Abstract
Chronic, low-grade inflammation increases the risk for atherosclerosis, cancer, and autoimmunity in diseases such as obesity and diabetes. Levels of CD4+ T helper 17 (Th17) cells, which secrete interleukin 17A (IL-17A), are increased in obesity and contribute to the inflammatory milieu; however, the relationship between signaling events triggered by excess nutrient levels and IL-17A–mediated inflammation is unclear. Here, using cytokine, quantitative real-time PCR, immunoprecipitation, and ChIP assays, along with lipidomics and MS-based approaches, we show that increased levels of the nutrient-responsive, post-translational protein modification, O-GlcNAc, are present in naive CD4+ T cells from a diet-induced obesity murine model and that elevated O-GlcNAc levels increase IL-17A production. We also found that increased binding of the Th17 master transcription factor RAR-related orphan receptor γ t variant (RORγt) at the IL-17 gene promoter and enhancer, as well as significant alterations in the intracellular lipid microenvironment, elevates the production of ligands capable of increasing RORγt transcriptional activity. Importantly, the rate-limiting enzyme of fatty acid biosynthesis, acetyl-CoA carboxylase 1 (ACC1), is O-GlcNAcylated and necessary for production of these RORγt-activating ligands. Our results suggest that increased O-GlcNAcylation of cellular proteins may be a potential link between excess nutrient levels and pathological inflammation.
Keywords: obesity, type 2 diabetes, inflammation, O-linked N-acetylglucosamine (O-GlcNAc), T helper cells, acetyl-CoA carboxylase (ACC), RAR-related orphan receptor gamma t variant, Th17, lipid metabolism
Introduction
CD4+ T cells orchestrate the adaptive immune response. Activation of naive CD4+ T cells in response to a pathogen prompts their differentiation into various effectors (e.g. T helper 1 (Th1),5 Th2, and Th17). Cytokines secreted by the effectors then lead to elimination of the pathogen (1). Major metabolic shifts occur during activation and are required for effector cell function. For example, activation induces a switch from oxidative phosphorylation to aerobic glycolysis (2, 3) and influx of glucose and glutamine necessary to meet the energetic requirements for rapid clonal proliferation of the T cell (4, 5). Furthermore, different effectors require different metabolic pathways. For example, Th1, Th2, and Th17 cells utilize glycolytic pathways for energy, whereas regulatory T cells (Tregs) require oxidative phosphorylation (6). Additionally, Th17 cells have a requirement for endogenous fatty acid synthesis, and pharmacological inhibition or genetic deletion of acetyl-CoA carboxylase 1 (ACC1) inhibits Th17 and favors Treg differentiation (7).
Metabolic abnormalities drive specific T cell effector pathology in several disease states. For example, the pro-inflammatory function of Th17 cells is enhanced in several autoimmune diseases, such as rheumatoid arthritis (8). Inflammatory Th17 cells infiltrating the synovium of joints in a rheumatoid arthritis model accumulate lipid droplets due to increased fatty acid metabolism (9). Additionally, extrinsic metabolic factors alter T cell function. In diseases of overnutrition, such as obesity and diabetes, Th1 and Th17 cells are increased in the peripheral blood and adipose tissue, contributing to atherosclerotic plaque formation and insulin resistance (10–13). However, mechanisms that clearly link excess nutrients with aberrant T cell function are unclear.
The post-translational protein modification O-GlcNAc (O-linked β-GlcNAc) is an intriguing candidate for a how a T cell can quickly interpret changes in nutrient status. O-GlcNAc is added to nuclear, cytoplasmic, and mitochondrial proteins by a single enzyme, O-GlcNAc transferase (OGT) and removed by O-GlcNAcase (OGA) (14). O-GlcNAc levels are exquisitely sensitive to the nutritional and environmental status of the cell. The substrate for OGT, UDP-GlcNAc, is made through the hexosamine biosynthetic pathway and requires input from all four major biomolecules: carbohydrate, amino acid, fatty acid, and nucleic acid (15). As nutrient flux changes throughout the cell, UDP-GlcNAc concentrations and O-GlcNAcylation by OGT change accordingly (16). Thus, in response to major nutrient shifts, rapid changes in O-GlcNAcylation of proteins and cellular function occur. This suggests that O-GlcNAcylation acts as a nutrient sensor to fine-tune cellular functions.
Whereas a physiological level of O-GlcNAcylation is essential for murine and human T cell activation (17, 18), abnormal levels of O-GlcNAcylation and the O-GlcNAc–processing enzymes contribute to T cell–mediated diseases. For example, hypomethylation of the X-linked OGT gene in female lupus patients increases OGT expression and contributes to increased inflammatory T cells (19), whereas an miRNA that targets OGT transcripts for degradation is decreased in T cells of multiple sclerosis patients (20). Additionally, an SNP leading to truncated, nonfunctional OGA in a Mexican–American population contributes to an increased incidence of diabetes (21). Thus, O-GlcNAc is essential for normal T cell function, and aberrant O-GlcNAcylation is linked to inflammation.
In this study, we demonstrate that elevated O-GlcNAc levels correlate with increased pro-inflammatory IL-17A cytokine secretion by murine and human CD4+ T cells. Additionally, we identify a metabolic shift in the lipid microenvironment that results in increased ligands capable of activating retinoic acid–related orphan receptor, t splice variant (RORγt) transcriptional activity. Our data suggest a critical role of O-GlcNAc nutrient sensing in regulating Th17 cell function, particularly in diet-induced obesity, and identify the O-GlcNAc modification as a potential contributor to how Th17 cells translate nutrient excess into amplified pathological inflammation.
Results
In murine CD4+ T cells, elevated O-GlcNAcylation correlates with increased pro-inflammatory IL-17A transcript and protein levels
To determine whether O-GlcNAc plays a role in the function of T cell effectors, we treated murine, splenic CD4+ T cells ex vivo with thiamet-G (TMG), a highly specific OGA inhibitor (22), for 6 h before activation under nonpolarizing conditions (Th0) or, in other words, without cytokines that would induce polarization toward a specific CD4+ T cell lineage (Th1, Th2, etc.). Our initial experiments using nonpolarizing conditions allowed us to determine how TMG treatment might alter proteins critical for differentiation of CD4+ T cells without the potentially dominating influence of polarizing cytokines. TMG treatment led to elevated O-GlcNAc levels over the 4 days of cell culture (Fig. 1A). As has been observed previously (14, 23), levels of OGT decreased and levels of OGA increased in response to elevated O-GlcNAcylation, allowing a return to steady-state levels of O-GlcNAc. This is a well-described compensatory phenomenon, suggesting that cells maintain a level of O-GlcNAcylation that is required for their function. Interestingly, restimulation of CD4+ T cells after 4 days of culture appears to reset or promote a restoration of the homeostatic levels of O-GlcNAc, OGT, and OGA—a phenomenon that confirms previous reports (17, 24). After 4 days of activation under nonpolarizing conditions, equal numbers of the total CD4+ T cell population were restimulated, and supernatants were harvested 24 h later. The levels of key cytokines produced by four main effector types (Th1, Th2, Th17, and Treg) were analyzed by ELISA. Strikingly, production of IL-17A, the major cytokine secreted by Th17 cells, doubled (Fig. 1B). IFNγ, the major cytokine secreted by Th1 cells, was also significantly increased. However, IL-4 and IL-10, cytokines predominantly produced by the Th2 and Treg lineages, respectively, showed no changes. We did not observe increased numbers of cells that produce IL-17A or IFNγ (data not shown), so increases in these cytokines were due to increased production per cell rather than an increase in cell number. The fact that both IL-17A and IFNγ are pro-inflammatory cytokines and IL-4 and IL-10 have immunomodulatory functions suggests that elevated O-GlcNAcylation promotes increased inflammatory function of CD4+ Th17 and potentially Th1 cells. Transcript levels of IL-17A, IFNγ, IL-4, and IL-10 followed similar trends as protein levels (Fig. 1C). Another critical pro-inflammatory Th17 marker, IL-23 receptor (IL-23R), was also significantly increased with TMG treatment. IL-23 binding and signaling through IL-23R stabilizes the Th17 phenotype (26, 27) and is also a marker of pathogenic Th17 function (28). The up-regulation of IL-23 receptor in nonpolarized T cells by TMG is suggestive of an increased percentage of Th17 cells with a pathogenic phenotype being induced by the drug. Overall, markers of Th17 function are increased with elevated O-GlcNAcylation.
Figure 1.
In murine, splenic CD4+ T cells, elevated O-GlcNAcylation increases pro-inflammatory IL-17A transcript and protein levels. A, TMG treatment increases O-GlcNAc levels over the course of splenic CD4+ T cell population differentiation and proliferation with corresponding decreases in OGT and increases in OGA. The red arrow indicates the time of restimulation. The blot is representative of three experiments. B, protein levels of cytokines secreted from splenic, total CD4+ T cells with or without TMG treatment. C, transcript levels of cytokines secreted from splenic, total CD4+ T cells with or without TMG treatment. D, O-GlcNAc levels remain elevated in Th17-polarized splenic CD4+ T cells on day 4 and normalize following 24 h of restimulation (day 5). The blot is representative of three experiments. E, protein level of IL-17A secreted by splenic naive CD4+ T cells polarized to Th17 lineage treated with or without TMG. F, transcript level of IL-17A secreted by splenic, naive CD4+ T cells polarized to Th17 lineage treated with or without TMG. G, frequency of live CD4+ IL-17+ cells is significantly increased with TMG treatment. 25,000 cells were analyzed per condition. Dead cells were excluded. A representative histogram is shown in Fig. S1. Bars, mean ± S.E. (error bars) of five biological replicates in B and C and four different biological replicates in E–G. Bars, median ± S.E. (error bars) of four biological replicates in G; *, p < 0.05; ***, p < 0.001.
Th17 cells make up less than 1% of all CD4+ T cells in the peripheral blood (29). To investigate the mechanism of O-GlcNAc regulation of Th17 function, we prepared a more homogeneous population of Th17 cells. We isolated splenic, naive CD4+ T cells (CD62LhiCD44lo) and treated them with or without TMG before activation and polarization toward the Th17 lineage. O-GlcNAc levels remain elevated in the TMG-treated Th17-polarized CD4+ T cells on the fourth day of culture, and as observed previously with the nonpolarized CD4+ T cells, restimulation for 24 h restored O-GlcNAc levels to steady-state levels (Fig. 1D). TMG treatment significantly increased both protein and transcript levels of IL-17A, even beyond the levels detected in cultures polarized toward Th17 in the absence of the drug (Fig. 1, E and F). Intracellular cytokine staining showed significant increase in CD4+IL-17+ cells, suggesting that there may be some effect on differentiation as well as functional cytokine output (Fig. 1G and Fig. S1; gating strategy shown in Fig. S2). However, this 5% increase in IL-17A–producing cells is unlikely to account for the full 30% increase in cytokine output, so the biological effect of this increase in cell percentage may be minimal. Together, elevated O-GlcNAcylation correlates with increased IL-17A production in CD4+ T cells polarized to the Th17 lineage, ex vivo.
