Abstract
Bearing compression from adjacent joints, the articular cartilage is cumulatively pressured in daily life, thus making it prone to injuries; however, once damaged, the self-healing capacity of articular cartilage is limited owing to its low metabolic property. Autologous chondrocyte implantation, a three-step repairing technique for articular lesions, has received satisfactory short-term clinical outcomes, whereas its long-term effect remains controversial. Currently, improved stem-cell therapies and novel biomaterials have shed new lights on autologous chondrocyte implantation. We would, therefore, synthesize these optimization strategies in order of their presences in the three-step protocol, seeking to find and amplify synergic effects between these strategies.
The translational potential of this article
Autologous chondrocytes implantation serves as an alternative for the treatment of articular cartilage lesions to avoid potentially detrimental effects of applying microfracture. The optimized ACI should improve the cost-effectiveness of repairing articular cartilage while circumventing latent complications like osteophyte. This article synthesized optimization strategies for ACI and provided appropriate applying approaches to maximize their synergic effects. It will be a pioneering trial for combinedly using stem cells and nanotechnology to regenerate cartilage.
Keywords: Autologous chondrocyte implantation, Scaffold, Stem cell, Nanotechnology
Introduction
Articular cartilage is a type of hyaline cartilage covering the synovial joints. It acts as a fulcrum to bear compression forces resulted from muscle lever systems; moreover, shearing forces induced by joint movements further abrade the articular surface [1], [2]. However, in the absence of vasculatures, lymphatics, and neural innervations, articular cartilage lacks reservoirs for stem cells and growth factors. Therefore, articular cartilage is barely capable of regenerating itself, making most injuries irreversible [2], [3].
Many surgical interventions, such as osteotomy, osteochondral transplantation, and microfracture, have been applied clinically to stop or reverse articular cartilage degeneration previously [4], [5]. These methods partially restore the function of articular cartilage and prevent further degeneration. However, they are associated with significant short-term (1- to 2-year follow-up) or long-term (9- to 15-year follow-up) clinical defects [2]. For instance, life-long pain and potential immune response have been reported to occur after osteotomy [4]. Also, microfracture is reported to predispose the affected joints to intralesional osteophyte because of subchondral penetration (51.5% of all cases) [5]. Above all, none of these methods mentioned previously could generate mechanically functional articular cartilage [5], [6].
Autologous chondrocyte implantation (ACI), a three-step regenerative therapy, is reported to effectively repair articular cartilage and restore its biomechanical function (85.1% successful cases in 4291 patients) [7], [8]. It mainly involves extracting chondrocytes from the host, expanding them in vitro, and reintroducing them back into the lesion sites (the three-step protocol). The postoperative follow-up studies revealed that ACI minimized surgery lesions, generated more hyaline-like cartilage with better anticompression properties, and had little postoperative virus infections. However, the drawbacks of ACI have also been abundantly indicated, for instance, the requirement of two surgeries, the long intraoperative time, poor tissue integration, and the emergence of biomechanically compromised fibrocartilage [2], [3], [7], [9].
Nine hundred ninety-six studies have been conducted during the past decades to improve the clinical outcomes of ACI further. Nevertheless, controversies and inconsistencies between these improving strategies emerge continuously. Here we categorize these optimization strategies according to their presences in the three-step procedure, comparing strategies within the same procedure and figuring out potential additive or synergic effects of different strategies. Apart from the three-step protocol, actions taken after implantation would be discussed as well.
Overview of ACI procedures, strengths, and weaknesses
ACI, the first cell-based treatment of articular cartilage defect, was applied clinically in 1994 by Britterg's team [10], [11]. The procedure includes, first, harvesting chondrocytes with a full-depth biopsy punch at the joint region where the weight-bearing function is lowest [2]. Chondrocytes are then cultivated and expanded in vitro for 14–21 days to generate a mass of hyaline cartilage with 12–48 million chondrocytes [2], [10], [11], [12]. Eventually, a second operation is performed to debride the injured tissue and implant neocartilage back into the defect joint; furthermore, to stabilize the newly implanted hyaline cartilage, the periosteum is used to cover the chondrocyte suspension [11], [13].
Compared with previously widely used clinical treatments (osteotomy, microfracture, and osteochondral allograft), ACI has many theoretical benefits listing as follows: (1) The natively derived neotissue effectively circumvent immune rejections and viral infections. (2) Only a small fracture of articular cartilage sample is collected, minimising intraoperative pain. (3) The autologous neotissue possesses more hyaline-like properties, strongly facilitating its maturation and enhancing its compatibility to native tissue with better biomechanical activities [2], [14].
The fully investigated short-term (up to 24 months) follow-up studies noted an overall similar postoperative effect between microfracture and ACI [14]. However, in terms of long-term clinical outcomes (15 years), the number of randomized comparative studies is limited (Knutsen et al. [17] and Saris et al. [16]), and the results are inconsistent. Knutsen et al. reported a nonsignificant difference of long-term clinical outcomes between ACI and microfracture and a higher risk of ACI surgery failure (42.5%) compared with microfracture (32.5%). However, in follow-ups by Knutsen et al., patients received other preliminary treatments such as microfracture and high tibial osteotomy, which potentially changed the subchondral plate and increased the risks of ACI failure and intralesional osteophyte [16]. Therefore, we adopt the result of the study by Saris et al. [5], whose participants strictly followed one therapy (either microscopy or ACI). Saris et al. indicated that ACI reduced the severity of pain and improved the patients' daily activities with a higher knee injury and osteoarthritis outcome score (KOOS) score (21.25 ± 3.60) compared with microscopy (15.83 ± 3.48), (P = 0.048) [5], [16]. In addition, subchondral osseous overgrowth was observed in the microfracture group under magnetic resonance imaging and arthroscopy, which could further develop into osteophyte and osteoarthritis [17]. However, in terms of tissue integrity, the results of ACI and microfracture are similar [5], [14].
The drawbacks of ACI are also clearly stated in previous clinical studies: (1) Surgical failures. Twenty-four percent of patients suffered unsatisfactory neotissue detachment and unwanted fibrocartilaginous biomechanical properties [14]. (2) The long postoperative recovery time. It takes between 10 and 21 months before the neotissue reaches full maturation with suitable biomechanical properties [2], [4], [15], which is detrimental for career athletes. (3) Two surgeries are needed [18], [19]. (4) The high cost. Recently reported cost of performing the total procedure of ACI is $14,400, and the majority of which is spent on in vitro cultivation [20]. (5) The complex procedure. The in vitro expansion step needs delicately modified cultural environment, which may otherwise lead to the induction of teratoma [21]. 6. Possible immune reactions may also occur owing to the allografted porcine membrane cover [2], [11], [13]. 7. The poor lateral integration between neotissue and native tissue resulted from the low metabolic rate and antiadhesive extracellular matrix (ECM) component [3]. (8) Postoperative chondrocytes dedifferentiation frequently occurs, inducing the development of biomechanical inferior articular cartilage [2].
