Abstract
Previous studies have shown that mild traumatic brain injury (mTBI) can cause abnormalities in clinically relevant magnetic resonance imaging (MRI) sequences. No large-scale study, however, has prospectively assessed this in athletes with sport-related concussion (SRC). The aim of the current study was to characterize and compare the prevalence of acute, trauma-related MRI findings and clinically significant, non-specific MRI findings in athletes with and without SRC. College and high-school athletes were prospectively enrolled and participated in scanning sessions between January 2015 through August 2017. Concussed contact sport athletes (n = 138; 14 female [F]; 19.5 ± 1.6 years) completed up to four scanning sessions after SRC. Non-concussed contact (n = 135; 15 F; 19.7 ± 1.6) and non-contact athletes (n = 96; 15 F; 20.0 ± 1.7) completed similar scanning sessions and served as controls. Board-certified neuroradiologists, blinded to SRC status, reviewed T1-weighted and T2-weighted fluid-attenuated inversion recovery and T2*-weighted and T2-weighted images for acute (i.e., injury-related) or non-acute findings that prompted recommendation for clinical follow-up. Concussed athletes were more likely to have MRI findings relative to contact (30.4% vs. 15.6%; odds ratio [OR] = 2.32; p = 0.01) and non-contact control athletes (19.8%; OR = 2.11; p = 0.04). Female athletes were more likely to have MRI findings than males (43.2% vs. 19.4%; OR = 2.62; p = 0.01). One athlete with SRC had an acute, injury-related finding; group differences were largely driven by increased rate of non-specific white matter hyperintensities in concussed athletes. This prospective, large-scale study demonstrates that <1% of SRCs are associated with acute injury findings on qualitative structural MRI, providing empirical support for clinical guidelines that do not recommend use of MRI after SRC.
Keywords: concussion, MRI, mTBI, sport, white matter hyperintensity
Introduction
Sport-related concussion (SRC) is thought to reflect a functional injury without distinct structural abnormalities on standard imaging.1 Clinically, acute imaging is generally only acquired to rule out more-severe brain injury or neurosurgical emergencies (e.g., traumatic hemorrhage, etc.), typically with computed tomography (CT) as the preferred modality.2 However, the robust sensitivity and increased utilization of magnetic resonance imaging (MRI) in research settings have resulted in frequent discovery of structural findings in both clinical and healthy populations.3, 4 In some studies, a sizable percentage of mild traumatic brain injury (mTBI) patients with normal CT are found to have significant traumatic abnormalities on more-sensitive brain MRI.5
For all diagnostic studies, the complement of sensitivity is specificity (e.g., the likelihood that a finding is specific to the suspected medical condition under interrogation). Specificity can be influenced by several factors, including frequency of incidental and non-specific findings. Studies in healthy cohorts have reported varying rates of MRI findings that are unrelated to the intended purpose of the exam, but are nonetheless of potential clinical significance (i.e., incidental findings).6,7 Some commonly encountered structural MRI findings, such as white matter hyperintensities (WMHs), have a non-specific etiology, with trauma being one of many possible causes. Other imaging abnormalities, such as hemorrhagic or non-hemorrhagic contusions, are more confidently associated with brain trauma and included in the National Institute of Neurological Disorders and Stroke Common Data Elements (CDE) in radiological imaging of TBI.2,8
Previous studies have characterized the prevalence of abnormal MRI findings in mTBI cohorts.5,9,10 However, few studies have characterized structural MRI findings in patients with SRC, and thus the typical rate of abnormal structural MRI findings associated with concussion or even exposure to repetitive head impacts without concussion is unknown. A retrospective study in children referred to a concussion clinic after SRC found that 79% of CT cases and 75% of MRI cases were negative.11 Moreover, in the positive MRI cases, findings were typically considered non-specific or incidental to the injury, such as arachnoid cysts and non-specific white matter changes. Similarly, of 427 pediatric patients with persistent symptoms after SRC, evidence of trauma-related injury (i.e., microhemorrhage) on MRI was evident in only 2 patients.12 A separate study in youth with post-concussion syndrome reported that 6 of 19 patients had MRI findings, with only 1 thought to be relevant to the current injury (i.e., not incidental).13 Bigler and colleagues observed neuroradiological findings in approximately 10% of cases from a mixed pediatric emergency department sample of patients with mTBI or orthopedic injury (37% SRC), many of which were non-specific WMH.9 Large-scale, prospective studies are needed to determine the typical rate of structural MRI abnormalities in SRC and contact sport populations.
The aim of the current study was to characterize and compare the prevalence of acute, trauma-related MRI findings and clinically significant, non-specific MRI findings in athletes with and without SRC. We combined data from two large-scale, prospective studies investigating the acute effects and recovery of SRC. Concussed contact sport athletes (CS-SRC) were compared to non-concussed control athletes from both contact (CS-Control) and non-contact sports (NCS-Control) across both studies to allow the differentiation of the acute effects of concussion from the effects of repetitive head impacts associated with contact sport participation.
Methods
Participants
Athletes were enrolled across two large, prospective studies assessing the acute and subacute effects of SRC from January 2015 through August 2017. The National Collegiate Athletic Association/Department of Defense (NCAA-DoD) Concussion Assessment, Research and Education (CARE) Consortium is the largest and most comprehensive study of acute concussion and recovery in NCAA student-athletes and U.S. Military Service Academy cadets. Project Head to Head II (H2H2) is a large-scale study of acute concussion in high school and college athletes in southeastern Wisconsin, with a protocol that parallels CARE. Adult participants and parents of minors provided written informed consent. Minors completed written assent. All aspects of each study were approved by a local institutional review board and the DoD Human Research Protections Office. A total of 138 concussed contact sport athletes, 135 control contact sport athletes, and 96 control non-contact sport athletes participated in this study (Table 1). Data from 35 of these athletes were previously included in a study of MRI metric stability,14 whereas data from 58 were included in a study of acute white matter abnormalities after SRC using diffusion MRI.15
Table 1.