In human CD4+ T cells, elevated O-GlcNAcylation increases pro-inflammatory IL-17A transcript and cytokine levels
Th17 cells infiltrate adipose tissue to a greater extent in obese humans (30) and secrete more IL-17A, contributing to adipose insulin resistance (31). We next determined whether TMG treatment increased IL-17A production from human CD4+ T cells. We obtained fresh, whole blood from human volunteers through the Biospecimen Repository Core Facility at the University of Kansas Medical Center. Characteristics of volunteers, including gender, age, and ethnicity, are detailed in Fig. 2A. CD4+ T cells were isolated from blood and treated with or without TMG before activation under Th17 conditions. IL-17A production was significantly increased in cells treated with TMG, as were O-GlcNAc levels (data not shown), recapitulating the results seen in mice (Fig. 2B). Variable percentages of naive CD4+ T cell populations in human samples likely contributed to the higher variability in IL-17A secretion that we observed. Transcript levels of IL-17A, IL-23R, and IFNγ were likewise elevated in response to TMG treatment (Fig. 2C). Thus, we have demonstrated a clear correlation between elevated O-GlcNAc levels and the production of several pro-inflammatory cytokines—particularly IL-17A—the major cytokine mediating the function of Th17 cells.
Figure 2.
In human CD4+ T cells isolated from peripheral blood, elevated O-GlcNAcylation increases pro-inflammatory IL-17A transcript and cytokine levels. A, clinical characteristics of human blood donors. B, TMG treatment increases IL-17A secretion from total human CD4+ cells isolated from peripheral blood and polarized under Th17 conditions. C, IL-17, IL-23R, and IFNγ transcript levels all significantly increase with TMG treatment. Bars, mean ± S.E. (error bars) of 9 and 7 human donors in B and C, respectively. *, p < 0.05; **, p < 0.01. BMI, body mass index.
Naive CD4+ T cells from mice fed a Western diet have elevated O-GlcNAc levels and increased IL-17A production, which is exacerbated by OGA inhibition
The previous data suggest that elevated O-GlcNAc increases pro-inflammatory cytokine production from CD4+ T cells. To investigate the physiological relevance of this effect in a disease model, we utilized a diet-induced obese mouse model. O-GlcNAc levels are sensitive to the nutritional state of the host (14, 32). Hence, we would expect them to be elevated in a diet-induced obese mouse model, phenocopying the effects of TMG in our in vitro studies. To test this hypothesis, we fed male, C57BL/6 mice high-fat and -cholesterol, “Western diet” (WD) chow for 16 weeks. As expected, WD-fed mice gained significantly more weight, and their blood glucose was significantly elevated 15 weeks after initiation of the diet, compared with mice fed standard chow (SC) (Fig. 3, A and B). Naive CD4+ T cells (CD62LhiCD44lo) isolated from WD-fed mice had significantly elevated O-GlcNAc levels compared with SC-fed mice (Fig. 3C). Interestingly, OGT and OGA levels were not different between WD- and SC-fed mice, suggesting that elevated O-GlcNAc levels in the WD-fed mice reach a new, albeit elevated and potentially pathogenic set point. This new, higher O-GlcNAc level possibly could be due to lack of changes in either OGT and OGA expression level or function. When naive CD4+ T cells from WD- and SC-fed mice were activated and polarized toward a Th17 lineage, cells from WD-fed mice secreted more IL-17A, as observed by previous groups (10, 12). When these cells were treated with TMG prior to activation and polarization, cells from SC-fed mice secreted levels of IL-17A that were comparable with levels produced by cells from WD-fed mice. IL-17A secretion by cells from WD-fed mice was even further exacerbated (Fig. 3D). The trends seen with IL-17A transcript levels were similar to the trends seen in protein levels (Fig. 3E). Together, these results demonstrate that in a type 2 diabetes model, elevated O-GlcNAc levels in CD4+ T cells lead to a phenotype similar to pharmacological OGA inhibition by TMG. This suggests that O-GlcNAc is a critical regulator of Th17 functional activity and, when aberrantly elevated—as in obesity—can contribute to the inflammatory milieu that drives the devastating pathology associated with type 2 diabetes.
Figure 3.
Western diet feeding in mice results in elevated O-GlcNAc levels in splenic, naive CD4+ T cells and increases IL-17A secretion from splenic, naive CD4+ T cells, which is exacerbated by TMG treatment. A, WD feeding results in significantly increased weight gain. B, WD feeding results in significantly elevated fasting blood glucose levels. C, WD-fed mice have significantly elevated O-GlcNAc levels. D, splenic, naive CD4+ T cells polarized to the Th17 lineage from WD-fed mice secrete significantly more IL-17A than cells from SC mice. Cells from SC-fed mice treated with TMG secrete levels similar to cells from WD mice, and TMG treatment of cells from WD-fed mice significantly exacerbates IL-17A secretion. E, IL-17A transcript levels mimic the trends seen with IL-17A protein secretion. In A and B, points represent average ± S.D. (error bars) and are calculated from four and five biological replicates (SC and WD, respectively). In C, the graph of densitometry is from eight biological replicates, and bars represent mean ± S.D. (error bars). Each lane in the blot represents whole-cell lysate from one mouse. In D and E, bars represent mean ± S.E. (error bars) of four and five biological replicates (SC and WD, respectively). HPRT is used an internal reference for gene expression. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Elevated O-GlcNAcylation has no effect on RORγt protein or transcript levels but does promote retention of RORγt at the IL-17 locus
RORγt is the master transcription factor that directs the Th17 lineage and is essential for IL-17A gene transcription (33). We found no differences in the expression of RORγt protein or transcript levels in the presence of TMG on the fourth day of cell culture (indicated as the zero time point (0)) or over a 24-h span after restimulation (Fig. 4, A and B). Furthermore, we saw no significant differences in RORγt protein or transcript levels at 24 h after restimulation in Th17-differentiated cells from mice fed SC or WD (Fig. S3). These results suggest that O-GlcNAc neither regulates the stability of RORγt or its transcripts; nor does O-GlcNAc affect transcription factors required for transcription of RORγt itself.
Figure 4.
Elevated O-GlcNAcylation has no effect on RORγt protein or transcript levels but does promote retention of RORγt at the IL-17 locus. A, splenic, naive CD4+ T cells were polarized to the Th17 lineage with and without TMG and cultured for 4 days. Prior to restimulation on the fourth day of culture (0) and over 24 h after restimulation, RORγt protein levels were unchanged. Densitometry was used to compare the RORγt/actin ratio at specified time points. The blot is representative of three biological replicates. Densitometry bars represent the mean ± S.E. (error bars) of three biological replicates. B, transcript levels of RORγt are unchanged 24 h after restimulation. RORγt gene expression was normalized to HPRT expression. Bars, mean ± S.E. (error bars) of five biological replicates. C, TMG treatment significantly increases RORγt binding at the IL-17 promoter and CNS2 enhancer regions in ChIP assays. Bars, mean ± S.E. (error bars) of four biological replicates. *, p < 0.05; **, p < 0.01.
Because RORγt levels did not change with TMG treatment, we speculated that RORγt was being retained at the IL-17A locus. We performed ChIP of RORγt at the IL-17 promoter and an enhancer, conserved noncoding sequence 2 (CNS-2), which is required for IL-17A transcription (34). TMG treatment resulted in increased RORγt binding at the IL-17 promoter and the CNS-2 enhancer region in Th17 cells differentiated ex vivo and fixed on the fourth day of cell culture (Fig. 4C). Together, TMG treatment increases the association of RORγt with the IL-17A promoter and enhancer regions.
In murine differentiated Th17 cells, elevated O-GlcNAc increases lipid ligands capable of increasing RORγt transcriptional activity
Among the lineage-defining transcription factors for CD4+ T cells, RORγt activity is uniquely regulated by the binding of lipid ligands. Cholesterol biosynthetic intermediates, such as 7β,27-dihydroxycholesterol and 7α,27-dihydroxycholesterol, are known endogenous ligands capable of binding the ligand binding domain of RORγt, strongly activating its transcriptional activity (35, 36). Additionally, fatty acids are critical for optimal RORγt activity (7, 37). The type of fatty acid ligand is also important for RORγt activity. An increased ratio of saturated fatty acids to polyunsaturated fatty acids (PUFAs) increases RORγt transcriptional activity at the IL-17A promoter while decreasing activity at the IL-10 promoter, thus promoting pathogenic Th17 differentiation (38). We investigated alterations in the lipidome that might increase RORγt transcriptional activity at the IL-17A gene. To assess how O-GlcNAc might affect the lipidome of T cells, we treated murine, splenic naive CD4+ T cells with or without TMG prior to activation and polarization toward the Th17 lineage. We then performed an unbiased analysis of the lipidome by MS. Of note, cholesterol and total sterol populations were both significantly elevated with TMG treatment (Fig. 5A; see raw data in Table S1). One possibility for these results is increased cholesterol synthesis and thus increased cholesterol biosynthetic intermediates capable of activating RORγt. Additionally, of the top 20 fatty acids detected in the cells, three saturated fatty acids, such as stearic acid (C18:0), were increased, and all PUFAs, such as arachidonic acid (C20:4), were decreased with TMG treatment (Fig. 5, B and C; see raw data in Tables S2 and S3). Interestingly, TMG treatment appears to have profound effects on choline metabolism. Whereas the total amount of phospholipids is unchanged, there is a significant decrease in phosphatidylcholine (PC) and compensatory increases in all other types of phospholipids with TMG treatment (Fig. 5D). Furthermore, sphingomyelin is synthesized from the enzymatic reaction combining ceramide and PC (39). Sphingomyelin is significantly decreased and ceramide is significantly increased in TMG-treated cells (Fig. 5D). These data suggest that choline is being shunted away from PC synthesis and toward other biosynthetic pathways, such as acetylcholine synthesis (40), and substrates, such as betaine, which is used in one-carbon metabolism required for DNA methylation (39). Recently, acetylcholine-producing T cells were reported to be necessary for vasodilation and tissue invasion of T cells responding to viral infection (41). This suggests that in addition to effects on IL-17A secretion, TMG-treated cells may also have improved tissue penetration, further exacerbating end organ inflammation. Additionally, elevated ceramides are indicative of an inflammatory phenotype, because knockout of a ceramide synthase in splenocytes results in improvement of colitis in a mouse model (42). Together, these results demonstrate that elevated O-GlcNAcylation correlates with significant alterations in the intracellular lipid microenvironment, promoting an inflammatory phenotype. Overall, several cholesterol intermediates and fatty acids capable of acting as activating ligands of RORγt at the IL-17A locus were increased. An increase in these lipid ligands potentially explains why RORγt is retained longer at the IL-17 locus.
Figure 5.
