Abstract
Background
Obesity can be characterized by low-grade chronic inflammation and is associated with an excesso production of reactive oxygen species, factors that contribute to coronary heart disease and other cardiomyopathies.
Objective
To verify the effects of resistance exercise training on oxidative stress and inflammatory parameters on mice with obesity induced by a high-fat diet (HFD).
Methods
24 Swiss mice were divided into 4 groups: standard diet (SD), SD + resistance exercise (SD + RE), diet-induced obesity (DIO), DIO + RE. The animals were fed SD or HFD for 26 weeks and performed resistance exercises in the last 8 weeks of the study. The insulin tolerance test (ITT) and body weight monitoring were performed to assess the clinical parameters. Oxidative stress and inflammation parameters were evaluated in the cardiac tissue. Data were expressed by mean and standard deviation (p < 0.05).
Results
The DIO group had a significant increase in reactive oxygen species levels and lipid peroxidation with reduction after exercise. Superoxide dismutase and the glutathione system showed no significant changes in DIO animals, with an increase in SD + RE. Only catalase activity decreased with both diet and exercise influence. There was an increase in tumor necrosis factor-alpha (TNF-α) in the DIO group, characterizing a possible inflammatory condition, with a decrease when exposed to resistance training (DIO+RE).
Conclusion
The DIO resulted in a redox imbalance in cardiac tissue, but the RE was able to modulate these parameters, as well as to control the increase in TNF-α levels.
Keywords: Exercise; Oxidative Stress; Obesity; Diet, High-Fat; Mice
Introduction
The World Health Organization (WHO)1 defines obesity as an abnormal or excessive accumulation of fat that brings health risks. The WHO brings data from the Global Health Observatory showing a worldwide prevalence of obesity of 39% in men and women over 18 years of age (2016 updated data). In this scenario, obesity is also a risk factor for lifestyle-related diseases, such as cardiovascular disease and type 2 diabetes mellitus.2 It can be characterized by low-grade chronic inflammation and is associated with increased levels of proinflammatory cytokines, as well as an excess production of reactive oxygen species.3,4
Studies have shown that hyperglycemia and alterations in glucose uptake in diabetes may lead to oxidative stress with consequent mitochondrial dysfunction, as well as to an inflammatory process with the presence of elevated proinflammatory cytokines, such as TNF-α. Both cases may be triggering factors for coronary heart disease and other cardiomyopathies5 (see review by Adeghate and Singh). Gamez-Mendez et al.,6 also showed that 8 weeks of high-fat diet (HFD) led to an increase in oxidative stress, resulting in an imbalance of vasoactive substances and consequent endothelial dysfunction of the coronary arteries in obese rats.
Studies point to physical exercise as an important ally in reducing the risks related to obesity due to its ability to reestablish the balance between pro-anti-inflammatory cytokines and regulate the cell redox state.7,8 According to Boardman et al.,9 physical exercise is not only an important therapeutic approach in obesity, but it is also crucial for cardiac function improvement and ischemic injury prevention in obese and/or diabetic animals.
Although the literature indicates that exercise is important to prevent or complement the treatment of obesity,10 one should consider the characteristics of the performed exercise, such as: intensity, duration, frequency and type.
Based on the aforementioned facts, this study aimed to identify whether resistance exercise (RE) modifies the oxidative stress and inflammation parameters caused by an HFD in an experimental model of obesity.
Methods
Animals
Male Swiss mice (40 days), obtained at the vivarium of Universidade do Extremo Sul Catarinense - UNESC (Criciúma/SC - Brazil), with an average weight of 35.45 g (± 1.29) were studied. The animals were kept at a 12/12h light/dark cycle at 22ºC in collective boxes (6 animals per box) and randomly divided into four groups (n = 6): standard diet (SD); diet-induced obesity (DIO); SD + RE; DIO + RE. The random selection procedure was carried out by arbitrarily or randomly allocating the animals to the respective groups, without prior performance evaluation or using any other indicator that allowed the groups to be divided.
Diet
The animals were fed ad libitium for 26 weeks with low-fat SD (SD: 27%, 23% and 50% of calories from proteins, fats and carbohydrates, respectively - 3.3kcal/g) or HFD (HFD: 15%, 59% and 26% of calories from proteins, fats and carbohydrates, respectively - 5.3kcal/g). The SD was purchased from the Puro Trato Nutrição Animal (Puro Lab 22PB) Santo Augusto/RS - Brazil, and the HFD from PragSoluções Biociência, Jaú/SP - Brazil.
