Abstract
The electromagnetic spectrum contains different frequency bands useful for medical imaging and therapy. Short wavelengths (ionizing radiation) are commonly used for radiological and radionuclide imaging and for cancer radiation therapy. Intermediate wavelengths (optical radiation) are useful for more localized imaging and for photodynamic therapy. Finally, longer wavelengths are the basis for magnetic resonance imaging and for hyperthermia treatments. Recently, there has been a surge of interest for new biomedical methods that synergize optical and ionizing radiation by exploiting the ability of ionizing radiation to stimulate optical emissions. These physical phenomena, together known as radioluminescence, are being used for applications as diverse as radionuclide imaging, radiation therapy monitoring, phototherapy, and nanoparticle-based molecular imaging. This review provides a comprehensive treatment of the physics of radioluminescence and includes simple analytical models to estimate the luminescence yield of scintillators and nanoscintillators, Cerenkov radiation, air fluorescence, and biologically endogenous radioluminescence. Examples of methods that use radioluminescence for diagnostic or therapeutic applications are reviewed and analyzed in light of these quantitative physical models of radioluminescence.
Keywords: Radioluminescence, biomedical imaging, radiation therapy, Cerenkov luminescence, scintillators, nanotechnology
1. Introduction
Radioluminescence is the production of optical photons from the interaction of ionizing radiation with matter. It is a broad term that encompasses many phenomena caused by different physics. These include scintillation, Cerenkov radiation, and the induction of fluorescence, phosphorescence, and delayed/persistent luminescence by ionizing radiation.
While radiation detectors have leveraged scintillator-produced radioluminescence for decades, most sources of radioluminescence are so weak that their potential utility in biomedicine has not been considered until recently. The prevalence of high sensitivity optical cameras now permits routine imaging of faint radioluminescent signals, even within living organisms.
Biomedical applications of radioluminescence broadly fall into two areas, depending on the intensity and duration of the radiation exposure: imaging or therapy. Imaging applications are feasible at low optical fluence rates while therapeutic ones necessitate high fluence rates over greater time durations to achieve a biological effect. The purpose of this review is to summarize the physics and applications of biomedically relevant sources of radioluminescence, to put fourth simple quantitative models for consideration of each phenomenon in the biomedical setting, and to speculate on their suitability for imaging and therapy applications.
The models described in this review are intended to serve as a quantitative framework for evaluating potential applications of radioluminescence. Due to their simplicity, they should be considered useful for order-of-magnitude estimation. Example calculations have been provided and different results may be obtained by changing the numerical values that have been assumed.
2. Cerenkov luminescence
2.1. Physics
Cerenkov radiation is an optical signal induced in dielectric media by fast, charged particles. It is a threshold effect occurring when a charged particle exceeds the phase velocity of light in that medium. The threshold is approximately 214 keV in water and 261 keV in tissue. Cerenkov radiation is induced by successive polarization and depolarization of the medium along the charged particle trajectory, which gives rise to constructive interferences (Jelley 1958).
The Cerenkov photon yield is computed using the Frank-Tamm formula (Mitchell et al 2011):
| (1) |
where is the number of photons emitted per fractional step length of a charged particle in a dielectric medium; n is the refractive index of the medium; is the ratio of the velocity of the charged particle to the vacuum speed of light; and is the fine structure constant. The emitted photons are, in practice, limited to wavelengths above 100 nm by refractive indices that decrease with wavelength in real dielectric materials (Jelley 1958).
Cerenkov radiation possesses unique spectral, spatial, and polarization properties. Its emission spectrum is continuous from the infrared to ultraviolet region and proportional to (Figure 1). Cerenkov photons are emitted anisotropically from the charged particle, following a cone-shaped distribution aligned with the direction of the travelling particle. The angle of the Cerenkov cone is directly related to the velocity of the charged particle, according to: .
Figure 1.
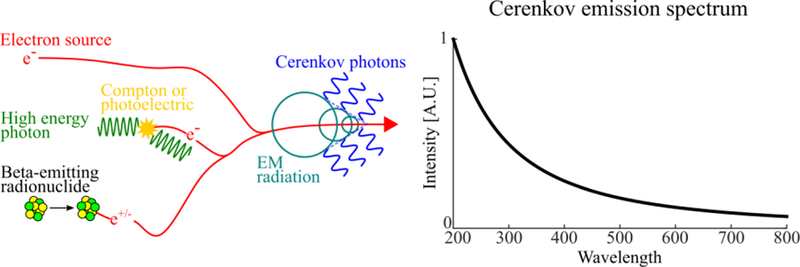
Physics of Cerenkov photon production (left) and emission spectrum (right). Cerenkov photons can be produced by a variety of medical sources including: linear accelerators (linacs) emitting megavoltage electrons or photons, and beta-emitting radionuclides used in radiopharmaceuticals. The emission spectrum is continuous from the ultraviolet to infrared with intensity proportional to λ−2
The physics and history of Cerenkov radiation have been exhaustively covered in (Jelley 1958), and biomedical applications are summarized in recent reviews (Grimm 2015, Tanha et al 2015, Ciarrocchi and Belcari 2017). The following section considers several pre-clinical and clinical applications, along with their current limitations.
2.2. Applications
2.2.1. Time-of-flight PET detectors
CL has been explored as a timing signal for time-of-flight (TOF) PET detectors. CL is generated promptly in the scintillator from recoil electrons and has lower transit time uncertainty compared to scintillation photons (Lecoq et al 2010). Proof-of-concept experiments have shown 71 ps timing resolution based on coincidence detection of the Cerenkov signal (Korpar et al 2011). BGO-based PET detectors have been developed (Kwon et al 2016, Brunner and Schaart 2017). They generate both Cerenkov and scintillation photons; the earliest arriving Cerenkov photons provide coincidence timing while later scintillation photons are used for energy discrimination. This hybrid detector harnesses the benefits of BGO (high stopping power, photoelectric fraction, no background radiation, lower cost), while overcoming timing resolution limitations.
2.2.2. Cerenkov luminescence imaging
Cerenkov luminescence imaging (CLI) is defined as optical imaging of Cerenkov radiation from radionuclides and radiotherapy beams. CLI is a direct method of imaging radionuclides otherwise only detectable with positron emission tomography (PET). All existing PET radionuclides and most beta-emitting radionuclides are sufficiently energetic to induce Cerenkov radiation in biological tissue.
CLI has been most prevalent in the pre-clinical domain, owing to the limited penetration depth of light in tissue, with planar imaging being the most common application (Robertson et al 2009, Liu et al 2010a, Ruggiero et al 2010, Mitchell et al 2011). Pre-clinical tomographic (Li et al 2010) and endoscopic (Liu et al 2012, Carpenter et al 2014) CLI have been reported. The benefit of CLI for pre-clinical imaging is the low cost and simplicity of the instrumentation, compared to PET.
CLI has also shown promise for important clinical applications, such as radiotherapy quality assurance, radiotherapy dosimetry, and surgical guidance.
Quality assurance and dosimetry are routinely performed to verify the quality of a radiotherapy treatment plan before it is delivered to the patient. Given the correlation between radiation dose and the intensity of the resulting CL, it is possible to use CLI to obtain a two-dimensional dose profile of a radiation beam (Glaser et al 2014). Multiple two-dimensional views can be combined to obtain three-dimensional beam profiles and dosimetry for electrons (Helo et al 2014) and photons (Glaser et al 2013a, 2013b). In addition, CLI can detect, in real-time, gross treatment errors during radiation treatment arising from hardware faults or patient mispositioning; it can also coarsely estimate skin surface dose (Jarvis et al 2014).
CLI of injected radiopharmaceuticals (e.g. 18F-fluorodeoxyglucose, FDG) is also being explored to guide surgeries such as sentinel lymph node biopsy, tumor margin assessment, and tumor detection; its potential utility is supported by both theoretical (Klein et al 2017) and experimental evidence (Thorek et al 2014b, Hu et al 2015, Grootendorst et al 2016b). Though briefly covered below, a comprehensive review has been written on the clinical applications of CLI (Grootendorst et al 2016a).
The first reported use of CLI in humans was for imaging a therapeutic dose of 131I in human thyroid (Spinelli et al 2013); 18F-FDG-positive lymph nodes have also been imaged in the axilla of breast cancer patients, using diagnostic levels of radiotracer (Thorek et al 2014b). Four patients were imaged and CLI signal correlated with positive lymph node status determined by PET.
Another pilot study of four patients showed promise for detecting human gastrointestinal (GI) tract tumors (Hu et al 2015). The study used a custom endoscope coupled to a sensitive EMCCD camera to image 18F-FDG during colonoscopy. The system made it possible to differentiate GI tumors from normal tissue, even when inflamed (occurring in one patient). The dark environment achievable inside internal body cavities makes endoscopy an attractive application of CLI due to the faint intensity of the signal.
An ongoing trial of CLI for margin detection during breast conserving surgery has demonstrated the potential of CLI for detecting positive tumor margins. Tissue samples from 12 patients were imaged with a commercial CLI imaging system, and the CLI signal correlated with histopathology in all patients with assessable margins (10 of 12). Two patients with small tumors and late imaging times had un-assessable margins due to absence of Cerenkov signal (Grootendorst et al 2016b).
Cerenkov luminescence can excite fluorophores in vivo, a process called Cerenkov radiation energy transfer or secondary Cerenkov-induced fluorescence imaging. High Stokes-shift nanoparticles (Dothager et al 2010, Liu et al 2010b, Volotskova et al 2015) or molecular fluorophores that emit at longer wavelengths provide increased imaging sensitivity deeper within tissues or unique molecular contrast (Thorek et al 2013). Negative contrast can also be achieved using light-absorbing contrast agents (Thorek et al 2014a). The combination of Cerenkov luminescence and nanoparticles has been reviewed in detail (Shaffer et al 2017).
Finally, a recent development in Cerenkov imaging is Cerenkov-excited luminescence scanned imaging (CELSI). CELSI uses a megavoltage photon sheet-beam to selectively induce Cerenkov luminescence in the imaged subject, similar to light-sheet microscopy or x-ray radioluminescence tomography techniques (Zhang et al 2015). When combined with a fluorescent oxygen-sensing contrast agent, spatial resolution <300 µm and nanomolar sensitivity, have been demonstrated up to 5 mm deep in tissue (Pogue et al 2018).
2.2.3. Cerenkov luminescence for cancer phototherapy
Photodynamic therapy (PDT) is a clinically deployed treatment in which a light-activated drug, called a photosensitizer, is administered and subsequently activated locally using an external light source. The photosensitizer preferentially accumulates in cancer tissue and, when excited with light, catalyzes the formation of reactive oxygen species (ROS) such as singlet oxygen (1O2) and hydroxyl radical (•OH) (Ochsner 1997). ROS exert a therapeutic effect through oxidative damage of cellular membranes, DNA and organelles, leading to cell death (Dougherty et al 1998, Wilson and Patterson 1986). PDT has better spatial specificity than systemic chemotherapy because its effect is only achieved in tissues under illumination. However, the possible applications of PDT are fundamentally limited by the limited penetration depth of light in tissue.
Cerenkov luminescence has garnered immense interest as a potential light source that could elegantly address the light penetration limitation of PDT. This combination could quickly enter the clinic by leveraging existing, approved photosensitizers, radiopharmaceuticals, and radiotherapy protocols.
Cerenkov luminescence-activated PDT has been reported using both radionuclides (Kotagiri et al 2015, Hartl et al 2016, Kamkaew et al 2016) and radiotherapy beams (Ouyang et al 2016, Yoon et al 2017). Notably, Kotagiri and colleagues reported a remarkable in vivo therapeutic effect using a TiO2-based nanoparticle (Kotagiri et al 2015).
However, theoretical and quantitative studies have cast doubt on the possibility of CL-activated PDT (Glaser et al 2015) and suggest that alternative mechanisms such as radiosensitization and direct beta excitation (Pratx 2017, Pratt et al 2018) explain the therapeutic effects reported in prior work. Our own quantitative analysis agrees with these findings. In the following section, we provide simple models for estimating: photon yield from Cerenkov-emitting sources, ROS yield, radiation dose, and expected therapeutic efficacy due to Cerenkov-PDT alone; these models have been applied to prior work and estimated values are summarized in Table 2.
