Abstract
Deletion of fatty acid amide hydrolase (FAAH), enzyme responsible for degrading endocannabinoids, increases alcohol consumption and preference. However, there is a lack of data on neurochemical events in mice exposed to alcohol in the absence of FAAH. Extracellular levels of endocannabinoids and relevant neurotransmitters were measured by in vivo microdialysis in the nucleus accumbens (NAc) of FAAH knockout (FAAH KO) and wild-type (WT) mice during an ethanol (EtOH, 2 g/kg ip) challenge in EtOH-naïve and repeated (r) EtOH-treated mice. In both genotypes, EtOH treatment caused no changes in baseline endocannabinoid levels, although FAAH KO mice displayed higher baseline N-arachidonoylethanolamine levels than WT mice. EtOH challenge caused a sustained increase in 2-arachidonoylglycerol (2-AG) levels in EtOH-naïve WT mice, but not in FAAH KO mice. In contrast, 2-AG levels were decreased following EtOH challenge in (r)EtOH-treated mice in both genotypes. Whereas (r)EtOH-treated mice showed higher baseline dopamine and serotonin levels than EtOH-naïve mice in WT mice, these differences were attenuated in FAAH KO mice. Significant differences in baseline GABA and glutamate levels by EtOH history were observed in WT mice, but not in FAAH KO mice. Moreover, opposed effects on glutamate response were observed after EtOH challenge in EtOH-naïve and (r)EtOH-treated FAAH KO mice. Finally, FAAH deletion failed to show EtOH-induced locomotion sensitivity. These data provide evidence of a potential influence of 2-AG in the neurochemical response to EtOH exposure in the NAc.
Keywords: Alcohol, endocannabinoid, fatty acid amide hydrolase (FAAH), nucleus accumbens, microdialysis
INTRODUCTION
Alcohol consumption affects the function of neurotransmitter systems in the central nervous system (CNS) and these alterations are involved in the pathophysiology and development of alcohol addiction (Koob and Volkow, 2016). Among these signaling systems, numerous studies have reported the participation of the endogenous cannabinoid system (ECS) in the behavioral and pharmacological effects of alcohol (Serrano and Parsons, 2011) and in the induction of addictive phenotype following chronic excessive alcohol exposure.
The ECS consist of two well-characterized cannabinoid receptors (CB1 and CB2), their endogenous ligands (endocannabinoids) and the enzymes responsible for endocannabinoid synthesis and metabolism. The best characterized endocannabinoids are N-arachidonoylethanolamine (anandamide, AEA) and 2-arachidonoylglycerol (2-AG), which are synthesized on demand through cleavage from membrane lipid precursors and immediately released from cells (Pertwee, 2015). Inactivation of endocannabinoid signaling is mediated by cellular reuptake and subsequent intracellular hydrolysis by multiple enzymes (Fezza et al., 2014). Fatty acid amide hydrolase (FAAH) (Cravatt et al., 1996) and monoacylglycerol lipase (Dinh et al., 2002) are the main enzymes responsible for the degradation of AEA and 2-AG, respectively. However, FAAH can also hydrolyze other fatty acid ethanolamides (e.g. N-palmitoylethanolamine and N-oleoylethanolamine) and 2-AG (Fowler et al., 2001).
A growing body of literature implicates CB1 receptors in the modulation of ethanol (EtOH) consumption (Henderson-Redmond et al., 2016). Exogenous CB1 receptor agonists dose-dependently increase EtOH consumption and the reinforcing properties of EtOH in both rats and mice (Colombo et al., 2002; Wang et al., 2003). Conversely, the CB1 receptor inverse agonist SR141716A reduces EtOH intake in rodents (Economidou et al., 2006; Freedland et al., 2001) and mice lacking CB1 receptor display lower EtOH preference and consumption than wild-type (WT) mice (Hungund et al., 2003; Thanos et al., 2005; Vinod et al., 2008b). Furthermore, EtOH tolerance involves the down regulation of the CB1 receptor and its function (Basavarajappa and Hungund, 2005).
The effect of CB1 receptor inactivation on EtOH intake suggests that endocannabinoids influence the motivation for EtOH through EtOH-induced increases in their formation and that long-term EtOH exposure can thereby lead to disruptions in endocannabinoid signaling. Consistent with this hypothesis are the observations that chronic EtOH exposure dose-dependently increases endocannabinoid formation in vitro (Basavarajappa and Hungund, 2005). However, much evidence demonstrates inconsistencies among in vivo studies, mainly in rats, to establish the direction of change and regional nature of the EtOH-induced effects on AEA and 2-AG (Pava and Woodward, 2012).
Manipulation of endocannabinoid clearance through genetic and/or pharmacological inhibition of FAAH has been extensively used in the literature to describe the interactions between EtOH and the ECS. Thus, FAAH knockout (KO) mice have a higher preference for EtOH and consume more EtOH than their WT counterparts (Basavarajappa et al., 2006; Blednov et al., 2007). FAAH KO mice also show reduced sensitivity to the sedative and hypothermic effects of EtOH and faster recovery from EtOH-induced motor incoordination (Blednov et al., 2007; Vinod et al., 2008a), suggesting that these mice may consume more EtOH as a result of decreased acute EtOH intoxication. Likewise, C57BL/6J mice treated with the FAAH inhibitor URB597 display increased EtOH preference and decreased sensitivity to EtOH-induced sedation and motor incoordination (Blednov et al., 2007). In addition to these effects, URB597 also reduces alcohol escalation and relapse drinking using a chronic intermittent access (Zhou et al., 2017). In humans, a FAAH variant (single polymorphism C385 to A converts Pro129 to Thr) has been reported to be associated with high prevalence of alcohol use disorders (Sipe et al., 2002; Sloan et al., 2018), and higher risk for alcohol problems in young individuals (Buhler et al., 2014). Therefore, these findings suggest that the behavioral response to EtOH is influenced by enhanced endocannabinoid tone or by heightened EtOH-induced increases in brain endocannabinoid levels. However, the neurochemical mechanisms affected by these alterations in the ECS have not been characterized in brain areas involved in addiction.
The present study is focused on the nucleus accumbens (NAc), a component of the basal ganglia that plays a critical role in mediating the rewarding properties of alcohol and other addictive substances. The NAc receives dopaminergic projections from ventral tegmental area (VTA) and integrates information from limbic and cortical structures through the participation of several signaling systems (Koob and Volkow, 2016). Our hypothesis is that enhanced endocannabinoid signaling by FAAH deletion promotes EtOH intake and preference through neurochemical events occurring in brain regions associated with rewarding and addictive behaviors. Consequently, we have investigated the influence of an acute EtOH challenge (2 g/kg EtOH) on relevant neurotransmitters in the NAc of FAAH KO and WT mice that were treated with EtOH (7 previous injections) or not (EtOH-naïve mice). In vivo microdialysis experiments were performed to determine interstitial levels of endocannabinoids (AEA and 2-AG), monoamine [dopamine (DA) and serotonin (5-HT)] and amino acids [γ-aminobutyric acid (GABA) and glutamate (GLU)]. Finally, we compared the EtOH-induced behavioral sensitization in the two genotypes.
METHODS AND MATERIALS
Animals
Null FAAH allele mice were created using homologous recombination as described previously and were maintained on original 129/SvJ×C57BL/6J genetic background (Cravatt et al., 2001). All in vivo microdialysis studies were performed on male homozygous FAAH KO mice and their WT littermates weighting 17–24 g and generated from crosses between heterozygous animals. The mice were group-housed (2–4 mice/cage) in a temperature-controlled vivarium (22°C) with a 12h light/dark cycle (lights off at 9:00AM) and were provided free access to food (PicoLab® Mouse Diet 20) and water. All procedures and animal experiments were conducted in strict adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978).
EtOH treatments
EtOH (20%, w/v) was prepared with 95% ethyl alcohol and water and mice were injected intraperitoneally (ip) with 2 g/kg EtOH in a volume of 10 mL/kg during the dark cycle at 9:30AM. FAAH KO and WT mice were given either a single EtOH injection or repeated EtOH injections.
