Abstract
The mTOR pathway has a central role in the regulation of cell metabolism, growth and proliferation. Studies involving selective gene targeting of mTOR complexes (mTORC1 and mTORC2) in renal cell populations and/or pharmacologic mTOR inhibition have revealed important roles of mTOR in podocyte homeostasis and tubular transport. Important advances have also been made in understanding the role of mTOR in renal injury, polycystic kidney disease and glomerular diseases, including diabetic nephropathy. Novel insights into the roles of mTORC1 and mTORC2 in regulation of immune cell homeostasis and function are helping to improve understanding of the complex effects of mTOR targeting on immune responses, including those that impact both de novo renal disease and renal allograft outcomes. Extensive experience in clinical renal transplantation has resulted in successful conversion of patients from calcineurin inhibitors to mTOR inhibitors at various times post-transplantation, with excellent long-term graft function. Widespread use of this practice has, however, been limited owing to mTOR-inhibitor-related toxicities. Unique attributes of mTOR inhibitors include reduced rates of squamous cell carcinoma and cytomegalovirus infection compared to other regimens. As understanding of the mechanisms by which mTORC1 and mTORC2 drive the pathogenesis of renal disease progresses, clinical studies of mTOR pathway targeting will enable testing of evolving hypotheses.
Introduction
Since the discovery of rapamycin (also known as sirolimus more than 40 years ago,1 advances in the understanding of its molecular mode of action as well as the functional biology of its primary target — mTOR — have permeated many areas of medicine, including cardiovascular disease, autoimmunity and cancer. mTOR is an evolutionarily-conserved serine-threonine kinase that regulates cell growth, proliferation and metabolism. Increasing evidence indicates that mTOR has an important role in the regulation of renal cell homeostasis and autophagy. Moreover, this kinase has been implicated in the development of glomerular disease, polycystic kidney disease (PKD), acute kidney injury (AKI) and kidney transplant rejection.
The development of rapamycin and its analogues (known as rapalogstemsirolimus and everolimus, has expanded the pharmacological armamentarium for treatment of renal disease. Owing to its ability to potently inhibit T cell proliferation, rapamycin was initially developed as an immunosuppressive agent in kidney transplantation.2 Rapalogs have now also been added to the immunosuppressive repertoire for glomerulonephritides (although not a therapeutic mainstay for these conditions) and renal cell carcinoma. In this Review, we discuss aspects of mTOR function and its inhibition in relation to renal physiology, kidney disease including malignancy, and the role of mTOR complexes and their inhibitors in renal transplantation.
mTOR complexes
mTOR operates in at least two distinct, multi-protein complexes: mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) (FIG. 1). Details of the structural biochemistry of mTOR and role in cellular signalling have been reviewed in detail elsewhere.3–5 mTORC1 is often described as a ‘nutrient sensor’ as it can be activated by amino acids and inhibited by severe oxidative stress and energy depletion. The primary roles of mTOR are to facilitate cell growth and anabolism as well as to prevent autophagy. Although mTORC1 was localized initially to the cytoplasm, this complex has since been identified in association with endosomal compartments (outer mitochondrial membranes and nuclei6–8) and has been shown to have a role in stress granule formation.9 These findings provide further evidence that mTOR is a ‘metabolic rheostat’ for eukaryotic cells.
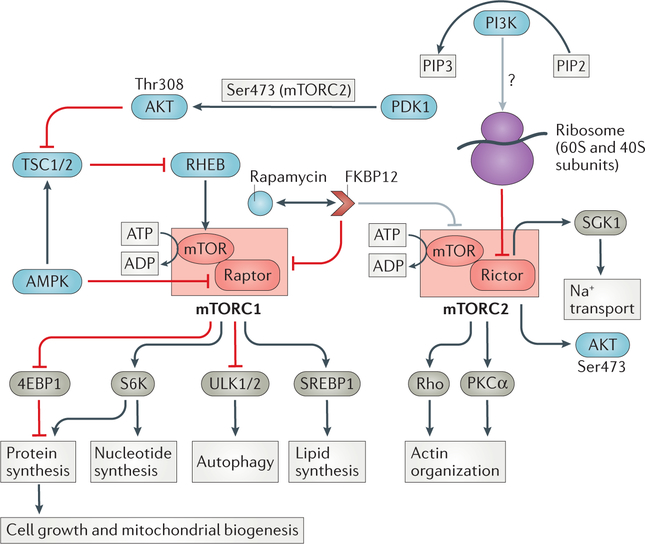
Figure 1. mTOR complex biology.
RAPA-sensitive mTOR complex 1 (mTORC1) is composed of mTOR in association with regulatory associated protein of mTOR (RAPTOR) as well as other proteins not shown here (mammalian lethal with Sec13 protein 8, proline-rich substrate of Akt of 40 kD and DEP domain-containing mTOR-interacting protein). mTORC1 is regulated by environmental cues (nutrients, growth factors and energy) to drive cell growth and metabolism. Many signalling pathways converge on the tumour suppressors tuberous sclerosis complex 1 (TSC1) and TSC2, a GTPase activating protein and major negative regulator of RHEB (Ras homologue enriched in brain), that directly stimulates mTORC1. The two main downstream targets of mTORC1 are p70 ribosomal S6 kinase (S6K) and 4E-binding protein 1 (4EBP1); their phosphorylation by mTORC1 drives ribosome synthesis, cap-dependent translation and cell growth. The transcription factor sterol regulatory element binding protein 1 (SREBP1) is also activated by mTORC1 and regulates lipid synthesis. Rapamycin-insensitive mTOR-containing complex 2 (mTORC2) lacks rAPTOR but has rapamycin-insensitive companion of mTOR (RICTOR) as an essential component. Known substrates of mTORC2 include AKT and the serum and glucocorticoid-induced kinase-1 (SGK1). PDK1 enhances Akt activity by phosphorylating the activation loop at threonine 308. mTORC2 uniquely stabilizes Akt via phosphorylation of the turn motif at serine 450 (not shown), and further stimulates Akt kinase activity by phosphorylating the hydrophobic motif at serine 473. mTORC2 controls fundamental cellular processes including metabolism, differentiation, cell cycle arrest and DNA repair. Ribosomes have been found to physically associate with mTORC2. Rapamycin and rapalogs form complexes with FKBP12 and acutely inhibit mTORC1 assembly, whereas inhibition of mTORC2 assembly requires chronic exposure and is inconsistent across cell types.
Growth factors and cytokines can activate mTORC1 via upstream signalling through phosphoinositide 3-kinase (PI3K). Generation of phosphatidylinositol (3,4,5) triphosphate (PIP3) by PI3K activates 3-phosphoinositide-dependent protein kinase-1 (PDK1), which enhances Akt (also known as protein kinase B) activity by phosphorylating the activation loop at threonine 308. Interestingly, mTORC2 uniquely stabilizes Akt via phosphorylation of the turn motif at serine 450, and further stimulates Akt kinase activity by phosphorylating the hydrophobic motif at serine 473. The activity of tuberous sclerosis complex 1 (TSC1) and TSC2, the major upstream inhibitors of mTORC1, is dampened directly by Akt-dependent phosphorylation and provides a functional link between the PI3K–Akt signalling axis and mTOR. TSC1 stabilizes and TSC2 inhibits the activity of the guanosine triphosphate (GTP)-ase Rheb (RAS homolog enriched in brain), through its GTPase-activating protein activity. Rheb enhances the catalytic activity of mTORC1 when they are in close proximity.
Tools to study mTORC2 are limited owing to a lack of mTORC2-specific inhibitors. Substrates of mTORC2 include Akt, protein kinase Cα (PKCα) and serum and glucocorticoid-induced kinase-1 (SGK-1). Furthermore, phosphorylation and cytoplasmic sequestration of the forkhead box proteins 01 (FOXO1) and FOXO3 by Akt has been shown to require mTORC2.10 Consistent with these signalling cascades, mTORC2-orchestrated processes include cell survival, protein synthesis, re-organization of the actin cytoskeleton and sodium homeostasis. Although active mTORC2 physically associates with ribosomes in mammalian cells, the intricacies of this pathway have not been completely characterized.11 Importantly, crosstalk between the mTORC1 and mTORC2 pathways12 adds an extra layer of complexity in dissecting mTORC1 and mTORC2 biology. Thus, mTORC1 inhibits the PI3K–mTORC2 pathway through a negative feedback mechanism.
mTOR inhibitors
Although rapamycin immediately inhibits mTORC1, its ability to destabilize and inhibit mTORC2 requires prolonged exposure and is more sensitive to fluctuations in concentrations of the immunophilin FK506 binding protein 12 (FKBP12), which binds rapamycin and mediates its interaction with mTOR 13 The negligible effect of rapamycin on mTORC2 function has been disputed, however, with evidence that this agent might inhibit mTORC2 assembly and signalling.14 The C-terminal part of Avo3, a subunit unique to TORC2 in yeast, is located close to the FKBP12/rapamycin-binding domain of mTORC2 and removal of Avo3 from TORC2 confers sensitivity to rapamycin.15 Although less is known about how rapalogs affect mTORC1 and 2, inhibition of mTORC2 has been demonstrated in acute myeloid leukemia cells exposed to everolimus and temsirolimus.16 Novel dual inhibitors of TORC1 and TORC2 (TORKinibs) that compete for the adenosine triphosphate (ATP)-binding site of mTOR were developed to limit activation of both mTOR complexes17 and provide broader clinical efficacy than the currently available rapalogs. These molecules were first described to inhibit P13K,17 a critical component upstream of the Akt/mTOR pathway, and subsequently also found to inhibit mTOR, presumably owing to sequence homology. Initial TORKinibs (mTOR and PI3K dual inhibitors) failed to selectively inhibit either signalling moiety,18 but newer molecular inhibitors (mTORC1 and mTORC2 dual inhibitors) exhibit greater potency for mTOR.19 These agents are being investigated for the treatment of malignancy in phase I and II clinical trials20 but have yet to be tested in clinical organ transplantation.
mTOR in renal physiology
Given the ubiquitous expression of mTORC1 and mTORC2 in the kidney, elucidating the roles of mTOR in renal physiology is a formidable undertaking. Nonetheless, pharmacologic inhibition and targeted cell-specific genetic deletion have enabled analyses of mTOR function in the rodent and human kidney (FIG 2). Studies of patients with TSC, a syndrome with autosomal dominant inheritance characterized by upregulation of mTORC1, have also increased understanding of the consequences of enhanced mTORC1 activity on renal physiology. Although much of the current understanding of mTOR function pertains to the podocyte or tubular epithelial cell, renal endothelial and immune cells are emerging as important players in the regulation of renal homeostasis and metabolism.
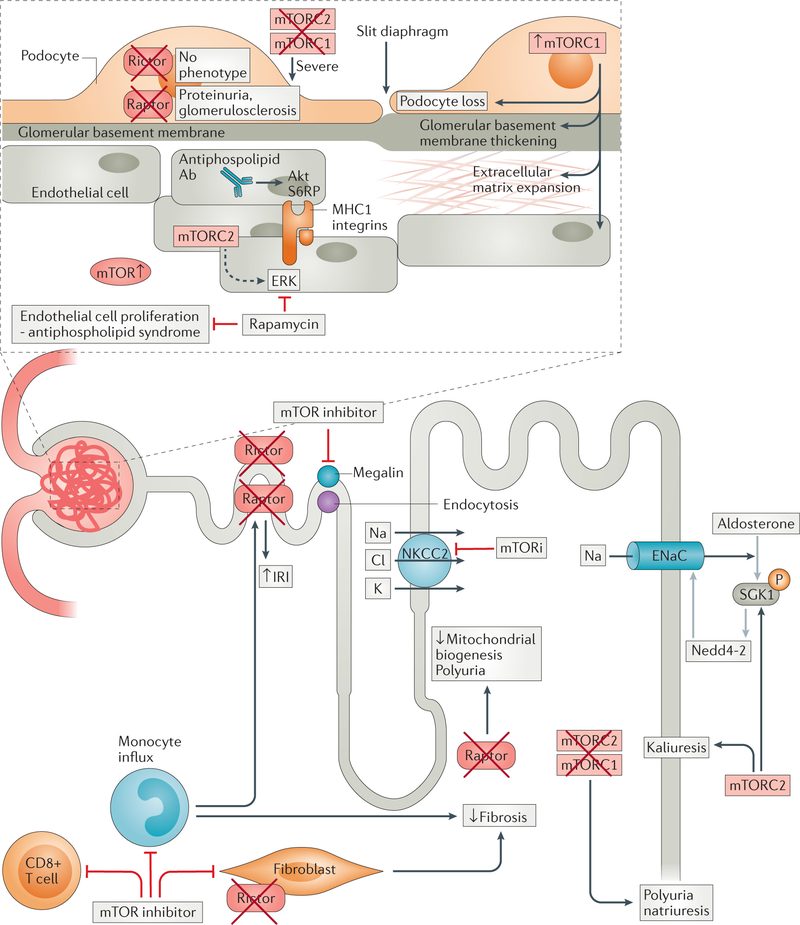
Figure 2. Roles of mTOR complexes in the kidney as revealed using pharmacological inhibition or cell-specific genetic deletion of mTOR1 or mTOR2.
mTOR complexes are expressed throughout the nephron and regulate homeostasis of all resident parenchymal and non-parenchymal cells. Disruption of mTOR signalling results in various pathologies that are dependent on cellular location and whether TORC1 or TORC2 is targeted: proximal TEC downregulate megalin resulting in proteinuria, while distal TEC exposure leads to polyuria and natriuresis, and inflammatory cell infiltrate and fibroblastic responses are reduced following IRI. Within the glomerulus, podocyte function is disrupted leading to frank proteinuria and glomerulosclerosis, while endothelial cell proliferation is limited. Abbreviations: ERK, extracellular signal-regulated kinase; NKCC, Na-K-Cl co-transporter; MR, mineralocorticoid receptor; SGK1, serum and glucocorticoid inducible kinase 1; ENaC, Epithelial sodium channel; IRI, ischemia-reperfusion injury; mTORi, mTOR inhibition.
Podocytes
Glomerular podocytes are highly-differentiated, voluminous cells with numerous foot processes that line the outer aspects of the glomerular basement membrane (GBM). The foot processes interdigitate with each other and form the slit diaphragm, an intercellular junction composed of several membrane proteins that regulate protein movement into the urinary space.21 Although glomerular diseases can be caused by disturbances in various barrier components, podocyte loss and effacement is a critical event that, if not constrained, leads to proteinuria, renal fibrosis and end-stage renal disease (ESRD).
Much of the understanding of podocyte mTORC1 and mTORC2 function stems from genetic deletion studies. In mice, podocyte-specific embryonic knockout of mTORC1 (owing to deletion of the regulatory-associated protein of mTOR (Raptor) (Ed: Yes) resulted in early albuminuria, later development of glomerulosclerosis, weight loss and increased mortality.22 Loss of podocyte mTORC1 in adult mice failed to induce similar deleterious effects. Surprisingly, heterozygous deletion of Raptor in podocytes expression and podocyte volume. Mice with podocyte-specific loss of mTORC2 (owing to deletion of rapamycin-insensitive companion of mTOR (Rictor) did not exhibit significant phenotypic differences compared to littermate controls, with the exception of transient albuminuria following protein overload. However, combined deletion of both mTOR complexes from podocytes precipitated an early (6 weeks of age) fulminant proteinuric phenotype, suggesting some degree of interaction between mTORC1 and mTORC2 in the regulation of podocyte development and homeostasis.
Excessive mTORC1 activity can also result in severe pathologic effects, including hallmarks of diabetic nephropathy. In a murine study, podocyte-specific deletion of Tsc1 led to aberrant mTORC1 activation, enhanced pS6 expression, mesangial expansion, GBM thickening, podocyte loss, podocyte foot-process effacement and proteinuria (that was attenuated by rapamycin), as well as glomerular expression of transforming growth factor β1 (TGF-β1), type IV collagen and fibronectin.23 The remaining podocytes of the transgenic mice had a fibroblastic phenotype with markers of endoplasmic reticulum stress. Immunofluorescence studies demonstrated altered distribution of podocyte nephrin and synaptopodin that was corrected by rapamycin administration.
Tubular epithelial cells
Tubular epithelial cells (TECs) are bathed in urine via their apical membranes and have an important role in maintaining salt and water balance, typically via their basolateral surfaces. This role is accomplished largely via energy-dependent cellular transporters. Initial evidence that mTOR might regulate tubular homeostasis came from repeated observations of electrolyte disturbances in renal transplant recipients treated with rapamycin, although the underlying cause of these disturbances is still disputed. The renal-related adverse effects of rapamycin are typically hypokalaemia,24 hypomagnesaemia and hypophosphataemia,25, 26 as well as decreased urinary calcium excretion.27 Sirolimus decreases the expression of the apical Na-K-2Cl co-transporter (NKCC2) in the thick ascending limb (TAL), resulting in renal tubular wasting of sodium, magnesium and potassium.26 The phosphaturic actions of rapamycin might be linked to the induction of klotho, Rictor and mTORC2, which has been shown in vivo and in an immortalized renal tubular cell line.28 Post-transplant phosphaturia is aggravated by administration of sirolimus, although this effect is reportedly not the result of altered phosphate uptake via sodium–phosphate co-transporters in the brush border of the proximal tubule.29 Nevertheless, in Xenopus oocytes expressing sodium–phosphate transporters, co-transfection with mTOR increased phosphate currents and this effect was abrogated by exposure to rapamycin.30 The past few years have seen several reports linking mTORC1 to proximal tubular transport mechanisms. Work performed mainly in Drosophila melanogaster showed that the proton pump V-ATPase responsible for intracellular compartment acidification and apical amino acid uptake requires mTORC1, This interplay seems to be regulated through the multi-ligand-binding receptor megalin.31 Indeed, in C57BL/6 mice, long-term rapamycin exposure reduced proximal tubular megalin expression and caused concurrent low molecular weight proteinuria, consistent with renal proximal TEC dysfunction.
Most recently, however, it has been demonstrated that mice deficient in proximal tubular mTORC1 present with a Fanconi Syndrome-like phenotype consisting of phosphaturia, glucosuria, low-molecular weight proteinuria, albuminuria and aminoaciduria.32 Deep proteomic and phosphoproteomic analysis shows that mTORC1 deficiency affects the translation and phosphorylation of specific transport systems, its regulators as well as proteins involved in endocytosis in a kinase-dependent manner. Interestingly, and in contrast to the earlier study,31 no alterations in the abundance of the two scavenger receptors cubilin and megalin could be detected.
In mice, specific deletion of Raptor (mTORC1) in distal TECs did not cause significant developmental defects, but these animals developed polyuria and hypercalciuria after weaning as a result of a defect in TAL countercurrent multiplication33. Consistent with these perturbations, transcriptional profiling identified reduced levels of NKCC2 in these mice. Generation of mTORC1/2-deficient animals resulted in aggravated pathology and increased mortality, suggesting that these complexes have complimentary roles.
