Abstract
Thrombospondins (TSPs) are cell adhesion molecules that play an important role in the maintenance of hearing and afferent synaptic connections. Based on their reported function in restoring synaptic connections after stroke, we tested a potential role for TSP1 and TSP2 genes in repairing cochlear synapses following noise injury. We observed a tonotopic gradient in the expression of TSP1 and TSP2 mRNA in control mouse cochleae and an upregulation of these genes following noise exposure. Examining the functional sequelae of these changes revealed that afferent synaptic counts and auditory brainstem responses (ABRs) in noise-exposed TSP1 and TSP2 knockout (−/−) mice exhibited a worst recovery when compared to controls. Consistent with their tonotopic expression, TSP1−/− mice showed greater susceptibility to noise-induced hearing loss (NIHL) at 8kHz and 16kHz frequencies, whereas NIHL in TSP2−/− mice occurred only at mid and high frequencies. Further analysis of the ABR waveforms indicated peripheral neuronal damage in TSP2−/− but not in TSP1−/− mice. Noise trauma affecting mid to high frequencies triggered severe seizures in the TSP2−/− mice. We found that decreased susceptibility to audiogenic seizures in TSP1−/− mice was correlated with increased TSP2 protein levels in their inner ears, suggesting that TSP2 might functionally compensate for the loss of TSP1 in these mice. Our data indicate that TSP1 and TSP2 are both involved in susceptibility to NIHL, with TSP2 playing a more prominent role.
Keywords: Auditory system, auditory synaptopathy, audiogenic seizure, noise-damaged
Introduction
Noise-induced hearing loss (NIHL) is a complex condition that is a growing epidemic worldwide. With over 1.6 million new cases every year (Leigh et al., 1999), NIHL is the second leading cause of sensorineural hearing loss after presbycusis (Stucken and Hong, 2014). Recent reports point to a significant genetic component in the susceptibility to NIHL since not all individuals exposed to similar noise levels are affected to the same extent (Sliwinska-Kowalska and Pawelczyk, 2013). In addition, strain-specific variation in noise trauma sensitivity has been demonstrated in mouse models of NIHL (Heinonen-Guzejev et al., 2005). While the extent of NIHL prevalence is well recognized, the multiple genetic factors underlying this condition are yet to be fully understood. Identifying these factors will be important in devising early diagnostic and intervention strategies for NIHL.
Several studies on animal experiments indicated that exposure to even moderate noise levels can result in immediate and irreversible damage to cochlear afferent synapses, also known as auditory synaptopathy (AS) (Lin et al., 2003; Kujawa and Liberman, 2009; Liberman et al., 2011; Hickox and Liberman, 2014). Other groups have reported that such noise damage to peripheral afferent input is associated with changes in central neuronal activity (Knipper et al., 2013), a feature observed in such deafness related conditions as hyperacusis and tinnitus. Converging evidence also points to similarities between the effects of moderate acoustic trauma experienced early in life and effects observed with age-related hearing loss (ARHL). For instance, both noise-exposed and aging rodents show considerable damaged or lost cochlear afferent synapses resulting in AS (Schmiedt, 1996; Furman et al., 2013). Therefore, understanding the genetic factors that contribute to NIHL susceptibility may also provide clues for the etiology of conditions such as tinnitus and ARHL. Our group previously reported that loss of the extracellular matrix proteins, thrombospondin 1 or 2 (TSP1 or TSP2), caused a defect in cochlear afferent synaptogenesis and resulted in progressive or ARHL in TSP1−/− and TSP2−/− mice, respectively (Mendus et al., 2014).
TSPs have also been shown to play an important role in repairing synaptic connections after brain injury (Lin et al., 2003; Liauw et al., 2008). Therefore, we hypothesized that these proteins are involved in responding to acoustic injury in the cochlea as well. Our data demonstrate a tonotopic trend within the cochlea for TSP1 and TSP2 gene expression and show elevation of TSP2 mRNA in the cochlea following noise exposure. Based on these results, we interrogated the role of TSP1 and TSP2 in the cochlear response to acoustic trauma by analyzing the differential response of TSP1 and TSP2 mutant mice to trauma. Given the tonotopic gradient in TSP expression, we hypothesized that the roles of TSP1 and TSP2 would be frequency-specific.
Results
Elevation in TSP2 mRNA levels in the cochlea following noise exposure
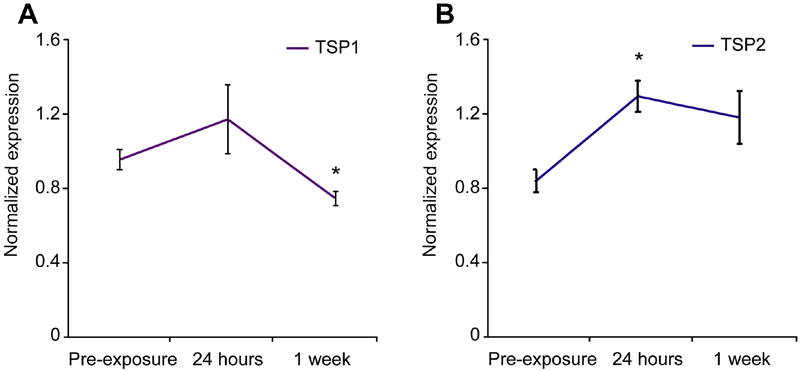
To investigate the potential role of TSP in protecting hearing upon noise injury, we exposed 3 month-old FVB mice (wild type (WT) control littermates of TSP mutants) to a 100 dB SPL noise ranging from 8–16 kHz (octave band) for 30 minutes. Previous studies have shown that this noise setting for a 2-hour duration causes reversible or temporary threshold shift (TTS) (Kujawa and Liberman 2009). For our experiment, we reduced the exposure time to 30 minutes because of the increased cochlear susceptibility of these mutants to noise injury. Moreover, we employed 3 month-old mice where expression of TSP1 and TSP2 genes was barely detectable in the cochlea (Mendus et al., 2014). qPCR of cochlear RNA revealed changes in TSP gene expression following noise exposure (Figure 1A,B). TSP2 mRNA was significantly upregulated 24 hours following exposure (p = 0.017), and it remained elevated a week after exposure (Figure 1B). TSP1 gene expression also showed a mild (but non-significant) elevation 24 hours following exposure that declined to significantly low levels (p=0.033) one week after noise trauma (Figure 1A). These data suggest important roles for TSP2 during the early phase and for TSP1 during the later phases after noise trauma.
Figure 1. Expression of TSP1 and TSP2 genes before and after noise exsposure (NE).
Quantitative RT-PCR was used to determine expression of TSP1 (A) TSP2 (B) genes in mouse cochlea before (pre-exposure) and after noise exposure (24 hours and 1 week). Significant upregulation of TSP2 (B), but not TSP1 (A), gene expression at 24 hours after noise exposure. TSP2 gene expression remains upregulated one week after noise (B). Results are expressed as mean ± standard error of the mean (SEM). Data shown is the expression of the gene of interest relative to the internal standard, GAPDH. Significant differences are indicated by * for p<0.05.
Synaptic loss in TSP mutants after noise exposure
TSPs were shown to play a role in repairing excitatory synaptic connectivity after stroke (Liauw et al., 2008). Based on these studies and our results above, we next tested the effect of noise trauma and the role of these genes in protecting cochlear afferent synapses. Previous studies have shown that despite the reversible hearing threshold following noise trauma, there can be a rapid (within 24h) and non-reversible loss of inner hair cell (IHC) afferent synapses (Kujawa and Liberman, 2009).