Repeated EtOH treatment was designed to evaluate the effect of chronic EtOH administration on neurochemical events in each genotype. We employed a dosing procedure based on previously published data that were shown to induce behavioral and neurochemical changes and sensitization (Kapasova and Szumlinski, 2008). Repeated (r) EtOH-treated mice were given EtOH (2 g/kg, ip) every other day at 9:30AM for 15 days (8 total injections).
For microdialysis studies, probes were implanted on the day before the last EtOH injection.
EtOH-induced locomotor sensitization
A total of 41 animals (n=20 FAAH KO mice, and n=21 WT mice) were used for EtOH-induced locomotor sensitization. Monitoring of locomotor activity was performed in 30×20×40 cm chambers equipped with infrared beams that were placed along the perimeter to detect and record horizontal locomotor activity (LISA lazer Monitoring System). EtOH-evoked locomotor sensitization was evaluated 1-day and 15-day immediately after EtOH injection to detect genotypic differences between acute and repeated EtOH treatments. Previously, all mice (8–10 per genotype and treatment) received three habituation sessions, spaced 2–4 days apart depending on the equipment availability. All habituation and test sessions were 10 min in duration, and data were collected in 1-min intervals. Activity testing was always performed in the same chambers between 9:30AM and 10:30AM.
Blood alcohol assay
Blood samples were collected immediately after the completion of the locomotor activity experiments (15 min after EtOH injection) from the same mice to determine blood alcohol concentration (BAC) (Serrano et al., 2012).
In vivo microdialysis studies and neurochemical determinations
All in vivo microdialysis sessions were performed using probes with 1 mm active membrane length aimed at the NAc after surgery.
Experiments 1.
Commercial probes of polyethyl sulfone membrane were used for determination of 2-AG and AEA interstitial content (SciPro Inc., Sanborn, NY, USA).
Experiments 2.
Probes of cellulose membrane were constructed as previously described for determination of monoamine (DA and 5-HT) and amino acid (GABA and GLU) neurotransmitters (Frantz et al., 2002).
Neurochemical determinations.
Dialysate levels of 2-AG and AEA were determined using liquid chromatography coupled with electrospray ionization mass spectrometry; DA and 5-HT were determined using high-performance liquid chromatography coupled with electrochemical detection; and GABA and GLU were determined using capillary electrophoresis with laser-induced fluorescence detection.
For details see Supporting Information (Supplementary Methods and Materials).
Statistical analysis
Between-group differences in basal dialysate levels (nM) of endocannabinoids, monoamines and amino acids were first compared by repeated-measures analysis of variance (ANOVA) in the NAc of FAAH KO and WT mice. For each group, the mean baseline level was calculated as the average of all dialysate samples that were collected before the EtOH administration (6 samples/animal). Subsequent analyses were conducted on dialysate data using two-way repeated-measures ANOVA, with EtOH history (EtOH-naïve and repeated EtOH treatments) as the between-subjects factor and sampling time (10-min intervals for endocannabinoids or 20-min intervals for monoamines and amino acids) as the within-subjects factor, to determine the impact of an injection of EtOH (2 g/kg) on NAc dialysate neurochemical levels.
Area under the curve (AUC) calculations (nM) were used for comparison of overall EtOH-induced alterations in control EtOH-naïve mice and (r)EtOH-treated mice using both genotypes. The AUC was calculated for each animal by subtracting the basal average concentration from the concentration value for each data point following EtOH administration, and subsequently summing all these data points. Significant differences in AUC values were determined by using unpaired Student’s t-tests and Welch’s t-test for unequal variances. Additionally, two-way ANOVA on AUC values were used when appropriate to determine the effects of genotype and EtOH history, and the interaction between the two factors.
Finally, differences in locomotor activity (crossovers) were determined by ANOVA. Therefore, EtOH history and genotype effects were analyzed on locomotor activity.
Test statistic values and degrees of freedom are indicated in the results where appropriate. Values of p<0.05 were considered statistically significant. All the statistical analyses were performed using Prism software (GraphPad, San Diego, CA, USA).
RESULTS
Effects of EtOH exposure on NAc dialysate endocannabinoid levels
In Experiments 1, microdialysis samples were used to assess the effect of an acute EtOH challenge in EtOH-naive (i.e., injection 1) and (r)EtOH-treated (i.e., injection 8) mice on 2-AG and AEA levels in NAc of WT and FAAH KO mice (Figure 1).
Figure 1.
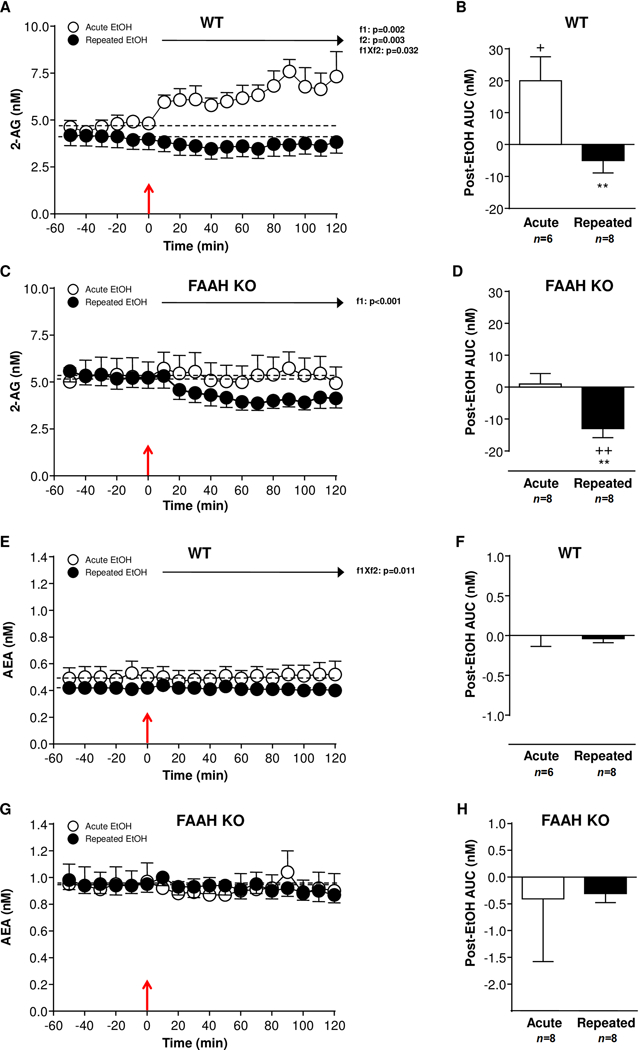
Effect of an EtOH challenge (2 mg/kg, i.p.) on endocannabinoid levels in NAc microdialysates of WT and FAAH KO mice that were treated with repeated EtOH treatment (filled circles) or not (open circles). (A) 2-AG levels in EtOH-naïve (n=6) and repeated EtOH-treated (n=8) WT mice. (B) Postinjection AUC values for 2-AG summarizes the effect of EtOH injection on dialysate 2-AG in WT mice. (C) 2-AG levels in EtOH-naïve (n=8) and repeated EtOH-treated (n=8) FAAH KO mice. (D) Postinjection AUC values for 2-AG summarizes the effect of EtOH injection on dialysate 2-AG in FAAH KO mice. (E) AEA levels in EtOH-naïve (n=6) and repeated EtOH-treated (n=6) WT mice. (F) Postinjection AUC values for AEA summarizes the effect of EtOH injection on dialysate AEA in WT mice. (G) AEA levels in EtOH-naïve (n=8) and repeated EtOH-treated (n=8) FAAH KO mice. (H) Postinjection AUC values for AEA summarizes the effect of EtOH injection on dialysate AEA in FAAH KO mice. Effects of sampling time (f1) and EtOH history (f2) on postinjection data were analyzed using two-way repeated-measures ANOVA. EtOH injection is indicated by the red arrow at time point zero. **p<0.01 denotes significant differences in repeated versus acute EtOH-treated mice. +p<0.05 and ++p<0.01 denote significant differences versus baseline (AUC=0).
