Abstract
Objective
To evaluate the cost-effectiveness of neuromuscular ultrasound (NMUS) for the evaluation of focal neuropathies.
Methods
A prior prospective, randomized, double-blind controlled trial demonstrated that NMUS, when added to electrodiagnostic testing, resulted in improved clinical outcomes after 6 months of follow-up. From this study, we abstracted quality-adjusted life-years (QALYs) from the 36-item Short Form Health Survey and entered this health-utility estimate into a mixed trial and model-based cost-effectiveness analysis from the societal perspective. Costs of intervention (NMUS) were estimated from Medicare payment rates for Current Procedural Terminology codes. Health care use was otherwise estimated to be equal, but sensitivity analyses further examined this and other key assumptions. Incremental cost-effectiveness ratio (ICER) was used as the primary outcome with a willingness-to-pay threshold of $50,000 per QALY.
Results
The predicted mean health outcome associated with use of NMUS was 0.079 QALY, and the mean cost was $37, resulting in an ICER of $463 per QALY. Results and conclusions remained robust across all sensitivity analyses, including variations in time horizon, initial distribution of health states, costs, and effectiveness.
Conclusions
From a societal perspective, the addition of NMUS to electrodiagnostic testing when evaluating a focal neuropathy is cost-effective. A study of longer follow-up incorporating total health care use would further quantify the value of NMUS.
ClinicalTrials.gov identifier:
Neuromuscular ultrasound (NMUS) is a diagnostic technique in which ultrasound is used to image the peripheral nervous system to assist in the diagnosis of a variety of neuromuscular conditions, and it is often used in combination with electrodiagnostic testing.1 NMUS has been demonstrated to be valid, reliable, sensitive, and specific for the evaluation of focal neuropathies,2–4 and it is more accurate than MRI when used to assess potential pathology in sonographically accessible regions.5 The use of NMUS in the most common focal neuropathy, carpal tunnel syndrome (CTS), is supported by a clinical practice guideline.6 In addition, the use of NMUS in the evaluation of focal neuropathies has been demonstrated to improve outcomes after 6 months of follow-up in a prospective, randomized, double-blind clinical trial.7 The goal of this current study is to use the data gathered in the previous clinical trial to determine whether NMUS is cost-effective, from a societal perspective, when combined with electrodiagnosis for the evaluation of focal neuropathies.
Methods
A detailed description of the original randomized, double-blind trial is reported elsewhere, and the trial is registered at ClinicalTrials.gov (NCT01394822).7 Briefly, between October 2011 and August 2013, all adult patients referred by nerve surgeons to the Wake Forest School of Medicine Diagnostic Neurology Laboratory were invited to participate if their clinical evaluation and electrodiagnostic testing were consistent with a focal neuropathy. Institutional review board approval was obtained before the original study, and all participants provided signed informed consent. The original study was designed to allow the investigators to break the blind if a potentially life-threatening condition was identified during the ultrasound study and the patient was randomized not to have the report sent, although this did not occur during the study. One hundred twenty individuals were randomized to “report sent” or “not sent” groups, depending on whether the NMUS report was sent to the referring surgeon. Of these, 100 participants completed the 6-month follow up (51 in report sent group and 49 in not sent group). Baseline, 3-month, and 6-month outcomes were measured, including the Inflammatory Cause and Treatment Overall Disability Sum Score, 36-Item Short-Form Health Survey (SF-36), Medical Research Council strength grading, sensory testing, and patient satisfaction. Results from this study were used as health-utility estimates to conduct a mixed trial and model-based cost-effectiveness analysis from the societal perspective.