Table 2:
Summary of reports of radionuclide-induced Cerenkov-activated photodynamic therapy.
| Reference | Source | Photosensitizer | Condition | Outcome | Cerenkov photon yield per cell (200 – 800 nm) | Estimated Cerenkov PDT effect [% tumor cells killed] | Estimated dose from radionuclide [Gy] |
|---|---|---|---|---|---|---|---|
| Kotagiri et al 2015 | 18F | TiO-Tf-Tc | I.V. injection 2x; HT1080 mouse tumor | Median survival increased from 15 to 50 days | 1.0×105 | 0.25 | 235 |
| 18F | TiO-Tf-Tc | I.V. injection 2x; A549 mouse tumor | ~6.5-fold tumor growth inhibition at day 30 compared to control | 1.0×105 | 0.25 | 235 | |
| 64Cu | TiO-Tf-Tc | Direct tumor injection; HT1080 mouse tumor | Complete regression at 30 days | 4.2×104 | 0.11 | 117 | |
| Kamkaew et al 2016 ‡ | 89Zr | HMSN-Ce6 | Direct injection; 4T1 mouse tumor | Tumor growth completely inhibited within 14 days post-injection | 8.6×105 | 2.13 | 973 |
| Hartl et al 2016 † | 90Y | TPPS2a | In vitro; C6 cells | Cell viability decreased ~22% compared to radiation effect alone | 1.4×107 | 29.6 | 592 |
| Nakamura et al 2017 | 18F | pan-IR700 (panitumumab conjugated to IR700 dye) | In vitro; A431-luc cells | Decreased A431-luc bioluminescence in dose-dependent manner (in vitro); no significant long-term therapeutic effect (in vivo) | 4.13×102 | 0 | 4 |
Expected photon yield, PDT effect, and radiation dose computed using 14 day experiment duration
Expected photon yield, PDT effect, and radiation dose computed using 72 hour reported incubation period
2.3. Models
2.3.1. Computed Cerenkov photon yield from radiation sources
Both analytical and Monte Carlo methods have been used to predict absolute Cerenkov yields from alpha- (Ackerman and Graves 2012) and beta-emitting radionuclides (Mitchell et al 2011, Beattie et al 2012, Gill et al 2015). There is general good agreement among computed values and experimental measurements. Most radionuclides emit 1 to 100 Cerenkov photons/decay, depending on the radionuclide and wavelength range considered (Gill et al 2015).
Cerenkov photon yields for alpha-emitting radionuclides have been computed using GEANT4 Monte Carlo simulation (Ackerman and Graves 2012). Due to their mass, alpha-emitting radionuclides have insufficient energy to directly generate CL (1673 MeV threshold) in tissue. The decay products of some alpha-emitting radionuclides produce CL, but they are not useful for biomedical imaging because CL is not proportional to radioactivity until the sample reaches equilibrium. 212Bi and 213Bi are considered the most suitable alpha-emitting radionuclides for imaging because they partially undergo beta decay, producing CL.
In vivo fluence rates have been estimated for radionuclides and radiotherapy sources (Glaser et al 2015). Monte Carlo simulations (GMOS) were used first to derive Green’s functions for Cerenkov light fluence over a range of biologically-relevant scattering and absorption coefficients. In vivo fluence rates were computed by convolving Green’s functions with a biologically relevant radionuclide activity concentration or radiotherapy beam dose rate. Typical in vivo fluence rates for radionuclides are on the order of nW/cm2/MBq/g, while those from radiotherapy sources are on the order of µW/cm2/Gy/s (Glaser et al 2015).
2.3.2. Cerenkov photon yield from radionuclides
The Cerenkov photon yield of a given radionuclide (unit: photons per decay) is tabulated in several reference reports (Ackerman and Graves 2012, Gill et al 2015). From these values, it is possible to estimate the number of Cerenkov photon emitted given the starting radioactivity (A0, in Bq), half-life (t½, in seconds) and decay time (t), by integrating the radioactive decay formula from time = 0 to t. This yields the following decay factor, which represents the mean number of decays per Bq of radioactivity:
| (1) |
This quantity can be multiplied by the relevant Cerenkov yield to determine the total number of emitted Cerenkov photons from an initial radionuclide activity of 1 Bq:
| (2) |
Photon yields for common medical radionuclides are computed using Equation 2 (Table 1). “Decay-adjusted Cerenkov yield” is the total number of photons emitted after decay of 1 Bq of initial activity over a duration t; “Decay factor” (computed from Equation 1) is computed as a function of t and t½; “Cerenkov yield” is the mean number of Cerenkov photons emitted per decay, factoring in the branching ratio (often <1).
Table 1:
Estimated photon yield and expected dose in tissue from various radionuclides.
| Radionuclide | Half-life [s] | Average emitted beta energy (Emean) [MeV/decay] | Cerenkov yield, 400 – 800 nm [photons/decay] | Decay factor [decays/Bq] | Decay-adjusted Cerenkov yield [photons/Bq] |
|---|---|---|---|---|---|
| 18F | 6,582 | 0.242 | 1.32 | 9,478 | 12,511 |
| 64Cu | 45,723 | 0.060 | 0.56 | 36,278 | 25,605 |
| 68Ga | 4,070 | 0.735 | 33.9 | 5,861 | 198,688 |
| 89Zr | 282,240 | 0.198 | 2.29 | 406,426 | 646,330 |
| 90Y | 230,400 | 0.934 | 47.3 | 331,776 | 10,897,920 |
Cerenkov photon yields from (Gill et al 2015) are computed in the 400 – 800 nm wavelength range and can be scaled into any valid wavelength range (λmin to λmax), according to the following scaling factor:
| (3) |
Radionuclides can impart significant radiation dose to tissue and cause biological effects. In this context, it is important to distinguish between radiotracer dose and radiation dose. Radiotracer dose corresponds to the amount of radioactivity administered to the subject and is given in units of Becquerel (Bq), which is defined the radioactivity of a substance that decays at a rate of one disintegration per second. In contrast, radiation dose is given in units of Gray (Gy) and is defined as the amount of ionizing energy (in Joules) deposited per kg of tissue. The relationship between radiotracer dose and radiation dose is complex and depends on many factors, such as the physical half-life of the radiotracer, its energy, its rate of uptake and excretion from different organs, and the type of radiation (alpha, beta, or gamma).
For the simple case of a beta-emitting radiotracer, radiation dose can be estimated by assuming that the entire kinetic energy of beta particles is deposited into the tissue volume of interest. This neglects the contribution of gamma rays that may be emitted from outside this volume. The radiation dose (in Gy, equivalent to J/kg) is:
| (4) |
where radioactivity concentration is that contained in the considered tissue; Emean is the mean kinetic energy of the emitted beta particles and is tissue density (typically 1 g/cm3). This formula is most accurate when the spatial dimensions of the volume of interest are large compared to particle range (typically ~mm); as volume becomes small, it will tend to overestimate dose due to the incorrect assumption that all energy is deposited within the volume.
2.3.3. Cerenkov photon yield from radiotherapy source
The Cerenkov photon yield (noted RE) has also been determined for radiotherapy source, as a function of energy (Glaser et al 2014); reproduced in Figure 3). The yield increases with photon energy until reaching a plateau around 100 photons/MeV.
Figure 3.
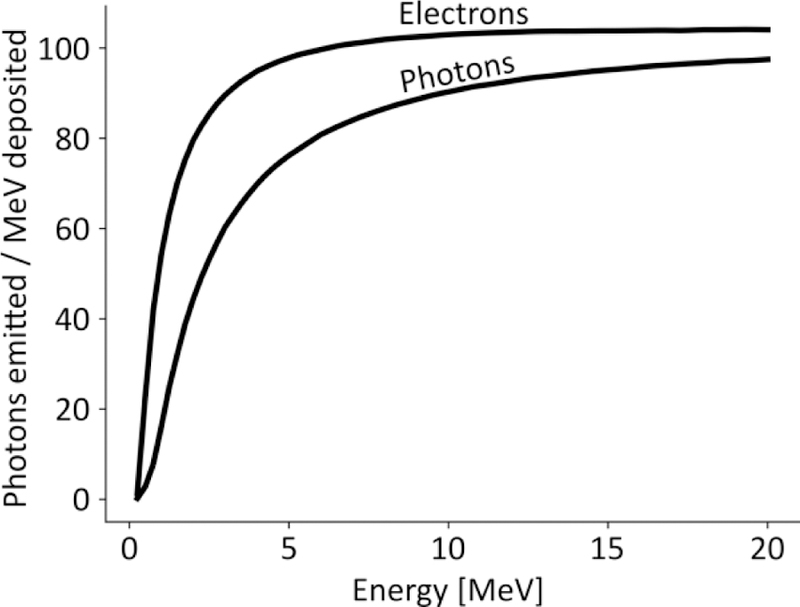
Estimated Cerenkov photon yield per energy deposited from photons and electron beams. Data are adapted from Glaser et al 2014
Cerenkov photon yield per tissue volume is determined by multiplying radiation dose and the experimentally-determined yield factor for either photons or electrons:
| (5) |
For example, tissue receiving 1 Gy from a 10 MV photon beam would generate Cerenkov photons per cm3 of irradiated tissue.
2.3.4. Model of Cerenkov-activated photodynamic therapy
Reported thresholds for effective PDT range from 107 to 109 1O2 molecules per cell (Patterson et al 1990, Georgakoudi et al 1997, Farrell et al 1998, Niedre et al 2003). We estimated cell killing probability using the lowest reported threshold, Nkill = 4×107 1O2 molecules per cell, which killed 63% of cells (one log) and was determined in vitro using protoporphyrin IX photosensitizer and OCI-AML5 cells (Niedre et al 2003).
For the numerical values listed in Table 2, Cerenkov photon yield, radiation dose and probability of cell killing due to PDT effects were estimated under the following optimistic assumptions: 1) the photosensitizer has 100% quantum efficiency at all wavelengths, 2) lowest killing threshold Nkill = 4×107 molecules / 63% cells); 3) cells are 10 µm-radius spheres; and 4) all ROS produced by Cerenkov-PDT are equally potent to 1O2.
The number of 1O2 molecules generated per cell from the excitation of the photosensitizer by Cerenkov light can be computed as:
| (6) |
Furthermore, the probability of a single cell being killed by a given dose of singlet oxygen can be approximated by a simple exponential relationship (Weston and Patterson 2008):
| (7) |
2.3.5. Summary of previous results
Reports of CL-activated PDT are summarized in Table 2 (radionuclides) and Table 3 (radiotherapy sources) and include estimations of Cerenkov photon yield, radiation dose, and probability of cell killing due to PDT effects. All extrapolated quantities are determined using methods and equations described in sections 2.3.2 and 2.3.3. The detailed calculations are also provided in a spreadsheet included as Supplemental Information.
Table 3:
Summary of reports of Cerenkov-activated radiotherapy induced photodynamic therapy
| Reference | X-ray energy [MV] | Photosensitizer | Condition | Outcome | Cerenkov photons absorbed per cell (200– 800 nm) | Estimated Cerenkov PDT effect [% tumor cells killed] | Reported dose to cell layer [Gy] |
|---|---|---|---|---|---|---|---|
| Ouyang et al 2016 | 6 | TiO2 anatase nanoparticles | In vitro, A549 | Decreased colony formation by ~20% compared to radiation group alone | 3.7×106 | 8.88 | 2 |
| Yoon et al 2017* | 6 | Psoralen | In vitro, B16 & 4T1 | Decreased colony formation of 20% and 9.5% for 4T1 and B16 cells, respectively | 2.8×105 | 0.69 | 12 |
Solid-water slab not included in model
For radionuclide sources: The radionuclide was assumed to fully decay, except for long-lived radionuclides such as 90Y, as noted in Table 2; the radiotracer accumulated entirely in the treatment volume (tumor or culture well), the emitted kinetic energy was entirely absorbed in the tumor volume or culture well media volume; cell killing was estimated for in vivo tumors by assuming that all photons generated in the tumor volume were absorbed by photosensitizer molecules in the tumor and converted to 1O2 with 100% efficiency; cell killing was estimated for in vitro cultures as before, but assuming that 50% of Cerenkov photons generated passed through a 10 µm cell monolayer and the photosensitizer optical absorption coefficient was µa = 1 cm-1.
For MV photon sources (e.g. linac): Cerenkov photon yield was computed from radiation dose (Gy) to the treatment volume (volume of liquid in culture well); photons absorbed by the cells were computed by assuming all generated photons passed through the 10 µm cell monolayer and the photosensitizer optical absorption coefficient was µa = 1 cm−1; cell killing was estimated by assuming absorbed photons were all converted to 1O2.
For (Kotagiri et al 2015), the estimated % of tumor cells killed from Cerenkov-activated PDT is 0.25% per 32 MBq 18FDG injection (assuming that the entire injected dose accumulates in the tumor). The starting volume for HT1080 tumors was ~50 mm3, and this would constitute an expected reduction of 0.13 mm3. This estimated therapeutic effect is small compared to the reported tumor suppression of >30 days, compared to untreated controls which grew to volumes >300 mm3. The corresponding estimated radiation dose to the tumor, assuming 100% of the injected dose in the tumor, is >100 Gy, one that should achieve excellent tumor control.
Additionally, it should be emphasized that cell killing is greatly overestimated in this model and only a small fraction of generated photons will result in production of an ROS molecule. The corresponding estimated radiation dose is 10–100 Gy for most studies examined, a significant one that would be expected to reproductively kill >99% of all cells. This simple model strongly suggests that the biological effects due to Cerenkov activated-PDT should be exceedingly small, even at tumoricidal radiation doses.
Critical analysis of results in both Table 2 and Table 3 reveals a similar trend for all experiments: minimal estimated Cerenkov-PDT-induced cell killing, even at very significant radiation dose levels. Based on these results, we conclude that Cerenkov activation of PDT is not the cause of the observed potent therapeutic effect. Most likely, it is a combination of direct excitation of the photosensitizer by ionizing radiation, physical radiosensitization by dense nanoparticles, and nanoparticle surface catalysis. Additionally, past studies have not always used the most appropriate assays for assessing radiation cell killing in vitro (discussed in section 2.3.7) and thus may underestimate the biological effects of ionizing radiation.
