Abstract
Objective:
To characterize the impact of TX-001HR on the relationship between vasomotor symptom (VMS) improvement and quality of life and sleep.
Methods:
REPLENISH (NCT01942668) was a phase 3, randomized, double-blind, placebo-controlled, multicenter trial, which evaluated four daily doses of 17β-estradiol and progesterone (E2/P4) combined in a single, oral, softgel capsule in postmenopausal women (40-65 years) with a uterus and moderate to severe VMS (≥7/day or ≥50/week). In post hoc analyses, growth models were used to examine relationships between linear changes in VMS frequency and severity over 12 weeks and changes from baseline in the Menopause-Specific Quality of Life (MENQOL; total score and VMS domain) and the Medical Outcomes Study-Sleep (total score, sleep problems indices I and II) questionnaire outcomes at 12 weeks with treatment compared with placebo.
Results:
Outcomes with all four E2/P4 doses were combined (n = 591) and compared with placebo (n = 135). In all 5 growth models, the effects of TX-001HR on MENQOL total score and vasomotor domain were significantly associated with changes in VMS frequency and severity observed over 12 weeks (all, P < 0.001). Treatment-mediated effects on MENQOL via VMS frequency and severity models were significant. Similar results were found with Medical Outcomes Study-Sleep total score and sleep problems indices.
Conclusions:
TX-001HR improvements in quality of life and sleep outcomes are associated with and may be mediated through improvements in VMS frequency and severity.
Keywords: Estradiol, Hot flushes, Menopause, Progesterone, Quality of life, sleep
Vasomotor symptoms (VMS) have been shown to negatively affect quality of life, increase sleep disturbances, impair social relationships, interfere with mood, and decrease work productivity, and are especially bothersome when severe.1-9 For example, women with moderate to severe VMS have increased frequency of nighttime wakefulness10-12 and chronic insomnia.13 They are also more than three times more likely to report impaired quality of life than those with mild or moderate VMS.14 Women with moderate to severe VMS were 2.8 times more likely to have moderate to severe depressive symptoms than women with no or mild VMS.15
The phase 3 REPLENISH trial, evaluated four doses of TX-001HR (TherapeuticsMD, Boca Raton, FL), an investigational, single, oral, softgel capsule containing 17β-estradiol and micronized progesterone (E2/P4) for the treatment of moderate to severe VMS associated with menopause. The 12-week VMS substudy of the REPLENISH trial showed that the two highest doses (1 mg E2/100 mg P4 and 0.5 mg E2/100 mg P4) significantly improved VMS frequency and severity, meeting the study's coprimary endpoints, and was the basis for FDA approval of the 1 mg E2/100 mg P4 dose (Bijuva, TherapeuticsMD, Boca Raton, FL) in October 2018.16 During the 12-month safety study of the REPLENISH trial, TX-001HR significantly improved menopause-specific quality of life, as measured with the Menopause-Specific Quality of Life (MENQOL) questionnaire, including the MENQOL overall and vasomotor domain scores for up to 12 months.17 TX-001HR also significantly improved sleep outcomes, as measured with the Medical Outcomes Study-Sleep (MOS-Sleep) questionnaire, including the MOS-Sleep total score and Sleep Problems Indices I and II scores for up to 12 months.18 Although an association between VMS and reduced quality of life and sleep disturbances have been reported,1-14 these relationships are not well understood. Thus, the objective of this analysis was to characterize the relationship between changes in VMS frequency and severity with TX-001HR and changes in the MENQOL questionnaire and the MOS-Sleep scale scores in the REPLENISH study.
METHODS
Study design
The REPLENISH study (NCT01942668) was a randomized, double-blind, placebo-controlled, multicenter trial that evaluated four doses of E2/P4 in a single, oral softgel capsule, in postmenopausal women with a uterus. Healthy postmenopausal women (aged 40-65 years; BMI ≤34 kg/m2) with ≥7/day or ≥50/week moderate to severe hot flushes were enrolled in a VMS substudy and randomized 1:1:1:1:1 to daily 1 mg E2/100 mg P4, 0.5 mg E2/100 mg P4, 0.5 mg E2/50 mg P4, 0.25 mg E2/50 mg P4, or placebo. Women who did not qualify for the VMS substudy were randomized 1:1:1:1 to active E2/P4 doses only. A computer-generated randomization schedule for treatment assignment was used to reduce the potential for selection bias. Other inclusion and exclusion criteria, which are typical of other trials assessing menopausal hormone therapy, are described elsewhere.16 All women took two different sized capsules to maintain study blind for the different sized pills of the different doses (two higher doses were a larger capsule than the two lower doses) utilizing a double-blind, double-dummy design. The capsules were otherwise identical.