Chondrocyte extraction and alternative cell sources
The initial step in ACI is to extract chondrocytes from a low-weight–bearing region in diarthrosis. Since Britterg performed the first ACI in human, the biopsy punch has been a conventional procedure with no alteration or improvement; however, failure in biopsy punch accounted for 20% of total ACI surgery failure during the past decades [5]. Hence, we would address some optimization strategies in biopsy punch, focusing on the optimal surface area and depth of the sample. Actually, apart from articular chondrocytes, other cell sources could also be used to perform ACI, as long as chondrocytes take up the majority in the neotissue implanted back. A summary of alternative cell sources together with clinical outcomes and cultivation conditions are listed in Table 1.
Table 1.
Alternative cell sources for ACI and their cultivation demand.
| Cell type | Exogenous stimuli in cultivation system | Epitope marker | Marker (genetic factors) for chondrogenic transdifferentiation | Clinical application | Reference | |
|---|---|---|---|---|---|---|
| Chondrocytes | AC | BMP-2/4, TGF-β1 | CD44/54/73+ CD164- |
no need | Most widely used, potential secondary degeneration during extraction | Makris et al. (2015) [2] Pelttari et al. (2017) [18] Campbell et al. (2012) [26] |
| NC | TGF-β1, FGF-2, IGF-1, GDF-5 | Hox (+) | Generate hyaline-like cartilage, capable of self-renewal | |||
| MSC | BMSC | TGF-β1, ascorbic acid, dexamethasone, sodium pyruvate, insulin–transferrin–selenium | CD44/73/29/90/105 (+) CD11/14/31/34/45 (−) |
Sox-9(+) FOXO3A (+) Hoxa2 (−) HOXD9 & HOXD13(+) ZNF145 (+) |
Bone marrow aspiration is painful, yielding small amount of MSC | Almalki and Agrawal (2017) [24] Perdisa et al. (2015) [27] |
| ADSC | Easy to isolate with large quantities; prone to misdifferentiation | |||||
| SSC | TGF-β1, Indian hedgehog (inducing hypertrophic chondrocytes) | PDPN (+); CD146 (−); CD73 (+); CD164 (+) | Sox-9(+) Runx2(−) PPARγ2(−) |
Newly identified, have not been applied clinically | Bianco & Robey (2015) [28] Chan et al. (2018) [27] |
|
| Pluripotent stem cells | ESC | Co-culture with mature chondrocytes | SSEA-3 (+) CD324/90/117/326 (+) |
Sox-9(+) Runx2(−) OSX (−) |
Cannot be extracted autologously; potential teratoma induction and immune response | Jukes, Blitterswijk and Boer (2010) [29] |
| iPSC | TGF-β1, Co-culture with mature chondrocytes | TRA-1-60 (+) TRA-1-81 (+) |
Teratoma induction and immune response due to incomplete reprogramming | Tapia & Schöler (2016) [30] | ||
AC = articular chondrocyte; ADSC = adipose-derived mesenchymal stem cell; BMSC = bone marrow–derived mesenchymal stem cells; BMP = bone morphogenic protein; ESC = embryonic stem cell; FGF = fibroblast growth factor; GDF = growth differentiation factor; IGF = insulin-like growth factor; iPSC = induced pluripotent stem cell; MSC = mesenchymal stem cell; NC = nasal chondrocyte; PPARγ2 = peroxisome proliferator–activated receptor γ2; Sox-9 = SRY-related high mobility group-box gene 9; SSC = skeletal stem cell; TGF = transforming growth factor; ZNF145 = zinc-finger protein 145; (+) = upregulation; (−) = downregulation
Improvements for biopsy punch
Too many concerns have been placed on the appropriate chondrocytes volume introduced back in clinical trials, while the optimal amount extracted during arthroscopy seems to be neglected. Saris et al. [5] suggest an optimal surface area of each sample ranging from 7 mm2 to 24 mm2, yielding few cases of surgery failure. The underlying reason of why this area range for biopsy is considered safe remains unclear. It is of no argument that increasing the extraction volume would accelerate tissue expansion and shortens intraoperative time; however, the large biopsy lesion also disrupts a massive number of mechanoreceptors, predisposes patients to greater intraoperative pain, and add up daily life inconvenience [5], [17].
Another question to address is whether the biopsy punch needs to be a full-depth one. As is indicated in previous clinical reports, one of the significant microfracture defects is the postoperatively- developed intralesional osteophyte (50% of all cases) [5], [15]. The intralesional osteophyte is primarily related to the subchondral penetration, which advances the tidemark of subchondral plate and makes the viscoelastic cartilage layer thinner [17]. The full-depth biopsy punch in ACI avoids altering the subchondral bone; instead, it exposes the subchondral plate directly. During the intraoperative time, abnormal ossification may occur on the naked subchondral plate which would further evolve into an advanced tidemark and increase the risk of developing intralesional osteophyte [5], [17]. Were the biopsy punch not a full-depth one, the subchondral plate would not be exposed. The depth of sampling is a convention of clinical performance, but the underlying rationale is, unfortunately, not mentioned before [2], [3], [5], [17]. Given that the flattened chondrocytes which possess higher self-renewal ability are mainly distributed at the superficial zone of the cartilage, taking a sample at superficial layer instead of a full-depth one would not compromise expansion efficiency [1]. Moreover, as tissue extracted at low-weight–bearing region would not be reintroduced back, maintaining tissue integrity there is of great importance [2]. Therefore, extracting sample only from the superficial zone is strongly recommended. If the depth of the sample is not altered, covering the subchondral plate with a layer of collagen membrane to restrict subchondral hyperplasia could ameliorate postoperative osteophyte.
Nasal chondrocytes vs. articular chondrocytes
There are two types of chondrocyte which produce hyaline cartilage: articular chondrocytes (ACs) and nasal chondrocytes (NCs) [22]. Different from mesoderm-developed ACs, NCs are derived from mes-ectoderm neural crest cells. HOX genes are downregulated in the ectoderm, which provides NCs with self-renewal ability and environmental reprogramming capacity [22]. When NCs are transplanted to the articular lesion site, the HOX-negative phenotype would be reversed, transforming NCs into ACs [22], [23]. As hyaline-like ECM could be produced by both NCs and ACs, the clinical outcomes of implanting NC-generated hyaline cartilage are usually better than those of directly transplanting NC pellets [23]. Moreover, because NCs are extracted by nasal biopsy, potential secondary degeneration resulted from AC extraction could be avoided [22].
Alternative cell sources substituting chondrocytes in ACI
Mesenchymal stem cells
Mesenchymal stem cells (MSCs) are the primary cell type functions in microfracture and other bone marrow stimulation therapies, which gain an advantage over chondrocytes for its high proliferation capacity [2]. They can be isolated from multiple sources (bone marrow, synovium, adipose tissue, and skeletal muscle) by identifying specific surface markers [24]. MSCs extraction circumvents secondary degeneration resulted from arthroscopy, which is beneficial for patients with large lesion areas or undetectable non–weight-bearing regions in articular cartilage [25]. However, considering the multiple downstream lineages of MSC (differentiation towards myocytes, chondrocytes, adipocytes, and osteocytes), the culture system for MSCs must be delicately controlled to induce a chondrogenic fate [24]. Moreover, cell sorting must be performed before MSC-derived chondrocytes producing its own ECM; otherwise, the cartilage generated will possess less hyaline-like phenotype [24].