Exercise
The exercise adaptation protocol was started in the 17thweek of the diet and the RE protocol in the 18th. The resistance training was performed in a 1-m ladder apparatus with 2cm steps and 85º-slope.11 The animals were familiarized with the climbing exercise on the steps for 5 consecutive days without load. The training protocol, adapted from Scheffer et al.,12,13 started 3 days after the last adaptation training, and was performed with a 48-h interval between sessions, for 8 weeks, totaling 28 training sessions. The exercise was performed with intensity progression by adding a weight to the animal's tail (load increase of 20% to 75% of body weight), and volume progression (5-10 series per session) (Table 1), with a 2-min interval between sessions in the rest area (closed box at the top of the steps measuring 20x20x20 cm). Each series was performed until the animals completed 5 repetitions/climbings (without interval), or could not climb the stairs even after encouragement (manual stimulation at the base of the tail).
Table 1.
Resistance training protocol
| Weeks | Load | Series | Interval between series |
|---|---|---|---|
| 1st | 20% | 5 | 1 (2min) |
| 2nd | 20% | 7 | 1 (2min) |
| 3rd | 50% | 5 | 1 (2min) |
| 4th | 50% | 7 | 1 (2min) |
| 5th | 50% | 10 | 1 (2min) |
| 6th | 50% | 10 | 1 (2min) |
| 7th | 75% | 7 | 1 (2min) |
| 8th | 75% | 10 | 1 (2min) |
Source: Study data. Adapted from Scheffer et al.12
Body weight and insulin tolerance test (ITT)
Individual body weight was measured at the start of the study and at weeks 3, 6, 10, 14, 18, 22 and 26. After 17 weeks on the diet, an ITT was performed to confirm insulin resistance. After 6 hours of fasting,14 all animals received a dose of 2 U/kg of insulin. Blood glucose was measured with a glycometer using a drop of blood collected from a small incision at the tip of the animal's tail. The same protocol was performed at the end of the experiment, 48 hours after the last exercise session.
Euthanasia
24 h after the last insulin tolerance test, euthanasia by decapitation was performed and the left ventricle of the heart was surgically extracted, immediately frozen in liquid nitrogen, and stored at -80ºC for biochemical analysis.
Biochemical analyses
For the biochemical assays described below and ELISA test, all samples were homogenized in 50 mM phosphate-buffered saline (PBS), with the addition of 10uM aprotinin. The homogenate was centrifuged for 10 min at 4ºC and the supernatant was stored at -80ºC. Protein levels were determined in all samples using the Bradford method.15
Oxidation of dichlorodihydrofluorescein (DCFH)
Reactive species levels were measured based on oxidation of the 2',7'-dichlorodihydrofluorescein diacetate (DCFH-DA) probe in a fluorescent 2',7'-dichlorodihydrofluorescein (DCF) compound as previously described.16 An aliquot of the lysate was incubated with 80 mM DCFH-DA at 37ºC for 15 minutes. The production of reactive species was quantified using a standard DCF curve and data were expressed as nM DCF/mg protein.
Antioxidant enzyme activity
The superoxide dismutase (SOD) activity was estimated by inhibiting the auto oxidation of adrenaline and read spectrophotometrically at 480 nm according to the method described by McCord and Fridovich.17 Catalase (CAT) activity was established based on the rate of hydrogen peroxide (H2O2) decomposition, generated by the enzyme present in the sample using a 10 mM H2O2 solution in potassium phosphate buffer with pH of 7.0. The maximum rate of H2O2 decomposition was measured at 240 nm.18 Values were expressed as units of SOD or CAT per mg of protein.
Total glutathione (GSH) levels
GSH levels were measured using the Hissin method.19 Samples were incubated in 0.6% sulfosalicylic acid followed by a reaction of the GSH present in the sample with 2-nitrobenzoic acid (5,5'-Dithiobis) (DTNB) producing an oxidized glutathione-TNB adduct (GS-TNB). The resulting color from the reaction between the DTNB and thiols compared to the standard curve for GSH was kinetically established at 412 nm for 10 min. Values were expressed as nmol/min/mg protein.
Lipoperoxidation
The concentrations of malondialdehyde (MDA) in the samples were determined by high-performance liquid chromatography (HPLC) (Agilent Technologies 1200 Series; Santa Clara, CA, USA),according to Grotto et al. (2007)20 using a derivative of thiobarbituric acid (TBA). A standard curve was created using MDA tetrabutylammonium salt at concentrations ranging from 0.5 to 5 µmol/L. The MDA was determined at 532 nm and the results were expressed as umol/L of MDA/milligram protein.
Inflammatory Parameter
The concentration of tumor necrosis factor-alpha (TNF-α) was assessed by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's recommendations (ThermoFisher Scientific, cat.KMC3011). The results were expressed in pg/mg of protein.