Sample Characteristics
| Variable | Value | CS-SRC | CS-Control | NCS-Control | p value |
|---|---|---|---|---|---|
| Total participants | 138 | 135 | 96 | ||
| H2H2: total subjects | 62 | 64 | 28 | ||
| H2H2: subjects at each visit | V1/V2/V3/V4 | 62/59/53/50 | 64/58/54/46 | 26/25/24/22 | |
| CARE: total subjects | 76 | 71 | 68 | ||
| CARE: subjects at each visit | V1/V2/V3/V4 | 57/62/51/38 | 69/70/65/47 | 64/65/63/34 | |
| MRI protocol | GE protocol 1 (32-channel) | 62 | 64 | 28 | |
| GE protocol 2 (32-channel) | 20 | 18 | 18 | ||
| Siemens protocol 1 (8-channel) | 6 | 6 | 7 | ||
| Siemens protocol 1 (12-channel)a | 12 | 8 | 3 | ||
| Siemens protocol 1 (32-channel) | 17 | 15 | 11 | ||
| Siemens protocol 2 (32-channel) | 21 | 24 | 29 | ||
| Sex (%) | Female | 14 (10.1) | 15 (11.1) | 15 (15.6) | 0.4163 |
| Race (%) | African American | 40 (29.0) | 34 (25.2) | 10 (10.4) | 0.014 |
| White | 90 (65.2) | 92 (68.1) | 76 (79.2) | ||
| All other | 8 (5.8) | 9 (6.7) | 10 (10.4) | ||
| Ethnicity (%) | Hispanic or Latino | 7 (5.1) | 9 (6.7) | 7 (7.3) | 0.761 |
| Sport (%) | Baseball | 0 (0.0) | 0 (0.0) | 37 (38.5) | |
| Basketball | 0 (0.0) | 0 (0.0) | 17 (17.7) | ||
| Cross country/track | 0 (0.0) | 0 (0.0) | 36 (37.5) | ||
| Football | 105 (76.1) | 105 (77.8) | 0 (0.0) | ||
| Ice hockey | 7 (5.1) | 6 (4.4) | 0 (0.0) | ||
| Lacrosse | 7 (5.1) | 5 (3.7) | 0 (0.0) | ||
| Soccer | 19 (13.8) | 19 (14.1) | 0 (0.0) | ||
| Softball | 0 (0.0) | 0 (0.0) | 6 (6.3) | ||
| Competition level (%) | High school | 15 (10.9) | 15 (11.1) | 12 (12.5) | 0.0185 |
| Division III | 47 (34.1) | 49 (36.3) | 16 (16.7) | ||
| Division I | 76 (55.1) | 71 (52.6) | 68 (70.8) | ||
| Medical history (%) | Any | 47 (34.1) | 27 (20.0) | 16 (16.7) | 0.0032 |
| Visual problems | 3 (2.2) | 4 (3.0) | 1 (1.0) | ||
| Memory problems | 5 (3.6) | 4 (3.0) | 0 (0.0) | ||
| Migraines | 12 (8.7) | 7 (5.2) | 3 (3.1) | ||
| Headaches (non-migraine) | 7 (5.1) | 3 (2.2) | 0 (0.0) | ||
| Learning disorder | 3 (2.2) | 1 (0.7) | 1 (1.0) | ||
| Psychiatric disorder | 3 (2.2) | 2 (1.5) | 6 (6.3) | ||
| Sleep disorder | 2 (1.4) | 0 (0.0) | 1 (1.0) | ||
| Balance disorder | 1 (0.7) | 0 (0.0) | 0 (0.0) | ||
| Diabetes | 1 (0.7) | 0 (0.0) | 0 (0.0) | ||
| ADHD | 19 (13.8) | 10 (7.4) | 6 (6.3) | ||
| Seizure | 1 (0.7) | 0 (0.0) | 1 (1.0) | ||
| TIA | 1 (0.7) | 0 (0.0) | 0 (0.0) | ||
| Hearing problems | 3 (2.2) | 2 (1.5) | 0 (0.0) | ||
| Meningitis | 1 (0.7) | 1 (0.7) | 0 (0.0) | ||
| Years in primary sport | 9.3 (3.8) | 9.5(3.5) | 10.9 (3.8) | 0.0037 | |
| No. of previous concussions | 0.9 (1.2) | 0.6 (0.9) | 0.1 (0.4) | <0.0001 | |
| Age at first scan | 19.5 (1.6) | 19.7 (1.6) | 20.0 (1.7) | 0.1271 | |
| Years of education | 13.3 (1.3) | 13.4 (1.3) | 13.9 (1.6) | 0.006 | |
| SES | 51.2 (10.6) | 48.2 (11.5) | 50.5 (10.5) | 0.0765 | |
| BMI | 28.1 (5.3) | 27.8 (4.9) | 23.4 (3.0) | <0.0001 |
Three concussed athletes used 12-channel TRIO on all time points except for 6-month visit, where 32-channel was used; 1 concussed athlete and 1 contact sport control initially used 12-channel, but used 32-channel at later visits.
CS-SRC, contact sport with sport-related concussion; CS-Control, contact sport control; NCS-Control, non-contact sport control; H2H2, Project Head to Head II; CARE, Concussion Assessment, Research and Education Consortium; V1, visit 1; V2, visit 2; V3, visit 3; V4, visit 4; GE, General Electric; ADHD, attention deficit hyperactivity disorder; TIA, transient ischemic attack; SES, socioeconomic status; BMI, body mass index.
Participants: Project Head to Head-II
Exclusion criteria for H2H2 included injury that would preclude study participation; current psychotic disorder or narcotic use; history or suspicion of conditions known to cause cognitive dysfunction such as epilepsy or moderate-to-severe TBI; and any contraindication to study procedures. Football players participating in H2H2 were enrolled at pre-season (baseline). Those sustaining a concussion (CS-SRC) completed neuroimaging sessions at 24–48 h, 8 days, 15 days, and 45 days post-concussion. Control groups completed the same imaging studies and clinical outcome assessments at similar time points. The CS-Control group included healthy football players matched on level (high school vs. college), school, team, age, estimated pre-morbid intelligence, race, handedness, concussion history, and position. The NCS-Control group consisted of non-contact sport athletes without current or high school football exposure matched on level (high school or college), age, estimated pre-morbid intelligence, and race.
H2H2 definition of concussion was based on the HEADS UP educational initiative from the Centers for Disease Control and Prevention: an injury resulting from a forceful bump, blow, or jolt to the head that results in rapid movement of the head and causes a change in the athlete's behavior, thinking, physical functioning, or the following symptoms: headache, nausea, vomiting, dizziness/balance problems, fatigue, difficulty sleeping, drowsiness, sensitivity to light/noise, blurred vision, memory difficulty, and difficulty concentrating. Certified athletic trainers and/or team physicians at each institution identified and diagnosed concussion. Study investigators then screened and triaged injuries to confirm that they met study definition and requirements.
