Abstract
Cynara scolymus L. (Artichoke) has been used for the treatment of metabolic disorders. The purpose of the present study was to investigate the hepatoprotective effect of Cynara scolymus leaves extract against a high fat diet (HFD) induced rats. This study investigated the most abundant phenolic compounds rich Cynara scolymus leaves extract and it is antihypercholesterolemic and antioxidative effects in vivo. The hypercaloric high fat diet (HFD) was treated with 200 mg/kg and 400 mg/kg of ethanol extract (EEA) from leaves of Cynara and atorvastatin (ATOR) (10 mg/kg/day) during an 8-week period. Lipid profile was measured and oxidative stress systematic in hepatic tissue was determined. Our data revealed that HFD-induced hepatic dysfunction manifested by significant abnormal levels of AST, ALT, ALP, LDH, and OCT was accompanied by increasing levels of oxidative stress biomarker (ROS, MDA, and AOPP) while decreasing in antioxidant status. Coadministration of EEA significantly reduced serum lipid profile and hepatic disorders which was confirmed to be histological by reducing the fatty liver deposition in hepatic lobule. These findings suggest that Cynara leaves exert antiobesity and antioxidant liver effects in HFD-induced obese rats.
1. Introduction
Nowadays, many researchers proved that obesity was a chronic metabolic disease. This explained the imbalance between the energy expenditure and intake, and it is caused seriously by increase of fat mass and lipid disposition in blood [1]. Kuboota et al. [2] and Holland et al. [3] reported that obesity is correlated with many metabolic diseases such as hypercholesterolemia, diabetes, and cardiovascular disease. According to Park et al. [4], obesity is the most consequence of liver damage, so we necessarily research therapeutic files such as herbs to advance the adequacy of the risk correlated with obesity [5, 6]. Among the medicinal plants identified from Mediterranean area (North Africa and southern Europe), there was Cynara scolymus (Asteraceae). According to Lattanzio et al. [7], this medicinal plant is cultivated almost around the world, because of its beneficial nutrition effects like cooking and medicinal properties. From traditional therapy, Cynara extract has been used as a drug in the treatment of several diseases effects of the biliary tract, digestive action, scurvy, and anemia and also has an antiartherosclerotic effect [8, 9]. Bonomi [10] explained that Cynara leaves extracts have been reported by many studies that it can be used alone or in association with other medicinal plants, to prepare herbal teas or medicinal plant-based capsule, according to Bonomi. Among the constituents of Cynara extract was the active compounds (polyphenols), which exhibited the potential antioxidants properties [11]. Many researchers reported that the treatment with various phenolic compounds achieved controlling the levels of lipid profile in HFD-fed rats [12, 13]. Yet, there is a great data available on in vivo research of Cynara leaves extract included liver complication. For that reason, we seriously plan our study to think of the greatest treatment with Cynara leaves extract on hepatic dysfunction and oxidative status on a model of HFD rats.
2. Materials and Methods
2.1. Preparation of C. scolymus Leaves Extract
Leaves around the stems of C. scolymus were obtained and cut into smaller pieces and then dried at room temperature under shade in order to obtain powder and to be subjected to extraction.
The protocol of extraction mentioned by 200 grams of powder leaves was extracted with different solvents and different polarities (1 L ×72 h): hexane, ethyl acetate, butanol, 75% v/v (ethanol/H2O), and water.
All extracts were filtered, evaporated, and removed pressure using a Rotovapor at 40°C and lyophilized by freeze-dryer (Alpha 1–2 LD plus Martin Christ®) to determine the weight of each extract and then stored at 4°C until analysis.
2.2. Determination of Total Phenol Content
Total phenol content of Cynara leaves extracts was determined using the method of Folin Ciocalteu by Fawole et al. [14]. The mixture contained 1 mL of Folin Ciocalteu reagent, 10 ml of NaCO3, and 1 mL of each extract. Then, the absorbance of each mixture was measured at 750 nm after 30 min. The total phenol content was expressed in terms of Gallic acid equivalent (mg of GAE/g of extract).
2.3. LC-MS/MS Analysis
The antioxidant compound rich EEA from leaves of Cynara was identified by LC-MS/MS analysis which composed an Agilent 1100 LC system (Agilent Technologies, Santa Clara, CA) containing degasser, binary pump, autosampler, and column heater. The column outlet was coupled to an Agilent MSD Ion Trap XCT mass spectrometer (Agilent Technologies, Santa Clara, CA) equipped with an ESI ion source.
The personal computer with Data Analysis software (Chemstations) evaluated data acquisition and mass spectrometric. For the chromatographic separation, we used a Zorbax 300 A° Extend-C-18 Column (2.1 150 mm; Phenomenex UK, Macclesfield, UK). The column was mixed by 95% solvent A (0.1% formic acid in water) and 5% solvent B (0.1% formic acid in acetonitrile) for 1 min, followed by an 11 min step gradient (5% B to 100% B); then it was kept for 4 min with 100% B. In the end, the elution was completed with a linear gradient from 100% to 5% B for 2 min. The flow rate was adjusted at 200 ml/ min and the volume was injected at 5 ml. The following parameters were regulated during all MS experiments: the polarity was charged with positive ion, the capillary voltage was established to 3.5 kV, the drying temperature was fixed to 350°C, the nebulizer pressure was maintained to 40 psi, and the drying gas flow was measured to 10 L/min. Moreover, the maximum accumulation time was fixed to 50 ms, the scan speed was 26 000 m/z/s (ultra scan mode) and the time of fragmentation was 30 ms.
The phenolic compounds were identified using a combination of two analytic methods: high-performance liquid chromatography (HPLC) with diode array detection and liquid chromatography with atmospheric pressure chemical ionization mass spectrometry (ESI- LC/MS/MS) on the basis of their ultraviolet (UV) spectra, mass spectra.
The mass spectra results were compared with those of available authentic standards.
2.4. Determination of Lipase Activity In Vitro
According to Nikai et al. [15], pancreatic lipase activity was measured using 4- methylumbelliferyl oleate (4-MU oleate) as a substrate. The mixture containing 25 µL of each extract dissolved in DMSO and 50 µL of 0.1 mM 4-MU solution dissolved in a buffer (13 mM Tris-HCl, 150 mM NaCl ) and 1.3 mM CaCl2 (pH 8.0) was mixed in the well of a microtiter plate and then 25 µL of the lipase solution (50 U/mL) in the above buffer was added in order to start the enzyme reaction. After incubation at 25°C for 30 min, 0.1 mL of 0.1 M sodium citrate (pH 4.2) was added to block the reaction. The amount of 4-methylumbelliferone released by lipase was measured with a fluorometric microplate reader (Fluoroskan Ascent C LabSystems, Inc.) at an excitation wavelength of 360 nm with a tolerance of ±40 nm and an emission wavelength of 460 nm with a tolerance of ± 20 nm.
Orlistat is used as a positive control. PI: percentage of lipase pancreatic activity inhibition.
| (1) |
2.5. Animals and Diet
Thirty Wistar female rats, weighing 180 ± 2g, were collected from the Tunisian Pharmaceutical Industries (SIPHAT, Tunisia). The environmental conditions of animals were kept in a controlled room (60% humidity, 25°C, and 12-h light-dark cycle) in the laboratory of Animal Ecophysiology of Sfax City, Tunisia. All these animal studies have occurred in this study guided by the International Guidelines for Animal Care and were accredited by the Tunisian Ethics Committee of the University of Sfax (Sfax, Tunisia).
Before animal experimentation, all animal were kept to acclimate for one week and fed on a standard diet (corn, soy, and vitamins) supplied by the Company of Animals Nutrition, Sfax, Tunisia.
2.6. Experimental Design
The creation of the model of high fat diet (HFD) was composed of 79.9% normal diet, 10% sheep fats, and 0.1% cholic acid. 30 Wistar rats were divided into 5 groups (n= 6 six):
Groups I: (C). Control rats fed on a standard diet.
