Supplemental Digital Content is available in the text.
Background.
Several risk models to predict outcome after liver transplantation (LT) have been developed in the last decade. This study compares the predictive performance of 7 risk models.
Methods.
Data on 62 294 deceased donor LTs performed in recipients ≥18 years old between January 2005 and December 2015 in the United Network for Organ Sharing region were used for this study. The balance of risk, donor risk index (DRI), Eurotransplant-DRI, donor-to-recipient model (DRM), simplified recipient risk index, Survival Outcomes Following Liver Transplantation (SOFT), and donor Model for End-stage Liver Disease scores were calculated, and calibration and discrimination were evaluated for patient, overall graft, and death-censored graft survival. Calibration was evaluated by outcome of high-risk transplantations (>80th percentile of the respective risk score) and discrimination by concordance index (c-index).
Results.
Patient survival at 3 months was best predicted by the SOFT (c-index: 0.68) and Balance of Risk score (c-index: 0.64), while the DRM and SOFT score had the highest predictive capacity at 60 months (c-index: 0.59). Overall, graft survival was best predicted by the SOFT score at 3-month follow-up (c-index: 0.65) and by the SOFT and DRM at 60-month follow-up (c-index: 0.58). Death-censored graft survival at 60-month follow-up is best predicted by the DRI (c-index: 0.59) and Eurotransplant-DRI (c-index: 0.58). For patient and overall graft survival, high-risk transplantations were best defined by the DRM. For death-censored graft survival, this was best defined by the DRI.
Conclusions.
This study shows that models dominated by recipient factors have the best performance for short-term patient survival. Models that also include sufficient donor factors have better performance for long-term graft survival. Death-censored graft survival is best predicted by models that predominantly included donor factors.
Nearly 14 000 patients are currently on the liver transplantion (LT) waiting list in the United States, and each year >10% of these patients die without a transplantation.1 Optimal use and allocation of livers available for transplantation are therefore essential. Such “optimal” allocation is, however, difficult to define. Currently, the majority of livers in the United States and Europe are allocated according to the Model for End-stage Liver Disease (MELD) or models derived from the MELD score (eg, MELD-Na).2,3 MELD is an objective score that includes 3 laboratory values of the recipient (creatinine, bilirubin, and international normalized ratio), validated for the prediction of 3-month waiting list mortality.4,5 Studies showed that it is less suitable to accurately predict outcome after transplantation.6
A model to predict outcome after transplantation should include all relevant characteristics of the donor, the recipient, and other relevant data relating to the transplantation. It would enable us to objectify and quantify the impact of several risk factors and could have numerous other applications. Over the past decade, several models for donor quality, recipient quality, or the combination have been developed. To predict outcome after LT, the Survival Outcomes Following Liver Transplantation (SOFT),6 donor MELD (D-MELD),7 and balance of risk (BAR) scores8 have been developed. While these models incorporate donor, recipient, and transplant characteristics, the donor risk index (DRI)9 and Eurotransplant-DRI (ET-DRI)10 include solely donor and transplant characteristics to measure donor and organ quality. The ET-DRI was developed and validated for the ET region in 2012. Later on, the simplified recipient risk index (sRRI) was developed.11 Both the donor model (ET-DRI) and recipient model (sRRI) were combined to predict outcome based on the combination of significant donor, transplantation, and recipient factors: the donor-to-recipient model (DRM).11 Although all models predict “outcome” after LT, there are several differences between them.12 Most importantly, the considered end point varies.
This study aims to compare the predictive capacity of 7 models on patient-, overall graft- and death-censored graft survival at different posttransplant follow-up periods after LT.
MATERIALS AND METHODS
Data Selection
This study used data on LTs from January 1, 2005, until December 31, 2015, from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donors, waitlisted candidates, and transplant recipients in the United States, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration, US Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. No ethical statement was required according to European guidelines and Dutch law. Follow-up data were available up to March 2017.
Study Population
In the study period, 71 429 LTs were performed. All LTs in recipients <18 years old were excluded (n = 6201) as well as those performed with livers from living donors (n = 2347) and auxiliary transplanted livers (n = 37). Any combinations of organs other than liver and kidney were also excluded (n = 550). This resulted in 62 294 transplantations included in the analysis.
Calculation of the BAR, SOFT, DRI, DRM, D-MELD, and Maximum C-statistic
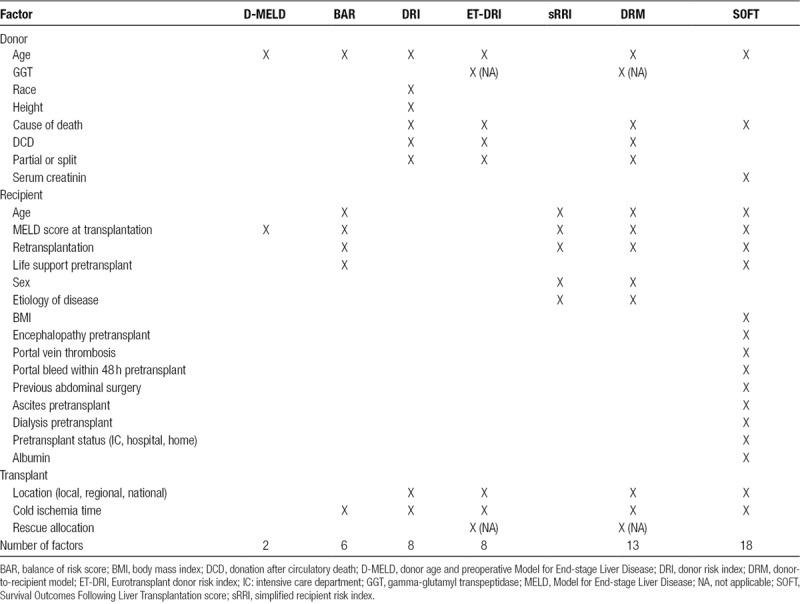
Variables incorporated in the respective models are shown in Table 1. Cold ischemic times were missing in 3% (n = 1562) and were singly imputed with the median cold ischemic time (6.3 h). Recipient body mass index was missing in 1552 cases and set at reference (body mass index <30) for calculation of the SOFT score. Gamma-glutamyl transpeptidase (GGT) and “rescue allocation” are required for calculation of the ET-DRI10 but were not available in the dataset. Rescue allocation can be considered a fast-track allocation that is used in the ET region for a “center-oriented” allocation after organs have not been accepted in “patient-oriented” allocation for medical or logistical reasons.13 They were therefore set at reference (GGT <50 U/L and rescue allocation “no”). Then, BAR score, SOFT score, DRI, ET-DRI, sRRI, DRM, and D-MELD scores were calculated for all transplantations as described before.6-11 The maximal c-statistic was calculated for a dynamic model including all factors that were incorporated in either the BAR, SOFT, DRI, ET-DRI, sRRI, DRM, or D-MELD score. The model is considered dynamic because the effects of each factor were estimated for each time point (per month follow-up period) separately.