Skeletal stem cells
With the advancement of single-cell sequencing, human skeletal stem cells (SSCs) are recently isolated from adult bones or bone morphogenic protein (BMP-2)- treated adipose tissue [27]. When transplanted into immune-deprived mice, these human SSCs (PDPN + CD146-CD73 + CD164+) can differentiate into chondrocytes (CD73 + CD164-) or even hypertrophic chondrocytes (CD73-CD164-), providing novel cell sources for repairing articular cartilage [27]. The chondrogenic stimuli for SSCs are not identified yet. Still, its cultivation system could mimic that of MSCs to upregulate chondrocyte-specific transcription factors such as Sox9 [28]. Similar to MSCs, SSCs have osteogenic and adipogenic lineages, which may hamper the production of hyaline cartilage. To surmount this, osteogenic factor (Runx2) and adipogenic factor (PPARγ2) should be silenced [28]. The amount of SSCs are relatively small, its extraction regarded as harmful as that of ACs [27]. Therefore, from our point of view, its clinical translation is not recommended; however, in situ activation of SSCs and guiding their chondrogenic differentiation could be tried.
Pluripotent stem cells (embryonic stem cells and induced pluripotent stem cells)
Compared with other adult stem cells, the proliferation and differentiation capacity of pluripotent stem cells is infinite [29], [30], [31]. There are two sources of pluripotent stem cells: embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) [29], [30]. ESCs are extracted from the inner cell mass in the human blastocyst, and its chondrogenic differentiation is achieved via coculturing with mature chondrocytes [29]. Unfortunately, autologous ESCs do not exist in postnatal individuals, making this technique impossible to be translated clinically [29].
Somatic tissue–derived iPSCs could also be used in ACI. Through transiently activating Oct4, c-Myc, Sox2, and Klf4 gene, pluripotency is acquired by somatic cells [30]. Those iPSCs are then reprogrammed to possess chondrogenic phenotype by upregulating SOX9 transcription and silencing RUNX2, OSX simultaneously [27]. Nevertheless, high risks of teratoma induction and immunogenicity are reported (79.6%), which are mainly attributed to the incomplete reprogramming and misdifferentiation [30], [31]. As the iPSCs used are cultured into terminally differentiated chondrocytes before reintroducing them back, the condition of teratoma induction and immune rejection should be ameliorated [31]. Overall, iPSC therapy is still under investigation, and few clinical trials are performed owing to its high tumorigenic risk; thus, the ability of iPSCs to produce the desired ECM component remains elusive.
In vitro cell cultivation
The successive procedure taken after extracting cells seeds from the host is to cultivate them in vitro. There are many aims to be achieved in this procedure: guiding chondrogenic differentiation, stimulating chondrocytes proliferation and maturation, increasing production of hyaline-like ECM, preventing dedifferentiation, and maintaining biomechanical properties. We would, therefore, list optimization strategies to achieve these aims by discussing the culture system, appropriate exogenous stimuli applied, and the utilization and modification of scaffolds.
2D vs. 3D culture system
ACs were initially cultivated in the monolayer (2D culture) of agarose gel in ages of immature cell expansion techniques [32]. This manipulation strongly improved chondrocyte viability in the presence of cell plate anchorages [32]. After serial cultivations, chondrocytes with a fibroblast-like flattened morphology were generated [33]. However, the flattened cell shape may sequester BMP-2 gene expression via methylating its regulatory sequence. With downregulated BMP-2, the fibrocartilaginous expression pattern was possessed by chondrocytes, where the expression level of proteoglycan and collagen II were reduced [33]. Moreover, to compensate for the decreased production of collagen II, the cultured chondrocytes upregulated collagen I to yield a complex collagen network, which further aggravated their dedifferentiation [34]. The dedifferentiated chondrocytes generated fibrocartilage with compromised biomechanical properties, which, if implanted back, would sharply restrict the locomotion of diarthrosis.
In contrast, when chondrocytes are cultivated in 3D culture, the chondrocytic morphology and phenotype would be retained [34]. However, a 20% reduction of cell viability is observed when chondrocyte pellets were transferred from monolayer plates into 3D matrices [32]. Moreover, owing to a lack of paracrine and juxtacrine signalling in 3D culture, the proliferative rate of chondrocytes is significantly decreased [32]. Although the final chondrocyte number is still acceptable for implantation, it directly prolongs intraoperative time. To overcome this, the number of supplemental growth factors provided should be slightly excessive [35]. In addition, chondrocytes with various stages (proliferating, quiescent, senescent, and apoptotic) coexist in the 3D culture after expansion [35]. If cells identities are not certified before implantation, dedifferentiation and progressive necrosis may still occur in vivo. The various cell stages mainly result from uneven exposure to matrix nutrition; thereby, dispersing single chondrocytes in the liquid agarose gel before solidification could be a solution to unify chondrocyte stages [35].
Layered distribution of appropriate exogenous stimuli
Appropriate exogenous stimuli, including biophysical stimuli and biochemical stimuli, are indispensable for chondrocytes expansion and hyaline cartilage generation. These exogenous stimuli, when used in combination, may exert synergic or counteractive effects. For instance, insulin-like growth factor-1 (IGF-1) facilitates hypertrophic maturation of chondrocytes, while BMP-4 inhibits the expression of hypertrophic protein collagen type X [36]. In previous clinical applications, researchers amplify synergic effects via establishing radial concentration gradient ladders for each stimulus; however, this method is theoretically not reliable as it never considers chondrocyte migration during cultivation.
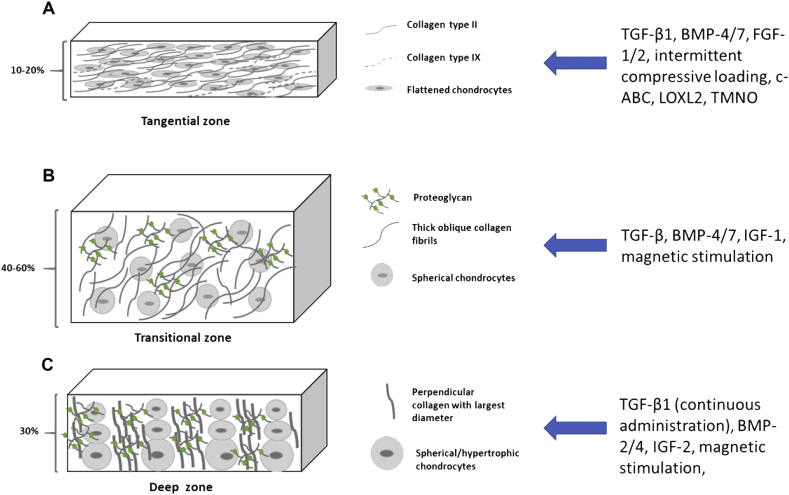
The full-depth articular cartilage tissue could be classified into three layers (zones), different layers contain chondrocytes with distinct stages and morphologies; moreover, ECM components in each layer varies, responsible for distinct biomechanical functions [1]. Through separating different layers during cultivation, exogenous stimuli with synergic effects could be added into the same layer, while interference between those with counteractive effects could be avoided [37]. In the following text, we would address the chondrocyte morphology, ECM component, biomechanical function, and respectively augmented stimuli in a layer-by-layer manner. A schematic overview is provided in Fig. 1.
Figure 1.