Effects of EtOH on NAc dialysate 2-AG levels
As shown in Fig. 1A, we observed no significant effect of EtOH history on baseline 2-AG levels (EtOH-naïve: 4.26±0.34 nM; (r)EtOH-treated: 4.09±0.54 nM) in WT mice. Overall, a two-way repeated-measures ANOVA during 120 min after an EtOH challenge revealed main effects of sampling time (F(11,132)=2.904;p=0.002) and EtOH history (F(1,12)=14.04;p=0.003) on 2-AG levels, and a significant interaction between the two factors (F(11,132)=2.016;p=0.032). In EtOH-naïve mice, acute EtOH injection produced a prolonged increase in dialysate 2-AG levels, but there was no significant effect in (r)EtOH-treated WT mice. The EtOH history effect was clearly evident in the AUC analysis for 2-AG after the EtOH challenge (Fig. 1B). Indeed, EtOH-naïve WT mice displayed greater AUC for dialysate 2-AG levels than (r)EtOH-treated WT mice following the EtOH challenge (t(12)=3.214;p=0.006). Interestingly, this 2-AG elevation in EtOH-naïve mice was statistically significant compared with baseline levels (AUC=0) (t(5)=2.644;p=0.046).
Similarly to WT, mice lacking FAAH showed no significant effect of EtOH history on baseline 2-AG levels (EtOH-naïve: 5.22±0.66 nM; (r)EtOH-treated: 5.32±0.52 nM) (Fig. 1C). A two-way repeated-measures ANOVA during 120 min after EtOH injection only revealed a main effect of sampling time (F(11,154)=3.313;p<0.001) on 2-AG levels. Although this statistical analysis did not reveal a significant effect of EtOH history or interaction effect, we found that (r)EtOH FAAH KO mice displayed lower dialysate 2-AG levels than EtOH-naïve mice (t(14)=3.143;p=0.007) in the postinjection AUC analysis (Fig. 1D). Also, when the AUC for 2-AG levels was compared with baseline levels (AUC=0), we observed a significant reduction in (r)EtOH-treated FAAH KO mice (t(7)=4.053;p=0.005).
We compared baseline 2-AG levels from FAAH KO mice versus WT mice to identify potential genotype differences using a two-way repeated-measures ANOVA. No differences in baseline 2-AG levels were observed in EtOH-naïve mice as compared FAAH KO and WT mice. Then, we used a two-way ANOVA to analyze all AUC measures of 2-AG by genotype and EtOH history (Figures 1B and 1D). The postinjection AUC analysis revealed a significant effect of genotype (F(1,26)=8.812;p=0.006) and EtOH history (F(1,26)=20.32;p<0.001), but there was no interaction between the two factors. Therefore, while FAAH KO mice showed lower AUC values for 2-AG levels than WT mice, (r)EtOH-treated mice had lower AUC values than EtOH-naïve mice after the EtOH injection.
Effects of EtOH on NAc dialysate AEA levels
WT mice displayed no effect of EtOH history on baseline AEA levels (EtOH-naïve: 0.49±0.08 nM; (r)EtOH-treated: 0.42±0.04 nM) (Fig. 1E). A subsequent statistical analysis of AEA levels after EtOH injection revealed a significant interaction between sampling time and EtOH history (F(11,132)=2.350;p=0.011). However, this interaction effect after an EtOH challenge was not associated with significant differences in AEA levels using multiple comparisons test. In fact, no difference in postinjection AUC was detected between (r)EtOH-treated mice and EtOH-naïve mice (Fig. 1F).
No significant effect of EtOH history on baseline AEA levels (EtOH-naïve: 1.00±0.19 nM; (r)EtOH-treated: 0.95±0.07 nM) was also observed in FAAH KO mice (Fig. 1G). Unlike WT mice, there were no main effects of sampling time or EtOH history on dialysate AEA levels after the EtOH challenge. Accordingly, further analyses on postinjection AUC data for AEA levels showed no differences between both EtOH treatments or after comparing each treatment with their respective baseline levels (AUC=0) (Figure 1H).
When baseline levels of AEA were compared between FAAH KO and WT mice, we found a significant main effect of genotype (F(1,11)=9.446;p=0.011). Thus, EtOH-naïve FAAH KO mice showed higher baseline AEA levels than EtOH-naïve WT mice. However, the statistical analysis of all AUC values showed no effects of genotype or EtOH history on the AEA response in the NAc after the EtOH challenge (Figures 1F and 1H).
Effects of EtOH exposure on NAc dialysate monoamine levels
In Experiments 2, microdialysis samples were collected to determine monoamine and amino acid levels in the NAc after an i.p. EtOH challenge. We first assessed the effect of an EtOH injection in EtOH-naïve and (r)EtOH-treated mice on DA and 5-HT levels in NAc dialysates of WT and FAAH KO mice (Figure 2).
Figure 2.
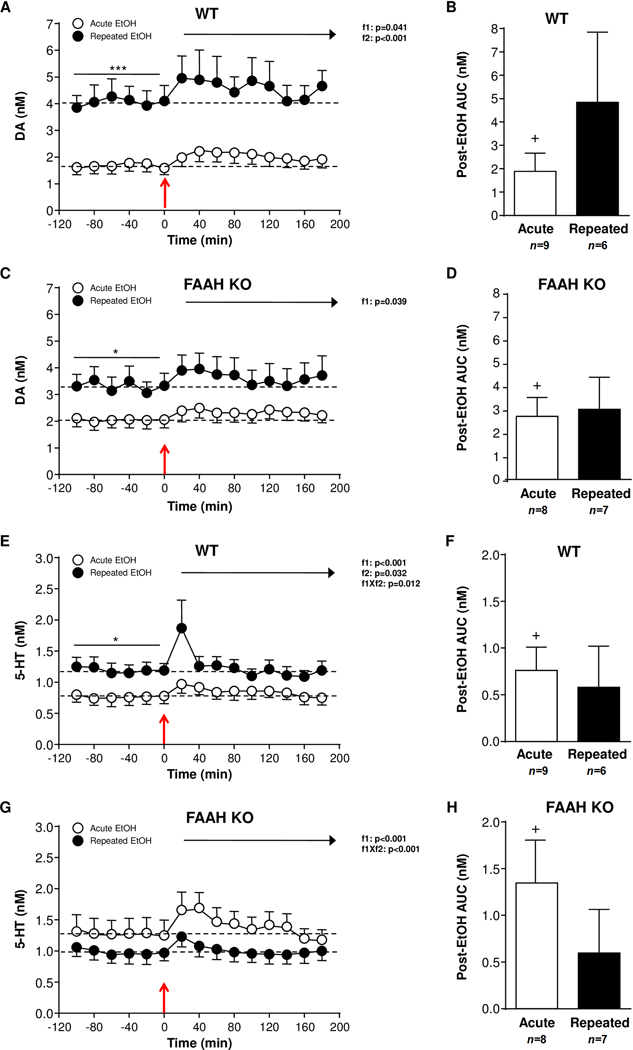
Effect of an EtOH challenge (2 mg/kg, i.p.) on monoamine levels in NAc microdialysates of WT and FAAH KO mice that were treated with repeated EtOH treatment (filled circles) or not (open circles). (A) DA levels in EtOH-naïve (n=9) and repeated EtOH-treated (n=6) WT mice. (B) Postinjection AUC values for DA summarizes the effect of EtOH injection on dialysate DA in WT mice. (C) DA levels in EtOH-naïve (n=8) and repeated EtOH-treated (n=7) FAAH KO mice. (D) Postinjection AUC values for DA summarizes the effect of EtOH injection on dialysate DA in FAAH KO mice. (E) 5-HT levels in EtOH-naïve (n=9) and repeated EtOH-treated (n=6) WT mice. (F) Postinjection AUC values for 5-HT summarizes the effect of EtOH injection on dialysate 5-HT in WT mice. (G) 5-HT levels in EtOH-naïve (n=8) and repeated EtOH-treated (n=7) FAAH KO mice. (H) Postinjection AUC values for 5-HT summarizes the effect of EtOH injection on dialysate 5-HT in FAAH KO mice. Effects of sampling time (f1) and EtOH history (f2) on postinjection data were analyzed using two-way repeated-measures ANOVA. EtOH injection is indicated by the red arrow at time point zero. *p<0.05 and **p<0.01 denote significant differences in repeated versus acute EtOH-treated mice. +p<0.05 denotes significant differences versus baseline (AUC=0).