In murine, Th17-differentiated cells, elevated O-GlcNAc increases lipid ligands capable of increasing RORγt transcriptional activity. A, splenic, naive CD4+ T cells were polarized to the Th17 lineage with and without TMG and cultured for 4 days and then restimulated for 8 h before harvesting for lipid analysis. The absolute value or normalized ion abundance of total sterols and cholesterol is increased with TMG treatment. B, of the top 20 fatty acids present in cells, the percentage of saturated fatty acids increased with TMG treatment. C, of the top 20 fatty acids present in cells, the percentage of polyunsaturated fatty acids decreased with TMG treatment. D, choline was shunted away from phosphatidylcholine and sphingomyelin synthesis, leading to a rise in ceramide levels. Bars, mean ± S.D. (error bars) of five biological replicates. *, p < 0.05; **, p < 0.01; ***, p < 0.001. PE, phosphatidylethanolamine; PG, phosphatidylglycerol; PI, phosphatidylinositol; PS, phosphatidylserine.
ACC1 is modified by O-GlcNAc
The substrates needed for both long-chain fatty acids and cholesterol synthesis originate from the activity of ACC1, which catalyzes the carboxylation of acetyl-CoA to form malonyl-CoA. Importantly, de novo fatty acid synthesis, and specifically ACC1 activity, is essential for Th17 differentiation (7). Because O-GlcNAcylation and phosphorylation can compete for the same sites and be reciprocal to one another (14), we looked at levels of inhibitory phosphorylation of ACC1 at serine 79 to determine whether TMG treatment altered ACC1 inhibition. AMP kinase (5′-adenosine monophosphate–activated protein kinase) senses low ATP levels in a cell and phosphorylates Ser-79 on ACC1 to inhibit biosynthetic activity (43). When we analyzed phospho-ACC1 levels at various times after restimulation of ex vivo differentiated Th17 cells, we observed no changes in ACC1-inhibitory phosphorylation when cells were treated with TMG (Fig. 6A).
Figure 6.
ACC1 is modified by O-GlcNAc. A, splenic, naive CD4+ T cells were polarized to the Th17 lineage with and without TMG and cultured for 4 days before restimulation for 24 h. Inhibitory phosphorylation of ACC1 at Ser-79 is unchanged with TMG treatment over the 24 h of restimulation. pACC1 and ACC1 levels were normalized to actin. Blot is representative of three biological replicates. Densitometry bars, mean ± S.E. (error bars) of three replicates. B, ACC1 or O-GlcNAc–modified proteins were immunoprecipitated (IP) from splenic CD4+ T cells and show that ACC1 is modified by O-GlcNAc. Blots are representative of three biological replicates each. C, representative mass spectra plot of high confidence O-GlcNAc–modified sites Ser-966 and Ser-976 on an ACC1 peptide. D, location of analogous O-GlcNAc–modified residues Ser-2129 and Ser-2323 in the carboxyl transferase domain on a purified human partial C-terminal crystal structure of ACC1 (Protein Data Bank entry 4ASI). E, palmitic acid significantly decreases with TMG treatment. Bars, mean ± S.D. (error bars) of five replicates; **, p < 0.01.
We next hypothesized that as a cellular nutrient sensor, O-GlcNAc might modify ACC1 and regulate its activity independently of AMPK phosphorylation. We first immunoprecipitated ACC1 from CD4+ T cells treated with or without TMG and observed that it was O-GlcNAcylated. The reverse procedure of pulling proteins down with an O-GlcNAc antibody also yielded ACC1 (Fig. 6B). We then confirmed this finding by performing MS on immunoprecipitated ACC1 from TMG-treated murine naive T cells polarized ex vivo to the Th17 lineage. Mass spectrometry analysis uncovered four high-confidence O-GlcNAc sites on murine ACC1: Ser-966, Ser-967, Ser-2091, Ser-2285 (Fig. 6, C and D; for raw data, see Tables S3 and S4). Two of these sites (Ser-2091 and Ser-2285) had homologous sites in a reported human partial C-terminal domain ACC1 crystal structure (Protein Data Bank entry 4ASI) (Fig. 6D). In the human crystal structure and analogous mouse domains, O-GlcNAc sites were found in the central region (Ser-966 and Ser-976) and carboxyl transferase domain (Ser-2091), suggesting that O-GlcNAc might regulate binding of protein partners in the central region (44) and/or the final step in the formation of malonyl-CoA, respectively, potentially altering ACC1-binding partner function or ACC1 catalytic activity directly.
In addition to no change in inhibitory phosphorylation of ACC1, palmitic acid (C16:0), which can negatively feedback to inhibit ACC1 activity, is significantly decreased with OGA inhibition (Fig. 6E). Thus, elevated O-GlcNAcylation results in increased lipid ligands that are synthesized downstream of ACC1 activity and leads to reduced inhibitory palmitic acid levels with no change in inhibitory phosphorylation of ACC1. Overall, OGA inhibition creates an environment conducive to ACC1 activity and generates lipid ligands capable of increasing RORγt activity at the IL-17 locus.
Discussion
Obesity has reached epidemic proportions in the developed world and is increasingly becoming a problem in less developed nations (45). Patients with diseases of overnutrition have a well-defined chronic, low-grade inflammation. This inflammation has been implicated in much of the downstream pathology associated with increased adiposity, such as atherosclerosis, insulin resistance, and increased risk of autoimmunity and cancer (11, 12, 46). Among this chronic inflammatory milieu, CD4+ T cell effectors, particularly Th1 and Th17 cells, are critical players in the development of atherosclerotic plaques and infiltrate adipose tissue to mediate insulin resistance (10, 12). In ApoE−/− mice predisposed to atherosclerotic lesions, administration of a neutralizing antibody to IL-17A reduced plaque size, whereas supplementation of recombinant IL-17A further increased plaque size (47). In the peripheral blood of type 2 diabetic humans, Th1 and Th17 cells are increased, whereas regulatory T cells are decreased (48). The serum level of IL-17A correlated with hemoglobin A1c in type 2 diabetics, suggesting that as IL-17A levels increase, so does blood glucose (48). In addition to peripheral blood, increased levels of IL-17A and IL-22 were found in the adipose of insulin-resistant, obese humans compared with lean or obese individuals with normal insulin resistance (49). Importantly, antibody neutralization of IL-17A ameliorated insulin resistance in a murine model of inflammation-induced insulin resistance (50). Thus, to lessen the morbidity, mortality, and financial burden consequent to obesity, a better understanding of the mechanisms that promote IL-17A-driven pathology related to excess adiposity is imperative.
In this study, we demonstrate that a homeostatic level of O-GlcNAcylation is necessary for the proper regulation of CD4+ T cell function. We cultured murine and human CD4+ T cells with TMG, a highly specific O-GlcNAcase inhibitor, to disrupt homeostatic O-GlcNAc levels. We observed a significant increase in pro-inflammatory cytokines, IL-17A and IFNγ, major cytokines secreted by the Th17 and Th1 lineages, respectively. We observed no change in IL-10 protein or transcript levels in CD4+ T cells treated with TMG, suggesting no significant decrease in Tregs. Under nonpolarizing, Th0 conditions, we did not consistently observe increased numbers of cells producing IL-17 in the presence of TMG (data not shown). In fact, under these conditions, we detected very few cells capable of producing the cytokine at all. Under Th17-polarizing conditions, we observed a modest, although significant, 4% increase in the number of cells producing IL-17A with TMG treatment. These results suggest that abnormally elevated O-GlcNAc results in more IL-17A being secreted per cell rather than skewing differentiation toward the Th17 lineage at the expense of Tregs.
Because excess O-GlcNAcylation increased Th17 and Th1 inflammatory cytokines, which are abnormally elevated in obesity, we wanted to determine the role of O-GlcNAc in a WD-fed mouse model. In agreement with O-GlcNAc acting as a nutritional sensor, O-GlcNAc levels were significantly increased in the naive CD4+ T cells of WD-fed mice. Interestingly, the levels of OGT and OGA were not different between mice fed SC and mice on the Western diet. Normally, O-GlcNAc levels are vigorously maintained at a level particular to the needs of a cell (23). For example, when O-GlcNAcylation is increased with TMG treatment, OGA levels will increase and OGT levels will decrease to return O-GlcNAc levels to baseline (23). However, our results suggest that long-term nutritional excess causes O-GlcNAc levels to reset to an abnormally high level. When this new, atypically high set point is pushed even further by TMG treatment, both cells from SC- and WD-fed mice produce significantly more IL-17A. These results agree with a known effect of an SNP in the OGA gene, which encodes OGA. In a Mexican–American population of individuals with these SNPs, OGA expression is decreased, and there is an increased incidence and an earlier age of onset of type 2 diabetes (21). Alterations to OGT or OGA levels could similarly contribute to an abnormal O-GlcNAc set point and pathogenicity. Thus, O-GlcNAcylation is a mechanism contributing to increased pro-inflammatory IL-17A secretion. This is particularly relevant in the context of diet-induced obesity, where O-GlcNAcylation attains a higher homeostatic level.
Lipid ligands, such as sterols and saturated fatty acids, capable of binding and activating RORγt at the IL-17 locus were increased with TMG treatment. Furthermore, we identified sites of O-GlcNAcylation on ACC1, which provides the building blocks for both long-chain fatty acid and cholesterol synthesis (51). In addition to increased fatty acid and sterol products that result from ACC1 activity, two other lines of evidence suggest increased ACC1 activity with TMG treatment. First, palmitic acid (C16:0) is the first fatty acid produced upon initiation of fatty acid biosynthesis by ACC1. As such, palmitic acid can serve as a negative feedback inhibitor of ACC1 (52). The significant decrease in palmitate with TMG treatment but significant increase in longer-chain saturated fatty acids suggests that palmitate is rapidly diverted into elongation pathways or acylation of proteins, so it is less available to inhibit ACC1 activity. Additionally, we were unable to detect a change in inhibitory phosphorylation of ACC1 by AMPK at serine 79. Thus, there is not increased inhibition of ACC1 with TMG treatment. Of note, lipidomic analysis of endothelial cells treated with soraphen A, a potent inhibitor of ACC1, resulted in effects opposite of what we observed (53). With inhibition of ACC1, PUFAs increased, whereas we observed a significant decrease in PUFAs in our T cell model. In addition to an O-GlcNAc site (Ser-2091) being present in the carboxyl transferase (CT) domain of ACC1, which catalyzes the final step of malonyl-CoA formation, sites were found in the central region of ACC1 (Ser-966 and Ser-976). In a crystal structure of yeast ACC1, the central region was found to be composed of five unique domains and was responsible for bringing the biotin carboxylase (BC) and CT domains together (54) during catalysis. Importantly, deletion of residues 940–972 in the first domain of the central region abolished catalytic activity. Overall, multiple lines of circumstantial evidence suggest that TMG treatment favors increased ACC1 activity and production of ligands that enhance RORγt activity.
