Abstract
There is more skeletal muscle tissue in the body than any other tissue and, as it is the organ of the majority of metabolic activity, muscle defect can affect the health of the entire body. Endoplasmic reticulum (ER) stress due to defects in protein folding/degradation balance, altered calcium and lipid levels and alterations in ER-mitochondria contacts has recently been recognised as the pathogenic cause of many different myopathies. In addition, a maladaptive ER stress response triggered by ER stress and mediated by three ER stress sensors (PERK, IRE1 and ATF6) is involved in a failure to relieve muscle tissue from this stress. Targeting ER stress and the ER stress response pathway offers a broad range of opportunities for treating myopathies but, as the inhibition of the three ER stress sensors may not be safe because it could lead to unexpected effects; it therefore calls for careful analysis of the changes in downstream signal transduction in the different myopathies so these sub-pathways can be pharmacologically targeted. This review summarises the known inhibitors of the ER stress response and the successful results obtained using some of them in mouse models of muscle diseases caused by ER stress/ER stress response.
Highlights
-
•
ER stress and the ER stress response are pathogenic causes of myopathies.
-
•
Pre-clinical models improve our understanding of the safest branch or sub-branch of the ER stress response to inhibit.
-
•
The inhibitors of signalling downstream of the three ER stress sensors is the safest pharmacological option.
-
•
Chemical chaperones are promising pharmacological means of treating myopathies.
1. Introduction
Recent evidence suggests that, although it is not a highly secretory tissue, skeletal muscle is subject to endoplasmic reticulum (ER) stress and the ER stress response because changes in environmental cues such as a high-fat diet and exhausting running induce markers of the ER stress response [1,2].
The ER stress response is generally a pathway that favours muscle adaptation following challenging exercise stimuli, but a maladaptive response is increasingly acknowledged to be the pathogenic cause of various types of muscle disorders [3]. Given the role of the three-armed ER stress response in regulating various aspects of skeletal muscle function and dysfunction, promising pharmacological targets for the treatment of muscle diseases include reducing ER stress and modulating the response to it [[3], [4], [5]].
The aim of this review is to describe recent advances in our understanding of the relationships between ER stress, ER stress response and muscle disorders, and analyse the pre-clinical models that have helped to clarify how disease-specific proteins affect ER homeostasis and trigger ER stress. It will also analyse the various steps of ER stress and stress responses that can safely be targeted pharmacologically, and critically discuss the success of targeted therapeutic strategies in pre-clinical models of myopathies.
2. ER stress response and its inhibitors
It is well known that ER stress is triggered by proteotoxic stimuli such as when the load of proteins to be folded exceeds the capacity of the ER folding machinery; consequently any perturbation in the ER milieu that compromises ER folding capacity, such as changes in redox and Ca2+ levels, can trigger ER stress. However, it has recently been discovered that, regardless of the levels of folded ER proteins, lipotoxic stimuli such as high levels of saturated fatty acids (FAs) can also trigger ER stress by directly acting on membrane fluidity [6]. Proteotoxic ER stress activates a homeostatic response (the ER stress response) that is initiated by the dissociation of binding immunoglobin protein (BIP) from the three proximal sensors inositil-requiring enzyme 1 [IRE1], protein kinase R-like ER kinase [PERK], and activating transcription factor [ATF6] of ER stress which are subsequently activated to start complex signal transduction [7]; in the case of lipotoxic stress, the three sensors are activated regardless of ER protein load [6,8,9].
The oldest of the three sensors is IRE1, a kinase and endoribonuclease that promotes the unconventional splicing of an intronic region of X box binding protein 1 (XBP1) that subsequently becomes a transcription factor of the genes involved in protein folding and ER-associated protein degradation (ERAD) [10,11]; activated PERK attenuates protein synthesis by phosphorylating eukaryotic initiation factor 2-alpha (eIF2-alpha) [12]; and activated ATF6 traffics to the Golgi, where it is proteolytically cleaved from its transmembrane domain, then migrates to the nucleus where it acts as a transcription factor and induces chaperones such as BIP/GRP78 and GRP94 [13]. The ER stress response therefore acts by inhibiting protein translation through the PERK pathway and favouring protein degradation and the induction of chaperones through the IRE1 and ATF6 pathways [14]. This coordinated action of protein degradation and the induction of chaperones relieves ER stress and re-establishes homeostasis.
However, a form of unrelieved chronic ER stress may occur as a result of the activation of maladaptive branches of the ER stress response leading to the failure of ER homeostasis and directing cells to apoptosis and dysfunction, thus making the process a sort of double-edged sword. For example, the IRE1 pathway is connected to pro-apoptotic signals via JNK [15], and all three pathways are connected to pro-apoptotic signals via the CHOP transcription factor (GADD153) involved in ER stress-induced apoptosis. It has been shown that CHOP regulates the expression of two genes that may also be involved in maladaptive responses: ERO1 alpha (henceforth ERO1) and GADD34 [16]. ERO1 is an ER protein disulphide oxidase that, together with PDI, introduces disulphide bonds in the nascent proteins promoting protein folding [17,18]; however, its activity is also associated with the stoichiometric production of H2O2, which is a dangerous oxidant at high concentrations and may explain why ERO1-deficient C. elegans are protected from the detrimental consequences of ER stress [19,20]. GADD34 recruits phosphatase PP1 to dephosphorylate eif2-alpha and reactivate protein synthesis. GADD34 appears only in vertebrates, which may represent an evolutionary advantage in the case of highly secretory cells whose protein secretion should be preserved, but is detrimental in cells expressing toxic gain-of-function protein mutants.
Given the multi-step process and multiple feedback mechanisms of the ER stress response, it should be possible to intervene pharmacologically, and this has led to wide-ranging efforts to find small molecules capable of modulating ER stress and/or one or more of the steps of the response to it. Here, we try to summarize all the known drugs available so far as inhibitors of the ER stress and its response.
2.1. Chemical chaperones
One of these is the small molecule 4-phenylbutyricacid (4-PBA), an FDA-approved drug that acts as a chemical chaperone to stabilise the conformation of folded protein and thus favour protein trafficking out of the ER and relieving ER stress [21]. Ursodeoxycholic acid (UDCA) together with its derivative tauroursodeoxycholic acid (TUDCA), is FDA-approved bile acid with chaperone properties that may act in a similar manner [22].