Participants: The National Collegiate Athletic Association/Department of Defense Concussion Assessment, Research and Education Consortium
The study design and methods of NCAA-DoD CARE Consortium have been previously detailed.16 The current work focuses on athletes enrolled in the CARE Advanced Research Core from sites in which the neuroimaging protocol is deployed. Athletes were enrolled at pre-season. Contact sport athletes sustaining a concussion (CS-SRC) participated in multiple follow-up visits. Neuroimaging data were collected at the following visits: within 24–48 h post-injury, after clearance to begin the return-to-play progression (i.e., when asymptomatic), 7 days after unrestricted return to play, and 6 months post-injury. Non-injured athletes from contact sports (e.g., football, hockey; CS-Controls) and non-contact sports (e.g., baseball, softball; NCS-Control) were matched on institution, sport (CS-Controls), sex, race/ethnicity, estimate of pre-morbid intelligence, concussion history (CS-Controls), years of participation (CS-Controls), status as a starter (CS-Controls), and head impact exposure estimate or data, if available (CS-Controls), and participated in similar time points individually matched to the schedule of matched injured athletes. NCS-Control athletes from CARE were not excluded if they had previous contact sport exposure (e.g., in high school or college).
Concussions were diagnosed by research and medical staff of each participating institution based on the following definition: “A change in brain function following a force to the head, which may be accompanied by temporary loss of consciousness, but is identified in awake individuals with measures of neurologic and cognitive dysfunction.”17
Data collection protocols for clinical and demographic information were matched between studies. Detailed information regarding demographics, health history, and injury history were obtained upon enrollment and subsequent follow-up visits for both studies. Socioeconomic status (SES) was estimated using the modified Hollingshead four-factor index.18 Injury characteristics were also collected, including presence of loss of consciousness (LOC), post-traumatic amnesia (PTA), and retrograde amnesia (RGA).
Imaging parameters
Anatomical images were acquired on 3 Tesla GE MR750 (GE Healthcare, Little Chalfont, UK), Siemens Tim Trio, or Siemens Prisma scanners (Siemens Healthcare, Erlangen, Germany). Clinical interpretations were performed on T1-weighted images, T2-weighted fluid-attenuated inversion recovery (FLAIR) images, multi-echo gradient recalled echo sequences to yield T2*-weighted images and subsequent susceptibility-weighted images, and T2-weighted images (if available). Specific scan parameters and the protocols deployed at various sites are listed in Table 2. Previous work has demonstrated the relative stability of MRI metrics across scanner sites and vendors.14
Table 2.
MRI Protocols and Parameters
| GE Protocol 1 (H2H2) | T1-weighted (MPRAGE) | T2-weigthed FLAIR | T2*-weighted | T2-weighted | GE Protocol 2 (CARE) | T1-weighted (BRAVO) | T2-weighted FLAIR | T2*-weighted | T2-weighted |
|---|---|---|---|---|---|---|---|---|---|
| Slices | 160 | 160 | 70 | 160 | Slices | 164 | 160 | 70 | 160 |
| Matrix | 256 × 256 | 256 × 256 | 384 × 240 | 256 × 256 | Matrix | 256 × 256 | 256 × 256 | 384 × 240 | 256 × 256 |
| TE (ms) | 3.008 | 69.82 | 13.53a | 64.26 | TE (ms) | 2.91 | 66.25 | 13.53a | 63.69 |
| TR (ms) | 7.592 | 6500 | 58.70 | 2500 | TR (ms) | 6.62 | 6500 | 58.70 | 2500 |
| TI (ms) | 900 | 1889 | NA | NA | TI (ms) | 450.0 | 1910 | NA | NA |
| FOV (mm) | 256 | 256 | 256 × 160 | 256 | FOV (mm) | 256 | 256 | 256 × 160 | 256 |
| Thickness (mm) | 1.0 | 1.0 | 3.0 | 1.0 | Thickness (mm) | 1.0 | 1.0 | 3.0 | 1.0 |
| Siemens Protocol 1 (TRIO) | T1-weighted (MPRAGE) | T2-weighted FLAIR | T2*-weighted | T2-weighted | Siemens Protocol 2 (PRISMA) | T1-weighted (MPRAGE) | T2-weighted FLAIR | T2*-weighted | T2-weighted |
|---|---|---|---|---|---|---|---|---|---|
| Slices | 176 | 80 | 56 | NA | Slices | 176 | 176 | 56 | NA |
| Matrix | 256 × 256 | 256 × 256 | 384 × 240 | NA | Matrix | 256 × 256 | 256 × 256 | 384 × 240 | NA |
| TE (ms) | 2.98 | 390.00 | 10.00a | NA | TE (ms) | 2.98 | 390.00 | 10.00a | NA |
| TR (ms) | 2300.0 | 5000.0 | 45.0 | NA | TR (ms) | 2300.0 | 5000.0 | 45.0 | NA |
| TI (ms) | 900 | 1800 | NA | NA | TI (ms) | 900 | 1800 | NA | NA |
| FOV (mm) | 256 | 256 | 256 × 160 | NA | FOV (mm) | 256 | 256 | 256 × 160 | NA |
| Thickness (mm) | 1.0 | 2.0 | 3.0 | NA | Thickness | 1.0 | 1.0 | 3.0 | NA |
T2*-weighted sequences acquired multiple echoes; the first echo is indicated.
H2H2, Project Head to Head II; CARE, Concussion Assessment, Research and Education Consortium; FLAIR, fluid-attenuated inversion recovery; MPRAGE, 3D magnetization prepared rapid gradient echo; BRAVO, 3D BRAin Volume; ms, milliseconds; mm, millimeters; TE, echo time; TR, repetition time; TI = inversion time; NA, not applicable.
Initial magnetic resonance imaging review protocol
Board-certified neuroradiologists conducted non-diagnostic clinical reads after every imaging session (A.K., J.U., and V.M.). Initial reviews of structural MRI scans were blinded to presence/absence of injury. Reviews of subsequent scans in participants with multiple sessions were not blinded to initial results to track progression of potential pathology. For the purposes of this study, an MRI abnormality was operationalized as any acute (i.e., injury related) or non-acute finding that prompted a recommendation for clinical follow-up.
