Abstract
Many animal experiments and clinical trials showed that probiotics are effective for the treatment of alcoholic liver disease. Alcohol disrupts the composition of intestinal flora; probiotics modulate the gut microbiota and reverse alcohol-associated intestinal barrier dysfunction by decreasing intestinal mucosal permeability and preventing intestinal bacteria from translocating. Probiotics enhance immune responses and reduce the levels of alcohol-induced inflammatory cytokines and reactive oxygen species (ROS) production in the liver and intestine. Probiotics also increase fatty acid β-oxidation and reduce lipogenesis, combating alcohol-induced hepatic steatosis. In this review, we summarize the current knowledge regarding the mechanism of action of probiotics for reducing the effects of alcoholic liver disease.
1. Introduction
With economic development and increased levels of social activity, alcohol consumption is increasing worldwide. An increasing number of patients suffer from alcoholic liver disease. According to statistics, 80% of drinkers exhibit characteristics of alcoholic liver injury, of which 10%–35% progress to alcoholic hepatitis and 10%–20% may develop alcoholic cirrhosis [1]. Alcohol abstinence can reverse mild alcoholic liver injury but not the irreversible injury of cirrhosis. To date, the details of alcoholic liver damage pathogenesis remain unclear, and effective drugs to treat the condition have not been discovered. Probiotics are functional products; their beneficial physiological and biochemical functions have been demonstrated in humans. Studies have demonstrated that probiotics effectively attenuate alcoholic liver disease as well as improve liver function [2–4]. In this paper, we summarize the effects of probiotics regarding improvement of alcoholic liver disease.
2. Alcoholic Liver Injury
Alcoholic liver disease (ALD) is a major of chronic liver disease, caused by excessive alcohol ingestion. In the initial stages, alcohol consumption induces development of fatty liver, gradually developing into hepatitis, liver fibrosis, and cirrhosis and often deteriorating to liver cancer [5–7]. Although abstinence can relieve alcoholic liver injury, it cannot completely reverse excessive alcohol consumption-induced liver injury. Treatments for alcoholic liver injury are few in number; therefore, it is urgent to find an effective method for the treatment of alcoholic liver injury. Alcohol exposure compromises intestinal barrier function, leading to increased intestinal permeability and accumulation of endotoxin in the blood, causing liver damage [8, 9]. Long-term or heavy drinking disrupts liver function, via mitochondrial injury and hepatic accumulation of acetaldehyde. Acetaldehyde combines with several proteins in the liver and causes functional protein denaturation and antigen exposure that stimulates the immune system to produce antibodies, causing liver damage [10, 11]. In recent years, the mechanisms of action of probiotics for alleviating alcoholic liver injury have been a subject of study.
3. Probiotics
Probiotics are living microorganisms that play beneficial roles, depending on the dose [12], directly maintaining the balance of intestinal microbiota [13]. The physiological functions of probiotics are achieved directly or indirectly by adjusting the composition of the host intestinal microbiota, activating the endogenous microbial community and regulating the immune system [14]. Oral probiotics can cure or alleviate a variety of gastrointestinal diseases. Some studies have confirmed that oral probiotics effectively alleviate lactose intolerance; prevent gastroenteritis, constipation, and diarrhea; and modulate the gut microbiota [15, 16]. Kirpich was the earliest to use probiotics to treat ALD patients, showing that probiotics (B. bifidum and L. plantarum 8PA3) significantly increased the number of Lactobacillus and Bifidobacterium in human feces and significantly altered the serum levels of ALT, low-density lipoprotein (LDL), and total bilirubin (STB) [4]. Therefore, many investigators began to pay attention on the role of probiotics in alcoholic liver disease. They used a variety of probiotic strains, most often Lactobacillus rhamnosus GG (LGG). LGG is a short Gram-positive heterofermentative facultative anaerobe which was isolated in 1983. LGG has been shown to be effective in the treatment of ALD.
4. Mechanisms of Probiotics in Improvement of Alcoholic Liver Injury
4.1. Probiotics and Antioxidant Activity
Reactive oxygen species (ROS) are highly reactive oxygen-containing molecules, ions, or groups that interact with one another and damage cellular molecule complexes, especially in the liver. In vivo, ROS causes oxidation of unsaturated fatty acids to produce lipid peroxides that induce fatty acid side-chain reactions, creating lipid hydroperoxides and malondialdehyde (MDA), which lead to damage of biological macromolecules (including protein and DNA), thereby affecting physiological metabolic activity and impairing cell structure and function [17–19]. ROS negatively regulate these mechanisms in alcoholic liver disease [20, 21]. Alcohol consumption induced the overproduction of ROS and inhibited fatty acid oxidation in the liver [22, 23], leading to ROS-mediated liver injury [24, 25] (Figure 1).
Figure 1.
The effect of alcohol on the gut-liver axis. Alcohol significantly changes intestinal microbiota diversity, reduces intestinal epithelial tight junction protein expression, and increases intestinal mucosal permeability, leading to barrier dysfunction and endotoxin translocated into the blood, inducing inflammatory cytokine and ROS production in the intestine and liver, causing hepatic steatosis and inflammation. Alcohol disorders the gut microbiota and decreases AhR ligand and IL-22 production. Alcohol exposure increased intestinal miR122a expression, decreasing occludin expression.