2.2. IRE1 alpha inhibitors
In relation to the three ER stress sensors, three selective inhibitors of the endonuclease activity of IRE1alpha (salicylaldehydes, 4μ8C and STF-083010) have been identified by means of high-throughput screening techniques and selectively inhibit IRE1 endonuclease activity without affecting its kinase activity in vitro and in vivo [[23], [24], [25]].
2.3. PERK inhibitors
Inhibitors of the kinase activity of PERK have also been identified: the first of these to be characterised was GSK2606414, which inhibits PERK activation in cells and impedes tumour growth [26], and further chemical optimisation led to the discovery of GSK2656157, which was found to inhibit the growth of tumour xenografts in mice [27]. However, it has recently been found that both also inhibit cellular RIPK1 at a lower concentration than that required to inhibit PERK activity, thus suggesting a lack of PERK specificity [28].
2.4. ATF6 alpha inhibitors
Finally, it has been found that the new class of ceapin molecules can inhibit the trafficking of ATF6 alpha to the nucleus by trapping it inside the ER. Interestingly these inhibitors are not toxic for unstressed cells but only increase their sensitivity to ER stress, thus suggesting a safety margin in drugging/inhibiting the ATF6 pathway in cells where it is activated [29].
2.5. eIF2-alpha dephosphorylation inhibitors
Most of the efforts that have been made to affect the steps of the ER stress response that occur downstream of the three sensors have concentrated on regulating eIF2-alpha phosphorylation/dephosphorylation. It has been shown that salubrinal selectively inhibits eIF2-alpha dephosphorylation [30], which is an appealing way of improving the course of protein misfolding disease. Guanabenz also delays translational recovery by binding and inhibiting the regulatory subunit of the stress-induced eIF2-alpha phosphatase consisting of GADD34 and PP1c [31], but its use is limited by its central hypotensive effect [32], something that is not related to the more recently discovered GADD34-inhibiting sephin 1 [33]. However, the findings of a recent report suggest that both drugs enfeeble the IRE1 branch of the ER stress response rather than alter eIF2-alpha phosphorylation, thus casting doubts on their activity as inhibitors of GADD34 phosphatase [34].
2.6. ERO1 alpha inhibitors
ERO1 inhibitors were discovered during attempts to modulate hyper-oxidation associated with ERO1-alpha activity. High-throughput screening led to the discovery of EN460, a small molecule that binds ERO1 and weakens its binding to its prosthetic group FAD, thus impeding ERO1 re-oxidation. EN460 is highly selective for ERO1-alpha, but its promiscuous reactivity with thiolate is a cause of toxicity that limits its in vivo use [35]. A recent study mapped an amino acid (Val 101) in ERO1 alpha crucial for its interaction with PDI and therefore proposes the use of peptides that target the region of interaction ERO1 alpha-PDI around this valine to impair ERO1 alpha activity [62]. This study offers a new perspective to target ERO1 inhibition and we hope it will fuel further efforts for the discovery of ERO1 alpha inhibitors for use in clinical practice (Fig. 1).
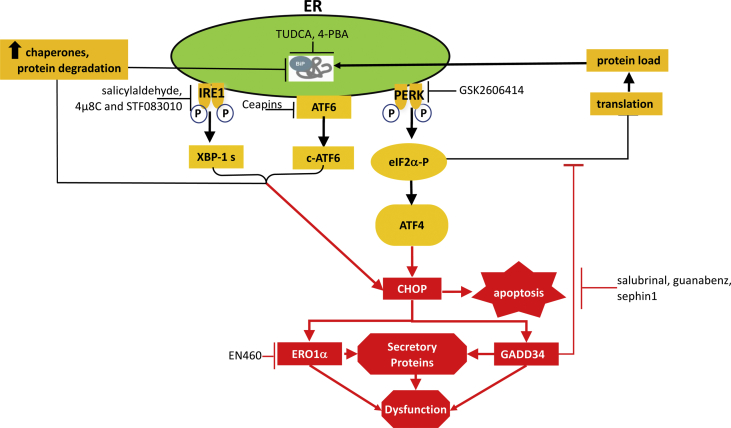
Fig. 1.
Rationale underlying the targeting of ER stress and the ER stress response.
Unfolded proteins in the ER cause ER stress and activate an ER stress response triggered by three sensors (IRE1, PERK and ATF6) which, together, attenuate protein synthesis and thus limit organelle load and promote the transcriptional up-regulation of the genes encoding the components of the ER protein handling machinery. This adaptive response is aimed at reducing ER stress and restoring ER homeostasis (black arrows). However, there is also a maladaptive response (red arrows) because the IRE1, ATF6 and PERK pathways are also connected to pro-apoptotic signals via the CHOP transcription factor (GADD153), which promotes the recovery of translation and oxidative protein folding by inducing GADD34 and ERO1 respectively, a process that leads to death due to the accumulation of malfolded proteins and the generation of excessive reactive oxygen species. Various inhibitors of ER stress and the ER stress response are available: the chemical chaperones TUDCA and 4-PBA help protein folding; salicylaldehydes, 4μ8C and STF083010 inhibit the endonuclease activity of IRE1-alpha; GSK2606414 inhibits the kinase activity of PERK; and the ceapin class of molecules inhibits the trafficking of ATF6-alpha to the nucleus by trapping it inside the ER. EN460 inhibits ERO1 alpha activity and salubrinal, gunabenz and sephin1 inhibit GADD34 activity.
3. ER stress response in muscle
Although it is not a highly secretory tissue, there is evidence suggesting that skeletal muscle undergoes ER stress because it can be induced by changes in environmental cues such as a high-fat diet or muscle exercise [1]; furthermore, the consequent ER stress response in healthy muscle is adaptive, as is demonstrated by the fact that the levels of ER stress markers decrease over time during periods of exercise training [2].