The four coprimary efficacy endpoints of the REPLENISH trial were changes from baseline in the weekly frequency and severity of moderate to severe hot flushes at weeks 4 and 12, as measured with a daily diary. Women entered in a daily diary the number of hot flushes experienced and their severity, which were scored as mild (score of 1), moderate (score of 2), or severe (score of 3); or checked a box if no hot flushes were experienced. Quality of life over the past month was measured using the MENQOL questionnaire19 composed of 29 items from four domains (vasomotor [3 items], psychosocial [7 items], physical [16 items], and sexual function [3 items]). If symptoms were experienced, they were scored using a seven-point Likert scale ranging from not at all bothered (analysis score of 2) to extremely bothered (analysis score of 8); if symptoms were not experienced, the analysis score was set to 1.
Sleep quality was measured using the MOS-Sleep scale20 consisting of 12 items that assess 6 dimensions of sleep over the past 4 weeks (sleep onset, sleep duration, maintenance, respiratory problems, perceived adequacy, and somnolence) and 9 subscales, which include sleep problems indices I and II.20 Participants provided times for sleep onset and duration and rated the remaining items on a six-point scale (1, all of the time; 6, none of the time). The MENQOL and MOS-Sleep questionnaires were self-administered at baseline, week 12, and months 6 and 12 visits.
Statistical analyses
Analyses were performed in the modified intent-to-treat (MITT)-VMS population, which was defined as women who were randomized to the VMS substudy, took at least one full dose (two capsules due to blinding) of the study medication, had ≥5 days of baseline VMS data, and ≥4 days of VMS data for 1 on-treatment week. Sample size for the VMS substudy was previously reported but was estimated to be 150 women per treatment group based on providing at least 90% power to test the primary VMS hypotheses and allowing a 20% discontinuation rate.
In post hoc analyses, growth models were used to summarize the longitudinal data and provide change estimates to examine the relationships between linear changes in frequency and severity of hot flushes over 12 weeks, and changes from baseline in MENQOL (total and VMS domain scores) and MOS-Sleep (total score, sleep problems indices I and II) outcomes at 12 weeks. All four TX-001HR doses were combined and compared with placebo. A fixed cubic term was used in the growth model for frequency. This term was fixed to a single estimated value across every individual in the sample and captured the flattening of the curvature in the frequency data over time. Similarly, a fixed quadratic term was included in the growth model for severity to account for some nonlinearity in the severity data over time. Growth model fit was evaluated by several fit statistics with respective adequate fit criteria, including the comparative fit index (CFI), root mean square error of approximation (RMSEA), and standardized root mean residual (SRMR), which are the most commonly used fit indexes to describe the fit of structural equation models. CFI scores >90, RMSEA scores <0.08, and SRMR <0.10 indicate that the data fit the structural equation model well. If the data do not fit the equation models, that would suggest that the hypotheses supported by the equation models are not accurate. All analyses were performed using Mplus version 8.21
RESULTS
Disposition and demographics
A total of 766 women were enrolled in the VMS substudy and 726 were eligible for analysis in the MITT-VMS population. The MITT-VMS population comprised 591 women randomized to one of the four E2/P4 doses (n = 141 for 1 mg E2/100 mg P4; n = 149 for 0.5 mg E2/100 mg P4; n = 147 for 0.5 mg E2/50 mg P4; n = 154 for 0.25 mg E2/50 mg P4) and 135 to the placebo group. Eighty-nine percent (89%) of the women completed the substudy at 12 weeks.
Women had a mean age of 55 years (40-65) and a mean BMI of 27 kg/m2 at study entry; 67% of the women were White and 31% were African American (Table 1). At baseline, the VMS severity score was 2.5 and the frequency of moderate to severe VMS ranged from 72.1 to 77.0 per week or 10.3 to 11.0 per day.
TABLE 1.