Studies demonstrated that probiotics enhance antioxidant activity to alleviate alcohol-induced liver injury (Figure 2). LGG treatment relieved alcohol-induced ROS accumulation in the ileum and Caco-2 cells [26]. LGG culture supernatant (LGG-s) pretreatment protected hepatic and ileal cells from oxidative stress and suppressed ROS formation [27]. Wang et al. showed that L. rhamnosus B10 significantly decreased the levels of ALT, LPS, and MDA; increased the activity of superoxide dismutase; and relieved alcohol-induced fatty degeneration and oxidative injury [28]. In the liver, ROS increased hepatic CYP2E1 protein and mRNA expression [24, 29] and substantially decreased Nrf-2 protein expression, all of which was ameliorated by LGG treatment [30]. These results suggested that probiotics reduce oxidative stress and promote the production of antioxidants to alleviate oxidative damage in the liver.
Figure 2.
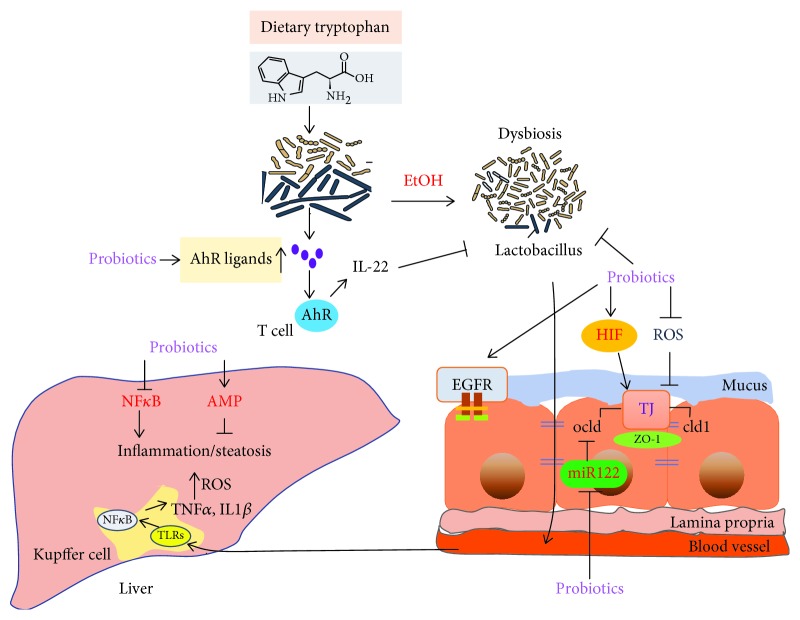
Probiotics function in gut-liver mechanisms. Probiotics and related products prevent ethanol-induced effects in the intestine and the liver via multiple mechanisms: (1) enhancement of antioxidant activity; (2) reduction of inflammatory cytokine expression; (3) hepatic AMPK and induction of lipid metabolism; (4) enhancement of intestinal tight junction ZO-1, claudin-1, and occludin expression via increased intestinal HIF signaling; (5) inhibition of miR122a expression leading to occludin upregulation; (6) activation of EGFR and preservation of barrier function in intestinal epithelial cells; and (7) positive modification of gut microbiota and increase AhR ligands.
4.2. Probiotics Improve Alcohol-Induced Lipid Metabolism
The AMP-activated protein kinase (AMPK) signaling pathway is an important energy metabolic pathway characterized by a series of cascade reactions that activate catabolism and deactivate anabolism [31]. AMPK regulates lipid metabolism by manipulation of several transcription factors, including peroxisome proliferator-activated receptor-α (PPARα) and sterol regulatory element-binding protein 1 (SREBP-1), which play important roles in lipogenesis and fatty acid oxidation [32, 33]. PPARα dominates the expression of lipid oxidation genes, including carnitine palmitoyltransferase-1 (CPT-1), acyl-CoA oxidase (ACO), acyl-CoA synthetase long-chain 1 (ACSL-1), and others. SREBP-1 regulates lipid synthesis by mediating its downstream genes, including acetyl-CoA carboxylase α (ACCα), fatty acid synthase (FAS), and stearoyl CoA desaturase 1 (SCD-1). Studies have shown that increased AMPK phosphorylation is effective in the treatment of alcoholic fatty liver [34, 35].
Chronic or acute alcohol consumption decreased AMPK and acetyl-CoA carboxylase (ACC) phosphorylation and increased malonyl-Co-A (MCA) production, leading to abnormal lipid metabolism in the liver [33, 36]. A study showed that LGG-s activated AMPK and suppressed alcohol-induced lipid accumulation via reduced expression of SREBP-1 and upregulated levels of CPT-1 and PPARα [37] (Figure 2). Zhang et al. observed that LGG-s stimulated adiponectin secretion and improved insulin sensitivity. LGG-s supplementation significantly increased the expression of Bcl-2 and inhibited the expression of Bax, suggesting that LGG-s had an important effect on protection against alcohol-induced hepatocyte apoptosis [37]. In short, LGG-s increases fatty acid oxidation to prevent chronic alcohol-induced liver steatosis and injury and decreases lipogenesis and hepatocyte apoptosis.