The levels of sarcoplasmic reticulum calcium are tightly regulated in skeletal muscle, and its release triggers muscle contraction through the process of excitation-contraction coupling. Defects in calcium regulation cause ER stress, and both can trigger skeletal muscle dysfunction by enfeebling the excitation contraction machinery and inducing muscle atrophy as a consequence of prolonged ER stress-dependent attenuation of protein translation [36]. In addition to being important for movement, skeletal muscle is also very important for the body's metabolism because it is where the majority of insulin-dependent glucose uptake (75–90%) takes place [37]. Skeletal muscle accumulation of lipids can be lipotoxic and induce ER stress, and the consequent ER stress response may impair insulin signalling. These findings suggest that ER stress and a maladaptive ER stress response may have two deleterious consequences: one relating to muscle atrophy/defective muscle force, and another relating to impaired muscle metabolism [3].
Mouse models of the various mediators of the three branches of the ER stress response can be very helpful in elucidating the role of the ER stress response in muscle diseases. Muscle-specific transgenic mice with inducible activated PERK show clear signs of muscle atrophy caused by the attenuation of protein translation mediated by eIF2-alpha phosphorylation [36,38], but as constant PERK activation, PERK inactivation also triggers skeletal muscle atrophy, it seems that both conditions are deleterious in skeletal muscles [39]. ATF6-alpha knock-out mice are exercise intolerant and show high levels of creatine kinase after one day of recovery from intense exercise, which suggests that the ATF6 alpha branch of the ER stress response is important for preserving muscle integrity [2].
Downstream of the three ER stress sensors, CHOP deletion improves running performance and reduces creatine kinase levels in exercise-intolerant PGC1-alpha muscle knock-out mouse [2]. It also improves diaphragm force in a SELENON KO mouse model and reduces its muscle insulin resistance by reducing ER stress levels in muscle [40,41]. These findings suggest that an attenuation of the CHOP pathway can be favourable in some muscle diseases. However, CHOP deletion worsens the pathological phenotype of AR113Q mice, a mouse model of spinal and bulbar muscle atrophy in which high levels of muscle ER stress have been detected, by accelerating denervation-induced muscle wasting [42].
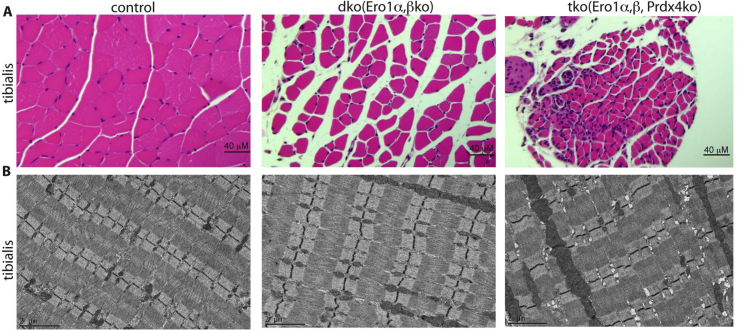
ERO1-alpha is located downstream from the CHOP signal [16], and it has been hypothesised that its inhibition may be advantageous in conditions of ER stress [19]. For example, ablation of ERO1 alpha protects SELENON deficient myotubes from ER-stress mediated lipotoxicity [41]. Previously unpublished data show that the deletion of protein disulphide oxidase ERO1 alpha alone does not trigger an overt pathological muscle phenotype in mice, which can only be triggered by the simultaneous deletion of ERO1 alpha, its homologue ERO1 beta and peroxiredin IV, an ER-resident peroxiredoxin that mediates oxidative protein folding [43] [20,44,45] (Fig. 2), thus suggesting that ERO1 alpha inhibition may be a promising therapeutic target in muscle myopathies caused by ER stress.
Fig. 2.
ERO1 deletion does not affect muscle.
A) Hematoxylin and eosin-stained transverse section of tibialis taken from a mouse model of serial deleted protein disulphide oxidases dko (Ero1-alpha and -beta) and tko (Ero1-alpha and -beta and PrdxIV) showing that wild-type muscle consists of cells that are densely packed into neat fascicles, whereas mutant dko tissue is more loosely packed and there is more space between the muscle fibres, and only tko tissue shows central nuclei and cell infiltration. B) Electron microscopy of longitudinal muscle sections reveals a tight, normally organised sarcoplasmic reticulum in wild-type and dko tissue, but conspicuous dilation of the sarcoplasmic reticulum in tko tissue.
4. Myopathies associated with chronic ER stress
Chronic ER stress has been observed in muscle biopsies of patients with congenital myotonic dystrophy type I: the atrophic muscles fibres showed the aberrant splicing of RYR1 and SERCA, as well as high levels of BIP, phosphorylated PERK and eIF2-alpha [46].
It has been hypothesised that ER stress may be the pathological cause of inclusion body myositis, a muscle disorder with an inflammatory component that typically affects the elderly people, but the exact cause of the chronic ER stress is still not known [47].
Mouse models have also proved to be very useful to understand the connection between ER stress and myopathy. Increased muscle ER stress (high levels of the active forms of XBP-1 and ATF6 and, to a lesser extent, phosphorylated eIF2-alpha and CHOP) has been detected in mouse models deficient in LPIN1, a protein whose loss of function leads to severe rhabdomyolysis in children. The accumulation of lipids in LPIN1-deficient skeletal muscle suggests that lipotoxicity may trigger ER stress and a maladaptive ER stress response [48]. ER stress has also been detected in the atrophic skeletal muscle of G93A*SOD1 (ALS-Tg) mice, a mouse mutant model of the genetic form of amyotrophic lateral sclerosis (ALS): mutant SOD1 binds to Derlin-1 on the cytosolic side of the ER membrane, thus blocking the degradation of ER proteins and triggering ER stress [49]. The ER stress sensors PERK and IRE1α were upregulated, downstream to them phospho-eIF2α and BIP were also upregulated together with the pro-apoptotic CHOP, thus suggesting a mechanism by means of which ER stress and a maladaptive ER stress response contribute to muscle atrophy in ALS [50].
Decreased resting cytosolic calcium levels have been detected in mice with a I4895T mutation in the type 1 ryanodine receptor/Ca2+ release channel (Ryr1) gene. This is the most frequent RYR1 mutation in humans and gives rise to a range of myopathies. Together with the alteration in calcium handling, the skeletal muscle of these mice showed increased levels of BIP, GRP94, CHOP and ERO1 alpha, and decreased protein translation, which suggests that the myopathy may be due to chronic ER stress and a maladaptive response leading to defective muscle force and atrophy [51].
