Abstract
A central question in comparative neurobiology concerns how evolution has produced brains with expanded neocortices, composed of more areas with unique connectivity and functional properties. Some mammalian lineages, such as primates, exhibit exceptionally large cortices relative to the size of sensory inputs from the dorsal thalamus, and this expansion is associated with a larger number of distinct cortical areas, composing a larger proportion of the cortical sheet. We propose a link between the organization of the neocortex and its expansion relative to the size of the dorsal thalamus, based on a combination of work in comparative neuroanatomy and experimental research.
Introduction
One of the defining features of the human brain is the exceptional size of the neocortex and the large number of cortical areas that compose it. Beyond the primary sensory and motor areas that we share with other mammals, humans and other primates exhibit additional functionally, anatomically, and architectonically distinct cortical areas involved in higher-order sensory processing and the generation of complex movements [1,2]. These changes in the human neocortex are, in part, responsible for many of our unique behavioral specializations such as precise manual control, cognitive flexibility, and language comprehension and production. Because of the important role that the neocortex plays in generating these behaviors, there has been a concerted effort to understand how the neocortex increases in size and why and how it fissures and folds [3,4,5].
A plethora of studies on cortical neurogenesis stemming from decades of elegant molecular experiments have detailed how progenitor cell pools have been modified to generate expansions in the cortical sheet, both in primates generally [6,7] and humans specifically [8,9]. Without question, these studies have increased our understanding of the evolution of the developmental mechanisms that generate an increase in the size of the cortical sheet over the course of mammalian evolution [10]. Nevertheless, how novel cortical areas emerge in evolution and become integrated with existing cortical networks remains one of the most puzzling and fundamental questions in neuroscience. Furthermore, developmental studies often overlook the important observation that cortical sheet expansion in mammals occurs in at least two different ways.
First, if we consider a particular mammalian lineage with a wide range of brain sizes – e.g. anthropoid primates, or carnivores – the neocortex tends to become disproportionately large as the whole brain, including non-cortical structures, also increases in size. These size-associated patterns of variation (i.e. allometric effects) are commonly called concerted brain evolution [11], and are thought to reflect systematic changes to neurodevelopmental schedules [12,13; but see 14]. Second, when comparing brains across mammalian lineages, it becomes apparent that some groups exhibit a disproportionate increase in the size of the neocortex relative to subcortical structures. These evolutionary features are observed irrespective of brain size, and are called mosaic brain evolution within particular lineages relative to others. Below we examine two ways in which the neocortex has expanded relative to the size of the structure that serves as the primary source of its sensory input: the dorsal thalamus. We also explore how these two types of expansion relate to alterations in cortical field number and cortical field size. We then review data that demonstrates that sensory input plays a prominent role in determining the functional organization of the cortical sheet, and postulate that this is particularly true for mammals that have a disproportionately large neocortex, like primates.
Concerted evolution of the neocortex and the dorsal thalamus
Mammalian brains vary in absolute size by five orders of magnitude, and the relative proportion of different brain components, such as the neocortex, dorsal thalamus and brain stem, have long been known to follow regular “concerted” patterns associated with total brain size [15]. Concerted effects have been linked to regular changes in the onset of neurogenesis from progenitor cell populations across species [12], and are associated with prenatal brain growth patterns [16] as well as differences in adult brains, such as gross structure size [12,13], neuron densities across the neocortex [17], rod/cone ratios in the retina [18], and even variation in brain structure size within a species, such as humans [19]. In many mammalian lineages studied, such as carnivores [20], dermoptera [21], primates, and insectivorous Afrotherian and Laurasiatherian mammals [15], concerted evolution accounts for how the neocortex composes a larger proportion of the brain in larger-brained species. The same relationship seems to apply to thalamus:cortex scaling – larger brains have exceptionally large neocortices relative to the size of the dorsal thalamus.
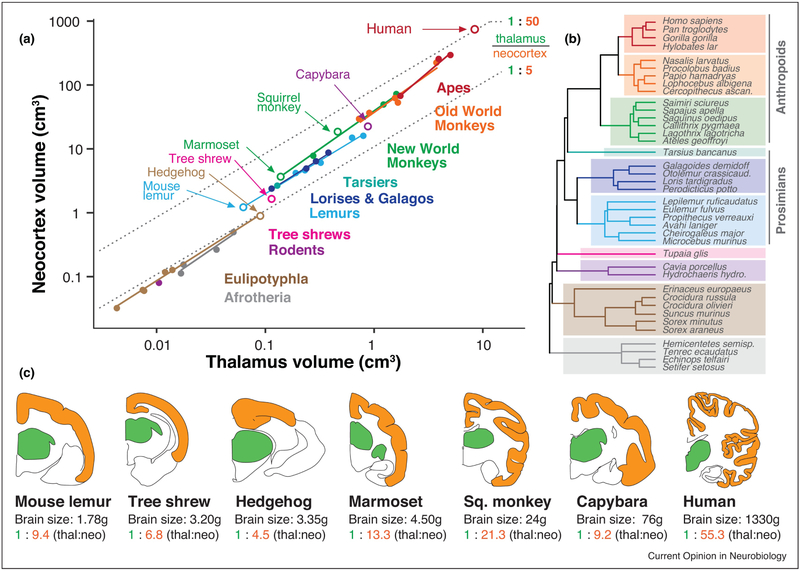
Figure 1 shows the scaling of the thalamus relative to the neocortex in 39 species (Figure 1A), with trend lines applied to diverse mammalian groups (Figure 1B). Cladistic levels were chosen to aid comparisons of major primate lineages against a number of shrews, tenrecs, and other species (Afrotheria and Laurasiatheria, previously “Insectivora”) for which data is available [15]. Every lineage exhibits a slope greater than 1 (apes: 1.45; Old World monkeys: 1.21; New World monkeys: 1.20; lemurs: 1.01; lorises and galagos: 1.07; Afrotheria: 1.17; Eulipotyphla: 1.09), indicating positive allometry. Simply put, as the dorsal thalamus gets larger, the neocortex outpaces it in size. For example, within New World monkeys, the small-brained pygmy marmoset (brain weight: 4.5g) exhibits a thalamus-to-neocortex volume proportion of 1:13, while the larger-brained spider monkey (brain weight: 108g) has nearly twice as much neocortex, proportionally speaking – 1:24 (Figure 1)[15]. Even after we account for evolutionary relationships (phylogenetic generalized least squares [PGLS] regression using a mammalian supertree from [22] and the {ape}, {gieger} and {caper} libraries for R), we see a robust and positive allometry between the thalamus and neocortex (slope=1.25, p<0.001, R2=0.98).
