Abstract
Despite the advancement of early childhood caries (ECC) prediction and treatment, ECC remains a significant public health burden in need of more effective preventive strategies. Pregnancy is an ideal period to promote ECC prevention given the profound influence of maternal oral health and behaviors on children’s oral health. However, studies have shown debatable results with respect to the effectiveness of ECC prevention by means of prenatal intervention. Therefore, this study systematically reviewed the scientific evidence relating to the association between prenatal oral health care, ECC incidence and Streptococcus mutans carriage in children. Five studies (3 randomized control trials, 1 prospective cohort study and 1 nested case-control study) were included for qualitative assessment. Tested prenatal oral health care included providing fluoride supplements, oral examinations/cleanings, oral health education, dental treatment referrals and Xylitol gum chewing. Four studies that assessed ECC incidence reduction were included in meta-analysis using an unconditional generalized linear mixed effects model with random study effects and age as a covariate. The estimated odds ratio and 95% confidence intervals suggested a protective effect of prenatal oral health care against ECC onset before 4 years of age, 0.12 (0.02, 0.77) at 1 year of age, 0.18 (0.05, 0.63) at 2 years of age, 0.25 (0.09, 0.64) at 3 years of age, and 0.35 (0.12, 1.00) at 4 years of age. Children’s S. mutans carriage was also significantly reduced in the intervention group. Future studies should consider testing strategies that restore an expectant mother’s oral health to a disease-free state during pregnancy.
Keywords: Prenatal oral health care, Odds ratio, Caries, ECC, Child dentistry, Clinical studies, Risk factor
Introduction
Although largely preventable, early childhood caries (ECC) remains the most common chronic childhood disease, with nearly 1.8 billion new cases per year globally [Disease et al., 2017; Dye et al., 2012; Dye et al., 2007], It afflicts approximately 37% of children aged 2-5 years in the US [Dye et al., 2012; Dye et al., 2007] and up to 73% of socioeconomically disadvantaged preschool children in both developing and industrialized countries [Dye et al., 2015]. ECC is defined as the presence of ≥1 decayed, missing (due to caries), or filled tooth surfaces in primary teeth in a child 71 months of age or younger [Colak et al., 2013]. Severe ECC (S-ECC) occurs in children <3 years of age with ≥1 decayed, missing (due to caries), or filled tooth surfaces and in children 4-6 years of age with elevated caries scores [Colak et al., 2013]. The short-term consequences of untreated ECC include pain, hospitalization and emergency room visits due to abscess and systemic infection, and even death [American Academy of Pediatric Dentistry Council on Clinical, 2005; Casamassimo et al., 2009]. Once decay has reached this stage, children often require total oral rehabilitation (TOR) under general anesthesia [Koo and Bowen, 2014] with multiple tooth extractions and restorations/crowns, at a cost of nearly $7000 per child (2009-2011 data in US) [Rashewsky et al., 2012]. In the long term, there is strong evidence that children who experienced ECC are much more likely to have diminished oral health-related quality of life and higher risk of caries lesions in permanent teeth [Heller et al., 2000; Powell, 1998].
Despite the advancement of ECC prediction and treatment strategies, ECC remains a public health burden. In the US; more than 1.5 billion dollars per year is spent on treatment. However, children remain at high risk for recurrent caries even after extensive TOR treatment. Up to 40% of children treated for S-ECC experience recurrent disease by the 6-month checkup post TOR[Berkowitz et al., 2011; Graves et al., 2004], despite pharmacologic interventions, such as topical fluoride/antimicrobial applications and dietary counseling to alter caries-promoting eating behaviors [Li and Tanner, 2015; O'Sullivan and Tinanoff, 1996]. Hence, more effective preventive strategies are critically needed.
Pregnancy is an ideal time to promote primary prevention of ECC in children given the profound influence of maternal health and behaviors on children’s oral health outcomes [Iida, 2017], ECC is a multifactorial bacterial disease with Streptococcus mutans as the prime cariogenic bacterium, and strongly influenced by diet [Caufield et al., 1993; Kanasi et al., 2010; Klein et al., 2004; Klinke et al., 2014; Li et al., 2005; Slayton, 2011; Zhan et al., 2012]. Studies have shown that maternal untreated caries and greater level of salivary S. mutans increase the risk of ECC in children. Children’s dietary and oral hygiene behaviors rely on parents or caregivers’ oral health knowledge, beliefs and behaviors [Finlayson et al., 2007; Wigen et al., 2011]. While revisiting the children’s dental caries risk model described by Fisher-Owens [Fisher-Owens et al., 2007] that included different levels of environmental elements, several factors that could potentially be influenced by mothers (underlined in red in Fig.1) including: 1) microflora and diet in the oral health element positioning at the oral health circle, 2) Health behaviors and practices, biological and genetic endowment, physical and demographic attributes, use of dental care, health behaviors and practices, and dental insurance, that are included in the child-level influences element; 3) family position, socioeconomic status physical safety, health status of parents, family function, culture and health behaviors, practices, and coping skills of family, that lie in the family-level influences element. These factors in the aforementioned dental caries risk prediction model further emphasize the maternal role in ECC development. Thus, in theory, oral health care intervention during pregnancy presents an ideal entry point to preventing ECC.
Figure 1. Modified Fisher-Owens conceptual model of child, family, and community influences on oral health outcomes of children.
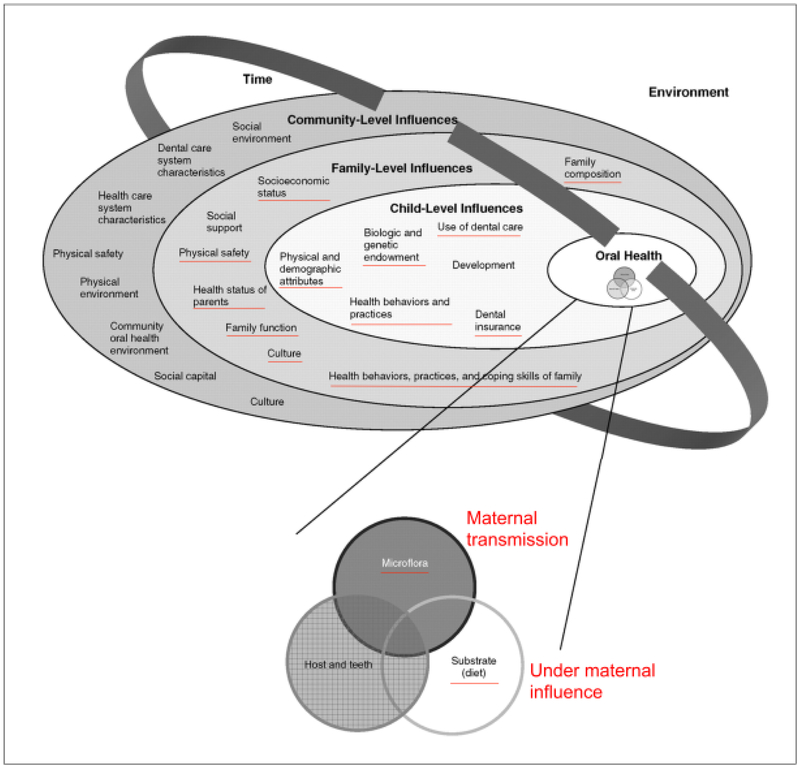
Factors underlined in red are those that could potentially be influenced by maternal attributes.
Previously, studies have shown a positive ECC prevention outcome by providing prenatal oral health education or intervention [Gunay et al., 1998; Nakai et al., 2010]; however, another study failed to show more effective ECC prevention when intervention during pregnancy was compared to the control group. Therefore, the aim of this study is to systematically review the scientific evidence relating to the association between prenatal oral health care, reduced carriage of S. mutans and ECC prevention.
Methods
Search strategy
Database searches were conducted in May 2018, to identify published studies on prenatal oral health care and ECC related outcome (onset of ECC and/or oral S. mutans colonization). A medical reference librarian developed individual search strategies and retrieved citations from PubMed, Embase, Scopus, Web of Science, LILACS, Cochrane Library, and ClinicalTrials.gov. A combination of text words and controlled vocabulary terms were used (Prenatal Care, Oral Health, Child, Infant, Breast Feeding, Newborn, Dental Caries). A detailed search strategy is shown in the Appendix 1.
Inclusion/Exclusion criteria
This systematic review included case-control studies, retrospective or prospective cohort studies, randomized or non-randomized controlled trials that examined the effect of oral health care during pregnancy on the incidence of ECC and/or oral carriage of S. mutans in children under the age of 6. Two trained independent reviewers completed the article selection in accordance with the inclusion/exclusion criteria. The agreement between reviewers was satisfactory (K=0.81). Disagreement were resolved by consensus between the two reviewers.