Mutations in the SELENON gene are associated with autosomic recessive SEPN1-related myopathy. It has been shown that SELENON protein redox-activates the ER calcium pump SERCA2, thus counteracting its ERO1 alpha-dependent inactivation and connecting SELENON function with ER calcium levels [52]. In addition, a SELENON KO mouse model shows diaphragmatic weakness, increased levels of BIP, CHOP and ERO1 alpha, and attenuated protein translation [40,53]. The lack of SELENON in mice also sensitises skeletal muscle to the ER stress-dependent consequence of lipotoxicity, thus compromising insulin sensitivity and muscle strength as seen in patients with SEPN1-related myopathy [41].
One important point to consider when exploring the mechanisms underlying muscle disease is the cross-talk between the ER and mitochondria, which are physically connected by means of proteinaceous tethers (mitochondria-associated membranes, MAMs). This interaction is a hub of a sub-cellular signalling node that influences the health of the two organelles in which ER calcium moves to mitochondria in order to influence the phosphorylative oxidation that affects protein folding inside the ER by generating ATP [54], thus suggesting that ER stress should also be considered in muscle diseases characterised by a primary defect in mitochondria.
One typical example of such cross-talk ER-mitochondria and its importance in determining muscle phenotypes is muscle-specific OPA1 knock-out mice. Human recessive mutations in the OPA1 gene, the protein of which is involved in the fusion of the outer and inner mitochondrial membranes, give rise to dominant optic atrophy, a disease with a myopathic phenotype. Interestingly, the acute deletion of OPA1 in mouse skeletal muscle (which resembles the human myopathic phenotype) triggers mitochondria alterations and ER stress with increased levels of ATF4, XBP-1s, CHOP and GADD34, which suggests that ER stress may be due to mitochondrial alterations in skeletal muscle [55].
5. The pharmacological treatment of myopathies with inhibitors of ER stress and the ER stress response
Targeting the ER stress response pathway offers a broad range of opportunities for treating myopathies, but it is very important to determine which sub-pathway is involved in which myopathy because inhibiting the three ER stress sensors (IRE1, PERK and ATF6) may not be safe as it could lead to unexpected side effects. Consequently, targeting more downstream mediators such as eIF2-alpha, GADD34 and ERO1 or favouring protein folding may be better options.
Chronic TUDCA treatment of lipin-1 deficient mice lowers BIP and active ATF6 (ATF6c) levels in skeletal muscle, decreases triglyceride levels and the number of lipid droplets. More importantly, it also improves muscle strength, which suggests that attenuating ER stress-dependent lipotoxicity is connected with the recovery of lipin-1 deficient skeletal muscle [48].
It has been shown that a derivative of UDCA has neuroprotective effects in a cellular model of superoxide dismutase1-dependent neurodegeneration by reducing matrix metalloproteinase 9 and caspase 9 activation [56]. However, the effects of UDCA and its derivatives have not been tested in ALS-Tg mice in which it may be important to determine whether the reduction in ER stress improves muscle function. A small clinical trial of TUDCA involving ALS patients found a number of responders, but there is a lack muscle biopsy data that would make it clearer whether the improvements were related a reduction in muscle ER stress [57]. It has been shown that chronic sephin 1 treatment prevents the motor deficit characterising ALS-Tg mice by reducing the levels of BIP, CHOP and XBP-1s in the spinal cord, but the effect of sephin 1 on the levels of ER stress markers in skeletal muscle was not tested [33].
Chronic TUDCA treatment of muscle OPA1 knock-out mice reduces ER stress, improves mitochondria numbers and prevents muscle wasting, thus suggesting that attenuating ER stress improves muscle function in a disease characterised by primary mitochondria deficiency [55].
Chronic treatment with the chemical chaperone 4PBA reduces GRP94, CHOP and BIP levels and improves muscle strength in mice carrying an I4895T mutation in the type I ryanodine receptor [51].
TUDCA preconditioning of SELENON devoid myotubes partially protects cells from palmitate-induced ER stress and improves the uptake of insulin-dependent glucose [41], and ongoing studies of chronic TUDCA treatment in SELENON KO mice will evaluate whether it is capable of rescuing diaphragmatic weakness (as preliminary results suggest) and the insulin-resistant muscle phenotype (Table 1).
Table 1.
Functional impact of ER stress/ER stress response on muscle diseases.
| Disease | Model | ER stress/ER stress response handling | Phenotype |
|---|---|---|---|
|
Spinal and bulbar muscular atrophy |
AR113Q mouse | CHOP KO | Accentuated muscle atrophy |
| Rhabdomyolysis in children | Lipin-1 muscle-deficient mouse | TUDCA (500 mg/kg twice a day) | Improved muscle force |
| Amyotrophic lateral sclerosis | G93A*SOD1 mouse | Sephin (5 mg/kg once a day) | Improved motor deficit |
| Optic atrophy | OPA1 muscle-deficient mouse | TUDCA (500 mg/kg twice a day) | Improved muscle wasting |
| SEPN1-related myopathy | SELENON KO mouse | AAV-ERO1 (gastrocnemius) | Reduced muscle force |
| CHOP KO | Improved muscle force, insulin resistance | ||
| RYR1-mediated core myopathy | RyR1I4895T/WT Knock-in mouse | 4-PBA (approximately 6–10 mg a day) | Improved muscle force |
5.1. Chemical chaperones in muscle diseases
On the basis of the pre-clinical studies described above, TUDCA and 4-PBA represent promising therapeutic options for the myopathies associated with ER stress, and deserve evaluation in human. Both have previously been shown to be effective in inhibiting the ER stress-mediated lipotoxicity of pancreatic beta-cells [58,59], and TUDCA has also been shown to improve the insulin sensitivity of muscle and liver of obese men and women [60]. However, there are still some aspects of TUDCA's mechanism of action as an ER stress inhibitor/chemical chaperone that need to be clarified. It is worth noting that it improves beta cell function in a mouse model of type I diabetes but, interestingly (and not intuitively if it is believed that TUDCA is only a chemical chaperone), this improvement depends on the ATF6 alpha branch of the ER stress response, and is lost in mice with the beta cell-specific deletion of ATF6 alpha [61]. This suggests that TUDCA may have an additional function and raises the thought-provoking question as to whether it alters the fluidity of the ER membrane, impedes the dimerisation and activation of the PERK and IRE1 pathways related to a maladaptive response, and favours the adaptive ATF6 pathway (the only ER stress sensor that does not need dimerisation to be activated) swinging into action and relieving ER stress? This may be a fruitful working hypothesis for future studies of the mechanism of action of this drug that is very promising and with an extraordinary safety profile.
