Abstract
Relapse and neurodegeneration are two of the major therapeutic targets in alcoholism. Fortuitously, the roles of glutamate/NMDA receptors (NMDARs) in withdrawal, conditioning and neurotoxicity mean that NMDAR inhibitors are potentially valuable for both targets. Preclinical studies further suggest that inhibitory modulators that specifically reduce the co-agonist effects of polyamines on NMDARs are potential non-toxic medications. Using agmatine as a lead compound, over 1000 novel compounds based loosely on this structure were synthesized using feedback from a molecular screen. A novel series of aryliminoguanidines with appropriate NMDAR activity in the molecular screen were discovered (US patent application filed 2007). The most potent and selective aryliminoguanidine, JR 220 [4(chlorobenzylidenamino)guanidine hydrochloride], has now been tested in a screening hierarchy for anti-relapse and neuroprotective activity, ranging from cell-based assay, through tissue culture to animal behavior. This hierarchy has been validated using drugs with known, or potential, clinical value at these targets (acamprosate (N-acetyl homotaurine), memantine and topiramate). JR220 was non-toxic and showed excellent activity in every screen with a potency 5–200x that of the FDA-approved anti-relapse agent, acamprosate. This chapter will present a review of the background and rationale for this approach and some of the findings garnered from this approach as well as patents targeting the glutamatergic system especially the NMDAR.
Keywords: NR2B neuroprotection, polyamine, relapse
INTRODUCTION
Alcohol Incidence and Cost:
Treating alcoholism continues to present considerable challenges. With current estimates of 3 – 4% of the population in the US being alcohol dependent and an estimated annual cost in excess of $200 billion dollars [1], this is clearly a significant financial, social and health issue. The current treatment approaches have limited success with high levels of relapse [2] and there appears to be only limited interest by the pharmaceutical industry in developing drugs for treating alcohol dependence. The reasons for this are complex and multifold. Almost certainly, contributing factors include concerns about assessing clinical efficacy and getting regulatory approval as well as concerns about market size and profitability not to mention that many treatment facilities do not approve/allow for a pharmacological component in treating alcohol dependence. Currently, there are only three drugs that have received FDA approval for treating alcohol dependence; disulfirum (Antabuse), naltrexone (ReVia and Vivetrol) and acamprosate (Campral) although there have been numerous other agents and approaches recently reported to reduce alcohol relapse and/or consumption (many of which are discussed in this special issue).
Alcohol and Glutamate:
While it is well known that alcohol affects many neurotransmitter systems [3], there is an extensive literature collected over the past 20+ years documenting the role of glutamate in alcohol’s actions. Glutamate is the major excitatory neurotransmitter in the CNS and glutamatergic nerves are distributed throughout the brain. There are both metabotropic and ionotropic GLU receptors. To date, three groups of G-protein coupled metabotropic GLU receptors (mGluRs) have been identified; Group I (mGluR1 and mGluR5) activates phospholipase C, producing diacylglycerol and inositol triphosphate as secondary messengers, while Groups II (mGluR2 and mGluR3) and III (mGluR4, mGluR6, mGluR7 and mGluR 8) are negatively coupled to adenylyl cyclase [see [4] for review]. The mGluRs are responsible for slow glutamate-mediated neurotransmission and modulation of transmitter release. These receptors couple with G-proteins and are located throughout the limbic and cortical brain regions that are implicated in alcoholism, and in particular, group I mGluRs (mGluR1 and mGluR5) appear important in regulating the effects of drugs of abuse [5].Acamprosate’s pharmacological targets may include mGluRs.
While mGluRs are involved in alcohol’s action, the ionotropic GLUrs have received far more attention. Three basic receptor families have been identified, including the N-methyl-D-aspartate receptor (NMDAR), the α-mino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAr) and the kainic acid receptor (KAr). All three are tetrameric complexes, and are inhibited by alcohol at physiologically relevant concentrations [6–8]. Fast synaptic transmission within this system is mediated by AMPAr and KAr, while NMDARs appear to mediate slower synaptic kinetics, involving Ca2+ and K+ flux [9].
AMPA receptors are composed of subunits GLUr1GLUr4, which all contain a GLU binding site. AMPAr are generally permeable to Ca2+, Na+ and K+, although many contain the Ca2+-impermeable GLUr2 subunit. Alcohol inhibition of AMPArs is well noted [10–13] and occurs over a wide range of concentrations (10–100mM). AMPAr undergo strong desensitization following agonist exposure [14,15], although comparably weaker and more rapid desensitization occurs following AMPA or GLU exposure [15–18]. Emerging evidence suggests that alcohol inhibits AMPArs by stabilizing this desensitized state [19]. Such inhibition is thought to be noncompetitive and distinct from channel blockade [20, 21], although interestingly, AMPAr do not appear to undergo adaptation following chronic alcohol exposure [22].
KA receptors are composed of subunits GLUr5–7 and KA1–2, and can form homomeric (GLUr only) or heterometric (GLUr5 and KA1–2) stoichiometries [23]. While a number of reports suggest that the KAr is less sensitive to alcohol than either AMPAr or NMDAR [for review see 7], such sensitivity appears to rely heavily on receptor localization; for instance, KAr in hippocampal CA3 neurons appear strongly affected by alcohol exposure [22]. Postsynaptic KArs appear to share a primary cellular function with AMPArs in that they both enable the voltage-dependant functioning of the NMDAR.
NMDARs are important in a variety of functions including learning and memory, synaptic plasticity and CNS development [24–27]. These receptors are tetrameric ligandgated cation channels, composed of two NR1 and two NR2 subunits [28, 29]. The receptor contains six major binding domains. The GLU binding site, which also binds NMDA, is located on NR2 subunits; the Mg2+ binding site is located within the channel which is blocked by Mg2+ under resting conditions; the MK801 binding site, which also binds phencyclidine-like compounds is found in the channel; the glycine (GLY) binding site is located on NR1 subunits; polyamine binding sites are located densely on, but not confined to, NR2B subunits (discussed below); the ifenprodil binding site, which also appears to bind several structurally related compounds (e.g., eliprodil) and is also found on NR2B subunits. X-ray crystallographic studies have demonstrated that NR1/NR2 dimers form within the receptor complex, providing allosteric modulation of gating activity. NR1 subunits are ubiquitous in brain, and expressed as at least eight splice variants [30]. Four NR2 transcripts have been identified (NR2A-D), and are implicated in the pharmacologic specificity of the receptor with various subunit combinations differing widely in their pharmacology [31–33]. NR2 subunits appear to determine the synaptic localization and function of the receptor [34].
The NMDAR appears to play a role in both acute and chronic effects of alcohol. In vitro studies have shown that acute alcohol exposure causes inhibition of NMDARmediated transmission [6, 35] in cortical slices [36, 37], amygdala [38], NAcc [39,40], dorsal striatum [41–43], and hippocampus [44–47]. Alcohol-inhibition of receptor function appears to depend, at least in part, on subunit composition. NMDARs containing NR2A or NR2B subunits display greater sensitivity to alcohol than those containing NR2C or NR2D subunits [48–50 also see 51, 52].
Following chronic alcohol exposure, a compensatory response occurs resulting in an upregulation of NMDAR [53–57]. Of particular interest for our focus, NR2B are upregulated in cortex [58–60] and hippocampus [58, 61–63] although there are some discrepancies [64]. Related increases in NR2B-specific antagonist binding has also been observed [65], and while much of the literature discussed here is from rodent models, alcohol-associated increases in NR2B subunit receptors have been noted in alcoholdependent human populations undergoing alcohol withdrawal [66].
NR2A subunit receptors are also increased in hippocampus following chronic alcohol exposure [58, 61, 67] although again there are some discrepancies in the literature [58, 62–64, 67, 68]. While these inconsistencies have not yet been fully explained, the basic finding that alcohol increases NMDAR subunit expression, resulting in enhanced NMDAR agonist sensitivity, is well supported. The implications of such increases are thought to be far-reaching for alcohol dependence, relapse, and alcohol-associated neurotoxicity.
Numerous studies have also implicated many of the GLU receptor subtypes in behavioral phenomena associated with alcohol, although again, our focus will be on data supporting the role of the NMDAR. NMDAR antagonists have been shown to reduce alcohol self-administration [69–72], the alcohol-deprivation effect”—a model of relapse [73], conditioned place preference (CPP) [74, 75] and sensitization to the activating effects of low doses of alcohol in rodents— which is thought to be important in the rewarding properties of alcohol [76, 77]. NMDAR antagonists have also been shown to reduce the excitotoxicity associated with alcohol withdrawal [78] providing support for pharmacological manipulations of this receptor in reducing alcohol’s rewarding and excitotoxic actions.
NMDARs and Neuroprotection:
Some of the early studies administered nonspecific NMDAR antagonists, such as the classic NMDAR channel-blocker, MK801 (dizocilpine). MK801 reduced seizures during alcohol withdrawal in rodents, [79] and both in vitro and in vivo studies have provided further evidence that MK801 is neuroprotective during alcohol withdrawal [80]. However, attenuation of alcohol effects by MK801 is highly sensitive to both timing and dose, with the wrong timing or dose resulting in an exacerbation of alcohol toxicity [81]. The clinical utility of MK801 is further limited by its lack of specificity, its abuse potential [82], its phencyclidine-like psychotomimetic and amnestic effects [83, 84], and potential neurotoxicity [85, 86]. Still, success in animal models has generated interest in alternative NMDAR antagonists that may be more viable. Various approaches have included the non-competitive NMDAR channel blocker, memantine, a drug currently used clinically for advanced stage Alzheimer’s Disease. Memantine appears neuroprotective in both in vitro [87] and in vivo [88] ETOH models. While memantine has unique properties due to its fast dissociation and lack of selectivity, compounds working outside the channel are also receiving attention. One approach of particular interest is the use of antagonists that demonstrate high specificity for the NMDAR subpopulations that are the most sensitive to ETOH withdrawal-associated damage including the NR2B subunit.
NR2B-specific Antagonism in Excitotoxic Models:
One of the most well-known and well-studied NR2B-specific antagonists is ifenprodil. Ifenprodil appears to act by binding to a modulatory site on the receptor reducing the affinity between polyamines and their binding sites [89, 90]. Bound GLU increases the affinity of the receptor for ifenprodil [91]. Ifenprodil appears neuroprotective during excitotoxic events, reducing edema and infarct volume in ischemia models [see [92]], improving outcomes in reserpine and MPTP models of Parkinson’s Disease [93, 94], models of neuropathic pain [see [95] for review], and attenuating excitotoxicity in vitro [96, 97]. In alcohol-associated models, ifenprodil reduces excitotoxic cell death during alcohol withdrawal in vitro [78], and reduces seizures during withdrawal in vivo [98]. Eliprodil is an ifenprodil analogue which has shown similar efficacy as a neuroprotectant in several models of excitotoxic injury [99–101]. While examination of both compounds returned promising results, their clinical development has been slowed due to secondary effects including Ca2+ channel blockade as well as alpha-adrenergic, 5HT1A, 5HT2, 5HT3 and sigma receptor inhibition [102–104].
CP-101,606 is also an ifenprodil analogue which reduces both the open dwell-time and frequency of channel opening of NR2B-containing receptors, but only modestly inhibits the channel activity of NR2A and NR2C-containing receptors [105, 106]. Interestingly, it is noted that among the NR2B antagonists, one class of compounds binds with high affinity as long as the NMDAR contains at least one NR2B subunit, however a second class binds with high affinity only if both NR2 subunits are NR2B subunits. CP-101,606 is a member of the latter class, and appears to demonstrate high affinity only for those receptors containing NR2B/NR2B subunits, and not NR2B combined with another NR2 subunit [107]. This high degree of specificity is suggested to limit its side effect profile in humans.
Thus far, CP-101,606 has received limited attention in alcohol research, but has been used in several other fields. CP-101,606 has shown efficacy as an antinociceptive agent [108], an anticonvulsant [109], and has demonstrated antiparkinsonian action [110, 111]. CP-101,606 attenuates the effects of traumatic brain injury and focal ischemia in animal models [112–114]. CP-101,606 also protects hippocampal neurons from glutamate toxicity in vitro [115], and reduces excitotoxic effects in a cortical cell culture during alcohol withdrawal [116].
Polyamines:
Polyamines are simple cationic compounds, derived from the amino acid arginine. Arginine can be converted into ornithine, which is then further converted to putrescine via ornithine decarboxylase (ODC). Alternatively, arginine can be converted to agmatine, then further converted to putrescine, however this pathway appears to account for only a small portion of polyamine production. Putrescine is the precursor for two other major polyamines, spermine and spermidine. These polyamines are ubiquitous in brain, and are involved in cell proliferation, differentiation, growth, and apoptosis [117, 118].
The Role of Polyamines in Alcohol’s effects:
Polyamines appear to play a critical role in alcoholassociated excitotoxicity. Increased polyamine expression has been observed in hippocampus, striatum, cortex, and cerebellum during alcohol withdrawal [119, 120]. Increases in ODC expression, the rate-limiting step in the synthesis of polyamines, have been reported following chronic alcohol exposure in hippocampus, cortex, striatum, and cerebellum [119, 121, 122]. Polyamine activity is positively correlated with the severity of alcohol withdrawal signs including alcohol withdrawal -induced tremors and seizures [119, 123] and in vitro, exogenous application of polyamines exacerbates damage during alcohol withdrawal [124, 190], while polyamine antagonists [120] have the opposite effect. Other pharmacological approaches that reduce polyamine levels or activity have similar effects. Inhibition of polyamine synthesis via difluoromethylornithine, an ODC inhibitor, inhibits WD-induced seizure, improves alcohol WD-associated outcomes in vivo [119], and attenuates cell death in vitro [120].
So, with our understanding of the effects of alcohol on NMDAR, particularly the NR2B subunit and the role of polyamines in modulating alcohol effects, the interaction of polyamines with NR2B subunits appears to be a viable target in medication development.
SPECIFIC THERAPEUTIC TARGETS
(a) Alcohol withdrawal:
Life-threatening aspects of alcohol withdrawal dissipate after one or two weeks, but less severe symptoms persist for months. These include anxiety, irritability, depression, hyperalgesia, and sleep disturbances [125]. Compensatory alterations in GABA and glutamate could account for symptoms of both acute and protracted withdrawal [126]. All these symptoms can be reversed by resumption of drinking, and so protracted withdrawal provides a neuropsychological “substrate” which can precipitate relapse [125]. This provides a target for anti-relapse drugs, thus, acamprosate inhibits acute withdrawal signs [127] and reverses sleep disturbances in protracted withdrawal [128]. Its mechanism is compatible with inhibition of the “hyperglutamatergic state” in acute and protracted withdrawal [129]. Withdrawal (both acute and protracted) is therefore an important therapeutic target for the development of antirelapse drugs.