TABLE 1.
Overview of all variables per risk model
Definitions
Primary outcomes were (1) patient, (2) overall graft, and (3) death-censored graft survival at follow-up periods of 3 months, 1 year, and 5 years after transplantation. Patient survival (1) was defined as the time period between transplantation and patient death. Overall graft survival (2) was evaluated as nondeath-censored graft survival and was defined as the time period between transplantation and either date of graft failure or patient death, whichever occurred first. Death-censored graft survival (3) was defined as the time period between transplantation and date of graft failure (note that patients were censored when deceased). Graft failure was, as specified in the OPTN follow-up forms, not entered for patients who died as a result of some other factor unrelated to graft failure. The individual scores were used to define risk groups of transplantations using increments of 20% in the quantiles of risk scores. High-risk transplantations were arbitrarily defined as scores above the 80th percentile according to the respective risk models.
Statistical Analysis
Clinical characteristics were summarized by median and 25% and 75% interquartile ranges (IQR) and number and percentage (N/%) for, respectively, continuous and categorical variables. Numerical and categorical factors between groups were compared using Kruskall–Wallis and Chi-square tests. Predictive performance of all models was compared by the area under the ROC curve or “c-statistic.”14 This c-statistic was calculated monthly up to 5 years for all 3 considered end points. Calculated c-statistics of individual models were compared in a boot-strapped 1000-fold database. Subsequently, transplantations were stratified by risk groups per score to evaluate the discriminative ability. Outcome of transplantations was stratified by risk groups using increments of 20% in the quantiles of risk scores in Kaplan–Meier analyses. Survival rate and rate of graft loss in the high-risk transplantations (above 80th percentile) were compared per end point between the several scores at 5-year follow-up. For death-censored graft survival, censoring by death was accounted for as a competing risk when calculating cumulative incidences.15
All analyses were performed with SPSS version 24 and R version 3.3.2. A P value below 0.05 was considered statistically significant. All analyses were performed in collaboration with the Department of Biomedical Data Sciences, Leiden University Medical Center.
RESULTS
Study Population
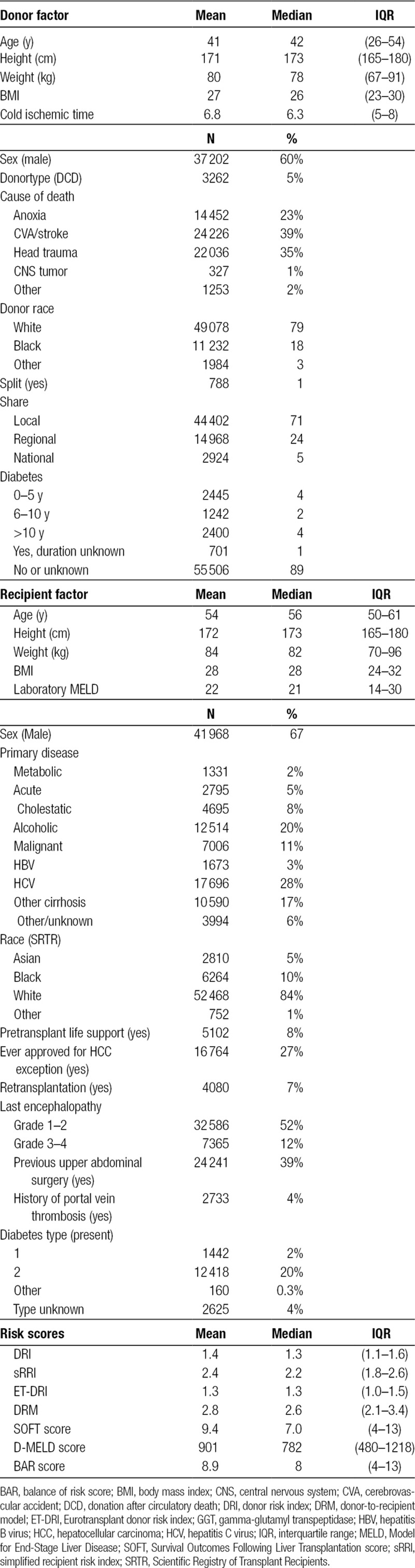
In the study period, 62 294 performed LTs were included. Mean transplant follow-up was 5.5 years for patient survival. Demographics of donors, patients, and transplantations are shown in Table 2. Most notably, donors had a median age of 42 years old (IQR: 26–54) and were transplanted with a median cold ischemic time of 6.3 hours (IQR: 5–8). Approximately 10% of all donors had diabetes mellitus, and about a third of all livers were shared either regionally (24%) or nationally (5%). Recipients had a median age of 56 years old and a median laboratory MELD score of 21 (IQR: 14–30). Most recipients were transplanted for hepatitis C–related disease (28%), followed by alcoholic cirrhosis (20%) or other causes of cirrhosis (17%).
