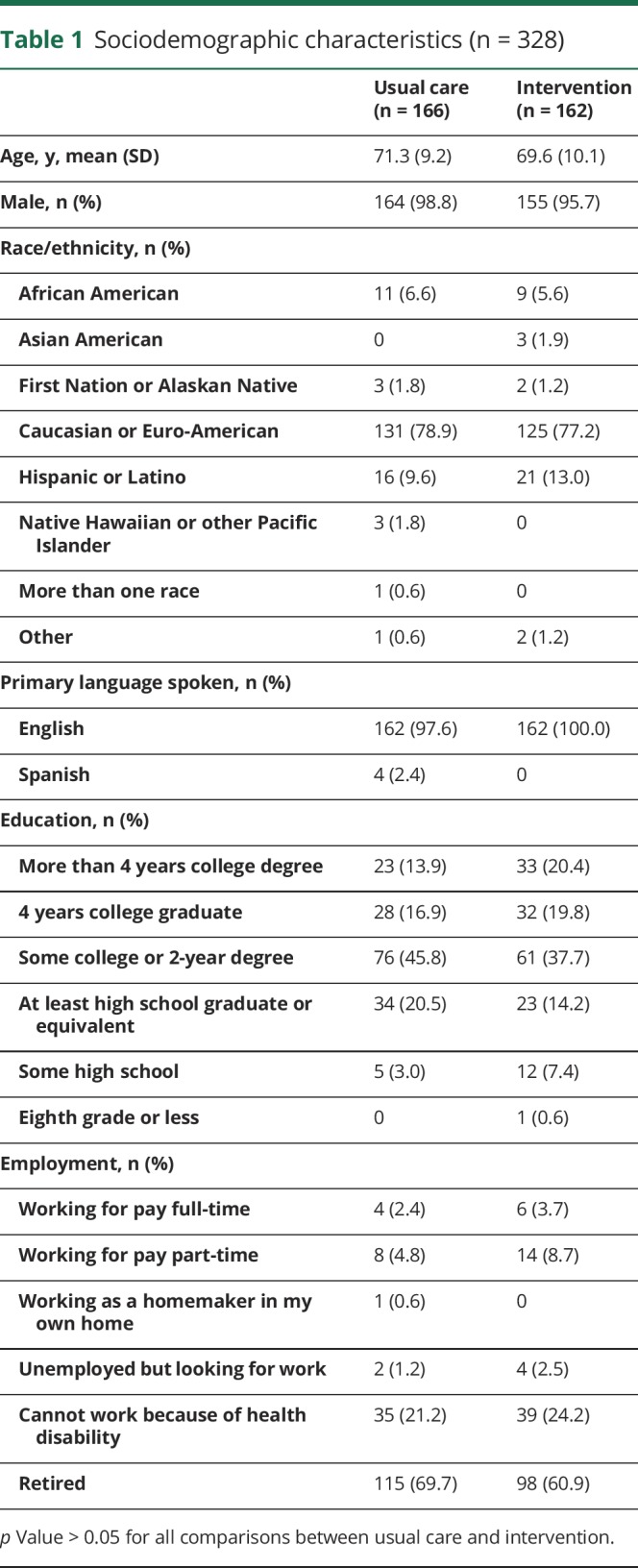
Table 2.
Secondary outcome measures at baseline (n = 328)
Intervention fidelity included 68% median NCM days of coverage (interquartile range, 47%–100%), and 86% of intervention arm participants (140 of 162) received an initial assessment. Participants had a median number of 3 contacts after the initial assessment (interquartile range, 1–4). However, 15% (n = 21) of the intervention arm veterans had no contact with an NCM after the initial assessment and 14% (n = 20) had only 1 additional contact. Health care notebooks were received by 100% of participants with initial assessments.
The mean time spent with veterans for the initial CHAPS assessment was 120 minutes (SD 78, range 10–352 minutes). Mean encounter time was 28 minutes (SD 20, range 3–120 minutes). The mean time for yearly structured reassessment was 34 minutes (SD 32, range 8–120 minutes).