Although the molecular pathways downstream of mTORC1 and mTORC2 that regulate ion channels in the TAL (such as NKCC2) remain to be determined, significant advances have occurred in the understanding of how mTOR regulates the aldosterone-sensitive epithelial sodium channel (ENaC), a protein that is most abundant in the distal tubule. Serum and glucocorticoid inducible kinase 1 (SGK1) acts as a central driver of this process by increasing cell surface expression of ENaC via inhibition of the E3 ubiquitin-protein ligase Nedd4–234 and mTORC2 has been found to activate SGK1 via phosphorylation of its hydrophobic motif.35
To investigate the physiological significance of the mTORC2–SGK1–ENaC axis in vivo, researchers compared the effect on electrolyte homeostasis of targeting mTORC1 (using rapamycin) with that of dual inhibition of mTORC1 and mTORC2 (using the TORKinibs PP242 and AZD8055).36 Interestingly, the TORKinibs uniquely induced polyuria and substantial natriuresis without kaliuresis (compared to rapamycin) in wild-type mice. This effect was lost, however, when PP242 and the ENaC blocker amiloride were administered together, suggesting that the natriuretic effects of TORKinibs occur upstream of the ENaC. Conflicting data came from a study that used a mouse genetic approach to examine the role of mTORC2 in the aldosterone-sensitive distal nephron.37 In contrast to the findings of the subtractive pharmacological inhibitor experiments, mice with mTORC2 deficiency (as a result of Rictor deletion) developed life-threatening hyperkalaemia when challenged with a high K+ diet or the K+-sparing diuretic triamterene. Patch-clamp recordings of cortical collecting ducts revealed an absence of barium-sensitive K+ currents. Phosphorylation of the hydrophobic motif of SGK1 and PKCα was virtually absent and membrane expression of renal outer medullary K+ channels greatly reduced in the Rictor-deficient animals. The findings of Grahammer et al37 essentially show that mTORC2 drives kaliuresis and is not a prerequisite for Na reabsorption. The equation: effect with TORKinib minus effect with rapamycin= mTORC2-specific effect, is certainly too simplistic and in most instances is wrong and misleading. It rather seems that inhibiting both mTORC1 and mTORC2 vitally challenges any cell examined so far. To obtain true insight into mTORC2-specific effects, the only possibility is to use a genetic approach, at least until mTORC2-specific inhibitors are available. The findings underline the necessity to combine genetic deletion and pharmacological inhibition approaches for a full appraisal of the functional importance of mTOR complexes.
Renal endothelial cells
Diseases such as thrombocytopaenic purpura (TTP), haemolytic uraemic syndrome (HUS), pre-eclampsia, anti-phospholipid antibody syndrome (APS) and antibody-mediated kidney allograft rejection are driven partially by endothelial cell activation and injury. Conceptualizing mTOR as a driver of vascular endothelial proliferation and immune activation has developed over the past ten years. Activation of the mTORC pathway has been observed in the intra-renal vessels of patients with APS nephropathy.38 Immunofluorescent staining of endothelium from the renal vessels (but excluding glomerular endothelium of individuals with primary or secondary APS identified phosphorylation of S6 ribosomal protein (S6RP, indicating mTORC1 activation) and Akt (indicating mTORC2 activation),38 often with colocalization. Incubation of human microvascular endothelial cells with antiphospholipid antibody led to upregulation of SR6P and Akt phosphorylation in vitro, a phenomenon that was prevented by treatment with the TORkinib PP242. Interestingly, patients who received sirolimus after transplantation showed reduced vascular proliferation and lesion recurrence on biopsy, compared to those receiving alternative immunosuppression. Use of mTOR inhibitors was also associated with a higher incidence of functioning allografts up to 12 years post-transplantation.
The development of transplant vasculopathy is induced by antibody binding to HLA class I molecules expressed by endothelial and smooth muscle cells, which promotes their proliferation. The effect of these antibodies on endothelial cell proliferation has been verified in vitro and is mediated by formation of mTORC1 and mTORC2.39 Downregulation of endothelial mTOR using small interfering RNA (siRNA) against Rictor or Raptor blocked HLA class I antibody-induced endothelial cell proliferation, whereas administration of rapamycin prevented HLA class I antibody-induced Akt phosphorylation. Additional studies have demonstrated TORC2-mediated extracellular kinase (ERK) phosphorylation induced by endothelial-cell-based MHC class I and integrin ligation,40 which was inhibited by rapamycin.
Although the role of mTOR in endothelial cell proliferation might imply a benefit of mTOR inhibitors in kidney transplant recipients, these agents have also been implicated in the development of donor-specific antibodies (DSAs) that might precipitate antibody-mediated rejection (ABMR). Both sirolimus41 and everolimus42 are associated with the development of de novo DSAs and subsequent onset of AMR.42, 43 Although endothelial mTOR pathway activation has been implicated in acute and chronic AMR in rodent and human studies,20, 39, 44, 45 the therapeutic efficacy of mTOR inhibitors in these settings remains unclear. Potential explanations for these conflicting findings might include reduced potency of mTOR inhibitors compared to CNIs, for T cell subsets that regulate AMR and higher rates of intolerable side effects with mTOR inhibitors that may limit compliance and, as a consequence, increase allograft rejection.
Regulation of autophagy
Autophagy is a catabolic process that degrades various substrates including misfolded proteins, damaged organelles and microorganisms. This material is sequestered in autophagosomes prior to breakdown in lysosomes. In most circumstances autophagy promotes cell survival and is important when a cell faces environmental stress and must use cellular components as an energy source.46 By contrast, the mTOR pathway is most active in nutrient-replete environments. Thus, autophagy and mTOR can be envisaged as opposing molecular pathways that negatively regulate each another.
Importantly, mechanisms by which mTOR regulates autophagy have been described over the past ten years. Three groups have demonstrated that mTORC1 inhibits the UNC-51-like autophagy-activating kinase (ULK) complex by phosphorylating ULK1/2 and other components47–49. In mammalian cells phosphorylation of ULK1/2 at Ser758 by mTORC1 prevents ULK1/2 activation by the energy sensor AMP-activated protein kinase (AMPK). mTORC1 has also been found to regulate autophagy at the transcriptional level via phosphorylation and cytoplasmic sequestration of transcription factor EB (a regulator of lysosomal and autophagy genes) and by inhibition of PI3K catalytic subunit type 3 (also known as VPS34), which has an important role in autophagosome formation.46 Whether mTORC2 regulates autophagy directly, independent of mTORC1, remains unclear.
Given the high metabolic demands and autophagic flux that exist in podocytes and TEC, the role of autophagy in renal health and disease is now under investigation.50 In ageing mice, podocyte-specific deletion of the autophagy inducer, autophagy protein 5 (Atg5), led to the accumulation of oxidized protein, podocyte loss, proteinuria and glomerulosclerosis.51 Similarly deletion of Atg5 from the renal epithelial cells (tubules and podocytes) of mice resulted in the development of a disease phenotype resembling human focal segmental glomerulosclerosis (FSGS) by the age of 2–4 months.52 Thus, the concept of ‘autophagic failure’ has become an attractive hypothesis to explain podocytopathies in humans. Renoprotective effects of autophagy in acute kidney injury (AKI) have also been characterized.53 Nonetheless, the beneficial effects of enhanced autophagy induced by mTOR inhibitors are likely to be offset by detrimental effects of these agents on cell growth and survival.
mTOR in renal ageing
Regulation of ageing by mTOR was first noted in studies of S. cerevisiae,54 C. elegans55 and Drosophila melanogaster.56 A subsequent report showed that feeding aged mice with rapamycin extended their lifespan.57 The role of mTOR in ageing has been reviewed previously.58 Age-related alterations in the kidney include accumulation of lipofuscin, damaged mitochondria and protein aggregates, as well as loss of podocytes and autophagosome formation. Atg5, a component of the autophagic pathway regulated by mTOR, is critical for autophagosome creation, and its deletion in specific renal compartments (podocytes or proximal tubules) phenocopies findings seen in aged kidneys.51, 53 Studies in ageing rodent kidneys have shown elevated mTOR expression in whole tissue and isolated primary mesangial cells,59 possibly through regulation of cell cycling by p21 and/or the class III histone deacetylase sirtuin.60 These features are ameliorated, at least in vitro, by treatment with rapamycin. Whether direct manipulation of renal mTOR expression and signal transduction ameliorates features of renal ageing remains to be shown.
mTOR in immune cells
The role of mTOR in regulation of immune cell metabolism, function and reactivity3, 4, 61 has implications for kidney transplant rejection, glomerulonephritides and their treatment.62, 63 Both innate (dendritic cells [DCs] and macrophages) and adaptive immune cells (T and B lymphocytes) reside in the kidney and can promote acute and chronic renal disease,64–66 but their dependence on mTOR activity remains unexplored. Our understanding of the role of mTOR in renal immunity must therefore be extrapolated largely from studies of lymphoid tissue. These observations (FIG. 3 and Table 1) provide insight into the potential of rapalogs, TORKinibs and other PI3K-mTOR pathway inhibitors in transplantation. In parallel, studies in experimental organ transplantation using rodent and non-human primate models, have increased understanding of the complex effects of rapamycin on allograft function.
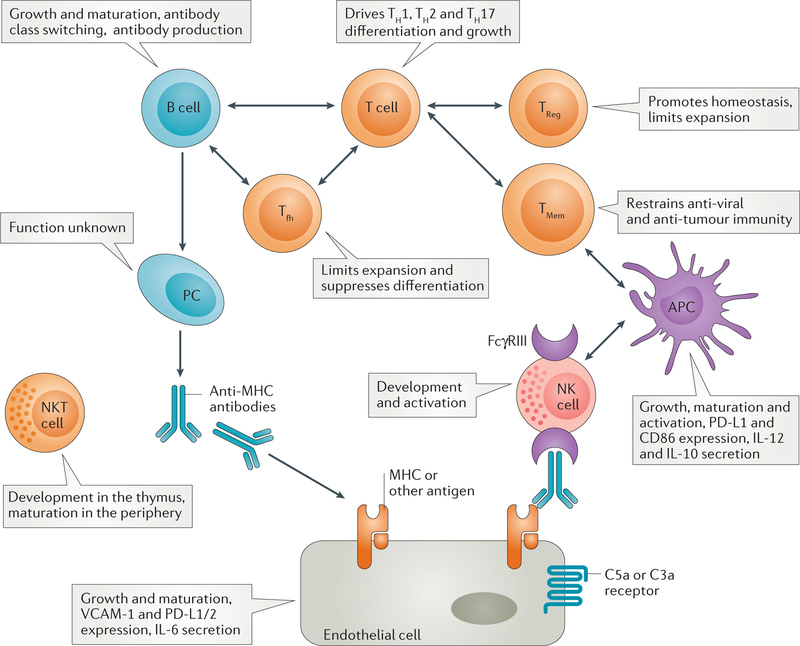
Figure 3. Roles of mTOR in shaping immune and vascular endothelial cell homeostasis.
mTOR shapes antigen-presenting cell (APC), natural killer (NK) cell, T cell, B cell and endothelial cell homeostasis, growth and function. Depicted is the role of mTOR in cells known to be key mediators in the pathogenesis of cell- and antibody-mediated rejection. While our knowledge of mTOR function in T cells and DC is extensive, less is known about how mTOR modulates B cells, Tfh and NK cells. As a result of the pivotal role of mTOR in metabolic programming of all immune cells, global inhibition of this pathway with pharmacologic inhibitors will modulate the immune response in a manner that remains incompletely understood. Given the importance of complement in AMR, C3a and C5a receptor engagement on endothelial cells may affect their proliferation via mTOR-dependent events. Abbreviations: PC=plasma cell; APC encompasses dendritic cells and macrophages; PD-L1, programmed death ligand-1; VCAM, vascular cell adhesion molecule.
Table 1:
Roles of mTOR complexes in immune cell populations and their putative roles in clinical organ transplantation
| Immune cell | Role of mTOR | Putative Role of mTOR in Clinical transplantation |
|---|---|---|
| cDC | Promotes growth, proliferation, maturation, alters pro/anti-inflammatory cytokine secretion,68, 70 suppresses inflammation (mTORC2)72, 297 and restrains T cell responses in human myeloid DC (mTORC1)298,299 | May either promote (eg rejection: ACR) or restrain (eg pneumonitis) immune hyper-responsiveness |
| pDC | Promotes activation (TLR9), type-I IFN production and restrains (TLR7) Tmem and Treg proliferation300, 301 | May regulate function including interaction with Treg |
| Effector T cells | Drives Th1 (mTORC1), Th2 (mTORC2+/−mTORC1), and Th17 (mTORC1) cell differentiation and growth81, 82, 302 |
Promotes expansion with role in rejection (ACR, AMR, CAMR) |
| TFH | Restrains TFH differentiation (mTORC1)83 | May prevent alloantibody formation and AMR |
| CD8 memory T cells | Restrains anti-viral and anti-tumor immunity124, 126, 303 | May promote viral infections, including CMV, human herpesvirus 8 and BK virus127, 304 |
| Treg | Promotes homeostasis and function (mTORC1)80, limits expansion (mTORC1+/−mTORC2)79 | May restrict role in promotion of tolerance |
| Neutrophils | Formation of neutrophil extracellular traps (NETs),305 promotes endothelial cell and extracellular matrix adhesion306 (mTORC1), promotes chemotaxis (mTORC2)307, 308 | May promote role in rejection (ACR, AMR, CAMR) |
| NK cells | Promotes development in the BM, activation in the periphery and IL-15-induced function90 | May promote role in rejection (ACR, AMR, CAMR) |
| NKT Cells | Promotes thymic and peripheral iNKT development (mTORC1)309, promotes thymic and peripheral iNKT development and fate of NKT17 cells (mTORC2)310 | May promote role in rejection (ACR,AMR,CAMR) and restrict role in tolerance |
| B cells | Promotes growth, proliferation, maturation, antibody class switching and production (mTORC1 +mTORC2)86, 87, 311, 312 but decreases IL-7 receptor and RAG recombinase gene expression (mTORC2)313 | May promote role in rejection (ACR, AMR, CAMR) |
| Breg | Unknown | Unknown |
| Plasma Cells | Unknown | Alloantibody generation in AMR/CAMR |
| Endothelial Cells | Promotes growth, proliferation, maturation, alters pro/anti-inflammatory cytokine secretion, negatively regulates Treg expansion and increases VCAM-1 expression (mTORC2)314, 315 | May promote role in rejection (ACR, AMR, CAMR) |
Abbreviations: ACR: acute cell-mediated rejection; AMR: antibody-mediated rejection; BM: bone marrow; Breg: regulatory B cell; CAMR: chronic antibody-mediated rejection; cDC: conventional dendritic cell; CMV: cytomegalovirus; EC: endothelial cell; IFN: interferon; iNKT: Invariant Natural Killer T cell; NK: Natural Killer; NKT: Natural Killer T cell; pDC: plasmacytoid DC; RAG: Recombination-activating gene; RAPA: Rapamycin; TFH: Follicular Helper CD4 T cells; Tmem: memory T cell; TLR: Toll-like receptor; Treg: regulatory T cell; VCAM-1: vascular cell adhesion molecule-1
Dendritic cells
DCs are professional APCs that link the innate and adaptive immune responses by regulating T-cell responses to antigen. In the context of transplantation, they orchestrate both direct and indirect allorecognition. DC density in biopsy samples has been reported to correlate with T-cell proliferation and diminished graft survival in human kidney transplantation.67 The understanding of the role of mTOR in shaping DC function stems largely from studies of the influence of rapamycin on these cells3, 61. Protracted mTORC1 inhibition by rapamycin inhibits maturation of DCs and their ability to promote effector T cell proliferation, while promoting regulatory T cell (Treg) enrichment.68, 69 In a rodent heart transplant model, rapamycin-treated DCs promoted allograft survival following their systemic administration. Paradoxically however, exposure to rapamycin can also enhance the T-cell stimulatory function of DCs with enhanced IL-12p40/p70 production following lipopolysaccharide stimulation.70
Evidence for an immunostimulatory effect of rapamycin on DC function also stems from studies of therapeutic vaccination. Pulsing DCs in vitro with a combination of rapamycin and Toll-like receptor (TLR) agonists enhanced antigen-specific CD8+ T-cell responses and anti-tumour immunity, as well as prolonged DC survival.71 Whether these effects are mediated by mTORC1 or mTORC2 remains unclear, but both genetic deletion and RNA targeting of mTORC2 (Rictor) in DCs induces a hyperinflammatory phenotype72, 73 and promotes alloreactive Th1 and Th17 responses in vitro and in vivo, suggesting that the effects may be mediated by mTORC2.74
T cells
The transition from naive to effector T cells in response to antigen stimulation involves a metabolic switch from primarily oxidative phosphorylation to a more glycolytic pathway.75 This switch enables activated T cells to consume large amounts of glucose and glutamine and undergo rapid proliferation and growth. By contrast, Treg cells, memory T cells and T follicular helper cells (Tfh; a T cell subset that stimulates germinal centre B cells) engage metabolism differently with a propensity to oxidize fatty acids and suppress mTOR through AMPK.
As understanding of T cell metabolism has progressed, distinct roles of mTORC1 and mTORC2 in T cell subset development have also emerged. In mice, both mTORC1 and mTORC2 promote thymic T cell development.76 Administration of rapamycin promotes thymic atrophy, accelerates apoptosis of CD4+CD8+ (immature) T cells and spares CD4+CD25+ Treg cells.77 The ‘sparing’ of Treg cells exposed to mTOR inhibitors has been observed in vivo, in mice and in the peripheral blood of kidney transplant recipients, and might be explained by the differing metabolism of these cells compared to effector T cells.78,79 Paradoxically, specific deletion of mTORC1 (Raptor) in Treg cells results in loss of suppressive activity and fatal inflammation in vivo.80 These results suggest that a basal level of mTOR activity, which might be rapamycin-resistant mTORC1 activity, is required by Treg cells to maintain their function. Furthermore, deletion of mTOR in T cells results in failure to generate Th1, Th2 or Th17 effector cells under skewing conditions, whereas Treg cell differentiation is preserved.81 Skewing of Th1 and Th17 cells has been attributed to mTORC1, whereas Th2 cell differentiation is attributed to mTORC2.82 The phenotype of mTORC2 deletion in T cells somewhat mirrors that in DCs and would be expected to promote rather than inhibit acute transplant rejection. Short hairpin RNA vectors targeted against mTORC1 (Raptor) and mTORC2 (Rictor) differentially altered the distribution of Th1 and Tfh cells; Raptor silencing augmented Tfh differentiation and reduced Th1 cell differentiation, whereas Rictor silencing minimally affected Tfh cells but promoted Th1 cell development.83 These results are relevant to renal transplantation as they could account for the elevations in DSA levels seen in some kidney transplant recipients on rapamycin.
B cells
Everolimus is a potent inhibitor of human B-cell activation and function (that is, T cell-dependent immunoglobulin production).84 Marginal zone (MZ) B cells and humoral immune responses are also affected by mTOR manipulation. Thus, conditional deletion of TSC1 in murine B cells leads to loss of MZ B cells,85 whereas conditional deletion of Rictor (mTORC2) impairs B cell survival and antibody responses.86 On the other hand, two studies that used mTOR inhibitors to dissect the role of mTOR in antibody class switching have produced conflicting results. In mice rapamycin administration promoted cross-strain protection against lethal influenza infection by inhibiting B cell class switching,87 whereas transient treatment with TORKinibs increased titres of class-switched high-affinity antibodies to a hapten-protein conjugate.88 Given these results, coupled with clinical data on the relationship between mTOR inhibitors and DSA production, more basic and clinical studies are necessary to ascertain whether mTOR pathway manipulation protects or promotes ABMR. Data suggest that everolimus inhibits anti-HLA class I antibody-mediated endothelial cell signalling, migration and proliferation more potently than does sirolimus89 pointing to a potential beneficial effect of everolimus in the prevention of chronic ABMR.