Statistical analysis
Initially, the data were assessed using Grubbs test to verify possible outliers followed by the Shapiro-Wilk normality test, and in both of them the data met the assumptions for the use of parametric tests. Then, the Two-way ANOVA test was performed, followed by the Bonferroni post-hoc test when necessary. For analysis of the ITT data (table 2), the one-way variance test (one-way ANOVA) of repeated measures was performed, followed by post-hoc Tukey test when necessary. The level of significance was set at p < 0.05. The Graph Pad Prism software, version 5, was used as the statistical package. All data were expressed as mean and standard deviation, except for figure 1A, expressed as mean and standard error of mean.
Results
Body weight and insulin resistance
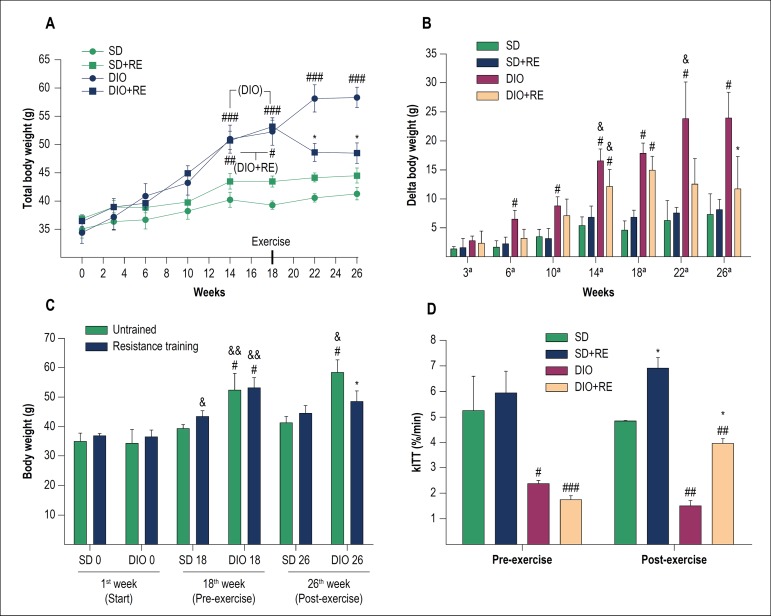
The RE had a beneficial effect, preventing the DIO + RE group from continuing to gain weight even with the HFD consumption, not characterizing weight loss, but rather weight maintenance, even without food intake control (Figure 1A-C).
Figure 1.
A – Total body weight during the study; B - Delta (body weight variation in relation to the start of the study); C - weight comparison between the 1st week (start), 18th week (pre-exercise) and 26th week (post-exercise); D - Glucose decay rate in the insulin tolerance test (kITT). Figure A, B and D - *p < 0.05 vs. respective untrained of the same period, #p < 0.05, ##p < 0.01 and ###p < 0,001 vs. respective SD of the same period; &p < 0.05 vs. same group of the previous week. Figure C - *p < 0.001 vs. respective untrained of the same period; #p < 0.01 and ##p < 0.001 vs. respective SD of the same period; &p < 0.05, &&p < 0.001 vs. same group of the previous week.
The pre-exercise ITT showed that the DIO animals had insulin resistance (p > 0.05) (Table 2). The results demonstrated that HFD can lead to a loss of glucose uptake even with an external insulin stimulus (2U/kg body weight). The RE (p < 0.05), even with HFD intake, was able to delay the progression of the disease, maintaining a better glucose decay rate when compared to the sedentary DIO group (p < 0.01). There was no significant difference between pre- and post-exercise in any group (Figure 1D).
Table 2.
Insulin tolerance test (ITT) – blood glucose curve
| Time of collection (Glucose mg/dL and variation in relation to time 0 min) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0 min | 5 min | 10 min | 15 min | 20 min | 25 min | 30 min | ||
| Pre-exercise | SD | 154.3 | 139.2 (-15.0) | 98.7 (-55.5) | 77.5 (-76.8) | 65.2 (-89.0) | 56.7 (-97.5) | 43.2 (-111.0) |
| SD + RE | 129.3 | 134.7 (+5.5) | 57.7 (-71.5) | 54.7 (-74.5) | 46.7 (-82.5) | 35.3 (-94.0) | 25.5 (-103.8) | |
| DIO | 191.0† | 186.0 (-5.0) | 125.5 (-65.5) | 118.0 (-73.0) | 106.5 (-84.5)† | 101.3 (-89.8)† | 99.0 (-92.0)† | |
| DIO + RE | 163.8*† | 164.5 (+0.8) | 128.0 (-35.8)† | 115.2 (-48.5)† | 107.3 (-56.5)† | 100.8 (-63.0)† | 99.5 (-64.3)† | |
| Post-exercise | SD | 129.0 | 97.5 (-31.5) | 71.0 (-58.0) | 54.5 (-74.5) | 59.0 (-70.0) | 37.5 (-91.5) | 27.0 (-102.0) |
| SD + RE | 122.3 | 102.0 (-20.3) | 66.2 (-56.0) | 53.5 (-68.8) | 42.0 (-80.3)* | 23.0 (-99.3) | 15.8 (-106.5) | |
| DIO | 150.8†‡ | 120.7 (-30.0)‡ | 110.5 (-40.3)† | 105.5 (-45.3)† | 97.0 (-53.8)† | 94.0 (-56.8)† | 92.0 (-58.8)† | |
| DIO + RE | 127.8*‡ | 133.5 (+5.8) | 93.7 (-34.0) | 68.0 (-59.8)‡ | 64.3 (-63.5)*‡ | 58.5 (-69.3)*†‡ | 46.5 (-81.3)*†‡ | |
Source: study data. Pre-adaptation/exercise (week 17) and post-exercise (week 26). Blood glucose was measured after 6 hours of fasting (data from the table) at times 0 min (baseline), followed by an intraperitoneal insulin injection (2 U/kg) and measurements at times 5-30 min.
