Abstract
Purpose:
High blood pressure is a relevant risk factor for vascular damage, leading to development of depressive symptoms and dementia in older adults. Moreover, subjective memory complaints are recognized as an early marker of cognitive impairment. However, it has been established that subjective memory complaints could also be a reflection of depressive symptoms. The objective of this paper is to assess the impact of depressive symptoms and subjective memory complaints on the incidence of cognitive impairment in older adults with high blood pressure.
Methods:
This is a secondary analysis of the Mexican Health and Aging Study, a representative cohort composed by individuals aged ≥ 50 years. Participants with cognitive impairment in 2012 were excluded since the outcome was incident cognitive impairment in 2015. Four groups were created according to depressive symptomatology and subjective memory complaints status, analyses were stratified according to blood pressure status. The odds incident cognitive impairment was estimated through logistic regression models.
Results:
A total of 6,327 participants were included, from which 6.44% developed cognitive impairment. No differences were seen regarding the development of cognitive impairment in participants without high blood pressure. However, increased risk was evident in those with both high blood pressure and depressive symptoms (OR=2.1, 95% CI 1.09 - 4.09, p =0.026) as with high blood pressure, depressive symptoms and subjective memory complaints (OR=1.91, 9% CI 1.4 - 3.2, p= 0.001).
Conclusion:
Individuals with high blood pressure have a higher risk of developing incident cognitive impairment when depressive symptoms and/or subjective memory complaints are present. Our results suggest that a sequence of events related to altered cerebral vascular dynamics is possible.
Keywords: Aged, Depressive symptoms, Cognition Disorders, Cognitive impairment, Hypertension, Subjective memory complaint
Introduction
Older people frequently live with chronic diseases, which interact with economic, cultural, familial and psychological features, particularly in this age group [1]. Moreover, one of the most important age-related challenges faced by both older persons and health professionals is the presence of dementia. The latter is associated with significant functional decline and loss of independence, leading to an increased usage of healthcare services and need for specialized care. It is also important to acknowledge that dementia has a high prevalence at a global scale, and is also an increasing issue in Latin America [2–4]. Furthermore, in order to prevent the progression of disease and further disability, it is imperative to identify modifiable risk factors that are present early or even precede the onset of cognitive symptomatology [4–6].
One of the conditions that are part of the spectrum of problems leading to dementia includes Subjective Memory Complaints (SMC). These may appear in the presence of normal cognitive tests or alongside mild cognitive impairment (MCI), which is defined by subjective and objective cognitive impairment with relatively preserved functioning [7–10]. However, SMC have also been associated with depressive symptoms (DS), not always reflecting symptoms of cognitive disorders [11].
More recently, DS have been described as an early manifestation of dementia since these symptoms could be related to the neuropathology of this disease, for instance, through vascular damage [12]. Furthermore, a well-known risk factor for cognitive impairment is high blood pressure (HBP); which is in line with the path that departs from vascular damage and ends in overt brain damage and dementia [13]. The exact mechanism behind this association is not clear. However, the presence of untreated chronic HBP has been associated with a variety of vascular structural and functional changes. These changes modify the ability of physiological self-regulation, leading to ischemia in vulnerable brain regions, such as those in charge of mood and affective control [13].
Thus, there is likely a mechanistic association between HBP, DS and cognitive impairment (CogI). As previously stated, this link is of major importance due to the potential for early identification, prevention and/or delay of the onset of dementia. This information becomes particularly relevant in Mexico, since high incidences of both HBP and dementia have been noted in the Mexican population [2,14]. The aim of this work was to assess whether an association exists between DS, SMC and CogI in patients with HBP and without HBP.
Materials and methods
Setting and participants
This is a secondary analysis of the Mexican Health and Aging Study (MHAS), a nation-wide representative cohort of community-dwelling Mexican older adults aged 50 years or older. [15][1] This study began in 2001, and currently includes information from three follow-up periods (2003, 2012 and 2015). The main objective of the MHAS was to determine which factors have an impact on the aging process in Mexican individuals. Thus, the study comprises a nationally representative sample of urban and rural areas of the 32 states of the country. Face-to-face interviews were performed by applying a set of questionnaires including topics from different dominions, such as: socio-demographic characteristics, health-related issues, accessibility and use of healthcare services, cognitive performance, functional status, and financial status. Further information regarding aims and procedures can be found elsewhere [15].
