Short abstract
Based on evidence extracted from a cross-sectional review of the literature, we sought to advance a novel conceptual framework that the physiology of hemorrhagic shock from exsanguination and maximal oxygen uptake (O2max), induced by physical exercise, shares key common features. As such, this review focuses on the notion that intolerance to inadequate oxygen delivery (DO2) resulting from associated states of hypovolemia appears to be a common physiological link that “connects” hemorrhagic shock to the physiology that limits maximal aerobic capacity. Our approach focuses on the similarities in a complex cascade of cardiopulmonary, metabolic and autonomic compensatory responses during hemorrhage and maximal physical exertion that ultimately function to avoid critical levels of DO2 (DO2crit) and are manifested by elevation in blood lactate levels. We introduce a paradigm of absolute (i.e. hemorrhage) versus relative (i.e. exercise) hypovolemia as a primary physiological factor that contributes to reaching DO2crit, and define the concept of “O2 deficit” to replace the clinical concept of O2 debt. Using the peer-reviewed literature, we provide human data obtained from patients who suffered hemorrhagic shock from severe blood loss and compare it to healthy subjects who performed maximal exercise. We include a novel conceptual framework of the continuum of metabolic relationship between DO2 and O2 that is manifested as the final step during both progressive blood loss leading to hemorrhagic shock and at O2max. We present evidence to support the contribution of utilizing “O2 extraction reserve” as the initial mechanism for developing an O2 deficit, and the notion of individual variability in compensatory responses. In the absence of reversing inadequate DO2, an increased reliance on O2 extraction reserve, cellular anaerobic glycolysis, and phosphocreatine stores to supplement the energy required by the tissues for normal function will deplete a finite capacity for compensation. In the end, acidity reflected by a blood pH ≤ ∼7.0 leads to disturbance of normal cell functioning of metabolic machinery manifested by irreversible shock in the case of hemorrhage or physical exhaustion when O2max is reached.
Impact statement
Disturbance of normal homeostasis occurs when oxygen delivery and energy stores to the body’s tissues fail to meet the energy requirement of cells. The work submitted in this review is important because it advances the understanding of inadequate oxygen delivery as it relates to early diagnosis and treatment of circulatory shock and its relationship to disturbance of normal functioning of cellular metabolism in life-threatening conditions of hemorrhage. We explored data from the clinical and exercise literature to construct for the first time a conceptual framework for defining the limitation of inadequate delivery of oxygen by comparing the physiology of hemorrhagic shock caused by severe blood loss to maximal oxygen uptake induced by intense physical exercise. We also provide a translational framework in which understanding the fundamental relationship between the body’s reserve to compensate for conditions of inadequate oxygen delivery as a limiting factor to O2max helps to re-evaluate paradigms of triage for improved monitoring of accurate resuscitation in patients suffering from hemorrhagic shock.
Keywords: Oxygen deficit, oxygen extraction reserve, blood pH, blood lactate, compensatory reserve
Introduction
Maintaining a balance between delivery and utilization of oxygen in various organs of the body represents a fundamental premise for sustaining “adequate” tissue oxygenation and cell function. As the requirement for oxygen uptake and utilization (O2) to meet cellular energy demand overwhelms the capacity of the respiratory and circulatory systems to deliver oxygen (DO2), the cells must rely increasingly on the metabolic transformation of glycogen to lactate known as anaerobic glycolysis, as well as tissue oxygen and phosphocreatine stores. Systemic DO2 is the product of cardiac output and arterial oxygen content.1 Mitigating the potential impact of failure to maintain an adequate DO2 on disrupting normal metabolic functioning at the cellular level relies on the integration of numerous compensatory mechanisms.
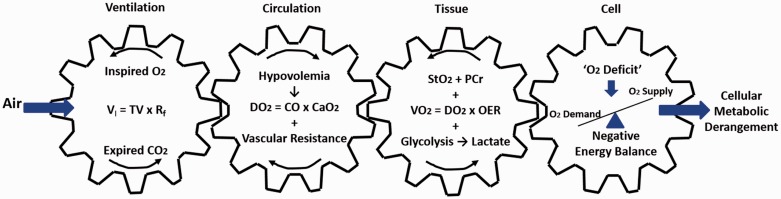
“Shock” has been described as a pathophysiological condition of “inadequate tissue oxygenation”1,2 that ensues when DO2 fails to meet the tissue O2 required to maintain aerobic energy production.2,3 In the context of this definition, maximal intensity exercise could be described as a type of “shock” in individuals, including endurance-trained athletes, whose O2max is limited by a failure of DO2 to meet the energy demand of working muscles.4–8 Within this context, progression of tissue acidity indicates failure to sustain a balance in oxygen supply and cellular energy demand that underlies the onset of both hemorrhagic shock and O2max. Although the magnitude of compensatory responses can differ between these two physiological states, we recognize that both hemorrhagic shock and O2max display qualitatively many similar hemodynamic, autonomic, metabolic, and compensatory patterns. Figure 1 represents our conceptual framework to illustrate the cascade coupling of ventilation, circulation, tissue metabolism, and energy balance in the mitochondria. Within this construct, an inability to maintain adequate DO2 to the cells can lead to disturbance of normal cellular metabolic functioning that defines a common pathway in both hemorrhagic shock and O2max. In this mini-review, we used this model in the context of young healthy humans, trained fit individuals, and/or athletes where, in general, DO2 sets the upper limit for O2max. Our primary comparison population from a conceptual perspective is young trauma victims, especially battlefield casualties. Our index populations are generally similar, so we explored relevant literature in an attempt to gain insight about the limiting factors that lead to hemorrhagic shock in bleeding patients by comparing the pathophysiological mechanisms of exsanguination to the physiological limits of individuals whose O2max is limited by inadequate DO2.
Figure 1.
Conceptual model to illustrate the cascade coupling of ventilation, circulation, tissue metabolism, and energy balance in the mitochondria to cell function that defines common pathways to hemorrhagic shock and V̇O2max. VI: inspiratory volume; TV: tidal volume; Rf: respiratory frequency; DO2: oxygen delivery; CO: cardiac output; CaO2: oxygen carrying capacity; V̇O2: oxygen uptake; OER: oxygen extraction ratio; StO2: tissue oxygen saturation; PCr: phosphocreatine. Conceptually adopted from Wasserman.9 (A color version of this figure is available in the online journal.)
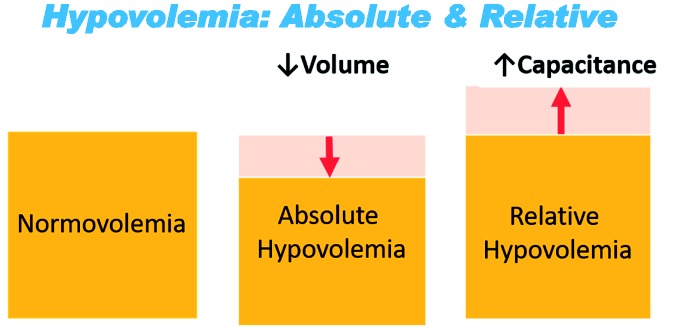
Absolute vs. relative hypovolemia
Central hypovolemia represents another common fundamental physiological state that underlies the inadequate DO2 resulting from both hemorrhage and many instances of physical exertion at O2max. Hypovolemia is defined as a reduction in circulating blood volume relative to the capacitance of the cardiovascular space. Figure 2 provides an illustration of the difference in the hypovolemic state caused by hemorrhage from that resulting from an acute exercise. During hemorrhage, an “absolute” hypovolemia occurs as a consequence of reduced circulating blood volume within the same vascular space (or smaller as ongoing compensatory vasoconstriction occurs).10,11 In contrast, increased vascular capacitance due to significant vasodilation in working muscles during acute exercise results in a “relative” hypovolemia defined by vascular space expansion with no or negligible change in circulating volume.10,11 Consequently, we can think of assessing limitations in compensatory adjustments to hemorrhage and O2max in DO2-limited individuals as a comparison in physiological differences in DO2 associated with absolute and relative hypovolemic conditions that eventually lead to under-perfused tissue.