Statistical analysis
Statistical analyses were conducted using SPSS software (version 21; SPSS, Inc., Chicago, IL). Group differences in demographic data were assessed using independent-samples t-tests, chi-square tests, or Kruskal-Wallis tests. Seven participants were injured after participating in the study as a CS-Control and subsequently enrolled as CS-SRC; these visits are treated as independent samples. The primary outcome measure of interest was the presence of an MRI finding that prompted recommendation for clinical follow-up at any of the available visits (i.e., yes or no). Ratings across multiple scans were collapsed into a single measure to improve confidence in MRI finding (i.e., utilize strength of repeated scanning sessions). The only MRI finding to show progression or resolution in any participant across multiple scans was sinus abnormalities (1 CS-SRC, 1 CS-Control, and 1 NCS-Control). Single predictor logistic regression analyses were used to determine the relationship between demographic variables and presence of MRI findings. Finally, multiple predictor logistic regression models were used to assess for group differences (i.e., CS-SRC, CS-Control, and NCS-Control) while accounting for variance explained by predictors that were significantly associated with MRI findings.
Results
Across the two study protocols, imaging data were available on 369 athletes. A total of 138 concussed athletes participated in at least one post-injury follow-up neuroimaging session. A total of 135 CS-Control and 96 NCS-Control athletes participated in similar follow-up visits and served as controls. Five (3.7%) of the 138 concussed athletes reported LOC, 19 (14%) reported PTA, and 10 (7.4%) reported RGA. Demographic comparisons are shown in Table S1. Positions for contact sport athletes are presented in Supplementary Table S1.
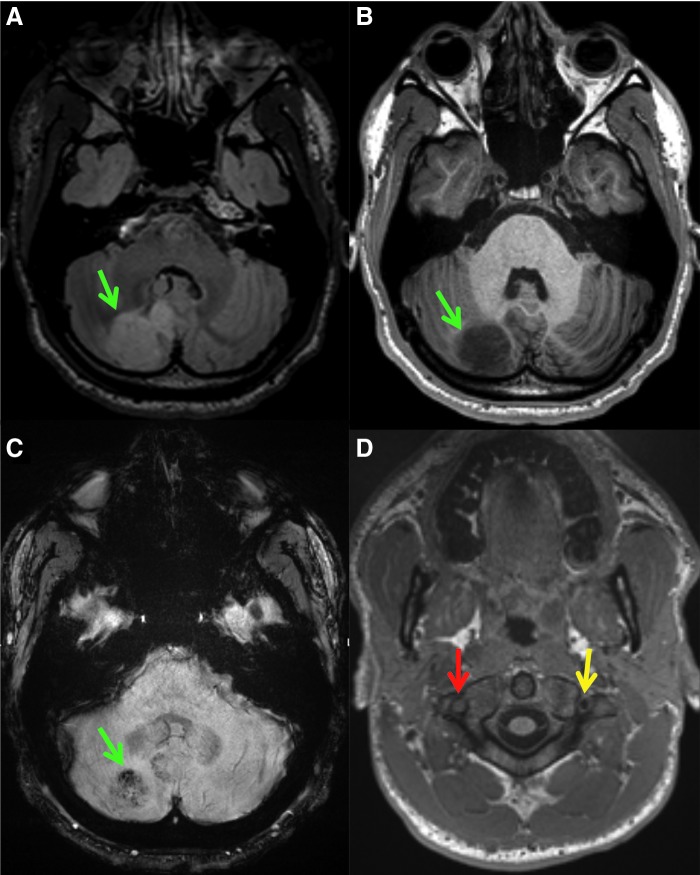
The percentage of athletes in each group with MRI findings requiring clinical follow-up is presented in Table 3. The most common potentially clinically significant finding across all groups was the presence of non-specific WMHs (Fig. 1). Of the 138 athletes with concussion, there was only 1 case of an acute finding on MRI that was attributed to the incident injury (discussed below). A 19-year-old male college football player demonstrated an acute finding on MRI after injury to the head and neck and was diagnosed with concussion. Initial post-injury MRI revealed a right vertebral artery dissection and concomitant right cerebellar infarct (Fig. 2).
Table 3.
Number and Percentage of Athletes With Positive MRI Findings
| CS-SRC (%) | CS-Control (%) | NCS-Control (%) | |
|---|---|---|---|
| Any finding | 42 (30.4) | 21 (15.6) | 19 (19.8) |
| White matter hyperintensity | 19 (13.8) | 9 (6.7) | 6 (6.3) |
| Any non-white-matter hyperintensity | 24 (17.4) | 14 (10.4) | 15 (15.6) |
| Pituitary abnormality | 4 (2.9) | 6 (4.4) | 5 (5.2) |
| Brain, head, or neck mass | 7 (5.1) | 2 (1.5) | 1 (1.0) |
| Sinus abnormality | 4 (2.9) | 1 (0.7) | 2 (2.1) |
| Chiari I malformation | 1 (0.7) | 1 (0.7) | 3 (3.1) |
| Chronic microhemorrhage or vascular malformation (e.g., cavernoma or capillary telangiectasia) | 3 (2.2) | 1 (0.7) | 1 (1.0) |
| Pineal cyst | 1 (0.7) | 2 (1.5) | 0 (0.0) |
| Heterotopia | 2 (1.4) | 0 (0.0) | 1 (1.0) |
| Brain infarct | 1 (0.7) | 0 (0.0) | 1 (1.0) |
| Cervical adenopathy | 1 (0.7) | 0 (0.0) | 0 (0.0) |
| Cervical cord Syrinx | 1 (0.7) | 0 (0.0) | 0 (0.0) |
| Geographical white matter abnormality | 0 (0.0) | 1 (0.7) | 0 (0.0) |
| Hydrocephalus | 1 (0.7) | 0 (0.0) | 0 (0.0) |
| Possible aneurysm | 0 (0.0) | 0 (0.0) | 1 (1.0) |
| Possible inflammatory cervical spine arthritis | 0 (0.0) | 0 (0.0) | 1 (1.0) |
| Cervical spinal canal stenosis | 0 (0.0) | 1 (0.7) | 0 (0.0) |
Note that athletes can have more than one finding. Brain, head, or neck masses include brain neoplasm/dysplasia, scalp or skull lesion/malformation, and juvenile nasopharyngeal angiofibroma (JNA).
