Abstract
Compartmentalization of biochemical processes is essential for cell function. While membrane-bound organelles are well-studied in this context, recent work has shown that phase separation is a key contributor to cellular compartmentalization through the formation of liquid-like membraneless organelles (MLOs). We first briefly discuss key mechanistic concepts that underlie MLO dynamics and function, including the relevant non-covalent interaction chemistry and polymer physical chemistry. Next, we discuss a few examples of MLOs and relevant proteins, and their functions, which highlight the relevance of the above concepts. We also discuss the developing area of active matter and non-equilibrium systems, which can give rise to unexpected effects in fluctuating cellular conditions. Finally, we discuss our thoughts for emerging and future directions in the field, including in vitro and in vivo studies of MLO physical chemistry and function.
Keywords: Membraneless Organelles, Liquid-liquid phase separation, Active matter, Biophysics
1. Introduction
Proper cell function requires specific physical and chemical interactions to take place among high concentrations of macromolecules, small molecules and ions. One important means by which this specificity is achieved is via compartmentalization of biochemistry. Until recently, work on cellular compartmentalization focused on membrane-bounded organelles such as the nucleus, vesicles, and mitochondria. In contrast, membraneless organelles (MLOs), including the nucleolus, stress granules, and P-bodies, represent a more dynamic form of compartmentalization.[1] While such bodies were observed and discussed more than 100 years ago,[2] these compartments have been the subject of increasing scrutiny over the past few years.[1] A solid body of evidence demonstrates that these “droplet” organelles are liquid-like and formed by a process of liquid-liquid phase separation (LLPS).[1] This physical mechanism affords dynamic compartments that can be regulated in both equilibrium and non-equilibrium ways.
General physical chemistry principles suggest that MLOs present a number of potential advantages for cell function.[1] Because MLOs concentrate biomolecules in their interiors, they may accelerate enzymatic reactions (see the example of the the miRISC complex in the outlook section). Alternatively, they can inhibit less-desirable reactions by selectively segregating reactants. Molecules concentrated in MLOs can exchange with the surrounding solution, which may facilitate turnover in enzymatic reactions or responses to changing cellular conditions. The crowded environment of the dense MLO phase can alter interactions (see the Ddx4 example below) and enzyme activity, potentially facilitating assembly of complexes and activation or inhibition of enzymes. MLOs present surfaces, gradients and substructures, which could alter biochemical processes (see the nucleophosmin example below). MLOs can store deactivated enzymes or other molecules by virtue of selective concentration, and also function as a buffer mechanism to maintain substrate concentrations and cellular homeostasis. The physics of MLOs also makes them particularly suited for rapidly responding to changing cellular conditions and needs (e.g. by forming, dissolving, or developing sub-structures).[3] Finally, energy-consuming processes (for example, those involving enzymes or motor proteins) can alter the characteristics of MLOs to impact cellular function. While some links have been made between this active matter concept and cellular observations, interesting predictions of theory will no doubt be the subject of future studies (see the active matter section 4 below for more details).
Since MLOs play key roles in cell biology, achieving a predictive understanding of cellular LLPS is of high interest. While the dynamic and crowded environment of cells enormously increases the complexity of MLO behavior, a basic understanding of physical chemistry principles can provide a mechanisitic basis for understanding and potentially manipulating MLOs and their associated functions. In this minireview, we first aim to briefly introduce the reader to key relevant chemical and physical principles. Specifically, we discuss molecular level forces and polymer physics involved in biomolecular condensation. We then discuss three examples of biological phase separation: the germ granule protein Ddx4; the nucleolus and protein nucleophosmin; and the pyrenoid and associated magic number effects. We follow with a section discussing active matter and non-equilibrium effects, which constitute a developing area particularly relevant for cellular conditions. Finally, we end with our thoughts on future directions in the field. We note that we have chosen to focus on and highlight a few interesting concepts and developments in this concise minireview. The interested reader is encouraged to refer to key reviews and original papers referenced here for further information.[1]
2. Phase separation fundamentals – a primer
The mechanistic underpinnings of phase separation can be broadly categorized into (i) the chemistry of non-covalent interactions and (ii) the polymer physics of phase transitions. Below, we briefly discuss a few key considerations in these two areas.
2.1. Multivalent non-covalent molecular interactions in LLPS
LLPS is often driven by networks of weak, multivalent contacts between conformationally dynamic molecules, though interactions with folded domains can also be involved.
Biomolecules make extensive contacts with the aqueous solvent in which they are suspended, and the properties of those contacts are determined by the chemical makeup of the solute (and solvent). Biomolecular condensation can be considered in terms of competition between solvation forces.[4] That is, phase separation occurs when it is more energetically favorable for polymers to be solvated partially by other polymers, rather than exclusively by the aqueous solvent. Thus, individually weak interactions can collectively lower the free energy of solvation (by the surrounding polymers) within a dense phase and hence drive phase separation.[5]
Electrostatic interactions in biology include hydrogen bonds between water, proteins, and nucleic acids; nucleic acid interactions with positively charged proteins, as in complex coacervation; and electrostatic patch interactions between proteins. All these interactions, which are involved in classical protein and nucleic acid folding and binding, are also important in LLPS to varying degrees. For example, complex coacervation is a form of phase separation that occurs between oppositely-charged particles or regions, as in the common interaction between arginine-rich protein regions and RNA.
Entropic forces also play a role in macromolecular conformations and interactions. The polarity of water drives the hydrophobic effect, causing nonpolar surfaces to cluster together: minimizing the surface area between nonpolar and polar surfaces minimizes the number of water molecules participating in a solvation shell, which maximizes the entropy of the solvent. Thus, nonpolar surfaces assume compact conformations due to this entropic effect. This is an important driving force behind much of classical protein folding, which often includes formation of a hydrophobic protein core. It also drives the phase separation of lipid droplets and of hydrophobic proteins like tropoelastin.[6]
However, tropoelastin’s hydrophobic self-assembly is also tuned by unstructured hydrophilic domains, which help limit aggregation.[7] As in this example, many interactions contributing to phase separation result from several types of forces which collectively provide favorable interaction energy.
Another such example is that of pi systems with both electrostatic and hydrophobic character. Many aromatic pi systems are quadrupoles, with polarized bonds evenly distributed so as to cancel out a dipole moment across the molecule. Thus, hydrophobic phenylalanine can participate in electrostatic interactions by attracting cations to its electronegative face.[8] This mechanism will be discussed below in regards to Ddx4 behavior. Pi quadrupoles can similarly associate with other dipoles or quadrupoles, and may play a role in carbohydrate-pi interactions by attraction to polarized C-H bonds.[9] Pi-pi stacking interactions have been suggested as a predictor of phase separation capacity.[10]
Pi systems are common in proteins, both in aromatic side chains and in the sp2-hybridized atoms of residues like glutamine, asparagine, and arginine.[10] Peptide bonds also contain sp2-hybridized atoms, which are relevant for small residues that expose those bonds for interaction.[10] Pi interactions are important both in protein structure and interactions among intrinsically disordered proteins.
