Abstract
This review is a call for mindfulness and precision when applying TNM 8 in oropharyngeal cancers. Implications, intentions, and weaknesses of TNM 8 are addressed in light of our own investigations and the published literature. In TNM 8, the impact of p16INK4A status on the staging of oropharyngeal SCC highlights i) that underlying evidence is scarce, ii) its stage grouping exclusively has prognostic intention, and iii) that a noncritical application of TNM 8 might negatively impact the patients' survival as the perception of TNM 8 as having therapeutic intention may lead to de-escalating treatment regimens in p16INK4A-positive cases, specifically when grouped into stage I despite the presence of neck metastasis. If other parameters from HPV positivity that also have a negative impact on the patient's survival, such as smoking or the presence of comorbidity, are neglected in therapy planning, survival outcomes might even become worse. Future studies applying TNM 8 and further investigating the value of p16INK4A as surrogate marker for active HPV infections will identify whether or not changes in TNM 8 should have therapeutic implications in HPV-associated, only p16INK4A-positive cases or whether this impact additionally holds true for nontonsillar cancers.
Background
The new edition of the TNM classification system (UICC/AJCC) became available January 1st, 2017. For the first time, HPV-positive and HPV-negative oropharyngeal carcinomas are classified as separate entities. However, the available data which this new classification is based on are not resilient. There is a profound risk for misclassification of cases and faulty treatment decision making, resulting in less favorable outcome for patients.
To raise awareness and to bring this issue in a practical setting, the authors will discuss a hypothetical case scenario resembling everyday clinical practice. In the lack of study results addressing the issue so far, a hypothetical patient case has been constructed and will lead through the points of interest concerning the application of TNM 8. The name of this constructed patient is Mrs. Doe.
Case
This is a 57-year-old female patient who was referred to the ENT clinic of a major academic medical center after being diagnosed with an oropharyngeal carcinoma for completing the diagnostic workup and treatment.
Her past medical history is unremarkable except for a well-controlled blood pressure. She smoked from age 20 up to the age of 50, one pack a day (i.e., 30 pack years in total). Her alcohol consumption is limited to moderate use.
The extension of her tumor is described as follows: in the right tonsillar fossa, measuring 3.5 cm in greatest dimension; there are 4 enlarged lymph nodes and up to 5.7 cm in diameter ipsilateral, no distant metastases, and no evidence of extranodular infiltration (ENI). Biopsy was taken from the primary tumor; pathology shows a moderately differentiated, nonkeratinizing squamous cell carcinoma (grade G2), p16INK4A-positive. According to the seventh edition of the TNM classification system, this is a T2N2bM0, UICC stage IVa tumor; according to the eighth edition, it is a T2N1M0 tumor, UICC stage I.
Discussion
Human Papillomavirus in the Eighth Edition of the TNM Classification System
Mrs. Doe suffers from a locally advanced tonsillar cancer, which classified according to the seventh edition of the TNM classification system (TNM 7) (1) would be a T2N2bM0, UICC stage IVa tumor. Tumor burden typically is reflected by stage and predicts survival for these patients. There are several therapeutic options for this patient based on histopathological findings, tumor stage, relatively young age, and no significant comorbidities. The first option would be primary radiochemotherapy. A second option would be transoral tumor resection (TLS, TORS) with neck dissection and risk-adapted adjuvant treatment [radio(chemo)therapy]. And last but not least, the patient could be treated by transcervical tumor resection (and possibly reconstruction using a flap) with neck dissection and risk-adapted adjuvant radio(chemo)therapy.
Our own recently published survival data on 126 patients with locally advanced tonsillar cancer (UICC stage III and IV according to TNM 7) show that a patient similar to the case has a 3- and 5-year survival probability of 67% and 62%, respectively, after transoral tumor resection, neck dissection, and adjuvant radio(chemo)therapy (2). These data confirm the excellent survival rates from the RTOG trials 0129 and 0522, which included similar radio(chemo)therapy regimens in their study design ([3], [4]).
However, these survival rates do not take into consideration the fact that the patient has—according to the new TNM classification system and its criteria for HPV diagnosis—an HPV-associated tonsillar carcinoma.