Figure 2.
Conceptual comparison of normal circulating blood volume (normovolemia) with absolute and relative hypovolemia. Orange indicates circulating blood volume; Pink indicates capacitance of vascular space. Adopted from M.N. Sawka with permission. (A color version of this figure is available in the online journal.)
Clinical concepts of oxygen deficit and debt
The terms oxygen “deficit” and or “debt” are routinely used in emergency and critical care medicine to reflect a mismatch between cellular energy requirement and O2.2,12–14 The conceptual use of the terms O2 deficit or debt by clinicians can be linked to the concept of a cumulative deficiency of DO2 needed to support cellular energy demands through oxidative metabolism that was initially described by A.V. Hill from experiments using strenuous physical activity performed by healthy individuals.15 Hill’s research included the measurement of O2 responses during steady-state and maximal exercise, laying the foundation for defining systemic O2 dynamics and the emergence of the concepts of O2 deficit and O2 debt.15 Subsequently, O2 deficit and or O2 debt have been used repeatedly in the clinical literature to describe the ultimate pathophysiological factor leading to shock.2,12,13,16 However, the use of “O2 debt” can be misleading and confusing to physiologists who recognize it as referring to a post-exercise O2 phenomenon that is quantitatively and physiologically different than O2 deficit.17,18 In an effort to alleviate confusion in terminology between clinicians and physiologists, we will use the term O2 deficit as a more appropriate term to describe cellular O2-energy requirement mismatch and “O2 extraction reserve” to reflect various sources of O2 available to cells to support their energy requirements (e.g. hemoglobin, myoglobin). Within this context, it is conceptually transparent that reduced total O2 availability resulting from lowered DO2 during severe blood loss or O2max will result in increased O2 deficit because O2 is “barrowed” from the O2 extraction reserve. Thus, O2 deficit represents a mechanism of compensation designed to avoid reaching the DO2crit. As such, we use this review to focus on lowered DO2 as the underpinning of progressive reduction in O2 extraction reserve in an effort to support increased O2 deficit that leads to failure in meeting cellular energy demands during either hemorrhagic shock (absolute hypovolemia) or O2max (relative hypovolemia).
Inadequate DO2 created by O2max
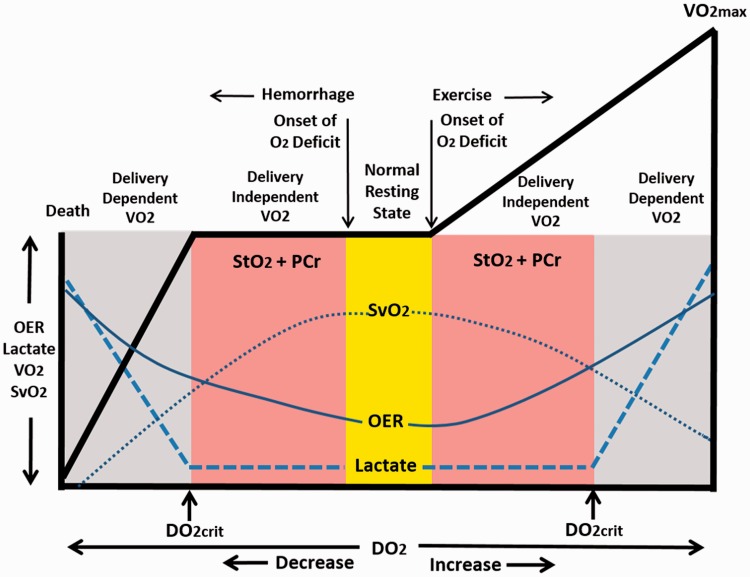
Inadequate DO2 can be defined as inability of O2 delivery to tissues to meet cellular energy demand. Figure 3 provides a conceptual schematic of the continuum of DO2 moving from normal rest. Moving to the right of resting baseline O2 (yellow bar in Figure 3) with the initiation of physical exercise, DO2 is progressively increased as a result of increased systemic blood flow (cardiac output). Despite higher absolute DO2 during exercise, there is an immediate development of an O2 deficit due to the time delay between immediate energy requirement and systemic O2. It is noteworthy that during the mismatch between energy requirement and O2 in the working muscle, blood lactate usually does not appear until the individual reaches a physiological work capacity above ∼50% of O2max.19 As such, an O2 deficit during physical exertion is initiated by two compensatory mechanisms that work in concert to mobilize O2 from the O2 extraction reserve in the presence of inadequate DO2: (1) increased extraction of hemoglobin-bound oxygen delivered through increased blood flow to the tissue (i.e. higher oxygen extraction ratio, OER in Figure 3) with a subsequent reduction in measured oxygen saturation of venous blood (SvO2); and (2) utilization of local tissue and O2 bound to myoglobin (SmO2) in addition to reserves of phosphocreatine (PCr) (Figure 3, right pink area).20 When increased DO2 and utilization of PCr/O2 reserves fail to support the cellular energy requirement, DO2crit is reached resulting in the production of energy through initiation of the transformation of glucose or glycogen to lactate. With limited amounts of oxygen available for aerobic glycolysis, an accumulation of lactate gradually appears in the blood in the presence of inadequate lactate clearance.4,21,22 When increasing energy demand can no longer be supported in the presence of accumulated O2 deficit, physical exhaustion is manifested by reaching O2max. In the end, the prevailing evidence indicates that O2max in healthy exercising humans is ultimately limited by inadequate DO2.4,23
Figure 3.
Conceptual representation of the continuum of metabolic relationship of oxygen delivery (DO2) to utilization (V̇O2) responses from hemorrhagic shock (left of Normal Resting State) to exertion at V̇O2max (right of Normal Resting State). OER: oxygen extraction ratio; SvO2: venous oxygen saturation; StO2: tissue oxygen saturation; PCr: phosphocreatine; DO2crit: critical oxygen delivery. Responses to progressive hemorrhage modified from Hooper et al.13 See text for explanations.
Inadequate DO2 created by hemorrhage
The extrapolation of metabolic events that lead to O2max induced by intense physical exercise to severe blood loss is reflected by a final common pathway that an inadequate DO2 limits tolerance of the body’s organs to sustain cellular energy requirements after reaching DO2crit. Similar to inadequate DO2 that limits the healthy exerciser from performing more physical work beyond O2max, is the arrival at a threshold of intolerable reduction in O2 extraction reserve with blood loss. This is due to the reduced DO2 that is not restored over time, which will lead to irreversible shock and death.13,14 Moving to the left from resting baseline O2 along the continuum with the initiation of hemorrhage (Figure 3), DO2 is reduced as a result of a loss of oxygen-carrying red blood cells and lower circulating volume for cardiac filling and output. Despite the progressive reduction in DO2 during the early stages of bleeding, O2 can be maintained at baseline levels by the capacity to utilize the O2 extraction reserve (i.e. increased OER); a cascade of events that is reflected by lowered SvO2. This notion of an early delivery-independent O2 in the presence of reduced DO2 was best illustrated by Weiskopf et al.24 who demonstrated no change in O2 or blood lactate (i.e. failed to reach DO2crit) following an experimentally-induced reduction in hemoglobin concentration (i.e. >60% lower DO2). Like the condition of physical exercise, it might be expected that the cells can draw upon tissue PCr and O2 extraction reserves (i.e. O2 deficit) during hemorrhage. If O2 extraction reserves contribute to the initial burden of an O2 deficit during the delivery-independent O2 phase of hemorrhage, higher O2 extraction (OER) and lower tissue O2 saturation (StO2) would be expected before an appearance of lactate in the blood. Consistent with this hypothesis is an immediate and progressive reduction in StO2 and elevation in OER during simulated human hemorrhage (Figure 4) while blood lactate remained within baseline levels (0.95 to 1.1 mmol/l) during lowered DO2.26
Figure 4.