NK cells and NKT cells
Studies of the role of mTOR in natural killer (NK) cells and NKT cells have extended our understanding of mTOR immunobiology. Given the important roles that these cells play in DSA formation and chronic ABMR, insights from these studies have direct implications for kidney transplant rejection.
mTOR is an essential component of the IL-15 signalling pathway in NK cells and drives both their development in the bone marrow and their activation in the periphery.90 However, only minimal effects of mTOR inhibition on anti-HLA antibody-dependent NK cell activation have been reported.91 On the other hand, exposure to either rapamycin or the dual mTORC1 and mTORC2 inhibitor PP242 inhibits inflammation-induced priming of induced (i) NK cells. Genetic deletion of either mTORC1 or mTORC2 in iNKT cells indicates that these complexes have important, non-overlapping roles. While mTORC2 is essential for transition from stage 1 to 2 development in the thymus, stage 3 thymic-resident iNKT cell subsets depend more on mTORC1 for survival.92
mTOR inhibitors in transplantation
Experimental organ transplantation
The majority of studies investigating mTOR in experimental organ transplantation have involved administration of rapamycin, either alone or in combination with other agents. Initially, rapamycin monotherapy was shown to prolong organ allograft survival in rodents.93, 94 Importantly, administration of rapamycin late post-transplant halted the progression of allograft vasculopathy in rats and non-human primates (NHPs).95, 96 Attributes of rapamycin reported in murine and NHP transplant models include an ability to induce myeloid-derived suppressor cells, preserve Treg half-life and phenotype post-infusion, and mixed chimerism when combined with costimulation blockade.97, 98
New generation mTOR inhibitors are of considerable potential interest in transplant models. TORKinibs were developed initially to overcome the limitations of rapamycin in clinical oncology. Thus “rapalog resistance”, seen in a variety of tumors treated with rapalogs, is largely attributed to incomplete inhibition of mTORC1 and compensatory activation of mTORC2 Despite rapid incorporation of these drugs into early phase clinical trials in oncology, little is known about their efficacy in transplantation. Theoretical advantages of TORKinbs over rapamycin in this setting include greater inhibition of mTORC2 in Tfh cells, B cells and endothelial cells, all of which orchestrate both DSA formation and chronic allograft vasculopathy. The dual mTOR inhibitor INK128 has been shown to regulate NF-κB signalling and suppress inflammatory cytokines in a murine macrophage cell line (RAW 264.7 cells).99 Moreover, the ATP-competitive mTOR inhibitor AZD8055 was shown to prevent acute rejection of mouse heart allografts and increase the numbers of graft-infiltrating Treg cells.100 In a rat transplant model, modulation of both mTORC1 and mTORC2 eliminated chronic rejection when combined with subtherapeutic ciclosporin.101 These data, coupled with the unacceptably high rates of late renal allograft failure secondary to chronic ABMR and vasculopathy, provide justification for further investigation of novel mTOR pathway inhibitors in transplantation.
Clinical organ transplantation
Initial studies that combined sirolimus (versus placebo or titrated dosage102) with ciclosporin and prednisolone in renal transplantation demonstrated lower biopsy-proven acute rejection (BPAR) with sirolimus compared to ciclosporin and prednisone alone,103, 104 even in the context of reduced ciclosporin dosage.104 Sirolimus was also clearly superior to azathioprine105 and has been investigated as a possible calcineurin-sparing agent.24, 106 However, a subsequent study found a higher rate of BPAR in patients on prednisone and sirolimus who withdrew ciclosporin at 3 months compared to those who remained on this agent.107 Furthermore, in the Elite-Symphony study, patients assigned to sirolimus, MMF and prednisone had higher BPAR rates (37.2%) than those treated with either low dose ciclosporin (24%) or tacrolimus (12.3%). Therefore, the use of Sirolimus as baseline immunosuppression in the absence of CNI has limitations, including higher rates of acute rejection.108,109, albeit offset by better GFR.107
The majority of studies and meta-analyses show that patient-based outcomes and the incidence of treatment withdrawal are worse following mTOR inhibition with sirolimus or everolimus compared to CNI-based regimens.110–112 It is generally accepted that mTOR inhibitors provide inferior initial post-transplant immunosuppression compared to conventional CNI-based regimens. In addition, the propensity of mTOR inhibitors to impair wound healing, induce wound dehiscence and promote lymphocoele development further precludes their use as a primary immunosuppressant. mTOR inhibitors might be best utilized for conversion therapy to avoid CNI toxicity, or to provide flexibility for patients who develop CNI-related complications. The documented adverse effects associated with mTOR inhibitors include bone marrow suppression, hypertriglyceridaemia, peripheral oedema, gastrointestional disturbance and stomatitis, new-onset diabetes after transplantation, proteinuria, gonadal toxicity, cough, dyspnoea and interstitial pneumonitis.113 The ACE inhibitor ramipril has proven effective in reducing the incidence of proteinuria for up to 1 year after conversion to sirolimus in renal transplant recipients on maintenance immunosuppression.114 However, the underlying pathobiology contributing to these adverse effects is incompletely understood. The development of aphthous ulcers might represent a breach of mucosal immunity and a lack of CD4+CD25+ Treg cells,115 but this mechanism is counterintuitive as treatment with mTOR inhibitors tends to spare Treg from depletion in pre-clinical models and human kidney graft recipients.116 Impaired wound healing through inhibition of cell proliferation and neoangiogenesis, or impaired glucose tolerance might be contributing factors. Similarly, interstitial pneumonitis might have an immunological aetiology, with increased CD4+ T cells found in bronchoalveolar lavage.117
Since the FDA approval of rapamycin (sirolimus) for use in clinical kidney transplantation in 1999, an extensive literature has emerged on its effects on graft survival, mortality and other important clinical outcomes, such as malignancy, cardiovascular disease and infection. The majority of studies of mTOR inhibitors involve conversion from CNI either early (2–6 months) or late (>6 months) post-transplantation. Few studies on the de novo use of mTOR inhibitors and mTOR inhibitor monotherapy post kidney transplant have been conducted (Table 3).
Table 3:
Summary of the effects of mTOR inhibitors in renal diseases
| Setting | Effect of mTOR inhibitor | |
|---|---|---|
| Pre-clinical models | Clinical studies | |
| Healthy kidney | No histologic abnormalities160 Deterioration in GFR in spontaneously hypertensive rat163 |
No effect on renal function (serum creatinine levels) after 8 weeks of treatment283 |
| Diabetes mellitus | Attenuates renal hypertrophy mitigates albuminuria175–177 | No direct studies; use of sirolimus post-islet transplant associated with proteinuria284 |
| Systemic lupus erythematosus | Preservation of renal mass and renal function, improved glomerular histological findings, decreased anti-double stranded DNA antibodies |
One human study, improvements in renal function and proteinuria in 3 of 5 patients184 |
| Adriamycin nephropathy | Preservation of renal function, amelioration of glomerulosclerosis and tubular dilatation180, 189 | No human disease equivalent |
| Anti-GBM disease, Goodpasture’s disease and crescentic GN | Concurrent with disease induction: improved proteinuria and renal histology After disease induction: worsening proteinuria and inflammatory infiltrates201 |
Case report of SRL reducing ANCA titre285, another case report suggesting limited utility owing to adverse events286 |
| Thrombotic microangiopathy | Impaired recovery190 | No human studies; SRL has been associated with TMA in renal allografts |
| Chronic glomerulonephritis | In Thy 1.1 nephritis, low dose prevents compensatory glomerular hypertrophy, renal inflammatory cell infiltration192 | 6 out of 11 patients with chronic glomerulonephritis and pre-existing proteinuria were treated with rapamycin developed acute renal failure287 |
| Chronic kidney disease | Induces proteinuria, interstitial fibrosis and glomerulosclerosis in a rat remnant kidney model251 | - No formal human studies |
| Membranous nephropathy | Mitigated proteinuria, and reduced immunoglobulin deposits in rats with Heymann nephritis199 | No formal human studies |
| IgA nephropathy | Protected kidney function, reduced IgA deposition and prevented proteinuria increase196 | Improved GFR, decreased endocapillary proliferation204 |
| FSGS | No studies | Evidence of complete and partial remission205, cases of nephrotoxicity reported287 |
| Minimal change nephropathy | No studies | Complete remission when combined with tacrolimus208 |
| Polycystic kidney disease | Decreased kidney enlargement and cyst volume; improved renal function 213 | Unimpressive results, high adverse effect profile288 |
| Acute kidney injury | Delayed recovery289 | Delayed recovery136, 137 |
| Angiomyolipoma | Decreased tumour burden, cyst size and increased survival in a mouse model of TSC290 | Long-term treatment effective in reducing tumour volume256, 263 Neoadjuvant use of sirolimus facilitates nephron-sparing resection261 |
| Renal cell carcinoma | Temsirolimus and the TORKinib Ku0063794 both inhibit tumor growth in a xenograft model of RCC291 | Several inhibitors tested without great success in advanced disease292 including temsirolimus,293 everolimus,294 deforolimus295 and CCI-779296 |
Abbreviations: ANCA, anti-neutrophil cytoplasmic antibody; FSGS, focal segmental glomerulosclerosis; GBM, glomerular basement membrane; GN, glomerulonephritis; NA, not applicable; TSC, tuberous sclerosis complex.
In the majority of the conversion trials published to date, mTOR inhibition has been compared with ciclosporin. In one study of renal transplant recipients, a ciclosporin-free regimen based on sirolimus reduced aortic stiffness, plasma endothelin-1 and oxidative stress, suggesting a protective effect on the arterial wall that might be translated into reduced cardiovascular risk.118 On the other hand, among patients randomly assigned to either sirolimus or ciclosporin at 3-months post-transplantation, the incidence of subclinical inflammation in protocol biopsy samples 1 year post-transplantation was greater in the sirolimus group.119 Tacrolimus is currently the most commonly used CNI in kidney transplantation. In a prospective randomized trial, kidney transplant recipients receiving rapid corticosteroid withdrawal, tacrolimus and mycophenolate mofetil (MMF) for 1 month were randomly assigned to either sirolimus plus MMF or tacrolimus plus MMF maintenance therapy.120 Although graft survival at 2 years was similar between the groups, withdrawal from the study owing to rejection or adverse effects was a major problem in the sirolimus group (63% versus 18% in the tacrolimus group). These data indicate that in the absence of steroids, sirolimus and tacrolimus are not interchangeable. Most studies comparing tacrolimus and sirolimus have involved de novo use of these agents rather than conversion.108, 109, 121
Other than their anti-neoplastic effects, a theoretical advantage of mTOR inhibitors in transplantation lies in their anti-viral properties. mTOR inhibitors stimulate CMV-specific Th1 cells and γδ T cells against CMV and translation of CMV viral proteins relies on host mTOR activity. 122–124 Furthermore, sirolimus inhibits and tacrolimus activates BK polyomavirus replication in renal TECs.125 Despite these paradoxical anti-viral properties, exposure to rapamycin enhances the quality and quantity of pathogen-specific but not graft-reactive CD8+ T cells.126 As BK and CMV infections cause considerable excess morbidity and mortality, the immunomodulatory effects of these mTOR inhibitors provide a rationale for conversion from CNIs post-transplantation. Unfortunately, many studies have not been adequately powered to compare the influence of different immunosuppressive regimens on the incidence of either CMV or BK virus infection. Nonetheless, many comparative studies have demonstrated that early use of mTOR-inhibitor-based regimens can reduce the incidence of CMV infection.127, 128 In a single centre case series that included 15 patients with BK nephropathy, suspension of mycophenolate and conversion from tacrolimus to everolimus immunotherapy was associated with decreased viraemia and increased graft survival.129 Although a role for mTOR inhibitors in biopsy-proven BK nephropathy is plausible, prospective large randomized studies are needed to adequately address this question.
Overall, mTOR inhibitors have some attractive characteristics, particularly their beneficial effects on kidney function by enabling CNI sparing and their association with reduced rates of malignancies and non-melanoma skin cancer,130 less viral infections and less weight gain131 compared to CNI-based regimens.132 Nonetheless, for the many renal transplant recipients with a documented history of sensitization, these agents remain inferior to CNIs. For these reasons, coupled with adverse effects and the availability of anti-viral agents, mTOR inhibitors are currently not used extensively post-transplantation. As malignancy and infection remain two of the most common causes of death among kidney transplant recipients, establishing novel and less toxic ways to manipulate the mTOR pathway is an important goal in transplantation.
mTOR in AKI and ischaemic injury
AKI is an important health problem that affects approximately one in five adults and one in three children who are hospitalized.133 As well as increasing morbidity and mortality in the acute setting, AKI increases the long-term risk of developing CKD.134 The underlying aetiologies that precipitate AKI are numerous and can be categorized into pre-renal, intrarenal and post-renal causes.135 When adequately addressed, pre-renal and post-renal AKI resolve rapidly. Management of intrarenal AKI is more difficult; this disease is often the result of ischaemic or toxic injury to renal tubules, which typically requires days to weeks to resolve. A special situation arises during kidney transplantation in which donated organs inevitably undergo both cold and warm ischaemia. Indeed, the potential positive and negative sequelae of mTOR inhibitor therapy first became evident in renal allograft recipients.136–138 The findings of delayed graft function (DGF) and cast nephropathy in humans by Smith et al. suggested that rapamycin therapy exerts increased toxicity on TEC and/or retards healing after ischaemic injury. 136 In all prospective randomized trials published to date, however, no association between rapamycin therapy and a reduction in GFR has been reported. To further elucidate the effects of mTOR inhibitors on TECs and vascular endothelial cells, as well as on local and systemic immune cells, rodent models of ischaemia–reperfusion (I/R) and various renal transplant models have been utilized.
Although several lines of evidence initially pointed to aggravation and delayed recovery from renal I/R injury with rapamycin,139, 140 this finding was later challenged.141, 142 The three most probable confounders contributing to these contradictory findings were likely the control group selected, the time of mTOR inhibitor administration and the timing of the analysis.141, 143 Most studies that used a placebo group have reported adverse outcomes after administration of mTOR inhibitor.140, 144 This delayed functional recovery could be reproduced in a mouse model by conditional deletion of mTORC1 in tubular cells before inflicting I/R injury.33 By contrast, studies that have compared mTORC1 inhibitors to calcineurin inhibitors (CNIs) have reported beneficial outcomes of mTOR inhibition that can be explained mainly as the result of avoidance of the deleterious effects of ciclosporin on the renal microcirculation.141, 145 Moreover, a preconditioning-like effect of mTOR inhibition prior to transplantation has been observed in organs such as the heart.146 This effect has also been reported in experimental renal transplantation; treatment of the donor with a single dose of rapamycin before nephrectomy improved allograft function.147
Another important confounder in studies of mTOR inhibitors in AKI is the timing of analysis after I/R. Studies that reported an adverse outcome of mTOR inhibition investigated early time-points (within several days of transplantation), whereas those that analysed much later time points (up to 16 weeks following the initial I/R) reported beneficial effects in normotensive rats and to a lesser degree in hypertensive animals.139, 145, 148, 149 Unfortunately, only a few studies have explored the mechanisms that underlie the observed effects. While reduced proliferation and increased apoptosis are suggested as the most likely underlying cause for the initial adverse effects in I/R33 directly linked to the role of the mTOR kinase, secondary effects of mTOR inhibition that influence dependent pathways (such as autophagy) or effects on the anti-oxidative system may be responsible for late beneficial effects of mTOR inhibitors in I/R.150, 151
Few studies have investigated the role of mTOR in AKI unrelated to transplantation. Interestingly, AKI concurrent with the start of mTOR inhibitor therapy has been noted in patients with cancer, in whom it can present as an acute-tubular-necrosis-type injury.152 By contrast, mTOR inhibition was found to be beneficial in a mouse model of AKI induced by HIV-associated nephropathy.153 This effect was most likely mediated by downregulation of mTOR-induced p53 expression resulting in a reduction in oxidative cell injury. Hence in this model, reduced apoptosis provided a functional and histological benefit.
In summary, the risk of delayed graft function seems to be increased in patients who are treated with mTOR inhibitors directly after transplantation. As this risk might complicate the immediate post-transplant phase with regard to the need for additional dialysis and graft biopsy to exclude acute rejection in the context of delayed graft function, early treatment with mTOR inhibitors should be avoided. Most injury mechanisms seem to be related to the anti-proliferative and pro-apoptotic effects of mTOR inhibitors on the renal epithelium, whereas specific effects on local and systemic immune cells that might affect the development of AKI have not been thoroughly explored.
mTOR in glomerular disease
The glomerulus regulates filtration by size and charge. Changes in glomerular permeability owing to hyperglycaemia,154 induction of reactive oxygen species155 or pharmacologic challenge156, 157 underpin a number of disease processes. Exposure of healthy rats to mTOR inhibitor (temsirolimus) increased early glomerular permeability, but reduced late permeability.158 In BALB/c mice without renal pathology, rapamycin induced a mild deterioration in renal function, increased albuminuria and podocyte foot-process width, and induced a short-term reduction in nephrin and podocin expression; these effects resolved after 8 weeks of treatment.159 Although previous studies had demonstrated no gross histologic changes in the renal glomerulus in response to mTOR inhibition,160, 161 pathologic abnormalities were seen in the rodent renal tubular compartment162 or vessels.163
Glomerulonephritides comprise a substantial proportion of human renal diseases and several rodent models have been developed to mimic these disorders. mTOR is increasingly recognized as having a fundamental role in the development of glomerular pathology, particularly in terms of dysregulation of rodent podocyte function.164 Activation of mTORC1 in podocytes led to the development of glomerular crescents,165 which was abolished following treatment with rapamycin. Podocyte homeostasis is disruptedby mTOR inhibitors that alter the cytoskeleton via RhoA signalling,166 reduce vascular endothelial growth factor (VEGF)167 synthesis and Akt phosphorylation,168 downregulate the expression of nephrin,169 podocin and synaptopodin168 and affect cell motility. Additional studies have identified activation of Akt2 downstream of mTORC2 as critical for podocyte survival.170
Diabetes mellitus
Diabetes mellitus is a leading cause of chronic kidney disease (CKD) in developed countries and the causative aetiology of ESRD in 30–40% of patients seeking renal replacement therapy.171 Early renal changes in response to hyperglycaemia include mesangial and basement membrane expansion, glomerular hypertrophy and loss of capillary surface area.172 Diabetes is now thought to represent a state of mTORC1 hyperactivation.173 The molecular mechanism underlying compensatory hypertrophy is not well-characterized, but involves increased phosphorylation of 40S ribosomal protein (rpS6), downstream of S6 kinase 1 (S6K1).174 This kinase in turn regulates protein synthesis and cellular growth downstream of mTORC1. The role of pS6 as a molecular signature of mTORC1 activation in podocytes has been confirmed in the glomeruli of patients22 and mice23 with diabetes. Moreover, knockout of S6K1 protected against the development of renal hypertrophy in diabetic mice.23 Inhibition of mTOR signalling has also been shown to ameliorate some renal compensatory mechanisms following induction of diabetes. In mice with streptozotocin-induced diabetes, renal hypertrophy was accompanied by upregulation of S6K1 kinase expression that could be attenuated by daily rapamycin administration.175 Similarly in diabetic rats, rapamycin ameliorated albuminuria (but had no effect on glomerular hypertrophy) and downregulated the expression of mTOR, pAkt, TGF-β1 and connective tissue growth factor176. These effects occurred without normalization of serum glucose and blood pressure levels and paralleled achievement of normoglycaemia177. High glucose levels have been shown to induce podocyte apoptosis, activate mTOR and promote NADPH oxidase (Nox)¼ expression; these effects are attenuated by rapamycin.178
Systemic lupus erythematosus
The chronic autoimmune disease systemic lupus erythematosus (SLE) commonly affects the kidney through immune complex deposition, complement activation and infiltration of innate immune cells. MRL/lpr and female NZBW/F1 mice develop overt SLE and renal disease that can be mitigated by rapamycin treatment.179–183 Activation of the P13K/Akt/mTOR pathway has been demonstrated in the affected glomeruli of NZBW/F1 mice, with upregulation of phosphorylated Akt (at residues 308 and 473) and concurrent downregulation of podocin and nephrin. In the glomerulus, expression of mTOR and Ser 2448 pTOR is co-localized in podocytes and endothelial cells. Mice treated with rapamycin (either 1 week after development of severe proteinuria or prior to SLE development) showed preservation of remaining renal mass and function, reduced levels of anti-double-stranded-DNA antibodies, mitigated pathognomonic histological lesions and maintenance of podocin and nephrin expression compared with untreated controls. Treatment with rapamycin or other mTOR inhibitors also suppressed interstitial inflammatory infiltrate (T cells, B cells and macrophages) in pre-clinical lupus models.179 Despite the anti-proliferative properties of rapamycin, few clinical studies have used this agent to treat lupus nephritis. A recent report of 7 patients suggests further investigation may be warrented.184 mTOR inhibitors have been investigated only sparingly in animal models of other glomerular diseases (Table 3). Morever few studies have explored the mechanisms involving mTOR and glomerular pathophysiology, as well as beneficial effects related to mTOR inhibitors. Proteinuria is a key clinical feature of glomerular disease and urinary protein loss is regulated predominantly by podocytes.186 Endoplasmic reticulum stress triggers the unfolded protein response, which is preceded by activation of mTORC1 and dysregulated energy production187; both of these effects can be inhibited by everolimus administration.