Effects of EtOH on NAc dialysate DA levels
The analysis of baseline DA levels in the WT mice revealed a main effect of EtOH history (F(1,13)=42.30;p<0.001), and (r)EtOH-treated WT mice displayed higher DA levels than EtOH-naïve mice (EtOH-naïve: 1.66±0.29 nM; (r)EtOH-treated: 4.03±0.58 nM) (Fig. 2A). When DA levels were analyzed during 120 min after EtOH injection we found main effects of sampling time (F(8,104)=2.111;p=0.041) and EtOH history (F(1,13)=33.75;p<0.001) on DA levels. Thus, WT mice exhibited a modestly enhanced increase in DA levels after an EtOH challenge and the analysis of AUC values showed a significant increase compared with baseline DA levels (AUC=0) for EtOH-naïve mice (t(8)=2.435;p=0.041) (Fig. 2B).
In FAAH KO mice, there was a main effect of EtOH history (F(1,13)=6.085;p=0.028) on baseline DA levels (EtOH-naïve: 2.05±0.31 nM; (r)EtOH-treated: 3.31±0.47 nM) (Fig. 2C) similarly to WT mice. However, a two-way repeated-measures ANOVA after EtOH injection revealed only a main effect of sampling time (F(8,104)=2.132;p=0.039) on DA levels, but no effect of EtOH history. Both groups of FAAH KO mice displayed comparable total dialysate DA levels following the EtOH injection, although higher DA levels were observed during the first 60 min of post-EtOH period. As shown Figure 2D, postinjection AUC data of DA were similar for both FAAH KO mice, but only EtOH-naïve mice displayed significantly higher AUC values after EtOH injection relative to baseline DA levels (AUC=0) (t(7)=3.359;p=0.012).
The baseline DA levels of EtOH-naïve mice were also compared and no differences were detected in the baseline DA levels by genotype. However, the repeated EtOH treatment (with previous 7 injections of EtOH) induced an enhanced increase in basal DA levels in the two genotypes, but this increase was modestly lower in FAAH KO mice than in WT mice. Regarding all AUC values for DA levels after the EtOH challenge, a two way ANOVA revealed no effects of genotype or EtOH history (Figures 2B and 2D).
Effects of EtOH on NAc dialysate 5-HT levels
As shown Fig. 2E, there was a main effect of EtOH history (F(1,13)=5.952;p=0.030) on baseline 5-HT levels in WT mice, and (r)EtOH-treated mice displayed higher 5-HT levels than EtOH-naïve mice (EtOH-naïve: 0.77±0.12n M; (r)EtOH-treated: 1.19±0.14n M). The analysis of 5-HT levels following an EtOH challenge revealed main effects of sampling time (F(8,104)=5.840;p<0.001) and EtOH history (F(1,13)=5.780;p=0.032), and a significant interaction effect (F(8,104)=2.607;p=0.012). Thus, the i.p. EtOH injection produced a rapid increase in NAc 5-HT levels that was immediately normalized to baseline levels, being more evident in (r)EtOH-treated mice. However, although the comparison of the postinjection AUC values of 5-HT (Fig. 2F) showed no differences between WT mice, only EtOH-naïve mice displayed AUC values significantly different from baseline 5-HT levels (AUC=0) (t(8)=3.097;p=0.015).
Unlike WT mice, we observed no effect of EtOH history on baseline 5-HT levels (EtOH-naïve: 1.27±0.24 nM; (r)EtOH-treated: 0.98±0.15 nM) in FAAH KO mice (Fig. 2G). After the EtOH injection, there was a main effect of sampling time (F(8,104)=14.15;p<0.001) on 5-HT levels, but also an interaction between sampling time and EtOH history (F(8,104)=3.772;p<0.001). Similar to WT littermates, 5-HT levels were increased after the EtOH challenge in both groups of FAAH KO mice, returning to baseline 5-HT levels immediately. The postinjection AUC values for 5-HT levels (Fig. 2H) showed no differences between FAAH KO mice, but only EtOH-naïve mice displayed AUC values significantly higher than baseline 5-HT levels (AUC=0) (t(7)=2.741;p=0.029).
Regarding the comparison between genotypes, a two-way repeated-measures ANOVA of baseline 5-HT levels in EtOH-naïve mice showed no significant differences between FAAH KO and WT mice. Despite different effects of repeated EtOH injections on baseline 5-HT levels in the two genotypes, a two way ANOVA of postinjection AUC values for 5-HT levels found no significant effects of genotype or EtOH history (Figures 2F and 2H).
Effects of EtOH exposure on NAc dialysate amino acid levels
In Experiments 2 we also assessed the effect of an EtOH injection in EtOH-naïve and (r)EtOH-treated mice on GABA and GLU levels in NAc dialysates of WT and FAAH KO mice (Figure 3).
Figure 3.
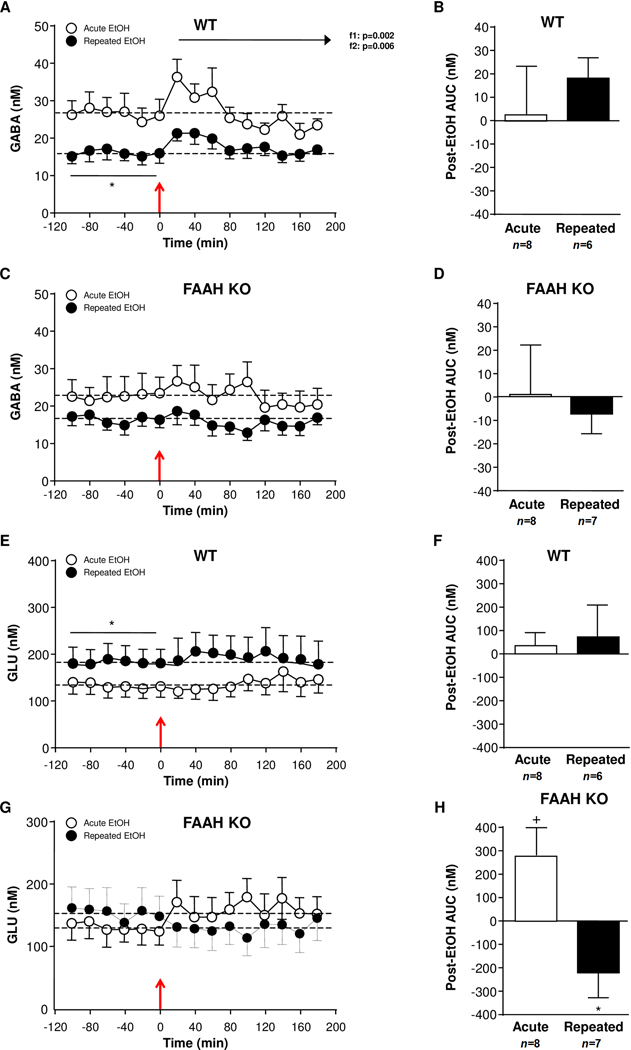
Effect of EtOH administration (2 mg/kg, i.p.) on amino acid levels in NAc microdialysates of WT and FAAH KO mice that were treated with repeated EtOH treatment (filled circles) or not (open circles). (A) GABA levels in EtOH-naïve (n=8) and repeated EtOH-treated (n=6) WT mice. (B) Postinjection AUC values for GABA summarizes the effect of EtOH injection on dialysate GABA in WT mice. (C) GABA levels in EtOH-naïve (n=8) and repeated EtOH-treated (n=7) FAAH KO mice. (D) Postinjection AUC values for GABA summarizes the effect of EtOH injection on dialysate GABA in FAAH KO mice. (E) GLU levels in EtOH-naïve (n=8) and repeated EtOH-treated (n=6) WT mice. (F) Postinjection AUC values for GLU summarizes the effect of EtOH injection on dialysate GLU in WT mice. (G) GLU levels in EtOH-naïve (n=8) and repeated EtOH-treated (n=7) FAAH KO mice. (H) Postinjection AUC values for GLU summarizes the effect of EtOH injection on dialysate GLU in FAAH KO mice. Effects of sampling time (f1) and EtOH history (f2) on postinjection data were analyzed using two-way repeated-measures ANOVA. EtOH injection is indicated by the red arrow at time point zero. *p<0.05 denotes significant differences in repeated versus acute EtOH-treated mice. +p<0.05 denotes significant differences versus baseline (AUC=0).