Energetic charged particles (created through beta decay or as secondary particles from energetic photons) deposit energy along their path, which can excite photosensitizers through direct excitation and ionization mechanisms (Pratt et al 2018). Furthermore, physical radiosensitization occurs when dense, high-Z nanoparticles absorb ionizing radiation and re-emit the absorbed energy in the form of short-range Auger electrons, which are highly damaging to cells due to their high linear energy transfer (T. Butterworth et al 2012). Finally, catalysis due to a structured water layer on the nanoparticle surface can weaken H-OH bonds and greatly enhances the efficiency of hydroxyl (•OH) production. A 1 nM concentration of 32.5 nm-diameter gold nanoparticles doubles the hydroxyl radical production efficiency (from 200 to 400 nM •OH/J) (Sicard-Roselli et al 2014).
It must also be noted that in vitro cell culture conditions do not appropriately model the optical absorption of Cerenkov light in the medium. Unlike in a tumor, cell culture medium is mostly transparent to visible light, therefore Cerenkov light emitted anywhere within the irradiation volume may propagate to the cell layer at the bottom of the culture vessel. Because of that, the amount of Cerenkov light reaching the cells is strongly dependent upon the volume of medium being irradiated. Furthermore, addition of material in the beam path (such as solid water, as in (Yoon et al 2017)), can increase the generation of Cerenkov radiation beyond what would be observed within a tumor.
Finally, photoreactivable DNA damage may also play a role in observed Cerenkov-induced cell killing, though it has not been explored in eukaryotic cells. This type of DNA damage is unique in that it can be repaired by a light-mediated, enzyme-driven process called photoreactivation. Cerenkov luminescence was shown to induce the majority (70%) of photoreactivable DNA damage from ionizing MV photon radiation (Moss and Smith 1980).
2.3.6. Direct ROS production through water radiolysis
These results should be considered in light of the ROS molecules that are produced directly through water radiolysis, the molecular decomposition of water through ionization and excitation mechanisms. Water radiolysis products are e−aq (solvated electron), •OH (hydroxyl radical), H• (hydrogen), HO2• (water radical), H3O+ (hydronium), −OH (hydroxide), H2O2 (peroxide), H2 (dihydrogen), and 1O2 (singlet oxygen – in aerated solutions) (Caër and Sophie 2011, Sharpatyi and Kraljić 1978). Water radiolysis creates 2.7×104 •OH radicals and 1000–3000 1O2 molecules per MeV of deposited energy (Schwarz 1981, Sharpatyi and Kraljić 1978). Radiolysis-generated •OH radicals are the primary mechanism of ionizing radiation induced cellular damage (Hall and Giaccia 2011). These yields are substantial when compared to Cerenkov luminescence, which yields no more than 100 photons per MeV of absorbed energy. As a result, the number of ROS molecules created through the direct action of ionizing radiation greatly exceeds the number of bioactive molecules potentially produced by Cerenkov luminescence.
2.3.7. Cell viability assays
The standard for assessing tumor therapy is clonogenicity, the capacity of a tumor cell to regenerate indefinitely (Hall and Giaccia 2011). Because the harmful consequences of a tumor stem from its proliferation, this standard is the most relevant from a clinical standpoint. Cancer cells that do not proliferate are not a threat to the patient. The traditional way to assay clonogenicity is the colony formation assay (Franken et al 2006), where a known number of cells are plated and the fraction forming colonies (defined to be 50 cells or more) are counted.
Many previous in vitro studies have relied on assays such as MTS, MTT, WST-1, and XTT, which reflects the number of metabolically active cells. These assays detect the activity of metabolic enzymes by measuring a colorimetric change brought about by the reduction of tetrazolium salts. Metabolic assays are insensitive to clonogenicity and generally inappropriate for assessing cell survival in the context of radiation treatment. A radiation dose of 10 Gy is considered sufficient to clonogenically kill most mammalian tumor cells, yet most cells would remain metabolically active for some time and appear “viable” using a metabolic assay; a dose of 100 Gy is required to halt cell functions (e.g. metabolism) (Hall and Giaccia 2011).
3. Scintillation
3.1. Physics
Scintillation has been defined as “luminescence induced by ionizing radiation in transparent, dielectric media” (Lecoq et al 2016). The additional characteristics of 1) generation by a scintillator via 2) a series of distinct processes capture how the word has traditionally been used and distinguish it from other phenomena such as Cerenkov radiation. Scintillators are the physical materials that facilitate scintillation by transforming absorbed ionizing radiation into lower energy photons (Lecoq et al 2016). Scintillators can be inorganic or organic materials.
3.1.1. Inorganic scintillators
Inorganic scintillators are typically single crystals doped with impurities that confer their luminescent properties, but they can also be semi-crystalline (ceramics) or amorphous (glasses). They are made of dense, high-Z materials, which have increased probability of absorbing ionizing radiation through photoelectric interactions. Unlike fluorescence, scintillation is not a property of a single molecule but an emergent bulk property of the inorganic scintillator material.
When ionizing radiation is absorbed by an inorganic scintillator, electrons are excited to the conduction band and leave positively charged holes in the valence band (Figure 4). After a process known as thermalization, these excited electron-hole pairs migrate within the conduction and valence band of the crystal lattice, respectively, and can enter luminescence centers, quenching centers or traps. Luminescence centers are excited when an electron-hole pair (exciton) recombines, and then emit light. Quenching centers can be similarly excited but they relax through non-radiative thermal dissipation. Traps capture and retain electrons or excitons until they return to the conduction band via acquisition of optical or thermal energy or to the valence band via a radiationless transition (Birks 1964).
Figure 4.
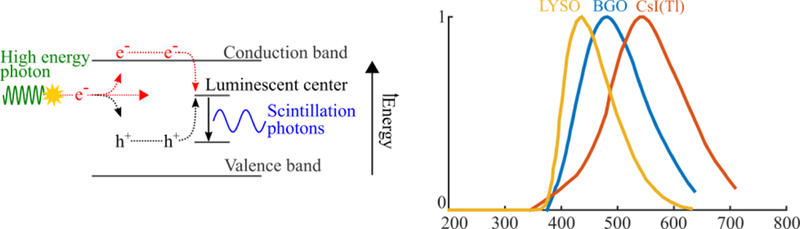
Physics of inorganic scintillation (left) and typical emission spectra for common scintillators (right). Ionizing radiation deposits energy in the scintillator, generating mobile electron-hole pairs that produce scintillation photons when they recombine in luminescent center.
3.1.2. Organic scintillators
Organic scintillators can be solid (crystal or polymer) or liquid; they can be composed of a single molecule (unary) or a system comprising a solvent and one (binary) or more (ternary, quaternary, etc.) solute molecules that are added to enhance fluorescence (Birks 1964). Organic scintillators, unlike inorganic ones, have scintillation properties that come from individual molecules and not from bulk properties of the material. Liquid and solid organic scintillators luminesce through the same physics: excitation by absorption of radiation, non-radiative Forster resonance transfer of excitation to a fluorophore, and luminescent emission.
Solid organic scintillators can be crystals (e.g. anthracene) or polymers (e.g. polystyrene, polyvinyltoluene) with solute (e.g. p-terphenyl) to enhance luminescence. Typical efficiency for solid organic scintillator is 104 photons / MeV (Cherry et al 2012).
Liquid scintillators are chemical cocktails composed of an aromatic solvent (such as toluene) and small quantity of dissolved luminophore solute. Ionizing radiation interacts in the solvent and can yield numerous products (e.g. ions, radicals, excited molecules, fragments, luminescence, x-rays), depending on energy and radiation type. These competing energy- and particle-dependent processes cause luminescent yield from a given energy to be greatest for electrons, then protons, followed by alpha particles. Typical efficiency for a liquid organic scintillator is 104 photons / MeV for electrons (Horrocks 1974).
3.2. Applications
3.2.1. Nuclear medicine and x-ray imaging
Bulk inorganic scintillators are a key component of nuclear imaging detectors, surgical gamma probes, X-ray imaging arrays, and well counters. In a nuclear imaging detector, incident gamma or annihilation photons are converted into light by solid inorganic scintillators. Sensitive optical detectors such as photomultiplier tubes or silicon photomultipliers sense the scintillation light, which is proportional to the absorbed photon energy. Typical inorganic scintillators used are: NaI(Tl), CsI(Tl), BGO, LYSO, or LSO. For comprehensive coverage of this topic see (Knoll 2010) and (Cherry et al 2012).
Organic scintillators are used in surgical beta probes, radiotherapy dosimeters, and in liquid scintillation counters. For surgical beta probes, they are favored compared to inorganic ones, due to decreased efficiency for gamma photon absorption (Daghighian et al 1994). Organic scintillator fibers have been explored as part of monitoring systems for quality assurance and real-time in vivo dosimetry (Beddar 2006). Finally, liquid scintillation counters are used for quantification of short-range alpha- or bets-emitting radionuclides, which are difficult to measure by other means (Horrocks 1974).
3.2.2. Radioluminescence microscopy
Radioluminescence microscopy (RLM) is an imaging modality that permits cellular-resolution imaging of beta-emitting radionuclides, which is otherwise not possible with PET or SPECT imaging. It consists of a high sensitivity camera coupled to a microscope objective that images a thin (typically 100–500 μm) CdWO4 scintillator placed above or below a culture monolayer (Kim et al 2017). By imaging beta particle scintillation tracks and applying a reconstruction algorithm (Pratx et al 2013), <20 μm resolution is achievable (Wang et al 2017). RLM has been used to study metabolism (18F-FDG), transgene expression (18F-FHBG) (Pratx et al 2012), and cell proliferation with 18F-FLT (Sengupta and Pratx 2016) in MDA-MB-231 human breast adenocarcinoma.
4. Radioluminescent nanoparticles
4.1. Physics
4.1.1. Interaction of tissue and ionizing radiation
The physics governing the interaction of nanoparticles, tissue, and ionizing radiation are depicted in Figure 7. Energetic photons (e.g. X-rays, gamma rays) interact with biological tissues primarily through two effects (Compton scattering and photoelectric absorption) and, by these processes, impart energy to an ejected electron (called recoil electron or photoelectron, respectively).
Figure 7.
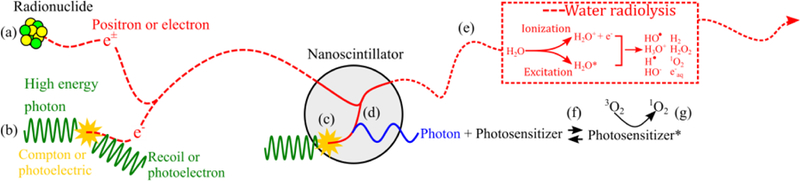
Physics of ionizing radiation interacting in scintillator-containing tissue. Electrons or positrons are emitted into tissue via (a) beta-emitting radionuclides or (b) as recoil or photoelectrons from the interactions of high-energy photons with tissue. Electrons or positrons passing through tissue generate reactive species through water radiolysis (e). Photons can directly interact in the nanoscintillator, generating electrons through Compton or photoelectric absorption (c). Electrons generated in tissue (a,b) or nanoscintillator (c) deposit a small fraction of their energy in nanoscintillators, producing scintillation photons (d). Scintillation photons absorbed by the photosensitizer (f) can generate singlet oxygen or other reactive oxygen species (g). The range of ionizing particles is several orders of magnitude larger than the diameter of nanoscintillators.
4.1.2. Interaction of nanoparticles and ionizing radiation
The introduction of high-Z nanoparticles increases the X-ray absorption cross section, particularly at lower energies (<100 keV). The radiation dose in the immediate vicinity of the nanoparticle is enhanced, a phenomenon called physical radiosensitization (T. Butterworth et al 2012). However, it important to note that the energy transferred from an X-ray photon to a nanoparticle is for the most part released into the surrounding medium due to the small size of nanoparticles compared to the range of ionizing charged particles. As a result, only a small fraction of the energy of the interacting X-ray is available to stimulate nanoparticle luminescence.
4.1.3. Materials and Physiochemical Properties
Radioluminescent nanoparticles are made of materials that luminesce when excited with ionizing radiation. Attachment of high affinity ligands, such as antibodies, peptides or small molecules, or physiological trafficking of these nanoparticles allow for optical imaging of specific molecular processes. Nanoparticle-based probes have drawn significant attention for their unique physiochemical properties when applied in these biological applications. Novel nanoscintillators, such as quantum dots (Nikolopoulos et al 2016), metal nanoclusters (Osakada et al 2014), metal organic frameworks (Wang et al 2014), and polymer dots (Osakada et al 2013) have been examined as radioluminescent probes. However, the most widely studied ones are nanophosphors, which have become synonymous with nanoscintillators. Phosphors are materials that luminesce in the form of either fluorescence or phosphorescence when excited with radiation. They are prevalent in the biomedical field in X-ray screens (Yaffe and Rowlands 1997) and, more recently, as contrast agents for X-ray luminescence imaging.
