Abstract
Objectives:
To compare the effects of inpatient enhanced multidisciplinary care (EMC) and multidisciplinary rehabilitation (MR) on the symptoms and quality of life (QOL) of patients with Parkinson disease (PD) and to clarify the relation between reduction in symptoms and the improved QOL.
Methods:
This study was a quasi-randomized controlled (alternate allocation), assessor-blinded, single-center study. We recruited 80 patients with idiopathic Parkinson disease, Hoehn and Yahr stage 2 to 4, on stable medication. Patients were included in an EMC or MR group. Both rehabilitation programs were performed for 8 weeks (17 h/wk). Main outcome measures were Parkinson’s Disease Questionnaire-39 and Unified Parkinson’s Disease Rating Scale.
Results:
The EMC induced significant improvements in QOL compared to MR. We found that body axis symptoms (rising from a chair, posture, postural stability, falling, and walking) as well as nonmotor symptoms (depression) in patients with PD were relieved by the inpatient EMC.
Conclusions:
Enhanced multidisciplinary care for patients with PD appears to be effective in improving the QOL. The improvement in motor and nonmotor symptoms, including depression, may contribute to the improved QOL.
Keywords: Parkinson disease, quality of life, multidisciplinary care, depression, aerobic exercise, nursing
Introduction
Parkinson disease (PD) is a progressive neurodegenerative disorder which presents with various symptoms, including resting tremor, rigidity, akinesia, and postural instability.1 Additionally, patients with PD frequently experience nonmotor symptoms, including depression, cognitive dysfunction, sleep/awakening disorder, pain, fatigue, olfactory disturbance, and autonomic nervous system disorders.2 Compared to normal elderly people, patients with PD report a lower quality of life (QOL). Furthermore, an association between reduced QOL and depression, insomnia, and decreased activities of daily living (ADLs) has been reported.3
The Parkinson’s Disease Questionnaire-39 (PDQ-39) has been frequently used as a disease-related QOL evaluation scale in PD. It addresses issues believed to be influenced in the lives of patients with PD and has a proven reliability and validity.4 A previous study that used the PDQ-39 identified depression, decreased ADL, postural instability, and cognitive dysfunction as factors independently influencing the QOL.5 Similarly, other studies have reported that gait disturbance, decreased ADL, and depression independently lead to reduced QOL.6,7 Altogether, these are important factors, potentially affecting the QOL in PD. Thus, it is crucial to provide appropriate treatment for these symptoms.
A recent study reported that rehabilitation can effectively reduce PD symptoms.8 Goodwin et al conducted a meta-analysis of 14 studies and reported that physical therapy (PT) effectively improves body function, QOL, muscular strength, balance, and walking speed.9 Moreover, it has been reported that occupational therapy (OT) can improve motor function and QOL in patients with PD.10,11 Speech and language therapy (ST) may be useful to improve dysphonia and dysphagia in these patients.12-15
Clinical and basic research studies support the effects of exercise on neuroplasticity in PD.16 Neuroplasticity is changes or adaptations in structure or function of the brain based on experience/exposure/practice. Plastic changes can occur on anatomical, molecular, genetic, structural, and functional levels within the brain. A possible neurobiological mechanism underlying the positive effects of exercise is the increased synthesis and release of neurotransmitters and neurotrophins, which could enhance neurogenesis, angiogenesis, and thus neuroplasticity.16
Furthermore, education, social support, psychological counseling, living guidance, and dietary counseling appear to play a major role in improving the patients’ QOL.6 The complexity of PD symptoms requires a multidisciplinary rehabilitation (MR) and, consequently, a multidisciplinary care is needed in order to obtain an improvement in QOL.17-19 Indeed, multidisciplinary care, including educational programs20 and group training,21 in addition to individual training, may effectively improve the QOL in patients with this disorder.
The aims of this study were to compare the effects of inpatient enhanced multidisciplinary care (EMC) and MR on the symptoms and QOL of patients with PD and to clarify the relationship between a reduction in symptoms and improved QOL.
Materials and Methods
Study Design and Patients
This study was a quasi-randomized controlled, single-blinded study performed in a single center. Patient recruitment started on April 2015 and was finalized 24 months later.
Patients were quasi-randomly assigned to an EMC or MR group after passing a phone screening procedure by a coordinator who did not know the contents of the study. This screening excluded patients who no longer met the inclusion criteria (see below) at 1 week before own admission. The procedure was concealed to the enrolling neurologist until the group assignment. We performed an alternate allocation method according to the order of admission to the study. The first patient to fulfill the inclusion criteria was included in the EMC, the second patient was included in the MR, and the allocation continued in this alternating way.
In this longitudinal study, we recruited patients with PD who had been diagnosed by 2 neurologists, according to the criteria set out by the UK Parkinson’s Disease Society Brain Bank.22 The inclusion criteria were the following: age 50 to 80, Hoehn and Yahr (H&Y)23 stage 2 to 4, ability to walk at least with physical assistance, and visual dysfunction limiting locomotion or balance at last hospital visit. Patients with neurological diseases other than PD, musculoskeletal diseases, heart disease, or respiratory disease were excluded from the study.
Written informed consent was obtained from all patients before participation. The study protocol was approved by the institutional review boards of the Hyogo Prefectural Rehabilitation Hospital at Nishi-Harima and performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. This trial was registered on University Hospital Medical Information Network Center website (UMIN000026127).