Demographic characteristics in the modified intent-to-treat vasomotor symptom population
| Total population n = 726 | |
| Age, y | 54.6 ± 4.4 |
| Race, n (%) | |
| White | 486 (66.9) |
| Black American | 225 (31.0) |
| Othera | 15 (2.1) |
| Body mass index, kg/m2 | 26.6 (4.0) |
| Years since last menstrual period | 5.9 (5.1) |
| Bilateral oophorectomy | 8 (1.1) |
Data expressed as mean ± SD.
aOthers includes Other (n = 10), American Indian or Alaska Native (n = 2), Native Hawaiian or Pacific Islander (n = 2), and Unknown (n = 1).
VMS, MENQOL, and MOS-Sleep assessments
Improvements from baseline to week 12 in the weekly frequency of moderate to severe VMS ranged from −50.2 to −55.1 with E2/P4 doses and were significantly greater than those with placebo (all, P < 0.01). Weekly severity of moderate to severe VMS improvements from baseline to week 12 ranged from −0.71 to −1.12 with E2/P4 doses and were significantly improved with E2/P4 1 mg/100 mg, 0.5 mg/100 mg, and 0.5 mg/50 mg doses compared with placebo (all, P < 0.05).
The MENQOL questionnaire was answered by 99.9% (725/726) of participants at baseline and by 89% (649/726) at week 12. Improvements from baseline to week 12 in the MENQOL overall and vasomotor domain scores ranged from −1.6 to −1.9 and from −3.2 to −3.8, respectively with E2/P4 doses; improvements were significantly improved with all E2/P4 doses compared with placebo (all, P < 0.05).
The MOS-Sleep questionnaire was answered by 99% (720/726) of participants at baseline and by 87% (634/720) at week 12. Improvements from baseline to week 12 in the MOS-Sleep overall score ranged from −13.1 to −18.5 for the E2/P4 doses versus −11.5 for placebo; improvements were significantly better with 1 mg E2/100 mg P4 and 0.5 mg E2/50 mg P4 than placebo. Similar results were also observed for the sleep problems index II. For the sleep problems index I, all E2/P4 doses improved the score with numeric improvement versus placebo; only the 0.5 mg E2/50 mg P4 was significantly different from placebo at 12 weeks.
Growth models