6. Concluding remarks
Chronic ER stress evoking a maladaptive ER stress response is the pathogenic cause of many myopathies, including those mitochondrial myopathies whose primary defect does not reside in the ER. This finding should encourage studies of ER stress and the response to it in animal and human mitochondrial myopathies.
Targeting ER stress and the ER stress response offers a broad range of therapeutic opportunities, but it is very important to determine which sub-pathway is altered in the different myopathies as inhibiting the three ER stress sensors (IRE1, PERK and ATF6) may not be safe as it could lead to unexpected side effects. Targeting more downstream mediators such as eIF2-alpha, GADD34 and ERO1, or favouring protein folding by means of the use of chemical chaperones, seems to be a better option.
Acknowledgments
I am indebted to Prof. David Ron as some reagents were developed in his lab.
This study was supported by a Telethon career award (TDEZ00112T), an ERC Cariplo grant (2014–1856), a Cariplo biomedical science for young scientist grant (2014–1075) and a Cure CMD/AFM telethon grant.
Abbreviations
- 4‐PBA
4‐phenylbutyrate
- AAV
Adenoassociated virus
- ATF6
Activating transcription factor 6
- BiP
Immunoglobulin heavy chain binding protein
- CHOP
C/EBP homologous protein
- eIF2α
Eukaryotic translation initiation factor 2α
- ER
Endoplasmic reticulum
- ERAD
ER‐associated degradation
- ERO1
Endoplasmic oxidoreductin 1
- GADD34
growth arrest and DNA damage‐inducible protein
- IRE1
Inositol‐requiring protein 1
- OPA1
Optic atrophy 1
- JNK
c‐Jun N‐terminal kinase
- PERK
Protein kinase R (PKR)‐like endoplasmic reticulum kinase
- PRDXIV
Peroxiredoxin IV
- RyR1
Ryanodine receptor type 1
- SELENON
Selenoprotein N1
- sXBP1
spliced X‐box‐binding protein 1
- TUDCA
Tauroursodeoxycolic acid
- XBP1
X‐box‐binding protein 1
References
- 1.Deldicque L., Cani P.D., Philp A., Raymackers J.M., Meakin P.J., Ashford M.L. The unfolded protein response is activated in skeletal muscle by high-fat feeding: potential role in the downregulation of protein synthesis. Am. J. Physiol. Endocrinol. Metab. 2010;299(5):E695–E705. doi: 10.1152/ajpendo.00038.2010. PubMed PMID: 20501874. [DOI] [PubMed] [Google Scholar]
- 2.Wu J, Ruas JL, Estall JL, Rasbach KA, Choi JH, Ye L, et al. The unfolded protein response mediates adaptation to exercise in skeletal muscle through a PGC-1alpha/ATF6alpha complex. Cell Metabol.. 13(2):160-169. Epub 2011/02/03. doi: S1550-4131(11)00004-0 [pii] 10.1016/j.cmet.2011.01.003. PubMed PMID: 21284983; PubMed Central PMCID: PMC3057411. [DOI] [PMC free article] [PubMed]
- 3.Afroze D., Kumar A. ER stress in skeletal muscle remodeling and myopathies. FEBS J. 2019;286(2):379–398. doi: 10.1111/febs.14358. PubMed PMID: 29239106; PubMed Central PMCID: PMCPMC6002870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohnert K.R., McMillan J.D., Kumar A. Emerging roles of ER stress and unfolded protein response pathways in skeletal muscle health and disease. J. Cell. Physiol. 2018;233(1):67–78. doi: 10.1002/jcp.25852. PubMed PMID: 28177127; PubMed Central PMCID: PMCPMC5548649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han J., Kaufman R.J. The role of ER stress in lipid metabolism and lipotoxicity. J. Lipid Res. 2016;57(8):1329–1338. doi: 10.1194/jlr.R067595. PubMed PMID: 27146479; PubMed Central PMCID: PMCPMC4959874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volmer R., Ron D. Lipid-dependent regulation of the unfolded protein response. Curr. Opin. Cell Biol. 2015;33:67–73. doi: 10.1016/j.ceb.2014.12.002. PubMed PMID: 25543896; PubMed Central PMCID: PMCPMC4376399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai E., Teodoro T., Volchuk A. Endoplasmic reticulum stress: signaling the unfolded protein response. Physiology. 2007;22:193–201. doi: 10.1152/physiol.00050.2006. PubMed PMID: 17557940. [DOI] [PubMed] [Google Scholar]
- 8.Tam A.B., Roberts L.S., Chandra V., Rivera I.G., Nomura D.K., Forbes D.J. The UPR activator ATF6 responds to proteotoxic and lipotoxic stress by distinct mechanisms. Dev. Cell. 2018;46(3):327–343. doi: 10.1016/j.devcel.2018.04.023. e7. PubMed PMID: 30086303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halbleib K., Pesek K., Covino R., Hofbauer H.F., Wunnicke D., Hanelt I. Activation of the unfolded protein response by lipid bilayer stress. Mol. Cell. 2017;67(4):673–684. doi: 10.1016/j.molcel.2017.06.012. e8. PubMed PMID: 28689662. [DOI] [PubMed] [Google Scholar]
- 10.Calfon M., Zeng H., Urano F., Till J.H., Hubbard S.R., Harding H.P. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415(6867):92–96. doi: 10.1038/415092a. PubMed PMID: 11780124. [DOI] [PubMed] [Google Scholar]
- 11.Acosta-Alvear D., Zhou Y., Blais A., Tsikitis M., Lents N.H., Arias C. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol. Cell. 2007;27(1):53–66. doi: 10.1016/j.molcel.2007.06.011. PubMed PMID: 17612490. [DOI] [PubMed] [Google Scholar]
- 12.Harding H.P., Zhang Y., Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397(6716):271–274. doi: 10.1038/16729. PubMed PMID: 9930704. [DOI] [PubMed] [Google Scholar]