Effects of EtOH on NAc dialysate GABA levels
As shown Fig. 3A, a two-way repeated-measures ANOVA revealed a main effect of EtOH history (F(1,12)=5.033;p=0.044) on baseline GABA levels in WT mice. Namely, (r)EtOH-treated mice displayed lower baseline GABA levels than EtOH-naïve mice (EtOH-naïve: 26.39±3.88 nM; (r)EtOH-treated: 15.83±2.11 nM). With respect to the effects of the EtOH challenge on GABA levels during 120 min, the statistical analysis revealed main effects of sampling time (F(8,96)=3.918;p<0.001) and EtOH history (F(1,12)=11.33;p=0.006), but no interaction effect. The EtOH injection produced a sustained increase in GABA levels in both groups of WT mice during the first 60 min of the post-EtOH period, then GABA levels decreased progressively. In addition, the differences in baseline GABA levels between EtOH-naïve and (r)EtOH-treated mice were unaltered after the EtOH injection. As shown Figure 3B, the analysis of postinjection AUC values for GABA levels showed no differences between both groups of WT mice, and no differences when each group of mice was compared with baseline levels (AUC=0).
In FAAH KO mice (Fig. 3C), modest differences in baseline GABA levels were observed between EtOH-naïve and (r)EtOH-treated mice, but the statistical analysis showed no significant effect of EtOH history (EtOH-naïve: 22.51±4.44 nM; (r)EtOH-treated: 16.37±2.29 nM). Furthermore, dialysate GABA levels after the EtOH injection in FAAH KO mice were not affected by sampling time or EtOH history during a 120-min post-EtOH period. In fact, further analyses on postinjection AUC values confirmed the lack of significant effects on NAc GABA levels (Fig. 3D).
On the other hand, the comparison of GABA levels between FAAH KO and WT mice during the microdialysis session showed no significant differences. Firstly, a two-way repeated-measures ANOVA indicated that there were no differences in baseline GABA levels in EtOH-naïve mice or (r)EtOH-treated mice when both genotypes were compared. Furthermore, additional the analysis of all AUC values for GABA levels showed no effects of genotype or EtOH history following the EtOH injection (Figures 3B and 3D).
Effects of EtOH on NAc dialysate GLU levels
In addition to GABA, GLU levels were also assessed in the NAc. In WT mice (Fig. 3E), the analysis of baseline GLU levels revealed a main effect of EtOH history (F(1,12)=5.122;p=0.043) and (r)EtOH-treated mice displayed higher GLU levels than EtOH-naïve mice (EtOH-naïve: 130.75±23.04 nM; (r)EtOH-treated: 182.67±30.53 nM). However, a two-way repeated-measures ANOVA of dialysate GLU levels after the EtOH challenge showed no significant effects of sampling time or EtOH history in WT mice. Accordingly, we observed no differences in postinjection AUC values for GLU levels between groups of EtOH treatment or significant changes when these AUC values were compared with their respective baseline levels (AUC=0) (Fig. 3F).
Unlike WT mice, we observed no effect of EtOH history on GLU levels in FAAH KO mice (EtOH-naïve: 130.60±24.41 nM; (r)EtOH-treated: 153.56±33.53 nM) (Fig. 3G). Furthermore, the analysis of GLU levels after the EtOH injection revealed no main effects of sampling time or EtOH history. Despite we found no statistical differences in postinjection GLU levels between both groups of FAAH KO mice, the AUC data analysis revealed significant differences. Thus, EtOH-naïve FAAH KO mice displayed greater AUC for GLU levels than (r)EtOH-treated mice following the EtOH injection (t(13)=2.969;p=0.011). Whereas postinjection AUC value of EtOH-naïve mice was statistically higher than the baseline (AUC=0) (t(7)=2.393;p=0.048), AUC value of (r)EtOH-treated mice was lower than AUC=0. However, this decrease of AUC was non-significant (Fig. 3D).
The dialysate GLU levels were also compared between genotypes. Similar to baseline GABA levels, no significant differences were observed in baseline GLU levels between FAAH KO and WT mice. Despite the differences in the postinjection AUC values for GLU levels in FAAH KO mice according to EtOH history, a two way ANOVA found no significant effects of genotype or EtOH history on AUC data (Figures 3F and 3H).
Effects of EtOH exposure on locomotor activity
EtOH-induced locomotion in FAAH KO and WT mice was assessed for 10 min immediately after an acute EtOH challenge (2 g/kg) in EtOH-naïve and (r)EtOH-treated mice. At the end of the locomotion session, BAC was determined.
In the two genotypes we observed that the EtOH injection induced a strong increase in locomotor activity (at the first 1-min period) with no differences between (r)EtOH-treated and EtOH-naïve mice. In the WT mice (Fig. 4A), a two-way repeated-measures ANOVA revealed a main effect of time (F(9,171)=22.02;p<0.001) and a significant interaction between time and EtOH history (F(9,171)=2.513;p=0.010) on EtOH-induced locomotor activity. Therefore, whereas EtOH-naïve WT mice displayed a rapid and progressive decrease in locomotion, (r)EtOH-treated WT mice displayed a high locomotion during the first 5-min period. However, we found no significant differences between both groups of WT mice in the total crossovers at the end of the experimental session (Fig. 4B). Unlike WT mice, there was only a significant main effect of time (F(9,162)=15.65;p<0.001) on EtOH-induced locomotor activity in FAAH KO mice (Fig. 4C). Furthermore, we found no significant differences between both EtOH treatment groups in total number of crossovers (Fig. 4D).
Figure 4.
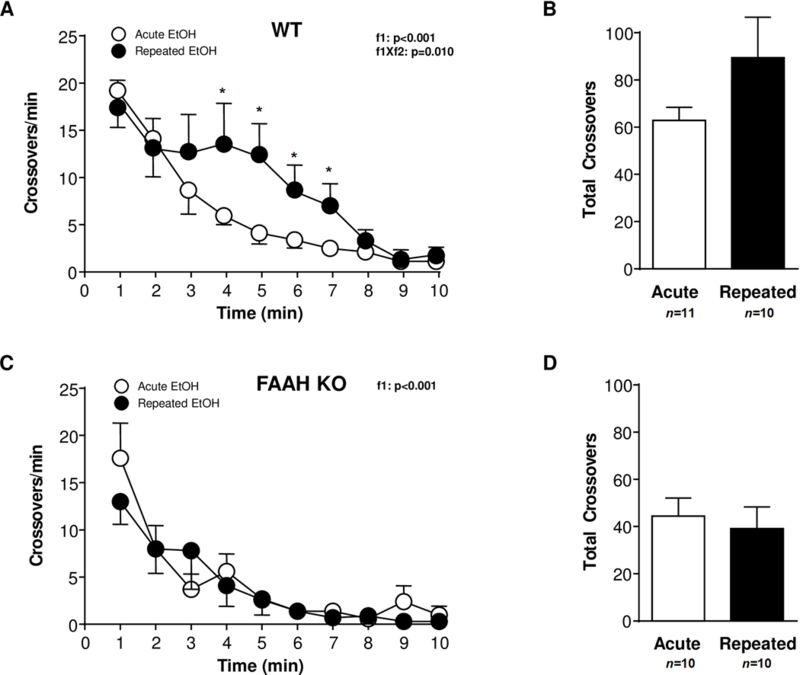
Effect of an EtOH challenge (2 mg/kg, i.p.) on locomotor activity in WT and FAAH KO mice that were treated with repeated EtOH treatment (filled circles) or not (open circles). (A) Locomotor activity (crossovers per min) during 10 min after EtOH injection in EtOH-naïve (n=11) and repeated EtOH-treated (n=10) WT mice. (B) Total locomotion (crossovers) after EtOH injection in EtOH-naïve and repeated EtOH-treated WT mice. (C) Locomotor activity (crossovers per min) during 10 min after EtOH injection in EtOH-naïve (n=10) and repeated EtOH-treated (n=10) FAAH KO mice. (D) Total locomotion (crossovers) after EtOH injection in EtOH-naïve and repeated EtOH-treated FAAH KO mice. Effects of sampling time (f1) and EtOH history (f2) on locomotion were analyzed using two-way repeated-measures ANOVA. *p<0.05 denotes significant differences in repeated versus acute EtOH-treated mice.