Figure 1. Primate “neocorticalization” relative to the dorsal thalamus.
Across a wide range of brain sizes, primates exhibit larger neocortices relative to the size of the dorsal thalamus than many mammalian groups. (A) Neocortex volume is plotted against dorsal thalamus volume in log-log coordinates, demonstrating effects of both concerted and mosaic evolution. Concerted evolution is demonstrated by each group having a slope greater than 1 (i.e. more than isometric scaling, or constant proportions across size variation). Mosaic evolution is exemplified by intercept shifts between mammalian lineages. Open circles indicate species shown in coronal section below. (B) A phylogenetic tree of included species, with colors corresponding to mammalian clades in (A). Even after correcting for phylogenetic relatedness, the neocortex always gets larger “faster” than the thalamus does (see text). (C) Coronal sections of selected brains in the dataset above, taken from a similar rostrocaudal level (ventral posterior [VP] nucleus of the thalamus), with associated brain sizes and thalamus:neocortex proportions. Primates, and particularly anthropoids (monkeys and apes) have an unusually large neocortex volume that dwarfs size-associated concerted effects. This mosaic evolution of the primate neocortex is so extreme (over & above size-related concerted effects) that only the largest rodent brain (capybara) has similar thalamus-to-neocortex proportions as the smallest primate brain (mouse lemur). Data from [15,22]. Coronal section outlines (dorsal thalamus in green, neocortex in orange) are from the Comparative Mammalian Brain Collection from the University of Wisconsin, and are not to scale. Thal = dorsal thalamus; neo = neocortex. Marmoset (Callithrix jacchus) thalamus size was estimated from the dataset in [15].
While concerted evolution describes the expansion of the neocortex associated with total brain size – the cortex getting larger “faster” than the medulla, for example – a second form of cortical expansion can be observed between mammalian lineages, even when subcortical structures, like the dorsal thalamus, are similar in size. Here, important differences emerge when we compare mammalian lineages to one another.
Mosaic evolution of the neocortex and dorsal thalamus
Comparing the trend lines of diverse mammalian lineages, it becomes apparent that some lineages have larger neocortices even when thalamus is similar in size (Figure 1A). This kind of disproportionate expansion beyond concerted effects is frequently called mosaic brain evolution, and plays a major role in the evolution of brain structure size in both birds [23,24] and fish [25,26,27]. In mammals, the evolution of different brain structures appears to be more constrained (i.e. concerted) than in non-mammalian vertebrates, except in certain structures such as the olfactory bulbs [15; but see 28,29]. Nevertheless, one of clearest cases of mosaic evolution in mammals is the disproportionate expansion of the neocortex or “neocorticalization.” Several mammalian clades, such as carnivores [20] and primates [15,30], exhibit larger neocortices than other mammalian lineages, after we control for subcortical brain structure size. For example, the mouse lemur, which has the smallest primate brain, has a higher neocortex:thalamus proportion than even the largest rodent, the capybara (Figure 1C). This is despite the fact that larger brains usually have proportionally larger neocortices (concerted expansion), and the capybara’s brain (~50g [31]) is 30 times larger than that of the mouse lemur (1.8g [15]).