Groups II: (HFD). Rats fed on the high fat diet, for 8 months.
Groups III: HFD + ATOR. Rates fed on HFD and treated with 10 mg/kg /bw a commercial drug ATOR (atorvastatin) by gastric gavages route in a volume of 1 ml during 2 months daily [16].
Groups IV: HFD+ EEA (200mg/kg/bw). Rats fed on HFD and treated with ethanol extract from leaves of Cynara scolymus at doses 200mg/kg/bw by gastric gavages route in a volume of 1 ml during 2 months daily [17].
Groups VI: HFD+ EEA (400mg/kg/bw). Rats fed on HFD and treated with ethanol extract from leaves of Cynara scolymus at doses 400mg/kg/bw by gastric gavages route in a volume of 1 ml during 2 months daily [17].
2.7. Measurement of Body Weights and Relative Organ Weights
Body weight was measured weekly during 2 months of each group and relative liver weight after sacrifice in the experiment period regularly.
2.8. Biochemical Analysis
The animal was sacrificed by decapitation and the trunk blood was collected. The serum of each rat was obtained by centrifugation (3000∗g, 15 min, 4°C) and stored at -20°C until biochemical analysis. The analyses of serum lipase and lipid profile (Triglycerides (TG), total cholesterol (T-Ch), and high-density lipoprotein cholesterol (HDL-C)) using the corresponding commercial kits (Biolabs, France) on an automatic biochemistry analyzer at the biochemical laboratory of CHU Habib Bourguiba Hospital of Sfax.
Low-density lipoprotein cholesterol concentration (LDL-c) was determined by Friedewald et al. formula [18].
Serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and lactate dehydrogenase (LDH) were measured in frozen aliquots of serum by standardized enzymatic procedures using commercial kits from (Biolabs, France) on an automatic biochemistry analyzer at the biochemical laboratory of CHU, Habib Bourguiba Hospital of Sfax. For the ornithine carbamoyltransferase (OCT) activity was determined by the colorimetric spectrophotometric methods [19].
2.9. Determination of Hematological Parameters
Hematological parameters including red blood cells (RBC), hemoglobin (Hb), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), white blood cell (WBC), and platelets were measured by Horiba ABX 80 Diagnostics (ABX pentra Montpellier, France).
2.10. Determination of Liver Oxidative Stress Markers
Oxidative stress was estimated through the following parameters: the extent of lipid peroxidation by measuring the thiobarbituric acid reactive substances (TBARS) in terms of malondialdehyde (MDA) formation according to Draper and Hadley [20] and expressed as nmol MDA/mg protein and the protein oxidation by measuring the advanced oxidation protein (AOPP) by Kayali et al. [21] method and expressed as nmol/mg protein.
2.11. Determination of Non- Enzymatic and Enzymatic Antioxidants Systems
Superoxide dismutase (SOD) activity was measured by Beyer and Fridovich [22] method and expressed as U/mg protein. Glutathione (GPx) activity was determined by the method described by Pagila and Valentine [23] and expressed as mmoles GSS/(min_mg protein). Reduced glutathione (GSH) activity was estimated according to the described method by Carlberg and Mannervik [24] and expressed as ug GSH/mg protein. The protein level was evaluated by Lowry et al. [25] using bovine serum albumin as the standard at 660 nm.
2.12. Determination of ROS Production
According to Gupta et al. [26] methods, the reactive oxygen species formation (ROS) in liver tissue was described with some modifications. Liver samples (200 mg) were homogenized in ice-cold Tris-HCl buffer (40 mM, pH 7.4) (1:10 w/v). Then, 100 mL of samples of tissue homogenate were mixed with Tris-HCl buffer (1 mL) and 5 mL of 20,70-dichlorofluorescein diacetate (10 mM) (Sigma-Aldrich, St. Louis, MO). The mixture then was incubated for 30 min in 37°C. After the incubation, the fluorescence intensity of the samples was assessed using a FLUOstar Omega multifunctional microplate reader (λexcitation 485 nm and λexcitation 535 nm).
2.13. Histopathological Liver Analysis
After the sacrifice of experimental rats, a small portion of each liver tissue of each rat was eliminated and fixed in 10% formaldehyde solution. Each washed tissues was dehydrated in increasing gradient of ethanol and finally cleared into toluene. The liver tissues were then embedded in molten paraffin wax. Sections were cut at 5 mm thickness and stained with hematoxylin and eosin (H&E).
3. Statistical Analysis
Data are expressed as mean ± standard deviation (mean ± SD). The one-way analysis of variance (ANOVA) and the Tukey post hoc test were performed on the data for intergroup comparisons. Database management and statistical analysis were performed using SPSS (SPSS Inc., Chicago, IL) statistical software package. The nominal statistical significance level was set at 0.05.
4. Results
4.1. Determination of Total Phenol Content
Figure 1 showed the highest content of total phenol content was obtained using 75% of ethanol extract from leaves of C. scolymus as the extraction solvent and corresponded to 54.54 ± 1.26 mg GAE/g dry extract, followed by ethyl acetate, aqueous, and butanol extracts, while the hexane extract was the lowest in the total phenol content (30.91 ± 9.36 mg GAE/ g dry extract).
Figure 1.
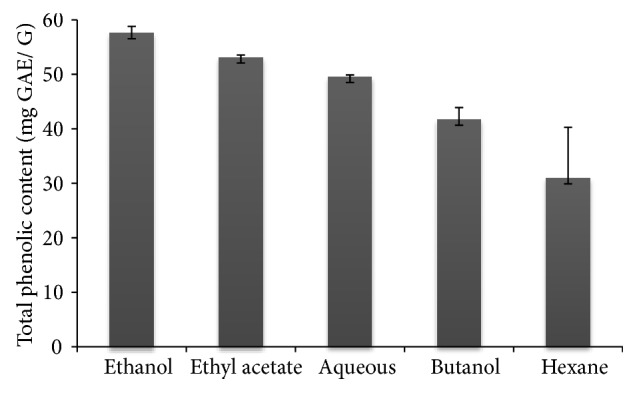
Total phenolic content of various extracts from leaves of Cynara scolymus. Values are given as means ± SD (n = 3).
4.2. LC-MS/MS Analysis
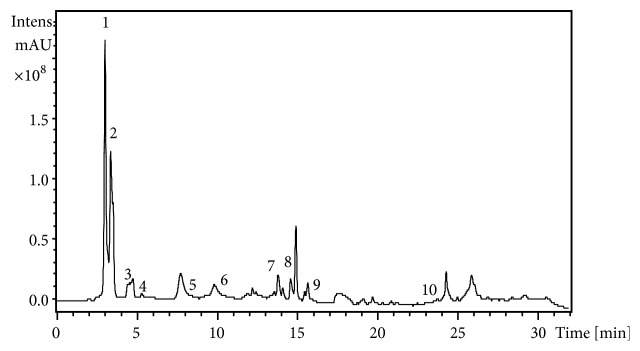
From the HPLC chromatogram of the EEA from leaves of Cynara scolymus, ten phenolic components were identified (Figure 2). Table 1 showed the retention times, UV max, the mode (+ or −) as well as the base peak (MS), and fragment ion (MS2). In the analysis of peak 1 by LC-MS/MS, a positive molecular ion at [MS+H]+ at m/z 122.4 as well as a fragment ion at m/z of 122.3, corresponding to salicylic acid-o-hexoside, was observed. Peak 2 exhibited from the same analysis a negative molecular ion [MS−H]- at m/z 117.2 as well as a fragment ion at an m/z 100, corresponding to chlorogenic acid. Peak 3 had a negative molecular ion [MS−H]- at m/z of 112 and three negative fragment ions at m/z of 214.8, 178.8, and 352.8 corresponding to a 3-mono-o-caffeoylquinic acid. Peak 4 exhibited a negative molecular ion at [MS−H]- at m/z 141 and the negative fragment ion at m/z of 265.9 which correspond to apigenin-7-glucoside. Peak 6 revealed a negative molecular ion [MS−H]- at m/z 275 and three negative fragment ions at m/z of 529.7, 350.8, and 284.7. The corresponding compound was identified as luteolin-7-o-rutinoside. Compounds in peak 7 had a negative molecular ion at m/z 327.1 and fragment at m/z of 170.9; this is suggestive of a dihydroxypropiophend-hexoside compound. The kaempferol 3-o-rutinoside was identified in peak 8 with a negative molecular ion at [MS−H]- at m/z of 395 and fragmentation products at m/z of 529.7, 288.9, and 307. Peak 9 revealed a negative molecular ion at [MS-H]- at m/z of 431 and three negative fragment ions at m/z of 434.7, 294.9, and 344.8; the corresponding compound was identified as quercetin-o-pentoside. Compounds in peak 10 had a negative molecular ion at m/z 623 and fragment at m/z of 255.1; this is suggestive as Caffeic acid compound. In the analysis of compound in peak 5 with referring to standards and literature, it was not well identified.