TABLE 2.
Study demographics (n = 62 294).
Discrimination
For the BAR, ET-DRI, DRI, DRM, sRRI, SOFT, and D-MELD scores, the change in predictive capacity (c-index) is demonstrated over time and per outcome type. For patient survival, this is shown in Figure 1A. In general, the ability to predict outcomes accurately decreases over time. Therefore, outcome at short-term follow-up can be more accurately predicted than at longer follow-up. Patient survival at 3-month follow-up was best predicted by the SOFT score (c-index: 0.68, P < 0.001) followed by the BAR (c-index: 0.64, P < 0.001) and DRM scores (c-index: 0.61, P < 0.001). From 3-year follow-up onward, the SOFT score has a comparable performance to the DRM. The initial high performance of the BAR score decreases rapidly to below 0.6 at 18-month follow-up. Patient survival at 60-month follow-up was best predicted by the DRM and SOFT score (c-index: 0.59 for both, P = 0.60). The maximal c-statistic for patient survival was higher at each time period than all other models (P < 0.001). The model with all factors included, calibrated monthly, reached a c-statistic of 0.70 at 3-month follow-up and decreased gradually to 0.66 and 0.63 at 12- and 60-month follow-up, respectively.
FIGURE 1.
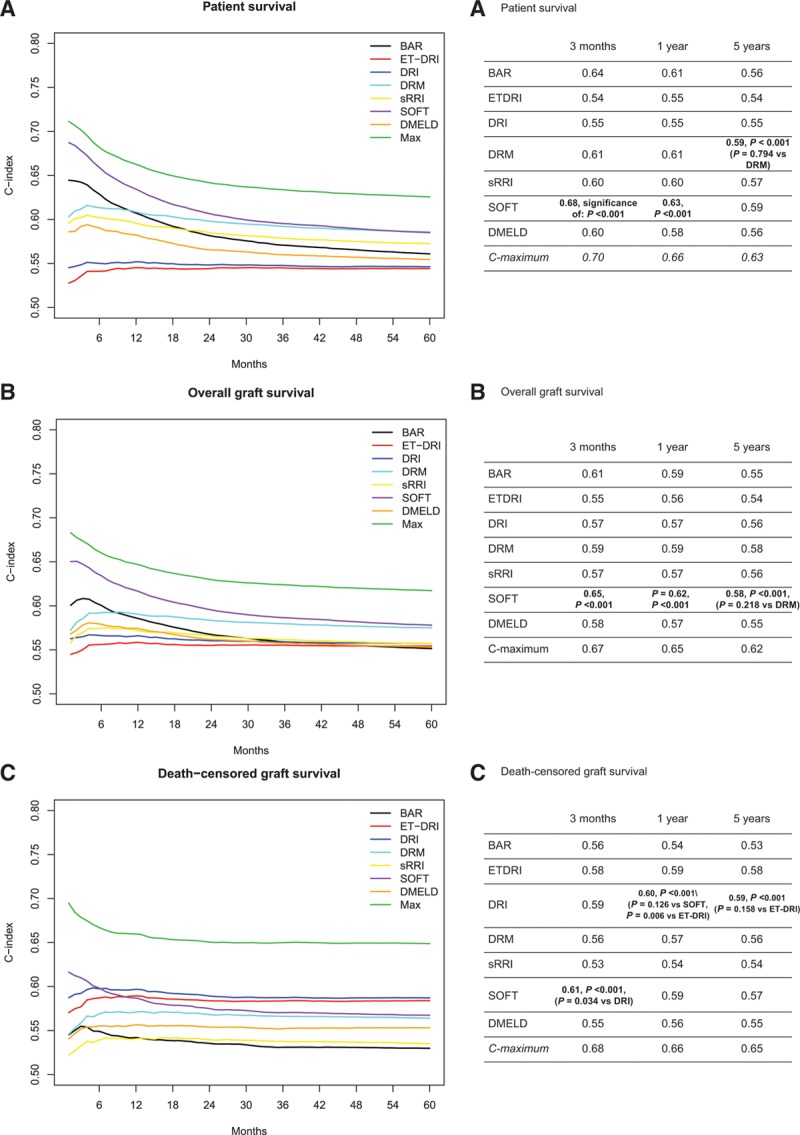
Performance of risk models. A, Patient survival. B, Overall graft survival. C, Death-censored graft survival. BAR, balance of risk score; D-MELD, donor age and preoperative model for end-stage liver disease; DRI, donor risk index; DRM, donor-to-recipient model; ET-DRI, Eurotransplant donor risk index; SOFT, Survival Outcomes Following Liver Transplantation score; sRRI, simplified recipient risk index.
To predict overall graft survival at short-term follow-up, the highest predictive value at 3 months was also achieved by the SOFT score (c-index: 0.65, P < 0.001), as is shown in Figure 1B. The BAR score and DRM performed reasonably when predicting overall graft survival at 3-month follow-up with c-indexes of 0.61 and 0.59, P ≤ 0.001, respectively. Overall graft survival at 60-month follow-up was again best predicted by the SOFT score and by the DRM with a similar c-index of 0.58 (P = 0.22). A notable difference between these 2 models is the performance at short term; the SOFT score had an optimal performance at approximately 2 months posttransplantation, whereas the DRM reached a peak after 6 months. Performance of the other risk scores for overall graft survival stabilized around a c-index of 0.56 after approximately 2 years. The maximal c-statistic for overall graft survival was 0.67 at 3-month follow-up and decreased to 0.65 and 0.62 at 12- and 60-month follow-up, respectively. These c-statistics were significantly higher than all other models at 3-, 12-, and 60-month follow-up (P < 0.001).