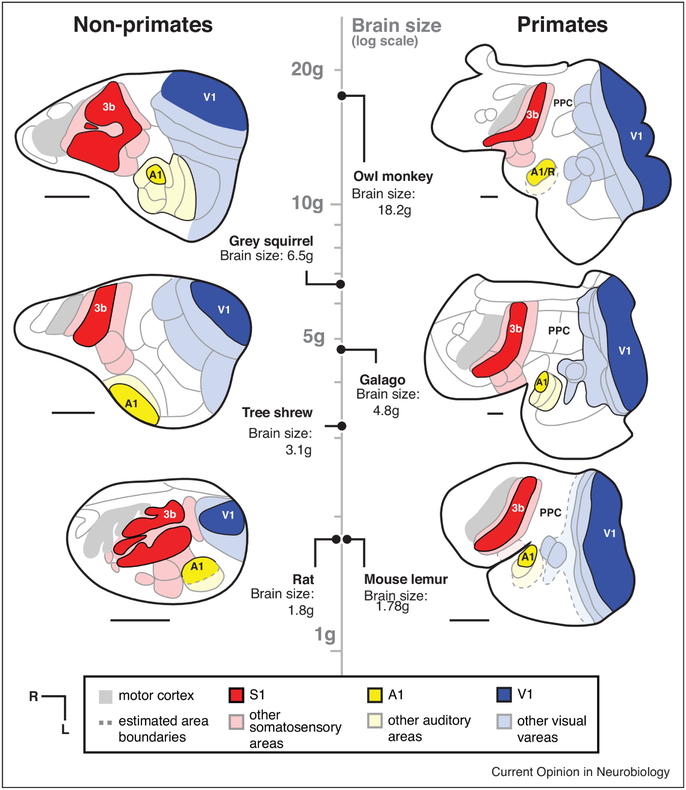
Of course, not all of the dorsal thalamus is composed of first-order sensory relay nuclei, and not all of the neocortex is composed of “primary” areas receiving these inputs (Figure 2). First-order sensory relay nuclei of the thalamus (e.g. ventral posterior nucleus (VP), dorsal lateral geniculate nucleus (dLGN), ventral medial geniculate nucleus (vMGN)) compose a smaller fraction of the dorsal thalamus in larger primate brains [15, 32]. A similar effect is observed as the neocortex increases in size: larger primate neocortices have relatively less space dedicated to primary sensory fields (with the exception of V1)[33,34]. Instead, a larger proportion of the neocortex is devoted to higher-order areas, such as posterior parietal (PPC) and inferotemporal (ITC) cortex, which are associated with multi-sensory integration, reaching and grasping, object recognition and other complex behaviors [35,36]. However, these effects have largely been observed among primate species, and it has yet to be determined whether these same principles of expansion are found in non-primate lineages.
Figure 2. Neocortical organization in primates and non-primate mammals with varying brain size.
Maps of cortical organization from flattened sections are drawn from architectonic and functional studies, and are aligned according to log-transformed brain size (vertical axis) with primates to the right, and closely-related euarchontoglire species to the left. Compared with mammals of similar brain size, primates have larger neocortices (parietal, temporal and frontal regions), as well as more non-primary sensory areas. While thalamus size is not available for every species included, larger brains have larger neocortex:thalamus proportions (concerted evolution), and primates have a larger neocortex:thalamus proportions across every brain size than do closely related species (mosaic evolution). All scale bars = 4mm. Maps are redrawn from [61,62,63,64].
Both concerted and mosaic evolution shape thalamocortical relationships and cortical organization.
Both concerted and mosaic forms of neocortical expansion result in a similar effect, with relatively smaller first-order thalamic nuclei sending projections to a relatively larger cortical sheet (Figure 1A). However, the two forms differ in important ways that are obscured by the general term “cortical expansion.” Recognizing these differences can inform research on the developmental mechanisms responsible for different types of neocortical expansion and allow us to appreciate the consequences of cortical sheet expansion on cortical organization.
First, whether cortical expansion is concerted or mosaic changes our perspective on the developmental mechanisms responsible for how the cortex has increased in size in different species. In concerted evolution, the cortex increases in size alongside other brain structures, suggesting that there are changes to global neurodevelopmental mechanisms that affect cortical development (i.e. the entire schedule of brain development [12, 37] rather than changes to cortex-specific mechanisms). By contrast, mosaic effects are likely to be the result of neurodevelopmental programs that are specific to the neocortex, and which do not alter subcortical structure size. Unfortunately, many of the species that serve as animal models represent mammalian lineages that reflect both types of evolution. For example, early comparative work on cortical neurogenesis in mouse vs. macaque demonstrated an expansion of the macaque embryonic subventricular zone (SVZ) and its differentiation into inner and outer subdivisions [38]. Is this transient embryonic phenotype in primates a signature of larger and more highly folded cortices in general (concerted evolution), or is it specific to primate “neocorticalization” (mosaic evolution)? While SVZ compartmentalization into outer (OSVZ) and inner (ISVZ) regions was originally thought to be primate-specific [38], subsequent work has shown that these developmental features are shared by various carnivores, rodents, and artiodactyls [6,39,40] and are linked to neocortex size rather than phylogeny. Interestingly, Garcia-Moreno and colleagues [41] compared the larger-brained (20.3g [15]) gyrencephalic rodent agouti (Dasyprocta) with the smaller-brained (7.9g [15]) lissencephalic marmoset (Callithrix) and found similar SVZ compartmentalization at mid-gestation in both species, although the size and the proportions of specific cell types in the OSVZ were different. This suggests that the presence of an OSVZ and some of its characteristics are more closely related to absolute neocortex size than they are to phylogeny or gyrification, and may reflect concerted evolutionary effects rather than mosaic neocorticalization. The approach of minimizing brain size differences in comparative developmental research (e.g. comparing larger-brained rodents with smaller-brained primates) allows us to untangle the mixed concerted and mosaic evolutionary processes at work.