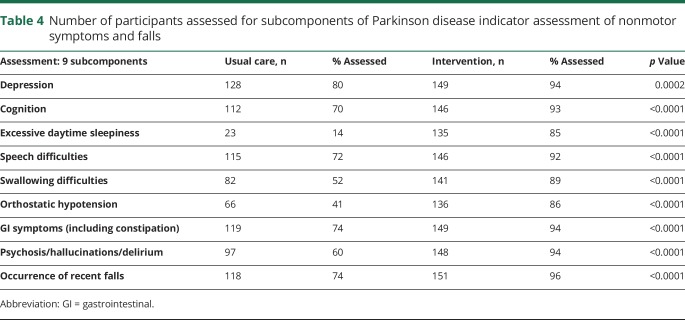
The mean of the proportion of indicators for which care was adherent per veteran at 18 months after enrollment was 0.77 for the intervention group and 0.58 for the usual care group (difference in mean proportion, 0.19 [95% CI 0.16, 0.22]) (table 3). A higher adherence for the intervention group was found in 6 of the 9 categories: assessment of nonmotor symptoms and falls (table 4); assessment and counseling on PD medication side effects; management of motor symptoms; management of nonmotor complications; use of nonpharmacologic approaches and therapies; and palliative care. Adherence for the usual care arm in the communication/education/continuity category was 0.72 compared to 0.67 in the intervention arm (95% CI −0.088, −0.002; p = 0.0371). A sensitivity analysis calculating mean proportion adherence at the arm level, as opposed to at the patient level, revealed similar results (analysis not shown).
Table 3.
Primary outcomes: mean proportion of recommended care indicators met per eligible participants
Table 4.
Number of participants assessed for subcomponents of Parkinson disease indicator assessment of nonmotor symptoms and falls
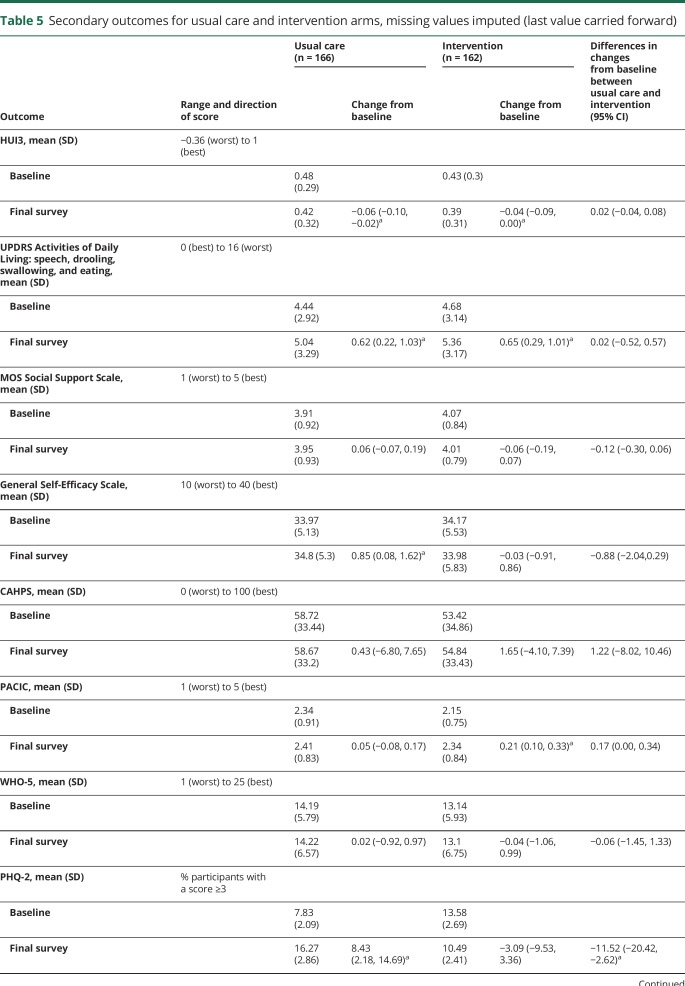
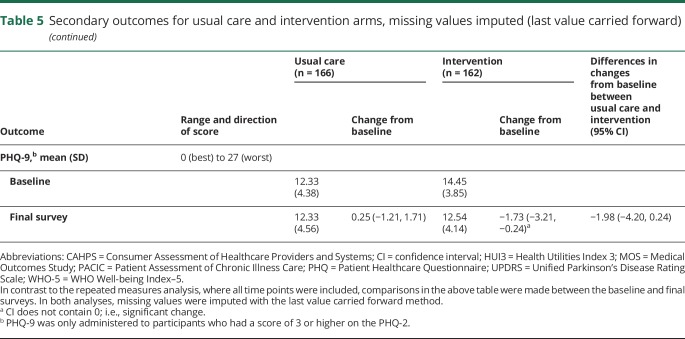
Of changes in secondary outcomes between baseline and final survey (either 18 or 24 months) (table 5). only the PHQ-2 (depression screener) was statistically significant, favoring the intervention arm (−11.52 [−20.42, −2.62]; p = 0.0130). Other secondary outcomes changed within each arm from baseline to the final survey, but not between arms.