Intrinsically disordered proteins and regions (IDPs and IDRs) are associated with many MLOs by virtue of low-complexity regions that exhibit multivalent motifs for many of the above-mentioned weak interactions.[1g, 5b, 11] However, associations involving folded protein domains may also provide sufficient multivalency to drive phase separation, as in the case of the tumor suppressor protein speckle-type POZ protein (SPOP) and linear motifs in its intrinsically disordered partner death-domain-associated protein (DAXX).[12] IDRs also can have weak structural propensities that are accentuated in droplets, allowing them to contribute folded motifs in specific contexts.[13] Many protein components of MLOs contain both IDRs and folded domains, and both can be involved in driving LLPS. Many of the interactions discussed above, including cation-pi, charge-charge, and pi-stacking, are also important for the folding, binding, and sequestering of RNA, another important component of many MLOs.
2.2. Polymer physical chemistry concepts
At equilibrium, phase separation is governed by minimization of the global free energy, which consists of enthalpic and entropic terms describing polymer, solvent, and polymer-solvent interactions.[1f] Environmental parameters such as temperature and pH can substantially influence phase separation. For example, the temperature dependence (or lack thereof) of interaction enthalpy and entropy in a given system can result in either an upper critical solution temperature (above which no phase separation occurs) or a lower critical solution temperature (below which no phase separation occurs). Various levels of theoretical treatment have been used to analyze these phase transitions. A basic overview of some of these treatments and related polymer physics concepts is provided below.
A simplified but widely used representation of phase separation processes is provided by Flory-Huggins theory.[1f, 14] The theory uses lattice model and mean field considerations to derive the entropic and enthalpic terms of the mixing free energy. For a binary polymer-solvent system, solvent and polymer molecules occupy NS (typically set to 1) and NP sites respectively on a lattice that represents the volume of the system. The entropic component (first two terms in Equation 1 below) was derived in terms of the volume fractions ϕS (solvent) and ϕP (polymer) and number of lattice sites occupied per molecule. The third term in equation 1 is an enthalpic term and is based on pairwise interactions in mean-field theory. The Flory-Huggins free energy of mixing per lattice site for this simple binary system is then given by
| (Equation 1) |
where R is the molar gas constant. χ is a parameter that incorporates the competition between different pairwise interactions between site occupants, and is given by
| (Equation 2) |
where Uij are the mean-field energies per site for the three types of pairwise interactions (polymer -solvent, solvent-solvent and polymer-polymer segment) and z is the coordination number of the lattice. Negative values of χ imply a good solvent for the polymer, while positive values imply a poor solvent. Therefore, above a critical values of χ, the enthalpic term outweighs the entropic one, resulting in an unstable region of negative curvature in the free energy and consequent phase separation. This theory has provided a conceptual framework for understanding LLPS, and has generally been used to model compositional effects and short-range interactions. See section 4 below for an example application.
Overbeek-Voorn theory extends the Flory-Huggins formalism by taking into account longer range electrostatic interactions, and is thus used to model complex coacervation (LLPS) of polyelectrolytes such as RNA and charged proteins that are constituents of many MLOs.[1f, 15] In this lattice model formalism, the free energy of mixing per lattice site for polycations and polyanoins, each occupying N lattice sites and a total volume fraction ϕ, is given by
| (Equation 3) |
Here, the first and second terms represent the mixing entropy. The third term is an electrostatic free energy term (Debye-Huckel). α is determined by charge per lattice site and the partial molar volume of the solvent, and σ is the linear charge density per polyion. Here again, the system will undergo phase separation above a certain value of the mixing enthalpy term that is determined by the balance of factors in equation 3.
The random phase approximation, adapted for polymers by de Gennes and Edwards, includes a treatment of polymer sequence dependence.[16] This theory can address why variations in sequence (with the same composition), can produce variations in phase separation behavior. Random phase approximation theory has been used to understand differences in phase separation propensities of sequence variants in the protein Ddx4 discussed later. Other theoretical advances aim to consider sequence effects, chain conformational preferences, and specific interactions at the atomic level.[16–17]
The existence of two or more solute components, as is the case in cells, complicates the theoretical analysis: components can segregate into a multicomponent dense phase or multiple homogenous dense phases.[18] One striking example is sub-compartmentalization in the nucleolus, discussed below.[18b] Finally, non-equilibrium and stoichiometry-dependent effects are relevant for many cellular processes. For example, recent work has explored how reentrant phase transition dynamics can give rise to sub-compartmentalized phases.[19] Additionally, active matter processes that consume energy (e.g. in the form of enzyme reactions or motor protein function), can result in complex behavior, and are likely important in several MLOs, as discussed later. Below, we discuss how these forces play out in biological systems.
3. Three examples: Proteins, MLOs and Function
Here, we discuss three protein/MLO systems with important cellular functions. Our main goal is to highlight physical chemistry principles and their influence on MLO properties and function. We discuss how interactions discussed above direct LLPS for Ddx4 and nucleophosmin, how functional sub-compartmentalization in the nucleolus can be explained by simple physics, and how magic numbers and valency matching may influence LLPS in the case of the pyrenoid.
3.1. Ddx4
The germ granule protein Ddx4 forms liquid-like droplets in vitro and in vivo.[20] Like many intrinsically disordered proteins, Ddx4 contains a large proportion of charged residues, which allow it to favorably interact with aqueous solvent in an unfolded, disperse state. Electrostatic interactions have been found to be the primary driving force of Ddx4’s phase separation, which is attenuated with increasing salt concentration as salt ions shield the protein’s charged residues from interaction.[20a]
Ddx4’s sequence is arranged in 8–10 residue blocks of alternating charge density, and this patterning is intrinsic to Ddx4’s behavior: a scrambled construct of identical composition was unable to phase separate under physiological conditions, despite accumulating to high concentrations in cells.[20a, 21] In addition, arginine methylation and phenylalanine mutation or disruption both abrogated droplet formation (Figure 1).[20a, 22] Together, this suggests pi-cation interactions between the positively charged arginine and the aromatic phenylalanine contribute to the electrostatic force that allows Ddx4 to condense from bulk solution. Furthermore, Ddx4 constructs in which all arginines were mutated to lysines, which preserve the positive charge but lack a pi system, failed to phase separate, suggesting a specific role for arginine in phase separation via pi-pi interactions.[21–22] The efficacy of these interactions at driving phase separation is apparently dependent on their sequence position within the alternating charge blocks.[20a]
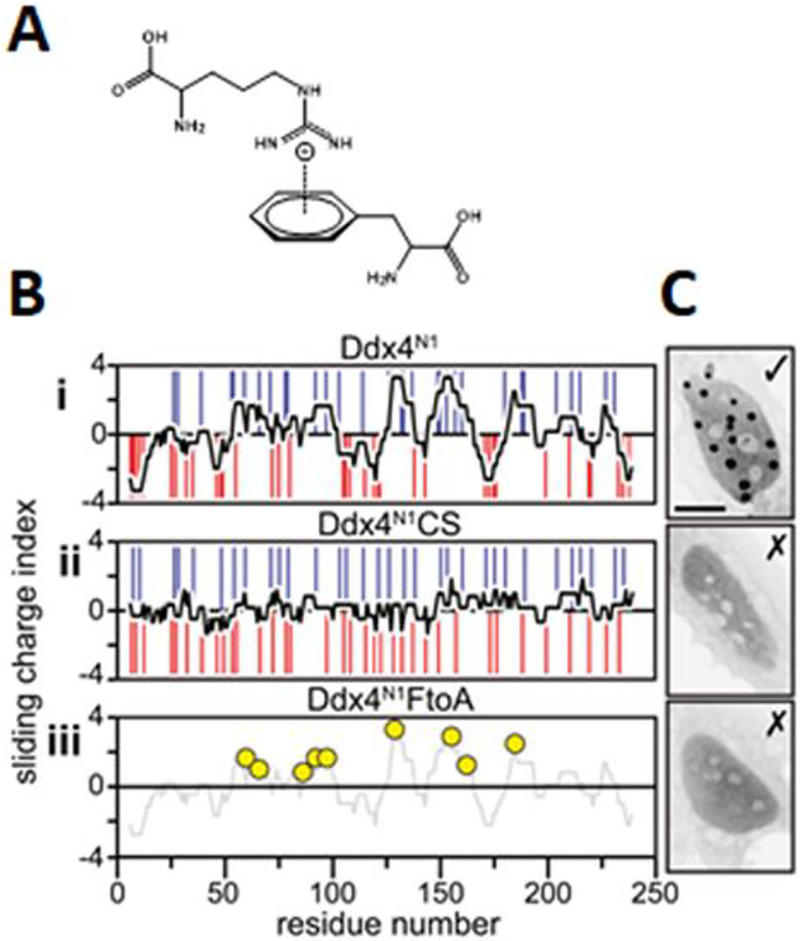
Figure 1.