Results for the five growth models are summarized in Figures 1 and 2. The specified models fit the observed data well with CFIs of 0.97, RMSEAs of 0.07, and SRMRs ranging from 0.05 to 0.08 for all five growth curves. Changes in the frequency and severity of VMS showed, among others, linear relationships with MENQOL total and vasomotor domain scores, as well as with MOS-Sleep total, and sleep problem index I and II scores. The fixed cubic term in the frequency growth model and the fixed quadratic term in the severity growth model were excluded from the figures because these terms did not show variance (fixed effects) on the models.
FIG. 1.
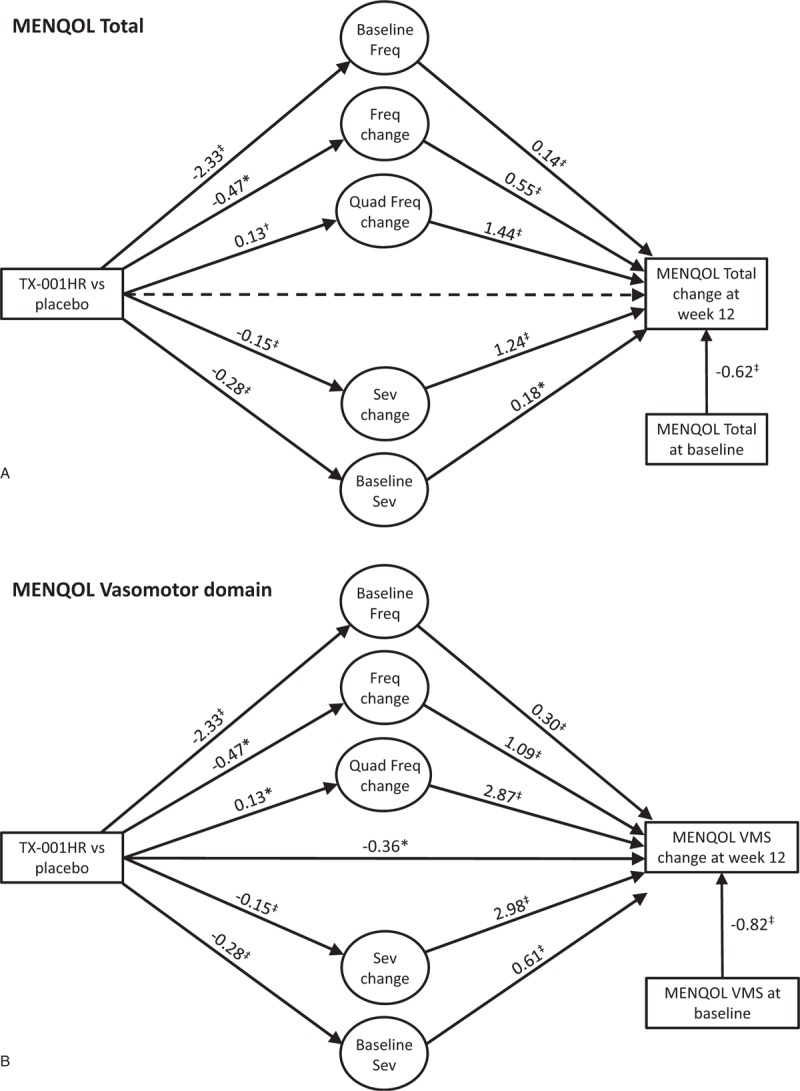
Relationship of treatment, hot flush frequency (Freq) and severity (Sev) changes, and changes in Menopause-Specific Quality of Life (MENQOL) questionnaire (A) overall; (B) vasomotor domain. ∗P < 0.05; †P < 0.01; ‡P < 0.001. Solid lines are statistically significant paths, dashed lines are not significant. Fixed cubic and quadratic (quad) terms were not included in the figures.
FIG. 2.
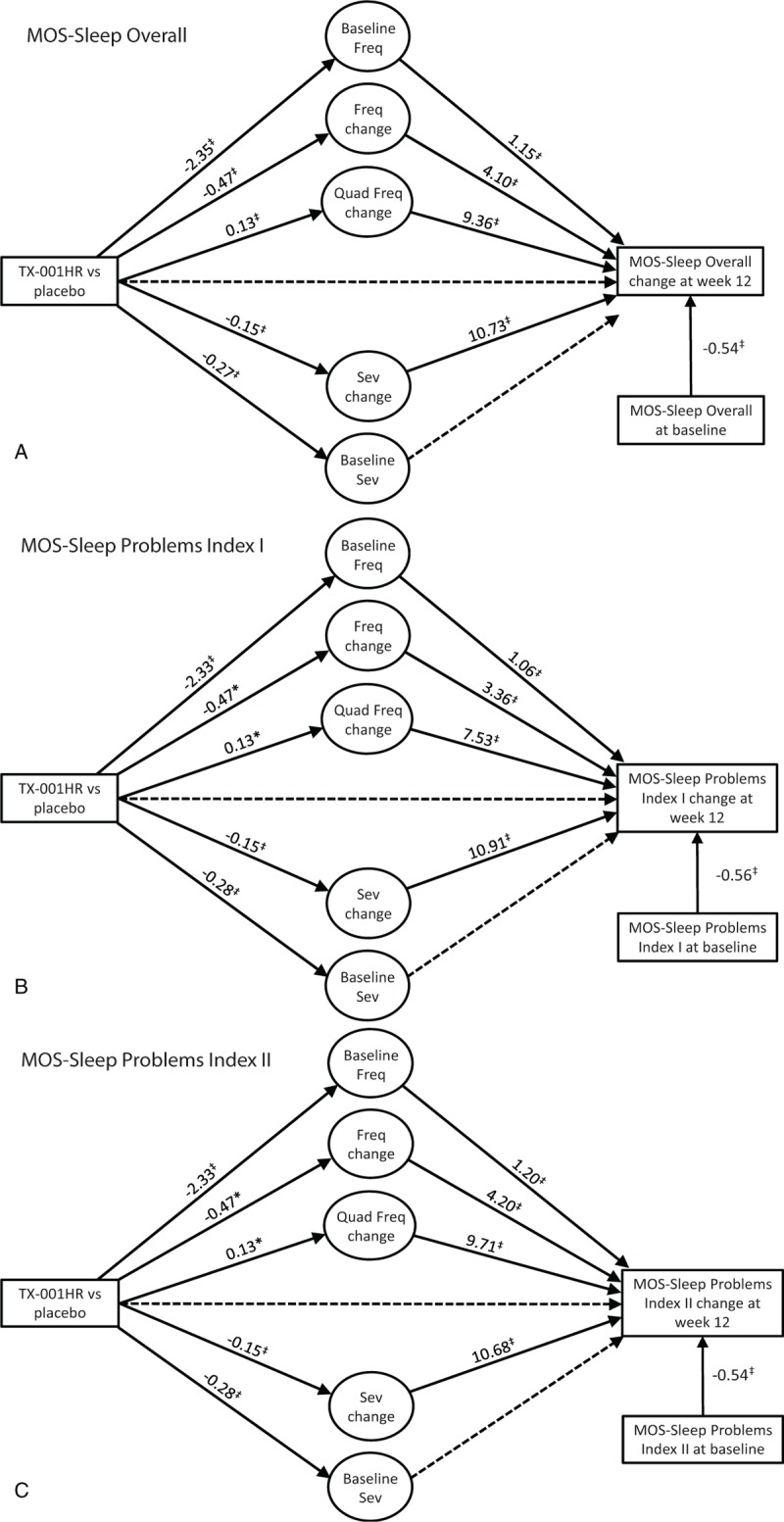
Relationship of treatment, hot flush frequency (Freq) and severity (Sev) changes, and changes in Medical Outcomes Study (MOS)-Sleep Scale (A) overall; (B) sleep problems index I; (C) sleep problems index II. ∗P < 0.05; †P < 0.01; ‡P < 0.001. Solid lines are statistically significant paths, dashed lines are not significant. Fixed cubic and quadratic (quad) terms were not included in the figures.
In women with moderate to severe VMS who received E2/P4, changes in both VMS frequency and severity over 12 weeks were significantly related to changes in MENQOL total and vasomotor scores from baseline (Fig. 1A and B). For both MENQOL total and VMS scores, the effect of treatment was significantly associated via changes in moderate to severe VMS frequency and severity (all P < 0.05). For the MENQOL vasomotor domain, treatment retained a direct effect (estimate −0.36; P < 0.05). Similar results were also observed for the evaluated MOS-Sleep parameters (Fig. 2A-C). The treatment effect on the three sleep outcomes (MOS-Sleep overall and sleep problems indices I and II) was associated with changes in VMS frequency and severity.
DISCUSSION
In this post hoc analysis of the REPLENISH trial, improvements observed in MENQOL total and vasomotor domain scores at 12 weeks with TX-001HR, as well as MOS-Sleep total and sleep problem index scores, appear to be mediated, for the most part, through improvements in VMS frequency and severity observed over 12 weeks.