(b) Relapse “triggers”:
The most common precipitants of relapse [130] are loosely classifiable into “priming”, “cues” and “stress” [131]. Priming implies that even a small “lapse” into drinking can provoke a major relapse by reminding the patient of the pleasurable effects of alcohol, causing “craving” to experience these effects again. Additionally, the taste and smell of alcohol act as conditioned stimuli signaling the expectation of alcohol, meaning that priming overlaps with cues. These are external and internal stimuli repeatedly associated with alcohol consumption, and which elicit conditioned responses in the brain which may be similar to the rewarding effects of the drug, or to the early signs of alcohol withdrawal, both of which can precipitate relapse [132, 133]. Finally, stress also overlaps with the other mechanisms, for example by interacting with protracted withdrawal to precipitate relapse [134]; equally, stress may provide an “internal cue” for alcohol consumption [133]. These relapse triggers provide a second important therapeutic target for anti-relapse agents.
The NMDAR as a Molecular Target in Relapse:
As stated above, NMDARs are implicated in acute and protracted withdrawal [126] and they also play an essential role in all conditioned responses [135]. For example, NMDARs in the amygdala and nucleus accumbens, are implicated in conditioned fear and anxiety [135–137], symptoms commonly associated with relapse [125, 138]. Inhibitors of NMDARs should therefore reduce protracted withdrawal, and the ability of cues to induce relapse via conditioned responses. In addition, drugs that indirectly modulate NMDARs have been shown to reduce stress-induced reinstatement of alcohol seeking behavior. It is possible therefore, that inhibitors of the NMDAR could impact all of the therapeutic targets that precipitate relapse.
Current Approved Medications for Relapse:
There are two CNS-acting drugs, naltrexone and acamprosate, that are FDA-approved and clinically useful in helping maintain abstinence [139–141]. Naltrexone reduces the rewarding effects of alcohol and may reduce cue-induced anticipation of reward [142]. In contrast, acamprosate has little effect on reward, and reduces relapse via a different mechanism. Thus, in rodents, acamprosate inhibits alcohol withdrawal-induced behaviors and brain c-fos expression [143, 144], and reduces alcohol consumption specifically after periods of alcohol “deprivation” [145, 146]. Acamprosate also suppresses alcohol-conditioned behavior [147] and inhibits cue-induced alcohol-seeking in operant models [148]. In patients, blunted response to cues and reductions in sleep disturbances [149] suggest similar mechanisms. The data suggest that the efficacy of acamprosate against relapse is related to inhibitory effects on protracted withdrawal and conditioned stimuli. While a recent meta-analysis suggests that acamprosate has only a moderate effect size [150], acamprosate remains one of the only two CNS acting drugs that is FDA approved for the clinical treatment of alcoholism.
Molecular Targets of Current Anti-Relapse Agents:
Naltrexone likely prevents relapse by antagonism of mu opioid receptors [151]. Many other potent mu receptor antagonists exist, so that direction in drug discovery seems superfluous, and medications development should probably focus on clinical efficacy of other naltrexone-like drugs. For acamprosate, the simple amino-acid structure suggests possible interactions with amino acid neurotransmitters [143] and radioligand binding and electrophysiological studies indicate that acamprosate inhibits NMDARs [143, 152, 153]. These actions are consistent with reduction in the “hyperglutamatergic state” that may underlie acute and protracted alcohol withdrawal [129, 154]. The precise molecular mechanism is unknown, but interactions with polyamine coagonist sites on the NMDAR protein complex [152] or with metabotropic glutamate receptors (mGluRs) have been suggested. Acamprosate, being a conformationally flexible structure of low potency, is unlikely to be specific [154, 155] and is therefore a useful lead compound by virtue of its proposed novel mechanism via the NMDAR rather than via its structure.
Other Glutamatergic Drugs with Anti-Relapse Potential
The low affinity uncompetitive NMDAR antagonist, memantine, is active in animal models in which alcohol withdrawal and conditioning are important [87, 143] and reduces cue-induced “craving” in recovering alcoholics [156]. The similar compound, neramexane is also effective in animal models, but a limited clinical trial was unsuccessful, perhaps related to dose [157]. Drugs that target metabotropic glutamate receptors (mGluRs), which indirectly affect NMDAR function, also have anti-relapse potential. Thus, MPEP (a mGluR5 antagonist) and LY379268 (a group 2 and 3 agonist) reduce alcohol consumption in animal models [158] and inhibit both stress and cueinduced reinstatement of alcohol-seeking behavior in operant models [159]. Finally, topiramate affects amino acid receptors [160] by actions that are superficially similar to those of acamprosate. Topiramate is active in all of our screens [161] including effects on stress-induced alcohol consumption, and reduces alcohol intake in clinical studies [162]. Thus, inhibition of the glutamatergic system, including the NMDAR, is a legitimate molecular target for anti-relapse agents and agents that are able to limit receptor activation via modulatory sites in a manner that maintains a basal level of function may be particularly effective therapies. This is the approach that we have taken.
NEUROPROTECTION AS A NOVEL THERAPEUTIC TARGET
Alcohol dependence is commonly associated with neurodegeneration and cognitive decline [163]. Alcohol acutely reduces neuronal viability and neurogenesis [164], but neurotoxicity also occurs during alcohol withdrawal [see 11]. Since alcohol withdrawal inevitably precedes “abstinence”, which must precede “relapse”, there are clearly potential interactions between neurotoxicity and relapse. First, frequent unsuccessful attempts at abstinence may cause cumulative neurodegeneration, and/or may “kindle” more severe seizures [165] and severe cognitive decline [166]. Second, neurodegeneration and cognitive deficits may increase the risk of relapse. Thus, even a single episode of withdrawal causes cognitive deficits and, because all anti-relapse treatments require “cognitive awareness”, this reduces the efficacy of anti-relapse treatments [167]. These interactions strongly suggest that anti-relapse drugs should either have neuroprotective properties, or should be supplemented with drugs that are neuroprotective.
Neuroprotective Effects of Anti-relapse Agents:
All of the inhibitory glutamatergic drugs described above are neuroprotective. Acamprosate is neuroprotective against alcohol withdrawal in vitro, in animal models [127, 154, 168, 169] and is effective clinically against other drugs of dependence. Whether these properties contribute to its efficacy in relapse is uncertain, but possible [168]. Memantine is active in several models of excitotoxicity [170] including alcohol withdrawal-induced neurotoxicity [87]. Topiramate is neuroprotective in models of excitoxicity [171] and mGluR ligands that produce inhibition of glutamatergic transmission are also neuroprotective [172]. This strengthens the assertion that glutamatergic targets are relevant both to relapse and neurotoxicity.
Molecular Target Selection for Neuroprotection:
There is a wealth of evidence implicating NMDARs in the neurotoxicity that is induced by alcohol withdrawal. Briefly, it is believed that the presence of alcohol is inhibitory to the function of both NMDARs and voltage operated Ca2+ channels (VOCCs). During the chronic presence of alcohol, neuroadaptive changes up regulate both NMDARs and VOCCs contributing to alcohol tolerance. On abrupt removal of alcohol, glutamate release activates the up-regulated NMDARs and excess Ca2+ enters neurons through these, and the upregulated VOCCs, causing excitotoxic neuronal damage. An additional factor is that the subunit expression of NMDARs is changed in favor of those including NR2B subunits, which are preferentially co-activated by endogenous polyamines. This is exacerbated by alcohol-induced fyn-kinase dependent phosphorylation of the NR2B subunit, conferring additionally increased sensitivity to co-activation by polyamines [42]. Chronic alcohol exposure also up-regulates ornithine decarboxylase (ODC), the rate-limiting step in polyamine synthesis, and alcohol withdrawal induces excess release of polyamines further activating the NMDARs to induce excitotoxicity. Inhibition of NMDARs, particularly via interactions with polyamines, is therefore an obvious molecular target for neuroprotection in alcohol withdrawal.
Molecular Target Validation for Neuroprotection:
There is considerable evidence validating NMDARs for this target in vitro, where direct NMDAR antagonists reduce alcohol withdrawal-induced toxicity [80], but experiments in vivo are equivocal, partly because these drugs are neurotoxic themselves [173]. In addition, the roles of NMDARs in learning and memory, and the abuse potential of NMDAR antagonists [174], suggest that direct antagonists of NMDARs might never be suitable as therapeutic agents. However, inhibitory modulators of the NMDAR inhibit function more subtly, ideally allowing glutamate to continue to activate the receptor normally, thus preserving physiological function, but inhibiting pathological over-activation. Drugs that are “modulators” also have much less abuse potential [174, 175]. Based on the preclinical findings, drugs that produce inhibitory modulation by inhibiting coactivation of the NMDAR by polyamines would be ideal neuroprotective candidates. However, validating this target is difficult because no potent or selective established drugs with this mechanism exist. Among the closest are agents which inhibit the synthesis of polyamines, such as difluoromethylornithine (DFMO). This agent has been shown to inhibit alcohol withdrawal neurotoxicity in vitro, but, because polyamines have many other beneficial roles in the CNS, DFMO and other ODC inhibitors will probably never be suitable for clinical use. An alternative group of agents are the NR2B-selective inhibitors of NMDARs, such as ifenprodil and CP-101,606, both of which inhibit alcohol withdrawal neurotoxicity in vitro. They are also active in many of the anti-relapse screens. However, these compounds inhibit the NR2B-containing sub-group of NMDARs whether polyamines are present or not, and so may suffer from some of the disadvantages of direct NMDAR antagonists in nerves which express mainly NR2B subunits (such as developing neurons). Nevertheless the efficacy of DFMO, ifenprodil and CP-101,606 suggests that this molecular target is legitimate. We therefore hypothesized that agents that inhibit the coactivation of NMDARs by polyamines were potentially nontoxic candidates for reducing alcohol withdrawal-induced neurotoxicity and preventing relapse.
SCREENING FOR NOVEL AGENTS
Inhibition of Polyamine Co-activation of NMDARs:
There are multiple modulatory sites for polyamines on the NMDAR [176]. These may include a “steric hindrance” site in which polyamines directly inhibit binding of channel ligands such as [3H]MK801 [177], and sites which enhance channel opening in response to glutamate [176]. One of these sites may increase the affinity for glycine at its site on the NR1 subunit [178], whereas another may be associated with the ifenprodil binding site on the NR2B subunit [176]. It is possible to screen for these types of modulatory activity on the NMDAR using the binding of channel ligands because their rate of association depends on the proportion of channels in the open configuration [177]. Thus, the presence of a positive modulator for the NMDAR (such as the polyamine, spermidine) accelerates binding of the “open channel” ligand [3H]MK801, and compounds that cause inhibitory modulation of the NMDAR reduce this acceleration. Therefore, compounds which selectively reduce the potentiating effects of spermidine (SP) on [3H]MK801 binding (SPMKB), without affecting binding in the absence of polyamine (MKB), are presumed to be NMDAR modulators with selectivity for the sites at which polyamines increase NMDAR function. This screen has previously been used by others to screen polyamine analogs for NMDAR activity [177, 179–181]. However, none of the simple polyamine-like inhibitory compounds [174, 177] are potent or selective and the industry has ignored them in favor of ifenprodil-like agents, which are more potent, with similar functional effects. Nevertheless the screen can clearly be used to identify lead compounds which might be modified synthetically to generate more selective and potent agents.
Interpretation of Molecular Screens:
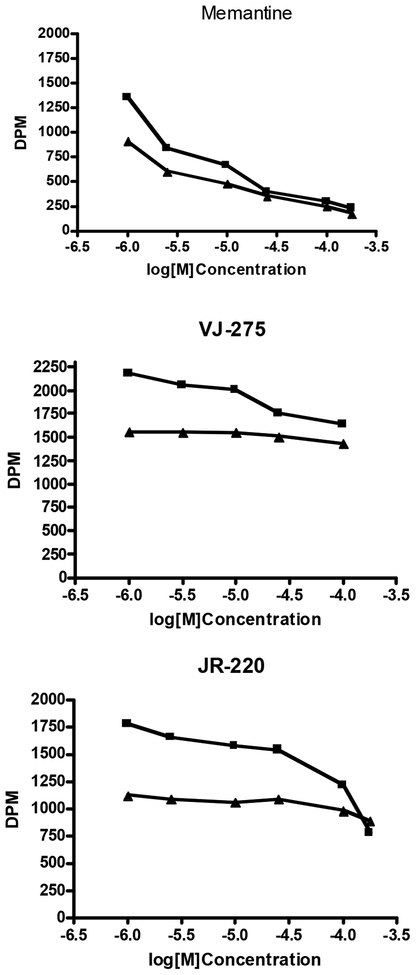
Compounds which show approximately equal effects on SPMKB and MKB, with monophasic inhibition curves, are assumed to act via direct competitive inhibition and/or steric hindrance. These include MK801, memantine, dextrorphan, ketamine, dextromethorphan, and putrescine as well as known glycine site inhibitors. Ifenprodil and eliprodil produce biphasic curves in which around 20% of both SPMKB and MKB are inhibited at concentrations in the low nM range (high affinity NR2B binding) whereas the remainder of the inhibition curve requires concentrations around 1uM. Polyaminedependent effects on [3H]MK801 binding are illustrated in the radioligand binding curves below. These show effects of compounds on SPMKB (upper curve) and [3H]MK801 binding alone (lower curve). Memantine produces a similar decrease in [3H]MK801 binding in the presence and absence of spermidine at all concentrations Fig (1). However, “positives” (e.g., VJ275 and JR220 in Fig. (1) completely prevent the potentiation of [3H]MK801 binding by spermidine at concentrations below those that have any significant effect on [3H]MK801 binding alone. Thus these compounds may selectively prevent polyamines from interacting with NMDARs at site(s) responsible for enhancing channel opening and accelerating [3H]MK801 binding.
Fig. (1).
Typical data from the “differential” molecular screen showing inhibition of [3H]MK801 binding in the presence (upper curves) and absence (lower curves) of potentiation by 100μM spermidine. The data are presented as untransformed DPM rather than as % specific binding, because the upper curves represent inhibition of time-dependent potentiation of [3H]MK801 binding by spermidine.
