Abstract
STUDY QUESTION
Does altering gut microbiota with antibiotic treatment have any impact on endometriosis progression?
SUMMARY ANSWER
Antibiotic therapy reduces endometriosis progression in mice, possibly by reducing specific gut bacteria.
WHAT IS KNOWN ALREADY
Endometriosis, a chronic condition causing abdominal pain and infertility, afflicts up to 10% of women between the ages of 25 and 40, ~5 million women in the USA. Current treatment strategies, including hormone therapy and surgery, have significant side effects and do not prevent recurrences. We have little understanding of why some women develop endometriosis and others do not.
STUDY DESIGN, SIZE, DURATION
Mice were treated with broad-spectrum antibiotics or metronidazole, subjected to surgically-induced endometriosis and assayed after 21 days.
PARTICIPANTS/MATERIALS, SETTING, METHODS
The volumes and weights of endometriotic lesions and histological signatures were analysed. Proliferation and inflammation in lesions were assessed by counting cells that were positive for the proliferation marker Ki-67 and the macrophage marker Iba1, respectively. Differences in faecal bacterial composition were assessed in mice with and without endometriosis, and faecal microbiota transfer studies were performed.
MAIN RESULTS AND THE ROLE OF CHANCE
In mice treated with broad-spectrum antibiotics (vancomycin, neomycin, metronidazole and ampicillin), endometriotic lesions were significantly smaller (~ 5-fold; P < 0.01) with fewer proliferating cells (P < 0.001) than those in mice treated with vehicle. Additionally, inflammatory responses, as measured by the macrophage marker Iba1 in lesions and IL-1β, TNF-α, IL-6 and TGF-β1 in peritoneal fluid, were significantly reduced in mice treated with broad-spectrum antibiotics (P < 0.05). In mice treated with metronidazole only, but not in those treated with neomycin, ectopic lesions were significantly (P < 0.001) smaller in volume than those from vehicle-treated mice. Finally, oral gavage of faeces from mice with endometriosis restored the endometriotic lesion growth and inflammation (P < 0.05 and P < 0.01, respectively) in metronidazole-treated mice.
LARGE-SCALE DATA
N/A.
LIMITATIONS, REASONS FOR CAUTION
These findings are from a mouse model of surgically-induced endometriosis. Further studies are needed to determine the mechanism by which gut bacteria promote inflammation, identify bacterial genera or species that promote disease progression and assess the translatability of these findings to humans.
WIDER IMPLICATIONS OF THE FINDINGS
Our findings suggest that gut bacteria promote endometriosis progression in mice. This finding if translated to humans, could aid in the development of improved diagnostic tools and personalised treatment strategies.
STUDY FUNDING AND COMPETING INTEREST(S)
This work was funded, in part, by: a National Institutes of Health (NIH)/ National Institute of Child Health and Human Development (NICHD) grant (R00HD080742) to RK; Washington University School of Medicine start-up funds to RK; an Endometriosis Foundation of America Research Award to R.K.; and an NIH/NICHD grant (R01HD091218) to IUM. The authors report no conflict of interest.
Keywords: endometriosis, inflammation, microbiome, gut bacteria, metronidazole
Introduction
Endometriosis causes pain in the pelvis and lower abdomen and afflicts up to 10% of women between the ages of 25 and 40, ~5 million women in the USA. Nearly, half of these women experience chronic pelvic pain that significantly diminishes their quality of life (Giudice, 2010). Factors implicated in establishment and expansion of endometriotic lesions include hormonal imbalance, immune dysfunction, epigenetic modifications triggered by environmental toxicants (Rier and Foster, 2003; Hsiao et al., 2017) and unopposed estrogen action coupled with progesterone resistance. The current treatments for endometriosis, principally hormone therapy and surgery, have negative side effects and do not prevent recurrences. Therefore, a new approach is needed to combat this disease (Falcone and Flyckt, 2018).
A well-accepted theory is that endometriosis is caused by endometrial tissue which enters the peritoneal cavity via retrograde menstruation and implants onto pelvic organs and peritoneal surfaces. However, up to 90% of women experience retrograde menstruation, yet only 10% of women develop endometriosis. This suggests that other factors contribute to the onset of endometriosis onset (Sourial et al., 2014). It is thought that the immune system usually clears the cells that enter the peritoneal cavity during retrograde menstruation, but when it is unable to do so, the lesions spread as a result of inflammation brought about by macrophages releasing pro-inflammatory cytokines and growth factors into the peritoneal cavity (Ahn et al., 2015b). This hypothesis is supported by findings in mouse models of endometriosis (Lin et al., 2006; Han et al., 2015). For example, macrophages drive lesion growth and vascularisation (Lin et al., 2006; Bacci et al., 2009; Capobianco et al., 2011) and enhance IL-1β signalling in response to inflammasome activation, which also promotes endometriotic angiogenesis (Lebovic et al., 2000; Bullon and Navarro, 2017).
Endometriosis may also be influenced by the microbiome. Distinct microbial communities have been identified in the reproductive tracts of reproductive-age women (Moreno et al., 2016; Chen et al., 2017), and some microbial compositions appear to correlate with reproductive pathologies such as preterm birth and infertility (Parnell et al., 2017b). Additionally, Cregger et al. (3–6) identified differences in the cervical and uterine microbiome communities between women with and without endometriosis (Cregger et al., 2017). Here, we tested the hypothesis that the gut microbiome, which encodes 150 times more genes than the host genome (O’Hara and Shanahan, 2006; Ursell et al., 2014), influences endometriosis disease progression. We demonstrate that treating mice with broad-spectrum antibiotics greatly curtailes the early growth and progression of endometriotic lesions. Whereas metronidazole treatment reduced endometriotic lesion growth, oral gavage of bacteria from mice with endometriosis restored endometriotic lesion growth and associated inflammatory responses. These results suggest that gut bacteria promote endometriosis disease progression and have implications for microbiota-based therapies to combat this painful disease.
Materials and Methods
Study approval
Animal studies were performed according to a protocol (number 20160227) approved by the Washington University School of Medicine Institutional Animal Care and Use Committee.
