Short abstract
This review provides a summary of recent discoveries in choline transport and the proteins mediating it with a specific focus on the choline transporter-like proteins (CTL)/solute carriers 44 A (SLC44A) and their role in phospholipid metabolism. Since its initial cloning, particularly, the CTL1/SLC44A1 transporter has been investigated further and its ubiquitous expression characterized in various cells and tissues of mouse, rat, and human origin. We describe the role of this choline transporter both in the plasma membrane and in the mitochondria and summarize novel aspects of choline transport regulation in the muscle, nervous system, and cancer.
Impact statement
This review will provide a summary of recent advances in choline transport research and highlight important novel areas of focus in the field.
Keywords: Choline, nutrition, transporters, variants, lipids, regulation
Introduction and purpose
In our review published in EBMin 2006,1we outlined the importance of choline as a nutrient that is crucial for the synthesis of the membrane building blocks phosphatidylcholine and sphingomyelin and as methyl group donor in the homocysteine-methionine cycle. Our review followed shortly after the initial cloning of the ubiquitous choline transporter solute carrier 44A1 (SLC44A1)/choline transporter-like protein (CTL1). The intent of this review is to provide an update on choline transport research since then, with a focus on discoveries related to the proteins mediating it.
Importance of choline
Choline cannot be synthesized by the human body and is therefore an essential nutrient. Adequate daily choline intake is recommended at 550 mg/day for men and 425 mg/day for women by the Institute of Medicine and 400 mg/day for all adults by the European Food Safety Authority. These recommendations are often not met, with a worldwide range in the daily intake from 284 mg/day to 468 mg/day for men and 263 mg/day to 374 mg/day for women.2Data obtained from the National Health and Nutrition Examination Survey between 2013 and 2014 also indicate lower than recommended choline intakes for North American men (402 mg) and women (278 mg),3and a food questionnaire in Canada revealed similar levels for Canadian men (372 mg/day) and women (292 mg/day).4In Europe, choline intake ranged from 269 to 468 mg/day in adults as determined in national food panels of seven European countries.5Pregnant and lactating women appear to often not meet the recommendations, which is of important as adequate choline intake may prevent neural tube defects and preeclampsia in this population group and is particularly important for the growth and development of the breastfed neonate.
If choline supply is deficient, dietary fats accumulate in the liver causing non-alcoholic steatohepatitis due to the lack of phospholipids that are building blocks of lipoproteins. This liver disease resulting from choline deficiency has been extensively characterized in animal models and human subjects.6–9At the molecular level, choline deficiency causes DNA strand breaks,10which is the consequence of impaired DNA methylation due to the lack of methyl groups, altered mitochondrial membrane composition and hence leakage of reactive oxygen species, and diminished thymidylate synthesis due to the lack of folate.11
The choline transporter family of SLC44A proteins
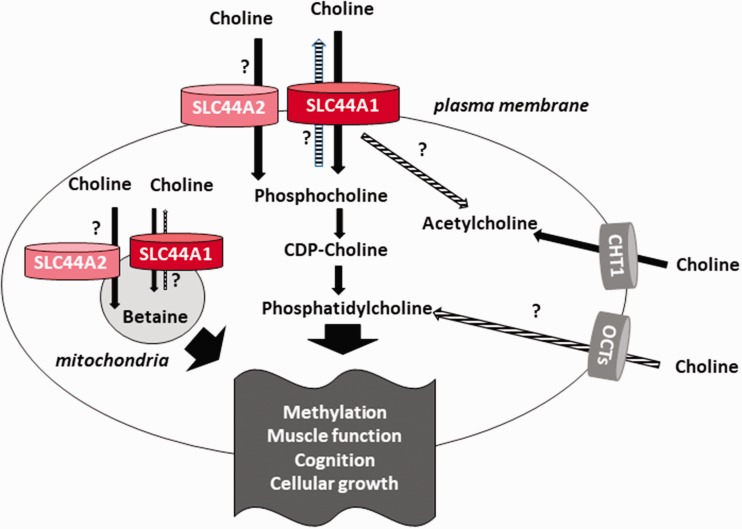
As a charged molecule, choline cannot freely cross the membrane lipid bilayer and depends on protein transporters to enter the cell. Three different choline transport systems have been identified (Figure 1). The high-affinity choline transporter CHT1 is structurally closely related to the SGLT-sodium glucose cotransporter family and mediates Na+-dependent choline transport across the plasma membrane in neuronal tissuesThe purpose of this transport is to provide choline for the synthesis of the neurotransmitter acetylcholine. Choline can also be transported by the members OCT1 and OCT2 of the organic cation transporter (OCT) family with a low affinity. These polyspecific transport proteins have a binding affinity for choline in the high micromolar range and depend on the membrane potential for choline transport.
Figure 1.
Choline transporters for betaine, acethylcholine, and phospholipid synthesis. CTLs (SLC44A1 and -2) and OCTs are widely expressed while choline transporter CHT1 in neuron-specific. Functions proposed but not confirmed are indicated (?). CDP: cytidine diphosphate; CDP-choline: citidyldiphosphocholine; CHT1: choline transporter CHT1 in neuron-specific; OCT: organic cation transporter; SLC44A: solute carriers 44 A.
The SLC44A family was only characterized in recent years and comprises five proteins termed SLC44A1 to SLC44A5, which are all proteins of approximately 70 kDa. Based on sequence homology, SLC44A1 and SLC44A3 form a subgroup, while SLC44A2, SLC44A4, and SLC44A5 share sequence identity between them.12Of these proteins, only SLC44A1 has been firmly established as a choline transporter (Figure 1), yet some data indicate that SLC44A2 might transport choline as well.13,14SLC44A4 appears to be involved in non-neuronal acetylcholine synthesis.15The function of SLC44A3 and A5 remains still unclear to date. In recent years, particularly SLC44A1 has been further studied.