Time course of responses of systemic oxygen delivery (DO2; Panel A), systemic oxygen uptake (V̇O2; Panel B), tissue oxygen saturation (StO2; Panel C), and oxygen extraction ratio (Panel D) during progressive central hypovolemia induced by lower body negative pressure (LBNP). Data are means ± SD; n = 18. Modified from Ward et al.25
Within this construct of timing, the clinical description of an onset of O2 deficit as the point in time at which tissue metabolism converts to anaerobic glycolysis to produce lactate in an effort to sustain the total energy requirement of the cell during hemorrhage has been misleading.1,13,14 Contrary to this hypothesis, failure to reach DO2crit in the presence of reduced O2 extraction reserves (Figure 4) supports the concept that O2 deficit, defined by reduced O2 extraction reserve, must occur in advance of reaching DO2crit. As such, the onset of O2 deficit during hemorrhage, like exercise, is initiated by a compensatory utilization of the O2 extraction reserves before there is increased anaerobic glycolysis. As such, we propose a revision in the biphasic relationship between DO2 and O213 to include stores of tissue PCr and O2 extraction reserve prior to the appearance of lactate in the blood (i.e. DO2crit; Figure 3, left pink area). In the end, the burden of a cellular O2 deficit beyond DO2crit must be “repaid” to restore adequate metabolic function and prevent organ dysfunction or injury at the cellular level whether describing DO2-limited strenuous physical activity that requires working at O2max or life-threatening exsanguination.
Physiologic similarities between hemorrhagic shock and O2max
The ultimate challenge of the cardiopulmonary and metabolic systems in both conditions of hemorrhagic shock and O2max is to maintain a sustainable DO2 so that a threshold of intolerable cellular O2 deficit and disturbance of normal cell functional metabolism can be avoided. This challenge requires a physiological capacity to recruit a complex network of compensatory mechanisms with the ultimate function of defending against inadequate DO2. A summary of compensatory responses and their outcomes is presented in Table 1 and described below.27,28
Table 1.
Similarities in qualitative and quantitative cardiopulmonary, metabolic and autonomic responses for hemorrhagic shock and exertion at O2max.
| Physiological responses | Directional change | Baseline rest | Hemorrhagic shock | References | Exertion @ V̇O2max | References |
|---|---|---|---|---|---|---|
| O2 deficit, mL kg−1 VO2 | ↑ | 0 | 104a | Siegel et al.16 | 50–80 | Linnarsson et al. 20Joyner and Coyle 19 |
| StO2, % | ↓ | 75 | 29–50 | Convertino and Sawka10 | 29–44 | Martin et al. 29 |
| PtO2, mmHg | ↓ | ∼34 | 5 | McKinley et al.30b | 3 | Richardson et al.31 |
| Heart rate, bpm | ↑ | 60–70 | 120–180 | Cloutier et al.32 Gutierrez et al.1 | 140–200 | Joyner and Casey33Tanaka et al. 34 |
| SNA, pg/mL | ↑ | 200–500 | 5–10 fold | Woolf 35 | > 7-fold | Engelke and Convertino 36 |
| OER, % | ↑ | 20–30 | > 50 | Ward et al. 25 | 85–90 | Joyner and Casey33Bassett and Howley23 |
| SvO2, % | ↓ | 65–75 | < 30 | Kasnitz et al. 37 | < 20 | Mortensen et al. 38 |
| Blood pH | ↓ | 7.4 | ≥ 7.0 | Cloutier et al. 32 | ≥ 7.0 | Osnes and Hermansen 39Goodwin et al.40 |
| Blood [LA], mmol/l | ↑ | 1–2 | > 3–13 | Bonanno et al. 41Vitek et al.27 | 15–25 | Osnes and Hermansen 39Ferguson et al.4 |
| Blood BD, mmol/l | ↑ | 0 | > 5–15 | Eastridge et al. 42 | ∼ 7 | Osnes and Hermansen 39 |
| Rf, breaths/min | ↑ | 10–20 | > 35 | Gutierrez et al.1 | > 40 | Forster et al. 43 |
| Vt, liters | ↑ | 0.5 | > 0.7 | Convertino et al.44 | > 2.5 | Forster et al. 43Younes and Burkes 45 |
| EtCO2, mmHg | ↓ | 35–40 | < 35 | Stone et al.46McManus et al.28 | < 35 | Hagberg et al. 47 |
| Compensatory Reserve, % | ↓ | 90–100 | < 15 | Convertino et al. 48 | < 20 | Convertino and Sawka 10 |
StO2: tissue oxygen saturation; PtO2: partial pressure of tissue oxygen; SNA: sympathetic nerve activity; OER: oxygen extraction ratio; SvO2: venous oxygen saturation; [LA]: lactate concentration; BD: base deficit; Rf: respiration frequency; Vt: tidal volume; EtCO2: end-tidal carbon dioxide.
aData from animal experiments.bCase study of one trauma patient.
Hemodynamic mechanisms
Despite stark differences in systemic arterial blood flow, blood pressure, DO2, and O2, one of the most obvious features between exercise and hemorrhage is pronounced elevation of heart rate that represents a compensatory attempt to maintain adequate DO2 in the face of a hypovolemia-induced accumulating O2 deficit. Indeed, inadequate systemic DO2 is viewed as the primary factor that ultimately leads to development of O2 deficit that limits O2max in most healthy exercising humans.5–8,24 as well as the onset of shock for bleeding patients.1,13,14
Similar to exercise, the cardiac response to hemorrhage relies on optimizing cardiac output in an effort to maintain perfusion (arterial blood) pressure. Despite vast differences in cardiac filling, severe hemorrhage can elicit tachycardia greater than 120–180 beats per minute (bpm) in shock1,32,49 similar to the pronounced elevation in heart rate of 140–200 bpm during exercise requiring maximal effort.33,34 Whether supporting the low cardiac filling states of acute hemorrhage or high cardiac filling during physical exercise, increased cardiac rate represents a common compensatory mechanism for optimizing cardiac output.
Autonomic mechanisms
Autonomically mediated elevations in heart rate are controlled by a combination of cardiac vagal withdrawal and sympathetic nerve activation.33 Although the direct measurement of parasympathetic nerve activity is not readily accessible in humans, the use of frequency-domain analysis R-R interval variability has provided an indirect metric for changes in cardiac vagal activity by demonstrating nearly complete elimination of the high-frequency spectra (0.15–0.40 Hz) during muscarinic receptor blockade.50 Calculating heart rate variability (HRV) from frequency-domain analysis has revealed that significant vagal withdrawal is an underlying mechanism of increased heart rate during both hemorrhagic shock51 and maximal exercise.33,52,53
Hypovolemia is a very potent stimulus for activation of sympathetic activity. Catecholamine levels in the blood have been used as an indicator for adrenergic response to hemorrhage and exercise since the relationship between HRV measures and sympathetic activity is not as established as that for cardiac vagal activity.52 In addition to vagal withdrawal, significant elevations in heart rate can be explained by blood levels of norepinephrine (NE) that become several times normal during both physical exercise at O2max and hemorrhagic shock (Table 1). Within minutes of the onset of hemorrhagic shock, blood levels of NE can reach as much as 10 times basal (>1800 pg/mL), enough to produce compensatory elevations in pulse rate and blood pressure.34 Likewise, maximal heart rate responses have been associated with average elevations in plasma NE from 183 to 1337 pg/mL during graded exercise that elicited O2max.36
The underlying mechanisms that elicit increased sympathetic nerve activation during physical exertion may provide insight to sympathetic control in conditions of blood loss. During exercise, heart rate can increase in the face of elevated arterial blood pressure because of a resetting of the cardiac baroreflex to a higher operating set point.54 In contrast, the elevation in sympathetic nerve activity and heart rate associated with the central hypovolemia of hemorrhage is accompanied by reduced cardiac baroreflex sensitivity.55 Since the pressure stimulus to arterial baroreceptors differs in direction between the hypotension associated with blood loss and the hypertension of exercise, metabolic stimuli that elicit activation of chemically-sensitive nerves in the tissues may represent a more likely common mechanism for activation of sympathetically mediated heart rate effects at O2max and in conditions of hemorrhagic shock. There is compelling evidence that an elevated sympathetic efferent response to activation of these so-called “metaboreflexes” results in increased perfusion (arterial) pressure as a consequence of chronotropic effects on the heart, acting to increase cardiac output as well as constriction of the vasculature.56 The end result of activating metaboreflexes in combination with baro- and chemoreflexes can result in similar magnitudes of sympathetic activation reflected by similar elevations in circulating catecholamines during hemorrhagic shock35 and maximal exercise.36
Oxygen extraction ratio and tissue oxygenation
Utilizing the O2 extraction reserve (e.g. hemoglobin bound O2, myoglobin-bound O2) represents a compensatory mechanism from which O2 can be provided to cells during hemorrhage and physical exercise when DO2 cannot meet the cellular requirement of O2. This lowering of the O2 extraction reserve inherently represents an O2 deficit. In this context, measurements of the oxygen extraction ratio (OER) and tissue oxygen saturation (StO2) can provide insights into the dynamics of O2 deficit as a compensatory mechanism during intense exercise or severe hemorrhage.