IDENTIFICATION OF NOVEL LEAD COMPOUNDS
We first investigated the effects of acamprosate in this screen because previous studies had suggested that it was active. However, acamprosate is probably a “false positive” because Ca2+ ions from the commercially available calcium salt of acamprosate interfered with the screen (the sodium salt was inactive). Nevertheless, in functional studies, the indirect modulation of the NMDAR by acamprosate was observed to be polyamine dependent [154] confirming that this is a legitimate molecular target. We next tested approximately two hundred miscellaneous known NMDARactive compounds unsuccessfully for their suitability as leads. None of these, including several polyamine analogs, were positive. However, we then found that the guanidinopolyamine natural product, agmatine, required ~5x lower concentrations to inhibit [3H]MK801 binding in the presence of spermidine compared with inhibition in the absence of spermidine [182]. Structurally, agmatine has similar flexibility, promiscuous binding, and low potency to spermidine, and is therefore not an ideal lead compound. However, it is neuroprotective [183], reduces self-administration of opiates and cocaine [184] and a simple analog has been reported to suppress alcohol intake in rats. We therefore chose agmatine as a lead compound and a large library of agmatine-like derivatives were synthesized and assessed in our molecular screen. In addition, plant indole alkaloids such as ibogaine inhibit NMDARs and are of potential value in drug dependence and alcoholism [185, 186]. A previously uninvestigated indole, 5-methoxy-tryptamine (D33) showed a small differential effect on [3H]MK801 binding in the presence of spermidine but, unlike agmatine, this effect was insensitive to pH [187]. This is typical of a polyamine interaction with the glycine site (177) and, in any event, suggests a subtle difference in mechanism to that of agmatine. We therefore chose 5-methoxy-tryptamine as a second lead compound.
The approach then was to generate a synthetic compound library that could be modified and screened based on feedback from the molecular screen. The agents that met screening criterion (i.e., the active compounds) would then be studied in cell–based assays for NMDAR modulation. Compounds with activity at the molecular and cellular level were tested in consecutively higher and more complex in vivo screens starting with a simple toxicological assay followed by behavioral screens of increasing complexity using rodent models that assessed neuroprotection during alcohol withdrawal, relapse and voluntary alcohol consumption as described below.
The Compound Library
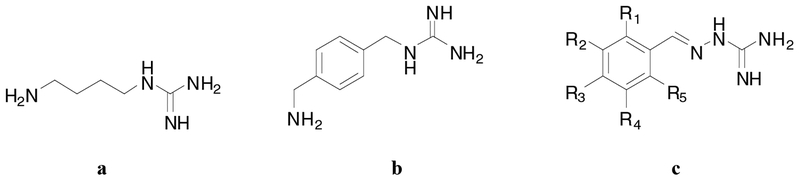
Only the structures loosely based on the agmatine molecule are described here Fig. (2). The other series of active compounds from the “indole” series are not yet fully characterized. Aromatic analogs of agmatine synthesized from the reaction of arylalkylamines with S-methylisothiopseudourea were active, but did not discriminate between the two binding conditions, i.e. the presence or absence of spermidine. We therefore synthesized a broader range of analogs, which included the aryliminoguanidines. These compounds were prepared from the condensation of aromatic and heteroaromatic aldehydes with aminoguanidine. Within this sublibrary of compounds, 7 analogs discriminated between the two differential binding conditions. Five of these analogs JR- 218, JR-220, JR-223, IG-14, and IG-18 inhibited the effect of spermidine (i.e., putative inhibitory modulation), e.g., JR- 220 was a potent analog with an IC50 of 3.6 mM in the spermidine-potentiated [3H]MK801 assay. However, JR-132 and JR126 enhanced the effect of spermidine. The concentration response curves indicate at least a 20-fold selectivity for inhibition of the polyamine-induced enhancement of NMDA function relative to direct (or steric hindrance) inhibition of [3H]MK801 binding. The most active aryliminoguanidine was JR-220, (>200x the potency of agmatine) which is now the lead compound for further investigation.
Fig. (2).
Structures of Agmatine (a), Arylalkylguanidine (b), Aryliminoguanidines (c)
IN VITRO SCREENS
Design of Cell-Based Screens for Functional Activity at NMDARs Using Differentiated SH-SY5Y Neuroblastoma Cells.
Differentiated SH-SY5Y cells express polyaminesensitive NMDARs. This cell line is useful for screening large libraries of compounds [188] and was used as our initial in vitro assay to examine the following: 1) Inhibition of NMDAR function as measured by Ca2+ entry. Briefly, the cells were pretreated for 5 min in a Ca2+ free buffer with the novel compound and then were exposed to NMDA in the presence of added glycine and spermidine in buffer containing CaCl2. Ca2+ uptake was terminated by rapid removal of the buffer by washing with ice-cold aliquots of buffer without NMDA or 45Ca2+. The cells were then lysed with 0.5M NaOH and 45Ca2+ entry was assessed using a scintillation plate counter. 2) Inhibition of NMDAR-mediated neuro- toxicity: The same cell line was used in a screen that assessed cell viability/damage estimated by MTT staining using a commercial kit (ATCC) with absorbance measured in a microplate reader; 3) Inhibition of NMDAR-mediated neurotoxicity when enhanced by alcohol withdrawal: (the presumed basis for alcohol-withdrawal -induced neurotoxicity). In primary neuronal cultures, alcohol withdrawal enhances NMDA-induced toxicity [e.g., 189]. In our screen chronic alcohol exposure to SH-SY5Y cells was followed by 200 M NMDA challenge during alcohol withdrawal. Neurotoxic effects of NMDA (using the MTT staining as above) was enhanced, and the effects of novel compounds or positive controls added 5 min prior to NMDA challenge were evaluated on this alcohol withdrawal enhanced neurotoxicity screen.
Results:
The use of 96 well plates containing SH-SY5Y neuroblastoma cells enabled 8 wells to be dedicated to each of 8 “unknown” compounds, with 8 wells for untreated controls, 8 wells for a standard concentration of NMDA (500μM), 8 wells for NMDA plus MK801 (20μM) and 8 wells for vehicle plus NMDA. Two concentrations of each novel compound (determined by “potency” in the molecular screen) were run in quadruplicate. Compounds that reduced neurotoxicity were then tested in the more complex in vitro organotypic hippocampal slice cultures.
Organotypic Hippocampal Cultures:
The organotypic hippocampal slice culture (OHC) model uses slices of hippocampus (typically 200 – 400 μm) from neonatal rats. The section contains an intact, living portion of hippocampus with its heterogeneity of neurons and glial cells. Thus, this model maintains a high level of complexity in regards to intact neuronal connections and is well suited as a model for predictions in vivo. This model is used in several neurotoxicity paradigms including that induced by NMDA. OHCs are the only in vitro models in which we have found reproducible “spontaneous” neurotoxicity during alcohol withdrawal (after 10 days exposure) [78, 80]. This alcohol withdrawal screen has been validated with NMDAR modulators, memantine [87], acamprosate [78] and agmatine [120]. Neurotoxicity is evaluated by propidium iodide (PI) uptake, and compounds are routinely screened for evidence of neurotoxicity alone, for inhibition of NMDA (5 M)-induced toxicity, and for inhibition of alcohol-withdrawal -neurotoxicity [see 78, 80, 190].
Results from the OHC Screen:
Alcohol-withdrawal induced toxicity was prevented by 10 M MK801, or memantine, 30μM. Acamprosate was also active against alcohol withdrawal, but exhibited a threshold of 200 M. Of interest, acamprosate was inactive against NMDA-induced toxicity, whether alone or enhanced by ethanol withdrawal [78] as would be predicted by an indirect action. Many novel compounds have been screened, including VJ156 (sulfur isostere of tryptamine) and VJ170 (harmine-beta-carboline) which were inhibitory to NMDAR function in the molecular screen. Both compounds were protective against NMDA and alcohol withdrawal as predicted. However, both were also neurotoxic alone at 250μM (similar to findings with MK801). In contrast, two aryliminoguanidine compounds (JR 223 and JR220) were protective against NMDA and ethanol withdrawal, but did not show any signs of neurotoxicity alone at 250μM (or 500 M). All of the aryliminoguanidines that were positive in the molecular screens produced the appropriate responses in the cell based screens.
BEHAVIORAL SCREENS
Criteria for Design of Behavioral Screens:
There are many excellent “models” of alcoholism that could be developed into “behavioral screens” but for this project there were two overriding criteria. The first was that the screens must ultimately predict clinical value, and therefore naltrexone and/or acamprosate should be active in the original models, and in the screens developed from these models. However, naltrexone and acamprosate have completely different mechanisms and the screens chosen must be compatible with the activity predicted by the molecular target. In this case, compounds targeted on NMDARs were predicted to have effects on alcohol withdrawal, conditioning and stressinduced consumption (i.e., effects similar to acamprosate, and other inhibitors of NMDAR function). It was also desirable that the screens have some face validity to relapse, but, in contrast to models, the major criteria for the value of screens were simplicity, reproducibility and potential predictive validity.
Some of the Behavioral Screens Used:
Seizure Susceptibility during Alcohol Withdrawal in Mice
Acamprosate [80] and all NMDAR antagonists and modulators previously tested including MK801, ifenprodil, cycloserine, and agmatine inhibited alcohol withdrawal induced seizures (“handling-induced convulsions”-HICs) in mice. Thus these models have predictive validity, but most require inhalation of ethanol to produce the long-lasting blood levels necessary for physical dependence [e.g., 191]. This makes them unsuitable as a rapid screen. Consequently, we used a simpler method that has some face validity to acute and protracted alcohol withdrawal.
Swiss Webster mice received daily injections of ethanol with 4-methylpyrazole (4MP) which inhibits alcohol dehydrogenase on 3 consecutive days. Thus, the mice treated with 4MP showed significantly increased blood alcohol levels and duration of exposure over the 3 days of treatment resulting in alcohol dependence and an increased susceptibility to HICs during alcohol withdrawal with only 3 days of treatment. This treatment produced consistent mild HICs (on an established 5 point scale) [191, 192] while alcohol administration alone had no effect on HIC scores.
Results:
This screen was validated with several compounds known to inhibit alcohol withdrawal HICs including diazepam, acamprosate, MK801 and memantine [87]. Acamprosate was active at doses of 100mg/kg IP and above. Other agents potentially valuable for clinical treatment (i.e., topiramate, memantine) also reduced alcohol withdrawal HICs. The NMDAR/NR2B-selective compounds, e.g., CP- 101,606, were also active and this is of particular interest because these compounds should exhibit very similar functional effects to the putative polyamine-dependent NMDAR inhibitory modulators. The majority of the novel compounds tested gave results as predicted by the primary molecular and cellular screens. However, some compounds that appeared to be inhibitors of the NMDAR actually increased seizure-like activity (e.g., VJ156 and VJ170). These differences can probably be explained by known tremorigenic and/or neurotoxic effects of these types of compound. Of the active aryliminoguanidines, the novel agent which appeared by far the most effective to date was JR220. JR220 significantly inhibited HICs at doses as low as 1 mg/kg IP (100x more potent than acamprosate, and similar to MK801 and CP- 101,606).
The Alcohol Deprivation Effect (ADE):
The ADE is the temporary increase in voluntary alcohol consumption (VAC) that occurs in rats when alcohol is available following a period of deprivation [145, 146]. The ADE has predictive validity since naltrexone and acamprosate can reduce or block the ADE. The ADE was endorsed as a potential screen at an NIAAA-sponsored workshop in 2002. It includes elements of alcohol withdrawal, as well as conditioning (the smell and taste of alcohol) and should therefore be sensitive to compounds acting on GLU and the NMDAR. Indeed, all NMDAR inhibitors have been reported to inhibit the ADE regardless of mechanism [73]. The ADE has been claimed to have face validity to craving [193] but its major value here is as a screen with potential predictive validity. SpragueDawley rats were trained to drink alcohol by a gradual “sucrose-fading” procedure in which the rats were exposed to decreasing levels of sucrose concurrently with increasing levels of ethanol up to 12%, in sipper tubes made from graduated cylinders. When ethanol consumption had stabilized, the drug was removed and, after 3 – 7 days ethanol was reintroduced. The ethanol consumed at this time was compared to the average stable baseline consumption with a statistically significant increase in VAC indicating an ADE. Drugs were injected once, immediately before the reintroduction of ethanol.
Results:
Acamprosate (100 or 200 mg/kg IP) significantly and consistently reduced the ADE. Topiramate (10mg/kg IP) also reduced the ADE [161] without affecting total fluid intake in the ADE screen. In repeated tests, JR220 consistently reduced VAC upon the re-institution of alcohol access after a period of alcohol deprivation. Saline injection did not affect the ADE which commonly results in an almost 100% increase in alcohol consumption above baseline during the 24h following re-introduction of the alcohol drinking bottle.
Conditioned Behavior in the Elevated Plus Maze (EPM):
The EPM is commonly used in screening for anxiolytic or anxiogenic activity in the pharmaceutical industry. Consequently, there is a large background literature on analysis of drug-induced behavior in the EPM [194]. It has been reported that repeated association between daily alcohol injections and the EPM can generate a characteristic anxietylike behavioral response (“stretched attend postures”) in mice in response to a saline injection in the same environment [147]. The potential predictive validity is supported by inhibition of the behavior by acamprosate (although not, as predicted, by naltrexone) [195]. Other interpretations are possible but based on its contingence to the EPM environment, this behavior has been suggested to be a consequence of conditioning between the EPM and the alcohol cue [147]. If this is correct, it has some face validity to “cue-induced relapse”, and the known roles of NMDARs in conditioning suggest that drugs with the mechanism sought should be active in this screen.
Swiss Webster mice received nine daily IP injections of saline or ethanol and were then placed on the EPM for 5 min daily. On day 10, ethanol (or saline) injection was replaced by the drug of interest or vehicle and the mouse returned to the maze for a 5 min videotaped session. The behaviors examined include number of closed or open arm entries, time spent in the central square, and number of “stretched attend postures” (SAPs). Substitution of saline for ethanol in the EPM-exposed animals produces anxiety-like behaviors (specifically SAPs) contingent on the EPM environment.
Results:
Acamprosate reduced this response at 200 mg/kg IP [195], and was used as the positive control. Topiramate (at both 10 mg/kg and 20 mg/kg IP) was also effective in inhibiting alcohol-conditioned SAPs [161]. The positive effects of these drugs suggest that the screen has some predictive validity. Acamprosate reduced the putatively alcohol-conditioned SAPs without having a significant effect on unconditioned (saline) SAPs, or on any other parameter in the EPM. The higher dose of topiramate (20mg/kg IP) also did show anxiolytic effects (increased entries into the open arms in controls) illustrating the value of the EPM screen. Because there is such a wealth of data on the effects of anxiolytic/sedative agents in this type of maze, considerable information on behavioral effects of novel potential antirelapse drugs can be obtained in a single screen. JR 220 also reduced conditioned SAPs relative to controls although it also reduced activity suggesting it could cause a sedative effect at the doses tested.