Intervention
The neurologist completed an appropriate drug adjustment plan within a month before intervention and, therefore, did not alter the medication until the intervention was complete. The physiatrist controlled the exercise strength of rehabilitation depending on the severity of the patient’s illness. The patients were instructed to exercise, in order to instill a habit of exercising regularly, and received feedback about the effects of rehabilitation, in order to improve their self-efficacy. Participants were guided in a problem-solving approach that focused on developing strategies to improve daily function and participation in self-identified roles in the society. The physiotherapist and occupational therapist provided individual rehabilitation, which aimed to improve 5 core areas: (1) transfers, (2) posture, (3) reaching and grasping, (4) balance, and (5) gait.24,25 The individual rehabilitation program included task-oriented training, gait training with cueing and cognitive movement strategies, and ADL, and instrumental ADL training (shopping, cooking, cleaning, using the telephone, washing, management of medicine, and use of a vehicle). Patients in the EMC group received PT and OT 6 days per week (40 min/d). Furthermore, the speech therapist provided individual rehabilitation to improve vocal speed, loudness, and pitch of speech12,13 and to enhance pharyngeal movement on swallowing.26 Patients in the EMC group received ST 6 days per week (40 min/d).
Nurses, music therapists, and physical education instructors provided exercise training during group rehabilitation, which included balance ball training, rhythmic exercise with dancing, and aerobic water exercise. Patients performed each exercise program for 3 days per week (60 min/d). A group educational program was provided by neurologists, physiatrists, nurses, pharmacists, and a nutritionist who provided, respectively, promotion of disease comprehension, directions for exercise methods, living guidance, patient compliance instructions, and nutritional education. Patients attended the educational program once a week (60 min/d). The psychologist promoted patients’ motivation for exercise and social participation through group exercise and educational programs. Finally, a social worker coordinated the provision of support after discharge.
Participants in the EMC group received 8 weeks of rehabilitation (17 h/wk), conducted by a neurologist, physiatrist, physiotherapist, occupational therapist, speech therapist, nurse, psychologist, pharmacist, nutritionist, music therapist, physical education instructor, and medical social worker (Table 1). In contrast, participants in the MR group received 8 weeks of rehabilitation (17 h/wk) that was conducted by only by 6 specialists: a neurologist, physiatrists, physiotherapist (60 min/d × 6 d/wk), occupational therapist (60 min/d × 6 d/wk), speech therapist (40 min/d × 6 d/wk), and medical social worker (60 min/d × 1 d/wk).
Table 1.
Overview of 2 Group Intervention.
| Enhanced Multidisciplinary Care (EMC) | Multidisciplinary Rehabilitation (MR) | ||
|---|---|---|---|
| Frequency | Total: 17 h/wk | Frequency | Total: 17 h/wk |
| Duration | 8 weeks | Duration | 8 weeks |
| Individual | Individual | ||
| Neurologists | An appropriate drug adjustment | Neurologists | An appropriate drug adjustment |
| Physiatrists | Control exercise strength | Physiatrists | Control exercise strength |
| PT | Five core areas | PT | Five core areas |
| Six d/wk (40 min/d) | Six days/week (60 min/d) | ||
| OT | Five core areas | OT | Five core areas |
| Six d/wk (40 min/d) | Six days/week (60 min/d) | ||
| ST | Speech, swallowing | ST | Speech, swallowing |
| Six d/wk (40 min/d) | Six d/wk (40 min/d) | ||
| MSW/psychologists | Once a week (60 min/d) | MSW | Once a week (60 min/d) |
| Group | |||
| Group rehabilitation | Three d/wk (60 min/d) | ||
| Nurse | Balance ball training | ||
| Music therapists | Rhythmic exercise with dancing | ||
| PEI | Aerobic water exercise | ||
| Group education | Once a week (60 min/d) | ||
| Neurologists | Promotion of disease comprehension | ||
| Physiatrists | Directions for exercise method | ||
| Nurses | Living guidance, foot care | ||
| Pharmacists | Patient compliance instructions | ||
| Nutritionist | Nutritional education | ||
Abbreviations: PDQ-39, Parkinson’s Disease Questionnaire-39; UPDRS, Unified Parkinson’s Disease Rating ScalePT, physical therapy; OT, occupational therapy; ST, speech and language therapy.
Assessment
Symptoms of PD were comprehensively assessed by evaluators who were blinded to treatment, using subscores of the Unified Parkinson’s Disease Rating Scale (UPDRS) at patients’ admission and at discharge.27 The UPDRS was composed of 4 main parts: (1) mention, behavior, and mood; (2) ADL; (3) motor examination; and (4) complications of therapy. Patients completed a PDQ-39 form to rate their QOL.4 In this questionnaire, patients were required to answer each question with regard to the previous month. This first questionnaire was given to patients upon hospital admission to assess their QOL prior to the hospitalization. The second questionnaire was given at 1 month after discharge, in order to avoid bias related to the hospitalization itself, which could affect patients’ response to the questionnaire. The Mini-Mental State Examination (MMSE) was used to assess cognitive function.28 Finally, all anti-parkinsonian medication used was expressed as levodopa-equivalent dose (LED).29
Sample Size Computation
We computed the sample size according to PDQ-39 (total) and UPDRS part 3 based on the outcome variables with standard error of measurements (SEMs) available from published studies. The published SEMs for PDQ-39 and UPDRS part 3 were 6.25 and 4, respectively.30,31 We expected an effect size of around 4.8 and 2.5 for the same variables (clinically minimal important difference).32 To detect this change with a 2-tailed type 1 error of 0.05 and a power of 80%, the estimated sample size (the largest between the 2 estimates) was 66 patients. The final conservative choice was 80 patients so that the study could be randomized.