The following inclusion and exclusion criteria were used for literature selection.
Inclusion criteria:
Types of participants
Pregnant women and their children under the age of 6
Types of intervention(s)/phenomena of interest
Prenatal oral health care utilization/intervention
Types of comparisons
Pregnant women who received and did not receive prenatal oral health care
Types of outcomes
Reduced dental caries in children
Reduced oral carriage of S. mutans
Types of studies
Case-control studies
Retrospective or prospective cohort studies
Randomized and non-randomized controlled trials
Types of statistical data
Odds ratios (OR)
Relative risk (RR)
Confidence intervals (CI)
p-values
Frequency of an absolute number of events vs. total number of individuals per group
Exclusion criteria:
In vitro studies
Animal studies
Papers with abstract only
Literature reviews
Letters to the editor
Editorials
Patient hand outs
Case report or case series
Cross-sectional studies
Data extraction
Descriptive data, including clinical and methodological factors such as country of origin, study design, study site, dental examination, dental examiner calibration, age of subjects, type of prenatal oral health care intervention, outcome measures (ECC and/or oral S. mutans), as well as results from statistical analyses were obtained using an extraction form (Appendix 2).
Qualitative assessment and quantitative analysis
The quality of the selected articles were assessed using two methodological validities: 1) Cochrane Collaboration’s tool for assessing risk of bias in randomized trials [Higgins et al., 2011]. Articles were scaled for the following bias categories: selection bias, performance bias, detection bias, attrition bias, reporting bias and other bias. 2) Adapted Down and Black scoring [Downs and Black, 1998] that assess the methodological quality of both randomized and non-randomized studies of health care interventions. A total score of 26 represents the highest study quality.
For the articles selected for quantitative analysis, R package metaphor was used for meta-analysis (https://cran.r-project.org/web/packages/metafor/). The OR and 95% CI and p-values were estimated using an unconditional generalized linear mixed effects model with random study effects. Children’s age at study endpoint was used as a covariate. Heterogeneity among the studies was evaluated using I2 statistics and tested using likelihood ratio test. A forest plot was created to summarize the meta-analysis study results.
Results
The literature analyses identified a total of 5,881 papers from the database search (Fig. 2). A total of 1,855 duplicate references were removed. The remaining 4026 studies were imported into an Endnote Library for further review. From those, 3854 studies were excluded after title screening, 128 studies were excluded after abstract screening. The remaining 44 articles were selected for a full text review. After the full text analysis, 40 studies were eliminated based on the exclusion criteria and 5 articles were chosen for qualitative assessment. For the quantitative assessment using meta-analysis to assess the effect of prenatal oral health care intervention on the onset of ECC, four out of 5 articles that received qualitative assessment were included. One article that was removed from meta-analysis only included oral S. mutans carriage in children, but not ECC as the outcome [Nakai et al., 2010]. The full list of excluded articles after full text review is shown in Appendix 3.
Figure 2. Flow diagram of study identification.
The four-phase Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram was used to determine the number of studies identified, screened, eligible, and included in the systematic review and meta-analysis (http://www.prisma-statement.org).
Study characteristics
The characteristics of studies included in the qualitative review are summarized in Table 1. All five studies were published between 1997-2016. One was conducted in the US [Leverett et al., 1997], one in Germany [Gunay et al., 1998] and one in Australia [Plutzer and Spencer, 2008], Two were conducted in Japan[Nakai et al., 2016; Nakai et al., 2010]. Among the 5 studies, three were randomized control trials [Leverett et al., 1997; Nakai et al., 2010; Plutzer and Spencer, 2008], one was a prospective cohort study [Gunay et al., 1998] and 1 was a nested case-control in a cohort study [Nakai et al., 2016]. Oral health care intervention adopted in all qualitative studies extended the intervention period from the prenatal to infant stage. The interventions included: a) Fluoride-based intervention, where fluoride supplement intake was provided to pregnant women and their infant in a population that was not exposed to optimal water fluoridation [Leverett et al., 1997]; b) Primary-Primary prevention originally proposed by Axelsson [Axelsson, 1988], where all prophylactic measures were carried out in pregnant women in order to prevent the transmission of cariogenic bacteria and improve feeding behaviors after birth [Gunay et al., 1998]; c) Oral health education promotion in pregnant women, which was used in Plutzer’s study [Plutzer and Spencer, 2008] and in Nakai’s study [Nakai et al., 2016], It was called antenatal health care in Nakai’s study; d) Xylitol gum chewing in pregnant women [Nakai et al., 2010]. Intervention approaches are further detailed in Table 1.
Table 1.
Characteristics of studies included in qualitative assessment
| Author Year |
City, country, study design |
Study site | Child age at exam |
Total subjects | Intervention | Control |
|---|---|---|---|---|---|---|
| Leverett 1997 | Maine, US, RCT | Private Obstetric practice and hospital prenatal clinics | 5 years | Subjects lived in an area without water fluoridation. Intervention: 585 pregnant women at baseline 398 children at 5 years Control: 590 pregnant women (baseline) 400 children at 5 years |
-Mother: daily intake of tablet contains 1 mg fluoride beginning with the 4th month of pregnancy until the end of pregnancy (approximately 6 mons). -Infant: daily drop of fluoride water from birth to 2 years of age. 0.5mg tablet from 2-3 years of age. |
No fluoride intake |
| Günay 1998 | Gennany, Prospective cohort study | Medical University of Hannover (Intervention group); Various kindergartens (control group) |
3 years and 4 years |
Intervention: 86 pregnant women; 54 mother-child dyads (3 years of age) 47 mother-child dyads (4 years of age) Control: 65 children (3 years of age) 45 children (4 years of age) |
-Primary-Primary prevention -Pregnancy 1stvisit: •Dental examination findings •Individual preventive self-care oral hygiene instruction (OHI) •Instruction on avoid microbes transmission •Caries etiology education •Referral for dental treatment if needed Pregnancy 2nd visit (>8 mons gestational age) •Education about infection related to caries maternal-child transmission -After birth visit (0-3 years): •Mother-Child dyads: •Exam •OHI -After birth visit (3-4 years): •OHI •Cleaning •Topical fluoride and chlorhexidine varnish |
Children from various kindergartens who were not in the intervention group |
| Plutzer 2008 | Adelaide, Australia, RCT | Adelaide public hospital | 20 ±2.5 mons |
Intervention: 327 pregnant women; 232 children Control: 322 pregnant women; 209 children |
-Oral health promotion information was given to mothers at a total of 3 rounds, 1 during pregnancy and 2 between 6–12 mons after birth -Two subgroups were included with additional structured telephone consultation 6–12 mons afterbirth in one subgroup. |
Oral health promotion information was NOT given. |
| Nakai 2010 | Okayama, Japan, RCT | Miyake obstetrics and Gynecology Clinic and Hello Dental Clinic | 15 mons |
Intervention: 56 pregnant women and 50 children examined at 6 mons, 46 children examined at 15 mons Control: 51 pregnant women and 35 children examined at 6 mons, 31 children examined at 15 mons |
-At 6 mons pregnant: basic prevention measures (Oral examination, OHI, Cleaning) -From 6 mons pregnant to 9 mons after birth: Xylitol gum (each gum pellet contains 1.32 g xylitol) chewing 4 times/day ≥5min |
At 6 mons pregnant: basic prevention measures (Oral examination, OHI, Cleaning) |
| Nakai 2016 | Okayama, Japan, nested case control in a cohort study | Miyake obstetrics and Gynecology Clinic and Hello Dental Clinic | 2.1±0.8 yrs |
Intervention: 125 children Control: 30 children |
-Antenatal health care (detail is not specified) | No antenatal health care |
| Author Year |
Dental examination calibration |
Outcome measurement |
Statistical analysis |
Study findings | Limitations |
|---|---|---|---|---|---|
| Leverett 1997 | Not documented | • DMFS/dfs •Fluorosis using Dean criteria |
Relative risk and 95% Confidence interval | -No statistical difference of caries incidence in children was seen between intervention (8%) and control group (9%). -There was no strong relationship between exposure to prenatal fluoride and fluorosis. -The tendency for deciduous maxillary second molars in females exposed to prenatal fluoride showed more fluorosis. |
There is no other prenatal oral health care intervention other than fluoride supplement |
| Günay 1998 | Not documented | • DMFS/dmfs • Proximal plaque index • Salivary S. mutans (Dentocult SM) |
t-test | -Caries and S. mutans reduction were significant between intervention and control groups Children at age 3: Intervention: • 0% caries (+) • 100% S. mutans score 0 Control: • 18.5% caries (+) with a 4.5 mean value of dmfs, • 38.5% S. mutans score 0 • 29.2% S. mutans score 1 • 20% S. mutans score 2 • 12.3% S. mutans score 3 Children at age 4: Intervention: • 8.5% caries (+) with a 1.5 mean value of dmfs • 42.6% S. mutans score 0 • 36.2% S. mutans score 1 • 19.1% S. mutans score 2 • 2.1% S. mutans score 3 Control: • 42.3% caries (+) with a 7.0 mean value of dmfs • 26.2% S. mutans score 0 • 13.3% S. mutans score 1 • 22.2% S. mutans score 2 • 37.7% S. mutans score 3 -Mothers showed a significant improvement in plaque index and reduction in S. mutans score |
Referral was given to mothers who need dental treatment, however Whether mothers received dental treatment was not noted. Whether pregnant women and their children in control group have received oral health care were unknown. |
| Plutzer 2008 | Not documented | Incidence of S-ECC (AAPD definition) | Fisher’s exact test | -Caries reduction was significant between intervention and control groups. • Intervention: S-ECC 1.7% • Control: S-ECC 9.6% -No difference between intervention subgroups with/without additional structured telephone consultation |
Dental examiners were blinded, but the subjects were randomized into intervention or control group without blinding. |
| Nakai 2010 | Intra-rate and inter-rate reliability tested. Kappa>0.80 | Salivary S. mutans (Dentocult SM) | t-test, Chi-square and Fisher’s exact tests | -Significantly more children in intervention group exhibited undetectable MS levels (score 0) on both the tongue and the gingival or tooth surfaces at 9, 12, and 24 mos. -The children in control group acquired S. mutans 8.8 mons earlier than those in intervention group (Mean, 12.0 vs. 20.8 mons) |
Caries was not evaluated in children. Study did not use a control gum. |
| Nakai 2016 | Not documented | dmft | Odds ratios and 95% confidence intervals | -Receiving antenatal health care (AOR, 3.27; 95% CI, 1.30-8.24) and child’s having regular check-ups (AOR, 3.42; 95% CI, 1.35-8.69) were significantly associated with caries-free status among three-year old children. | The subjects in control group is much less than the intervention |
DMFS: Decayed, missing, filled surfaces in permanent dentition.