A unique feature of nanomaterials, including nanoscintillators, is their high surface area-to-volume ratio, which allows for extensive surface loading and potential multivalent binding to facilitate specificity. The role of the surface chemistry also has a significant impact on the biological interaction of the nanoparticle in the bloodstream for in vivo applications. Biocompatible coatings are necessary to avoid rapid clearance by the immune system; however, these may also affect energy transfer in PDT applications in which photosensitizers are grafted via linker molecules. Another important consideration is the surface of the nanocrystals, as these sites and defects may negatively impact the luminescent output of the material in comparison to their bulk properties (Dujardin et al 2010). To address this potential surface quenching, nanophosphors comprised of core-shell architectures have been employed to improve radioluminescence efficiency (Naczynski et al 2015).
4.2. Applications
4.2.1. Radioluminescent nanoparticle imaging
Tomographic radioluminescence imaging modalities are inspired from fluorescence x-ray computed tomography (XFCT; (Cesareo and Mascarenhas 1989), a method that forms images by exciting and detecting X-ray fluorescence. However, unlike XFCT, they form images by detecting optical, rather than X-ray, photons.
X-ray luminescence computed tomography (XLCT) uses an X-ray pencil beam to excite nanophosphor contrast agents, which emit near-infrared (NIR) photons that are detected with an optical detector. The approach takes advantage of weak scattering in tissue of the X-ray excitation beam and enables reconstruction of high quality, tomographic images regardless of optical scatter (Pratx et al 2010a, 2010b, Li et al 2013).
A critical factor in the development of these imaging platforms is the uptake or total quantity of materials accumulating at sites of interest. Few studies have demonstrated target-specific accumulation of probes with subsequent in vivo imaging. Often, concentrations administered via intravenous injection are close to the limit of detection. However, depending on the imaging application (e.g. tumor cell detection), the accumulation of probes can increase if preferential uptake by target cells is achieved. An additional factor to consider is the local aggregation of the material in endosomal and lysosomal compartments, which could impact local nanoparticle densities and subsequent energy transfer between different excited nanoparticles. Unique applications of X-ray luminescence imaging include cancer cell imaging in the 1000–1500 nm window (Naczynski et al 2015), pH monitoring (Chen et al 2013), and drug delivery (Moore Thomas L. et al 2014).
4.2.2. X-ray activated photodynamic therapy
There is growing interest in combining nanoparticle scintillators with radiotherapy to activate photodynamic therapy (Morgan et al 2009, Scaffidi et al 2011, Chen et al 2015, Clement et al 2016). The general reasoning is that these nanoparticles will efficiently transduce X-ray energy into light for photosensitizer activation and thereby provide additional efficacy during radiotherapy with no additional radiation dose.
In vitro evidence of nanoparticle efficacy has been reported using psoralen-conjugated Y2O3 nanoparticles (Scaffidi et al 2011). However, the reported biological effect is small, with physical radiosensitization, due to the presence of nanoscintillator alone, being the dominant factor. X-ray-activated PDT has also been tested in vitro using 8 keV X-rays and a CeF3 nanoparticle conjugated to vetereporfin (Clement et al 2016). This low energy used in this experiment is favorable for observing an effect, due to the greatly enhanced X-ray absorption cross-section of the nanoparticle, yet low energy X-rays are not practical for radiotherapy. In the following Models section, we review the theoretical basis for this idea and provide an appropriate model for estimating its efficacy.
4.2.3. Clinical evidence of PDT-activation through non-light mechanism
Therapeutic enhancement has been reported by combining a photosensitizer with radiotherapy; significant necrosis and inhibition of mouse osteosarcoma tumors, compared with sham-treated mice, was reported using a 5 Gy dose and acridine orange photosensitizer (Hashiguchi et al 2002). A subsequent uncontrolled clinical trial treated six synovial sarcoma patients with a 5 Gy radiotherapy beam dose and acradine orange and none had local recurrence during follow up (ranged from 19–52 months) (Kusuzaki et al 2005). A semi-controlled study found better outcomes with minimally invasive surgery followed by radiotherapy and acridine orange, than wide limb resection alone (Matsubara et al 2010). None of these studies was rigorously controlled so as to generate the highest level of medical evidence. However, their consistency and results are encouraging and suggest the possibility of a non-light-based mechanism of PDT activation.
4.3. Models
4.3.1. Limitations of previous models
Previous studies have theoretically investigated the use of nanoparticles to transduce ionizing radiation into optical photons for activation of PDT (Morgan et al 2009, Clement et al 2016). Based on the assumption that X-ray photons impinging on a nanoparticle would transfer all of their energy to that nanoparticle, these studies have concluded that this strategy would be effective for photon sources <300 kV.
An alternative model for nanoscintillator-mediated PDT recognizes that secondary electrons generated in the nanoscintillator have ranges much greater than the size of the nanoscintillator (Bulin et al 2015). Therefore, contrary to prior assumptions, only a small fraction of the X-ray energy will generate light within the nanoscintillator. At physiological nanoparticle concentrations (i.e. <1 mg/cm3), biological effects of radiation are primarily mediated by water, the most abundant molecule in biological tissues. Upon absorbing X-ray photons, water releases energetic electrons (mostly Compton recoil electrons) that propagate through the tissue and deposit energy along their path. When such an electron encounters a nanoparticle, some of its energy is transferred to the nanoparticle, stimulating the emission of light (Figure 7). The amount of energy transferred is a function of the electron energy, nanoparticle size, and nanoparticle composition (electron stopping power and density; (Berger et al 2005). The typical energy transfer between an electron and 20 nm diameter nanoparticle is on the order of 10 eV. This indirect transfer of energy from ionizing photon to scintillator molecule is the primary mechanism for X-ray luminescence.
4.3.2. Proposed electron absorption cross section-based model
Because X-ray are predominantly absorbed by water molecules, the efficiency of X-ray luminescence is nearly independent of nanoparticle X-ray stopping power. The luminescence yield can be approximated as a function of the radiation dose to tissue (unit: Gy), the nanoparticle mass concentration CNP (unit: g/cm3), the scintillator light yield Ysc (assumed here to be 105 photons/MeV) and the electron cross-sections for tissue and the nanoparticle material. The modified model for estimating the density of scintillation photons emitted by the nanoparticle is:
| (8) |
A derivation of this equation is provided as Online Supplemental Information. The stopping power coefficients for electrons can be obtained from the ESTAR database, which is maintained by the National Institute of Standards and Technologies (NIST). Singlet oxygen concentration is calculated by assuming that all photons are converted to singlet oxygen molecules:
| (9) |
The number of singlet oxygen molecules per cell is calculated using the cell volume estimated assuming 10 µm diameter spherical cells:
| (10) |
The model based on electron stopping power should more accurately predict the efficacy of X-ray PDT, compared to previous models. It should be effective for predicting PDT efficacy within the correct order of magnitude. However, it should be noted that values obtained with this model represent an upper bound for the actual number of scintillation photons emitted, as explained in the supplemental information, and thus are approximate. The model also does not account for enhanced radiation dose (physical radiosensitization) near nanoparticles, and therefore it is valid mainly for low nanoparticle concentrations (< 10 mg/cm3), where particles are relatively far from one another. For greater accuracy, Monte Carlo simulations, which model photon-nanoscintillator interactions in greater detail, are necessary (Bulin et al 2015).
Sample photon yields are computed in Table 4 for 1 Gy radiation delivered to tissue containing 1 mg/cm3 LaF3 nanoscintillator. Over a large energy range, photon production by the nanoscintillator is relatively inefficient, yielding <100 photons per MeV of energy deposited. Although less efficient than Cerenkov luminescence, nanoparticle radioluminescence is localized to the nanoparticle, and therefore the scintillation light may be easier to harvest for efficient photosensitizer excitation. These calculations are also provided in a convenient spreadsheet included as Supplemental Information.
Table 4:
Estimated photon yield from 1 mg/cm3 LaF3 nanoscintillator in tissue receiving 1 Gy radiation dose using different photon energies
| Photon energy [MV] | Electron mass attenuation coefficient [MeV∙cm2/g] | Photons emitted | ||
|---|---|---|---|---|
| LaF3 | Tissue | Photons / cm3 | Photons / MeV deposited | |
| 0.001 | 41.9 | 121 | 2.15 × 1011 | 34 |
| 0.01 | 12.3 | 22.6 | 3.37 × 1011 | 54 |
| 0.1 | 2.55 | 4.11 | 3.85 × 1011 | 62 |
| 1 | 1.26 | 1.85 | 4.22 × 1011 | 68 |
| 10 | 2.09 | 2.13 | 6.08 × 1011 | 97 |
The table also shows that LaF3 (and other high-Z materials) have a lower mass-energy attenuation coefficient for electrons than water. This reflects the fact that the nucleus of high-Z atoms contains a higher fraction of neutrons than protons, compared to lower-Z nuclei. Nevertheless, the high density of nanoparticles ultimately results in increased absorption cross-section and enhanced energy absorption compared to water.
4.3.3. Comparison of proposed electron cross section model with previous photon-based model
The impact of electron cross sections can be demonstrated by substituting electron for photon cross sections into the model that predicts singlet oxygen molecules per cell (Morgan et al 2009). Here, the volume of a cell is taken to be 4.2×10−9 cm3 (10 µm-diameter sphere) and a concentration of 3 mg/cm3 of LaF3 NP is assumed. The modified model predicts ~106 1O2 per cell over a wide energy range and is factor of 101 to 103 less than the original model (Figure 8). This result is much less than the needed 107 1O2 per cell PDT threshold (Niedre et al 2003) and suggests that even a 60 Gy dose would be insufficient for an enhancement effect.
Figure 8.
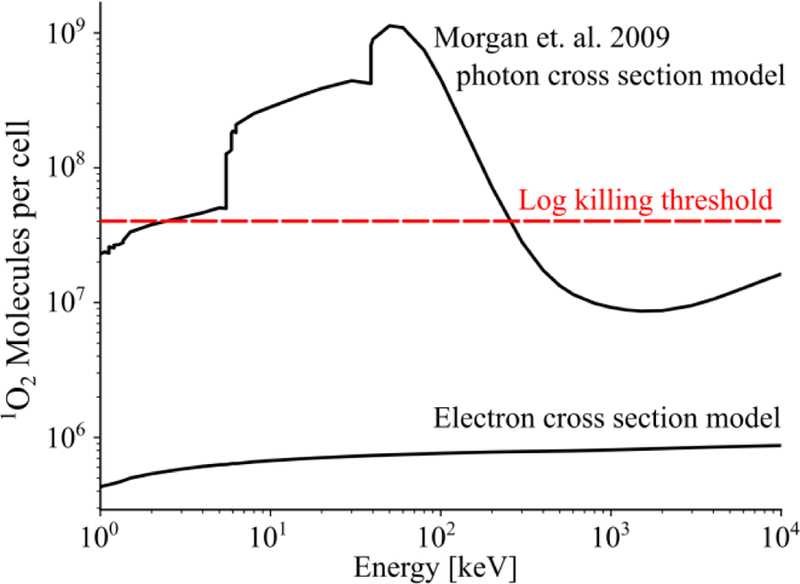
Estimated molecules of O2 generated per cell for a 60 Gy photon dose. Models that use either photon or electron cross secitons are plotted, along with an established log killing threshold (dashed).
5. Air fluorescence
5.1. Physics
Air fluorescence, also known as air scintillation, is fluorescence emitted by atmospheric gases excited and/or ionized by radiation. The three most abundant atmospheric gases—nitrogen (78% v/v), oxygen (21% v/v) and argon (1% v/v)—determine the fluorescent yield. Other atmospheric gases such as CO2 and CH4 are potent fluorescent quenchers (Morii et al 2004), but not present in quantities (US EPA 2016) sufficient to appreciably influence fluorescent yield.
Nitrogen fluoresces primarily via , , or (where * denotes an excited state and + denotes positive charge due electron loss) reactions which yield of 141 photon/MeV of deposited energy (Morii et al 2004). Argon fluoresces brightly, yielding 104 photon/MeV via primary (Suzuki and Kubota 1979) and secondary three-body:, reactions (Monteiro et al 2008). Oxygen is weakly fluorescent, yielding ~0.5 photons/MeV of deposited energy (Morii et al 2004), yet it is also a powerful quencher of nitrogen and argon fluorescence via and reactions. Consequently, the spectral properties of air fluorescence are dominated by nitrogen while the fluorescent yield, 25 photons/MeV deposited energy, is dominated by the quenching action of oxygen.
5.2. Applications
Air fluorescence has been used to detect the interaction of high-energy cosmic radiation with the Earth’s upper atmosphere, which produce an abundance of secondary radiations dispersed over hundreds of square kilometers.
Air fluorescence can be imaged during radiotherapy, providing monitoring capabilities that could prevent gross dosimetry errors during treatment (Fahimian et al 2014). This approach is relatively simple to implement and would consist of a sensitive camera optimized for ultraviolet imaging and short-pass filters to block ambient room lighting; gating image acquisition with radiotherapy source pulse could also greatly improve signal-to-noise ratio.
Air fluorescence can be a significant source of background during X-ray luminescence imaging, especially in the kV range. Fortunately, this emission is primarily at wavelengths below 430 nm, thus it can be blocked using a suitable long-pass filter.
5.3. Models
5.3.1. Air fluorescence yield from typical radiotherapy source
Air fluorescence yield from a radiotherapy source is estimated at a point along the beam as follows. The considered geometry is depicted in Figure 11.
Figure 11.