p < 0.05 versus respective untrained;
p < 0.05 versus respective standard diet;
p < 0.05 versus respective pre-exercise. SD: standard diet; RE: resistance exercise; DIO: diet-induced obesity.
DCFH oxidation
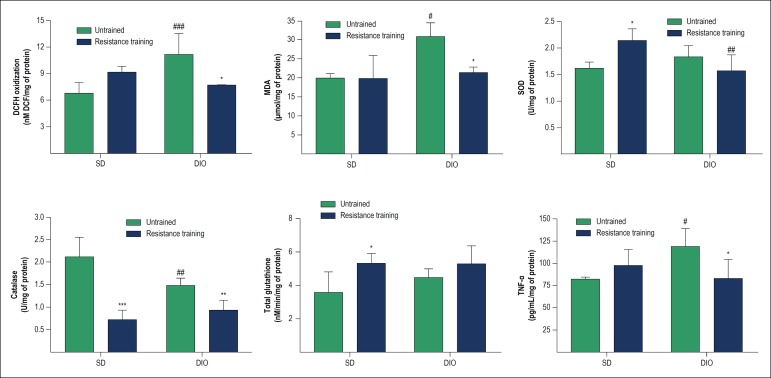
DCFH oxidation levels were measured as indicators of the reactive species production, especially hydrogen peroxide, and the results showed that in untrained animals, DIO caused an increase in DCF levels (p < 0.001) in comparison to animals with SD. HFD-fed animals submitted to resistance training (DIO + RE) showed a significant decrease in DCF levels compared to the DIO group (p < 0.01) (Figure 2A).
Figure 2.
Redox balance and inflammatory parameters in cardiac tissue of animals fed standard or high-fat diet and subsequently submitted to resistance training. A - DCFH oxidation; B - MDA content; C - SOD enzyme activity; D - CAT enzyme activity; E - Total glutathione content (reduced and oxidized); F - levels of TNF-α. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. respective untrained; #p < 0.05, ##p < 0.01 and ###p < 0.001 vs. respective SD.
Lipoperoxidation
As shown in Figure 2B, MDA levels suggest an increase in lipoperoxidation in DIO animals (p < 0.05), with reversion of the condition (p < 0.05) with resistance training (DIO + RE).
SOD Activity
The results observed in Figure 2C show that DIO did not alter SOD activity, but resistance training was able to increase its activity in the SD + RE group (p < 0.05), an increase not observed when exercise was performed in the obese group.
CAT Activity
The results observed in Figure 2D show a reduction in CAT activity in trained animals (SD + RE, p < 0.001 and SD, DIO + RE, p < 0.01 vs. untrained animals). On the other hand, the HFD animals also showed a decrease in CAT (p < 0.05), but only when compared to the SD animals.
GSH
Total glutathione levels were not significantly altered in both the interventions used in the present study (diet and exercise) (Figure 2E).
Inflammatory Parameter
The levels of TNF-α were used as an inflammatory indicator in cardiac tissue and the results observed in figure 2F showed an increase in TNF-α content in DIO animals (p < 0.05), which was significantly reduced (p < 0.05) after the intervention with resistance physical exercise (DIO + RE).