Model inputs: Intervention effects
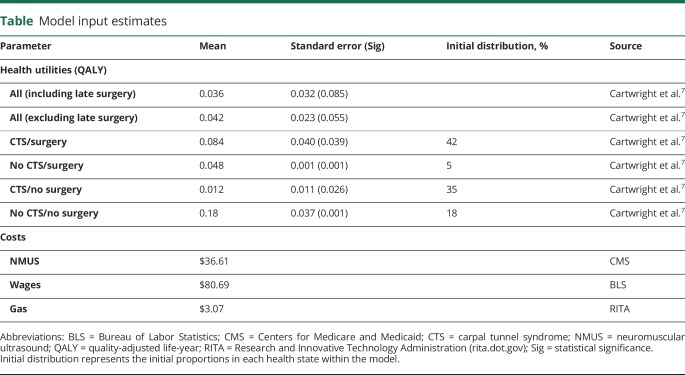
Measures of health-related quality of life such as the SF-368 are frequently used in clinical research but are not designed for economic evaluation. Quality-adjusted life-year9 (QALY) is the summary health outcome metric of choice and considers both quality and quantity of life. Through assessing QALYs and costs, we can hold interventions economically accountable on a level playing field in a world with limited resources. SF-6D10 is a published algorithm that converts SF-36 into QALYs, allowing scrutiny of any intervention with associated SF-36 data. This allowed us to quantify the differences between the NMUS report sent and not sent groups using the QALY paradigm. A priori, we excluded 21 patients who had surgery within the 3-month period before the final visit to prevent contamination by reduced postsurgical quality of life. Once QALY data had been abstracted at each time point, the report sent and not sent groups were compared by use of a generalized estimating equation, with a priori unstructured working correlation and model-based covariance matrices. The difference between the NMUS report sent and not sent groups was analyzed in several important subgroups who were used as the model inputs (table), including those who had surgical intervention, those with CTS, and combinations thereof.
Table.
Model input estimates
Model inputs: Intervention costs
Direct and indirect costs are detailed in the table. NMUS cost was based on Current Procedural Terminology codes obtained from the Centers for Medicare and Medicaid (cms.gov; 2017 conversion factor $35.89) in US dollars (Current Procedural Terminology code 76882, $36.61). Private insurance costs were not included because Medicare costs most closely represent the opportunity cost of resources in society.11 Wages were derived from the Bureau of Labor Statistics (bls.gov; $23.86/h, 3.5 hours) and adjusted for the unemployment rate for a 53-year-old individual (bls.gov, 3.375%). Transportation costs were based on gasoline at $2.25 per gallon, with a 30-mile round trip at 22 miles per gallon (rita.dot.gov). Patient time, caregiver time, and extra travel were not included in the model because NMUS was added at the time of electrodiagnostic testing. Productivity and other indirect costs are assumed equal, and productivity is typically included in QALYs.11 For a hypothetical separate NMUS visit, wages lost and transportation costs were included. Direct costs of electrodiagnostic testing were assumed equivalent.
Cost-effectiveness analysis
The health-utility estimates (QALY change) obtained from the general estimating equation model were combined with cost data to perform the analyses using TreeAge Pro (TreeAge Software Inc, Williamstown, MA). The time horizon was modeled as 3 years, with a 50%/y convergence rate. This implies that any relative benefits gained from having the ultrasound report sent diminish by 50% each year and are zero by year 4. The base case was set as a 53-year-old (mean trial age) with a life expectancy of 29 years (based on ssa.gov data). Discounting for costs was not required, and mortality was accounted for with published rates (ssa.gov).
One-way sensitivity analyses included variation of effectiveness by 3 standard errors, variation in the number of participants undergoing surgery (25%–75%), variation in costs beyond the willingness-to-pay threshold, and variation of a time horizon between 6 months and 3 years. Two-way sensitivity analyses assessed the interaction between effectiveness and costs. Incremental cost-effectiveness ratio (ICER) in US dollars was the primary outcome. ICER is used to define cost-effectiveness in health care and represents the cost of gaining 1 QALY when 1 intervention is compared to another. When this cost is below the willingness-to-pay threshold, it can be used to justify use of the intervention. The willingness-to-pay threshold used in these analyses was set at $50,000 per QALY.
Data availability
Anonymized data will be shared by request with qualified investigators.