In addition to O-GlcNAc regulation of fatty acid synthesis necessary for Th17 function, several other metabolic pathways affecting IL-17A production and other inflammatory mediators could be regulated by O-GlcNAc. Because there was a clear decrease in the percentage of polyunsaturated fatty acids and increase in the percentage of saturated fatty acids, O-GlcNAc might act to directly inhibit desaturases or decrease transcription factor activity necessary for desaturase expression. This could be another mechanism that favors saturated fatty acid ligands that increase RORγt activity and increased IL-17A expression. One of the PUFAs significantly decreased in our lipidomic analysis, linoleic acid, is an essential fatty acid (55) and thus would not be susceptible to changes in ACC1 synthetic activity. The significant decrease in this PUFA might be due to decreased cellular uptake or possibly increased shunting of linoleic acid toward arachidonic acid synthesis (55). However, we also saw a significant decrease in arachidonic acid, suggesting that elevated O-GlcNAcylation might also be triggering arachidonic acid catabolism, potentially leading to increased prostaglandin and leukotriene production. Finally, because AMP kinase activity—specifically inhibition of ACC1 at Ser-79—was unaffected by TMG treatment, another major metabolic regulator, mTOR, which is also inhibited by AMP kinase, could be influenced by O-GlcNAc (56). mTOR is a kinase that serves as a critical nutrient-sensing node in the cell, regulating cell growth, translation, and numerous other cellular processes. mTOR complex 1 (mTORC1) contains the Raptor scaffold protein and is activated in part by Rheb, a small GTP-binding protein (57, 58). Interestingly, both Rheb and Raptor are required for Th1 and Th17 differentiation but have little effect on Th2 differentiation (59). mTORC1 activation specifically increases nuclear translocation of RORγt and prevents inhibition of RORγt, resulting in increased Th17 differentiation (60). Of note, increased mTORC1 activity increases expression of HIF1α (hypoxia inducible factor 1α), which is a transcription factor necessary for RORγt expression and Foxp3 degradation, favoring Th17 differentiation (61, 62). Thus, further investigation of how O-GlcNAc regulates the mTORC1 and HIF1α signaling pathways in the context of CD4+ T cell differentiation might reveal other mechanisms for increased IL-17A secretion and Th17 differentiation in the context of aberrant O-GlcNAcylation.
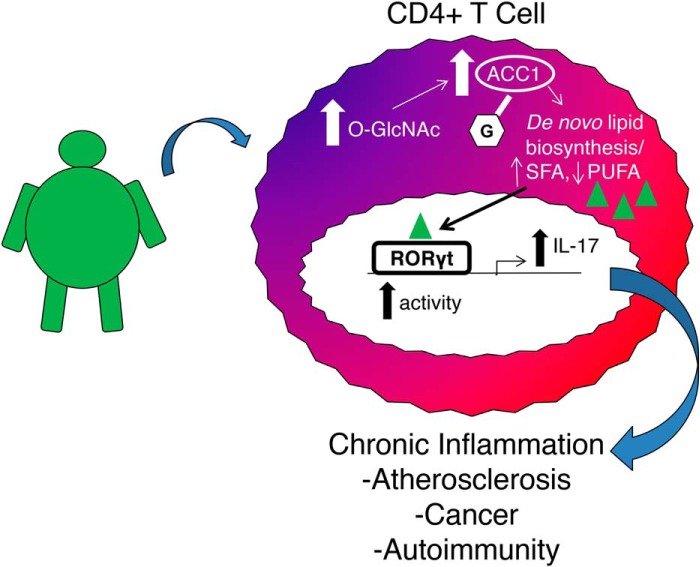
Obesity and diabetes are major public health concerns, and the immune system is a critical driver of pathogenesis in these diseases. Intervening at the level of inflammation is of critical importance in preventing pathological complications due to obesity. Unfortunately, the mechanisms driving chronic inflammation have been difficult to elucidate. The results of this study represent the first investigation of how the O-GlcNAc post-translational modification regulates CD4+ T cell function and differentiation and support a role for aberrant O-GlcNAcylation in mediating a pro-inflammatory, Th17 response in a diet-induced obesity mouse model. (Fig. 7). Potentially, aberrant O-GlcNAcylation in T cells explains in part why diet influences flare-ups in autoimmunity and why pathogenic inflammatory cytokine production increases in diseases of overnutrition.
Figure 7.
O-GlcNAc regulates pro-inflammatory Th17 cell function. This schematic represents diet-induced changes in O-GlcNAc levels leading to increased ACC1 O-GlcNAcylation and alterations in the lipidome. Changes in lipid expression activate RORγt, leading to more IL-17 production.
Materials and methods
Mouse T cell isolation, activation, and differentiation
C57BL/6 male and female mice (Jackson Laboratories) between 6 and 24 weeks of age were used in these studies. Mice were humanely euthanized using CO2 asphyxiation according to an Institutional Animal Care and Use Committee–approved protocol. CD4+ T cells were isolated from spleen using the CD4+ T cell isolation kit (Stem Cell Technologies). CD4+ T cells alone or naive cells obtained by staining with APC-CD62L and FITC-CD44 (BD Biosciences) were sorted and treated with 10 μm thiamet-G (SD Chemmolecules, LLC) or vehicle control for 6 h. Cells (2.5 × 105 cells/well) were then activated on anti-CD3 and anti-CD28 (Bio X Cell) antibody-coated plates. Plates were prepared by the addition of 2 μg of anti-CD3 and 1 μg of anti-CD28 per well of a 48-well plate in PBS containing calcium and magnesium incubated overnight at 4 °C. At the time of activation, polarizing cytokines were added to cells to promote differentiation toward specific lineages as follows: Th0, no cytokines added; Th17, mouse IL-6 (20 ng/ml; Miltenyi), human TGFβ1 (2 ng/ml; Miltenyi), anti-IL-4 (10 μg/ml), and anti-IFNγ (10 μg/ml). All cells were incubated at 37 °C in 5% CO2 in a 95% humidified incubator. On the second day of culture, mouse IL-2 (eBioscience) at 20 ng/ml was added to cultures, and the cells were resuspended. On the fourth day of culture, cells were resuspended, dead cells were removed by gradient centrifugation with Lymphocyte Separation Medium (Corning), and remaining living cells were counted with a hemocytometer. Activation was confirmed by a 6–8-fold expansion of cell number after 4 days of cell culture as well as characteristic changes in O-GlcNAcylation during T cell activation as described previously (63, 64). 2.5 × 105 cells/well were then restimulated on plates containing 3 μg of anti-CD3 and 1 μg of anti-CD28 per well, which were prepared as previously described. 24 h after restimulation, supernatants and cells were harvested. Activation was confirmed by characteristic increase in O-GlcNAc levels between 0 h (unactivated) and cells activated after 24 h. Mouse IL-17A, IFNγ, IL-4, or IL-10 levels in the supernatants were quantified using the cytokine's corresponding ELISA kit (eBioscience).
Western diet mouse model
For Western diet studies, 6–8-week-old male C57BL/6 mice (Jackson Laboratories) were fed a 42% kcal from fat (>60% saturated fat), 0.2% cholesterol, and high-sucrose “Western diet” (Teklad TD.88137) for 16 weeks. Control 6–8-week-old male C57BL/6 mice were fed standard chow, containing 14% kcal from fat (Teklad 8604). Mice were weighed at 0, 2, 4, 6, 8, 10, 12, 14, and 16 weeks. At 15 weeks, control and Western diet–fed mice were fasted overnight for 15 h. At the end of the fast, tail vein blood was used to determine the blood glucose level (mg/dl) using One Touch Ultra Blue test strips and the One Touch UltraMini blood glucose monitoring system (LifeScan). At 16 weeks, mice were sacrificed following a 15-h overnight fast, and splenic CD4+ T cells were used for subsequent studies as described.
Intracellular cytokine staining
CD4+ T cells were isolated and cultured as described previously (26). After 4 days of culture, cells were resuspended, any dead cells were removed by gradient centrifugation with Lymphocyte Separation Medium (Corning), and viable cells were resuspended at a concentration of 1–10 million cells/ml. Cells were stimulated with 50 ng/ml phorbol 12-myristate 13-acetate and 1 μg/ml ionomycin (both Sigma) for 5 h. Protein secretion was inhibited by the addition of 4 μl of BD GolgiStopTM protein transport inhibitor (containing monensin) per 6 ml of culture. After stimulation, cells were washed twice with PBS + 2% fetal bovine serum and counted. One million cells per condition were added to an Eppendorf tube, spun, and resuspended in cold BD CytofixTM buffer from the Mouse Th1/Th17 Phenotyping Kit (BD Biosciences) and incubated for 20 min at room temperature. Cells were then washed twice with PBS + 2% FBS and diluted in BD Perm/Wash BufferTM for 15 min at room temperature. Cells were centrifuged and then resuspended in 50 μl of BD Perm/Wash BufferTM plus 20 μl/tube of staining mixture (mouse PerCP-Cy5.5-CD4, PE-IL-17A, FITC-IFNγ) or BD Perm/Wash BufferTM as a negative control or mouse PerCP-Cy5.5-CD4 and isotype controls for IL-17A and IFNγ for 30 min at room temperature. Cells were washed twice with PBS + 2% fetal bovine serum, and data were acquired on an LSR II (BD Biosciences) and analyzed with FlowJo (Tree Star).
Quantitative real-time PCR (qPCR)
Mouse or human RNA was extracted by dissolving 2 × 106 cells in TRI reagent (Sigma) followed by the addition of chloroform. The mixture was mixed by inversion and centrifuged, and the aqueous layer was taken. Isopropyl alcohol was added to the aqueous layer to precipitate the RNA. The RNA was pelleted by centrifugation, washed with 75% ethanol, and allowed to air-dry before adding diethyl pyrocarbonate–treated, RNase-free water (Ambion). RNA of high purity (A260/280 and A260/230 > 1.7) was used for further analysis. 0.5–0.75 μg of RNA was converted to cDNA using iScript reverse transcriptase reaction mix (Bio-Rad) in a thermal cycler (model 2720, Applied Biosystems) using the following protocol: priming, 5 min at 25 °C; reverse transcription, 30 min at 42 °C; and reverse transcription inactivation, 5 min at 85 °C. For qPCRs, 10 μl of SsoAdvanced Universal SYBR Green Supermix (Bio-Rad), 10–100 ng of cDNA, 0.2 μl each of forward and reverse primers (100 μm stock concentration), and water to a final reaction volume of 20 μl were added per well to 96-well plates (Midsci). The reactions were run on a CFX96 real-time PCR detection system (Bio-Rad), using the following conditions: polymerase activation and DNA denaturation, 30 s at 95 °C; amplification denaturation, 5 s at 95 °C; and amplification annealing and extension, 30 s at 60 °C or 62 °C for 40 cycles. The dynamic range of reverse transcription and the amplification efficiency was determined for each primer pair and cell culture condition. Thus, Cq values within these ranges were reliably used to calculate the -fold change in gene expression compared with an internal standard using the ΔΔCq method. The following primers were used: mHPRT, GGCCAGACTTTGTTGGATTTG (forward) and CGCTCATCTTAGGCTTTGTATTTG (reverse); mIL-17A, CGCAATGAAGACCCTGATAGAT (forward) and CTCTTGCTGGATGAGAACAGAA (reverse); mIL-23, GAGCCAGACAGCAAGTATGT (forward) and CAGTTTCTTGGGAAGTTTGGTG (reverse); mIFNγ, GGCCATCAGCAACAACATAAG (forward) and GTTGACCTCAAACTTGGCAATAC (reverse); mIL-4, GAAGAACACCACAGAGAGTGAG (forward) and TGCAGCTCCATGAGAACAC (reverse); mIL-5, CCCAACCTTAGCATCCTTTCT (forward) and AGGGAGTTGAGGAGAGATTGA (reverse); mIL-10, TGCACTACCAAAGCCACAA (forward) and GATCCTCATGCCAGTCAGTAAG (reverse); hHPRT, CTTTCCTTGGTCAGGCAGTATAA (forward) and AGTCTGGCTTATATCCAACACTTC (reverse); hIL-17A, ACCGGAATACCAATACCAATCC (forward) and GGATATCTCTCAGGGTCCTCAT (reverse); hIL-23R, GCTGGTGTCATGGAGGAATTA (forward) and TTCCTTGGTTGGCAGTTCTTA (reverse); hIFNγ, ATGTCCAACGCAAAGCAATAC (forward) and ACCTCGAAACAGCATCTGAC (reverse).