4.3. Probiotics Reduce Inflammatory Cytokine Expression in the Liver and Intestine
Alcohol-induced barrier dysfunction results from local and systemic production of proinflammatory cytokines such as TNF-α and IL-1β [38–40] (Figure 1). Once the gut barrier function is compromised, there is translocation of bacteria and bacterial products released into the blood; subsequently, a large number of Kupffer cells accumulate and toll-like receptors (TLRs) on the surface of liver Kupffer cells combine with endotoxin to activate mitogen-activated protein kinase (MAPK) and nuclear factor κB (NF-κB), producing inflammatory cytokines, including TNF-α and interleukin (IL-6, IL-1β) [16, 40, 41]. Our group demonstrated that LGG-s treatment decreased alcohol-induced inflammatory cytokine (TNF-a) production via inhibition of TLR4- and TLR5-mediated endotoxin activation [30] (Figure 2). Bajaj et al. showed that probiotic administration significantly decreased the amount of endotoxin and TNF-α in patients with mild hepatic encephalopathy [42].
NF-κB also is activated in intestinal TLRs, important promoters of intestinal immunity [43–45]; probiotics act on the immune system through TLRs. In 2009, Miyauchi et al. showed that LGG reduced the secretion levels of TNF-α from the intestinal mucosa and restored the integrity and barrier function of epithelial cells [46]. Probiotic supplementation decreased TNF-a and TLR (TLR4, TLR5) expression in the intestine and decreased the phosphorylation of p38 MAP kinase in mice with ALD [30, 40]. LGG-fermented milk contains two soluble proteins, p40 and p75, both of which have been reported to promote survival and growth of intestinal epithelial cells through activation of the epidermal growth factor receptor (EGFR). EGFR and Akt activation prevented cytokine-induced inflammation and intestinal epithelial cell apoptosis [47] (Figure 2).
4.4. Probiotics Enhance Intestinal Barrier Function and Modulate the Mucosal Immune System
In 2006, Ewaschuk and Dieleman showed that LGG directly or indirectly affected intestinal epithelial cells, strengthening intestinal mucosal barrier function and regulating the immune system [48] (Figure 2). Probiotics significantly increase the content of short-chain fatty acids (SCFA) that provide the energy metabolic substrate in the intestine and promote absorption of sodium ions, proliferation of colon cells, and intestinal mucous growth [49, 50]. A recent study found that the probiotic Akkermansia muciniphila promoted intestinal barrier integrity via enhancing the expression of tight junction (TJ) proteins claudin-3 and occludin, thereby ameliorating alcohol liver injury [51].
Alcohol consumption decreased the expression of the hypoxia-inducible factor (HIF). HIF is a master transcription factor involved in maintaining barrier function. It increases the expression of the intestinal trefoil factor (ITF), xenobiotic clearance by P-glycoprotein (P-gp), and various other nucleotide signaling pathways [52]. Wang et al. used a C57BL/6N mouse model to show that chronic alcohol treatment significantly decreased the expression levels of ITF and TJ proteins claudin-1, ZO-1, and occludin, while LGG treatment significantly improved these conditions [26]. HIF-1α regulated a variety of genes in the intestine, including antimicrobial peptides, β-defensin 1, and CRAMP [53–55]. LGG and LGG-s increased HIF expression in chronic and acute alcohol liver diseases in mice; we also demonstrated that knockdown of HIF1/2α resulted in increased permeability and decreased trans-epithelial electrical resistance in Caco-2 cells [26, 27]. Alcohol (EtOh) impaired epithelial integrity, and probiotic preserved barrier function by maintaining HIF activity and mucus molecules.
Probiotics also prevent and mitigate alcohol-induced disruption of colonic epithelial tight junctions, endotoxemia, and liver damage by an epidermal growth factor receptor- (EGFR-) dependent mechanism [56]. EGF receptor transactivation, prevented apoptosis, and preserved barrier function in intestinal epithelial cells [57].
There are many mechanisms of probiotics that regulate tight junction proteins. MicroRNA 122a (miR122a) is a target of tight junction occludin [58]. Alcohol induced the overexpression of miR122a in the intestine, decreasing occludin expression (Figure 1). Probiotic LGG-s suppressed miR122a expression and restored intestinal occludin protein expression in ALD mice [59] (Figure 2).
4.5. Probiotics Regulate Intestinal Flora
There is a close relationship between gut microbiota and human health. They are crucial actors in the pathogenesis of liver disease [26, 60, 61]. Many of these effects are mediated by metabolites that are produced by microbes. Tryptophan is an amino acid that is metabolized into aryl hydrocarbon receptor (AhR) ligands by intestinal microbiota in mice and humans [62]. AhR activation-mediated intestinal T cells secrete IL-22 that modulates the gut microbiota [63, 64]. Both chronic and acute alcohol consumption leads to intestinal dysbiosis and pathogenic bacterial overgrowth [24, 65–68] and decreased AhR ligand production [69]. Disturbance of gut microbiota increased the intestinal permeability and decreased TJ protein expression [63] (Figure 1).