In rodents with adriamycin nephropathy, rapamycin reduces proteinuria, preserves renal function and ameliorates glomerulosclerosis and tubular dilatation, with reductions in the intrarenal expression of C-C motif chemokine 5 (CCL5, also known as RANTES) and collagen.188 These renoprotective and antiproteinuric effects can be recapitulated with everolimus, which also restores glomerular nephrin and podocin expression.189
Administration of everolimus slowed recovery from endothelial cell injury in a mouse model of thrombotic microangiopathy; this agent inhibited endothelial cell proliferation, but had no effect on endothelial cell apoptosis.190 These data are in keeping with in vitro findings of dose-dependent inhibition of glomerular endothelial cell proliferation. Everolimus also inhibits the expression of VEGF and S6K in cultured podocytes and in vivo.
The rat chronic Thy1.1 nephritis model (generated by injection of antibodies against glomerular mesangial cells) mimics membranoproliferative glomerulonephritis.191 In this model, administration of everolimus, particularly at low doses, prevented compensatory glomerular hypertrophy, reduced fibronectin and VEGF expression and reduced renal influx of monocytic lineage cells.192 Rapamycin similarly abrogated a rise in blood pressure attributable to CKD in these rats, but had a detrimental effect at high doses in the acute injury phase193, 194 owing to inhibition of rat endothelial cell proliferation and glomerular neoangiogenesis.100,101 A deleterious effect of everolimus has also been reported in a rat remnant kidney model; this agent induced proteinuria, interstitial fibrosis and glomerulosclerosis.195 In a rat model of established IgAN, low-dose rapamycin reduced IgA deposition, prevented progression of proteinuria and limited deterioration of renal function.196 These effects correlated with cell cycle arrest, upregulation of cyclin-dependent kinase inhibitor 1B and presumably inhibition of mesangial cell proliferation.
In Heymann nephritis197 — a rodent model of human membranous nephropathy — production of antibodies directed against the target antigens megalin and receptor-associated protein198 leads to the development of glomerular deposits. Rapamycin mitigates proteinuria199 and histologic lesions in this model,200 including CD8+ T cell inflammation, with restoration of glomerular expression of podocin and nephrin200 and a reduction in immunoglobulin deposits.199
A nephrotoxic serum nephritis model, induced by immunization with heterologous (sheep or rabbit) GBM proteins, is analogous to Goodpasture’s disease in humans. In this model the development of glomerular crescents is preceded by mTORC1 activation.201 Rapamycin administration concurrent with disease initiation decreased proteinuria, inflammatory infiltrate and type 1 T helper (Th1) cytokine production. By contrast, when commenced after disease induction,202,203 rapamycin treatment led to increased albuminuria and accompanying macrophage and CD4+ T cell infiltration with increased Foxp3 transcripts suggesting accumulation of Treg cells, and glomerular pro-inflammatory cytokine production. These divergent, time-dependent effects are thought to be related to an initial, beneficial, down-regulation of T and B cell responses, followed by inhibition of podocyte VEGF-A production, reducing endothelial proliferation and thus the integrity of the glomerulus.
Few clinical studies have investigated the influence of mTOR modulation in glomerular disease (Table 1), possibly owing to concern about exacerbation of proteinuria. Moreover, low numbers of recruited patients limit the interpretation and generalizability of existing studies. Twenty-three patients with poor prognosis IgA nephropathy (>1g proteinuria per 24 h and glomerular filtration rate (GFR) 30–60 ml/min/1.73 m2) treated with angiotensin-converting-enzyme (ACE) inhibitor and statin were also assigned sirolimus or placebo for 12 months.204 Those treated with sirolimus showed improvements in GFR and decreased endocapillary proliferation compared with the placebo group. Proteinuria, glomerulosclerosis and interstitial fibrosis were unchanged, regardless of treatment. The efficacy of sirolimus has also been evaluated in 21 patients with FSGS;205 the therapy induced complete remission in 19% of patients and partial remission in 38%, with maintenance of GFR and reduction in proteinuria. A phase II open-label trial in 6 patients with FSGS, however, reported a lack of responsiveness to sirolimus and an association of this therapy with nephrotoxicity.206 In some kidney transplant recipients, high-dose sirolimus has been reported to induce de novo FSGS, characterized by decreased expression of synaptopodin and p57.207 A case of successful treatment of minimal change nephropathy using combined tacrolimus and rapamycin has also been reported.208
mTOR in polycystic kidney disease
Autosomal dominant polycystic kidney disease (ADPKD), the most common renal monogenetic disease, is caused by mutations in PKD1 or PKD2.209 Although this disease primarily affects the kidneys, the liver, cerebral blood vessels, gut, pancreas and cardiac valves can also be affected; PKD is thus a systemic disease.210 Approximately half of affected patients require renal replacement therapy in their sixth or seventh decade.209 In addition, several juvenile cystic kidney diseases caused by various gene defects commonly lead to renal failure before adulthood.211 Although key insights into disease mechanisms at a cellular level have been gained over the past two decades, treatment choices remain limited.212
mTOR has an important role during initiation and enlargement in PKD.213, 214 Initial studies aimed to establish whether mTOR inhibitors could ameliorate PKD in rodent models.213, 214 With very few exceptions (female Han:SPRD rats, PCK rats),215, 216 these agents elicited a profound and long-lasting reduction in kidney size and an improvement in renal function. The gene product of PKD1, polycystin-1, interacts with TSC2, which forms a complex with the GTP-binding protein RHEB upstream of mTOR. When polycystin-1 was mutated this multiprotein complex led to aberrant activation of mTORC1 in tubular epithelial cells in polycystic mice and in patients with PKD.214 Loss of PKD1 is also associated with increased expression and activity of ERK, which phosphorylates TSC2 and thereby increases the stimulatory effect of the TSC2/RHEB complex on mTORC1.217 This molecular mechanism brings together several known signalling and cellular perturbations related to PKD1 insufficiency: increased proliferation, increased cell size and increased apoptosis217.
Aberrant signalling in the primary cilia is a hallmark and initiating feature of PKD. Loss of cilia inhibits the basal body compartment and reduces serine/threonine-protein kinase STK11–AMPK-mediated inhibition of mTORC1.218 This reduction in inhibition in turn, leads to increased activation of mTORC1 and increased cell size. Reduced AMPK signalling, together with increased ERK signalling, have also been identified as the cause of the high anaerobic glycolytic activity observed in human PKD1 knockout cells and kidneys of PKD1 knockout mice.219 Administration of metformin, AICAR (an analogue of AMP) or 2-deoxyglucose reduced glycolytic activity and restored AMPK phosphorylation, thereby inhibiting mTORC1 and ameliorating cyst growth and functional decline inPKD.219, 220
Clinical trials of mTOR inhibitors in PKD were initiated based on the central pathomechanistic role of mTORC1 in this disease, encouraging preclinical results in rodent models, and anecdotal reports of shrinkage of enlarged polycystic kidneys in transplant recipients receiving mTOR inhibitors.214, 221 The two largest clinical trials to date enrolled a total of >500 patients. The Swiss ADPKD study focused on patients with early stage ADPKD who had only slightly limited renal function and fairly small kidneys222, whereas the Everolimus in ADPKD Study enrolled patients with progressive disease, moderate to severe impairment of renal function and enlarged kidneys223. Both trials failed to demonstrate improvement in renal function with mTOR inhibition. Moreover, in the everolimus in ADPKD Study, an initial significant increase in GFR followed by a trend towards worse renal function was reported. In the Swiss ADPKD study, change in kidney size did not differ between mTOR inhibitor and placebo after 18 months; the everolimus in ADPKD Study demonstrated a reduction in kidney size with everolimus versus placebo after 6 months and a trend towards a reduction in kidney size at 12 months, which likely failed to reach significance owing to the drop-out rate. Several explanations as to why these bench-to-bedside approaches did not yield positive results have been suggested,224, 225 these relate to the short follow-up period for a slowly-progressive disease and, in the Swiss ADPKD study, low blood trough levels of rapamycin that might have been insufficient to achieve inhibition of mTORC1 within the cystic tissue.222, 226 In the Everolimus in ADPKD trial, the heterogeneous patient population with advanced disease, frequent adverse effects (angioedema, aphthous stomatitis, proteinuria) and hence a high drop-out rate, might have obscured subgroups that could have benefitted from an mTOR-targeted approach.223 A smaller Italian study (SIRENA) in 21 patients with ADPKD reported positive outcomes with sirolimus in terms of arresting kidney enlargement and reducing loss of renal parenchymal tissue.227 Again possibly due to the short study period and small sample size, no improvement in renal function was detected. Another caveat was the high blood trough levels of rapamycin that would certainly cause substantial adverse events in the long-term.
Despite the rather disappointing clinical outcomes of the adequately powered studies published to date, several smaller trials entailing different treatment protocols and more specific patient cohorts have been initiated.228, 229 Despite the compelling pre-clinical data, mTOR inhibitors cannot currently be advocated as a therapeutic option in ADPKD outside of clinical trials. Further experimental work is needed to define optimal treatment time-points and appropriate drug levels for mTOR inhibitors during the course of ADPKD. Organ-specific drug targeting approaches would likely enable a reduction in adverse events and could enhance the desired effects of mTOR inhibitors within polycystic kidneys. Whether combined mTORC1/2 inhibition could have a role in future trials remains to be determined.230
mTOR in renal fibrosis and CKD
The role of mTOR in the induction and progression of pathology in fibrotic kidney disease is less well described than the role of mTOR in AKI. Concurrent findings indicate that the use of rapamycin following kidney injury, regardless of aetiology, reduces the subsequent development of interstitial fibrosis and/or the expression of fibrosis-related genes in IRI,231 transplantation,232 adriamycin nephropathy,233 unilateral ureteral obstruction (UUO)234 and glomerulopathy.235, 236
Treatment of normal rat kidney cells (NRK-49F cell line) with TGF-β1 activates Rictor and mTORC2 signalling in a time-dependent fashion, whereas knockdown of Rictor (using siRNA) inhibits the expression of fibronectin and smooth muscle actin237. In rats subjected to UUO (a standard model of renal fibrosis that causes phenotypic transition of renal tubular epithelial cells, endothelial cells and pericytes), Rictor was upregulated in comparison to the levels in sham-operated controls and co-localized with cells expressing smooth muscle actin, consistent with myofibroblast expression. Fibroblast-specific deletion of Rictor produced phenotypically and functionally normal kidneys that demonstrated lower collagen content and fibrosis, apoptosis and inflammatory cell infiltration following UUO237. Despite the utility of this animal model, one must consider that UUO results in acute fibrosis, whereas interstitial fibrosis, at least in humans, usually occurs as a chronic consequence of renal injury.238
Initial findings of mTOR activation in kidney fibrosis were limited to macrophages and myofibroblasts.239 ). Administration of rapamycin mitigated the fibrotic and macrophage-based inflammation in response to UUO, and in vitro induction of fibroblast-mediated fibrogenic activity in response to TGF-β. Indeed, TGF-β activates mTOR exclusively through the TSC2 pathway in fibroblasts240 and upregulation of TGF-β-induced Rheb/mTORC1 signalling in interstitial myofibroblasts was abolished by rapamycin.241 Activation of mTOR also inhibited microRNA-21 suppression of mesangial cell protein synthesis and hypertrophy by TGF-β.242
Fully-mismatched rodent models of kidney transplantation have also been used to evaluate the role of mTOR in chronic allograft nephropathy. As in other chronic kidney injury models, mTOR inhibition in these animals resulted in improvements in proteinuria and glomerulosclerosis,243, 244 reduced inflammatory infiltrate245 and decreased expression of fibrotic factors (TGF-β, platelet-derived growth factor and CTGF).245, 246 Multiple mechanisms that might underlie these beneficial effects of mTOR inhibitors have been reported. Several studies noted alterations in podocyte VEGF transcript and protein expression in vivo following treatment with mTOR inhibitors, and this finding has been replicated in vitro.247 Treatment with sirolimus reduced VEGF-C/VEGFR-3 signalling (which is critical to lymphangiogenesis and lymphatic endothelial cells, and inhibited lymphangiogenesis might represent the mechanism of nephroprotection.248 Plasmin activator inhibitor (PAI)-1 has been implicated in fibrosis and is thought to have a critical role in extracellular matrix deposition and matrix metalloproteinase activity.249 In renal transplant recipients with chronic allograft nephropathy treated with rapamycin, glomerular and tubulointerstitial expression of plasmin activator inhibitor (PAI)-1 was reduced compared to those maintained on calcineurin inhibitors.250
The rodent remnant kidney model involves unilateral nephrectomy and partial infarction of the remaining kidney leading to CKD, which is characterized by a progressive rise in systolic blood pressure and serum creatinine levels. In this model, everolimus and sirolimus have both been reported to limit the progression of renal disease,251, 252 with decreased proteinuria, fibrosis and TGF-β expression, fibroblast activation252 and VEGF signalling.251 However, deleterious effects of everolimus (induction of proteinuria, interstitial fibrosis and glomerulosclerosis) have also been demonstrated in the same model.195 - These divergent findings are puzzling and it is not clear whether the observed differences are simply related to the rodent genotype.
mTOR in malignancies
Renal angiomyolipomas
Angiomyolipomas (AMLs) resulting from clonal proliferation of epithelioid cells, are the most common renal lesions in patients with TSC. These lesions manifest as multiple and bilateral benign kidney tumours that can bleed spontaneously or cause life-threatening haemorrhage if >4 cm in diameter.253 Less common renal manifestations of TSC include renal cysts, renal cell carcinomas, oncocytomas and FSGS. TSC is caused by mutations in either TSC1 on chromosome 9 or TSC2 on chromosome 16.254, 255 Their gene products, hamartin and tuberin respectively, dimerize and function as a GTPase-activating protein for Rheb, explaining the variety of benign tumors that form across multiple organs.
Given the genetic and molecular basis of TSC, use of mTOR inhibitors has been investigated as a systemic treatment.253, 256 Early mTOR inhibiton in patients with TSC might prevent development of TSC lesions and alter the natural history of the disease.257 Multiple non-randomized studies have found that sirolimus reduces AML tumour volume.258, 259 In one study, rapamycin treatment reduced mean tumour volume by 55% at 6 months and 66% at 1 year,260 with no difference in mean tumour volume seen between years 1 and 2. Neoadjuvant use of mTOR inhibitors to facilitate nephron-sparing resection has also been reported.261
The largest study of mTOR inhibitors in TSC to date is the EXIST-II trial.262 In this study, 118 patients were randomly assigned in 2:1 ratio to receive everolimus or placebo. Inclusion criteria included age >18 years, diagnosis of TSC (or sporadic lymphangioleiomyomatosis (LAM)) and at least one AML ≥3 cm in diameter. The rate of response (defined as a 50% reduction in total AML volume) was 42% for everolimus and 0% for placebo. As a result of these findings, everolimus was FDA-approved for treatment of adults with renal AMLs and TSC who do not require immediate surgery. Long-term everolimus treatment seems to be safe and effective in patients with TSC or sporadic LAM-associated renal AML-related bleeding.263 Currently, mTOR inhibitors are recommended as first-line therapy for patients with AML volume >3 cm who are not candidates for immediate surgery.
Renal cell carcinoma
Renal cell carcinomas (RCCs) originate in the renal cortex and constitute 80–85% of primary renal neoplasms. The role of mTOR inhibitors in the treatment of advanced RCC has been studied extensively,264 although their use has been restricted largely to patients refractory to anti-VEGF therapy or with mutations in PI3K. In the INTORSECT trial, 500 patients refractory to the protein kinase inhibitor (PKI) sunitinib were randomly assigned to receive either the PKI sorafenib or temsirolimus.265 Overall survival was shorter with temsirolimus. In the BEST trial, treatment-naive patients were randomly assigned to various molecular therapies including combinations of mTOR and VEGF pathway inhibitors266. However, none of these combinations outperformed anti-VEGF (bevacizumab) monotherapy. In the RECORD-1 trial, everolimus therapy prolonged progression-free survival (PFS) in comparison to placebo in patients with metastatic RCC that had progressed on sunitinib, sorafenib, both therapies, or cytokines208. In the RECORD-4 trial everolimus was assessed in a second-line setting that confirmed the PFS benefit209.
Several TORKinibs have emerged as attractive alternatives to rapalogs in advanced RCC, owing to their dual inhibition of mTORC1 and mTORC2 and their favourable pharmacokinetic profile. Targeting of both complexes is considered theoretically advantageous, as this approach would prevent ‘rapalog resistance’ caused by activation of feedback loops that arise from mTORC1 inhibition alone and produce greater cytotoxicity against malignant cells.267 The novel TORKinib AZD2014 inhibited RCC cell survival and growth and enhanced autophagy in vitro, and inhibited RCC cell growth in a xenograft model.268 However, a randomized Phase II study of AZD2014 versus everolimus in anti-VEGF-refractory metastatic RCC, showed inferior PFS and overall survival with the TORKinib despite favourable toxicity and pharmacokinetic profiles.269 At present, a role for these novel agents in advanced anti-VEGF-refractory RCC remains uncertain. With the advent of molecular profiling of tumours, targeted mTOR inhibition might, however, still prove beneficial in subgroups of patients with mTOR-driven neoplasms.
Post-transplant malignancy
In the setting of post-transplant malignancy mTOR inhibitors have theoretical advantages over other established immunosuppressive agents owing to their simultaneous stimulatory effects on certain immune cells and growth suppressive effects on malignant cells. In various animal models, sirolimus suppresses the growth and proliferation of tumours.270 As a nutrient sensor and regulator of cell growth, tumours rely on the mTOR pathway to acquire and catabolize nutrients. Indeed, many tumours are characterized by upregulated mTOR signalling as a result of mutations in tumour suppressors (PTEN, TSC1/2) or oncogenes (PI3K, S6K, eIF4E).271 Hence, most tumours are susceptible to mTOR inhibitors to varying degrees. The ability of mTOR inhibitors to directly inhibit the replication of viruses such as cytomegalovirus (CMV) and human herpesvirus 8 is another potential advantage of these agents.272, 273
Despite the potential advantages of mTOR inhibitors and some evidence of anti-cancer effects in transplant recipients,274, 275 including an anti-tumour effect of sirolimus early after transplantation in subgroups of liver recipients with hepatocellular carcinoma,276 these agents have not replaced CNIs in the clinic. Reasons for this failure include a plethora of adverse effects that limit tolerability and a lack of randomized control trials showing a clear benefit of conversion to mTOR inhibitors on hard clinical outcomes, such as de novo malignancy or cancer-related death. Nonetheless, conversion to mTOR inhibitors seems to be a reasonable choice for patients with either prior non-melanoma skin cancer277 or newly-diagnosed Kaposi’s sarcoma.278 Several randomized controlled trials have demonstrated lower recurrence rates of squamous cell carcinoma (SCC) in patients with skin tumours converted to sirolimus than in those remaining on CNIs.277, 279 In one study, conversion of kidney transplant recipients with at least one cutaneous SCC to sirolimus reduced the incidence of new SCC at 2 years (22% in the sirolimus group versus 39% in the CNI group)280. In all studies, however, discontinuation of sirolimus because of adverse events was common (20–50%). Given these high discontinuation rates, balancing the optimal therapeutic dose to prevent graft rejection whilst avoiding dose-related toxicities remains a challenge.