Figure 2.
LC-MS/MS chromatogram of ethanol extract from leaves of Cynara scolymus at two different wavelengths (200 and 700 nm).
Table 1.
Identification of phenolic compounds in ethanol extract from leaves of C. scolymus using their retention times, LC/MS, and LC–MS/MS data.
| Peaka | RT (min) | m/z | Mode (+/-) | ƛ max (min) | MS/MS | Tentative ID |
|---|---|---|---|---|---|---|
| 1 | 3.00 | 122.3 | + | 200-700 | 82 | Salicylic acid-o-hexoside |
| 2 | 3.7 | 117.2 | - | 200-700 | 100 | Chlorogenic acid |
| 3 | 4.2 | 112 | - | 200-700 | 214.8, 178.8, 352.8 | 3-mono-o-cafeoylquinic acid |
| 4 | 5.3 | 141 | - | 200-700 | 265.9 | Apigenin-7-glucoside |
| 5 | 8.4 | 223 | - | 200-700 | 331.4 | Unknown |
| 6 | 10.30 | 275 | - | 200-700 | 529.7, 350.8, 284.7 | Luteolin-7-o-rutinoside |
| 7 | 13.5 | 327.1 | - | 200-700 | 170.9 | Dihydroxypropiophend-hexoside |
| 8 | 14.8 | 395 | - | 200-700 | 529.7, 288. 9,307 | Kampferol 3-o-rutinoside |
| 9 | 16.2 | 431 | - | 200-700 | 434.7, 294.9, 344.8 | Quercetin-o-pentoside |
| 10 | 23.3 | 623 | - | 200-700 | 255.1 | Caffeic acid |
Identification was aided by comparison with reference standards that were available and by correlation with previous literature reports.
a Peak numbers and retention times (Rt) refer to LC/MS chromatograms in Figure 2.
4.3. Lipase Pancreatic Inhibition Activity
Table 2 showed that EEA presented a great pancreatic lipase inhibition with an IC50 value of 61.50 µg/mL as compared to Orlistat specific inhibitor (IC50 = 17.76 µg/mL). The potential in in vitro inhibitory effect of lipase was observed to EEA compared to the other extracts that leads us to apply it for in vivo investigation.
Table 2.
The inhibitory capacity of C. scolymus leaves extracts against lipase pancreatic activity.
| Concentration (ug/ml) | % Inhibition | CI50 (ug/ml) | |
|---|---|---|---|
| Orlistat | 25 | 80.25 ± 1.08 | 17.76 |
| 50 | 97.21 ± 0.59 | ||
| 100 | 90.35 ± 1.20 | ||
|
| |||
| Hexane | 25 | 17.46 ± 0.05 | 200.15 |
| 50 | 20.13 ± 1.19 | ||
| 100 | 49.81 ± 0.28 | ||
|
| |||
| Ethylacetate | 25 | - | - |
| 50 | |||
| 100 | |||
|
| |||
| Butanol | 25 | 23.09 ± 1.88 | 101.50 |
| 50 | 30.13± 1.02 | ||
| 100 | 70.18 ± 0.07 | ||
|
| |||
| Ethanol | 25 | 60.38 ± 1.58 | 61.50 |
| 50 | 69.23 ± 0.84 | ||
| 100 | 73.90 ± 1.77 | ||
|
| |||
| Aqueous | 25 | 36.81 ± 0.07 | 95.78 |
| 50 | 62.10 ± 1.02 | ||
| 100 | 67.24 ± 0.13 | ||
All data are expressed as mean ± SD. (-): inactive.
4.4. Evaluation of Body Absolute and Relative Organ Weights
HFD groups showed a significant increase in body weight and liver relative weight (p<0.001) compared with the control groups, while HFD groups treated with EEA at doses (200-400 mg/kg/bw) observed a significant decrease (p<0.001) when compared to HFD groups (Table 3, Figure 3).
Table 3.
Effect of treatment with C. scolymus leaves extract on body weight.
| Groups | Cont | HFD | HFD+EEA (200mg/kg/bw) | HFD+EEA (400mg/kg/bw) | HFD+ATOR |
|---|---|---|---|---|---|
| Day 0 | 210.00 ± 0.67 | 265.66 ± 3.45∗∗∗ | 260.45 ± 2.21 | 266.00 ± 2.13 | 255.78 ± 2.30∗∗ |
| Day 65 | 214.50±10.40 | 280.10±13.93∗∗∗ | 222.5 ± 15.00### | 234.33 ±10.11###¥ | 216.66 ±18.00### |
HFD groups were orally administered with ethanol extract (EEA) from leaves of C. scolymus and atorvastatin (ATOR) at the doses mentioned earlier for 60 days. Values are given as means ± SD (n = 6).∗∗ p ≤ 0.01 and ∗∗∗ p ≤ 0.001 were considered significant compared to control groups; ### p ≤ 0.001 was considered significant compared to HFD groups; ¥p ≤ 0.05 was considered significant compared to HFD groups treated with ATOR.
Figure 3.
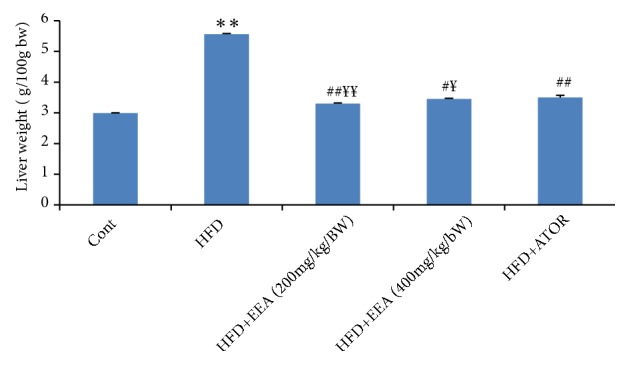
Effect of C. scolymus leaves extract on the liver weight of control and HFD groups. HFD groups were orally administered with ethanol extract (EEA) from leaves of C. scolymus and atorvastatin (ATOR) at the doses mentioned earlier for 60 days. Values are given as means ± SD (n = 6). ∗∗p ≤ 0.01 was considered significant compared to control groups; # p ≤ 0.05; ## p ≤ 0.01 were considered significant compared to HFD groups; ¥p ≤ 0.05; ¥¥ p ≤ 0.01 were considered significant compared to HFD groups treated with atorvastatin.
4.5. Effect of a High Fat Diet on Lipase Pancreatic in Serum
Our results indicated that treatment with HFD for 8 months showed a significantly elevated (p<0.001) in lipase pancreatic activity in serum by 75 % in compared with control groups, while the treatment with EEA from leaves of C. scolymus and ATOR decreased significantly (p<0.001) these levels by 56 % in serum compared with HFD groups (Figure 4).