Considering the two genotypes, the i.p. EtOH challenge induced different effects on locomotor activity during a postinjection 10-min period, particularly in (r)EtOH-treated mice. These apparent differences in locomotion between FAAH KO and WT mice were confirmed with a two-way ANOVA of total crossovers that revealed a main effect of genotype (F(1,37)=6.352;p=0.016). Indeed, FAAH KO mice displayed lower EtOH-induced locomotion than WT littermates (Figures 4B and 4D).
BAC in WT and FAAH KO mice
BAC was determined for FAAH KO and WT mice 15 min after the i.p. EtOH injection (EtOH-naïve WT mice: 293.4±14.2 mg%; (r)EtOH-treated WT mice: 528.6±21.1 mg%; EtOH-naïve FAAH KO mice: 295.0±23.4 mg% and (r)EtOH-treated FAAH KO mice: 583.7±22.3 mg%) when EtOH-induced locomotion experiments were concluded. A two-way ANOVA revealed a main effect of EtOH history on BAC (F(1,37)=166.3;p<0.001), but no effect of genotype or interaction between the two factors.
DISCUSSION
Previous studies have reported that mice lacking FAAH exhibit increased EtOH intake and preference, which are consistent with the results of pharmacological data using FAAH inhibitors (Basavarajappa et al., 2006; Blednov et al., 2007). In addition, FAAH KO mice show decreased sensitivity to EtOH-induced hypothermia, sedation and locomotor incoordination relative to their WT littermates (Blednov et al., 2007; Vinod et al., 2008a). However, there is a lack of neurochemical data linking these EtOH-induced behavioral responses to changes in endocannabinoids and other neurotransmission systems in the mouse brain.
In the present study, we have investigated the effect of EtOH exposure and FAAH deletion on interstitial levels of neurotransmitters involved in rewarding and addictive behaviors in the NAc, a component of the basal ganglia that plays a critical role in mediating the rewarding properties of EtOH. To explore the relationship between both genotypes in EtOH-induced neuroadaptations within the NAc, in vivo microdialysis experiments were conducted in WT and FAAH KO mice following a repeated EtOH injection regimen previously used to characterize neurochemical changes in the NAc of C57BL/6J mice (Kapasova and Szumlinski, 2008).
EtOH-induced changes in NAc endocannabinoid levels by genotype and EtOH history
Firstly, we observed a 2-fold increase in baseline AEA levels in FAAH KO mice but no concurrent changes in 2-AG levels compared with WT mice, which is consistent with literature (Cravatt et al., 2001).
The baseline endocannabinoid levels in the NAc were not affected by EtOH history in the two genotypes but there were significant differences in the effects on endocannabinoid levels following an i.p. EtOH challenge (injection 1 for acute treatment and injection 8 for repeated treatment). Notably, the EtOH injection in WT mice produced a sustained increase in 2-AG levels in EtOH-naïve mice but a modest decrease in 2-AG levels in mice with repeated EtOH treatment. Conversely, dialysate AEA levels were not altered after the EtOH challenge. Although contradictory data in AEA and 2-AG levels have been reported with EtOH exposure (Pava and Woodward, 2012; Serrano and Parsons, 2011), our results are in agreement with previous microdialysis studies in the NAc of Wistar rats. These studies showed that self-administration of EtOH dose-dependently increases 2-AG but not AEA (Caille et al., 2007), and that acute systemic injection of EtOH increases 2-AG but decreases AEA in EtOH-naïve rats (Alvarez-Jaimes et al., 2009b; Ferrer et al., 2007). Furthermore, a recent study from our lab in the central amygdala of EtOH-dependent and non-dependent Wistar rats has shown increases in 2-AG and no changes in AEA after 30-min EtOH self-administration (Serrano et al., 2018). Taken together, these data suggest that 2-AG is mainly responsible for endocannabinoid neuroadaptations in the NAc in response to repeated EtOH treatment. However, we cannot to indicate whether these changes in extracellular 2-AG levels after acute EtOH administration are associated with a reduced 2-AG clearance or enhanced 2-AG formation.
In contrast to WT mice, the EtOH injection in FAAH KO mice produced no changes in 2-AG levels in EtOH-naïve FAAH KO mice. However, an enhanced decrease was observed in FAAH KO mice treated with repeated EtOH exposure compared with WT mice. We hypothesize that these differences in the 2-AG response after EtOH administration could be related to an inhibitory effect of the increased AEA tone in FAAH KO mice on 2-AG formation and/or release because both endocannabinoids share common molecular pathways and targets, and cooperate to modulate synaptic transmission via CB1 receptors. Accordingly, previous studies have reported that elevation of AEA concentrations by pharmacological or genetic inhibition of AEA degradation reduce the metabolism and physiological effects of 2-AG (Maccarrone et al., 2008). Consequently, these endocannabinoid alterations in FAAH KO mice exposed to EtOH could be associated with lower sensitivity to EtOH-induced toxic and physiological effects and higher preference for EtOH (Blednov et al., 2007; Vinod et al., 2008a) through a CB1 mechanism. In fact, several studies in rodents have demonstrated changes in expression and activity of CB1 receptors following EtOH exposures: while CB1 receptor availability is increased in the NAc after acute EtOH treatment (Ceccarini et al., 2013), a decrease in CB1 binding and coupling is observed after chronic EtOH (Basavarajappa et al., 2006).
Evidence suggests a critical role of CB1 receptors in EtOH-motivated behavior and implicates the participation of several neurotransmitters in the brain (Henderson-Redmond et al., 2016). For example, the blockade of CB1 receptors abolishes EtOH-induced increases in DA neuron firing in the NAc (Alvarez-Jaimes et al., 2009a; Cheer et al., 2007; Perra et al., 2005). On the other hand, the activation of CB1 receptors regulates the strength and plasticity of GABA and GLU synapses on dopaminergic neurons in the VTA (Haj-Dahmane and Shen, 2010) and, therefore, modulates DA content in response to alcohol in the NAc (Wang and Lupica, 2014).
EtOH-induced changes in NAc monoamine and amino acid levels by genotype and EtOH history
Several published findings provide evidence that EtOH stimulates the release of DA and 5-HT in the mesolimbic system, which mediates the reinforcing effects (Imperato and Di Chiara, 1986; Weiss et al., 1996; Yim et al., 1998; Yoshimoto et al., 1992). In the present study, an EtOH challenge produced increases in NAc monoamine levels in WT mice, which is in agreement with previous microdialysis data in the NAc of C57BL/6J mice exposed to systemic EtOH injections (Kapasova and Szumlinski, 2008). However, while these authors found no differences in basal levels of monoamines between mice treated with acute and repeated EtOH exposure, we observed that (r)EtOH-treated mice exhibited higher baseline monoamine levels than EtOH-naïve mice. In addition to this enhanced DA response to repeated EtOH exposure, decreases in baseline GABA levels and increases in baseline GLU levels were observed in (r)EtOH-treated WT mice. Accordingly, previous studies in rats and mice reported that systemic EtOH administration induces similar alterations in the basal content of GLU and GABA in the NAc (Kapasova and Szumlinski, 2008; Melendez et al., 2005). On the contrary, FAAH deletion showed EtOH-induced neurochemical differences in the NAc because the increase in baseline DA levels did not parallel changes in baseline levels of GABA and GLU. Moreover, EtOH injection exerted the opposite effects on GLU levels with a significant elevation in EtOH-naïve mice and a significant decrease in (r)EtOH-treated mice relative to their basal GLU levels. The majority of NAc neurons are GABAergic medium spiny neurons, and their activity is heavily modulated by afferent glutamatergic terminals from the prefrontal cortex, amygdala and hippocampus expressing CB1 receptors (Robbe et al., 2001). Thus, increased NAc 2-AG formation in GABAergic neurons through a DA receptor (D2R)-mediated activation can suppress excitatory inputs (for review (Parsons and Hurd, 2015)). In our study, increased GLU levels after EtOH challenge in naïve FAAH KO mice could be associated with a disruption of the 2-AG/CB1-mediated suppression of excitatory signaling associated with an endocannabinoid dysregulation.