Statistical Analysis
We used a mixed-design analysis of variance (ANOVA), with intervention as between factor (EMC vs MR) and time (pre and post) as within factor (repeated measure). If a significant interaction effect for time and intervention was found, post hoc analyses were performed using paired t tests to compare post- and prerehabilitation in both groups of patients. For within-group comparisons, changes in the scores of the PDQ-39 (total) and UPDRS (total and subscores) were analyzed before and after interventions using paired t-test, while between-group comparisons were assessed with unpaired t test. Statistical significance was defined as P < .05. Statistical analyses were conducted with the JMP pro software (version 13.1.0; SAS Institute, Cary, North Carolina).
Results
Effects of EMC
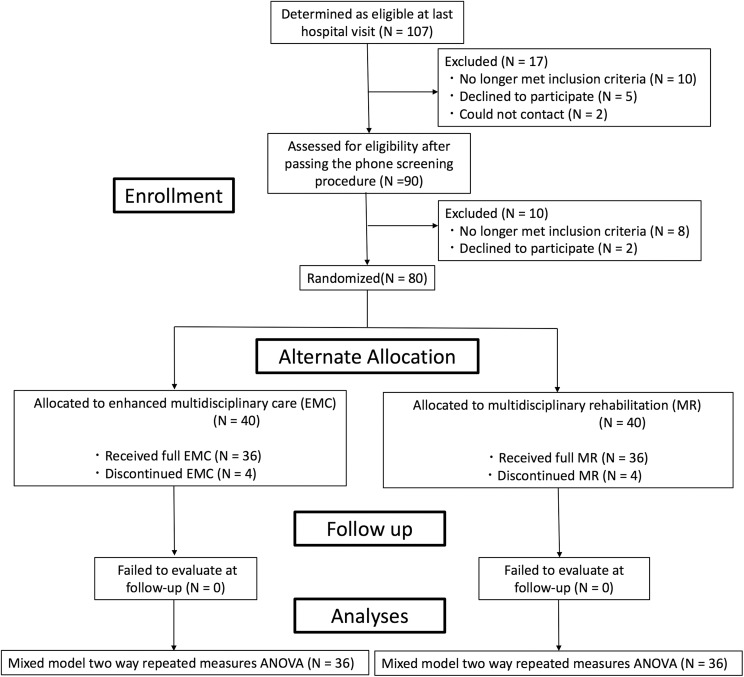
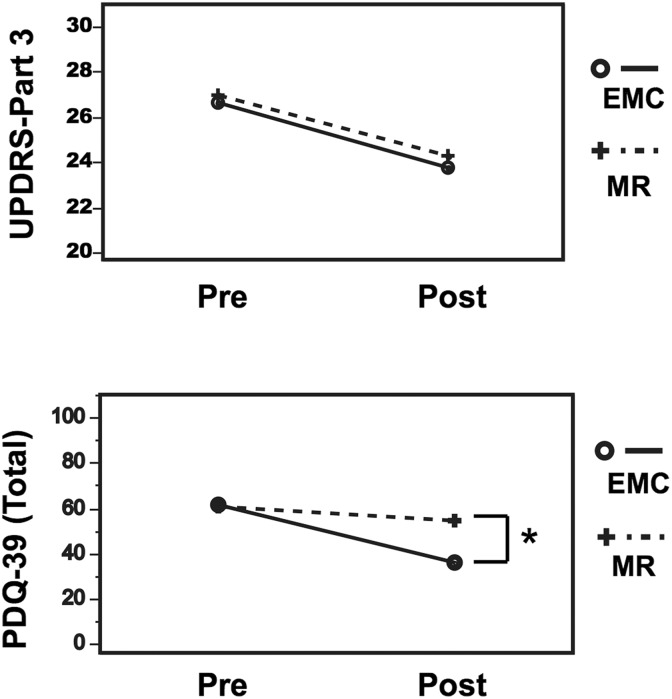
In the EMC group, a total of 36 patients completed the study, with 2 dropouts for respiratory disease and 2 for musculoskeletal disease. In the MR group, 36 patients completed the study, with 2 dropouts for other neurological diseases and 2 for musculoskeletal disease (Figure 1). No difference was observed in the 2 groups of patients in terms of age, disease duration, H&Y stage, MMSE, and baseline LED (Table 2). Results of the ANOVA for all variables are summarized in Table 3. The time course of PDQ-39 was different between the EMC and MR groups, as revealed by the significant interaction for time × group in the mixed model of 2-way repeated-measures ANOVA (Table 3, last column). In the same way, there were no significant interaction for time × group in UPDRS part 3 (Table 3, last column). Between-group comparisons showed that improvement scores of PDQ-39 were greater in the EMC than the MR group posttreatment (P = .0019; Figure 2).
Figure 1.
Study design. Patients were quasi-randomly assigned to an enhanced multidisciplinary care (EMC) or multidisciplinary rehabilitation (MR) group after passing the phone screening procedure. ANOVA indicates analysis of variance
Table 2.
