Supplemental digital content is available in the text.
Key Words: RINm5F, pancreatic cell, iron overload, green tea; EGCG, insulin
Abstract
Objectives
We have investigated the efficacy of mono- and combined therapy with green tea extract (GTE) in mobilizing redox iron, scavenging reactive oxygen species (ROS), and improving insulin production in iron-loaded pancreatic cells.
Methods
Rat insulinoma pancreatic β-cells were iron-loaded using culture medium supplemented with either fetal bovine serum or ferric ammonium citrate and treated with various doses of GTE for epigallocatechin-3-gallate (EGCG) equivalence and in combination with iron chelators. Cellular iron, ROS, and secretory insulin were measured.
Results
The rat insulinoma pancreatic cells took up iron from fetal bovine serum more rapidly than ferric ammonium citrate. After treatment with GTE (0.23–2.29 μg EGCG equivalent), cellular levels of iron and ROS were dose dependently decreased. Importantly, secretory insulin levels were increased nearly 2.5-fold with 2.29 μg of EGCG equivalent GTE, indicating a recovery in insulin production.
Conclusions
Green tea EGCG ameliorated oxidative damage of iron-loaded β-cells by removing redox iron and free radicals and attenuating insulin production. The impact can result in the restoration of pancreatic functions and an increase in insulin production. Green tea extract exerts iron-chelating, free-radical scavenging, and pancreato-protective effects in the restoration of β-cell functions, all of which we believe can increase insulin production in diabetic β-thalassemia patients.
In β-thalassemia with iron overload, the unusual forms of iron including the cytosolic labile iron pool, the plasma nontransferrin bound iron, the labile plasma iron, and membrane nonheme iron appear to be redox active and toxic to the cells.1–5 These various forms of iron, particularly ferrous ion (Fe2+), catalyze the generation of reactive oxygen species (ROS) via Fenton reaction.6–8 Excessive iron is primarily accumulated in the liver, heart, and endocrine glands, consequently causing organ dysfunction.9,10 Iron chelators including deferoxamine (DFO), deferiprone (DFP), and deferasirox (DFX) are used to treat patients with iron overload to control tissue iron levels and to prevent oxidative tissue damage.11
Insulin deficiency and resistance have been reported in β-thalassemia patients with iron overload, possibly resulting from impaired pancreatic β-cell function.12,13 Such changes can lead to the disturbance of glucose homeostasis.14–16 Importantly, iron chelation therapy improves glucose homeostasis in diabetic β-thalassemia patients.17–21 Recently, we have demonstrated that Eltrombopag (a thrombopoietin receptor agonist with iron chelating properties) decreased cellular iron and ROS while increasing insulin production in a rat pancreatic cell line.22
Green tea extract (GTE) containing catechin (C), epicatechin (EC), epicatechin-3-gallate (ECG), epigallocatechin (EGC), gallocatechin-3-gallate (GCG), and epigallocatechin-3-gallate (EGCG) has been shown to possess iron-chelating properties as well as pancreatoprotective activities.23–26 The influence of green tea was investigated in pancreatic cells induced by high glucose, cytokine, and dioxin.27–29 In this work, we have reported on the effects of GTE on iron mobilization, ROS scavenging, and insulin production in iron-loaded pancreatic cells.
MATERIALS AND METHODS
Chemicals and Reagents
2′,7′-Dichlorohydrofluorescin diacetate (DCFH-DA), ferric ammonium citrate (FAC), bovine serum albumin (BSA), fetal bovine serum (FBS), l-ascorbic acid, and 3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-p,p′-disulfonic acid disodium salt hydrate (ferrozine) were acquired in the purest form available and purchased from Sigma-Aldrich Chemicals, Company (St Louis, Mo). In addition, DFO, DFP, DFX, C, EC, ECG, EGC, EGCG, gallic acid (GA), gallocatechin (GC), and GAG were purchased from Sigma-Aldrich Chemicals, Company. Lactate dehydrogenase (LDH) assay kit was purchased from Takara Bio Inc (Shiga, Japan). Gibco RPM1 1640 medium, Krebs Ringer bicarbonate (KRB) buffer pH 7.4, phosphate buffered saline (PBS) pH 7.0, and protease inhibitor cocktail were obtained from Thermo-Fisher Scientific Company (Waltham, Mass). A sandwich ELISA kit for rat insulin was purchased from Life Technologies Limited Company (Paisley, United Kingdom). Rat insulinoma pancreatic (RINm5F) cell line (ATCC CRL-11605) was obtained from American Type Culture Collection (Manassas, Va). High-performance liquid chromatography (HPLC) column (ZORBAX Eclipse Plus C18 type, 150 mm × 3.0 mm, 5 μm pore size) was purchased from Agilent Technologies Company (Santa Clara, Calif).
GTE Preparation and Analysis
Tea (Camellia sinensis) shoots were freshly harvested from the Doi Angkhang Tea Fields, Chiang Mai, Thailand, and polyphenol oxidase was immediately inactivated using a microwave oven (800 Watts, 3 minutes, 120°C). Dry tea leaves were ground in a blender, extracted with hot water (50 g/500 mL) at 80°C for 10 minutes, filtered through Whatman's cellulose-type paper (Kent, United Kingdom), and lyophilized by being freeze-dried. Green tea catechins were analyzed using reverse-phase HPLC under the following conditions: HPLC column, mobile-phase solvent (0.05% H2SO4/acetonitrile/ethyl acetate, 86:12:2 by volume), flow rate 1.0 mL/min, and wavelength detection 280 nm. Eluents were identified from their specific retention times (TR), and their concentrations were calculated in comparison with standard concentrations of authentic catechins including GA, EGC, C, ECG, GCG, EC, GC, and EGCG (TR = 2.305, 3.74, 4.95, 6.19, 6.76, 8.04, 10.55, and 15.81 minutes, respectively).23,30 Four major peaks were detected in GTE, and they were found to be EGC (TR = 3.705 minutes), ECG (TR = 6.054 minutes), EGCG (TR = 16.070 minutes), and one unidentified phenolic compound (TR = 20.714 minutes) (Fig. 1).31 Our GTE contained 29.0 mg of EGC, 90.75 mg of ECG, and 24.75 mg of EGCG (a total of 144.5 mg catechins) per gram dry weight and used in this study.