Collectively, these data support the hypothesis that accumbal endocannabinoids can modulate both inhibitory and excitatory transmissions and provide a direct or indirect influence on DA neurons in regions innervated by the NAc, such as VTA (Alvarez-Jaimes et al., 2009a). In addition, differences in GABA and GLU transmissions in FAAH KO mice could contribute to the distinctive traits within EtOH exposure (Basavarajappa et al., 2006; Blednov et al., 2007; Vinod et al., 2008a).
Lack of EtOH-induced Behavioral Sensitization in FAAH KO mice
Repeated EtOH treatment can produce behavioral sensitization in rodents, which is characterized by a progressive increase in locomotion or stereotypes (Camarini and Pautassi, 2016). Behavioral sensitization reflects neuroadaptations in the mesolimbic reward circuitry, and neurotransmitters such as DA and GLU have been reported to be essential in the induction and subsequent expression of sensitization (Vanderschuren and Kalivas, 2000). Consistently, our results revealed an increased EtOH-induced locomotion in WT mice treated with repeated EtOH exposure, and this behavioral sensitization was accompanied by increased baseline levels of DA and GLU in the NAc and rapid responses to the EtOH injection, as above described. In contrast, FAAH KO mice displayed no differences in EtOH-induced locomotion when EtOH-naïve and (r)EtOH-treated mice were compared. In this case, while EtOH-induced DA levels were similar to WT mice, GLU levels were differently affected. Thus, basal GLU content was unaltered after repeated EtOH exposure although a transient decrease was immediately observed after the last EtOH injection. Taken together, changes in GLU transmission in the NAc can be critical to the induction and expression of EtOH-induced locomotor sensitization following a repeated EtOH treatment (Nona and Nobrega, 2018).
Limitations
In addition to the EtOH effects on endocannabinoid levels, it is known that the stress response induced by i.p. injection may influence endocannabinoid formation in the brain (Gorzalka et al., 2008). For example, acute stress induces selective decreases in mouse brain AEA but not in 2-AG (Rademacher et al., 2008). In contrast, we recently reported that restraint stress induces increased 2-AG levels and no changes in AEA levels in the amygdala of non-dependent rats (Serrano et al., 2018). Although these studies are performed in the amygdala, a key region in the regulation of negative emotional states and stress, previous studies strongly suggest that both amygdala and NAc are involved in the primary reinforcing effects of EtOH receiving dopaminergic projections from the VTA (Koob and Volkow, 2016).
Finally, we cannot disregard the influence of additional fatty acid ethanolamides in both EtOH-induced behavioral and neurochemical consequences in FAAH KO mice, since lipid transmitters such as N-oleoylethanolamine has been reported to be involved in the behavioral effects of alcohol exposure in rodents and humans (Bilbao et al., 2016; Garcia-Marchena et al., 2017). The existence of interactions with other signaling systems or developmental changes in these animals should be also considered.
Further pharmacological research (e.g., using FAAH inhibitors prior EtOH challenge) is needed to elucidate the molecular and functional cross-talk between 2-AG and AEA signaling in EtOH exposure in reward-relevant brain regions such as NAc.
Supplementary Material
ACKNOWLEDGEMENTS
We dedicate this manuscript to Dr. Loren H Parsons in loving memory to our departed friend, colleague and mentor. He was a recognized researcher in the field of endocannabinoid signaling and drug addiction.
This is manuscript number 29714 from The Scripps Research Institute. The present study has been supported by the Pearson Center for Alcoholism and Addiction Research and the following grants: National Institute on Alcohol Abuse and Alcoholism (NIAAA) (grant no. AA020404, AA022249 and AA024146 to RM-F; AA017447 to MR; and ARC AA006420 to RM-F and MR); Ministerio de Economía y Competitividad, Instituto de Salud Carlos III (ISCIII) and European Regional Development Funds-European Union (ERDF-EU) (Subprograma RETICS Red de Trastornos Adictivos RD12/0028/0001, PI16/01953, PI16/01689 and PI17/02026); Ministerio de Sanidad, Asuntos Sociales e Igualdad and Plan Nacional sobre Drogas (PND2017/043); Junta de Andalucía, Plan Andaluz de Investigación, Desarrollo e Innovación and ERDF-EU (PAIDI CTS-433). FJP and AS hold a “Miguel Servet” research contract funded by ISCIII and ERDF-EU (CP14/00212 and CP14/00173, respectively).
REFERENCES
- Alvarez-Jaimes L, Polis I, Parsons LH (2009a) Regional Influence of Cannabinoid CB1 Receptors in the Regulation of Ethanol Self-Administration by Wistar Rats. Open Neuropsychopharmacol J 2:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Jaimes L, Stouffer DG, Parsons LH (2009b) Chronic ethanol treatment potentiates ethanol-induced increases in interstitial nucleus accumbens endocannabinoid levels in rats. J Neurochem 111:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavarajappa BS, Hungund BL (2005) Role of the endocannabinoid system in the development of tolerance to alcohol. Alcohol Alcohol 40:15–24. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Yalamanchili R, Cravatt BF, Cooper TB, Hungund BL (2006) Increased ethanol consumption and preference and decreased ethanol sensitivity in female FAAH knockout mice. Neuropharmacology 50:834–844. [DOI] [PubMed] [Google Scholar]
- Bilbao A, Serrano A, Cippitelli A, Pavon FJ, Giuffrida A, Suarez J, Garcia-Marchena N, Baixeras E, Gomez de Heras R, Orio L, Alen F, Ciccocioppo R, Cravatt BF, Parsons LH, Piomelli D, Rodriguez de Fonseca F (2016) Role of the satiety factor oleoylethanolamide in alcoholism. Addict Biol 21:859–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Cravatt BF, Boehm SL 2nd, Walker D, Harris RA(2007) Role of endocannabinoids in alcohol consumption and intoxication: studies of mice lacking fatty acid amide hydrolase. Neuropsychopharmacology 32:1570–1582. [DOI] [PubMed] [Google Scholar]
- Buhler KM, Huertas E, Echeverry-Alzate V, Gine E, Molto E, Montoliu L, Lopez-Moreno JA (2014) Risky alcohol consumption in young people is associated with the fatty acid amide hydrolase gene polymorphism C385A and affective rating of drug pictures. Mol Genet Genomics 289:279–289. [DOI] [PubMed] [Google Scholar]
- Caille S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH (2007) Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J Neurosci 27:3695–3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarini R, Pautassi RM (2016) Behavioral sensitization to ethanol: Neural basis and factors that influence its acquisition and expression. Brain Res Bull 125:53–78. [DOI] [PubMed] [Google Scholar]
- Ceccarini J, Casteels C, Koole M, Bormans G, Van Laere K (2013) Transient changes in the endocannabinoid system after acute and chronic ethanol exposure and abstinence in the rat: a combined PET and microdialysis study. Eur J Nucl Med Mol Imaging 40:1582–1594. [DOI] [PubMed] [Google Scholar]
- Colombo G, Serra S, Brunetti G, Gomez R, Melis S, Vacca G, Carai MM, Gessa L (2002) Stimulation of voluntary ethanol intake by cannabinoid receptor agonists in ethanol-preferring sP rats. Psychopharmacology (Berl) 159:181–187. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH (2001) Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A 98:9371–9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB (1996) Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384:83–87. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, Phillips PE, Wightman RM (2007) Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci 27:791–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D (2002) Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A 99:10819–10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Mattioli L, Cifani C, Perfumi M, Massi M, Cuomo V, Trabace L, Ciccocioppo R (2006) Effect of the cannabinoid CB1 receptor antagonist SR-141716A on ethanol self-administration and ethanol-seeking behaviour in rats. Psychopharmacology (Berl) 183:394–403. [DOI] [PubMed] [Google Scholar]