One of the major mechanisms of probiotic function is alteration of gut microbiota. Gut microbiota play a key role in the immune system; imbalance of the intestinal microbiota stimulates the immune system, promoting chronic liver inflammation [8, 9]. Bifidobacteria and Lactobacillus are the predominant genera when there is a significant difference between the host and individual bacterial species [70, 71], which prevents the growth of anaerobic Gram-positive bacteria, inhibits the growth of Gram-negative bacteria, enhances phagocytic activity, and promotes the secretion of IgA, thereby enhancing cellular immune function [72]. Supplementation with probiotics naturally produced AhR ligands, promoting IL-22 production; intestinal regenerating islet-derived 3 gamma (Reg3g) expression was mediated by IL-22 which improved alcohol-induced liver injury and prevented microbiota disorder [69] (Figure 2).
Mutlu and colleagues used a rat model with ALD and found that microbiota composition in the colon did not change with 4–6-week alcohol consumption until the 10th week, and this was prevented by probiotic treatment [73]. In 1984, Bode was the first to study changes in gut microbiota in patients with alcoholic liver disease. They found that, compared with the normal control group, the number of Gram-negative anaerobic bacteria was significantly greater, and the same situation was observed for aerobic bacteria. This conclusion was helpful in the analysis of the function of the small intestine in patients with alcoholic liver [74]. Alcohol consumption decreased the number of Bacteroides and Firmicutes and increased the number of Proteobacteria and Actinobacteria in the intestine [75]. Alcohol induced the greatest expansion of Corynebacterium and Alcaligenes; the proliferation of Alcaligenes increased intestinal pH and led to pathogenic bacteria overgrowth. The addition of LGG suppressed the overgrowth of Gram-negative bacteria Alcaligenes and Gram-positive bacteria Corynebacterium [75].
Akkermansia muciniphila constitutes 1% to 4% of fecal microbiota in healthy individuals [76, 77]. A. muciniphila is a Gram-negative anaerobic commensal that utilizes host-derived mucins such as carbon and nitrogen [78], producing SCFA propionates. SCFA propionates act as histone deacetylase inhibitors, suggesting that they can epigenetically influence host gene expression [79]. Alcohol exposure diminished intestinal A. muciniphila abundance in both mice and humans, and A. muciniphila supplementation reversed alcohol-mediated liver injury and gut microbiota dysbiosis [51].
5. Future Perspectives of Microbial-Associated Therapy in ALD
Alcohol-induced liver disease significantly changed the gut microbial composition. In preclinical models, investigators found that fecal microbiota transfer caused the transmissibility of alcohol-related liver disease [80, 81]. Modulation of gut microbial composition and fecal microbiota transplantation (FMT) from healthy individuals will be two of the logical treatments in the future. Philips et al. showed daily FMT from several donors for 7 days improved liver function and reduced potentially pathogenic species [82]. Clinical trials found that FMT was beneficial in severe alcoholic hepatitis, hepatic encephalopathy, nonalcoholic fatty liver disease, hepatitis B-related chronic liver disease, and primary sclerosing cholangitis [83].
Restoration of microbial metabolites will be another logical treatment approach in the future. Supplementation with probiotics increases AhR ligand, SCFA, and LCFA production by modulating the gut microbiota; bioengineered bacteria can deliver therapeutics to the microbiota or host. Alternatively, drugs can be used to change and modulate bacterial enzymes or pathways [83].
Although we have some understanding of the interactions between the intestinal microbiome and the host, we lack a comprehensive understanding of the function of the intestinal microbiome in alcoholic liver disease. Larger and long-term clinical trials on the modification of intestinal microorganisms are required.
6. Conclusion
A large number of studies have found and confirmed that probiotics have the effect on alleviating alcoholic liver injury. Probiotics increase TJ protein expression and prevent disturbances of the intestinal barrier function in ALD. Intestinal flora is closely related to human health. Probiotics modulate the gut microbiota and immune responses, decreasing inflammatory cytokine and ROS expression in the intestine and liver. Probiotics also reduce fat accumulation in the liver by increasing fatty acid β-oxidation.
Acknowledgments
This work was partly supported by the National Key R&D Program of China (2017YFE0105400).
Abbreviations
- ALD:
Alcoholic liver disease
- LGG:
L. rhamnosus GG
- LGG-s:
L. rhamnosus GG culture supernatant
- HIF:
Hypoxia-inducible factor
- ITF:
Intestinal trefoil factor
- P-gp:
P-glycoprotein
- TJ:
Tight junction
- CRAMP:
Cathelin-related antimicrobial peptide
- TNF-α:
Tumor necrosis factor-α
- ROS:
Reactive oxygen species
- MAPK:
Mitogen-activated protein kinase
- TLRs:
Toll-like receptors
- NF-κB:
Nuclear factor κB
- AMPK:
Adenosine monophosphate-activated protein kinase
- EGFR:
Epidermal growth factor receptor
- AhR:
Aryl hydrocarbon receptor
- Reg3g:
Regenerating islet-derived 3 gamma.
Conflicts of Interest
The authors declare that they have no conflicts of interest (financial or otherwise) related to the data presented in this manuscript.