A. Cation-pi interactions involved in phase separation of Ddx4. B. Sliding charge analysis of Ddx4, showing charge blocks in the WT (i) but not in a charge scrambled mutant (ii). iii shows positions of phenylalanine to alanine mutants in another mutant Ddx4. C. Images showing that the above mutants reduce phase-separation propensity in cells. B and C from Nott et al. Molecular Cell (2015) 57:936–947.[20a, 23]
Although electrostatic forces are the primary driver of Ddx4’s phase separation, theoretical calculations showed that theirs is not the only contribution to phase separation. Electrostatics-only models failed to match the experimental phase diagrams for Ddx4, and the equations only recapitulated the data once aromatic interactions were considered.[21] Furthermore, the free energy contribution from entropy was also calculated to favor droplet formation under all conditions, but the contribution was comparatively small, allowing droplets to condense and dissolve primarily according to electrostatic tuning.[20a]
Ddx4 highlights an increasing understanding of the role that amino acid sequence, not just composition, plays in phase separation. Developments in random-phase approximation theory describe this role in terms of electrostatic interactions, finding that phase separation propensity is directly dependent on the number of alternating charge blocks in a protein.[24] Unlike Flory-Huggins or Overbeek-Voorn theories, random-phase approximation theory accounts for residue sequence and sequence-correlated interactions, allowing the theory to recapitulate the experimental result that charge-scrambled Ddx4 fails to phase separate. Further development of the theory predicts that polymers with similar charge patterning are most likely to phase separate together, whereas charge pattern mismatches are more likely to disrupt phase separation.[25] Efforts continue to rationalize ternary phase coexistence, as observed in nucleophosmin systems discussed below.
The interactions that produce Ddx4 phase separation also influence the incorporation and stability of other species. Ddx4 droplets have been shown to preferentially incorporate single-stranded nucleic acids and destabilize double-stranded DNA.[20b] This preference is relevant for Ddx4’s RNA helicase, and Ddx4 organelles have been proposed to play a role in RNA processing, like many other MLOs.[20a] [26] By partially excluding water, these droplets form a distinct solvent that may passively alter the concentrations and conformations of partner molecules. [20b, 22]
3.2. Nucleophosmin and the nucleolus
Although the nucleolus was observed in cells over 100 years ago, only recently have studies demonstrated its liquid-like properties such as fusion and flowing.[27] The nucleolus is an MLO, best known as the center of ribosome biogenesis, though it has been associated with numerous other roles including ribonucleoprotein formation, genome stability and cancer.[28] Nucleoli are spatially associated with nucleolar organizer regions on chromosomal DNA, and show dissociation and regrowth during different stages of the cell cycle. Internally, nucleoli are not spatially uniform and instead show more complex sub-structure.
Nucleophosmin (NPM1) is an abundant nucleolar protein involved in several cellular functions including ribosome biogenesis, stress response and tumor suppression.[29] The protein consists of an N-terminal oligomerization domain (NTD), C-terminal nucleic-acid binding domain (CTD) and central disordered region. It interacts with a variety of nucleolar partners including proteins and RNA, and recent studies have suggested that it helps organize the liquid phase of nucleoli.[30]
Charge interactions play roles at several levels of NPM1 organization. The protein forms a pentamer via its NTD, though repulsion between negatively-charge patches on the NTD necessitates shielding by higher salt concentrations for oligomerization in vitro (Figure 2a).[31] The disordered region and CTD also contain multiple charged patches that, together with pentamerization, provide NPM1 with interaction multivalency and allow it to coordinate with partners with complementary charge patterning. NPM1 can phase separate in combination with either RNA (negatively charged) or arginine-rich protein partners (postiively charged regions) through different types of electrostatic interactions (Figure 2). The protein can also phase separate on its own due to its multivalency and different types of electrostatic interaction possibilities (Figure 2c). This complex interplay of intra- and inter-molecular interactions has been suggested as a key modulator of nucleolar LLPS.[18b, 30a]
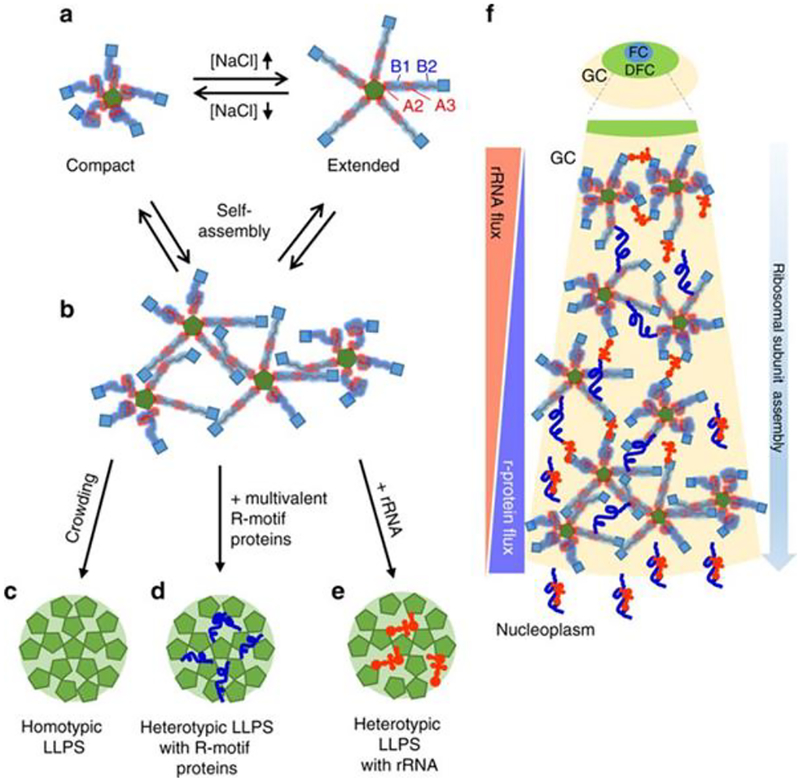
Figure 2.