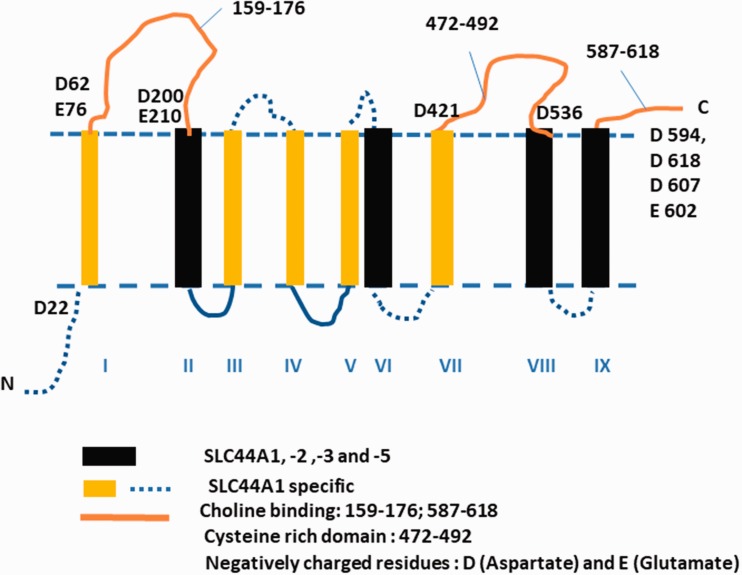
In 2000, CTL1 (now termed SLC44A1) was cloned from Torpedo marmorata and rat,16followed by cloning of the human17and we cloned a mouse18transporter. The first available antibody we used for the CTL1/CDw92 human antigen17recognized a protein smaller than the predicted size of CTL1. Therefore, we developed two new antibodies termed LV-58 and EN-627, referring to the amino acid from which the peptide sequence starts in the protein. Peptide sequences were chosen, which are conserved across mouse, human, and rat CTL1/SLC44A1.19Under native conditions, both LV-58 and EN-627 recognize a protein of approximately 70 kDa, which is close to the predicted 72 kDa size of CTL1/SLC44A1. These antibodies also allowed for immunostaining experiments on cultured cells and potentially hint on the alignment of CTL1 protein in the membrane. Prediction programs yield a protein with 9 or 10 transmembrane (TM) domains and give varying results on the localization of the N- and C-terminal ends. In our first review,1we showed a model for the 10 TM domain variants using a prediction program and the mouse CTL1 sequence. Judging from our immunostaining experiments with the new antibodies, it appears that the N-terminus is located intracellularly and the C-terminus extracellularly, since both LV-58, which binds in the predicted first extracellular loop, and the C-terminal antibody EN-627, which binds at the very end of the C-terminus, recognize the protein in live cells with an unpermeabilized plasma membrane. These observations would agree with the nine TM models of mouse and human CTL1.19Here, we compared CTL1 protein structure with the structure of other family members (SLC44A 1–5) and with better characterized choline proteins, choline kinase and neuronal choline transporter CHT1. Figure 2shows the CTL1 membrane topology, which includes features we identified to be highly conserved among different species and within the SLC44A family. CTL1 protein is composed of nine TM domains, the intracellular N-terminus and the extracellular C-terminus. The sequence alignment of rat, mouse, and human SLC44A proteins establishes protein homology for four TM domains (2, 6, 8, and 9) with the highest conservation in TM8 and TM9, suggesting that they are surrounded with the most critical functional domains, perhaps including the substrate-choline binding site(s) in the entire family. It is also highly likely that the negatively charged Asp (D) and Glu (E) residues abundant at the C-terminus are involved in the binding and transport of positively charged choline and protons. This information was based on the protein sequence homology, and it could be used as a reference point in future research since no further experimental information on the membrane organization or substrate biding mechanism for CTL1 or other family members is currently available.
Figure 2.
CTL1 protein structure. The CTL1 membrane topology and features identified to be highly conserved among different species and within the SLC44A family. SLC44A: solute carriers 44 A.
Both the dietary requirements for choline and therefore choline metabolism are influenced by single nucleotide polymorphisms (SNPs).10,20–22CTL1/SLC44A1 SNPs (rs7873937, rs2771040, rs6479313, and rs3199966) were identified in those individuals that developed muscle damage on a choline-deficient diet.22These alleles varied among the ethnic groups analyzed in the study: rs7873937 was present in approximately one-quarter of study participants of African descent, Mexican Americans, and European Americans, while it was entirely absent in Asian Americans. Interestingly, the Asian American population had none of the SLC44A1 alleles that were discovered to be linked to muscle disease in choline deficiency. Furthermore, both the distribution of rs2771040 and rs3199966 was at around 71% in those study participants of African descent, while approximately a quarter of the European Americans featured these alleles and a third of those of Mexican descent. These polymorphisms render carriers to a particular sensitivity to choline efficiency in the muscle. The rs3199966, which results in a serine to alanine substitution genotype, was further evaluated in a recent study,20where participants with this genotype as well as those with the rs7873937 SNP were linked to greater turnover of betaine to methionine on a subgroup receiving high choline supplementation.
Choline uptake studies have established CTL1/SLC44A1 as an Na+-independent choline transporter at the plasma membrane of various cells and tissues.18,23,24CTL1/SLC44A1-mediated choline transport appears to be linked to both phospholipid and betaine synthesis but might also mediate choline transport for non-neuronal acetycholine synthesis25–27(Figure 1). CTL1/SLC44A1 has an intermediate affinity for choline, with a Kmin the low micromolar range. Similar to the high-affinity choline transporter CHT1, CTL1 is selectively inhibited by the choline analogue hemicholinium-3 (CH-3), yet with a much lower sensitivity. In recent years, further choline uptake studies confirmed the expression and choline transporter function of CTL1/SLC44A1 in human lung alveolar cells,28mouse bone marrow-derived macrophages,42rat renal tubule epithelial cells,30human keratinocytes,31human brain microvascular endothelial cells,14rheumatoid arthritis synovial fibroblasts,40mouse muscle and liver cell lines,32,33and numerous cancer cell lines26,28,34–39,41as summarized in Table 1.