Discussion
Studies have shown that the consumption of a diet rich in fat, concomitant with a sedentary lifestyle, can trigger several health problems21,22 with a significant impact on the cardiovascular system. Therefore, experimental studies have been used to study the cellular effects of a high fat diet.6,23,24 The results of body weight and ITT showed that the adopted experimental model was effective in increasing weight and causing insulin resistance. Increased body weight has been associated to inflammatory changes and oxidative stress, and both these alterations to insulin resistance in skeletal muscle,25,26 but recent studies have also shown that cardiac cells are also susceptible to weight gain, by elevating inflammatory mediators and oxidative stress.6,23,24,27,28 In this context, previous studies suggested an important role of physical exercise, specifically aerobic or endurance, on the biochemical and molecular changes occurring in the myocardium as the result of a diet rich in fat.3,7 However, these effects depend on the characteristics of the exercise, such as duration, frequency, intensity and type.
The initial results of our study show that DIO animals have elevated levels of DCF, an indirect indicator of hydrogen peroxide production.29 These data were also observed by a recent study published in 2017 by Zeng et al.23 The authors showed high myocardial susceptibility to oxidative stress, with a significant increase in DCFH oxidation, both in vitro and in vivo, mediated by a high-fat diet. These increased DCF values, observed in DIO animals, were significantly reduced after resistance training, which suggests an important role of this type of training in the regulation of cell oxidant levels. Such effect may be associated to the fact that resistance training has a modulatory role on endogenous antioxidant enzymes. This observation is based on previous studies of our group in other experimental models of inflammation, which show the important role of resistance training on the enzymatic antioxidant system in different tissues.30,31
SOD and CAT are two enzymes that act synergistically in the formation (via superoxide radical dismutation) and catalysis of hydrogen peroxide, respectively. DIO animals showed no changes in SOD activity, suggesting that an increased production of DCF may be associated with other stimuli independent from SOD. One of the factors responsible for it is that, although the oxidation of DCFH to DCF is widely used as an indicator of hydrogen peroxide production, studies have observed that DCFH can also be oxidized by other reactive species, on a smaller scale, such as hydroxyl, peroxyl, nitric oxide and peroxide nitrite.29 It is also noteworthy that the formation of hydrogen peroxide is not totally dependent on SOD activity. On a smaller scale, auto-oxidation events of biomolecules also contribute to the formation of hydrogen peroxide.32 These conditions would limit SOD activity, which may justify the results found.
Catalases constitute a group of enzymes that catalyze the decomposition of hydrogen peroxide into water and oxygen. Our results show a reduction in the activity of this enzyme after resistance training in the group exposed to SD. As observed, SOD activity was increased in this same group, thus generating higher levels of hydrogen peroxide. However, the decrease in enzyme activity suggests a lower hydrogen peroxide catalysis, but it is worth noting that hydrogen peroxide can be catalyzed under these conditions by other cell detoxification systems, such as glutathione and peroxins,33 which could justify our results since the glutathione system showed an increase in this group (SD + RE). Furthermore, we observed reduced levels of CAT after resistance training in the DIO + RE group and, therefore, considering that the diet significantly increases the production of cell oxidants such as hydrogen peroxide, a reduced CAT activity could have an impact on the possible oxidative damages in the myocardium, if hydrogen peroxide were not catalyzed by other aforementioned systems (not investigated in the present study, although they deserve attention in future studies).
Aiming to observe the effects of RE on oxidative damage in the myocardium induced by the DIO model, we evaluated the levels of MDA, a byproduct of lipoperoxidation, and observed that DIO animals showed greater lipid damage in relation to the SD group and that the RE was able to reverse these effects. These effects of the DIO model on lipoperoxidation levels were also observed in a study with BL6/C57 mice performed by Muthulakshmi and Saravanan (2013).34 Positive RE results are possibly associated with the exercise capacity to promote the modulation of antioxidant systems, in addition to the activity of primary antioxidant enzymes such as SOD and CAT. One of the mechanisms that can be mediated by RE is the Nuclear Factor Erythroid 2 (NRF2) translocation to the nucleus and expression of several antioxidant enzymes such as NADH: quinone oxidoreductase 1 (NQO1) and Heme Oxygenase-1 (HO1). which help to detoxify the biological system and contribute to the reduction of oxidative stress.35 Merry and Ristow (2016)36 suggest that exercise may stimulate NRF2 translocation to functionally regulate mitochondrial biogenesis of skeletal muscle and the expression of antioxidants defense genes. Although these results are obtained from aerobic training and in skeletal muscle, it is believed that such effects may also occur via resistance training, as resistance training activates the Adenosine Monophosphate-Activated Protein Kinase (AMPK)37, which enhances the phosphorylation of NRF2 in the cell and increases the level of phosphorylated NRF2 in the nucleus.38
The increased production of oxidants in the myocardium can be mediated by a possible inflammatory response induced by obesity/HFD with the secretion of different mediators. In this context, TNF-α is a mediator sensitive to the HFD model that shows a wide range of pro-inflammatory actions. Our results showed a significant increase in TNF-α levels in DIO animals with a consequent reduction after resistance training. Increased levels of TNF-α in the myocardium induced by a HFD have also been observed in previous studies.3,28 The effects of exercise may be related to the fact that aerobic and resistance exercises promote increased secretion of anti-inflammatory cytokines and regulate TNF-α levels.30,31 During the exercise, the muscles release myosins, which are involved in tissue growth, repair and anti-inflammatory responses.39 IL-6 is the primary myosin released in response to exercise and increases IL-10 and decreases TNF-α levels.40 IL-10 reduces cardiac dysfunction by decreasing cardiac fibrosis.39 In this scenario, HFD-induced obesity decreases IL-10 protein levels, but physical training significantly increases the IL-10 levels in cardiac tissues.3
According to data from previous studies, the control of TNF-α secretion in the myocardium by RE is considered an important factor in the cardioprotection mechanisms related to oxidative stress.