dmft: decayed, missing, filled teeth in primary dentition.
S. mutans scoring in Günay’s study: 0=0-103 cfu (colony forming unit)/ml, 1=103-105 cfu/ml, 2=105-106 cfu/ml; 3= >106 cfu/ml.
Study outcomes were assessed when children reached 2-5 years of age. The onset of ECC and salivary S. mutans carriage are the two primary outcomes evaluated in these 5 studies. Quality and risk of bias for all 5 studies was assessed and shown in Fig. 3. Two studies with randomized controlled trial design have high quality based on Cochrane risk of bias assessment tool [Higgins et al., 2011] and Down and Black scoring system [Downs and Black, 1998], the other 3 studies have moderate quality.
Figure 3. Summary of quality and risk of bias assessment using Cochrane Collaboration’s tool for assessing risk of bias in randomized trials and adapted Down and Black scoring tool.
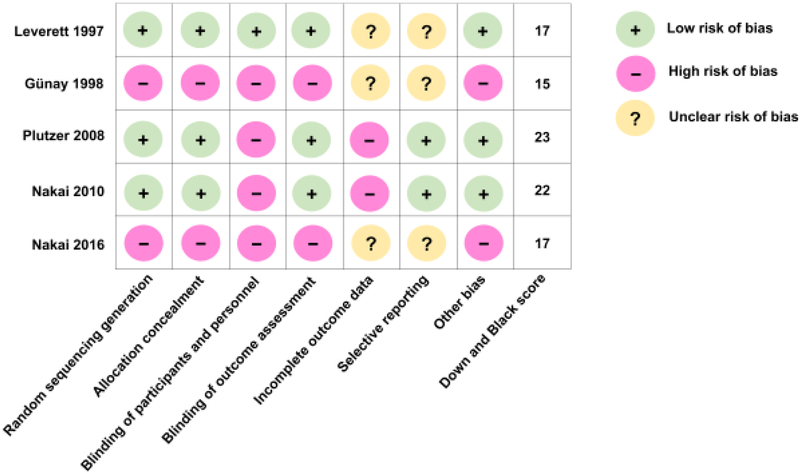
The quality of the selected articles were assessed using two methodological validities: 1) Cochrane Collaboration’s tool for assessing risk of bias in randomized trials [Higgins et al., 2011]. 2) Adapted Down and Black scoring [Downs and Black, 1998] that assess the methodological quality of both randomized and non-randomized studies of health care interventions. A total score of 26 represents the highest study quality.
Prenatal oral health care and ECC prevention
Three studies [Gunay et al., 1998; Nakai et al., 2016; Plutzer and Spencer, 2008] revealed lower ECC incidence in the group that received oral health care intervention during pregnancy and early infancy, when compared to the control group. Prenatal oral health care intervention approaches used in these 3 studies were Primary-Primary prevention, oral examination and cleaning, and oral health education. One study [Leverett et al., 1997] investigating fluoride supplement use during pregnancy showed no statistical difference (p>0.05) in caries incidence in children between the intervention (8%) and control group (9%).
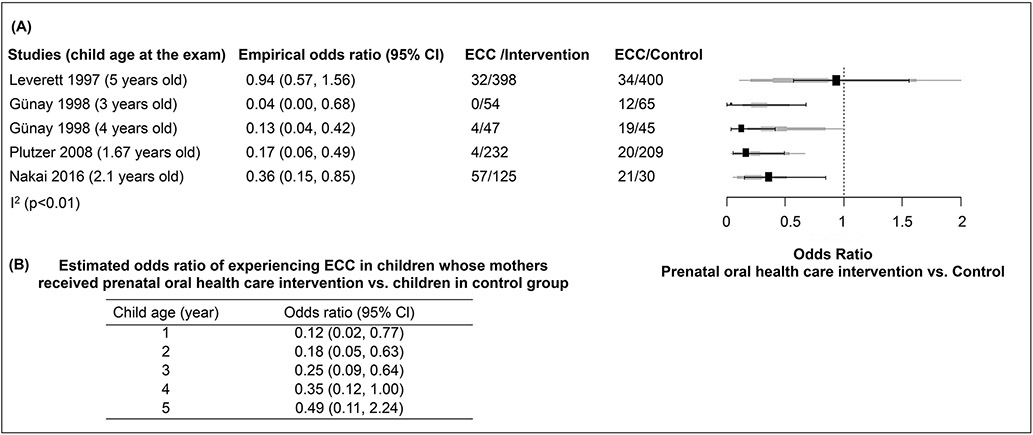
Meta-analysis was performed on four studies that assessed ECC incidence (results shown in Fig. 4). In particular, Gunay et al [Gunay et al., 1998] examined the same cohort of children at two time points, when they reached 3- and 4-years of age; their results were included as two data sets in the meta-analysis. Study heterogeneity (I2=75.06%) and the related p-value were calculated using likelihood ratio test (p<0.0001).
Figure 4. Odds Ratio of ECC events in prenatal oral health care intervention group and control group.
Meta-analysis was performed on four studies that assessed ECC incidence. In particular, Günay et al, 1998 examined the same cohort of children at two time points, when they reached 3- and 4- years of age; their results were included as two data sets in the meta-analysis. Study heterogeneity (I2=75.06%) and the related p-value were calculated using likelihood ratio test (p<0.0001). The empirical odds ratio (OR) and 95% confidence interval (CI) of each study included in the meta-analysis was shown in (A). Based on the generalized linear mixed effect model with covariate age, the estimates of OR and 95% CI shown in (B) indicate that regarding ECC incidence, there is a statistically significant difference between the intervention and control groups for children younger than 4 years of age. The solid line indicates when OR=1.
The empirical ORs and 95% CIs of the studies included in meta-analysis are shown in Fig. 4A. When compared to the control group, the empirical odds ratio (95% CI) of ECC in children whose mothers received Primary-Primary prevention is 0.04 (0.00, 0.68) at 3 years of age [Gunay et al., 1998] and 0.13 (0.04, 0.42) at 4 years of age [Gunay et al., 1998]. Compared to the control group, the empirical odds ratio (95% CI) of ECC is 0.17 (0.06, 0.49) in children whose mothers received oral health education [Plutzer and Spencer, 2008], 0.36 (0.15, 0.85) in children whose mothers received antenatal health care [Nakai et al., 2016], and 0.94 (0.57, 1.56) in children whose mothers received a fluoride supplement [Leverett et al., 1997].