The survival probability of the patient improves after stratification for HPV positivity according to the mentioned own study results (2) in terms of Mrs. Doe's 3- and 5-year survival to 80% and 78%, which again is in line with the results of RTOG 0129 and TAX 324 (5) and other clinical studies with post hoc analyses of HPV status (6). The latter and various other retrospective study results being in line with the results from RTOG 0129, 0522 and TAX 324 such as own previous studies ([2], [7], [8], [9], [10], [11]) on the positive impact of HPV-infections on the course of disease of patients with squamous cell carcinoma of the head and neck (HNSCC) form the evidence on which the innovations of TNM 8 have been based on.
The fact that patients with HPV-related carcinoma of the tonsil show a significantly better survival probability has been incorporated into the new TNM classification as a new tumor entity, and Mrs. Doe's tumor is now classified as a UICC stage I tumor.
At the same time, HPV-negative tonsillar tumors of the same extent with a survival probability at 3 and 5 years of 60% and 52% remain in stage IVa. Of note, data from our own patients (2) and summarized studies by Masterson and coworkers (6) are derived from standard treatment protocols, i.e., without treatment de-escalation strategies. This is also the case for all cited own HPV-based studies ([2], [7], [8], [9], [10], [11]) implementing full-dose therapy regimens.
Important point: The excellent survival of patients with HPV-related oropharyngeal carcinoma was shown in patients treated with standard therapies.
Most Important Changes in the Eighth Edition of the TNM Classification
The resulting “downstaging” of HPV-associated tumors with the TNM 8 is based on a trial with 1907 patients. Results showed that prognosis of these patients was better reflected with the new stage grouping (12). Major changes in the TNM 8 (13) are discussed by Lydiatt et al. (14) and summarized in Table 1. It is important to notice that, in p16INK4A-positive tumors, the clinical nodal (cN) and pathological nodal status (pN) differ. After surgery, the pathological staging apparently enables other prognosticators to be more relevant than in clinically staged nonsurgical patients. The latter is in line with own data showing that the HPV-positive impact on survival also can be observed in patients exclusively treated by surgery without any adjuvant treatment (2), meaning that prognosis changes already alone with surgery. Previous own studies showing a high positive correlation of HPV status in primary tumors and their corresponding lymph node metastases of the lateral neck ([15], [16]) might explain the different biological behavior of HPV-negative and HPV-positive tumors due to hampered virus activity following surgery in HPV-positive cases.
Table 1.
Important Findings and Consequences: Changes in the Eighth edition of TNM Classification for Oropharyngeal Carcinoma (OPC)
| Findings | Consequences in TNM Classification |
|---|---|
| With increasing incidence of HPV-related OPC, stage grouping in TNM 7 does not adequately differentiate survival prognosis. | p16+ and p16− OPCs were introduced as separate entities. |
| No basal membrane in Waldeyer's ring; no difference in survival in p16+ OPC T4a and T4b | p16+ OPC: no Tis; no subgroups in T4 |
| In p16+ OPC; no effect of lymph node metastases <6 cm: influence on survival after neck dissection | p16+ OPC: cN and pN categories |
| In p16+ OPC, Ipsilateral lymph node metastases <6 cm: same survival independent of number of lymph node metastases | p16+ OPC: cN1: ipsilateral one or more lymph nodes metastases |