MRI, magnetic resonance imaging; CS-SRC, contact sport with sport-related concussion; CS-Control, contact sport control; NCS-Control, non-contact sport control.
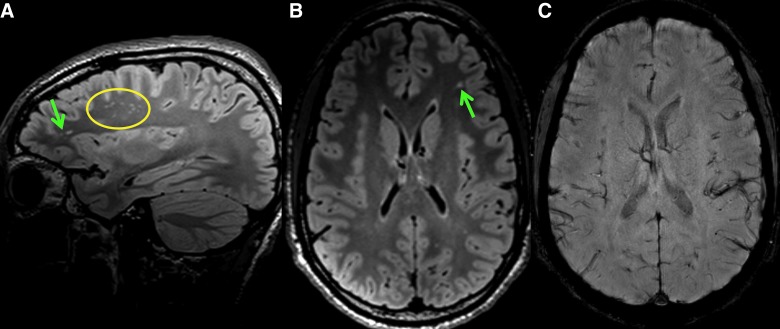
FIG. 1.
Sagittal FLAIR (A), axial FLAIR (B), and axial susceptibility-weighted (C) images of a non-concussed soccer player demonstrate numerous FLAIR hyperintense white matter lesions (green arrows and yellow circle), more than expected for the subject's age. No susceptibility is present to suggest associated blood products. These white matter lesions are non-specific and have been associated with various medical conditions, as well as increasing age. FLAIR, fluid-attenuated inversion recovery. Color image is available online.
FIG. 2.
Axial FLAIR (A), axial T1-weighted (B and D), and axial susceptibility-weighted (C) images demonstrate a rounded area of abnormal signal and susceptibility within the right cerebellum (green arrows) of a concussed football player. At the C1–C2 level, there is loss of the normal right vertebral artery flow void (red arrow), suggestive of slow flow or occlusion. There is a normal left vertebral artery flow void (yellow arrow). The subject sustained a traumatic right vertebral artery dissection and resultant right cerebellar infarct with internal petechial hemorrhage. FLAIR, fluid-attenuated inversion recovery. Color image is available online.
Single predictor logistic regression analyses were performed to determine the association of demographic variables on the incidence of potentially clinically significant MRI findings. As seen in Table 4, group (CS-SRC), female sex, higher SES, years of participation in primary sport, and competition level (division I) were significantly associated with a higher incidence of MRI findings that prompted a recommendation for follow-up using single predictor models. These variables were carried forward into a multiple predictor model. The multiple variable model for MRI findings was significant (χ2(7) = 27.66; p < 0.001; Nagelkerke R2 = 0.12), with group and sex being significant predictors (Table 4). Specifically, female athletes were more likely to have MRI findings relative to males (odds ratio [OR] = 2.62), and CS-SRC athletes were more likely to have MRI findings relative to CS-Control athletes (OR = 2.32) and NCS-Control athletes (OR = 2.11). There was no significant difference between CS-Control and NCS-Control athletes in frequency of MRI findings. SES, years of participation, and competition level did not significantly contribute to the model.
Table 4.
Association of Variables with Any Positive MRI Finding
| Single predictor model | Multiple predictor model | |||
|---|---|---|---|---|
| Variable | p value | Odds ratio [95% CI] | p value | Odds ratio [95% CI] |
| Group | 0.011 | NA | 0.018 | NA |
| Group (CS-SRC vs. CS-Control) | 0.004 | 2.37 [1.32–4.28] | 0.011 | 2.32 [1.22–4.41] |
| Group (CS-SRC vs. NCS-Control) | 0.07 | 1.77 [0.95–3.29] | 0.035 | 2.11 [1.06–4.20] |
| Group (CS-Control vs. NCS-Control) | 0.40 | 0.75 [0.38–1.48] | 0.80 | 0.91 [0.43–1.91] |
| Competition level | 0.017 | NA | 0.42 | NA |
| Competition level (D1 vs. HS) | 0.040 | 2.80 [1.05–7.46] | 0.23 | 1.93 [0.66–5.63] |
| Competition level (D1 vs. D3) | 0.023 | 1.98 [1.10–3.55] | 0.42 | 1.33 [0.67–2.65] |
| Competition level (D3 vs. HS) | 0.52 | 1.42 [0.49–4.10] | 0.52 | 1.45 [0.47–4.44] |
| Sex (female) | 0.001 | 3.16 [1.64–6.10] | 0.014 | 2.62 [1.21–5.67] |
| SES | 0.006 | 1.04 [1.01–1.07] | 0.15 | 1.02 [0.99–1.05] |
| Years in primary sport | 0.048 | 1.07 [1.00–1.14] | 0.37 | 1.04 [0.96–1.12] |
| Years education | 0.81 | 1.02 [0.86–1.22] | — | — |
| No. of past concussion | 0.82 | 0.97 [0.74–1.26] | — | — |
| Medical history (Y) | 0.24 | 1.39 [0.80–2.40] | — | — |
| Migraines (Y) | 0.27 | 1.69 [0.67–4.30] | — | — |
| Headache (Y) | 0.86 | 0.87 [0.18–4.19] | — | — |
| ADHD (Y) | 0.60 | 1.24 [0.56–2.76] | — | — |
| BMI | 0.072 | 0.95 [0.90–1.00] | — | — |
| Age at first scan | 0.69 | 1.03 [0.89–1.20] | — | — |
| Race | 0.50 | NA | — | — |
| Black/Af-Amer. versus white | 0.28 | 1.37 [0.77–2.43] | — | — |
| Black/Af-Amer. versus other | 0.98 | 1.01 [0.38–2.72] | — | — |
| Other versus white | 0.52 | 1.35 [0.54–3.37] | — | — |
| Ethnicity (Hispanic or Latino) | 0.65 | 1.25 [0.48–3.29] | — | — |
MRI, magnetic resonance imaging; CS-SRC, contact sport with sport-related concussion, CS-Control, contact sport control; NCS-Control, non-contact sport control; D1, division I; HS, high school; SES, socioeconomic status; y, yes; D3, division III; ADHD, attention deficit hyperactivity disorder; BMI, body mass index; Af-Amer., African-American; CI, confidence interval; NA, not applicable.