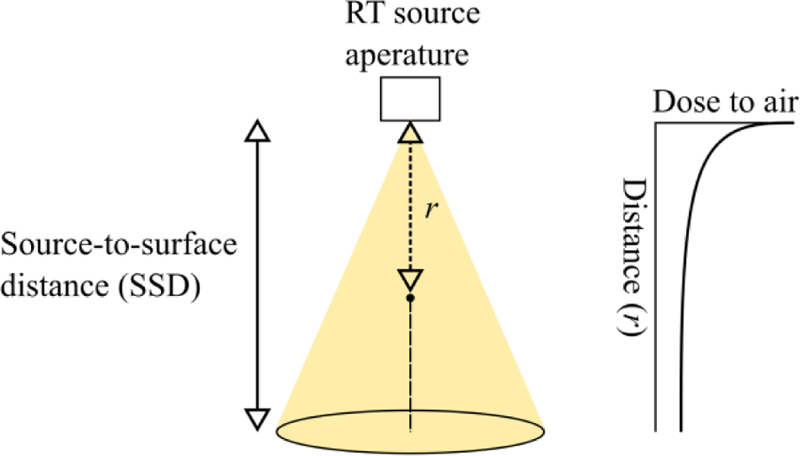
Air fluorescence radiotherapy geometry. Dose to air falls with the inverse square of the distance from source (right).
Dose to air is the related to dose to water according to the ratio of their respective mass attenuation coefficients. Furthermore, if we assume that luminescence is observed at a distance r from the source and that dose to water is for a given source-to-surface distance (SSD; r<SSD; Figure 11), the divergence of the beam emanating from the linear accelerator can be modeled by incorporating a 1/r2 factor into the formula:
| (11) |
The energy deposited in air (MeV/cm3) is computed by multiplying the dose by air density (ρair = 1.23∙10−6 kg/cm3) and MeV per joule unit conversion factor:
| (12) |
Finally, light production (photons/cm3) is determined by multiplying the deposited energy by experimentally-determined air fluorescence yield (Morii et al 2004):
| (13) |
Estimated photon yield for electron and photon sources of typical radiotherapy energies are listed in Table 5, assuming 1 Gy to tissue at SSD = 100 cm. Mass-energy attenuation coefficients for photon and electrons in air and tissue were sourced from online databases (Berger et al 2005, 2009). The calculations are also provided in a spreadsheet included as Supplemental Information.
Table 5:
Energy deposited in air and computed luminescence from 6 and 10 MeV electrons or photons emitted from a radiotherapy source.
| Energy [MeV] | Particle type | Mass-energy attenuation coefficient [cm2/g or MeV∙cm2/g] | Dose to air (r = 50 cm) per 1 Gy dose to tissue (SSD = 100 cm) [Gy] | Photons generated (SSD = 50 cm) [photons/cm3] | |
|---|---|---|---|---|---|
| Air | Tissue | ||||
| 6 | Photon | 0.0165 | 0.0179 | 3.69 | 7.00×108 |
| 10 | Photon | 0.0145 | 0.0155 | 3.74 | 7.11×108 |
| 6 | e− | 1.97 | 2.00 | 3.94 | 7.49×108 |
| 10 | e− | 2.16 | 2.13 | 4.05 | 7.69×108 |
6. Persistent/delayed luminescence
6.1. Physics
Persistent luminescence is a phenomenon that is caused by slow liberation of radiation-induced trapped charged carriers (Van den Eeckhout et al 2010). Thermoluminescence is the heating of persistent luminescent materials to liberate radiation-induced electrons that were trapped at metastable sites. Luminescence is produced when these electrons recombine with corresponding holes and is used to reveal trap levels in a given material (Van den Eeckhout et al 2010). Optically-stimulated luminescence, similar to thermoluminescence, is optically induced liberation of trapped electrons. These electrons similarly recombine with localized holes, producing luminescence (Boetter-Jensen et al 2003). The emitted luminescence is proportional to radiation dose accumulated by the material (Nelson et al 1967), a useful relationship that has been used for dating of archaeological and geological materials, biological dosimetry, and in photostimulable phosphor plates used for X-ray medical imaging (Rowlands 2002).
In the in vivo setting, detectable in irradiated animals and persisting for minutes following irradiation, this phenomenon has been called radiobioluminescence (Rao 2015). This effect has been observed both in live and dead tissues, suggesting that its origin is not biological but physical. The mechanism is likely related to food thermoluminescence, caused by presence of thermoluminescent minerals (e.g. sand and dust; (Sanderson et al 1989, Soika and Delincée 2000).
6.2. Applications
Persistent luminescent nanoparticles, both silicate- and polymer-based, have been developed for preclinical imaging and in vivo PDT activation. They have favorable properties for sensitive in vivo imaging (Chermont et al 2007, Maldiney et al 2012, Palner et al 2015). These are: 1) can be excited with visible, ultraviolet, or x-ray photons before administration or in vivo, repeatedly; 2) emission wavelengths in the 600–800 nm range, which is favorable for imaging owing to weak attenuation by biological tissues; 3) can luminescence for >10 hours (Maldiney et al 2013), eliminating background signal induced by excitation source (e.g. autofluorescence).
Persistent luminescence nanoparticles have been developed that can be repeatedly excited in vivo by external X-ray irradiation for imaging and activation of PDT (Chen et al 2017, Song Liang et al 2018), as shown in Figure 13.
Figure 13.
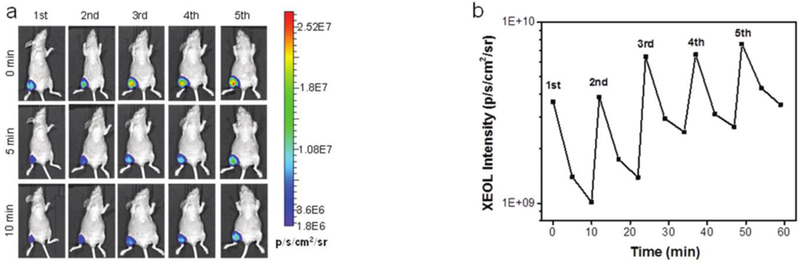
Imaging (left) of repeated x-ray activation of persistent luminescence nanoparticles in vivo and ROI quantitation following repeated excitation (right) from Chen et al 2017.
7. Radioluminescence of biologically-endogenous molecules
7.1. Physics
7.1.1. Protein and amino acid luminescence
Biological molecules such as trypsin, tyrosine, phenyalanine, and tryptophan exhibit both immediate luminescence and long afterglow following X-ray irradiation. Immediate luminescence emitted is fluorescence caused by electron excitation; it decreases with absorbed dose likely due to temporary molecular damage (Nelson et al 1967).
7.1.2. Water radioluminescence
Radioluminescence has been observed in pure water (Sitharamarao and Duncan 1963) at energies below the Cerenkov threshold (Quickenden 1971, Tarasov et al 2007, Spinelli et al 2011, Yamamoto et al 2016). Mechanisms for experimentally-observed water radioluminescence are fluorescence and chemiluminescence related to trace impurities as well as fluorescence of water and radiolysis products.
Impurity-related luminescence can be caused by direct excitation of endogenous luminophores dissolved in water (e.g. aromatic amino acids, humic compounds; (Belovolova et al 2009) or chemiluminescence resulting from the reaction of water radiolysis products with trace impurities (Vasil’ev, R.F. 1970). Impurity-related luminescence is not observed in purified water.
Water is inherently fluorescent (Quickenden 1971, Belovolova et al 2009) and water-radiolysis products, including hydroxyl radicals (•OH), singlet oxygen (1O2), hydrated electrons (eaq−) are also fluorescent (Sitharamarao and Duncan 1963, Quickenden 1971).
Water radioluminescence is ~60× less efficient than air fluorescence, equating to a yield of <1 photon/MeV of deposited energy (Tarasov et al 2007).
7.2. Models
Similar to air fluorescence (see section 5.3), the radioluminescence of biological molecules is proportional to the energy deposited into them.
7.3. Applications
Water radioluminescence, used in combination with a water phantom and sensitive camera, has been suggested as a means of dose estimation for lower energy x-ray beams (Yamamoto et al 2016). It could supplement or replace ionization chamber point measurements. Compared to Cerenkov luminescence, the signal is not hampered by threshold effects. It could also be used to measure the range of protons and ion beams in water, given that these particles do not generate Cerenkov luminescence at clinically relevant energies (Yamamoto et al 2016).
Endogenous radioluminophores such as aromatic amino acids (e.g. tryptophan) can also be imaged with XLCT, using suitable emission filters. However, in most situations, they create an unwanted luminescent background that impede the detection of low concentrations of administered molecular probes. Spectral or temporal filtering approaches must be implemented to reject this “auto-radioluminescence” and achieve high sensitivity with XLCT.
8. Summary
8.1. Summary of radioluminescence sources
Radioluminescence has been explored for a multitude of biomedical applications and this review has endeavored to summarize the physics and applications of all relevant phenomena. We note a recent and complementary review that focuses on biomedical applications at the intersection of optical and ionizing radiation (Pogue and Wilson 2018). This summary quantitatively tabulates the strength of each phenomenon in Table 6 and speculates on their utility for either imaging or therapy in the biomedical setting.
Table 6:
Summary of radioluminescent sources
| Source | Photon yield (photons / MeV energy deposited) | Spectral range (nm) | Mechanism | References | |||
|---|---|---|---|---|---|---|---|
| Inorganic scintillators | 104 – 105 | 400 – 600 | Scintillation | (Cherry et al 2012) | |||
| Organic scintillators | 104 | 300 – 450 | Fluorescence / phosphorescence | (Birks 1964) | |||
| Liquid scintillators | 104 | 300 – 450 | Fluorescence / phosphorescence | (Horrocks 1974) | |||
| Air scintillation | N2 | 120 | 100 – 500 (peaks) | Ionization / fluorescence | (Suzuki and Kubota 1979, Morii et al 2004) | ||
| O2 | <1 (quencher) | ||||||
| Ar | 104 | ||||||
| Air | 25 | ||||||
| Cerenkov radiation | Radionuclides | 1 – 100 (decay−1) | 200 – 800 | Cerenkov radiation | (Ackerman and Graves 2012, Gill et al 2015) | ||
| Radiotherapy | 6 MeV e− | 100 | 200 – 800 | Cerenkov radiation | (Glaser et al 2014) | ||
| 10 MeV e− | 103 | ||||||
| 6 MeV photon | 81 | ||||||
| 10 MeV photon | 91 | ||||||
| Nanoparticle scintillators (1 mg/cm3) | 10 – 100 | 400 – 600 | Scintillation | (Bulin et al 2015) | |||
| Endogenous biological molecules (Tyrosine, Trypsin, Phenylalanine, Tryptophan) † | Immediate | Thermo-luminescence | 300 – 600 | Fluorescence / persistent luminescence | (Nelson et al 1967) | ||
| 8×103 - 6×104 | 4.5 – 310 | ||||||
| Water radioluminescence | < 1 | 200 – 600 (500 peak) | Fluorescence | (Tarasov et al 2007) | |||
Reported values are for molecules in their pure, crystalized form; typical concentrate is ~µg/cm3 in vivo (Madras et al 1974)
8.1.1. Imaging
Single-photon-sensitivity cameras can image all radioluminescence signals, given sufficient integration time. However, some phenomena are impractically weak to be useful in the biomedical setting, especially where better methods are already available.
Scintillator-based detectors have proven their utility for radionuclide and radiological imaging, owing to their ability to efficiently transduce ionizing photons into detectable optical ones. Though advances in scintillator materials and optical detectors have improved their performance over past decades, the general concept has changed little. Recently, scintillators have found new uses as in vivo nanoscintillator contrast agents for x-ray luminescence computed tomography and in radioluminescence microscopy for cellular-resolution imaging of beta-emitting radiotracers.
Cerenkov luminescence provides a means for simple, direct imaging of radionuclides via optical signals. It is a suitable substitute for in vivo PET imaging when the trade-off of decreased in vivo imaging resolution, lack of penetration and absolute quantitation are minor compared to benefits of cost, speed and simplicity. Consequently, preclinical, radiotherapy, and surgical applications appear to be most useful for CLI.
Persistent luminescence has been harnessed extensively in radiation dosimetry and in storage phosphors for X-ray medical imaging. It is also a source of noise emitted by biologically-endogenous molecules during sensitive, prolonged imaging of living organisms. Recently, persistent luminescence nanoparticles have been used for in vivo imaging. These materials can be repeatedly excited in vivo via X-ray radiation and luminesce for a sustained duration.
Water radioluminescence is the weakest form of radioluminescence. It has been explored for radiotherapy applications, however other methods could likely accomplish the same goal. For example, direct excitation of a fluorescent solution could provide a brighter, more readily detectable signal for kV X-ray dosimetry.
8.1.2. Therapy
Clinical phototherapies require bright, sustained sources of light. Most radioluminescence sources are too weak to provide sufficient radiant exposure to activate these therapies. Our analysis shows that radionuclide-generated Cerenkov luminescence is too weak to activate photodynamic therapy at reasonable radioactivity concentrations. Radiotherapy-generated Cerenkov luminescence is brighter, but still unlikely as a means of activating phototherapy.
Nanoscintillators have been suggested as therapeutic agents to efficiently transduce radiotherapy photons into light for phototherapy. While these high-Z nanoparticles are certainty effective physical radiosensitizers, our analysis shows their potential contributions to therapy to be exceedingly small at physiological concentrations.