Conclusion
Our results showed an important effect of RE on the control/stabilization of body weight even without food intake control. It was also demonstrated that there is a redox alteration in the cardiac tissue with an obesity model, but it does not seem to be mediated mainly by the classic antioxidant production and control of hydrogen peroxide, but rather by other reactive species. However, RE was able to reverse lipid damage and the production of reactive species, even with the consumption of a HFD, as well as positively modulate one of the main cytokines responsible for the activation of the inflammatory process.
Therefore, RE can be a great ally in the health process regarding the therapeutic approach to obesity. Some limitations found in these studies were the data related to the quantification of serum insulin to better confirm insulin resistance, and the evaluation of other molecules that can alter the cell redox balance. These tests were not performed for technical reasons.
Finally, other studies should be performed aiming to better explain how RE promotes these effects, particularly in the regulation of reactive species such as hydroxyl, peroxyl, nitric oxide and peroxide nitrite, as well as other inflammatory and anti-inflammatory parameters in cardiac tissue.
Footnotes
Sources of Funding
This study was funded by CNPq and Capes.
Study Association
This article is part of the thesis of Doctoral submitted by Pauline Souza Effting, from Universidade do Extremo Sul Catarinense.
Ethics approval and consent to participate
This study was approved by the Ethics Committee on Animal Experiments of the Universidade do Extremo Sul Catarinense under the protocol number 067/2014-2.
Author contributions
Conception and design of the research: Ceddia RB, Pinho RA; Acquisition of data: Brescianini SMS, Fernandes BB, Fidelis GSP, da Silva PRL, Nesi RT; Analysis and interpretation of the data: Effting PS, Brescianini SMS, Silveira PCL, Nesi RT, Pinho RA; Statistical analysis: Effting PS, Silveira PCL, Pinho RA; Obtaining financing: Pinho RA; Writing of the manuscript: Effting PS, Pinho RA; Critical revision of the manuscript for intellectual content: Ceddia RB, Pinho RA.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
References
- 1.World Healt Organization. Global Health Observatory . Overweight and obesity [Internet] Copenhagen: WHO; 2018. [30 jul. 2018]. Disponível em: http://www.who.int/gho/ncd/risk_factors/overweight_obesity/obesity_adults/en/ [Google Scholar]
- 2.Fernández-Sánchez A, Madrigal-Santillán E, Bautista M, Esquivel-Soto J, Morales-González Á, Esquivel-Chirino C, et al. Inflammation, oxidative stress, and obesity. Int J Mol Sci. 2011;12(5):3117–3132. doi: 10.3390/ijms12053117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kesherwani V, Chavali V, Hackfort BT, Tyagi SC, Mishra PK. Exercise ameliorates high fat diet induced cardiac dysfunction by increasing interleukin 10. Front Physiol. 2015 Apr 22;6:124–124. doi: 10.3389/fphys.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakurai T, Izawa T, Kizaki T, Ogasawara J, Shirato K, Imaizumi K, et al. Exercise training decreases expression of inflammation-related adipokines through reduction of oxidative stress in rat white adipose tissue. Biochem Biophys Res Commun. 2009;379(2):605–609. doi: 10.1016/j.bbrc.2008.12.127. [DOI] [PubMed] [Google Scholar]
- 5.Adeghate E, Singh J. Structural changes in the myocardium during diabetes-induced cardiomyopathy. Heart Fail Rev. 2014;19(1):15–23. doi: 10.1007/s10741-013-9388-5. [DOI] [PubMed] [Google Scholar]
- 6.Gamez-Mendez AM, Vargas-Robles H, Ríos A, Escalante B. Oxidative stress-dependent coronary endothelial dysfunction in obese mice. PLoS One. 2015;10(9):e0138609. doi: 10.1371/journal.pone.0138609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Touati S, Montezano AC, Meziri F, Riva C, Touyz RM, Laurant P. Exercise training protects against atherosclerotic risk factors through vascular NADPH oxidase, extracellular signal-regulated kinase 1/2 and stress-activated protein kinase/c-Jun N-terminal kinase downregulation in obese rats. Clin Exp Pharmacol Physiol. 2015;42(2):179–185. doi: 10.1111/1440-1681.12338. [DOI] [PubMed] [Google Scholar]