Based on the generalized linear mixed effect model with covariate age, the estimates of ORs and 95% CIs indicate that regarding ECC incidence, there is a statistically significant difference between the intervention and control groups for children younger than 4 years old, regardless of intervention modalities (detailed in Fig 4B). The odds of experiencing ECC among the children younger than 4 whose mothers received prenatal oral health care is significantly less than those children in the control group, indicating a protective effect of prenatal oral health care against ECC development with 95% confidence intervals whose upper bounds smaller than 1. For instance, the estimated odds ratios (95% CI) are 0.12 (0.02, 0.77) for children at 1 year of age, 0.18 (0.05, 0.63) for children of 2 years of age, 0.25 (0.09, 0.64) at 3 years of age, and 0.35 (0.12, 1.00) at 4 years of age, respectively. For children 5 years of age or older, the estimated odds ratio is still smaller than 1, but the 95% confidence interval contains 1, indicating the protective effect becomes insignificant.
Prenatal oral health care and reduction of S. mutans carriage in children
The effect of prenatal oral health care intervention on the reduction of children’s S. mutans carriage was assessed in two studies [Gunay et al., 1998; Nakai et al., 2010], In the study by Günay et al [Gunay et al., 1998], S. mutans reduction was significant between the intervention and control groups; 100% of children in the intervention group remained S. mutans free by the age of 3, whereas only 38.5% of children in the control group remained S. mutans free by the age of 3. Moreover, mothers in the intervention group also showed a significant improvement in plaque index and reduction in S. mutans score. The study by Nakai et al [Nakai et al., 2010] showed that significantly more children in the xylitol chewing group remained S. mutans free at 9-, 12- and 24-months. Furthermore, pre- and perinatal xylitol chewing by mothers delayed S. mutans carriage in children. The children’s S. mutans acquisition age in the xylitol chewing group was 8.8 months later than that of the control group (Mean age, 20.8 vs. 12.0 months).
Discussion
The results of this review have shown a reduced ECC incidence in children whose mothers received prenatal oral health care. ECC is a multifactorial disease with complex socioeconomic, genetic, oral hygiene behaviors, bacterial and diet factors that affect risk for this disease [Ruby and Goldner, 2007; Wang et al., 2012]. S. mutans and, more recently, Candida species have been implicated as potential major etiological microorganisms that may be involved in the initiation and development of ECC [Gross et al., 2012; Tanzer et al., 2001; Xiao et al., 2018]. Studies have shown an association between maternal poor oral health and increased risk for ECC [Chaffee et al., 2014]. The association between mother’s and child’s oral health could possibly be explained by: 1) mothers’ oral health behavior, e.g. perception and knowledge influences the dental health of their children [Goettems et al., 2012; Olak et al., 2018; Saied-Moallemi et al., 2008]; 2) mother might be a main source of her children’s acquisition of oral S. mutans and Candida sp. [Bliss et al., 2008; Caufield et al., 2005; Childers et al., 2017; Waggoner-Fountain et al., 1996; Xiao et al., 2016].
The following points should be considered when interpreting the results of this review: 1) various intervention modalities and frequencies were used across the 5 studies, which produced challenges for data analysis, e.g., the heterogeneity of studies included in the meta-analysis is significant (p<0.01). 2) The timing of the main outcome measurement (ECC incidence) with respect to children’s age lacks consistency throughout the 5 studies. The peak of ECC onset is 3 years of age; there is a significant increase in incidence between age 2 and 3. Kopycka [Kopycka-Kedzierawski et al., 2008] reported a 26% ECC prevalence among 2 years olds in Rochester, NY; Quinonez [Quinonez et al., 2001] reported a 20% ECC prevalence in children 18-36 months of age in North Carolina, US; Rosenblatt [Rosenblatt and Zarzar, 2002] reported a 46% S-ECC prevalence rate among Brazilian children 25-36 months of age. Two studies included in the quantitative analysis only monitored study children until age 2, which might have underestimated the preventive effect of prenatal oral health care on ECC. 3) As we were not able to collect study subjects’ data on other caries determinants, e.g. demographic, socioeconomic, sugar consumption, etc., the meta-analysis performed in this review did not use multivariate analyses to consider potential confounders mentioned above. Given the multifactorial nature of ECC, the ORs calculated might have under- or overestimated the effectiveness of prenatal oral health care. 4) For the strategies that used prenatal oral health education or Primary-Primary prevention, it was not clear to what degree the prenatal oral intervention had improved or restored pregnant women’s oral health. Therefore, it is challenging to make recommendations on how much oral health care a pregnant woman needs to receive and how much oral health education is needed to demonstrate effective ECC prevention in children. Taking the aforementioned limitations into account, future randomized clinical trials are desired to test prenatal oral health care strategies that maintain or restore an expectant mother’s oral health and that measure improvements in oral health knowledge.
Moreover, another dilemma that needs to be considered is that: although routine oral care during pregnancy has been demonstrated to be safe, and recommendations for prenatal oral care have been disseminated globally, utilization of prenatal oral health care is limited in both developed and developing countries [Rocha et al., 2018]. In contrast to the limited utilization of prenatal dental care, over 76% of US women admitted to suffering from oral health problems (pain, bleeding gums and oral infection) during pregnancy while more than 43% did not have a dental checkup during pregnancy [Editorial, 2015]. Furthermore, dental care utilization during pregnancy was lower among black women [Thompson et al., 2013], ethnic minorities [Marchi et al., 2010] and women with socioeconomic disadvantages [Singhal et al., 2014]. Thus, oral health represents an important often-neglected heath disparity during pregnancy among minority women and women with socioeconomic disadvantages [Azofeifa et al., 2014; Guamizo-Herreno and Wehby, 2012]. In order to use successfully prenatal oral health care to prevent ECC, future efforts need to gain a better understanding of the factors that enable or inhibit the use of prenatal dental care at both the community and individual levels. Effective strategies might derive from collaborations among dental and medical providers involved in women’s and children’s dental and medical health, policy makers and community social workers.
Conclusions
This review reports a reduced ECC incidence and S. mutans carriage in children whose mothers received prenatal oral health care. Maintaining oral health and improving oral health care knowledge during pregnancy is a critical and promising step towards ECC prevention. Future studies should consider testing strategies that maintains an expectant mother’s oral health or restores an expectant mother’s oral health to a disease free state during pregnancy.
Acknowledgements
This study was supported in part by Jin Xiao’s faculty start-up funds from the Eastman Institute for Oral Health, University of Rochester and the National Institute for Dental and Craniofacial Research/National Center for Advancing Translational Sciences grant KL2 TR001999 and K23 DE027412. The funding agencies had no role in the study design, data collection, analyses, decision to publish, or preparation of the manuscript.
JX contributed to the study design, JX, NA, DAC, TTW performed the data acquisition and analysis. JX, DAC, DTK, RJB, LR, HM, EE and YR contributed to the data interpretation, manuscript writing and critical revision of the manuscript.