Table 1.
Studies assessing choline uptake in cells with SLC44A1 as the main choline transporter.
| Cell type | Origin | Organelle | References |
|---|---|---|---|
| THP-1 monocytes | Human | Plasma membrane | Fullerton et al.23 |
| Lung adenocarcinoma A549, H1299, and SPC-A-1 | Human | Plasma membrane | Wang et al.29 |
| A549 lung alveolar cells | Human | Plasma membrane | Ishiguro et al.28 |
| Alveolar type II | Rat | Plasma membrane | Ishiguro et al.28 |
| NG108-15 neuroblastoma × glioma cell line | Mouse | Plasma membrane | Machova et al.36 |
| Keratinocytes | Human | Plasma membrane | Uchida et al.31 |
| C2C12 myotubes | Mouse | Mitochondria | Michel and Bakovic32 |
| FL83B hepatocytes | Mouse | Mitochondria | Michel and Bakovic32 |
| Liver | Mouse | Mitochondria | Michel and Bakovic32 |
| MCF7 breast cancer cells | Human | Mitochondria | Michel and Bakovic32 |
| C2C12 myotubes | Mouse | Mitochondria, plasma membrane | Michel et al.33 |
| SH-SY5Y and LA-N-2 neuroblastoma cells | Human | Plasma membrane | Yamada et al.37 |
| MCF7 breast cancer cells | Human | Plasma membrane | Ward et al. |
| NCI-H69 small-cell lung carcinoma cells | Human | Plasma membrane | Inazu et al.26 |
| A-172 and U-251MG glioblastoma cell lines | Human | Plasma membrane | Taguchi et al.38 |
| C2C12 myotubes | Mouse | Mitochondria, plasma membrane | Schenkel and Bakovic46 |
| Skin fibroblasts | Human | Plasma membrane | Schenkel et al.43 |
| hBMEC brain microvascular epithelial cells | Human | Plasma membrane | Iwao et al.14 |
Mitochondrial choline transporters
Despite the fact that the conversion of choline to betaine is long known to take place in the mitochondrial matrix of liver and kidney, it was unclear how the charged molecule choline can cross the mitochondrial membrane. Using subcellular fractionation and immunostaining techniques, we discovered that SLC44A1 is also present in the mitochondrial membrane of mouse and human tissues.32Organelle preparations of isolated mitochondria showed that radiolabeled 3H-choline (HC3) is transported into the mitochondria, and that this transport is selectively inhibited by micromolar concentrations of HC3 and by an excess of choline. Incubation of isolated mitochondria with CTL1/SLC44A1-specific antibodies also significantly blocked mitochondrial choline transport, demonstrating the function of the transporter in the mitochondrial membrane. Overexpression of a His-tagged CTL1/SLC44A1 in cultured mouse muscle cells resulted in the localization of His-tagged protein in the mitochondria and a doubling of mitochondrial choline transport. An obvious explanation for the necessity of a choline transporter in liver and kidney mitochondria is the oxidation of choline to betaine inside the mitochondrial matrix of these tissues (Figure 1). Nevertheless, mitochondrial CTL1/SLC44A1 could be detected in murine heart tissue and cultured mouse muscle and human breast cancer cells mitochondria as well. In choline-deficient skin fibroblast from the postural orthostatic tachycardia syndrome (POTS) patient mentioned above, CTL1/SLC44A1 protein is reduced, and at the same time, oxygen consumption, mitochondrial potential, and glycolytic activity are diminished and mitochondrial function therefore impaired.43Recent studies could also localize CTL2/SLC44A2 protein to the mitochondria of human esophageal cells,41renal tubule epithelial cells,30human tongue carcinoma cells,39and human brain microvascular epithelial cells.14The purpose of a widely expressed choline transporter in the mitochondrial membrane is not clear yet, but it is likely that there is a link between CTL1/SLC44A1-mediated choline transport and mitochondrial phospholipid metabolism. Furthermore, the presence of a choline transporter in the mitochondrial membrane might link choline transport to phospholipid synthesis in the endoplasmic reticulum (ER) because free choline can be generated from phosphatidylcholine in the mitochondria and become available for the synthesis of phospholipids in the ER. An interesting question to answer in the future is whether mitochondrial CTL1/SLC44A1 could function as a shuttle to transport choline both ways across the mitochondrial membrane and potentially serve as a transporter for other substrates as well (Figure 1).
Novel importance of choline in the muscle
Research of recent years indicates that the lack of choline does not only affect hepatic lipid metabolism but disturbs skeletal muscle lipid homeostasis as well. Choline-deficient muscle cells have fragile membranes caused by an inappropriate phospholipid ratio and by apoptosis.44,45We33have shown that in cultured mouse C2C12 muscle cells, choline deficiency alters intracellular lipid metabolism by reducing phosphatidylcholine synthesis, modifying phosphatidylcholine fatty acid side chains, and slowing down triglyceride metabolism resulting in lipid droplet accumulation inside the muscle cells. This lipid accumulation is distinct from that observed in the liver because skeletal muscles do not produce lipoproteins to shuttle lipids away from the organ and neither do they have the ability to methylate phosphatidylethanolamine to phosphatidylcholine. Choline-deficient muscle rather has impaired Kennedy pathway for phosphatidylcholine synthesis (Figure 1) and therefore lipid precursors accumulate inside the muscle cells. In this pathway, choline entering the cell is rapidly phosphorylated by choline kinase to phosphocholine, which is then activated to CDP-choline by CDP-choline: DAG (diacylglycerol) cholinephosphotransferase. The synthesis of phosphatidylcholine results from the addition of diacylglycerol to CDP-choline and the removal of the CDP group, a reaction catalyzed by CTP (cytidine triphosphate): phosphocholine cytidyltransferase. If this reaction is impaired, diacylglyerols remain unincorporated and are stored inside the cell, leading to storage lipid droplet accumulation in the muscle cells.