Clinical Profiles of Patients With Parkinson Disease.a
| Variable | Enhanced Multidisciplinary Care (EMC) | Multidisciplinary Rehabilitation (MR) | P Value |
|---|---|---|---|
| Mean ± SEM | Mean ± SEM | ||
| Age, years | 69.0 ± 0.93 | 68.2 ± 1.40 | .634 |
| Disease duration, months | 127 ± 10.9 | 113 ± 9.76 | .326 |
| H&Y stage (1-5) | 2.97 ± 0.11 | 3.05 ± 0.12 | .609 |
| MMSE (0-30) | 26.8 ± 0.29 | 26.9 ± 0.34 | .805 |
| LED, mg | 634 ± 57.9 | 662 ± 66.7 | .76 |
Abbreviations: H&Y stage, Hoehn and Yahr stage; LED, levodopa equivalent dose; MMSE, Mini-Mental State Examination; SEM, standard error of the mean.
an = 72.
Table 3.
Effect of Enhanced Multidisciplinary Care (EMC) or Multidisciplinary Rehabilitation (MR) for Parkinson Disease.a
| Group Effect | Time Effect | Interaction (Time × Group) | ||||
|---|---|---|---|---|---|---|
| F 1, 70 | P | F 1, 70 | P | F 1, 70 | P | |
| UPDRS part 1 | 0.625 | .432 | 6.36 | .014b | 2.66 | .108 |
| UPDRS part 2 | 0.732 | .396 | 5.78 | .019b | 2.65 | .109 |
| UPDRS part 3 | 0.021 | .885 | 16 | .0002b | 0.02 | .889 |
| UPDRS part 4 | 2.33 | .132 | 13.8 | .0004b | 1.27 | .264 |
| PDQ-39 total | 3.123 | .083 | 47.4 | <.0001b | 17.56 | .0001b |
Abbreviations: PDQ-39, Parkinson’s Disease Questionnaire-39; UPDRS, Unified Parkinson’s Disease Rating Scale.
aN = 72. Statistical results obtained using a mixed model 2-way repeated-measures analysis of variance.
bP < .05.
Figure 2.
Effects of enhanced multidisciplinary care (EMC). Results of analysis of variance (ANOVA) showed the time course of PDQ-39 was different between the EMC and multidisciplinary rehabilitation (MR) groups. Between-group comparisons showed that improvement scores of PDQ-39 were greater in the EMC than in the MR group posttreatment (P = .0019). N = 36/36, *P < .05. PDQ-39 indicates Parkinson’s Disease Questionnaire-39; UPDRS, Unified Parkinson’s Disease Rating Scale.
Relationship Between Reduction in Symptoms and Improved QOL
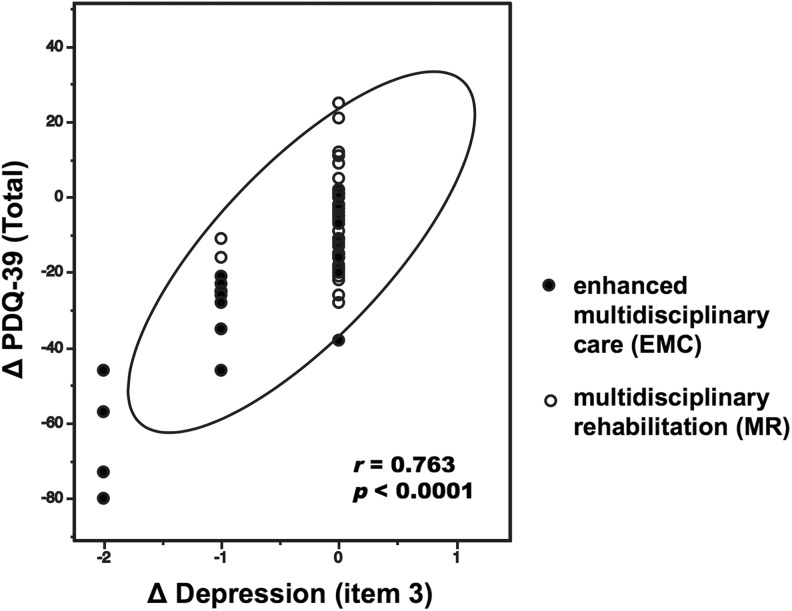
Tables 4 and 5 show the mean of significantly improved UPDRS subscores and PDQ-39 total and subscores, respectively, before and after the EMC and MR, as well as the mean change in these scores. Table 4 shows the mean UPDRS total and subscores before and after the EMC. After EMC, we observed a significant reduction in depression, as measured by UPDRS part 1 (psychic function, action, and mood) subscore. Falling (unrelated to freezing), walking, and cutting food as well as handling utensils significantly improved when evaluated using UPDRS part 2 (ADL). There were further significant improvements in rising from a chair, posture, and postural stability, as evaluated using UPDRS part 3 (motor). Furthermore, dyskinesia duration, morning dystonia, and the duration of “off” periods were significantly reduced as evaluated using UPDRS part 4 (complications of treatment). There were significant improvements in all subscores of PDQ-39 (Table 5). Table 4 shows the mean UPDRS total and subscores before and after MR. We observed a significant reduction in falling, as measured by the UPDRS part 2 (ADL) after MR. There were further significant improvements in rising from a chair and postural stability, as evaluated using the UPDRS part 3 (motor). There was significant improvement in social support, as evaluated with a subscore of the PDQ-39 (Table 5). Assessing the relationship between PDQ-39 (total) and UPDRS (total) at admission in EMC, we found no significant association between both measurements (Spearman r = 0.13, P = .496). This association was also not significant at the second time point (Spearman r = 0.31, P = .127). When comparing the delta change (post–pre) between EMC and MR, many dimensions (mobility, ADL, emotional well-being, cognition, and bodily discomfort) were significantly different (as measured via t test between groups in the PDQ-39). In addition, only 2 dimensions (depression and morning dystonia) were significantly different in the UPDRS evaluation. Importantly, we found that the care-induced improvements in depression (item 3) scores of UPDRS part 1 were associated with improved QOL (total; r = 0.763, P < .0001; Figure 3).