References
- 1.Gao B., Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141(5):1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ewaschuk J., Endersby R., Thiel D., et al. Probiotic bacteria prevent hepatic damage and maintain colonic barrier function in a mouse model of sepsis. Hepatology. 2007;46(3):841–850. doi: 10.1002/hep.21750. [DOI] [PubMed] [Google Scholar]
- 3.Chávez-Tapia N. C., González-Rodríguez L., Jeong M. S., et al. Current evidence on the use of probiotics in liver diseases. Journal of Functional Foods. 2015;17:137–151. doi: 10.1016/j.jff.2015.05.009. [DOI] [Google Scholar]
- 4.Kirpich I. A., Solovieva N. V., Leikhter S. N., et al. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol. 2008;42(8):675–682. doi: 10.1016/j.alcohol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beier J. I., McClain C. J. Mechanisms and cell signaling in alcoholic liver disease. Biological Chemistry. 2010;391(11):1249–1264. doi: 10.1515/BC.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frazier T. H., Stocker A. M., Kershner N. A., Marsano L. S., McClain C. J. Treatment of alcoholic liver disease. Therapeutic Advances in Gastroenterology. 2010;4(1):63–81. doi: 10.1177/1756283X10378925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McClain C. J., Song Z., Barve S. S., Hill D. B., Deaciuc I. Recent advances in alcoholic liver disease IV. Dysregulated cytokine metabolism in alcoholic liver disease. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2004;287(3):G497–G502. doi: 10.1152/ajpgi.00171.2004. [DOI] [PubMed] [Google Scholar]
- 8.Mutlu E. A., Gillevet P. M., Rangwala H., et al. Colonic microbiome is altered in alcoholism. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2012;302(9):G966–G978. doi: 10.1152/ajpgi.00380.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bajaj J. S., Hylemon P. B., Ridlon J. M., et al. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2012;303(6):G675–G685. doi: 10.1152/ajpgi.00152.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuma J., Casey C. A. Dangerous byproducts of alcohol breakdown-focus on adducts. Alcohol Research and Health. 2003;27(4):285–290. [PMC free article] [PubMed] [Google Scholar]
- 11.Teare J. P., Carmichael A., Burnett F., Rake M. O. Detection of antibodies to acetaldehyde-albumin conjugates in alcoholic liver disease. Alcohol and Alcoholism. 1993;28(1):11–16. [PubMed] [Google Scholar]
- 12.Neish A. S. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136(1):65–80. doi: 10.1053/j.gastro.2008.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambert J. C., Zhou Z., Wang L., Song Z., McClain C., Kang Y. J. Prevention of alterations in intestinal permeability is involved in zinc inhibition of acute ethanol-induced liver damage in mice. Journal of Pharmacology and Experimental Therapeutics. 2003;305(3):880–886. doi: 10.1124/jpet.102.047852. [DOI] [PubMed] [Google Scholar]
- 14.Lata J., Jurankova J., Kopacova M., Vitek P. Probiotics in hepatology. World Journal of Gastroenterology. 2011;17(24):2890–2896. doi: 10.3748/wjg.v17.i24.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adachi Y., Moore L. E., Bradford B. U., Gao W., Thurman R. G. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108(1):218–224. doi: 10.1016/0016-5085(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 16.Inokuchi S., Tsukamoto H., Park E. J., Liu Z. X., Brenner D. A., Seki E. Toll-Like Receptor 4 Mediates Alcohol-Induced Steatohepatitis Through Bone Marrow-Derived and Endogenous Liver Cells in Mice. Alcoholism: Clinical and Experimental Research. 2011;35:1509–1518. doi: 10.1111/j.1530-0277.2011.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin M. Y., Yen C. L. Reactive oxygen species and lipid peroxidation product-scavenging ability of yogurt organisms. Journal of Dairy Science. 1999;82(8):1629–1634. doi: 10.3168/jds.S0022-0302(99)75391-9. [DOI] [PubMed] [Google Scholar]
- 18.Halliwell B., Gutteridge J. M. C. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochemical Journal. 1984;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toyokuni S. Reactive oxygen species-induced molecular damage and its application in pathology. Pathology International. 1999;49(2):91–102. doi: 10.1046/j.1440-1827.1999.00829.x. [DOI] [PubMed] [Google Scholar]
- 20.Pan J. H., Lim Y., Kim J. H., et al. Root bark of Ulmus davidiana var. japonica restrains acute alcohol-induced hepatic steatosis onset in mice by inhibiting ROS accumulation. PloS One. 2017;12(11, article e0188381) doi: 10.1371/journal.pone.0188381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cederbaum A. I., Lu Y., Wu D. Role of oxidative stress in alcohol-induced liver injury. Archives of Toxicology. 2009;83(6):519–548. doi: 10.1007/s00204-009-0432-0. [DOI] [PubMed] [Google Scholar]
- 22.García-Villafranca J., Guillén A., Castro J. Ethanol consumption impairs regulation of fatty acid metabolism by decreasing the activity of AMP-activated protein kinase in rat liver. Biochimie. 2008;90(3):460–466. doi: 10.1016/j.biochi.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 23.You M., Liang X., Ajmo J. M., Ness G. C. Involvement of mammalian sirtuin 1 in the action of ethanol in the liver. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2008;294(4):G892–G898. doi: 10.1152/ajpgi.00575.2007. [DOI] [PubMed] [Google Scholar]