OER represents the ratio of O2 to DO2 as the fraction of oxygen delivered to the microcirculation that is taken up by the tissues (OER = O2/DO2 – Figure 1).57 Despite differences between hemorrhage and exercise in O2 magnitude and direction, both conditions require increased OER as a contributing compensatory mechanism for maintenance of adequate O2 in the face of inadequate DO2. This was best demonstrated by Weiskopf et al.24 when they found that the failure to reduce O2 or elevate blood lactate in healthy humans who underwent >65% reductions in O2–carrying capacity with whole blood withdrawal. The Weiskopf observation actually supports the contention that the only way that O2 and lactate could remain constant in the face of reduced DO2 was to incur an O2 deficit by borrowing from the O2 extraction reserve.22 The insight to be gained from exercise is that significant O2 deficit can be incurred without inducing significant injury in the form of organ damage or failure. In this context, O2 deficit is indeed an adaptive and protective response as part of the compensatory reserve measurement.
The premise that DO2crit occurs well after the onset and accumulation of O2 deficit is a fundamental relationship that evolved from the early work in exercise physiology that demonstrated an immediate development of an O2 deficit because of the difference between energy requirement and O2 at the onset of exercise. It is the O2 kinetics data obtained from physical exercise that provides the evidence that an O2 deficit is defined by the immediate reduction in O2 extraction reserve. Given that fact, the data in Figure 4 clearly demonstrate that, like exercise, O2 deficit resulting from a “borrowing” of oxygen from the O2 extraction reserve is increased immediately upon the onset of absolute central hypovolemia (e.g. hemorrhage) in the form of reduced StO2 with concurrent reduction in DO2.25 As such, the early increase in O2 deficit represents the compensatory part of hemorrhagic shock defined as Class I and II shock by the American College of Surgeons58 well before the onset of decompensatory Class III shock (i.e. DO2crit).
The reliance on elevations in OER is reflected by normal resting levels of 20% to 30%57 rising to as high as >50% during blood loss25 and > 85–90% at O2max.23,33 The increase in OER estimated for hemorrhagic shock is most likely underestimated since it does not represent any reported measurements made near the point of death when OER would be maximal (far left in Figure 3). Low mixed venous oxygen saturation (SvO2= DO2–O2) has been proposed to identify anaerobic glycolysis and global tissue hypoxia.41 As a result of increased OER, SvO2 is precipitously reduced from its normal resting value of 65–75%59 to less than 20% to 30% in both hemorrhage37 and exercise.38
StO2 represents the amount (%) of myoglobin-bound O2. The myoglobin disassociation curve requires a significant reduction in the partial pressure of oxygen (pO2) in the tissue before O2 will be disassociated from myoglobin. Indeed, tissue pO2 has been reported to be as low as ∼5 mmHg compared to a baseline level of ∼34 mmHg in both hemorrhage30 and maximal exercise.31 A reduction in StO2 reflects a compensatory increase in the availability of O2 to support cellular O2 in the presence of inadequate DO2. This was best illustrated in humans undergoing progressive central hypovolemia without changing systemic O2 or blood lactate despite significant reductions in DO2 because of mobilization of myoglobin-bound O2 (i.e. decreased StO2) and hemoglobin-bound O2 (Figure 4).25 This compensatory mechanism for increasing cellular O2 availability is reflected by a dramatic reduction in StO2 from normal resting levels of ∼70% to as low as ∼30% during progressive central hypovolemia similar to blood loss10 and at O2max.29
Glycolysis and lactate
When O2 can no longer meet the cellular energy demand alone, the cell becomes more reliant on energy sources of phosphocreatine and glycolysis to meet its ATP demand. Consequently, increased levels of blood lactate in the absence of adequate clearance are common to both hemorrhage and intense physical exercise. Although blood lactate is commonly measured by clinicians as a metric of shock, study of the physiology of O2max has revealed that glycolysis and lactate production per se rarely accumulate because of inadequate DO2.4 The benign presence of blood lactate is best reflected by the observation that a high risk of shock and death is associated with elevated arterial blood lactate levels of only > 4 mmol/L during hemorrhage,41 while blood lactate levels have been reported to be >15–25 mmol at O2max.40 The mechanism underlying the physiological tolerance to higher levels of blood lactate accumulation during muscle contraction compared to hemorrhage is reflected by the use of lactate as an oxidizable fuel during intense exercise, i.e. increased lactate clearance.4,22 As such, the dynamics of lactate metabolism during intense exercise challenges the paradigm that measures of systemic blood lactate fail to provide an accurate assessment of cellular O2 deficit in a patient with severe hemorrhage. During the absolute hypovolemia of hemorrhage, blood flow is redirected away from tissues with high mass but low energy demand (e.g. skeletal muscle) to support DO2 to critical organs like the brain, heart, gut, liver, and kidney. Significant levels of lactate can accumulate in the brain during hemorrhage and diffuse into the blood.60 However, the dilution of highly concentrated blood lactate leaving the brain in blood from other less metabolically-active tissues will lead to underestimation of brain lactate as an accurate measure of hemorrhagic shock. Thus, local measures of blood lactate in blood leaving specific tissues may prove to provide a more accurate clinical measure of hemorrhagic shock. Ultimately, it is not the amount of accumulated systemic blood lactate that reflects the limitation of tolerance to severe blood loss or intense exercise, but rather emergence of intolerable O2 deficit that results from inadequate DO2.
Acid–base buffering
In contrast to lactate, the ultimate challenge during hemorrhagic shock and O2max is impaired removal of accumulated H+ created by a complex number of metabolic byproducts61 in the cells and blood that are associated with increased acidity (reduced pH). It is tissue acidity that may define the common critical factor emerging from inadequate DO2 that leads to intolerable disturbance of cellular metabolic function. The deleterious effect of reduced cellular pH may be best reflected by the similarity in maximal arterialized blood pH of ≤ 7.0 that has been reported during bot hemorrhagic shock32 and exercise at O2max39,40,61 despite a large discrepancy in accumulated blood lactate. Thus, the ability to sustain acid-base balance during progressive blood loss or intense physical exercise depends on the capacity to buffer accumulated H+.