Table 4.
Improved Unified Parkinson’s Disease Rating Scale Subscores Before and After Enhanced Multidisciplinary Care (EMC) and Multidisciplinary Rehabilitation (MR).a.
| Item | Pre | Post | ΔPost-Pre | Pre-Post P value | ΔPost-Pre P Value | |
|---|---|---|---|---|---|---|
| EMC | EMC | EMC | EMC | |||
| (MR) | (MR) | (MR) | (MR) | EMC vs MR | ||
| Part 1 | 1.00 | 0.45 | −0.55 | .022b | .108 | |
| (1.00) | (0.88) | (−0.12) | (.422) | |||
| 3 | Depression | 0.53 | 0.01 | −0.52 | <.0001b | .0003b |
| (0.33) | (0.28) | (−0.05) | (.160) | |||
| Part 2 | 8.23 | 5.94 | −2.29 | .003b | .1086 | |
| (8.50) | (8.06) | (−0.44) | (.612) | |||
| 9 | Cutting food, handling utensils | 0.45 | 0.26 | −0.19 | .031b | .2695 |
| (0.53) | (0.53) | (0.00) | (1.00) | |||
| 13 | Falling | 0.67 | 0.32 | −0.35 | .032b | .7072 |
| (0.76) | (0.32) | (−0.44) | (.011b) | |||
| 15 | Walking | 0.84 | 0.55 | −0.29 | .037b | .1995 |
| (0.82) | (0.79) | (−0.03) | (.845) | |||
| Part 3 | 26.6 | 23.6 | −3 | .008b | .8890 | |
| (26.9) | (24.2) | (−2.7) | (.008b) | |||
| 27 | Rising from a chair | 0.64 | 0.35 | −0.29 | .005b | .4175 |
| (0.94) | (0.53) | (−0.41) | (.001b) | |||
| 28 | Posture | 1.55 | 1.32 | −0.23 | .017b | .7474 |
| (1.58) | (1.39) | (−0.19) | (.083) | |||
| 30 | Postural stability | 1.48 | 1.19 | −0.29 | .005b | .3562 |
| (1.47) | (1.29) | (−0.18) | (.032b) | |||
| Part 4 | 2.06 | 1.13 | −0.93 | .001b | .2638 | |
| (2.59) | (2.09) | (−0.50) | (.084) | |||
| 32 | Dyskinesia duration | 0.29 | 0.16 | −0.13 | .043b | .3285 |
| (0.24) | (0.21) | (−0.03) | (.711) | |||
| 35 | Morning dystonia | 0.13 | 0.01 | −0.12 | .044b | .0306b |
| (0.09) | (0.15) | (0.06) | (.325) | |||
| 39 | “Off” period duration | 0.58 | 0.32 | −0.26 | .018b | .6291 |
| (0.85) | (0.68) | (−0.17) | (.184) | |||
| Total | 37.9 | 31.3 | −6.6 | .0002b | .2016 | |
| (39.1) | (35.2) | (−3.9) | (.0071b) |
aN = 36/36. Statistical results obtained using paired t tests and t tests.
bP < .05.
Table 5.
Parkinson’s Disease Questionnaire-39 Subscores Before and After Enhanced Multidisciplinary Care (EMC) and Multidisciplinary Rehabilitation (MR).a
| Item | Pre | Post | ΔPost-Pre | Pre-Post P Value | ΔPost-Pre P Value | |
|---|---|---|---|---|---|---|
| EMC | EMC | EMC | EMC | |||
| (MR) | (MR) | (MR) | (MR) | EMC vs MR | ||
| 1 | Mobility (0-40) | 21.3 ± 1.9 | 14.0 ± 1.8 | −7.3 | .0007b | .0242b |
| (20.9 ± 1.5) | (18.6 ± 1.6) | (−2.30) | (.0675) | |||
| 2 | ADL (0-24) | 10.2 ± 1.0 | 6.3 ± 1.0 | −3.9 | .0008b | .0185b |
| (9.58 ± 0.8) | (8.91 ± 0.9) | (−0.67) | (.4369) | |||
| 3 | Emotional well-being (0-24) | 10.3 ± 1.0 | 5.9 ± 0.9 | −4.4 | <.0001b | .0049b |
| (9.36 ± 0.6) | (8.12 ± 0.5) | (−1.24) | (.0804) | |||
| 4 | Stigma (0-16) | 3.10 ± 0.6 | 1.83 ± 0.4 | −1.27 | .0228b | .7823 |
| (4.29 ± 0.6) | (3.24 ± 0.4) | (−1.05) | (.069) | |||
| 5 | Social support (0-12) | 1.96 ± 0.4 | 0.75 ± 0.3 | −1.21 | .0233b | .5692 |
| (2.66 ± 0.4) | (1.82 ± 0.3) | (−0.84) | (.0436b) | |||
| 6 | Cognition (0-16) | 6.96 ± 0.8 | 4.86 ± 0.7 | −2.1 | .0047b | .0061b |
| (5.62 ± 0.5) | (5.79 ± 0.5) | (−0.17) | (.701) | |||
| 7 | Communication (0-12) | 3.48 ± 0.5 | 1.79 ± 0.4 | −1.69 | .0004b | .0684 |
| (3.41 ± 0.5) | (2.91 ± 0.4) | (−0.50) | (.2995) | |||
| 8 | Bodily discomfort (0-12) | 4.96 ± 0.5 | 1.96 ± 0.4 | −3 | <.0001b | .0065b |
| (4.30 ± 0.6) | (3.52 ± 0.6) | (−0.78) | (.1501) | |||
| Total (0-156) | 61.3 ± 4.8 | 35.9 ± 4.2 | −25.4 | .0001b | .0001b | |
| (60.5 ± 3.2) | (54.3 ± 3.2) | (−6.2) | (.0109b) | |||
Abbreviation: ADL, activities of daily living.