Immunoblotting
Cells were lysed on ice in 20 mm Tris, pH 7.4, 150 mm NaCl, 40 mm GlcNAc, 2 mm EDTA, 1 mm DTT, 1% Nonidet P-40 lysis buffer with protease inhibitors (1 mm β-glycerophosphate, 1 mm NaF, 2 mm phenylmethylsulfonyl fluoride, 1× inhibitor mixture composed of 1 μg/ml leupeptin, 1 μg/ml antipain, 10 μg/ml benzamidine, and 0.1% aprotinin (Sigma)) added immediately before lysis. Lysates were incubated on ice for 20 min and vortexed every 5 min. Lysates were then centrifuged for 20 min at 4 °C, and the supernatant was removed to another tube. Protein concentration of the lysate was determined using protein assay dye reagent (Bio-Rad), using known concentrations of BSA (Bio-Rad) as the standard. Lysates were then denatured by the addition of 4× protein solubility mixture (100 mm Tris, pH 6.8, 10 mm EDTA, 8% SDS, 50% sucrose, 5% β-mercaptoethanol, 0.08% Pyronin Y) and boiling for 2 min. Equal amounts of lysates were loaded onto 4–15% Criterion precast TGX gels (Bio-Rad). Electrophoresis occurred at 125 V, and then the gel proteins were transferred to polyvinylidene difluoride membranes at 0.4 A. Membranes were blocked with 3% BSA, 0.01% sodium azide in TBST (25 mm Tris, pH 7.6, 150 mm NaCl, 0.05% Tween 20) for at least 20 min. Blots were then probed overnight at 4 °C with primary antibody to the protein of interest at 1:1,000 dilution. The next day, blots were washed five times in TBST for 5 min each. HRP-conjugated secondary antibody at 1:10,000 dilution was added for 1 h at room temperature, followed by washing five times for 5 min each. Blots were then developed using the chemiluminescence HRP antibody detection method (HyGlo Spray, Denville Scientific). Commonly, blots were striped with 200 mm glycine, pH 2.5, for 1 h at room temperature, blocked, and reprobed with antibodies to other proteins of interest. Where shown, ImageJ (65) was used to quantify the density of protein bands compared with an internal standard protein band such as actin. Antibodies used in these studies were as follows: pACC1 (Ser-79) and ACC1 (Cell Signaling Technologies); O-GlcNAc (clone: RL2) and RORγt (Abcam); and actin (Sigma). OGT (clone: AL-34) and OGA (clone: 345) antibodies were a generous gift from the laboratory of Dr. Gerald Hart (Department of Biological Chemistry, The Johns Hopkins University).
Immunoprecipitation
CD4+ T cells were lysed with lysis buffer (50 mm Tris, pH 7.4, 150 mm NaCl, 1 mm DTT, 1% NP-40, 40 mm GlcNAc), and protein concentration was quantified using Protein Assay Dye Reagent (Bio-Rad). One μg of antibody for the protein of interest or 1 μg of isotype-matched IgG was added to equal amounts of whole-cell lysate diluted to an equal volume in Nonidet P-40 lysis buffer and rotated overnight at 4 °C. As an additional control, 1 μg of antibody to the protein of interest was added to lysis buffer only and rotated overnight at 4 °C. Protein G–Sepharose beads (Millipore) were added and rotated at 4 °C for 2 h. The protein G beads were then washed three times with NP-40 lysis buffer containing 600 mm NaCl followed by two PBS washes, briefly vortexing beads between washes. Washed beads were resuspended in Laemmli buffer, boiled for 2 min, and run on 4–15% gradient Criterion TGX gels (Bio-Rad). Gel proteins were transferred to polyvinylidene difluoride membranes and blocked with 3% BSA, 0.01% sodium azide in TBST (25 mm Tris, pH 7.6, 150 mm NaCl, 1% Tween 20). Blots were then probed overnight at 4 °C with antibodies to O-GlcNAc (RL2, Abcam) followed by immunoprecipitated protein (ACC1 (CST) and RORγt (Abcam)) and developed using the chemiluminescence HRP antibody detection method (HyGlo Spray, Denville Scientific Inc.). For RORγt immunoprecipitation, samples were treated with 5 units of IdeZ protease (Promega) in PBS for 30 min at 37 °C after washing the beads.
ChIP
Mouse naive CD4+ T cells were isolated from mice fed standard chow. TMG was added to naive CD4+ T cells for 6 h before activation and differentiated toward the Th17 lineage as described previously (23). After 4 days of culture, cells were harvested and fixed with 2 mm ethylene glycol bis(succinimidyl succinate) (Thermo Scientific) in PBS for 30 min followed by 1% formaldehyde (Thermo Scientific) for 10 min. Nuclei were extracted by resuspending fixed cells in hypotonic cell lysis buffer (10 mm Tris, pH 8.0, 10 mm NaCl, 3 mm MgCl2, 40 mm GlcNAc, 0.5% NP-40) with freshly added protease inhibitors (1 mm β-glycerophosphate, 1 mm NaF, 1× protease inhibitor mixture (containing aprotinin, leupeptin, antipain, and benzamidine), and 2 mm phenylmethylsulfonyl fluoride) for 20 min on ice. Nuclear lysis buffer (50 mm Tris, pH 8.0, 10 mm EDTA, 1% SDS, 25% glycerol) with freshly added protease inhibitors and the chromatin was sonicated using the QSonica sonicator and cooling system (model numbers Q800R and Oasis 180, respectively) for 200 cycles of 15 s on at 75% amplitude and 45 s off. Sonication efficiency was checked by treating sheared chromatin diluted in ChIP buffer (50 mm Tris, pH 7.5, 150 mm NaCl, 5 mm EDTA, 0.5% NP-40, 1% Triton X-100) with 1 μg of RNase A (Invitrogen) overnight at 65 °C, followed by 2 μg of Proteinase K (Sigma) for 6 h at 65 °C, before the addition of phenol/chloroform/isoamyl alcohol (Acros Organics). The top aqueous layer was taken, and 20 μg of glycogen (Invitrogen) followed by 1 ml of cold 100% ethanol (Sigma) was added and incubated overnight at −20 °C to precipitate the DNA. The DNA was pelleted by centrifuging for 30 min at 4 °C and then washed with 75% ethanol and centrifuged for an additional 10 min at 4 °C. The supernatant was aspirated, and the DNA pellet was dried for at least 6 h. DNA was resuspended in diethyl pyrocarbonate–treated, DNase-free water (Ambion) and run on a 1.5% agarose gel. Bands of 100–300 bp in length indicated a successful sonication cycle. After ensuring adequate shearing of the chromatin, 2 μg of antibody to the protein of interest or 2 μg of isotype control was then added to equal amounts of sheared chromatin diluted 4-fold with ChIP buffer and rotated overnight at 4 °C. The next day, 20 μl of isotype-matched Dynabeads (Life Technologies, Inc.) were added and rotated for another 2 h. Beads were then washed with 1 ml of cold ChIP buffer five times using the DynaMag-2 Magnet (12321D, Invitrogen), rotating for 5 min at 4 °C between washes. 100 μl of 10% Chelex 100 slurry was added to the beads and boiled for 10 min. 1 μg of RNase A was added and incubated at 65 °C for 15 min minimum, followed by the addition of 2 μg of Proteinase K for 30 min at 65 °C. The samples were boiled again for 10 min to inactivate Proteinase K and then centrifuged at 12,000 × g for 1 min at 4 °C. The supernatant (70 μl) was transferred to a new tube, and the remaining Chelex resin was washed with 130 μl of water and re-spun, and the resulting supernatant was added to the first one. 10% of the chromatin used for the immunoprecipitate was reserved as input for the qPCR. DNA from inputs was extracted using the same method to determine sonication efficiency. 10 μl of the immunoprecipitated DNA or DNA purified from inputs was used per reaction for quantitative PCR and run on the CFX96 real-time PCR detection system as described previously (23). The Cq values were normalized to percentage of input. Antibodies used in these studies were as follows: rabbit control IgG and RORγt (Abcam). Primers used were as follows: mRORC, ATCTGGAGGAAGGACAACTTTC (forward) and CCTAGGGATACCACCCTTCATA (reverse); mIL-17 prom, CAGCTCCCAAGAAGTCATGC (forward) and GCAACATCTGTCTCGAAGGTAG (reverse); mCNS2, CACATGGACAATGTAATGGATCA (forward) and CAACTCTAAGAAGCAAACCTAACACA (reverse).
Lipidomics
Mouse naive CD4+ T cells were isolated, activated, and differentiated toward the Th17 lineage as described previously from mice fed standard chow with and without TMG treatment. After 4 days of culture, cells were restimulated with plate-bound anti-CD3 and anti-CD28 for 8 h before harvesting, washing with PBS, and snap-freezing the cell pellet.
Lipid extraction
Frozen cell pellets were subjected to monophasic lipid extraction in methanol/chloroform/water (2:1:0.74, v/v/v) as described previously (66). During lipid extraction, each sample was spiked with synthetic dimyristoyl phosphatidylcholine obtained from Avanti Polar Lipids (Alabaster, AL) and D7-cholesterol obtained from Steraloids (Newport, RI) at 1 nmol/mg protein as an internal standard for relative quantitation of lipids and sterols. Dried lipid extracts were washed three times with 10 mm ammonium bicarbonate, dried under vacuum, and resuspended in methanol containing 0.01% butylated hydroxytoluene. Samples were stored under a blanket of nitrogen at −80 ºC until further analysis.