Appendix 1: Search strategy
PubMed Strategy
("Oral Health"[Mesh] OR "Oral Hygiene"[Mesh] OR "Dental Health Services"[Mesh] OR "Dental Care"[Mesh] OR (Oral Health) OR (Oral Hygiene) OR (Dental Health Services) OR (Dental Care) OR (Dental Health) OR (Dental Hygiene) OR (Dental Procedure) OR (Mouth Hygiene) OR (Tooth Hygiene) OR (Teeth Hygiene) OR (Oral Care) OR (Tooth Care) OR (Teeth Care) OR (Mouth Care)) AND ("Prenatal Care"[Mesh] OR "Pregnancy"[Mesh] OR "Pregnant Women"[Mesh] OR (Prenatal Care) OR Pregnancy OR (Pregnant Women) OR Prenatal OR Antenatal OR Gestation OR Pregnant) AND ("Dental Caries"[Mesh] OR "Infant, Low Birth Weight"[Mesh] OR "Premature Birth"[Mesh] OR "Infant, Premature"[Mesh] OR "Fetal Death"[Mesh] OR "Attitude to Health"[Mesh] OR "Health Behavior"[Mesh] OR "Health Education, Dental"[Mesh] OR Caries OR (Tooth Decay) OR (Teeth Decay) OR (Dental Decay) OR (Dental Fissure) OR (Dental Fissures) OR (Tooth Fissure) OR (Tooth Fissures) OR (Teeth Fissure) OR (Teeth Fissures) OR (Carious Dentin) OR (Carious Dentine) OR (White Spot) OR (White Spots) OR Cavity OR Cavities OR (Low Birth Weight) OR Underweight OR Premature OR (Pre Mature) OR Prematurity OR Prematuritas OR Preterm OR (Pre Term) OR (Fetus Death) OR (Fetus Death) OR (Fetus Deaths) OR (Fetus Demise) OR (Fetus Mummification) OR (Fetus Resorption) OR (Fetus Resorptions) OR (Fetal Deaths) OR (Fetal Demise) OR (Fetal Mummification) OR (Fetal Resorption) OR (Fetal Resorptions) OR Stillbirth OR Stillbirths OR Stillborn OR (Health Attitude) OR (Health Attitudes) OR (Health Knowledge) OR (Health Behavior) OR (Health Behaviors) OR (Health Behaviour) OR (Health Behaviours) OR (Health Education)) AND (randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tiab] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab] NOT (animals [mh] NOT humans [mh]))
Embase Strategy
('Mouth Hygiene'/exp OR 'Dental Procedure'/exp OR 'Dental health'/exp OR (Oral Health) OR (Oral Hygiene) OR (Dental Health Services) OR (Dental Care) OR (Dental Health) OR (Dental Hygiene) OR (Dental Procedure) OR (Mouth Hygiene) OR (Tooth Hygiene) OR (Teeth Hygiene) OR (Oral Care) OR (Tooth Care) OR (Teeth Care) OR (Mouth Care)) AND ('Prenatal Care'/exp OR 'Pregnancy'/exp OR 'Pregnant Woman'/exp OR (Prenatal Care) OR Pregnancy OR (Pregnant Women) OR Prenatal OR Antenatal OR Gestation OR Pregnant) AND ('Dental Caries'/exp OR 'Low Birth Weight'/exp OR 'Prematurity'/exp OR 'Fetus Death'/exp OR 'Attitude to Health'/exp OR 'Health Behavior'/exp OR 'Dental Health Education'/exp OR Caries OR (Tooth Decay) OR (Teeth Decay) OR (Dental Decay) OR (Dental Fissure) OR (Dental Fissures) OR (Tooth Fissure) OR (Tooth Fissures) OR (Teeth Fissure) OR (Teeth Fissures) OR (Carious Dentin) OR (Carious Dentine) OR (White Spot) OR (White Spots) OR Cavity OR Cavities OR (Low Birth Weight) OR Underweight OR Premature OR (Pre Mature) OR Prematurity OR Prematuritas OR Preterm OR (Pre Term) OR (Fetus Death) OR (Fetus Death) OR (Fetus Deaths) OR (Fetus Demise) OR (Fetus Mummification) OR (Fetus Resorption) OR (Fetus Resorptions) OR (Fetal Death) OR (Fetal Deaths) OR (Fetal Demise) OR (Fetal Mummification) OR (Fetal Resorption) OR (Fetal Resorptions) OR Stillbirth OR Stillbirths OR Stillborn OR (Health Attitude) OR (Health Attitudes) OR (Health Knowledge) OR (Health Behavior) OR (Health Behaviors) OR (Health Behaviour) OR (Health Behaviours) OR (Health Education)) AND ('crossover procedure':de OR 'double-blind procedure':de OR 'randomized controlled trial':de OR 'single-blind procedure':de OR (random* OR factorial* OR crossover* OR cross NEXT/1 over* OR placebo* OR doubl* NEAR/1 blind* OR singl* NEAR/1 blind* OR assign* OR allocat* OR volunteer*):de,ab,ti)
Web of Science Strategy
((Oral Health) OR (Oral Hygiene) OR (Dental Health Services) OR (Dental Care) OR (Dental Health) OR (Dental Hygiene) OR (Dental Procedure) OR (Mouth Hygiene) OR (Tooth Hygiene) OR (Teeth Hygiene) OR (Oral Care) OR (Tooth Care) OR (Teeth Care) OR (Mouth Care)) AND ((Prenatal Care) OR Pregnancy OR (Pregnant Women) OR Prenatal OR Antenatal OR Gestation OR Pregnant) AND (Caries OR (Tooth Decay) OR (Teeth Decay) OR (Dental Decay) OR (Dental Fissure) OR (Dental Fissures) OR (Tooth Fissure) OR (Tooth Fissures) OR (Teeth Fissure) OR (Teeth Fissures) OR (Carious Dentin) OR (Carious Dentine) OR (White Spot) OR (White Spots) OR Cavity OR Cavities OR (Low Birth Weight) OR Underweight OR Premature OR (Pre Mature) OR Prematurity OR Prematuritas OR Preterm OR (Pre Term) OR (Fetus Death) OR (Fetus Death) OR (Fetus Deaths) OR (Fetus Demise) OR (Fetus Mummification) OR (Fetus Resorption) OR (Fetus Resorptions) OR (Fetal Death) OR (Fetal Deaths) OR (Fetal Demise) OR (Fetal Mummification) OR (Fetal Resorption) OR (Fetal Resorptions) OR Stillbirth OR Stillbirths OR Stillborn OR (Health Attitude) OR (Health Attitudes) OR (Health Knowledge) OR (Health Behavior) OR (Health Behaviors) OR (Health Behaviour) OR (Health Behaviours) OR (Health Education)) AND (random* OR factorial* OR crossover* OR (cross over*) OR placebo* OR (doubl* AND blind*) OR (singl* AND blind*) OR assign* OR allocat* OR volunteer*)
LILACS Database Strategy
((Oral Health) OR (Oral Hygiene) OR (Dental Health Services) OR (Dental Care) OR (Dental Health) OR (Dental Hygiene) OR (Dental Procedure) OR (Mouth Hygiene) OR (Tooth Hygiene) OR (Teeth Hygiene) OR (Oral Care) OR (Tooth Care) OR (Teeth Care) OR (Mouth Care)) AND ((Prenatal Care) OR Pregnancy OR (Pregnant Women) OR Prenatal OR Antenatal OR Gestation OR Pregnant) AND (Caries OR (Tooth Decay) OR (Teeth Decay) OR (Dental Decay) OR (Dental Fissure) OR (Dental Fissures) OR (Tooth Fissure) OR (Tooth Fissures) OR (Teeth Fissure) OR (Teeth Fissures) OR (Carious Dentin) OR (Carious Dentine) OR (White Spot) OR (White Spots) OR Cavity OR Cavities OR (Low Birth Weight) OR Underweight OR Premature OR (Pre Mature) OR Prematurity OR Prematuritas OR Preterm OR (Pre Term) OR (Fetus Death) OR (Fetus Death) OR (Fetus Deaths) OR (Fetus Demise) OR (Fetus Mummification) OR (Fetus Resorption) OR (Fetus Resorptions) OR (Fetal Death) OR (Fetal Deaths) OR (Fetal Demise) OR (Fetal Mummification) OR (Fetal Resorption) OR (Fetal Resorptions) OR Stillbirth OR Stillbirths OR Stillborn OR (Health Attitude) OR (Health Attitudes) OR (Health Knowledge) OR (Health Behavior) OR (Health Behaviors) OR (Health Behaviour) OR (Health Behaviours) OR (Health Education)) AND (random* OR factorial* OR crossover* OR (cross over*) OR placebo* OR (doubl* AND blind*) OR (singl* AND blind*) OR assign* OR allocat* OR volunteer*)
Cochrane Database Strategy
((Oral Health) OR (Oral Hygiene) OR (Dental Health Services) OR (Dental Care) OR (Dental Health) OR (Dental Hygiene) OR (Dental Procedure) OR (Mouth Hygiene) OR (Tooth Hygiene) OR (Teeth Hygiene) OR (Oral Care) OR (Tooth Care) OR (Teeth Care) OR (Mouth Care)) AND ((Prenatal Care) OR Pregnancy OR (Pregnant Women) OR Prenatal OR Antenatal OR Gestation OR Pregnant) AND (Caries OR (Tooth Decay) OR (Teeth Decay) OR (Dental Decay) OR (Dental Fissure) OR (Dental Fissures) OR (Tooth Fissure) OR (Tooth Fissures) OR (Teeth Fissure) OR (Teeth Fissures) OR (Carious Dentin) OR (Carious Dentine) OR (White Spot) OR (White Spots) OR Cavity OR Cavities OR (Low Birth Weight) OR Underweight OR Premature OR (Pre Mature) OR Prematurity OR Prematuritas OR Preterm OR (Pre Term) OR (Fetus Death) OR (Fetus Death) OR (Fetus Deaths) OR (Fetus Demise) OR (Fetus Mummification) OR (Fetus Resorption) OR (Fetus Resorptions) OR (Fetal Death) OR (Fetal Deaths) OR (Fetal Demise) OR (Fetal Mummification) OR (Fetal Resorption) OR (Fetal Resorptions) OR Stillbirth OR Stillbirths OR Stillborn OR (Health Attitude) OR (Health Attitudes) OR (Health Knowledge) OR (Health Behavior) OR (Health Behaviors) OR (Health Behaviour) OR (Health Behaviours) OR (Health Education)) AND (random* OR factorial* OR crossover* OR (cross over*) OR placebo* OR (doubl* AND blind*) OR (singl* AND blind*) OR assign* OR allocat* OR volunteer*)