- 13.Haze K., Yoshida H., Yanagi H., Yura T., Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell. 1999;10(11):3787–3799. doi: 10.1091/mbc.10.11.3787. PubMed PMID: 10564271; PubMed Central PMCID: PMCPMC25679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walter P., Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–1086. doi: 10.1126/science.1209038. Epub 2011/11/26. doi: 334/6059/1081 [pii] 10.1126/science.1209038. PubMed PMID: 22116877. [DOI] [PubMed] [Google Scholar]
- 15.Urano F., Wang X., Bertolotti A., Zhang Y., Chung P., Harding H.P. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287(5453):664–666. doi: 10.1126/science.287.5453.664. PubMed PMID: 10650002. [DOI] [PubMed] [Google Scholar]
- 16.Marciniak S.J., Yun C.Y., Oyadomari S., Novoa I., Zhang Y., Jungreis R. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18(24):3066–3077. doi: 10.1101/gad.1250704. Epub 2004/12/17. doi: 18/24/3066 [pii] 10.1101/gad.1250704. PubMed PMID: 15601821; PubMed Central PMCID: PMC535917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frand A.R., Kaiser C.A. The ERO1 gene of yeast is required for oxidation of protein dithiols in the endoplasmic reticulum. Mol. Cell. 1998;1(2):161–170. doi: 10.1016/s1097-2765(00)80017-9. Epub 1998/07/11. doi: S1097-2765(00)80017-9 [pii]. PubMed PMID: 9659913. [DOI] [PubMed] [Google Scholar]
- 18.Pollard M.G., Travers K.J., Weissman J.S. Ero1p: a novel and ubiquitous protein with an essential role in oxidative protein folding in the endoplasmic reticulum. Mol. Cell. 1998;1(2):171–182. doi: 10.1016/s1097-2765(00)80018-0. Epub 1998/07/11. doi: S1097-2765(00)80018-0 [pii]. PubMed PMID: 9659914. [DOI] [PubMed] [Google Scholar]
- 19.Harding H.P., Zhang Y., Zeng H., Novoa I., Lu P.D., Calfon M. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell. 2003;11(3):619–633. doi: 10.1016/s1097-2765(03)00105-9. Epub 2003/04/02. doi: S1097276503001059 [pii]. PubMed PMID: 12667446. [DOI] [PubMed] [Google Scholar]
- 20.Zito E. ERO1: a protein disulfide oxidase and H2O2 producer. Free Radic. Biol. Med. 2015;83:299–304. doi: 10.1016/j.freeradbiomed.2015.01.011. Epub 2015/02/06. doi: S0891-5849(15)00018-0 [pii] 10.1016/j.freeradbiomed.2015.01.011. PubMed PMID: 25651816. [DOI] [PubMed] [Google Scholar]
- 21.Welch W.J., Brown C.R. Influence of molecular and chemical chaperones on protein folding. Cell Stress Chaperones. 1996;1(2):109–115. doi: 10.1379/1466-1268(1996)001<0109:iomacc>2.3.co;2. PubMed PMID: 9222596; PubMed Central PMCID: PMCPMC248462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie Q., Khaoustov V.I., Chung C.C., Sohn J., Krishnan B., Lewis D.E. Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress-induced caspase-12 activation. Hepatology. 2002;36(3):592–601. doi: 10.1053/jhep.2002.35441. PubMed PMID: 12198651. [DOI] [PubMed] [Google Scholar]
- 23.Cross B.C., Bond P.J., Sadowski P.G., Jha B.K., Zak J., Goodman J.M. The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule. Proc. Natl. Acad. Sci. U. S. A. 2012;109(15):E869–E878. doi: 10.1073/pnas.1115623109. PubMed PMID: 22315414; PubMed Central PMCID: PMCPMC3326519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papandreou I., Denko N.C., Olson M., Van Melckebeke H., Lust S., Tam A. Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood. 2011;117(4):1311–1314. doi: 10.1182/blood-2010-08-303099. PubMed PMID: 21081713; PubMed Central PMCID: PMCPMC3056474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Volkmann K., Lucas J.L., Vuga D., Wang X., Brumm D., Stiles C. Potent and selective inhibitors of the inositol-requiring enzyme 1 endoribonuclease. J. Biol. Chem. 2011;286(14):12743–12755. doi: 10.1074/jbc.M110.199737. PubMed PMID: 21303903; PubMed Central PMCID: PMCPMC3069474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Axten J.M., Medina J.R., Feng Y., Shu A., Romeril S.P., Grant S.W. Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H-indol-5-yl)-7H-p yrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) J. Med. Chem. 2012;55(16):7193–7207. doi: 10.1021/jm300713s. PubMed PMID: 22827572. [DOI] [PubMed] [Google Scholar]
- 27.Atkins C., Liu Q., Minthorn E., Zhang S.Y., Figueroa D.J., Moss K. Characterization of a novel PERK kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 2013;73(6):1993–2002. doi: 10.1158/0008-5472.CAN-12-3109. PubMed PMID: 23333938. [DOI] [PubMed] [Google Scholar]
- 28.Rojas-Rivera D., Delvaeye T., Roelandt R., Nerinckx W., Augustyns K., Vandenabeele P. When PERK inhibitors turn out to be new potent RIPK1 inhibitors: critical issues on the specificity and use of GSK2606414 and GSK2656157. Cell Death Differ. 2017;24(6):1100–1110. doi: 10.1038/cdd.2017.58. PubMed PMID: 28452996; PubMed Central PMCID: PMCPMC5442476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallagher C.M., Garri C., Cain E.L., Ang K.K., Wilson C.G., Chen S. Ceapins are a new class of unfolded protein response inhibitors, selectively targeting the ATF6alpha branch. Elife. 2016;5 doi: 10.7554/eLife.11878. PubMed PMID: 27435960; PubMed Central PMCID: PMCPMC4954757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyce M., Bryant K.F., Jousse C., Long K., Harding H.P., Scheuner D. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307(5711):935–939. doi: 10.1126/science.1101902. PubMed PMID: 15705855. [DOI] [PubMed] [Google Scholar]
- 31.Tsaytler P., Harding H.P., Ron D., Bertolotti A. Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science. 2011;332(6025):91–94. doi: 10.1126/science.1201396. PubMed PMID: 21385720. [DOI] [PubMed] [Google Scholar]