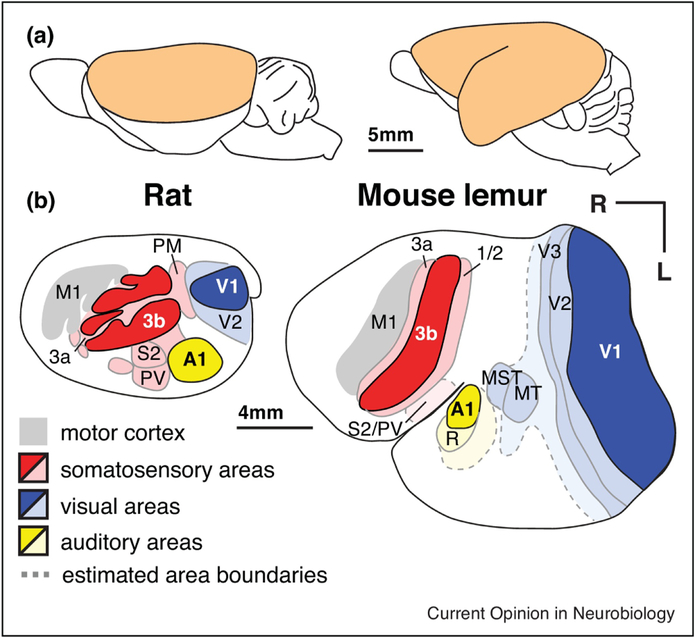
Second, the mosaic evolution of the primate neocortex relative to its thalamic inputs may be especially important for understanding the exceptional increase in the number of cortical areas observed in even the smallest primate brains. Both the rat and the prosimian mouse lemur share a similar brain size, but mouse lemurs have larger neocortices that are composed of more cortical areas and expanded parietal and temporal regions (Figures 2, 3). In fact, a larger number of neocortical areas is a characteristic of primates in general [1], particularly in parietal, temporal, and frontal regions that are proportionally larger in primates (Figure 2)[35,36,42], Deacon [43] proposed that changes in the relative size of brain structures – a consequence of either concerted or mosaic evolution – might necessarily result in alterations in their connections. Since axons compete for targets during development, an exceptionally large axonal source might out-compete other axons for the same target, thereby “displacing” ancestral connections. Alternatively, an expanded innervation target (e.g. an expanded cortex relative to its thalamic inputs) might relax the process of competition, allowing for novel combinations of connections to form on an expanded target. How much of the increased complexity in primate neocortical organization might be a consequence of a disproportionate expansion of the cortical sheet relative to the structure which provides its main source of sensory inputs, the dorsal thalamus (Figure 2)? Could an expansion of the target of thalamocortical axons be sufficient to generate novel combinations of inputs and thus a new cortical field?
Figure 3. Neocortex of a rat and mouse lemur drawn to scale.
Although rats and mouse lemurs have a similar size brain (~1.8g), the neocortex of the mouse lemur has a surface area ~3 times that of the rat. The mouse lemur also has a larger number of distinct cortical fields in the expanded area of cortex between S1, V1 and A1. The white region in mouse lemur between area 1/2 and other visual areas (light blue) is likely composed of multiple parietal subdivisions (as in the galago; see Fig 2) that have not been fully characterized. Temporal and frontal cortex have also expanded in mouse lemurs. Modified from [61,62] (rat) and [63] (mouse lemur).
While it is true that neocortical expansion relative to the dorsal thalamus is associated with a larger number of cortical fields (at least in mammals for which both allometric and functional data are available, e.g. euarchontoglires), other mechanisms are involved in the emergence of new cortical fields over the course of evolution. These include alterations in the expression of genes associated with axon guidance, as well as alterations in the types and relative ratio of incoming sensory inputs. As discussed above, the capybara brain exhibits similar proportions of thalamus to neocortex as does the mouse lemur brain (~1:9), but the brain of the capybara appears to have a simpler cortical organization and a smaller number of higher-order cortical fields. In fact, capybara neocortical organization looks like a scaled-up version of the guinea pig [31], despite the fact that the capybara has a thalamus:neocortex proportion within the range of prosimian primates (Figure 1A). Exceptional cases such as the capybara – which has a large neocortex but relatively simple organization – allow us to appreciate that cortical field evolution does not involve only simple scaling rules, but is more complicated and can only be fully understood using a comparative approach.
The role of sensory input in determining the functional organization of the neocortex.
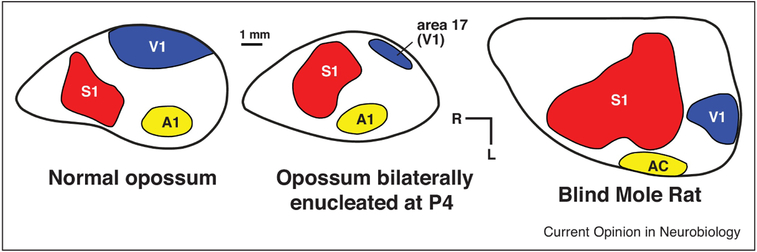
Primary sensory fields (V1, S1, A1) and several other sensory areas (V2, S2, PV, R) are ubiquitous across every investigated mammal [44]. Each of these fields has retained specific patterns of thalamocortical and corticocortical projections from the common mammalian ancestor, and each has a number of readily identified architectonic features, including a dense layer IV in primary areas. For example, all mammals possess a primary visual area with dense projections from the dorsal lateral geniculate nucleus of the thalamus, even when the animal is eyeless (enucleated or anophthalmic mice [45,46]) or does not engage in visually mediated behavior (blind mole rat [47])(Figure 4). V1 in these animals is reduced in size and the subcortical connections of the lost or reduced sensory system have been altered, such that the dLGN receives input from other sensory systems. Despite the absence of a functional visual system, basic aspects of the connection network are maintained, as are features of cytoarchitecture, even as “V1” becomes functionally dedicated to auditory processing [48].
Figure 4. Neocortex in normal and bilaterally enucleated opossums and blind mole rats.
