Visual Abstract
Keywords: peritoneal dialysis, fluid status, fluid overload, bioimpedance, renal dialysis, Confidence Intervals, Prospective Studies, Follow-Up Studies, Proportional Hazards Models, Water-Electrolyte Imbalance, Renal Replacement Therapy, Treatment Outcome, diabetes mellitus, Hypertonic Solutions, Spectrum
Abstract
Background and objectives
Volume overload is frequent in prevalent patients on kidney replacement therapies and is associated with outcome. This study was devised to follow-up volume status of an incident population on peritoneal dialysis (PD) and to relate this to patient-relevant outcomes.
Design, setting, participants, & measurements
This prospective cohort study was implemented in 135 study centers from 28 countries. Incident participants on PD were enrolled just before the actual PD treatment was started. Volume status was measured using bioimpedance spectroscopy before start of PD and thereafter in 3-month intervals, together with clinical and laboratory parameters, and PD prescription. The association of volume overload with time to death was tested using a competing risk Cox model.
Results
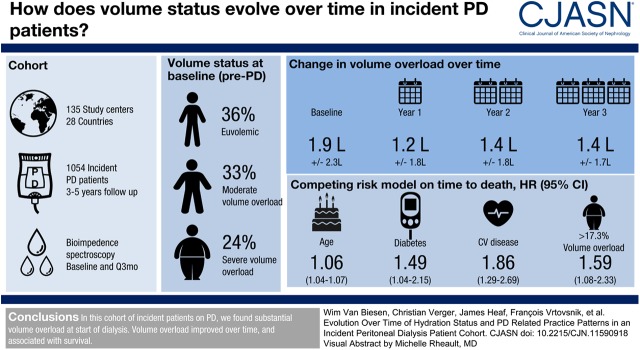
In this population of 1054 participants incident on PD, volume overload before start of PD amounted to 1.9±2.3 L, and decreased to 1.2±1.8 L during the first year. At all time points, men and participants with diabetes were at higher risk to be volume overloaded. Dropout from PD during 3 years of observation by transfer to hemodialysis or transplantation (23% and 22%) was more prevalent than death (13%). Relative volume overload >17.3% was independently associated with higher risk of death (adjusted hazard ratio, 1.59; 95% confidence interval, 1.08 to 2.33) compared with relative volume overload ≤17.3%. Different practice patterns were observed between regions with respect to proportion of patients on PD versus hemodialysis, selection of PD modality, and prescription of hypertonic solutions.
Conclusions
In this large cohort of incident participants on PD, with different treatment practices across centers and regions, we found substantial volume overload already at start of dialysis. Volume overload improved over time, and was associated with survival.
Introduction
Peritoneal dialysis (PD) allows an individualized dialysis prescription by combining different techniques (automated versus manual), dialysis solutions (tonicity, biocompatibility, type of osmotic agent), and number and duration of dwells to optimize maintenance of residual kidney function, preservation of the peritoneal membrane, and control of uremic symptoms, volume, and nutritional status, with the aim of prolonging technique and patient survival (1–4).
Elevated systolic BP and volume overload are associated with higher risk of mortality in patients on kidney replacement therapy (5–7), and both factors occur equally in patients on hemodialysis (HD) and PD (8). In a large cohort of incident patients on HD, baseline and cumulative volume overload over 1 year as assessed by bioimpedance spectroscopy was associated with mortality, independent of baseline BP (5). In PD, volume overload is frequent among prevalent patients (9), and associated with mortality (7). However, data in incident patients on PD were lacking until the international, multicentric study Initiative for Patient Outcomes in Dialysis-Peritoneal Dialysis (IPOD-PD) was started, studying incident participants on PD (10). Whereas relying on clinical observation might lead to an underestimation of volume overload in patients on PD (10), objective measurement of volume status as assessed by bioimpedance spectroscopy offers the opportunity to diagnose volume overload from the beginning of kidney replacement therapy, and to implement the appropriate treatment. It is postulated that the active management of volume overload may reduce the risk of technique failure (transfer to HD) (1) and improve survival in patients on PD (11,12). However, most of the strategies used to reduce volume overload also carry a risk of undesired side effects, such as faster degradation of the peritoneal membrane by the use of hypertonic exchanges, or faster decline of residual kidney function when volume depletion ensues. It is thus also important to take into account the strategies used to maintain euvolemia. With this in mind, the IPOD-PD study investigates volume status in an incident patient population to relate patient characteristics and practice patterns over a long-term follow-up to volume status and patient-relevant outcomes.
Materials and Methods
The study was registered in January 2011 with Clinicaltrials.gov, under identifier NCT01285726.
Study Objectives and Design
This international, prospective, observational cohort study was designed to assess volume status of incident participants on PD and its evolution over time using bioimpedance spectroscopy measurement. Consecutive incident participants on PD from involved centers were invited to be recruited between January 2011 and December 2012 (Supplemental Table 1); no specific recruitment procedure has been requested for the centers. Participant follow-up was until December 2015, and lasted a minimum of 3 and maximum of 5 years.
The objective of this analysis is to describe the population and PD-related practice patterns by geographical region, with focus on volume status and time to death.
Study Procedures
Data of the latest body composition monitor (BCM; Fresenius Medical Care, Bad Homburg, Germany) measurement, together with clinical data, laboratory parameters, planned PD prescription, clinical assessment of volume status, and medication ≤3 days before start of PD therapy, were registered as baseline values (Supplemental Table 2). The same parameters were collected 1 and 3 months after the actual start of PD, and from then onward every 3 months until the participant changed kidney replacement modality, terminated the study prematurely for other reasons, or died (10). Clinicians were free to decide whether to prescribe PD regimens according to results from BCM measurement.
Routine laboratory parameters were on the basis of data provided by the laboratories of the respective centers. GFR was calculated as the average of creatinine and urea clearance on a 24-hour urine collection when available.
Center characteristics, including the number of prevalent participants on PD and HD, were defined at the inclusion of the first participant in the respective center.
Bioimpedance Spectroscopy with the BCM
Body composition, including volume status, was assessed using multifrequency bioimpedance spectroscopy with the BCM device, measuring total body water, extracellular water, and intracellular water (ICW). The BCM measures the impedance at 50 different frequencies (minimum 5 kHz and maximum 1 Mhz). This allows for measurements of extracellular volume with low-frequency current while high-frequency currents flow through both the extracellular and intracellular volume. With this method, volume status in the different body compartments (extracellular and intracellular) can be estimated. Volume status, lean tissue, and fat tissue are calculated from the impedance data derived from the three compartment model described by Chamney et al. (13), which contains normohydrated lean tissue, normohydrated fat tissue, and excess fluid. Volume depletion or volume overload is the expected difference between the extracellular volume and the expected amount of volume in the euvolemic tissue as estimated by a previously published algorithm (13,14), expressed in absolute values (liters) or in relative terms (percentage of extracellular volume).
Patients are considered volume depleted or volume overloaded when their relative fluid volume is below the 10th or above the 90th percentile of a presumed healthy reference population (corresponding to −7% or +7% relative volume overload) (15,16).