- 24.Albano E. Oxidative mechanisms in the pathogenesis of alcoholic liver disease. Molecular Aspects of Medicine. 2008;29(1-2):9–16. doi: 10.1016/j.mam.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Lluis J. M., Colell A., García–Ruiz C., Kaplowitz N., Fernández–Checa J. C. Acetaldehyde impairs mitochondrial glutathione transport in HepG2 cells through endoplasmic reticulum stress. Gastroenterology. 2003;124(3):708–724. doi: 10.1053/gast.2003.50089. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y., Kirpich I., Liu Y., et al. Lactobacillus rhamnosus GG treatment potentiates intestinal hypoxia-inducible factor, promotes intestinal integrity and ameliorates alcohol-induced liver injury. The American Journal of Pathology. 2011;179(6):2866–2875. doi: 10.1016/j.ajpath.2011.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y., Liu Y., Sidhu A., Ma Z., McClain C., Feng W. Lactobacillus rhamnosus GG culture supernatant ameliorates acute alcohol-induced intestinal permeability and liver injury. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2012;303(1):G32–G41. doi: 10.1152/ajpgi.00024.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WANG Y.-h., GAO J., ZHANG J., Yao-hui H. U. Lactobacillus rhamnosus B10 treatment ameliorates ethanol-induced mouse liver injury by antioxidant pathways. Food Science. 2012;33:270–274. [Google Scholar]
- 29.Parola M., Robino G. Oxidative stress-related molecules and liver fibrosis. Journal of Hepatology. 2001;35(2):297–306. doi: 10.1016/S0168-8278(01)00142-8. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y., Liu Y., Kirpich I., et al. Lactobacillus rhamnosus GG reduces hepatic TNFα production and inflammation in chronic alcohol-induced liver injury. The Journal of Nutritional Biochemistry. 2013;24(9):1609–1615. doi: 10.1016/j.jnutbio.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winder W. W., Hardie D. G. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. American Journal of Physiology-Endocrinology And Metabolism. 1999;277(1):E1–E10. doi: 10.1152/ajpendo.1999.277.1.E1. [DOI] [PubMed] [Google Scholar]
- 32.Li Y., Xu S., Mihaylova M. M., et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell metabolism. 2011;13(4):376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.You M., Matsumoto M., Pacold C. M., Cho W. K., Crabb D. W. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology. 2004;127(6):1798–1808. doi: 10.1053/j.gastro.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 34.Ajmo J. M., Liang X., Rogers C. Q., Pennock B., You M. Resveratrol alleviates alcoholic fatty liver in mice. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2008;295(4):G833–G842. doi: 10.1152/ajpgi.90358.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shearn C. T., Smathers R. L., Jiang H., Orlicky D. J., Maclean K. N., Petersen D. R. Increased dietary fat contributes to dysregulation of the LKB1/AMPK pathway and increased damage in a mouse model of early-stage ethanol-mediated steatosis. The Journal of Nutritional Biochemistry. 2013;24(8):1436–1445. doi: 10.1016/j.jnutbio.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Purohit V., Gao B., Song B. J. Molecular mechanisms of alcoholic fatty liver. Alcoholism: Clinical and Experimental Research. 2009;33(2):191–205. doi: 10.1111/j.1530-0277.2008.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang M., Wang C., Wang C., et al. Enhanced AMPK phosphorylation contributes to the beneficial effects of Lactobacillus rhamnosus GG supernatant on chronic-alcohol-induced fatty liver disease. The Journal of Nutritional Biochemistry. 2015;26(4):337–344. doi: 10.1016/j.jnutbio.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang B., Sang L., wang Y., Tong J., Zhang D., Wang B. The protective effect of VSL# 3 on intestinal permeability in a rat model of alcoholic intestinal injury. BMC Gastroenterology. 2013;13(1) doi: 10.1186/1471-230X-13-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Segawa S., Wakita Y., Hirata H., Watari J. Oral administration of heat-killed Lactobacillus brevis SBC8803 ameliorates alcoholic liver disease in ethanol-containing diet-fed C57BL/6N mice. International Journal of Food Microbiology. 2008;128(2):371–377. doi: 10.1016/j.ijfoodmicro.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 40.Hong M., Kim S. W., Han S. H., et al. Probiotics (Lactobacillus rhamnosus R0011 and acidophilus R0052) reduce the expression of toll-like receptor 4 in mice with alcoholic liver disease. PLoS One. 2015;10(2, article e0117451) doi: 10.1371/journal.pone.0117451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao B. Hepatoprotective and anti-inflammatory cytokines in alcoholic liver disease. Journal of Gastroenterology and Hepatology. 2012;27:89–93. doi: 10.1111/j.1440-1746.2011.07003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bajaj J. S., Heuman D. M., Hylemon P. B., et al. Randomised clinical trial: Lactobacillus GG modulates gut microbiome, metabolome and endotoxemia in patients with cirrhosis. Alimentary Pharmacology & Therapeutics. 2014;39(10):1113–1125. doi: 10.1111/apt.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galdeano C. M., Perdigon G. The probiotic bacterium Lactobacillus casei induces activation of the gut mucosal immune system through innate immunity. Clinical and Vaccine Immunology. 2006;13(2):219–226. doi: 10.1128/CVI.13.2.219-226.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tlaskalová-Hogenová H., Štěpánková R., Hudcovic T., et al. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunology Letters. 2004;93(2-3):97–108. doi: 10.1016/s0165-2478(04)00037-9. [DOI] [PubMed] [Google Scholar]