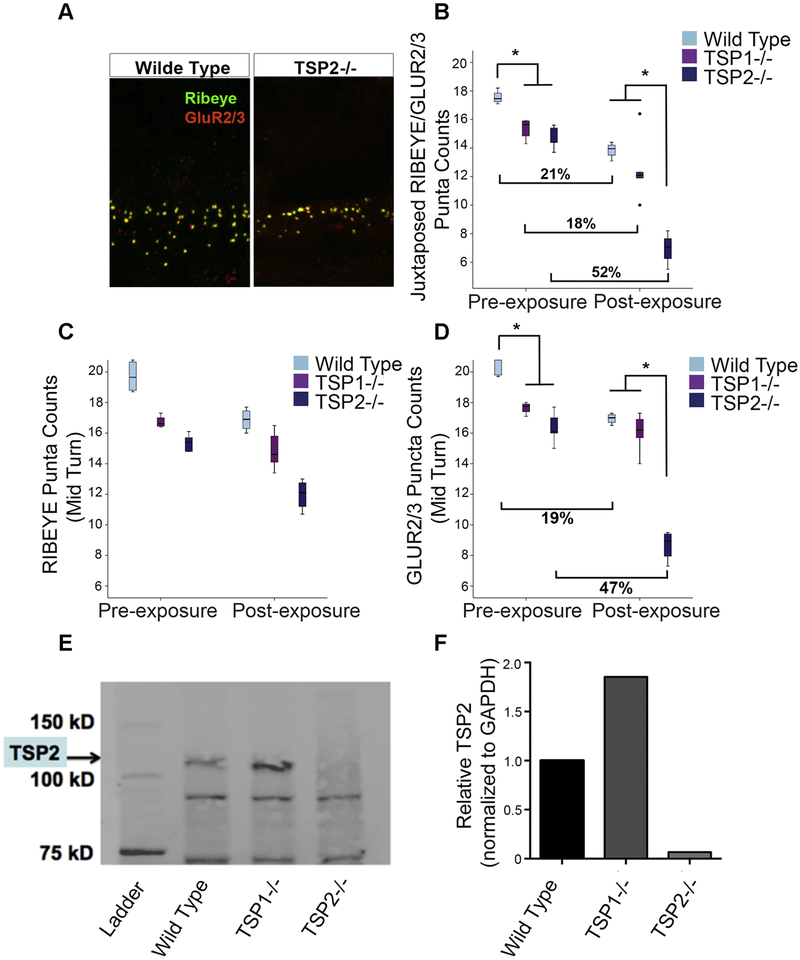
TSP mutants and WT FVB controls were exposed to a similar TTS noise for 30 minutes and the cochlear region corresponding to 16 kHz was examined. We observed significant changes in synaptic counts (p=0.00005) for both TSP2−/− and WT mice 8 weeks after NE (Figure 2A). There was a 52.7% and a 21.1% reduction in the number of synaptic puncta in TSP2−/− and WT mice respectively after NE, compared to their pre-exposure levels (Figure 2B). There was a strong trend (18.5%) towards a decrease in synaptic counts in TSP1−/− mice before and after NE, even though this was not statistically significant (p=0.055) (Figure 2B). When we compared baseline levels of synaptic counts, we found that TSP1−/− and TSP2−/− mice had a relatively lower number of synaptic puncta in the pre-exposure baseline compared the WT group (p =0.001). However, after NE, both TSP1−/− and WT mice had similar levels of synaptic puncta, whereas the TSP2−/− mice had significantly fewer puncta than both WT (p=0.0005) and TSP1−/− mice (p =0.002). This result indicates that TSP2−/− mice suffered greater damage to their synapses in the cochlear mid-turn than both TSP1−/− and WT mice (Figure 2B).
Figure 2. Evaluation of synapse numbers in the wild type, TSP1 and TSP2 mutants before and after noise exposure.
(A) A representative image of immunolabeling of RIBEYE (pre-synaptic marker) and GluR2/3 (post-synaptic marker) in IHCs of wild type and TSP2−/− mutants in the 16 kHz region 8 weeks following noise exposure. (B) Juxtaposed pre- and post-synaptic puncta in the 16 kHz region are reduced in pre-exposed TSP1−/− and TSP2−/− as compared to wild type controls. Significant loss of synaptic puncta in noise exposed TSP2−/− when compared to both WT and TSP1−/− mice. (C) No significant difference in the synaptic ribbon counts at 16 kHz was observed between wild type and TSP mutant mice at any time point tested. (D) Significant difference in postsynaptic marker GluR2/3 between WT and TSP mutants before noise trauma. After noise exposure only TSP2−/− showed significant reduction in the number of GluR2/3 puncta when compared to wild type control mice. The solid line in the middle of each box represents the median. The box represents the middle 50% and the whiskers represent the upper and lower 25%. Circles represent mild outliers. Results are expressed as mean ± SEM. Significant differences are indicated by * = p < 0.05. (E, F) Western Blot for TSP2 in wild type and TSP mutant cochlear lysates. A band for TSP2 (145 kDa) is observed in cochlear lysate from wild type and TSP1−/− mice. Other observed bands are probably not specific. Bands were normalized to GAPDH using ImageJ software (NIH). All samples are derived from the same experiment and blots were processed in parallel.
We further examined the synaptic terminals to determine whether the synaptic damage was localized to either pre- (RIBEYE positive) or post-synaptic (GLUR2/3 positive) structures in these mice. A two-way ANOVA with time points and genotype as factors and RIBEYE or GLUR2/3 puncta counts as dependent variables showed a significant main effect of exposure by genotype interaction (F2,20=19.605, p=0.00002). We then performed planned comparisons of RIBEYE and GLUR2/3 counts. These analyses showed no reduction in TSP1−/− and a small (but non-significant) reduction in both TSP2−/− and WT presynaptic RIBEYE counts after NE (Figure 2C). Interestingly, WT and TSP2−/− mice both showed a marked reduction in postsynaptic puncta as suggested by the GLUR2/3 counts after NE (Figure 2D). The number of GLUR2/3 puncta in TSP1−/− and TSP2−/− mice at the pre-exposure baseline was lower than WT mice (p=0.008, Welch’s ANOVA) (Figure 2D). After NE, TSP1−/− and WT mice revealed similar levels of GLUR2/3 puncta, whereas the TSP2−/− mice had significantly lower levels (p=0.00004,). We recorded a 19.5% loss of GLUT2/3 counts in WT mice and a 47.3% reduction in TSP2−/− mice (p=0.009 for FVB; p=0.00001 for TSP2−/−, Welch’s ANOVA) (Figure 2D). Changes in TSP1−/− for GLUT2/3 were not statistically significant. These results indicate that TSP1−/− mice suffered less damage to their synapses from NE than either the TSP2−/− or even the WT mice. Moreover, the loss of synaptic puncta after NE in the WT and TSP2−/− mice is largely due to postsynaptic damage. The described trends observed in the mid turn were also seen in the apical turn although the numbers there were not statistically significant (data not shown).
These results are in agreement with the significant up-regulation of TSP2 gene expression (and not TSP1) 24 h after noise trauma and the potentially important role this gene is playing in protection and/or recovery of afferent synapse connectivity following noise trauma. To explain the reduced susceptibility (synaptic loss) of the TSP1−/− mutants to noise injury, we next considered whether TSP2 might be upregulated in the TSP1−/− mice. Such an upregulation would suggest that TSP2 might functionally compensate for the loss of TSP1 in these mice. To test this compensation hypothesis, we compared the level of TSP2 protein in the cochlear lysate from TSP1−/−, FVB (positive control), and TSP2−/− (negative control) mice by Western blot. Our anti-TSP2 antibody detected a specific TSP2 band at ~ 150 kDa (Figure 2E). This band was more intense in the TSP1−/− mice than in the FVB mice qualitatively and quantitatively when normalized to GAPDH using ImageJ software (NIH). As expected, the TSP2−/− mice did not show any TSP2 protein expression (Figure 2F).