Figure 4.
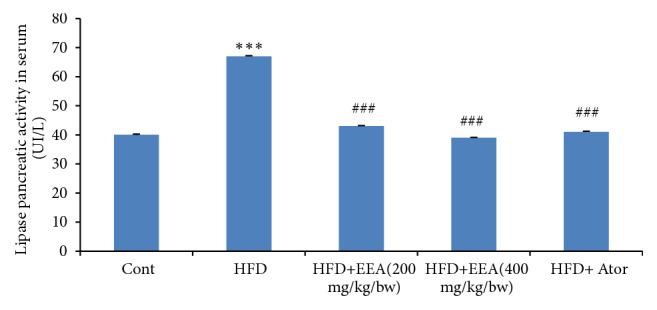
Effect of treatment with C. scolymus leaves extract on lipase activity in serum of control and HFD groups. HFD groups were orally administered with ethanol extract from leaves of C. scolymus and atorvastatin (ATOR) at the doses mentioned earlier for 60 days. Values are given as means ± SD (n = 6). ∗∗∗p ≤ 0.001 was considered significant compared to control groups; ### p ≤ 0.001 was considered significant compared to HFD groups.
4.6. Effect of a High Fat Diet on Serum Lipid Profile
The effect of HFD on serum biochemical was shown in Table 4. The levels of TG, TC, and LDL-c showed a significant (p<0.001) increase by 46.06 %, 69.36 %, and 66.66 % as compared to control groups. Meanwhile, the serum level of HDL-c significantly (p<0.001) decreased by 59% in HFD groups.
Table 4.
Effect of treatment with C. scolymus leaves extract on serum lipid profile.
| Groups | Cont | HFD | HFD+ EEA (200mg/kg / bw) |
HFD+EEA (400mg/kg/bw) | HFD+ATOR |
|---|---|---|---|---|---|
| TG (mmol/L) | 1.00 ± 0.29 | 2.50±0.40∗∗∗ | 1.13 ± 0.20### | 0.8± 0.42### | 1.00 ± 0.29### |
| T-Ch (mmol/L) | 1.37 ± 0.12 | 3.50±0.55∗∗∗ | 1.60 ± 0.15### | 1.70 ± 0.15### | 1.62 ± 0.08### |
| LDL- c (mmol/L) | 0.60 ± 0.19 | 1.80±0.07∗∗∗ | 1.30± 0.35### | 0.80 ± 0.01### | 0.45 ± 0.40### |
| HDL-c (mmol/L) | 1.44 ± 0.33 | 0.50±0.10∗∗∗ | 0.80 ± 0.33### | 1.19± 0.07###¥ | 0.76 ± 0.08### |
| AIP | 0.95 ± 0.17 | 6.88±0.14∗∗∗ | 2.00 ± 0.12### | 1.48 ±0.19###¥¥ | 2.13 ± 0.20### |
| CRI-I | 0.951 | 7 | 2 | 1.42 | 2.13 |
HFD groups were orally administered with ethanol extract (EEA) from leaves of C. scolymus and atorvastatin (ATOR) at the doses mentioned earlier for 60 days. Values are given as means ± SD (n = 6). ∗∗∗ p ≤ 0.001 was considered significant compared to control groups; ### p ≤ 0.001 was considered significant compared to HFD groups; ¥p ≤ 0.05 and ¥¥ p ≤ 0.01 were considered significant compared to HFD groups treated with ATOR.
The administration of EEA (200-400 mg/kg/bw) restored normal levels of TC by 45.67 %, 67.78 %, and 36.22 %, TG by 54.92 %, 68.08 %, and 60.11 %, and LDL-c by 27.27 %, 33.33 %, and 44.44 % (p<0.001) and caused significant increase in serum HDL-c level by 44.27 %, 60.79 %, and 38.76 % (p<0.001) compared to HFD groups.
Our results indicated that HFD for a duration of 8 months has significantly increased the risk of atherosclerosis indicated by the atherogenic index of plasma (AIP) and Castelli's risk index-I (CRI-I). However the therapy with EEA (200-400mg/kg/bw) significantly (p<0.001) decreased the AIP and (CRI-I) levels compared to HFD groups.
4.7. Effect of Cynara scolymus Leaves Extract in Hepatic Dysfunction Enzymes
HFD caused severe hepatotoxicity as evidenced by a significant elevation (p<0.001) of serum AST, ALT, LDH, ALP, and OCT levels, compared to control groups. However, the coadministration of EEA (200-400mg/kg/bw) and ATOR completely recovered the impaired liver functions by decreasing hepatic parameters compared with HFD groups (Table 5).
Table 5.
Effect of treatment with C. scolymus leaves extract on liver dysfunction enzymes.
| Groups | AST (UI/L) | ALT (UI/L) | LDH (UI/L) | ALP (UI/L) | OCT (mg / g protein) |
|---|---|---|---|---|---|
| Cont | 131.9 ± 1.50 | 42.76 ± 1.78 | 76.2 ± 2.5 | 64.78 ± 2.50 | 10.5±1.3 |
| HFD | 273.7 ±2.33∗∗∗ | 76.03±7.31∗∗∗ | 137±8.03∗∗∗ | 206.66± 6.52∗∗∗ | 7.9±0.5∗ |
| HFD+EEA(200mg/kg/bw) | 128.56 ±7.87### | 41.51 ± 8.03### | 70.18 ±8.03## | 72.83 ± 8.00## | 5.7±0.2# |
| HFD+EEA(400mg/kg/bw) | 112.18 ±5.47### | 36.56 ±1.85###¥ | 63.2 ±1.85### | 72.20 ± 3.00## | 6.0±0.1 |
| HFD+ATOR | 169.18 ±4.37### | 49.36±3.73###¥¥ | 84.5 ± 1.22## | 89.2 ± 4.18## | 6.4±0.7 |
HFD groups were orally given ethanol extract (EEA) from leaves of C. scolymus and atorvastatin (ATOR) at the doses mentioned earlier for 60 days. Values are given as means ± SD (n = 6). ∗p ≤ 0.05 and ∗∗∗ p ≤ 0.001 were considered significant compared to control groups; # p ≤ 0.05, ## p ≤ 0.01, and ### p ≤ 0.001 were considered significant compared to HFD groups; ¥p ≤ 0.05 and ¥¥ p ≤ 0.01 were considered significant compared to HFD groups treated with ATOR.
4.8. Effect of Cynara scolymus Leaves Extract on Hematological Parameters
The results indicated that, compared to control groups, there was a significant decrease in the levels of RBC, Hb, MCV, MCH, MCHC, WBC, and platelets observed in HFD groups. However, these hematological markers were considerably increased to near normal values after the treatment with EEA from leaves of C. scolymus (200-400 mg/kg/bw) (p < 0.001) compared with HFD groups (Table 6).
Table 6.