Table 5.
Secondary outcomes for usual care and intervention arms, missing values imputed (last value carried forward)
Changes in secondary outcomes in the repeated-measures analysis also showed less depressive symptomatology as measured by the PHQ-2 in the intervention arm relative to usual care arm (p = 0.005, data not shown). Each time point in the intervention arm showed decreased symptoms compared to baseline (data not shown). In contrast, in the usual care arm, only at 12 months were symptoms less than baseline. All the remaining time points (6, 18, and 24 months) had increased depressive symptoms compared to baseline (data not shown). Sensitivity analyses for the repeated-measures analysis of the PHQ-2, controlling for baseline age, race, HUI3, and MOS Social Support, showed the findings remained significant in all analyses (data not shown).
The MOS Social Support scale was better in usual care compared to intervention in the primary repeated-measures analysis (p = 0.037), but there were no between-group differences for any of the 4 sensitivity analyses (data not shown). None of the other 6 secondary outcome measures were different in either the primary repeated-measures analysis or any sensitivity analyses (data not shown).
The effect size for the primary outcome, average proportion of indicators met, was 0.49 (95% CI 0.39–0.59). For the secondary outcome, PHQ-2 depression screener, it was −0.62 (95% CI −1.11 to −0.13). The effect sizes for all other secondary outcomes were nonsignificant.
For process evaluation, surveys of 28 intervention arm veterans indicated that 70% (19 of 28) reported their NCM helped them to manage their PD and 77% (20 of 28) reported their NCM helped them to be safe and active. Clinician feedback surveys were received from 7 out of 8 NCMs and 10 of 12 neurologist/PD specialists who interacted with the NCMs. Overall, 82% (14 of 17) reported “CHAPS assessments, administered by CHAPS Nurse Care Managers, have provided information that will improve how I take care of my patients” and 71% (12 of 17) reported the “CHAPS Program (i.e., intervention) provides recommendations that are useful to help me care for my patients with PD.”
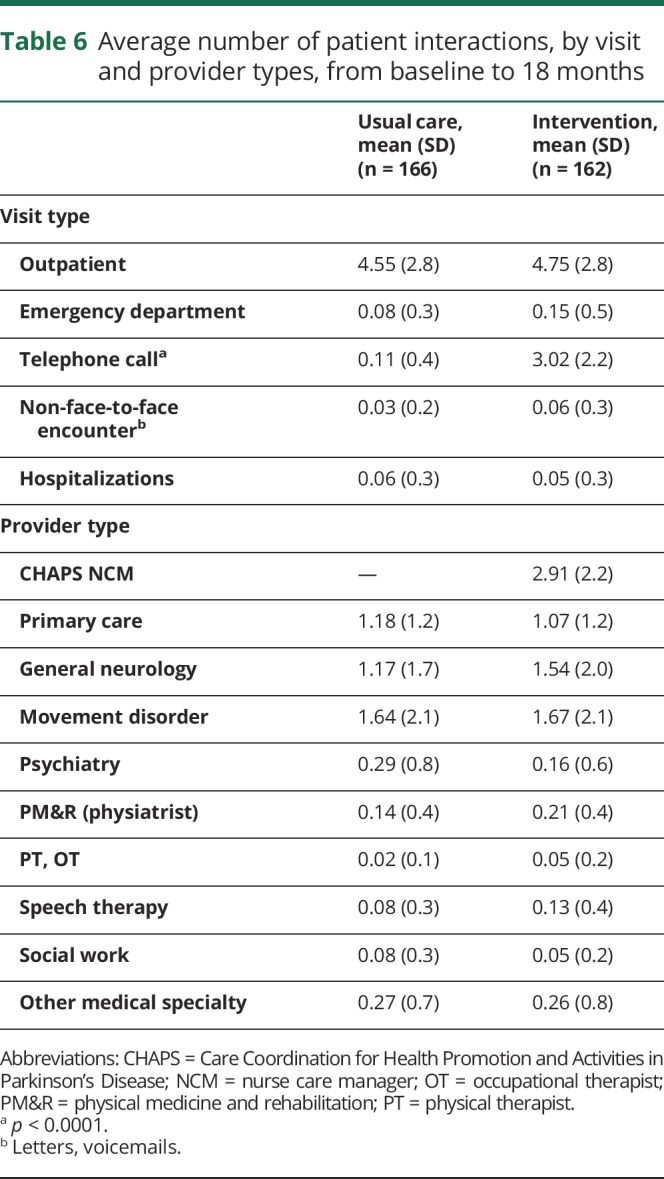
The only difference in utilization was a higher number of average telephone calls per patient in the intervention arm (3.02 [2.2]) compared to usual care (0.11 [0.4]) (p < 0.0001) (table 6).