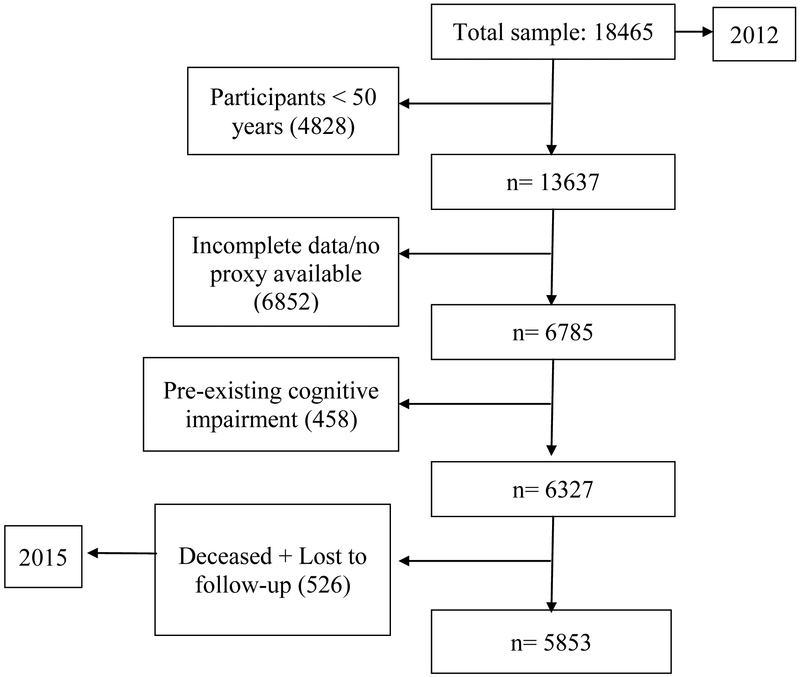
For the purpose of this work we excluded participants with a past medical history remarkable for previous strokes or psychiatric diseases, as well as those with incomplete data. Participants found to have CogI in 2012 were also excluded from the sample, yielding a total subset of 6,327 participants. Nevertheless, 472 participants were lost to follow-up due to absenteeism, difficulties in location or refusal to continue participating. Thus, a final sample of 5,853 older persons was analyzed (see Figure 1).
Figure 1.
Flowchart of the study sample
Variables
Dependent variable
The dependent variable was cognitive impairment, which was assessed through the Cross-Cultural Cognitive Examination test (CCCE). This test has a maximum score of 80 points and includes the evaluation of several cognitive domains: a) primary verbal memory, b) selective attention; c) secondary verbal memory; d) executive function and motor control; e) visual memory. The CCCE has shown high sensitivity (100%) and specificity (83%) for detection of dementia in a previous publication [18]. Standardization with z-scores was used dividing the studied participants in three groups according to years of schooling (0, 1-7 and 7 years or more) and three age categories (60-69, 70-79 and 80 years and above). The cut-off value for CogI was established through normative data generated according to these categories, and was set at −1.5 standard deviations. [16,17]
Independent variable
Depressive symptoms
A validated depression questionnaire was used to assess depressive symptomas. The MHAS depression questionnaire has shown to be valid for the detection of clinical depression, with a sensitivity and specificity of 80.7% and 68.7%, respectively. The MHAS screening questionnaire for depression consists of nine items with "yes" or "no" answers: (Within the past week, was the respondent: 1. Depressed? 2. Experiencing difficulty performing? 3. Experiencing restless sleep? 4. Happy? 5. Lonely? 6. Enjoying life 7. Sad? 8. Feeling tired? 9. Energetic?). The cut-off value for defining depression is any score ≥5 points [18-20].
Subjective memory complaints
The presence of SMC was evaluated with the single question: “How would you evaluate your memory in the present?”.
The answer options were: excellent, very good, good, fair and poor; and further collapsed into only two groups: no-SMC (Excellent, very good, good) and SMC (fair and poor).
In order to assess the effect of SMC (with or without DS) on incident CogI, four groups were defined in the baseline assessment (2012):
Without SMC/without DS (noSMC/noDS)
With SMC/without DS (SMC/noDS)
Without SMC/with DS (noSMC/DS)
With SMC/with DS (SMC/DS)
High Blood Pressure
Sample was then stratified according to HBP status. This variable was assessed through self-report, with the question: “Have you ever been told by a doctor or medical provider that you have HBP?” If the subject answered yes, they were considered to have HBP.