Effect of treatment with C. scolymus leaves extracts on hematological parameters.
| Groups | Cont | HFD | HFD+EEA (200mg/kg/bw) | HFD+EEA (400mg/kg/bw) | HFD+ATOR |
|---|---|---|---|---|---|
| GR (×106) | 6.96 ± 2.37 | 9.13 ± 0.09 | 8.91 ± 0.04¥¥ | 9.00 ± 0.07#¥¥¥ | 8.48 ± 0.04### |
| Hb (g/dL) | 12.55 ± 0.91 | 15.15± 0.91∗∗∗ | 14.85± 0.63 | 15.00 ± 0.21 | 14.80 ± 0.14 |
| HCT(fL) | 36.25± 0.96 | 44.35 ± 1.90∗∗∗ | 43.45 ± 1.90 | 38.55± 0.35#¥¥¥ | 35.75 ± 0.07# |
| VGM(fL) | 50.55 ± 0.63 | 54.9±3.53 | 48.75 ± 2.61 | 50.9 ± 0.00 | 50.55 ± 0.63 |
| TCMH (g/dL) | 13.45± 4.73 | 33.7± 0.15∗∗ | 16.65 ± 0.91## | 16.75 ± 0.07###¥¥¥ | 17.45 ± 0.07## |
| MCHC (g/dL) | 31.6± 1.97 | 33.7± 1.27 | 30.2 ± 0.00 | 32.85 ± 0.21 | 32.85 ± 0.21 |
| GB (×103 /uL) | 6.82 ± 2.06 | 17.45 ± 2.04∗∗ | 10.41 ± 2.06# | 11.2 ±1.12# | 9.46 ±1.08## |
| PLT (×103/uL) | 859 ± 9.23 | 1260 ± 9.34∗∗∗ | 839.5± 8.45## | 914.5± 11.87## | 879 ± 8.21## |
HFD groups were orally administered with ethanol extract (EEA) from leaves of C. scolymus and atorvastatin (ATOR) at the doses mentioned earlier for 60 days. Values are given as means ± SD (n = 6). ∗∗ p ≤ 0.01 and ∗∗∗ p ≤ 0.001 were considered significant compared to control groups; # p ≤ 0.05, ## p ≤ 0.01, and ### p ≤ 0.001 were considered significant compared to HFD groups; ¥¥ p ≤ 0.01 and ¥¥¥ p ≤ 0.001 were considered significant compared to HFD groups treated with ATOR.
4.9. Effect of Cynara scolymus Leaves Extract in Oxidative Stress Status
HFD has significantly (p < 0.001) increased TBARS levels by 52.29 %, AOPP levels by 55.58 %, and liver ROS intracellular production by 53.33 % as compared with HFD groups (Table 7). After all, treatment with EEA at both doses (200-400mg/kg/bw) in HFD rats has improved the antioxidant capacities significantly (p< 0.001) in the liver by increasing SOD, GP-x, and GSH activities as compared to HFD groups.
Table 7.
Effect of treatment with C. scolymus leaves extract on liver oxidative stress parameters.
| Groups | Liver ROS (fluorescence intensity, FI) | TBARS (nmol MDA/ mg protein) | AOPP (nmol/mg protein) | SOD (U SOD/mg protein) | GSH (ug/mg protein) | GPx (nmol GSH min/mg protein) |
|---|---|---|---|---|---|---|
| Cont | 70.02 ±11.23 | 8.64 ± 0.43 | 4.62 ± 0.01 | 3.79±0.31 | 5.45 ± 0.41 | 6.46 ± 0.60 |
| HFD | 150.30±20.34∗∗∗ | 18.15±0.92∗∗∗ | 8.85±0.08∗∗∗ | 1.91±0.04∗∗∗ | 2.06 ± 0.14∗∗∗ | 4.55 ± 0.28∗∗∗ |
| HFD+EEA(200mg/kg/bw) | 80.23±15.17## | 10.51± 0.56### | 5.12 ± 0.05### | 2.84±0.19### | 4.17± 0.28###¥¥¥ | 5.81 ± 0.17### |
| HFD+EEA(400mg/kg/bw) | 84.17± 16.57### | 9.25 ± 0.61### | 4.53± 0.04### | 2.91±0.04### | 5.10 ± 0.15### | 6.00 ± 0.56###¥ |
| HFD+ATOR | 72.49±13.45### | 10.44 ±1.03### | 4.37 ± 0.05### | 2.49±0.49### | 4.09 ± 0.33### | 5.45 ± 0.15### |
HFD groups were orally administered with ethanol extract (EEA) from leaves of C. scolymus and atorvastatin (ATOR) at the doses mentioned earlier for 60 days. Values are given as means ± SD (n = 6). ∗∗∗ p ≤ 0.001 was considered significant compared to control groups; ### p ≤ 0.001 was considered significant compared to HFD groups; ¥ p ≤ 0.05 and ¥¥¥ p ≤ 0.001 were considered significant compared to HFD groups treated with
ATOR.
4.10. Histopathological Analysis of Liver
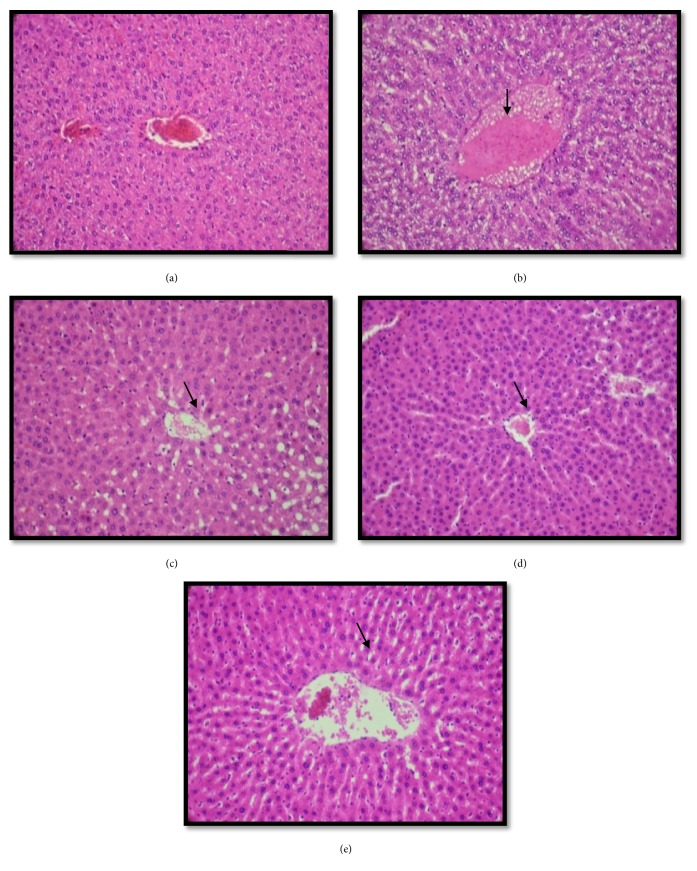
To approve our results on the biochemical analysis, we examined the pathologic changes in liver microscopically. Our study revealed that the histological findings in the liver indicated the presence of lipid accumulation in hepatic lobule in the case of the HFD groups, indicated by arrows (Figure 5(b)). However, the treatment with EEA from leaves of C. scolymus (200-400 mg/kg/bw) and ATOR showed a protective effect in the form of the liver histoarchitecture (Figures 5(c), 5(d), and 5(e)).
Figure 5.
Histopathological examination of liver in experimental groups of rats. (a) represents a normal hepatic lobules of the control groups; (b) represents histopathological investigation of liver tissue in rats fed on HFD groups which showed severe macrovascular fatty changes distributed throughout the liver lobules; (c and d) represent HFD+ EEA (200- 400mg/kg/bw) groups, which showed a reduction in accumulation of fatty throughout the liver lobules; (e) represents HFD+ ATOR groups in which a potential protective action was shown (H&E × 400).
5. Discussion
One of the most chronic metabolic diseases was obesity, including severe human complication. It is closely related to type 2 diabetes, hypertension and cardiovascular disease according to Wilis et al. [27], cancer, respiratory complications, and osteoarthritis [28], which has been increased at an alarming percentage around the world.
According to Mnafgui et al. [29], the detection of antiobesity drugs without undesirable side effect has become a big problem. Nowadays, the medicinal plant has been used as a complementary treatment, in order to decrease the undesirable effect of drugs treated by patients.
This research was carried out to appreciate the potentiality effect of C. scolymus leaves extract on obese rat induced HFD and to assess its impact therapeutic about obesity.
Chakrabarti [30] demonstrated that the use of safer lipase inhibitors is considered as an efficacious therapeutic approach to overcome obesity and hyperlipidemia by the delay of the digestion and absorption of fat.