According to physiologic plausibility, a BCM measurement was considered as invalid and excluded from statistical analysis if one of the following five criteria was present: volume depletion <−4 L, fat tissue mass/body weight not between 0.02 and 0.62, extracellular water/ICW not between 0.61 and 2.5, BCM quality indicator <50% and volume overload >6 L, and ICW/body weight not between 0.08 and 0.42.
Ethical Considerations
The study was carried out in accordance with the Declaration of Helsinki, and according to national laws and regulations submitted to ethics committees and/or national authorities. Before enrolling a participant, the individual was informed orally and in writing about the study, and provided written informed consent according to applicable law.
Statistical Analyses
Because of the explorative character of this observational study, no formal sample size estimation was performed; only available data were considered and no substitution procedure for missing data were applied. All analyses were done with SAS v9.4 (SAS Institute Inc., Cary, NC). Baseline data were analyzed descriptively and are given as percentages for categorical variables, mean±SD for normally distributed variables, and median (25th and 75th quantile) for non-normally distributed continuous variables.
To analyze associations between factors (measured at month 1) and relative volume status at the same time point and the effect of these factors on the course of relative volume status in the next 5 months, a linear mixed model was applied using SAS MIXED procedure. All available values of relative volume at month 1, 3, and 6 were used as outcome in the model. The variable time was calculated describing the time in months since month 1 and used as covariate. Additional factors as given in Table 1 were included in the model as main effects and by interaction with time. Participant and country were used as random effects and an autoregressive process of first order for irregular time intervals was applied as covariance.
Table 1.
Baseline characteristics of 1054 participants in the study, by volume status
| Characteristic | Categorized Rel FO | Total | |||
|---|---|---|---|---|---|
| Volume Depleted | Euvolemic | Moderate Volume Overload | Severe Volume Overload | ||
| N | 73 | 377 | 351 | 253 | 1054 |
| Age, yr | 56±16 | 56±16 | 59±15 | 61±14 | 58±15 |
| Men, % | 40 | 49 | 64 | 65 | 57 |
| Height, cm | 163±10 | 165±10 | 168±10 | 166±10 | 166±10 |
| Weight, kg | 73±18 | 73±17 | 72±16 | 70±15 | 72±16 |
| Body mass index, kg/m2 | 27.4±6.0 | 26.5±4.9 | 25.7±4.7 | 25.1±4.2 | 26.0±4.8 |
| Systolic BP, mm Hg | 136±20 | 134±20 | 141±20 | 144±24 | 139±21 |
| Diastolic BP, mm Hg | 81±13 | 79±12 | 81±13 | 80±15 | 80±13 |
| Primary kidney disease, % | |||||
| Diabetes | 16 | 16 | 26 | 51 | 28 |
| Glomerulonephritis | 21 | 23 | 19 | 11 | 19 |
| Hypertension | 19 | 17 | 15 | 16 | 17 |
| Hereditary/congenital disease | 8 | 12 | 11 | 2 | 9 |
| Other | 22 | 20 | 15 | 13 | 17 |
| Unknown | 14 | 11 | 13 | 7 | 11 |
| Comorbidities, % | |||||
| Hypertension | 88 | 88 | 89 | 89 | 88 |
| Diabetes (type 1+2) | 26 | 24 | 33 | 62 | 36 |
| Cardiovascular disease (NYHA stage I, II, III, IV, unknown) | 14 | 22 | 25 | 36 | 26 |
| Medication | |||||
| Use of RAAS blockers, % | 47 | 54 | 54 | 56 | 54 |
| Use of diuretics | 66 | 62 | 67 | 76 | 67 |
| Transport status, % (first assessment) | |||||
| High (fast) | 3 | 4 | 8 | 12 | 7 |
| High average | 21 | 25 | 26 | 28 | 26 |
| Low average | 19 | 19 | 17 | 15 | 17 |
| Low (slow) | 19 | 17 | 13 | 13 | 15 |
| Missing | 38 | 35 | 37 | 32 | 35 |
| Dialysis solution, % | |||||
| Polyglucose | 11 | 15 | 17 | 13 | 15 |
| Biocompatible solution | 74 | 75 | 76 | 66 | 73 |
| Standard solution | 26 | 25 | 25 | 34 | 27 |
| Volume overload | |||||
| Absolute volume overload, L | n=73 | n=377 | n=351 | n=253 | n=1054 |
| −0.8±2.4 | 0.2±0.6 | 2.0±0.7 | 4.8±2.2 | 1.9±2.3 | |
| Relative volume overload, % | n=65 | n=377 | n=351 | n=253 | n=1046 |
| −10.4±2.9 | 1.4±3.7 | 11.6±2.9 | 24.4±6.8 | 9.7±11.1 | |
| GFR at month 1 (if available), mL/min | n=26 | n=127 | n=126 | n=104 | n=383 |
| 8±4 | 9±5 | 7±4 | 6±3 | 7±4 | |
| 24 h urine output, ml | n=73 | n=375 | n=350 | n=253 | n=1051 |
| 1644±866 | 1633±754 | 156±741 | 1375±727 | 1550±757 | |
| Blood parameter | |||||
| Albumin, g/dl | n=69 | n=341 | n=318 | n=233 | n=961 |
| 3.9±0.5 | 3.9±0.5 | 3.8±0.5 | 3.4±0.6 | 3. ± 0.6 | |
| CRP, mg/L | n=61 | n=291 | n=275 | n=203 | n=830 |
| 3.0 [1.0; 6.6] | 4.2 [1.0; 8.2] | 4.0 [1.1; 8.0] | 5.0 [1.0; 13] | 4.1 [1.0; 9.0] | |
Data are given as mean±standard deviation, percentage or median and interquartile range (CRP).
Volume depleted: rel FO < −7%, euvolemic: −7% ≤ rel FO ≤7%, moderate volume overload: 7% < rel FO ≤17.3%, severe volume overload: rel FO >17.3%. Rel FO, relative fluid overload; NYHA, New York Heart Association; RAAS, renin-angiotensin-aldosteron-system; CRP, C-reactive protein.
The association between variables measured at baseline or month 1 on time to death in the following 3 years was analyzed with a competing risk model (Fine and Gray regression model for the subdistribution hazard ratios [17]) with the SAS PHREG procedure. Change to HD and transplantation were considered as competing risks, and dropouts for other reasons were censored. Backward variable selection according to the Akaike Information Criteria (18) were used to reduce the number of fixed effects and to find a good trade-off between the goodness of fit and the complexity of the model. Candidate variables for both models were region, age, sex, presence of liver disease and cardiovascular disease at baseline, and the following variables measured at month 1: diabetes status, PD modality, transport status, ultrafiltration, and volume overload (yes/no). Volume overload was defined as >17.3% (corresponding to the 75% quantile of relative volume status at month 1). In case of an omitted visit at month 1, baseline values were used instead in all three models described above.