- 32.Holmes B., Brogden R.N., Heel R.C., Speight T.M., Avery G.S., Guanabenz A review of its pharmacodynamic properties and therapeutic efficacy in hypertension. Drugs. 1983;26(3):212–229. doi: 10.2165/00003495-198326030-00003. PubMed PMID: 6352237. [DOI] [PubMed] [Google Scholar]
- 33.Das I., Krzyzosiak A., Schneider K., Wrabetz L., D'Antonio M., Barry N. Preventing proteostasis diseases by selective inhibition of a phosphatase regulatory subunit. Science. 2015;348(6231):239–242. doi: 10.1126/science.aaa4484. PubMed PMID: 25859045; PubMed Central PMCID: PMCPMC4490275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crespillo-Casado A., Chambers J.E., Fischer P.M., Marciniak S.J., Ron D. PPP1R15A-mediated dephosphorylation of eIF2alpha is unaffected by Sephin1 or Guanabenz. Elife. 2017;6 doi: 10.7554/eLife.26109. PubMed PMID: 28447936; PubMed Central PMCID: PMCPMC5429092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blais J.D., Chin K.T., Zito E., Zhang Y., Heldman N., Harding H.P. A small molecule inhibitor of endoplasmic reticulum oxidation 1 (ERO1) with selectively reversible thiol reactivity. J. Biol. Chem. 2010;285(27):20993–21003. doi: 10.1074/jbc.M110.126599. Epub 2010/05/06. doi: M110.126599 [pii] 10.1074/jbc.M110.126599. PubMed PMID: 20442408; PubMed Central PMCID: PMC2898301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyake M., Nomura A., Ogura A., Takehana K., Kitahara Y., Takahara K. Skeletal muscle-specific eukaryotic translation initiation factor 2alpha phosphorylation controls amino acid metabolism and fibroblast growth factor 21-mediated non-cell-autonomous energy metabolism. FASEB J. 2016;30(2):798–812. doi: 10.1096/fj.15-275990. PubMed PMID: 26487695; PubMed Central PMCID: PMCPMC4945323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdul-Ghani M.A., DeFronzo R.A. Pathogenesis of insulin resistance in skeletal muscle. J. Biomed. Biotechnol. 2010;2010:476279. doi: 10.1155/2010/476279. PubMed PMID: 20445742; PubMed Central PMCID: PMCPMC2860140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyake M., Kuroda M., Kiyonari H., Takehana K., Hisanaga S., Morimoto M. Ligand-induced rapid skeletal muscle atrophy in HSA-Fv2E-PERK transgenic mice. PLoS One. 2017;12(6) doi: 10.1371/journal.pone.0179955. PubMed PMID: 28644884; PubMed Central PMCID: PMCPMC5482508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gallot Y.S., Bohnert K.R., Straughn A.R., Xiong G., Hindi S.M., Kumar A. PERK regulates skeletal muscle mass and contractile function in adult mice. FASEB J. 2019;33(2):1946–1962. doi: 10.1096/fj.201800683RR. PubMed PMID: 30204503; PubMed Central PMCID: PMCPMC6338633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pozzer D., Varone E., Chernorudskiy A., Schiarea S., Missiroli S., Giorgi C. A maladaptive ER stress response triggers dysfunction in highly active muscles of mice with SELENON loss. Redox Biol. 2018;20:354–366. doi: 10.1016/j.redox.2018.10.017. PubMed PMID: 30391828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varone E., Pozzer D., Di Modica S., Chernorudskiy A., Nogara L., Baraldo M. SELENON (SEPN1) protects skeletal muscle from saturated fatty acid-induced ER stress and insulin resistance. Redox Biol. 2019;24:101176. doi: 10.1016/j.redox.2019.101176. PubMed PMID: 30921636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu Z., Wang A.M., Adachi H., Katsuno M., Sobue G., Yue Z. Macroautophagy is regulated by the UPR-mediator CHOP and accentuates the phenotype of SBMA mice. PLoS Genet. 2011;7(10) doi: 10.1371/journal.pgen.1002321. PubMed PMID: 22022281; PubMed Central PMCID: PMCPMC3192827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zito E., Melo E.P., Yang Y., Wahlander A., Neubert T.A., Ron D. Oxidative protein folding by an endoplasmic reticulum-localized peroxiredoxin. Mol. Cell. 2010;40(5):787–797. doi: 10.1016/j.molcel.2010.11.010. Epub 2010/12/15. doi: S1097-2765(10)00848-8 [pii] 10.1016/j.molcel.2010.11.010. PubMed PMID: 21145486; PubMed Central PMCID: PMC3026605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zito E., Hansen H.G., Yeo G.S., Fujii J., Ron D. Endoplasmic reticulum thiol oxidase deficiency leads to ascorbic acid depletion and noncanonical scurvy in mice. Mol. Cell. 2012 doi: 10.1016/j.molcel.2012.08.010. Epub 2012/09/18. doi: S1097-2765(12)00697-1 [pii] 10.1016/j.molcel.2012.08.010. PubMed PMID: 22981861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zito E. PRDX4, an ER-localised peroxiredoxin at the crossroads between enzymatic oxidative protein folding and non-enzymatic protein oxidation. Antioxidants Redox Signal. 2012 doi: 10.1089/ars.2012.4966. Epub 2012/10/03. PubMed PMID: 23025503. [DOI] [PubMed] [Google Scholar]
- 46.Ikezoe K., Nakamori M., Furuya H., Arahata H., Kanemoto S., Kimura T. Endoplasmic reticulum stress in myotonic dystrophy type 1 muscle. Acta Neuropathol. 2007;114(5):527–535. doi: 10.1007/s00401-007-0267-9. PubMed PMID: 17661063. [DOI] [PubMed] [Google Scholar]