Different NPM1 states and LLPS mechanisms. a. NPM1 has different folded and disordered regions with charge-patch interactions modulating conformational properties. b-e. Interactions with self or complementary molecules can result in different LLPS mechanisms. f. Suggested assembly-dependent partitioning of rRNA during ribosome biogenesis. See text for additional details. Figure from Mitrea et al. Nature Communications (2018) 9:842.[23, 30a]
Based on results of experiments using a series of biophysical tools including SAXS, microscopy, analytical ultracentrifugation and single-molecule FRET, it has been suggested that ribosomal rRNA could experience an assembly-dependent partitioning within the nucleolus (Figure 2f).[30a] Assembly intermediates with a larger fraction of unbound RNA can participate in more interactions with NPM1 and other nucleolar elements. As assembly proceeds, interactions with the nucleolar scaffold will decrease, resulting in eventual dissociation from the nucleolus.[30a]
A particularly interesting feature of the nucleolus is its sub-compartmentalized structure, featuring droplets within droplets (Figure 3). A recent study showed that NPM1 and other nucleolar components can form such sub-compartmentalized droplets in vitro.[18b] Moreover, simulations with a simplified model of a ternary mixture including NPM1, rRNA and a nucleolar protein FIB1 recapitulate this sub-structure. The authors conclude that surface tension and molecular charge-charge interactions play key roles.
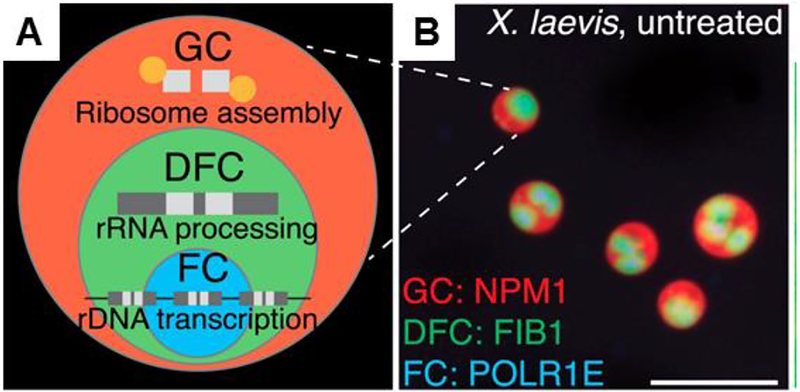
Figure 3.
Sub-compartmentalization in the nucleolus. (A) Cartoon of nucleolar substructure and (B) images of droplet within droplet structures in the nucleolus. Figure adapted with permission from Feric et al. Cell (2016) 165:1686.
3.3. Pyrenoids and the magic number effect
Pyrenoids were first identified over 200 years ago, but details of their internal structure are still emerging. An adaptation of certain eukaryotic algae, pyrenoids concentrate the enzyme Rubisco and its substrate CO2 to improve the specificity of the first step of photosynthesis.[32] While initial conflicting evidence from electron micrographs favored various solid-assembly (though non-membrane-bound) organizations, recent studies have instead suggested a dynamic and liquid-like pyrenoid model.[32–33]
Essential Pyrenoid Component 1 (EPYC1) is required for Rubisco localization to the pyrenoid and is proposed to link Rubisco holoenzymes to form the pyrenoid matrix.[34] EPYC1 is highly disordered and likely forms weak interactions with Rubisco. Though both components were found to diffuse within the pyrenoid, they did so at different rates, suggesting that the diffusing unit is not a stable EPYC1-Rubisco complex.[33b]
The stability of an EPYC1-Rubisco complex may lead to unique macroscopic effects. In computational models, eight binding sites of the Rubisco holoenzyme may be fully occupied by two EPYC1 proteins, each thought to contain four sites.[33b] This stoichiometry gives rise to a magic number effect in the simulations. It is noteworthy that magic number effects have long been discussed as playing important roles in chemistry,[35] including the physical chemistry of the (atomic) nucleus.[35c] For EPYC1-Rubisco, the simulations indicate that when the stoichiometry is satisfied, only small complexes and oligomers are formed, thus avoiding phase separation. However, when this stoichiometry is disturbed, the unsatisfied valences lead to widespread co-association (Figure 4). For example, if EPYC1 is modified to have 3 or 5 binding sites, no small EPCY1-Rubisco complex can satisfy every site, allowing other partners to be recruited by the unsatisfied valances.
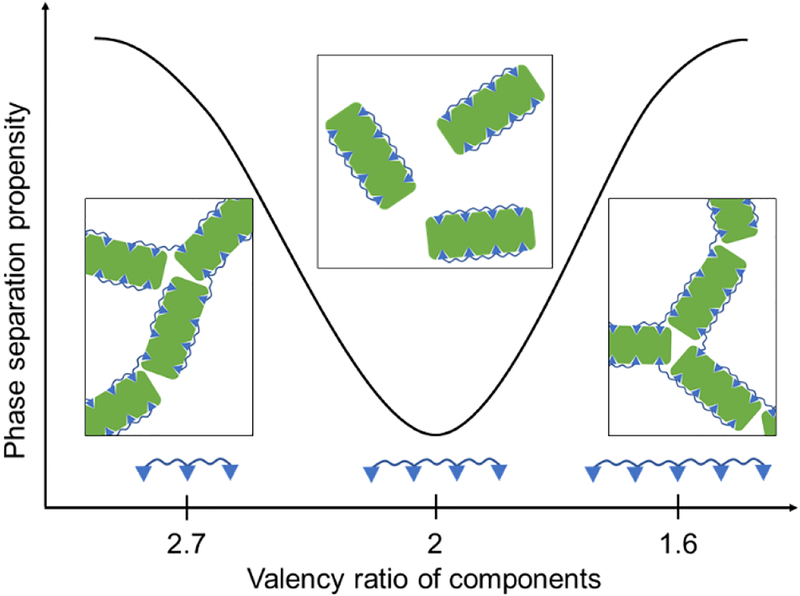
Figure 4.
Cartoon of the magic number effect. When the number of valences on one of the components (here 8, green) is a multiple of those on the other (here 4, blue), a small oligomer satisfies all valences and reduces phase separation. When this condition is not satisfied, larger networks of interacting species are formed leading to LLPS.
Interestingly, the simulations provided evidence for multiple magic numbers as a function of the number of EPYC1 binding sites. These observed magic numbers (2, 4, or 8) occurred whenever all valencies on both interacting components could be satisfied within the context of a small oligomer. In pyrenoids, the magic number effect may allow rapid phase transitions (condensation and dissolution), regulated by post-translational modifications that change the valency of either molecule. Notably, EPYC1 phosphorylation is affected by CO2 availability, potentially modifying EPYC1’s affinity for Rubisco. A magic number system can produce rapid phase changes in response to slight stoichiometry changes, allowing carefully tunable regulation.