FIGURE 1.
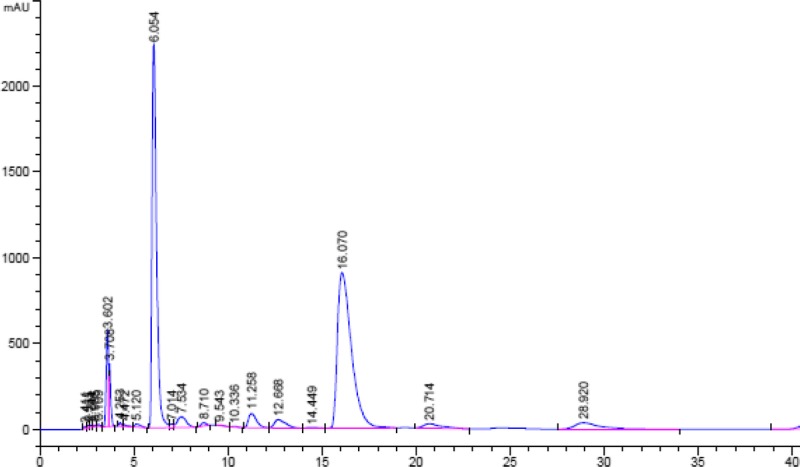
High-performance liquid chromatography analysis of C content in GTE (10 mg/mL).
Pancreatic Cell Culture and Iron Loading
Rat insulinoma pancreatic β-cells were cultured in RPM1 1640 medium containing 10% FBS, 100 U/mL of penicillin, and 100 μg/mL of streptomycin.22 Cells (1 × 106 cells/well) were plated on culture plates in a humidified chamber (5% CO2 atmosphere, 37°C) for 24 hours awaiting 80% confluence, and the medium was then removed. The cells were incubated once with RPMI 1640 medium containing antibiotics and 10% FBS for 8 to 10 hours. After that, the medium was removed, the cells were again combined using the same medium and incubated for 8 to 10 hours. In comparison, 80% confluent RINm5F cells were incubated with FAC (1–30 μM) for 24 hours. In both iron-loading methods, the cells were washed with PBS in the final step before proceeding with the cellular iron measurement experiments. Viability of cells was assessed by using an LDH assay according to the manufacturer's instructions. Viable cells were also numerated with Automated Coulter cell counters. Cell preparations with viability of greater than 80% were used in all experiments.
Cellular iron was quantitated using a modified colorimetric ferrozine assay.22 The treated cells were lysed with 200 mM of NaOH overnight. Lysate was neutralized with 10 mM of HCl and incubated with an iron-releasing agent comprised of 1.4 M of HCl and 4.5% (w/v) KMnO4 (1:1, v/v) for 2 hours at room temperature. The reaction mixture was incubated with an iron chromogenic agent (6.5 mM ferrozine, 6.5 mM neocuproine, 2.5 M ammonium acetate, and 1 M ascorbic acid) for 30 minutes, and the absorbance was photometrically measured at 562 nm. Protein concentration of the cell lysate was quantitated using the Coomassie Brilliant Blue cytophotometric method with BSA as a standard.32 Cellular iron concentration was normalized against lysate protein content and expressed as nmol/mg protein.
Iron Mobilization in RINm5F Cells
In monotherapy, iron-loaded RIN5mF cells were treated with different concentrations of GTE (1 and 10 μM EGCG), and iron chelators (10 μM each) as DFO, DFP, and DFX at 37°C for 0 to 12 hours. In the combined therapy, iron-loaded RIN5mF cells were treated with GTE (1–10 μM EGCG) together with the chelators (10 μM each) for 12 hours. Cellular iron concentration was determined by using a modified ferrozine assay.33 The treated cells were lysed with 200 mM of NaOH overnight, then neutralized with 10 mM of HCl, and incubated with an iron-releasing agent constituting 1.4 M of HCl and 4.5% (w/v) KMnO4 (1:1, v/v) for 2 hours at room temperature. The lysate was incubated with an iron chromogenic agent (6.5 mM ferrozine, 6.5 mM neocuproine, 2.5 M ammonium acetate, and 1 M ascorbic acid) for 30 minutes, and absorbance was measured at 562 nm. Iron concentration was normalized against protein content and expressed as nmol/mg protein.
Reduction of ROS in RINm5F Cells
Iron-loaded RIN5mF cells were washed with sterile PBS, labeled with 9 μM of DCFH-DA solution for 30 minutes and treated with GTE (1–10 μM EGCG), and the chelators (10 μM each) were treated for 60 minutes at 37°C under the kinetics system in a microplate reader. Fluorescence intensity was measured with a spectrofluorometer (λex = 493 nm/λem = 522 nm).34
Evaluation for Response of Insulin Secretion in RINm5F Cells
To optimize cellular iron overload with minimal effect on insulin production, cells were loaded with iron using a recently published protocol.22 Rat insulinoma pancreatic cells that were sensitive to glucose challenges were used to investigate insulin secretion. After being loaded with 10% FBS or 25% FBS (once or twice), the cells were challenged with KRB buffer (119 mM NaCl, 4.74 mM KC1, 2.54 mM CaC12, 1.19 mM MgC12, 1.19 mM KH2PO4, 25 mM NaHCO3, pH 7.4) containing 0.5% BSA and 2.8 mM of glucose for 1 hour. After that, the remaining buffer was switched to the KRB buffer containing 16.7 mM of glucose for 1 hour. The supernatant was then collected for the quantification of insulin using a standard rat insulin ELISA kit. The remaining cells were washed once with ice-cold PBS (pH 7.4) containing the protease inhibitor cocktail. Iron and protein concentrations of the cell lysates were measured using the established methods as described previously. Cellular iron concentrations were normalized with cellular protein concentrations and are expressed as nmol/mg protein.
In another experiment, RINm5F cells were iron loaded with 25% FBS twice and treated with GTE (1–20 μM EGCG) or iron chelators (1 and 10 μM each) for 8 hours. The treated cells were switched to be incubated in KRB buffer containing 0.5% BSA and 2.8 mM of glucose for 1 hour and then consecutively in 16.7 mM glucose for 1 hour. Secretory insulin concentrations in the medium were determined using the ELISA method as described previously and are expressed as pU/mL.