Table 6.
Average number of patient interactions, by visit and provider types, from baseline to 18 months
Discussion
The CHAPS intervention achieved improved adherence to a wide-ranging set of PD quality indicators, thus supporting our primary study hypothesis. Almost all PD indicators based on medical record review showed statistically significant improvement whereas those collected by veteran survey did not. Better documentation may have been one component in meeting these PD indicators, as CHAPS NCMs' documentation was guided by the SDMM, assessment algorithms targeting specific problems, and associated care plan/intervention suggestions.9
Improvements on the PHQ-2, a depression screen, was the only secondary patient-centered outcome that was better in the intervention arm relative to the usual care arm, although the WHO-5 showed no difference across trial arms. This may be because the WHO-5 assesses for well-being or positive symptoms rather than being framed as endorsing negative symptoms as with the PHQ-2.
This study population had a high level of disability given the mean HUI3 score of 0.46. While similar to another PD VA study population (HUI3 0.45), it differs from a large community-dwelling non-VA PD population (HUI3 0.61).25–27 Therefore, generalizability of these findings may be limited. The magnitude of the primary outcome findings may be conservative. Some intervention participants had limited NCM support for periods during the medical record abstraction time frame, 18 months after baseline. Also, through the standardized assessment process, more patients were assessed for potential need of nonmotor complications and nonpharmacologic approaches, thereby increasing the denominator of eligible patients. Thus, the difference in proportion of indicators met between intervention and usual care for these PD indicators represents a conservative estimate of intervention effect. Because some EMR notes used the term “CHAPS,” the research nurse abstractor was not completely blinded. However, indicator adherence information was being collected from both arms (table 3) using a standardized tool that required the abstractor to look for these data in any EMR notes.
Baseline PD quality indicator adherence measures were not obtained. Nearly all of these care quality indicators were specified as applicable over a time frame of 12 months or as ongoing in the context of management changes or disease course. So the baseline scores would provide information on whether care quality differed at baseline across the usual care and intervention arms, but the primary outcome of adherence to the care quality indicators would not need to take into account analytically the adherence over the period prior to enrollment, as the goal for each and every indicator was a benchmark of full adherence over the postrandomization interval (as long as the indicator was applicable to a given enrollee over that interval). Given this conceptualization and the scope of study resources, we therefore focused on the adherence to the PD quality indicators over the period through 18 months postrandomization.
Interestingly, the PD quality indicator of communication/education/continuity scored higher in the usual care. The VA has been working extensively on improved oral and written communication (i.e., printed after-visit sheets with care plan are provided to many veterans in Parkinson’s Disease Research, Education and Clinical Centers and outpatient primary care sites). However, such activities would have affected both of our study arms equally. Another possibility is that this is a chance finding since we made a number of comparisons and did not adjust for multiple comparisons.
Implementation challenges were several. Non-PD nurses required transitioning into a proactive and PD-specialist environment, necessitating CHAPS process education (structured assessments, algorithms, templates, health care notebooks) that was new to them. Two externally related factors were that the CHAPS care management Microsoft Access program was not integrated within the VA EMR and a VA hiring freeze during part of the study precluded full implementation of CHAPS.