ClinicalTrials.gov Strategy
((Oral OR Dental OR Mouth OR Touth OR Teeth) AND (Health OR Hygiene OR Care OR Procedure)) AND (Pregnancy OR Pregnant OR Prenatal OR Antenatal OR Gestation) AND (Caries OR Carious OR ((Tooth OR Teeth OR Dental) AND (Decay OR Fissure*)) OR (White Spot*) OR Cavit*) / ((Low Birth Weight) OR Underweight OR Premature OR (Pre Mature) OR Prematurity OR Preterm OR (Pre Term) OR ((Fetus OR Fetal) AND (Death* OR Demise OR Mummification OR Resorption*)) OR Stillbirth* OR Stillborn) / ((Health Attitude*) OR (Health Knowledge) OR (Health Behavior*) OR (Health Behaviour*) OR (Health Education))
Appendix 2: Data extraction form
Appendix 3: Excluded articles after full-text review
- 1.Kanellis MJ, Logan HL, Jakobsen J. Changes in maternal attitudes toward baby bottle tooth decay. Pediatric dentistry. 1997;19(1):56–60. [PubMed] [Google Scholar]
- 2.Lydon-Rochelle MT, Krakowiak P, Hujoel PP, Peters RM. Dental care use and self-reported dental problems in relation to pregnancy. American Journal of Public Health. 2004;94(5):765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez NJ, Da Silva I, Ipinza J, Gutierrez J. Periodontal therapy reduces the rate of preterm low birth weight in women with pregnancy-associated gingivitis. Journal of periodontology. 2005;76(11 Suppl):2144–53. [DOI] [PubMed] [Google Scholar]
- 4.Castaldi JL, Bertin MS, Gimenez F, Lede R. Periodontal disease: Is it a risk factor for premature labor, low birth weight or preeclampsia? Revista Panamericana De Salud Publica-Pan American Journal of Public Health. 2006;19(4):253–8. [DOI] [PubMed] [Google Scholar]
- 5.Sadatmansouri S, Sedighpoor N, Aghaloo M. Effects of periodontal treatment phase I on birth term and birth weight. Journal of Indian Society of Pedodontics and Preventive Dentistry. 2006;24(1):23–6. [DOI] [PubMed] [Google Scholar]
- 6.Lopez R Periodontal treatment in pregnant women improves periodontal disease but does not alter rates of preterm birth. Evidence-based dentistry. 2007;8(2):38. [DOI] [PubMed] [Google Scholar]
- 7.Tarannum F, Faizuddin M. Effect of periodontal therapy on pregnancy outcome in women affected by periodontitis. Journal of periodontology. 2007;78(11):2095–103. [DOI] [PubMed] [Google Scholar]
- 8.Lopez R Periodontal treatment during pregnancy did not reduce the occurrence of poor pregnancy outcomes. Evidence-based dentistry. 2009;10(4):105. [DOI] [PubMed] [Google Scholar]
- 9.Lucey SM. Oral health promotion initiated during pregnancy successful in reducing early childhood caries. Evidence-based dentistry. 2009;10(4):100–1. [DOI] [PubMed] [Google Scholar]
- 10.Newnham JP, Newnham IA, Ball CM, Wright M, Pennell CE, Swain J, et al. Treatment of periodontal disease during pregnancy: a randomized controlled trial. Obstetrics and gynecology. 2009;114(6):1239–48. [DOI] [PubMed] [Google Scholar]
- 11.Novak T, Radnai M, Gorzo I, Urban E, Orvos H, Eller J, et al. Prevention of preterm delivery with periodontal treatment. Fetal diagnosis and therapy. 2009;25(2):230–3. [DOI] [PubMed] [Google Scholar]
- 12.Offenbacher S, Beck JD, Jared HL, Mauriello SM, Mendoza LC, Couper DJ, et al. Effects of periodontal therapy on rate of preterm delivery: a randomized controlled trial. Obstetrics and gynecology. 2009;114(3):551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pare E, Macones G, Srinivas S, Jeffcoat M, Appleby D, Sammel M, et al. Periodontal disease in pregnancy: Does it affect neonatal outcome? American Journal of Obstetrics and Gynecology. 2009;201(6):S83–S4. [Google Scholar]
- 14.Wandera MN, Engebretsen IM, Rwenyonyi CM, Tumwine J, Åstrøm AN. Periodontal status, tooth loss and self-reported periodontal problems effects on oral impacts on daily performances, OIDP, in pregnant women in Uganda: A cross-sectional study. Health and Quality of Life Outcomes. 2009;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Preterm births not reduced by periodontal treatment. Journal of the National Medical Association. 2010;102(3):259–60. [Google Scholar]
- 16.Cruz SS, Costa Mda C, Gomes-Filho IS, Barreto ML, dos Santos CA, Martins AG, et al. Periodontal therapy for pregnant women and cases of low birthweight: an intervention study. Pediatrics international : official journal of the Japan Pediatric Society. 2010;52(1):57–64. [DOI] [PubMed] [Google Scholar]
- 17.Gomes-Filho IS, Cruz SS, Da Conceigao NCM, Passos JS, Cerqueira EMM, Sampaio FP, et al. Periodontal therapy and low birth weight: Preliminary results from an alternative methodologic strategy. Journal of periodontology. 2010;81(12):1725–33. [DOI] [PubMed] [Google Scholar]
- 18.Macones GA, Parry S, Nelson DB, Strauss JF, Ludmir J, Cohen AW, et al. Treatment of localized periodontal disease in pregnancy does not reduce the occurrence of preterm birth: results from the Periodontal Infections and Prematurity Study (PIPS). American Journal of Obstetrics and Gynecology. 2010;202(2):147.e1–.e8. [DOI] [PubMed] [Google Scholar]
- 19.Meyer K, Geurtsen W, Gunay H. An early oral health care program starting during pregnancy: results of a prospective clinical long-term study. Clinical oral investigations. 2010;14(3):257–64. [DOI] [PubMed] [Google Scholar]
- 20.Niederman R. Periodontal treatment did not prevent complications of pregnancy. Evidence-based dentistry. 2010;11(1):18–9. [DOI] [PubMed] [Google Scholar]
- 21.Hart R, Doherty DA, Newnham IA, Pennell CE, Newnham JP. Periodontal disease n a further potentially modifiable risk factor limiting conception n a case for a pre-pregnancy dental checkup? Human Reproduction. 2011;26:i70. [DOI] [PubMed] [Google Scholar]
- 22.Jeffcoat M, Parry S, Gerlach RW, Doyle MJ. Use of alcohol-free antimicrobial mouth rinse is associated with decreased incidence of preterm birth in a high-risk population. Am J Obstet Gynecol. 2011;205(4):382.e1–6. [DOI] [PubMed] [Google Scholar]
- 23.Jeffcoat M, Parry S, Sammel M, Clothier B, Catlin A, Macones G. Periodontal infection and preterm birth: successful periodontal therapy reduces the risk of preterm birth. BJOG : an international journal of obstetrics and gynaecology. 2011;118(2):250–6. [DOI] [PubMed] [Google Scholar]
- 24.Sant'Ana AC, Campos MR, Passanezi SC, Rezende ML, Greghi SL, Passanezi E. Periodontal treatment during pregnancy decreases the rate of adverse pregnancy outcome: a controlled clinical trial. Journal of applied oral science : revista FOB. 2011;19(2):130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clifford H, Johnson NW, Brown C, Battistutta D. When can oral health education begin? Relative effectiveness of three oral health education strategies starting pre-partum. Community dental health. 2012;29(2):162–7. [PubMed] [Google Scholar]
- 26.Plutzer K, Spencer AJ, Keirse MJ. How first-time mothers perceive and deal with teething symptoms: a randomized controlled trial. Child: care, health and development. 2012;38(2):292–9. [DOI] [PubMed] [Google Scholar]
- 27.Reisine S, Douglass J, Aseltine R, Shanley E, Thompson C, Thibodeau E. Prenatal nutrition intervention to reduce mutans streptococci among low-income women. Journal of public health dentistry. 2012;72(1):75–81. [DOI] [PubMed] [Google Scholar]
- 28.Arrow P, Raheb J, Miller M. Brief oral health promotion intervention among parents of young children to reduce early childhood dental decay. BMC public health. 2013;13:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milgrom P, Riedy CA, Weinstein P, Mancl LA, Garson G, Huebner CE, et al. Design of a community-based intergenerational oral health study: "Baby Smiles". BMC oral health. 2013;13:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weidlich P, Moreira CH, Fiorini T, Musskopf ML, da Rocha JM, Oppermann ML, et al. Effect of nonsurgical periodontal therapy and strict plaque control on preterm/low birth weight: a randomized controlled clinical trial. Clinical oral investigations. 2013;17(1):37–44. [DOI] [PubMed] [Google Scholar]