Mouse surgical endometriosis model
We used a well-established endometriosis model in which uterine tissue from estrus-stage mice is autologously transplanted onto the peritoneal wall. After 3 weeks, the resulting endometriotic lesions are composed of a single cyst (Cummings and Metcalf, 1995; Pelch et al., 2012) and resemble those observed in human endometriosis (Fainaru et al., 2008; Umezawa et al., 2009; Korbel et al., 2010). Briefly, one uterine horn from 10-week-old, estrus-stage mice (C57BL/6, Taconic, n = 4 to 15 per group) was excised and cut longitudinally. Next, a dermal biopsy punch was used to isolate a 3-mm endometrial fragment, which was sutured to the peritoneal wall in the same mouse through a midline incision (Fainaru et al., 2008; Schreinemacher et al., 2012; Machado et al., 2016; Kiani et al., 2018). For the sham surgery, a similar procedure was performed except that a thread was sutured onto the peritoneal wall without an endometrial fragment.
Antibiotic treatment
Twenty-four hours after endometriosis-induction surgery, mice were provided drinking water containing 0.5 g/l vancomycin, 1 g/l neomycin, 1 g/l metronidazole and 1 g/l ampicillin (VNMA) for 21 days as described previously (Rakoff-Nahoum et al., 2004). To mask the taste of the antibiotics, 2 g/l aspartame was added to the VNMA-containing water. Control mice received drinking water containing aspartame alone (Huang et al., 2015). In other experiments, mice received water contatining only 1 g/l metronidazole or 1 g/l neomycin plus aspartame, or water containing only aspartame. Then, mice were euthanised, faecal samples were collected and eutopic endometrium and endometriotic lesions were isolated. Peritoneal fluid was collected by washing the peritoneum with 1ml sterile PBS. Lesions were weighed (mg), and lesion volumes (mm3) were measured with a Vernier Calliper (VCB001, United scientific Supplies Waukegan, IL, USA) by an investigator blinded to treatment groups.
Faecal transplantation
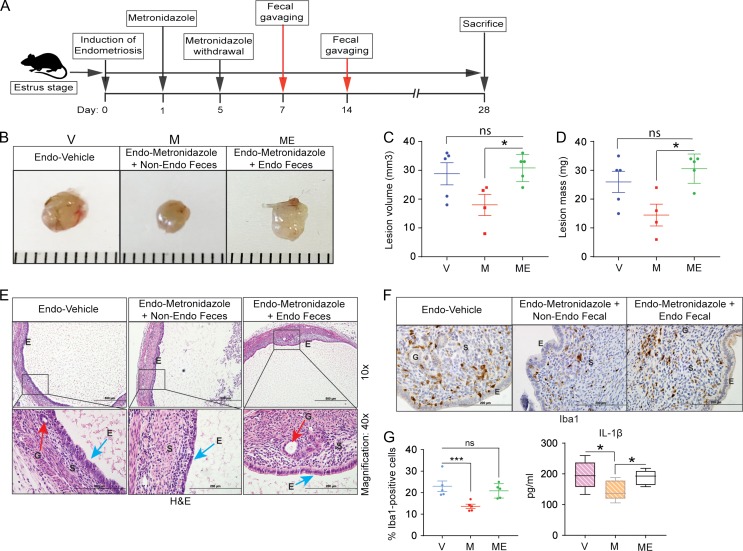
Faecal pellets were immediately frozen at −80°C as reported previously (Hintze et al., 2014). Faecal pellets were resuspended in phosphate-buffered saline (PBS) (one faecal pellet/0.1 ml of PBS), and 200 μl of the suspension was given by oral gavage to each mouse (Wong et al., 2017) as indicated in Fig. 5A. The numbers of mice were as follows: endo-metronidazole + non-endo faeces, n = 4 and endo-metronidazole + endo faeces, n = 4.
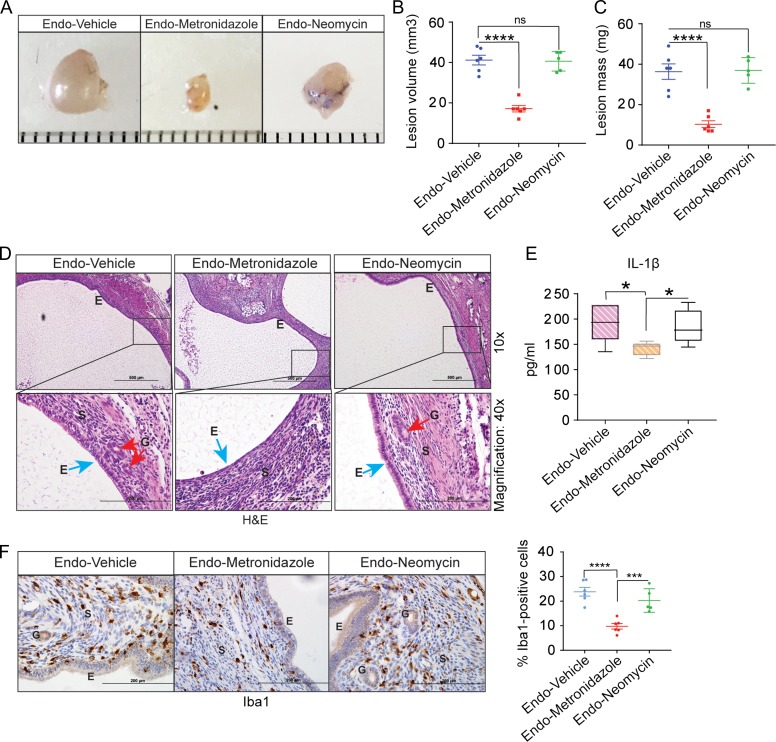
Figure 5.
Oral gavage of faeces from endometriotic mice promotes endometriotic lesion growth in antibiotic-treated mice. (A) Schematic of experimental timeline and procedures. (B–D) Representative gross images (B), volumes (C) and masses (D) of ectopic endometriotic lesions from the indicated treatment groups 28 days after surgical induction of endometriosis; ‘V’, ‘M’ and ‘ME’ denote endo-vehicle, endo-metronidazole + non-endo faeces, and endo-metronidazole + endo faeces, respectively; n = 4–5. (E) Representative Hematoxylin and Eosin-stained cross-section images of ectopic lesions from the indicated treatment groups; n = 4–5. Scale bars represent 200 μm (upper panel) and 500 μm (lower panel). (F) Representative cross-sectional images of ectopic lesions stained for Iba1. (G) Quantification of Iba1-positive cells counted in at least five different areas in ectopic lesions and plotted as percent positive cells relative to total cells (left panel) and quantification of IL-1β concentration in peritoneal fluid from the indicated treatment groups (right panel). Data are presented as mean ± SE (n = 4–5). ‘E’, ‘G’ and ‘S’ denote epithelia, glands and stroma, respectively. *P < 0.05, ***P < 0.001, and ns, non-significant.