Although the neocortex in bilaterally enucleated opossums never receives either spontaneous or sensory driven input from the eyes, an architectonically defined primary visual area (V1 or area 17) is still present. Likewise, the blind mole rat has anophthalmic eyes with skin grown over them, yet they still possess an architectonically defined V1. In blind opossums, what would have been visual cortex is co-opted by the somatosensory and auditory system, and in blind mole rats V1 is activated by auditory inputs. Figures redrawn from [47,49].
Experimental studies in which visual input to the developing brain is removed via bilateral enucleation very early in development also supports the important role of sensory driven activity for anatomical and functional specification of primary sensory areas. In opossums in which the eyes are removed prior to the onset of spontaneous activity (and well before the onset of sensory driven activity) a greatly reduced primary visual area, defined architectonically, is still present as are aspects of thalamocortical and corticocortical connections. However, neurons in V1 are activated by both auditory and somatosensory stimulation, and the reorganized V1 receives new input from somatosensory and auditory structures of the cortex and the dorsal thalamus [49,50]. The annexation of visual cortex by the spared sensory systems has also been demonstrated in congenitally blind humans. Visual cortex in these individuals is activated when performing a variety of non-visual tasks such as Braille reading [51]. sound localization [52,53], and language processing [54, see 55 for review]. While primary cortical areas are generally defined by a combination of criteria including their architectonic appearance, connectivity, and functional organization, the available evidence suggests that these features can be decoupled when sensory input is altered, either in evolution or in experimental studies, emphasizing the central role that sensory input plays in generating “normal” cortical fields.
The persistence of these primary fields – even when their input is reduced or removed – is likely due to the contingent nature in which genes are expressed during the development of the neocortex. A wide range of genetic cascades involved in cell proliferation, adhesion, axon fasciculation and guidance have been well described for the development of primary and secondary cortical fields [56]. Alterations in the expression of these genes can alter the size and relative location of these cortical areas, but never result in their total absence [56,57]. This temporal and contingent deployment of genes early in development acts to constrain cortical evolution and explains the ubiquity of these fields in all mammals. However, our knowledge of how higher-order cortical areas develop and how new cortical fields are added to this evolutionarily old, genetically specified network is not well understood.
We propose that the addition of non-primary cortical fields in primates may reflect the disproportionate expansion of the cortical sheet relative to its primary inputs from the dorsal thalamus, rather than the type of genetic specification that has been described for primary fields. There are several lines of evidence that indicate that the portions of the neocortex that have greatly expanded within the primate order include temporal cortex, posterior parietal cortex (PPC) and prefrontal cortex [35,36,42]. Certain features of non-primary sensory cortical fields – e.g. the mirror reversals of visual field representation across visual fields, or topographic representation across somatosensory areas – suggest a role for self-organization in generating network structure during development [58]. This indicates that the functional role played by cortical areas in the expanded posterior parietal and temporal cortex are, to a large extent, contingent on their functional inputs during development. Much of the evidence for this comes from studies in humans. For example, the fusiform face area in the temporal lobe of humans, which normally is activated by faces, is instead activated by particular voices in congenitally blind individuals [59], suggesting that this area is only loosely specified for person perception and is built by the available inputs that signal a given individual’s identity. Another example comes from studies of individuals born without hands, and who develop remarkable dexterity with their feet which serve as their major effectors [60]. As we might expect, the regions of primary somatosensory and motor cortex that would normally represent the missing hand instead represent adjacent body parts. However, posterior parietal cortex, a region normally associated with reaching and grasping and generating coordinated hand/eye movements, is activated by the foot in these individuals, rather than by the forelimb as it is in individuals with hands. Thus, these expanded, higher-order regions of cortex are, in a sense, mimicking what happens via traditional evolutionary mechanisms, with their functional role emerging from pervasive exposure to unique combinations of sensory input that are dependent on context and use. In short, the functional organization of these areas is built during development.
Conclusions
The available comparative data suggests that there is a relationship between the proliferation of cortical areas and the disproportionate expansion of the neocortex relative to its sensory inputs from the thalamus, especially in “neocorticalized” lineages such as primates. The functional plasticity of the neocortex during development is supported by experimental studies and studies in naturally blind animals in which sensory inputs are completely lost or reduced, in which the neocortex that normally subserves the lost input is functionally re-specified. Nevertheless, the question of how cortical areas are added or functionally altered in evolution and development remains a central question in neurobiology. One clue to this puzzle may lie in distinguishing between the two forms of neocortical expansion – concerted vs. mosaic – relative to the size of the dorsal thalamus, or to the size of other structures that provide inputs to the cortex. Primates of every brain size have unusually large neocortices relative to the size of the dorsal thalamus, and this expansion is associated with a larger number of non-primary cortical fields involved in higher-order processing such as multisensory integration, the generation of body-centered coordinates for manipulating objects with the forelimbs, and face recognition (e.g. PPC and ITC).
Undoubtedly genes intrinsic to the early developing neocortex play a central role in the process of cortical arealization. In fact, the genetic regulation of ephrins, which guide axons from thalamic nuclei to their cortical targets, plays an essential role in organizing thalamocortical networks. However, the emergence of cortical areas that are not strictly specified by genes intrinsic to the developing cortex – and that are not tightly specified by peripheral input from the primary sensory nuclei of the dorsal thalamus – may be more plastic in their functional role and, to a large extent, may be built within the lifetime of an individual. This increased functional plasticity for areas of cortex that are differentially expanded in primates (e.g. PPC, ITC) may be one of the driving forces in the behavioral flexibility that is characteristic of humans, and the primate lineage to which we belong.