Our approach to determine the relationship between VMS frequency and severity with quality of life and sleep is consistent with the results of other analyses showing strong associations between VMS and quality of life and sleep outcomes.5,6,9,10 One study on health-related quality of life found that bothersome VMS negatively affected women's social functioning, emotional functioning, mental health, energy levels, and bodily pain.6 Another reported that women with VMS were significantly more likely to have poor sleep, reduced sexual function/satisfaction, anxiety and fears, somatic symptoms, and decreased memory and concentration compared with those without VMS.9 In addition, women with no VMS at baseline, had no improvements in health-related quality of life with hormone therapy.9 Similar data have been reported for sleep outcomes. In SWAN, women with moderate to severe VMS were almost three times more likely to report problems of frequent nocturnal awakenings compared with women with no VMS.22
Pinkerton and colleagues performed an analysis similar to ours using the results of the SMART-2 trial, which evaluated the effect of conjugated equine estrogens with bazedoxifene on hot flushes.23 Consistent with our results, the data showed that VMS frequency and severity had approximately linear relationships with MENQOL and sleep parameters, and therefore improvements in hot flushes were associated with improvements in menopause-specific quality of life and sleep. Furthermore, they also reported that improvement in VMS severity was more strongly related to improvements in MENQOL outcomes than VMS frequency. This would suggest that the impact of VMS on quality of life is driven more by the intensity than frequency of the VMS. These results are in line with another study, which found that VMS problem rating or “bothersomeness” was a better predictor of VMS impact on health-related quality of life than frequency.6
A limitation of this analysis is that it included all participants of the VMS substudy, including women who took the lowest dose of E2/P4 (0.25 mg/50 mg), which was included as a noneffective dose, and likely dampened the strength of the VMS frequency and severity relationships observed. Regardless, significant relationships were still found between VMS changes and quality of life and sleep parameters. Another limiting factor for interpretation of the data is that the correlations were only performed at week 12.