Schematic overview of layered distribution of appropriate exogenous stimuli. The pseudostratified structure of articular cartilage (the three layers) and their cellular and molecular components listed in the left panel. Appropriated exogenous stimuli added into corresponding layer listed in the right panel (blue arrow). (A) Tangential zone; (B) transitional zone; (C) deep zone. BMP = bone morphogenic protein; FGF = fibroblast growth factor; LOXL2 = lysyl oxidase–like 2; TGF = transforming growth factor; TMNO = trimethylamine N-oxide.
The tangential zone (superficial layer) adjacent to periosteum incorporates immature flattened chondrocytes which are undergoing rapid proliferation [1]. To further amplify chondrogenic differentiation and proliferation, fibroblast growth factor-2, IGF-1, and BMP-7 could be used in combined, which have already been proven to increase chondrocyte proliferation by sixfolds [38]. Collagen II/IX in that layer are packed tightly and parallelly aligned along the articular surface, mainly responsible for its tensile and antishearing properties [1]. Chondroitinase-ABC effectively improves tensile properties via reconstitution of the collagen matrix. When it is simultaneously applied with trimethylamine N-oxide and lysyl oxidase–like 2, the antishearing ability improved by two times [39]. Also, biophysical stimuli such as hydrostatic pressure and intermittently applied compressive loading further increase the antishearing forces by stimulating the expression of collagen type II [36]. (Fig. 1a).
Directly below the tangential zone lies the transitional zone (intermediate layer). Spherical mature chondrocytes are sparsely distributed in that layer with seldom cell–cell communications visualized [1]. BMP-4/7 and transforming growth factor (TGF)-β which promote chondrocytes maturation are, therefore, suggested to be added [36], [40], [41]. Obliquely arranged type II collagen still predominantly take up the ECM, bearing progressively increased compression forces [1]. To further increase the anticompression ability, neotissue is treated with magnetic stimulation; however, the underlying mechanism remains unclear [39]. IGF-1 has a dual role in that layer. It primarily prevents chondrocyte apoptosis via inhibiting the expression of the parathyroid hormone–related protein. Apart from this, it preserves anticompression feature of that layer by rescuing the expression of glycosaminoglycans (GAGs) [36]. When chondrocytes are cultivated on the polyglycolic acid scaffold, the amount of secreted GAGs significantly reduced; nevertheless, when the IGF-1 is applied on that scaffold, the GAG amount increased by fivefolds (Fig. 1b) [37], [38].
The deep zone hiding beneath the tangential zone and transitional zone account for the last 30% of the whole tissue [1]. In that layer, chondrocytes are aligned in a columnar manner, the cell size increasing with depth. Some mature chondrocytes become hypertrophic, possessing the potential of osteogenic transdifferentiation [1]. Therefore, BMP-2 and BMP-4 should be sequentially applied, which simultaneously allow hypertrophic maturation, while preventing transdifferentiation [36]. Collagen with largest diameters is aligned perpendicular to the articular surface, fighting against the largest compression forces applied on it [1]. For the sake of maximising the anticompression properties, the collagen fibrils and GAGs must be densely allocated, which demands a continuously administered TGF-β1 [39], [40]. TGF-β1 significantly increase the expression of SOX-9, a transcription factor responsible for upregulating type II/IX/XI collagen and GAGs [36]. In addition, similar to the manipulation performed in the transitional zone, IGF-2, BMP-2/4, and magnetic stimuli could be synergically added [36]. (Fig. 1c).
Scaffold-based techniques and nanoscale modification
As mentioned before, the 3D culture system compromises chondrocyte viability as a consequence of decreased cell adhesion. To overcome this, scaffolds are applied, which efficiently rescue chondrocyte detachment while maintaining chondrogenic differentiation.
Regarding the functional site, scaffolds could be classified into two subtypes: hydrogels and membranes [32]. Hydrogels are composed of hydrophilic macromolecular polymers, which encapsulate chondrocytes in their cross-linked networks [42]. The highly porous structure of hydrogel makes it convenient for the delivery of exogenous biochemical stimuli, which facilitates the chondrogenic differentiation and production of hyaline-like ECM [43]. Moreover, hydrogels perfectly recapitulate the water assimilation properties and the physiochemical composition of native ECM, satisfactory biocompatibility thusly ensured [32]. Hydrogel cross-links could be established and reversibly break down in a biomechanically dependent manner (PH, temperature, and electric field); therefore, the cultivation system could be altered at any desired time [42].
Membranes cover the surface of neotissue, maintaining its structural stability and enhancing tissue integration [32]. The first generation of membrane used in ACI is a layer of periosteum sealing the lesion site [2]. However, as it is allografted, immune responses are observed postoperatively. Furthermore, the allografted periosteal flap contains cytokines favouring the cell growth; therefore, the original flattened chondrocytes lying in the tangential zone become hypertrophic, which eventually result in surgery failure [2]. These drawbacks could be overcome by substituting the periosteum with a collagen membrane [32].
Both hydrogels and membranes derive their sources from natural materials and synthesized polymers. Natural biomaterials are easily obtained with higher tissue compatibility and cell adhesion capacity, while synthesized polymers possess immediate biomechanical properties and controllable degradation rate [44]. A summary of classification of scaffolds, examples, clinical comments, and potential modifications are provided in Table 2.
Table 2.
Classification of scaffolds, examples, clinical comments, and current modifications.
| Functional sites | Natural |
Synthesized polymer |
References | ||||
|---|---|---|---|---|---|---|---|
| Examples | Clinical comments | Modifications | Examples | Clinical comments | Modifications | ||
| Hydrogels | Agarose | Support chondrogenesis, facilitate function of biomechanical stimuli | Not indicated yet | PEG | Biocompatible, suitable for chondrocytes and MSCs cultivation | Lactic acid, RGD residue, combinedly used with other natural materials | Kim et al. (2012) [42] Dewan et al. (2014) [32] Stevens and George (2005) [45] Wang et al. (2011) [47] |
| Alginate | Low stability and degradation rate | RGD peptides (improve adhesion) | |||||
| Hyaluronic acid | Facilitate chondrogenic differentiation | MMP-sensitive peptide (controllable degradation) | |||||
| Collagen | Low biomechanical stability, easily contracted during expansion, great biocompatibility | Nanoscale detail addition via electrospinning | |||||
| Fibrin | Support chondrogenesis, compromised biomechanical property | Not indicated yet | |||||
| Membrane | Periosteum | Immune response Hypertrophy |
Not indicated yet | Aliphatic polyesters | Toxic degradation by-product | Matching degradation with local metabolic clearance | |
| Collagen membrane | No hypertrophy Efficiently seal lesion site |
Coculture with chondrocytes during cell expansion | |||||
PEG = polyethylene glycol; RGD peptide = repeated sequence of arginine, glycine, and aspartame.