Cacna2d1, neuronal receptor for cochlear TSP2, modulates synaptogenesis
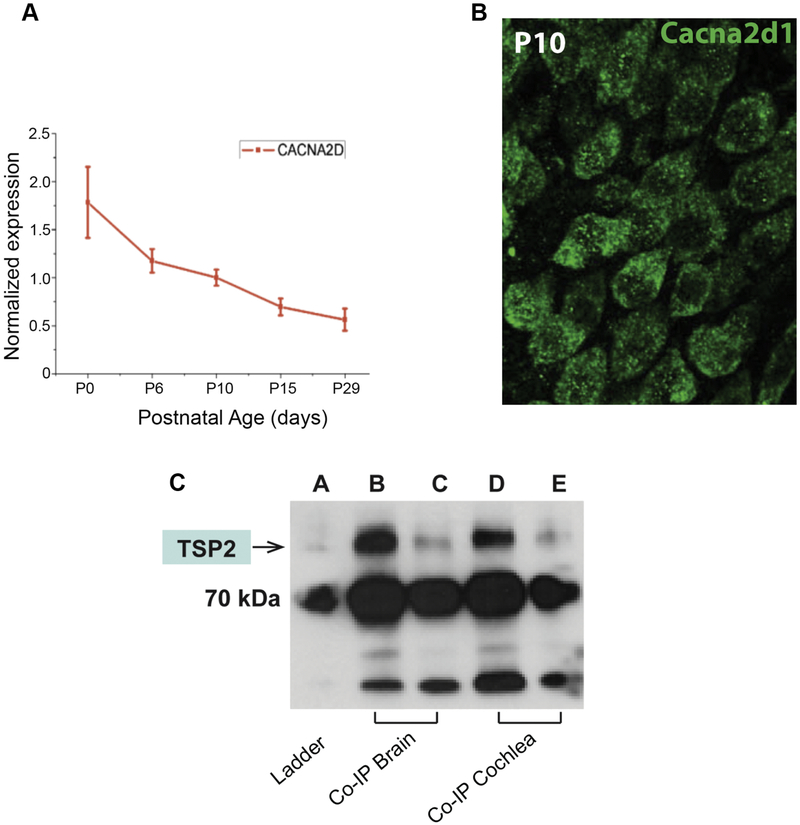
Recent reports have shown that TSP mediates synaptogenic activity through its neuronal receptor, the calcium channel Cacna2d1 subunit (Eroglu and Barres, 2010). To determine whether the mechanism by which TSP mediates synaptogenic effects is the same in the cochlea, Cacna2d1 expression was first examined in the cochlea. Both qPCR and immunohistochemistry demonstrated that Cacna2d1 is expressed in the cochlea and produced by cochlear spiral ganglion neurons (SGNs) (Figure 3A, B). To determine whether Cacna2d1 interacts with TSP2, we immunoprecipitated Cacna2d1 from P10 mouse cochlear lysate. Brain tissue was used as a positive control for the interaction. We prepared two dilutions for both cochlear and brain lysates and detected TSP2 protein in the fractions obtained after immunoprecipitation of Cacna2d1 (Figure 3C). These observations demonstrate an in vivo interaction between Cacna2d1 and TSP2 in the cochlea. This finding suggests an important role for Cacna2d1 in cochlear synapse formation and/or function that may be worth investigating in future studies.
Figure 3. Immunohistochemistry and qPCR assessment of Cacna2d1 expression and interaction with TSP2.
(A) Cacna2d1 mRNA is expressed in the dissected spiral ganglion neurons during development. (B) Cacna2d1 protein is detected in the spiral ganglion neurons. (C) Co-immunoprecipitation followed by western blot experiments. Brain and cochlear lysates (n=7) were immunoprecipitated with anti-Cacna2d1 antibody and blotted for the presence of TSP2. Two brain lysates (lane b: no dilution; lane c: 1/10 dilution) and cochlear lysates (lane d: no dilution; lane e: 1/10 dilution). 70kDa is marked by ladder (lane a) and TSP2 band (145 kDa) is identified by the arrow.
Frequency-specific threshold shifts in noise-exposed TSP mutants
We next investigated how changes in TSP gene expression in cochlea following noise exposure may impact hearing function. Our previous study (Mendus et al., 2014) revealed normal Auditory Brainstem Response (ABR) threshold in 3-month old TSP mutant mice at 16kHz. For this study, we used 3 month-old mice (TSP1−/−, TSP2−/−, and WT FVB) and exposed them to noise (8–16 kHz octave band, 100 dB SPL) for 30 minutes. We assessed the functional consequences of noise trauma in WT mice and in mice lacking TSP1 and/or TSP2 by examining ABRs before noise exposure (pre-exposure), at 24 hours and 8 weeks after noise exposure. We determined the auditory thresholds at three different frequencies representing each turn of the cochlea; 8 kHz (low frequency), 16 kHz (mid frequency), and 32 kHz (high frequency) to assess function at the apical, middle and the basal (data not shown) turns of the cochlea, respectively.
A two-way ANOVA of the ABR data was performed, with ABR threshold as dependent variable and genotype (WT, TSP1−/− and TSP2−/−) and time point (before and after noise trauma) as independent factors. This analysis showed a significant main effect of genotype × time point interaction for all three frequencies (8, 16, and 32 kHz) (F6,104 =3.564, p = 0.003 for 8 kHz; F6,104 =6.853, p = 4E-06 for 16 kHz; F6,104 =7.326, p = 2E-06 for 32 kHz). However, baseline ABR thresholds at 32 kHz were shifted in TSP2−/− (p=2.01E-10) with respect to WT control mice even without NE (data not shown).
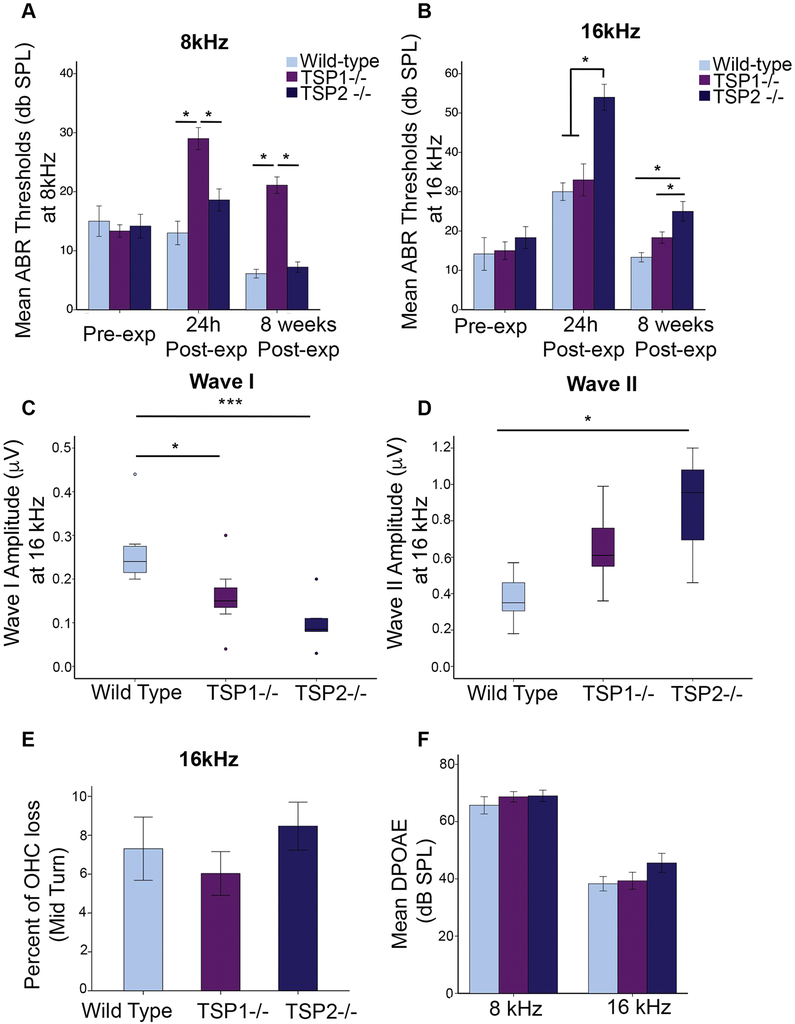
At 8 kHz, WT mice did not show a threshold shift 24 h after NE (Figure 4A). At 16 kHz, however, we recorded a marked ABR threshold shifts 24 h post-NE compared to pre-NE levels (p =0.0003) (Figure 4B). These mice recovered completely by 8 weeks and ABR thresholds were then comparable to pre-NE levels (Figure 4B). TSP1−/− mice had elevated ABR thresholds at both 8 kHz and 16 kHz at the 24 h time point post-NE compared to pre-NE levels (p=0.0003 for 8kHz; p=0.001 for 16kHz). At 8 weeks post-NE, ABR thresholds had recovered in 16kHz but not in 8kHz where it was still significantly elevated (p=0.004) (Figure 4A, B). TSP2−/− mice did not show a threshold shift at 8kHz and 24 h post-NE. Interestingly, TSP2−/− mice had a small decrease in ABR thresholds at 8 weeks post-NE compared to pre-NE levels at 8kHz (p=0.023) (Figure 4A). At 16kHz, these mice showed marked threshold shifts 24 h post-NE that recovered partially by 8 weeks, but still remained elevated compared to pre-NE levels (p=3.09E-13 for 24h vs. pre-NE; p=0.038 for 8wk post-NE vs. pre-NE) (Figure 4B).