- 47.Nogalska A., Wojcik S., Engel W.K., McFerrin J., Askanas V. Endoplasmic reticulum stress induces myostatin precursor protein and NF-kappaB in cultured human muscle fibers: relevance to inclusion body myositis. Exp. Neurol. 2007;204(2):610–618. doi: 10.1016/j.expneurol.2006.12.014. PubMed PMID: 17261282; PubMed Central PMCID: PMCPMC1909753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rashid T., Nemazanyy I., Paolini C., Tatsuta T., Crespin P., de Villeneuve D. Lipin1 deficiency causes sarcoplasmic reticulum stress and chaperone-responsive myopathy. EMBO J. 2019;38(1) doi: 10.15252/embj.201899576. PubMed PMID: 30420558; PubMed Central PMCID: PMCPMC6315296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishitoh H., Kadowaki H., Nagai A., Maruyama T., Yokota T., Fukutomi H. ALS-linked mutant SOD1 induces ER stress- and ASK1-dependent motor neuron death by targeting Derlin-1. Genes Dev. 2008;22(11):1451–1464. doi: 10.1101/gad.1640108. PubMed PMID: 18519638; PubMed Central PMCID: PMCPMC2418582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen D., Wang Y., Chin E.R. Activation of the endoplasmic reticulum stress response in skeletal muscle of G93A*SOD1 amyotrophic lateral sclerosis mice. Front. Cell. Neurosci. 2015;9:170. doi: 10.3389/fncel.2015.00170. PubMed PMID: 26041991; PubMed Central PMCID: PMCPMC4435075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee C.S., Hanna A.D., Wang H., Dagnino-Acosta A., Joshi A.D., Knoblauch M. A chemical chaperone improves muscle function in mice with a RyR1 mutation. Nat. Commun. 2017;8:14659. doi: 10.1038/ncomms14659. PubMed PMID: 28337975; PubMed Central PMCID: PMCPMC5376670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marino M., Stoilova T., Giorgi C., Bachi A., Cattaneo A., Auricchio A. SEPN1, an endoplasmic reticulum-localized selenoprotein linked to skeletal muscle pathology, counteracts hyper-oxidation by means of redox-regulating SERCA2 pump activity. Hum. Mol. Genet. 2015;24:1843–1855. doi: 10.1093/hmg/ddu602. Epub 2014/12/03. doi: ddu602 [pii] 10.1093/hmg/ddu602. PubMed PMID: 25452428. [DOI] [PubMed] [Google Scholar]
- 53.Pozzer D., Favellato M., Bolis M., Invernizzi R.W., Solagna F., Blaauw B. Endoplasmic reticulum oxidative stress triggers tgf-beta-dependent muscle dysfunction by accelerating ascorbic acid turnover. Sci. Rep. 2017;7:40993. doi: 10.1038/srep40993. Epub 2017/01/21. doi: srep40993 [pii] 10.1038/srep40993. PubMed PMID: 28106121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bravo R., Vicencio J.M., Parra V., Troncoso R., Munoz J.P., Bui M. Increased ER-mitochondrial coupling promotes mitochondrial respiration and bioenergetics during early phases of ER stress. J. Cell Sci. 2011;124(Pt 13):2143–2152. doi: 10.1242/jcs.080762. Epub 2011/06/02. doi: jcs.080762 [pii] 10.1242/jcs.080762. PubMed PMID: 21628424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tezze C., Romanello V., Desbats M.A., Fadini G.P., Albiero M., Favaro G. Age-associated loss of OPA1 in muscle impacts muscle mass, metabolic homeostasis, systemic inflammation, and epithelial senescence. Cell Metabol. 2017;25(6):1374–13789 e6. doi: 10.1016/j.cmet.2017.04.021. PubMed PMID: 28552492; PubMed Central PMCID: PMCPMC5462533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vaz A.R., Cunha C., Gomes C., Schmucki N., Barbosa M., Brites D. Glycoursodeoxycholic acid reduces matrix metalloproteinase-9 and caspase-9 activation in a cellular model of superoxide dismutase-1 neurodegeneration. Mol. Neurobiol. 2015;51(3):864–877. doi: 10.1007/s12035-014-8731-8. PubMed PMID: 24848512. [DOI] [PubMed] [Google Scholar]
- 57.Elia A.E., Lalli S., Monsurro M.R., Sagnelli A., Taiello A.C., Reggiori B. Tauroursodeoxycholic acid in the treatment of patients with amyotrophic lateral sclerosis. Eur. J. Neurol. 2016;23(1):45–52. doi: 10.1111/ene.12664. PubMed PMID: 25664595; PubMed Central PMCID: PMCPMC5024041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choi S.E., Lee Y.J., Jang H.J., Lee K.W., Kim Y.S., Jun H.S. A chemical chaperone 4-PBA ameliorates palmitate-induced inhibition of glucose-stimulated insulin secretion (GSIS) Arch. Biochem. Biophys. 2008;475(2):109–114. doi: 10.1016/j.abb.2008.04.015. PubMed PMID: 18455496. [DOI] [PubMed] [Google Scholar]
- 59.Chen Y.Y., Sun L.Q., Wang B.A., Zou X.M., Mu Y.M., Lu J.M. Palmitate induces autophagy in pancreatic beta-cells via endoplasmic reticulum stress and its downstream JNK pathway. Int. J. Mol. Med. 2013;32(6):1401–1406. doi: 10.3892/ijmm.2013.1530. PubMed PMID: 24142192. [DOI] [PubMed] [Google Scholar]
- 60.Kars M., Yang L., Gregor M.F., Mohammed B.S., Pietka T.A., Finck B.N. Tauroursodeoxycholic Acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes. 2010;59(8):1899–1905. doi: 10.2337/db10-0308. PubMed PMID: 20522594; PubMed Central PMCID: PMCPMC2911053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Engin F., Yermalovich A., Nguyen T., Hummasti S., Fu W., Eizirik D.L. Restoration of the unfolded protein response in pancreatic beta cells protects mice against type 1 diabetes. Sci. Transl. Med. 2013;5(211):211ra156. doi: 10.1126/scitranslmed.3006534. PubMed PMID: 24225943; PubMed Central PMCID: PMCPMC4169117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y. Targeting the functional interplay between endoplasmic reticulum oxidoreductin-1α and protein disulfide isomerase suppresses the progression of cervical cancer. EBioMedicine. 2019 doi: 10.1016/j.ebiom.2019.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]