aN = 36/36. Statistical results obtained using paired t-tests and t-tests.
bP < .05.
Figure 3.
Analysis of improved symptom factors in relation to changes in quality of life (QOL). The reduction in depression (item 3) of UPDRS part1 correlated with the improved PDQ-39 total scores (r = .763, P < .0001). N =72. PDQ-39 indicates Parkinson’s Disease Questionnaire-39; UPDRS, Unified Parkinson’s Disease Rating Scale.
Discussion
Compared to an MR, the EMC induced significant improvements in the QOL. In this study, we provided an EMC program for patients with PD in order to examine its effects on various PD symptoms using subscores of the UPDRS. This is a conventional rating system used worldwide; however, only few studies have examined the effects of rehabilitation on each separate UPDRS subscore. This research showed that EMC ameliorates PD symptoms, as assessed with subscores of both the UPDRS and PDQ-39. Furthermore, our study suggests that body axis symptoms (rising from a chair, posture, postural stability, falling, and walking) as well as nonmotor symptoms (depression) in patients with PD can be relieved by inpatient EMC administered for 8 weeks. The improvement in such symptoms, including depression, may contribute to improved QOL. Previous cross-sectional studies have shown that depression, gait disturbance, postural instability, functional decline in recognition, and insomnia are related to the QOL.3-7 In agreement, the results of our longitudinal approach support this relationship between QOL and depression.
Our EMC program contained a wide variety of interventions compared to previous intervention studies, which may have been enabled by the patients’ hospitalization. Inpatient rehabilitation could be performed in a more intensive way when compared to outpatient rehabilitation. Furthermore, patients could practice ADL by directly observing differences in ADL state between nighttime and daytime. Three previous studies also implemented intensive MR during hospitalization, similar to our study. However, 2 of these examined patients with more advanced PD,33,34 while the other examined patients at an earlier stage of the illness.35 It is thus difficult to directly compare these findings because the aims and methods of rehabilitation, as well as the stage of PD, were different among the different studies.
Our EMC program has the unique feature of including 12 different specialists in the intervention and an intensive program during hospitalization. Monticone et al reported a similar inpatient rehabilitation program lasting 8 weeks,33 but our program was more intense (17 h/wk) than the one reported by Monticone et al33 (12 h/wk) and also than those reported by Ellis et al34 (15 h/wk) and Frazzitta et al35 (15 h/wk). More specifically, our EMC group patients received PT, OT, and ST for 6 days a week (40 min/d), while group exercise programs, provided by nurses, music therapists, and physical education instructors, were conducted 3 days a week for 60 minutes per day. Moreover, neurologists, physiatrists, nurses, and pharmacists provided group-based education once a week for 60 minutes.
In contrast to the MR program, our multidisciplinary intervention included group rehabilitation, in addition to individual one. Our psychological methods promoted patients’ motivation for exercise and social participation through group exercise and educational programs; the program was devised so that patients could continue to exercise enjoyably throughout the group program. During the group educational program, we provided a place where the patients could discuss among themselves what they had learned. Five theme lectures (promotion of disease comprehension, directions for exercise method, living guidance/foot care, patient compliance instructions, and nutritional education) on health education specific to PD were delivered to the intervention group. The group education program appeared to be a beneficial and practical intervention by complementing the rehabilitation intervention for patients with PD and meeting the growing demand for long-term care.20 To establish good exercise habits and increase self-efficacy, each patient was interviewed by a psychologist and received positive feedback regarding the effects of his or her rehabilitation.36 It is particularly worth noting that our EMC program led to improvements in all items, including ADL, communication, and physical pain, as well as in QOL motor items. These factors may contribute to improvements in the QOL of patients with PD, as also reported by Guo et al.20 Importantly, recent studies have suggested that aerobic exercise improved depression,37 while rhythmic exercise that incorporates dance might contribute to improvements associated with rising from a chair, postural stability, walking, falling, depression, and QOL.38,39 Moreover, water exercise might also contribute to improved posture and postural stability, as assessed by the UPDRS,40 and improved QOL.41 Recent studies have shown that exercise-induced general brain health might promote conditions for neuroplasticity important for facilitating motor learning, cognitive function, and overall behavioral performance (mood/motivation).16
Studies that have incorporated motor–cognitive training and aerobic exercise have supported the potential for maintaining motor improvements.16 A recent review has suggested that psychosocial factors are important modulators of motor learning.42 Motor learning may be important for the long-term maintenance of rehabilitation effects and its generalization to ADL. There were many opinions regarding how the motivation for daily activity was improved by group exercise and educational programs according to our questionnaire. The group intervention was an effective and positive psychiatric aspect for all patients.