For both Cerenkov luminescence and radiation-activated nanoscintillators, the delivered radiation dose must exceed tumoricidal levels to observe even the smallest PDT effect. We conclude that therapeutic uses of radioluminescence, based on available literature, are unlikely with current nanoparticles and photosensitizers.
Supplementary Material
Figure 2.
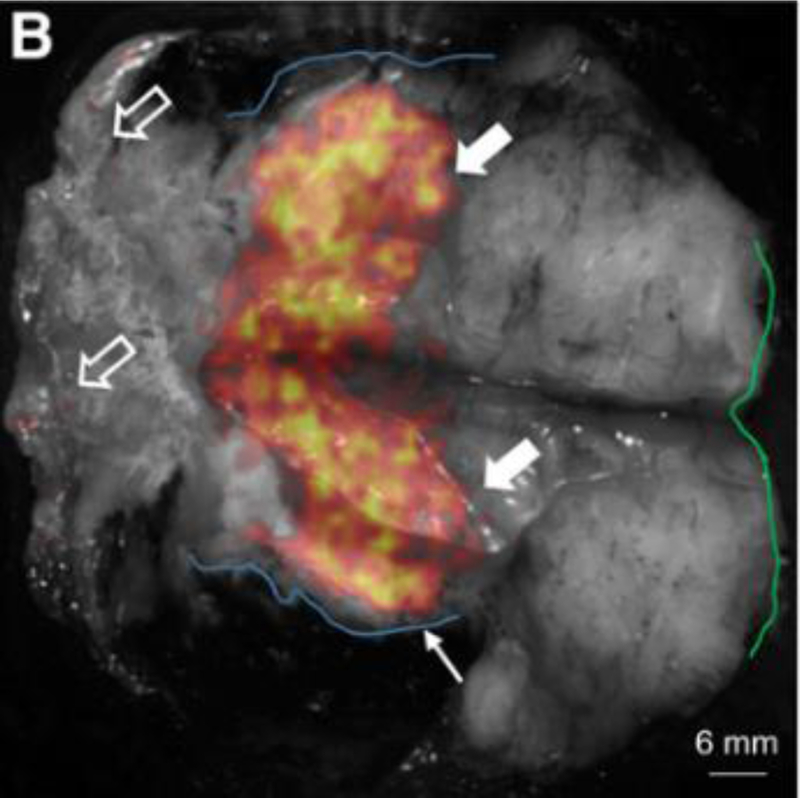
Ex vivo specimen imaging of excised breast tissue. Black and white is reflectance tissue image and overlaid heatmap is CL image. This research was originally published in JNM. Grootendorst M. R. et al. A 2016 Intraoperative Assessment of Tumor Resection Margins in Breast-Conserving Surgery using 18F-FDG Cerenkov Luminescence Imaging – A First-in-Human Feasibility Study. J Nucl Med. 2016. Jun;58(6):891–898. © SNMMI.
Figure 5.
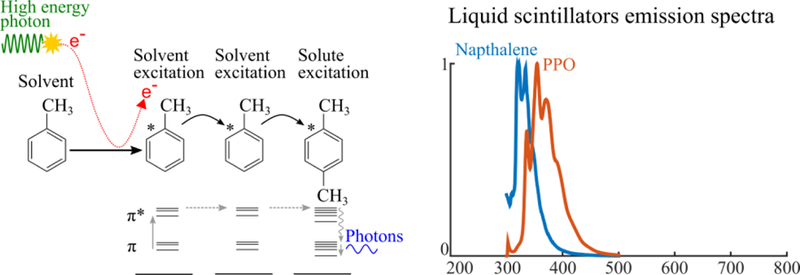
Physics of organic scintillators (left) and emission spectra (right). Primary or secondary charged particles deposit energy in the solvent, exciting solvent molecules. Excitation energy transfers non-radiatively from solvent to solvent molecule. Luminescence is produced when excitation energy is transferred to a solute molecule.
Figure 6.
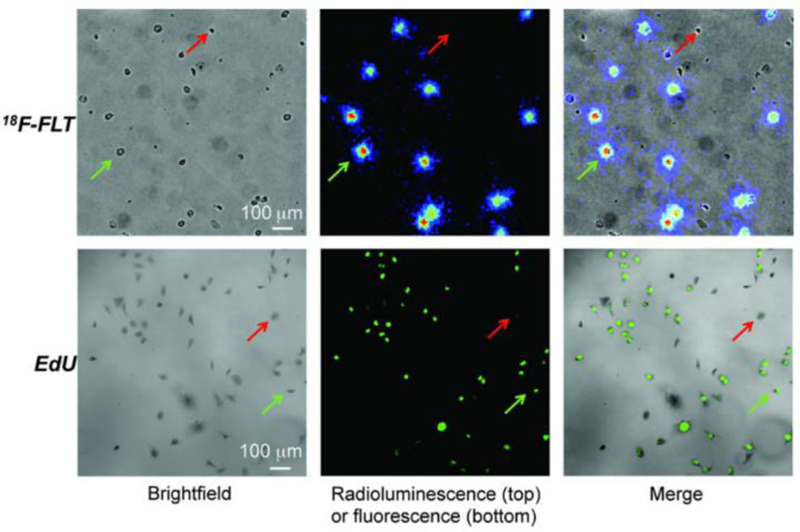
MDA-MB-231 cells imaged using 18F-FLT RLM and EdU fluorescence microscopy. This research was originally published in JNM. Sengupta, D. and Pratx, G. Single-cell characterization of FLT uptake with radioluminescence microscopy. J Nucl Med. 2016;Jul;57(7):1136–40. © SNMMI
Figure 9.
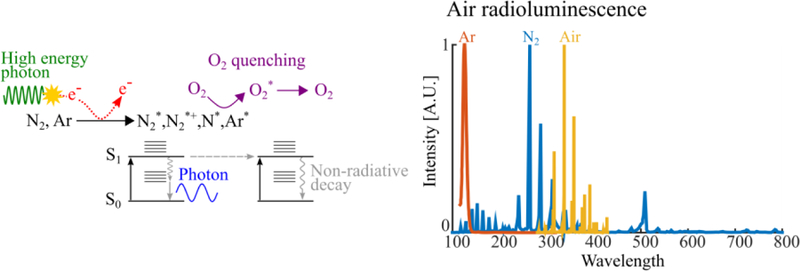
Air fluorescence physics (left) and emission spectra (right). Electrons generated from the interaction of ionizing radiation with air generates electrons that excite its fluorescent constituents (primarily N2).
Figure 10.
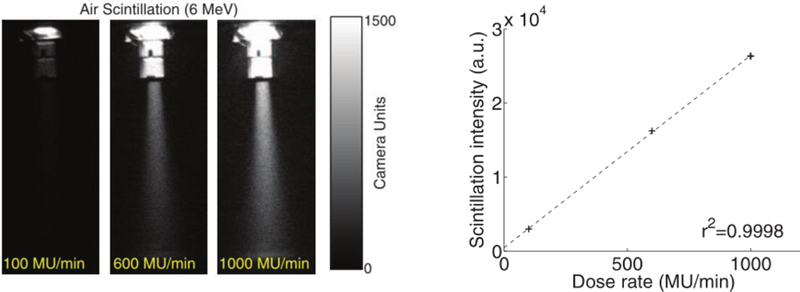
Air fluorescence images from a 6 MeV electron beam at various dose rates (left) and linear relationship between fluorescence intensity and dose rate (right), from Fahimian et al 2014.
Figure 12.
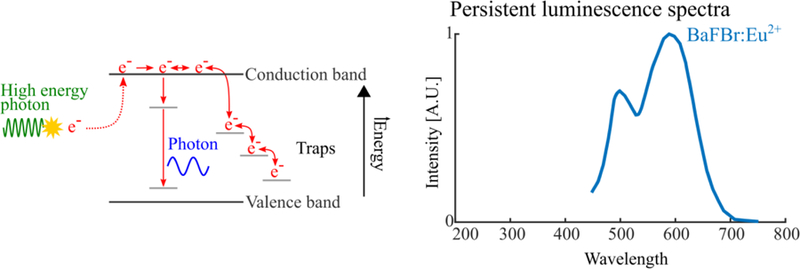
Persistent luminescence physics (left) and spectra of fluorescence and persistently luminescent biological molecules (right). Absorption of energy excites electrons into the conduction band of the persistent luminescent material. Electrons can fall into traps and also return to the conduction band, eventually leading to luminescence.
Figure 14.
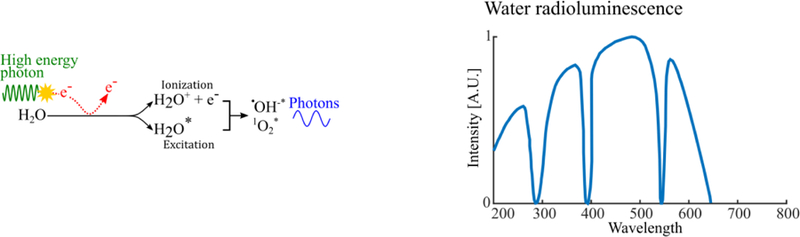
Water radioluminesce physics (left) and typical spectrum (right). In pure water, radioluminescence is induced by excitation of water and water-radiolysis products by ionizing radiation.
9. Acknowledgments
Justin Klein is a Stanford Molecular Imaging Scholar under NIH grant T32 CA1186810. Conroy Sun acknowledges support from NIH Grant 5R35GM119839. Guillem Pratx receives support from NIH under grants 5R01CA186275 and 5R21CA193001.