Global lipidomics analysis
For each analysis, lipid extracts were transferred to an Eppendorf twin.tec® 96-well plate (Sigma-Aldrich) and evaporated under nitrogen. The dried lipid film was then resuspended in isopropyl alcohol/methanol/chloroform (4:2:1, v/v/v) containing 20 mm ammonium formate and sealed with Teflon Ultra-Thin Sealing Tape (Analytical Sales and Services, Pompton Plains, NJ). Appropriate sample dilution to minimize ion suppression effects was determined as described previously (66). Global lipidomics analysis was performed by direct-infusion high-resolution/accurate MS and tandem MS. Samples were introduced to the mass spectrometer by nanoelectrospray ionization using an Advion Triversa Nanomate nESI source (Advion, Ithaca, NY) with a spray voltage of 1.4 kV and a gas pressure of 0.3 p.s.i. A Thermo Scientific LTQ-Orbitrap Velos (San Jose, CA) mass spectrometer was used for lipid detection. High-resolution MS and MS/MS spectra were acquired for each sample in positive and negative ionization modes using the Orbitrap FT analyzer operating at 100,000 mass resolving power (defined at m/z 400).
Analysis of free and total sterol content
Sterols and oxysterols were analyzed by high-resolution/accurate mass LC-MS using a Shimadzu Prominence HPLC equipped with an in-line solvent degassing unit, autosampler, column oven, and two LC-20AD pumps, coupled to a Thermo Scientific LTQ-Orbitrap Velos mass spectrometer. Lipid extracts were used directly for analysis of “free” sterols, or subjected to alkaline hydrolysis of sterol esters for analysis of total cellular sterols as described previously (67). Ten-microliter injections of each total or hydrolyzed lipid extract were separated using a Phenomenex (Torrance, CA) Synergi Hydro-RP C18 column, 2 mm × 150 mm, with 3-μm particles and 80-μm pore size, at 50 ºC. The gradient elution method was modified from (67) and utilized A (water containing 0.1% formic acid) and B (methanol containing 0.1% formic acid), with a 13-min gradient from 85% B to 100% B at a flow rate of 250 μl/min. The column eluent was introduced to the LTQ-Orbitrap Velos by a heated electrospray ionization source, using a spray voltage of 4.5 kV, sheath gas pressure of 30 p.s.i., and auxiliary gas pressure of 10 p.s.i. The source heater and MS capillary inlet tube were held at 350 ºC. Sterols and oxysterols were analyzed by full-scan positive ion mode MS using the Orbitrap detector at a resolution of 60,000 (defined at m/z 400) to detect sterol in-source fragmentation-derived [M − H2O + H]+ and [M − 2H2O + H]+ ions, which were the most abundant sterol ions formed under the conditions utilized. Targeted high-resolution HCD-MS/MS at 45% normalized collision energy was employed on known sterol-related ions and their product ions (67) to verify the identity of each sterol peak.
Peak finding, analyte identification, and quantification
For global lipidomics analysis, lipids were identified using the Lipid Mass Spectrum Analysis (LIMSA) version 1.0 software linear fit algorithm, in conjunction with a user-defined database of hypothetical lipid compounds for automated peak finding and correction of 13C isotope effects. Relative quantification of lipid abundance between samples was performed by normalization of target lipid ion peak areas to the dimyristoyl phosphatidylcholine internal standard as described previously (68). For free and total sterol analysis, chromatographic peak alignment, compound identification, and relative quantitation against the D7-cholesterol internal standard was performed using MAVEN software (69).
Mass spectrometry
Mouse naive CD4+ T cells were isolated, activated, and differentiated toward the Th17 lineage as described previously from mice fed standard chow. After 4 days of culture, cells were harvested and lysed, and ACC1 was immunoprecipitated as described previously. Immunopurified complexes bound to agarose beads were trypsin-digested as described previously (25).
LC-MS analysis
Following enzymatic digestion, the resulting peptides were concentrated on a Centrivac concentrator (Labconco) to a final volume of 20 μl. The peptide extracts were analyzed using an Orbitrap Fusion Lumos mass spectrometer (Thermo Fisher Scientific) coupled to a uHPLC (nLC 1200, Thermo Fisher Scientific). In each run, the sample was injected directly into the reversed-phase column (Acclaim PepMap RSLC, 50 μm × 15 cm, C18 reversed phase (2 μm, 100 Å)) and washed with 0.1% formic acid for 5 min at 500 nl/min. The column was mounted on the electrospray stage of the mass spectrometer, and the peptides were eluted with a gradient of acetonitrile (solvent B: 90% acetonitrile, 0.1% formic acid) in 0.1% formic acid in water (solvent A). The gradient profile was as follows: 0–5 min, 5–15% B; 5–60 min, 15–40% B; 60–70 min, 40% B; 70–75 min, 40–75% B; 75–83 min 75% B. At the end of each chromatographic run, the column was washed with 100% B. The flow rate was maintained at 300 nl/min.
MS acquisition
The mass spectrometer was operated in positive ionization mode with a nanospray flex ionization source operated at 2.6 kV and source temperature at 250 ºC. Multiple runs were performed using different fragmentation methods as described below. The instrument was operated in data-dependent acquisition mode, with full MS scans over a mass range of m/z 350–1,900 with detection in the Orbitrap (120 K resolution) and with automatic gain control set to 100,000. In each cycle of data-dependent acquisition analysis, following each survey scan, the most intense ions above a threshold ion count of 30,000 were selected for fragmentation at normalized collision energy of 28% (higher-energy collisional dissociation) or 35% (collision-induced dissociation). The number of selected precursor ions for fragmentation was determined by the “Top Speed” acquisition algorithm and a dynamic exclusion of 60 s. Fragment ion spectra were acquired in the linear ion trap, with an automatic gain control of 4,000 and a maximum injection time of 300 ms for ion trap MS2 detection. All data were acquired with Xcalibur software version 3.0.63 (Tune version 2.0 1258).
Data analysis
For data analysis, all MS/MS scans were searched using Proteome Discoverer (version 2.2, Thermo Fisher Scientific) running the Sequest HT algorithm. A database search was conducted against a human protein database derived from the NIBInr repository as of January 9, 2017. The refined data were subjected to a database search using trypsin cleavage specificity, with a maximum of two missed cleavages. The following variable modifications were selected: pyroglutamination from Q and E (N-terminal), oxidation of M, and deamidation of N and Q. Carboxymethylation of C was selected as a fixed modification. HexNac was selected as variable modification. A maximum of three modifications/peptide was allowed. Estimation of false positive rate was conducted by searching all spectra against a decoy database consisting of the inverted sequences of all proteins in the original (direct) database. For peptide identification, a false positive rate < 1 was defined, and a minimum of two unique peptides per protein were required for protein identification. Amino acid sequence assignment of all peptides of interest was subsequently inspected manually.
Human T cell assays
Human whole-blood samples were obtained with consent from patient volunteers by the Biospecimen Repository Core Facility at the University of Kansas Medical Center. Inclusion criteria for volunteers was a body mass index (calculated as weight in kg/height in m2) between 18 and 29 and no prior type 1 or type 2 diabetes diagnoses. Exclusion criteria for samples included patient history of or current immunotherapy for cancer, autoimmune disorder, or current use of steroids or immune-modifying drugs (i.e. methotrexate). Whole blood was diluted 1:1 with PBS and gently layered on top of lymphocyte separation medium (Corning) and then centrifuged at 2,000 × g for 15 min at room temperature with the lowest level of brake. Samples of plasma were collected and snap-frozen, and the remaining plasma was aspirated. The white blood cell layer was collected and washed twice with PBS. CD4+ T cells were isolated from the washed white blood cell layer using anti-human CD4 particles (BD Biosciences). CD4+ T cells were treated with TMG or vehicle for 6 h before activation with anti-CD3– and anti-CD28–conjugated beads from the Human T Cell Activation/Expansion Kit (Miltenyi). Cells were cultured in RPMI 1640 medium (Sigma) containing 10% FBS (Gemini), 1% penicillin-streptomycin (Life Technologies), 2 mm GlutaMAX (Life Technologies), 10 mm HEPES, 1 mm sodium pyruvate, and 1 mm minimum Eagle's medium nonessential amino acids (Sigma). CD4+ T cells were cultured under Th17 conditions (human IL-6 (50 ng/ml), human IL-1β (10 ng/ml), human IL-23 (10 ng/ml), human TGFβ1 (3 ng/ml) (all Miltenyi), and neutralizing antibodies to IFNγ (clone: XMG1.2) and IL-4 (clone: 11B11) at 10 μg/ml (Bio X Cell)). Cells were incubated at 37 °C in 5% CO2 in a 95% humidified incubator. On day 3 of culture, 20 ng/ml human IL-2 (Miltenyi) was added to cells. On day 7 of culture, cells and supernatants were harvested. IL-17A levels in supernatants were quantified using a human IL-17A ELISA (Invitrogen) and normalized to number of cells present at day 7. Protein and RNA were harvested from cells and used for Western blotting or qPCR analyses, respectively, as described previously.
Statistics
Student's t test was performed to compare means between two groups. p values < 0.05 (*), less than 0.01 (**), and less than 0.001 (***) were statistically significant. S.D. or S.E. is shown by error bars as indicated in the figure legends.
Author contributions
M. M., H. S., T. Li, C. S., and P. E. F. conceptualization; M. M., T. Li, C. S., and P. E. F. supervision; M. M., T. Li, C. S., and P. E. F. funding acquisition; M. M., H. S., Z. Z., E. P. T., J. L., M. T. V., A. A., T. Lydic, G. C., and C. S. investigation; M. M., H. S., Z. Z., T. Li, T. Lydic, G. C., C. S., and P. E. F. methodology; M. M., H. S., T. Li, C. S., and P. E. F. writing-original draft; M. M., H. S., T. Li, C. S., and P. E. F. writing-review and editing; T. Li resources; A. A. and T. Lydic validation.
Supplementary Material
Acknowledgments
We acknowledge the Flow Cytometry Core Laboratory, which is supported, in part, by an NIGMS, National Institutes of Health, COBRE grant, and we especially thank Dr. Rich Hastings, Flow Core Director, for invaluable assistance and expertise. We acknowledge support from the staff of the University of Kansas Cancer Center Biospecimen Repository Core Facility (supported by the University of Kansas Cancer Center's Cancer Center Support Grant (NCI, National Institutes of Health, Grant P30 CA168524)) for helping to obtain human specimens. Finally, we are indebted to the volunteers who generously donated blood in support of this study.
This work was supported in part by the Molecular Regulation of Cell Development and Differentiation COBRE P30GM122731 (to P. E. F. and C. S.) and a University of Kansas Medical Center Biomedical Research Training Program grant (to M. M.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Figs. S1–S3 and Tables S1–S4.
- Th
- T helper
- Treg
- T regulatory cell
- ACC1
- acetyl-CoA carboxylase 1
- OGT
- O-GlcNAc transferase
- OGA
- O-GlcNAcase
- TMG
- thiamet-G
- IL
- interleukin
- IL-23R
- IL-23 receptor
- IFN
- interferon
- WD
- Western diet
- SC
- standard chow
- RORγt
- RAR-related orphan receptor γ t variant
- PUFA
- polyunsaturated fatty acid
- PC
- phosphatidylcholine
- CT
- carboxyl transferase
- BC
- biotin carboxylase
- mTORC1
- mTOR complex 1
- HRP
- horseradish peroxidase
- NP-40
- Nonidet P-40
- HPRT
- hypoxanthine-guanine phosphoribosyltransferase
- WB
- Western blotting.