Highlights.
Mammals differ in the size of the neocortex and number of areas that compose it
Neocortical expansion occurs in different ways relative to inputs from the thalamus
Species with expanded cortices relative to thalamic inputs have more cortical areas
Acknowledgments
Thanks to Mary K.L. Baldwin, Jon Kaas, and the editors for helpful comments that improved the scope and clarity of the article.
Footnotes
Conflicts of Interest
We declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Recommended Reading
- 1.Kaas J: The evolution of neocortex in primates. Prog Br Res 2012, 195:91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]; Synthetic review of decades of work on primate neocortical organization, with a focus on novel cortical fields observed in primates in comparison to other mammalian lineages.
- 2.Goldring A, Krubitzer L: Evolution of parietal cortex in mammals: from manipulation to tool use In Evolution of Nervous Systems, Second Edition, Volume 3 Edited by Krubitzer L, Kaas J. Elsevier; 2017:259–286. [Google Scholar]
- 3.Lui JH, Hansen DV, Kriegstein AR: Development and evolution of the human neocortex. Cell 2011, 146:18–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Striedter GF, Srinivasan S, Monuki ES: Cortical folding: when, where, how and why? Annu Rev Neurosci 2015, 38:291–307. [DOI] [PubMed] [Google Scholar]; A synthetic review of the diverse mechanisms involved in cortical folding in mammalian brain evolution, including a new proposal for the role of radial intercalation in generating tangential expansion.
- 5.Mota B, Herculano-Houzel S: Cortical folding scales universally with surface area and thickness, not number of neurons. Science 2015, 349:74–77. [DOI] [PubMed] [Google Scholar]
- 6.Martínez-Cerdeño V, Cunningham CL, Camacho J, Antczak JL, Prakash AN, Cziep ME, Walker AI, Noctor SC: Comparative analysis of the subventricular zone in rat, ferret, and macaque: Evidence for an outer subventricular zone in rodents. PLoS One 2012, 7:e30178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kriegstein A, Alvarez-Buylla A: The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 2009, 32:149–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Florio M, Huttner WB: Neural progenitors, neurogenesis and the evolution of the neocortex. Development 2014, 141:2182–2194. [DOI] [PubMed] [Google Scholar]; Review of evolutionary diversity in the neurodevelopment of the neocortex, including specific reference to primate neocorticalization and its possible developmental origins.
- 9.Nowakowski TJ, Pollen AA, Sandoval-Espinosa C, Kriegstein AR: Transformation of the radial glia scaffold demarcates two stages of human cerebral cortical development. Neuron 2016, 91:1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molnár Z, Kaas JH, de Carlos JA, Hevner RF, Němec ELP: Evolution and development of the mammalian cerebral cortex. Br Behav Evol 2014, 83:126–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Striedter GF: Principles of Brain Evolution. Sinauer Associates; 2005. [Google Scholar]
- 12.Finlay BL, Darlington RB: Linked regularities in the development and evolution of mammalian brains. Science 1995, 268:1578–1584. [DOI] [PubMed] [Google Scholar]
- 13.Finlay BL, Darlington RB, Nicastro N: Developmental structure and evolution. Behav Brain Sci 2001, 24:263–308. [PubMed] [Google Scholar]
- 14.Montgomery SH, Mundy NI, Barton RA: Brain evolution and development: adaptation, allometry, and constraint. Proc Roy Soc Lond B Biol Sci 2016, 283:20160433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stephan H, Frahm H, Baron G: New and revised data on volumes of brain structures in insectivores and primates. Folia Primatol 1981, 35:1–29. [DOI] [PubMed] [Google Scholar]
- 16.Halley AC: Minimal variation in eutherian brain growth rates during fetal neurogenesis. Proc Roy Soc Lond B Biol Sci 2017, 284:20170219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charvet CJ, Cahalane DJ, Finlay BL: Systematic, cross-cortex variation in neuron numbers in rodents and primates. Cereb Cort 2015, 25:147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finlay BL, Franco ECS, Yamada ES, Crowley JC, Parsons M, Muniz JAPC, Silveira LCL: Number and topography of cones, rodes and optic nerve axons in New and Old World primates. Vis Neurosci 2008, 25:289–299. [DOI] [PubMed] [Google Scholar]
- 19.Charvet CJ, Darlington RB, Finlay BL: Variation in human brains may facilitate evolutionary change toward a limited range of phenotypes. Br Behav Evol 2013, 81:74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reep RL, Finlay BL, Darlington RB: The limbic system in mammalian brain evolution. Br Behav Evol 2007, 70:57–70. [DOI] [PubMed] [Google Scholar]
- 21.Pirlot P, Kamiya T: Relative size of brain and brain components in three gliding placentals (Dermoptera: Rodentia). Can J Zool 1982, 60:565–572. [Google Scholar]
- 22.Bininda-Emonds ORP, Cardillo M, Jones KE, MacPhee RDE, Beck RMD, Grenyer R, Price SA, Vox RA, Gittleman JL, Purvis A: The delayed rise of present-day mammals. Nature 2007, 446:507–512. [DOI] [PubMed] [Google Scholar]