Discussion
To our knowledge, this is the first study to prospectively characterize the prevalence of acute, trauma-related MRI findings and potentially clinically significant, non-specific MRI findings in high school and collegiate athletes with and without concussion. Concussed contact sport athletes had more MRI findings that prompted recommendation for clinical follow-up than both non-concussed contact and non-contact sport controls. Of the 138 acutely concussed athletes with remarkable findings on MRI, however, only 1 case of an acute TBI-related finding was observed. Thus, the observed effect was driven by an overall higher rate of non-specific MRI findings in concussed contact sport athletes, such as WMH, rather than MRI findings that can be definitively attributed to the acute SRC (e.g., radiological CDE). This large, prospective study informs future work about the rate at which common MRI findings may be expected in SRC populations and how these findings compare to studies in civilian mTBI patients.
The findings from this SRC study have important implications. Foremost, these findings support the current clinical guidelines that MRI is not a standard diagnostic procedure after SRC.1 Only 1 athlete of 138 concussions (0.7%) demonstrated an obvious acute traumatic finding on MRI after concussion. Radiological CDE have been developed to characterize these findings that are more common in moderate and severe TBI (e.g., hemorrhages, contusions, and skull fracture).8 Further, the low rate of acute findings in the current sample is in contrast to data on mTBI patients recruited from trauma centers, where 28% of CT-negative patients had MRI findings attributable to injury.5 Our results suggest that these CDE are less applicable to the large majority of SRC cases and highlight potential differences in the incidence of CDE based on point of care.
Second, these findings provide the first prospective evidence suggesting that SRC is associated with a higher incidence of non-specific MRI findings requiring clinical follow-up relative to both contact and non-contact control athletes. Interestingly, this result was not driven by findings obviously associated with acute injury, but rather non-specific and non-trauma-related findings. For example, sinus disease, heterotopia, tumor, aneurysm, and pineal cyst are examples of non-trauma-related findings observed in the current sample. Other observed non-specific structural abnormalities, including WMH, chronic microhemorrhages, and pituitary abnormalities, have been previously associated with previous TBI.10 In the current study, individuals in the SRC group were 2.7–3.8 times more likely to have WMH than control groups. WMHs have been associated with moderate and severe TBI2 as well as chronic mTBI.10 WMHs, which are generally presumed to have a vascular origin, are a common finding in clinical and research populations19 with known associations with advancing age,6 migraines,20 and vascular risk factors, including hypertension, smoking, and diabetes.21 The WMHs observed in our study were presumed to be chronic and not related to the acute injury because of the absence of associated acute traumatic abnormalities such as microhemorrhage, contusion, and extra-axial hemorrhage. One concussed athlete did have multiple WMHs and a single FLAIR hypointensity with magnetic susceptibility abnormality (T2*-weighted) in the left frontal white matter that was interpreted during the blinded radiological read as chronic blood products. The distinction between acute and chronic incidence of WMH, however, cannot be made with absolute certainty. Therefore, it is possible that the increased rate of WMH in concussed athletes at least partially reflects acute injury. An alternative possibility is that WMHs represent a pre-disposing factor for concussion or are secondary to other risk factors for concussion not accounted for in the current study. Of note, a recent study showed that the presence of WMHs in active-duty service members with mTBI was associated with worse performance on cognitive testing.22 Additional work is ongoing to more systematically assess the number and volume of WMHs observed in the current study and their relationship with pre- and post-injury variables.
The current study enrolled both contact sport and non-contact sport athletes as controls to allow the differentiation of acute effects of SRC and the cumulative effects of contact sport exposure. The lack of difference between contact and non-contact controls suggests that contact-sport exposure is not associated with potentially clinically significant structural MRI abnormalities, despite growing concern of the effects of repetitive head impacts and concussion on long-term neurologic health in contact sport athletes. For example, recent work in retired, symptomatic professional football players has shown that younger age of first exposure to repetitive head impacts is associated with smaller thalamic volumes, altered white matter integrity, and cognitive impairments.23–25 In the current study, no effect of contact sport participation was observed even though contact sport controls had, on average, 9.5 years of exposure in their primary sport (e.g., football). The current work, however, did not include quantitative analyses of structural images, such as comparisons of cortical thickness or brain volume, which may be more-sensitive measures for assessing the effects of contact-sport exposure and repetitive head impacts. In addition, there are alternative ways to assess exposure, such as the use of head impact sensors. Future work is needed to determine the complex relationship between exposure, previous concussion, and the risk for structural abnormalities on MRI or whether these relationships may be present on other imaging modalities (e.g., diffusion MR).
Finally, current results have important implications for research. Clinical MRI reads can be accomplished with modest financial commitments,4 and the ethical considerations associated with incidental MRI findings for research studies have been extensively discussed.26 We identified urgent findings that resulted in swift clinical intervention, which demonstrates the importance of completing clinical over-reads for all research scans. From a methodological viewpoint, our results also highlight the importance of documenting MRI abnormalities attributed to their potential effects on advanced neuroimaging metrics. For example, the presence of WMH has been associated with differences in commonly used diffusion MR metrics.27
The current results should be interpreted within their limitations. First, the sex effect in the current study, with females having higher incidence of non-specific, clinically significant MRI findings than males, should be taken with caution given the relatively small number of women athletes included. However, other studies have reported similar findings. For example, greater incidence of WMH has been reported in women in a large study of older subjects.28 Second, there is inherent subjectivity and cross-reader variability in the determination of what constitutes a significant MRI finding that merits clinical follow-up. This is especially true on research scans that are not intended for clinical evaluation as well as with a blinded approach to the presence or absence of TBI and symptomatology, factors that are critical in exam interpretation in any clinical radiology setting. Additionally, the thinner image slices used for the current study relative to routine clinical protocols are likely to increase the detection of findings, which limits the extrapolation of our results to clinical populations as well as other research populations using different MR protocols. Moreover, the three neuroradiologists were from the same institution, which may introduce some institutional preferences or bias. MRIs from the CARE study were labeled in a manner that allowed the inference of the sport of the participant; thus, radiologists were not blinded to sport for those participants. It should be noted, however, that these factors are unlikely to account for the observed group differences, given that raters were blinded to presence of SRC. Finally, additional factors associated with exposure may impact MRI findings, such as the varying levels of exposure to contact sports at the youth or high school level in the non-contact athletes and the positions played for each sport. Future, targeted studies are needed to isolate the effects of these factors on potentially clinically significant MRI findings.