Drinking in the Dark by C57/Bl Mice:
Rodent models of voluntary alcohol consumption (VAC) are very common in alcohol research, but it is rare for animals to drink to intoxication; reducing face-validity for modeling human alcohol abuse. However, a model based on genetically-determined high VAC, referred to as “drinking in the dark” (DID) paradigm has been developed using C57BL/6J mice [196]. These mice are given access to a 20% v/v ethanol solution for a limited number of hours (typically 2–4h/day) daily during the dark cycle. With this schedule, these mice will drink to the point of behavioral impairment, consuming 2–3 g/kg ethanol resulting in average BACs over 100mg% [196]. The value of this model of high VAC as a screen is supported by the observation that both naltrexone and acamprosate [197] are active and reduce DID, so that DID may have predictive validity for anti-relapse agents. C57BL/6J male mice were placed on a reverse light dark cycle and given access to 20% ethanol for 4 hours into the dark cycle daily. Mice consistently drank comparable amounts of alcohol (3–4 g/kg in 4 hour access) and drugs of interest were injected 5 minutes before the 4hr alcohol access.
Results:
Acamprosate has been shown to reduce DID [197]. In addition, the novel NR2B-selective NMDAR inhibitor CP-101,606 and JR 220 both significantly reduced VAC at 10 mg/kg IP.
Summary:
Our findings provide compelling support for the role of polyamine antagonists and modulation of NMDARs in reducing neurotoxicity during alcohol withdrawal as well as relapse and voluntary alcohol consumption in a number of rodent models (see Table 1 for a summary of the results discussed). Furthermore, the development of a novel aryliminoguanidine compound JR 220 is particularly exciting given that the compound appears to be effective in numerous screens related to alcohol dependence. While JR 220 does not affect ETOH pharmacokinetics, additional information on this promising drug candidate is still needed and the plan is to submit this compound to the “rapid access to investigational drug” (RAID) program at NIH to determine the potential value of JR 220 as a therapeutic agent.
Table 1.
Summary of Behavioral Studies (our work and others using similar paradigms)
| Agent | SS1 | ADE2 | EPM3 | DID4 |
|---|---|---|---|---|
| Acamprosate | +[190] | +[145,146] | +[194] | +[196] |
| Agmatine | +[219] | ? | - | ? |
| CP 101,606 | + | ? | ? | + |
| Ifenprodil | + | +[73] | ? | ? |
| Memantine | +[87] | +[220] | ? | ? |
| MK-801 | + | ? | ? | ? |
| Naltrexone | ? | +[217] | −[194] | +[195] |
| Topiramate | +[160] | + | +[160] | +[218] |
| JR 220 | + | + | + | + |
TERATOGENIC SCREENS
Additional Studies with Developmental Alcohol Exposure and Polyamine Manipulations:
Fetal alcohol exposure is the leading preventable cause of mental retardation in the Western world, affecting up to 9.1 of every 1000 live births in the U.S. and Canada, with higher estimates for specific vulnerable populations [198,199]. Fetal alcohol-related healthcare costs amount to approximately 3.4 billion dollars annually, although if variables such as residential care and lost productivity are included, estimates rise to 11 billion dollars [200], making the consequences of FASD a serious socioeconomic concern, as well as a significant health and societal issue.
As we gain a better understanding of how alcohol affects the developing brain, there have been many approaches including pharmacological, nutritional and environmental manipulations that attempt to reduce some of the consequences of alcohol on the developing brain. At least in animal models, these have met with some success [see 201–203 for examples]. Considering the role that polyamines typically play in normal brain development and the effects that alcohol has on polyamines, a natural offshoot of the medication development project was to examine how known and novel compounds that modulate NMDAR function via the polyamine site could aid the developing brain exposed to alcohol.
Polyamines are ubiquitous during early brain development, playing an important and complex role in cell proliferation, differentiation, growth, communication and apoptosis [for review see 117,204]. High levels of ODC are associated with periods of cellular proliferation and differentiation. In the rat brain, regions that mature relatively early (midbrain and brainstem) experience peaks in ODC activity prenatally, while regions that mature later (cortex and cerebellum) peak peri/postnatally and then decline over the neonatal period [for review see 117]. During this perinatal period, polyamines influence the developmental plasticity of the NMDAR [205], suggesting a critical role in CNS development.
Chronic fetal alcohol exposure can result in persistent, widespread disruptions in ODC/polyamine activity, however these effects appear to be dependent on the timing of the exposure, severity of withdrawal and brain region [206,207]. As mentioned earlier in this article, polyamine levels are elevated in neonatal hippocampal slices during alcohol withdrawal corresponding with increased cell damage/death [120]. Certain brain regions such as the cerebellum and hippocampus are particularly sensitive to alcohol and to polyamines during the neonatal period and these regions are rich in NR2B subunits [49,208–210]. Alcohol also increases NR2B subunit expression in the hippocampus, cortex, and cerebellum, while delaying the developmental transfer from NR2B to NR2A subunits, which may make these cells more vulnerable to alcohol’s effects, [58,210]. The combination of increased NR1:NR2B expression and elevated polyamine levels during this period may contribute to fetal alcohol neurotoxic effects, suggesting that pharmacological modulation of polyamines during this period should be beneficial.
Preliminary Studies on Screens for Neuroprotection against Alcohol Withdrawal Toxicity in Neonates
Examination of Alcohol and Polyamine Modulation In vitro as a Developmental Model.
The organotypic hippocampal slice preparation serves as a very useful model to study the effects of alcohol on the developing brain. The majority of studies that have looked at alcohol exposure and/or withdrawal in this model use hippocampal slices derived from neonatal rats although only occasionally is the developmental aspect of the results considered. There are definite age dependent differences in sensitivity to the damaging effects of alcohol and/or polyamines even between hippocampi within the first neonatal week [211]. As discussed above and as predicted, the neurotoxicity observed following alcohol withdrawal can be potentiated by exogenous polyamines [120,190,211] and reduced by agents that directly or indirectly reduce polyamine activity including agmatine, [120], ifenprodil, [78,120, 211], CP-101,606 [212], and of particular interest for us, our novel compound JR 220 [submitted].
Examination of Alcohol and Polyamine Modulation In vivo in our Developmental Model
Alcohol Exposure:
The usual exposure period used in our laboratory involves exposure to alcohol during the first weeks after birth as a model to overlap the CNS “brain growth spurt” that occurs during the 3rd trimester of human pregnancy. Sprague Dawley rat pups are intubated with either alcohol or a control diet usually from postnatal days (PND) 1 – 7 in two daily administrations. A non-intubated control group is also included. After chronic alcohol exposure, within the first 24 hr of alcohol withdrawal, the pup receives a single treatment of the drug or vehicle. Behavioral assessments are then conducted at various time points to assess activity, learning and memory, motor coordination and/or other behaviors shown to be sensitive to prenatal/neonatal alcohol exposure.
Pharmacological Manipulations in the Neonatal Alcohol Exposure Model.
With our neonatal exposure model, we have shown that DFMO, which blocks the synthesis of polyamines, and agmatine, which modulates NMDAR activity, eliminates deficits in isolation-induced ultrasonic vocalizations in neonatal rats and reduces balance deficits in adolescent rats – deficits that are typically observed following neonatal alcohol exposure [214–216]. CP-101,606 also reduced a variety of behavioral deficits following neonatal alcohol exposure including hyperactivity, balance deficits and spatial memory [212]. Perhaps the most interesting and exciting data stems from our recent work with JR 220. A single administration of JR 220 approximately 10 hours after the last alcohol administration on PND 7 results in improvements in all of the behavioral endpoints examined thus far including isolation-induced ultrasonic vocalizations, hyperactivity, balance and spatial learning and memory [213].
Overview of existing patents:
As reviewed in the present chapter, the glutamatergic/NMDA system is a key player in alcohol’s deleterious effects and a number of patents have been granted for the treatment of alcoholism. Patents for precursors of acamprosate have increased substantially in the last few years [221–225]. As discussed above, endogenous polyamines (putrescine, spermidine, spermine), 1,3propanediamine including bioprecursor amides have been patented for treating alcohol abuse and dependence [226–228]. In addition, general NMDA antagonists such as pyrido (4,3-B) indole derivatives [229, 230], as well as treatments targeting NR1and/or NR2[231–233] associated receptors also have been patented for treating alcohol addiction. Given the basic research indicating mGluR5’s role in alcohol addiction, antagonists for this receptor have been patented as well [234–236]. A number of pyrrolidine derivatives act as powerful glutamate uptake blockers, with specificity for certain EAAT subtypes across the compounds [237]. These pyrrolidine derivatives also have been patented for treating alcoholism [238–245]. Similarly, benzoyl piperidines and pyrrolidines, as functional agonists via enhancement of synaptic responses mediated by AMPA receptors, have been patented to treat alcohol dependence [246, 247]. AMPA receptor antagonists, atropisomers of 3-aryl-4(3H)-quinazolinones [248] or thieno-pyrimidin-4-one [249], also have been patented for treating alcohol dependence. Adenosine receptors appear to control glutamatergic transmission [250] and treatments targeting the A2a receptor [251, 252] have been patented to treat alcohol addiction. Also, a number of patents to reduce extracellular glutamate [253–255], reduce presynaptic glutamate release [256] or modulate glutamate carboxypeptidase [257] have been patented.
CURRENT AND FUTURE DEVELOPMENTS
In this article, we have presented the rationale and some background literature for our underlying hypothesis that novel NMDAR modulators that work via the polyamine site on the NMDAR may be a useful medication development approach for alcohol dependence. We have also discovered a novel aryliminoguanidine, JR220, that appears to be beneficial in screens ranging from molecular all the way to complex in vivo behavioral screens. The potential value of compounds such as JR 220 in preventing those aspects of FASD which are a consequence of alcohol withdrawal is an unexpected bonus. Whether this basic research can be translated into a therapeutic intervention remains to be seen but in any event the availability of compounds with this degree of selectivity should facilitate research that addresses the role(s) of polyamines and NMDARs in FASD. Given the wide spectrum of glutamatergic modulators that have been patented, a combinational approach that includes polyamine modulators appears to be a promising treatment strategy targeting alcohol abuse and dependence.
ACKNOWLEDGEMENTS
This work was supported, in part, by NIH AA014032 to SB and AA12600 to JM. JR-220 and the other arylidenaminoguanidines discussed in th is application are protected under U.S. Patent Application Serial Number 11/972,576.
Footnotes
CONFLICT OF INTEREST
The authors do not have any conflicts of interest associated with this material.