In kidney transplant recipients with Kaposi’s sarcoma, conversion from ciclosporin to sirolimus was associated with complete regression of lesions by 3 months.274 Patients with Kaposi’s sarcoma show markedly increased basal P70 (S6K) activation and depressed Akt phosphorylation in peripheral blood mononuclear cells, and long-term treatment with rapamycin is associated with marked inhibition of basal and stimulated phosphorylation of both Akt and P70 (S6K) in parallel with regression of the skin neoplasm.281
Two meta-analyses have examined the efficacy of mTOR inhibitors for prevention of de novo malignancy in kidney transplant recipients. In the first study, Knoll et al showed a decreased incidence of cancer with sirolimus therapy (40% reduction in risk of malignancy and 56% reduction in risk of non-melanoma skin cancer)130. However, excess mortality largely owing to cardiovascular disease and infection was also seen in the sirolimus group. By contrast, the second analysis (of cancer registry data) showed no benefit of sirolimus on cancer incidence, although this finding was attributed largely to an unexplained significantly increased incidence of prostate cancer in patients who recieved this therapy.282 These findings, particularly the excess mortality in the first study, have dampened enthusiasm for mTOR inhibitors.
Thus, although mTOR inhibitors continue to show promise for the treatment of post-kidney-transplant malignancies, their use for malignancies other than Kaposi’s sarcoma and non-melanoma skin cancer remains controversial and understudied in well-powered randomized controlled trials. Furthermore, no role is apparent for the de novo use of these agents in the prevention of cancer.
Conclusions and future perspectives
Although considerable insights have been gained, much remains to be uncovered regarding the roles of mTOR in renal physiology and disease. Moreover, the potential of mTOR inhibitors to alleviate kidney pathology and improve outcomes in kidney transplantation needs more extensive assessment, both in the laboratory and in the clinic. The advent of mTOR complex gene targeting in specific renal and immune cell populations and the availability of new generation pharmacologic mTOR inhibitors is enhancing our understanding of the diverse roles of mTORC1 and mTORC2 in regulation of kidney function, pathology and the autoimmune and alloimmune responses that impact on renal disease, patient care and transplant survival. Areas particularly worthy of investigation (Box 1) and future directions (Box 2) include the roles of mTOR in control of tissue ageing, growth of certain cancers and resistance to specific viral infections, as well as the optimal ways to use mTOR antagonists in conjunction with other therapeutic agents in the clinic.
Box 1 |. Gaps in knowledge regarding mTOR.
A complete interactome of mTORC1 and mTORC2 within the different cells of the kidney is lacking and hence physiological function of these two complexes is only partially known
Several findings regarding mTOR inhibition e.g. promotion of immune regulation and tolerance are well-documented in pre-clinical models of transplantation, yet proof in patients is lacking
Influence of mTOR inhibitors on immune cells, for example B cells and Breg cells
The underlying mechanisms by which mTOR inhibition promotes donor-specific antibody generation in transplant recipients need further exploration.
Prospective CRTs are lacking to substantiate the benefits of using mTOR inhibitors to avoid or reduce certain cancers and viral infections in transplant patients.
While there is evidence in invertebrate and rodent models that mTOR inhibition can prolong life by ameliorating ageing-associated cellular decline, it is unclear whether this also applies in humans
Box 2 |. Future directions.
From a pathophysiological perspective it is paramount to improve understanding of how mTORC1 and mTORC2 drive pathogenesis of renal disease
Clinical trials in transplantation should aim at delineating the optimal drug combinations accompanying mTOR inhibition on both a temporal and intensity basis
Future drug development should not only aim to test the potential superiority of TORKinibs over mTOR inhibitors, but should also identify downstream mediators of mTORC1 and mTORC2 action which might improve the side effect profile by specifically targeting pathological activation loops
Table 2:
Clinical trials of mTORi in renal transplantation
| Study | Type and duration of follow-up | n | Treatment groups | Outcomes |
|---|---|---|---|---|
| Groth et al (1999)24 | Multi-centre, open-label (1 year) | 83 | Steroid + AZA + CsA or SRL | Graft survival, patient survival and BPAR similar; serum creatinine lower in SRL group; higher pneumonia rates in SRL group |
| Kahan et al (1999)104 | Phase II trial (1 year) | 149 | Steroid + CsA + placebo or low- or high-dose SRL, steroid + low-dose CsA + low- or high-dose SRL | Addition of SRL reduced BPAR in standard dose CsA group; no difference in graft or patient survival; haematologic and lipid abnormalities in SRL group, HT and NODAT in CsA group |
| Kreis et al (2000)106 | Multi-centre, open-label (1 year) | 78 | Steroid + MMF + CsA or SRL | Graft survival, patient survival and BPAR similar; serum creatinine lower in SRL group |
| Rapamune US (2000)105 | (1 year) | 719 | Steroid + CsA + AZA or SRL | Reduced occurrence and severity of BPAR in SRL group at 6 months |
| Rapamune Global (2001)103 | Phase III (1 year) | 576 | Steroid + CsA + placebo, low- or- high-dose SRL | Addition of SRL reduced acute rejection rates |
| Johnson et al (2001)316 | Open-label (1 year) | 525 | Steroid + CsA + SRL, 3 month withdrawal CsA or CsA maintenance | Improved renal function and lower BP when CsA withdrawn; thrombocytopenia, hypokalemia and abnormal LFTS in SRL group |
| Gonwa et al (2001)317 | Phase II, open-label (1 year) | 246 | CsA + SRL or reduced-dose CsA (taper at 2 months)+ SRL | Renal function better in CsA elimination arm; graft and patient survival similar, BPAR similar |
| Rapamune Maintenance Study (2003318, 2005319) | Phase III (4 years) | 525 | Steroid + CsA + SRL, random assignment to CsA withdrawal at 3 months | 2 years: CsA withdrawal showed improved renal function and blood pressure, no change in graft loss or late acute rejection rates; 4 years: Non-significant increase in acute rejection rates with CsA withdrawal; higher incidence of adverse effects with triple therapy |
| Larson et al (2006)320 | (1 year) | 165 | Steroid + MMF + TAC or Steroid + MMF + SRL | Similar acute rejection, graft survival and renal function |
| SPIESSER Study (2007321, 2012322, 2016323) | (8 years) | 1 year: 145 5 years: 133 8 years: 119 |
Polyclonal antilymphocyte antibodies + steroid + MMF + CsA or SRL | 1 year: BPAR, graft survival and patient survival not different; SRL group had higher adverse events (bronchopneumonia, proteinuria) and discontinuation rates 5 years: eGFR higher in SRL group; graft and patient survival no different, adverse effects more common in SRL group 8 years: No difference in graft survival, eGFR greater in SRL group, no detrimental impact in patients in whom SRL was withdrawn. No difference in malignancy |
| Symphony (2007108, 2009324) | (3 years) | 1 year: 1,645 3 years: 958 |
Steroid + CsA + MMF or daclizumab + MMF + low-dose CsA/low-dose TAC or low-dose SRL |
1 year: GFR and allograft survival highest and BPAR lowest in low-dose TAC; adverse effects most common in low-dose SRL 3 years: highest GFR and graft survival in MMF+TAC group |
| CONCEPT Study (2009325, 2011326) | (4 years) | 1 year: 192 (237 enrolled) 4 years: 162 |
Steroid+MMF + CsA, ± converted to SRL at 3 months | 1 year: patient and graft survival similar, GFR better in SRL group, ACR rates not significantly higher in SRL group, more adverse events in SRL group 4 years: mean benefits in renal function maintained |
| Glotz et al (2010)121 | 1 year | 141 | Steroid + MMF + SRL or TAC | No difference in GFR or patient survival, graft loss, withdrawal and adverse events higher in SRL group |
| SMART trial (2010327, 2012328) | 3 years | 1 year: 141 2 & 3 years: 132 |
ATG induction, steroids + MMF + CsA, conversion to SRL at 10–24 days | 1 year: GFR better in SRL group, BPAR, patient and graft survival not different, lower incidence CMV infection, more adverse events in SRL group 2 & 3 years: SRL conversion associated with sustained improvement of renal function; discontinuation of SRL due to adverse events was common |
| ZEUS Study (2011329, 2015330) | 5 years | 1 year: 503 enrolled, 5 year follow- up: 245 |
Basiliximab induction, steroids + MMF + CsA, at 4–5 months conversion to EVL or stay on CsA | 1 year: GFR better in EVL group, higher BPAR, lipidaemia and proteinuria, lower haemoglobin and greater adverse events 5 years: EVL group: better GFR Higher BPAR (grade I), did not affect long-term graft function. No difference in graft loss, mortality, adverse events and neoplasm |
| ASCERTAIN study (2011)331 | Multi-centre, open-label, 2 years | n=394 | Randomization at >6 months to EVL with CNI elimination, EVL with CNI mimization or CNI unchanged | Conversion to EVL with CNI elimination or minimization had no renal benefit; more frequent adverse events and discontinuation |
| Heilman et al (2011)120 | (2 years) | 122 | MMF+TAC or MMF+SRL | 63% withdrawal from SRL group |
| STN Study (2011332, 2016333) | (8 years) | 2 years: 229 8 years: 128 |
MMF+CNI MMF+SRL | 2 years: Similar renal function between groups 8 years: Improved long-term renal function with SRL/MMF compared to CNI/MMF |
| Orion (2011)109 | Phase IV trial (2 years) | 443 | (1) SRL+TAC, TAC eliminated week 13; (2) SRL+MMF; (3) TAC+MMF All patients also received steroids and daclizumab | Group 2 had high BPAR (>30%), SRL assoc with hyperlipidemia and delayed wound healing, SRL assoc w greater proteinuria and discontinuation, TAC assoc with NODAT, SRL not associated with improved outcomes |
| Mjörnstedt et al (2012334, 2015)335 | (3 years) | 1 year: 202 3 years: 182 |
Steroid+MMF + CsA, at 6 weeks convert to EVL or stay on CsA | 1 year: GFR better in EVL group, but higher incidence of BPAR and adverse events leadng to discontinuation 3 years: EVL associated with significant benefit in renal function but drug discontinuation more common |
| APOLLO Study (2015)336 |
(1 year) | 93 | Remain on CsA or convert to EVL | Premature termination due to slow recruitment. Higher rate of discontinuation with EVL |
Abbreviations: ACR, acute cellular rejection; ATG, anti-thymocyte globulin; AZA, azathioprine; BPAR, biopsy proven acute rejection; CMV, choriomeningitis virus; CsA, cyclosporine; GFR, glomerular filtration rate; LFTS, liver function tests; MMF, mycophenolate mofetil; NODAT, new onset diabetes after transplantation; EVL, Everolimus; SRL, Sirolimus; TAC, Tacrolimus
Key points.
The mTOR pathway has a central role in regulation of cell metabolism, growth and proliferation
Pharmacological inhibition of mTOR and selective gene targeting of mTORC1 or mTORC2 in podocytes and tubular epithelial cells has begun to elucidate their role in renal cell homeostasis, including autophagy
mTOR is increasingly recognized as having a fundamental role in the development of glomerular disease and in acute kidney injury; its role in fibrotic kidney disease is less certain
Mechanistic insights into the roles of mTOR complexes in regulating immune cell function are helping to improve understanding of the effects of mTOR inhibitors in renal disease and transplant rejection
New generation dual mTORC1 and mTORC2 inhibitors offer potential for treatment of renal cell carcinoma
mTOR inhibitors are associated with reduced rates of skin cancer and cytomegalovirus infection in renal transplant recipients
Acknowledgements
D.F.’s work is supported by an American Society of Nephrology Ben Lipps Research Fellowship. N.M.R.’s work is supported by an NH & MRC C.J. Martin Fellowship, an American Society of Transplantation Basic Science Fellowship, an American Heart Association award (13 POST1452003), and by a Joseph A. Patrick Fellowship from the Starzl Transplantation Institute. A.W.T. is in receipt of research grants from the National Institutes of Health (R56 AI126377; U01 AI91197 and U01 AI102456) and the US Department of Defense (W81XWH-15-2-0027). The authors’ work is also supported by the German Research Foundation (D.F.G): CRC 1140 KidGEM (F.G., T.B.H.), Heisenberg program and CRC 992 (T.B.H.); by the European Research Council (T.B.H.); and by the Excellence Initiative of the German Federal and State Governments (EXC 294 to T.B.H.). We thank Ms. Miriam Freeman for excellent administrative support.
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.Vezina C, Kudelski A & Sehgal SN Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo) 28, 721–6 (1975). [DOI] [PubMed] [Google Scholar]
- 2.Calne RY et al. Rapamycin for immunosuppression in organ allografting. Lancet 2, 227 (1989). [DOI] [PubMed] [Google Scholar]
- 3.Thomson AW, Turnquist HR & Raimondi G Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol 9, 324–37 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powell JD, Pollizzi KN, Heikamp EB & Horton MR Regulation of immune responses by mTOR. Annu Rev Immunol 30, 39–68 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimobayashi M & Hall MN Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat Rev Mol Cell Biol 15, 155–62 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Flinn RJ, Yan Y, Goswami S, Parker PJ & Backer JM The late endosome is essential for mTORC1 signaling. Mol Biol Cell 21, 833–41 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SG et al. Metabolic stress controls mTORC1 lysosomal localization and dimerization by regulating the TTT-RUVBL1/2 complex. Mol Cell 49, 172–85 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yadav RB et al. mTOR direct interactions with Rheb-GTPase and raptor: sub-cellular localization using fluorescence lifetime imaging. BMC Cell Biol 14, 3 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fournier MJ et al. Inactivation of the mTORC1-eukaryotic translation initiation factor 4E pathway alters stress granule formation. Mol Cell Biol 33, 2285–301 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guertin DA et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell 11, 859–71 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Zinzalla V, Stracka D, Oppliger W & Hall MN Activation of mTORC2 by association with the ribosome. Cell 144, 757–68 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Xie J & Proud CG Crosstalk between mTOR complexes. Nat Cell Biol 15, 1263–5 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Schreiber KH et al. Rapamycin-mediated mTORC2 inhibition is determined by the relative expression of FK506-binding proteins. Aging Cell 14, 265–73 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarbassov DD et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell 22, 159–68 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Gaubitz C et al. Molecular basis of the rapamycin insensitivity of target of rapamycin complex 2. Mol Cell 58, 977–88 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Zeng Z et al. Rapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AML. Blood 109, 3509–12 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldman ME et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol 7, e38 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunn GJ et al. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J 15, 5256–67 (1996). [PMC free article] [PubMed] [Google Scholar]
- 19.Yu K et al. Biochemical, cellular, and in vivo activity of novel ATP-competitive and selective inhibitors of the mammalian target of rapamycin. Cancer Res 69, 6232–40 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Bendell JC et al. A phase I dose-escalation study to assess safety, tolerability, pharmacokinetics, and preliminary efficacy of the dual mTORC1/mTORC2 kinase inhibitor CC-223 in patients with advanced solid tumors or multiple myeloma. Cancer 121, 3481–90 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pavenstadt H, Kriz W & Kretzler M Cell biology of the glomerular podocyte. Physiol Rev 83, 253–307 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Godel M et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Invest 121, 2197–209 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoki K et al. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest 121, 2181–96 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groth CG et al. Sirolimus (rapamycin)-based therapy in human renal transplantation: similar efficacy and different toxicity compared with cyclosporine. Sirolimus European Renal Transplant Study Group. Transplantation 67, 1036–42 (1999). [DOI] [PubMed] [Google Scholar]
- 25.Schwarz C, Bohmig GA, Steininger R, Mayer G & Oberbauer R Impaired phosphate handling of renal allografts is aggravated under rapamycin-based immunosuppression. Nephrol Dial Transplant 16, 378–82 (2001). [DOI] [PubMed] [Google Scholar]
- 26.da Silva CA et al. Rosiglitazone prevents sirolimus-induced hypomagnesemia, hypokalemia, and downregulation of NKCC2 protein expression. Am J Physiol Renal Physiol 297, F916–22 (2009). [DOI] [PubMed] [Google Scholar]
- 27.Wu MS, Hung CC & Chang CT Renal calcium handling after rapamycin conversion in chronic allograft dysfunction. Transpl Int 19, 140–5 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Tataranni T et al. Rapamycin-induced hypophosphatemia and insulin resistance are associated with mTORC2 activation and Klotho expression. Am J Transplant 11, 1656–64 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Haller M et al. Sirolimus induced phosphaturia is not caused by inhibition of renal apical sodium phosphate cotransporters. PLoS One 7, e39229 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kempe DS et al. Rapamycin-induced phosphaturia. Nephrol Dial Transplant 25, 2938–44 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Gleixner EM et al. V-ATPase/mTOR signaling regulates megalin-mediated apical endocytosis. Cell Rep 8, 10–9 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Grahammer F et al. mTOR Regulates Endocytosis and Nutrient Transport in Proximal Tubular Cells. J Am Soc Nephrol In press (2016). [DOI] [PMC free article] [PubMed]
- 33.Grahammer F et al. mTORC1 maintains renal tubular homeostasis and is essential in response to ischemic stress. Proc Natl Acad Sci U S A 111, E2817–26 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lang F & Pearce D Regulation of the epithelial Na+ channel by the mTORC2/SGK1 pathway. Nephrol Dial Transplant (2015). [DOI] [PMC free article] [PubMed]
- 35.Lu M et al. mTOR complex-2 activates ENaC by phosphorylating SGK1. J Am Soc Nephrol 21, 811–8 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gleason CE et al. mTORC2 regulates renal tubule sodium uptake by promoting ENaC activity. J Clin Invest 125, 117–28 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grahammer F et al. mTORC2 critically regulates renal potassium handling. J Clin Invest 126, 1773–82 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canaud G et al. Inhibition of the mTORC pathway in the antiphospholipid syndrome. N Engl J Med 371, 303–12 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Jindra PT, Jin YP, Rozengurt E & Reed EF HLA class I antibody-mediated endothelial cell proliferation via the mTOR pathway. J Immunol 180, 2357–66 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Jindra PT, Jin YP, Jacamo R, Rozengurt E & Reed EF MHC class I and integrin ligation induce ERK activation via an mTORC2-dependent pathway. Biochem Biophys Res Commun 369, 781–7 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Sandes-Freitas TV et al. Subclinical lesions and donor-specific antibodies in kidney transplant recipients receiving tacrolimus-based immunosuppressive regimen followed by early conversion to sirolimus. Transplantation 99, 2372–81 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Liefeldt L et al. Donor-specific HLA antibodies in a cohort comparing everolimus with cyclosporine after kidney transplantation. Am J Transplant 12, 1192–8 (2012). [DOI] [PubMed] [Google Scholar]