Results
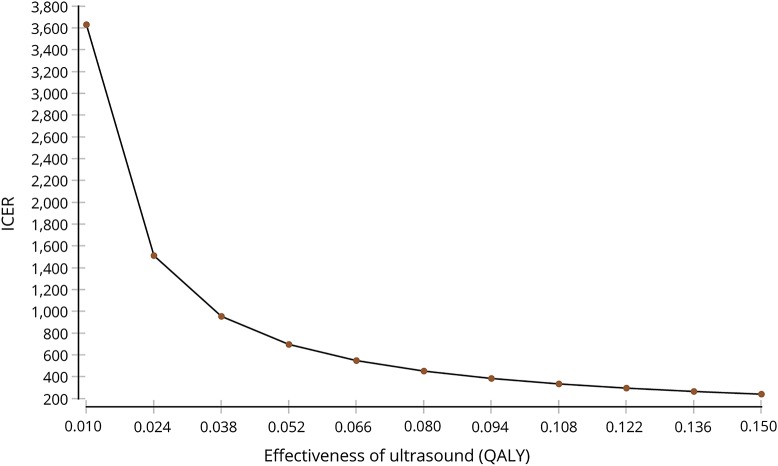
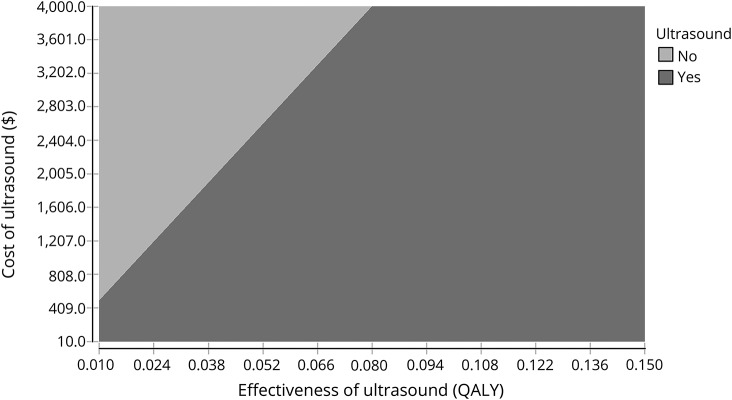
The main analysis demonstrates NMUS as cost-effective with an ICER of $463. One-way effectiveness sensitivity analyses (figure 1) demonstrates that the ICER remains within the willingness-to-pay threshold when effectiveness is reduced by 3 standard errors (0.011 QALY, ICER $3,454), which covers the scenario of a 6-month time horizon (equivalent to effectiveness of 0.025 QALY, ICER $1,464). One-way cost sensitivity analyses revealed NMUS costs can increase to $3,913 before being considered not cost-effective, a range that covers a separate visit for diagnostic testing (indirect cost $84, ICER $1,524). The dominance of NMUS under most conditions is graphically displayed in the 2-way cost-effectiveness sensitivity analyses (figure 2). Variation of the proportion who had surgery showed that ICER increases minimally as the proportion decreases (ICER $383 at 75%, $646 at 25%).
Figure 1. Effectiveness sensitivity analysis.
ICER = incremental cost-effectiveness ratio; QALY = quality-adjusted life-year.
Figure 2. Cost and effectiveness 2-way sensitivity analysis.
QALY = quality-adjusted life-year.
Discussion
The main analysis demonstrates an ICER of $463, representing the cost of gaining 1 QALY when NMUS is used in complement with electrodiagnostic testing for the evaluation of a focal neuropathy. This is far below our willingness-to-pay threshold of $50,000 and supports the cost-effectiveness of NMUS in this setting. While this is significant, it is important to note that the original study may not generalize because of the shortage of neurodiagnostic laboratories providing NMUS and because patients with focal neuropathies are not always referred by nerve surgeons. Other potential limitations include the health-utility inputs being based on a study that was not powered to detect changes in SF-36, which could be exacerbated further in abstracted scores such as the SF-6D, and that the study included few lower extremity focal neuropathies.
Despite these limitations, sensitivity analyses suggest that NMUS remains robustly cost-effective. Increasing costs to account for a second visit, as may be needed if NMUS is not completed at the time of neurophysiology testing, still does not approach the willingness-to-pay threshold. In fact, the costs associated with NMUS can increase from $36.61 to $3,913 before it is no longer cost-effective, which is an amount that could absorb sizable health care use and indirect costs not accounted for in this study, including potentially substantially greater private insurance charges. There is also uncertainty around the time horizon of effectiveness, chosen as convergent over 3 years, which is potentially a conservative estimate. Nevertheless, when effect duration is reduced to only 6 months, NMUS continues to be cost-effective with an ICER of $1,464. In addition, the effectiveness of NMUS may be significantly greater than our estimate if the 6-month study duration failed to capture the full benefit of NMUS.