4. Active matter and non-equilibrium effects
Research on the biophysics of MLOs has mainly focused on equilibrium conditions or temporally averaged properties. As in small-molecule physical chemistry, reactions and dynamics can introduce effects that are not observed at equilibrium. Active matter is distinguished by the presence of molecular species that consume energy and carry out chemical reactions, as is the case in cells. Recent work has resulted in interesting implications for LLPS within the conceptual framework of active matter physics.[36]
Ongoing chemical reactions can bias the localization of MLOs by influencing protein concentration gradients. P granule localization during germline formation in C. Elegans embryos presents an interesting case of this phenomenon. P granules are formed by interactions of RNA with various proteins, and phosphorylation and dephosphorylation reactions are believed to contribute to polarized growth and dissolution.[37] A recent single-molecule imaging study provided new insight into how cellular concentration gradients are formed for the case of a key P-granule protein.[38] By tracking individual molecules, the work shows that proteins rapidly switch between fast- and slow-diffusing states, and that the concentration of the slow-diffusing state is polarized towards one end of the cell. These experimental results and accompanying simulations support a simple model for protein gradient formation. Post-translational modifications change the affinity of the protein for a less mobile partner in the cell. The gradient is formed due to a polarized distribution of the modifying enzymes, rather than a localized source or sink of the protein or a gradient in the partner concentration. This mechanism provides a way for gradients to form even across the relatively short length scales of typical mammalian cells, and in turn may lead to anisotropic phase separation across the cell.
Chemical reactions can also alter the physics of droplet size and number in ways that are likely important for cell function.[39] In an equilibrium picture, a system containing several droplets will generally evolve by larger droplets growing at the expense of smaller ones, a process termed Ostwald ripening. Larger droplets are more stable than smaller ones because of their smaller surface to volume ratio. Since molecules are constantly exchanging between droplets and solution, ripening occurs by a net diffusion of molecules out of smaller droplets and into more stable larger ones. Furthermore, droplets nucleate at random in solution, and they can fuse but do not generally split into smaller species. Recent work has studied how chemical reactions can change these characteristics.
The influence of Ostwald ripening can be opposed by chemical reactions that produce or deplete droplet material.[39–40] In one example, a Flory-Huggins approach that included chemical reactions was used to model the dynamics of centrosomes, which are important in organizing networks of microtubules in cells.[41] Autocatalytic chemical reactions which created droplet material at centrioles were a key aspect of the model that permitted co-existence of centrosomes in cells by suppressing random nucleation and Ostwald ripening.
Molecular fluxes due to chemical reactions can result in other non-equilibrium effects in LLPS. As discussed above, cellular MLOs are often formed by complex coacervation of RNA and proteins featuring arginine-rich motifs. A recent study from our lab demonstrated that monotonic addition of RNA to arginine-rich motifs gives rise to phase separation at lower concentrations but will result in droplet dissolution at higher concentrations.[19] This behavior could be induced by producing RNA using an in vitro transcription reaction. Analysis of this reentrant dissolution transition revealed dynamic formation, growth and loss of vacuolar substructures within the main droplet. This observation is important given that such sub-structures are observed in cellular MLOs, and that RNA fluxes can be induced by processes such as transcription and cell stress.[3b] We note that dissolution in this simple model of such reentrant behavior is mainly predicated on saturation of valences in one of the two interacting components, thereby preventing further non-covalent crosslinking and LLPS. Indeed, this type of formation and dissolution has been included in multiple papers on LLPS.[42] Our model therefore provides a simple physical mechanism for such reentrant behavior as well as formation of dynamic substructures. We also note that the magic number mechanism discussed above may be superimposed on and modulate the overall reentrant phase transition in some cases, though this interesting idea remains to be studied.
5. Outlook
In the past few years, the study of LLPS has grown from initial demonstrations of liquid-like behavior of MLOs to studies of their chemistry, physics and biology. We anticipate more detailed progress in multiple directions. Here, we briefly discuss several interesting ongoing and future directions. Citations to initial or related work are included for the readers’ benefit.
Continued advances are expected in understanding the unique chemical and physical characteristics of known MLOs. MLOs generally have complex and changing compositions comprising a number of protein, RNA and other components. While major components of key MLOs are known and have been intensely studied recently, it is clear that even relatively minor components could perturb phase transitions. Indeed, this is one of the potential functional advantages provided by these organelles, making detailed studies of composition especially important. Along these lines, the influence of small molecule metabolites is also of interest. For example, the influence of ATP on phase separation has recently been discussed.[43] Another recent paper has discussed how the chemical chaperone (osmolyte) TMAO differentially affects the phase separation and fibril formation from the ALS-linked protein TDP-43.[44] More detailed measurements of MLO properties like fluidity, dynamics of formation and dissolution, partitioning ability, and diffusional properties will provide us with more complete pictures of the effects of composition on MLO function.
New important functions of MLOs continue to be discovered (or existing bodies rediscovered as liquid-like, as with the nucleolus and pyrenoid discussed above).[45] As a recent example, in a study on the miRISC complex, the intrinsically disordered TNRC6B was found to recruit Argonaute2, the catalytic member of the complex, into phase separated droplets in cells.[45b] Furthermore, Argonaute2 was able to selectively partition its RNA targets into the droplets, where several processing reactions proceeded or were even accelerated in the phase-separated environment. In another example, recent work has suggested that phase separation is important in gene silencing via heterochromatin.[46] Heterochromatin protein 1 (HP1) can undergo LLPS controlled by phosphorylation and DNA/ligand binding, and the droplets can selectively compartmentalize other heterochromatin factors.
In addition to micron-sized MLOs that have been studied thus far, we expect that smaller sub-micron condensates will also be discovered and characterized. As one potential example, Hnisz et al. proposed a model of transcriptional regulation by phase separation, arguing that aspects of super-enhancer behavior can be explained within a framework describing the enhancer as a small, dense, distinct molecular phase.[47] In support of their phase separation hypothesis, Hnisz et al. observed that super-enhancers may assemble cooperatively around a nucleation point and dissipate upon inhibition of key components; that single enhancers are able to drive synchronous bursting of multiple target genes, likely requiring physical proximity; and that low-complexity protein domains and nucleic acids are available at enhancers in the form of bound transcription factors, enhancer RNA, and other noncoding RNA which has been shown to play a role in localizing transcription factors.[47–48] Other recent studies have also supported an LLPS model of transcription.[49] We anticipate continued growth in the discovery and studies of such liquid-like organizations of cellular machinery even at the sub-micron scale, potentially geometrically constrained by scaffolding species like duplex DNA.
Cellular studies will continue to be complemented with in vitro studies. Experiments with purified proteins, model peptides and RNA can tease out the many molecular details of the driving forces for LLPS. The insights and predictions of these experiments can then be tested in cells. For example, recent work using theory and experiment has shed light on the rules that govern various features of LLPS including spatial patterning of droplets.[18a] Other recent work has used mutagenesis to reveal the roles of side-chain interactions underlying phase separation of the family of proteins that includes ALS-linked protein Fused in Sarcoma (FUS).[50] The results demonstrated a major contribution of tyrosine-arginine interactions to LLPS, with other sequence features leading to changes in the liquidity of droplets.