Statistical Analysis
Data were analyzed using IBM SPSS Statistics version 22 (Armonk, NY) program and are presented as mean ± standard error of the mean (SEM). Statistical significance was determined using 1-way analysis of variance, in which P < 0.05 was considered significant.
RESULTS
Iron Loading in Pancreatic Cell Culture
The RIN5mF pancreatic β-cell line was iron overloaded using 2 iron sources, 10% FBS and FAC. Cellular iron levels were markedly increased in proportion to the frequency of the FBS change (1 and 2 times) and the dose dependence of FAC. Fetal bovine serum loaded iron into the cells more efficiently than FAC (Fig. 2). The result suggests that FBS is an appropriate source of iron, consistent with the work of Kakuta el al.35
FIGURE 2.

Cellular iron in RINm5F cells incubated with medium supplemented with FBS (10%, once and twice) or FAC (1–30 µM). Data obtained from 3 independent experiments are expressed as mean ± SEM. *P < 0.05 when compared with PBS; #P < 0.05 when compared with 1×.
Fetal bovine serum and FAC (1–10 μM, except 30 μM) were found to not be harmful to the cells based on LDH assay. This finding was consistent with the automated cell counter assay, where cell viability was greater than 80% when cultured in the medium containing the FBS and the FAC (data not shown). Green tea extract treatment (1–30 μM EGCG) tended to be toxic to the cells, as judged by LDH release in a dose-dependent manner. Increased release of LDH into the medium was observed and is shown in Supplemental Figure 1 (http://links.lww.com/MPA/A721). We conducted experiments where only viability exceeded 80% (1%–20% LDH leakage). The concentrations of GTE between 1 and 20 μM of EGCG showed levels of LDH equivalence up to 20%. This concentration range was therefore chosen to perform further experiments. Viability of the cells treated with GTE (1 μM EGCG equivalence) and justified by LDH leaving was well maintained.
Iron Mobilization in Iron-Loaded Cells by GTE
At 8 hours, GTE at quantities of 1 and 10 μM EGCG displayed high levels of efficacy in the mobilizing of cellular iron in iron-overloaded RINm5F cells, in which the iron mobilization was dependent upon the concentrations during 2 to 6 hours of treatment (Fig. 3). Similarly, reference iron chelators (10 μM each) presented effective iron mobilization over 8 hours, and this outcome remained steady after this time point. The relative degree of initial iron mobilization in the treated cells was 10 μM DFX > 10 μM DFO > 10 μM DFP, 10 μM-EGCG GTE > 1 μM-EGCG GTE. Thus, not only were standard chelators able to gain access to iron overloaded cells and remove chelatable iron but green tea also displayed this function.
FIGURE 3.
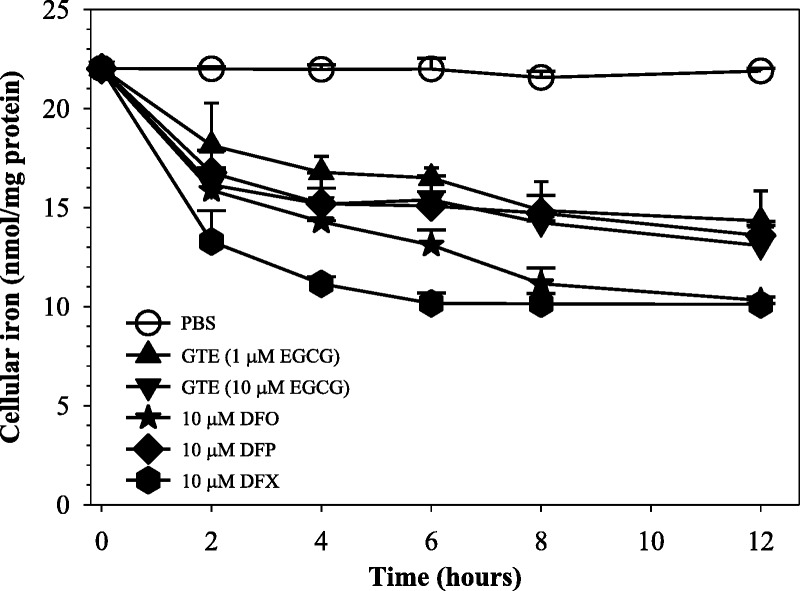
Time-course mobilization of iron in iron-loaded RINm5F cells treated with GTE (1 and 10 μM EGCG) and DFO, DFP, and DFX (10 μM each). Data obtained from 3 independent experiments are expressed as mean ± SEM.
As is shown in Figure 4, all 3 chelators (10 μM each) effectively removed iron from RINm5F cells (P < 0.05) with the relative degree of DFX > GTE > DFP ≈ DFO. Interestingly, GTE (1 and 10 μM EGCG) monotherapy decreased the amount of cellular iron in a concentration-dependent manner (P < 0.05), whereas GTE at 10 μM EGCG showed almost a 2-fold decrease in intracellular iron when compared with the nontreatment. At 1 μM EGCG, iron release was significant but without substantial effect on LDH release. The combinations (GTE [1 μM EGCG] + 10 μM DFO) and (GTE [10 μM EGCG] + 10 μM DFO) presented a synergistic effect of intracellular iron mobilization. However, DFP and DFX only showed trends of synergism when combinations of GTE (10 μM EGCG) with the chelators (10 μM each) were used, respectively.
FIGURE 4.
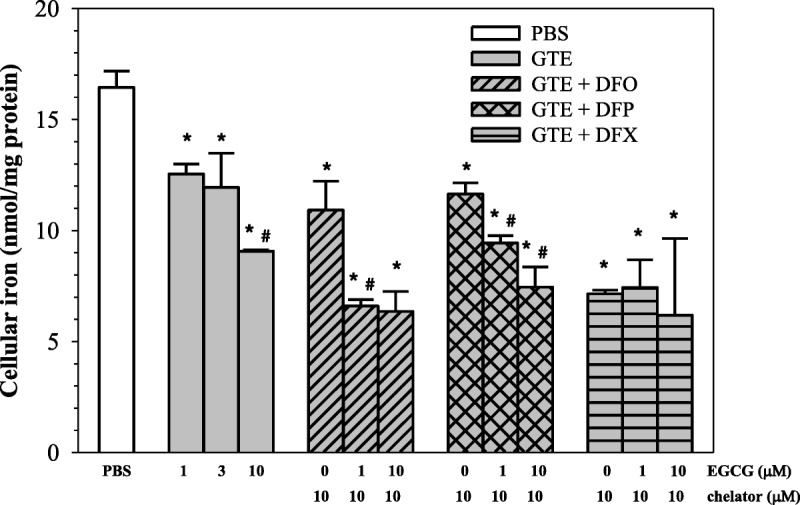
Dose-response mobilization of iron in iron-loaded RINm5F cells treated with PBS, GTE, and a combination of GTE with iron chelators as DFO, DFP, and DFX (10 μM each). Data obtained from 3 independent experiments are expressed as mean ± SEM. *P < 0.05 when compared with PBS; #P < 0.05 when compared with consecutive lower concentrations.