- 8.Sharma NM, Rabeler B, Zheng H, Raichlin E, Patel KP. Exercise training attenuates upregulation of p47(phox) and p67(phox) in hearts of diabetic rats. Oxid Med Cell Longev. 2016;2016:5868913–5868913. doi: 10.1155/2016/5868913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boardman NT, Hafstad AD, Lund J, Rossvoll L, Aasum E. Exercise of obese mice induces cardioprotection and oxygen sparing in hearts exposed to high-fat load. Am J Physiol Circ Physiol. 2017;313(5):H1054–H1062. doi: 10.1152/ajpheart.00382.2017. [DOI] [PubMed] [Google Scholar]
- 10.Di Meo S, Iossa S, Venditti P. Improvement of obesity-linked skeletal muscle insulin resistance by strength and endurance training. J Endocrinol. 2017;234(3):R159–R181. doi: 10.1530/JOE-17-0186. [DOI] [PubMed] [Google Scholar]
- 11.Hornberger TA Jr, Farrar RP. Physiological hypertrophy of the FHL muscle following 8 weeks of progressive resistance exercise in the rat. Can J Appl Physiol. 2004;29(1):16–31. doi: 10.1139/h04-002. [DOI] [PubMed] [Google Scholar]
- 12.Scheffer DL, Silva LA, Tromm CB, da Rosa GL, Silveira PC, de Souza CT, et al. Impact of different resistance training protocols on muscular oxidative stress parameters. Appl Physiol Nutr Metab. 2012;37(6):1239–1246. doi: 10.1139/h2012-115. [DOI] [PubMed] [Google Scholar]
- 13.Vilela TC, Effting PS, Dos Santos PG, Farias H, Paganini L, Rebelo SH, et al. Aerobic and strength training induce changes in oxidative stress parameters and elicit modifications of various cellular components in skeletal muscle of aged rats. Exp Gerontol. 2018 Jun;106:21–27. doi: 10.1016/j.exger.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Ayala JE, Samuel VT, Morton GJ, Obici S, Croniger CM, Shulman GI, et al. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech. 2010;3(9-10):525–534. doi: 10.1242/dmm.006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 07;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 16.Dong J, Sulik KK, Chen SY. The role of NOX enzymes in ethanol-induced oxidative stress and apoptosis in mouse embryos. Toxicol Lett. 2010;193(1):94–100. doi: 10.1016/j.toxlet.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244(22):6049–6055. [PubMed] [Google Scholar]
- 18.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 19.Hissin PJ, Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem. 1976;74(1):214–226. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- 20.Grotto D, Santa Maria LD, Boeira S, Valentini J, Charão MF, Moro AM, et al. Rapid quantification of malondialdehyde in plasma by high performance liquid chromatography-visible detection. J Pharm Biomed Anal. 2007;43(2):619–624. doi: 10.1016/j.jpba.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 21.Barba I, Miró-Casas E, Torrecilla JL, Pladevall E, Tejedor S, Sebastián-Pérez R, et al. High-fat diet induces metabolic changes and reduces oxidative stress in female mouse hearts. J Nutr Biochem. 2017 Feb;40:187–193. doi: 10.1016/j.jnutbio.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Carbone S, Mauro AG, Mezzaroma E, Kraskauskas D, Marchetti C, Buzzetti R, et al. A high-sugar and high-fat diet impairs cardiac systolic and diastolic function in mice. Int J Cardiol. 2015 Nov 01;198:66–69. doi: 10.1016/j.ijcard.2015.06.136. [DOI] [PubMed] [Google Scholar]
- 23.Zeng C, Zhong P, Zhao Y, Kanchana K, Zhang Y, Khan ZA, et al. Curcumin protects hearts from FFA-induced injury by activating Nrf2 and inactivating NF-κB both in vitro and in vivo. J Mol Cell Cardiol. 2015 Feb;79:1–12. doi: 10.1016/j.yjmcc.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Chen F, Chen D, Zhao X, Yang S, Li Z, Sanchis D, et al. Interleukin-6 deficiency facilitates myocardial dysfunction during high fat diet-induced obesity by promoting lipotoxicity and inflammation. Biochim Biophys Acta Mol Basis Dis. 2017;1863(12):3128–3141. doi: 10.1016/j.bbadis.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 25.Farias JM, Bom KF, Tromm CB, Luciano TF, Marques SO, Tuon T, et al. Effect of physical training on the adipose tissue of diet-induced obesity mice: interaction between reactive oxygen species and lipolysis. Horm Metab Res. 2013;45(3):190–196. doi: 10.1055/s-0032-1323740. [DOI] [PubMed] [Google Scholar]