Refinement or development of methods will continue to be important for both in vitro and in cell experiments. Improved fluorescence imaging, single-molecule, cryo-EM (for example using FIB milling technology), NMR, mass-spectral tools in conjunction with chemical biology techniques are expected to provide information from the molecular to droplet scales.[31c, 51] In recent work, advanced fluorescence correlation spectroscopy measurements were used to understand the diffusion of molecules and corresponding organization of MLOs.[51c] In other work, an advanced application of FRET methodology enabled measurements of nucleation for prion-like proteins in live cells.[51d] The FRET method used controlled photoconversion of a fluorescent protein from donor to acceptor species directly in cells, thus generating the FRET dye pair in situ and avoiding variations with protein concentration and individual cells. In other studies, single-molecule FRET studies on NPM1 shed light on protein conformational changes during phase separation.[31c] Single-molecule FRET is particularly useful for such studies as it allows distributions of conformational states and dynamics to be directly measured, while avoiding loss of information due to ensemble averaging.[51a] This method has been extensively used for studies of conformational complexity in proteins and nucleic acids. Since little is known about the intra- and intermolecular structural features of proteins and RNA within MLOs, adaptation and development of experimental tools for this purpose are an important direction. Another novel development is the ability to activate droplet formation in cells using light, by conjugating the phase separating protein of interest with a protein whose oligomerization is light-activated.[52] This development permits direct mesaurements of various properties of LLPS directly in cells.
Studies closely linking LLPS to cellular functions are expected to also be an important focus. In this regard, we anticipate an increase in studies of how the physicochemical characteristics of droplets tune functional properties of proteins including by partitioning, conformational biasing, and modulating enzyme activity. Since the cell is a non-equilibrium system, novel features introduced due to dynamics and active matter effects will be especially relevant.[53] Theoretical studies and work with other systems such as colloids are already providing the field with novel characteristics of LLPS that can be explored within a cellular context. For example, theoretical work has shown that chemical reactions could result in spontaneous fission of droplets, in contrast with the equilibrium picture.[54] While the authors discuss this model in terms of prebiotic life, it could also be relevant and explored in modern cells. As an additional example, active motion can give rise to phase separation even in the absence of the kinds of molecular interactions that we have discussed above, if the diffusion of molecular species slows substantially when they are clustered.[55] Such non-equilibrium effects could be important for cellular phase separation involving protein motors.
Finally, continued studies of cellular LLPS associated with disease states will also be important endeavors.[56] For example, for the tumor suppressor SPOP, multivalent associations with DAXX allow phase separation that may be central to SPOP’s role preventing oncogenesis.[12, 57] Proteins such as FUS that are linked to ALS have been shown to be important components of stress granules and have been linked with toxic aggregation and aging of of these MLOs.[13, 42a, c, 57–58] A more detailed understanding of the physicochemical parameters governing abberant effects in this context may also facilitate the development of small-molecule drugs to combat related diseases.
Rather than comprehensively exploring every cellular phase-separating system in this minireview, we have instead discussed principles universally underlying their physical and chemical properties. As the field advances, theoretical strides hand in hand with experimental discoveries are expected to increase our understanding and predictive power of LLPS behavior and its role in cell function.
Acknowledgements
We gratefully acknowledge Anthony Milin, Paulo Onuchic and Priya Banerjee for their contributions to our work on LLPS. We also gratefully acknowledge the US NIGMS/NIH (Grants RO1 GM066833 and RO1 GM115634) and US National Science Foundation (Grant 1818385) for support of our lab’s related work.
References
- [1].a) Hyman AA, Weber CA and Julicher F, Annu Rev Cell Dev Biol 2014, 30, 39–58; [DOI] [PubMed] [Google Scholar]; b) Meng F, Na I, Kurgan L and Uversky VN, Int J Mol Sci 2015, 17, 1–26; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Hyman AA and Brangwynne CP, Dev Cell 2011, 21, 14–16; [DOI] [PubMed] [Google Scholar]; d) Mitrea DM and Kriwacki RW, Cell Commun Signal 2016, 14, 1; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Holehouse AS and Pappu RV, Biochemistry 2018, 57, 2415–2423; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Brangwynne CP, Tompa P and Pappu RV, Nature Physics 2015, 11, 899–904; [Google Scholar]; g) Boeynaems S, Alberti S, Fawzi NL, Mittag T, Polymenidou M, Rousseau F, Schymkowitz J, Shorter J, Wolozin B, Van Den Bosch L, Tompa P and Fuxreiter M, Trends Cell Biol 2018, 28, 420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].a) Wagner R, Müllers Archiv Anat Physiol Wissenschaft Med. 1835, 373–377; [Google Scholar]; b) Valentin G, Verlag Veit Comp Berl. 1836, 1, 1–293; [Google Scholar]; c) Wilson EB, Science 1899, 10, 33–45. [DOI] [PubMed] [Google Scholar]
- [3].a) Ruff KM, Roberts S, Chilkoti A and Pappu RV, J Mol Biol 2018, 430, 4619–4635; [DOI] [PubMed] [Google Scholar]; b) Milin AN and Deniz AA, Biochemistry 2018, 57, 2470–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zaslavsky BY and Uversky VN, Biochemistry 2018, 57, 2437–2451. [DOI] [PubMed] [Google Scholar]
- [5].a) Anslyn EV and Dougherty DA, Modern physical organic chemistry, University Science, Sausalito, CA, 2006; [Google Scholar]; b) Mittag T and Parker R, J Mol Biol 2018, 430, 4636–4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vrhovski B, Jensen S and Weiss AS, Eur J Biochem 1997, 250, 92–98. [DOI] [PubMed] [Google Scholar]
- [7].a) Bisaccia F, Castiglione-Morelli MA, Spisani S, Ostuni A, Serafini-Fracassini A, Bavoso A and Tamburro AM, Biochemistry 1998, 37, 11128–11135; [DOI] [PubMed] [Google Scholar]; b) Jensen SA, Vrhovski B and Weiss AS, J Biol Chem 2000, 275, 28449–28454. [DOI] [PubMed] [Google Scholar]