Conclusion
This study provides prospective evidence that the large majority of SRCs are not associated with acute injury findings on structural MRI. Athletes with SRC, however, still had higher prevalence of non-specific MRI findings, largely driven by WMH. The association, and direction of association, between concussion and non-specific MRI findings requires further study. In the meantime, clinical neuroradiologists can benefit from being aware of the association as they interpret MRI scans in athletes with SRC. Results also underscore the need for the development of radiological CDE for describing and reporting non-specific or incidental MRI findings in trauma, which would be particularly relevant for research studies in which radiological reads are performed blinded to clinical information.
Supplementary Material
Acknowledgments
This project was funded with support from the Grand Alliance Concussion Assessment, Research, and Education (CARE) Consortium, funded, in part by the National Collegiate Athletic Association (NCAA) and the Department of Defense (DOD). The U.S. Army Medical Research Acquisition Activity, (820 Chandler Street, Fort Detrick, MD 21702-5014) is the awarding and administering acquisition office. This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Psychological Health and Traumatic Brain Injury Program under Award No. W81XWH-14-2-0151. This work was also supported by the Defense Health Program under the Department of Defense Broad Agency Announcement for Extramural Medical Research through Award No. W81XWH-14-1-0561. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the DOD (DHP funds). The REDCap electronic database used for this project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Award No. UL1TR001436. This research was completed, in part, with computational resources and technical support provided by the Research Computing Center at the Medical College of Wisconsin.
The authors thank Jody Harland, Michelle Leigh LaPradd, Janetta Matesan, and Larry Riggen (Indiana University); Ashley Rettmann (University of Michigan); Melissa Koschnitzki, Ashley LaRoche, Brad Swearingen, and Alexa Wild (Medical College of Wisconsin); Michael Jarrett, Vibeke Brinck, and Bianca Byrne (Quesgen); Thomas Dompier, Melissa Niceley Baker, and Sara Dalton (Datalys Center for Sports Injury Research and Prevention); and the research and medical staff at each participating site.
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. McCrory P., Meeuwisse W., Dvorak J., Aubry M., Bailes J., Broglio S., Cantu R.C., Cassidy D., Echemendia R.J., Castellani R.J., Davis G.A., Ellenbogen R., Emery C., Engebretsen L., Feddermann-Demont N., Giza C.C., Guskiewicz K.M., Herring S., Iverson G.L., Johnston K.M., Kissick J., Kutcher J., Leddy J.J., Maddocks D., Makdissi M., Manley G.T., McCrea M., Meehan W.P., Nagahiro S., Patricios J., Putukian M., Schneider K.J., Sills A., Tator C.H., Turner M., and Vos P.E. (2017). Consensus statement on concussion in sport—the 5th International Conference on Concussion in Sport held in Berlin, October 2016. Br. J. Sports Med. 51, 838–847 [DOI] [PubMed] [Google Scholar]
- 2. Amyot F., Arciniegas D.B., Brazaitis M.P., Curley K.C., Diaz-Arrastia R., Gandjbakhche A., Herscovitch P., Hinds S.R., II, Manley G.T., Pacifico A., Razumovsky A., Riley J., Salzer W., Shih R., Smirniotopoulos J.G., and Stocker D. (2015). A review of the effectiveness of neuroimaging modalities for the detection of traumatic brain injury. J. Neurotrauma 32, 1693–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Katzman G.L., Dagher A.P., and Patronas N.J. (1999). Incidental findings on brain magnetic resonance imaging from 1000 asymptomatic volunteers. JAMA 282, 36–39 [DOI] [PubMed] [Google Scholar]
- 4. Shoemaker J.M., Holdsworth M.T., Aine C., Calhoun V.D., de La Garza R., Feldstein Ewing S.W., Hayek R., Mayer A.R., Kiehl K.A., Petree L.E., Sanjuan P., Scott A., Stephen J., and Phillips J.P. (2011). A practical approach to incidental findings in neuroimaging research. Neurology 77, 2123–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yuh E.L., Mukherjee P., Lingsma H.F., Yue J.K., Ferguson A.R., Gordon W.A., Valadka A.B., Schnyer D.M., Okonkwo D.O., Maas A.I., Manley G.T. and Investigators T.-T. (2013). Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury. Ann. Neurol. 73, 224–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morris Z., Whiteley W.N., Longstreth W.T., Jr, Weber F., Lee Y.C., Tsushima Y., Alphs H., Ladd S.C., Warlow C., Wardlaw J.M., and Al-Shahi Salman R. (2009). Incidental findings on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 339, b3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bos D., Poels M.M., Adams H.H., Akoudad S., Cremers L.G., Zonneveld H.I., Hoogendam Y.Y., Verhaaren B.F., Verlinden V.J., Verbruggen J.G., Peymani A., Hofman A., Krestin G.P., Vincent A.J., Feelders R.A., Koudstaal P.J., van der Lugt A., Ikram M.A., and Vernooij M.W. (2016). Prevalence, clinical management, and natural course of incidental findings on brain MR images: the population-based Rotterdam Scan Study. Radiology 281, 507–515 [DOI] [PubMed] [Google Scholar]
- 8. Haacke E.M., Duhaime A.C., Gean A.D., Riedy G., Wintermark M., Mukherjee P., Brody D.L., DeGraba T., Duncan T.D., Elovic E., Hurley R., Latour L., Smirniotopoulos J.G., and Smith D.H. (2010). Common data elements in radiologic imaging of traumatic brain injury. J. Magn. Reson. Imaging 32, 516–543 [DOI] [PubMed] [Google Scholar]
- 9. Bigler E.D., Abildskov T.J., Goodrich-Hunsaker N.J., Black G., Christensen Z.P., Huff T., Wood D.M., Hesselink J.R., Wilde E.A., and Max J.E. (2016). Structural neuroimaging findings in mild traumatic brain injury. Sports Med. Arthrosc. 24, e42–e52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Riedy G., Senseney J.S., Liu W., Ollinger J., Sham E., Krapiva P., Patel J.B., Smith A., Yeh P.H., Graner J., Nathan D., Caban J., French L.M., Harper J., Eskay V., Morissette J., and Oakes T.R. (2016). Findings from structural MR imaging in military traumatic brain injury. Radiology 279, 207–215 [DOI] [PubMed] [Google Scholar]