Results
Participants
A total of 1092 participants were recruited in 135 centers from 28 countries grouped into Western Europe, Eastern Europe and Middle East, Asia Pacific, and Latin America (Table 2). Of note, all except two participants from Asia Pacific were recruited in South Korea. After exclusion because of violation of inclusion criteria (n=2), missing follow-up visits (n=6), and missing valid measurements of volume status at baseline (n=30), the final analysis population consisted of 1054 participants (Supplemental Figure 1), being slightly more than in the interim analysis (10). Whereas 36% of participants were euvolemic at start of PD, the majority showed either moderate or severe volume overload (33% and 24%, respectively), with some differences between regions (Table 2).
Table 2.
Proportion of participants at baseline by region of enrollment and categories of volume overload within the 1054 participants in the study
| Regiona | Countries | Clinics | Participants | Volume Depleted | Euvolemic | Moderately Volume Overloaded | Severely Volume Overloaded | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | N | N | N | % | N | % | N | % | N | % | |
| Western Europe | 16 | 101 | 715 | 54 | 8 | 279 | 39 | 244 | 34 | 138 | 19 |
| Eastern Europe and Middle East | 7 | 15 | 80 | 5 | 6 | 26 | 33 | 32 | 40 | 17 | 21 |
| Latin America | 3 | 13 | 130 | 10 | 8 | 38 | 29 | 31 | 24 | 51 | 39 |
| Asia Pacific | 2 | 6 | 129 | 4 | 3 | 34 | 26 | 44 | 34 | 47 | 36 |
| Total | 28 | 135 | 1054 | 73 | 7 | 377 | 36 | 351 | 33 | 253 | 24 |
Volume depleted: relative fluid overload < −7%, euvolemic: −7% ≤ relative fluid overload ≤7%, moderately volume overloaded: 7% < relative fluid overload ≤17.3%, severely volume overloaded: relative fluid overload >17.3%.
Western Europe: Belgium, Czech Republic, Finland, France, Germany, Greece, Italy, Netherlands, Portugal, Spain, Sweden, Switzerland, UK, Norway, Denmark, and Austria. Eastern Europe and Middle East: Bosnia, Croatia, Israel, Estonia, Latvia, Lithuania, and Turkey. Latin America: Brazil, Cuba, and Venezuela. Asia Pacific: Korea and India.
The characteristics of the overall population at baseline by category of volume status are given in Table 1. In more than one third of participants, no peritoneal membrane status test was documented within the first 6 months of dialysis. More than 30% were identified at the first test as high/high average transporter.
PD Prescription
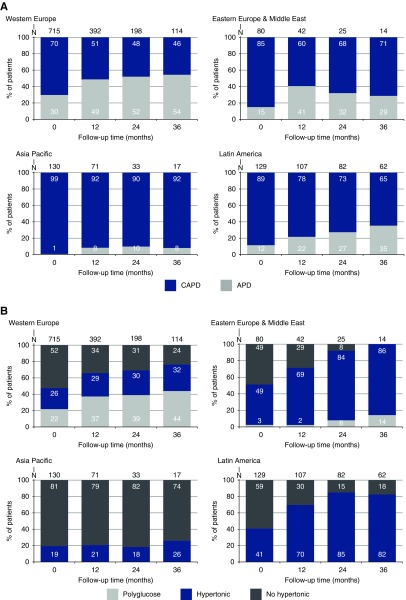
At study start, 77% of the participants were treated with continuous ambulatory peritoneal dialysis (CAPD). The proportion of automated peritoneal dialysis (APD) increased in all regions to 38% during the first 3 years on dialysis (Figure 1A). Biocompatible solutions (defined as PD fluids prepared in two-chamber bags) were prescribed to the majority of participants (73% at baseline), with some differences between the different regions.
Figure 1.
Patterns of prescription practice by region, with (A) use of APD versus CAPD and (B) use of polyglucose and hypertonic solution.
At baseline, 31% of the entire cohort were prescribed hypertonic PD solutions, defined as at least one exchange with a dextrose concentration >1.5%. This percentage increased steadily to 51% at month 36. Hypertonic solutions were prescribed at baseline to 34% and 28%, and at month 36 to 50% and 53%, of volume-overloaded and nonvolume-overloaded participants, respectively. Use of hypertonic exchanges was substantially different between regions (Figure 1B). The percentage of participants using polyglucose as an osmotic agent increased from 15% at baseline to 25% at month 36, but prescription was very heterogeneous across regions, likely because of differences in local availability.
Dropout from PD
The cumulative dropout during 3 years was 74%, with the main cause being transfer to HD (23%), followed by transplantation (22%). Overall, dropout for any reason was lowest in the Asia Pacific region (60% of the participants were still on PD after 3 years versus 26%, 23%, and 16% in Western Europe, Eastern Europe and Middle East, and Latin America).
Volume Status
Mean volume overload as measured before start of the PD treatment was 1.9±2.3 L, with a reduction to 1.2±1.8 L during the first year, and it remained rather stable at year 2 and 3, at 1.4±1.8 L and 1.4±1.7 L, respectively. This corresponds to a relative volume overload of 9.7%±11.1% at baseline, 6.6%±9.5% after 1 year, 7.4%±9.6% after 2 years, and 7.7%±8.9% after 3 years. The course of BP, GFR, and urine output over 3 years is given in Supplemental Figures 2–4.
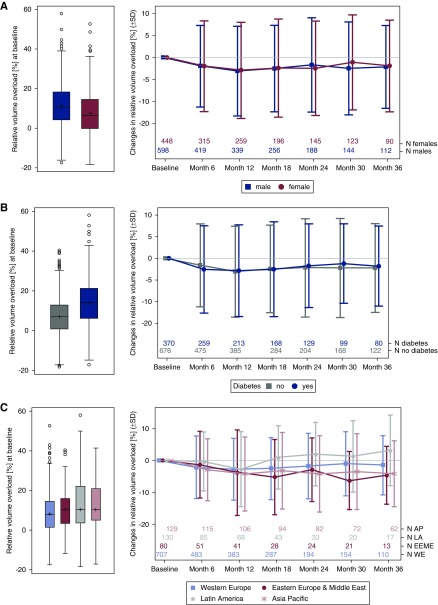
Volume overload was at all time points higher in men than in women, and higher in participants with versus without diabetes. The course of relative volume overload showed a slight and similar decrease over time in all groups (Figure 2, A and B). After 3 years of follow-up, the mean relative volume overload in the remaining cohort was lower than at baseline in participants from all regions, except those from Latin America, where it increased (Figure 2C).
Figure 2.
Relative volume overload at baseline (left panel) and mean change during the study (right panel) by (A) sex, (B) diabetes status, and (C) region. The Δ of relative volume overload/depletion in percent points was calculated for each patient and visit (baseline, month 1, month 3, month 6, and so on, until month 36) as the absolute difference between relative volume overload or depletion at the respective visit and at baseline. The plots show the interquartile range (IQR; box), mean (cross), median (line), minimum and maximum value within the IQR±1.5 IQR (whiskers), and outliers (circles). AP, Asia Pacific; LA. Latin America; EEME, Eastern Europe & Middle East; WE, Western Europe.