We have recently established that muscle choline transport is regulated by fatty acids, implicating a correlation between glycerolipid metabolism and choline availability.46In this study, treatment of mouse myotubes with palmitic acid reduced total SLC44A1 protein content and SLC44A1 at the plasma membrane, while this SLC44A1 fraction was unaffected by oleic acid treatment. In turn, oleic acid reduced the amount of SLC44A1 protein in the mitochondrial membrane, while palmitic acid treatments had no effect on this fraction. Consequently, palmitic acid reduced choline uptake across the plasma membrane by 50%.
Several study participants consuming a diet low in choline presented with muscle damage rather than liver damage in a recent study,22which further supports the notion that functional choline metabolism is crucial for normal skeletal muscle function. Interestingly, there was an association between muscle dysfunction and polymorphisms of the choline transport protein SLC44A1, which is abundant in skeletal muscle18and is upregulated in numerous myopathies.47In mice lacking one allele of CTP:phosphoethanolamine cytidylyl transferase (Pcyt2+/−mice), choline supplementation was able to restore lipid metabolism and therefore alleviate the disturbances in glucose metabolism in the skeletal muscle of these mice.48Choline reduced triglyceride accumulation by decreasing fatty acid synthesis and lipogenesis in the muscle of these mice, increased fatty acid oxidation, and improved insulin signaling. In summary, these recent data show that choline is not only important for lipid metabolism in the liver, but that choline deficiency may alter phosphatidylcholine metabolism and in consequence all glycerolipid metabolism in muscle cells.
In addition to phospholipid synthesis, CTL1/SLC44A1 and CTL2/SLC44A2 may mediate choline transport for non-neuronal cholinergic systems, as both proteins were recently found to be the major choline transporters in macrophages and fibroblasts of the synovium and cartilage of the hip joint in arthritis patients.49Rheumatoid arthritis appears to be linked to the cholinergic anti-inflammatory system and the choline transporters in the hip joint appear to mediate choline transport for acetylcholine synthesis.
The importance of functional choline transport in fibroblasts was further obviated by analysis of skin fibroblasts from a patient with POTS and apparent choline deficiency.43CTL1/SLC44A1 expression was reduced in these cells, and hence choline transport was diminished. Membrane homeostasis was disturbed in the fibroblasts, as reflected by reduced phosphatidylcholine:phosphatidylethanolamine and sphingomyelin:cholesterol ratios and altered phospholipid fatty acid composition. This study presented the first known case of reduced CTL1/SLC44A1 expression in a patient and the resulting mitochondrial dysfunction, reduced choline transport, and impaired membrane homeostasis.
Link between CTL1/SLC44A1 and cancer
Fast-growing cells such as cancerous cells depend on a rapid phospholipid synthesis to provide building blocks for their plasma membrane assembly and consequently their proliferation. Brain, breast, prostate, and colon cancer cells have been characterized by elevated choline and phosphocholine levels and abnormal choline metabolism, which correlates with malignancy of the cancer. In recent years, CTL/SLC44A protein overexpression was detected in various cancer cell lines25,39,41,50, and the proteins are involved in choline transport in these cells.15For example, expression of CTL1/SLC44A1, CTL2/SLC44A2, and CTL4/SLC44A4 messenger RNA is higher in cancerous MCF7 breast epithelial cells than in the MCF10A non-cancerous comparative cell line.25At the same time, the ratio of phosphocholine to glycerophosphocholine as well as the total choline-containing metabolites is elevated in these breast cancer cells compared to non-cancerous cells lines.51In human esophageal cancer cells, both CTL1/SLC44A1 and CTL2/SLC44A2 are expressed and apparently mediate an Na+-independent, HC3-inhibitable choline transport in these cells.41The increased expression of the choline transporter in cancer cells has been shown to be accompanied by a high expression of the Na+/H+exchanger protein to elevate H+ levels inside the cell and Na+ outside.26,37The acidification of the extracellular milieu by inhibition of the exchanger was shown to block choline transport in human colon carcinoma cells34and small cell lung carcinoma cells.26Hence, CTL1/SLC44A1 apparently requires a proton gradient to drive choline transport inside the cell. The inhibition of CTL1/SLC44A1-mediated choline transport with HC3 appears to inhibit the growth of small cell lung carcinoma,26human leukemic T-cells,25and colon cancer25,34cell lines. In all of these cells, CTL1/SLC44A1 has been linked to non-neuronal acetylcholine synthesis. The mechanism for cancer cell death following CTL1/SLC44A1 inhibition is not yet clear, but the activity of caspase-3/7 is increased and apoptosis ensues. When choline transport inside the cell is limited and intracellular choline is deficient, cells increase alternative pathways to generate phospholipids. The side effect of these pathways is an elevated ceramide production, which is a promoter of apoptosis.52The inhibition of choline transport in cancer cells should be further elucidated as a target for cancer therapy in the future.
Emerging role of CTL1/SLC44A1 in the nervous system
The importance of choline for adequate phospholipid supply in the brain is reflected in the benefits of choline intake on cognition. The brains of Alzheimer disease patients have reduced phosphatidylcholine and phosphatidylethanolamine levels and increased levels of their metabolites, glycerophosphocholine, and glycerophosphoethanolamine.53What is more, additional phosphatidylcholine could be synthesized through methylation of phosphatidylethanolamine by the enzyme phosphatidylethanolamine-N-methyltransferase (PEMT). This enzyme is primarily expressed in the liver, but there is also PEMT activity in the brain which is highest in the perinatal period. The methyl groups for this reaction originate from S-adenosylmethionine (SAM), which is generated through the methylation of homocysteine to methionine followed by adenylation of methionine to form SAM. Methyltetrahydrofolate and betaine can provide methyl groups for this pathway. Hence, choline supply to the brain is crucial to ensure adequate phospholipid synthesis in this organ.