Bacterial 16S rRNA gene sequencing and diversity analysis
DNA was extracted from faecal pellets (0.1 gm) by using the QIAmp Power Faecal DNA Kit (12850-50, Qiagen) as per the manufacturer’s protocol. The numbers of mice were as follows: non-endo, n = 5; endo-vehicle, n = 5; and endo-VNMA, n = 4. Amplicon generation and sequencing were performed by the Genome Technology Access Center in the Department of Genetics at Washington University School of Medicine as described previously (Parnell et al., 2017a). Fastq sequences were uploaded to the NCBI Sequence Read Archive (SUB3753571). Quantitative Insights Into Microbial Ecology analysis of variable region four was used to remove operational taxonomic units (OTUs) that were only observed once and rarefy tables to 600 OTUs. This dataset was used for downstream alpha and beta diversity analysis, and identification of the top 10 OTUs was as described previously (Parnell et al., 2017a).
Histological analysis
Tissues were fixed in 4% paraformaldehyde, and sections were immunostained (n = 4–5 per group) as described previously (Kommagani et al., 2016). Briefly, tissue sections were blocked with 5% goat serum and then incubated overnight at 4°C in 2% goat serum containing the following primary antibodies from Abcam: Rabbit anti-ER-α (ab75635), anti-Ki-67 (ab16667) or anti-Iba1 (ab178847). Sections were then incubated with biotinylated secondary antibody and counter-stained with hematoxylin. Finally, sections were dehydrated and mounted in Permount histological mounting medium (Fisher Scientific Inc.). Ki-67- and Iba1-positive cells were counted manually in images taken at 400X magnification by two independent investigators blinded to treatment groups. Cells were counted in at least five different areas in ectopic lesions and plotted as percent positive cells relative to total number of cells as described previously (Kommagani et al., 2013). For hematoxylin and eosin staining, tissue sections were fixed, processed, embedded, deparaffinized and stained as described previously (Kommagani et al., 2016).
Enzyme-linked immunosorbent assays
Enzyme-linked immunosorbent assays (ELISA) kits were used to measure the peritoneal concentrations of IL-1β (KMC0011, Invitrogen, Life Technologies), TNF-α (ab208348, Abcam), IL-6 (ab100712, Abcam), IL-10 (ab108870, Abcam) and TGF-β1 (ab119557, Abcam), according to the manufacturer’s instructions (n = 10 per group). The intra- and inter-assay coefficients of variation for the IL-1β ELISA were 8.4% and 4.9%, respectively. Peritoneal concentrations were deduced from standard curves, and the final concentration was calculated by normalising with the total protein concentration in peritoneal fluid.
Statistics
A two-tailed paired Student's t-test was used for statistical significance testing for all data except the 16 S sequencing data, which did not follow a normal distribution. The non-parametric Kruskal–Wallis test was used to compare the Shannon diversities of microbiota from non-endo, endo-vehicle and endo-VNMA groups and the Mann–Whitney test was used to compare Shannon diversities between groups. For multidimentional scaling (MDS) plots, the R ‘Phyloseq’ package was used to perform permutation analysis of variance. P < 0.05 was considered significant.
Results
Treatment with broad-spectrum antibiotics reduces endometriotic lesion growth, proliferation and inflammation
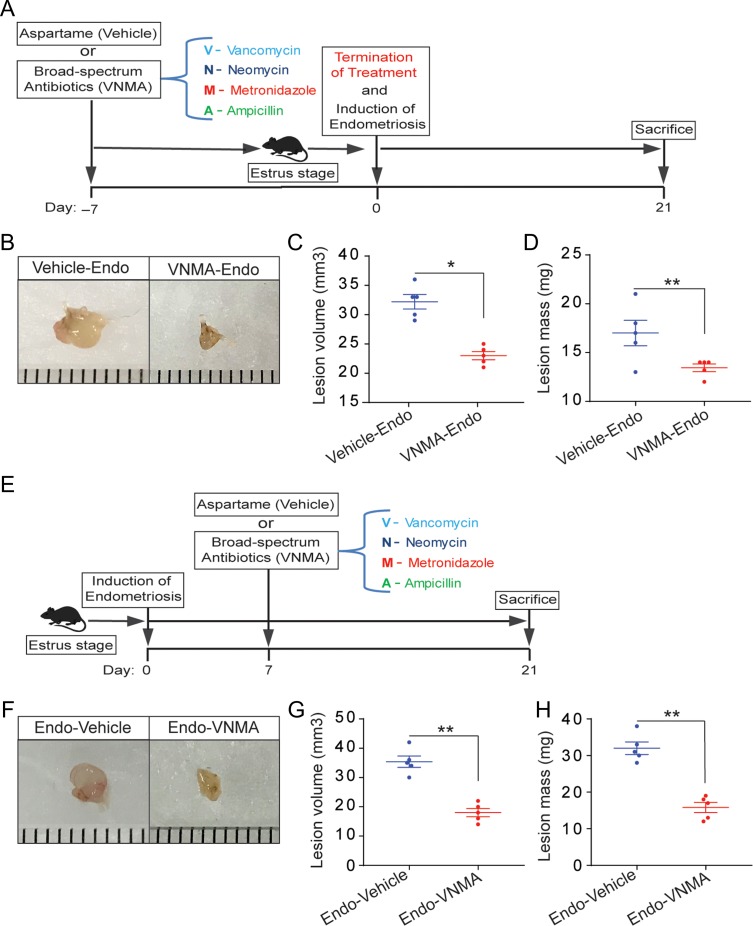
To determine whether antibiotics affect early endometriotic lesion growth, we treated mice with the broad-spectrum antibiotics VNMA in drinking water containing aspartame to mask the antibiotics taste. Control mice received drinking water containing aspartame alone. We then performed endometriosis-induction surgery (Fig. 1A). Mice that consumed VNMA (VNMA-endo) had smaller endometriotic lesions than those that consumed vehicle alone (vehicle-endo) (Fig. 1B–D). In a second experiment aimed at assessing progression of established endometriotic lesions, we treated mice with antibiotics after endometriosis surgery (Fig. 1E). Lesions were smaller in mice that consumed VNMA (endo-VNMA) than in those that consumed vehicle (endo-vehicle) (Fig. 1F–H). These two experiments indicated that antibiotic treatment reduced both early growth and progression of endometriotic lesions.