ROS Levels in Iron-Loaded Cells After GTE Treatment
Green tea extract (1–10 μM EGCG) monotherapy clearly reduced cellular ROS levels in a concentration-dependent manner (P < 0.05) (Fig. 5). When compared with standard chelator (10 μM) monotherapy, the higher GTE dose was more efficient at removing cellular iron and gave better results than the chelators at equivalent concentrations (P < 0.05). When iron chelators were added to GTE, decrement in ROS was still observed along with increasing GTE values (P < 0.05). However, with the exception of DFP, ROS was not found to be significantly less than with GTE. Thus, a combination of GTE and DFP displayed higher efficacy in lowering cellular ROS than the other 2 chelators (P < 0.05). Our findings suggest that green tea possesses high antioxidant activity and a powerful free-radical scavenging capacity.
FIGURE 5.
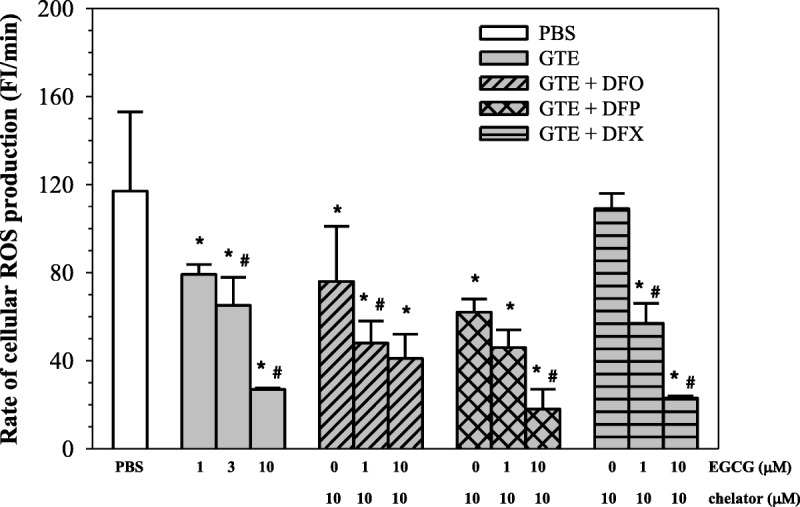
Reactive oxygen species levels in iron-loaded RINm5F cells treated with PBS, GTE, and GTE combined with DFO, DFP, and DFX (10 μM each). Data obtained from 3 independent experiments are expressed as mean ± SEM. *P < 0.05 when compared with PBS; #P < 0.05 when compared with consecutive lower concentrations.
Response of Pancreatic β-Cell Function to GTE Treatment
When RINm5F cells were loaded with iron and became subject to oxidative stress, insulin synthesis would be affected. We wish to determine whether this leads to decreased insulin production while cell viability was unchanged. We predicted that GTE and the chelator treatment might restore β-cell functions in iron overloaded RINm5F cells and consequently increase insulin production/secretion. As is shown in Figure 6, cellular iron concentrations were increased, and secretory insulin levels were decreased sequentially after being loaded with 10% FBS (once and twice) or 25% FBS (once and twice). Ten percent FBS medium (twice) was found to be more suitable than 25% FBS for loading iron into RINm5F cells, thereby generating the highest value of insulin secretion.
FIGURE 6.
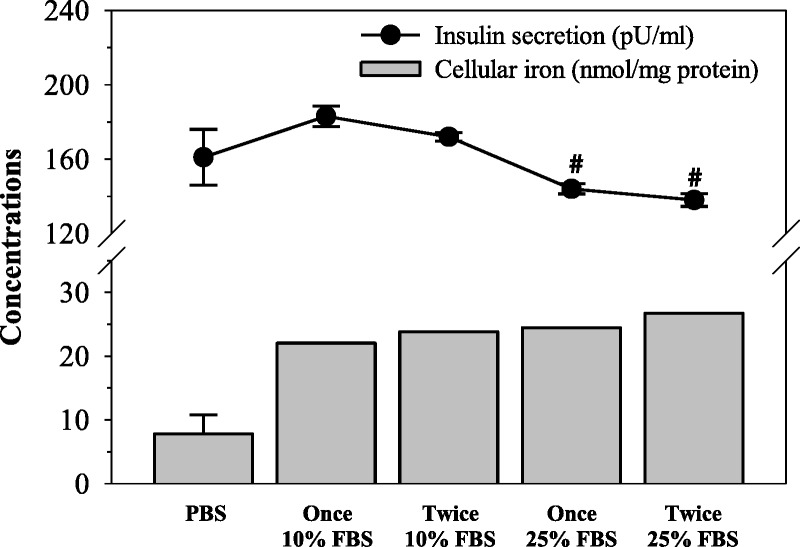
Response of insulin production in RINm5F cells incubated in FBS-supplemented RPMI medium once or twice. Data obtained from 3 independent experiments are expressed as mean ± SEM. #P < 0.05 when compared with PBS.
Importantly, monotherapy with GTE (1–20 μM EGCG) was found to have dose increased insulin secretion from iron loaded RINm5F cells (P < 0.05), but the viability of the cells was still well maintained (Fig. 7). Green tea extract and the 3 other chelators increased the insulin production in a concentration-dependent manner (P < 0.05). In this case, GTE was less efficient than 1 μM of DFX but most effective at 10 μM (Fig. 8). The results suggest that GTE itself and its combination with iron chelators could restore pancreatic β-cell function from oxidative damage and improve insulin secretion under iron overloaded conditions.