- 26.Pinho RA, Sepa-Kishi DM, Bikopoulos G, Wu M V, Uthayakumar A, Mohasses A, et al. High-fat diet induces skeletal muscle oxidative stress in a fiber type-dependent manner in rats. Free Radic Biol Med. 2017 Sep;110:381–389. doi: 10.1016/j.freeradbiomed.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Pieri BL, Souza DR, Luciano TF, Marques SO, Pauli JR, Silva AS, et al. Effects of physical exercise on the P38MAPK/REDD1/14-3-3 pathways in the myocardium of diet-induced obesity rats. Horm Metab Res. 2014;46(9):621–627. doi: 10.1055/s-0034-1371824. [DOI] [PubMed] [Google Scholar]
- 28.Farhangi MA, Nameni G, Hajiluian G, Mesgari-Abbasi M. Cardiac tissue oxidative stress and inflammation after vitamin D administrations in high fat- diet induced obese rats. BMC Cardiovasc Disord. 2017;17(1):161–161. doi: 10.1186/s12872-017-0597-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X, Zhong Z, Xu Z, Chen L, Wang Y. 2',7'-Dichlorodihydrofluorescein as a fluorescent probe for reactive oxygen species measurement: forty years of application and controversy. Free Radic Res. 2010;44(6):587–604. doi: 10.3109/10715761003709802. [DOI] [PubMed] [Google Scholar]
- 30.Tuon T, Souza PS, Santos MF, Pereira FT, Pedroso GS, Luciano TF, et al. Physical training regulates mitochondrial parameters and neuroinflammatory mechanisms in an experimental model of Parkinson's disease. Oxid Med Cell Longev. 2015;2015:261809–261809. doi: 10.1155/2015/261809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Souza PS, Gonçalves ED, Pedroso GS, Farias HR, Junqueira SC, Marcon R, et al. Physical exercise attenuates experimental autoimmune encephalomyelitis by inhibiting peripheral immune response and blood-brain barrier disruption. Mol Neurobiol. 2017;54(6):4723–4737. doi: 10.1007/s12035-016-0014-0. [DOI] [PubMed] [Google Scholar]
- 32.Yi Y, Wang L, Li G, Guo H. A review on research progress in the direct synthesis of hydrogen peroxide from hydrogen and oxygen: noble-metal catalytic method, fuel-cell method and plasma method. Catal Sci Technol. 2016;6(6):1593–1610. [Google Scholar]
- 33.Lennicke C, Rahn J, Lichtenfels R, Wessjohann LA, Seliger B. Hydrogen peroxide - production, fate and role in redox signaling of tumor cells. Cell Commun Signal. 2015 Sep 14;13:39–39. doi: 10.1186/s12964-015-0118-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muthulakshmi S, Saravanan R. Protective effects of azelaic acid against high-fat diet-induced oxidative stress in liver, kidney and heart of C57BL/6J mice. Mol Cell Biochem. 2013;377(1-2):23–33. doi: 10.1007/s11010-013-1566-1. [DOI] [PubMed] [Google Scholar]
- 35.Done AJ, Traustadóttir T. Nrf2 mediates redox adaptations to exercise. Redox Biol. 2016 Dec;10:191–199. doi: 10.1016/j.redox.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merry TL, Ristow M. Nuclear factor erythroid-derived 2-like 2 (NFE2L2, Nrf2) mediates exercise-induced mitochondrial biogenesis and the anti-oxidant response in mice. J Physiol. 2016;594(18):5195–5207. doi: 10.1113/JP271957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williamson D, Gallagher P, Harber M, Hollon C, Trappe S. Mitogen-activated protein kinase (MAPK) pathway activation: effects of age and acute exercise on human skeletal muscle. J Physiol. 2003;547(3):977–987. doi: 10.1113/jphysiol.2002.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joo MS, Kim WD, Lee KY, Kim JH, Koo JH, Kim SG. AMPK facilitates nuclear accumulation of Nrf2 by phosphorylating at serine 550. Mol Cell Biol. 2016;36(14):1931–1942. doi: 10.1128/MCB.00118-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petersen AMW, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98(4):1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 40.Benatti FB, Pedersen BK. Exercise as an anti-inflammatory therapy for rheumatic diseases-myokine regulation. Nat Rev Rheumatol. 2015;11(2):86–97. doi: 10.1038/nrrheum.2014.193. [DOI] [PubMed] [Google Scholar]