Confounding variables
Multivariate regression models were adjusted for confounders from different dominions, such as: sociodemographic (age, sex, marital status, years of schooling); health-related (self-reported vision, stroke, acute myocardial infarction, arthropathy, diabetes mellitus, cancer, chronic pulmonary disease and alcohol use). Importantly, alcohol use was assessed through the CAGE questionnaire, [21] an acronym that stands for four yes/no questions: 1. “Have you ever felt you should Cut down on your drinking?”, 2. “Have people Annoyed you by criticizing your drinking?”, 3. “Have you ever felt bad or Guilty about your drinking?”, 4. “Have you ever had a drink as an Eye-opener first thing in the morning to steady your nerves or help a hangover?”. A total score of 2 or greater was considered clinically significant and a score of 4 was categorized as alcohol dependence [21].
Statistical analysis
Initially, we used a univariate analysis to explore extreme values and normal distribution in order to adjust and categorize variables. Regarding descriptive statistics, frequencies were used for presenting categorical variables, while standard deviations and means were employed for continuous variables. Chi2 test was used for finding differences between groups for categorical variables, and ANOVA was used for continuous variables. Finally, for the multivariate analysis, logistic regression models were fitted in order to obtain odds ratio (OR) with 95% confidence intervals (CIs) of having incident CogI according to DS/SMC groups (using the group noSMC/noDS as reference). Adjusted and non-adjusted estimates are presented. The statistical level of significance was set at p-value <0.05. Data was analyzed using STATA 14® software.
Ethical issues
The Institutional Review Boards of Ethics Committees of the University of Texas Medical Branch in the United States, the Instituto Nacional de Estadística y Geografía and the Instituto Nacional de Salud Pública in México approved the study. All study participants signed an informed consent. The study adhered to the ethical guidelines of the Declaration of Helsinki.
Declaration of Sources of Funding
The MHAS was sponsored by the National Institutes of Health/National Institute on Aging (Grant NIH R01AG018016), as well as the Sealy Center on Aging at the University of Texas Medical Branch in Galveston and by the Health of Older Minorities T32AG00270 training Grant from the National Institute on Aging.
Results
At baseline from the 6,327 participants, the mean age was 68.59 (± SD 6.78) years, 57.7% (n=3,385) were female p <0.001, 60.57% (n= 3814) were married p <0.001, 43.4% (n=2760) had visual deficits, p <0.001, 43.83% (n=2773) had SMC, 6.89% (n=436) had DS and 17.57% (n=1111) had both SMC and DS p= 0.474. The prevalence of HBP was 42.86%. The sample is described in more detail in Table 1.
Table 1.
Descriptive sample and Bivariate analysis
| Age mean ± SD |
Sex (women) n (%) |
Married n (%) |
Schooling (years) mean ± SD |
Alcohol consumption n (%) |
Visual deficits n (%) |
Comorbidities mean ± SD |
Total n (%) | Incidence of cognitive impairment n (%) |
|
|---|---|---|---|---|---|---|---|---|---|
| No-HBP | |||||||||
| 1 | 67.72 ± 6.59 | 462 (39.55) | 794 (67.98) | 6.65 ± 5.24 | 0.22 ± 0.65 | 355 (30.39) | 0.28 (0.52) | 1168 (33.58) | 60 (6.37) |