- 31.Kopycka-Kedzierawski DT. Maternal Salivary Bacterial Challenge is Associated With Oral Infection Among Children and Predicts Early Childhood Caries (ECC) Incidence in a High-risk Cohort of 36-month-old Children. Journal of Evidence-Based Dental Practice. 2014;14(3):147–8. [DOI] [PubMed] [Google Scholar]
- 32.Meyer K, Khorshidi-Boehm M, Geurtsen W, Guenay H. An early oral health care program starting during pregnancy-a long-term study-phase V. Clinical oral investigations. 2014;18(3):863–72. [DOI] [PubMed] [Google Scholar]
- 33.Mohebbi SZ, Yazdani R, Sargeran K, Tartar Z, Janeshin A. Midwifery students training in oral care of pregnant patients: an interventional study. Journal of dentistry (Tehran, Iran). 2014;11(5):587–95. [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang H, Xiong X, Buekens P, Su Y, Qian X. Use of mouth rinse during pregnancy to improve birth and neonatal outcomes: a randomized controlled trial. BMC pregnancy and childbirth. 2015;15:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson M, George A, Dahlen H, Ajwani S, Bhole S, Blinkhorn A, et al. The midwifery initiated oral health-dental service protocol: an intervention to improve oral health outcomes for pregnant women. BMC oral health. 2015;15:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang H, Xiong X, Su Y, Peng J, Zhu X, Wang J, et al. Use of antiseptic mouthrinse during pregnancy and pregnancy outcomes: a randomised controlled clinical trial in rural China. BJOG : an international journal of obstetrics and gynaecology. 2016;123 Suppl 3:39–47. [DOI] [PubMed] [Google Scholar]
- 37.Al Khamis S, Asimakopoulou K, Newton T, Daly B. The effect of dental health education on pregnant women's adherence with toothbrushing and flossing - A randomized control trial. Community dentistry and oral epidemiology. 2017;45(5):469–77. [DOI] [PubMed] [Google Scholar]
- 38.Vilella KD, Fraiz FC, Benelli EM, Assuncao LR. Oral Health Literacy and Retention of Health Information Among Pregnant Women: A Randomised Controlled Trial. Oral health & preventive dentistry. 2017;15(1):41–8. [DOI] [PubMed] [Google Scholar]
- 39.George A, Dahlen HG, Blinkhorn A, Ajwani S, Bhole S, Ellis S, et al. Evaluation of a midwifery initiated oral health-dental service program to improve oral health and birth outcomes for pregnant women: A multi-centre randomised controlled trial. International journal of nursing studies. 2018;82:49–57 [DOI] [PubMed] [Google Scholar]
Footnotes
Declaration of interests
The authors declare no conflict of interests.
Reference:
- American Academy of Pediatric Dentistry Council on Clinical A: Policy on early childhood caries (ecc): Unique challenges and treatment options. Pediatric dentistry 2005;27:34–35. [PubMed] [Google Scholar]
- Axelsson P: Preventive programs Preventive Dental Health Center, Karlstad, Sweden: 1988. [Google Scholar]
- Azofeifa A, Yeung LF, Alverson CJ, Beltran-Aguilar E: Oral health conditions and dental visits among pregnant and nonpregnant women of childbearing age in the united states, national health and nutrition examination survey, 1999-2004. Prev Chronic Dis 2014;11:E163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz RJ, Amante A, Kopycka-Kedzierawski DT, Billings RJ, Feng C: Dental caries recurrence following clinical treatment for severe early childhood caries. Pediatric dentistry 2011;33:510–514. [PubMed] [Google Scholar]
- Bliss JM, Basavegowda KP, Watson WJ, Sheikh AU, Ryan RM: Vertical and horizontal transmission of candida albicans in very low birth weight infants using DNA fingerprinting techniques. The Pediatric infectious disease journal 2008;27:231–235. [DOI] [PubMed] [Google Scholar]
- Casamassimo PS, Thikkurissy S, Edelstein BL, Maiorini E: Beyond the dmft: The human and economic cost of early childhood caries. J Am Dent Assoc 2009;140:650–657. [DOI] [PubMed] [Google Scholar]
- Caufield PW, Cutter GR, Dasanayake AP: Initial acquisition of mutans streptococci by infants: Evidence for a discrete window of infectivity. Journal of dental research 1993;72:37–45. [DOI] [PubMed] [Google Scholar]
- Caufield PW, Li Y, Dasanayake A: Dental caries: An infectious and transmissible disease. Compend Contin Educ Dent 2005;26:10–16. [PubMed] [Google Scholar]
- Chaffee BW, Gansky SA, Weintraub JA, Featherstone JD, Ramos-Gomez FJ: Maternal oral bacterial levels predict early childhood caries development. Journal of dental research 2014;93:238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childers NK, Momeni SS, Whiddon J, Cheon K, Cutter GR, Wiener HW, Ghazal TS, Ruby JD, Moser SA: Association between early childhood caries and colonization with streptococcus mutans genotypes from mothers. Pediatr Dent 2017;39:130–135. [PMC free article] [PubMed] [Google Scholar]
- Colak H, Dulgergil CT, Dalli M, Hamidi MM: Early childhood caries update: A review of causes, diagnoses, and treatments. Journal of natural science, biology, and medicine 2013;4:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disease GBD, Injury I, Prevalence C: Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: A systematic analysis for the global burden of disease study 2016. Lancet 2017;390:1211–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs SH, Black N: The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye BA, Hsu KL, Afful J: Prevalence and measurement of dental caries in young children. Pediatric dentistry 2015;37:200–216. [PubMed] [Google Scholar]
- Dye BA, Li X, Thorton-Evans G: Oral health disparities as determined by selected healthy people 2020 oral health objectives for the united states, 2009-2010. NCHS Data Brief 2012:1–8. [PubMed] [Google Scholar]
- Dye BA, Tan S, Smith V, Lewis BG, Barker LK, Thornton-Evans G, Eke PI, Beltran-Aguilar ED, Horowitz AM, Li CH: Trends in oral health status: United states, 1988-1994 and 1999-2004. Vital and health statistics Series 11, Data from the national health survey 2007:1–92. [PubMed] [Google Scholar]
- Editorial: Majority of pregnant women have oral health problems, yet 43% don't seek dental treatment; in: Dentistry IQ. 2015. [Google Scholar]
- Finlayson TL, Siefert K, Ismail AI, Sohn W: Maternal self-efficacy and 1-5-year-old children's brushing habits. Community dentistry and oral epidemiology 2007;35:272–281. [DOI] [PubMed] [Google Scholar]
- Fisher-Owens SA, Gansky SA, Platt LJ, Weintraub JA, Soobader MJ, Bramlett MD, Newacheck PW: Influences on children's oral health: A conceptual model. Pediatrics 2007;120:e510–520. [DOI] [PubMed] [Google Scholar]
- Goettems ML, Ardenghi TM, Demarco FF, Romano AR, Torriani DD: Children's use of dental services: Influence of maternal dental anxiety, attendance pattern, and perception of children's quality of life. Community Dent Oral Epidemiol 2012;40:451–458. [DOI] [PubMed] [Google Scholar]
- Graves CE, Berkowitz RJ, Proskin HM, Chase I, Weinstein P, Billings R: Clinical outcomes for early childhood caries: Influence of aggressive dental surgery. Journal of dentistry for children (Chicago, Ill) 2004;71:114–117. [PubMed] [Google Scholar]
- Gross EL, Beall CJ, Kutsch SR, Firestone ND, Leys EJ, Griffen AL: Beyond streptococcus mutans: Dental caries onset linked to multiple species by 16s rrna community analysis. PloS one 2012;7:e47722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnizo-Herreno CC, Wehby GL: Explaining racial/ethnic disparities in children's dental health: A decomposition analysis. Am J Public Health 2012;102:859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunay H, Dmoch-Bockhorn K, Gunay Y, Geurtsen W: Effect on caries experience of a long-term preventive program for mothers and children starting during pregnancy. Clinical oral investigations 1998;2:137–142. [DOI] [PubMed] [Google Scholar]