Although many believe that sleep (or quality of life) improvements are directly related to VMS relief, the idea that estrogens may affect sleep via the central nervous system has been proposed.24 It has also been postulated that P4 induces GABAergic effects, having a beneficial effect on sleep. Studies have shown that hormone therapy containing P4 improved sleep parameters better than those containing medroxyprogesterone acetate.25,26 In the REPLENISH trial, women treated with E2/P4 showed improvement in measured sleep outcomes and had low incidences of somnolence (0.2%-1.2%).18 However, the analysis presented here suggest an indirect effect of TX-001HR on sleep and quality of sleep through VMS reduction rather than a direct effect on these outcomes. In fact, the only direct effect of TX-001HR treatment on outcomes was observed on the MENQOL vasomotor domain, which may not be surprising as the MENQOL vasomotor domain specifically evaluates symptoms of hot flushes, night sweats, and sweating; and TX-001HR significantly improved the frequency and severity of hot flushes.16
CONCLUSIONS
The REPLENISH trial was a large, phase 3, randomized controlled trial in which oral continuous combined doses of E2/P4 significantly reduced the frequency and severity of VMS, with no endometrial hyperplasia, in postmenopausal women with a uterus. The analysis reported here extends those results by showing that TX-001HR improved quality of life and sleep outcomes via improvements in VMS frequency and severity.
Acknowledgments
The authors would also like to acknowledge the medical writing assistance of Dominique Verlaan, PhD, CMPP of Precise Publications, LLC, which was supported by TherapeuticsMD.
Footnotes
Source (s) of the work or study: TherapeuticsMD.
Trial Registration: https://clinicaltrials.gov/ct2/show/NCT01942668.
Funding/support: TherapeuticsMD sponsored the study and provided support for the medical writing assistance of Dominique Verlaan, PhD, CMPP (Precise Publications, LLC).
Financial disclosures/conflicts of interest: Dr Mirkin, Dr Graham, and Dr Bernick are employees of TherapeuticsMD with stock/stock options. Dr Bernick is also a board member of TherapeuticsMD. Dr Revicki is an employee of Evidera, who consults for TherapeuticsMD. Dr Bender was an employee of Evidera, which consults for TherapeuticsMD. Dr Constantine consults to multiple pharmaceutical companies including but not limited to TherapeuticsMD and has stock options from TherapeuticsMD.
REFERENCES
- 1.Blumel JE, Chedraui P, Baron G, et al. A large multinational study of vasomotor symptom prevalence, duration, and impact on quality of life in middle-aged women. Menopause 2011; 18:778–785. [DOI] [PubMed] [Google Scholar]
- 2.Blumel JE, Cano A, Mezones-Holguin E, et al. A multinational study of sleep disorders during female mid-life. Maturitas 2012; 72:359–366. [DOI] [PubMed] [Google Scholar]
- 3.Whiteley J, Wagner JS, Bushmakin A, Kopenhafer L, Dibonaventura M, Racketa J. Impact of the severity of vasomotor symptoms on health status, resource use, and productivity. Menopause 2013; 20:518–524. [DOI] [PubMed] [Google Scholar]
- 4.Kleinman NL, Rohrbacker NJ, Bushmakin AG, Whiteley J, Lynch WD, Shah SN. Direct and indirect costs of women diagnosed with menopause symptoms. J Occup Environ Med 2013; 55:465–470. [DOI] [PubMed] [Google Scholar]
- 5.Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: a comprehensive review. Health Qual Life Outcomes 2005; 3:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayers B, Hunter MS. Health-related quality of life of women with menopausal hot flushes and night sweats. Climacteric 2013; 16:235–239. [DOI] [PubMed] [Google Scholar]
- 7.Hunter MS, Gentry-Maharaj A, Ryan A, et al. Prevalence, frequency and problem rating of hot flushes persist in older postmenopausal women: impact of age, body mass index, hysterectomy, hormone therapy use, lifestyle and mood in a cross-sectional cohort study of 10,418 British women aged 54-65. BJOG 2012; 119:40–50. [DOI] [PubMed] [Google Scholar]