Death-censored graft survival showed a different picture; models that are dominated by donor factors like the DRI as well as the ET-DRI had best predictive capability as from 1 year onward, as shown in Figure 1C. The DRI and ET-DRI achieved c-indexes at 12 months of 0.60 and 0.59 (P = 0.01), respectively, and at 60 months of 0.59 and 0.58 (P = 0.16). The maximal c-statistic for death-censored graft survival was significantly higher as compared with each other model at the respective time points (P < 0.001); it varied from 0.68 to 0.66 and 0.65 at 3-, 12-, and 60-month follow-up, respectively.
Calibration
As a measure of calibration, outcome of transplantations was stratified by risk groups defined by increments of 20% of the several risk models (Table 3). The lowest patient survival rate in high-risk transplantations was observed in the group defined by the DRM with a survival rate of 64% at 5-year follow-up (Figure 2). Patient survival stratified by other risk models is shown in Figure S1A–F, SDC, http://links.lww.com/TXD/A209. Also, for overall graft survival, the lowest survival rate in high-risk transplantations was observed in the group defined by the DRM (Figure 3) and by the SOFT score with a survival rate of 62% (Figure 4). Overall graft survival stratified by other risk models is shown in Figure S2A–E, SDC, http://links.lww.com/TXD/A209. Death-censored graft survival was best predicted by models that were dominated by donor characteristics, such as the DRI and ET-DRI. In high-risk transplantations defined by these models, a graft loss rate of 15% was observed (Figures 5 and 6). Death-censored graft survival stratified by other risk models is shown in Figure S3A–E, SDC, http://links.lww.com/TXD/A209.
TABLE 3.
Outcome by risk groups at 5-year follow-up
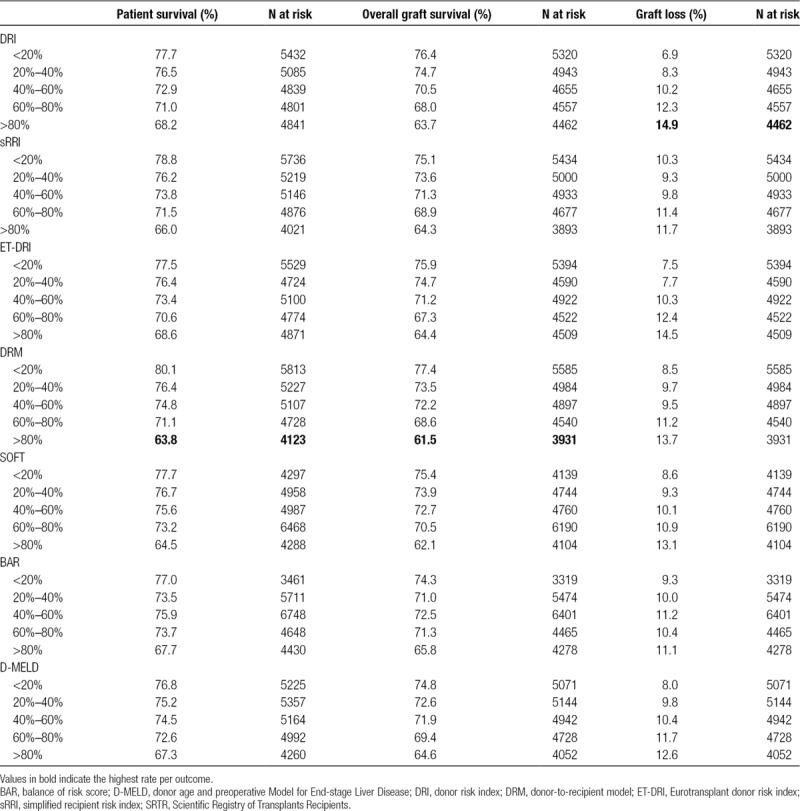
FIGURE 2.
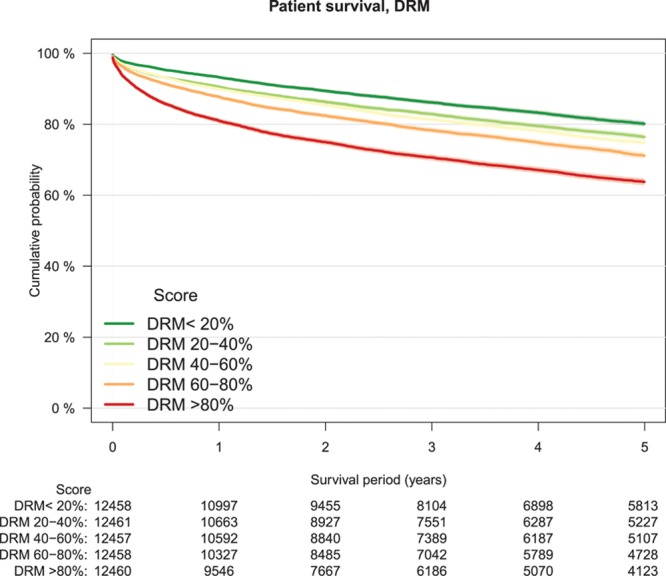
Patient survival by DRM risk groups, Kaplan–Meier analysis. DRM, donor-to-recipient model.
FIGURE 3.