Figure 1.
Treatment with broad-spectrum antibiotics prevents early endometriotic lesion growth and progression. (A and E) Schematic of experimental timeline and procedures. (B–D and F–H) Representative gross images (B and F), volumes (C and G), and masses (D and H) of ectopic endometriotic lesions from the indicated treatment groups 21 days after surgical induction of endometriosis. Data are presented as mean ± SE(n = 5) *P < 0.05, **P < 0.01.
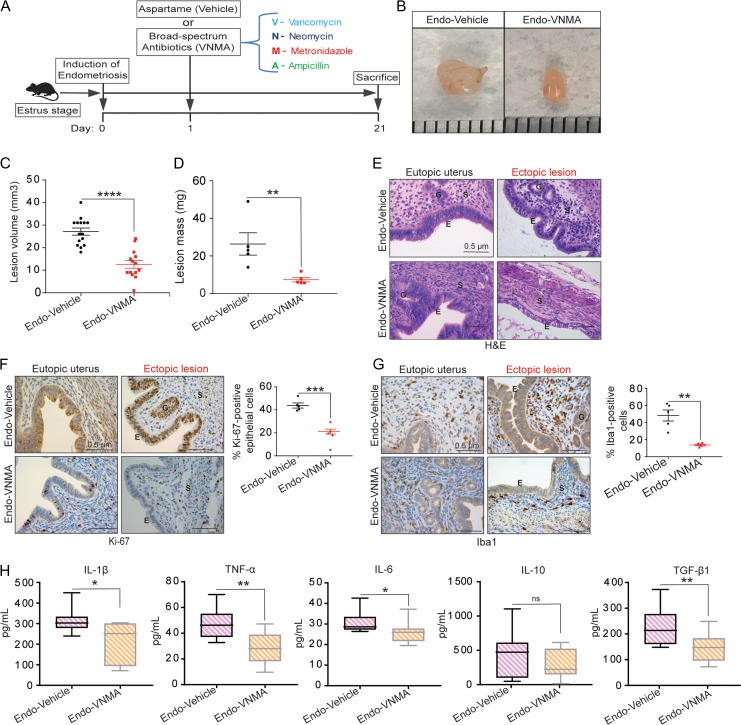
To begin to uncover the mechanism by which antibiotics affected endometriotic lesion progression, we treated mice with antibiotics immediately after endometriosis surgery and performed a series of analyses (Fig. 2). First, we confirmed that neither surgery nor antibiotic treatment had any effect on water consumption or body weight (Supplementary Figure S1). Second, hematoxylin and eosin staining revealed that whereas lesions from endo-vehicle mice had typical endometriosis-like structures, including a thick epithelial layer and glandular areas, lesions from endo-VNMA mice had thinner epithelial areas and no glands (Fig. 2E). Additionally, consistent with reports that stromal tissue volume correlates with lesion growth (Korbel et al., 2010), lesions from endo-VNMA mice had smaller stromal areas than lesions from endo-vehicle mice (Fig. 2E). Importantly, the eutopic uteri had similar epithelial, glandular and stromal areas in both endo-vehicle and endo-VNMA mice (Fig. 2E). Third, we stained the lesions with an antibody specific to estrogen receptor alpha (ERα), which is thought to promote proliferation and inflammation and thus drive endometriotic lesion growth and expansion (Huhtinen et al., 2012). However, ERα expression was similar between lesions from endo-vehicle and endo-VNMA mice (Figure S2). Furthermore, consistent with a report that stage of estrous had no impact on lesion growth (Fainaru et al., 2008; Schreinemacher et al., 2012; Machado et al., 2016; Kiani et al., 2018), lesion volumes did not appear to correlate with the stage of estrous at sacrifice (data not shown).
Figure 2.
Treatment with broad-spectrum antibiotics reduces endometriotic lesion proliferation and inflammation. (A) Schematic of experimental timeline and procedures. (B–D) Representative gross images (B), volumes (C) and masses (D) of ectopic endometriotic lesions from the indicated treatment groups 21 days after surgical induction of endometriosis. Data are presented as mean ± SE; endo-vehicle (n = 15) and endo-VNMA (n = 14). (E) Representative Hematoxylin and Eosin-stained cross-section images of the eutopic uteri and ectopic lesions from the indicated treatment groups. The scale bar (0.5 μm) applies to all images; n = 5. (F–G) Representative cross-sectional images (left) of the eutopic uteri and ectopic lesions stained for Ki-67 (F) and Iba1 (G); respective graphs on the right show positively stained cells counted in at least five different areas in ectopic lesions and plotted as percent positive cells relative to total cells. The scale bar (0.5 μm) applies to all images; n = 5. ‘E’, ‘G’ and ‘S’ denote epithelia, glands and stroma, respectively. (H) ELISA-based quantification of IL-1β, TNF-α, IL-6, IL-10 and TGF-β1 levels in peritoneal fluid from the indicated treatment groups. Data are presented as mean ± SE (n = 5). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 and ns, non-significant.
Fourth, we assessed epithelial proliferation, which is a hallmark of endometriosis in women and is widely used to assess disease progression in rodent models of endometriosis (Wu et al., 2006; Celik et al., 2008; Burney and Giudice, 2012; Han et al., 2012, 2015; Ozer et al., 2013; Song et al., 2014; Zhao et al., 2015). Consistent with their larger size, lesions from endo-vehicle mice had significantly more epithelial cells that were positive for the proliferation marker Ki-67 than did lesions from endo-VNMA mice (Fig. 2F). Fifth, we examined macrophage infiltration in lesions because macrophages drive lesion growth and vascularisation in a mouse model of endometriosis. As illustrated by the macrophage marker Iba1 (Lin et al., 2006; Bacci et al., 2009; Capobianco et al., 2011), lesions from endo-vehicle mice contained significantly more macrophages than did lesions from endo-VNMA mice (Fig. 2G). Finally, we measured peritoneal concentrations of IL-1β, as this cytokine is elevated in the peritoneal fluid and peritoneal macrophages of women with endometriosis (Mori et al., 1992; Lebovic et al., 2000). Endo-vehicle mice had higher peritoneal IL-1β than did endo-VNMA mice (Fig. 2H left panel). Similarly, endo-vehicle mice had higher peritoneal concentrations of TNF-α, IL-6 and TGF-β1 than did endo-VNMA mice (Fig. 2H). Together, these data indicate that treatment with broad-spectrum antibiotics reduces endometriotic lesion proliferation and peritoneal inflammation.