- 43.Croze LE et al. Conversion to mammalian target of rapamycin inhibitors increases risk of de novo donor-specific antibodies. Transpl Int 27, 775–83 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Lepin EJ et al. Phosphorylated S6 ribosomal protein: a novel biomarker of antibody-mediated rejection in heart allografts. Am J Transplant 6, 1560–71 (2006). [DOI] [PubMed] [Google Scholar]
- 45.Jindra PT et al. Anti-MHC class I antibody activation of proliferation and survival signaling in murine cardiac allografts. J Immunol 180, 2214–24 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim YC & Guan KL mTOR: a pharmacologic target for autophagy regulation. J Clin Invest 125, 25–32 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ganley IG et al. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J Biol Chem 284, 12297–305 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hosokawa N et al. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell 20, 1981–91 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jung CH et al. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell 20, 1992–2003 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fougeray S & Pallet N Mechanisms and biological functions of autophagy in diseased and ageing kidneys. Nat Rev Nephrol 11, 34–45 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Hartleben B et al. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest 120, 1084–96 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawakami T et al. Deficient autophagy results in mitochondrial dysfunction and FSGS. J Am Soc Nephrol 26, 1040–52 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kimura T et al. Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J Am Soc Nephrol 22, 902–13 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fabrizio P, Pozza F, Pletcher SD, Gendron CM & Longo VD Regulation of longevity and stress resistance by Sch9 in yeast. Science 292, 288–90 (2001). [DOI] [PubMed] [Google Scholar]
- 55.Vellai T et al. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature 426, 620 (2003). [DOI] [PubMed] [Google Scholar]
- 56.Kapahi P et al. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol 14, 885–90 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harrison DE et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–5 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson SC, Rabinovitch PS & Kaeberlein M mTOR is a key modulator of ageing and age-related disease. Nature 493, 338–45 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhuo L et al. Expression and mechanism of mammalian target of rapamycin in age-related renal cell senescence and organ aging. Mech Ageing Dev 130, 700–8 (2009). [DOI] [PubMed] [Google Scholar]
- 60.Zhang S et al. SIRT1 is required for the effects of rapamycin on high glucose-inducing mesangial cells senescence. Mech Ageing Dev 133, 387–400 (2012). [DOI] [PubMed] [Google Scholar]
- 61.Weichhart T, Hengstschlager M & Linke M Regulation of innate immune cell function by mTOR. Nat Rev Immunol 15, 599–614 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fantus D & Thomson AW Evolving perspectives of mTOR complexes in immunity and transplantation. Am J Transplant 15, 891–902 (2015). [DOI] [PubMed] [Google Scholar]
- 63.Lo YC, Lee CF & Powell JD Insight into the role of mTOR and metabolism in T cells reveals new potential approaches to preventing graft rejection. Curr Opin Organ Transplant 19, 363–71 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Turner JE, Paust HJ, Steinmetz OM & Panzer U The Th17 immune response in renal inflammation. Kidney Int 77, 1070–5 (2010). [DOI] [PubMed] [Google Scholar]
- 65.Rogers NM, Ferenbach DA, Isenberg JS, Thomson AW & Hughes J Dendritic cells and macrophages in the kidney: a spectrum of good and evil. Nat Rev Nephrol 10, 625–43 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park CO & Kupper TS The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med 21, 688–97 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Batal I et al. Dendritic cells in kidney transplant biopsy samples are associated with T cell infiltration and poor allograft survival. J Am Soc Nephrol 26, 3102–13 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Turnquist HR et al. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J Immunol 178, 7018–31 (2007). [DOI] [PubMed] [Google Scholar]
- 69.Hackstein H et al. Rapamycin inhibits IL-4--induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo. Blood 101, 4457–63 (2003). [DOI] [PubMed] [Google Scholar]
- 70.Turnquist HR et al. mTOR and GSK-3 shape the CD4+ T-cell stimulatory and differentiation capacity of myeloid DCs after exposure to LPS. Blood 115, 4758–69 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Amiel E et al. Inhibition of mechanistic target of rapamycin promotes dendritic cell activation and enhances therapeutic autologous vaccination in mice. J Immunol 189, 2151–8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brown J, Wang H, Suttles J, Graves DT & Martin M Mammalian target of rapamycin complex 2 (mTORC2) negatively regulates Toll-like receptor 4-mediated inflammatory response via FoxO1. J Biol Chem 286, 44295–305 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Raich-Regue D et al. mTORC2 deficiency in myeloid dendritic cells enhances their allogeneic Th1 and Th17 stimulatory ability after TLR4 ligation in vitro and in vivo. J Immunol 194, 4767–76 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Raich-Regue D et al. Intratumoral delivery of mTORC2-deficient dendritic cells inhibits B16 melanoma growth by promoting CD8C effector T cell responses. Oncoimmunology (In Press). [DOI] [PMC free article] [PubMed]
- 75.Chi H Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol 12, 325–38 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee K et al. Vital roles of mTOR complex 2 in Notch-driven thymocyte differentiation and leukemia. J Exp Med 209, 713–28 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tian L, Lu L, Yuan Z, Lamb JR & Tam PK Acceleration of apoptosis in CD4+CD8+ thymocytes by rapamycin accompanied by increased CD4+CD25+ T cells in the periphery. Transplantation 77, 183–9 (2004). [DOI] [PubMed] [Google Scholar]
- 78.Haxhinasto S, Mathis D & Benoist C The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med 205, 565–74 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Battaglia M, Stabilini A & Roncarolo MG Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood 105, 4743–8 (2005). [DOI] [PubMed] [Google Scholar]
- 80.Zeng H et al. mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature 499, 485–90 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Delgoffe GM et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 30, 832–44 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Delgoffe GM et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol 12, 295–303 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ray JP et al. The Interleukin-2-mTORc1 Kinase Axis Defines the Signaling, Differentiation, and Metabolism of T Helper 1 and Follicular B Helper T Cells. Immunity 43, 690–702 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matz M et al. Effects of sotrastaurin, mycophenolic acid and everolimus on human B-lymphocyte function and activation. Transpl Int 25, 1106–16 (2012). [DOI] [PubMed] [Google Scholar]
- 85.Benhamron S & Tirosh B Direct activation of mTOR in B lymphocytes confers impairment in B-cell maturation andloss of marginal zone B cells. Eur J Immunol 41, 2390–6 (2011). [DOI] [PubMed] [Google Scholar]
- 86.Lee K et al. Requirement for Rictor in homeostasis and function of mature B lymphoid cells. Blood 122, 2369–79 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Keating R et al. The kinase mTOR modulates the antibody response to provide cross-protective immunity to lethal infection with influenza virus. Nat Immunol 14, 1266–76 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Limon JJ et al. mTOR kinase inhibitors promote antibody class switching via mTORC2 inhibition. Proc Natl Acad Sci U S A 111, E5076–85 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jin YP, Valenzuela NM, Ziegler ME, Rozengurt E & Reed EF Everolimus inhibits anti-HLA I antibody-mediated endothelial cell signaling, migration and proliferation more potently than sirolimus. Am J Transplant 14, 806–19 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marcais A et al. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nat Immunol 15, 749–57 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shin BH et al. Regulation of anti-HLA antibody-dependent natural killer cell activation by immunosuppressive agents. Transplantation 97, 294–300 (2014). [DOI] [PubMed] [Google Scholar]
- 92.Prevot N et al. Mammalian target of rapamycin complex 2 regulates invariant NKT cell development and function independent of promyelocytic leukemia zinc-finger. J Immunol 194, 223–30 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ochiai T et al. Effects of rapamycin in experimental organ allografting. Transplantation 56, 15–9 (1993). [DOI] [PubMed] [Google Scholar]
- 94.Stepkowski SM, Chen H, Daloze P & Kahan BD Rapamycin, a potent immunosuppressive drug for vascularized heart, kidney, and small bowel transplantation in the rat. Transplantation 51, 22–6 (1991). [DOI] [PubMed] [Google Scholar]
- 95.Poston RS et al. Rapamycin reverses chronic graft vascular disease in a novel cardiac allograft model. Circulation 100, 67–74 (1999). [DOI] [PubMed] [Google Scholar]
- 96.Ikonen TS et al. Sirolimus (rapamycin) halts and reverses progression of allograft vascular disease in non-human primates. Transplantation 70, 969–75 (2000). [DOI] [PubMed] [Google Scholar]
- 97.Nakamura T, Nakao T, Yoshimura N & Ashihara E Rapamycin prolongs cardiac allograft survival in a mouse model by inducing myeloid-derived suppressor cells. Am J Transplant 15, 2364–77 (2015). [DOI] [PubMed] [Google Scholar]
- 98.Page A et al. CD40 blockade combines with CTLA4Ig and sirolimus to produce mixed chimerism in an MHC-defined rhesus macaque transplant model. Am J Transplant 12, 115–25 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pan H et al. The second-generation mTOR kinase inhibitor INK128 exhibits anti-inflammatory activity in lipopolysaccharide-activated RAW 264.7 cells. Inflammation 37, 756–65 (2014). [DOI] [PubMed] [Google Scholar]
- 100.Rosborough BR et al. Adenosine triphosphate-competitive mTOR inhibitors: a new class of immunosuppressive agents that inhibit allograft rejection. Am J Transplant 14, 2173–80 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang L et al. Abrogation of chronic rejection in rat model system involves modulation of the mTORC1 and mTORC2 pathways. Transplantation 96, 782–90 (2013). [DOI] [PubMed] [Google Scholar]
- 102.Murgia MG, Jordan S & Kahan BD The side effect profile of sirolimus: a phase I study in quiescent cyclosporine-prednisone-treated renal transplant patients. Kidney Int 49, 209–16 (1996). [DOI] [PubMed] [Google Scholar]
- 103.MacDonald AS A worldwide, phase III, randomized, controlled, safety and efficacy study of a sirolimus/cyclosporine regimen for prevention of acute rejection in recipients of primary mismatched renal allografts. Transplantation 71, 271–80 (2001). [DOI] [PubMed] [Google Scholar]
- 104.Kahan BD, Julian BA, Pescovitz MD, Vanrenterghem Y & Neylan J Sirolimus reduces the incidence of acute rejection episodes despite lower cyclosporine doses in caucasian recipients of mismatched primary renal allografts: a phase II trial. Rapamune Study Group. Transplantation 68, 1526–32 (1999). [DOI] [PubMed] [Google Scholar]
- 105.Kahan BD Efficacy of sirolimus compared with azathioprine for reduction of acute renal allograft rejection: a randomised multicentre study. The Rapamune US Study Group. Lancet 356, 194–202 (2000). [DOI] [PubMed] [Google Scholar]
- 106.Kreis H et al. Sirolimus in association with mycophenolate mofetil induction for the prevention of acute graft rejection in renal allograft recipients. Transplantation 69, 1252–60 (2000). [DOI] [PubMed] [Google Scholar]
- 107.Kreis H et al. Long-term benefits with sirolimus-based therapy after early cyclosporine withdrawal. J Am Soc Nephrol 15, 809–17 (2004). [DOI] [PubMed] [Google Scholar]
- 108.Ekberg H et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 357, 2562–75 (2007). [DOI] [PubMed] [Google Scholar]
- 109.Flechner SM et al. The ORION study: comparison of two sirolimus-based regimens versus tacrolimus and mycophenolate mofetil in renal allograft recipients. Am J Transplant 11, 1633–44 (2011). [DOI] [PubMed] [Google Scholar]
- 110.Webster AC, Lee VW, Chapman JR & Craig JC Target of rapamycin inhibitors (TOR-I; sirolimus and everolimus) for primary immunosuppression in kidney transplant recipients. Cochrane Database Syst Rev, CD004290 (2006). [DOI] [PubMed]
- 111.Lim WH et al. A systematic review of conversion from calcineurin inhibitor to mammalian target of rapamycin inhibitors for maintenance immunosuppression in kidney transplant recipients. Am J Transplant 14, 2106–19 (2014). [DOI] [PubMed] [Google Scholar]
- 112.Su L et al. Everolimus-based calcineurin-inhibitor sparing regimens for kidney transplant recipients: a systematic review and meta-analysis. Int Urol Nephrol 46, 2035–44 (2014). [DOI] [PubMed] [Google Scholar]
- 113.Murakami N, Riella LV & Funakoshi T Risk of metabolic complications in kidney transplantation after conversion to mTOR inhibitor: a systematic review and meta-analysis. Am J Transplant 14, 2317–27 (2014). [DOI] [PubMed] [Google Scholar]
- 114.Mandelbrot DA et al. Effect of Ramipril on Urinary Protein Excretion in Maintenance Renal Transplant Patients Converted to Sirolimus. Am J Transplant 15, 3174–84 (2015). [DOI] [PubMed] [Google Scholar]
- 115.Lewkowicz N et al. Dysfunction of CD4+CD25high T regulatory cells in patients with recurrent aphthous stomatitis. J Oral Pathol Med 37, 454–61 (2008). [DOI] [PubMed] [Google Scholar]
- 116.Sabbatini M et al. Oscillatory mTOR inhibition and Treg increase in kidney transplantation. Clin Exp Immunol 182, 230–40 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Morelon E et al. Characteristics of sirolimus-associated interstitial pneumonitis in renal transplant patients. Transplantation 72, 787–90 (2001). [DOI] [PubMed] [Google Scholar]
- 118.Joannides R et al. Immunosuppressant regimen based on sirolimus decreases aortic stiffness in renal transplant recipients in comparison to cyclosporine. Am J Transplant 11, 2414–22 (2011). [DOI] [PubMed] [Google Scholar]
- 119.Thierry A et al. Long-term impact of subclinical inflammation diagnosed by protocol biopsy one year after renal transplantation. Am J Transplant 11, 2153–61 (2011). [DOI] [PubMed] [Google Scholar]
- 120.Heilman RL et al. Results of a prospective randomized trial of sirolimus conversion in kidney transplant recipients on early corticosteroid withdrawal. Transplantation 92, 767–73 (2011). [DOI] [PubMed] [Google Scholar]
- 121.Glotz D et al. Thymoglobulin induction and sirolimus versus tacrolimus in kidney transplant recipients receiving mycophenolate mofetil and steroids. Transplantation 89, 1511–7 (2010). [DOI] [PubMed] [Google Scholar]
- 122.Li H & Pauza CD Rapamycin increases the yield and effector function of human gammadelta T cells stimulated in vitro. Cancer Immunol Immunother 60, 361–70 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Clippinger AJ, Maguire TG & Alwine JC The changing role of mTOR kinase in the maintenance of protein synthesis during human cytomegalovirus infection. J Virol 85, 3930–9 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Araki K et al. mTOR regulates memory CD8 T-cell differentiation. Nature 460, 108–12 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hirsch HH, Yakhontova K, Lu M & Manzetti J BK polyomavirus replication in renal tubular epithelial cells is inhibited by sirolimus, but activated by tacrolimus through a pathway involving FKBP-12. Am J Transplant (2015). [DOI] [PMC free article] [PubMed]
- 126.Ferrer IR et al. Cutting edge: Rapamycin augments pathogen-specific but not graft-reactive CD8+ T cell responses. J Immunol 185, 2004–8 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nashan B et al. Review of cytomegalovirus infection findings with mammalian target of rapamycin inhibitor-based immunosuppressive therapy in de novo renal transplant recipients. Transplantation 93, 1075–85 (2012). [DOI] [PubMed] [Google Scholar]
- 128.Tedesco-Silva H et al. Reduced incidence of cytomegalovirus infection in kidney transplant recipients receiving everolimus and reduced tacrolimus doses. Am J Transplant 15, 2655–64 (2015). [DOI] [PubMed] [Google Scholar]
- 129.Polanco N et al. Everolimus-based immunosuppression therapy for BK virus nephropathy. Transplant Proc 47, 57–61 (2015). [DOI] [PubMed] [Google Scholar]
- 130.Knoll GA et al. Effect of sirolimus on malignancy and survival after kidney transplantation: systematic review and meta-analysis of individual patient data. BMJ 349, g6679 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Diekmann F et al. Treatment with sirolimus is associated with less weight gain after kidney transplantation. Transplantation 96, 480–6 (2013). [DOI] [PubMed] [Google Scholar]
- 132.Halleck F et al. An evaluation of sirolimus in renal transplantation. Expert Opin Drug Metab Toxicol 8, 1337–56 (2012). [DOI] [PubMed] [Google Scholar]
- 133.Susantitaphong P et al. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol 8, 1482–93 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rimes-Stigare C et al. Evolution of chronic renal impairment and long-term mortality after de novo acute kidney injury in the critically ill; a Swedish multi-centre cohort study. Crit Care 19, 221 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Himmelfarb J & Ikizler TA Acute kidney injury: changing lexicography, definitions, and epidemiology. Kidney Int 71, 971–6 (2007). [DOI] [PubMed] [Google Scholar]
- 136.Smith KD et al. Delayed graft function and cast nephropathy associated with tacrolimus plus rapamycin use. J Am Soc Nephrol 14, 1037–45 (2003). [DOI] [PubMed] [Google Scholar]
- 137.McTaggart RA et al. Sirolimus prolongs recovery from delayed graft function after cadaveric renal transplantation. Am J Transplant 3, 416–23 (2003). [DOI] [PubMed] [Google Scholar]
- 138.Loverre A et al. Ischemia-reperfusion induces glomerular and tubular activation of proinflammatory and antiapoptotic pathways: differential modulation by rapamycin. J Am Soc Nephrol 15, 2675–86 (2004). [DOI] [PubMed] [Google Scholar]
- 139.Goncalves GM et al. The role of immunosuppressive drugs in aggravating renal ischemia and reperfusion injury. Transplant Proc 39, 417–20 (2007). [DOI] [PubMed] [Google Scholar]
- 140.Fuller TF et al. Sirolimus delays recovery of rat kidney transplants after ischemia-reperfusion injury. Transplantation 76, 1594–9 (2003). [DOI] [PubMed] [Google Scholar]