Scaffold modification has long been focussed on macroscopic alterations, while nanoscale modifications seem to be neglected [45]. Cell fates and expression pattern are partially associated with their nanoscale morphology; for instance, when the number of filopodia and microspikes is increased on the MSC surface, cell responsiveness would be strongly elevated, which favours chondrogenic differentiation and proliferation [46]. With the development of the electrospinning technique, nanoscale details could be added to scaffolds. By applying a powerful electric field, the diameters of artificial collagen fibrils are decreased to 67–100 nm [46]. The thinner, tightly aligned collagen fibres provide better anticompression forces [43], [45]. However, it is still not comparable to the ultranano structure in native cartilage ECM, which demands spaces between adjacent collagen fibrils to be within 6 nm [46]. Another benefit of electrospinning is that it allows aligning polymer scaffolds along a determined axis [46]. As seen in Fig. 1, the multiple zones of articular cartilage contain collagen fibrils with distinct diameters aligning in different directions. When the correctly unified collagen alignment is achieved, the subtle biomechanical properties of different cartilage zones would be improved [45]. In addition, uniformly distributed integrins on these nanoscale-modified collagen scaffolds exert tensile forces on chondrocytes and trigger their expansion, the chondrocytes proliferation rate, therefore, increased [45].
In vivo implantation and manipulations being taken afterwards
As the mature and compatible neotissue is yielded, the next and final step is to reintroduce the neotissue back into the defect site. Before this, the damaged tissue should be delicately removed from the defect site as senescent or necrotic chondrocytes incorporated in defect tissue may exert a degenerative effect to neighbouring tissue. Also, antisenescent drugs such as FOXO4-DRI should be sprayed on the lesion surface [48]. Approximately 45.45 chondrocytes are observed per square millimetre in the native articular cartilage [49]. Because apoptosis usually occurs after tissue implantation, the optimum chondrocyte density in neotissue should be a little bit higher (50–52 cells/mm2) [50]. Implantation is the last step of ACI but merely the beginning of rehabilitation. Here, we would address how to maintain tissue integrity after implantation in detail. Moreover, to pursue an effect of “once for all”, methods used to improve local self-healing capacity would be mentioned.
Improvement of lateral cartilage integration
The poor tissue integrity is always a major defect of ACI, which has not been fully resolved yet. Cartilage integration could be classified into two types: vertical integration and lateral integration [3]. The previous one refers to the amalgamation of the deep zone cartilage tissue and the subchondral plate. Given the relatively high metabolic rate at the subchondral interface, ECM reconstitution and collagen interweave between the calcified zone and deep zone are easily achieved, satisfactory vertical integration thusly yielded [3]. In contrast, lateral integration, the fixation between neocartilage and native cartilage, is still unachievable [3], [51]. The conditions even grow worse for the layered cultivation strategy we proposed. Some factors hindering cartilage–cartilage integration could be avoided when the cultivation system is well designed, for instance, chondrocyte dedifferentiation [37]. In the following text, the postoperatively applied methods for rescuing lateral integration would be addressed.
One week after implanting the neotissue, necrosis occurred at the interface of the neotissue and native tissue, accounting for a 100- to 200-μm necrotic space [50]. These necrotic cells sent apoptotic signals to neighbouring chondrocytes to induce progressive apoptosis, which expanded the necrotic space into a 400-μm acellular fissure [52]. Previous studies have applied antiapoptotic molecules such as ZVAD-fmk, a caspase inhibitor, to the lesion boundary to cease apoptosis; however, the antiapoptotic molecules seemed to perturb the expression of matrix-producing genes and compromised the hyaline-like ECM, yielding unwanted biomechanical properties [50]. At the end of the apoptosis, surrounding surviving chondrocytes repopulate, but they seldom migrate to the 400-μm fissure, making the acellular zone persist [53]. The low migration rate is not induced by the persistent apoptotic signals residing in the acellular zone. Instead, the restricted motility resulted from the densely allocated collagen/aggrecan network is the cause [50], [53], [54]. Researchers attempted to fill the acellular zone with interweaved collagen/proteoglycan to maintain biomechanical function there; however, without the refreshing and turning over effect exerted by chondrocytes, this acellular network eventually ends up with mechanical fatigue [50].
Collagen is one of the hindering factors for chondrocytes migration; simultaneously, collagen II handles the hyaline-like property to fight against compression [3], [53]. Hence, collagenase could be applied in a pulse-chase manner to induce a transient degradation of collagen fibrils, allowing chondrocyte passage [50]. After chondrocyte migration, collagen networks would be reconstituted, facilitated by 17β-oestradiol [50], [53]. The remaining question is how to monitor the completion of migration [54]. Moreover, lysyl oxidase, which mediates the formation of collagen cross-links, has different activity in neocartilage and native cartilage, making collagen cross-links between these two tissues hard to achieve [50]. Applying the lysyl oxidase inhibitor would accelerate the accumulation of cross-linked precursor and therefore promote collagen interweave [54].
Proteoglycans also inhibit chondrocyte migration and inhibit integration [50]. They are distributed in two locations, the transitional/deep zones of neotissue and synovial fluid [55]. Small proteoglycan present in transitional/deep zone directly construct a meshwork to prevent chondrocyte migration. Superficial zone proteoglycan and proteoglycan-4 in the synovial fluid produce ineffective hyaluronan to prohibit chondrocyte migration indirectly [55]. Similar to collagen, proteoglycans are also essential for the biomechanical function of articular cartilage [1], [3], [56]. Therefore, transiently applying hyaluronidase and chondroitinase-ABC could be a solution [50].
As articular cartilage is an avascular, alymphatic tissue, transiently injecting other catabolic molecules (interleukin-1β) can also enhance integration [56]. Interleukin-1β should be delicately delivered; otherwise, it will induce local inflammation and degenerate cartilage for a second time [56]. To avoid inflammation, steroid hormones such as testosterone and dehydroepiandrosterone are alternative choices [57], [58].
Improve intrinsic self-healing capacity via ambushing self-healing units
Even when biocompatible tissue with excellent biomechanical properties is inserted into the defect site, risks of secondary lesion persist due to daily repetitive compression and uric acid accumulation [59], [60], [61]. To avoid performing a secondary ACI, the best way is to enable the articular cartilage to possess self-healing capacity. Chondrocytes with self-renewal ability do exist in the matrix of articular cartilage, responsible for curing small lesions; however, considering their limited number and reduced motility, larger lesions cannot be intrinsically reversed [2], [3]. Thereby, we proposed to construct several self-healing units (Fig. 2) ambushed to large weight-bearing sites, which actively respond to trauma and initiate regeneration.
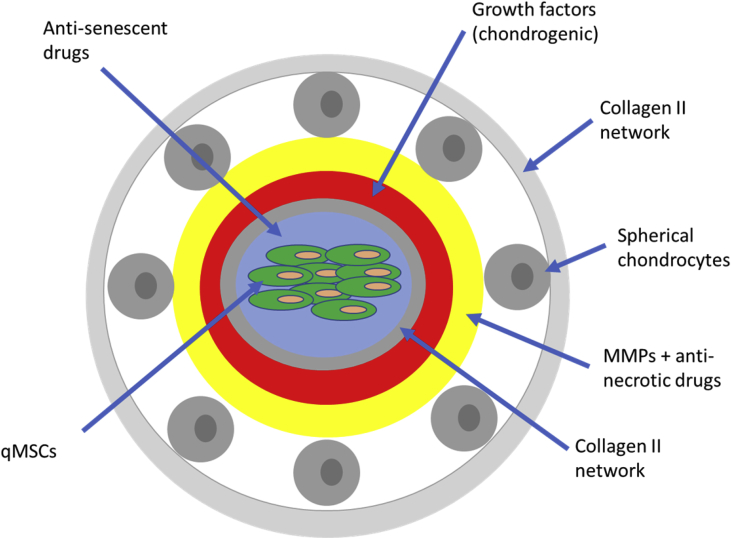
Figure 2.