- 45.Chassaing B., Etienne-Mesmin L., Gewirtz A. T. Microbiota-liver axis in hepatic disease. Hepatology. 2014;59(1):328–339. doi: 10.1002/hep.26494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miyauchi E., Morita H., Tanabe S. Lactobacillus rhamnosus alleviates intestinal barrier dysfunction in part by increasing expression of zonula occludens-1 and myosin light-chain kinase in vivo. Journal of Dairy Science. 2009;92(6):2400–2408. doi: 10.3168/jds.2008-1698. [DOI] [PubMed] [Google Scholar]
- 47.Yoda K., Miyazawa K., Hosoda M., Hiramatsu M., Yan F., He F. Lactobacillus GG-fermented milk prevents DSS-induced colitis and regulates intestinal epithelial homeostasis through activation of epidermal growth factor receptor. European Journal of Nutrition. 2014;53(1):105–115. doi: 10.1007/s00394-013-0506-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ewaschuk J. B., Dieleman L. A. Probiotics and prebiotics in chronic inflammatory bowel diseases. World Journal of Gastroenterology. 2006;12(37):5941–5950. doi: 10.3748/wjg.v12.i37.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cani P. D., Everard A., Duparc T. Gut microbiota, enteroendocrine functions and metabolism. Current Opinion in Pharmacology. 2013;13(6):935–940. doi: 10.1016/j.coph.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Flint H. J., Scott K. P., Louis P., Duncan S. H. The role of the gut microbiota in nutrition and health. Nature Reviews Gastroenterology and Hepatology. 2012;9(10):577–589. doi: 10.1038/nrgastro.2012.156. [DOI] [PubMed] [Google Scholar]
- 51.Grander C., Adolph T. E., Wieser V., et al. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut. 2018;67(5):891–901. doi: 10.1136/gutjnl-2016-313432. [DOI] [PubMed] [Google Scholar]
- 52.Colgan S. P., Taylor C. T. Hypoxia: an alarm signal during intestinal inflammation. Nature Reviews Gastroenterology and Hepatology. 2010;7(5):281–287. doi: 10.1038/nrgastro.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fan D., Coughlin L. A., Neubauer M. M., et al. Activation of HIF-1α and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nature Medicine. 2015;21(7):808–814. doi: 10.1038/nm.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kelly C. J., Glover L. E., Campbell E. L., et al. Fundamental role for HIF-1α in constitutive expression of human β defensin-1. Mucosal Immunology. 2013;6(6):1110–1118. doi: 10.1038/mi.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shao T., Zhao C., Li F., et al. Intestinal HIF-1α deletion exacerbates alcoholic liver disease through inducing intestinal dysbiosis and barrier dysfunction. Journal of Hepatology. 2018;69(4):886–895. doi: 10.1016/j.jhep.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shukla P. K., Meena A. S., Manda B., et al. Lactobacillus plantarum prevents and mitigates alcohol-induced disruption of colonic epithelial tight junctions, endotoxemia, and liver damage by an EGF receptor–dependent mechanism. The FASEB Journal. 2018;32(11):6274–6292. doi: 10.1096/fj.201800351r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yan F., Liu L., Dempsey P. J., et al. A Lactobacillus rhamnosus GG-derived Soluble Protein, p40, Stimulates Ligand Release from Intestinal Epithelial Cells to Transactivate Epidermal Growth Factor Receptor. Journal of Biological Chemistry. 2013;288(42):30742–30751. doi: 10.1074/jbc.m113.492397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ye D., Guo S., al–Sadi R., Ma T. Y. MicroRNA regulation of intestinal epithelial tight junction permeability. Gastroenterology. 2011;141(4):1323–1333. doi: 10.1053/j.gastro.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao H., Zhao C., Dong Y., et al. Inhibition of miR122a by Lactobacillus rhamnosus GG culture supernatant increases intestinal occludin expression and protects mice from alcoholic liver disease. Toxicology letters. 2015;234(3):194–200. doi: 10.1016/j.toxlet.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 60.Cesaro C., Tiso A., del Prete A., et al. Gut microbiota and probiotics in chronic liver diseases. Digestive and Liver Disease. 2011;43(6):431–438. doi: 10.1016/j.dld.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 61.Loguercio C., Federico A., Tuccillo C., et al. Beneficial effects of a probiotic VSL# 3 on parameters of liver dysfunction in chronic liver diseases. Journal of Clinical Gastroenterology. 2005;39(6):540–543. doi: 10.1097/01.mcg.0000165671.25272.0f. [DOI] [PubMed] [Google Scholar]