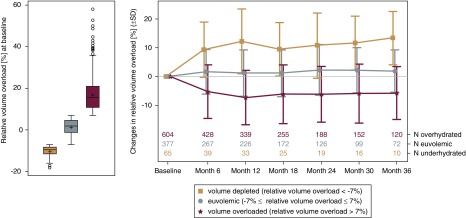
Before the start of PD, 57% of the participants showed a relative volume overload of >7%. This proportion decreased to 48%, 49%, and 53% after 1, 2, and 3 years of follow-up, respectively. Relative fluid depletion (<−7%) was found in approximately 3%–8% of the participants at all time points. On average, mean relative volume increased in the first year in participants with volume depletion at baseline, whereas in participants with volume overload at baseline, a decreasing trend in mean relative volume in the first year was observed (Figure 3). The follow-up observations showed that both groups (with volume depletion and volume overload) tended toward euvolemia.
Figure 3.
Relative volume overload at baseline (left panel) and mean change of relative volume overload (right panel) of participants in the following categories: volume depleted (<−7%), euvolemic (−7% ≤ relative volume status ≤7%), and volume overloaded (>7%). The plots show the interquartile range (IQR; box), mean (cross), median (line), minimum and maximum value within IQR±1.5 IQR (whiskers), and outliers (circles).
In a linear mixed model increasing age, male sex, the regions Asia Pacific and Eastern Europe and Middle East, intake of diuretics, presence of diabetes were associated with elevated relative volume overload at month 1. For participants with a slow peritoneal membrane transport, relative volume was estimated at 6% higher for participants using APD than CAPD (P=0.05). No significant effect of any factor on the course of relative volume until month 6 could be found (Table 3).
Table 3.
Associations of clinical characteristics with volume overload and its change over time
| Fixed Effects | Baseline Relative Volume Overload (%), Mean (95% CI) at 1 mo | Adjusted Difference (95% CI) in Baseline Relative Volume Overload (%) at 1 mo | Change in Relative Volume Overload (% per mo), Mean (95% CI) | Adjusted Difference (95% CI) in Change in Relative Volume Overload (% per mo) | |
|---|---|---|---|---|---|
| Age | 1 yr increase in age | 0.1 (0.0–0.1)a | 0.0 (0.0–0.0) | ||
| Sex | Women | 10.6 (8.4 to 12.9) | 0 (ref) | −0.4 (−1.1 to 0.4) | 0 (ref) |
| Men | 14.0 (11.8 to 16.2) | 3.4 (2.1 to 4.7)a | −0.3 (−0.1 to 0.4) | 0.1 (−0.2 to 0.4) | |
| Region | Western Europe | 9.6 (7.6 to 11.6) | 0 (ref) | −0.3 (−1.0 to 0.4) | 0 (ref) |
| Eastern Europe, Middle East | 14.2 (11.0 to 17.5) | 4.6 (1.7 to 7.5)a | −0.8 (−1.6 to 01) | −0.5 (−1.1 to 0.1) | |
| Latin America | 12.2 (9.1 to 15.2) | 2.6 (−0.1 to 5.3) | 0.0 (−0.7 to 0.8) | 0.3 (−0.1 to 0.8) | |
| Asia Pacific | 13.3 (9.8 to 16.8) | 3.7 (0.4 to 7.0)a | −0.3 (−1.0 to 0.5) | 0.0 (−0.5 to 0.5) | |
| Diuretics | No | 11.4 (9.0 to 13.8) | 0 (ref) | −0.3 (−1.0 to 0.5) | 0 (ref) |
| Yes | 13.3 (11.1 to 15.4) | 1.8 (0.4 to 3.3)a | −0.4 (−1.1 to 0.3) | −0.1 (−0.5 to 0.2) | |
| Diabetes | No | 9.9 (7.7 to 12.2) | 0 (ref) | −0.3 (−1.0 to 0.4) | 0 (ref) |
| Yes | 14.7 (12.5 to 17.0) | 4.8 (3.3 to 6.2)a | −0.3 (−1.1 to 0.4) | 0.0 (−0.3 to 0.3) | |
| Polyglucose | No | 12.5 (10.4 to 14.5) | 0 (ref) | −0.4 (−1.1 to 0.3) | 0 (ref) |
| Yes | 12.2 (9.6 to 14.8) | −0.3 (−2.1 to 1.6) | −0.3 (−1.0 to 0.5) | 0.1 (−0.3 to 0.5) | |
| Transport status dialysis modalitya | CAPD/slow | 8.4 (5.6 to 11.2) | 0 (ref) | 0.1 (−0.7 to 0.9) | 0 (ref) |
| CAPD/average | 11.8 (9.9 to 13.8) | 3.4 (0.6 to 6.3)a | −0.4 (−1.0 to 0.3) | −0.5 (−1.1 to 0.1) | |
| CAPD/fast | 14.9 (11.5 to 18.4) | 6.5 (2.4 to 10.6)a | −0.4 (−1.3 to 0.6) | −0.5 (−1.4 to 0.4) | |
| CAPD/missing | 13.1 (11.5 to 14.7) | 4.7 (1.9 to 7.4)a | −0.4 (−1.0 to 0.3) | −0.5 (−1.1 to 0.1) | |
| APD/slow | 14.5 (8.7 to 20.3) | 6.1 (0.0 to 12.2) | −0.7 (−2.2 to 0.7) | −0.9 (−2.3 to 0.6) | |
| APD/average | 12.6 (9.1 to 16.1) | 4.2 (0.2 to 8.2)a | −0.4 (−1.3 to 0.5) | −0.5 (−1.4 to 0.4) | |
| APD/fast | 10.9 (0.0 to 1.8) | 2.5 (−8.7 to 13.7) | −0.2 (−2.5 to 2.1) | −0.4 (−2.7 to 1.9) | |
| APD/missing | 12.4 (10.1 to 14.6) | 4.0 (0.9 to 7.1)a | 0.3 (−0.9 to 0.4) | −0.4 (−1.1 to 0.3) |
Linear mixed-model analyzing the association between variables measured at month 1 and relative volume overload (VO) at the same time point as well as the potential influence of these variables on changes in relative volume overload until month 6. Least square means for month 0 and for changes in 1 month were calculated for each categorical variable via least square means and estimate statement in PROC MIXED procedure in SAS. The adjusted differences in mean and in change of relative VO are always comparing the named categories with the reference category. The reference categories are marked as (ref). 95% CI, 95% confidence interval; ref, reference; CAPD, continuous ambulatory peritoneal dialysis; APD, automated peritoneal dialysis.
Significant difference between category and reference category.