REFERENCES
- [1].Multiple Authors. National Epidemiologic Survey on Alcohol and Related Conditions. Summarized in Alcohol Alert. National Institute on Alcohol Abuse and Alcoholism. October 2006. [PMC free article] [PubMed] [Google Scholar]
- [2].Moos RH, Moos BS. Rates and predictors of relapse after natural and treated remission from alcohol use disorders. Addiction 2006; 101: 212–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nevo I, Hamon M. Neurotransmitter and neuromodulatory mechanisms involved in alcohol abuse and alcoholism. Neurochem Int 1995; 26: 305–36. [DOI] [PubMed] [Google Scholar]
- [4].Lau A, Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch 2010; 460: 525–42. [DOI] [PubMed] [Google Scholar]
- [5].Kenny PJ, Markou A. The ups and downs of addiction: role of metabotropic glutamate receptors. Trends Pharmacol Sci 2004; 25: 265–72. [DOI] [PubMed] [Google Scholar]
- [6].Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA activated ion current in hippocampal neurons. Sci 1989; 243: 1721–4. [DOI] [PubMed] [Google Scholar]
- [7].Weiner JL, Dunwiddie TV, Valenzuela CF. Ethanol inhibition of synaptically evoked kainate responses in rat hippocampal CA3 pyramidal neurons. Mol Pharmacol 1999; 56: 85–90. [DOI] [PubMed] [Google Scholar]
- [8].Tsai G Glutamatergic neurotransmission in alcoholism. J Biomed Sci 1998; 5: 309–20. [DOI] [PubMed] [Google Scholar]
- [9].Hoffman PL. NMDA receptors in alcoholism. Int Rev Neurobiol 2003; 56: 35–82. [DOI] [PubMed] [Google Scholar]
- [10].Dildy-Mayfield JE, Harris RA. Comparison of ethanol sensitivity of rat brain kainate, DL-alpha-amino-3-hydroxy-5-methyl-4- isoxalone proprionic acid and N-methyl-D-aspartate receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther 1992; 262: 487–94. [PubMed] [Google Scholar]
- [11].Lovinger DM. Excitotoxicity and alcohol-related brain damage. Alcohol Clin Exp Res 1993; 17: 19–27. [DOI] [PubMed] [Google Scholar]
- [12].Dildy-Mayfield JE, Harris RA. Ethanol inhibits kainate responses of glutamate receptors expressed in Xenopus oocytes: role of calcium and protein kinase C. J Neurosci 1995; 15: 3162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wirkner K, Eberts C, Poelchen W, Allgaier C, Illes P. Mechanism of inhibition by ethanol of NMDA and AMPA receptor channel functions in cultured rat cortical neurons. Naunyn Schmiedebergs Arch Pharmacol 2000; 362: 568–76. [DOI] [PubMed] [Google Scholar]
- [14].Trussell LO, Thio LL, Zorumski CF, Fischbach GD. Rapid desensitization of glutamate receptors in vertebrate central neurons. Proc Natl Acad Sci USA 1988; 85: 4562–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tang CM, Dichter M, Morad M. Quisqualate activates a rapidly inactivating high conductance ionic channel in hippocampal neurons. Sci 1989; 243: 1474–7. [DOI] [PubMed] [Google Scholar]
- [16].Trussell LO, Fischbach GD. Glutamate receptor desensitization and its role in synaptic transmission. Neuron 1989; 3: 209–18. [DOI] [PubMed] [Google Scholar]
- [17].Activation Hestrin S. and desensitization of glutamate-activated channels mediating fast excitatory synaptic currents in the visual cortex. Neuron 1992; 9: 991–9. [DOI] [PubMed] [Google Scholar]
- [18].Barbour B, Keller BU, Llano I, Marty A. Prolonged presence of glutamate during excitatory synaptic transmission to cerebellar Purkinje cells. Neuron 1994; 12: 1331–43. [DOI] [PubMed] [Google Scholar]
- [19].Moykkynen TP, Coleman SK, Keinanen K, Lovinger DM, Korpi ER. Ethanol increases desensitization of recombinant GluR-D AMPA receptor and TARP combinations. Alcohol 2009; 43: 277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Peoples RW, White G, Lovinger DM, Weight FF. Ethanol inhibition of N-methyl-D-aspartate-activated current in mouse hippocampal neurones: whole-cell patch-clamp analysis. Br J Pharmacol 1997; 122: 1035–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wirkner K, Poelchen W, Koles L, Muhlberg K, Scheibler P, Allgaier C, et al. Ethanol-induced inhibition of NMDA receptor channels. Neurochem Int 1999; 35: 153–62. [DOI] [PubMed] [Google Scholar]
- [22].Davis KM, Wu JY. Role of glutamatergic and GABAergic systems in alcoholism. J Biomed Sci 2001; 8: 7–19. [DOI] [PubMed] [Google Scholar]
- [23].Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev 1999; 51: 7–61. [PubMed] [Google Scholar]
- [24].Hetman M, Kharebava G. Survival signaling pathways activated by NMDA receptors. Curr Top Med Chem. 2006; 6: 787–99. [DOI] [PubMed] [Google Scholar]
- [25].Lee YS, Silva AJ. The molecular and cellular biology of enhanced cognition. Nat Rev Neurosci 2009; 10: 126–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Collingridge GL, Lester RA. Excitatory amino acid receptors in the vertebrate central nervous system. Pharmacol Rev 1989; 41: 143–210. [PubMed] [Google Scholar]
- [27].Collingridge GL, Singer W. Excitatory amino acid receptors and synaptic plasticity. Trends Pharmacol Sci 1990; 11: 290–6. [DOI] [PubMed] [Google Scholar]
- [28].McBain CJ, Mayer ML. N-methyl-D-aspartic acid receptor structure and function. Physiol Rev 1994; 74: 723–60. [DOI] [PubMed] [Google Scholar]
- [29].Schorge S, Colquhoun D. Studies of NMDA receptor function and stoichiometry with truncated and tandem subunits. J Neurosci 2003; 23: 1151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nakanishi S Molecular diversity of the glutamate receptors. Clin Neuropharmacol 1992; 15 Suppl 1 Pt A: 4A–5A. [DOI] [PubMed] [Google Scholar]
- [31].Wafford KA, Bain CJ, Le Bourdelles B, Whiting PJ, Kemp JA. Preferential co-assembly of recombinant NMDA receptors composed of three different subunits. Neuroreport 1993; 4: 1347–9. [DOI] [PubMed] [Google Scholar]
- [32].Monaghan DT, Andaloro VJ, Skifter DA. Molecular determinants of NMDA receptor pharmacological diversity. Prog Brain Res 1998; 116: 171–90. [DOI] [PubMed] [Google Scholar]
- [33].Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol 2001; 11: 327–35. [DOI] [PubMed] [Google Scholar]
- [34].Chung HJ, Huang YH, Lau LF, Huganir RL. Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand. J Neurosci 2004; 24: 10248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hoffman PL, Rabe CS, Moses F, Tabakoff B. N-methyl-D- aspartate receptors and ethanol: inhibition of calcium flux and cyclic GMP production. J Neurochem 1989; 52: 1937–40. [DOI] [PubMed] [Google Scholar]
- [36].Wright JM, Peoples RW, Weight FF. Single-channel and whole- cell analysis of ethanol inhibition of NMDA-activated currents in cultured mouse cortical and hippocampal neurons. Brain Res 1996; 738: 249–56. [DOI] [PubMed] [Google Scholar]
- [37].Woodward JJ, Pava MJ. Effects of ethanol on persistent activity and up-States in excitatory and inhibitory neurons in prefrontal cortex. Alcohol Clin Exp Res 2009; 33: 2134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Calton JL, Wilson WA, Moore SD. Magnesium-dependent inhibition of N-methyl-D-aspartate receptor-mediated synaptic transmission by ethanol. J Pharmacol Exp Ther 1998; 287: 1015–9. [PubMed] [Google Scholar]
- [39].Maldve RE, Zhang TA, Ferrani-Kile K, Schreiber SS, Lippmann MJ, Snyder GL, et al. DARPP-32 and regulation of the ethanol sensitivity of NMDA receptors in the nucleus accumbens. Nat Neurosci 2002; 5: 641–8. [DOI] [PubMed] [Google Scholar]
- [40].Nie Z, Madamba SG, Siggins GR. Ethanol inhibits glutamatergic neurotransmission in nucleus accumbens neurons by multiple mechanisms. J Pharmacol Exp Ther 1994; 271: 1566–73. [PubMed] [Google Scholar]
- [41].Yin HH, Park BS, Adermark L, Lovinger DM. Ethanol reverses the direction of long-term synaptic plasticity in the dorsomedial striatum. Eur J Neurosci 2007; 25: 3226–32. [DOI] [PubMed] [Google Scholar]
- [42].Wang J, Carnicella S, Phamluong K, Jeanblanc J, Ronesi JA, Chaudhri N, et al. Ethanol induces long-term facilitation of NR2B- NMDA receptor activity in the dorsal striatum: implications for alcohol drinking behavior. J Neurosci 2007; 27: 3593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Popp RL, Lickteig R, Browning MD, Lovinger DM. Ethanol sensitivity and subunit composition of NMDA receptors in cultured striatal neurons. Neuropharmacology 1998; 37: 45–56. [DOI] [PubMed] [Google Scholar]
- [44].Siggins GR, Martin G, Roberto M, Nie Z, Madamba S, De Lecea L. Glutamatergic transmission in opiate and alcohol dependence. Ann N Y Acad Sci 2003; 1003: 196–211. [DOI] [PubMed] [Google Scholar]
- [45].Lovinger DM, White G, Weight FF. NMDA receptor-mediated synaptic excitation selectively inhibited by ethanol in hippocampal slice from adult rat. J Neurosci 1990; 10: 1372–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Morrisett RA, Martin D, Oetting TA, Lewis DV, Wilson WA, Swartzwelder HS. Ethanol and magnesium ions inhibit N-methyl- D-aspartate-mediated synaptic potentials in an interactive manner. Neuropharmacol 1991; 30: 1173–8. [DOI] [PubMed] [Google Scholar]
- [47].Kolb JE, Trettel J, Levine ES. BDNF enhancement of postsynaptic NMDA receptors is blocked by ethanol. Synapse 2005; 55: 52–7. [DOI] [PubMed] [Google Scholar]
- [48].Kuner T, Schoepfer R, Korpi ER. Ethanol inhibits glutamate- induced currents in heteromeric NMDA receptor subtypes. Neuroreport 1993; 5: 297–300. [DOI] [PubMed] [Google Scholar]
- [49].Mirshahi T, Woodward J. Ethanol sensitivity of heteromeric NMDA receptors: effects of subunit assembly, glycine and NMDAR1 Mg(2+)-insensitive mutants. Neuropharmacol 1995; 34: 347–55. [DOI] [PubMed] [Google Scholar]
- [50].Masood K, Wu C, Brauneis U, Weight FF. Differential ethanol sensitivity of recombinant N-methyl-D-aspartate receptor subunits. Mol Pharmacol 1994; 45: 324–9. [PubMed] [Google Scholar]
- [51].Allgaier C Ethanol sensitivity of NMDA receptors. Neurochem Int 2002; 41: 377–82. [DOI] [PubMed] [Google Scholar]
- [52].Sucher NJ, Awobuluyi M, Choi YB, Lipton SA. NMDA receptors: from genes to channels. Trends Pharmacol Sci 1996; 17: 348–55. [PubMed] [Google Scholar]
- [53].Tabakoff B, Hoffman PL. Alcohol addiction: an enigma among us. Neuron 1996; 16: 909–12. [DOI] [PubMed] [Google Scholar]
- [54].Kumari M, Ticku MK. Regulation of NMDA receptors by ethanol. Prog Drug Res 2000; 54: 152–89. [PubMed] [Google Scholar]
- [55].Grant KA, Valverius P, Hudspith M, Tabakoff B. Ethanol withdrawal seizures and the NMDA receptor complex. Eur J Pharmacol 1990; 176: 289–96. [DOI] [PubMed] [Google Scholar]
- [56].Gulya K, Grant KA, Valverius P, Hoffman PL, Tabakoff B. Brain regional specificity and time-course of changes in the NMDA receptor-ionophore complex during ethanol withdrawal. Brain Res 1991; 547: 129–34. [PubMed] [Google Scholar]
- [57].Snell LD, Tabakoff B, Hoffman PL. Radioligand binding to the N- methyl-D-aspartate receptor/ionophore complex: alterations by ethanol in vitro and by chronic in vivo ethanol ingestion. Brain Res 1993; 602: 91–8. [DOI] [PubMed] [Google Scholar]
- [58].Follesa P, Ticku MK. Chronic ethanol treatment differentially regulates NMDA receptor subunit mRNA expression in rat brain. Mol Brain Res 1995; 29: 99–106. [DOI] [PubMed] [Google Scholar]
- [59].Kalluri HS, Mehta AK, Ticku MK. Up-regulation of NMDA receptor subunits in rat brain following chronic ethanol treatment. Mol Brain Res 1998; 58: 221–4. [DOI] [PubMed] [Google Scholar]
- [60].Hardy PA, Chen W, Wilce PA. Chronic ethanol exposure and withdrawal influence NMDA receptor subunit and splice variant mRNA expression in the rat cerebral cortex. Brain Res 1999; 819: 33–9. [DOI] [PubMed] [Google Scholar]
- [61].Pian JP, Criado JR, Milner R, Ehlers CL. N-methyl-D-aspartate receptor subunit expression in adult and adolescent brain following chronic ethanol exposure. Neurosci 2010; 170: 645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Maler JM, Esselmann H, Wiltfang J, Kunz N, Lewczuk P, Reulbach U, et al. Memantine inhibits ethanol-induced NMDA receptor up-regulation in rat hippocampal neurons. Brain Res 2005; 1052: 156–62. [DOI] [PubMed] [Google Scholar]
- [63].Follesa P, Ticku MK. Chronic ethanol-mediated up-regulation of the N-methyl-D-aspartate receptor polypeptide subunits in mouse cortical neurons in culture. J Biol Chem 1996; 271: 13297–9. [DOI] [PubMed] [Google Scholar]
- [64].Chandler LJ, Sutton G, Norwood D, Sumners C, Crews FT. Chronic ethanol increases N-methyl-D-aspartate-stimulated nitric oxide formation but not receptor density in cultured cortical neurons. Mol Pharmacol 1997; 51: 733–40. [DOI] [PubMed] [Google Scholar]
- [65].Narita M, Soma M, Mizoguchi H, Tseng LF, Suzuki T. Implications of the NR2B subunit-containing NMDA receptor localized in mouse limbic forebrain in ethanol dependence. Eur J Pharmacol 2000; 401: 191–5. [DOI] [PubMed] [Google Scholar]
- [66].Biermann T, Reulbach U, Lenz B, Frieling H, Muschler M, Hillemacher T, et al. N-methyl-D-aspartate 2b receptor subtype (NR2B) promoter methylation in patients during alcohol withdrawal. J Neural Transm 2009; 116: 615–22. [DOI] [PubMed] [Google Scholar]
- [67].Snell LD, Nunley KR, Lickteig RL, Browning MD, Tabakoff B, Hoffman PL. Regional and subunit specific changes in NMDA receptor mRNA and immunoreactivity in mouse brain following chronic ethanol ingestion. Mol Brain Res 1996; 40: 71–8. [DOI] [PubMed] [Google Scholar]
- [68].Chandler LJ, Norwood D, Sutton G. Chronic ethanol upregulates NMDA and AMPA, but not kainate receptor subunit proteins in rat primary cortical cultures. Alcohol Clin Exp Res 1999; 23: 363–70. [PubMed] [Google Scholar]