| Contralateral or bilateral lymph node metastases <6 cm: unfavorable effect on survival | p16+ OPC: cN2: contralateral or bilateral lymph node metastases <6 cm |
| Lymph node metastases >6 cm: unfavorable effect on survival | p16+ OPC: cN3: any lymph node metastases >6 cm |
| In p16+ OPC, size and laterality of lymph node metastases have no effect on survival prognosis after neck dissection; up to 4 positive lymph nodes | p16+ OPC: pN1: 1-4 lymph node metastases pN2: 5 lymph node metastases independent of size and laterality no category pN3 |
| ENI is a high-risk factor for all head and neck tumors, except for p16+ OPC | p16− OPC: cN = pN N categories dependent of number of positive nodes, laterality, size, ENI different categories |
| p16+ OPCs have a better prognosis compared to p16− OPCs despite higher tumor burden | p16+ OPC: Downstaging to reflect survival prognosis; stage IV: distant metastases |
In TNM 8, nodal staging in HPV-negative patients is like TNM 7 except for “extranodular infiltration” (ENI), which was incorporated in cN as well as in pN as the most important negative prognostic factor. In nonsurgical p16INK4A-positive patients who usually undergo primary radio(chemo)therapy, N stage is grouped in ipsi- versus bilateral nodal metastases of more than 6 cm as important negative prognostic factor. Contralateral nodes and size of lymph node metastases do not seem to influence prognosis in HPV-positive patients who underwent primary surgery. Only the absolute number of lymph node metastases is important. Therefore, there are only pN0 and pN1 (four or less positive lymph nodes) and pN2 (more than four lymph node metastases). In patients with resectable p16INK4A-positive tonsillar tumors, surgery becomes a positive predictive factor. This was also shown in our own series: Thirty-nine patients (31%) of 126 patients who underwent primary surgery did not receive adjuvant radiochemotherapy even if 13 patients had stage III and IVa disease (TNM 7). Figure 1 shows that there is no difference in survival between patients with or without adjuvant radio(chemo)therapy. Within the group of patients who underwent primary surgery, the same positive effect on survival is seen in patients with HPV-associated tumors compared to surgically treated patients with HPV-negative tumors (2). Both results suggest that better outcomes in patients with HPV-positive oropharyngeal carcinoma are most likely not exclusively due to a better response to radiation therapy, but to any therapy, especially surgery. The role of surgery in times of a generally favored predominance of radiation treatment protocols in HPV-positive and HPV-negative HNSCC already has been addressed by us in 2010 and on ([2], [17]).
Fig. 1.
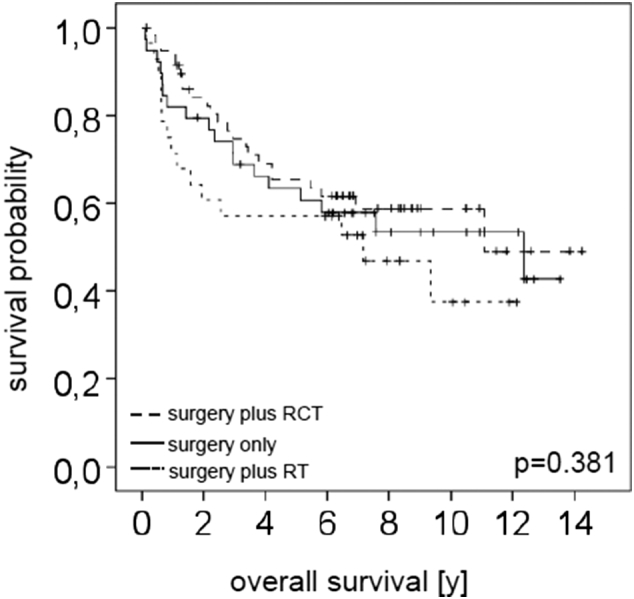
Influence of therapy on the overall survival of the patients.
In total, 39 patients were treated by surgery only, with a median follow-up time of 6.16 years (range 0.12-13.53 years). Overall survival after 3 years was 67.2%, after 5 years 61.3%, and after 10 years 53.6%. Further 59 patients were after surgery treated with radiochemotherapy (RCT). Here the median follow-up time was 5.88 years with a range from 0.16 to 14.24 years. Overall survival was 74.7%, 65.4%, and 58.8% after 3, 5, and 10 years, respectively. The remaining 28 patients received, after initial surgery, radiotherapy (RT). The median follow-up period in this group was 6.26 years (range 0.14-12.12 years), with overall survival rates of 56.1%, 57.1%, and 37.5% after 3, 5, and 10 years, respectively.