References
- Ackerman NL and Graves EE 2012. The potential for Cerenkov luminescence imaging of alpha-emitting radionuclides Phys. Med. Biol 57 771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie BJ, Thorek DLJ, Schmidtlein CR, Pentlow KS, Humm JL and Hielscher AH 2012. Quantitative Modeling of Cerenkov Light Production Efficiency from Medical Radionuclides PLOS ONE 7 e31402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beddar AS 2006. Plastic scintillation dosimetry and its application to radiotherapy Radiat. Meas 41 S124–33 [Google Scholar]
- Belovolova LV, Glushkov MV, Vinogradov EA, Babintsev VA and Golovanov VI 2009. Ultraviolet fluorescence of water and highly diluted aqueous media Phys. Wave Phenom 17 21–31 [Google Scholar]
- Berger MJ, Coursey JS, Zucker MA and Chang J 2005. NIST Stopping-Power and Range Tables: Electrons, Protons, Helium Ions Online: http://www.nist.gov/pml/data/star/index.cfm
- Berger MJ, Hubbell JH, Seltzer SM, Chang J, Coursey JS, Sukumar R, Zucker DS and Olsen K 2009. XCOM: Photon Cross Section Database (version 1.5). [Online] NIST Stand. Ref. Database 8 XGAM Online: https://www.nist.gov/pml/xcom-photon-cross-sections-database
- Birks JB 1964. The Theory and Practice of Scintillation Counting: International Series of Monographs in Electronics and Instrumentation (Elsevier; ) [Google Scholar]
- Boetter-Jensen L, McKeever SWS and Wintle AG 2003. Optically Stimulated Luminescence Dosimetry (Elsevier; ) [Google Scholar]
- Brunner SE and Schaart DR 2017. BGO as a hybrid scintillator / Cherenkov radiator for cost-effective time-of-flight PET Phys. Med. Biol 62 4421. [DOI] [PubMed] [Google Scholar]
- Bulin A-L, Vasil’ev A, Belsky A, Amans D, Ledoux G and Dujardin C 2015. Modelling energy deposition in nanoscintillators to predict the efficiency of the X-ray-induced photodynamic effect Nanoscale 7 5744–51 [DOI] [PubMed] [Google Scholar]
- Caër L and Sophie 2011. Water Radiolysis: Influence of Oxide Surfaces on H2 Production under Ionizing Radiation Water 3 235–53 [Google Scholar]
- Carpenter CM, Ma X, Liu H, Sun C, Pratx G, Wang J, Gambhir SS, Xing L and Cheng Z 2014. Cerenkov Luminescence Endoscopy: Improved Molecular Sensitivity with β−-Emitting Radiotracers J. Nucl. Med 55 1905–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesareo R and Mascarenhas S 1989. A new tomographic device based on the detection of fluorescent x-rays Nucl. Instrum. Methods Phys. Res. Sect. Accel. Spectrometers Detect. Assoc. Equip 277 669–72 [Google Scholar]
- Chen H, Moore T, Qi B, Colvin DC, Jelen EK, Hitchcock DA, He J, Mefford OT, Gore JC, Alexis F and Anker JN 2013. Monitoring pH-Triggered Drug Release from Radioluminescent Nanocapsules with X-ray Excited Optical Luminescence ACS Nano 7 1178–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Sun X, Wang GD, Nagata K, Hao Z, Wang A, Li Z, Xie J and Shen B 2017. LiGa5O8:Cr-based theranostic nanoparticles for imaging-guided X-ray induced photodynamic therapy of deep-seated tumors Mater. Horiz 4 1092–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Wang GD, Chuang Y-J, Zhen Z, Chen X, Biddinger P, Hao Z, Liu F, Shen B, Pan Z and Xie J 2015. Nanoscintillator-Mediated X-ray Inducible Photodynamic Therapy for In Vivo Cancer Treatment Nano Lett 15 2249–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chermont Q le M de, Chanéac C, Seguin J, Pellé F, Maîtrejean S, Jolivet J-P, Gourier D, Bessodes M and Scherman D 2007. Nanoprobes with near-infrared persistent luminescence for in vivo imaging Proc. Natl. Acad. Sci 104 9266–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry SR, Sorenson JA and Phelps ME 2012. Physics in Nuclear Medicine (Fourth Edition) Physics in Nuclear Medicine (Fourth Edition) (Philadelphia: W.B. Saunders; ) pp 63–85 Online: http://www.sciencedirect.com/science/article/pii/B978141605198500006X [Google Scholar]
- Ciarrocchi E and Belcari N 2017. Cerenkov luminescence imaging: physics principles and potential applications in biomedical sciences EJNMMI Phys 4 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement S, Deng W, Camilleri E, Wilson BC and Goldys EM 2016. X-ray induced singlet oxygen generation by nanoparticle-photosensitizer conjugates for photodynamic therapy: determination of singlet oxygen quantum yield Sci. Rep 6 srep19954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daghighian F, Mazziotta JC, Hoffman EJ, Shenderov P, Eshaghian B, Siegel S and Phelps ME 1994. Intraoperative beta probe: A device for detecting tissue labeled with positron or electron emitting isotopes during surgery Med. Phys 21 153–7 [DOI] [PubMed] [Google Scholar]
- Dothager RS, Goiffon RJ, Jackson E, Harpstrite S and Piwnica-Worms D 2010. Cerenkov Radiation Energy Transfer (CRET) Imaging: A Novel Method for Optical Imaging of PET Isotopes in Biological Systems PLOS ONE 5 e13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J and Peng Q 1998. Photodynamic Therapy J. Natl. Cancer Inst 90 889–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin C, Amans D, Belsky A, Chaput F, Ledoux G and Pillonnet A 2010. Luminescence and Scintillation Properties at the Nanoscale IEEE Trans. Nucl. Sci 57 1348–54 [Google Scholar]
- Fahimian B, Ceballos A, Türkcan S, Kapp DS and Pratx G 2014. Seeing the invisible: Direct visualization of therapeutic radiation beams using air scintillation Med. Phys 41 010702. [DOI] [PubMed] [Google Scholar]
- Farrell TJ, Wilson BC, Patterson MS and Olivo MC 1998. Comparison of the In Vivo Photodynamic Threshold Dose for Photofrin, Mono- and Tetrasulfonated Aluminum Phthalocyanine Using a Rat Liver Model Photochem. Photobiol 68 394–9 [PubMed] [Google Scholar]
- Franken NAP, Rodermond HM, Stap J, Haveman J and van Bree C 2006. Clonogenic assay of cells in vitro Nat. Protoc 1 2315–9 [DOI] [PubMed] [Google Scholar]
- Georgakoudi I, Nichols MG and Foster TH 1997. The Mechanism of Photofrin Photobleaching and Its Consequences for Photodynamic Dosimetry Photochem. Photobiol 65 135–44 [DOI] [PubMed] [Google Scholar]
- Gill RK, Mitchell GS and Cherry SR 2015. Computed Cerenkov luminescence yields for radionuclides used in biology and medicine Phys. Med. Biol 60 4263–80 [DOI] [PubMed] [Google Scholar]
- Glaser AK, Davis SC, McClatchy DM, Zhang R, Pogue BW and Gladstone DJ 2013a. Projection imaging of photon beams by the Čerenkov effect Med. Phys 40 012101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser AK, Davis SC, Voigt WHA, Zhang R, Pogue BW and Gladstone DJ 2013b. Projection imaging of photon beams using Čerenkov-excited fluorescence Phys. Med. Biol 58 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser AK, Zhang R, Andreozzi JM, Gladstone DJ and Pogue BW 2015. Cherenkov radiation fluence estimates in tissue for molecular imaging and therapy applications Phys. Med. Biol 60 6701–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser AK, Zhang R, Gladstone DJ and Pogue BW 2014. Optical dosimetry of radiotherapy beams using Cherenkov radiation: the relationship between light emission and dose Phys. Med. Biol 59 3789. [DOI] [PubMed] [Google Scholar]
- Grimm J 2015. Cerenkov Luminescence Imaging Imaging and Visualization in The Modern Operating Room ed Fong Y, Giulianotti PC, Lewis J, Koerkamp BG and Reiner T (Springer; New York: ) pp 107–20 Online: http://link.springer.com/chapter/10.1007/978-1-4939-2326-7_8 [Google Scholar]
- Grootendorst MR, Cariati M, Kothari A, Tuch DS and Purushotham A 2016a. Cerenkov luminescence imaging (CLI) for image-guided cancer surgery Clin. Transl. Imaging 4 353–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootendorst MR, Cariati M, Pinder S, Kothari A, Douek M, Kovacs T, Hamed H, Pawa A, Nimmo F, Owen J, Ramalingam V, Sethi S, Mistry S, Vyas K, Tuch D, Britten A, Hemelrijck MV, Cook G, Sibley-Allen C, Allen S and Purushotham A 2016b. Intraoperative Assessment of Tumor Resection Margins in Breast-Conserving Surgery using 18F-FDG Cerenkov Luminescence Imaging – A First-in-Human Feasibility Study J. Nucl. Med. jnumed 116181032 [DOI] [PubMed] [Google Scholar]
- Hall EJ and Giaccia AJ 2011. Radiobiology for the Radiologist (Philadelphia, United States STATES: Wolters Kluwer Health; ) Online: http://ebookcentral.proquest.com/lib/stanford-ebooks/detail.action?docID=2031840 [Google Scholar]
- Hartl BA, Hirschberg H, Marcu L and Cherry SR 2016. Activating Photodynamic Therapy in vitro with Cerenkov Radiation Generated from Yttrium-90 J. Environ. Pathol. Toxicol. Oncol 35 185–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiguchi S, Kusuzaki K, Murata H, Takeshita H, Hashiba M, Nishimura T, Ashihara T and Hirasawa Y 2002. Acridine Orange Excited by Low-Dose Radiation Has a Strong Cytocidal Effect on Mouse Osteosarcoma Oncology 62 85–93 [DOI] [PubMed] [Google Scholar]
- Helo Y, Rosenberg I, D’Souza D, MacDonald L, Speller R, Royle G and Gibson A 2014. Imaging Cerenkov emission as a quality assurance tool in electron radiotherapy Phys. Med. Biol 59 1963. [DOI] [PubMed] [Google Scholar]
- Horrocks DL 1974. Applications of Liquid Scintillation Counting Applications of Liquid Scintillation Counting (Academic Press; ) p xiii Online: http://www.sciencedirect.com/science/article/pii/B9780123562401500056 [Google Scholar]
- Hu H, Cao X, Kang F, Wang M, Lin Y, Liu M, Li S, Yao L, Liang J, Liang J, Nie Y, Chen X, Wang J and Wu K 2015. Feasibility study of novel endoscopic Cerenkov luminescence imaging system in detecting and quantifying gastrointestinal disease: first human results Eur. Radiol 25 1814–22 [DOI] [PubMed] [Google Scholar]
- Jarvis LA, Zhang R, Gladstone DJ, Jiang S, Hitchcock W, Friedman OD, Glaser AK, Jermyn M and Pogue BW 2014. Cherenkov Video Imaging Allows for the First Visualization of Radiation Therapy in Real Time Int. J. Radiat. Oncol 89 615–22 [DOI] [PubMed] [Google Scholar]
- Jelley JV 1958. Cerenkov Radiation and Its Applications (Pergamon Press; ) [Google Scholar]
- Kamkaew A, Cheng L, Goel S, Valdovinos HF, Barnhart TE, Liu Z and Cai W 2016. Cerenkov Radiation Induced Photodynamic Therapy Using Chlorin e6-Loaded Hollow Mesoporous Silica Nanoparticles ACS Appl. Mater. Interfaces 8 26630–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TJ, Türkcan S and Pratx G 2017. Modular low-light microscope for imaging cellular bioluminescence and radioluminescence Nat. Protoc 12 1055–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JS, Mitchell GS and Cherry SR 2017. Quantitative assessment of Cerenkov luminescence for radioguided brain tumor resection surgery Phys. Med. Biol 62 4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll GF 2010. Radiation Detection and Measurement (John Wiley & Sons; ) [Google Scholar]
- Korpar S, Dolenec R, Križan P, Pestotnik R and Stanovnik A 2011. Study of TOF PET using Cherenkov light Nucl. Instrum. Methods Phys. Res. Sect. Accel. Spectrometers Detect. Assoc. Equip 654 532–8 [Google Scholar]
- Kotagiri N, Sudlow GP, Akers WJ and Achilefu S 2015. Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers Nat. Nanotechnol 10 370–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusuzaki K, Murata H, Matsubara T, Miyazaki S, Shintani K, Seto M, Matsumine A, Hosoi H, Sugimoto T and Uchida A 2005. Clinical Outcome of a Novel Photodynamic Therapy Technique Using Acridine Orange for Synovial Sarcomas Photochem. Photobiol 81 705–9 [DOI] [PubMed] [Google Scholar]
- Kwon SI, Gola A, Ferri A, Piemonte C and Cherry SR 2016. Bismuth germanate coupled to near ultraviolet silicon photomultipliers for time-of-flight PET - IOPscience Phys. Med. Biol 61 L38–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecoq P, Auffray E, Brunner S, Hillemanns H, Jarron P, Knapitsch A, Meyer T and Powolny F 2010. Factors Influencing Time Resolution of Scintillators and Ways to Improve Them IEEE Trans. Nucl. Sci 57 2411–6 [Google Scholar]
- Lecoq P, Gektin A and Korzhik M 2016. Inorganic Scintillators for Detector Systems: Physical Principles and Crystal Engineering (Springer; ) [Google Scholar]
- Li C, Di K, Bec J and Cherry SR 2013. X-ray luminescence optical tomography imaging: experimental studies Opt. Lett 38 2339–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Mitchell GS and Cherry SR 2010. Cerenkov luminescence tomography for small-animal imaging Opt. Lett 35 1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Carpenter CM, Jiang H, Pratx G, Sun C, Buchin MP, Gambhir SS, Xing L and Cheng Z 2012. Intraoperative Imaging of Tumors Using Cerenkov Luminescence Endoscopy: A Feasibility Experimental Study J. Nucl. Med 53 1579–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Ren G, Miao Z, Zhang X, Tang X, Han P, Gambhir SS and Cheng Z 2010a. Molecular Optical Imaging with Radioactive Probes PLOS ONE 5 e9470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zhang X, Xing B, Han P, Gambhir SS and Cheng Z 2010b. Radiation-Luminescence-Excited Quantum Dots for in vivo Multiplexed Optical Imaging Small 6 1087–91 [DOI] [PubMed] [Google Scholar]
- Madras BK, Cohen EL, Messing R, Munro HN and Wurtman RJ 1974. Relevance of free tryptophan in serum to tissue tryptophan concentrations Metabolism 23 1107–16 [DOI] [PubMed] [Google Scholar]
- Maldiney T, Kaikkonen MU, Seguin J, le Masne de Chermont Q, Bessodes M, Airenne KJ, Ylä-Herttuala S, Scherman D and Richard C 2012. In Vitro Targeting of Avidin-Expressing Glioma Cells with Biotinylated Persistent Luminescence Nanoparticles Bioconjug. Chem 23 472–8 [DOI] [PubMed] [Google Scholar]