While CHT1 is the mediator of high-affinity choline transport for acetylcholine synthesis in cholinergic nerve endings, the ubiquitous CTL1/SLC44A1 choline transporter is also expressed in human47and mouse18brain, in neurons and oligodendrocytes,54and in cultured astrocytes,24neurons,55as well as brain microvascular endothelial cells14(see Michel and Bakovic19for summary of CTL1/SLC44A1 nervous system expression profile). Of the SLC44A proteins, only CTL1 is significantly expressed in the nervous system, while SLC44A2 is barely detectable and SLC44A3-5 are not expressed. In 2009, CTL1/SLC44A1 knockdown was shown to prevent the growth of a cholinergic neuroblastoma cell line.36This cell line does not express CHT1 yet features a high-affinity choline transport mediated by CTL1/SLC44A1, pointing further to a role of the transporter in the nervous system. We are currently further investigating the role of CTL1/SLC44A1 in the brain. Judging from our results, the choline transporter is linked to neurogenerative disease and its lack in the brain results in disturbance of phospholipid metabolism and in consequence very long chain fatty acid homeostasis.
Gene transcription and regulation with choline availability
CTL1/SLC44A1 is a choline/H+-antiporter, the function that could be particularly relevant in the electrogenic tissues such as skeletal muscle and heart. We produced evidence showing that CTL1/SLC44A1 is differently regulated with muscle cell differentiation33and choline availability.43Choline supplementation restores the level of CTL1/SLC44A1 mRNA and protein and improve choline transport at the plasma membrane and mitochondria in CTL1/SLC44A1-deficient cells43and in the skeletal muscle of obese mice.48CTL1/SLC44A1 protein is a biomarker of monocytic cell differentiation and is elevated in differentiated dendritic cells.17Deregulated choline biochemistry is a hallmark of malignant transformations and CTL1/SLC44A1 gene became activated by anomalous cell-cycle regulators.25Based on the microarray data, CTL1/SLC44A1 is transcriptionally upregulated during myoblast to myotube differentiation, and the cell differentiation to a cardiac lineage substantially induces CTL1/SLC44A1 transcription during later stages of differentiation period when beating cardiomyocytes appear (GEO database, ID:68413151). In the first and the most essential step in studying transcriptional regulation, we have identified and isolated the regulatory promoter of the human CTL1/SLC44A1 gene.47There is a high structural conservation among mammalian CTL1/SLC44A1 genes, and they share a strong promoter immediately upstream from the transcriptional start site. The initiator of transcription is the transcription factor Sp1 bound to a longer stretch of guanosine-cytosine (GC) rich elements. The most conserved regions include the binding sites for the ubiquitous factors Sp1, E2F, and NF1 and the cell-specific zinc finger transcription factor GATA (immune cells and heart muscle) and MyoD-class I myosine (skeletal muscle).47The regulation of CTL1/SLC44A1 promoter with those and other factors in the context of the cell type, differentiation, and choline availability needs to be further elucidated.
Methylation plays a central role in development, gene imprinting, and gene silencing, and there is an adaptive epigenetic response to choline availability. Choline oxidation product betaine is a direct methyl group donor for the formation of SAM, the principal histone, and DNA methylation agent.56Mounting evidence suggest that choline exposure during pregnancy alters histone and DNA methylation in placenta and embryo that persist postnatally and have life-time effects. Maternal choline intake affects the epigenetic state of fetal cortisol regulated genes in humans.57Human intergenerational studies showed that maternal methyl donors influence infant DNA methylation in genes related to metabolism, growth, and maintenance of DNA methylation. Betaine specifically increases methylation of human DNMT1-DNA methyltransferase 1, POMC-proopiomelanocortin, and RXRA-retinoic acid receptor alpha.58,59Gestational choline deficiency upregulates Dnmt1 demethylase and causes global and Igf2 (insilin-like growth factor 2) DNA hypermethylation.60Igf2 is an imprinted gene and maternal choline and betaine decreased the Igf2 expression and fetal adiposity.61On the other hand, gestational choline supply increases the histone methylation by simultaneously activating Kmt1c and Kmt1a methyltransferases and increasing the concentration of SAM.62Therefore, choline has a long-term epigenomic impacts on human health as presented in numerous benefits of prenatal choline supplementation on neurogenesis, gestational diabetes, and non-alcoholic liver disease.63
CTL1/SLC44A1 regulation by methylation is not established. The CTL1/SLC44A1 promoter is rich in CpG sequence, and they could be hypo–hypermethylated at some stages of development and/or by choline availability. The global CpG-methylation and degree of methylation at specific CpG sites in CTL1/SLC44A1 promoter need to be established. The methylation status of the CTL1/SLC44A1 gene segments for which we already established to contain several functional CpG islands47could be monitored together with the methylation of the insulin-like growth factor (Igf2) gene as a choline sensitive control since the Igf2 regulation by methylation is well characterized and known to be regulated by choline.60–62
Conclusions and prospects
Since the initial cloning, particularly the CTL1/SLC44A1 protein has been characterized as an Na+-independent, HC3-sensitive choline transporter that is ubiquitously expressed. It mediates choline transport both across the plasma membrane and the mitochondrial membrane and is linked to all aspects of choline metabolism, e.g. betaine, phospholipid, and acetylcholine synthesis. In addition to its role in classical organs of phospholipid metabolism such as the liver, exciting emerging data elucidate novel roles of the protein in the muscle and the nervous system. The observed overexpression of CTL1/SLC44A1 in cancer cells could be linked to the high demand for phospholipids of these cells, and future research should elucidate a potential for manipulation of this choline transporter to diminish cancer cell growth. Future epigenetic studies should complement the promoter/transcription factor analyses and to give more insights about the regulation of CTL1/SLC44A1 expression according to strict demands for choline during the cell growth, differentiation, and development.