- 11. Ellis M.J., Leiter J., Hall T., McDonald P.J., Sawyer S., Silver N., Bunge M., and Essig M. (2015). Neuroimaging findings in pediatric sports-related concussion. J. Neurosurg. Pediatr. 16, 241–247 [DOI] [PubMed] [Google Scholar]
- 12. Bonow R.H., Friedman S.D., Perez F.A., Ellenbogen R.G., Browd S.R., Mac Donald C.L., Vavilala M.S., and Rivara F.P. (2017). Prevalence of abnormal magnetic resonance imaging findings in children with persistent symptoms after pediatric sports-related concussion. J. Neurotrauma 34, 2706–2712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morgan C.D., Zuckerman S.L., King L.E., Beaird S.E., Sills A.K., and Solomon G.S. (2015). Post-concussion syndrome (PCS) in a youth population: defining the diagnostic value and cost-utility of brain imaging. Childs Nerv. Syst. 31, 2305–2309 [DOI] [PubMed] [Google Scholar]
- 14. Nencka A.S., Meier T.B., Wang Y., Muftuler L.T., Wu Y.C., Saykin A.J., Harezlak J., Brooks M.A., Giza C.C., Difiori J., Guskiewicz K.M., Mihalik J.P., LaConte S.M., Duma S.M., Broglio S., McAllister T., McCrea M.A., and Koch K.M. (2018). Stability of MRI metrics in the advanced research core of the NCAA-DoD Concussion Assessment, Research and Education (CARE) consortium. Brain Imaging Behav. 12, 1121–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mustafi S.M., Harezlak J., Koch K.M., Nencka A.S., Meier T., West J.D., Giza C.C., DiFiori J., Guskiewicz K.K., Mihalik J., LaConte S.M., Duma S.M., Broglio S.P., Saykin A.J., McCrea M., McAllister T., and Wu Y.C. (2018). Acute white-matter abnormalities in sports-related concussion: a diffusion tensor imaging study from the NCAA-DoD CARE Consortium. J. Neurotrauma 35, 2653–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Broglio S.P., McCrea M., McAllister T., Harezlak J., Katz B., Hack D., andHainline B.; CARE Consortium Investigators. (2017). A national study on the effects of concussion in collegiate athletes and US Military Service Academy members: the NCAA-DoD Concussion Assessment, Research and Education (CARE) Consortium structure and methods. Sports Med. 47, 1437–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carney N., Ghajar J., Jagoda A., Bedrick S., Davis-O'Reilly C., du Coudray H., Hack D., Helfand N., Huddleston A., Nettleton T., and Riggio S. (2014). Concussion guidelines step 1: systematic review of prevalent indicators. Neurosurgery 75, Suppl. 1, S3–15 [DOI] [PubMed] [Google Scholar]
- 18. Hollingshead A. (1975). Four factor index of social status. Yale University Department of Psychology, New Haven, CT [Google Scholar]
- 19. Wardlaw J.M., Valdes Hernandez M.C., and Munoz-Maniega S. (2015). What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J. Am. Heart Assoc. 4, 001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hamedani A.G., Rose K.M., Peterlin B.L., Mosley T.H., Coker L.H., Jack C.R., Knopman D.S., Alonso A., and Gottesman R.F. (2013). Migraine and white matter hyperintensities: the ARIC MRI study. Neurology 81, 1308–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jeerakathil T., Wolf P.A., Beiser A., Massaro J., Seshadri S., D'Agostino R.B., and DeCarli C. (2004). Stroke risk profile predicts white matter hyperintensity volume: the Framingham Study. Stroke 35, 1857–1861 [DOI] [PubMed] [Google Scholar]
- 22. Tate D.F., Gusman M., Kini J., Reid M., Velez C.S., Drennon A.M., Cooper D.B., Kennedy J.E., Bowles A.O., Bigler E.D., Lewis J.D., Ritter J., and York G.E. (2017). Susceptibility weighted imaging and white matter abnormality findings in service members with persistent cognitive symptoms following mild traumatic brain injury. Mil. Med. 182, e1651–e1658 [DOI] [PubMed] [Google Scholar]
- 23. Schultz V., Stern R.A., Tripodis Y., Stamm J., Wrobel P., Lepage C., Weir I., Guenette J.P., Chua A., Alosco M.L., Baugh C.M., Fritts N.G., Martin B.M., Chaisson C.E., Coleman M.J., Lin A.P., Pasternak O., Shenton M.E., and Koerte I.K. (2018). Age at first exposure to repetitive head impacts is associated with smaller thalamic volumes in former professional American football players. J. Neurotrauma 35, 278–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stamm J.M., Bourlas A.P., Baugh C.M., Fritts N.G., Daneshvar D.H., Martin B.M., McClean M.D., Tripodis Y., and Stern R.A. (2015). Age of first exposure to football and later-life cognitive impairment in former NFL players. Neurology 84, 1114–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stamm J.M., Koerte I.K., Muehlmann M., Pasternak O., Bourlas A.P., Baugh C.M., Giwerc M.Y., Zhu A., Coleman M.J., Bouix S., Fritts N.G., Martin B.M., Chaisson C., McClean M.D., Lin A.P., Cantu R.C., Tripodis Y., Stern R.A., and Shenton M.E. (2015). Age at first exposure to football is associated with altered corpus callosum white matter microstructure in former professional football players. J. Neurotrauma 32, 1768–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wolf S.M., Lawrenz F.P., Nelson C.A., Kahn J.P., Cho M.K., Clayton E.W., Fletcher J.G., Georgieff M.K., Hammerschmidt D., Hudson K., Illes J., Kapur V., Keane M.A., Koenig B.A., Leroy B.S., McFarland E.G., Paradise J., Parker L.S., Terry S.F., Van Ness B., and Wilfond B.S. (2008). Managing incidental findings in human subjects research: analysis and recommendations. J. Law Med. Ethics 36, 219–248 , 211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lange R.T., Shewchuk J.R., Heran M.K., Rauscher A., Jarrett M., Brubacher J.R., and Iverson G.L. (2014). To exclude or not to exclude: further examination of the influence of white matter hyperintensities in diffusion tensor imaging research. J. Neurotrauma 31, 198–205 [DOI] [PubMed] [Google Scholar]
- 28. Haberg A.K., Hammer T.A., Kvistad K.A., Rydland J., Muller T.B., Eikenes L., Garseth M., and Stovner L.J. (2016). Incidental intracranial findings and their clinical impact; the HUNT MRI Study in a general population of 1006 participants between 50–66 years. PLoS One 11, e0151080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.