Lack of Precision in Using the Eighth Edition of the TNM Classification System
TNM 8 harbors pitfalls when used without caution. It should be used for risk stratification and to identify patients who potentially benefit from de-escalated treatment strategies. A few critical aspects which in part have been addressed by us ([18], [19], [20], [21]) previously are discussed in the following paragraphs.
TNM 8 introduces HPV-positive and HPV-negative carcinomas of the oropharynx. The significantly higher rates of HPV infection and therefore higher incidence of HPV prevalence are exclusively seen in regions which belong to the characteristic lymphoepithelial tissue of the tonsils of the Waldeyer ring (tonsillae lingualis and palatinae) ([7], [8], [9], [11]). The Waldeyer ring, except the adenoids, is in fact part of the oropharynx in addition to other regions such as the posterior pharyngeal wall and soft palate. Carcinomas of the soft palate are as rarely HPV-positive as hypopharyngeal carcinomas ([7], [11]). Having stated this, one has to be clear about the fact that the chapter in TNM 8 which deals with HPV-positive oropharyngeal carcinomas cannot necessarily be transferred to tumors other than oropharyngeal primary sites without caution.
The cellular protein of the gene CDKN2A, p16INK4A, belongs to the group of the INK4 proteins. It inhibits cyclin D–dependent kinases and is upregulated through a negative feedback mechanism. Cyclin-dependent kinases become phosphorylated through the retinoblastoma gene product (pRB), resulting in the release of the proliferation factor E2F. The viral oncogene E7 is imitating cyclin D–dependent kinase activity and causes uncontrolled replication of mucosa cells which are needed by human papillomaviruses since they do not have their own replication machinery. This uncontrolled replication results in proliferation and malignant transformation depending on the malignant potential of the human papillomavirus (22). HPV16 causes more than 90% of HPV infections in the head and neck. In SCC of the tonsils which, due to its lymphoepithelial character, seems susceptible for HPV infections, HPV16 prevalence rates vary between 30% in the Netherlands and 90% in Scandinavia depending on the applied HPV-detection methods and, even more important, the geographical region the patients live in (11). The reason for the latter is only poorly understood until today. Together with types 18, 33, and 35, HPV16 belongs to the group with the highest potential to cause malignant transformation (high-risk types). p16INK4A positivity indirectly shows supposed viral infection in tumor tissue ([8], [23]). According to TNM 8, p16INK4A immunohistochemistry (IHC) is recommended to detect HPV-associated SCCs in exclusively oropharyngeal cancers. The decision of the AJCC/UICC to consider p16INK4A as surrogate marker for HPV infection is based on studies which showed a strong correlation between HPV DNA and RNA (mRNA of oncogenes E6 and E7) and a strong positive result for p16INK4A on IHC. Strong diffuse staining for p16INK4A-IHC is considered positive for HPV infection with active oncogenes. This method is widely available and affordable. However, there are numerous studies from several regions of the world showing that there is a considerable discrepancy between p16INK4A results and the molecular proof of HPV DNA and/or RNA in tumor tissues ([2], [9], [11]) which even holds true when African and Caucasian Americans are compared (24). There is even uncertainty whether or not the cutoff value in p16INK4A IHC detecting HPV positivity has been determined precisely (25). We and other research groups have repeatedly shown these discrepancies. For instance, of the 126 tonsillar cancers, we showed a rate of 18% (2). In addition, it was shown that patients with p16INK4A-positive and HPV DNA-negative tumors had a better survival than patients being negative for both parameters ([11], [26], [27]). This observation is still discussed in the community and not well understood. The authors of TNM 8 for oropharyngeal carcinomas and the accompanying publications ([12], [13], [14], [28]) need to be open to the criticism that only publications which support p16INK4A as surrogate marker for HPV infection were considered. The authors want to make readers aware that based on p16INK4A IHC as sole method for HPV detection as recommended in TNM 8, there is a fair chance of misclassification of patients as false positive or false negative. In the best case scenario, this leads to a wrong estimation of survival prognosis only. In any case, TNM 8 lacks sufficient information from different populations and geographic regions in the world.