Our study has some limitations that should be considered for further interpretation of our results. First, our study investigated the effects of such intervention in moderately ill patients and not in those at earlier or more advanced stages. Thus, it is difficult to generalize the results to other patient groups. Second, we did not follow-up the effects after discharge, which means that it is difficult to assess the effects in the home environment or for how long they persisted. Finally, no correlation between PDQ39 (total) and UPDRS (total) was found at admission and second time point in EMC. A potential placebo effect might occur because PDQ-39 was subjective evaluation.
In conclusion, an EMC appears to be effective for improving the QOL and managing both motor and nonmotor symptoms in moderately severe cases of PD. Further detailed studies are necessary to examine the mechanism by which rehabilitation improves the QOL.
Clinical Messages
Intervention with EMC decreases depression and improves physical parameters, including walking, falling, and posture.
Enhanced multidisciplinary care in patients with PD is effective in improving their quality of life.
Acknowledgments
The authors would like to thank Editage (www.editage.jp) for English-language editing.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: Part of this research was supported by Grant-in-Aid for Young Scientists, Japan Society for the Promotion of Science (KAKENHI [18K17778]).
ORCID iD: Kohei Marumoto  https://orcid.org/0000-0001-5781-0782
https://orcid.org/0000-0001-5781-0782
References
- 1. Lang AE, Lozano AM. Parkinson’s disease. First of two parts. N Engl J Med. 1998;339(15):1044–1053. [DOI] [PubMed] [Google Scholar]
- 2. Pont-Sunyer C, Hotter A, Gaig C, et al. The onset of nonmotor symptoms in Parkinson’s disease (the ONSET PD study). Mov Disord. 2015;30(2):229–237. [DOI] [PubMed] [Google Scholar]
- 3. Karlsen KH, Larsen J, Tandberg E, Maeland JG. Influence of clinical and demographic variables on quality of life in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1999;66(4):431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peto V, Jenkinson C, Fitzpatrick R, Greenhall R. The development and validation of a short measure of functioning and well being for individuals with Parkinson’s disease. Qual Life Res. 1995;4(3):241–248. [DOI] [PubMed] [Google Scholar]
- 5. Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson’s disease? J Neurol Neurosurg Psychiatry. 2000;69(3):308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Global Parkinson’s Disease Survey Steering Committee. Factors impacting on quality of life in Parkinson’s disease: results from an international survey. Mov Disord. 2002;17(1):60–67. [DOI] [PubMed] [Google Scholar]
- 7. Grosset D, Taurah L, Burn DJ, et al. A multicentre longitudinal observational study of changes in self reported health status in people with Parkinson’s disease left untreated at diagnosis. J Neurol Neurosurg Psychiatry. 2007;78(5):465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fox SH, Katzenschlager R, Lim SY, et al. The movement disorder society evidence-based medicine review update: treatments for the motor symptoms of Parkinson’s disease. Mov Disord. 2011;26(suppl 3):S2–S41. [DOI] [PubMed] [Google Scholar]
- 9. Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL. The effectiveness of exercise interventions for people with Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2008;23(5):631–640. [DOI] [PubMed] [Google Scholar]
- 10. Dixon L, Duncan D, Johnson P, et al. Occupational therapy for patients with Parkinson’s disease. Cochrane Database Syst Rev. 2007;(3):CD002813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rao AK. Enabling functional independence in Parkinson’s disease: update on occupational therapy intervention. Mov Disord. 2010;25(suppl 1):S146–S151. [DOI] [PubMed] [Google Scholar]
- 12. Herd CP, Tomlinson CL, Deane KH, et al. Comparison of speech and language therapy techniques for speech problems in Parkinson’s disease. Cochrane Database Syst Rev. 2012;(8):CD002814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herd CP, Tomlinson CL, Deane KH, et al. Speech and language therapy versus placebo or no intervention for speech problems in Parkinson’s disease. Cochrane Database Syst Rev. 2012;(8):CD002812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sapir S, Ramig LO, Fox CM. Intensive voice treatment in Parkinson’s disease: Lee Silverman voice treatment. Expert Rev Neurother. 2011;11(6):815–830. [DOI] [PubMed] [Google Scholar]
- 15. van Hooren MR, Baijens LW, Voskuilen S, Oosterloo M, Kremer B. Treatment effects for dysphagia in Parkinson’s disease: a systematic review. Parkinsonism Relat Disord. 2014;20(8):800–807. [DOI] [PubMed] [Google Scholar]
- 16. Petzinger GM, Fisher BE, McEwen S, Beeler JA, Walsh JP, Jakowec MW. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson’s disease. Lancet Neurol. 2013;12(7):716–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rochester L, Espay AJ. Multidisciplinary rehabilitation in Parkinson’s disease: a milestone with future challenges. Mov Disord. 2015;30(8):1011–1003. [DOI] [PubMed] [Google Scholar]