FIGURE 7.
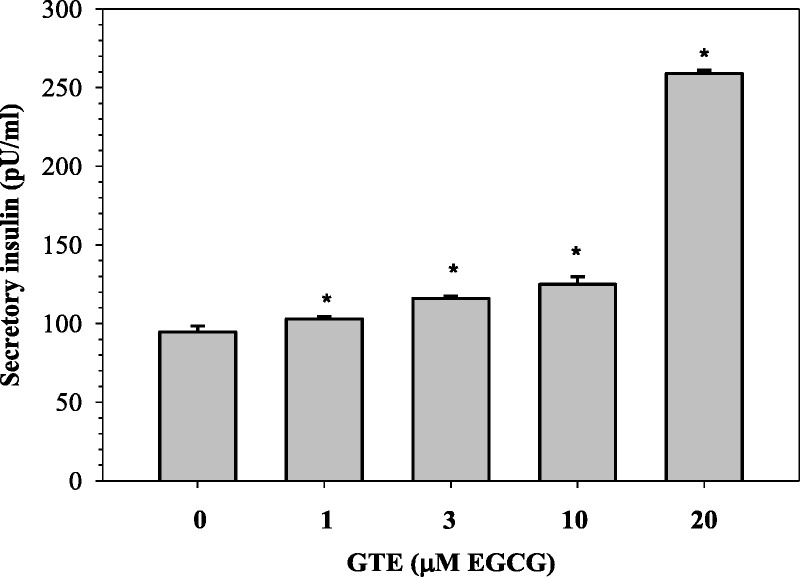
Levels of insulin secreted from iron-loaded RINm5F cells treated with various concentrations of GTE. Data obtained from 3 independent experiments are expressed as mean ± SEM. *P < 0.05 when compared nontreated cells.
FIGURE 8.
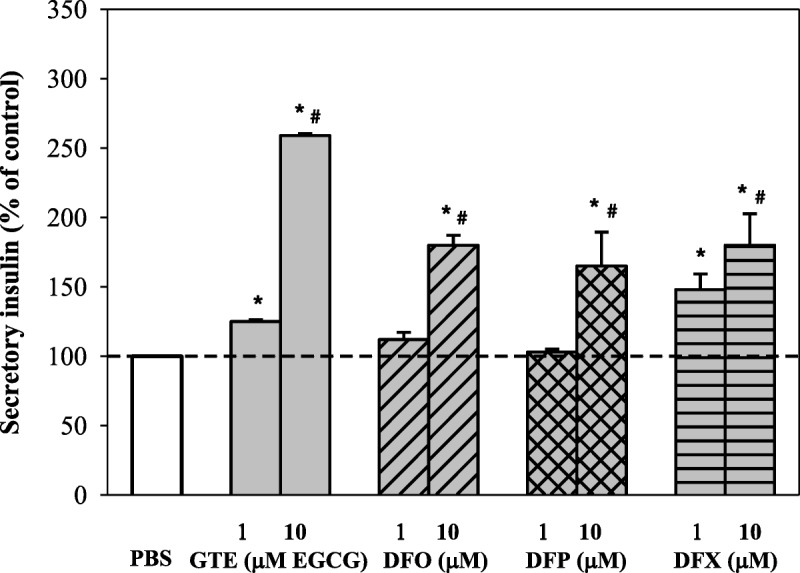
Percent change of secretory insulin when compared with GTE (1 and 10 μM EGCG) and the chelators (1 and 10 μM each). Data obtained from 3 independent experiments are expressed as mean ± SEM. *P < 0.05 when compared with PBS; #P < 0.05 when compared with 1 μM.
CONCLUSIONS
Diabetes is an endocrinopathy complication commonly found in nontransfusion-dependent and transfusion-dependent β-thalassemia patients who have accumulated iron in the pancreatic islets of β-cells.13,17 Plasma membrane metal transporters such as divalent metal transporters Zip14 and Zip1836,37 and/or L-type voltage dependent calcium channel38 contribute to taking up iron, mostly nontransferrin bound iron, into pancreatic β-cells. Two rat insulinoma pancreatic β-cell lines, RIM5mF and INS-1, are usually used for investigating the effects of glucose-induced insulin secretion. Bovine serum contains sources of iron including ferritin, whereas transferrin can supply iron to cultured RINm5F cells.39 In this study, we used fetal bovine serum, which was less toxic to RINm5F cells than ferric ammonium citrate, while achieving the iron overload of our pancreatic cell lines.
Green tea extract and the EGCG fraction were found to ameliorate iron overload and oxidative stress levels in the livers and hearts of β-thalassemia mice.23,40 Here, this work has been extended to pancreatic β-cells. Epigallocatechin-3-gallate can delay the onset of insulin-dependent diabetic mellitus in mice and relieve the inflammation of pancreatic β-cells.27,41,42 However, it has also been reported that green tea EGCG is a potential prooxidant that can induce oxidative damage to the HIT-T15 pancreatic β-cell line.43 This is relevant to our findings where we show that EGCG-rich GTE can cause cell damage, as is seen by high extracellular LDH levels when high doses of GTE were used. Because GTE is associated with increased LDH release at higher concentrations, the possibility of cell damage leading to the release of preformed rather than active insulin cannot be excluded. However, some evidence and experiments are mentioned in support of this. Lactate dehydrogenase expression and activity were found to be low in the pancreatic insulinoma (INS-1) β-cells; however, LDH overexpression was capable of highly increasing cellular LDH activity but did not influence glucose-stimulated insulin secretion.44 In addition, oxidative human islet amyloid polypeptide was found to induce toxicity and LDH release of the INS-1E cells, whereas Se-enriched Spirulina extract exerted such protective effects by preventing the ROS overproduction.45 Another study has demonstrated that pretreatment of interleukin-1β-induced human chondrocyte culture with EGCG was found to decrease NO• production and LDH leakage in a concentration-dependent manner.46
Green tea EGCG was found to protect against ROS and the cytokine-induced β-cell destruction of RINm5F cells by reducing levels of inducible nitric oxide synthase expression and NO• production.27 Furthermore, EGCG was found to increase levels of plasma insulin and anti-inflammatory cytokine interleukin-6 in nonobese diabetic mice.42 These findings demonstrate that EGCG could be a possible natural product that is capable of inhibiting ROS generation and decreasing diabetic mellitus progression. Importantly, we have recently reported that EGCG-rich GTE was able to reduce pancreatic iron accumulation and lipid-peroxidation products in β-thalassemic mice with iron overload, leading to an increase in plasma insulin production.47 In contrary, treatment of the HIT-T15 pancreatic β-cell line with EGCG (5–100 μM) was found to increase apoptotic cell death and cellular ROS (especially H2O2) production but decreased cell viability. This suggests that EGCG may mediate Fe2+ formation and H2O2 generation via Haber-Weiss reaction.43 Another study revealed that intraperitoneal administration of EGCG (5 mg kg−1d−1) for 4 days impaired the β-cell response in diabetic rats, suggesting a prooxidant effect of EGCG.48 Consistently, our study supports green tea polyphenols, mainly EGCG and ECG; reverse impaired function; and decreased insulin secretion in iron overloaded-pancreatic β-cells through chelating extracellular iron (such as transferrin-bound iron in FBS), mobilizing cellular iron, and inhibiting ROS production.