Mrs. Doe is a smoker. Since the widely recognized publication by Ang et al., it is well accepted that smoking has a negative effect on survival in head and neck cancer patients independent of HPV status (3). Compared to HPV-positive nonsmokers (with very good prognosis) and HPV-negative smokers (with poor prognosis), HPV-positive smokers and HPV-negative nonsmokers showed an intermediate survival probability. The positive effect of HPV infection on survival was completely void with a positive smoking history in our own patient population with 126 tonsillar cancer patients (2). Smoking habit/history was not considered in the TNM 8. The authors want to raise concern because this might mislead professionals in estimating prognosis in patients with HPV-positive cancers with a smoking history. It is important to keep in mind that the number of patients (with HPV-positive tumors) actively smoking is significantly larger outside the United States, especially in certain parts of Southern Europe. Currently, there are no studies or data supporting the understanding or interpretation of this issue.
The incidence of cancer of the upper aerodigestive tract shows a male to female ratio of 1:5. Based on differences between woman and men concerning HPV infection latencies, infection rates, HPV uptake according to lifetime sexual partners, and others, it might be assumed that there is a major difference in immunocompetence between women and men (detailed information on HPV infection in men is given in references [29], [30], [31]: HPV infection of the cervix is cleared without any treatment by about 75% of women, and only 25% develop a permanent infection. These phenomena have not been described in the mucosa of the aerodigestive tract where it has been repeatedly shown that, in the head and neck, there is no detectable HPV in the mucosa without a lesion ([29], [30]) and vice versa, and that the presence of HPV in mucosa of the head and neck region is detected only when lesions are present ([2], [8], [9], [10], [11], [30], [31]). Due to the latter, studies observing the course of latent infections in mucosae of the head and neck are not feasible. Moreover, in men, the rate of new infections increases proportionally with increasing number of sexual partners, while in women, it plateaus at the number of 6 ([32], [33], [34], [35]). It is unclear whether the immunological aspects in women result in different tumor biology in HPV-driven cancers. It remains questionable whether TNM 8 is applicable in men and women alike. However, to date, there is no scientific evidence to argue against it.
HPV Status and Therapy
The excellent survival of patients with HPV-related tumors suggests the need for adjustment of therapy, such as reduction of radiation dose and/or chemotherapy or replacement of classic chemotherapies with biologicals (anti-EGFR or anti-PD1/-PDL1). Currently, there are several prospective clinical trials recruiting patients (6) looking into de-escalated treatment strategies to minimize treatment related toxicities while maintaining outcome. However, some issues discussed here are part of the ongoing trials and will probably make interpretation of the results challenging: i) some trials recruited patients with tumors of several anatomic sites of the upper aerodigestive tract, ii) only p16INK4A IHC is required as method for HPV detection in the majority of studies, and (iii) smoking habit is considered in only a few studies. Moreover, most of the trials were designed between 2010 and 2014. TNM 7 was then introduced; now TNM 8 has become available and is used. Reclassification should be done to make data comparable. Currently, only two de-escalation trials (DeESACALaTE and RTOG1016) have been published ([36], [37]). Both noninferiority trials tested cisplatin against cetuximab in the setting of radiochemotherapy, resulting in significantly worse survival of patients in the cetuximab arm, therefore classifying both trials to be negative. All other available data on patients with HPV-related oropharyngeal cancer showing the survival advantage were derived from trials originally designed for different endpoints with post hoc HPV analyses. Again, all patients received treatment according to standard of care. Unfortunately, neither DeESCALaTE nor RTOG1016 included HPV (p16INK4A)-negative cases, preventing the chance to show the survival advantage of HPV-positive cases in a prospective setting.
The here discussed hypothetical patient with a p16INK4A-positive tonsillar cancer, cT2N1M0, stage I (TNM 8), would currently have an excellent survival prognosis despite being a former smoker if she received a standard, not de-escalated, treatment regimen.