It is known that the major role of pancreatic lipase is the digestion of fats by dividing dietary triglycerides into monoacylglycerides and free fatty acids and then is the absorption by enterocytes. Among the drugs used for the prevention of obesity and hyperlipidemia by inhibition of the pancreatic lipase activity by the Orlistat [31], from our results, the pancreatic lipase activity was inhibited by EEA by hydrolyzing of dietary triglycerides nonabsorbable into monoglycerides and free fatty acids absorbable by the intestine. Our results were consistent with the observation by Mnafgui et al. [29]. Accordingly, we can explore the mentioned ethanol extract in vivo. The inhibition of pancreatic lipase activity was one of the solutions to avoid obesity complication [32]. The present research evidenced that EEA inhibited lipase activity in the plasma in HFD groups, which make a decrease in serum T-Ch, LDL-c, and TG levels as well as a significant reduction in the calculated atherogenic index (AI). Consequently, it showed an increase in HDL-c and a decrease in body and liver weight like antiobesity action [33]. These results were confirmed by histological findings which proved a fatty disposition throughout the liver lobules. So it is clear that the administration of EEA from leaves of C. scolymus for two months caused a suppressive effect on fat accumulation in all experimental groups.
These results were confirmed to Abdel Magied et al. [34] who showed the strongest hypotriglyceridemic and hypocholesterolemic effects of C. scolymus leaves extract from Egypt; moreover, this study showed the hypocholesterolemic effect of aqueous extract from leaves of C. scolymus at doses (150, 300, and 600 mg/kg bw) for one month of treatment, similar to that reported by Küskü-Kiraz et al. [35] who revealed a significant decrease in Ch-T and TG level in C. scolymus leaves extract at doses of 1,5mg/kg bw for two weeks in hypercholesterolemic diet rats. Meanwhile, any reduction was observed in LDL- c, TG, and VLDL-c level with a hydroalcoholic extract from leaves of C. scolymus at doses of 100-400 mg/kg/bw for two weeks of treatment.
The efficacious hypolipidemia effect of EEA might be associated with a high content of active compounds richness Cynara leaves such as phenols, flavonoids, and tannins. Among these active constituents identified by LC-MS/MS, it was mentioned that cynarin and luteolin play a crucial role in inhibiting cholesterol and triglycerides synthesis according to Ben Salem et al. [36]. Some studies showed that luteolin compound could inhibit 60% of cholesterol synthesis in the digestive tract, by regulation of HMG-CoA reductase activity [9, 37]. Moreover, Qiang et al. [38] mentioned that C. scolymus leaves extract is able to diminish hypercholesterolemia by increasing the fecal excretion of bile acids and by inhibition of HMG-CoA reductase activity, following a significant LDL-c decreasing effect [39].
Our results were in accordance with the results of Kucukgergin et al. [40] who reported that Cynara leaves extract treatment for hypercholesterolemic rats was useful for decreasing serum cholesterol and triglycerides levels of rats. On the other hand, Cynara extract has been proposed to be antiatherogenic, due to its lipid-reducing and antioxidant effect [41, 42].
According to Gebhardt [8], it was reported the hepatoprotective effect of Cynara extract by reducing the cholesterol biosynthesis and the oxidation of LDL [41, 43, 44]. Among the biological activities of C. scolymus, the antimicrobial properties in the gut disorder the intestinal microflora; this allows affecting the absorption of cholesterol [45]. Moreover, Brown and Rice-Evans [46] state that chlorogenic acid and luteolin may prevent atherosclerosis disease by inhibiting LDL oxidation.
The results of the biochemical analysis were confirmed by histopathological of liver tissue which showed that EEA at doses 200 mg/kg bw-400 mg/kg bw from leaves of C. scolymus treated HFD revealed a reduction in fatty infiltration in the liver. From these results, we can conclude that Cynara leaves extract may be useful for the preventive treatment of hypercholesterolemia.
Moreover, many markers proposed include mitochondrial markers, such as ornithine carbamoyl transferase, since this marker has been considered less sensitive in the detection of cellular damage than cytosolic markers such as ALT and AST [47]. However, Murayama et al. [48] showed that OCT marker was recently proved to be more sensitive than ALT and AST in the detection of hepatotoxicities induced by several toxicants. Therefore, to evaluate better the hepatotoxicity in HFD model, ALT, AST, ALP, LDH, and OCT enzymes are analyzed, and their elevation in circulation suggests significant hepatocellular damage [49, 50].
Our results revealed that EEA treatment significantly reduced these hepatic enzymatic; therefore the extract ameliorated the potential liver damage caused by HFD.
These results are in accordance with the results of Raida et al. [32] and Ben Abdallah et al. [51]. Many researchers showed the hepatoprotective effect of C. scolymus leaves extract, related to its active compounds. However, more researches were needed to fully explain the hepatoprotective mechanisms and cellular.
Ouchi et al. [52] state that obesity is related to an increase in inflammatory markers which characterized a low-grade chronic inflammation. In this study, we noted that HFD provokes increase in inflammatory markers. However, the treatment with EEA (200- 400mg/kg/bw) showed a significant decrease in inflammatory markers. The anti-inflammatory properties of Cynara were confirmed by Ben Salem et al. [36] in the report of nonalcoholic fatty liver disease.
Vial et al. [53] found a relationship between HFD and increased reactive oxygen species (ROS) production in the liver. ROS causes cell damage via the mechanism implying lipid peroxidation and protein oxidation that disposes tissue damage, especially in the liver [54]. In the current investigation, we found that HFD caused oxidative stress in the liver as revealed by increasing in ROS levels production as mentioned in Table 7, accompanied by increasing in lipid peroxidation and decreasing in antioxidant activities. Ohara et al. [55] reported that HFD was a set of chronic pathologies that can cause hypercholesterolemia and oxidative stress.
Among the antioxidants systems, antioxidant enzymes such as GSH, SOD, and GPx present great defense mechanisms, which play a pivotal role in maintaining the ROS under adequate concentrations. In the current study, EEA (200-400 mg/kg/bw) treatment provokes an increase of GSH, SOD, and GPx activities. As similar results found in Dianita et al. [16] and Abdel Majid et al. [34] studies, it has also been reported that Cynara leaves extract treatment caused significant increases in GSH-Px activities in the liver [56]. Kuçükgergin et al. [40] reported that Cynara leaves extract caused significant decreases in MDA levels in the liver tissues with an increase in hepatic GSH-Px activities in hypercholesterolemic rats. Moreover, Ben Salem et al. [11] proved the antioxidative effect of EEA (200-400 mg/kg bw) in the liver tissues of the diabetic rat.
The report of Mehmetcik et al. [56] showed a decrease in hepatic MDA and conjugated dienes levels and an increase in GSH and GSH-Px activities after treatment with Cynara leaves extract. An increase of antioxidant activity in erythrocytes was also found by Küçükgergin et al. [40], after treatment with water extract from Cynara which confirmed the antioxidant properties of this plant.
Many in vitro studies have shown that the antioxidant potential of Cynara is dependent on radical scavenging and metal ion chelating effect of its active constituents such as cynarin, chlorogenic acid, and flavonoids [46, 57]. Nevertheless, the in vivo efficiency has not been confirmed sufficiently. Most in vivo studies correlated to the antioxidative capacity of Cynara and the hepatoprotective effect [58].
6. Conclusion
Cynara scolymus leaves extracts, especially their ethanol extract, exert a potential effect on rats induced by a high fat-rich diet. The administration of antioxidant-rich Cynara extract ameliorates the undesirable effect of a high fat diet on lipid accumulation and hepatic disorders.