- [69].Holter SM, Danysz W, Spanagel R. Novel uncompetitive Nmethyl-D-aspartate (NMDA)-receptor antagonist MRZ 2/579 suppresses ethanol intake in long-term ethanol-experienced rats and generalizes to ethanol cue in drug discrimination procedure. J Pharmacol Exp Ther 2000; 292: 545–52. [PubMed] [Google Scholar]
- [70].Shelton KL, Balster RL. Effects of gamma-aminobutyric acid agonists and N-methyl-D-aspartate antagonists on a multiple schedule of ethanol and saccharin self-administration in rats. J Pharmacol Exp Ther 1997; 280: 1250–60. [PubMed] [Google Scholar]
- [71].Piasecki J, Koros E, Dyr W, Kostowski W, Danysz W, Bienkowski P. Ethanol-reinforced behaviour in the rat: effects of uncompetitive NMDA receptor antagonist, memantine. Eur J Pharmacol 1998; 354: 135–43. [DOI] [PubMed] [Google Scholar]
- [72].Bienkowski P, Koros E, Kostowski W, Danysz W. Effects of Nmethyl-D-aspartate receptor antagonists on reinforced and nonreinforced responding for ethanol in rats. Alcohol 1999; 18: 131–7. [DOI] [PubMed] [Google Scholar]
- [73].Vengeliene V, Bachteler D, Danysz W, Spanagel R. The role of the NMDA receptor in alcohol relapse: a pharmacological mapping study using the alcohol deprivation effect. Neuropharmacol 2005; 48: 822–9. [DOI] [PubMed] [Google Scholar]
- [74].Boyce-Rustay JM, Cunningham CL. The role of NMDA receptor binding sites in ethanol place conditioning. Behav Neurosci 2004; 118: 822–34. [DOI] [PubMed] [Google Scholar]
- [75].Biala G, Kotlinska J. Blockade of the acquisition of ethanolinduced conditioned place preference by N-methyl-D-aspartate receptor antagonists. Alcohol Alcohol 1999; 34: 175–82. [DOI] [PubMed] [Google Scholar]
- [76].Broadbent J, Weitemier AZ. Dizocilpine (MK-801) prevents the development of sensitization to ethanol in DBA/2J mice. Alcohol Alcohol 1999; 34: 283–8. [DOI] [PubMed] [Google Scholar]
- [77].Meyer PJ, Phillips TJ. Bivalent effects of MK-801 on ethanolinduced sensitization do not parallel its effects on ethanol-induced tolerance. Behav Neurosci 2003; 117: 641–9. [DOI] [PubMed] [Google Scholar]
- [78].Mayer S, Harris BR, Gibson DA, Blanchard JA, Prendergast MA, Holley RC, et al. Acamprosate, MK-801, and ifenprodil inhibit neurotoxicity and calcium entry induced by ethanol withdrawal in organotypic slice cultures from neonatal rat hippocampus. Alcohol Clin Exp Res 2002; 26: 1468–78. [DOI] [PubMed] [Google Scholar]
- [79].Morrisett RA, Rezvani AH, Overstreet D, Janowsky DS, Wilson WA, Swartzwelder HS. MK-801 potently inhibits alcohol withdrawal seizures in rats. Eur J Pharmacol 1990; 176: 103–5. [DOI] [PubMed] [Google Scholar]
- [80].Prendergast MA, Harris BR, Mullholland PJ, Blanchard JA, 2nd, Gibson DA, Holley RC, et al. Hippocampal CA1 region neurodegeneration produced by ethanol withdrawal requires activation of intrinsic polysynaptic hippocampal pathways and function of N-methyl-D-aspartate receptors. Neurosci 2004; 124: 869–77. [DOI] [PubMed] [Google Scholar]
- [81].Thomas J, Fleming And S, Riley E. MK-801 can exacerbate or attenuate behavioral alterations associated with neonatal alcohol exposure in the rat, depending on the timing of administration. Alcohol Clin Exp Res 2001; 25: 764–73. [PubMed] [Google Scholar]
- [82].Grant KA, Knisely JS, Tabakoff B, Barrett JE, Balster RL. Ethanol-like discriminative stimulus effects of non-competitive nmethyl-d-aspartate antagonists. Behav Pharmacol. 1991. 2: 87–95. [PubMed] [Google Scholar]
- [83].Morita T, Sonoda R, Nakato K, Koshiya K, Wanibuchi F, Yamaguchi T. Phencyclidine-induced abnormal behaviors in rats as measured by the hole board apparatus. Psychopharmacol (Berl) 2000; 148: 281–8. [DOI] [PubMed] [Google Scholar]
- [84].Klein M, Calderon S, Hayes B. Abuse liability assessment of neuroprotectants. Ann N Y Acad Sci 1999; 890: 515–25. [DOI] [PubMed] [Google Scholar]
- [85].Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Sci 1999; 283: 70–4. [DOI] [PubMed] [Google Scholar]
- [86].Bender C, de Olmos S, Bueno A, de Olmos J, Lorenzo A. Comparative analyses of the neurodegeneration induced by the non-competitive NMDA-receptor-antagonist drug MK801 in mice and rats. Neurotoxicol Teratol 2010; 32: 542–50. [DOI] [PubMed] [Google Scholar]
- [87].Stepanyan TD, Farook JM, Kowalski A, Kaplan E, Barron S, Littleton JM. Alcohol withdrawal-induced hippocampal neurotoxicity in vitro and seizures in vivo are both reduced by memantine. Alcohol Clin Exp Res 2008; 32: 2128–35. [DOI] [PubMed] [Google Scholar]
- [88].Volbracht C, van Beek J, Zhu C, Blomgren K, Leist M. Neuroprotective properties of memantine in different in vitro and in vivo models of excitotoxicity. Eur J Neurosci 2006; 23: 2611–22. [DOI] [PubMed] [Google Scholar]
- [89].Kew JN, Kemp JA. An allosteric interaction between the NMDA receptor polyamine and ifenprodil sites in rat cultured cortical neurones. J Physiol 1998; 512: 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Berger ML, Rebernik P. Zinc and ifenprodil allosterically inhibit two separate polyamine-sensitive sites at N-methyl-D-aspartate receptor complex. J Pharmacol Exp Ther 1999; 289: 1584–91. [PubMed] [Google Scholar]
- [91].Kew JN, Richards JG, Mutel V, Kemp JA. Developmental changes in NMDA receptor glycine affinity and ifenprodil sensitivity reveal three distinct populations of NMDA receptors in individual rat cortical neurons. J Neurosci 1998; 18: 1935–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wang CX, Shuaib A. NMDA/NR2B selective antagonists in the treatment of ischemic brain injury. Curr Drug Targets CNS Neurol Disord 2005; 4: 143–51. [DOI] [PubMed] [Google Scholar]
- [93].Nash JE, Hill MP, Brotchie JM. Antiparkinsonian actions of blockade of NR2B-containing NMDA receptors in the reserpinetreated rat. Exp Neurol 1999; 155: 42–8. [DOI] [PubMed] [Google Scholar]
- [94].Nash JE, Fox SH, Henry B, Hill MP, Peggs D, McGuire S, et al. Antiparkinsonian actions of ifenprodil in the MPTP-lesioned marmoset model of Parkinson’s disease. Exp Neurol 2000; 165: 136–42. [DOI] [PubMed] [Google Scholar]
- [95].Chizh BA, Headley PM. NMDA antagonists and neuropathic pain-multiple drug targets and multiple uses. Curr Pharm Des 2005; 11: 2977–94. [DOI] [PubMed] [Google Scholar]
- [96].Tamura Y, Sato Y, Yokota T, Akaike A, Sasa M, Takaori S. Ifenprodil prevents glutamate cytotoxicity via polyamine modulatory sites of N-methyl-D-aspartate receptors in cultured cortical neurons. J Pharmacol Exp Ther 1993; 265: 1017–25. [PubMed] [Google Scholar]
- [97].Bath CP, Farrell LN, Gilmore J, Ward MA, Hicks CA, O’Neill MJ, et al. The effects of ifenprodil and eliprodil on voltage-dependent Ca2+ channels and in gerbil global cerebral ischaemia. Eur J Pharmacol 1996; 299: 103–12. [DOI] [PubMed] [Google Scholar]
- [98].Pawlak R, Melchor JP, Matys T, Skrzypiec AE, Strickland S. Ethanol-withdrawal seizures are controlled by tissue plasminogen activator via modulation of NR2B-containing NMDA receptors. Proc Natl Acad Sci USA 2005; 102: 443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Toulmond S, Serrano A, Benavides J, Scatton B. Prevention by eliprodil (SL 82.0715) of traumatic brain damage in the rat. Existence of a large (18 h) therapeutic window. Brain Res 1993; 620: 32–41. [DOI] [PubMed] [Google Scholar]
- [100].Thomas JD, Garcia GG, Dominguez HD, Riley EP. Administration of eliprodil during ethanol withdrawal in the neonatal rat attenuates ethanol-induced learning deficits. Psychopharmacology (Berl) 2004; 175: 189–95. [DOI] [PubMed] [Google Scholar]
- [101].Reyes M, Reyes A, Opitz T, Kapin MA, Stanton PK. Eliprodil, a non-competitive, NR2B-selective NMDA antagonist, protects pyramidal neurons in hippocampal slices from hypoxic/ischemic damage. Brain Res 1998; 782: 212–8. [DOI] [PubMed] [Google Scholar]
- [102].Chenard BL, Shalaby IA, Koe BK, Ronau RT, Butler TW, Prochniak MA, et al. Separation of alpha 1 adrenergic and Nmethyl-D-aspartate antagonist activity in a series of ifenprodil compounds. J Med Chem 1991; 34: 3085–90. [DOI] [PubMed] [Google Scholar]
- [103].McCool BA, Lovinger DM. Ifenprodil inhibition of the 5- hydroxytryptamine3 receptor. Neuropharmacology 1995; 34: 621–9. [DOI] [PubMed] [Google Scholar]
- [104].Biton B, Granger P, Carreau A, Depoortere H, Scatton B, Avenet P. The NMDA receptor antagonist eliprodil (SL 82.0715) blocks voltage-operated Ca2+ channels in rat cultured cortical neurons. Eur J Pharmacol 1994; 257: 297–301. [DOI] [PubMed] [Google Scholar]
- [105].Brimecombe JC, Boeckman FA, Aizenman E. Functional consequences of NR2 subunit composition in single recombinant N-methyl-D-aspartate receptors. Proc Natl Acad Sci U S A 1997; 94: 11019–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Brimecombe JC, Gallagher MJ, Lynch DR, Aizenman E. An NR2B point mutation affecting haloperidol and CP101,606 sensitivity of single recombinant N-methyl-D-aspartate receptors. J Pharmacol Exp Ther 1998; 286: 627–34. [PubMed] [Google Scholar]
- [107].Chazot PL, Lawrence S, Thompson CL. Studies on the subtype selectivity of CP-101,606: evidence for two classes of NR2B- selective NMDA receptor antagonists. Neuropharmacology 2002; 42: 319–24. [DOI] [PubMed] [Google Scholar]
- [108].Boyce S, Wyatt A, Webb JK, O’Donnell R, Mason G, Rigby M, et al. Selective NMDA NR2B antagonists induce antinociception without motor dysfunction: correlation with restricted localisation of NR2B subunit in dorsal horn. Neuropharmacology 1999; 38: 611–23. [DOI] [PubMed] [Google Scholar]
- [109].Brackett RL, Pouw B, Blyden JF, Nour M, Matsumoto RR. Prevention of cocaine-induced convulsions and lethality in mice: effectiveness of targeting different sites on the NMDA receptor complex. Neuropharmacology 2000; 39: 407–18. [DOI] [PubMed] [Google Scholar]
- [110].Nash JE, Ravenscroft P, McGuire S, Crossman AR, Menniti FS, Brotchie JM. The NR2B-selective NMDA receptor antagonist CP- 101,606 exacerbates L-DOPA-induced dyskinesia and provides mild potentiation of anti-parkinsonian effects of L-DOPA in the MPTP-lesioned marmoset model of Parkinson’s disease. Exp Neurol 2004; 188: 471–9. [DOI] [PubMed] [Google Scholar]
- [111].Steece-Collier K, Chambers LK, Jaw-Tsai SS, Menniti FS, Greenamyre JT. Antiparkinsonian actions of CP-101,606, an antagonist of NR2B subunit-containing N-methyl-d-aspartate receptors. Exp Neurol 2000; 163: 239–43. [DOI] [PubMed] [Google Scholar]
- [112].Okiyama K, Smith DH, White WF, McIntosh TK. Effects of the NMDA antagonist CP-98,113 on regional cerebral edema and cardiovascular, cognitive, and neurobehavioral function following experimental brain injury in the rat. Brain Res 1998; 792: 291–8. [DOI] [PubMed] [Google Scholar]
- [113].Tsuchida E, Rice M, Bullock R. The neuroprotective effect of the forebrain-selective NMDA antagonist CP101,606 upon focal ischemic brain damage caused by acute subdural hematoma in the rat. J Neurotrauma 1997; 14: 409–17. [DOI] [PubMed] [Google Scholar]
- [114].Kundrotiene J, Cebers G, Wagner A, Liljequist S. The NMDA NR2B subunit-selective receptor antagonist, CP-101,606, enhances the functional recovery the NMDA NR2B subunit-selective receptor and reduces brain damage after cortical compressioninduced brain ischemia. J Neurotrauma 2004; 21: 83–93. [DOI] [PubMed] [Google Scholar]
- [115].Menniti F, Chenard B, Collins M, Ducat M, Shalaby I, White F. CP-101,606, a potent neuroprotectant selective for forebrain neurons. Eur J Pharmacol 1997; 331: 117–26 [DOI] [PubMed] [Google Scholar]
- [116].Nagy J The NR2B subtype of NMDA receptor: a potential target for the treatment of alcohol dependence. Curr Drug Targets CNS Neurol Disord 2004; 3: 169–79. [DOI] [PubMed] [Google Scholar]
- [117].Slotkin T, Bartolome J. Role of ornithine decarboxylase and the polyamines in nervous system development: a review. Brain Res Bull 1986; 17: 307–20. [DOI] [PubMed] [Google Scholar]
- [118].Slotkin TA, Ferguson SA, Cada AM, McCook EC, Seidler FJ. Neonatal polyamine depletion by alpha-difluoromethylornithine: effects on adenylyl cyclase cell signaling are separable from effects on brain region growth. Brain Res 2000; 887: 16–22. [DOI] [PubMed] [Google Scholar]
- [119].Davidson M, Wilce P. Chronic ethanol treatment leads to increased ornithine decarboxylase activity: implications for a role of polyamines in ethanol dependence and withdrawal. Alcohol Clin Exp Res 1998; 22: 1205–11. [PubMed] [Google Scholar]
- [120].Gibson DA, Harris BR, Prendergast MA, Hart SR, Blanchard JA, 2nd, Holley RC, et al. Polyamines contribute to ethanol withdrawal-induced neurotoxicity in rat hippocampal slice cultures through interactions with the NMDA receptor. Alcohol Clin Exp Res 2003; 27: 1099–106. [DOI] [PubMed] [Google Scholar]
- [121].Thadani PV, Lau C, Slotkin TA, Schanberg SM. Effect of maternal ethanol ingestion on neonatal rat brain and heart ornithine decarboxylase. Biochem Pharmacol 1977; 26: 523–7. [DOI] [PubMed] [Google Scholar]
- [122].Shibley IA, Jr., Gavigan MD, Pennington SN. Ethanol’s effect on tissue polyamines and ornithine decarboxylase activity: a concise review. Alcohol Clin Exp Res 1995; 19: 209–15. [DOI] [PubMed] [Google Scholar]
- [123].Davidson MD, Wilce P, Shanley BC. Increased sensitivity of the hippocampus in ethanol-dependent rats to toxic effect of N-methylD-aspartic acid in vivo. Brain Res 1993; 606: 5–9. [DOI] [PubMed] [Google Scholar]