| 2 | 68.79 ± 7.22 | 654 (44.58) | 964 (65.71) | 4.86 ± 3.97 | 0.25 ± 0.75 | 702 (47.85) | 0.38 (0.61) | 1467 (42.18) | 60 (5.07) |
| 3 | 69.09 ± 6.66 | 107 (55.44) | 112 (58.03) | 5.11 ± 4.73 | 0.16 ± 0.56 | 88 (45.60) | 0.4 (0.58) | 193 (5.55) | 10 (7.14) |
| 4 | 67.91 ± 6.17 | 262 (62.53) | 250 (59.67) | 4.34 ± 3.57 | 0.21 ± 0.68 | 244 (58.23) | 0.61 (0.73) | 419 (12.36) | 15 (5.57) |
| P value | <0.001 | <0.001 | 0.003 | <0.001 | <0.001 | <0.001 | <0.001 | 0.567 | 0.367 |
| Total | 68.37 ± 6.66 | 1,485 (50.52) | 2,120 (62.84) | 5.24 ± 4.37 | 0.21 ± 0.66 | 1,389 (45.51) | 1.67 (0.61) | 3,247 (93.67) | 145 (6.03) |
| HBP | |||||||||
| 1 | 68.8 ± 6.64 | 448 (53.46) | 541 (64.56) | 6.51 ± 4.95 | 0.17 ± 0.57 | 257 (30.67) | 0.61 ± 0.71 | 838 (25.42) | 26 (3.99) |
| 2 | 69.07 ± 6.72 | 761 (58.49) | 848 (65.18) | 4.85 ± 3.84 | 0.17 ± 0.61 | 606 (46.58) | 0.62 ± 0.73 | 1301 (39.47) | 53 (5.42) |
| 3 | 68.59 ± 6.31 | 175 (72.92) | 122 (50.83) | 5.12 ± 4.38 | 0.15 ± 0.56 | 101 (42.08) | 0.93 ± 0.91 | 240 (7.28) | 16 (9.20) |
| 4 | 68.59 ± 6.29 | 508 (73.62) | 381 (55.22) | 3.97 ± 3.38 | 0.15 ± 0.61 | 401 (58.12) | 0.85 ± 0.82 | 690 (20.93) | 46 (8.57) |
| P value | 0.456 | <0.001 | <0.001 | <0.001 | 0.573 | <0.001 | <0.001 | 0.241 | 0.002 |
| Total | 68.7 ± 6.49 | 1,892 (64.62) | 1,892 (58.94) | 5.11 ± 4.13 | 0.16 ± 0.58 | 1,365 (44.36) | 3.01 ± 0.79 | 3,069 (23.27) | 141 (6.7) |
| Total sample | |||||||||
| 1 | 68.17 ± 6.69 | 911 (45.27) | 1,335 (66.47) | 6.59 ± 5.12 | 0.21 ± 0.62 | 613 (30.54) | 0.23 ± 0.69 | 2007 (31.72) | 86 (5.40) |
| 2 | 68.91 ± 6.99 | 1,418 (51.15) | 1,812 (65.32) | 4.86 ± 3.91 | 0.21 ± 0.69 | 1,310 (47.24) | 0.17 ± 0.57 | 2773 (43.83) | 113 (5.22) |
| 3 | 68.8 ± 6.78 | 285 (65.26) | 234 (53.63) | 5.10 ± 4.54 | 0.15 ± 0.57 | 190 (43.58) | 0.12 ± 0.56 | 436 (6.89) | 26 (8.21) |
| 4 | 68.4 ± 6.64 | 771 (69.42) | 633 (56.88) | 4.12 ± 3.45 | 0.17 ± 0.64 | 647 (58.24) | 0.18 ± 0.62 | 1,111 (17.57) | 61 (6.96) |
| P value | 0.0018 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.474 | 0.061 |
| Total | 68.59 ± 6.78 | 3,385 (57.7) | 3,814 (60.57) | 5.16 ± 4.26 | 0.185 ± 0.63 | 2,760 (43.40) | 0.7 (0.61) | 6,327 (25) | 286 (6.44) |
Sample description according to baseline characteristics: Chi2 test used for categorical variables and ANOVA used for continuous variables..
HBP=High Blood Pressure, 1=without SMC/ without DS, 2=with SMC/ without DS, 3=without SMC/ with DS, 4=with SMC/ with DS
The 3-year incidence of cognitive impairment was 6.44% (n=286); 6.7% (n=141) for those with HBP, and 6.03% (n=145) in participants without HBP.
The differences in the incidence of CogI were only statistically significant in older persons with HBP. Group 3 + HBP had the greater incidence of CogI corresponding to 9.2% (n=16), followed by group 4 + HBP 8.5% (n=46), the group 2 + HBP 5.4% (n=53) and the group 1 + HBP (reference group) with 3.9% (n=26) (p=0.002)(Table 1).
Participants with HBP from group 3 (OR=2.43, 95% CI 1.27-4.64, p=0.007) and group 4 (OR=2.25, 95% CI 1.37 - 3.69, p=0.001) displayed significant associations with the incidence of CogI. After the adjusted analysis, the associations in participants with HBP from the group 3 (OR=2.11, 95% CI 1.09-4.09, p=0.026) and group 4 (OR=1.91, 95% CI 1.41-3.22, p=0.001) remained significant. The analysis performed on subjects without HBP showed no statistically significant associations (Table 2).
Table 2.