Conclusions
This article is a call for mindfulness and precision when applying TNM 8 in oropharyngeal cancers. In TNM 8, the impact of p16INK4A status on the staging of oropharyngeal SCC highlights i) that underlying evidence is scarce, ii) that it's stage grouping exclusively has prognostic intention, and iii) that a noncritical application of TNM 8 might negatively impact the patients' survival as the perception of TNM 8 as having therapeutic intention may lead to de-escalating treatment regimens in p16INK4A-positive cases, specifically when grouped into stage I despite the presence of neck metastasis. If other parameters from HPV positivity that also have a negative impact on the patient's survival, such as smoking or the presence of comorbidity, are neglected in therapy planning, survival outcomes might even become worse. Future studies applying TNM 8 and further investigating the value of p16INK4A as surrogate marker for active HPV infections will identify whether or not changes in TNM 8 should have therapeutic implications in HPV-associated, only p16INK4A-positive cases or whether this impact additionally holds true for nontonsillar cancers.
The authors do not have any conflicts of interests to declare.
Contributor Information
Markus Hoffmann, Email: markus.hoffmann@uksh.de.
Silke Tribius, Email: s.tribius@asklepios.com.
References
- 1.Edge SB, Byrd DR, Compton CC, editors. AJCC cancer staging manual. 7th ed. Springer; New York: 2010. [Google Scholar]
- 2.Hoffmann M, Quabius ES, Tribius S, Gebhardt S, Görögh T, Hedderich J, Huber K, Dunst J, Ambrosch P. Influence of HPV-status on survival of patients with tonsillar carcinomas (TSCC) treated by CO2-laser surgery plus risk adapted therapy — a 10 year retrospective single centre study. Cancer Lett. 2017;413:59–68. doi: 10.1016/j.canlet.2017.10.045. [DOI] [PubMed] [Google Scholar]
- 3.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, Westra WH, Chang CH, Jordan RC, Lu C. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan PF, Sherman EJ, Weber RS, Galvin JM, Bonner JA, Harris J, El-Naggar AK. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014;32:2940–2950. doi: 10.1200/JCO.2013.53.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Posner MR, Lorch JH, Goloubeva O, Tan M, Schumaker LM, Sarlis NJ, Haddad RI, Cullen KJ. Survival and human papillomavirus in oropharynx cancer in TAX 324: a subset analysis from an international phase III trial. Ann Oncol. 2011;22:1071–1077. doi: 10.1093/annonc/mdr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masterson L, Moualed D, Liu ZW, Howard JE, Dwivedi RC, Tysome JR, Benson R, Sterling JC, Sudhoff H, Jani P. De-escalation treatment protocols for human papillomavirus-associated oropharyngeal squamous cell carcinoma: a systematic review and meta-analysis of current clinical trials. Eur J Cancer. 2014;50:2636–2648. doi: 10.1016/j.ejca.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann M, Görögh T, Gottschlich S, Lohrey C, Rittgen W, Ambrosch P, Schwarz E, Kahn T. Human papillomaviruses in head and neck cancer: 8 year-survival-analysis in 73 patients. Cancer Lett. 2005;18:199–206. doi: 10.1016/j.canlet.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann M, Ihlhoff AS, Görögh T, Weise JB, Fazel A, Krams M, Rittgen W, Schwarz E, Kahn T. p16(INK4A) overexpression predicts translational active human papillomavirus infection in tonsillar cancer. Int J Cancer. 2010;127:1595–1602. doi: 10.1002/ijc.25174. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann M, Tribius S, Quabius ES, Henry H, Pfannenschmidt S, Burkhardt C, Görögh T, Halec G, Hoffmann AS, Kahn T, Röcken C, Haag J, Waterboer T, Schmitt M: HPV DNA, E6*I-mRNA expression and p16INK4A immunohistochemistry in head and neck cancer — how valid is p16INK4A as surrogate marker? Cancer Lett 2012;323:388–96. [DOI] [PubMed]