Figure 4. Assessment of ABR thresholds in wild type and TSP mutant mice before and after noise exposure.
Compared ABR thresholds between WT and TSP mutants before and after noise exposure and at both frequencies 8kHz (A) and 16 kHz (B) At 8kHz only TSP1−/− exhibited a PTS when compared to WT mice (A). At 16kHz all genotypes revealed ABR threshold shift 24h after exposure but only TSP2−/− ABR threshold remained shifted 8 weeks after exposure (B). ABR wave I amplitude measured 30 dB above the threshold and at 16 kHz was decreased in noise exposed TSP2−/− (C). (D) ABR wave II was increased in noise exposed TSP2-/−. The solid line in the middle of each box represents the median. The box represents the middle 50% and the whiskers represent the upper and lower 25%. Circles represent mild outliers. (E) OHC counts in the mid turn or around the 16 kHz region. (F) DPOAE readings (8 weeks post-exposure) at 8 and 16 kHz frequencies. DPOAE thresholds and OHC counts were comparable to those of the FVB control mice. Results are expressed as mean ± SEM. Significant differences are indicated by *p < 0.05, ***p<0.001.
We then compared ABR thresholds between WT and TSP mutants before and after NE. At both 8 and 16 kHz, all three genotypes initially displayed similar thresholds. At 8kHz, the TSP1−/− mice showed a significant elevation in ABR threshold at 24 h post-NE compared to WT (p=0.001) and to TSP2−/− (p=0.004). Even though ABR thresholds for TSP2−/− also appeared mildly elevated at 24 h post-NE compared to WT, this effect was not statistically significant. At 8 weeks post-NE, thresholds in the TSP1−/− were still elevated with respect to both WT and TSP2−/− (p=0.00011 for TSP1−/− vs. WT, p=0.00018 for TSP1−/− vs. TSP2−/−) (Figure 4A). At the 16kHz frequency, only TSP2−/− showed a significant shift in thresholds at 8 weeks post-NE compared to WT controls (p =3.8E-7) (Figure 4B). These results suggest that unlike their WT littermates, in both TSP1−/− and TSP2−/− mice the NE resulted in permanent threshold shifts at the 8kHz and 16 kHz. The above ABR data, taken together with the synaptic quantification results, indicate that while TSP1 may be important for protection from NE in the lower frequency, TSP2 may be required at the mid frequency (16kHz).
As previously described, the ABR waveform shows the summation of all neuronal responses to a given auditory stimulus (Kujawa and Liberman, 2009; Liberman et al., 2011). To have an understanding of the number of neurons that are firing and to quantify the speed of transmission, we analyzed the peak amplitude and latency of waves I and II from ABRs at 30 dB above hearing threshold levels. We used data from the 16 kHz measurements since this represents the most sensitive hearing region in mice. We analyzed the peak and latency data from TSP1−/−, TSP2−/− and WT mice, followed by a Scheffe post hoc test. We found a significant decrease in wave I amplitudes for both TSP1−/− and TSP2−/− mice compared to WT mice, indicating reduced afferent synaptic activity in these mutants (*p= 0.03 for TSP1−/− vs. WT; ***p= 0.0002 for TSP2−/− vs. WT) (Figure 4C). The amplitude of wave II, representing activity of the proximal fibers of the auditory nerve as it enters the cochlear nucleus, was not changed in TSP1−/− group. However, wave II amplitudes in the TSP2−/− mice were significantly higher (p=0.001) compared to WT controls (Figure 4D), indicating that TSP2−/− group had increased neuronal responses in the cochlear nucleus. Latency measurements did not show any significant differences between TSP1−/−, TSP2−/−, and WT mice (data not shown) suggesting the speed of stimulus transmission was not impaired in either mutant group. We also assessed the effect of noise trauma on outer hair cells (OHC) using functional and histological tests. OHC were long considered the most vulnerable inner ear cell type to noise. Our morphological data revealed similar OHC counts in both 8kHz (data not shown) and 16kHz region of WT and both TSP1−/− and TSP2−/− mutants (Figure 4E). Distortion product autoacoustic emission (DPOAE) levels in TSP1−/− and TSP2−/− mutants were also comparable to WT controls, 8 weeks post-NE at both frequencies 8kHz and 16kHz (Figure 4F). These data suggest that OHCs were largely unaffected by the level and duration of the noise injury applied in TSP mutants.
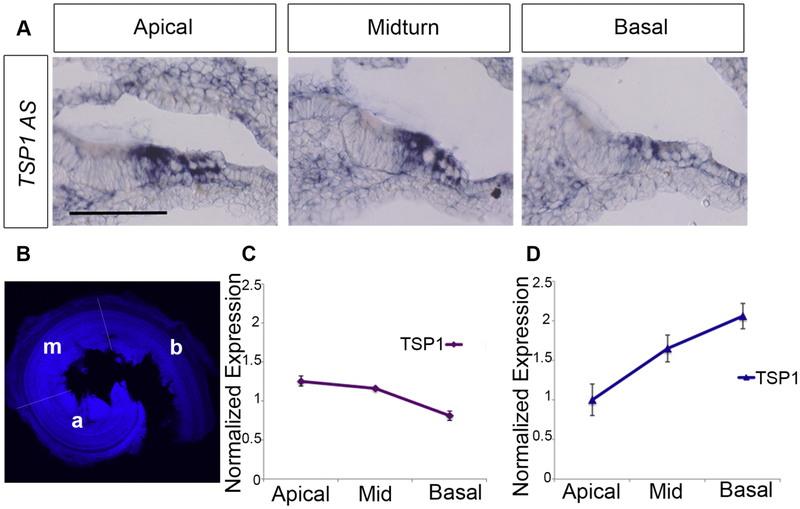
Tonotopic expression of TSP1 and TSP2 mRNAs in the mouse cochlea
Based on the hearing test results, we speculated whether the expression level of TSP1 and TSP2 genes would correlate with their differential roles in the various frequency regions. To investigate this hypothesis, we examined mRNA levels for these genes at P5 using in situ hybridization on cochlear tissue for TSP1, which suggested its expression by the supporting cells (Figure 5A) (Mendus et al., 2014). We also quantified TSP mRNA levels using qPCR to examine three regions of the organ of Corti microdissected into - apical, mid, and basal turns (Figure 5B), for both TSP transcripts. TSP1 mRNA tended to be more abundant in the apical and mid turns compared to the basal turn (Figure 5C). The reverse was true for TSP2 mRNA, with higher levels in the basal turn corresponding to high frequency regions of the cochlea (Figure 5D). These results are in agreement with our ABR data that suggest greater susceptibility to NE in the low and mid frequencies for the TSP1−/− mice, and in the mid to high frequency range for the TSP2−/− mice (Figure 4A, B).
Figure 5. Tonotopic expression of TSP1 and TSP2 from cochleae of P5 mice.
(A) In situ hybridization for TSP1 in cross-sections from the cochleae of P5 mice suggests an apical to basal gradient of TSP1 mRNA (AS for Antisense probe). TSP1 and TSP2 mRNA levels were quantified using qPCR from microdissected organ of Corti from three regions: apical, middle, and basal turns (B) TSP1 mRNA tended to be more abundant in the apical and mid turns compared to the basal turn (C). The pattern was opposite for TSP2 mRNA, with higher levels in the basal turn which represents the high frequency region (D).