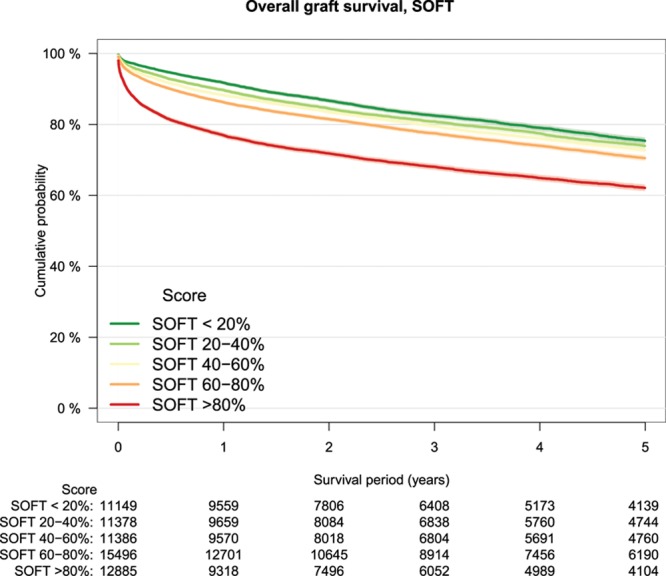
Overall graft survival by SOFT risk groups, Kaplan–Meier analysis. SOFT, Survival Outcomes Following Liver Transplantation score.
FIGURE 4.
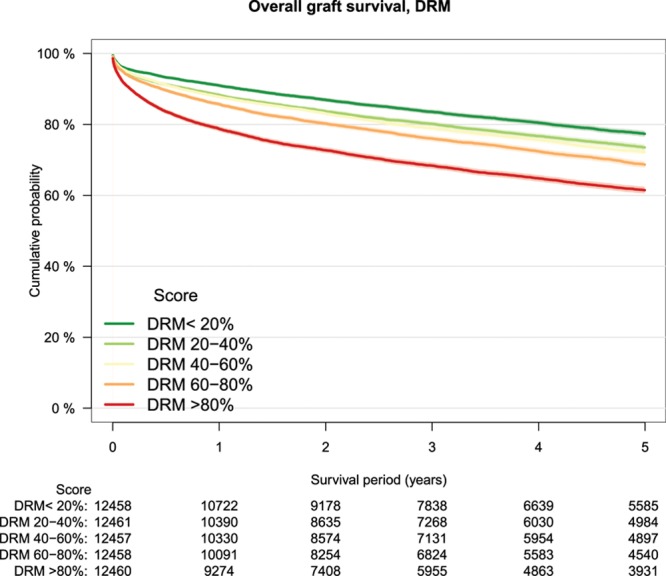
Overall graft survival by DRM risk groups, Kaplan–Meier analysis. DRM, donor-to-recipient model.
FIGURE 5.
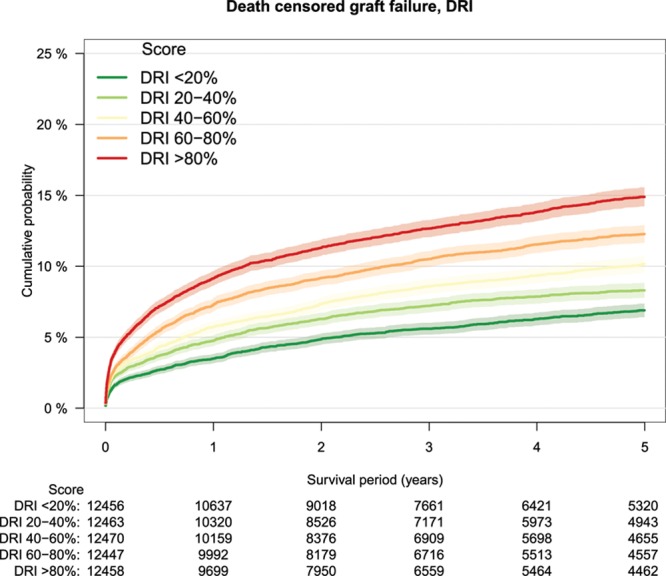
Death-censored graft survival by DRI risk groups, Kaplan–Meier analysis. DRI, donor risk index.
FIGURE 6.
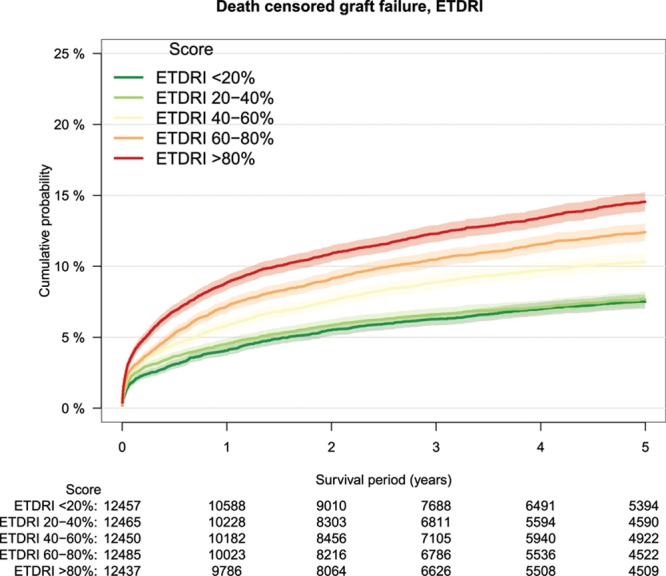
Death-censored graft survival by ET-DRI risk groups, Kaplan–Meier analysis. ET-DRI, Eurotransplant donor risk index.
DISCUSSION
Predicting outcome after LT is important for issues varying from quality control to decision-making for liver offers. It could even be important for improving allocation algorithms. Therefore, several prediction models have been proposed in the past decade. This study has evaluated their performance with SRTR data, when applied to patient-, overall graft-, and death-censored graft survival at different posttransplant follow-up periods. Our results show that models that predominantly constitute of recipient characteristics have the best performance at predicting (short-term) patient survival. Models that include a combination of donor and recipient characteristics, like the SOFT and DRM, have a better performance for predicting overall graft survival. Death-censored graft survival is best predicted by a model that predominantly constitutes of donor factors, as in the DRI and ET-DRI.