Time to Death
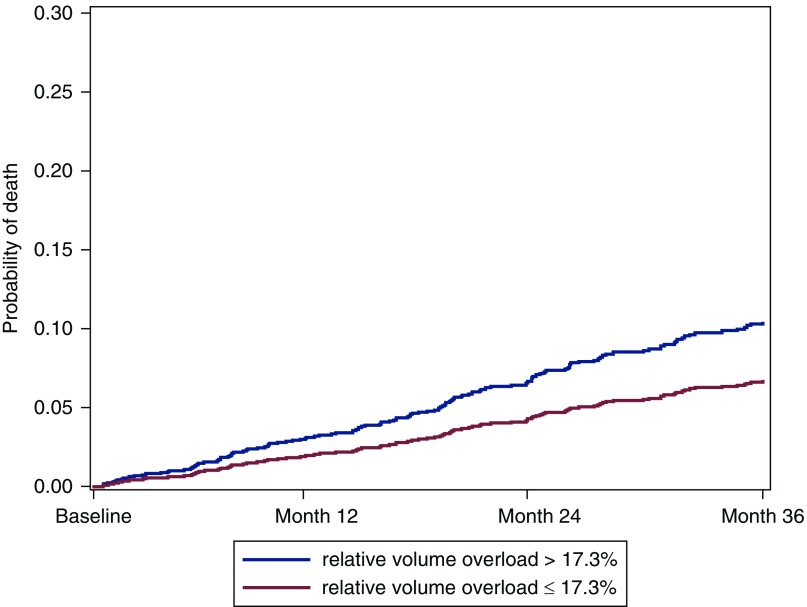
In a competing risk model on time to death, controlling for change to HD and transplantation, the variables volume overload (defined as >17.3%; the 75th percentile of relative volume overload at month 1 in our cohort), age, cardiovascular disease, liver disease, and diabetes were used (Table 4). The subdistributional hazard ratio for volume-overloaded participants is 1.59 compared with nonvolume-overloaded participants (95% confidence interval, 1.08 to 2.33; P=0.02). The cumulative incidence curve is visualized in Figure 4.
Table 4.
Competing risk analysis for death, with censoring for dropout because of transfer to hemodialysis or transplantation.
| Parameter | Subdistribution Hazard Ratio | Lower 95% CI | Upper 95% CI | P Value | |
|---|---|---|---|---|---|
| Relative volume overload | Relative volume overload >17.3% | 1.59 | 1.08 | 2.33 | 0.02 |
| Age | per 1 year | 1.06 | 1.04 | 1.07 | <0.001 |
| Cardiovascular disease | Yes | 1.86 | 1.29 | 2.68 | <0.001 |
| Liver disease | Yes | 2.11 | 1.14 | 3.90 | 0.02 |
| Diabetes | Yes | 1.49 | 1.04 | 2.15 | 0.03 |
Participants with dropout before month 1 (n=44) were excluded from this analysis. Volume overload is defined as relative volume overload >17.3% (75th percentile of the cohort). 95% CI, 95% confidence interval.
Figure 4.
Cumulative incidence of death by volume status 1 month after initiating PD, adjusted for competing risks of transfer to HD and transplantation in participants with relative volume overload >17.3% versus ≤17.3% for a participant with median age, no diabetes, and no cardiovascular disease.
Discussion
The IPOD-PD study represents the largest cohort of incident patients on PD providing data on volume status as measured by bioimpedance spectroscopy, dialysis practices, and outcome. Overall, we found a substantial volume overload, which tended to improve over time of observation, but was at all times higher in men versus women and in participants with versus without diabetes. The results also indicate a variation in PD-related practices across regions, with an effect on volume status and dropout from PD. In a competing risk analysis, a relative volume overload >17.3% at month 1 was associated with time to death over the 3-year observation period.
Although volume overload is frequent in patients on kidney replacement therapy, the IPOD-PD study demonstrates that this volume overload is already present before the start of kidney replacement therapy. Volume overload tended to improve from baseline over the next 6 months, and stabilized afterward. It is difficult to determine whether this is a true improvement, rather a regression to the mean or selection bias by informative dropout of those who are volume overloaded. Participants who were euvolemic at start mostly remained euvolemic, whereas the extent of volume depletion or volume overload tended to decrease.
As in prevalent patients, age, diabetes, and male sex are risk factors for being more volume overloaded in incident patients. Participants with diabetes are at risk for volume overload as they have more upregulation of vasopressin because of the hyperosmolar state induced by the hyperglycemia. The use of hypertonic exchanges can induce hyperglycemia, even in patients without diabetes (19). Adequate control of glycaemia is thus essential to maintain volume status in patients on PD. Fernandes et al. (20) also found a higher level of volume overload in patients with versus without diabetes, and associated this with a lower transperitoneal ultrafiltration capacity in patients with diabetes.
Several studies document higher odds of volume overload in men versus women on kidney replacement therapy. A recent small cohort study found a negative association between low testosterone and volume overload (21), and other cohort studies report a more substantial loss of lean tissue in men versus women on dialysis (8), suggesting that hormonal disturbances might play an important role in the observed volume overload in men. Poor adherence with therapy is a less likely explanation because our data indicate that the sex disparity in volume status is already present before start of kidney replacement therapy.
There were substantial differences in volume status between regions and clinical practices, which in turn may have an effect on volume status. In Asia Pacific, the usage of hypertonic bags and polyglucose was low; however, despite this, the initial volume overload could be successfully reduced. In contrast, in Latin America, volume overload even increased over time despite a high percentage using hypertonic solutions after 2 and 3 years. An influence on volume overload by the use of hypertonic exchanges might be only limited and possibly superimposed by differences in adherence to salt and fluid restriction. However, our results indicate that it is possible to maintain good volume status even without the use of hypertonic exchanges, polyglucose, or APD.
Patients with a faster transport status are at highest risk of volume overload, and this exists before the start of PD (10). Thus, the volume overload observed in fast transporters is not only due to PD-related factors, suggesting that common factors explain both the fast transport status and volume overload, such as inflammation (22) and/or salt intake (23).
In Asia Pacific, PD dropout was substantially lower than in other regions, despite a limited usage of APD, hypertonic exchanges, and/or polyglucose. It could be speculated that this might be due to physiognomy, e.g., a lower body weight and body mass index in participants from Asia Pacific versus the other regions. Furthermore, the centers from Asia Pacific are characterized by the highest percentage of patients on PD versus HD and the highest number of incident patients on PD. Center size and number of incident patients on PD per year seem to have an effect on technique success (24).
The choice of modality (CAPD versus APD) and type of solution was certainly also governed by local availability. This is reflected in the absence of polyglucose prescription, the low use of APD, and the difference in use of biocompatible solutions in Asia Pacific and Latin America, with practically 100% use in Asia Pacific and nonusage in Latin America. The proportion of APD versus CAPD increased over time, but was always highest in Western Europe and lowest in Asia Pacific, in line with recent epidemiologic analysis on PD use worldwide (25).
In a recent meta-analysis (26), volume overload as assessed by bioimpedance was independently associated with mortality or hospitalization in prevalent kidney replacement therapy. This relationship remained after adjustment for nutritional status and inflammation, contradicting the hypothesis that the association between bioimpedance-defined volume overload and survival only reflects cachexia, and supporting a direct toxic effect of hypervolemia. Our data confirm that this association is also present in incident kidney replacement therapy.