Composition of the gut microbiota is altered in mice with endometriotic lesions
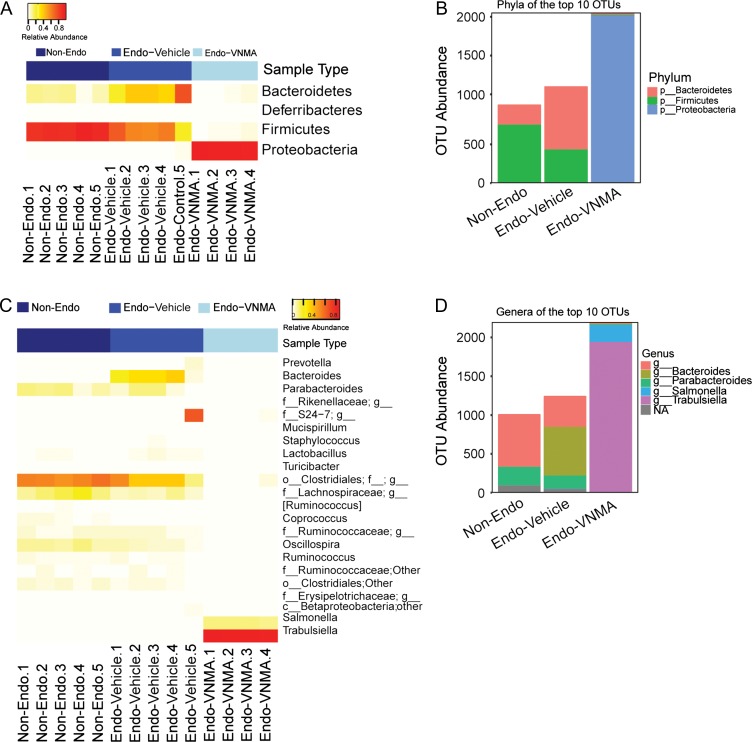
To determine the effect of broad-spectrum antibiotics on gut microbial composition, we performed 16 S rRNA gene sequencing of DNA isolated from faecal samples from endo-vehicle and endo-VNMA mice. Additionally, we included mice that did not undergo endometriosis-inducing surgery (non-endo). As shown in Supplementary Figure S3A, microbial diversity (alpha, or Shannon, Diversity) was higher in faeces from non-endo and endo-vehicle mice than in faeces from endo-VNMA mice. MDS analysis uniquely clustered each group, suggesting distinct bacterial community profiles in non-endo, endo-vehicle and endo-VNMA faecal samples (Supplementary Figure S3B). We calculated three metrics of between-group diversity (beta diversity) and noted the greatest microbial diversity in endo-vehicle mice and lowest diversity in endo-VNMA mice (Supplementary Figure S3C). Furthermore, the faecal bacterial composition of endo-VNMA mice was broadly dissimilar from that of either non-endo or endo-vehicle mice (Supplementary Figure S3B–C). This analysis demonstrated that antibiotic treatment altered the enteric bacterial diversity.
To determine whether the unique enteric bacterial profiles were attributed to specific taxa, we profiled the phyla across samples in each group. Faecal samples from endo-vehicle mice contained a higher abundance of Bacteroidetes and lower abundance of Firmicutes than samples from non-endo mice (Fig. 3A). In contrast, faecal samples from endo-VNMA mice contained negligible abundance of Bacteroidetes and Firmicutes but had increased abundance of Proteobacteria (Fig. 3A). We confirmed these findings by analysing the 10 most abundant OTUs in the datasets (Fig. 3B). We next examined bacteria at the genus level and detected Bacteroides genera in the endo-vehicle mice but not in non-endo or endo-VNMA mice (Fig. 3 C–D). The Bacteroides genus are gram-negative, non-spore-forming, anaerobic bacteria that are part of the endogenous microbiota of humans and other mammals (Brook, 1989). Finally, to assess whether surgery altered faecal microbial composition, we performed sham surgery on a group of mice. After 3 weeks, the abundances of Bacteroidetes and Firmicutes in these mice were similar to those in non-endo mice (Supplementary Figure S4A–B), indicating that surgery had no effect on gut bacteria composition. We conclude that the gut microbial composition was altered in mice with endometriosis.
Figure 3.
Bacteroides are enriched in faeces from mice with endometriotic lesions. (A) Heat map representation of relative abundances of the phyla in faecal samples from non-endo (n = 5), endo-vehicle (n = 5) and endo-VNMA (n = 4) mice. (B) Stacked bar plots of the phyla belonging to the 10 most abundant OTUs. (C) Heat map depiction of the relative abundances of the genera in each faecal sample. (D) The genera belonging to the 10 most abundant OTUs across each group are shown as stacked bar plots.
Metronidazole-sensitive gut bacteria may promote endometriotic lesion growth
Because members of the Bacteroides genus are highly susceptible to metronidazole and are resistant to neomycin (Ingham et al., 1968; Sutter et al., 1973; Yehya et al., 2013), we examined the effects of metronidazole and neomycin individually on endometriotic lesion growth. Mice treated with metronidazole alone (endo-metronidazole) developed ectopic lesions that were significantly smaller in volume and mass than those that developed in endo-vehicle mice (Fig. 4A–C). In contrast, mice treated with neomycin alone (endo-neomycin) developed similarly sized ectopic lesions as endo-vehicle mice (Fig. 4A–C). Histological analysis revealed that lesions from endo-metronidazole mice lacked the typical endometriosis-like appearance (e.g. glands and thick epithelial layer) seen in lesions from endo-vehicle and endo-neomycin mice (Fig. 4D). Consistent with endometriotic lesion growth, metronidazole-treated mice had fewer macrophages in lesions and less IL-1β in the peritoneal fluid than vehicle- or neomycin-treated mice (Fig. 4E–F). Together, these data indicate that metronidazole suppresses endometriotic lesion growth in mice, possibly by reducing Bacteroides growth.