To evaluate the efficacy of LT, overall graft survival might be the most suitable outcome measure. This end point covers patient mortality as well as survival of the graft, which is as important in the light of the current organ shortage. Both the DRM and SOFT scores, which include donor and recipient characteristics, have the highest predictive value for this outcome at long-term follow-up (c-index: 0.60). However, the highest overall predictive performance was observed for short-term patient survival. Both the SOFT and BAR scores achieved c-indexes of 0.68 and 0.64, respectively, for predicting patient survival at 3-month follow-up.
Our results show that when the follow-up period increases, the accuracy of the prediction of posttransplant outcome decreases. This increasing uncertainty is most likely the result of the input for the models; the prediction is based on factors that are defined at the time of or just before the transplantation. The initial strong relation with short-term complications or early mortality after transplantation decreases rapidly after the transplantation. Issues like changes in therapy, unexpected events, or medical compliance are therefore not taken into account. Models that predict short-term outcomes are therefore more likely to achieve higher c-indexes as compared with models that focus on long-term survival.16 Our results also show that the performance of posttransplant outcome decreases when used for other end points than they were developed for. This applies to the respective outcome as well as the considered follow-up period.
The maximal c-indexes that can be achieved by incorporating all factors of the respective models are promising and indicate that current models may be further improved. It is to be noted that in these maximum models, the effects of each factor are calibrated for each time point separately. The SRTR has made an effort to do so by analyzing their entire dataset and all variables.17 They have developed models for patient and overall graft survival at 1- and 3-year follow-up. These 4 models include between 40 and 48 factors and incorporate between 165 and 204 coefficients.17 They are updated periodically and can be used to correct center-specific outcomes.18 Although the extent of the data and analyses are impressive, the number of coefficients and the required data pose challenges for other transplant organizations to use them. The 1-year SRTR models for patient and graft survival in adults achieved c-indexes at 1-year follow-up of 0.677 and 0.664, respectively (data SRTR).19
Our results are in line with published results on the performance of all models when they are applied to their initial end points. For patient survival at 90-day follow-up, the SOFT score has a reported predictive capacity of 0.76,8 (c-index of 0.68 in this study) and the BAR score of 0.66–0.748,20-25 (c-index of 0.64 in this study). In one study, a c-index of 0.8 was reported for both the BAR and SOFT scores.26 The D-MELD was also developed for patient survival. It has a relatively low reported predictive capacity, most likely because of its simplicity and because it is often applied to short-term outcomes.8,23,24,27-29 To predict graft survival at long-term follow-up, the DRM model has been developed in the ET region. It has a reported c-index of 0.62 to predict 5-year graft survival11 in the ET database (c-index of 0.58 in this study). In calculating the DRM, GGT and rescue allocation were not available and were therefore set at reference in this study. Most likely the c-index would be higher if these factors had been available to get a more accurate DRM value. Models that solely include donor factors like the ET-DRI and DRI provide a suboptimal predictive capacity for long-term overall graft survival when used without adjustment for recipient characteristics as indicated by a c-index below 0.6.8,23,24,30-32 These models, however, have the best performance for predicting death-censored graft survival. Such donor models can therefore be considered as a measure for the quality of the organ itself.
We have chosen to validate the risk models in the United Network for Organ Sharing database, because it is the most complete and extensive database available. Therefore, most risk models could be calculated correctly, except for the ET-DRI. The ET-DRI, also used for the DRM, contains 2 factors (rescue allocation and GGT) that were not available. While most studies focus on patient survival at short-term follow-up, this study has analyzed patient, overall, and death-censored graft survival with the follow-up period as a continuous variable. The findings from this study, an objective comparison of models in a large dataset, may be used as a reference to choose an appropriate model.
In comparing center-specific outcomes, risk models may be used to take potential differences in donor and recipient characteristics (case-mix) into account.18,33 When outcomes of individual transplant centers are not adjusted for donor quality, available “high-risk” liver allografts are likely less used. Effects of a focus on absolute outcomes seems to be already more present in the United States than in Europe; although utilization rates of available livers seem to be similar between both, the quality of transplanted livers is not.34-36 European transplant centers tend to accept livers that have a higher mean donor age and have more comorbidities on average.37,38
Besides an application in evaluating center-specific outcomes, risk models could also have a great value for improving allocation algorithms. The modest discriminative accuracy of risk prediction models is currently the most important concern.22,39 It is important to note that c-statistics represent the accuracy of a model to predict in what order individual patients will experience an event. Models may therefore have limited use for individual patients but might define risk factor strata very well. Such findings have been published for the widely used Gail model for breast cancer. It is reported to have a modest discriminatory accuracy (c-index: 0.58) but a good fit in the dataset.40,41 Currently, liver allocation in the United States and Europe is performed using the (Na-)MELD score.3 This algorithm does not take into account outcome after transplantation. Models for outcome after LT could therefore increase the overall survival benefit42 by balancing the estimated posttransplantation outcome with the expected outcome on the waiting list by the MELD score.43 For LT, the risk models may not be perfect, but they might represent the most accurate objective prediction of outcome that is currently available. Therefore, incorporating estimated survival at 3-month follow-up (with a c-statistic over 0.7) might provide a good start. We should, however, strive to further improve the performance of these models. This might be done by including more direct (bio) data. Such data may become available with the introduction of machine perfusion.20,44 Also, a more detailed characterization of patients may be incorporated, for example, by including the frailty index or the degree of sarcopenia.45-48
CONCLUSIONS
This study has validated the performance of 7 risk models in the perspective of different LT end points. The accuracy of predicting posttransplant outcome decreases when the follow-up period increases. Models dominated by recipient variables have the best performance for predicting short-term patient survival. Overall graft survival is best predicted by the DRM and SOFT scores, models that combine donor and recipient characteristics. The DRI and ET-DRI best predict death-censored graft survival and can therefore best describe donor quality.