Acknowledgments
The authors dedicate this work to Dr. Zongfei Yuan who pioneered the CTL1 work in our laboratory.
Author Contributions
Both authors wrote, compiled, and edited the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This study was supported by an operating grant from the Natural Sciences and Engineering Research Council of Canada (to M Bakovic).
References
- 1.Michel V, Yuan Z, Ramsubir S, Bakovic M. Choline transport for phospholipid synthesis. Exp Biol Med (Maywood) 2006; 231:490–504 [DOI] [PubMed] [Google Scholar]
- 2.Wiedeman AM, Barr SI, Green TJ, Xu Z, Innis SM, Kitts DD, Dietary choline intake: current state of knowledge across the life cycle. Nutrients 2018; 1:pii:, E1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallace TC, Fulgoni VL., III. Assessment of total choline intakes in the United States. J Am Coll Nutr 2016; 35:108–12 [DOI] [PubMed] [Google Scholar]
- 4.Gao X, Wang Y, Randell E, Pedram P, Yi Y, Gulliver W, Sun G. Higher dietary choline and betaine intakes are associated with better body composition in the adult population of Newfoundland, Canada. PLoS One 2016; 11:e0155403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.EFSA Panel on Dietetic Products NaAN. Dietary reference values for choline. EFSA J 2016; 16:e04484 [Google Scholar]
- 6.Lombardi B, Pani P, Schlunk FF. Choline-deficiency fatty liver: impaired release of hepatic triglycerides. J Lipid Res 1968; 9:437–46 [PubMed] [Google Scholar]
- 7.Lombardi B, Ugazio G, Raick AN. Choline-deficiency fatty liver: relation of plasma phospholipids to liver triglycerides. Am J Physiol 1966; 210:316. [DOI] [PubMed] [Google Scholar]
- 8.Williams WL, Cardle JB, Meader RD. The nature of dietary fat and the pattern of hepatic liposis in choline-deficient mice. Yale J Biol Med 1959; 31:263–70 [PMC free article] [PubMed] [Google Scholar]
- 9.Zeisel SH. Choline deficiency. J Nutr Biochem 1990; 1:332–49 [DOI] [PubMed] [Google Scholar]
- 10.da Costa KA, Kozyreva OG, Song J, Galanko JA, Fischer LM, Zeisel SH. Common genetic polymorphisms affect the human requirement for the nutrient choline. FASEB J. 2006; 20:1336–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeisel SH. Dietary choline deficiency causes DNA strand breaks and alters epigenetic marks on DNA and histones. Mutat Res 2012; 733:34–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Traiffort E, O’Regan S, Ruat M. The choline transporter-like family SLC44: properties and roles in human diseases. Mol Aspects Med 2013; 34:646–54 [DOI] [PubMed] [Google Scholar]
- 13.Kommareddi PK, Nair TS, Thang LV, Galano MM, Babu E, Ganapathy V, Kanazawa T, McHugh JB, Carey TE. Isoforms, expression, glycosylation, and tissue distribution of CTL2/SLC44A2. Protein J 2010; 29:417–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwao B, Yara M, Hara N, Kawai Y, Yamanaka T, Nishihara H, Inoue T, Inazu M. Functional expression of choline transporter like-protein 1 (CTL1) and CTL2 in human brain microvascular endothelial cells. Neurochem Int 2016; 93:40–50 [DOI] [PubMed] [Google Scholar]
- 15.Song P, Rekow SS, Singleton CA, Sekhon HS, Dissen GA, Zhou M, Campling B, Lindstrom J, Spindel ER. Choline transporter-like protein 4 (CTL4) links to non-neuronal acetylcholine synthesis. J Neurochem 2013; 126:451–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Regan S, Traiffort E, Ruat M, Cha N, Compaore D, Meunier FM. An electric lobe suppressor for a yeast choline transport mutation belongs to a new family of transporter-like proteins. Proc Natl Acad Sci U S A 2000; 97:1835–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wille S, Szekeres A, Majdic O, Prager E, Staffler G, Stockl J, Kunthalert D, Prieschl EE, Baumruker T, Burtscher H, Zlabinger GJ, Knapp W, Stockinger H. Characterization of CDw92 as a member of the choline transporter-like protein family regulated specifically on dendritic cells. J Immunol 2001; 167:5795–804 [DOI] [PubMed] [Google Scholar]
- 18.Yuan Z, Wagner L, Poloumienko A, Bakovic M. Identification and expression of a mouse muscle-specific CTL1 gene. Gene 2004; 341:305–12 [DOI] [PubMed] [Google Scholar]
- 19.Michel V, Bakovic M. The ubiquitous choline transporter SLC44A1. Cent Nerv Syst Agents Med Chem 2012; 12:70–81 [DOI] [PubMed] [Google Scholar]
- 20.Ganz AB, Cohen VV, Swersky CC, Stover J, Vitiello GA, Lovesky J, Chuang JC, Shields K, Fomin VG, Lopez YS, Mohan S, Ganti A, Carrier B, Malysheva OV, Caudill MA. Genetic variation in choline-metabolizing enzymes alters choline metabolism in young women consuming choline intakes meeting current recommendations. Int J Mol Sci 2017; 18: pii:, E252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohlmeier M, da Costa KA, Fischer LM, Zeisel SH. Genetic variation of folate-mediated one-carbon transfer pathway predicts susceptibility to choline deficiency in humans. Proc Natl Acad Sci U S A 2005; 102:16025–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.da Costa KA, Corbin KD, Niculescu MD, Galanko JA, Zeisel SH. Identification of new genetic polymorphisms that alter the dietary requirement for choline and vary in their distribution across ethnic and racial groups. FASEB J 2014; 28:2970–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fullerton MD, Wagner L, Yuan Z, Bakovic M. Impaired trafficking of choline transporter-like protein-1 at plasma membrane and inhibition of choline transport in THP-1 monocyte-derived macrophages. Am J Physiol Cell Physiol 2006; 290:C1230–8 [DOI] [PubMed] [Google Scholar]