- Heller KE, Eklund SA, Pittman J, Ismail AA: Associations between dental treatment in the primary and permanent dentitions using insurance claims data. Pediatr Dent 2000;22:469–474. [PubMed] [Google Scholar]
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods G, Cochrane Statistical Methods G: The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida H: Oral health interventions during pregnancy. Dental clinics of North America 2017;61:467–481. [DOI] [PubMed] [Google Scholar]
- Kanasi E, Johansson I, Lu SC, Kressin NR, Nunn ME, Kent R Jr., Tanner AC: Microbial risk markers for childhood caries in pediatricians' offices. Journal of dental research 2010;89:378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein MI, Florio FM, Pereira AC, Hofling JF, Goncalves RB: Longitudinal study of transmission, diversity, and stability of streptococcus mutans and streptococcus sobrinus genotypes in brazilian nursery children. Journal of clinical microbiology 2004;42:4620–4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinke T, Urban M, Luck C, Hannig C, Kuhn M, Kramer N: Changes in candida spp., mutans streptococci and lactobacilli following treatment of early childhood caries: A 1-year follow-up. Caries research 2014;48:24–31. [DOI] [PubMed] [Google Scholar]
- Koo H, Bowen WH: Candida albicans and streptococcus mutans: A potential synergistic alliance to cause virulent tooth decay in children. Future microbiology 2014;9:1295–1297. [DOI] [PubMed] [Google Scholar]
- Kopycka-Kedzierawski DT, Bell CH, Billings RJ: Prevalence of dental caries in early head start children as diagnosed using teledentistry. Pediatric dentistry 2008;30:329–333. [PubMed] [Google Scholar]
- Leverett DH, Adair SM, Vaughan BW, Proskin HM, Moss ME: Randomized clinical trial of the effect of prenatal fluoride supplements in preventing dental caries. Caries research 1997;31:174–179. [DOI] [PubMed] [Google Scholar]
- Li Y, Caufield PW, Dasanayake AP, Wiener HW, Vermund SH: Mode of delivery and other maternal factors influence the acquisition of streptococcus mutans in infants. Journal of dental research 2005;84:806–811. [DOI] [PubMed] [Google Scholar]
- Li Y, Tanner A: Effect of antimicrobial interventions on the oral microbiota associated with early childhood caries. Pediatric dentistry 2015;37:226–244. [PMC free article] [PubMed] [Google Scholar]
- Marchi KS, Fisher-Owen SA, Weintraub JA, Yu Z, Braveman PA: Most pregnant women in california do not receive dental care: Findings from a population-based study. Public Health Rep 2010;125:831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai Y, Mori Y, Tamaoka I: Antenatal health care and postnatal dental check-ups prevent early childhood caries. Tohoku J Exp Med 2016;240:303–308. [DOI] [PubMed] [Google Scholar]
- Nakai Y, Shinga-Ishihara C, Kaji M, Moriya K, Murakami-Yamanaka K, Takimura M: Xylitol gum and maternal transmission of mutans streptococci. Journal of Dental Research 2010;89:56–60. [DOI] [PubMed] [Google Scholar]
- O'Sullivan DM, Tinanoff N: The association of early dental caries patterns with caries incidence in preschool children. Journal of public health dentistry 1996;56:81–83. [DOI] [PubMed] [Google Scholar]
- Olak J, Nguyen MS, Nguyen TT, Nguyen BBT, Saag M: The influence of mothers' oral health behaviour and perception thereof on the dental health of their children. EPMA J 2018;9:187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plutzer K, Spencer AJ: Efficacy of an oral health promotion intervention in the prevention of early childhood caries. Community dentistry and oral epidemiology 2008;36:335–346. [DOI] [PubMed] [Google Scholar]
- Powell LV: Caries prediction: A review of the literature. Community Dent Oral Epidemiol 1998;26:361–371. [DOI] [PubMed] [Google Scholar]
- Quinonez RB, Keels MA, Vann WF Jr., McIver FT, Heller K, Whitt JK: Early childhood caries: Analysis of psychosocial and biological factors in a high-risk population. Caries research 2001;35:376–383. [DOI] [PubMed] [Google Scholar]
- Rashewsky S, Parameswaran A, Sloane C, Ferguson F, Epstein R: Time and cost analysis: Pediatric dental rehabilitation with general anesthesia in the office and the hospital settings. Anesth Prog 2012;59:147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha JS, Arima LY, Werneck RI, Moyses SJ, Baldani MH: Determinants of dental care attendance during pregnancy: A systematic review. Caries Res 2018;52:139–152. [DOI] [PubMed] [Google Scholar]
- Rosenblatt A, Zarzar P: The prevalence of early childhood caries in 12- to 36-month-old children in recife, brazil. ASDC journal of dentistry for children 2002;69:319–324, 236. [PubMed] [Google Scholar]
- Ruby J, Goldner M: Nature of symbiosis in oral disease. J Dent Res 2007;86:8–11. [DOI] [PubMed] [Google Scholar]
- Saied-Moallemi Z, Virtanen JI, Ghofranipour F, Murtomaa H: Influence of mothers' oral health knowledge and attitudes on their children's dental health. Eur Arch Paediatr Dent 2008;9:79–83. [DOI] [PubMed] [Google Scholar]
- Singhal A, Chattopadhyay A, Garcia AI, Adams AB, Cheng D: Disparities in unmet dental need and dental care received by pregnant women in maryland. Matern Child Health J 2014;18:1658–1666. [DOI] [PubMed] [Google Scholar]
- Slayton RL: Reducing mutans streptococci levels in caregivers may reduce transmission to their children and lead to reduced caries prevalence. The journal of evidence-based dental practice 2011;11:27–28. [DOI] [PubMed] [Google Scholar]
- Tanzer JM, Livingston J, Thompson AM: The microbiology of primary dental caries in humans. J Dent Educ 2001;65:1028–1037. [PubMed] [Google Scholar]
- Thompson TA, Cheng D, Strobino D: Dental cleaning before and during pregnancy among maryland mothers. Matern Child Health J 2013;17:110–118. [DOI] [PubMed] [Google Scholar]
- Waggoner-Fountain LA, Walker MW, Hollis RJ, Pfaller MA, Ferguson JE 2nd, Wenzel RP, Donowitz LG: Vertical and horizontal transmission of unique candida species to premature newborns. Clin Infect Dis 1996;22:803–808. [DOI] [PubMed] [Google Scholar]
- Wang X, Shaffer JR, Zeng Z, Begum F, Vieira AR, Noel J, Anjomshoaa I, Cuenco KT, Lee MK, Beck J, Boerwinkle E, Cornelis MC, Hu FB, Crosslin DR, Laurie CC, Nelson SC, Doheny KF, Pugh EW, Polk DE, Weyant RJ, Crout R, McNeil DW, Weeks DE, Feingold E, Marazita ML: Genome-wide association scan of dental caries in the permanent dentition. BMC Oral Health 2012;12:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigen TI, Espelid I, Skaare AB, Wang NJ: Family characteristics and caries experience in preschool children. A longitudinal study from pregnancy to 5 years of age. Community dentistry and oral epidemiology 2011;39:311–317. [DOI] [PubMed] [Google Scholar]
- Xiao J, Huang X, Alkhers N, Alzamil H, Alzoubi S, Wu TT, Castillo DA, Campbell F, Davis J, Herzog K, Billings R, Kopycka-Kedzierawski DT, Hajishengallis E, Koo H: Candida albicans and early childhood caries: A systematic review and meta-analysis. Caries Res 2018;52:102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Moon Y, Li L, Rustchenko E, Wakabayashi H, Zhao X, Feng C, Gill SR, McLaren S, Malmstrom H, Ren Y, Quivey R, Koo H, Kopycka-Kedzierawski DT: Candida albicans carriage in children with severe early childhood caries (s-ecc) and maternal relatedness. PloS one 2016;11:e0164242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan L, Tan S, Den Besten P, Featherstone JD, Hoover CI: Factors related to maternal transmission of mutans streptococci in high-risk children-pilot study. Pediatric dentistry 2012;34:e86–91. [PubMed] [Google Scholar]