- [124].Butler TR, Self RL, Smith KJ, Sharrett-Field LJ, Berry JN, Littleton JM, et al. Selective vulnerability of hippocampal cornu ammonis 1 pyramidal cells to excitotoxic insult is associated with the expression of polyamine-sensitive N-methyl-D-asparate-type glutamate receptors. Neuroscience 2010; 165: 525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res 2003; 27: 232–43. [DOI] [PubMed] [Google Scholar]
- [126].Littleton J Neurochemical mechanisms underlying alcohol withdrawal. Alcohol Health Res World 1998; 22: 13–24. [PMC free article] [PubMed] [Google Scholar]
- [127].Littleton J, Zieglgansberger W. Pharmacological mechanisms of naltrexone and acamprosate in the prevention of relapse in alcohol dependence. Am J Addict 2003; 12 Suppl 1: S3–11. [DOI] [PubMed] [Google Scholar]
- [128].Staner L, Boeijinga P, Danel T, Gendre I, Muzet M, Landron F, et al. Effects of acamprosate on sleep during alcohol withdrawal: A double-blind placebo-controlled polysomnographic study in alcohol-dependent subjects. Alcohol Clin Exp Res 2006; 30: 1492–9. [DOI] [PubMed] [Google Scholar]
- [129].Tambour S, Quertemont E. Preclinical and clinical pharmacology of alcohol dependence. Fundam Clin Pharmacol 2007; 21: 9–28. [DOI] [PubMed] [Google Scholar]
- [130].Miller WR. What is a relapse? Fifty ways to leave the wagon. Addiction 1996; 91 Suppl: S15–27. [PubMed] [Google Scholar]
- [131].Connors GJ, Maisto SA, Donovan DM. Conceptualizations of relapse: a summary of psychological and psychobiological models. Addiction 1996; 91 Suppl: S5–13. [PubMed] [Google Scholar]
- [132].Solomon RL. The opponent-process theory of acquired motivation: the costs of pleasure and the benefits of pain. Am Psychol 1980; 35: 691–712. [DOI] [PubMed] [Google Scholar]
- [133].O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol 1998; 12: 15–22. [DOI] [PubMed] [Google Scholar]
- [134].Valdez GR, Zorrilla EP, Roberts AJ, Koob GF. Antagonism of corticotropin-releasing factor attenuates the enhanced responsiveness to stress observed during protracted ethanol abstinence. Alcohol 2003; 29: 55–60. [DOI] [PubMed] [Google Scholar]
- [135].Kelley AE, Smith-Roe SL, Holahan MR. Response-reinforcement learning is dependent on N-methyl-D-aspartate receptor activation in the nucleus accumbens core. Proc Natl Acad Sci USA 1997; 94: 12174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Gewirtz JC, Davis M. Second-order fear conditioning prevented by blocking NMDA receptors in amygdala. Nature 1997; 388: 471–4. [DOI] [PubMed] [Google Scholar]
- [137].Maren S Synaptic transmission and plasticity in the amygdala. An emerging physiology of fear conditioning circuits. Mol Neurobiol 1996; 13: 1–22. [DOI] [PubMed] [Google Scholar]
- [138].Stout RL, Longabaugh R, Rubin A. Predictive validity of Marlatt’s relapse taxonomy versus a more general relapse code. Addiction 1996; 91 Suppl: S99–110. [PubMed] [Google Scholar]
- [139].Paille FM, Guelfi JD, Perkins AC, Royer RJ, Steru L, Parot P. Double-blind randomized multicentre trial of acamprosate in maintaining abstinence from alcohol. Alcohol Alcohol 1995; 30: 239–47. [PubMed] [Google Scholar]
- [140].Sass H, Soyka M, Mann K, Zieglgansberger W. Relapse prevention by acamprosate. Results from a placebo-controlled study on alcohol dependence. Arch Gen Psychiatry 1996; 53: 673–80. [DOI] [PubMed] [Google Scholar]
- [141].O’Brien CP, Volpicelli LA, Volpicelli JR. Naltrexone in the treatment of alcoholism: a clinical review. Alcohol 1996; 13: 35–9. [DOI] [PubMed] [Google Scholar]
- [142].Modesto-Lowe V, Burleson JA, Hersh D, Bauer LO, Kranzler HR. Effects of naltrexone on cue-elicited craving for alcohol and cocaine. Drug Alcohol Depend 1997; 49: 9–16. [DOI] [PubMed] [Google Scholar]
- [143].Spanagel R, Zieglgansberger W. Anti-craving compounds for ethanol: new pharmacological tools to study addictive processes. Trends Pharmacol Sci 1997; 18: 54–9. [PubMed] [Google Scholar]
- [144].Putzke J, Spanagel R, Tolle TR, Zieglgansberger W. The anticraving drug acamprosate reduces c-fos expression in rats undergoing ethanol withdrawal. Eur J Pharmacol 1996; 317: 39–48. [DOI] [PubMed] [Google Scholar]
- [145].Spanagel R, Holter SM, Allingham K, Landgraf R, Zieglgansberger W. Acamprosate and alcohol: I. Effects on alcohol intake following alcohol deprivation in the rat. Eur J Pharmacol 1996; 305: 39–44. [DOI] [PubMed] [Google Scholar]
- [146].Heyser CJ, Schulteis G, Durbin P, Koob GF. Chronic acamprosate eliminates the alcohol deprivation effect while having limited effects on baseline responding for ethanol in rats. Neuropsychopharmacology 1998; 18: 125–33. [DOI] [PubMed] [Google Scholar]
- [147].Cole JC, Littleton JM, Little HJ. Effects of repeated ethanol administration in the plus maze; a simple model for conditioned abstinence behaviour. Psychopharmacology (Berl) 1999; 142: 270–9. [DOI] [PubMed] [Google Scholar]
- [148].Bachteler D, Economidou D, Danysz W, Ciccocioppo R, Spanagel R. The effects of acamprosate and neramexane on cue-induced reinstatement of ethanol-seeking behavior in rat. Neuropsychopharmacology 2005; 30: 1104–10. [DOI] [PubMed] [Google Scholar]
- [149].Mason BJ, Crean R. Acamprosate in the treatment of alcohol dependence: clinical and economic considerations. Expert Rev Neurother 2007; 7: 1465–77. [DOI] [PubMed] [Google Scholar]
- [150].Rosner S, Hackl-Herrwerth A, Leucht S, Lehert P, Vecchi S, Soyka M. Acamprosate for alcohol dependence. Cochrane Database Sys Rev 2010; CD004332. [DOI] [PubMed] [Google Scholar]
- [151].Honkanen A, Vilamo L, Wegelius K, Sarviharju M, Hyytia P, Korpi ER. Alcohol drinking is reduced by a mulbut not by a delta-opioid receptor antagonist in alcohol-preferring rats. Eur J Pharmacol 1996; 304: 7–13. [DOI] [PubMed] [Google Scholar]
- [152].Naassila M, Hammoumi S, Legrand E, Durbin P, Daoust M. Mechanism of action of acamprosate. Part I. Characterization of spermidine-sensitive acamprosate binding site in rat brain. Alcohol Clin Exp Res 1998; 22: 802–9. [PubMed] [Google Scholar]
- [153].al Qatari M, Bouchenafa O, Littleton J. Mechanism of action of acamprosate. Part II. Ethanol dependence modifies effects of acamprosate on NMDA receptor binding in membranes from rat cerebral cortex. Alcohol Clin Exp Res 1998; 22: 810–4. [PubMed] [Google Scholar]
- [154].Littleton JM. Acamprosate in alcohol dependence: Implications of a unique mechanism of action. Journal of Addiction Medicine 2007; 1: 115–25 10 [DOI] [PubMed] [Google Scholar]
- [155].Harris BR, Prendergast MA, Gibson DA, Rogers DT, Blanchard JA, Holley RC, et al. Acamprosate inhibits the binding and neurotoxic effects of trans-ACPD, suggesting a novel site of action at metabotropic glutamate receptors. Alcohol Clin Exp Res 2002; 26: 1779–93. [DOI] [PubMed] [Google Scholar]
- [156].Krupitsky EM, Neznanova O, Masalov D, Burakov AM, Didenko T, Romanova T, et al. Effect of memantine on cue-induced alcohol craving in recovering alcohol-dependent patients. Am J Psychiatry 2007; 164: 519–23. [DOI] [PubMed] [Google Scholar]
- [157].Rammes G, Schierloh A. Neramexane (Merz Pharmaceuticals/Forest Laboratories). Drugs 2006; 9: 128–35. [PubMed] [Google Scholar]
- [158].McMillen BA, Crawford MS, Kulers CM, Williams HL. Effects of a metabotropic, mGlu5, glutamate receptor antagonist on ethanol consumption by genetic drinking rats. Alcohol Alcohol 2005; 40: 494–7. [DOI] [PubMed] [Google Scholar]
- [159].Zhao Y, Dayas CV, Aujla H, Baptista MA, Martin-Fardon R, Weiss F. Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. J Neurosci 2006; 26: 9967–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Czapinski P, Blaszczyk B, Czuczwar SJ. Mechanisms of action of antiepileptic drugs. Curr Top Med Chem 2005; 5: 3–14. [DOI] [PubMed] [Google Scholar]
- [161].Farook JM, Morrell DJ, Lewis B, Littleton JM, Barron S. Topiramate (Topamax) reduces conditioned abstinence behaviours and handling-induced convulsions (HIC) after chronic administration of alcohol in Swiss-Webster mice. Alcohol Alcohol 2007; 42: 296–300. [DOI] [PubMed] [Google Scholar]
- [162].Johnson BA. An overview of the development of medications including novel anticonvulsants for the treatment of alcohol dependence. Exp Opin Pharmacother 2004; 5: 1943–55. [DOI] [PubMed] [Google Scholar]
- [163].Harper C, Dixon G, Sheedy D, Garrick T. Neuropathological alterations in alcoholic brains. Studies arising from the New South Wales Tissue Resource Centre. Prog Neuropsychopharmacology Biol Psychiatry 2003; 27: 951–61. [DOI] [PubMed] [Google Scholar]
- [164].Crews FT, Collins MA, Dlugos C, Littleton J, Wilkins L, Neafsey EJ, et al. Alcohol-induced neurodegeneration: when, where and why? Alcohol Clin Exp Res 2004; 28: 350–64. [DOI] [PubMed] [Google Scholar]
- [165].Booth BM, Blow FC. The kindling hypothesis: further evidence from a U.S. national study of alcoholic men. Alcohol Alcohol 1993; 28: 593–8. [PubMed] [Google Scholar]
- [166].Duka T, Townshend JM, Collier K, Stephens DN. Impairment in cognitive functions after multiple detoxifications in alcoholic inpatients. Alcohol Clin Exp Res 2003; 27: 1563–72. [DOI] [PubMed] [Google Scholar]
- [167].McCrady BS, Smith DE. Implications of cognitive impairment for the treatment of alcoholism. Alcohol Clin Exp Res 1986; 10: 145–9. [DOI] [PubMed] [Google Scholar]
- [168].Koob GF, Mason BJ, De Witte P, Littleton J, Siggins GR. Potential neuroprotective effects of acamprosate. Alcohol Clin Exp Res 2002; 26: 586–92. [PubMed] [Google Scholar]
- [169].al Qatari M, Khan S, Harris B, Littleton J. Acamprosate is neuroprotective against glutamate-induced excitotoxicity when enhanced by ethanol withdrawal in neocortical cultures of fetal rat brain. Alcohol Clin Exp Res; 2001: 1276–83. [DOI] [PubMed] [Google Scholar]
- [170].Lipton SA. Pathologically-activated therapeutics for neuroprotection: mechanism of NMDA receptor block by memantine and S-nitrosylation. Curr Drug Targets 2007; 8: 621–32. [DOI] [PubMed] [Google Scholar]
- [171].Schubert S, Brandl U, Brodhun M, Ulrich C, Spaltmann J, Fiedler N, et al. Neuroprotective effects of topiramate after hypoxiaischemia in newborn piglets. Brain Res 2005; 1058: 129–36. [DOI] [PubMed] [Google Scholar]
- [172].Harris BR, Gibson DA, Prendergast MA, Blanchard JA, Holley RC, Hart SR, et al. The neurotoxicity induced by ethanol withdrawal in mature organotypic hippocampal slices might involve cross-talk between metabotropic glutamate type 5 receptors and N-methyl-D-aspartate receptors. Alcohol Clin Exp Res 2003; 27: 1724–35. [DOI] [PubMed] [Google Scholar]
- [173].Low SJ, Roland CL. Review of NMDA antagonist-induced neurotoxicity and implications for clinical development. Int J Clin Pharmacol Ther 2004; 42: 1–14. [DOI] [PubMed] [Google Scholar]
- [174].Parsons CG, Danysz W, Quack G. Glutamate in CNS disorders as a target for drug development: an update. Drug News Perspect 1998; 11: 523–69. [DOI] [PubMed] [Google Scholar]
- [175].Hundt W, Danysz W, Holter SM, Spanagel R. Ethanol and Nmethyl-D-aspartate receptor complex interactions: a detailed drug discrimination study in the rat. Psychopharmacology (Berl) 1998; 135: 44–51. [DOI] [PubMed] [Google Scholar]
- [176].Rock DM, Macdonald RL. Polyamine regulation of N-methyl-Daspartate receptor channels. Annu Rev Pharmacol Toxicol 1995; 35: 463–82. [DOI] [PubMed] [Google Scholar]
- [177].Bergeron RJ, Weimar WR, Wu Q, Feng Y, McManis JS. Polyamine analogue regulation of NMDA MK-801 binding: a structure-activity study. J Med Chem 1996; 39: 5257–66. [DOI] [PubMed] [Google Scholar]
- [178].Ransom RW, Deschenes NL. Polyamines regulate glycine interaction with the N-methyl-D-aspartate receptor. Synapse 1990; 5: 294–8. [DOI] [PubMed] [Google Scholar]
- [179].Lewin AH, Sun G, Fudala L, Navarro H, Zhou LM, Popik P, et al. Molecular features associated with polyamine modulation of NMDA receptors. J Med Chem 1998; 41: 988–95. [DOI] [PubMed] [Google Scholar]
- [180].Romano C, Williams K, DePriest S, Seshadri R, Marshall GR, Israel M, et al. Effects of mono-, di-, and triamines on the Nmethyl-D-aspartate receptor complex: a model of the polyamine recognition site. Mol Pharmacol 1992; 41: 785–92. [PubMed] [Google Scholar]
- [181].Berger ML, Seifriz I, Letschnig M, Schodl C, Noe CR. Interaction of long chain n-alkyl diamines with the NMDA receptor complex. Neurosci Lett 1992; 142: 85–8. [DOI] [PubMed] [Google Scholar]
- [182].Gibson DA, Harris BR, Rogers DT, Littleton JM. Radioligand binding studies reveal agmatine is a more selective antagonist for a polyamine-site on the NMDA receptor than arcaine or ifenprodil. Brain Res 2002; 952: 71–7. [DOI] [PubMed] [Google Scholar]
- [183].Gilad GM, Salame K, Rabey JM, Gilad VH. Agmatine treatment is neuroprotective in rodent brain injury models. Life Sci 1996; 58: 41–6. [DOI] [PubMed] [Google Scholar]
- [184].Morgan AD, Campbell UC, Fons RD, Carroll ME. Effects of agmatine on the escalation of intravenous cocaine and fentanyl self-administration in rats. Pharmacol Biochem Behav 2002; 72: 873–80. [DOI] [PubMed] [Google Scholar]
- [185].Levi MS, Borne RF. A review of chemical agents in the pharmacotherapy of addiction. Curr Med Chem 2002; 9: 1807–18. [DOI] [PubMed] [Google Scholar]
- [186].Skolnick P Ibogaine as a glutamate antagonist: relevance to its putative antiaddictive properties. Alkaloids Chem Biol 2001; 56: 55–62. [DOI] [PubMed] [Google Scholar]