Frequency-specific audiogenic seizures followed by sudden death due to epilepsy (SUDEP) in TSP2 mutants
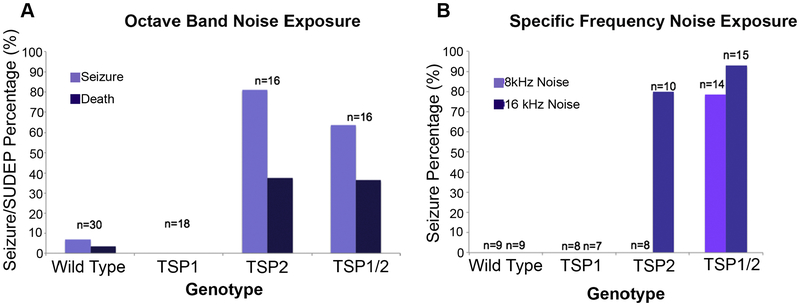
During NE many TSP2−/− mice exhibited seizures. Monitoring these mice during noise exposure revealed that 80% of TSP2−/− mice had wild running and tonic-clonic activity lasting <20 seconds with ~38% of these mutants undergoing sudden death due to epilepsy (SUDEP) (Figure 6A). Interestingly, the WT littermates of these mice had an 8% risk of audiogenic seizure, but none of the TSP1−/− mice tested (n=18) experienced an audiogenic seizure during noise exposure (Figure 6A). We then monitored the TSP1/2−/− double mutants behavior under noise trauma and observed similar seizure behavior to the TSP2−/− mice.
Figure 6. Audiogenic epilepsy and sudden unexpected death in epilepsy (SUDEP) in TSP2−/− and double TSP1/2−/− mutant mice.
Data from videotape collected during noise exposure of wild type, TSP single, and TSP double mutant mice. (A) Percentage of animals per genotype that had audiogenic seizures and SUDEP after exposure to octave band noise. 70% of TSP2−/− and 80% and TSP1/2−/− mice exhibited audiogenic seizures. About 38% of the mutant mice showed SUDEP. While WT mice had a 8% risk of audiogenic seizure, none of the TSP1−/− mice tested (n=18) experienced an audiogenic seizure during noise exposure. (B) Percentage of animals per genotype that manifested seizure at a specific frequency. 80% of TSP2−/− mice had seizure at 16kHZ but none at 8kHz. 78% of TSP1/2−/− mice exhibited seizures at 8kHz and 93% at 16kHZ.
These findings, together with the hearing test results, suggested a functional overlap between TSP1 and TSP2, where TSP2 may compensate for the loss of TSP1 and protect the TSP1−/− mice from audiogenic seizures. Also, given the qPCR data that showed tonotopic expression for TSP1 and TSP2 in the cochlea, we next examined if the susceptibility to audiogenic seizures and SUDEP was frequency-specific. We exposed 3 month-old TSP1−/−, TSP-/−2, and TSP1/2−/− double mutants and their WT controls to 100 dB SPL sound at either 8 kHz or 16 kHz (Figure 6B). Surprisingly, none of the single mutants (TSP1−/− and TSP2−/−, n=8 for both mutants) seized at 8 kHz (Figure 6B). However, 78% of TSP1/2−/− double mutants (n=14) had seizures (Figure 6B). When these mice were exposed at 16 kHz to a100 dB SPL tone, only TSP2−/− (80%) and TSP1/2−/− (93%) mutants seized (Figure 6B). These data suggested that TSP2 mutants were protected from audiogenic seizure at 8kHz, possibly by the upregulation of TSP1 in this cochlear region following NE. This hypothesis is also supported by the seizure behavior exhibited by the TSP1/2−/− double mutants at both frequencies. However, due to the unavailability of a suitable TSP1 antibody, we were unable to confirm this hypothesis.
We next examined whether the observed seizure behavior was auditory-specific or if it could be triggered by another peripheral sensory system. We checked for seizure response to visual stimuli by applying a flashlight at different frequencies (at both 15–20 flashes per second and 50 flashes per second) for up to 10 minutes. No seizure-like response was triggered with this type of stimulus in the TSP mutants. Therefore, we focused on the auditory pathway, and we next tested the central auditory pathway for changes in TSP1 and TSP2 gene expression following NE.
Noise-induced changes in TSP gene expression in the auditory brain regions
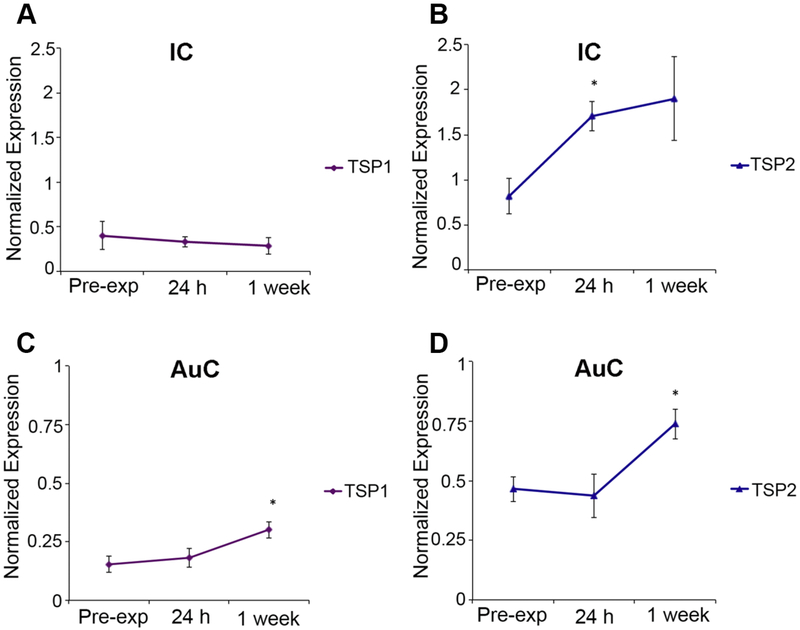
First, we examined the levels of TSP1 and TSP2 mRNA after NE in the auditory cortex (AuC) and inferior colliculus (IC). In the IC, TSP1 mRNA did not show a significant change at 24 hours or at 1 week after NE (p=0.79; Welch’s ANOVA) (Figure 7A). In contrast, TSP2 mRNA showed a 2-fold elevation at 24 hours compared to pre-exposure levels in the IC (p=0.029; Welch’s ANOVA) (Figure 7B). This elevation appeared to extend to the 1-week after NE time point as well.
Figure 7. Noise-induced changes in TSP gene expression in auditory brain regions.
Quantitative qPCR was used to determine expression of TSP1 (A, C) and TSP2 (B, D) genes in mouse inferior colliculus (IC) and auditory cortex (AuC) before (pre-exposure) and after noise exposure (24 hours and 1 week). In the IC significant upregulation of TSP2 (B), but not TSP1 (A), gene expression was observed 24 hours after noise exposure. TSP2 gene expression remains upregulated one week after noise. In the AuC, both TSP1 and TSP2 mRNA levels were significantly higher at 1 week after noise exposure compared to pre-exposure levels (C and D respectively). Data shown is the expression of the gene of interest relative to the internal standard, GAPDH. Results are expressed as mean ± standard error of the mean (SEM). Significant differences are indicated by * = p<0.05.
In the AuC, TSP1 mRNA levels gradually rose after NE and were significantly higher at 1 week after NE compared to pre-exposure levels (p=0.037; Welch’s ANOVA) (Figure 7C). Similarly, TSP2 mRNA levels also increased after NE and were significantly elevated at 1 week after NE compared to controls in the AuC (p=0.022; Welch’s ANOVA) (Figure 7D). These results mirrored the earlier changes in TSP2 mRNA levels seen in the cochlea soon after NE at 24 h (Figure 1), suggesting an important role for this gene in the central auditory regions as well in response to acoustic trauma. This result is especially significant given the acute vulnerability of the TSP2−/− mice to audiogenic seizures. The findings suggest that TSP2 may play a role in dampening spontaneous neuronal activity.
Discussion
We have previously shown that TSP1−/− and TSP2−/− mice exhibit synaptic deficits as well as progressive and age-related hearing loss (Mendus et al., 2014). In the current study we found that loss of TSPs leads to a greater susceptibility to NIHL. We observed statistically significant differences between noise-exposed controls and TSP-deficient mice in several experimental procedures. The protection conferred by TSPs was frequency-specific -- TSP1 played a greater role at the low frequencies and TSP2 in the mid to high range. We performed histological, physiological, and imaging experiments to examine the mutant phenotype in detail.