Acknowledgments
This research was supported by the Tunisian Ministry of Higher Education and Scientific Research.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Fujioka K. Management of obesity as a chronic disease: Nonpharmacologic, pharmacologic, and surgical options. Obesity Research. 2002;10:116–123. doi: 10.1038/oby.2002.204. [DOI] [PubMed] [Google Scholar]
- 2.Kubota N., Terauchi Y., Miki H., Tamemoto H., Yamauchi T., Komeda K., et al. PPAR gamma mediates high-fat diet-induce adipocyte hypertrophy and insulin resistance. Molecular Cell. 1999;4 doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- 3.Holland W. L., Brozinick J. T., Wang L. P., et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metabolism. 2007;5(3):167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Park E. J., Lee J. H., Yu G.-Y., et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140(2):197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim Y. J., Bae Y. C., Suh K. T., Jung J. S. Quercetin, a flavonoid, inhibits proliferation and increases osteogenic differentiation in human adipose stromal cells. Biochemical Pharmacology. 2006;72(10):1268–1278. doi: 10.1016/j.bcp.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 6.Kim S. Y., Jin Y. J., Choi Y. S., Park T. Resveratrol exerts anti-obesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochemical Pharmacology. 2011;81(11):1343–1351. doi: 10.1016/j.bcp.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Lattanzio V., Kroon P. A., Linsalata V., Cardinali A. Globe artichoke: A functional food and source of nutraceutical ingredients. Journal of Functional Foods. 2009;1(2):131–144. doi: 10.1016/j.jff.2009.01.002. [DOI] [Google Scholar]
- 8.Gebhardt R. Antioxidative and protective properties of extracts from leaves of the artichoke (Cynara scolymus L.) against hydroperoxide-induced oxidative stress in cultured rat hepatocytes. Toxicology and Applied Pharmacology. 1997;144(2):279–286. doi: 10.1006/taap.1997.8130. [DOI] [PubMed] [Google Scholar]
- 9.Kraft K. Artichoke leaf extract — Recent findings reflecting effects on lipid metabolism, liver and gastrointestinal tracts. Phytomedicine. 1997;4(4):369–378. doi: 10.1016/S0944-7113(97)80049-9. [DOI] [PubMed] [Google Scholar]
- 10.Bonomi B. M. L’impiego della farina di foglie di carciofo disidratate (Cinara scolymus L.) nell’alimentazione delle manzette e delle manze. La Rivista di Scienza dell’Alimentazione. 2001;30:361–370. [Google Scholar]
- 11.Ben Salem M., Ben Abdallah Kolsi R., Dhouibi R., et al. Protective effects of Cynara scolymus leaves extract on metabolic disorders and oxidative stress in alloxan-diabetic rats. BMC Complementary and Alternative Medicine. 2017;17, article no. 328 doi: 10.1186/s12906-017-1835-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu L.-X., Yan H.-X., Liu Q., et al. Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. Hepatology. 2010;52(4):1322–1333. doi: 10.1002/hep.23845. [DOI] [PubMed] [Google Scholar]
- 13.Zhang S., Zheng L., Dong D., et al. Effects of flavonoids from Rosa laevigata Michx fruit against high-fat diet-induced non-alcoholic fatty liver disease in rats. Food Chemistry. 2013;141(3):2108–2116. doi: 10.1016/j.foodchem.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 14.Fawole O. A., Opara U. L., Theron K. I. Chemical and phytochemical properties and antioxidant activities of three pomegranate cultivars grown in South Africa. Food and Bioprocess Technology. 2012;5(7):2934–2940. doi: 10.1007/s11947-011-0533-7. [DOI] [Google Scholar]
- 15.Nakai M., Fukui Y., Asami S., et al. Inhibitory effects of oolong tea polyphenols on pancreatic lipase in vitro. Journal of Agricultural and Food Chemistry. 2005;53(11):4593–4598. doi: 10.1021/jf047814+. [DOI] [PubMed] [Google Scholar]
- 16.Dianita R., Jantan I., Jalil J., Amran A. Z. Effects of Labisia pumila var alata extracts on the lipid profile, serum antioxidant status and abdominal aorta of high-cholesterol diet rats. Phytomedicine. 2016;23(8):810–817. doi: 10.1016/j.phymed.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Heidarian E., Soofiniya Y. Hypolipidemic and hypoglycemic effects of aerial part of Cynara scolymus in streptozotocin-induced diabetic rats. Journal of Medicinal Plants Research. 2011;5(13):2717–2723. [Google Scholar]
- 18.Friedwald W. T., Levy R. I., Fredrickson D. S. Estimation of concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 19.Tsuchiya R., Fujise H., Nishizono K., Ashida Y., Yamada T., Kobayashi K. Assay of ornithine carbamoyl transferase activity: modification for application to bovine serum. Journal of Veterinary Medical Science. 1994;56(1):21–26. doi: 10.1292/jvms.56.21. [DOI] [PubMed] [Google Scholar]
- 20.Draper H. H., Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods in Enzymology. 1990;186:421–431. doi: 10.1016/0076-6879(90)86135-I. [DOI] [PubMed] [Google Scholar]
- 21.Kayali R., Çakatay U., Akçay T., Altuğ T. Effect of alpha-lipoic acid supplementation on markers of protein oxidation in post-mitotic tissues of ageing rat. Cell Biochemistry & Function. 2006;24(1):79–85. doi: 10.1002/cbf.1190. [DOI] [PubMed] [Google Scholar]
- 22.Beyer W. F., Jr., Fridovich I. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Analytical Biochemistry. 1987;161(2):559–566. doi: 10.1016/0003-2697(87)90489-1. [DOI] [PubMed] [Google Scholar]
- 23.Paglia D. E., Valentine W. N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. Journal of Laboratory and Clinical Medicine. 1967;70:158–169. [PubMed] [Google Scholar]
- 24.Carlberg I., Mannervik B. Glutathione reductase in Glutamate, Glutamine, Glutathione, and Related Compounds, of Methods in Enzymology. Enzymology. 1985;113:484–490. doi: 10.1016/S0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- 25.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. Protein measurement with the folin phenol reagent. The Journal of Biological Chemistry. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 26.Gupta R., Dubey D. K., Kannan G. M., Flora S. J. S. Concomitant administration of Moringa oleifera seed powder in the remediation of arsenic-induced oxidative stress in mouse. Cell Biology International. 2007;31(1):44–56. doi: 10.1016/j.cellbi.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Wisse B. E., Kim F., Schwartz M. W. Physiology: An integrative view of obesity. Science. 2007;318(5852):928–929. doi: 10.1126/science.1148032. [DOI] [PubMed] [Google Scholar]
- 28.Kopelman P. G. Obesity as a medical problem. Nature. 2000;404(6778):635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 29.Mnafgui K., Derbali A., Sayadi S., Gharsallah N., Elfeki A., Allouche N. Anti-obesity and cardioprotective effects of cinnamic acid in high fat diet- induced obese rats. Journal of Food Science and Technology. 2014;52(7):4369–4377. doi: 10.1007/s13197-014-1488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chakrabarti R. Pharmacotherapy of obesity: Emerging drugs and targets. Expert Opinion on Therapeutic Targets. 2009;13(2):195–207. doi: 10.1517/14728220802637063. [DOI] [PubMed] [Google Scholar]
- 31.Carrière F., Renou C., Ransac S., et al. Inhibition of gastrointestinal lipolysis by Orlistat during digestion of test meals in healthy volunteers. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2001;281(1):G16–G28. doi: 10.1152/ajpgi.2001.281.1.G16. [DOI] [PubMed] [Google Scholar]