Construct self-healing units and ambush them in the neotissue. Self-healing units are constructed via electrospinning. Rings labelled with different colours contain different drugs or cells which are marked and pointed in the blue arrow. The diameter of each ring and component concentration are yet to be determined. MSCs = mesenchymal stem cells.
Quiescent MSCs (qMSCs), either autologously derived or extracted from young hosts, are placed in the centre of the self-healing unit, their quiescence specifically identified via surface markers (CD44/73/90) [62]. As long-term inactivation of these qMSCs would make them prone to undergo senescent transformations, which furthermore aggravate surrounding tissues, these qMSCs are enveloped with a layer of antisenescent drugs such as ABT-737 and Dasatinib [63], [64], [65]. A layer of collagen type II then encapsulates the inner cell complex, which also functions to isolate them from the external chondrogenic growth factors. These chondrogenic factors, including TGF-β1/2, BMP-2/4/7, and IGF-1, are used to activate qMSCs and guide their chondrogenic differentiation when lesion occurs [24], [37]. Afterwards, several spherical chondrocytes adhere to the surface of the self-healing unit, covered by another collagen II network.
During secondary trauma, the outer collagen capsule would be disrupted, spherical chondrocytes adjacent to it wandering somewhere else. To avoid necrosis of these spherical chondrocytes, a layer of antinecrotic drugs is incorporated inside. Matrix metalloproteinase (MMPs) are also contained in that layer, responsible for degrading inner and outer collagen matrix for releasing qMSCs and enabling their migration, respectively. Chondrogenic growth factors then infiltrate through the disrupted internal collagen matrix to induce the activation and differentiation of qMSCs, which eventually repair the lesion site. For the sake of minimising alteration to the biomechanical properties, the self-healing units are ambushed in a number corresponding to the local chondrocyte density.
The multilayered self-healing units are constructed with the help of electrospinning techniques, which allows delicate distribution of cells and drugs [46], [66]. This novel approach has not been applied clinically, and its clinical outcome remains unclear, but it could, at least, improve the efficiency of intrinsic self-healing and target middle-sized trauma.
Conclusions and future perspectives
This review lists previously found ways to optimize ACI in a step-chronicle way. While the detailed procedure of ACI is addressed to give an overview of the theoretical and clinical feasibility and drawbacks of this regeneration treatment, the emphasis is placed on recently developed artificial adjustments in the process of cell extraction, in vitro cell cultivation, and in vivo implantation. Among all these three steps, manipulations after tissue implantation are barely mentioned in other studies; thus, we provided additional details to address its significance in clinical applications and offer new prospects to improve tissue integration and intrinsic self-healing capacity. Some of these optimization strategies have been discussed before, but we thought that categorising these optimization strategies with their respective functioning procedure could help researchers pinpoint their functions and use them appropriately. We have also noted several potential novel therapies which have not been attempted before, for instance, distributing exogenous stimuli in a layer-dependent manner, constructing self-healing units, and ambushing it in large weight-bearing sites.
As cell-based therapies evolved, ACI is not confined to only using autologously extracted chondrocytes as cell seeds, and other stem cells such as MSCs, SSCs, ESCs, and iPSCs could also be cell sources. However, whichever cell type being used as seeds, the neotissue implanted back should mainly incorporate chondrocytes; otherwise, misdifferentiation may occur as a consequence of uncontrollable endogenous stimuli. Nanoscale manipulations significantly improve the biomimetic properties of synthesized scaffold matrix; for instance, we have mentioned the powerful usages of electrospinning in modifying scaffolds and constructing self-healing units. The structural specificity could be furthermore refined if 3D printing technique is applied.
There is still a long way to go before humans overcome articular cartilage–related diseases. However, developing science and tech make it possible to solve the weaknesses of current therapies. Combined with synthetic biology, cartilage resurfacing can be a promising way to improve neotissue integration, the satisfactory clinical outcome of photoreactive chondroitin sulphate hydrogel on local adhesion serving as a good example [67]. Besides, some recently developed repairing methods (cell-free treatments and scaffold-less treatments) choose to rely on the intrinsic organogenesis, which achieved by injecting growth factors and other small molecules into the lesion site, without in vitro cell culture and twice surgeries. Moreover, in vivo cartilage functional assessment system demands improvement with great urgency; for instance, tissue integration could only be detected via functional magnetic resonance imaging currently, which unfortunately fails to assess lateral integration between cartilages due to their avascular nature. Finally, we hope ACI together with these new strategies will make it easier to cure articular cartilage lesions.
Conflict of interest
None.
Acknowledgement
This work is supported by the National Key R&D Program of China (2017YFA0104900), and the NSFC grants (31830029, 81630065). The authors gratefully acknowledge support from NIH, and Dr. Li Dak Sum & Yip Yio Chin for stem cell and regenerative medicine.
Contributor Information
Yuchen Xiang, Email: 3170111457@zju.edu.cn.
Varitsara Bunpetch, Email: V.bunpetch@gmail.com.
Wenyan Zhou, Email: 11618114@zju.edu.cn.
Hongwei Ouyang, Email: hwoy@zju.edu.cn.