The strength of this study is the size of the cohort, with data on volume status from the start of kidney replacement therapy for a period equivalent to or even longer than the median survival time on PD. Furthermore, it comprises participants from a broad geographical origin and thus treatment practices.
The limitations of this study are the observational nature of the study, limiting the derivation of cause-effect associations. Our ability to derive associations between prescription patterns and volume status might be limited by the range of such patterns over the regions and countries, and by our sample size. The generalizability of our observations is hampered by the fact that the percentage of patients on PD versus HD is much higher than average in that region, indicating that there might be a selection bias in favor of more experienced PD centers.
In conclusion, our study found that substantial volume overload was present in this incident cohort of participants on PD, with men and patients with diabetes being more affected. Volume overload was associated with mortality. The study revealed different treatment practices across centers and regions. Despite not using hypertonic exchanges, APD, or polyglucose, the best technique survival was noted in Asia Pacific.
Disclosures
Dr. Brito, Ms. de los Rios, Dr. Gauly, and Ms. Ihle report full-time employment at Fresenius Medical Care. Dr. Heaf reports grants and personal fees from Fresenius, during the conduct of the study; grants and personal fees from Baxter, outside the submitted work. Dr. Pérez Martínez reports travel grants from Fresenius during the conduct of the study and receiving personal fees from Baxter, outside the submitted work. Dr. Van Biesen received travel grants and speaker fees from Fresenius Medical Care and Baxter Healthcare. Dr. Verger is a resident of the French Language Peritoneal Dialysis Registry (RDPLF), which gets financial support from Baxter, Fresenius, Theradial, and Physidia. Dr. Crepaldi, Dr. Do, Dr. Prieto-Velasco, Dr. Ronco, and Dr. Vrtovsnik have nothing to disclose.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11590918/-/DCSupplemental.
Supplemental Table 1. Inclusion and exclusion criteria.
Supplemental Table 2. Documented parameters by visit.
Supplemental Figure 1. Flow diagram of the analysis population.
Supplemental Figure 2. Course of (A) systolic and (B) diastolic BP.
Supplemental Figure 3. Course of GFR during the study.
Supplemental Figure 4. Course of 24-hour urine output during the study.
Supplementary Material
Acknowledgments
The following centers participating in the study are gratefully acknowledged for their contribution and dedication to the study: Austria: Klinikum Wels-Grieskirchen GmbH, Wels (Dr. M. Windpessl). Belgium: Algemeen Stedelijk Ziekenhuis Campus Geraardsbergen, Geraardsbergen (Prof. van Biesen); Universitair Ziekenhuis Gent, Gent (Prof. van Biesen); Centre Hospitalier Universitaire Sart Tilman, Liège (Dr. C. Bovy); Algemeen Stedelijk Ziekenhuis Campus Aalst, Aalst (Prof. N. Veys); Universitair Ziekenhuis Leuven, Leuven (Prof. B. Bammens); Centre Hospitalier Universitaire de Charleroi, Charleroi (Dr. S. Treille); and H.-Hartziekenhuis Roeselare-Menen vzw, Roeselare (Dr. Bart Maes). Brazil: Clinica de Doenças Renais, Pirai (Dr. A. Ferreira Teixeira); Centro De Terapia Nefrologica, Sao Paulo (Dr. Britto); and Instituto Mineiro De Nefrologia, Belo Horizonte (Dr. V. Ladeira Rodrigues). Bosnia: Klinička Bolnica Mostar, Mostar (Dr. D. Rončević); and Univerzitetski Klinički Centar Tuzla, Tuzla (Dr. E. Mesic). Czech Republic: Fresenius Medical Care Most, Most (Dr. P. Machek); Institut Klinické a Experimentální Medicíny, Praha (Dr. A. Parikova); Všeobecná fakultní Nemocnice Karlovo namesti, Praha (Dr. V. Bednarova); Všeobecná fakultní Nemocnice Strahov, Praha (Dr. V. Polakovic); Faculty Hospital Plzen, Plzen (Dr. T. Reischig); University Hospital Fakultní Nemocnice Brno Bohunice, Brno (Dr. J. Řehořová); Fakultní Nemocnice Hradec Kralove, Kralove (Dr. B. Hájková); and Třebíč Hospital, Trebic (Dr. H. Chmelíčková). Croatia: University Hospital Merkur, Zagreb (Dr. M. Knotek); University Hospital Center Osijek, Osijek (Dr. M. Jakic); and University Hospital Center Split, Split (Dr. J. Radic). Cuba: Instituto de Nefrología, La Habana (Dr. R. Bohorques). Denmark: Herlev Hospital, Herlev (Dr. Heaf); Aarhus University Hospital, Aahrus (Prof. J. Povlsen); Roskilde Hospital, Roskilde (Dr. B. Ekelund); and Hillerød Hospital, Hillerød (Dr. H. Mollerup). Estonia: West Talinn Central Hospital, Tallinn (Dr. M. Muliin); North Estonian Regional Hospital, Tallinn (Dr. E. Kuzmina); and Tartu University Hospital, Tartu (Dr. K. Kõlvald). Finland: Satakunnan keskussairaala, Pori (Dr. K. Laine); Kymenlaakson keskussairaala, Kotka (Dr. M. Huuskonen); Keski-Suomen keskussairaala, Jyväskylä (Dr. M. Miettinen); Helsinki University Hospital, Helsinki (Dr. V. Rauta); Oulu University Hospital, Oulu (Dr. M. Tamminen); Tampere University Hospital, Tampere (Dr. H. Saha); Central Hospital in Joensuu, Joensuu (Dr. K. Jääskeläinen); and Päijät-Hämeen Central Hospital, Lahti (Dr. M. Vilpakka). France: Centre Hospitalier Rene Dubos, Pontoise (Dr. Verger); Hôpital Civil de Strasbourg, Strasbourg (Dr. F. Heibel); AUB Santé Quimper, Quimper (Dr. P.Y. Durand); Centre Hospitalier Dunkerque, Dunkerque (Dr. R. Azar); CHU Bichat, Paris (Prof. Vrtovsnik); Centre Hospitalier Universitaire de Bordeaux, Bordeaux (Dr. C. Moreau); and Expansion des Centres d'Hémodialyse de l'Ouest Nantes, Rezé (Dr. I. Oancea). Germany: Nierenzentrum Heidelberg, Heidelberg (Prof. V. Schwenger); Nephrologisches Zentrum