- 23.Iwaniuk AN, Dean KM, Nelson JE: A mosaic pattern characterizes the evolution of the avian brain. Proc R Soc Lond B Biol Sci Suppl 2004, 271 :S148–S151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gutiérrez-Ibáñez C, Iwaniuk AN, Moore BA, Fernández-Juricic E, Corfield JR, Krilow JM, Kolominsky J, Wylie DR: Mosaic and concerted evolution in the visual system of birds. PLoS One 2014, 9:e90102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noreikiene K, Herczeg G, Gonda A, Balázs G, Husby A, Perilä J: Quantitative genetic analysis of brain size variation in sticklebacks: support for the mosaic model of brain evolution. Proc R Soc Lond B Biol Sci 2015, 282:20151008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yopak KE, Lisney TJ, Collin SP: Not all sharks are “swimming noses”: variation in olfactory bulb size in cartilaginous fishes. Br Struct Funct 2015, 220:1127–1143. [DOI] [PubMed] [Google Scholar]
- 27.Kotrschal A, Zeng H-L, van der Bijl W, Öhman-Mägi C, Kotrschal K, Pelckmans K, Kolm N: Evolution of brain region volumes during artifial selection for relative brain size. Evolution 2017, 71:2942–2951. [DOI] [PubMed] [Google Scholar]
- 28.Smaers JB, Soligo C: Brain reorganization, not relative brain size, primarily characterizes anthropoid brain evolution. Proc R Soc Lond B Biol Sci 2013, 280:20130269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gómez-Robles A, Hopkins WD, Sherwood CC: Modular structure facilitates mosaic evolution of the brain in chimpanzees and humans. Nature Comm 2014, 5:4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barton RA, Harvey PH: Mosaic evolution of brain structures in mammals. Nature 2000, 405:1055–1058. [DOI] [PubMed] [Google Scholar]
- 31.Campos GB, Welker WI: Comparisons between brains of a large and a small Hystricomorph rodent: capybara, Hydrochoerus and guinea pig, Cavia; neocortical projection regions and measurements of brain subdivisions. Br Behav Evol 1976, 13:243–266. [DOI] [PubMed] [Google Scholar]
- 32.Armstrong E: A quantitative comparison of the hominoid thalamus: I. Specific sensory relay nuclei. Am J Phys Anthrop 1979, 51:365–382. [DOI] [PubMed] [Google Scholar]
- 33.Kaskan PM, Franco ECS, Yamada ES, Silbeira LCL, Darlington RB, Finlay BL: Peripheral variability and central constancy in mammalian visual system evolution. Proc Roy Soc Lond B Biol Sci 2005, 272:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Changizi MA, Shimojo S: Parcellation and area-area connectivity as a function of neocortical size. Br Behav Evol 2005, 66:88–98. [DOI] [PubMed] [Google Scholar]
- 35.Chaplin TA, Yu H-H, Soares JGM, Gattass R, Rosa MGP: A conserved pattern of differential expansion of cortical areas in simian primates. J Neurosci 2013, 33:15120–15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reardon PK, Seidlitz J, Vandekar S, Liu S, Patel R, Park MTM, Alexander-Bloch A, Clasen LS, Blumenthal JD, Lalonde FM, et al. Normative brain size variation and brain shape diversity in humans. Science 2018, 360:1222–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Workman AD, Charvet CJ, Clancy B, Darlington RB, Finlay BL: Modeling transformations of neurodevelopmental sequences across mammalian species. J Neurosci 2013, 33:7368–7383. [DOI] [PMC free article] [PubMed] [Google Scholar]; Comparative modeling of neurodevelopment in diverse species demonstrating a highly conserved order of neurogenic events, deployed across varying temporal windows and thereby generating concerted evolutionary effects on the size of brain structures.
- 38.Smart IHM, Dehay C, Giroud P, Berland M, Kennedy H. Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb Cort 2002, 12:37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fietz SA, Kelava I, Vogt J, Wilsch-Bräuninger M, Stenzel D, Fish JL, Corbeil D, Riehn A, Distler W, Nitsch R, Huttner WB: OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nature Neurosci 2010, 13:690–701. [DOI] [PubMed] [Google Scholar]
- 40.Reillo I, de Juan Romero C, Ángel García-Cabezas M, Borrell V: A role for intermediate radial glia in the tangential expansion of the mammalian cerebral cortex. Cereb Cortex 2011, 21:1674–1694. [DOI] [PubMed] [Google Scholar]
- 41.García-Moreno F, Vasistha NA, Trevia N, Bourne JA, Molnár Z: Compartmentalization of cerebral cortical germinal zones in a lissencephalic primate and gyrencephalic rodent. Cereb Cortex 2012, 22:482–492. [DOI] [PubMed] [Google Scholar]
- 42.Van Essen DC, Dierker DL: Surface-based and probabilistic atlases of primate cerebral cortex. Neuron 2007, 56:209–225. [DOI] [PubMed] [Google Scholar]
- 43.Deacon TW: Rethinking mammalian brain evolution. Amer Zool 1990, 30:629–705. [Google Scholar]; A critical history of theories of mammalian brain evolution, including Deacon’s “displacement hypothesis” of how brain area scaling impacts neuronal connectivity and function.
- 44.Krubitzer LA, Prescott TJ: The combinatorial creature: cortical phenotypes within and across lifetimes. Trends Neurosci 2018:744–762. [DOI] [PubMed] [Google Scholar]; Review of both intrinsic and activity-dependent influences on the neocortical phenotype drawing on experimental studies and evolutionary variation.