- 141.Inman SR et al. Rapamycin preserves renal function compared with cyclosporine A after ischemia/reperfusion injury. Urology 62, 750–4 (2003). [DOI] [PubMed] [Google Scholar]
- 142.Cicora F et al. Sirolimus in kidney transplant donors and clinical and histologic improvement in recipients: rat model. Transplant Proc 42, 365–70 (2010). [DOI] [PubMed] [Google Scholar]
- 143.Lieberthal W et al. Rapamycin impairs recovery from acute renal failure: role of cell-cycle arrest and apoptosis of tubular cells. Am J Physiol Renal Physiol 281, F693–706 (2001). [DOI] [PubMed] [Google Scholar]
- 144.Lui SL et al. Effect of rapamycin on renal ischemia-reperfusion injury in mice. Transpl Int 19, 834–9 (2006). [DOI] [PubMed] [Google Scholar]
- 145.Yang B et al. Inflammation and caspase activation in long-term renal ischemia/reperfusion injury and immunosuppression in rats. Kidney Int 68, 2050–67 (2005). [DOI] [PubMed] [Google Scholar]
- 146.Khan S et al. Rapamycin confers preconditioning-like protection against ischemia-reperfusion injury in isolated mouse heart and cardiomyocytes. J Mol Cell Cardiol 41, 256–64 (2006). [DOI] [PubMed] [Google Scholar]
- 147.Cicora F et al. Preconditioning donor with a combination of tacrolimus and rapamacyn to decrease ischaemia-reperfusion injury in a rat syngenic kidney transplantation model. Clin Exp Immunol 167, 169–77 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Viklicky O et al. Effect of sirolimus on renal ischaemia/reperfusion injury in normotensive and hypertensive rats. Transpl Int 17, 432–41 (2004). [DOI] [PubMed] [Google Scholar]
- 149.Goncalves GM et al. The role of heme oxygenase 1 in rapamycin-induced renal dysfunction after ischemia and reperfusion injury. Kidney Int 70, 1742–9 (2006). [DOI] [PubMed] [Google Scholar]
- 150.Nakagawa S, Nishihara K, Inui K & Masuda S Involvement of autophagy in the pharmacological effects of the mTOR inhibitor everolimus in acute kidney injury. Eur J Pharmacol 696, 143–54 (2012). [DOI] [PubMed] [Google Scholar]
- 151.Kezic A, Thaiss F, Becker JU, Tsui TY & Bajcetic M Effects of everolimus on oxidative stress in kidney model of ischemia/reperfusion injury. Am J Nephrol 37, 291–301 (2013). [DOI] [PubMed] [Google Scholar]
- 152.Izzedine H et al. Acute tubular necrosis associated with mTOR inhibitor therapy: a real entity biopsy-proven. Ann Oncol 24, 2421–5 (2013). [DOI] [PubMed] [Google Scholar]
- 153.Rai P et al. mTOR plays a critical role in p53-induced oxidative kidney cell injury in HIVAN. Am J Physiol Renal Physiol 305, F343–54 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Axelsson J, Rippe A & Rippe B Acute hyperglycemia induces rapid, reversible increases in glomerular permeability in nondiabetic rats. Am J Physiol Renal Physiol 298, F1306–12 (2010). [DOI] [PubMed] [Google Scholar]
- 155.Kuwabara A, Satoh M, Tomita N, Sasaki T & Kashihara N Deterioration of glomerular endothelial surface layer induced by oxidative stress is implicated in altered permeability of macromolecules in Zucker fatty rats. Diabetologia 53, 2056–65 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.McCarthy ET et al. TNF-alpha increases albumin permeability of isolated rat glomeruli through the generation of superoxide. J Am Soc Nephrol 9, 433–8 (1998). [DOI] [PubMed] [Google Scholar]
- 157.Axelsson J, Rippe A, Oberg CM & Rippe B Rapid, dynamic changes in glomerular permeability to macromolecules during systemic angiotensin II (ANG II) infusion in rats. Am J Physiol Renal Physiol 303, F790–9 (2012). [DOI] [PubMed] [Google Scholar]
- 158.Axelsson J, Rippe A & Rippe B mTOR inhibition with temsirolimus causes acute increases in glomerular permeability, but inhibits the dynamic permeability actions of puromycin aminonucleoside. Am J Physiol Renal Physiol 308, F1056–64 (2015). [DOI] [PubMed] [Google Scholar]
- 159.Stylianou K et al. Rapamycin induced ultrastructural and molecular alterations in glomerular podocytes in healthy mice. Nephrol Dial Transplant 27, 3141–8 (2012). [DOI] [PubMed] [Google Scholar]
- 160.DiJoseph JF, Sharma RN & Chang JY The effect of rapamycin on kidney function in the Sprague-Dawley rat. Transplantation 53, 507–13 (1992). [DOI] [PubMed] [Google Scholar]
- 161.Di Joseph JF & Sehgal SN Functional and histopathologic effects of rapamycin on mouse kidney. Immunopharmacol Immunotoxicol 15, 45–56 (1993). [DOI] [PubMed] [Google Scholar]
- 162.Andoh TF, Burdmann EA, Fransechini N, Houghton DC & Bennett WM Comparison of acute rapamycin nephrotoxicity with cyclosporine and FK506. Kidney Int 50, 1110–7 (1996). [DOI] [PubMed] [Google Scholar]
- 163.DiJoseph JF, Mihatsch MJ & Sehgal SN Renal effects of rapamycin in the spontaneously hypertensive rat. Transpl Int 7, 83–8 (1994). [DOI] [PubMed] [Google Scholar]
- 164.Grahammer F, Wanner N & Huber TB mTOR controls kidney epithelia in health and disease. Nephrol Dial Transplant 29 Suppl 1, i9–i18 (2014). [DOI] [PubMed] [Google Scholar]
- 165.Mao J et al. Mammalian target of rapamycin complex 1 activation in podocytes promotes cellular crescent formation. Am J Physiol Renal Physiol 307, F1023–32 (2014). [DOI] [PubMed] [Google Scholar]
- 166.Jeruschke S et al. Protective effects of the mTOR inhibitor everolimus on cytoskeletal injury in human podocytes are mediated by RhoA signaling. PLoS One 8, e55980 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Letavernier E et al. Sirolimus interacts with pathways essential for podocyte integrity. Nephrol Dial Transplant 24, 630–8 (2009). [DOI] [PubMed] [Google Scholar]
- 168.Muller-Krebs S et al. Cellular effects of everolimus and sirolimus on podocytes. PLoS One 8, e80340 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vollenbroker B et al. mTOR regulates expression of slit diaphragm proteins and cytoskeleton structure in podocytes. Am J Physiol Renal Physiol 296, F418–26 (2009). [DOI] [PubMed] [Google Scholar]
- 170.Canaud G et al. AKT2 is essential to maintain podocyte viability and function during chronic kidney disease. Nat Med 19, 1288–96 (2013). [DOI] [PubMed] [Google Scholar]
- 171.Narres M et al. The incidence of end-stage renal disease in the diabetic (compared to the non-diabetic) population: A systematic review. PLoS One 11, e0147329 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ibrahim HN & Hostetter TH Diabetic nephropathy. J Am Soc Nephrol 8, 487–93 (1997). [DOI] [PubMed] [Google Scholar]
- 173.Mori H et al. The mTOR pathway is highly activated in diabetic nephropathy and rapamycin has a strong therapeutic potential. Biochem Biophys Res Commun 384, 471–5 (2009). [DOI] [PubMed] [Google Scholar]
- 174.Chen JK, Chen J, Thomas G, Kozma SC & Harris RC S6 kinase 1 knockout inhibits uninephrectomy- or diabetes-induced renal hypertrophy. Am J Physiol Renal Physiol 297, F585–93 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sakaguchi M et al. Inhibition of mTOR signaling with rapamycin attenuates renal hypertrophy in the early diabetic mice. Biochem Biophys Res Commun 340, 296–301 (2006). [DOI] [PubMed] [Google Scholar]
- 176.Yang Y et al. Rapamycin prevents early steps of the development of diabetic nephropathy in rats. Am J Nephrol 27, 495–502 (2007). [DOI] [PubMed] [Google Scholar]
- 177.Lloberas N et al. Mammalian target of rapamycin pathway blockade slows progression of diabetic kidney disease in rats. J Am Soc Nephrol 17, 1395–404 (2006). [DOI] [PubMed] [Google Scholar]
- 178.Eid AA et al. Mammalian target of rapamycin regulates Nox4-mediated podocyte depletion in diabetic renal injury. Diabetes 62, 2935–47 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Stylianou K et al. The PI3K/Akt/mTOR pathway is activated in murine lupus nephritis and downregulated by rapamycin. Nephrol Dial Transplant 26, 498–508 (2011). [DOI] [PubMed] [Google Scholar]
- 180.Lui SL et al. Rapamycin attenuates the severity of established nephritis in lupus-prone NZB/W F1 mice. Nephrol Dial Transplant 23, 2768–76 (2008). [DOI] [PubMed] [Google Scholar]
- 181.Lui SL et al. Rapamycin prevents the development of nephritis in lupus-prone NZB/W F1 mice. Lupus 17, 305–13 (2008). [DOI] [PubMed] [Google Scholar]
- 182.Ramos-Barron A et al. Prevention of murine lupus disease in (NZBxNZW)F1 mice by sirolimus treatment. Lupus 16, 775–81 (2007). [DOI] [PubMed] [Google Scholar]
- 183.Warner LM, Adams LM & Sehgal SN Rapamycin prolongs survival and arrests pathophysiologic changes in murine systemic lupus erythematosus. Arthritis Rheum 37, 289–97 (1994). [DOI] [PubMed] [Google Scholar]
- 184.Yap DY, Ma MK, Tang CS & Chan TM Proliferation signal inhibitors in the treatment of lupus nephritis: preliminary experience. Nephrology (Carlton) 17, 676–80 (2012). [DOI] [PubMed] [Google Scholar]
- 185.Fernandez D, Bonilla E, Mirza N, Niland B & Perl A Rapamycin reduces disease activity and normalizes T cell activation-induced calcium fluxing in patients with systemic lupus erythematosus. Arthritis Rheum 54, 2983–8 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Greka A & Mundel P Cell biology and pathology of podocytes. Annu Rev Physiol 74, 299–323 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ito N et al. mTORC1 activation triggers the unfolded protein response in podocytes and leads to nephrotic syndrome. Lab Invest 91, 1584–95 (2011). [DOI] [PubMed] [Google Scholar]
- 188.Lui SL et al. Rapamycin attenuates the severity of murine adriamycin nephropathy. Am J Nephrol 29, 342–52 (2009). [DOI] [PubMed] [Google Scholar]
- 189.Ramadan R et al. Early treatment with everolimus exerts nephroprotective effect in rats with adriamycin-induced nephrotic syndrome. Nephrol Dial Transplant 27, 2231–41 (2012). [DOI] [PubMed] [Google Scholar]
- 190.Keller K et al. Everolimus inhibits glomerular endothelial cell proliferation and VEGF, but not long-term recovery in experimental thrombotic microangiopathy. Nephrol Dial Transplant 21, 2724–35 (2006). [DOI] [PubMed] [Google Scholar]
- 191.Bagchus WM, Hoedemaeker PJ, Rozing J & Bakker WW Glomerulonephritis induced by monoclonal anti-Thy 1.1 antibodies. A sequential histological and ultrastructural study in the rat. Lab Invest 55, 680–7 (1986). [PubMed] [Google Scholar]
- 192.Wittmann S et al. The mTOR inhibitor everolimus attenuates the time course of chronic anti-Thy1 nephritis in the rat. Nephron Exp Nephrol 108, e45–56 (2008). [DOI] [PubMed] [Google Scholar]
- 193.Daniel C, Ziswiler R, Frey B, Pfister M & Marti HP Proinflammatory effects in experimental mesangial proliferative glomerulonephritis of the immunosuppressive agent SDZ RAD, a rapamycin derivative. Exp Nephrol 8, 52–62 (2000). [DOI] [PubMed] [Google Scholar]
- 194.Daniel C et al. Mechanisms of everolimus-induced glomerulosclerosis after glomerular injury in the rat. Am J Transplant 5, 2849–61 (2005). [DOI] [PubMed] [Google Scholar]
- 195.Vogelbacher R, Wittmann S, Braun A, Daniel C & Hugo C The mTOR inhibitor everolimus induces proteinuria and renal deterioration in the remnant kidney model in the rat. Transplantation 84, 1492–9 (2007). [DOI] [PubMed] [Google Scholar]
- 196.Tian J, Wang Y, Liu X, Zhou X & Li R Rapamycin ameliorates IgA nephropathy via cell cycle-dependent mechanisms. Exp Biol Med (Maywood) 240, 936–45 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Heymann W, Hackel DB, Harwood S, Wilson SG & Hunter JL Production of nephrotic syndrome in rats by Freund’s adjuvants and rat kidney suspensions. Proc Soc Exp Biol Med 100, 660–4 (1959). [DOI] [PubMed] [Google Scholar]
- 198.Farquhar MG, Saito A, Kerjaschki D & Orlando RA The Heymann nephritis antigenic complex: megalin (gp330) and RAP. J Am Soc Nephrol 6, 35–47 (1995). [DOI] [PubMed] [Google Scholar]
- 199.Naumovic R et al. Effects of rapamycin on active Heymann nephritis. Am J Nephrol 27, 379–89 (2007). [DOI] [PubMed] [Google Scholar]
- 200.Stratakis S et al. Rapamycin ameliorates proteinuria and restores nephrin and podocin expression in experimental membranous nephropathy. Clin Dev Immunol 2013, 941893. [DOI] [PMC free article] [PubMed]
- 201.Kurayama R et al. Role of amino acid transporter LAT2 in the activation of mTORC1 pathway and the pathogenesis of crescentic glomerulonephritis. Lab Invest 91, 992–1006 (2011). [DOI] [PubMed] [Google Scholar]
- 202.Hochegger K et al. Differential effects of rapamycin in anti-GBM glomerulonephritis. J Am Soc Nephrol 19, 1520–9 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kirsch AH et al. The mTOR-inhibitor rapamycin mediates proteinuria in nephrotoxic serum nephritis by activating the innate immune response. Am J Physiol Renal Physiol 303, F569–75 (2012). [DOI] [PubMed] [Google Scholar]
- 204.Cruzado JM et al. Low-dose sirolimus combined with angiotensin-converting enzyme inhibitor and statin stabilizes renal function and reduces glomerular proliferation in poor prognosis IgA nephropathy. Nephrol Dial Transplant 26, 3596–602 (2011). [DOI] [PubMed] [Google Scholar]
- 205.Tumlin JA et al. A prospective, open-label trial of sirolimus in the treatment of focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 1, 109–16 (2006). [DOI] [PubMed] [Google Scholar]
- 206.Cho ME, Hurley JK & Kopp JB Sirolimus therapy of focal segmental glomerulosclerosis is associated with nephrotoxicity. Am J Kidney Dis 49, 310–7 (2007). [DOI] [PubMed] [Google Scholar]
- 207.Letavernier E et al. High sirolimus levels may induce focal segmental glomerulosclerosis de novo. Clin J Am Soc Nephrol 2, 326–33 (2007). [DOI] [PubMed] [Google Scholar]
- 208.Patel P, Pal S, Ashley C, Sweny P & Burns A Combination therapy with sirolimus (rapamycin) and tacrolimus (FK-506) in treatment of refractory minimal change nephropathy, a clinical case report. Nephrol Dial Transplant 20, 985–7 (2005). [DOI] [PubMed] [Google Scholar]
- 209.Ong AC, Devuyst O, Knebelmann B, Walz G & Diseases E.-E.W.G.f.I.K. Autosomal dominant polycystic kidney disease: the changing face of clinical management. Lancet 385, 1993–2002 (2015). [DOI] [PubMed] [Google Scholar]
- 210.Grantham JJ Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med 359, 1477–85 (2008). [DOI] [PubMed] [Google Scholar]
- 211.Hildebrandt F, Benzing T & Katsanis N Ciliopathies. N Engl J Med 364, 1533–43 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Torres VE et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367, 2407–18 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tao Y, Kim J, Schrier RW & Edelstein CL Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease. J Am Soc Nephrol 16, 46–51 (2005). [DOI] [PubMed] [Google Scholar]
- 214.Shillingford JM et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A 103, 5466–71 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Renken C, Fischer DC, Kundt G, Gretz N & Haffner D Inhibition of mTOR with sirolimus does not attenuate progression of liver and kidney disease in PCK rats. Nephrol Dial Transplant 26, 92–100 (2011). [DOI] [PubMed] [Google Scholar]
- 216.Belibi F, Ravichandran K, Zafar I, He Z & Edelstein CL mTORC1/2 and rapamycin in female Han:SPRD rats with polycystic kidney disease. Am J Physiol Renal Physiol 300, F236–44 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Distefano G et al. Polycystin-1 regulates extracellular signal-regulated kinase-dependent phosphorylation of tuberin to control cell size through mTOR and its downstream effectors S6K and 4EBP1. Mol Cell Biol 29, 2359–71 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Boehlke C et al. Primary cilia regulate mTORC1 activity and cell size through Lkb1. Nat Cell Biol 12, 1115–22 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Boletta A Emerging evidence of a link between the polycystins and the mTOR pathways. Pathogenetics 2, 6 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Takiar V et al. Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc Natl Acad Sci U S A 108, 2462–7 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Huber TB, Walz G & Kuehn EW mTOR and rapamycin in the kidney: signaling and therapeutic implications beyond immunosuppression. Kidney Int 79, 502–11 (2011). [DOI] [PubMed] [Google Scholar]
- 222.Serra AL et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med 363, 820–9 (2010). [DOI] [PubMed] [Google Scholar]
- 223.Walz G et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med 363, 830–40 (2010). [DOI] [PubMed] [Google Scholar]
- 224.Grantham JJ, Bennett WM & Perrone RD mTOR inhibitors and autosomal dominant polycystic kidney disease. N Engl J Med 364, 286–7; author reply 287–9 (2011). [DOI] [PubMed] [Google Scholar]
- 225.Levey AS & Stevens LA mTOR inhibitors and autosomal dominant polycystic kidney disease. N Engl J Med 364, 287; author reply 287–9 (2011). [DOI] [PubMed] [Google Scholar]
- 226.Novalic Z et al. Dose-dependent effects of sirolimus on mTOR signaling and polycystic kidney disease. J Am Soc Nephrol 23, 842–53 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Perico N et al. Sirolimus therapy to halt the progression of ADPKD. J Am Soc Nephrol 21, 1031–40 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Riegersperger M, Herkner H & Sunder-Plassmann G Pulsed oral sirolimus in advanced autosomal-dominant polycystic kidney disease (Vienna RAP Study): study protocol for a randomized controlled trial. Trials 16, 182 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Braun WE, Schold JD, Stephany BR, Spirko RA & Herts BR Low-dose rapamycin (sirolimus) effects in autosomal dominant polycystic kidney disease: an open-label randomized controlled pilot study. Clin J Am Soc Nephrol 9, 881–8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ravichandran K et al. An mTOR anti-sense oligonucleotide decreases polycystic kidney disease in mice with a targeted mutation in Pkd2. Hum Mol Genet 23, 4919–31 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Jain S, Bicknell GR, Whiting PH & Nicholson ML Rapamycin reduces expression of fibrosis-associated genes in an experimental model of renal ischaemia reperfusion injury. Transplant Proc 33, 556–8 (2001). [DOI] [PubMed] [Google Scholar]
- 232.Jolicoeur EM et al. Combination therapy of mycophenolate mofetil and rapamycin in prevention of chronic renal allograft rejection in the rat. Transplantation 75, 54–9 (2003). [DOI] [PubMed] [Google Scholar]
- 233.Rangan GK & Coombes JD Renoprotective effects of sirolimus in non-immune initiated focal segmental glomerulosclerosis. Nephrol Dial Transplant 22, 2175–82 (2007). [DOI] [PubMed] [Google Scholar]
- 234.Wu MJ et al. Rapamycin attenuates unilateral ureteral obstruction-induced renal fibrosis. Kidney Int 69, 2029–36 (2006). [DOI] [PubMed] [Google Scholar]
- 235.Bonegio RG et al. Rapamycin ameliorates proteinuria-associated tubulointerstitial inflammation and fibrosis in experimental membranous nephropathy. J Am Soc Nephrol 16, 2063–72 (2005). [DOI] [PubMed] [Google Scholar]
- 236.Kramer S et al. Low-dose mTOR inhibition by rapamycin attenuates progression in anti-thy1-induced chronic glomerulosclerosis of the rat. Am J Physiol Renal Physiol 294, F440–9 (2008). [DOI] [PubMed] [Google Scholar]
- 237.Li J et al. Rictor/mTORC2 signaling mediates TGFbeta1-induced fibroblast activation and kidney fibrosis. Kidney Int 88, 515–27 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Grahammer F Halting renal fibrosis: an unexpected role for mTORC2 signaling. Kidney Int 88, 437–9 (2015). [DOI] [PubMed] [Google Scholar]