References
- 1.Fox A.J.S., Bedi A., Rodeo S.A. The basic science of articular cartilage: structure, composition, and function. Sport Health. 2009;1(6):461–468. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makris E.A., Gomoll A.H., Malizos K.N., Hu J.C., Athanasiou K.A. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11:21–34. doi: 10.1038/nrrheum.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huey D.J., Hu J.C., Athanasiou K.A. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338(6109):917–921. doi: 10.1126/science.1222454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brouwer R.W., Tom V.R., Bierma-Zeinstra S., Verhagen A., Jakma T.S.C., Verhaar J. Osteotomy for treating knee osteoarthritis. Cochrane Database Syst Rev. 2014;12 doi: 10.1002/14651858.CD004019.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Saris D.B.F., Vanlauwe J., Victor J., Haspl M., Bohnsack M., Fortems Y. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008;36(2):235–246. doi: 10.1177/0363546507311095. [DOI] [PubMed] [Google Scholar]
- 6.Gomoll A.H., Madry H., Knutsen G., Dijk N.V., Seil R., Brittberg M. The subchondral bone in articular cartilage repair: current problems in the surgical management. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):434–447. doi: 10.1007/s00167-010-1072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nawaz S.Z., Bentley G., Briggs T.W.R., Carrington R., Skinner J.A., Gallagher K.R. Autologous chondrocyte implantation in the knee: mid-term to long-term results. The Journal of bone and joint surgery. American Volume. 2014;96(10):824–830. doi: 10.2106/JBJS.L.01695. [DOI] [PubMed] [Google Scholar]
- 8.Behery O.A., Harris J.D., Karnes J.M., Siston R.A., Flanigan D.C. Factors influencing the outcome of autologous chondrocyte implantation: a systematic review. J Knee Surg. 2013;26(3):203–211. doi: 10.1055/s-0032-1329231. [DOI] [PubMed] [Google Scholar]
- 9.Harris J.D., Siston R.A., Brophy R.H., Lattermann C., Carey J.L., Flanigan D.C. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779–791. doi: 10.1016/j.joca.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Peterson L., Vasiliadis H.S., Brittberg M., Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38(6):1117–1124. doi: 10.1177/0363546509357915. [DOI] [PubMed] [Google Scholar]
- 11.Brittberg M., Lindahl A., Nilsson A., Ohlsson C., Isaksson O., Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 12.Brittberg M., Nilsson A., Lindahl A., Ohlsson C., Peterson L. Rabbit articular cartilage defects treated with autologous cultured chondrocytes. Curr Orthop Prac. 1996;326:270–283. doi: 10.1097/00003086-199605000-00034. [DOI] [PubMed] [Google Scholar]
- 13.O'Driscoll S.W., James F. The role of periosteum in cartilage repair. Clin Orthop Relat Res. 2011;391(Suppl):190–207. doi: 10.1097/00003086-200110001-00019. [DOI] [PubMed] [Google Scholar]
- 14.Minas T., Bryant T. The role of autologous chondrocyte implantation in the patellofemoral joint. Clin Orthop Relat Res. 2005;436:30–39. doi: 10.1097/01.blo.0000171916.40245.5d. [DOI] [PubMed] [Google Scholar]
- 15.Saris D.B.F., Vanlauwe J.J., Victor J., Almqvist K.F., Verdonk R., Bellemans J. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 Months in a randomized trial compared to microfracture. Am J Sports Med. 2009;X(XX):1–10. doi: 10.1177/0363546509350694. [DOI] [PubMed] [Google Scholar]
- 16.Knutsen G., Drogset J.O., Engebretsen L., GrØntvedt T., Ludvigsen T.C., LeØken S. Randomized multicenter trial comparing autologous chondrocyte implantation with microfracture long-term follow-up at 14 to l5 years. J Bone Joint Sun Am. 2016;98:1332–1339. doi: 10.2106/JBJS.15.01208. [DOI] [PubMed] [Google Scholar]
- 17.Saris D.B., Vanlauwe J., Victor J., Almqvist K.F., Verdonk R., Bellemans J. Study Group. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;Suppl:10–19. doi: 10.1177/0363546509350694. [DOI] [PubMed] [Google Scholar]
- 18.Perdisa F., Gostyńska N., Roffi A., Filardo G., Marcacci M., Kon E. Adipose-derived mesenchymal stem cells for the treatment of articular cartilage: a systematic review on preclinical and clinical evidence. Stem Cell Int. 2015;12(1):39. doi: 10.1155/2015/597652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benthien J.P., Behrens P. The treatment of chondral and osteochondral defects of the knee with autologous matrix-induced chondrogenesis (AMIC): method description and recent developments. Knee Surg Sports Traumatol Arthrosc. 2011;19(8):1316–1319. doi: 10.1007/s00167-010-1356-1. [DOI] [PubMed] [Google Scholar]
- 20.Clar C., Cummins E., McIntyre L., Lamb J., Bain L., Jobanputra P. Clinical and cost-effectiveness of autologous chondrocyte implantation for cartilage defects in knee joints: systematic review and economic evaluation. Health Technol Assess. 2005;21(6):1–294. doi: 10.3310/hta9470. [DOI] [PubMed] [Google Scholar]
- 21.Kafienah W., Mistry S., Dickinson S.C., Sims T.J., Learmonth I., Hollander A.P. Three-dimensional cartilage tissue engineering using adult stem cells from osteoarthritis patients. Arthritis Rheum. 2007;56:177–187. doi: 10.1002/art.22285. [DOI] [PubMed] [Google Scholar]
- 22.Pelttari K., Mumme M., Barbero A., Martin I. Nasal chondrocytes as a neural crest-derived cell source for regenerative medicine. Curr Opin Biotechnol. 2017;47:1–6. doi: 10.1016/j.copbio.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Mumme M., Barbero A., Miot S., Wixmerten A., Feliciano S., Wolf F. Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: an observational first-in-human trial. Lancet. 2016;388(10055):1985–1994. doi: 10.1016/S0140-6736(16)31658-0. [DOI] [PubMed] [Google Scholar]
- 24.Almalki S.G., Agrawal D.K., Science T. Key transcription factors in the differentiation of mesenchymal stem cells. Differentiation. 2017;92:41–51. doi: 10.1016/j.diff.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nejadnik H., Hui J.H., Feng Choong E.P., Tai B.C., Lee E.H. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med. 2010;38(6):1110–1116. doi: 10.1177/0363546509359067. [DOI] [PubMed] [Google Scholar]
- 26.Campbell D.D., Pei M. Surface markers for chondrogenic determination: a highlight of synovium-derived stem cells. Cells. 2012;1:1107–1120. doi: 10.3390/cells1041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan C.K.F., Gulati G.S., Sinha R., Weissman I.L., Chang H.Y., Longaker M.T. Identification of the human skeletal stem cell. Cell. 2018;175:43–56. doi: 10.1016/j.cell.2018.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bianco P., Robey P.G. Skeletal stem cells. Development. 2015;142(6):1023–1027. doi: 10.1242/dev.102210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jukes J.M., Van Blitterswijk C.A., De Boer J. Skeletal tissue engineering using embryonic stem cells. J Tissue Eng Regen Med. 2010;4:165–180. doi: 10.1002/term.234. [DOI] [PubMed] [Google Scholar]