The level of insulin release in the cell culture has been used previously as a biochemical marker to predict pancreatic iron overload and to assess the efficacy of iron chelators.49 Our findings show that iron loading by bovine serum is associated with decreased insulin secretion to levels below or above the control levels, whereas EGCG could restore the insulin secretion in glucose-stimulated RIN5mF cells with iron overload. In comparison, the inhibitory effect of insulin secretion in an INS-1D β-cell line by EGCG was reported.50 High doses of free radical scavengers and iron chelators were ineffective in relieving oxidative stress and were even found to be toxic to rat pancreatic cells.51 Moreover, RIM5mF cells with a weak antioxidant defense and enhanced autophagocytosis were very sensitive to oxidants (eg, alloxan) while DFO prevented such toxic effects.52 Here, the clinically used chelators DFO, DFP, and DFX also restored insulin release while having no effect on LDH release. Depending upon the concentration of GTE, it could act either as a potential antioxidant or prooxidant. This effect may also be influenced by the relative concentration and redox state of iron. The type of glucose sensitive/insensitive insulinoma pancreatic β-cell line involved in insulin secretion would need to be indicated as well.
Further studies are needed to assess the relationship between the antioxidative and prooxidative effects of GTE under controlled conditions of excess iron (II) and iron (III). Subsequent studies would be of interest to assess whether the addition of GTE to clinically established iron chelation regimes could improve insulin secretion in an iron overloaded mice model and ultimately in clinical practice. In conclusion, green tea, particularly EGCG, could decrease cellular iron, decrease ROS, and increase insulin production in iron-loaded pancreatic cells (Fig. 9). The traditional use of supplementary green tea could be helpful for glucose homeostasis in diabetic β-thalassemia patients with iron overload.
FIGURE 9.
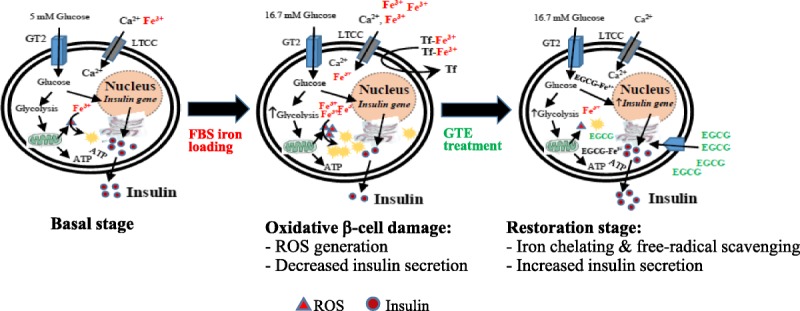
Diagrammatic conclusion for restoration of iron-overloaded pancreatic β-cells by green tea treatment. ATP indicates adenosine 5′-triphosphate; EGCG-Fe3+, ferric-epigallocatechin-3-gallate complex; GT2, glucose transporter-2; LTCC, L-type voltage dependent calcium channel; Tf, transferrin.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the Department of Biochemistry, Faculty of Medicine, Chiang Mai University, Thailand, and the Department of Haematology, University College London, London, United Kingdom, for providing the laboratory facilities and Emeritus Professor Robert C. Hider, PhD, King's College London, United Kingdom, for English proofreading and scientific comments.
Footnotes
This work was supported by the Royal Golden Jubilee PhD Program through Miss Pimpisid Koonyosying (PHD/0060/2554), Thailand Research Fund; Faculty of Medicine Research Fund, Chiang Mai University; and Chair Professor Grant through Professor Suthat Fucharoen, MD, of National Science and Technology Development Agency, Thailand.
P.K. designed and conducted the experiments, analyzed data, and wrote a draft manuscript. C.U. designed experiments and shared comments. S.F. partially supported funding and had a consultant/advisory role. E.V.K. conducted experiments and provided data discussion. J.B.P. supervised assay techniques and revised the manuscript. S.S. conceived and designed the experiments and had a consultant/advisory role. All authors have read and approved of the manuscript. The authors are fully responsible for all content and editorial decisions associated with this article.
The authors declare no conflict of interest.
Animals/humans were not used in this study.
Presented at the 14th Conference for Thalassemia and Haemoglobinopathies (TIF), November 17–19, 2017, Thesseloniki, Greece.
Supplemental digital contents are available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.pancreasjournal.com).