ACKNOWLEDGMENTS
The authors thank Mr. Bryn Thompson for help with the SRTR database. The data reported here have been supplied by the Minneapolis Medical Research Foundation as the contractor for the SRTR. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government.
Supplementary Material
Footnotes
Published online 22 May, 2019.
J.D.B., A.E.B., J.J.B., and H.P. participated in study concept and design. J.D.B. participated in acquisition of data. J.D.B., A.E.B., and H.P. participated in statistical analysis and analysis and interpretation of data. J.D.B., J.J.B., and A.E.B. participated in drafting of the manuscript. J.J.B., H.P., I.P.J.A., and B.H. participated in critical revision of the manuscript. A.E.B. participated in study supervision.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
The authors declare no funding or conflicts of interest.
REFERENCES
- 1.Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2016 annual data report: liver. Am J Transplant. 2018;18Suppl 1172–253.. [DOI] [PubMed] [Google Scholar]
- 2.Malinchoc M, Kamath PS, Gordon FD, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–871.. [DOI] [PubMed] [Google Scholar]
- 3.Wiesner R, Edwards E, Freeman R, et al. ; United Network for Organ Sharing Liver Disease Severity Score Committee. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91–96.. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki H, Bartlett AS, Muiesan P, et al. High model for end-stage liver disease score as a predictor of survival during long-term follow-up after liver transplantation. Transplant Proc. 2012;44:384–388.. [DOI] [PubMed] [Google Scholar]
- 5.Desai NM, Mange KC, Crawford MD, et al. Predicting outcome after liver transplantation: utility of the model for end-stage liver disease and a newly derived discrimination function. Transplantation. 2004;77:99–106.. [DOI] [PubMed] [Google Scholar]
- 6.Rana A, Hardy MA, Halazun KJ, et al. Survival outcomes following liver transplantation (SOFT) score: a novel method to predict patient survival following liver transplantation. Am J Transplant. 2008;8:2537–2546.. [DOI] [PubMed] [Google Scholar]
- 7.Halldorson JB, Bakthavatsalam R, Fix O, et al. D-MELD, a simple predictor of post liver transplant mortality for optimization of donor/recipient matching. Am J Transplant. 2009;9:318–326.. [DOI] [PubMed] [Google Scholar]
- 8.Dutkowski P, Oberkofler CE, Slankamenac K, et al. Are there better guidelines for allocation in liver transplantation? A novel score targeting justice and utility in the model for end-stage liver disease era. Ann Surg. 2011;254:745–53.; discussion 753. [DOI] [PubMed] [Google Scholar]
- 9.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–790.. [DOI] [PubMed] [Google Scholar]
- 10.Braat AE, Blok JJ, Putter H, et al. ; European Liver and Intestine Transplant Association (ELITA) and Eurotransplant Liver Intestine Advisory Committee (ELIAC). The eurotransplant donor risk index in liver transplantation: ET-DRI. Am J Transplant. 2012;12:2789–2796.. [DOI] [PubMed] [Google Scholar]
- 11.Blok JJ, Putter H, Rogiers X, et al. ; Eurotransplant Liver Intestine Advisory Committee. Combined effect of donor and recipient risk on outcome after liver transplantation: research of the eurotransplant database. Liver Transpl. 2015;21:1486–1493.. [DOI] [PubMed] [Google Scholar]
- 12.Blok JJ, Putter H, Metselaar HJ, et al. Identification and validation of the predictive capacity of risk factors and models in liver transplantation over time. Transplant Direct. 2018;4:e382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapter 5-ET Liver Allocation System (ELAS). Eurotransplant manual [Eurotransplant Web site]. Available at https://www.eurotransplant.org/cms/index.php?page=et_manual. Accessed February 2018.
- 14.van Houwelingen HC, Putter H. Dynamic Prediction in Clinical Survival Analysis. 20121st ed CRC Press. [Google Scholar]
- 15.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26:2389–2430.. [DOI] [PubMed] [Google Scholar]
- 16.Ma Y, Wang Q, Yang J, et al. Comparison of different scoring systems based on both donor and recipient characteristics for predicting outcome after living donor liver transplantation. Plos One. 2015;10:e0136604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.SRTR. Risk adjustment models posttransplant outcomes [SRTR Web site]. Available at https://www.srtr/org/reports-tools/risk-adjustment-models-posttransplant-outcomes/. Accessed October 23, 2018.