- [8].Kumar K, Woo SM, Siu T, Cortopassi WA, Duarte F and Paton RS, Chem Sci 2018, 9, 2655–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Spiwok V, Molecules 2017, 22, 1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Vernon RM, Chong PA, Tsang B, Kim TH, Bah A, Farber P, Lin H and Forman-Kay JD, Elife 2018, 7, e31486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].a) van der Lee R, Buljan M, Lang B, Weatheritt RJ, Daughdrill GW, Dunker AK, Fuxreiter M, Gough J, Gsponer J, Jones DT, Kim PM, Kriwacki RW, Oldfield CJ, Pappu RV, Tompa P, Uversky VN, Wright PE and Babu MM, Chem Rev 2014, 114, 6589–6631; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wright PE and Dyson HJ, Nat Rev Mol Cell Biol 2015, 16, 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].a) Bouchard JJ, Otero JH, Scott DC, Szulc E, Martin EW, Sabri N, Granata D, Marzahn MR, Lindorff-Larsen K, Salvatella X, Schulman BA and Mittag T, Mol Cell 2018, 72, 19–36; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Marzahn MR, Marada S, Lee J, Nourse A, Kenrick S, Zhao H, Ben-Nissan G, Kolaitis RM, Peters JL, Pounds S, Errington WJ, Prive GG, Taylor JP, Sharon M, Schuck P, Ogden SK and Mittag T, Embo j 2016, 35, 1254–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang A, Conicella AE, Schmidt HB, Martin EW, Rhoads SN, Reeb AN, Nourse A, Ramirez Montero D, Ryan VH, Rohatgi R, Shewmaker F, Naik MT, Mittag T, Ayala YM and Fawzi NL, EMBO J 2018, 37, e97452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].a) Huggins ML, Journal of Chemical Physics 1941, 9, 440–440; [Google Scholar]; b) Flory P, Principles of Polymer Chemistry, 1953; [Google Scholar]; c) Flory PJ, Journal of Chemical Physics 1941, 9, 660–661. [Google Scholar]
- [15].Overbeek JT and Voorn MJ, J Cell Physiol Suppl 1957, 49, 7–22; discussion, 22–26. [PubMed] [Google Scholar]
- [16].Lin YH, Forman-Kay JD and Chan HS, Biochemistry 2018, 57, 2499–2508. [DOI] [PubMed] [Google Scholar]
- [17].a) Harmon TS, Holehouse AS, Rosen MK and Pappu RV, Elife 2017, 6, e30294; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Chang LW, Lytle TK, Radhakrishna M, Madinya JJ, Velez J, Sing CE and Perry SL, Nature Communications 2017, 8, Article 1273; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Lytle TK and Sing CE, Molecular Systems Design & Engineering 2018, 3, 183–196; [Google Scholar]; d) Sing CE, Advances in Colloid and Interface Science 2017, 239, 2–16; [DOI] [PubMed] [Google Scholar]; e) Martin EW and Mittag T, Biochemistry 2018, 57, 2478–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].a) Simon JR, Carroll NJ, Rubinstein M, Chilkoti A and Lopez GP, Nat Chem 2017, 9, 509–515; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Feric M, Vaidya N, Harmon TS, Mitrea DM, Zhu L, Richardson TM, Kriwacki RW, Pappu RV and Brangwynne CP, Cell 2016, 165, 1686–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Banerjee PR, Milin AN, Moosa MM, Onuchic PL and Deniz AA, Angewandte Chemie-International Edition 2017, 56, 11354–11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].a) Nott TJ, Petsalaki E, Farber P, Jervis D, Fussner E, Plochowietz A, Craggs TD, Bazett-Jones DP, Pawson T, Forman-Kay JD and Baldwin AJ, Mol Cell 2015, 57, 936–947; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Nott TJ, Craggs TD and Baldwin AJ, Nat Chem 2016, 8, 569–575. [DOI] [PubMed] [Google Scholar]
- [21].Lin YH, Forman-Kay JD and Chan HS, Phys Rev Lett 2016, 117, 178101. [DOI] [PubMed] [Google Scholar]
- [22].Brady JP, Farber PJ, Sekhar A, Lin YH, Huang R, Bah A, Nott TJ, Chan HS, Baldwin AJ, Forman-Kay JD and Kay LE, Proc Natl Acad Sci U S A 2017, 114, E8194–E8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. https://creativecommons.org/licenses/by/4.0/.
- [24].Lin YH, Forman-Kay JD and Chan HS, Physical Review Letters 2016, 117, 178101. [DOI] [PubMed] [Google Scholar]
- [25].Lin YH, Brady JP, Forman-Kay JD and Chan HS, New Journal of Physics 2017, 19, Article 115003. [Google Scholar]
- [26].Banani SF, Lee HO, Hyman AA and Rosen MK, Nat Rev Mol Cell Biol 2017, 18, 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Brangwynne CP, Mitchison TJ and Hyman AA, Proc Natl Acad Sci U S A 2011, 108, 4334–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lindstrom MS, Jurada D, Bursac S, Orsolic I, Bartek J and Volarevic S, Oncogene 2018, 37, 2351–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Grisendi S, Mecucci C, Falini B and Pandolfi PP, Nat Rev Cancer 2006, 6, 493–505. [DOI] [PubMed] [Google Scholar]
- [30].a) Mitrea DM, Cika JA, Stanley CB, Nourse A, Onuchic PL, Banerjee PR, Phillips AH, Park CG, Deniz AA and Kriwacki RW, Nat Commun 2018, 9, 842; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Mitrea DM, Cika JA, Guy CS, Ban D, Banerjee PR, Stanley CB, Nourse A, Deniz AA and Kriwacki RW, Elife 2016, 5, 13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].a) Mitrea DM, Grace CR, Buljan M, Yun MK, Pytel NJ, Satumba J, Nourse A, Park CG, Madan Babu M, White SW and Kriwacki RW, Proc Natl Acad Sci U S A 2014, 111, 4466–4471; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Banerjee PR, Mitrea DM, Kriwacki RW and Deniz AA, Angew Chem Int Ed Engl 2016, 55, 1675–1679; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Mitrea DM, Cika JA, Guy CS, Ban D, Banerjee PR, Stanley CB, Nourse A, Deniz AA and Kriwacki RW, Elife 2016, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Meyer MT, Genkov T, Skepper JN, Jouhet J, Mitchell MC, Spreitzer RJ and Griffiths H, Proceedings of the National Academy of Sciences of the United States of America 2012, 109, 19474–19479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].a) Holdsworth RH, J Cell Biol 1968, 37, 831–837; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Freeman Rosenzweig ES, Xu B, Kuhn Cuellar L, Martinez-Sanchez A, Schaffer M, Strauss M, Cartwright HN, Ronceray P, Plitzko JM, Forster F, Wingreen NS, Engel BD, Mackinder LCM and Jonikas MC, Cell 2017, 171, 148–162 e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mackinder LC, Meyer MT, Mettler-Altmann T, Chen VK, Mitchell MC, Caspari O, Freeman Rosenzweig ES, Pallesen L, Reeves G, Itakura A, Roth R, Sommer F, Geimer S, Muhlhaus T, Schroda M, Goodenough U, Stitt M, Griffiths H and Jonikas MC, Proc Natl Acad Sci U S A 2016, 113, 5958–5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].a) Copp SM, Schultz D, Swasey S, Pavlovich J, Debord M, Chiu A, Olsson K and Gwinn E, J Phys Chem Lett 2014, 5, 959–963; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wagner J and Zandi R, Biophys J 2015, 109, 956–965; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Mayer MG, in Nobel Lecture - The Shell Model. URL: https://www.nobelprize.org/prizes/physics/1963/mayer/lecture/. [Google Scholar]
- [36].Berry J, Brangwynne CP and Haataja M, Rep Prog Phys 2018, 81, 046601. [DOI] [PubMed] [Google Scholar]
- [37].a) Smith J, Calidas D, Schmidt H, Lu T, Rasoloson D and Seydoux G, Elife 2016, 5, 21337; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wang JT, Smith J, Chen BC, Schmidt H, Rasoloson D, Paix, Lambrus BG, Calidas D, Betzig E and Seydoux G, Elife 2014, 3, e04591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wu Y, Han B, Li Y, Munro E, Odde DJ and Griffin EE, Proc Natl Acad Sci U S A 2018, 115, E8440–E8449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wurtz JD and Lee CF, Phys Rev Lett 2018, 120, 078102. [DOI] [PubMed] [Google Scholar]