- 239.Chen G et al. Rapamycin ameliorates kidney fibrosis by inhibiting the activation of mTOR signaling in interstitial macrophages and myofibroblasts. PLoS One 7, e33626 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Rahimi RA et al. Distinct roles for mammalian target of rapamycin complexes in the fibroblast response to transforming growth factor-beta. Cancer Res 69, 84–93 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Jiang L et al. Rheb/mTORC1 signaling promotes kidney fibroblast activation and fibrosis. J Am Soc Nephrol 24, 1114–26 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Dey N, Ghosh-Choudhury N, Kasinath BS & Choudhury GG TGFbeta-stimulated microRNA-21 utilizes PTEN to orchestrate AKT/mTORC1 signaling for mesangial cell hypertrophy and matrix expansion. PLoS One 7, e42316 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Koch M, Mengel M, Poehnert D & Nashan B Effects of everolimus on cellular and humoral immune processes leading to chronic allograft nephropathy in a rat model with sensitized recipients. Transplantation 83, 498–505 (2007). [DOI] [PubMed] [Google Scholar]
- 244.Viklicky O et al. SDZ-RAD prevents manifestation of chronic rejection in rat renal allografts. Transplantation 69, 497–502 (2000). [DOI] [PubMed] [Google Scholar]
- 245.Lutz J, Zou H, Liu S, Antus B & Heemann U Apoptosis and treatment of chronic allograft nephropathy with everolimus. Transplantation 76, 508–15 (2003). [DOI] [PubMed] [Google Scholar]
- 246.Luo L, Sun Z & Luo G Rapamycin is less fibrogenic than Cyclosporin A as demonstrated in a rat model of chronic allograft nephropathy. J Surg Res 179, e255–63 (2013). [DOI] [PubMed] [Google Scholar]
- 247.Liu Q et al. Characterization of Torin2, an ATP-competitive inhibitor of mTOR, ATM, and ATR. Cancer Res 73, 2574–86 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Palin NK, Savikko J & Koskinen PK Sirolimus inhibits lymphangiogenesis in rat renal allografts, a novel mechanism to prevent chronic kidney allograft injury. Transpl Int 26, 195–205 (2013). [DOI] [PubMed] [Google Scholar]
- 249.Ghosh AK & Vaughan DE PAI-1 in tissue fibrosis. J Cell Physiol 227, 493–507 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Pontrelli P et al. Rapamycin inhibits PAI-1 expression and reduces interstitial fibrosis and glomerulosclerosis in chronic allograft nephropathy. Transplantation 85, 125–34 (2008). [DOI] [PubMed] [Google Scholar]
- 251.Diekmann F et al. Mammalian target of rapamycin inhibition halts the progression of proteinuria in a rat model of reduced renal mass. J Am Soc Nephrol 18, 2653–60 (2007). [DOI] [PubMed] [Google Scholar]
- 252.Kurdian M et al. Delayed mTOR inhibition with low dose of everolimus reduces TGFbeta expression, attenuates proteinuria and renal damage in the renal mass reduction model. PLoS One 7, e32516 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Budde K & Gaedeke J Tuberous sclerosis complex-associated angiomyolipomas: focus on mTOR inhibition. Am J Kidney Dis 59, 276–83 (2012). [DOI] [PubMed] [Google Scholar]
- 254.Hallett L, Foster T, Liu Z, Blieden M & Valentim J Burden of disease and unmet needs in tuberous sclerosis complex with neurological manifestations: systematic review. Curr Med Res Opin 27, 1571–83 (2011). [DOI] [PubMed] [Google Scholar]
- 255.Osborne JP, Fryer A & Webb D Epidemiology of tuberous sclerosis. Ann N Y Acad Sci 615, 125–7 (1991). [DOI] [PubMed] [Google Scholar]
- 256.Curatolo P et al. The eole of mTOR inhibitors in the treatment of patients with tuberous sclerosis complex: Evidence-based and expert opinions. Drugs 76, 551–65 (2016). [DOI] [PubMed] [Google Scholar]
- 257.Kotulska K, Borkowska J & Jozwiak S Possible prevention of tuberous sclerosis complex lesions. Pediatrics 132, e239–42 (2013). [DOI] [PubMed] [Google Scholar]
- 258.Bissler JJ et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med 358, 140–51 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Dabora SL et al. Multicenter phase 2 trial of sirolimus for tuberous sclerosis: kidney angiomyolipomas and other tumors regress and VEGF-D levels decrease. PLoS One 6, e23379 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Cabrera-Lopez C et al. Assessing the effectiveness of rapamycin on angiomyolipoma in tuberous sclerosis: a two years trial. Orphanet J Rare Dis 7, 87 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Staehler M et al. Nephron-sparing resection of angiomyolipoma after sirolimus pretreatment in patients with tuberous sclerosis. Int Urol Nephrol 44, 1657–61 (2012). [DOI] [PubMed] [Google Scholar]
- 262.Bissler JJ et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 381, 817–24 (2013). [DOI] [PubMed] [Google Scholar]
- 263.Bissler JJ et al. Everolimus for renal angiomyolipoma in patients with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis: extension of a randomized controlled trial. Nephrol Dial Transplant 31, 111–9 (2016). [DOI] [PubMed] [Google Scholar]
- 264.Pal SK & Quinn DI Differentiating mTOR inhibitors in renal cell carcinoma. Cancer Treat Rev 39, 709–19 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Hutson TE et al. Randomized phase III trial of temsirolimus versus sorafenib as second-line therapy after sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol 32, 760–7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Flaherty KT et al. BEST: A Randomized Phase II study of vascular endothelial growth factor, RAF kinase, and mammalian target of rapamycin combination targeted therapy with bevacizumab, sorafenib, and temsirolimus in advanced renal cell carcinoma--a trial of the ECOG-ACRIN Cancer Research Group (E2804). J Clin Oncol 33, 2384–91 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Wander SA, Hennessy BT & Slingerland JM Next-generation mTOR inhibitors in clinical oncology: how pathway complexity informs therapeutic strategy. J Clin Invest 121, 1231–41 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Zheng B et al. Pre-clinical evaluation of AZD-2014, a novel mTORC1/2 dual inhibitor, against renal cell carcinoma. Cancer Lett 357, 468–75 (2015). [DOI] [PubMed] [Google Scholar]
- 269.Powles T et al. A randomised Phase 2 Study of AZD2014 versus everolimus in patients with VEGF-refractory metastatic clear cell renal cancer. Eur Urol (2015). [DOI] [PubMed]
- 270.Guba M et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med 8, 128–35 (2002). [DOI] [PubMed] [Google Scholar]
- 271.Ghosh AP et al. Point mutations of the mTOR-RHEB pathway in renal cell carcinoma. Oncotarget 6, 17895–910 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Nichols LA, Adang LA & Kedes DH Rapamycin blocks production of KSHV/HHV8: insights into the anti-tumor activity of an immunosuppressant drug. PLoS One 6, e14535 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Roy S & Arav-Boger R New cell-signaling pathways for controlling cytomegalovirus replication. Am J Transplant 14, 1249–58 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Stallone G et al. Sirolimus for Kaposi’s sarcoma in renal-transplant recipients. N Engl J Med 352, 1317–23 (2005). [DOI] [PubMed] [Google Scholar]
- 275.Geissler EK Post-transplantation malignancies: here today, gone tomorrow? Nat Rev Clin Oncol 12, 705–17 (2015). [DOI] [PubMed] [Google Scholar]
- 276.Geissler EK et al. Sirolimus Use in Liver Transplant Recipients With Hepatocellular Carcinoma: A Randomized, Multicenter, Open-Label Phase 3 Trial. Transplantation 100, 116–25 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Halleck F & Budde K Transplantation: Sirolimus for secondary SCC prevention in renal transplantation. Nat Rev Nephrol 8, 687–9 (2012). [DOI] [PubMed] [Google Scholar]
- 278.Hosseini-Moghaddam SM, Soleimanirahbar A, Mazzulli T, Rotstein C & Husain S Post renal transplantation Kaposi’s sarcoma: a review of its epidemiology, pathogenesis, diagnosis, clinical aspects, and therapy. Transpl Infect Dis 14, 338–45 (2012). [DOI] [PubMed] [Google Scholar]
- 279.Campbell SB, Walker R, Tai SS, Jiang Q & Russ GR Randomized controlled trial of sirolimus for renal transplant recipients at high risk for nonmelanoma skin cancer. Am J Transplant 12, 1146–56 (2012). [DOI] [PubMed] [Google Scholar]
- 280.Euvrard S et al. Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med 367, 329–39 (2012). [DOI] [PubMed] [Google Scholar]
- 281.Di Paolo S, Teutonico A, Ranieri E, Gesualdo L & Schena PF Monitoring antitumor efficacy of rapamycin in Kaposi sarcoma. Am J Kidney Dis 49, 462–70 (2007). [DOI] [PubMed] [Google Scholar]
- 282.Yanik EL et al. Sirolimus use and cancer incidence among US kidney transplant recipients. Am J Transplant 15, 129–36 (2015). [DOI] [PubMed] [Google Scholar]
- 283.Reitamo S et al. Efficacy of sirolimus (rapamycin) administered concomitantly with a subtherapeutic dose of cyclosporin in the treatment of severe psoriasis: a randomized controlled trial. Br J Dermatol 145, 438–45 (2001). [DOI] [PubMed] [Google Scholar]
- 284.Senior PA, Paty BW, Cockfield SM, Ryan EA & Shapiro AM Proteinuria developing after clinical islet transplantation resolves with sirolimus withdrawal and increased tacrolimus dosing. Am J Transplant 5, 2318–23 (2005). [DOI] [PubMed] [Google Scholar]
- 285.Constantinescu AR, Liang M & Laskow DA Sirolimus lowers myeloperoxidase and p-ANCA titers in a pediatric patient before kidney transplantation. Am J Kidney Dis 40, 407–10 (2002). [DOI] [PubMed] [Google Scholar]
- 286.Koening CL, Hernandez-Rodriguez J, Molloy ES, Clark TM & Hoffman GS Limited utility of rapamycin in severe, refractory Wegener’s granulomatosis. J Rheumatol 36, 116–9 (2009). [DOI] [PubMed] [Google Scholar]
- 287.Fervenza FC et al. Acute rapamycin nephrotoxicity in native kidneys of patients with chronic glomerulopathies. Nephrol Dial Transplant 19, 1288–92 (2004). [DOI] [PubMed] [Google Scholar]
- 288.Kim HJ & Edelstein CL Mammalian target of rapamycin inhibition in polycystic kidney disease: From bench to bedside. Kidney Res Clin Pract 31, 132–8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Lieberthal W, Fuhro R, Andry C, Patel V & Levine JS Rapamycin delays but does not prevent recovery from acute renal failure: role of acquired tubular resistance. Transplantation 82, 17–22 (2006). [DOI] [PubMed] [Google Scholar]
- 290.Lee L et al. Efficacy of a rapamycin analog (CCI-779) and IFN-gamma in tuberous sclerosis mouse models. Genes Chromosomes Cancer 42, 213–27 (2005). [DOI] [PubMed] [Google Scholar]
- 291.Zhang H et al. A comparison of Ku0063794, a dual mTORC1 and mTORC2 inhibitor, and temsirolimus in preclinical renal cell carcinoma models. PLoS One 8, e54918 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Chiarini F, Evangelisti C, McCubrey JA & Martelli AM Current treatment strategies for inhibiting mTOR in cancer. Trends Pharmacol Sci 36, 124–35 (2015). [DOI] [PubMed] [Google Scholar]
- 293.Hudes G et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 356, 2271–81 (2007). [DOI] [PubMed] [Google Scholar]
- 294.Motzer RJ et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 372, 449–56 (2008). [DOI] [PubMed] [Google Scholar]
- 295.Mita MM et al. Phase I trial of the novel mammalian target of rapamycin inhibitor deforolimus (AP23573; MK-8669) administered intravenously daily for 5 days every 2 weeks to patients with advanced malignancies. J Clin Oncol 26, 361–7 (2008). [DOI] [PubMed] [Google Scholar]
- 296.Atkins MB et al. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol 22, 909–18 (2004). [DOI] [PubMed] [Google Scholar]
- 297.Raich-Regue D, Rosborough BR, Turnquist HR & Thomson AW Myeloid dendritic cell-specific mTORC2 deficiency enhances alloreactive Th1 and Th17 cell responses and skin graft rejection. Am J Transplant 14, 18 (Abstract #584) (2014). [Google Scholar]
- 298.Weichhart T et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity 29, 565–77 (2008). [DOI] [PubMed] [Google Scholar]
- 299.Haidinger M et al. A versatile role of mammalian target of rapamycin in human dendritic cell function and differentiation. J Immunol 185, 3919–31 (2010). [DOI] [PubMed] [Google Scholar]
- 300.Cao W et al. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat Immunol 9, 1157–64 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Boor PP, Metselaar HJ, Mancham S, van der Laan LJ & Kwekkeboom J Rapamycin has suppressive and stimulatory effects on human plasmacytoid dendritic cell functions. Clin Exp Immunol 174, 389–401 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Lee K et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity 32, 743–53 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Berezhnoy A, Castro I, Levay A, Malek TR & Gilboa E Aptamer-targeted inhibition of mTOR in T cells enhances antitumor immunity. J Clin Invest 124, 188–97 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Liacini A, Seamone ME, Muruve DA & Tibbles LA Anti-BK virus mechanisms of sirolimus and leflunomide alone and in combination: toward a new therapy for BK virus infection. Transplantation 90, 1450–7 (2010). [DOI] [PubMed] [Google Scholar]
- 305.McInturff AM et al. Mammalian target of rapamycin regulates neutrophil extracellular trap formation via induction of hypoxia-inducible factor 1 alpha. Blood 120, 3118–25 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Vitiello D, Neagoe PE, Sirois MG & White M Effect of everolimus on the immunomodulation of the human neutrophil inflammatory response and activation. Cell Mol Immunol 12, 40–52 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.He Y et al. Mammalian target of rapamycin and Rictor control neutrophil chemotaxis by regulating Rac/Cdc42 activity and the actin cytoskeleton. Mol Biol Cell 24, 3369–80 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Liu L, Gritz D & Parent CA PKCbetaII acts downstream of chemoattractant receptors and mTORC2 to regulate cAMP production and myosin II activity in neutrophils. Mol Biol Cell 25, 1446–57 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Zhang L et al. Mammalian target of rapamycin complex 1 orchestrates invariant NKT cell differentiation and effector function. J Immunol 193, 1759–65 (2014). [DOI] [PubMed] [Google Scholar]
- 310.Wei J, Yang K & Chi H Cutting edge: Discrete functions of mTOR signaling in invariant NKT cell development and NKT17 fate decision. J Immunol 193, 4297–301 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Zhang S et al. Constitutive reductions in mTOR alter cell size, immune cell development, and antibody production. Blood 117, 1228–38 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Zhang Y et al. Rictor is required for early B cell development in bone marrow. PLoS One 9, e103970 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Lazorchak AS et al. Sin1-mTORC2 suppresses rag and il7r gene expression through Akt2 in B cells. Mol Cell 39, 433–43 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Wang C et al. Rapamycin-treated human endothelial cells preferentially activate allogeneic regulatory T cells. J Clin Invest 123, 1677–93 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Wang C et al. Rapamycin antagonizes TNF induction of VCAM-1 on endothelial cells by inhibiting mTORC2. J Exp Med 211, 395–404 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Johnson RW et al. Sirolimus allows early cyclosporine withdrawal in renal transplantation resulting in improved renal function and lower blood pressure. Transplantation 72, 777–86 (2001). [DOI] [PubMed] [Google Scholar]
- 317.Gonwa TA, Hricik DE, Brinker K, Grinyo JM & Schena FP Improved renal function in sirolimus-treated renal transplant patients after early cyclosporine elimination. Transplantation 74, 1560–7 (2002). [DOI] [PubMed] [Google Scholar]
- 318.Oberbauer R et al. Long-term improvement in renal function with sirolimus after early cyclosporine withdrawal in renal transplant recipients: 2-year results of the Rapamune Maintenance Regimen Study. Transplantation 76, 364–70 (2003). [DOI] [PubMed] [Google Scholar]
- 319.Oberbauer R et al. Early cyclosporine withdrawal from a sirolimus-based regimen results in better renal allograft survival and renal function at 48 months after transplantation. Transpl Int 18, 22–8 (2005). [DOI] [PubMed] [Google Scholar]
- 320.Larson TS et al. Complete avoidance of calcineurin inhibitors in renal transplantation: a randomized trial comparing sirolimus and tacrolimus. Am J Transplant 6, 514–22 (2006). [DOI] [PubMed] [Google Scholar]
- 321.Buchler M et al. Sirolimus versus cyclosporine in kidney recipients receiving thymoglobulin, mycophenolate mofetil and a 6-month course of steroids. Am J Transplant 7, 2522–31 (2007). [DOI] [PubMed] [Google Scholar]
- 322.Lebranchu Y et al. Five-year results of a randomized trial comparing de novo sirolimus and cyclosporine in renal transplantation: the SPIESSER study. Am J Transplant 12, 1801–10 (2012). [DOI] [PubMed] [Google Scholar]
- 323.Gatault P et al. Eight-year results of the Spiesser study, a randomized trial comparing de novo sirolimus and cyclosporine in renal transplantation. Transpl Int 29, 41–50 (2016). [DOI] [PubMed] [Google Scholar]
- 324.Ekberg H et al. Calcineurin inhibitor minimization in the Symphony study: observational results 3 years after transplantation. Am J Transplant 9, 1876–85 (2009). [DOI] [PubMed] [Google Scholar]
- 325.Lebranchu Y et al. Efficacy on renal function of early conversion from cyclosporine to sirolimus 3 months after renal transplantation: concept study. Am J Transplant 9, 1115–23 (2009). [DOI] [PubMed] [Google Scholar]
- 326.Lebranchu Y et al. Efficacy and safety of early cyclosporine conversion to sirolimus with continued MMF-four-year results of the Postconcept study. Am J Transplant 11, 1665–75 (2011). [DOI] [PubMed] [Google Scholar]
- 327.Guba M et al. Renal function, efficacy, and safety of sirolimus and mycophenolate mofetil after short-term calcineurin inhibitor-based quadruple therapy in de novo renal transplant patients: one-year analysis of a randomized multicenter trial. Transplantation 90, 175–83 (2010). [DOI] [PubMed] [Google Scholar]
- 328.Guba M et al. Early conversion to a sirolimus-based, calcineurin-inhibitor-free immunosuppression in the SMART trial: observational results at 24 and 36months after transplantation. Transpl Int 25, 416–23 (2012). [DOI] [PubMed] [Google Scholar]
- 329.Budde K et al. Everolimus-based, calcineurin-inhibitor-free regimen in recipients of de-novo kidney transplants: an open-label, randomised, controlled trial. Lancet 377, 837–47 (2011). [DOI] [PubMed] [Google Scholar]
- 330.Budde K et al. Five-year outcomes in kidney transplant patients converted from cyclosporine to everolimus: the randomized ZEUS study. Am J Transplant 15, 119–28 (2015). [DOI] [PubMed] [Google Scholar]
- 331.Holdaas H et al. Conversion of long-term kidney transplant recipients from calcineurin inhibitor therapy to everolimus: a randomized, multicenter, 24-month study. Transplantation 92, 410–8 (2011). [DOI] [PubMed] [Google Scholar]
- 332.Weir MR et al. Mycophenolate mofetil-based immunosuppression with sirolimus in renal transplantation: a randomized, controlled Spare-the-Nephron trial. Kidney Int 79, 897–907 (2011). [DOI] [PubMed] [Google Scholar]
- 333.Weir MR et al. Long-term follow-up of kidney transplant recipients in the spare-the-nephron-trial. Transplantation In press (2016). [DOI] [PubMed]
- 334.Mjornstedt L et al. Improved renal function after early conversion from a calcineurin inhibitor to everolimus: a randomized trial in kidney transplantation. Am J Transplant 12, 2744–53 (2012). [DOI] [PubMed] [Google Scholar]
- 335.Mjornstedt L et al. Renal function three years after early conversion from a calcineurin inhibitor to everolimus: results from a randomized trial in kidney transplantation. Transpl Int 28, 42–51 (2015). [DOI] [PubMed] [Google Scholar]
- 336.Budde K et al. Renal, efficacy and safety outcomes following late conversion of kidney transplant patients from calcineurin inhibitor therapy to everolimus: the randomized APOLLO study. Clin Nephrol 83, 11–21 (2015). [DOI] [PubMed] [Google Scholar]