References
- 1. Geginat J., Paroni M., Facciotti F., Gruarin P., Kastirr I., Caprioli F., Pagani M., and Abrignani S. (2013) The CD4-centered universe of human T cell subsets. Semin. Immunol. 25, 252–262 10.1016/j.smim.2013.10.012 [DOI] [PubMed] [Google Scholar]
- 2. Frauwirth K. A., Riley J. L., Harris M. H., Parry R. V., Rathmell J. C., Plas D. R., Elstrom R. L., June C. H., and Thompson C. B. (2002) The CD28 signaling pathway regulates glucose metabolism. Immunity 16, 769–777 10.1016/S1074-7613(02)00323-0 [DOI] [PubMed] [Google Scholar]
- 3. Chang C.-H., Curtis J. D., Maggi L. B. Jr., Faubert B., Villarino A. V., O'Sullivan D., Huang S. C., van der Windt G. J., Blagih J., Qiu J., Weber J. D., Pearce E. J., Jones R. G., and Pearce E. L. (2013) Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153, 1239–1251 10.1016/j.cell.2013.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Macintyre A. N., Gerriets V. A., Nichols A. G., Michalek R. D., Rudolph M. C., Deoliveira D., Anderson S. M., Abel D. E., Chen B. J., Hale L. P., and Rathmell J. C. (2014) The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 20, 61–72 10.1016/j.cmet.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Swamy M., Pathak S., Grzes K. M., Damerow S., Sinclair L. V., van Aalten D. M. F., and Cantrell D. A. (2016) Glucose and glutamine fuel protein O-GlcNAcylation to control T cell self-renewal and malignancy. Nat. Immunol. 17, 712–720 10.1038/ni.3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Michalek R. D., Gerriets V. A., Jacobs S. R., Macintyre A. N., MacIver N. J., Mason E. F., Sullivan S. A., Nichols A. G., and Rathmell J. C. (2011) Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 186, 3299–3303 10.4049/jimmunol.1003613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berod L., Friedrich C., Nandan A., Freitag J., Hagemann S., Harmrolfs K., Sandouk A., Hesse C., Castro C. N., Bähre H., Tschirner S. K., Gorinski N., Gohmert M., Mayer C. T., Huehn J., et al. (2014) De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat. Med. 20, 1327–1333 10.1038/nm.3704 [DOI] [PubMed] [Google Scholar]
- 8. Stadhouders R., Lubberts E., and Hendriks R. W. (2018) A cellular and molecular view of T helper 17 cell plasticity in autoimmunity. J. Autoimmun. 87, 1–15 10.1016/j.jaut.2017.12.007 [DOI] [PubMed] [Google Scholar]
- 9. Shen Y., Wen Z., Li Y., Matteson E. L., Hong J., Goronzy J. J., and Weyand C. M. (2017) Metabolic control of the scaffold protein TKS5 in tissue-invasive, proinflammatory T cells. Nat. Immunol. 18, 1025–1034 10.1038/ni.3808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Winer S., Paltser G., Chan Y., Tsui H., Engleman E., Winer D., and Dosch H. M. (2009) Obesity predisposes to Th17 bias. Eur. J. Immunol. 39, 2629–2635 10.1002/eji.200838893 [DOI] [PubMed] [Google Scholar]
- 11. Schindler T. I., Wagner J.-J., Goedicke-Fritz S., Rogosch T., Coccejus V., Laudenbach V., Nikolaizik W., Härtel C., Maier R. F., Kerzel S., and Zemlin M. (2017) TH17 cell frequency in peripheral blood is elevated in overweight children without chronic inflammatory diseases. Front. Immunol. 8, 1543 10.3389/fimmu.2017.01543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chehimi M., Vidal H., and Eljaafari A. (2017) Pathogenic role of IL-17-producing immune cells in obesity, and related inflammatory diseases. J. Clin. Med. 6, E68 10.3390/jcm6070068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kanneganti T.-D., and Dixit V. D. (2012) Immunological complications of obesity. Nat. Immunol. 13, 707–712 10.1038/ni.2343 [DOI] [PubMed] [Google Scholar]
- 14. Hart G. W., Slawson C., Ramirez-Correa G., and Lagerlof O. (2011) Cross-talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu. Rev. Biochem. 80, 825–858 10.1146/annurev-biochem-060608-102511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Slawson C., Copeland R. J., and Hart G. W. (2010) O-GlcNAc signaling: a metabolic link between diabetes and cancer? Trends Biochem. Sci. 35, 547–555 10.1016/j.tibs.2010.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kreppel L. K., and Hart G. W. (1999) Regulation of a cytosolic and nuclear O-GlcNAc transferase: role of the tetratricopeptide repeats. J. Biol. Chem. 274, 32015–32022 10.1074/jbc.274.45.32015 [DOI] [PubMed] [Google Scholar]
- 17. Golks A., Tran T. T. T., Goetschy J. F., and Guerini D. (2007) Requirement for O-linked N-acetylglucosaminyltransferase in lymphocytes activation. EMBO J. 26, 4368–4379 10.1038/sj.emboj.7601845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lund P. J., Elias J. E., and Davis M. M. (2016) Global analysis of O-GlcNAc glycoproteins in activated human T cells. J. Immunol. 197, 3086–3098 10.4049/jimmunol.1502031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hewagama A., Gorelik G., Patel D., Liyanarachchi P., McCune W. J., Somers E., Gonzalez-Rivera T., Michigan Lupus Cohort, Strickland F., and Richardson B. (2013) Overexpression of X-linked genes in T cells from women with lupus. J. Autoimmun. 41, 60–71 10.1016/j.jaut.2012.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu R., Ma X., Chen L., Yang Y., Zeng Y., Gao J., Jiang W., Zhang F., Li D., Han B., Han R., Qiu R., Huang W., Wang Y., and Hao J. (2017) MicroRNA-15b suppresses Th17 differentiation and is associated with pathogenesis of multiple sclerosis by targeting O-GlcNAc transferase. J. Immunol. 198, 2626–2639 10.4049/jimmunol.1601727 [DOI] [PubMed] [Google Scholar]
- 21. Lehman D. M., Fu D.-J., Freeman A. B., Hunt K. J., Leach R. J., Johnson-Pais T., Hamlington J., Dyer T. D., Arya R., Abboud H., Göring H. H. H., Duggirala R., Blangero J., Konrad R. J., and Stern M. P. (2005) A single nucleotide polymorphism in MGEA5 encoding O-GlcNAc–selective N-acetyl-β-d-glucosaminidase is associated with type 2 diabetes in Mexican Americans. Diabetes 54, 1214–1221 10.2337/diabetes.54.4.1214 [DOI] [PubMed] [Google Scholar]
- 22. Yuzwa S. A., Macauley M. S., Heinonen J. E., Shan X., Dennis R. J., He Y., Whitworth G. E., Stubbs K. A., McEachern E. J., Davies G. J., and Vocadlo D. J. (2008) A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat. Chem. Biol. 4, 483–490 10.1038/nchembio.96 [DOI] [PubMed] [Google Scholar]
- 23. Zhang Z., Tan E. P., VandenHull N. J., Peterson K. R., and Slawson C. (2014) O-GlcNAcase expression is sensitive to changes in O-GlcNAc homeostasis. Front. Endocrinol. (Lausanne) 5, 206 10.3389/fendo.2014.00206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kearse K. P., and Hart G. W. (1991) Topology of O-linked N-acetylglucosomaine in murine lymphocytes. Arch. Biochem. Biophys. 290, 543–548 10.1016/0003-9861(91)90579-8 [DOI] [PubMed] [Google Scholar]
- 25. Mohammed H., D'Santos C., Serandour A. A., Ali H. R., Brown G. D., Atkins A., Rueda O. M., Holmes K. A., Theodorou V., Robinson J. L., Zwart W., Saadi A., Ross-Innes C. S., Chin S. F., Menon S., et al. (2013) Endogenous purification reveals GREB1 as a key estrogen receptor regulatory factor. Cell Rep. 3, 342–349 10.1016/j.celrep.2013.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aggarwal S., Ghilardi N., Xie M.-H., de Sauvage F. J., and Gurney A. L. (2003) Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278, 1910–1914 10.1074/jbc.M207577200 [DOI] [PubMed] [Google Scholar]
- 27. Chen Z., Tato C. M., Muul L., Laurence A., and O'Shea J. J. (2007) Distinct regulation of interleukin-17 in human T helper lymphocytes. Arthritis Rheumatism 56, 2936–2946 10.1002/art.22866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ghoreschi K., Laurence A., Yang X.-P., Tato C. M., McGeachy M. J., Konkel J. E., Ramos H. L., Wei L., Davidson T. S., Bouladoux N., Grainger J. R., Chen Q., Kanno Y., Watford W. T., Sun H.-W., et al. (2010) Generation of pathogenic TH17 cells in the absence of TGF-β signalling. Nature 467, 967–971 10.1038/nature09447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shen H., Goodall J. C., and Hill Gaston J. S. (2009) Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 60, 1647–1656 10.1002/art.24568 [DOI] [PubMed] [Google Scholar]
- 30. McLaughlin T., Liu L.-F., Lamendola C., Shen L., Morton J., Rivas H., Winer D., Tolentino L., Choi O., Zhang H., Hui Yen Chng M., and Engleman E. (2014) T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arterioscler. Thromb. Vasc. Biol. 34, 2637–2643 10.1161/ATVBAHA.114.304636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eljaafari A., Robert M., Chehimi M., Chanon S., Durand C., Vial G., Bendridi N., Madec A.-M., Disse E., Laville M., Rieusset J., Lefai E., Vidal H., and Pirola L. (2015) Adipose tissue–derived stem cells from obese subjects contribute to inflammation and reduced insulin response in adipocytes through differential regulation of the Th1/Th17 balance and monocyte activation. Diabetes 64, 2477–2488 10.2337/db15-0162 [DOI] [PubMed] [Google Scholar]
- 32. Butkinaree C., Park K., and Hart G. W. (2010) O-Linked β-N-acetylglucosamine (O-GlcNAc): extensive crosstalk with phosphorylation to regulate signaling and transcription in response to nutrients and stress. Biochim. Biophys. Acta 1800, 96–106 10.1016/j.bbagen.2009.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ivanov I. I., McKenzie B. S., Zhou L., Tadokoro C. E., Lepelley A., Lafaille J. J., Cua D. J., and Littman D. R. (2006) The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 10.1016/j.cell.2006.07.035 [DOI] [PubMed] [Google Scholar]