Study findings may have meaningful implications for clinical care. Given that multiple PD quality indicators were met more frequently in the intervention than usual care, implementing CHAPS improves meeting quality of care process measures. For example, as PD medication benefits become more limited, meeting nonpharmacologic process measures and discussions of palliative care become important.28–33 Also, reduction in practice variation and better nursing role definition facilitates PD specialists' and neurologists' understanding of the CHAPS nursing process. Regarding patient-centered outcomes, the positive PHQ-2 findings merit additional study since depression has a negative effect on function and quality of life.34,35 However, that CHAPS did not improve other patient outcome measures could reflect the lack of full implementation of the model due to external factors, a lack of association between the process of care measures we targeted and patient outcomes, and/or inefficacy of the model for having an effect on outcomes at all or within the time frame we followed patients. Process evaluation responses by CHAPS veterans, providers, and NCMs support iterative research. Given no major increase in health services utilization through CHAPS over usual care except for additional telephone calls by NCMs, a feasibility study is needed to evaluate technology, staffing, and resource costs vs benefits. Overall, this study provides insights on care coordination and care management in PD.28,36–40
A coordinated, nurse-led chronic care management intervention, CHAPS, substantially increased adherence to PD quality of care indicators among veterans with PD, as documented in the EMR. Of 8 secondary outcomes assessed, a screening measure for depressive symptomatology was the only measure that was better in the intervention compared to usual care arms. The higher number of telephone calls in CHAPS was the only utilization difference over usual care. As a result, CHAPS appears promising for improving PD care.
In the implementation science field, a major focus is to elucidate how to strengthen the process-to-outcome effect. Three factors relate to finding this effect in health care intervention trials in which process measures are shown to be improved: (1) How strong is the evidence/relationship between the process quality indicator and an outcome? (2) How appropriate/valid are the outcome measures for the process quality indicators being acted on by the particular intervention? (3) How long is the timeframe of a study for capturing the effect of improving a process measure of care quality on outcomes? These factors can be examined further to refine the CHAPS model in routine clinical care so that CHAPS measurably improves patient-centered outcomes.
Acknowledgment
The authors thank the following people for their advice and guidance on this project: Donna Benton, PhD; Dorothy Cole, MD; Erik Ernst, DNP; Emad Farag, MD; Dennis Fujikawa, MD; Virginia Janovsky, MS, MN; Donna McNeese-Smith, RN, EdD; Selina Parveen, MD; Jo Rosen; Steven Schreiber, MD; Evelyn Tacoma, MD, PhD; Mary J. Tichacek, MN, APRN-BC; and members of the project's Advisory Board: Edna Ball; John Ball; Jeff Bronstein, MD, PhD; Michele Popadynec, RN, MPS; Robert Ruff, MD, PhD; Peter Schmidt, PhD; Sharon Valente, RN, PhD; and Fran Weaver, PhD.
Glossary
- CAHPS
Consumer Assessment of Healthcare Providers and Systems
- CHAPS
Care Coordination for Health Promotion and Activities in Parkinson's Disease
- CI
confidence interval
- EMR
electronic medical record
- HUI3
Health Utilities Index 3
- ICD-9
International Classification of Diseases–9
- IRB
institutional review board
- MOS
Medical Outcomes Study
- NCM
nurse care manager
- OR
odds ratio
- PACIC
Patient Assessment of Chronic Illness Care
- PD
Parkinson disease
- PHQ
Patient Healthcare Questionnaire
- PM
project manager
- SDMM
Siebens Domain Management Model
- VA
Veterans Affairs
- WHO-5
WHO Well-being Index–5
Appendix. Authors
Footnotes
Editorial, page 739
Class of Evidence: NPub.org/coe
Study funding
Supported by the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service (NRI 11–126). This work does not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Disclosure
K. Connor, E. Cheng, and F. Barry report no disclosures relevant to the manuscript. H. Siebens discloses that she uses materials in this manuscript (Siebens Domain Management Model, Siebens Health Care Notebook) in consulting work with health care organizations and others. M. Lee, D. Ganz, B. Mittman, M. Connor, L. Edwards, M. McGowan, and B. Vickrey report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Naqvi E. Parkinson's disease statistics. Available at: parkinsonsnewstoday.com/parkinsons-disease-statistics/. Accessed April 25, 2018.