Another sensitive means of assessing tissue oxygenation and O2 deficit is by measuring the base deficit, which represents the number of millimoles of base required to correct the pH of one liter of whole blood to 7.4.41,49 Indeed, the capacity to buffer blood acidity is reflected by a high correlation between increased base deficit and reduced arterialized blood pH,32 with base deficits > 5 mmol/L being associated with both hemorrhagic shock32,42 and O2max.39
Relatively small base deficits of only 6 to 10 mmol/L observed during hemorrhagic shock have been associated with “metabolic decompensation” when there is increasing requirements for blood transfusion42 and the probability of death rises exponentially.12 Although the reliance on tissue oxygen extraction reserves, phosphocreatine, and glycolysis exists with inadequate DO2 during both hemorrhage and O2max, it is important to recognize that intense physical activity can result in metabolic acidosis with base deficits three to five times higher than those associated with hemorrhagic shock. This disparity of higher base deficits created by exercise is an indication that extreme changes in the acid-base balance seem to be well tolerated in healthy exercising subjects39 compared to patients with severe blood loss. These comparisons reaffirm the notion that high levels of blood lactate are not by themselves detrimental as long as cellular metabolism is high enough to oxidize the lactate as a source of energy.4 Thus, it is the reduction in O2 as a consequence of lowered DO2 during severe hemorrhage that leads to inadequate blood lactate clearance after reaching DO2crit that can result in circulatory shock in the presence of relatively low blood lactate levels.
Mechanisms of pulmonary ventilation
Pulmonary ventilation is usually associated with gas exchange and is critical to the maintenance of adequate DO2 by maintaining optimal hemoglobin saturation of the blood in both hemorrhagic shock and exercise at O2max. However, pulmonary ventilation function also contributes significantly to acid–base balance and hemodynamics necessary for enhancing DO2 and attenuating the accumulation of O2 deficit at the cellular level.
Comparisons of pulmonary ventilation responses during hemorrhagic shock and O2max are presented in Table 1. Respiration rate (breaths per minute) increases from a resting level ranging from 10 to 20 to more than 35–40 in conditions of hemorrhagic shock1 and O2max.43 This hyperventilatory response acts to create a respiratory alkalosis by removing carbon dioxide (CO2) from the blood as a way to buffer the accumulation of H+ and reduction in pH.61 The respiratory alkalosis is reflected by concomitant reductions in end-tidal CO2 below a threshold of 35 mmHg in both individuals in hemorrhagic shock46 and exercising at O2max.47
Like respiration rate, the time kinetics of the tidal volume (VT) response to progressively increasing intensity of exercise leading to O2max44,45 is similar to that reported during progressive reductions in central blood volume associated with hemorrhage and leading to decompensation.44 In both physiological conditions, VT remains relatively unchanged until a level >80% of tolerance is reached, at which time there is an exponential increase in VT characterized by deeper inspirations. Inspiration lowers intrathoracic pressure.62 The greater vacuum created in the thorax by this “respiratory pump” increases venous return, cardiac filling, and cardiac output62 which in turn may prove instrumental in sustaining systemic DO2 through elevations in perfusion pressure during conditions of hypovolemia10 represented by both blood loss and O2max. Perhaps it is no surprise that patients with severe conditions of hypovolemia who gasp (i.e. the so-called “last gasp”) to increase VT have better mortality outcomes than those who do not.48
Compensatory reserve
The physiological parameters listed in Table 1 represent responses of compensation to the combination of accumulated tissue O2 shortfall and inadequate DO2 associated with hypovolemic states of hemorrhage or exercise at maximal intensity. The capacity for compensation that “protects” against low tissue perfusion during states of compromised DO2 is known as the compensatory reserve.10,63–65 A historical challenge has been to develop a capability to measure the integration of compensatory mechanisms, particularly as it relates to DO2, rather than their physiological outcomes (e.g. blood pressure, SpO2, blood pH). Novel monitoring technologies have been developed that include the application of advanced real-time signal analysis and machine learning of hundreds of thousands of photoplethysmographic waveforms during progressive states of central hypovolemia. Application of such advanced computer processing algorithms has demonstrated that measures of arterial waveform features reflect the integration of all compensatory mechanisms recruited to sustain adequate DO2 during conditions of progressive hypovolemia with greater specificity and sensitivity compared to standard vital signs and metabolic markers.66–70 Specifically, changing features of the ejection wave reflect the sum of all compensatory mechanisms that control myocardial function, while reflected wave features reflect the sum of all mechanisms associated with compensation for compromised cellular energy requirements.63
From the initial onset of hemorrhage or exercise (illustrated in Figure 3, broken lines), recruitment of the entirety of all compensatory mechanisms (e.g. autonomically-mediated tachycardia and blood flow redistribution, O2 extraction reserve, respiration, etc.) is required to maintain adequate DO2. With progression along the DO2 time continuum (Figure 3), the reserve capacity to compensate would be expected to gradually deplete until energy requirements for oxygen at the cellular level can no longer be sustained during progressively severe hypovolemia. This concept is supported by a linear relationship between compensatory reserve and DO2 (Figure 5). Ultimately, it is the depletion of compensatory reserve that leads to an inability to sustain energy requirements of the cells in the face of inadequate DO2 that leads to impending hemodynamic decompensation from blood loss63,72 or failure to continue muscular work at O2max.10
Figure 5.
Linear regression of the relationship between systemic oxygen delivery (DO2) and compensatory reserve measurement (CRM) expressed by DO2 (mL O2·kg−1 ·min−1) = 0.08 × CRM (%) + 5.3. Open circles represent extension of the regression line to 0% and 100% CRM. Data, collected from non-human primates, are expressed as mean ± SEM; N = 12. Modified from Koons et al.71
Individual variability in compensatory responses: A different perspective
Although the physiological responses listed in Table 1 represent generalizable direction and magnitude of changes in both conditions of hemorrhagic shock and physical work at O2max, it is recognized that there exists significant individual variability in tolerance to tissue deoxygenation and O2 deficit. This variability is most apparent from the data presented in Figure 6 that illustrates large differences in tissue oxygen saturation across levels of hypovolemia, making it impossible to distinguish some individuals with >85% of compensatory reserve from some individuals with <5% reserve to compensate. The data presented in Figure 6 also indicates that there remains >30% of tissue oxygen saturation in most individuals who have very little remaining capacity to compensate. Consistent with these data, actual measured O2max has been reported to be only ∼90% of the theoretical O2max calculated from mitochondrial oxidative capacity with significant individual variability.73 Taken together, these observations support the notion suggested by others4 that tissue oxygen per se is not necessarily a primary limiting factor in development of intolerable O2 deficit.
Figure 6.