- 8.Duffy OK, Iversen L, Hannaford P. The impact and management of symptoms experienced at midlife: a community-based study of women in northeast Scotland. BJOG 2012; 119:554–564. [DOI] [PubMed] [Google Scholar]
- 9.Savolainen-Peltonen H, Hautamaki H, Tuomikoski P, Ylikorkala O, Mikkola TS. Health-related quality of life in women with or without hot flashes: a randomized placebo-controlled trial with hormone therapy. Menopause 2014; 21:732–739. [DOI] [PubMed] [Google Scholar]
- 10.Ensrud KE, Stone KL, Blackwell TL, et al. Frequency and severity of hot flashes and sleep disturbance in postmenopausal women with hot flashes. Menopause 2009; 16:286–292. [DOI] [PubMed] [Google Scholar]
- 11.Lampio L, Polo-Kantola P, Polo O, Kauko T, Aittokallio J, Saaresranta T. Sleep in midlife women: effects of menopause, vasomotor symptoms, and depressive symptoms. Menopause 2014; 21:1217–1224. [DOI] [PubMed] [Google Scholar]
- 12.De Zambotti M, Colrain IM, Javitz HS, Baker FC. Magnitude of the impact of hot flashes on sleep in perimenopausal women. Fertil Steril 2014; 102:1708–1715.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohayon MM. Severe hot flashes are associated with chronic insomnia. Arch Intern Med 2006; 166:1262–1268. [DOI] [PubMed] [Google Scholar]
- 14.Williams RE, Levine KB, Kalilani L, Lewis J, Clark RV. Menopause-specific questionnaire assessment in US population-based study shows negative impact on health-related quality of life. Maturitas 2009; 62:153–159. [DOI] [PubMed] [Google Scholar]
- 15.Worsley R, Bell RJ, Gartoulla P, Robinson PJ, Davis SR. Moderate-severe vasomotor symptoms are associated with moderate-severe depressive symptoms. J Womens Health (Larchmt) 2017; 26:712–718. [DOI] [PubMed] [Google Scholar]
- 16.Lobo RA, Archer DF, Kagan R, et al. A 17β-estradiol–progesterone oral capsule for vasomotor symptoms in postmenopausal women: A randomized controlled trial. Obstet Gynecol 2018; 132:161–170. [DOI] [PubMed] [Google Scholar]
- 17.Simon JA, Kaunitz A, Kroll R, et al. Oral 17β-estradiol-progesterone (TX-001HR) and quality of life in postmenopausal women with vasomotor symptoms. Menopause 2019; 26:506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kagan R, Constantine G, Kaunitz AM, Bernick B, Mirkin S. Improvement in sleep outcomes with a 17β-estradiol-progesterone oral capsule (TX-001HR) for postmenopausal women. Menopause 2018; [In press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilditch JR, Lewis J, Peter A, et al. A menopause-specific quality of life questionnaire: development and psychometric properties. Maturitas 1996; 24:161–175. [DOI] [PubMed] [Google Scholar]
- 20.Hays RD, Stewart AL. Stewart AL, Ware JE. Sleep measures. Measuring Functioning and Well-being: The Medical Outcomes Study Approach. Durham, NC: Duke University Press; 1992. 235–259. [Google Scholar]
- 21.Muthén LK, Muthén BO. Mplus User's Guide. Muthén and Muthén, Eighth ed.Los Angeles, CA: 1998-2017. [Google Scholar]
- 22.Kravitz HM, Joffe H. Sleep during the perimenopause: a SWAN story. Obstet Gynecol Clin North Am 2011; 38:567–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinkerton JV, Abraham L, Bushmakin AG, Cappelleri JC, Komm BS. Relationship between changes in vasomotor symptoms and changes in menopause-specific quality of life and sleep parameters. Menopause 2016; 23:1060–1066. [DOI] [PubMed] [Google Scholar]
- 24.Polo-Kantola P. Sleep problems in midlife and beyond. Maturitas 2011; 68:224–232. [DOI] [PubMed] [Google Scholar]
- 25.Montplaisir J, Lorrain J, Denesle R, Petit D. Sleep in menopause: differential effects of two forms of hormone replacement therapy. Menopause 2001; 8:10–16. [DOI] [PubMed] [Google Scholar]
- 26.Gambacciani M, Ciaponi M, Cappagli B, et al. Effects of low-dose, continuous combined hormone replacement therapy on sleep in symptomatic postmenopausal women. Maturitas 2005; 50:91–97. [DOI] [PubMed] [Google Scholar]