- 2.Prizer LP, Browner N. The integrative care of Parkinson's disease: a systematic review. J Parkinsons Dis 2012;2:79–86. [DOI] [PubMed] [Google Scholar]
- 3.Starfield B. Is patient-centered care the same as person-focused care? Permanente J 2011;15:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Geriatrics Society Expert Panel on Person-Centered Care. Person-centered care: a definition and essential elements. J Am Geriatr Soc 2016;64:15–18. [DOI] [PubMed] [Google Scholar]
- 5.Cheng EM, Siderowf A, Swarztrauber K, Eisa M, Lee M, Vickrey BG. Development of quality of care indicators for Parkinson's disease. Mov Disord 2004;19:136–150. [DOI] [PubMed] [Google Scholar]
- 6.Cheng EM, Tonn S, Swain-Eng R, Factor SA, Weiner WJ, Bever CT. Quality improvement in neurology: AAN Parkinson disease quality measures: report of the quality measurement and reporting subcommittee of the American Academy of Neurology. Neurology 2010;75:2021–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q 1996;74:511–544. [PubMed] [Google Scholar]
- 8.Vickrey BG, Mittman BS, Connor KI, et al. The effect of a disease management intervention on quality and outcomes of dementia care: a randomized, controlled trial. Ann Intern Med 2006;145:713–726. [DOI] [PubMed] [Google Scholar]
- 9.Connor K, Cheng E, Siebens HCet al. Study protocol of “CHAPS”: a randomized controlled trial protocol of care coordination for health promotion and activities in Parkinson's disease to improve the quality of care for individuals with Parkinson's disease. BMC Neurol 2015;15:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott K, McSherry R. Evidence-based nursing: clarifying the concepts for nurses in practice. J Clin Nurs 2009;18:1085–1095. [DOI] [PubMed] [Google Scholar]
- 11.Siebens H. Proposing a practical clinical model. Top Stroke Rehabil 2011;18:60–65. [DOI] [PubMed] [Google Scholar]
- 12.Siebens HC. The Siebens Health Care Notebook. Seal Beach. CA: Siebens Patient Care Communications; 2008. [Google Scholar]
- 13.Parkinson's Disease Care Management Indicator Rating Booklet: Quality Indicators for PD Care Management. Washington, DC: Department of Veterans Affairs; 2009:1–251. [Google Scholar]
- 14.Horsman J, Furlong W, Feeny D, Torrance G. The Health Utilities Index (HUI): concepts, measurement properties and applications. Health Qual Life Outcomes 2003;1:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrison MB, Wylie SA, Frysinger RC, et al. UPDRS activity of daily living score as a marker of Parkinson's disease progression. Mov Disord 2009;24, 224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sherbourne CD, Stewart AL. The MOS Social Support survey. Soc Sci Med 1991;32:705–714. [DOI] [PubMed] [Google Scholar]
- 17.Fujii C, Aoshima T, Sato S, Mori N, Ohkoshi N, Oda S. Self-efficacy and related factors related in Parkinson's disease patients. Nippon Koshu Eisei Zasshi 1997;44:817–826. [PubMed] [Google Scholar]
- 18.Crofton C, Darby C, Farquhar M, Clancy CM. The CAHPS Hospital Survey: development, testing, and use. Jt Comm J Qual Patient Saf 2005;31:655–601. [DOI] [PubMed] [Google Scholar]
- 19.Glasgow RE, Wagner EH, Schaefer J, Mahoney LD, Reid RJ, Greene SM. Development and validation of the Patient Assessment of Chronic Illness Care (PACIC). Med Care 2005;43:436–444. [DOI] [PubMed] [Google Scholar]
- 20.Staehr Johansen K. The use of well-being measures in primary health care: the DepCare project. In: World Health Organization, Regional Office for Europe: Well-Being Measures in Primary Health Care: the DepCare Project. Geneva: World Health Organization; 1998, vol 12:E60246. [Google Scholar]
- 21.Kroenke K, Spitzer RL, Williams JBW. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care 2003;41:1284–1292. [DOI] [PubMed] [Google Scholar]
- 22.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akaike H. A new look at the statistical model identification, IEEE Trans Automatic Control 1974;19:716–723. [Google Scholar]