- 34. Wang X., Zhang Y., Yang X. O., Nurieva R. I., Chang S. H., Ojeda S. S., Kang H. S., Schluns K. S., Gui J., Jetten A. M., and Dong C. (2012) Transcription of Il17 and Il17f is controlled by conserved noncoding sequence 2. Immunity 36, 23–31 10.1016/j.immuni.2011.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Santori F. R., Huang P., van de Pavert S. A., Douglass E. F. Jr., Leaver D. J., Haubrich B. A., Keber R., Lorbek G., Konijn T., Rosales B. N., Rozman D., Horvat S., Rahier A., Mebius R. E., Rastinejad F., Nes W. D., and Littman D. R. (2015) Identification of natural RORγ ligands that regulate the development of lymphoid cells. Cell Metab. 21, 286–298 10.1016/j.cmet.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Soroosh P., Wu J., Xue X., Song J., Sutton S. W., Sablad M., Yu J., Nelen M. I., Liu X., Castro G., Luna R., Crawford S., Banie H., Dandridge R. A., Deng X., et al. (2014) Oxysterols are agonist ligands of RORγt and drive Th17 cell differentiation. Proc. Natl. Acad. Sci. U.S.A. 111, 12163–12168 10.1073/pnas.1322807111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Endo Y., Asou H. K., Matsugae N., Hirahara K., Shinoda K., Tumes D. J., Tokuyama H., Yokote K., and Nakayama T. (2015) Obesity drives Th17 cell differentiation by inducing the lipid metabolic kinase, ACC1. Cell Rep. 12, 1042–1055 10.1016/j.celrep.2015.07.014 [DOI] [PubMed] [Google Scholar]
- 38. Wang C., Yosef N., Gaublomme J., Wu C., Lee Y., Clish C. B., Kaminski J., Xiao S., Meyer Zu Horste G., Pawlak M., Kishi Y., Joller N., Karwacz K., Zhu C., Ordovas-Montanes M., et al. (2015) CD5L/AIM regulates lipid biosynthesis and restrains Th17 cell pathogenicity. Cell 163, 1413–1427 10.1016/j.cell.2015.10.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zeisel S. H., and Blusztajn J. K. (1994) Choline and Human Nutrition. Annu. Rev. Nutr. 14, 269–296 10.1146/annurev.nu.14.070194.001413 [DOI] [PubMed] [Google Scholar]
- 40. Rinner I., and Schauenstein K. (1993) Detection of choline-acetyltransferase activity in lymphocytes. J. Neurosci. Res. 35, 188–191 10.1002/jnr.490350209 [DOI] [PubMed] [Google Scholar]
- 41. Cox M. A., Duncan G. S., Lin G. H. Y., Steinberg B. E., Yu L. X., Brenner D., Buckler L. N., Elia A. J., Wakeham A. C., Nieman B., Dominguez-Brauer C., Elford A. R., Gill K. T., Kubli S. P., Haight J., Berger T., Ohashi P. S., Tracey K. J., Olofsson P. S., and Mak T. W. (2019) Choline acetyltransferase–expressing T cells are required to control chronic viral infection. Science 363, 639–644 10.1126/science.aau9072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Scheffel M. J., Helke K., Lu P., Bowers J. S., Ogretmen B., Garrett-Mayer E., Paulos C. M., and Voelkel-Johnson C. (2017) Adoptive transfer of ceramide synthase 6 deficient splenocytes reduces the development of colitis. Sci. Rep. 7, 15552 10.1038/s41598-017-15791-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ha J., Daniel S., Broyles S. S., and Kim K. H. (1994) Critical phosphorylation sites for acetyl-CoA carboxylase activity. J. Biol. Chem. 269, 22162–22168 [PubMed] [Google Scholar]
- 44. Shen Y., and Tong L. (2008) Structural evidence for direct interactions between the BRCT domains of human BRCA1 and a phospho-peptide from human ACC1. Biochemistry 47, 5767–5773 10.1021/bi800314m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C., Mullany E. C., Biryukov S., Abbafati C., Abera S. F., Abraham J. P., Abu-Rmeileh N. M. E., Achoki T., AlBuhairan F. S., Alemu Z. A., et al. (2014) Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384, 766–781 10.1016/S0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ridker P. M., Everett B. M., Thuren T., MacFadyen J. G., Chang W. H., Ballantyne C., Fonseca F., Nicolau J., Koenig W., Anker S. D., Kastelein J. J. P., Cornel J. H., Pais P., Pella D., Genest J., et al. (2017) Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 47. Gao Q., Jiang Y., Ma T., Zhu F., Gao F., Zhang P., Guo C., Wang Q., Wang X., Ma C., Zhang Y., Chen W., and Zhang L. (2010) A critical function of Th17 proinflammatory cells in the development of atherosclerotic plaque in mice. J. Immunol. 185, 5820–5827 10.4049/jimmunol.1000116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jagannathan-Bogdan M., McDonnell M. E., Shin H., Rehman Q., Hasturk H., Apovian C. M., and Nikolajczyk B. S. (2011) Elevated proinflammatory cytokine production by a skewed T cell compartment requires monocytes and promotes inflammation in type 2 diabetes. J. Immunol. 186, 1162–1172 10.4049/jimmunol.1002615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fabbrini E., Cella M., McCartney S. A., Fuchs A., Abumrad N. A., Pietka T. A., Chen Z., Finck B. N., Han D. H., Magkos F., Conte C., Bradley D., Fraterrigo G., Eagon J. C., Patterson B. W., et al. (2013) Association between specific adipose tissue CD4+ T-cell populations and insulin resistance in obese individuals. Gastroenterology 145, 366–374.e1–3 10.1053/j.gastro.2013.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chuang H.-C., Sheu W. H. H., Lin Y.-T., Tsai C.-Y., Yang C.-Y., Cheng Y.-J., Huang P.-Y., Li J.-P., Chiu L.-L., Wang X., Xie M., Schneider M. D., and Tan T.-H. (2014) HGK/MAP4K4 deficiency induces TRAF2 stabilization and Th17 differentiation leading to insulin resistance. Nat. Commun. 5, 4602 10.1038/ncomms5602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kidani Y., and Bensinger S. J. (2017) Reviewing the impact of lipid synthetic flux on Th17 function. Curr. Opin. Immunol. 46, 121–126 10.1016/j.coi.2017.03.012 [DOI] [PubMed] [Google Scholar]
- 52. Nikawa J., Tanabe T., Ogiwara H., Shiba T., and Numa S. (1979) Inhibitory effects of long-chain acyl coenzyme a analogues on rat liver acetyl coenzyme a carboxylase. FEBS Lett. 102, 223–226 10.1016/0014-5793(79)80005-8 [DOI] [PubMed] [Google Scholar]
- 53. Glatzel D. K., Koeberle A., Pein H., Löser K., Stark A., Keksel N., Werz O., Müller R., Bischoff I., and Fürst R. (2018) Acetyl-CoA carboxylase 1 regulates endothelial cell migration by shifting the phospholipid composition. J. Lipid Res. 59, 298–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wei J., and Tong L. (2015) Crystal structure of the 500-kDa yeast acetyl-CoA carboxylase holoenzyme dimer. Nature 526, 723–727 10.1038/nature15375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rieckehoff I. G., Holman R. T., and Burr G. O. (1980) Polyethenoid fatty acid metabolism: effect of dietary fat on polyethenoid fatty acids of rat tissues. Nutr. Rev. 38, 247–250 [DOI] [PubMed] [Google Scholar]
- 56. Gwinn D. M., and Shaw R. J. (2010) 3-AMPK control of mTOR signaling and growth. in The Enzymes (Tamanoi F., and Hall M. N. eds.) pp. 49–75, Academic Press, Inc., New York [Google Scholar]
- 57. Hara K., Maruki Y., Long X., Yoshino K., Oshiro N., Hidayat S., Tokunaga C., Avruch J., and Yonezawa K. (2002) Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110, 177–189 10.1016/S0092-8674(02)00833-4 [DOI] [PubMed] [Google Scholar]
- 58. Aspuria P.-J., and Tamanoi F. (2004) The Rheb family of GTP-binding proteins. Cell. Signal. 16, 1105–1112 10.1016/j.cellsig.2004.03.019 [DOI] [PubMed] [Google Scholar]
- 59. Delgoffe G. M., Pollizzi K. N., Waickman A. T., Heikamp E., Meyers D. J., Horton M. R., Xiao B., Worley P. F., and Powell J. D. (2011) The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat. Immunol. 12, 295–303 10.1038/ni.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kurebayashi Y., Nagai S., Ikejiri A., Ohtani M., Ichiyama K., Baba Y., Yamada T., Egami S., Hoshii T., Hirao A., Matsuda S., and Koyasu S. (2012) PI3K-Akt-mTORC1-S6K1/2 axis controls Th17 differentiation by regulating Gfi1 expression and nuclear translocation of RORγ. Cell Rep. 1, 360–373 10.1016/j.celrep.2012.02.007 [DOI] [PubMed] [Google Scholar]
- 61. Dang E. V., Barbi J., Yang H.-Y., Jinasena D., Yu H., Zheng Y., Bordman Z., Fu J., Kim Y., Yen H.-R., Luo W., Zeller K., Shimoda L., Topalian S. L., Semenza G. L., Dang C. V., Pardoll D. M., and Pan F. (2011) Control of TH17/Treg balance by hypoxia-inducible factor 1. Cell 146, 772–784 10.1016/j.cell.2011.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shi L. Z., Wang R., Huang G., Vogel P., Neale G., Green D. R., and Chi H. (2011) HIF1α–dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of Th17 and Treg cells. J. Exp. Med. 208, 1367–1376 10.1084/jem.20110278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Torres C. R., and Hart G. W. (1984) Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes: evidence for O-linked GlcNAc. J. Biol. Chem. 259, 3308–3317 [PubMed] [Google Scholar]
- 64. Kearse K. P., and Hart G. W. (1991) Lymphocyte activation induces rapid changes in nuclear and cytoplasmic glycoproteins. Proc. Natl. Acad. Sci. U.S.A. 88, 1701–1705 10.1073/pnas.88.5.1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schneider C. A., Rasband W. S., and Eliceiri K. W. (2012) NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lydic T. A., Busik J. V., and Reid G. E. (2014) A monophasic extraction strategy for the simultaneous lipidome analysis of polar and nonpolar retina lipids. J. Lipid Res. 55, 1797–1809 10.1194/jlr.D050302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. McDonald J. G., Thompson B. M., McCrum E. C., and Russell D. W. (2007) Extraction and analysis of sterols in biological matrices by high performance liquid chromatography electrospray ionization mass spectrometry. Methods Enzymol. 432, 145–170 10.1016/S0076-6879(07)32006-5 [DOI] [PubMed] [Google Scholar]
- 68. Lydic T. A., Townsend S., Adda C. G., Collins C., Mathivanan S., and Reid G. E. (2015) Rapid and comprehensive “shotgun” lipidome profiling of colorectal cancer cell derived exosomes. Methods 87, 83–95 10.1016/j.ymeth.2015.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Clasquin M. F., Melamud E., and Rabinowitz J. D. (2002) LC-MS data processing with MAVEN: a metabolomic analysis and visualization engine. Curr. Protoc. Bioinformatics, Chapter 14, Unit 14.11 10.1002/0471250953.bi1411s37 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.