- 30.Tapia N., Schöler H.R. Molecular obstacles to clinical translation of iPSCs. Cell Stem Cell. 2016;19(3):298–309. doi: 10.1016/j.stem.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 31.Wu J., Ocampo A., Belmonte J.C.I. Cellular metabolism and induced pluripotency. Cell. 2016;166(6):1371–1385. doi: 10.1016/j.cell.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Dewan A.K., Gibson M.A., Elisseeff J.H., Trice M.E. Evolution of autologous chondrocyte repair and comparison to other cartilage repair techniques. BioMed Res Int. 2014:272481. doi: 10.1155/2014/272481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benya P.D., Shaffer J.D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30(1):215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 34.Ma B., Leijten J.C.H., Wu L., Kip M., van Blitterswijk C.A., Post J.N. Gene expression profiling of dedifferentiated human articular chondrocytes in monolayer culture. Osteoarthr Cartil. 2013;21(4):599–603. doi: 10.1016/j.joca.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 35.Edmondson R., Broglie J.J., Adcock A.F., Yang L. Three-Dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol. 2014;12(4):207–218. doi: 10.1089/adt.2014.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwon H., Paschos N.K., Hu J.C., Athanasiou K. Articular cartilage tissue engineering: the role of signalling molecules. Cell Mol Life Sci. 2016;73:1173–1194. doi: 10.1007/s00018-015-2115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jahr H., Matta C., Mobasheri A. Physicochemical and biomechanical stimuli in cell-based articular cartilage repair. Curr Rheumatol Rep. 2015;17(22):1–12. doi: 10.1007/s11926-014-0493-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Athanasiou K., Responte D.J., Brown W.E., Hu J.C. Harnessing biomechanics to develop cartilage regeneration strategies. J Biomech Eng. 2015;137(2):020901. doi: 10.1115/1.4028825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Darling E.M., Athanasiou K.A. Growth factor impact on articular cartilage subpopulations. Cell Tissue Res. 2005;322(3):463–473. doi: 10.1007/s00441-005-0020-4. [DOI] [PubMed] [Google Scholar]
- 40.Yang X., Chen L., Xu X., Li C., Huang C., Deng C.X. TGFbeta/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol. 2001;153(1):35–46. doi: 10.1083/jcb.153.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiao B., Padilla S.R., Benya P.D. Transforming growth factor (TGF)-beta-activated kinase 1 mimics and mediates TGF-beta-induced stimulation of type II collagen synthesis in chondrocytes independent of Col2a1 transcription and Smad3 signaling. J Biol Chem. 2005;280:17562–17571. doi: 10.1074/jbc.M500646200. [DOI] [PubMed] [Google Scholar]
- 42.Kim I., Mauck R.L., Burdick J.A. Hydrogel design for cartilage tissue engineering: a case study with hyaluronic acid. Biomaterials. 2012;32(34):8771–8782. doi: 10.1016/j.biomaterials.2011.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuo C.K., Li W.J., Mauck R.L., Tuan R.S. Cartilage tissue engineering: its potential and uses. Curr Opin Rheumatol. 2006;18(1):64–73. doi: 10.1097/01.bor.0000198005.88568.df. [DOI] [PubMed] [Google Scholar]
- 44.Cao Z., Dou C., Dong S. Scaffolding biomaterials for cartilage regeneration. J Nanomater. 2014;2014:1–8. [Google Scholar]
- 45.Stevens M.M., George J.H. Exploring and engineering the cell surface interface. Science. 2005;310(5751):1135–1138. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- 46.Matthews J.A., Wnek G.E., Simpson D.G., Bowlin G.L. Electrospinning of collagen nanofibers. Biomacromolecules. 2002;3:232–238. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 47.Wang C.C., Yang K.C., Lin K.H., Liu H.C., Lin F.H. A highly organized three-dimensional alginate scaffold for cartilage tissue engineering prepared by microfluidic technology. Biomaterials. 2011;32(29):7118–7126. doi: 10.1016/j.biomaterials.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 48.Baar M.P., Brandt R.M.C., Putavet D.A., Klein J.D.D., Derks K.W.J., Bourgeois B.R.M. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell. 2017;169:132–147. doi: 10.1016/j.cell.2017.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stockwell R.A. The cell density of human articular and costal cartilage. J Anat. 1967;101(Pt 4):753–763. [PMC free article] [PubMed] [Google Scholar]
- 50.Khan I.M., Gilbert S.J., Singhrao S.K., Duance V.C., Archer C.W. Cartilage integration: evaluation of the reasons for failure of integration during cartilage repair. A review. Eur Cells Mater. 2008;16:26–39. doi: 10.22203/ecm.v016a04. [DOI] [PubMed] [Google Scholar]
- 51.Theodoropoulos J.S., Amritha De Croos J.N., Park S.S., Pilliar R., Kandel R.A. Integration of tissue-engineered cartilage with host cartilage: an in vitro model. Clin Orthop Relat Res. 2011;469:2785–2795. doi: 10.1007/s11999-011-1856-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Archer C.W., Redman S., Khan I., Bishop J., Richardson K. Enhancing tissue integration in cartilage repair procedures. J Anat. 2006;209:481–493. doi: 10.1111/j.1469-7580.2006.00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DiMicco M.A., Sah R.L. Integrative cartilage repair: adhesive strength is correlated with collagen deposition. J Orthop Res. 2001;19:1105–1112. doi: 10.1016/S0736-0266(01)00037-7. [DOI] [PubMed] [Google Scholar]
- 54.Silverman R.P., Bonassar L.J., Passaretti D., Randolph M.A., Yaremchuk M.J. Adhesion of tissue-engineered cartilage to native cartilage. Plast Reconstr Surg. 2000;105:1393–1398. doi: 10.1097/00006534-200004040-00019. [DOI] [PubMed] [Google Scholar]
- 55.Schaefer D.B., Wendt D., Moretti M., Jakob M., Jay G.D., Heberer M. Lubricin reduces cartilage— cartilage integration. Biorheology. 2004;41:503–508. [PubMed] [Google Scholar]
- 56.Khan I.M., Gonzalez L.G., Francis L., Conlan R.S., Gilbert S.J., Singhrao S.K. Interkeulin-1β enhances cartilage-to-cartilage integration. Eur Cells Mater. 2011;22:190–201. doi: 10.22203/ecm.v022a15. [DOI] [PubMed] [Google Scholar]
- 57.Englert C., Blunk T., Fierlbeck J., Kaiser J., Stosiek W., Angele P. Steroid hormones strongly support bovine articular cartilage integration in the absence of interleukin-1beta. Arthritis Rheum. 2010;54(12):3890–3897. doi: 10.1002/art.22250. [DOI] [PubMed] [Google Scholar]
- 58.Krishnan Y., Grodzinsky A.J. Cartilage diseases. Matrix Biol J Int Soc Matrix Biol. 2018;71–72:51–69. doi: 10.1016/j.matbio.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Higuchi Y., Hasegawa K., Yamashita M., Tanaka H., Tsukahara H. A novel mutation in the COL2A1 gene in a patient with Stickler syndrome type 1: a case report and review of the literature. J Med Case Rep. 2017;11(1):237. doi: 10.1186/s13256-017-1396-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hainer B.L., Matheson E., Wilkes R.T. Diagnosis, treatment, and prevention of gout. Am Fam Physician. 2014;90(12):831–836. [PubMed] [Google Scholar]
- 61.Øiestad B.E., Juhl C.B., Eitzen I., Thorlund J.B. Knee extensor muscle weakness is a risk factor for the development of knee osteoarthritis. A systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23(2):171–177. doi: 10.1016/j.joca.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 62.Ramos T.L., Sánchez-Abarca L.I., Muntión S., Preciado S., Puig N., López-Ruano G. MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry. Cell Commun Signal. 2016;14:2. doi: 10.1186/s12964-015-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leeman D.S., Hebestreit K., Ruetz T., Webb A.E., McKay A., Elizabeth A. Lysosome activation clears aggregates and enhances quiescent neural stem cell activation during aging. Science. 2018;359(6381):1277–1283. doi: 10.1126/science.aag3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang J., Lu X., Sakk V., Klein C.A., Rudolph K.L. Senescence and apoptosis block hematopoietic activation of quiescent hematopoietic stem cells with short telomeres. Blood. 2014;124(22):3237–3240. doi: 10.1182/blood-2014-04-568055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Childs B.G., Gluscevic M., Baker D.J., Laberge R.M., Marquess D., Dananberg J. Senescent cells: an emerging target for diseases of ageing. Nat Rev Drug Discov. 2017;16(10):718–735. doi: 10.1038/nrd.2017.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao Z., Liu Y., Zhang K., Zhuo S., Fang R., Zhang J. Biphasic synergistic gel materials with switchable mechanics and self-healing capacity. Angew Chem Int Ed. 2017;56(43):13464–13469. doi: 10.1002/anie.201707239. [DOI] [PubMed] [Google Scholar]
- 67.Sharma B., Fermanian S., Gibson M., Unterman S., Herzka D.A., Cascio B. Human cartilage repair with a photoreactive adhesive-hydrogel composite. Sci Transl Med. 2013;5(167):1–9. doi: 10.1126/scitranslmed.3004838. [DOI] [PMC free article] [PubMed] [Google Scholar]