Figure 4.
Metronidazole treatment reduces endometriotic lesion growth. (A–C) Representative gross images (A), volumes (B) and masses (C) of ectopic endometriotic lesions from the indicated treatment groups; (n = 5). (D) Representative Hematoxylin and Eosin-stained cross-section images of ectopic lesions from the indicated treatment groups; n = 5. Scale bars represent 200 μm (upper panel) or 500 μm (lower panel). (E) Quantification of IL-1β concentration in peritoneal fluid from the indicated treatment groups. (F) Representative cross-sectional images of ectopic lesions stained for Iba1; graph on the right shows the number of positively stained cells counted in at least five different areas in ectopic lesions and plotted as percent positive cells relative to total cells. ‘E’, ‘G’ and ‘S’ denote epithelia, glands and stroma, respectively. Data are presented as mean ± SE; (n = 5). *P < 0.05, ***P < 0.001, ****P < 0.0001, and ns, non-significant.
Faeces from endometriotic mice promotes endometriotic lesion progression
Given our observation that faeces from endo-vehicle mice contained more Bacteroides than faeces from non-endo mice, we wondered whether this altered gut bacteria in the faeces from mice with endometriosis was sufficient to drive endometriosis progression. To address this possibility, we performed endometriosis-induction surgery on Day 0, provided mice with metronidazole in drinking water on Days 1 through 5, orally gavaged the mice with PBS containing faeces from mice with or without endometriosis on Days 7 and 14, and examined lesions on Day 28 (illustrated in Fig. 5A). Endo-metronidazole mice gavaged with faeces from mice with endometriosis (endo-faeces) developed endometriotic lesions that were similar in mass and volume to those in endo-vehicle mice. In contrast, endo-metronidazole mice gavaged with faeces from mice without endometriosis (non-endo faeces) developed significantly smaller lesions (Fig. 5B–D). As a control, we examined endometriotic lesion growth in mice that were not gavaged with faeces but were allowed to recover from metronidazole until Day 28. As expected, endometriotic lesions were significantly smaller in these mice than in those that did not receive metronidazole (Supplementary Figure S5). We observed typical endometriosis-like histology (presence of glands and thick epithelial layer) in lesions from endo-metronidazole mice gavaged with faeces from endo-mice (Fig. 5E). In contrast, lesions from endo-metronidazole mice gavaged with faeces from non-endo mice lacked glands and had a thin epithelial layer (Fig. 5E). Furthermore, endo-metronidazole mice that received endo-faeces contained more macrophages in lesions and more IL-1β in the peritoneal fluid than endo-metronidazole mice that received non-endo faeces (Fig. 5F–G). Taken together, these findings suggest a role for gut microbiota in endometriosis disease progression.
Discussion
Given that their ability to influence systemic and peritoneal inflammation and estrogen regulation, gut microbiota could contribute to endometriosis. Here, we showed that antibiotic treatment reduced endometriotic lesions in a mouse model of endometriosis. Additionally, mice with endometriosis had more Bacteroidetes and less Firmicutes in their guts than mice without endometriosis. Finally, metronidazole, which targets Bacteroides genus, reduced endometriotic lesion growth, but lesion growth was restored in mice gavaged with faeces from mice with endometriosis, suggesting that gut bacteria promote endometriotic lesion progression.
Once an initial endometriotic lesion is established, pro-inflammatory cytokines and growth factors are released into the peritoneal cavity, and the resulting inflammation promotes lesion spread (Ahn et al., 2015a,b). Additionally, macrophages drive lesion growth and vascularisation in a mouse model of endometriosis (Lin et al., 2006; Bacci et al., 2009; Capobianco et al., 2011). Inflammasomes and IL-1β also contribute to endometriotic lesion growth (Snider et al., 2010; Goncalves et al., 2017). We showed that mice treated with VNMA or metronidazole alone had fewer macrophages in their lesions and lower peritoneal IL-1β concentration than vehicle-treated mice. Additionally, metronidazole-treated mice that were orally gavaged with faeces from mice with endometriosis had a similar number of lesion macrophages and peritoneal IL-1β concentration as vehicle-treated mice. Gut bacteria can modulate systemic inflammatory responses (Borody and Khoruts, 2011; Ellekilde et al., 2014; Rose et al., 2015), and release of bacterial products into the peritoneal cavity promotes auto-immunity (Luckey et al., 2013). Thus, we suggest that gut bacteria promote endometriosis by promoting inflammation. Future work should further test this model and define the mechanism by which this occurs.
We found that microbial diversity was altered in faeces from mice with endometriotic lesions and that mice with endometriosis had a higher abundance of Bacteroidetes and lower abundance of Firmicutes in their guts than mice without endometriosis. Our results differ somewhat from those of Yuan et al. (2018), who reported that, along with changes in Firmicutes and Bacteroidetes, Bifidobacterium was altered in mice with endometriosis. This difference perhaps reflects the origin of the mice and differences in diet.
In summary, our findings suggest that gut bacteria promote endometriosis disease progression in mice. If our findings are translated to humans, they may lead to new diagnostic strategies and microbiota-based therapies to treat this debilitating disease.
Supplementary Material
Acknowledgements
We thank Dr Jeffrey I. Gordon (Department of Pathology and Immunology, Washington University) for helpful advice and Dr Deborah J. Frank, Marina N. Rowen and Gwendalyn L. Krekeler (Department of Obstetrics and Gynecology, Washington University) for assistance with manuscript editing.
Authors’ roles
SBC and RK designed experiments, conducted most of the studies, and analysed the data. MC assisted with animal surgeries, collected tissues and fluids, and generated some of the reagents. LAP, YY, and AS analysed metagenomics data. IUM analysed some data, provided reagents, and reviewed the final draft of the manuscript. RK conceived the project, supervised the work, and wrote the manuscript.