Velbert, Velbert (Prof. M. Koch); Nieren und Hochdruckzentrum Kiel, Kiel (Dr. J. Struck); Robert Bosch Krankenhaus, Stuttgart (Prof. D. Alscher); Nephrologische Praxis Wiesbaden, Wiesbanden (Prof. T. Mettang); Klinikum Braunschweig, Braunschweig (Dr. R. Wanninger); KfH Zentrum Bottrop, Bottrop (Prof. M. Hollenbeck); and Nephrology – Universitätsklinikum Gießen-Marburg Giessen, Gießen (Prof. H.W. Birk). Greece: General Hospital G. Gennimatas, Athens (Dr. G. Tsirpanlis); General University Hospital of Alexandroupolis, Alexandroupolis (Dr. M. Theodoridis); General University Hospital of Thessaloniki Thessaloniki (Dr. V. Liakopoulos); General University Hospital of Ioannina, Ioannina (Dr. O. Balafa); and General Hospital of Ioannina, Ioannina (Dr. A. Andrikos). India: Madras Medical Mission, Chennai (Dr. G. Abraham). Israel: Western Galilee Hospital, Naharyia (Dr. H. Kamal). Italy: Ospedale San Bortolo, Vicenza (Dr. Crepaldi); Azienda Ospedaliera Brotzu, Cagliari (Dr. G. Cabiddu); UO Nefrologia Policlinico BARI, Bari (Prof. R. Russo); and Ospedale Civico Palermo, Palermo (Dr. F. Caputo). Korea: NHIS Ilsan Hospital, Koyang (Dr. Sug-Kyun Shin); Yeungnam University Hospital, Daegu (Prof. Jun-Young Do); Seoul National University Hospital, Seoul (Prof. Kook-Hwan Oh); Severance Hospital, Seoul (Prof. Shin Wook Kang); and Konkuk University Medical Center, Seoul (Prof. Young-Il Jo). Latvia: Nephrology Centre, P. Stradins Clinical University Hospital, Riga (Dr. I. Puide); and Med Alfa Ltda, Riga (Dr. I. Busmane). Lithuania: Klaipeda Republic Hospital, Klaipeda (Dr. I. Puide); Lithuanian University of Health Sciences Hospital Kaunas Clinics, Kaunas (Dr. N. Kusleikaite); and Vilnius University Antakalnis Hospital, Vilnius (Dr. S. Dalia). Netherlands: University Hospital Maastricht, Maastricht (Prof. F. van der Sande). Norway: St. Olavs Hospital, Sluppen (Dr. M. Radtke); Nordlandssykehuset Bodø, Bodø (Dr. A.K. Fagerheim); Sykehuset i Møre og Romsdal, Aalesund (Dr. A.B. Tafford); and Sykehuset Levanger, Levanger (Dr. J. Rocke). Portugal: Hospital Santa Cruz, Carnaxide (Dr. M.A. Gaspar); and CHP Hospital Santo Antonio, Porto (Dr. A. Soares Rodrigues). Spain: Complejo Asistencial de León, León (Dr. M. Prieto); Hospital Clinic i Provincial de Barcelona, Barcelona (Dr. M. Vera Rivera); Hospital de Mollet, Mollet del Valles (Dr. R. Samon Guasch); Hospital General de Vic, Vic (Dr. J. Feixas); Hospital Universitario Central, Oviedo (Dr. C. Rodríguez); Hospital Josep Trueta, Girona (Dr. I. Garcia); Hospital Xeral Cies de Vigo, Vigo (Dr. M. Moreiras-Plaza); Hospital San Pedro de Logroño, Logroño (Dr. M. Sierra); Hospital Universitario de Puerto Real, Puerto Real (Dr. C. Orellana); Hospital Universitario Rio Hortega, Valladolid (Dr. A. Molina); Complejo Hospital Universitario de Santiago, Santiago de Compostela (Dr. R. Valente); Fundació Puigvert, Barcelona (Dr. T. Doñate); Fundación Hospital Manacor, Manacor (Dr. D. Tura); Hospital de Basurto, Bilbao (Dr. O. González); Hospital General Universitario Gregorio Marañon, Madrid (Dr. J.M. López); Hospital Universitario Ramón y Cajal, Madrid (Dr. M. Rivera); Hospital Virgen de la Macarena, Sevilla (Dr. N. Areste); Hospital Universitario Puerta del Mar, Cádiz (Dr. F. Tejuca); Complejo Hospitalario de Jaén, Jaén (Dr. J.M. Gil); Hospital de Txagoritxu, Vitoria (Dr. J.I. Minguela); Hospital Universitario A Coruña, Coruña (Dr A. Rodriguez-Carmona); Hospital Universitario Marques de Valdecilla, Santander (Dr. R. Palomar); Hospital universitario Fundación Alcorcón, Alcorcón (Dr. A.M. Tato); Hospital Clínico San Carlos, Madrid (Dr. R. Valero); Hospital Carlos Haya, Málaga (Dr. S. Ros); CHU Albacete, Albacete (Dr. J. Pérez); Hospital Clínico de Valencia, Valencia (Dr. M.A. González); Hospital de Torrecardenas, Almeria (Dr. F.J. González); Hospital General de Castellón, Castellón (Dr. J.J. Sánchez); Hospital Universitario Puerta de Hierro, Madrid (Dr. J.M. Portoles); Fundación Jiménez Díaz, Madrid (Dr. A. Ortiz); Hospital Lucus Augusti/Hospital de Lugo, Lugo (Dr. B. Millán); Hospital de Galdakano, Bilbao (Dr. J. Montenegro); and Hospital Severo Ochoa, Legane, Madrid (Dr. P. Gallar). Sweden: Sahlgrenska PD-mottagningen, Göteborg (Dr. A. Aldenbratt); Skanes University Hospital Malmo, Malmo (Dr. A.C. Johansson); Hallands Hospital Halmstad, Halmstad (Dr. K.H. Gydell); Kungsholmsdialysen, Stockholm (Dr. O. Heimbürger); Danderyds Hospital, Stockholm (Dr. G.F. Germanis); Karolinska University Hospital Solna, Stockholm (Dr. B. Hylander); Sunderby Sjukhus, Luleå (Dr. M. Isaksson); and Mälarsjukhuset Eskilstuna, Eskilstuna (Dr. K.C. Gröntoft). Switzerland: Luzern Kantonspital, Luzern (Dr. A. Fischer). Turkey: Akdeniz Üniversitesi Tıp Fakültesi Nefroloji kliniği, Antalya (Prof. F. Fevzi Ersoy). UK: Western Infirmary Glasgow, Glasgow (Dr. M. Gorrie); and Barts and The London NHS Trust, London (Dr. S. Fan). Venezuela: FME Zulia, Maracaibo (D. Nava); FME Maracay, Turmero (I. Martínez); Instituto Docente de Urologia, Valencia (A. Román); Cenesuca, Cumana (F. Velásquez); FME Puerto de la Cruz, Puerto la Cruz (Dr. A. Gonzalez); FME El Tigre, El tigre (Dr. M. Alvarez); FME Caracas, Caracas (J.M. González); FME Charallave, Charallave (M.C. Navas); and FME Maturin, Maturin (Dr. D. Rodríguez). The valuable support by Fresenius Medical Care colleagues in all participating countries is gratefully acknowledged.
The study has been funded by Fresenius Medical Care Deutschland GmbH and Fresenius Medical Care Asia Pacific Ltd.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related Patient Voice, “Managing Fluid Control in the Peritoneal Dialysis Population,” on pages 783–784.