- Ferrer B, Bermudez-Silva FJ, Bilbao A, Alvarez-Jaimes L, Sanchez-Vera I, Giuffrida A, Serrano A, Baixeras E, Khaturia S, Navarro M, Parsons LH, Piomelli D, Rodriguez de Fonseca F (2007) Regulation of brain anandamide by acute administration of ethanol. Biochem J 404:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fezza F, Bari M, Florio R, Talamonti E, Feole M, Maccarrone M (2014) Endocannabinoids, related compounds and their metabolic routes. Molecules 19:17078–17106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CJ, Jonsson KO, Tiger G (2001) Fatty acid amide hydrolase: biochemistry, pharmacology, and therapeutic possibilities for an enzyme hydrolyzing anandamide, 2-arachidonoylglycerol, palmitoylethanolamide, and oleamide. Biochem Pharmacol 62:517–526. [DOI] [PubMed] [Google Scholar]
- Frantz KJ, Hansson KJ, Stouffer DG, Parsons LH (2002) 5-HT(6) receptor antagonism potentiates the behavioral and neurochemical effects of amphetamine but not cocaine. Neuropharmacology 42:170–180. [DOI] [PubMed] [Google Scholar]
- Freedland CS, Sharpe AL, Samson HH, Porrino LJ (2001) Effects of SR141716A on ethanol and sucrose self-administration. Alcohol Clin Exp Res 25:277–282. [PubMed] [Google Scholar]
- Garcia-Marchena N, Pavon FJ, Pastor A, Araos P, Pedraz M, Romero-Sanchiz P, Calado M, Suarez J, Castilla-Ortega E, Orio L, Boronat A, Torrens M, Rubio G, de la Torre R, Rodriguez de Fonseca F, Serrano A (2017) Plasma concentrations of oleoylethanolamide and other acylethanolamides are altered in alcohol-dependent patients: effect of length of abstinence. Addict Biol 22:1366–1377. [DOI] [PubMed] [Google Scholar]
- Gorzalka BB, Hill MN, Hillard CJ (2008) Regulation of endocannabinoid signaling by stress: implications for stress-related affective disorders. Neurosci Biobehav Rev 32:1152–1160. [DOI] [PubMed] [Google Scholar]
- Haj-Dahmane S, Shen RY (2010) Regulation of plasticity of glutamate synapses by endocannabinoids and the cyclic-AMP/protein kinase A pathway in midbrain dopamine neurons. J Physiol 588:2589–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson-Redmond AN, Guindon J, Morgan DJ (2016) Roles for the endocannabinoid system in ethanol-motivated behavior. Prog Neuropsychopharmacol Biol Psychiatry 65:330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungund BL, Szakall I, Adam A, Basavarajappa BS, Vadasz C (2003) Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J Neurochem 84:698–704. [DOI] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G (1986) Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther 239:219–228. [PubMed] [Google Scholar]
- Kapasova Z, Szumlinski KK (2008) Strain differences in alcohol-induced neurochemical plasticity: a role for accumbens glutamate in alcohol intake. Alcohol Clin Exp Res 32:617–631. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2016) Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3:760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, Rossi S, Bari M, De Chiara V, Fezza F, Musella A, Gasperi V, Prosperetti C, Bernardi G, Finazzi-Agro A, Cravatt BF, Centonze D (2008) Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nat Neurosci 11:152–159. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Hicks MP, Cagle SS, Kalivas PW (2005) Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcohol Clin Exp Res 29:326–333. [DOI] [PubMed] [Google Scholar]
- Nona CN, Nobrega JN (2018) A role for nucleus accumbens glutamate in the expression but not the induction of behavioural sensitization to ethanol. Behav Brain Res 336:269–281. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Hurd YL (2015) Endocannabinoid signalling in reward and addiction. Nat Rev Neurosci 16:579–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pava MJ, Woodward JJ (2012) A review of the interactions between alcohol and the endocannabinoid system: implications for alcohol dependence and future directions for research. Alcohol 46:185–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perra S, Pillolla G, Melis M, Muntoni AL, Gessa GL, Pistis M (2005) Involvement of the endogenous cannabinoid system in the effects of alcohol in the mesolimbic reward circuit: electrophysiological evidence in vivo. Psychopharmacology (Berl) 183:368–377. [DOI] [PubMed] [Google Scholar]
- Pertwee RG (2015) Endocannabinoids and Their Pharmacological Actions. Handb Exp Pharmacol 231:1–37. [DOI] [PubMed] [Google Scholar]
- Rademacher DJ, Meier SE, Shi L, Ho WS, Jarrahian A, Hillard CJ (2008) Effects of acute and repeated restraint stress on endocannabinoid content in the amygdala, ventral striatum, and medial prefrontal cortex in mice. Neuropharmacology 54:108–116. [DOI] [PubMed] [Google Scholar]
- Robbe D, Alonso G, Duchamp F, Bockaert J, Manzoni OJ (2001) Localization and mechanisms of action of cannabinoid receptors at the glutamatergic synapses of the mouse nucleus accumbens. J Neurosci 21:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano A, Parsons LH (2011) Endocannabinoid influence in drug reinforcement, dependence and addiction-related behaviors. Pharmacol Ther 132:215–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano A, Pavon FJ, Buczynski MW, Schlosburg J, Natividad LA, Polis IY, Stouffer DG, Zorrilla EP, Roberto M, Cravatt BF, Martin-Fardon R, Rodriguez de Fonseca F, Parsons LH (2018) Deficient endocannabinoid signaling in the central amygdala contributes to alcohol dependence-related anxiety-like behavior and excessive alcohol intake. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano A, Rivera P, Pavon FJ, Decara J, Suarez J, Rodriguez de Fonseca F, Parsons LH (2012) Differential effects of single versus repeated alcohol withdrawal on the expression of endocannabinoid system-related genes in the rat amygdala. Alcohol Clin Exp Res 36:984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe JC, Chiang K, Gerber AL, Beutler E, Cravatt BF (2002) A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc Natl Acad Sci U S A 99:8394–8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan ME, Gowin JL, Yan J, Schwandt ML, Spagnolo PA, Sun H, Hodgkinson CA, Goldman D, Ramchandani VA (2018) Severity of alcohol dependence is associated with the fatty acid amide hydrolase Pro129Thr missense variant. Addict Biol 23:474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Dimitrakakis ES, Rice O, Gifford A, Volkow ND (2005) Ethanol self-administration and ethanol conditioned place preference are reduced in mice lacking cannabinoid CB1 receptors. Behav Brain Res 164:206–213. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW (2000) Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 151:99–120. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Sanguino E, Yalamanchili R, Manzanares J, Hungund BL (2008. a) Manipulation of fatty acid amide hydrolase functional activity alters sensitivity and dependence to ethanol. J Neurochem 104:233–243. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Yalamanchili R, Thanos PK, Vadasz C, Cooper TB, Volkow ND, Hungund BL (2008. b) Genetic and pharmacological manipulations of the CB(1) receptor alter ethanol preference and dependence in ethanol preferring and nonpreferring mice. Synapse 62:574–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Lupica CR (2014) Release of endogenous cannabinoids from ventral tegmental area dopamine neurons and the modulation of synaptic processes. Prog Neuropsychopharmacol Biol Psychiatry 52:24–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu J, Harvey-White J, Zimmer A, Kunos G (2003) Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc Natl Acad Sci U S A 100:1393–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Parsons LH, Schulteis G, Hyytia P, Lorang MT, Bloom FE, Koob GF (1996) Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J Neurosci 16:3474–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim HJ, Schallert T, Randall PK, Gonzales RA (1998) Comparison of local and systemic ethanol effects on extracellular dopamine concentration in rat nucleus accumbens by microdialysis. Alcohol Clin Exp Res 22:367–374. [PubMed] [Google Scholar]
- Yoshimoto K, McBride WJ, Lumeng L, Li TK (1992) Alcohol stimulates the release of dopamine and serotonin in the nucleus accumbens. Alcohol 9:17–22. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Schwartz BI, Giza J, Gross SS, Lee FS, Kreek MJ (2017) Blockade of alcohol escalation and “relapse” drinking by pharmacological FAAH inhibition in male and female C57BL/6J mice. Psychopharmacology (Berl) 234:2955–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