Funding
This work was funded, in part, by: a National Institutes of Health (NIH)/National Institute of Child Health and Human Development (NICHD) grant (R00HD080742) to Ramakrishna Kommagani (RK); Washington University School of Medicine start-up funds to Ramakrishna Kommagani (RK); an Endometriosis Foundation of America Research Award to Ramakrishna Kommagani (R.K.); and an National Institutes of Health (NIH)/ National Institute of Child Health and Human Development (NICHD) grant (R01HD091218) to Indira U Mysorekar (IUM). Additionally, the Genome Technology Access Center is supported by a National Cancer Institute Grant (P30 CA91842) to the Siteman Cancer Center and by a Clinical and Translational Sciences Award Grant (UL1TR002345) from the National Institutes of Health (NIH) National Center for Advancing Translational Sciences.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
- Ahn SH, Edwards AK, Singh SS, Young SL, Lessey BA, Tayade C. IL-17A contributes to the pathogenesis of endometriosis by triggering proinflammatory cytokines and angiogenic growth factors. J Immunol 2015. a;195:2591–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn SH, Monsanto SP, Miller C, Singh SS, Thomas R, Tayade C. Pathophysiology and immune dysfunction in endometriosis. Biomed Res Int 2015. b;2015:795976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacci M, Capobianco A, Monno A, Cottone L, Di Puppo F, Camisa B, Mariani M, Brignole C, Ponzoni M, Ferrari S et al. Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. Am J Pathol 2009;175:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol 2011;9:88–96. [DOI] [PubMed] [Google Scholar]
- Brook I. Pathogenicity of the Bacteroides fragilis group. Ann Clin Lab Sci 1989;19:360–376. [PubMed] [Google Scholar]
- Bullon P, Navarro JM. Inflammasome as a key pathogenic mechanism in endometriosis. Curr Drug Targets 2017;18:997–1002. [DOI] [PubMed] [Google Scholar]
- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril 2012;98:511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capobianco A, Monno A, Cottone L, Venneri MA, Biziato D, Di Puppo F, Ferrari S, De Palma M, Manfredi AA, Rovere-Querini P. Proangiogenic Tie2(+) macrophages infiltrate human and murine endometriotic lesions and dictate their growth in a mouse model of the disease. Am J Pathol 2011;179:2651–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik O, Hascalik S, Elter K, Tagluk ME, Gurates B, Aydin NE. Combating endometriosis by blocking proteasome and nuclear factor-kappaB pathways. Hum Reprod 2008;23:2458–2465. [DOI] [PubMed] [Google Scholar]
- Chen C, Song X, Wei W, Zhong H, Dai J, Lan Z, Li F, Yu X, Feng Q, Wang Z et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat Commun 2017;8:875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cregger MA, Braundmeier A, Lenz K, Leary E, Leach R et al.. Reproductive microbiomes: using the microbiome as a novel diagnostic tool for endometriosis. Reprod Immunol 2017;2:36. [Google Scholar]
- Cummings AM, Metcalf JL. Induction of endometriosis in mice: a new model sensitive to estrogen. Reprod Toxicol 1995;9:233–238. [DOI] [PubMed] [Google Scholar]
- Ellekilde M, Selfjord E, Larsen CS, Jakesevic M, Rune I, Tranberg B, Vogensen FK, Nielsen DS, Bahl MI, Licht TR et al. Transfer of gut microbiota from lean and obese mice to antibiotic-treated mice. Sci Rep 2014;4:5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fainaru O, Adini A, Benny O, Adini I, Short S, Bazinet L, Nakai K, Pravda E, Hornstein MD, D’Amato RJ et al. Dendritic cells support angiogenesis and promote lesion growth in a murine model of endometriosis. FASEB J 2008;22:522–529. [DOI] [PubMed] [Google Scholar]
- Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol 2018;131:557–571. [DOI] [PubMed] [Google Scholar]
- Giudice LC. Clinical practice. Endometriosis. N Engl J Med 2010;362:2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves GA, Invitti AL, Parreira RM, Kopelman A, Schor E, Girao MJ. p27(kip1) overexpression regulates IL-1beta in the microenvironment of stem cells and eutopic endometriosis co-cultures. Cytokine 2017;89:229–234. [DOI] [PubMed] [Google Scholar]
- Han SJ, Hawkins SM, Begum K, Jung SY, Kovanci E, Qin J, Lydon JP, DeMayo FJ, O’Malley BW. A new isoform of steroid receptor coactivator-1 is crucial for pathogenic progression of endometriosis. Nat Med 2012;18:1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SJ, Jung SY, Wu SP, Hawkins SM, Park MJ, Kyo S, Qin J, Lydon JP, Tsai SY, Tsai MJ et al. Estrogen receptor beta modulates apoptosis complexes and the inflammasome to drive the pathogenesis of endometriosis. Cell 2015;163:960–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintze KJ, Cox JE, Rompato G, Benninghoff AD, Ward RE, Broadbent J, Lefevre M. Broad scope method for creating humanized animal models for animal health and disease research through antibiotic treatment and human fecal transfer. Gut Microbes 2014;5:183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao KY, Wu MH, Tsai SJ. Epigenetic regulation of the pathological process in endometriosis. Reprod Med Biol 2017;16:314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YL, Chassard C, Hausmann M, von Itzstein M, Hennet T. Sialic acid catabolism drives intestinal inflammation and microbial dysbiosis in mice. Nat Commun 2015;6:8141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhtinen K, Stahle M, Perheentupa A, Poutanen M. Estrogen biosynthesis and signaling in endometriosis. Mol Cell Endocrinol 2012;358:146–154. [DOI] [PubMed] [Google Scholar]
- Ingham HR, Selkon JB, Codd AA, Hale JH. A study in vitro of the sensitivity to antibiotics of Bacteroides fragilis. J Clin Pathol 1968;21:432–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiani K, Movahedin M, Malekafzali H, Mirfasihi F, Sadati SN, Moini A, Ostad S, Aflatoonian R. Effect of the estrus cycle stage on the establishment of murine endometriosis lesions. Int J Reprod Biomed (Yazd) 2018;16:305–314. [PMC free article] [PubMed] [Google Scholar]