TSP’s role in the peripheral auditory system following noise exposure
The molecular mechanisms that underlie susceptibility to noise-induced hearing impairment and, in particular, auditory synaptopathy are poorly understood. The upregulation of cochlear TSP2 gene expression following moderate noise trauma suggested an important role of this gene in recovery of cochlear function after injury. TSP2 mutants indeed suffered greater synaptic loss in the middle turn of the cochlea compared to the apical turn. Interestingly, we found that the cochlear ribbon/afferent synapses in the TSP1 mutants were the least affected by NE with a greater number of synapses than either WT or TSP2 mutants. The functional significance of this observation is, however, currently unclear. The rapid upregulation of TSP2 mRNA levels soon after NE in addition to the compensatory upregulation of TSP2 protein in TSP1 mutants (as reflected by western blot analysis) likely serve to protect the TSP1 mutants from noise damage.
Frequency-specific susceptibility to NIHL in TSP mutants
In contrast, our functional and histological data following the moderate noise exposure suggest that OHCs were largely unaffected in WT and TSP mutants. However, analysis of the ABR data showed a permanent threshold shift (PTS) in TSP2 mutants in the mid and high frequencies. These data correlate well with differential afferent synapse numbers across mutants. In line with our hypothesis, TSP1−/− showed PTS at the low but not at mid and high frequencies despite the normal number of synapses. This frequency-specific susceptibility of TSP1−/− and TSP2−/− to NIHL may be explained by their tonotopic expression pattern in the cochlea. Greater TSP2 mRNA expression in the mid and basal turns correlates with a greater role in protection from noise in the mid to high frequency regions. Likewise, the expression of the TSP1 mRNA was higher in the apical and mid turns corresponding to the “low to mid” frequencies, suggesting a greater role for TSP1 in this range. Increased levels of TSP2 protein in the cochlea of the TSP1−/− mice also suggests that TSP2 might functionally compensate for the lack of TSP1 in these mice indicating some redundancy in this system. In this case, higher TSP2 levels would serve to protect the TSP1−/− mice from noise damage at the mid and high frequencies as suggested by our data. Whether the TSP1 gene is also elevated in TSP2−/− cochleae will be the focus of future studies once a reliable TSP1 antibody is available.
Cacna2d1 is the neuronal TSP2 receptor in the cochlea
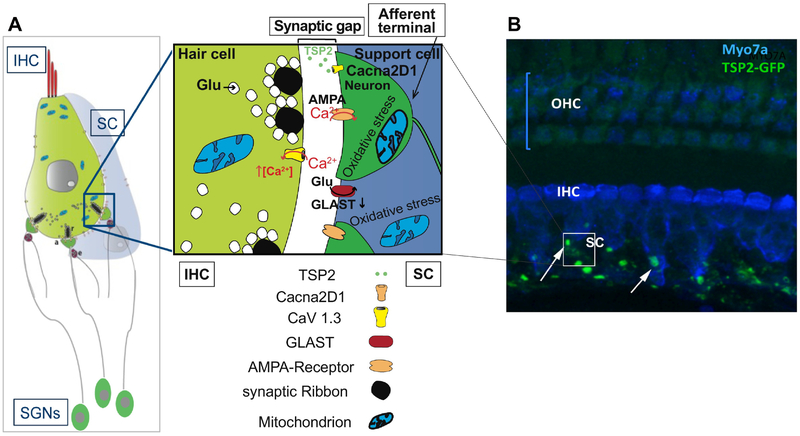
The molecular interactions that regulate afferent synapse formation and maintenance in the cochlea are not well characterized. Here we have shown an interaction between TSP2 and its neuronal receptor Cacna2d1 using co-immunoprecipitation from cochlear lysate. This interaction was shown to be required for mediating synaptogenic effects of TSP in the brain (Eroglu and Barres, 2010). Our findings raise the questions about how this interaction promotes synapse formation and/or maintenance with age and following noise-injury (as summarized in Figure 8) and provide a number of important implications including therapeutic strategies to be investigated in future studies.
Figure 8.
(A) Schematic representing the potential molecular mechanism by which TSP2 is mediating its synaptogenic effects in the inner hair cells (IHC). TSP2 is secreted by the supporting cells (SC). Interaction between TSP2 and Cacna2d1, expressed by the afferent terminals of the spiral ganglion neurons (SGNs), triggers the assembly of elements necessary for synapse formation or repair after injury. (B) A representative z-stack projection confocal image of immunolabeling of Myo7A hair cell marker (blue) on a whole mount of the organ of Corti of TSP2_eGFP mouse. TSP2 or eGFP-expression is detected at the postsynaptic region of IHCs and where the afferent synaptic terminals are located.
TSP dependent cochlear afferent input predicts NIHL susceptibility
The noise induced PTS in the TSP1−/− and TSP2−/− mice was accompanied by distinct changes in the amplitude of wave I and wave II ABR waveforms, providing some insight into the neural changes accompanying noise trauma. The amplitude of wave I in the ABR waveform at 16 kHz, was significantly lower in the TSP2 mutants compared to WT, when measured 8 weeks post NE. The importance of these studies is underscored by the correlation with studies in human patients which indicate that increased age and hearing thresholds are associated with reductions in wave I amplitude (Bramhall et al., 2015). These observations are similar to what has been reported for hyperacusis and tinnitus, other conditions that can result from cochlear damage due to PTS and TTS noise exposures (Knipper et al., 2013; Manzoor et al., 2013; Mulders and Robertson, 2013).
We previously showed that TSP1−/− and TSP2−/− deficient mice had synaptic loss and a phenotype associated with progressive and age-related hearing loss, respectively (Mendus et al., 2014). Our current data demonstrate that afferent synaptic counts and auditory brainstem response (ABR) thresholds in noise-exposed TSP mutants are irreversibly damaged. Based on these data, we infer that TSPs are involved in response to acoustic trauma and in the cochlea, similar to their proposed role in the brain (Liauw et al., 2008). We propose that a defect in a gene critical for synaptic “health”, such as TSP2, could render an individual less protected from noise trauma, and more vulnerable to NIHL. In light of our results, we suggest TSP2 as a candidate gene for diagnostic screening of vulnerable populations such as those living or working in a noisy environment, for example military personnel. Early detection of defects in genes critical for synaptic protection and recovery will aid in devising prophylactic (use of noise dampening devices) and therapeutic strategies (gene therapy) to mitigate the effects of noise exposure by overexpressing TSP2.
Experimental Procedures
Animals
Mice lacking TSP1 (TSP1−/−) (Lawler et al., 1998), TSP2 (TSP2−/−) (Kyriakides et al., 1998) or double mutants (TSP1/2 −/−) (Agah et al., 2002) and their littermate FVB WT controls were used for the experiments. These mice with FVB/NJ genetic background were time-mated and the day of birth named postnatal day zero (P0). In addition, we have used TSP2 green fluorescent protein (GFP) reporter mice (TSP2-eGFP) generously provided by Dr. Kurt (Palenski et al., 2013). Three or more animals per genotype were used per experiment. Each experiment was repeated two to three times for validation. All animal procedures were approved by the Stanford University Administrative Panel on Laboratory Animal Care and conducted in accord with the principles and procedures outlined in the National Institutes of Health Guidelines for the Care and Use of Experimental Animals. Animals were treated in accordance with the Animal Welfare Act and DHHS “Guide for the Care and Use of Laboratory Animals.”
RNA extraction and quantitative RT-PCR (qPCR).