- [187].Worthen DR, Gibson DA, Rogers DT, Bence AK, Fu M, Littleton JM, et al. Endogenous indoles as novel polyamine site ligands at the N-methyl-D-aspartate receptor complex. Brain Res 2001; 890: 343–6. [DOI] [PubMed] [Google Scholar]
- [188].Mundy WR, Radio NM, Freudenrich TM. Neuronal models for evaluation of proliferation in vitro using high content screening. Toxicol 2010; 270: 121–30. [DOI] [PubMed] [Google Scholar]
- [189].Ahern KB, Lustig HS, Greenberg DA. Enhancement of NMDA toxicity and calcium responses by chronic exposure of cultured cortical neurons to ethanol. Neurosci Lett 1994; 165: 211–4. [DOI] [PubMed] [Google Scholar]
- [190].Prendergast MA, Harris BR, Blanchard JA, 2nd, Mayer S, Gibson DA, Littleton JM. In vitro effects of ethanol withdrawal and spermidine on viability of hippocampus from male and female rat. Alcohol Clin Exp Res 2000; 24: 1855–61. [PubMed] [Google Scholar]
- [191].Farook JM, Krazem A, Lewis B, Morrell DJ, Littleton JM, Barron S. Acamprosate attenuates the handling induced convulsions during alcohol withdrawal in Swiss Webster mice. Physiol Behav 2008; 95: 267–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Crabbe JC, Young ER, Janowsky J, Rigter H. Pyrazole exacerbates handling-induced convulsions in mice. Neuropharmacology 1981; 20: 605–9. [DOI] [PubMed] [Google Scholar]
- [193].Sinclair JD, Senter RJ. Increased preference for ethanol in rats following alcohol deprivation. Psychonomic Science 1967; 8: 11–2. [Google Scholar]
- [194].File SE. New strategies in the search for anxiolytics. Drug Des Deliv 1990; 5: 195–201. [PubMed] [Google Scholar]
- [195].Cole JC, Littleton JM, Little HJ. Acamprosate, but not naltrexone, inhibits conditioned abstinence behaviour associated with repeated ethanol administration and exposure to a plus-maze. Psychopharmacology (Berl) 2000; 147: 403–11. [DOI] [PubMed] [Google Scholar]
- [196].Kamdar NK, Miller SA, Syed YM, Bhayana R, Gupta T, Rhodes JS. Acute effects of naltrexone and GBR 12909 on ethanol drinking-in-the-dark in C57BL/6J mice. Psychopharmacology (Berl) 2007; 192: 207–17. [DOI] [PubMed] [Google Scholar]
- [197].Gupta T, Syed YM, Revis AA, Miller SA, Martinez M, Cohn KA, et al. Acute effects of acamprosate and MPEP on ethanol Drinkingin-the-Dark in male C57BL/6J mice. Alcohol Clin Exp Res 2008; 32: 1992–8. [DOI] [PubMed] [Google Scholar]
- [198].Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, et al. Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology 1997; 56: 317–26. [DOI] [PubMed] [Google Scholar]
- [199].Williams R, Odaibo F, McGee J. Incidence of fetal alcohol syndrome in northeastern Manitoba. Can J Public Health 1999; 90: 192–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Lupton C, Burd L, Harwood R. Cost of fetal alcohol spectrum disorders. Am J Med Genet C Semin Med Genet 2004; 127C: 42–50. [DOI] [PubMed] [Google Scholar]
- [201].Helfer JL, Goodlett CR, Greenough WT, Klintsova AY. The effects of exercise on adolescent hippocampal neurogenesis in a rat model of binge alcohol exposure during the brain growth spurt. Brain Res 2009; 1294: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Thomas JD, Idrus NM, Monk BR, Dominguez HD. Prenatal choline supplementation mitigates behavioral alterations associated with prenatal alcohol exposure in rats. Birth Defects Res A Clin Mol Teratol 2010; 88: 827–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Wilkemeyer MF, Menkari CE, Charness ME. Novel antagonists of alcohol inhibition of l1-mediated cell adhesion: multiple mechanisms of action. Mol Pharmacol 2002; 62: 1053–60. [DOI] [PubMed] [Google Scholar]
- [204].Seiler N, Raul F. Polyamines and apoptosis. J Cell Mol Med 2005; 9: 623–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Sircar R Developmental maturation of the N-methyl-D-aspartic acid receptor channel complex in postnatal rat brain. Int J Dev Neurosci 2000; 18: 121–31. [DOI] [PubMed] [Google Scholar]
- [206].Davidson M, Bedi K, Wilce P. Ethanol inhibition of brain ornithine decarboxylase activity in the postnatal rat. Neurotoxicol Teratol 1998; 20: 523–30. [DOI] [PubMed] [Google Scholar]
- [207].Thadani P, Slotkin T, Schanberg S. Effects of late prenatal or early postnatal ethanol exposure on ornithine decarboxylase activity in brain and heart of developing rats. Neuropharmacology 1977; 16: 289–93. [DOI] [PubMed] [Google Scholar]
- [208].Williams K, Zappia A, Pritchett D, Shen Y, Molinoff P. Sensitivity of the N-methyl-D-aspartate receptor to polyamines is controlled by NR2 subunits. Mol Pharmacol 1994; 45: 803–9. [PubMed] [Google Scholar]
- [209].Naassila M, Daoust M. Effect of prenatal and postnatal ethanol exposure on the developmental profile of mRNAs encoding NMDA receptor subunits in rat hippocampus. J Neurochem 2002; 80: 850–60. [DOI] [PubMed] [Google Scholar]
- [210].Williams K, Russell SL, Shen YM, Molinoff PB. Developmental switch in the expression of NMDA receptors occurs in vivo and in vitro. Neuron 1993; 10: 267–78. [DOI] [PubMed] [Google Scholar]
- [211].Barron S, Mulholland PJ, Littleton JM, Prendergast MA. Age and gender differences in response to neonatal ethanol withdrawal and polyamine challenge in organotypic hippocampal cultures. Alcohol Clin Exp Res 2008; 32: 929–36. [DOI] [PubMed] [Google Scholar]
- [212].Lewis B, Wellmann KA, Kehrberg AMH, Carter ML, Baldwin T, Cohen M, et al. Behavioral deficits and cellular damage following developmental ethanol exposure in rats are attenuated by CP- 101,606, an NMDAR antagonist with unique NR2B specificity. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Wellmann KA, Lewis B, Carter LG, Carter ML, McGahan J, Crooks PA, et al. JR 220, a novel aminoguanadino compound, ameliorates ultrasonic vocalization and balance deficits in rats following “3rd trimester” ethanol exposure. Submitted. [Google Scholar]
- [214].Rubin MA, Wellmann KA, Lewis B, Overgaauw BJ, Littleton JM, Barron S. Difluoromethylornithine (DFMO) reduces deficits in isolation-induced ultrasonic vocalizations and balance following neonatal ethanol exposure in rats. Pharmacol Biochem Behav 2009; 92: 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Wellmann K, Lewis B, Barron S. Agmatine reduces ultrasonic vocalization deficits in female rat pups exposed neonatally to ethanol. Neurotoxicol Teratol 2010; 32: 158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Lewis B, Wellmann KA, Barron S. Agmatine reduces balance deficits in a rat model of third trimester binge-like ethanol exposure. Pharmacol Biochem Behav 2007; 88: 114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Heyser CJ, Moc K, Koob, GF. Effects of naltrexone alone and in combination with acamprosate on the alcohol deprivation effect in rats. Neuropsychopharmacol 2003; 28: 1463–71. [DOI] [PubMed] [Google Scholar]
- [218].Gabriel KI, Cunningham CL. Effects of topiramate on ethanol and saccharin consumption and preferences in C57BL/6J mice. Alcohol Clin Exp Res 2005; 29: 75–80. [DOI] [PubMed] [Google Scholar]
- [219].Uzbay IT, Ye ilyurt O, Celik T, Ergün H, I imer A. Effects of agmatine on ethanol withdrawal syndrome in rats. Behav Brain Res 2000; 107: 153–9. [DOI] [PubMed] [Google Scholar]
- [220].Zakharova E, Malyshkin A, Kashkin V, Neznanova O, Sukhotina I, Danysz W, et al. The NMDA receptor channel blocker memantine and opioid receptor antagonist naltrexone inhibit the saccharin deprivation effect in rats. Behav Pharmacol 2004; 15: 273–8. [DOI] [PubMed] [Google Scholar]
- [221].Jandeleit B, Li Y, Gallop MA, Zerangue N, Virsik PA, Fischer W-N Masked carboxylate neopentyl sulfonyl ester cyclization release prodrugs of acamprosate, compositions thereof, and methods of use. US0069419 (2009). [Google Scholar]
- [222].Jandeleit B, Li Y, Gallop MA, Zerangue N, Virsik PA, Fischer W-N Complex pantoic acid ester neopentyl sulfonyl ester cyclization release prodrugs of acamprosate, compositions thereof, and methods of use. US0076147 (2009). [Google Scholar]
- [223].Jandeleit B, Li Y, Gallop MA, Zerangue N, Virsik PA, Fischer W-N Externally masked neopentyl sulfonyl ester cyclization release prodrugs of acamprosate, compositions thereof, and methods of use. US0082464 (2009). [Google Scholar]
- [224].Jandeleit B, Li Y, Gallop MA, Zerangue N, Virsik PA, Fischer W-N Simple pantoic acid ester neopentyl sulfonyl ester cyclization release prodrugs of acamprosate, compositions thereof, and methods of use. US7994218 (2011). [Google Scholar]
- [225].Li Y, Jandeleit B, Gallop MA, Zerangue N, Virsik PA, Fischer W-N Internally masked neopentyl sulfonyl ester cyclization release prodrugs of acamprosate, compositions thereof, and methods of use. US0099253 (2009). [Google Scholar]
- [226].Arellano MB F. Compounds used to treat alcoholism. MX011864 (2005). [Google Scholar]
- [227].Bilbeny LN, Garcia MH Amines with antialcoholic agents. WO03026634 (2003). [Google Scholar]
- [228].Bilbeny LN, Garcia MH Amines as anti-alcoholism agents. US7232846 (2007). [Google Scholar]
- [229].Bachurin SO, Gavrilova SI, Grigoriev VV, Beznosko BK, Zefirov NS Means for improving cognitive functions and memory based on hydrogenated pyrido (4,3-B) indoles (variants), pharmacological means based thereon and method for the use thereof. WO2008069963 (2008). [Google Scholar]
- [230].Gerlach M, Przewosny M, Englberger W, Reissmueller E, Bloms-Funke P, Maul C, Jagusch U-P Substituted 1,2,3,4 tetrahydroquinoline-2-carboxylic acid derivatives. US6699877 (2004). [Google Scholar]
- [231].Kawamura M 3,4-dihydroquinolin-2(1H)-one compounds as NR2B receptor antagonists. US6713490 (2004). [Google Scholar]
- [232].Masui M, Adachi M, Mikamiyama H, Matsumura A, Tsuno N Nitrogen-containing heterocycle derivatives substituted with cyclic group. US7935706 (2011). [Google Scholar]
- [233].Wong AJ Alternative splice forms of proteins as basis for multiple therapeutic. US8021658 (2011). [Google Scholar]
- [234].Kuehnert S, Zemolka S, Hauraud M, Schiene K Substituted imidazo[2,1-b]thiazole compounds and uses thereof. US7893069 (2011). [Google Scholar]
- [235].Haurand M, Schiene K, Kiihnert S, Reich M, Zemolka S Substituted propiolic acid amides and their use for producing drugs. US8008304 (2011a). [Google Scholar]
- [236].Haurand M, Schiene K, Kühnert Reich M Substituted thiazoles and their use for producing drugs. US7871999 (2011b). [Google Scholar]
- [237].Bridges RJ, Esslinger CS. The excitatory amino acid transporters: pharmacological insights on substrate and inhibitor specificity of the EAAT subtypes. Pharmacol Ther 2005; 107:271–285. [DOI] [PubMed] [Google Scholar]
- [238].Bohm A, Dubroeucq M-C, Fratta W, Guyon C, Imperato A, Manfre F Application of pyrrolidine derivatives to the preparation of medicaments intended to the treatment of drug abuse. EP0879054 (2007). [Google Scholar]
- [239].Imperato A Use of pyrrolidine derivatives for treating alcoholism. NZ310488 (2000). [Google Scholar]
- [240].Imperato A The use of pyrrolidine derivatives for the treatment of alcoholism. BG0063189 (2001a). [Google Scholar]
- [241].Imperato A Use of pyrrolidine derivatives for preparation of medicament for treating alcoholism. SK0282063 (2001b). [Google Scholar]
- [242].Imperato A Method for treating chronic alcoholism or conditions caused by alcohol abuse. RO0117297 (2002a). [Google Scholar]
- [243].Imperato A Use of pyrrolidine derivatives for treating alcoholism. SI0828486 (2002b). [Google Scholar]
- [244].Imperato A Use of pyrrolidine derivatives for treating alcoholism. EP0828486 (2002c). [Google Scholar]
- [245].Imperato A Use of pyrrolidine derivatives for treating alcoholism. CA2219455 (2009). [Google Scholar]
- [246].Nilsson L, Rogers GA Benzoyl piperidines/pyrrolidines for enhancing synaptic response. CA2222976 (2001). [Google Scholar]
- [247].Rogers GA, Nilsson L Benzoyl piperidines/pyrrolidines for enhancing synaptic response. EP0839134 (2004). [Google Scholar]
- [248].Welch WM 2nd. Atropisomers of 3-aryl-4(3H)-quinazolinones and their use as AMPA-receptor antagonists. EA0001963 (2001). [Google Scholar]
- [249].Chenard BL, Welch WM, Weinhold AR Thieno-pyrimidin-4 one AMPA antagonists. US6921764 (2005). [Google Scholar]
- [250].Ciruela F, Gomez-Soler M, Guidolin D, Borroto-Escuela DO, Agnati LF, Fuxe K, et al. Adenosine receptor containing oligomers: their role in the control of dopamine and glutamate neurotransmission. Biochimica et Biophysica Acta 2011; 1808:1245–55. [DOI] [PubMed] [Google Scholar]
- [251].Liang BT, Jacobson KA Methods and compositions for reducing ischemic injury of the heart by administering adenosine receptor agonists and antagonists. US6586413 (2003). [Google Scholar]
- [252].Shook BC, Jackson PF Arylindenopyrimidines compound and use as an adenosine A2a receptor antagonists. US8017614 (2011). [Google Scholar]
- [253].Eisenbach-Schwartz M, Yoles E, Sorek N, Hauben E PolyGlu,Tyr for neuroprotective therapy. US7399740 (2008). [Google Scholar]
- [254].Feuerstein TJ, Knoerle R Position-4 substituted 2-pyrrolidinone derivatives to reduce the level of extracellular glutamate. US6384069 (2002). [Google Scholar]
- [255].Feuerstein TJ, Knoerle R 2-pyrrolidinone derivatives substituted at position 4 for reducing the extracellular glutamate level. US6984659 (2006). [Google Scholar]
- [256].Slusher BS, Tsukamoto T Thiolactones. US7125907 (2006). [Google Scholar]
- [257].Jackson PF, Tsukamoto T, Slusher BS, Wang E Benzenedicarboxylic acid derivatives. US6452044 (2002). [Google Scholar]