- Kommagani R, Szwarc MM, Kovanci E, Gibbons WE, Putluri N, Maity S, Creighton CJ, Sreekumar A, DeMayo FJ, Lydon JP et al. Acceleration of the glycolytic flux by steroid receptor coactivator-2 is essential for endometrial decidualization. PLoS Genet 2013;9:e1003900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kommagani R, Szwarc MM, Vasquez YM, Peavey MC, Mazur EC, Gibbons WE, Lanz RB, DeMayo FJ, Lydon JP. The promyelocytic leukemia zinc finger transcription factor is critical for human endometrial stromal cell decidualization. PLoS Genet 2016;12:e1005937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korbel C, Menger MD, Laschke MW. Size and spatial orientation of uterine tissue transplants on the peritoneum crucially determine the growth and cyst formation of endometriosis-like lesions in mice. Hum Reprod 2010;25:2551–2558. [DOI] [PubMed] [Google Scholar]
- Lebovic DI, Bentzien F, Chao VA, Garrett EN, Meng YG, Taylor RN. Induction of an angiogenic phenotype in endometriotic stromal cell cultures by interleukin-1beta. Mol Hum Reprod 2000;6:269–275. [DOI] [PubMed] [Google Scholar]
- Lin YJ, Lai MD, Lei HY, Wing LY. Neutrophils and macrophages promote angiogenesis in the early stage of endometriosis in a mouse model. Endocrinology 2006;147:1278–1286. [DOI] [PubMed] [Google Scholar]
- Luckey D, Gomez A, Murray J, White B, Taneja V. Bugs & us: the role of the gut in autoimmunity. Indian J Med Res 2013;138:732–743. [PMC free article] [PubMed] [Google Scholar]
- Machado DE, Rodrigues-Baptista KC, Alessandra-Perini J, Soares de Moura R, Santos TA, Pereira KG, Marinho da Silva Y, Souza PJ, Nasciutti LE, Perini JA. Euterpe oleracea Extract (Acai) is a promising novel pharmacological therapeutic treatment for experimental endometriosis. PLoS One 2016;11:e0166059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno I, Codoner FM, Vilella F, Valbuena D, Martinez-Blanch JF, Jimenez-Almazan J, Alonso R, Alama P, Remohi J, Pellicer A et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am J Obstet Gynecol 2016;215:684–703. [DOI] [PubMed] [Google Scholar]
- Mori H, Sawairi M, Nakagawa M, Itoh N, Wada K, Tamaya T. Expression of interleukin-1 (IL-1) beta messenger ribonucleic acid (mRNA) and IL-1 receptor antagonist mRNA in peritoneal macrophages from patients with endometriosis. Fertil Steril 1992;57:535–542. [DOI] [PubMed] [Google Scholar]
- O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep 2006;7:688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer H, Boztosun A, Acmaz G, Atilgan R, Akkar OB, Kosar MI. The efficacy of bevacizumab, sorafenib, and retinoic acid on rat endometriosis model. Reprod Sci 2013;20:26–32. [DOI] [PubMed] [Google Scholar]
- Parnell LA, Briggs CM, Cao B, Delannoy-Bruno O, Schrieffer AE, Mysorekar IU. Microbial communities in placentas from term normal pregnancy exhibit spatially variable profiles. Sci Rep 2017. a;7:11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnell LA, Briggs CM, Mysorekar IU. Maternal microbiomes in preterm birth: recent progress and analytical pipelines. Semin Perinatol 2017. b;41:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelch KE, Sharpe-Timms KL, Nagel SC. Mouse model of surgically-induced endometriosis by auto-transplantation of uterine tissue. J Vis Exp 2012;59:e3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 2004;118:229–241. [DOI] [PubMed] [Google Scholar]
- Rier S, Foster WG. Environmental dioxins and endometriosis. Semin Reprod Med 2003;21:145–154. [DOI] [PubMed] [Google Scholar]
- Rose C, Parker A, Jefferson B, Cartmell E. The characterization of feces and urine: a review of the literature to inform advanced treatment technology. Crit Rev Environ Sci Technol 2015;45:1827–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreinemacher MH, Backes WH, Slenter JM, Xanthoulea S, Delvoux B, van Winden L, Beets-Tan RG, Evers JL, Dunselman GA, Romano A. Towards endometriosis diagnosis by gadofosveset-trisodium enhanced magnetic resonance imaging. PLoS One 2012;7:e33241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider AJ, Orr Gandy KA, Obeid LM. Sphingosine kinase: Role in regulation of bioactive sphingolipid mediators in inflammation. Biochimie 2010;92:707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WW, Lu H, Hou WJ, Xu GX, Zhang JH, Sheng YH, Cheng MJ, Zhang R. Expression of vascular endothelial growth factor C and anti-angiogenesis therapy in endometriosis. Int J Clin Exp Pathol 2014;7:7752–7759. [PMC free article] [PubMed] [Google Scholar]
- Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med 2014;2014:179515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter VL, Kwok Y, Finegold SM. Susceptibility of Bacteroides fragilis to six antibiotics determined by standardized antimicrobial disc susceptibility testing. Antimicrob Agents Chemother 1973;3:188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa M, Tanaka N, Tainaka H, Takeda K, Ihara T, Sugamata M. Microarray analysis provides insight into the early steps of pathophysiology of mouse endometriosis model induced by autotransplantation of endometrium. Life Sci 2009;84:832–837. [DOI] [PubMed] [Google Scholar]
- Ursell LK, Haiser HJ, Van Treuren W, Garg N, Reddivari L, Vanamala J, Dorrestein PC, Turnbaugh PJ, Knight R. The intestinal metabolome: an intersection between microbiota and host. Gastroenterology 2014;146:1470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SH, Zhao L, Zhang X, Nakatsu G, Han J, Xu W, Xiao X, Kwong TNY, Tsoi H, Wu WKK et al. Gavage of fecal samples from patients with colorectal cancer promotes intestinal carcinogenesis in germ-free and conventional mice. Gastroenterology 2017;153:1621–1633.e1626. [DOI] [PubMed] [Google Scholar]
- Wu Y, Strawn E, Basir Z, Halverson G, Guo SW. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in endometriosis. Epigenetics 2006;1:106–111. [DOI] [PubMed] [Google Scholar]
- Yehya M, Hamze M, Mallat H, Dabbousi F. Prevalence and antibiotic susceptibility of Bacteroides fragilis group isolated from stool samples in North Lebanon. Braz J Microbiol 2013;44:807–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Li D, Zhang Z, Sun H, An M, Wang G. Endometriosis induces gut microbiota alterations in mice. Hum Reprod 2018;33:607–616. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Gong P, Chen Y, Nwachukwu JC, Srinivasan S, Ko C, Bagchi MK, Taylor RN, Korach KS, Nettles KW et al. Dual suppression of estrogenic and inflammatory activities for targeting of endometriosis. Sci Transl Med 2015;7:271ra279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.