The increase in health utility associated with NUMS was greatest in those who underwent surgical intervention, particularly in those with CTS. This may indicate that NMUS appropriately identifies individuals who will most benefit from surgical intervention, which is one of the goals of adding imaging to electrodiagnostic testing.
Further exploration of the use of NMUS in regard to cost-effectiveness is needed. Specifically, a prospective evaluation of all health care expenditures associated with NMUS would provide the optimal estimate of costs. In addition, including NMUS as the initial diagnostic test may decrease or eliminate the need for some electrodiagnostic testing, which should also be explored through cost-effectiveness research.
Glossary
- CTS
carpal tunnel syndrome
- ICER
incremental cost-effectiveness ratio
- NMUS
neuromuscular ultrasound
- QALY
quality-adjusted life-year
- SF-36
36-Item Short-Form Health Survey
Appendix. Authors
Footnotes
Editorial, page 1081
Study funding
No targeted funding reported.
Disclosure
R. Mandeville performs NMUS in his clinical practice (5% effort) and bills for this procedure. A. Wali is funded through the NIH TL1 predoctoral grants 1TL1TR001443 and TL1TR00098. C. Park is funded through the NIH TL1 predoctoral grants 1TL1TR001443 and TL1TR00098. E. Groessl reports no disclosures relevant to the manuscript. F. Walker performs NMUS in his clinical practice (5% effort) and bills for this procedure and receives royalties from the publication of Neuromuscular Ultrasound. M. Cartwright had funding from the NIH/National Institute of Neurological Disorders and Stroke (1K23NS062892) to study NMUS, performs NMUS in his clinical practice (5% effort) and bills for this procedure, and receives royalties from the publication of Neuromuscular Ultrasound. Go to Neurology.org/N for full disclosures.
References
- 1.Walker FO, Alter KE, Boon AJ, et al. . Neuromuscular Ultrasound Qualifications: AANEM Position Statement [online]. American Association of Neuromuscular & Electrodiagnostic Medicine; 2009. Available at: aanem.org/getmedia/d0c7eb14-8a11-4b1a-ae33-1d9601cef2cf/Neuromuscular_Ultrasound_CLEAN.pdf.aspx. Accessed October 4, 2017. [Google Scholar]
- 2.Walker FO, Cartwright MS, Wiesler ER, Caress J. Ultrasound of nerve and muscle. Clin Neurophysiol 2004;115:495–507. [DOI] [PubMed] [Google Scholar]
- 3.Padua L, Aprile I, Pazzaglia C, et al. Contribution of ultrasound in a neurophysiological lab in diagnosing nerve impairment: a one-year systematic assessment. Clin Neurophysiol 2007;118:1410–1416. [DOI] [PubMed] [Google Scholar]
- 4.Cartwright MS, Walker FO. Neuromuscular ultrasound in common entrapment neuropathies. Muscle Nerve 2013;48:696–704. [DOI] [PubMed] [Google Scholar]
- 5.Zaidman CM, Seelig MJ, Baker JC, Mackinnon SE, Pestronk A. Detection of peripheral nerve pathology. Neurology 2013;80:1634–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cartwright MS, Hobson-Webb LD, Boon AJ, et al. Evidence-based guideline: neuromuscular ultrasound for the diagnosis of carpal tunnel syndrome. Muscle Nerve 2012;46:287–293. [DOI] [PubMed] [Google Scholar]
- 7.Cartwright MS, Griffin LP, Dowlen H, et al. A randomized trial of diagnostic ultrasound to improve outcomes in focal neuropathies. Muscle Nerve 2015;52:746–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ware JE, Sherbourne CD. The MOS 36-ltem Short-Form Health Survey (SF-36), I: conceptual framework and item selection. Med Care 1992;30:473–483. [PubMed] [Google Scholar]
- 9.Lipscomb J, Drummond M, Fryback D, Gold M, Revicki D. Retaining, and enhancing, the QALY. Value Health 2009;12:S18–S26. [DOI] [PubMed] [Google Scholar]
- 10.Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271–292. [DOI] [PubMed] [Google Scholar]
- 11.Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG. Cost-Effectiveness in Health and Medicine, 2nd ed. Oxford: Oxford University Press; 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request with qualified investigators.