- 18. Tan SB, Williams AF, Kelly D. Effectiveness of multidisciplinary interventions to improve the quality of life for people with Parkinson’s disease: a systematic review. Int J Nurs Stud. 2014;51(1):166–174. [DOI] [PubMed] [Google Scholar]
- 19. Prizer LP, Browner N. The integrative care of Parkinson’s disease: a systematic review. J Parkinsons Dis. 2012;2(2):79–86. [DOI] [PubMed] [Google Scholar]
- 20. Guo L, Jiang Y, Yatsuya H, Yoshida Y, Sakamoto J. Group education with personal rehabilitation for idiopathic Parkinson’s disease. Can J Neurol Sci. 2009;36(1):51–59. [DOI] [PubMed] [Google Scholar]
- 21. Trend P, Kaye J, Gage H, Owen C, Wade D. Short-term effectiveness of intensive multidisciplinary rehabilitation for people with Parkinson’s disease and their carers. Clin Rehabil. 2002;16(7):717–725. [DOI] [PubMed] [Google Scholar]
- 22. Litvan I, Bhatia KP, Burn DJ, et al. Movement Disorders Society Scientific Issues Committee Report: SIC Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov Disord. 2003;18(5):467–486. [DOI] [PubMed] [Google Scholar]
- 23. Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–442. [DOI] [PubMed] [Google Scholar]
- 24. Keus SH, Bloem BR, Hendriks EJ, et al. Evidence-based analysis of physical therapy in Parkinson’s disease with recommendations for practice and research. Mov Disord. 2007;22(4):451–460. [DOI] [PubMed] [Google Scholar]
- 25. Keus SH, Munneke M, Nijkrake MJ, Kwakkel G, Bloem BR. Physical therapy in Parkinson’s disease: evolution and future challenges. Mov Disord. 2009;24(1):1–14. [DOI] [PubMed] [Google Scholar]
- 26. Baijens LW, Speyer R. Effects of therapy for dysphagia in Parkinson’s disease: systematic review. Dysphagia. 2009;24(1):91–102. [DOI] [PubMed] [Google Scholar]
- 27. Fahn S, Elton RL. Unified Parkinson’s Disease Rating Scale In: Fahn S, Marsden CD, Calne D, Goldstein M, eds. Recent Developments in Parkinson’s Disease. Florham Park, NJ: Macmillan Health Care Information; 1987:153–163. [Google Scholar]
- 28. Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 29. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25(15):2649–2653. [DOI] [PubMed] [Google Scholar]
- 30. Ferrazzoli D, Ortelli P, Zivi I, et al. Efficacy of intensive multidisciplinary rehabilitation in Parkinson’s disease: a randomised controlled study. J Neurol Neurosurg Psychiatry. 2018;89(8):828–835. doi:10.1136/jnnp-2017-316437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Steffen T, Seney M. Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-item Short-Form Health Survey, and the Unified Parkinson Disease Rating Scale in people with parkinsonism. Phys Ther. 2008;88(6):733–746. [DOI] [PubMed] [Google Scholar]
- 32. Shulman LM, Gruber-Baldini AL, Anderson KE, Fishman PS, Reich SG, Weiner WJ. The clinically important difference on the Unified Parkinson’s Disease Rating Scale. Arch Neurol. 2010;67(1):64–70. [DOI] [PubMed] [Google Scholar]
- 33. Monticone M, Ambrosini E, Laurini A, Rocca B, Foti C. In-patient multidisciplinary rehabilitation for Parkinson’s disease: a randomized controlled trial. Mov Disord. 2015;30(8):1050–1058. [DOI] [PubMed] [Google Scholar]
- 34. Ellis T, Katz DI, White DK, DePiero TJ, Hohler AD, Saint-Hilaire M. Effectiveness of an inpatient multidisciplinary rehabilitation program for people with Parkinson disease. Phys Ther. 2008;88(7):812–819. [DOI] [PubMed] [Google Scholar]
- 35. Frazzitta G, Maestri R, Bertotti G, et al. Intensive rehabilitation treatment in early Parkinson’s disease: a randomized pilot study with a 2-year follow-up. Neurorehabil Neural Repair. 2015;29(2):123–131. [DOI] [PubMed] [Google Scholar]
- 36. Ellis T, Cavanaugh JT, Earhart GM, et al. Factors associated with exercise behavior in people with Parkinson disease. Phys Ther. 2011;91(12):1838–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Uc EY, Doerschug KC, Magnotta V, et al. Phase I/II randomized trial of aerobic exercise in Parkinson disease in a community setting. Neurology. 2014;83(5):413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sharp K, Hewitt J. Dance as an intervention for people with Parkinson’s disease: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2014;47:445–456. [DOI] [PubMed] [Google Scholar]
- 39. Hashimoto H, Takabatake S, Miyaguchi H, Nakanishi H, Naitou Y. Effects of dance on motor functions, cognitive functions, and mental symptoms of Parkinson’s disease: a quasi-randomized pilot trial. Complement Ther Med. 2015;23(2):210–219. [DOI] [PubMed] [Google Scholar]
- 40. Vivas J, Arias P, Cudeiro J. Aquatic therapy versus conventional land-based therapy for Parkinson’s disease: an open-label pilot study. Arch Phys Med Rehabil. 2011;92(8):1202–1210. [DOI] [PubMed] [Google Scholar]
- 41. Ayán C, Cancela J. Feasibility of 2 different water-based exercise training programs in patients with Parkinson’s disease: a pilot study. Arch Phys Med Rehabil. 2012;93(10):1709–1714 [DOI] [PubMed] [Google Scholar]
- 42. Zemankova P, Lungu O, Bares M. Psychosocial modulators of motor learning in Parkinson’s disease. Front Human Neurosci. 2016;10:74. [DOI] [PMC free article] [PubMed] [Google Scholar]