RNA extraction and cDNA preparation from cochleae were performed as previously described (Mendus et al., 2014). The intact cochlea, including the inferior colliculus (IC) and the auditory cortex (AC) were dissected from nine 12 week-old adult WT FVB mice and placed in RNA lysis buffer for each time point (before, 24hours (h) and 1 week after noise exposure). RNAqueous-Micro and PCR Kits from Ambion were used according to the manufacturer instructions for RNA extraction. cDNA was prepared using a High Capacity RNA to cDNA kit (ABI), as per the protocol supplied with the kit. To quantify mRNA expression levels, the cDNA was amplified using TaqMan PCR assays (Applied Biosystems, Foster City, CA). We used proprietary probes and primers from Applied Biosystems for TSP1 (assay ID Mm01335418_m1) and TSP2 (assay ID Mm01279240_m1). GAPDH (assay ID Mm03302249_g1) was used as the internal standard. At least four independent experiments were run with all samples in triplicate. Error bars represent standard error of the mean (SEM). For the noise-exposure experiment, qPCR data was analyzed by an ANOVA (SPSS Statistics Premium Grad Pack, IBM Corp.) to determine if there were significant differences in TSP1 or TSP2 gene expression after acoustic trauma. Relative expression level for a gene was used as the variable and the time point (before, 24h, or 1 week after noise trauma) was used as the independent factor. If this comparison was significant (p<0.05), then a Scheffe post hoc test was performed to identify specific differences between the groups. For cochlear regional gene expression studies of TSP1 and TSP2 mRNA levels, we pooled cochlear tissue that was microdissected from either the apical, mid, or basal turns (n=9) from P5 mice.
Immunohistochemistry
Inner ears were dissected into cold 1X phosphate buffered saline (PBS). Cochleae were perfused with 4% paraformaldehyde and post-fixed for 10 minutes. Samples were then washed 3 times in PBS for 10 minutes each and blocked in 5% bovine serum albumin and 0.5% Triton X-100 for 40 minutes at room temperature. Same blocking buffer was used for diluting antibodies. Primary antibodies were incubated at 4°C for 48 hours followed by three washes in 0.1% PBS-Tween. Secondary antibodies were added for 1 hour at room temperature, followed by three washes in 0.1 %PBS-Tween. The following primary antibodies were used: goat anti-CtBP2 (1:200, Santa Cruz Biotechnology), rabbit anti-Glutamate Receptor 2&3 (1:100, Millipore Biosciences Research Reagents) and rabbit anti-MYOSIN VIIa (1:300, Proteus Biosciences). Secondary antibodies used were Alexa Fluor 488-conjugated anti-goat, Alexa Fluor 546-conjugated anti-rabbit (1:500, Invitrogen). Cochleae were washed in PBS-Tween and mounted on slides in ProLong (Invitrogen) anti-fading media.
Cochlear z-stacks images were taken from a selected frequency region for each sample. Images were analyzed using Volocity 3D Image Analysis Software. Quantification of hair cells and synaptic puncta was performed as previously described (Meng et al., 2009; Mustapha et al., 2009). For inner hair cell afferent synapse counts, a minimum of 30 inner hair cells per cochlea and five to seven animals per genotype and/or noise exposed group were counted for each marker.
Confocal analysis of outer hair cells number
Images were collected on a Zeiss AxioVert confocal inverted microscope. A minimum of 30 outer hair cells (OHCs) per ear and an “n” of six to eleven animals were counted for MYOSIN VIIA marker. Cochlear frequency map (Muller et al., 2005) was estimated for every sample in order to localize hair cells from different frequency regions. At least one Cochlear z-stacks from a selected frequency region were taken for each sample. The length of each region was 20μm and contained at least 30 OHCs. More then one 20μm images were considered for some samples. Hair cells were manually counted using Z-stack images and Volocity 3D Image Analysis Software.
Co-immunoprecipitation and Western blotting
Brain and cochleae plasma membranes were prepared for co-immunoprecipitation. Tissues were harvested from seven P10 pups in cold 1× phosphate buffered saline (PBS) and then resuspended in ice-cold hypotonic buffer (10mM Tris pH 7.4, 1mM CaCl2 and 1mM MgCl2) with protease inhibitors (Complete EDTA-free, Roche). After homogenization in a glass-on-glass douncer (5 times), minced tissues were incubated on ice for 15 min for cells to swell. The nuclei and unbroken cells were removed by centrifugation at 300g for 5 min. The post-nuclear supernatant was centrifuged for 20 min at 20,000g to pellet the membranes. The membranes were then resuspended in solubilization buffer (25mM Tris pH 7.2,150mM NaCl, 250mM Sucrose, 1mM CaCl2 and 1mM MgCl2) with protease inhibitors and 0.5% Surfact-Amps NP-40 (Pierce) and incubated at 4°C for 10 min to allow for solubilization. The insoluble debris was removed by centrifugation (20,000g for 10 min) and the soluble fraction was pre-incubated with 2μg of Cacna2d1 antibody (V21, Santa Cruz) overnight with gentle rocking. Two dilutions of each lysate were used for the experiment. The lysate and antibody complex was then added to 50μl of the washed A/G magnetic bead pellet (Active Motifs) and incubated with gentle rocking for 15 min at room temperature. The bead pellet was washed 3 times with 500 μl of 1X cell lysis buffer and resuspended in 20–40 μl 3X SDS sample buffer. Samples were heated to 95–100°C for 5 min and beads eliminated by microcentrifuge for 1 min at 14,000 × g. Thirty five microliters of each sample was loaded on SDS-PAGE gel and analyzed by Western blotting. Blots were blocked 2h in Blocker/Diluent buffer (Invitrogen), probed overnight with rabbit anti-TSP2 (BD biosciences) After incubation with an anti-rabbit peroxidase-labelled secondary antibody (1/5000 (GE)), blots were visualized using Luminata Forte HRP substrate (Millipore) and analyzed using the integrated density approach in ImageJ (NIH). TSP2 expression was normalized to the GAPDH loading control and expressed as relative fold changes in TSP1 and 2−/− compared to the Wild Type.
Noise exposure
Mice were exposed to one of three sound paradigms: 1) octave band noise, 8 kHz - 16 kHz at 100 dB SPL for 30 minutes 2) a tone of 8 kHz at 100 dB SPL for 30 minutes or 3) a tone of 16 kHz at 100 dB SPL for 30 minutes. Animals were awake and unrestrained during noise exposure in lidless individual boxes. Three animals were placed directly under the horn of the loudspeaker. Noise calibration to the target SPL was performed immediately before each exposure session using a sound level meter with a 60–120 dB range (Radioshack). SPL variations between experiments and across the cages were less than 1 dB.
Light exposure
Mice were exposed to visual stimuli by applying a flashlight at different frequencies (at both 15–20 flashes per second and 50 flashes per second) for up to 10 minutes. Animals were awake and unrestrained during light exposure in lidless individual boxes placed in a dark room.
Hearing tests
Auditory Brainstem Responses (ABR)
ABR recordings were performed using the Auditory Core of the Department of Otolaryngology, Stanford University. Mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) administered intraperitoneally. Core body temperature was kept at 37.0 C for all recordings using a homeothermic heating pad (FHC). The presentation of stimuli, ABR acquisition, equipment control and data management were coordinated using the computerized Intelligent Hearing System (IHS; Miami, FL). Three needle electrodes were placed under the skin. 8–16- and 32-kHz tone-bursts were channeled through plastic tubes into the animal’s ear canals. Sound levels were incremented in 5 dB steps from 10–20 dB below threshold to 80 dB (for 8 and 16 kHz) or 100 dB (for 32 kHz). Threshold for ABR was defined as the lowest stimulus level at ABR waves could be identified in the response waveform. Amplitude and latency measurements for waves I and II of the ABR waveform were performed at 30 dB above hearing threshold levels at the 16 kHz frequency as previously described (Kujawa and Liberman, 2009). The amplitude analysis was done by peak-to-peak measurement of the ABR waveform and latency was calculated as time delay from the onset of the stimulus (0 ms) until the occurrence of the ABR response peak.
Statistical analyses were performed using an SPSS software package. The wave I amplitude measurements were not normally distributed therefore a rank test- the Kruskal-Wallis test with Dunn’s test for multiple comparison correction was performed. For the wave II measurements, a two-way ANOVA of the ABR data was performed, with ABR threshold as dependent variable and genotype (WT, TSP1−/− and TSP2−/−) and time point (before and after noise trauma) as independent factors.