- 10.Tribius S, Hoffmann AS, Bastrop S, Görögh T, Haag J, Röcken C, Clauditz T, Grob T, Wilczak W, Tennstedt P. HPV status in patients with head and neck carcinoma of unkown primary site: HPV, tobacco, smoking, and outcome. Oral Oncol. 2012;48:1178–1184. doi: 10.1016/j.oraloncology.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 11.Quabius ES, Haag J, Kühnel A, Henry H, Hoffmann AS, Görögh T, Hedderich J, Evert M, Beule AG, Maune S. Geographical and anatomical influences on human papillomavirus prevalence diversity in head and neck squamous cell carcinoma in Germany. Int J Oncol. 2015;46:414–422. doi: 10.3892/ijo.2014.2697. [DOI] [PubMed] [Google Scholar]
- 12.O'Sullivan B, Huang SH, Su J, Garden AS, Sturgis EM, Dahlstrom K, Lee N, Riaz N, Pei X, Koyfman SA. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol. 2016;17:440–451. doi: 10.1016/S1470-2045(15)00560-4. [DOI] [PubMed] [Google Scholar]
- 13.Amin MB, Edge SB, Greene FL, editors. AJCC cancer staging manual. 8th ed. Springer; New York: 2017. [Google Scholar]
- 14.Lydiatt WM, Patel SG, O'Sullivan B, Brandwein MS, Ridge JA, Migliacci JC, Loomis AM, Shah JP. Head and neck cancers—major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:122–137. doi: 10.3322/caac.21389. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann M, Gottschlich S, Görögh T, Lohrey C, Ambrosch P, Schwarz E, Kahn T. Human papillomaviruses in lymph node neck metastases of head and neck cancers. Acta Otolaryngol. 2005;218:199–206. doi: 10.1080/00016480510028528. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann M, Orlamünder A, Sucher J, Gottschlich S, Görögh T, Fazel A, Ambrosch P, Rittgen W, Schwarz E, Kahn T. HPV16 DNA in histologically confirmed tumour-free neck lymph nodes of head and neck cancers. Anticancer Res. 2006;26:663–670. [PubMed] [Google Scholar]
- 17.Ihloff AS, Petersen C, Hoffmann M, Knecht R, Tribius S. Human papilloma virus in locally advanced stage III/IV squamous cell cancer of the oropharynx and impact on choice of therapy. Oral Oncol. 2010;46:705–711. doi: 10.1016/j.oraloncology.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Tribius S, Ihloff AS, Rieckmann T, Petersen C, Hoffmann M. Impact of HPV status on treatment of squamous cell cancer of the oropharynx: what we know and what we need to know. Cancer Lett. 2011;304:71–79. doi: 10.1016/j.canlet.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann M, Hoffmann AS, Tribius S. Public awareness of human papillomavirus infection in the head and neck area: an appeal for precision in diagnostic and for public health awareness. HNO. 2012;60:968–973. doi: 10.1007/s00106-012-2552-8. [DOI] [PubMed] [Google Scholar]
- 20.Tribius S, Hoffmann M. Human papillomavirus infection n head and neck cancer. Dtsch Arztebl Int. 2013;110:184–190. doi: 10.3238/arztebl.2013.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.HNO, editor. Relevance of HPV infections in head and neck cancers: highlights of the 2016 ASCO Annual Meeting. Vol. 64. 2016. pp. 731–735. [DOI] [PubMed] [Google Scholar]
- 22.Münger K, Basile JR, Duensing S, Eichten A, Gonzales SL, Grace M, Zacny VL. Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene. 2001;20:7888–7898. doi: 10.1038/sj.onc.1204860. [DOI] [PubMed] [Google Scholar]
- 23.Chung CH, Zhang Q, Kong CS, Fertig EJ, Harari PM, Wang D, Remond KP, Shenouda G, Trotti A, Raben D. p16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. J Clin Oncol. 2014;32:3930–3938. doi: 10.1200/JCO.2013.54.5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chernock RD, Zhang Q, El-Mofty SK, Thorstad WL, Lewis JS., Jr. Human papillomavirus-related squamous cell carcinoma of the oropharynx: a comparative study in whites and African Americans. Arch Otolaryngol Head and Neck Surg. 2011;137:163–169. doi: 10.1001/archoto.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis JS, Jr., Chernock RD, Ma XJ, Flanagan JJ, Luo Y, Wang X, El-Mofty SK. Partial p16 staining in oropharyngeal squamous cell carcinoma: extent and pattern correlate with human papillomavirus RNA status. Mod Pathol. 2012;25:1212–1220. doi: 10.1038/modpathol.2012.79. [DOI] [PubMed] [Google Scholar]