REFERENCES
- 1.Brissot P, Ropert M, Le Lan C, et al. Non-transferrin bound iron: a key role in iron overload and iron toxicity. Biochim Biophys Acta. 2011;1820:403–410. [DOI] [PubMed] [Google Scholar]
- 2.Pootrakul P, Breuer W, Sametband M, et al. Labile plasma iron (LPI) as an indicator of chelatable plasma redox activity in iron-overloaded beta-thalassemia/HbE patients treated with an oral chelator. Blood. 2004;104:1504–1510. [DOI] [PubMed] [Google Scholar]
- 3.Fibach E, Rachmilewitz EA. Iron overload in hematological disorders. Presse Med. 2017;46:e296–e305. [DOI] [PubMed] [Google Scholar]
- 4.Kruszewski M. Labile iron pool: the main determinant of cellular response to oxidative stress. Mutat Res. 2003;531:81–92. [DOI] [PubMed] [Google Scholar]
- 5.Repka T, Shalev O, Reddy R, et al. Nonrandom association of free iron with membranes of sickle and beta-thalassemic erythrocytes. Blood. 1993;82:3204–3210. [PubMed] [Google Scholar]
- 6.Andrews NC. Disorders of iron metabolism. N Engl J Med. 1999;341:1986–1995. [DOI] [PubMed] [Google Scholar]
- 7.Cabantchik ZI, Breuer W, Zanninelli G, et al. LPI-labile plasma iron in iron overload. Best Pract Res Clin Haematol. 2005;18:277–287. [DOI] [PubMed] [Google Scholar]
- 8.Kohgo Y, Ikuta K, Ohtake T, et al. Body iron metabolism and pathophysiology of iron overload. Int J Hematol. 2008;88:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fibach E, Rachmilewitz EA. Pathophysiology and treatment of patients with beta-thalassemia - an update. F1000Res. 2017;6:2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berdoukas V, Nord A, Carson S, et al. Tissue iron evaluation in chronically transfused children shows significant levels of iron loading at a very young age. Am J Hematol. 2013;88:E283–E285. [DOI] [PubMed] [Google Scholar]
- 11.Porter JB, Shah FT. Iron overload in thalassemia and related conditions: therapeutic goals and assessment of response to chelation therapies. Hematol Oncol Clin North Am. 2010;24:1109–1130. [DOI] [PubMed] [Google Scholar]
- 12.de Assis RA, Ribeiro AA, Kay FU, et al. Pancreatic iron stores assessed by magnetic resonance imaging (MRI) in beta thalassemic patients. Eur J Radiol. 2012;81:1465–1470. [DOI] [PubMed] [Google Scholar]
- 13.Kosaryan M, Rahimi M, Darvishi-Khezri H, et al. Correlation of pancreatic iron overload measured by T2*-weighted magnetic resonance imaging in diabetic patients with β-thalassemia major. Hemoglobin. 2017;41:151–156. [DOI] [PubMed] [Google Scholar]
- 14.Cario H, Holl RW, Debatin KM, et al. Insulin sensitivity and beta-cell secretion in thalassaemia major with secondary haemochromatosis: assessment by oral glucose tolerance test. Eur J Pediatr. 2003;162:139–146. [DOI] [PubMed] [Google Scholar]
- 15.De Sanctis V, Soliman A, Yassin M. Iron overload and glucose metabolism in subjects with β-thalassaemia major: an overview. Curr Diabetes Rev. 2013;9:332–341. [DOI] [PubMed] [Google Scholar]
- 16.Noetzli LJ, Mittelman SD, Watanabe RM, et al. Pancreatic iron and glucose dysregulation in thalassemia major. Am J Hematol. 2011;87:155–160. [DOI] [PubMed] [Google Scholar]
- 17.Chuansumrit A, Pengpis P, Mahachoklertwattana P, et al. Effect of iron chelation therapy on glucose metabolism in non-transfusion-dependent thalassaemia. Acta Haematol. 2016;137:20–26. [DOI] [PubMed] [Google Scholar]
- 18.Gomber S, Dabas A, Bagmar S, et al. Glucose homeostasis and effect of chelation on beta cell function in children with beta-thalassemia major. J Pediatr Hematol Oncol. 2017;40:56–59. [DOI] [PubMed] [Google Scholar]
- 19.Belhoul KM, Bakir ML, Saned MS, et al. Serum ferritin levels and endocrinopathy in medically treated patients with β-thalassemia major. Ann Hematol. 2012;91:1107–1114. [DOI] [PubMed] [Google Scholar]
- 20.Capra L, Atti G, De Sanctis V, et al. Glucose tolerance and chelation therapy in patients with thalassaemia major. Haematologica. 1983;68:63–68. [PubMed] [Google Scholar]
- 21.Christoforidis A, Perifanis V, Tsatra I, et al. Evolution of OGTT in patients with beta-thalassaemia major in relation to chelation therapy. Diabetes Res Clin Pract. 2007;76:6–11. [DOI] [PubMed] [Google Scholar]
- 22.Vlachodimitropoulou E, Chen YL, Garbowski M, et al. Eltrombopag: a powerful chelator of cellular or extracellular iron(III) alone or combined with a second chelator. Blood. 2017;130:1923–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thephinlap C, Ounjaijean S, Khansuwan U, et al. Epigallocatechin-3-gallate and epicatechin-3-gallate from green tea decrease plasma non-transferrin bound iron and erythrocyte oxidative stress. Med Chem. 2007;3:289–296. [DOI] [PubMed] [Google Scholar]
- 24.Chan S, Kantham S, Rao VM, et al. Metal chelation, radical scavenging and inhibition of Aβ42 fibrillation by food constituents in relation to Alzheimer's disease. Food Chem. 2016;199:185–194. [DOI] [PubMed] [Google Scholar]
- 25.Mandel SA, Avramovich-Tirosh Y, Reznichenko L, et al. Multifunctional activities of green tea catechins in neuroprotection. Modulation of cell survival genes, iron-dependent oxidative stress and PKC signaling pathway. Neurosignals. 2005;14:46–60. [DOI] [PubMed] [Google Scholar]
- 26.Cao T, Zhang X, Yang D, et al. Antioxidant effects of epigallocatechin-3-gallate on the aTC1-6 pancreatic alpha cell line. Biochem Biophys Res Commun. 2017;495:693–699. [DOI] [PubMed] [Google Scholar]
- 27.Han MK. Epigallocatechin gallate, a constituent of green tea, suppresses cytokine-induced pancreatic beta-cell damage. Exp Mol Med. 2003;35:136–139. [DOI] [PubMed] [Google Scholar]