- 18.Kasiske BL, Wey A, Salkowski N, et al. Seeking new answers to old questions about public reporting of transplant program performance in the united states. Am J Transplant. 2019;19:317–323.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wey A, Salkowski N, Kasiske BL, et al. The relationship between the C-statistic and the accuracy of program-specific evaluations. Am J Transplant. 2019;19:407–413.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golse N, Guglielmo N, El Metni A, et al. Arterial lactate concentration at the end of liver transplantation is an early predictor of primary graft dysfunction. Ann Surg. 2018. doi:10.1097/SLA.0000000000002726. [DOI] [PubMed] [Google Scholar]
- 21.de Campos Junior ID, Stucchi RS, Udo EY, et al. Application of the BAR score as a predictor of short- and long-term survival in liver transplantation patients. Hepatol Int. 2015;9:113–119.. [DOI] [PubMed] [Google Scholar]
- 22.Schrem H, Platsakis AL, Kaltenborn A, et al. Value and limitations of the BAR-score for donor allocation in liver transplantation. Langenbecks Arch Surg. 2014;399:1011–1019.. [DOI] [PubMed] [Google Scholar]
- 23.Schlegel A, Linecker M, Kron P, et al. Risk assessment in high- and low-MELD liver transplantation. Am J Transplant. 2017;17:1050–1063.. [DOI] [PubMed] [Google Scholar]
- 24.Åberg F, Nordin A, Mäkisalo H, et al. Who is too healthy and who is too sick for liver transplantation: external validation of prognostic scores and survival-benefit estimation. Scand J Gastroenterol. 2015;50:1144–1151.. [DOI] [PubMed] [Google Scholar]
- 25.Jochmans I, Monbaliu D, Pirenne J. The balance of risk score for allocation in liver transplantation. Ann Surg. 2014;259:e34. [DOI] [PubMed] [Google Scholar]
- 26.Conjeevaram Selvakumar PK, Maksimak B, Hanouneh I, et al. Survival outcomes scores (SOFT, BAR, and pedi-SOFT) are accurate in predicting post-liver transplant survival in adolescents. Pediatr Transplant. 2016;20:807–812.. [DOI] [PubMed] [Google Scholar]
- 27.Schrem H, Reichert B, Frühauf N, et al. The donor-risk-index, ECD-score and D-MELD-score all fail to predict short-term outcome after liver transplantation with acceptable sensitivity and specificity. Ann Transplant. 2012;17:5–13.. [DOI] [PubMed] [Google Scholar]
- 28.Briceño J, Cruz-Ramírez M, Prieto M, et al. Use of artificial intelligence as an innovative donor-recipient matching model for liver transplantation: results from a multicenter Spanish study. J Hepatol. 2014;61:1020–1028.. [DOI] [PubMed] [Google Scholar]
- 29.Costabeber AM, Lionço LC, Marroni C, et al. D-MELD does not predict post-liver transplantation survival: a single-center experience from Brazil. Ann Hepatol. 2014;13:781–787.. [PubMed] [Google Scholar]
- 30.Reichert B, Kaltenborn A, Goldis A, et al. Prognostic limitations of the eurotransplant-donor risk index in liver transplantation. J Negat Results Biomed. 2013;12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salgia RJ, Goodrich NP, Marrero JA, et al. Donor factors similarly impact survival outcome after liver transplantation in hepatocellular carcinoma and non-hepatocellular carcinoma patients. Dig Dis Sci. 2014;59:214–219.. [DOI] [PubMed] [Google Scholar]
- 32.Winter A, Féray C, Audureau E, et al. External validation of the donor risk index and the eurotransplant donor risk index on the french liver transplantation registry. Liver Int. 2017;37:1229–1238.. [DOI] [PubMed] [Google Scholar]
- 33.Blok JJ, de Boer JD, Putter H, et al. ; Eurotransplant Liver Intestine Advisory Committee. The center effect in liver transplantation in the eurotransplant region: a retrospective database analysis. Transpl Int. 2018;31:610–619.. [DOI] [PubMed] [Google Scholar]
- 34.Halazun KJ, Rana AA, Fortune B, et al. No country for old livers? Examining and optimizing the utilization of elderly liver grafts. Am J Transplant. 2018;18:669–678.. [DOI] [PubMed] [Google Scholar]
- 35.Rana A, Sigireddi RR, Halazun KJ, et al. Predicting liver allograft discard: the discard risk index. Transplantation. 2018;102:1520–1529.. [DOI] [PubMed] [Google Scholar]
- 36.Blok JJ, Braat AE, Adam R, et al. ; European Liver Intestine Transplant Association Eurotransplant Liver Intestine Advisory Committee; Eurotransplant Liver Intestine Advisory Committee. Validation of the donor risk index in orthotopic liver transplantation within the eurotransplant region. Liver Transpl. 2012;18:112–119.. [DOI] [PubMed] [Google Scholar]
- 37.Ghinolfi D, Lai Q, Pezzati D, et al. Use of elderly donors in liver transplantation: A paired-match analysis at a single center. Ann Surg. 2018;268:325–331.. [DOI] [PubMed] [Google Scholar]
- 38.de Boer JD, Blok JJ, Putter H, et al. ; Eurotransplant Liver and Intestine Advisory Committee. Optimizing the use of geriatric livers for transplantation in the eurotransplant region. Liver Transpl. 2019;25:260–274.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Avolio AW, Halldorson JB, Burra P, et al. Balancing utility and need by means of donor-to-recipient matching: a challenging problem. Am J Transplant. 2013;13:522–523.. [DOI] [PubMed] [Google Scholar]
- 40.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–1886.. [DOI] [PubMed] [Google Scholar]
- 41.Rockhill B, Spiegelman D, Byrne C, et al. Validation of the Gail et al. Model of breast cancer risk prediction and implications for chemoprevention. J Natl Cancer Inst. 2001;93:358–366.. [DOI] [PubMed] [Google Scholar]
- 42.Schaubel DE, Sima CS, Goodrich NP, et al. The survival benefit of deceased donor liver transplantation as a function of candidate disease severity and donor quality. Am J Transplant. 2008;8:419–425.. [DOI] [PubMed] [Google Scholar]
- 43.Magder LS, Regev A, Mindikoglu AL. Comparison of seven liver allocation models with respect to lives saved among patients on the liver transplant waiting list. Transpl Int. 2012;25:409–415.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faitot F, Besch C, Battini S, et al. Impact of real-time metabolomics in liver transplantation: graft evaluation and donor-recipient matching. J Hepatol. 2018;68:699–706.. [DOI] [PubMed] [Google Scholar]
- 45.Englesbe MJ, Patel SP, He K, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211:271–278.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lai JC, Feng S, Terrault NA, et al. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant. 2014;14:1870–1879.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kahn J, Wagner D, Homfeld N, et al. Both sarcopenia and frailty determine suitability of patients for liver transplantation—A systematic review and meta-analysis of the literature. Clin Transplant. 2018;32:e13226. [DOI] [PubMed] [Google Scholar]
- 48.Hamaguchi Y, Kaido T, Okumura S, et al. Impact of quality as well as quantity of skeletal muscle on outcomes after liver transplantation. Liver Transpl. 2014;20:1413–1419.. [DOI] [PubMed] [Google Scholar]