- Maldiney T, Viana B, Bessière A, Gourier D, Bessodes M, Scherman D and Richard C 2013. In vivo imaging with persistent luminescence silicate-based nanoparticles Opt. Mater 35 1852–8 [Google Scholar]
- Matsubara T, Kusuzaki K, Matsumine A, Murata H, Nakamura T, Uchida A and Sudo A 2010. Clinical outcomes of minimally invasive surgery using acridine orange for musculoskeletal sarcomas around the forearm, compared with conventional limb salvage surgery after wide resection J. Surg. Oncol 102 271–5 [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Gill RK, Boucher DL, Li C and Cherry SR 2011. In vivo Cerenkov luminescence imaging: a new tool for molecular imaging Philos. Transact. A Math. Phys. Eng. Sci 369 4605–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro CMB, Lopes JAM, Veloso JFCA and dos Santos JMF 2008. Secondary scintillation yield in pure argon Phys. Lett. B 668 167–70 [Google Scholar]
- Moore L. Thomas, Fenglin Wang, Hongyu Chen, Grimes W. Stuart, Anker Jeffrey N. and Frank Alexis 2014. Polymer‐Coated Radioluminescent Nanoparticles for Quantitative Imaging of Drug Delivery Adv. Funct. Mater 24 5815–23 [Google Scholar]
- Morgan NY, Kramer-Marek G, Smith PD, Camphausen K and Capala J 2009. Nanoscintillator Conjugates as Photodynamic Therapy-Based Radiosensitizers: Calculation of Required Physical Parameters Radiat. Res 171 236–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morii H, Mizouchi K, Nomura T, Sasao N, Sumida T, Kobayashi M, Murayama Y and Takashima R 2004. Quenching effects in nitrogen gas scintillation Nucl. Instrum. Methods Phys. Res. Sect. Accel. Spectrometers Detect. Assoc. Equip 526 399–408 [Google Scholar]
- Moss SH and Smith KC 1980. Cerenkov Ultraviolet Radiation (137Cs γ-rays) and Direct Excitation (137Cs γ-rays and 50 kVp X-rays) Produce Photoreactivable Damage in Escherichia Coli Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med 38 323–34 [DOI] [PubMed] [Google Scholar]
- Naczynski DJ, Sun C, Türkcan S, Jenkins C, Koh AL, Ikeda D, Pratx G and Xing L 2015. X-ray-Induced Shortwave Infrared Biomedical Imaging Using Rare-Earth Nanoprobes Nano Lett 15 96–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DR, Carter JG, Birkhoff RD, Hamm RN and Augenstein LG 1967. Yield of Luminescence from X-Irradiated Biochemicals Radiat. Res 32 723–43 [PubMed] [Google Scholar]
- Niedre MJ, Secord AJ, Patterson MS and Wilson BC 2003. In Vitro Tests of the Validity of Singlet Oxygen Luminescence Measurements as a Dose Metric in Photodynamic Therapy Cancer Res 63 7986–94 [PubMed] [Google Scholar]
- Nikolopoulos D, Valais I, Michail C, Bakas A, Fountzoula C, Cantzos D, Bhattacharyya D, Sianoudis I, Fountos G, Yannakopoulos P, Panayiotakis G and Kandarakis I 2016. Radioluminescence properties of the CdSe/ZnS Quantum Dot nanocrystals with analysis of long-memory trends Radiat. Meas 92 19–31 [Google Scholar]
- Ochsner M 1997. Photophysical and photobiological processes in the photodynamic therapy of tumours J. Photochem. Photobiol. B 39 1–18 [DOI] [PubMed] [Google Scholar]
- Osakada Y, Pratx G, Hanson L, Solomon PE, Xing L and Cui B 2013. X-ray excitable luminescent polymer dots doped with an iridium(iii) complex Chem. Commun 49 4319–21 [DOI] [PubMed] [Google Scholar]
- Osakada Y, Pratx G, Sun C, Sakamoto M, Ahmad M, Volotskova O, Ong Q, Teranishi T, Harada Y, Xing L and Cui B 2014. Hard X-ray-induced optical luminescence via biomolecule-directed metal clusters Chem. Commun 50 3549–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Z, Liu B, Yasmin-Karim S, Sajo E and Ngwa W 2016. Nanoparticle-aided external beam radiotherapy leveraging the Čerenkov effect Phys. Med 32 944–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palner M, Pu K, Shao S and Rao J 2015. Semiconducting Polymer Nanoparticles with Persistent Near-Infrared Luminescence for In Vivo Optical Imaging Angew. Chem 127 11639–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson MS, Wilson BC and Graff R 1990. In vivo tests of the concept of photodynamic threshold dose in normal rat liver photosensitized by aluminum chlorosulphonated phthalocyanine Photochem. Photobiol 51 343–9 [DOI] [PubMed] [Google Scholar]
- Pogue BW, Feng J, LaRochelle EP, Bruža P, Lin H, Zhang R, Shell JR, Dehghani H, Davis SC, Vinogradov SA, Gladstone DJ and Jarvis LA 2018. Maps of in vivo oxygen pressure with submillimetre resolution and nanomolar sensitivity enabled by Cherenkov-excited luminescence scanned imaging Nat. Biomed. Eng 2 254–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogue BW and Wilson BC 2018. Optical and x-ray technology synergies enabling diagnostic and therapeutic applications in medicine J. Biomed. Opt 23 121610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt EC, Shaffer TM, Zhang Q, Drain CM and Grimm J 2018. Nanoparticles as multimodal photon transducers of ionizing radiation Nat. Nanotechnol 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratx G 2017. A tale of two photons: radioluminescence and its application in molecular imaging Molecular-Guided Surgery: Molecules, Devices, and Applications III Molecular-Guided Surgery: Molecules, Devices, and Applications III vol 10049 (International Society for Optics and Photonics; ) p 1004916 Online: https://www.spiedigitallibrary.org/conference-proceedings-of-spie/10049/1004916/A-tale-of-two-photons--radioluminescence-and-its-application/10.1117/12.2256702.short [Google Scholar]
- Pratx G, Carpenter CM, Sun C, Rao RP and Xing L 2010a. Tomographic molecular imaging of x-ray-excitable nanoparticles Opt. Lett 35 3345–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratx G, Carpenter CM, Sun C and Xing L 2010b. X-Ray Luminescence Computed Tomography via Selective Excitation: A Feasibility Study IEEE Trans. Med. Imaging 29 1992–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratx G, Chen K, Sun C, Axente M, Sasportas L, Carpenter C and Xing L 2013. High-Resolution Radioluminescence Microscopy of 18F-FDG Uptake by Reconstructing the β-Ionization Track J. Nucl. Med 54 1841–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratx G, Chen K, Sun C, Martin L, Carpenter CM, Olcott PD and Xing L 2012. Radioluminescence Microscopy: Measuring the Heterogeneous Uptake of Radiotracers in Single Living Cells PLOS ONE 7 e46285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quickenden TI 1971. The Luminescence of Water Excited by Ambient Ionizing Radiation Radiat. Res 46 28–35 [PubMed] [Google Scholar]
- Rao P VL. Radiobioluminescence, cerenkov luminescence-God light in likes, a potential in radiation therapy imaging. 2015. Online: http://www.cancerjournal.net/article.asp?issn=0973-1482;year=2015;volume=11;issue=1;spage=241;epage=242;aulast=Rao. [DOI] [PubMed]
- Robertson R, Germanos MS, Li C, Mitchell GS, Cherry SR and Silva MD 2009. Optical imaging of Cerenkov light generation from positron-emitting radiotracers Phys. Med. Biol 54 N355–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands JA. The physics of computed radiography. Phys. Med. Biol. 2002;47:R123. doi: 10.1088/0031-9155/47/23/201. [DOI] [PubMed] [Google Scholar]
- Ruggiero A, Holland JP, Lewis JS and Grimm J 2010. Cerenkov Luminescence Imaging of Medical Isotopes J. Nucl. Med 51 1123–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson DCW, Slater C and Cairns KJ 1989. Thermoluminescence of foods: Origins and implications for detecting irradiation Int. J. Radiat. Appl. Instrum. Part C Radiat. Phys. Chem 34 915–24 [Google Scholar]
- Scaffidi JP, Gregas MK, Lauly B, Zhang Y and Vo-Dinh T 2011. Activity of Psoralen-Functionalized Nanoscintillators against Cancer Cells upon X-ray Excitation ACS Nano 5 4679–87 [DOI] [PubMed] [Google Scholar]
- Schwarz HA. Free radicals generated by radiolysis of aqueous solutions. J. Chem. Educ. 1981;58:101. [Google Scholar]
- Sengupta D and Pratx G 2016. Single-cell characterization of FLT uptake with radioluminescence microscopy J. Nucl. Med. jnumed 115167734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer TM, Pratt EC and Grimm J 2017. Utilizing the power of Cerenkov light with nanotechnology Nat. Nanotechnol 12 106–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpatyi VA and Kraljić I 1978. Detection of Singlet Oxygen in Radiolysis of Aerated Aqueous Solutions Photochem. Photobiol 28 587–90 [Google Scholar]
- Sicard-Roselli C, Brun E, Gilles M, Baldacchino G, Kelsey C, McQuaid H, Polin C, Wardlow N and Currell F 2014. A New Mechanism for Hydroxyl Radical Production in Irradiated Nanoparticle Solutions Small 10 3338–46 [DOI] [PubMed] [Google Scholar]
- Sitharamarao DN and Duncan JF 1963. Molecular Excitation of Water by $gamma$-Irradiation J. Phys. Chem. US Vol: 67 Online: https://www.osti.gov/scitech/biblio/4633308 [Google Scholar]
- Soika C and Delincée H 2000. Thermoluminescence Analysis for Detection of Irradiated Food—Luminescence Characteristics of Minerals for Different Types of Radiation and Radiation Doses LWT - Food Sci. Technol 33 431–9 [Google Scholar]
- Liang Song, Pei‐Pei Li, Wen Yang, Xia‐Hui Lin, Hong Liang, Xiao‐Feng Chen, Gang Liu, Juan Li and Huang‐Hao Yang 2018. Low‐Dose X‐ray Activation of W(VI)‐Doped Persistent Luminescence Nanoparticles for Deep‐Tissue Photodynamic Therapy Adv. Funct. Mater 0 1707496 [Google Scholar]
- Spinelli AE, Calandrino R, Meo SL, Sbarbati A and Boschi F 2011. Optical imaging of Tc-99m-based tracers: in vitro and in vivo results J. Biomed. Opt 16 116023. [DOI] [PubMed] [Google Scholar]
- Spinelli AE, Ferdeghini M, Cavedon C, Zivelonghi E, Calandrino R, Fenzi A, Sbarbati A and Boschi F 2013. First human Cerenkography J. Biomed. Opt 18 020502–020502 [DOI] [PubMed] [Google Scholar]
- Suzuki M and Kubota S 1979. Mechanism of proportional scintillation in argon, krypton and xenon Nucl. Instrum. Methods 164 197–9 [Google Scholar]
- Tanha K, Pashazadeh AM and Pogue BW 2015. Review of biomedical Čerenkov luminescence imaging applications Biomed. Opt. Express 6 3053–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasov MD, El’yash SL, Goncharova VF, Petrushin ON, Savel’ev YA, Tarakanov MY and Shigaev YS 2007. Efficiency of radioluminescence of water under the action of accelerated electrons Instrum. Exp. Tech 50 761–3 [Google Scholar]
- Butterworth K T, McMahon S J, Currell F J and Prise K M 2012. Physical basis and biological mechanisms of gold nanoparticle radiosensitization Nanoscale 4 4830–8 [DOI] [PubMed] [Google Scholar]
- Thorek DLJ, Das S and Grimm J 2014a. Molecular Imaging Using Nanoparticle Quenchers of Cerenkov Luminescence Small 10 3729–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorek DLJ, Ogirala A, Beattie BJ and Grimm J 2013. Quantitative imaging of disease signatures through radioactive decay signal conversion Nat. Med 19 1345–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorek DLJ, Riedl CC and Grimm J 2014b. Clinical Cerenkov Luminescence Imaging of 18F-FDG J. Nucl. Med 55 95–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- US EPA O. Climate Change Indicators: Atmospheric Concentrations of Greenhouse Gases US EPA. 2016 Online: https://www.epa.gov/climate-indicators/climate-change-indicators-atmospheric-concentrations-greenhouse-gases.
- Van den Eeckhout K, Smet PF and Poelman D 2010. Persistent Luminescence in Eu2+-Doped Compounds: A Review Materials 3 2536–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasil’ev RF. Chemiluminescence Excitation Mechanisms. Russ. Chem. Rev. 1970;39:529. [Google Scholar]
- Volotskova O, Sun C, Stafford JH, Koh AL, Ma X, Cheng Z, Cui B, Pratx G and Xing L 2015. Efficient Radioisotope Energy Transfer by Gold Nanoclusters for Molecular Imaging Small 11 4002–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Volotskova O, Lu K, Ahmad M, Sun C, Xing L and Lin W 2014. Synergistic Assembly of Heavy Metal Clusters and Luminescent Organic Bridging Ligands in Metal–Organic Frameworks for Highly Efficient X-ray Scintillation J. Am. Chem. Soc 136 6171–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Sengupta D, Kim TJ and Pratx G 2017. Performance evaluation of 18F radioluminescence microscopy using computational simulation Med. Phys 44 1782–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston MA and Patterson MS 2008. Simple Photodynamic Therapy Dose Models Fail to Predict the Survival of MLL Cells After HPPH–PDT In Vitro Photochem. Photobiol 85 750–9 [DOI] [PubMed] [Google Scholar]
- Wilson BC and Patterson MS 1986. The physics of photodynamic therapy Phys. Med. Biol 31 327. [DOI] [PubMed] [Google Scholar]
- Yaffe MJ and Rowlands JA 1997. X-ray detectors for digital radiography Phys. Med. Biol 42 1. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Koyama S, Komori M and Toshito T 2016. Luminescence imaging of water during irradiation of X-ray photons lower energy than Cerenkov- light threshold Nucl. Instrum. Methods Phys. Res. Sect. Accel. Spectrometers Detect. Assoc. Equip 832 264–70 [Google Scholar]
- Yoon SW, Tsvankin V, Shrock Z, Meng B, Zhang X, Dewhirst M, Fecci P, Adamson J and Oldham M 2017. Enhancing Radiation Therapy through Cherenkov Light Activated Phototherapy Int. J. Radiat. Oncol Online: http://www.sciencedirect.com/science/article/pii/S0360301617341226 [DOI] [PMC free article] [PubMed]
- Zhang R, D’souza AV, Gunn JR, Esipova TV, Vinogradov SA, Glaser AK, Jarvis LA, Gladstone DJ and Pogue BW 2015. Cherenkov-excited luminescence scanned imaging Opt. Lett 40 827–30 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