- 28.Martino L, Masini M, Novelli M, et al. The aryl receptor inhibitor epigallocatechin-3-gallate protects INS-1E beta-cell line against acute dioxin toxicity. Chemosphere. 2013;93:1447–1455. [DOI] [PubMed] [Google Scholar]
- 29.Pournourmohammadi S, Grimaldi M, Stridh MH, et al. Epigallocatechin-3-gallate (EGCG) activates AMPK through the inhibition of glutamate dehydrogenase in muscle and pancreatic ss-cells: a potential beneficial effect in the pre-diabetic state? Int J Biochem Cell Biol. 2017;88:220–225. [DOI] [PubMed] [Google Scholar]
- 30.Srichairatanakool S, Ounjaijean S, Thephinlap C, et al. Iron-chelating and free-radical scavenging activities of microwave-processed green tea in iron overload. Hemoglobin. 2006;30:311–327. [DOI] [PubMed] [Google Scholar]
- 31.Upanan S, Pangjit K, Uthaipibull C, et al. Combined treatment of 3-hydroxypyridine-4-one derivatives and green tea extract to induce hepcidin expression in iron-overloaded β-thalassemic mice. Asian Pac J Trop Biomed. 2015;5:1010–1017. [Google Scholar]
- 32.Tas J, van der Ploeg M, Mitchell JP, et al. Protein staining methods in quantitative cytochemistry. J Microsc. 1980;119:295–311. [DOI] [PubMed] [Google Scholar]
- 33.Vlachodimitropoulou Koumoutsea E, Garbowski M, Porter J. Synergistic intracellular iron chelation combinations: mechanisms and conditions for optimizing iron mobilization. Br J Haematol. 2015;170:874–883. [DOI] [PubMed] [Google Scholar]
- 34.Barbu A, Welsh N, Saldeen J. Cytokine-induced apoptosis and necrosis are preceded by disruption of the mitochondrial membrane potential (Deltapsi(m)) in pancreatic RINm5F cells: prevention by Bcl-2. Mol Cell Endocrinol. 2002;190:75–82. [DOI] [PubMed] [Google Scholar]
- 35.Kakuta K, Orino K, Yamamoto S, et al. High levels of ferritin and its iron in fetal bovine serum. Comp Biochem Physiol A Physiol. 1997;118:165–169. [DOI] [PubMed] [Google Scholar]
- 36.Hansen JB, Moen IW, Mandrup-Poulsen T. Iron: the hard player in diabetes pathophysiology. Acta Physiol (Oxf). 2014;210:717–732. [DOI] [PubMed] [Google Scholar]
- 37.Coffey R, Knutson MD. The plasma membrane metal-ion transporter ZIP14 contributes to nontransferrin-bound iron uptake by human β-cells. Am J Physiol Cell Physiol. 2016;312:C169–C175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oudit GY, Sun H, Trivieri MG, et al. L-type Ca2+ channels provide a major pathway for iron entry into cardiomyocytes in iron-overload cardiomyopathy. Nat Med. 2003;9:1187–1194. [DOI] [PubMed] [Google Scholar]
- 39.Geertsema RS, Kimball RA, Corbeil LB. Bovine plasma proteins increase virulence of Haemophilus somnus in mice. Microb Pathog. 2007;42:22–28. [DOI] [PubMed] [Google Scholar]
- 40.Saewong T, Ounjaijean S, Mundee Y, et al. Effects of green tea on iron accumulation and oxidative stress in livers of iron-challenged thalassemic mice. Med Chem. 2010;6:57–64. [DOI] [PubMed] [Google Scholar]
- 41.Li C, Allen A, Kwagh J, et al. Green tea polyphenols modulate insulin secretion by inhibiting glutamate dehydrogenase. J Biol Chem. 2006;281:10214–10221. [DOI] [PubMed] [Google Scholar]
- 42.Fu Z, Zhen W, Yuskavage J, et al. Epigallocatechin gallate delays the onset of type 1 diabetes in spontaneous non-obese diabetic mice. Br J Nutr. 2010;105:1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suh KS, Chon S, Oh S, et al. Prooxidative effects of green tea polyphenol (−)-epigallocatechin-3-gallate on the HIT-T15 pancreatic beta cell line. Cell Biol Toxicol. 2009;26:189–199. [DOI] [PubMed] [Google Scholar]
- 44.Ishihara H, Wang H, Drewes LR, et al. Overexpression of monocarboxylate transporter and lactate dehydrogenase alters insulin secretory responses to pyruvate and lactate in beta cells. J Clin Invest. 1999;104:1621–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li XL, Wong YS, Xu G, et al. Selenium-enriched Spirulina protects INS-1E pancreatic beta cells from human islet amyloid polypeptide-induced apoptosis through suppression of ROS-mediated mitochondrial dysfunction and PI3/AKT pathway. Eur J Nutr. 2014;54:509–522. [DOI] [PubMed] [Google Scholar]
- 46.Ahmed S, Rahman A, Hasnain A, et al. Green tea polyphenol epigallocatechin-3-gallate inhibits the IL-1 beta-induced activity and expression of cyclooxygenase-2 and nitric oxide synthase-2 in human chondrocytes. Free Radic Biol Med. 2002;33:1097–1105. [DOI] [PubMed] [Google Scholar]
- 47.Koonyosying P, Kongkarnka S, Uthaipibull C, et al. Green tea extract modulates oxidative tissue injury in beta-thalassemic mice by chelation of redox iron and inhibition of lipid peroxidation. Biomed Pharmacother. 2018;108:1694–1702. [DOI] [PubMed] [Google Scholar]
- 48.Yun SY, Kim SP, Song DK. Effects of (−)-epigallocatechin-3-gallate on pancreatic beta-cell damage in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2006;541:115–121. [DOI] [PubMed] [Google Scholar]
- 49.Bas M, Gumruk F, Gonc N, et al. Biochemical markers of glucose metabolism may be used to estimate the degree and progression of iron overload in the liver and pancreas of patients with β-thalassemia major. Ann Hematol. 2015;94:1099–1104. [DOI] [PubMed] [Google Scholar]
- 50.Kaneko YK, Takii M, Kojima Y, et al. Structure-dependent inhibitory effects of green tea catechins on insulin secretion from pancreatic β-cells. Biol Pharm Bull. 2015;38:476–481. [DOI] [PubMed] [Google Scholar]
- 51.Schulz HU, Niederau C. Oxidative stress-induced changes in pancreatic acinar cells: insights from in vitro studies. Hepatogastroenterol. 1994;41:309–312. [PubMed] [Google Scholar]
- 52.Zhang H, Olejnicka B, Ollinger K, et al. Starvation-induced autophagocytosis enhances the susceptibility of insulinoma cells to oxidative stress. Redox Rep. 1996;2:235–247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