- 24.Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med 2000;19:3127–3131. [DOI] [PubMed] [Google Scholar]
- 25.Kleiner-Fisman G, Stern MB, Fisman DN. Health-related quality of life in Parkinson disease: correlation between Health Utilities Index III and Unified Parkinson's Disease Rating Scale (UPDRS) in US male veterans. Health Qual Life Outcomes 2010;8:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pohar SL, Jones AC. The burden of Parkinson disease (PD) and concomitant comorbidities. Arch Gerontol Geriatr 2009;49:317–321. [DOI] [PubMed] [Google Scholar]
- 27.Feng Y, Bernier J, McIntosh C, Orpana H. Validation of disability categories derived from Health Utilities Index mark 3 scores. Health Rep 2009;20:43–50. [PubMed] [Google Scholar]
- 28.Bloem B, Munneke M. Revolutionizing management of chronic disease: the Parkinson Net approach. BMJ 2014;348:g1838. [DOI] [PubMed] [Google Scholar]
- 29.Munneke M, Nijkrake MJ, Keus SHM, et al. Efficacy of community-based physiotherapy networks for patients with Parkinson's disease: a cluster-randomized trial. Lancet Neurol 2010;9:46–54. [DOI] [PubMed] [Google Scholar]
- 30.Van de Marck MA, Bloem BR, Borm GF, Overeem S, Munneke M, Guttman M. Effectiveness of multidisciplinary care for Parkinson's disease: a randomized, controlled trial. Mov Disord 2013;26:605–611. [DOI] [PubMed] [Google Scholar]
- 31.Van der Marck MA, Munneke M, Mulleners W, et al. Integrated multidisciplinary care in Parkinson's disease: a non-randomized, controlled trial (IMPACT). Lancet Neurol 2013;12:947–956. [DOI] [PubMed] [Google Scholar]
- 32.Gray BH, Sarnak DO, Tanke M. ParkinsonNet: An Innovative Dutch Approach to Patient-Centered Care for a Degenerative Disease. New York: Commonwealth Fund; 2016:1–11. [Google Scholar]
- 33.Richfield EW, Jones EJ, Alty JE. Palliative care for Parkinson's disease: a summary of the evidence and future directions. Palliat Med 2013;27:805–810. [DOI] [PubMed] [Google Scholar]
- 34.Skorvanek M, Gdovinova Z, Rosenberger J, et al. The associations between fatigue, apathy, and depression in Parkinson's disease. Acta Neurol Scand 2015;131:80–87. [DOI] [PubMed] [Google Scholar]
- 35.Xie CL, Xiao-Dan W, Jie C, et al. A systematic review and meta-analysis of cognitive behavioral and psychodynamic therapy for depression in Parkinson's disease patients. Neurol Sci 2015;36:833–843. [DOI] [PubMed] [Google Scholar]
- 36.Bodenheimer T. Coordinating care: a perilous journey through the health care system. N Engl J Med 2008;358:1064–1071. [DOI] [PubMed] [Google Scholar]
- 37.van der Marck MA, Bloem BR. How to organize multispecialty care for patients with Parkinson's disease. Parkinsonism Relat Disord 2014;20(suppl 1):S167–S173. [DOI] [PubMed] [Google Scholar]
- 38.Woskinski J, Delmas P, Bouwers B, Stormacq C, Kiszio B. Feasibility, appropriateness, meaningfulness, and effectiveness of nursing interventions on the well-being of people with Parkinson's disease and their caregivers living in the community: a mixed-methods systematic review protocol. JBI Database Sys Rev Implement Rep 2015;13:14–29. [DOI] [PubMed] [Google Scholar]
- 39.Hellqvist C, Berterö C. Support supplied by Parkinson's disease specialist nurses to Parkinson's disease patients and their spouses. Appl Nurs Res 2015;28:86–91. [DOI] [PubMed] [Google Scholar]
- 40.Lennaerts H, Groot M, Rood B, et al. A guideline for Parkinson's disease nurse specialists, with recommendations for clinical practice. J Parkinson's Dis 2017;7:749–754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We cannot share adequately de-identified data (i.e., by the time we de-identify data to the degree that would be acceptable, too many key covariates are taken out, given how one can re-identify veterans with enough social or personal demographic and area information).