- 32.Zouari R., Hamden K., Feki A. E., et al. Protective and curative effects of Bacillus subtilis SPB1 biosurfactant on high-fat-high-fructose diet induced hyperlipidemia, hypertriglyceridemia and deterioration of liver function in rats. Biomedicine & Pharmacotherapy. 2016;84:323–329. doi: 10.1016/j.biopha.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 33.Subramaniam S., Subramaniam R., Rajapandian S., Uthrapathi S., Gnanamanickam V. R., Dubey G. P. Antiatherogenic activity of ethanolic fraction of Terminalia arjuna bark on hypercholeterolemic rabbits. Evidence-Based Complementary and Alternative Medicine. 2011;2011:8. doi: 10.1093/ecam/neq003.487916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdel Magied M. M., Salah EL Din H., Sahar M. Z., Rania M. S. Artichoke (Cynara scolymus L.) Leaves and Heads Extracts as Hypoglycemic and Hypocholesterolemic in Rats. Journal of Food and Nutrition Research. 2016;41:60–68. [Google Scholar]
- 35.Küskü-Kiraz Z., Mehmetçik G., Dogru-Abbasoglu S., Uysal M. Artichoke leaf extract reduces oxidative stress and lipoprotein dyshomeostasis in rats fed on high cholesterol diet. Phytotherapy Research. 2010;24(4):565–570. doi: 10.1002/ptr.2985. [DOI] [PubMed] [Google Scholar]
- 36.Salem M. B., Affes H., Ksouda K., et al. Pharmacological studies of artichoke leaf extract and their health benefits. Plant Foods for Human Nutrition. 2015;70(4):441–453. doi: 10.1007/s11130-015-0503-8. [DOI] [PubMed] [Google Scholar]
- 37.Gebhardt R. Inhibition of cholesterol biosynthesis in HepG2 cells by artichoke extracts is reinforced by glucosidase pretreatment. Phytotherapy Research. 2002;16(4):368–372. doi: 10.1002/ptr.960. [DOI] [PubMed] [Google Scholar]
- 38.Qiang Z., Lee S.-O., Ye Z., Wu X., Hendrich S. Artichoke extract lowered plasma cholesterol and increased fecal bile acids in Golden Syrian hamsters. Phytotherapy Research. 2012;26(7):1048–1052. doi: 10.1002/ptr.3698. [DOI] [PubMed] [Google Scholar]
- 39.Hosseinzadeh M., Shekari F., Janmohammadi M., Sabaghnia N. Effect of sowing date and fliar application of salicylia acid on forage yields and quality of globe artichoke (cynara scolymus L.) Annales. 2013;8:50–59. [Google Scholar]
- 40.Küçükgergin C., AydIn A. F., Özdemirler-Erata G., Mehmetçik G., Koçak-Toker N., Uysal M. Effect of artichoke leaf extract on hepatic and cardiac oxidative stress in rats fed on high cholesterol diet. Biological Trace Element Research. 2010;135(1-3):264–274. doi: 10.1007/s12011-009-8484-9. [DOI] [PubMed] [Google Scholar]
- 41.Wang M., Simon J. E., Aviles I. F., He K., Zheng Q.-Y., Tadmor Y. Analysis of antioxidative phenolic compounds in artichoke (Cynara scolymus L.) Journal of Agricultural and Food Chemistry. 2003;51(3):601–608. doi: 10.1021/jf020792b. [DOI] [PubMed] [Google Scholar]
- 42.Joy J. F., Haber S. L. Clinical uses of artichoke leaf extract. American Journal of Health-System Pharmacy. 2007;64(18):1904–1909. doi: 10.2146/ajhp070013. [DOI] [PubMed] [Google Scholar]
- 43.Jiménez-Escrig A., Dragsted L. O., Daneshvar B., Pulido R., Saura-Calixto F. In vitro antioxidant activities of edible artichoke (Cynara scolymus L.) and effect on biomarkers of antioxidants in rats. Journal of Agricultural and Food Chemistry. 2003;51(18):5540–5545. doi: 10.1021/jf030047e. [DOI] [PubMed] [Google Scholar]
- 44.Zapolska-Downar D., Zapolski-Downar A., Naruszewicz M., Siennicka A., Krasnodbska B., Kolodziej B. Protective properties of artichoke (Cynara scolymus) against oxidative stress induced in cultured endothelial cells and monocytes. Life Sciences. 2002;71(24):2897–2908. doi: 10.1016/s0024-3205(02)02136-7. [DOI] [PubMed] [Google Scholar]
- 45.Zhu X., Zhang H., Lo R. Phenolic compounds from the leaf extract of artichoke (Cynara scolymus L.) and their antimicrobial activities. Journal of Agricultural and Food Chemistry. 2004;52(24):7272–7278. doi: 10.1021/jf0490192. [DOI] [PubMed] [Google Scholar]
- 46.Brown J. E., Rice-Evans C. A. Luteolin-rich artichoke extract protects low density lipoprotein from oxidation in vitro. Free Radical Research. 1998;29(3):247–255. doi: 10.1080/10715769800300281. [DOI] [PubMed] [Google Scholar]
- 47.Zimmerman H. J., Kodera Y., West M. Rate of increase in plasma levels of cytoplasmic and mitochondrial enzymes in experimental carbon tetrachloride hepatotoxicity. Journal of Laboratory and Clinical Medicine. 1965;66:315–323. [PubMed] [Google Scholar]
- 48.Murayama H., Ikemoto M., Fukuda Y., Nagata A. Superiority of serum type-I arginase and ornithine carbamyltransferase in the detection of toxicant-induced acute hepatic injury in rats. Clinica Chimica Acta. 2008;391(1-2):31–35. doi: 10.1016/j.cca.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 49.Srivastava A. R., Kumar S., Agarwal G. G., Ranjan P. Blunt abdominal injury: Serum ALT-A marker of liver injury and a guide to assessment of its severity. Injury. 2007;38(9):1069–1074. doi: 10.1016/j.injury.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 50.Chapman S. E., Hostutler R. A. A laboratory diagnostic approach to hepatobiliary disease in small animals. Veterinary Clinics of North America - Small Animal Practice. 2013;43(6):1209–1225. doi: 10.1016/j.cvsm.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 51.Ben Abdallah K. R., Ben Gara A., Chaaben R., El Feki A. Anti-obesity and lipid lowering effects of Cymodocea nodosa sulphated polysaccharide on high cholesterol-fed-rats. Archives of Physiology and Biochemistry. 2015 doi: 10.3109/13813455.1105266 [DOI] [PubMed] [Google Scholar]
- 52.Ouchi N., Parker J. L., Lugus J. J., Walsh K. Adipokines in inflammation and metabolic disease. Nature Reviews Immunology. 2011;11(2):85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vial G., Dubouchaud H., Couturier K., et al. Effects of a high-fat diet on energy metabolism and ROS production in rat liver. Journal of Hepatology. 2011;54(2):348–356. doi: 10.1016/j.jhep.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 54.Tian L., Shi X., Yu L., Zhu J., Ma R., Yang X. Chemical composition and hepatoprotective effects of polyphenol-rich extract from houttuynia cordata tea. Journal of Agricultural and Food Chemistry. 2012;60(18):4641–4648. doi: 10.1021/jf3008376. [DOI] [PubMed] [Google Scholar]
- 55.Ohara Y., Peterson T. E., Harrison D. G. Hypercholesterolemia increases endothelial superoxide anion production. The Journal of Clinical Investigation. 1993;91(6):2546–2551. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mehmetçik G., Özdemirler G., Koçak-Toker N., Çevikbaş U., Uysal M. Effect of pretreatment with artichoke extract on carbon tetrachloride-induced liver injury and oxidative stress. Experimental and Toxicologic Pathology. 2008;60(6):475–480. doi: 10.1016/j.etp.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 57.Pérez-García F., Adzet T., Cañigueral S. Activity of artichoke leaf extract on reactive oxygen species in human leukocytes. Free Radical Research. 2000;33(5):661–665. doi: 10.1080/10715760000301171. [DOI] [PubMed] [Google Scholar]
- 58.Aktay G., Deliorman D., Ergun E., Ergun F., Yeşilada E., Çevik C. Hepatoprotective effects of Turkish folk remedies on experimental liver injury. Journal of Ethnopharmacology. 2000;73(1-2):121–129. doi: 10.1016/S0378-8741(00)00286-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.