- [40].Zwicker D, Hyman AA and Julicher F, Phys Rev E Stat Nonlin Soft Matter Phys 2015, 92, 012317. [DOI] [PubMed] [Google Scholar]
- [41].Zwicker D, Decker M, Jaensch S, Hyman AA and Julicher F, Proc Natl Acad Sci U S A 2014, 111, E2636–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].a) Burke KA, Janke AM, Rhine CL and Fawzi NL, Mol Cell 2015, 60, 231–241; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Conicella AE, Zerze GH, Mittal J and Fawzi NL, Structure 2016, 24, 1537–1549; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Lee KH, Zhang P, Kim HJ, Mitrea DM, Sarkar M, Freibaum BD, Cika J, Coughlin M, Messing J, Molliex A, Maxwell BA, Kim NC, Temirov J, Moore J, Kolaitis RM, Shaw TI, Bai B, Peng J, Kriwacki RW and Taylor JP, Cell 2016, 167, 774–788 e717; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Li P, Banjade S, Cheng HC, Kim S, Chen B, Guo L, Llaguno M, Hollingsworth JV, King DS, Banani SF, Russo PS, Jiang QX, Nixon BT and Rosen MK, Nature 2012, 483, 336–340; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Zhang H, Elbaum-Garfinkle S, Langdon EM, Taylor N, Occhipinti P, Bridges AA, Brangwynne CP and Gladfelter AS, Mol Cell 2015, 60, 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Patel A, Malinovska L, Saha S, Wang J, Alberti S, Krishnan Y and Hyman AA, Science 2017, 356, 753–756. [DOI] [PubMed] [Google Scholar]
- [44].Choi KJ, Tsoi PS, Moosa MM, Paulucci-Holthauzen A, Liao SJ, Ferreon JC and Ferreon ACM, Biochemistry 2018, doi: 10.1021/acs.biochem.1028b01051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].a) Franzmann TM, Jahnel M, Pozniakovsky A, Mahamid J, Holehouse AS, Nuske E, Richter D, Baumeister W, Grill SW, Pappu RV, Hyman AA and Alberti S, Science 2018, 359, eaao5654; [DOI] [PubMed] [Google Scholar]; b) Sheu-Gruttadauria J and MacRae IJ, Cell 2018, 173, 946–957.e916; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Bergeron-Sandoval, et and al, bioRxiv 2017, doi: 10.1101/145664; [DOI] [Google Scholar]; d) Dine E, Gil AA, Uribe G, Brangwynne CP and Toettcher JE, Cell Syst 2018, 6, 655–663 e655; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Larson AG and Narlikar GJ, Biochemistry 2018, 57, 2540–2548; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Janssen A, Colmenares SU and Karpen GH, Annu Rev Cell Dev Biol 2018, 34, 265–288. [DOI] [PubMed] [Google Scholar]
- [46].Larson AG, Elnatan D, Keenen MM, Trnka MJ, Johnston JB, Burlingame AL, Agard DA, Redding S and Narlikar GJ, Nature 2017, 547, 236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hnisz D, Shrinivas K, Young RA, Chakraborty AK and Sharp PA, Cell 2017, 169, 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].a) Engreitz JM, Haines JE, Perez EM, Munson G, Chen J, Kane M, McDonel PE, Guttman M and Lander ES, Nature 2016, 539, 452–455; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lai F, Orom UA, Cesaroni M, Beringer M, Taatjes DJ, Blobel GA and Shiekhattar R, Nature 2013, 494, 497–501; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Pefanis E, Wang J, Rothschild G, Lim J, Kazadi D, Sun J, Federation A, Chao J, Elliott O, Liu ZP, Economides AN, Bradner JE, Rabadan R and Basu U, Cell 2015, 161, 774–789; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Sigova AA, Abraham BJ, Ji X, Molinie B, Hannett NM, Guo YE, Jangi M, Giallourakis CC, Sharp PA and Young RA, Science 2015, 350, 978–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].a) Sabari BR, Dall’Agnese A, Boija A, Klein IA, Coffey EL, Shrinivas K, Abraham BJ, Hannett NM, Zamudio AV, Manteiga JC, Li CH, Guo YE, Day DS, Schuijers, Vasile E, Malik S, Hnisz D, Lee TI, Cisse II, Roeder RG, Sharp PA, Chakraborty AK and Young RA, Science 2018, 361, eaar3958; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Chong S, Dugast-Darzacq C, Liu Z, Dong P, Dailey GM, Cattoglio C, Heckert A, Banala S, Lavis L, Darzacq X and Tjian R, Science 2018, 361, eaar2555; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Cho WK, Spille JH, Hecht M, Lee C, Li C, Grube V and Cisse II, Science 2018, 361, 412–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wang J, Choi JM, Holehouse AS, Lee HO, Zhang X, Jahnel M, Maharana S, Lemaitre R, Pozniakovsky A, Drechsel D, Poser I, Pappu RV, Alberti S and Hyman AA, Cell 2018, 174, 688–699 e616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].a) Deniz AA, Mukhopadhyay S and Lemke EA, J R Soc Interface 2008, 5, 15–45; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Mitrea DM, Chandra B, Ferrolino MC, Gibbs EB, Tolbert M, White MR and Kriwacki RW, J Mol Biol 2018, 430, 4773–4805; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Wei MT, Elbaum-Garfinkle S, Holehouse AS, Chen CC, Feric M, Arnold CB, Priestley RD, Pappu RV and Brangwynne CP, Nat Chem 2017, 9, 1118–1125; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Khan T, Kandola TS, Wu J, Venkatesan S, Ketter E, Lange JJ, Rodriguez Gama A, Box A, Unruh JR, Cook M and Halfmann R, Mol Cell 2018, 71, 155–168 e157; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Konig I, Zarrine-Afsar A, Aznauryan M, Soranno A, Wunderlich B, Dingfelder F, Stuber JC, Pluckthun A, Nettels D and Schuler B, Nat Methods 2015, 12, 773–779. [DOI] [PubMed] [Google Scholar]
- [52].Shin Y, Berry J, Pannucci N, Haataja MP, Toettcher JE and Brangwynne CP, Cell 2017, 168, 159–171 e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].a) Kumar KV, Bois JS, Julicher F and Grill SW, Physical Review Letters 2014, 112, 208101; [Google Scholar]; b) Soroldoni D, Jorg DJ, Morelli LG, Richmond DL, Schindelin J, Julicher F and Oates AC, Science 2014, 345, 222–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zwicker D, Seyboldt R, Weber CA, Hyman AA and Julicher F, Nature Physics 2017, 13, 408–413. [Google Scholar]
- [55].a) Lee CF, Soft Matter 2017, 13, 376–385; [DOI] [PubMed] [Google Scholar]; b) Digregorio P, Levis D, Suma A, Cugliandolo LF, Gonnella G and Pagonabarraga I, Phys Rev Lett 2018, 121, 098003. [DOI] [PubMed] [Google Scholar]
- [56].Li XH, Chavali PL, Pancsa R, Chavali S and Babu MM, Biochemistry 2018, 57, 2452–2461. [DOI] [PubMed] [Google Scholar]
- [57].Molliex A, Temirov J, Lee J, Coughlin M, Kanagaraj AP, Kim HJ, Mittag T and Taylor JP, Cell 2015, 163, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Naumann M, Pal A, Goswami A, Lojewski X, Japtok J, Vehlow A, Naujock M, Gunther R, Jin M, Stanslowsky N, Reinhardt P, Sterneckert J, Frickenhaus M, Pan-Montojo F, Storkebaum E, Poser I, Freischmidt A, Weishaupt JH, Holzmann K, Troost D, Ludolph AC, Boeckers TM, Liebau S, Petri S, Cordes N, Hyman AA, Wegner F, Grill SW, Weis J, Storch A and Hermann A, Nat Commun 2018, 9, 335. [DOI] [PMC free article] [PubMed] [Google Scholar]