Relationship between tissue oxygen saturation and compensatory reserve. Symbols are average (±95% CI) values generated from 55 healthy volunteer subjects exposed to 0, −15, −30, −45, −60, −70, −80 and −90 mmHg LBNP. Amalgamated r2 = 0.965. Modified from Convertino and Sawka.10
One approach to test this hypothesis is to compare individual variability in global compensatory response to hypovolemia in normal sedentary people to exercise-trained individuals and world class endurance athletes who have extraordinarily high blood volume, capillary density, cellular mitochondrial content, and aerobic enzyme activities.19,23 If tissue capacity for storing and utilizing oxygen contributes to mitigating the accumulation of O2 deficit, it might be expected that the endurance athlete’s physiology designed to support high O2max would be reflected by high tolerance to absolute central hypovolemia such as severe hemorrhage. Contrary to this notion, there is consistently reported observations in the literature that individuals who participate in endurance exercise activities (e.g. marathon runners) demonstrate some degree of physiological compromise to their blood pressure regulation leading to a higher incidence of symptomatic syncope in conditions of central hypovolemia compared to non-athletes.74–76 This observation is reasonable when consideration is given to the evidence that endurance-trained athletes demonstrate a steeper slope of the Frank–Starling relation that translates to greater reductions in stroke volume for equal falls in left ventricular filling volume.75 Consequently, the steep Frank–Starling curve that provides endurance athletes a great physiological advantage during competitive running can predispose them to decompensation in the face of severe central hypovolemia such as during hemorrhage by compromising DO2. The comparison of physiological functions in endurance athletes, sedentary individuals, and chronic heart failure patients support the notion that, in the end, the limiting factor to reaching hemorrhagic shock or O2max is inadequate DO2 to meet the need to maintain cellular O2 demand.4,73 Thus, it is not enough to measure either tissue oxygen or DO2, but individual variability demands measurements that reflect both factors to assure a most accurate assessment of which combination of compensatory mechanisms dictates either hemorrhagic shock or O2max in each individual. It is for this reason that measurements of arterial waveform features provide the most accurate assessment of either hemorrhagic shock or O2max since features of the ejected wave represent the sum total of all mechanisms that dictate systemic DO2, while features of the reflected wave represent the impact of metabolism that influences cellular O2 and accumulated tissue O2 deficit.63
Hemorrhagic shock vs. O2max conceptual framework: Other factors to consider
In addition to sharing key common physiological features, it is important to consider that reaching the threshold of hemorrhagic shock or O2max will depend on the rate of accumulated O2 deficit during blood loss or maximal oxygen uptake. For instance, a patient suffering from a slow venous bleed may sustain a progressively increasing acidotic state for an extended period of time without succumbing to hemorrhage. This slow hemorrhage condition would be physiologically equivalent to a healthy individual who performs a prolonged period of submaximal exercise above the threshold for blood lactate accumulation. On the other hand, an injury to a major artery that results in rapid exsanguination and imminent death would be analogous to a sprint exercise that reaches O2max in a short time period, both conditions resulting in rapid imbalance between oxygen demand and oxygen supply that cannot be resolved because of a limitation to DO2. The physiology of hemorrhagic shock is also complicated by the notion that individual tissues (e.g. gut, brain, muscle) during blood loss varies in their tolerance to low DO2, which may be analogous to the varying patterns of tissue oxygen demands during physical exercise that are defined by the inverse relationship between power and speed duration.
Clinical translation from the exercise experience
Accurate guidance for resuscitation of patients suffering from hemorrhagic shock represents a significant challenge to emergency medicine caregivers in their efforts to avoid the sequela associated with over- or under-resuscitation. A metric of inadequate DO2 reflects the most sensitive quantifier of the degree of shock,13 but accurate assessment of DO2 has been limited by technology gaps in the ability to measure a bleeding trauma patient’s O2 shortfall and repayment O2 extraction reserve in real time.13 As a result of this monitoring limitation, use of devices in pre-hospital critical care medicine that provide measures of blood lactate or tissue oxygen saturation has been proposed.13,77 However, challenges associated with the use of blood lactate or tissue oxygen as surrogate measures of compromised DO2 for guidance to clinical intervention are numerous. First, blood lactate levels do not necessarily reflect pathophysiology since their rise at O2max in young healthy individuals is multiple times that observed with hemorrhagic shock (Table 1) without clinical consequence. Second, DO2 during hemorrhage can be decreased during the early stages of compensatory shock in the absence of elevated blood lactate. Third, although tissue oxygen is reduced during hemorrhage and at O2max, there remains adequate cellular O2 levels indicated by ∼30% to 50% of resting baseline (Table 1). As such, clinical and exercise evidence indicates that it is likely that measures of blood lactate or tissue oxygen saturation may misrepresent the DO2 status of a patient with hemorrhagic shock.
The key to effective resuscitation in patients with hemorrhagic shock is an appreciation “that it is not enough to simply halt the accumulation of oxygen debt (i.e. shortfall); it (O2 shortfall) must be repaid.”13 The challenge, however, is that the clinical community has not realized a way to assess O2 shortfall in real time.13 Figure 5 provides data obtained from experiments conducted on healthy baboons illustrating that measurement of the capacity to compensate (i.e. compensatory reserve) during whole blood resuscitation is closely correlated to DO2.71 These results reflect the ability of changing features of the arterial waveform to provide a real-time measurement of integrated mechanisms that contribute to control of physiological factors that influence the development of O2 deficit. As such, it is compensatory reserve data collected with measures of exercise O2 deficit during exercise that provide a basis for using such technology for early identification of bleeding patients with hemorrhagic shock and for guiding accurate fluid or whole blood resuscitation leading to O2 deficit repayment.
Summary
Evidence provided by a cross-sectional review of the literature supports our conceptual framework that hemorrhagic shock in bleeding patients and O2max of endurance athletes shares similar physiological responses initiated by a requirement to avoid critical levels of accumulated tissue O2 deficit in response to inadequate DO2 associated with states of hypovolemia (Figure 1). In both physiological conditions, hypovolemia initiates a similar complex cascade of compensatory responses that include (but are not limited to) vagally- and sympathetically-mediated tachycardia, increased oxygen extraction from the blood and tissue O2 extraction reserves, and hyperventilation acting to optimize acid–base balance and central hemodynamics. With a finite capacity to maintain adequate DO2, the sum total of all compensatory responses eventually can be depleted without repayment of the O2 deficit. With this progression of events, and individual variability, a critical threshold of DO2 is reached, requiring a marked increase in the energy contribution from glycolysis to supplement the energy required by the tissues for normal function that can no longer be provided by aerobic metabolism alone. The resulting impairment in removal of accumulated H+ leads to a reduction in pH (cellular acidity) that, if not reversed, will lead to a disturbance of normal cell metabolic function as reflected by irreversible shock in the case of hemorrhage or physical exhaustion when O2max is reached.
ACKNOWLEDGMENTS
The authors thank Dr. Taylor Schlotman, Ms. Aiyana Helme, and Ms. Denise Woods for their technical assistance in the preparation of this manuscript.
Authors’ contribution
All authors contributed to parts of the conceptualization, drafting or revising the article critically for important intellectual content, and final review and approval of the version submitted for publication.
DECLARATION OF CONFLICTING INTERESTS
The authors have no conflict of interests to report. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
FUNDING
Funding for this work was provided in part by appointments to the Internship/Research Participation Program at the United States Army Institute of Surgical Research, administered by the Oak Ridge Institute for Science and Education (KRL, NJK) through an interagency agreement between the U.S. Department of Energy and Environmental Protection Agency, and grants from the US Army Medical Research and Materiel Command Combat Casualty Care Research Program (D-023–2011-USAISR; D-009–2014-USAISR; STO R.MED.2016.20).