References
- 1.Jager KJ, Merkus MP, Dekker FW, Boeschoten EW, Tijssen JG, Stevens P, Bos WJ, Krediet RT; NECOSAD Study Group : Mortality and technique failure in patients starting chronic peritoneal dialysis: Results of The Netherlands cooperative study on the adequacy of dialysis. Kidney Int 55: 1476–1485, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Htay H, Cho Y, Pascoe EM, Darssan D, Nadeau-Fredette AC, Hawley C, Clayton PA, Borlace M, Badve SV, Sud K, Boudville N, McDonald SP, Johnson DW: Center effects and peritoneal dialysis peritonitis outcomes: Analysis of a national registry. Am J Kidney Dis 71: 814–821, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Beduschi Gde C, Figueiredo AE, Olandoski M, Pecoits-Filho R, Barretti P, de Moraes TP; All Centers that Contributed to the BRAZPD : Automated peritoneal dialysis is associated with better survival rates compared to continuous ambulatory peritoneal dialysis: A propensity score matching analysis. PLoS One 10: e0134047, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatoth DK, Golper TA, Gokal R: Morbidity and mortality in redefining adequacy of peritoneal dialysis: A step beyond the national kidney foundation dialysis outcomes quality initiative. Am J Kidney Dis 33: 617–632, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Zoccali C, Moissl U, Chazot C, Mallamaci F, Tripepi G, Arkossy O, Wabel P, Stuard S: Chronic fluid overload and mortality in ESRD. J Am Soc Nephrol 28: 2491–2497, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wizemann V, Wabel P, Chamney P, Zaluska W, Moissl U, Rode C, Malecka-Masalska T, Marcelli D: The mortality risk of overhydration in haemodialysis patients. Nephrol Dial Transplant 24: 1574–1579, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Lone EL, Visser A, Finney H, Fan SL: Clinical significance of multi-frequency bioimpedance spectroscopy in peritoneal dialysis patients: Independent predictor of patient survival. Nephrol Dial Transplant 29: 1430–1437, 2014 [DOI] [PubMed] [Google Scholar]
- 8.van Biesen W, Claes K, Covic A, Fan S, Lichodziejewska-Niemierko M, Schoder V, Verger C, Wabel P: A multicentric, international matched pair analysis of body composition in peritoneal dialysis versus haemodialysis patients. Nephrol Dial Transplant 28: 2620–2628, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Van Biesen W, Williams JD, Covic AC, Fan S, Claes K, Lichodziejewska-Niemierko M, Verger C, Steiger J, Schoder V, Wabel P, Gauly A, Himmele R; EuroBCM Study Group : Fluid status in peritoneal dialysis patients: The European Body Composition Monitoring (EuroBCM) study cohort. PLoS One 6: e17148, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ronco C, Verger C, Crepaldi C, Pham J, De Los Ríos T, Gauly A, Wabel P, Van Biesen W; IPOD-PD Study Group : Baseline hydration status in incident peritoneal dialysis patients: The initiative of patient outcomes in dialysis (IPOD-PD study). Nephrol Dial Transplant 30: 849–858, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan S, Davenport A: The importance of overhydration in determining peritoneal dialysis technique failure and patient survival in anuric patients. Int J Artif Organs 38: 575–579, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Jotterand Drepper V, Kihm LP, Kälble F, Diekmann C, Seckinger J, Sommerer C, Zeier M, Schwenger V: Overhydration is a strong predictor of mortality in peritoneal dialysis patients - independently of cardiac failure. PLoS One 11: e0158741, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chamney PW, Wabel P, Moissl UM, Müller MJ, Bosy-Westphal A, Korth O, Fuller NJ: A whole-body model to distinguish excess fluid from the hydration of major body tissues. Am J Clin Nutr 85: 80–89, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Moissl UM, Wabel P, Chamney PW, Bosaeus I, Levin NW, Bosy-Westphal A, Korth O, Müller MJ, Ellegård L, Malmros V, Kaitwatcharachai C, Kuhlmann MK, Zhu F, Fuller NJ: Body fluid volume determination via body composition spectroscopy in health and disease. Physiol Meas 27: 921–933, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Wabel P, Moissl U, Chamney P, Jirka T, Machek P, Ponce P, Taborsky P, Tetta C, Velasco N, Vlasak J, Zaluska W, Wizemann V: Towards improved cardiovascular management: The necessity of combining blood pressure and fluid overload. Nephrol Dial Transplant 23: 2965–2971, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Wieskotten S, Heinke S, Wabel P, Moissl U, Becker J, Pirlich M, Keymling M, Isermann R: Bioimpedance-based identification of malnutrition using fuzzy logic. Physiol Meas 29: 639–654, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
- 18.Akaike H: A new look at the statistical model identification. IEEE Trans Automat Contr 19: 716–723, 1973 [Google Scholar]
- 19.Pletinck A, Verbeke F, Van Bortel L, Dequidt C, Vijt D, Van Biesen W, Vanholder R: Acute central haemodynamic effects induced by intraperitoneal glucose instillation. Nephrol Dial Transplant 23: 4029–4035, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Fernandes A, Ribera-Sanchez R, Rodríguez-Carmona A, López-Iglesias A, Leite-Costa N, Pérez Fontán M: Peritoneal water transport characteristics of diabetic patients undergoing peritoneal dialysis: A longitudinal study. Am J Nephrol 46: 47–54, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Hassan K, Elimeleh Y, Shehadeh M, Fadi H, Rubinchik I: The relationship between hydration status, male sexual dysfunction and depression in hemodialysis patients. Ther Clin Risk Manag 14: 523–529, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Biesen W, Van der Tol A, Veys N, Dequidt C, Vijt D, Lameire N, Vanholder R: The personal dialysis capacity test is superior to the peritoneal equilibration test to discriminate inflammation as the cause of fast transport status in peritoneal dialysis patients. Clin J Am Soc Nephrol 1: 269–274, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Pletinck A, Consoli C, Van Landschoot M, Steppan S, Topley N, Passlick-Deetjen J, Vanholder R, Van Biesen W: Salt intake induces epithelial-to-mesenchymal transition of the peritoneal membrane in rats. Nephrol Dial Transplant 25: 1688–1696, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Htay H, Cho Y, Pascoe EM, Darssan D, Nadeau-Fredette AC, Hawley C, Clayton PA, Borlace M, Badve SV, Sud K, Boudville N, McDonald SP, Johnson DW: Multicenter registry analysis of center characteristics associated with technique failure in patients on incident peritoneal dialysis. Clin J Am Soc Nephrol 12: 1090–1099, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain AK, Blake P, Cordy P, Garg AX: Global trends in rates of peritoneal dialysis. J Am Soc Nephrol 23: 533–544, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tabinor M, Elphick E, Dudson M, Kwok CS, Lambie M, Davies SJ: Bioimpedance-defined overhydration predicts survival in end stage kidney failure (ESKF): Systematic review and subgroup meta-analysis. Sci Rep 8: 4441, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.