- 24.Inazu M, Takeda H, Matsumiya T. Molecular Functional characterization of an Na+-independent choline transporter in rat astrocytes. J Neurochem 2005; 94:1427–37 [DOI] [PubMed] [Google Scholar]
- 25.Inazu M. Choline transporter-like proteins CTLs/SLC44 family as a novel molecular target for cancer therapy. Biopharm Drug Dispos 2014; 35:431–49 [DOI] [PubMed] [Google Scholar]
- 26.Inazu M, Yamada T, Kubota N, Yamanaka T. Functional expression of choline transporter-like protein 1 (CTL1) in small cell lung carcinoma cells: a target molecule for lung cancer therapy. Pharmacol Res 2013; 76:119–31 [DOI] [PubMed] [Google Scholar]
- 27.Damm MMB, Jensen TSR, Mahmood B, Lundh M, Poulsen SS, Bindslev N, Hansen MB. Acetylcholine-related proteins in non-neoplastic appearing colonic mucosa from patients with colorectal neoplasia. Mol Carcinog 2017; 56:2223–33 [DOI] [PubMed] [Google Scholar]
- 28.Ishiguro N, Oyabu M, Sato T, Maeda T, Minami H, Tamai I. Decreased biosynthesis of lung surfactant constituent phosphatidylcholine due to inhibition of choline transporter by gefitinib in lung alveolar cells. Pharm Res 2008; 25:417–27 [DOI] [PubMed] [Google Scholar]
- 29.Wang T, Li J, Chen F, Zhao Y, He X, Wan D, Gu J. Choline transporters in human lung adenocarcinoma: expression and functional implications. Acta Biochim Biophys Sin (Shanghai) 2007; 39:668–74 [DOI] [PubMed] [Google Scholar]
- 30.Yabuki M, Inazu M, Yamada T, Tajima H, Matsumiya T. Molecular and functional characterization of choline transporter in rat renal tubule epithelial NRK-52E cells. Arch Biochem Biophys 2009; 485:88–96 [DOI] [PubMed] [Google Scholar]
- 31.Uchida Y, Inazu M, Takeda H, Yamada T, Tajima H, Matsumiya T. Expression and functional characterization of choline transporter in human keratinocytes. J Pharmacol Sci 2009; 109:102–9 [DOI] [PubMed] [Google Scholar]
- 32.Michel V, Bakovic M. The solute carrier 44A1 is a mitochondrial protein and mediates choline transport. FASEB J 2009; 23:2749–58 [DOI] [PubMed] [Google Scholar]
- 33.Michel V, Singh RK, Bakovic M. The impact of choline availability on muscle lipid metabolism. Food Funct 2011; 2:53–62 [DOI] [PubMed] [Google Scholar]
- 34.Kouji H, Inazu M, Yamada T, Tajima H, Aoki T, Matsumiya T. Molecular and functional characterization of choline transporter in human colon carcinoma HT-29 cells. Arch Biochem Biophys 2009; 483:90–8 [DOI] [PubMed] [Google Scholar]
- 35.Brandes AH, Ward CS, Ronen SM. 17-allyamino-17-demethoxygeldanamycin treatment results in a magnetic resonance spectroscopy-detectable elevation in choline-containing metabolites associated with increased expression of choline transporter SLC44A1 and phospholipase A2. Breast Cancer Res 2010; 12:R84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Machova E, O’Regan S, Newcombe J, Meunier FM, Prentice J, Dove R, Lisá V, Dolezal V. Detection of choline transporter-like 1 protein CTL1 in neuroblastoma × glioma cells and in the CNS, and its role in choline uptake. J Neurochem 2009; 110:1297–309 [DOI] [PubMed] [Google Scholar]
- 37.Yamada T, Inazu M, Tajima H, Matsumiya T. Functional expression of choline transporter-like protein 1 (CTL1) in human neuroblastoma cells and its link to acetylcholine synthesis. Neurochem Int 2011; 58:354–65 [DOI] [PubMed] [Google Scholar]
- 38.Taguchi C, Inazu M, Saiki I, Yara M, Hara N, Yamanaka T, Uchino H. Functional analysis of [methyl-(3)H]choline uptake in glioblastoma cells: influence of anti-cancer and central nervous system drugs. Biochem Pharmacol 2014; 88:303–12 [DOI] [PubMed] [Google Scholar]
- 39.Nishiyama R, Nagashima F, Iwao B, Kawai Y, Inoue K, Midori A, Yamanaka T, Uchino H, Inazu M. Identification and functional analysis of choline transporter in tongue cancer: a novel molecular target for tongue cancer therapy. J Pharmacol Sci 2016; 131:101–9 [DOI] [PubMed] [Google Scholar]
- 40.Seki M, Kawai Y, Ishii C, Yamanaka T, Odawara M, Inazu M. Functional analysis of choline transporters in rheumatoid arthritis synovial fibroblasts. Mod Rheumatol 2017; 27:995–1003 [DOI] [PubMed] [Google Scholar]
- 41.Nagashima F, Nishiyama R, Iwao B, Kawai Y, Ishii C, Yamanaka T, Uchino H, Inazu M. Molecular and functional characterization of choline transporter-like proteins in esophageal cancer cells and potential therapeutic targets. Biomol Ther (Seoul) 2018; 26:399–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snider SA, Margison KD, Ghorbani P, LeBlond ND, O’Dwyer C, Nunes JRC, Nguyen T, Xu H, Bennett SAL, Fullerton MD. Choline transport links macrophage phospholipid metabolism and inflammation. J Biol Chem 2018; 293:11600–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schenkel LC, Singh RK, Michel V, Zeisel SH, da Costa KA, Johnson AR, Mudd HS, Bakovic M. Mechanism of choline deficiency and membrane alteration in postural orthostatic tachycardia syndrome primary skin fibroblasts. FASEB J 2015; 29:1663–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.da Costa KA, Badea M, Fischer LM, Zeisel SH. Elevated serum creatine phosphokinase in choline-deficient humans: mechanistic studies in C2C12 mouse myoblasts. Am J Clin Nutr 2004; 80:163–70 [DOI] [PubMed] [Google Scholar]