References
- 1.Gutierrez G, Reines HD, Wulf-Gutierrez ME. Clinical review: hemorrhagic shock. Crit Care 2004; 8:373–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbee RW, Reynolds PS, Ward KR. Assessing shock resuscitation strategies by oxygen debt repayment. Shock 2010; 33:113–22 [DOI] [PubMed] [Google Scholar]
- 3.Schumacker PT, Cain SM. The concept of a critical oxygen delivery. Intensive Care Med 1987; 13:223–9 [DOI] [PubMed] [Google Scholar]
- 4.Ferguson BS, Rogatzki MJ, Goodwin ML, Kane DA, Rightmire Z, Gladdin LB. Lactate metabolism: historical context, prior misinterpretations, and current understanding. Eur J Appl Physiol 2018; 118(4):691–728 [DOI] [PubMed] [Google Scholar]
- 5.Wagner PD. Systemic oxygen transport and utilization. J Breath Res 2008; 2:1–12 [DOI] [PubMed] [Google Scholar]
- 6.Roca J, Agustia G, Alonso A. Effects of training on muscle O2 transport at VO2max. J Appl Physiol 1992; 73:1067–76 [DOI] [PubMed] [Google Scholar]
- 7.Gifford RJ, Garten RS, Nelson AD. Symmorphosis and skeletal muscle VO2max: in vivo and in vitro measaures reveal differing constraints in the exercise-trained and untrained human. J Physiol 2016; 594:1741–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knight DR, Schaffartzik W, Poole C, Hogan MC, Bebout DE, Wagner PD. Effects of hyperoxia on maximal leg O2 supply and utilization in men. J Appl Physiol 1993; 75:2586–94 [DOI] [PubMed] [Google Scholar]
- 9.Wasserman K. Breathing during exercise. N Engl J Med 1978; 298:780–5 [DOI] [PubMed] [Google Scholar]
- 10.Convertino VA, Sawka MN. Wearable compensatory reserve measurement for hypovolemia sensing. J Appl Physiol 2018; 124:442–51 [DOI] [PubMed] [Google Scholar]
- 11.Sawka MN, Cheuvront SN, Kenefick RW. Hypohydration and human performance: impact of environment and physiological mechanisms. Sports Med 2015; 45:S51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rixen D, Siegel JH. Bench-to-bedside review: oxygen debt and its metabolic correlates as quantifiers of the severity of hemorrhagic and post-traumatic shock. Crit Care 2005; 9:441–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hooper TJ, De Pasquale M, Strandenes G, Sunde G, Ward KR. Challenges and possibilities in forward resuscitation. Shock 2014; 41:13–20 [DOI] [PubMed] [Google Scholar]
- 14.White NJ, Ward KR, Pati S, Strandenes G, Cap AP. Hemorrhagic blood failure: oxygen debt, coagulopathy, and endothelial damage. J Trauma Acute Care Surg 2017; 82:S41–S9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bassett DR. Scientific contributions of A.V. Hill: exercise physiology pioneer. J Appl Physiol 2002; 93:1567–82 [DOI] [PubMed] [Google Scholar]
- 16.Siegel J, Fabian M, Smith J, Kingston E, Steele K, Wells M. Oxygen debt criteria quantify the effectiveness of early partial resuscitation after hypovolemic hemorrhagic shock. J Trauma 2003; 54:862–80 [DOI] [PubMed] [Google Scholar]
- 17.Gaesser G, Brooks GA. Metabolic bases of excess post-exercise oxygen consumption: a review. Med Sci Sports Exerc 1984; 16:29–43 [PubMed] [Google Scholar]
- 18.Gladden LB. Lactate metabolism: a new paradigm for the third millennium. J Physiol 2004; 558:5–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joyner MJ, Coyle EF. Endurance exercise performance: the physiology of champions. J Physiol 2008; 586:35–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linnarsson D, Karlsson J, Fagraeus L, Saltin B. Muscle metabolites and oxygen deficit with exercise in hypoxia and hyperoxia. J Appl Physiol 1974; 36:399–402 [DOI] [PubMed] [Google Scholar]
- 21.Bergman BC, Wolfel EE, Butterfield GE, Lopaschuk GD, Casazza GA, Horning MA, Brooks GA. Active muscle and whole body lactate kinetics after endurance training in men. J Appl Physiol 1999; 87:1684–96 [DOI] [PubMed] [Google Scholar]
- 22.Brooks GA. The science and translation of lactate shuttle theory. Cell Metab 2018; 27:757–85 [DOI] [PubMed] [Google Scholar]
- 23.Bassett DR, Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc 2000; 32:70–84 [DOI] [PubMed] [Google Scholar]
- 24.Weiskopf RB, Viele MK, Feiner J, Kelley S, Lieberman J, Noorani M, Leung JM, Fisher DM, Murray WR, Toy P, Moore MA. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA 1998; 279:217–21 [DOI] [PubMed] [Google Scholar]
- 25.Ward K, Tiba M, Ryan K, Torres-Filho I, Rickards C, Witten T, Soller B, Ludwig D, Convertino V. Oxygen transport characterization of a human model of progressive hemorrhage. Resuscitation 2010; 81:987–93 [DOI] [PubMed] [Google Scholar]
- 26.Soller BR, Soyemi OO, Yang Y, Ryan KL, Rickards CA, Walz JM, Heard SO, Convertino VA. Noninvasively measured muscle oxygen saturation is an early indicator of central hypovolemia in humans. J Appl Physiol 2008; 104:475–81 [DOI] [PubMed] [Google Scholar]
- 27.Vitek V, Cowley RA. Blood lactate in the prognosis of various forms of shock. Ann Surg 1971; 173:308–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McManus J, Ryan K, Morton M, RIckards A, Cooke W, Convertino V. Limitations of end-tidal CO2 as an early indicator of central hypovolemia in humans. Prehosp Emerg Care 2008; 12:199–205 [DOI] [PubMed] [Google Scholar]
- 29.Martin D, Levett D, Mythen M, Gocott M. Changes in skeletal muscle oxygenation during exercise measured by near-infrared spectroscopy on ascent to altitude. Crit Care 2009; 13:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKinley B, Ware D, Marvin R, Moore F. Skeletal muscle pH, PCO2, and PO2 during resuscitation of severe hemorrhagic shock. J Trauma 1998; 45:633–6 [DOI] [PubMed] [Google Scholar]
- 31.Richardson R, Duteil S, Wary C, Wray D, Hoff J, Carlier P. Human skeletal muscle intracellular oxygenation: the impact of ambient oxygen availability. J Physiol 2006; 571:415–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cloutier CT, Lowery BD, Carey LC. Acid-base disturbances in hemorrhagic shock. Arch Surg 1969; 98:551–7 [DOI] [PubMed] [Google Scholar]
- 33.Joyner MJ, Casey DP. Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev 2015; 95:549–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka H, Monahan KD, Seals DR. Age-related maximal heart rate revisited. J Am Coll Cardiol 2001; 37:153–6 [DOI] [PubMed] [Google Scholar]
- 35.Woolf PD. Endocrinology of shock. Ann Emerg Med 1986; 15:1401–5 [DOI] [PubMed] [Google Scholar]
- 36.Engelke KA, Convertino VA. Catecholamine response to maximal exercise following 16 days of simulated microgravity. Aviat Space Environ Med 1996; 67:243–7 [PubMed] [Google Scholar]
- 37.Kasnitz P, Druger GL, Yorra F, Simmons DH. Mixed venous oxygen tension and hyperlactatemia. JAMA 1976; 236:570–4 [PubMed] [Google Scholar]
- 38.Mortensen SP, Damsgaard R, Dawson EA, Secher N, Gonzalez-Alonso J. Restrictions in systemic and locomotor skeletal muscle perfusion, oxygen supply and VO2 during high intensity whole-body exercise in humans. J Physiol 2008; 586:2621–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osnes J-B, Hernansen L. Acid-base balance after maximal exercise of short duration. J Appl Physiol 1972; 32:59–63 [DOI] [PubMed] [Google Scholar]
- 40.Goodwin ML, Harris JE, Hernandez A, Gladden LB. Blood lactate measurements and analysis during exercise: a guide for clinicians. J Diabetes Sci Technol 2007; 1:558–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonanno FG. Clinical pathology of the shock syndromes. J Emerg Trauma Shock 2011; 4:233–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eastridge BJ, Malone D, Holcomb JB. Early predictors of transfusion and mortality after injury: a review of the data-based literature. J Trauma 2006; 60:620–5 [DOI] [PubMed] [Google Scholar]
- 43.Forster HV, Haouzi P, Dempsey JA. Control of breathing during exercise. Comput Physiol 2012; 2:743–77 [DOI] [PubMed] [Google Scholar]