- 26.Lewis JS, Jr., Thorstad WL, Chernock RD, Haughey BH, Yip JH, Zhang Q, El-Mofty SK. p16 positive oropharyngeal squamous cell carcinoma:an entity with a favorable prognosis regardless of tumour HPV status. Am J Surg Pathol. 2010;34:1088–1096. doi: 10.1097/PAS.0b013e3181e84652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephen JK, Divine G, Chen KM, Chitale D, Havard S, Worsham MJ. Significance of p16 in site-specific HPV-positive and HPV-negative head and neck squamous cell carcinoma. Cancer Clin Oncol. 2013;2:51–61. doi: 10.5539/cco.v2n1p51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang SH, O'Sullivan B. Overview of the 8th edition TNM-classification for head and neck cancer. Curr Treat Options in Oncol. 2017;18(7):40. doi: 10.1007/s11864-017-0484-y. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann M, Kahn T, Goeroegh T, Lohrey C, Gottschlich S, Meyer J, Rudert H, Maune S. Tracing human papillomavirus DNA in nasal polyps by polymerase chain reaction. Acta Otolaryngol. 2000;15:815–818. doi: 10.1080/000164800750061750. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann M, Kahn T, Mahnke CG, Lippert BM, Werner JA. Prevalence of human papillomavirus in squamous cell carcinoma of the head and neck determined by polymerase chain reaction and Southern blot hybridization: proposal for optimized diagnostic requirements. Acta Otolaryngol (Stckh) 1998;118:138–144. doi: 10.1080/00016489850155279. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann M, Klose N, Gottschlich S, Görögh T, Fazel A, Lohrey C, Rittgen W, Ambrosch P, Schwarz E, Kahn T. Detection of human papillomavirus DNA in benign and malignant sinonasal neoplasms. Cancer Lett. 2006;28:64–70. doi: 10.1016/j.canlet.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 32.Chaturvedi AK, Graubard BI, Broutian T, Pickard RK, Tong ZY, Xiao W, Kahle L, Gillison ML. NHANES 2009-2012 findings: association of sexual behaviors with higher prevalence of oral oncogenic human papillomavirus infections in U.S. men. Cancer Res. 2015;75:2468–2477. doi: 10.1158/0008-5472.CAN-14-2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giuliano AR, Nyitray AG, Kreimer AR, Pierce Campbell CM, Goodman MT, Sudenga SL, Monsonego J, Franceschi S. EUROGIN 2014 roadmap: differences in human papillomavirus infection natural history, transmission and human papillomavirus-related cancer incidence by gender and anatomic site of infection. Int J Cancer. 2015;136:2752–2760. doi: 10.1002/ijc.29082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor S, Bunge E, Bakker M, Castellsagué X. The incidence, clearance and persistence of non-cervical human papillomavirus infections: a systematic review of the literature. BMC Infect Dis. 2016;16:293. doi: 10.1186/s12879-016-1633-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viscidi RP, Kotloff KL, Clayman B, Russ K, Shapiro S, Shah KV. Prevalence of antibodies to human papillomavirus (HPV) type 16 virus-like particles in relation to cervical HPV-infection among college women. Clin Diagn Lab Immunol. 1997;4:122–126. doi: 10.1128/cdli.4.2.122-126.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehanna H, Robinson M, Hartley A, Kong A, Foran B, Fulton-Lieuw T, Dalby M, Mistry P, Sen M, O'Toole L. De-ESCALaTE HPV Trial Group. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet. 2019;5:51–60. doi: 10.1016/S0140-6736(18)32752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gillison ML, Trotti AM, Harris J, Eisbruch A, Harari PM, Adelstein DJ, Sturgis EM, Burtness B, Ridge JA, Ringash J. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet. 2019;5:40–50. doi: 10.1016/S0140-6736(18)32779-X. [DOI] [PMC free article] [PubMed] [Google Scholar]