- 45.Chabot N, Robert S, Tremblay R, Miceli D, Boire D, Bronchti G: Audition differently activates the visual system in neonatally enucleated mice compared with anophthalmic mutants. Eur J Neurosci 2007, 26:2334–2348. [DOI] [PubMed] [Google Scholar]
- 46.Charbonneau V, Laramée M-E, Boucher V, Bronchti G, Boire D: Cortical and subcortical projections to primary visual cortex in anophthalmic, enucleated and sighted mice. Eur J Neurosci 2012, 36:2949–2963. [DOI] [PubMed] [Google Scholar]
- 47.Bronchti G, Heil P, Sadka R, Hess A, Scheich H, Wollberg Z: Auditory activation of ‘visual’ cortical areas in the blind mole rat (Spalax ehrenbergi). Eur J Neurosci 2002, 16:311–329. [DOI] [PubMed] [Google Scholar]
- 48.Sadaka RS, Wollberg Z: Response properties of auditory activated cells in occipital cortex of the blind mole rat: an electrophysiological study. J Comp Physiol 2004, 190:403–413. [DOI] [PubMed] [Google Scholar]
- 49.Karlen SJ, Kahn DM, Krubitzer L: Early blindness results in abnormal corticocortical and thalamocortical connections. Neurosci 2006, 142:843–858. [DOI] [PubMed] [Google Scholar]
- 50.Dooley JC, Krubitzer L: Alterations in cortical and thalamic connections of somatosensory cortex following early loss of vision. J Comp Neurol 2018, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gizewski ER, Gasser T, de Greiff A, Boehm A, Forsting M: Cross-modal plasticity for sensory and motor activation patterns in blind subjects. Neuroimage 2003, 19:968–975. [DOI] [PubMed] [Google Scholar]
- 52.Arnott SR, Thaler L, Milne JL, Kish D, Goodale MA: Shape-specific activation of occipital cortex in an early blind echolocation expert. Neuropsychologia 2013, 51:938–949 [DOI] [PubMed] [Google Scholar]
- 53.Gougoux F, Zatorre RJ, Lassonde M, Voss P, Lepore F: A functional neuroimaging study of sound localization: visual cortex activity predicts performance in early-blind individuals. PLoS Biol 2005, 3:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bedny M, Richardson H, Saxe R: “Visual” cortex responds to spoken language in blind children. J Neuro 2015, 35:11674–11681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ricciardi E, Bonino D, Pellegrini S, Pietrini P: Mind the blind brain to understand the sighted one! Is there a supramodal cortical functional architecture? Neurosci Biobehav Rev 2014, 41:64–77. [DOI] [PubMed] [Google Scholar]
- 56.Greig LC, Woodworth MB, Galazo MJ, Padmanabhan H, Macklis JD: Molecular logic of neocortical projection neuron specification, development and diversity. Nat Rev Neurosci 2013, 14:755–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Antón-Bolaños N, Espinosa A, López-Bendito G: Developmental interactions between thalamus and cortex: a true reciprocal love story. Curr Opin Neurobiol 2018, 52:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]; A detailed look at both genetic and activity-dependent mechanisms involved in generating reciprocal connectivity between neocortical fields and thalamic nuclei, emphasizing their mutual roles in establishing adult connectivity.
- 58.Finlay BL, Uchiyama R: Developmental mechanisms channeling cortical evolution. Trends Neurosci 2015, 38:69–76. [DOI] [PubMed] [Google Scholar]; Overview of the driving developmental factors involved in neocortical organization in evolution, with a focus on “primary” inputs from the thalamus and the role of self-organization in cortical evolution.
- 59.Fairhall FL, Porter KB, Bellucci C, Mazzetti M, Cipolli C, Gibboni MI: Plastic reorganization of neural systems for perception of others in the congenitally blind. Neuroimage 2017, 158:126–135. [DOI] [PubMed] [Google Scholar]
- 60.Striem-Amit E, Vannuscorps G, Caramazza A: Plasticity based on compensatory effector use in the association but not primary sensorimotor cortex of people born without hands. Proc Natl Acad Sci USA 2018, 115:7801–7806. [DOI] [PMC free article] [PubMed] [Google Scholar]; A study on cortical reorganization in individuals born without hands, showing that while primary motor and sensory areas have a limited capacity for functional reorganization, posterior parietal cortex is dominated by hindlimb movements associated with compensatory use.
- 61.Krubitzer L, Campi KL, Cooke DF: All rodents are not the same: a modern synthesis of cortical organization. Br Behav Evol 2011, 78:51–93. [DOI] [PMC free article] [PubMed] [Google Scholar]; A review of variation in neocortical organization and cortical area scaling in diverse rodent species.
- 62.Remple MS, Henry EC, Catania KC: Organization of somatosensory cortex in the laboratory rat (Rattus norvegicus): evidence for two lateral areas joined at the representation of the teeth. J Comp Neurol 2003, 467:105–118. [DOI] [PubMed] [Google Scholar]
- 63.Saraf MP, Balaram P, Pifferi F, Gamanut R, Kennedty H, Kaas JH: Architectonic features and relative locations of primary sensory and related areas of neocortex in mouse lemurs. J Comp Neurol 2018, preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krubitzer L, Huffman KJ: Arealization of the neocortex in mammals: genetic and epigenetic contributions to the phenotype. Br Behav Evol 2000, 55:322–335. [DOI] [PubMed] [Google Scholar]
- 65.Kahn DM, Krubitzer L: Massive cross-modal cortical plasticity and the emergence of a new cortical area in developmentally blind animals. Proc Natl Acad Sci USA 2002, 99:11429–11434. [DOI] [PMC free article] [PubMed] [Google Scholar]