- 44.Convertino VA, Rickards CA, Lurie KG, Ryan KL. Hyperventilation in response to progressive reduction in central blood volume to near syncope. Aviat Space Environ Med 2009; 80:1012–7 [DOI] [PubMed] [Google Scholar]
- 45.Younes M, Jones J. Breathing pattern during and after exercise of different intensities. J Appl Physiol 1985; 59:898–908 [DOI] [PubMed] [Google Scholar]
- 46.Stone ME, Kalata S, Liveris A, Adomo Z, Yellin S, Chao E, Reddy SH, Jones M, Vargas C, Teperman S. End-tidal CO2 on admission is associated with hemorrhagic shock and predicts the need for massive transfusion as defined by the critical administration threshold: a pilot study. Injury 2017; 48:51–7 [DOI] [PubMed] [Google Scholar]
- 47.Hagberg JM, Coyle EF, Carroll JE, Miller JM, Martin WH, Brooke MH. Exercise hyperventilation in patients with McArdle's disease. J Appl Physiol Respir Environ Exerc Physiol 1982; 52:991–4 [DOI] [PubMed] [Google Scholar]
- 48.Convertino VA, Ryan KL, Rickards CA, Glorsky SL, Idris AH, Yannopoulos D, Metzger A, Lurie KG. Optimizing the respiratory pump: harnessing inspiratory resistance to treat systemic hypotension. Respir Care 2011; 56:846–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El Sayad M, Noureddine H. Recent advances of hemorrhage management in severe trauma. Emerg Med Intl 2014; 2014:638956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akselrod S, Gordon D, Madwed JB, Snidman NC, Shannon DC, Cohen RJ. Hemodynamic regulation: investigation by spectral analysis. Am J Physiol 1985; 249:H867–H75 [DOI] [PubMed] [Google Scholar]
- 51.Cooke WH, Salinas J, Convertino VA, Ludwig DA, Hinds D, Duke JH, Moore FA, Holcomb JB. Heart rate variability and its association with mortality in pre-hospital trauma patients. J Trauma 2006; 60:363–70 [DOI] [PubMed] [Google Scholar]
- 52.Michael S, Graham KS, Davis GM. Cardiac autonomic responses during exercise and post-exercise recovery during heart rate variability and systolic time intervals – a review. Front Physiol 2017; 8:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gourine G, Ackland C. Cardiac vagus and exercise. Physiology 2019; 34:71–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joyner MJ. Baroreceptor function during exercise: resetting the record. Exp Physiol 2006; 91:27–36 [DOI] [PubMed] [Google Scholar]
- 55.Schiller AM, Howard JT, Convertino VA. The physiology of blood loss and shock: new insights from a human laboratory model of hemorrhage. Exp Biol Med 2017; 242:874–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boushel R. Muscle metaboreflex control of the circulation during exercise. Acta Physiol 2010; 199:367–83 [DOI] [PubMed] [Google Scholar]
- 57.McLellan SA, Walsh TS. Oxygen delivery and hemoglobin. Cont Ed Anesthes Crit Care Pain 2004; 4:123–6 [Google Scholar]
- 58.American College of Surgeons Committee on Trauma. Advanced trauma life support for doctors (student course manual). 8th ed Chicago, IL: American College of Surgeons; 2012. p.61 [Google Scholar]
- 59.Van Beest P, Wietasch G, Scheeren T, Spronk P, Kuiper M. Clinical review: use of venous oxygen saturations as a goal – a yet unfinished puzzle. Crit Care 2011; 15:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dienel G. Brain lactate metabolism: the discoveries and the controversies. J Cereb Blood Flow Metab 2012; 32:1107–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Strickland M, Lindinger M, Olfert I, Heigenhauser G, Hopkins S. Pulmonary gas exchange and acid-base balance during exercise. Comput Physiol 2013; 3:693–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moreno AH, Burchell AR, Van der Woude R, Burke JH. Respiratory regulation of splanchnic and systemic venous return. Am J Physiol 1967; 213:455–65 [DOI] [PubMed] [Google Scholar]
- 63.Convertino VA, Cardin S, Batchelder P, Grudic GZ, Mulligan J, Moulton SL, MacLeod D. A novel measurement for accurate assessment of clinical status in patients with significant blood loss: the compensatory reserve. Shock 2015; 44:27–32 [DOI] [PubMed] [Google Scholar]
- 64.Convertino VA, Wirt MD, Glenn JF, Lein BC. The compensatory reserve for early and accurate prediction of hemodynamic compromise: a review of the underlying physiology. Shock 2016; 45:580–90 [DOI] [PubMed] [Google Scholar]
- 65.Convertino VA, Schiller AM. Measuring the compensatory reserve to identify shock. J Trauma Acute Care Surg 2017; 82:S57–6 [DOI] [PubMed] [Google Scholar]
- 66.Howard JT, Janak JC, Hinojosa-Laborde C, Convertino VA. Specificity of compensatory reserve and tissue oxygenation as early predictors of tolerance to progressive reductions in central blood volume. Shock 2016; 46:68–73 [DOI] [PubMed] [Google Scholar]
- 67.Janak JC, Howard JT, Goei KA, Weber R, Muniz GW, Hinojosa-Laborde C, Convertino VA. Predictors of the onset of hemodynamic decompensation during progressive central hypovolemia: comparison of the peripheral perfusion index, pulse pressure variability, and compensatory reserve index. Shock 2015; 44:548–53 [DOI] [PubMed] [Google Scholar]
- 68.Schiller AM, Howard JT, Lye KR, Magby CG, Convertino VA. Comparisons of traditional metabolic markers and compensatory reserve as early predictors of tolerance to central hypovolemia in humans. Shock 2018; 50:71–7 [DOI] [PubMed] [Google Scholar]
- 69.Benov A, Yaslowitz O, Hakin T, Amir-Keret R, Nadler R, Brand A, Glassberg E, Yitzhak A, Convertino VA, Paran H. The effect of blood transfusion on compensatory reserve. A prospective clinical trial. J Trauma Acute Care Surg 2017; 83:S71–S6 [DOI] [PubMed] [Google Scholar]
- 70.Johnson MC, Alarhayem A, Convertino VA, Carter IIR, Chung K, Stewart R, Myers J, Dent D, Liao L, Cestero R, Nicholson S, Muir M, Schwaca M, Wampler D, deRosa M, Eastridge BJ. Compensatory reserve index: performance of a novel monitoring technology to identify bleeding trauma patients. Shock 2018; 49:295–300 [DOI] [PubMed] [Google Scholar]
- 71.Koons N, Nguyen B, Suresh M, Hinojosa-Laborde C, Convertino V. Tracking DO2 with compensatory reserve during whole blood resuscitation following controlled hemorrhage in baboons. Shock 2019. (In press). [DOI] [PubMed]
- 72.Convertino VA, Grudic GZ, Mulligan J, Moulton SL. Estimation of individual-specific progression to impending cardiovascular instability using arterial waveforms. J Appl Physiol 2013; 115:1196–202 [DOI] [PubMed] [Google Scholar]
- 73.van Der Zwaard S, De Ruiter CJ, Noordhof DA, Sterrenburg R, Bloemers FW, De Koning JJ, Jaspers RT, Van der Laarse WJ. Maximal oxygen uptake is proportional to muscle fiber oxidative capacity, from chronic heart failure patients to professional cyclists. J Appl Physiol 2016; 121:636–45 [DOI] [PubMed] [Google Scholar]
- 74.Syncope in the athlete. A primer on the autonomic nervous system. Amsterdam: Elsevier Sci. Publ, 2004. pp.252–3 [Google Scholar]
- 75.Levine BD. Regulation of central blood-volume and cardiac filling in endurance athletes - the frank-starling mechanism as a determinant of orthostatic tolerance. Med Sci Sports Exerc 1993; 25:727–32 [PubMed] [Google Scholar]
- 76.Raven PB, Pawelczyk JA. Chronic endurance exercise training: a condition of inadequate blood pressure regulation and reduced tolerance to LBNP. Med Sci Sports Exerc 1993; 25:713–21 [PubMed] [Google Scholar]
- 77.Butler FK, Holcomb JB, Schreiber MA, Kotwal RS, Jenkins DA, Champion HR, Bowling F, Cap AP, Dubose JJ, Dorlac WC, Dorlac GR, McSwain NE, Timby JW, Blackbourne LH, Stockinger ZT, Strandenes G, Weiskopf RB, Gross KR, Bailey JA. Fluid resuscitation for hemorrhagic shock in tactical combat casualty care: TCCC guidelines change 14-01-2 June 2014. J Spec Oper Med 2014; 14:13–38 [DOI] [PubMed] [Google Scholar]