- 62.Agus A., Planchais J., Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host & Microbe. 2018;23(6):716–724. doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 63.Natividad J. M., Agus A., Planchais J., et al. Impaired Aryl hydrocarbon receptor ligand production by the gut microbiota is a key factor in metabolic syndrome. Cell Metabolism. 2018;28(5):737–749.e4. doi: 10.1016/j.cmet.2018.07.001. e4. [DOI] [PubMed] [Google Scholar]
- 64.Teng Y., Ren Y., Sayed M., et al. Plant-derived exosomal microRNAs shape the gut microbiota. Cell Host & Microbe. 2018;24(5):637–652.e8. doi: 10.1016/j.chom.2018.10.001. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McLoughlin R. M., Mills K. H. G. Influence of gastrointestinal commensal bacteria on the immune responses that mediate allergy and asthma. Journal of Allergy and Clinical Immunology. 2011;127(5):1097–1107. doi: 10.1016/j.jaci.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 66.MCC C., NL L., CM F., et al. Comparing the effects of acute alcohol consumption in germ-free and conventional mice: the role of the gut microbiota. BMC microbiology. 2014;14(1):p. 240. doi: 10.1186/s12866-014-0240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bajaj J. S., Heuman D. M., Hylemon P. B., et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. Journal of hepatology. 2014;60(5):940–947. doi: 10.1016/j.jhep.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gabbard S. L., Lacy B. E., Levine G. M., Crowell M. D. The impact of alcohol consumption and cholecystectomy on small intestinal bacterial overgrowth. Digestive Diseases and Sciences. 2014;59(3):638–644. doi: 10.1007/s10620-013-2960-y. [DOI] [PubMed] [Google Scholar]
- 69.Hendrikx T., Duan Y., Wang Y., et al. Bacteria engineered to produce IL-22 in intestine induce expression of REG3G to reduce ethanol-induced liver disease in mice. Gut. 2018;(article gutjnl-2018-317232) doi: 10.1136/gutjnl-2018-317232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bäckhed F. Host responses to the human microbiome. Nutrition reviews. 2012;70:S14–S17. doi: 10.1111/j.1753-4887.2012.00496.x. [DOI] [PubMed] [Google Scholar]
- 71.Tap J., Mondot S., Levenez F., et al. Towards the human intestinal microbiota phylogenetic core. Environmental Microbiology. 2009;11(10):2574–2584. doi: 10.1111/j.1462-2920.2009.01982.x. [DOI] [PubMed] [Google Scholar]
- 72.Zhang W., Gu Y., Chen Y., et al. Intestinal flora imbalance results in altered bacterial translocation and liver function in rats with experimental cirrhosis. European Journal of Gastroenterology & Hepatology. 2010;22(12):1481–1486. doi: 10.1097/MEG.0b013e32833eb8b0. [DOI] [PubMed] [Google Scholar]
- 73.Mutlu E., Keshavarzian A., Engen P., Forsyth C. B., Sikaroodi M., Gillevet P. Intestinal dysbiosis: a possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcoholism: Clinical and Experimental Research. 2009;33(10):1836–1846. doi: 10.1111/j.1530-0277.2009.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bode J., Bode C., Heidelbach R., Dürr H. K., Martini G. A. Jejunal microflora in patients with chronic alcohol abuse. Hepato-gastroenterology. 1984;31(1):30–34. [PubMed] [Google Scholar]
- 75.Bull-Otterson L., Feng W., Kirpich I., et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PloS One. 2013;8(1, article e53028) doi: 10.1371/journal.pone.0053028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Collado M. C., Derrien M., Isolauri E., de Vos W. M., Salminen S. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Applied and Environmental Microbiology. 2007;73(23):7767–7770. doi: 10.1128/AEM.01477-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Derrien M., Collado M. C., Ben-Amor K., Salminen S., de Vos W. M. The Mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Applied and Environmental Microbiology. 2008;74(5):1646–1648. doi: 10.1128/AEM.01226-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Derrien M., Vaughan E. E., Plugge C. M., de Vos W. M. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. International Journal of Systematic and Evolutionary Microbiology. 2004;54(5):1469–1476. doi: 10.1099/ijs.0.02873-0. [DOI] [PubMed] [Google Scholar]
- 79.Gentile C. L., Weir T. L. The gut microbiota at the intersection of diet and human health. Science. 2018;362(6416):776–780. doi: 10.1126/science.aau5812. [DOI] [PubMed] [Google Scholar]
- 80.Fouts D. E., Torralba M., Nelson K. E., Brenner D. A., Schnabl B. Bacterial translocation and changes in the intestinal microbiome in mouse models of liver disease. Journal of Hepatology. 2012;56(6):1283–1292. doi: 10.1016/j.jhep.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Llopis M., Cassard A. M., Wrzosek L., et al. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut. 2016;65(5):830–839. doi: 10.1136/gutjnl-2015-310585. [DOI] [PubMed] [Google Scholar]
- 82.Philips C. A., Pande A., Shasthry S. M., et al. Healthy donor fecal microbiota transplantation in steroid-ineligible severe alcoholic hepatitis: a pilot study. Clinical Gastroenterology and Hepatology. 2017;15(4):600–602. doi: 10.1016/j.cgh.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 83.Sarin S. K., Pande A., Schnabl B. Microbiome as a therapeutic target in alcohol-related liver disease. Journal of Hepatology. 2019;70(2):260–272. doi: 10.1016/j.jhep.2018.10.019. [DOI] [PubMed] [Google Scholar]