Distortion-Product Otoacoustic Emission (DPOAE)
To test the function of outer hair cells we measured DPOAEs as previously described (Calton et al., 2014), using the computerized National Hearing Instruments conducted in a sound-attenuating room at the Auditory Core for Department of Otolaryngology, Stanford University. DPOAE thresholds were calculated by interpolating the data and identifying when the signal was >−5 dB SPL and greater than two standard deviations above the noise floor. If no DPOAE response was detected even at our equipment limits of 80 dB SPL, we arbitrarily defined the threshold to be 80 dB.
Quantification and Statistical Analysis
Several experiments were performed to address each question. In addition, each experiment contained two to three replicates to distinguish technical from biological variability. Counts are shown as bar chart or box and whisker plots that represent each data point in the set. The whiskers illustrate the minimum and maximum values in the specific group examined. Outliers in the data set are also shown.
A two-way ANOVA was performed for the immunohistochemistry imaging and remaining hearing related data with synaptic or outer hair cells counts as the dependent variable with genotype (FVB, TSP1−/−, or TSP2−/−) and noise exposure condition (pre-exposure or time post-exposure) or cochlear region/frequency as independent factors for these tests. Similar statistical method was used for the hearing test; planned comparisons were performed with a one-way ANOVA or Welch’s ANOVA, with ABR threshold as dependent variable and either time point (before, 24h, or 8 weeks after acoustic trauma) or genotype as independent factor. This was followed by Scheffe post hoc test to identify specific differences between genotypes, or across the time point tested, at a given frequency (8, 16, or 32 kHz).
On the basis of significant main effects and interactions from this statistical test, planned comparisons were performed either by Student’s t test or one-way ANOVA for dependent variables, followed by Scheffe, or Tukey HSD for post hoc comparisons to identify differences between genotypes, across noise exposure groups, or at different cochlear regions/frequencies (SPSS, and Microsoft Excel). For data sets that were not normally distributed, as verified by Shapiro-Wilk test, we have used a non-parametric statistical analysis, Kruskal-Wallis test with Dunn’s test for multiple comparison correction. In all statistical tests, a p < 0.05 was considered statistically significant. Specific p values for significant comparisons are stated in the appropriate results section.
Highlights.
Hearing threshold of TSP mutants did not recover following a moderate noise injury.
TSP mutants exhibited differential susceptibility to noise-induced hearing loss.
Differential expression of TSP genes in peripheral and central auditory systems.
Potential synaptogenic activity of TSP mediated through Cacna2d1 neuronal receptor.
Acknowledgments
The authors would like to thank Dr. Holt, Associate Professor at Wayne State University School of Medicine for spending her valuable time in teaching us the microdissection and isolation of the different central auditory regions, for reading and discussing the research and the manuscript. The authors also would like to thank Dr. Kurt, Professor at Michigan State University, who generously shared his valuable TSP2 transgenic mouse and experience.
Funding source
This work was supported by the National Institute in Deafness and other Communicative Disorders: R01 DC09590 (M. Mustapha).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agah A, Kyriakides TR, Lawler J, Bornstein P (2002) The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice. Am J Pathol 161:831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhall N, Ong B, Ko J, Parker M (2015) Speech Perception Ability in Noise is Correlated with Auditory Brainstem Response Wave I Amplitude. Journal of the American Academy of Audiology 26:509–517. [DOI] [PubMed] [Google Scholar]
- Calton MA, Lee D, Sundaresan S, Mendus D, Leu R, Wangsawihardja F, Johnson KR, Mustapha M (2014) A lack of immune system genes causes loss in high frequency hearing but does not disrupt cochlear synapse maturation in mice. PLoS One 9:e94549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu C, Barres BA (2010) Regulation of synaptic connectivity by glia. Nature 468:223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman AC, Kujawa SG, Liberman MC (2013) Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. Journal of neurophysiology 110:577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen-Guzejev M, Vuorinen HS, Mussalo-Rauhamaa H, Heikkila K, Koskenvuo M, Kaprio J (2005) Genetic component of noise sensitivity. Twin research and human genetics: the official journal of the International Society for Twin Studies 8:245–249. [DOI] [PubMed] [Google Scholar]
- Hickox AE, Liberman MC (2014) Is noise-induced cochlear neuropathy key to the generation of hyperacusis or tinnitus? Journal of neurophysiology 111:552–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipper M, Van Dijk P, Nunes I, Ruttiger L, Zimmermann U (2013) Advances in the neurobiology of hearing disorders: recent developments regarding the basis of tinnitus and hyperacusis. Progress in neurobiology 111:17–33. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC (2009) Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. The Journal of neuroscience : the official journal of the Society for Neuroscience 29:14077–14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakides TR, Zhu YH, Smith LT, Bain SD, Yang Z, Lin MT, Danielson KG, Iozzo RV, LaMarca M, McKinney CE, Ginns EI, Bornstein P (1998) Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. J Cell Biol 140:419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J, Sunday M, Thibert V, Duquette M, George EL, Rayburn H, Hynes RO (1998) Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J Clin Invest 101:982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh J, Macaskill P, Kuosma E, Mandryk J (1999) Global burden of disease and injury due to occupational factors. Epidemiology 10:626–631. [PubMed] [Google Scholar]
- Liauw J, Hoang S, Choi M, Eroglu C, Choi M, Sun GH, Percy M, Wildman-Tobriner B, Bliss T, Guzman RG, Barres BA, Steinberg GK (2008) Thrombospondins 1 and 2 are necessary for synaptic plasticity and functional recovery after stroke. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 28:1722–1732. [DOI] [PubMed] [Google Scholar]
- Liberman LD, Wang H, Liberman MC (2011) Opposing gradients of ribbon size and AMPA receptor expression underlie sensitivity differences among cochlear-nerve/hair-cell synapses. The Journal of neuroscience : the official journal of the Society for Neuroscience 31:801–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TN, Kim GM, Chen JJ, Cheung WM, He YY, Hsu CY (2003) Differential regulation of thrombospondin-1 and thrombospondin-2 after focal cerebral ischemia/reperfusion. Stroke; a journal of cerebral circulation 34:177–186. [DOI] [PubMed] [Google Scholar]
- Manzoor NF, Gao Y, Licari F, Kaltenbach JA (2013) Comparison and contrast of noise-induced hyperactivity in the dorsal cochlear nucleus and inferior colliculus. Hearing research 295:114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendus D, Sundaresan S, Grillet N, Wangsawihardja F, Leu R, Muller U, Jones SM, Mustapha M (2014) Thrombospondins 1 and 2 are important for afferent synapse formation and function in the inner ear. The European journal of neuroscience 39:1256–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, Zhang X, Hankenson KD, Wang MM (2009) Thrombospondin 2 potentiates notch3/jagged1 signaling. The Journal of biological chemistry 284:7866–7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulders WH, Robertson D (2013) Development of hyperactivity after acoustic trauma in the guinea pig inferior colliculus. Hearing research 298:104–108. [DOI] [PubMed] [Google Scholar]
- Müller M, Hünerbein von K, Hoidis S, Smolders JWT (2005) A physiological place-frequency map of the cochlea in the CBA/J mouse. Hear Res 202: 63–73 [DOI] [PubMed] [Google Scholar]
- Mustapha M, Fang Q, Gong TW, Dolan DF, Raphael Y, Camper SA, Duncan RK (2009) Deafness and permanently reduced potassium channel gene expression and function in hypothyroid Pit1dw mutants. The Journal of neuroscience : the official journal of the Society for Neuroscience 29:1212–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palenski TL, Gurel Z, Sorenson CM, Hankenson KD, and Sheibani (2013). Cyp1B1 expression promotes angiogenesis by suppressing NF-kappaB activity. Am. J. Physiol. Cell Physiol. 305:C1170–C1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedt RA (1996) Effects of aging on potassium homeostasis and the endocochlear potential in the gerbil cochlea. Hearing research 102:125–132. [DOI] [PubMed] [Google Scholar]
- Sliwinska-Kowalska M, Pawelczyk M (2013) Contribution of genetic factors to noise-induced hearing loss: a human studies review. Mutation research 752:61–65. [DOI] [PubMed] [Google Scholar]
- Stucken EZ, Hong RS (2014) Noise-induced hearing loss: an occupational medicine perspective. Current opinion in otolaryngology & head and neck surgery 22:388–393. [DOI] [PubMed] [Google Scholar]