- 45.Fischer LM, daCosta KA, Kwock L, Stewart PW, Lu TS, Stabler SP, Allen RH, Zeisel SH. Sex and menopausal status influence human dietary requirements for the nutrient choline. Am J Clin Nutr 2007; 85:1275–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schenkel LC, Bakovic M. Palmitic acid and oleic acid differentially regulate choline transporter-like 1 levels and glycerolipid metabolism in skeletal muscle cells. Lipids 2014; 49:731–44 [DOI] [PubMed] [Google Scholar]
- 47.Yuan Z, Tie A, Tarnopolsky M, Bakovic M. Genomic organization, promoter activity, and expression of the human choline transporter-like protein 1. Physiol Genomics 2006; 26:76–90 [DOI] [PubMed] [Google Scholar]
- 48.Taylor A, Schenkel LC, Yokich M, Bakovic M. Adaptations to excess choline in insulin resistant and Pcyt2 deficient skeletal muscle. Biochem Cell Biol 2017; 95:223–31 [DOI] [PubMed] [Google Scholar]
- 49.Beckmann JSJ, Morhenn HG, Grau V, Schnettler R, Lips KS. Expression of choline and acetylcholine transporters in synovial tissue and cartilage of patients with rheumatoid arthritis and osteoarthritis. Cell Tissue Res 2015; 359:465–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Penet MF, Shah T, Bharti S, Krishnamachary B, Artemov D, Mironchik Y, Wildes F, Maitra A, Bhujwalla ZM. Metabolic imaging of pancreatic ductal adenocarcinoma detects altered choline metabolism. Clin Cancer Res 2015; 21:386–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aboagye EO, Bhujwalla ZM. Malignant transformation alters membrane choline phospholipid metabolism of human mammary epithelial cells. Cancer Res 1999; 59:80–4 [PubMed] [Google Scholar]
- 52.Yen CL, Mar MH, Zeisel SH. Choline deficiency-induced apoptosis in PC12 cells is associated with diminished membrane phosphatidylcholine and sphingomyelin, accumulation of ceramide and diacylglycerol, and activation of a caspase. FASEB J 1999; 13:135–42 [PubMed] [Google Scholar]
- 53.Blusztajn JK, Slack BE, Mellott TJ. Neuroprotective actions of dietary choline. Nutrients 2017; 9:pii:, E815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Golan N, Adamsky K, Kartvelishvily E, Brockschnieder D, Mobius W, Spiegel I, Roth AD, Thomson CE, Rechavi G, Peles E. Identification of Tmem10/Opalin as an oligodendrocyte enriched gene using expression profiling combined with genetic cell ablation. Glia 2008; 56:1176–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fujita T, Shimada A, Okada N, Yamamoto A. Functional characterization of Na+-independent choline transport in primary cultures of neurons from mouse cerebral cortex. Neurosci Lett 2006; 393:216–21 [DOI] [PubMed] [Google Scholar]
- 56.Zeisel S. Choline, other methyl-donors and epigenetics. Nutrients 2017; 9:pii:, E445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang X, Yan J, West AA, Perry CA, Malysheva OV, Devapatla S, Pressman E, Vermeylen F, Caudill MA. Maternal choline intake alters the epigenetic state of fetal cortisol-regulating genes in humans. FASEB J 2012; 26:3563–74 [DOI] [PubMed] [Google Scholar]
- 58.James P, Sajjadi S, Tomar AS, Saffari A, Fall CHD, Prentice AM, Shrestha S, Issarapu P, Yadav DK, Kaur L, Lillycrop K, Silver M, Chandak GR. Candidate genes linking maternal nutrient exposure to offspring health via DNA methylation: a review of existing evidence in humans with specific focus on one-carbon metabolism. Int J Epidemiol 2018; 47:1910–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pauwels S, Ghosh M, Duca RC, Bekaert B, Freson K, Huybrechts I, Langie SAS, Koppen G, Devlieger R, Godderis L. Maternal intake of methyl-group donors affects DNA methylation of metabolic genes in infants. Clin Epigenetics 2017; 9:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kovacheva VP, Mellott TJ, Davison JM, Wagner N, Lopez-Coviella I, Schnitzler AC, Blusztajn JK. Gestational choline deficiency causes global and Igf2 gene DNA hypermethylation by up‐regulation of Dnmt1 expression. J Biol Chem 2007; 282:31777–88 [DOI] [PubMed] [Google Scholar]
- 61.Joselit Y, Nanobashvili K, Jack-Roberts C, Greenwald E, Malysheva OV, Caudill MA, Saxena A, Jiang X. Maternal betaine supplementation affects fetal growth and lipid metabolism of high-fat fed mice in a temporal-specific manner. Nutr Diabetes 2018; 8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davison JM, Mellott TJ, Kovacheva VP, Blusztajn JK. Gestational choline supply regulates methylation of histone H3, expression of histone methyltransferases G9a (Kmt1c) and Suv39h1 (Kmt1a), and DNA methylation of their genes in rat fetal liver and brain. J Biol Chem 2009; 284:1982–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ideraabdullah FY, Zeisel SH. Dietary modulation of the epigenome. Physiol Rev 2018; 98:667–95 [DOI] [PMC free article] [PubMed] [Google Scholar]