Logistic regression model
| TOTAL | NO-HBP | HBP | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Study groups | OR | p-value | CI | OR | p-value | CI | OR | p value | CI |
| Unadjusted | |||||||||
| 1 | |||||||||
| 2 | 0.96 | 0.81 | (0.72 - 1.28) | 0.78 | 0.19 | (0.54 - 1.13) | 1.37 | 0.19 | (0.85 - 2.22) |
| 3 | 1.56 | 0.05 | (0.99 - 2.47) | 1.13 | 0.72 | (0.56 - 2.26) | 2.43 | 0.007 | (1.27 - 4.64) |
| 4 | 1.31 | 0.11 | (0.93 - 1.84) | 0.68 | 0.2 | (0.38 - 1.22) | 2.25 | 0.001 | (1.37 - 3.69) |
| Adjusted | |||||||||
| 1 | |||||||||
| 2 | 0.86 | 0.34 | (0.64 - 1.16) | 0.69 | 0.059 | (0.47 - 1.01) | 1.29 | 0.31 | (0.79 - 2.12) |
| 3 | 1.34 | 0.21 | (0.84 - 2.13) | 1.01 | 0.97 | (0.51 - 2.04) | 2.11 | 0.03 | (1.09 - 4.09) |
| 4 | 1.04 | 0.80 | (0.73 - 1.49) | 0.53 | 0.041 | (0.29 - 0.97) | 1.91 | 0.01 | (1.14 - 3.22) |
Logistic regression model which displays the variables when assessed by SMC and DS status. Adjusted by sex, age, number of years in school, marital status, comorbidity, alcoholism, and visual deficits.
HBP=High Blood Pressure, 1=without SMC/ without DS, 2=with SMC/ without DS, 3=without SMC/ with DS, 4=with SMC/ with DS
Discussion
According to our results, DS were significantly associated with an increase on the 3-year incidence of CogI, only in those participants with HBP. This finding is consistent with several previous studies that have disclosed an important association between development of CogI and early manifestations of dementia such as DS. [22–24] For instance, a meta-analysis recently reported that late-life depression was associated with a significant risk for all-cause dementia, vascular dementia and in particular, Alzheimer’s Disease. [25] Large community-based studies have shown independent associations between late-life depression, small basal ganglia lesions and white matter abnormalities visualized as hyperintense regions on MRI, [24,26] particularly ischemic lesions in the fronto-striatal region; [27] which give rise to the plausible pathway arising from depressive symptoms and leading to CogI in those individuals with vascular risk factors such as HBP.
Moreover, in the Cardiovascular Health Study, the severity of DS independently predicted the diagnosis of MCI 6 years later. [28] Studies have shown that major depression confers a greater relative risk for CogI than the diagnosis of dysthymia or self-reported DS, suggesting a possible dose-response relationship between severity of depressive disease, and risk of cognitive deterioration. [29] Other studies suggest that when depressive and cognitive symptoms appear close in time they likely arise from common neuropathological processes. [30] In this paper, we assess self-report of DS and we show the effect of these over CogI in a period of 3 years. We believe that DS are not cognitive symptoms. Nonetheless, the combination of DS with vascular risk factors such as HBP could predict further cognitive involvement.
Also, studies have shown that the risk for cognitive deterioration and dementia persists latent even after treating depression, which suggests that irreversible vascular damage is the real risk factor and DS just a manifestation. [31,32] Furthermore, as previously described, untreated chronic HBP has been associated with CogI, [6] and even linked to pathological findings related to Alzheimer's disease (AD). [13] For instance, in the Framingham Heart Study, the first wave of patients had an incidence of dementia of 2.8%, whilst the second and third waves of patients had incidences of 2.2% and 2% respectively. It is not entirely clear why the incidence of dementia in this study declined, but it seems to be associated with a healthy lifestyle, control and prevention of HBP. [31]
Our study has some limitations, self-report is one of the main issues, leading toward potential memory bias, and under-diagnosis of HBP. It is a secondary analysis from a study not designed specifically for solving our hypothesis. Besides, it was not possible to determine the intensity or duration of DS, thus we were not able to differentiate between dysthymia and major depression. Also, the treatment status of DS was unknown. Moreover, CCCE is a screening tool, therefore, a diagnosis of overt dementia and subtype thereof was not possible. Regarding HBP, because the MHAS was conducted as a survey, it was not possible to disclose whether HBP was controlled at the time (data regarding blood pressure assessment at the time was not available). In spite of the fact that 70% of the participants were receiving treatment at the time of data collection, having therapy is not equal to be controlled, data in the Latin-American region and other reports in México for uncontrolled hypertension range from 12% to 41%. [33,34] At certain point, all HBP participants (controlled or not) had been exposed to HBP levels. Thus, the risk for CogI was present. However, uncontrolled participants might have been at greater risk (harmful exposures still present).
Although the treatment of HBP is very useful in the prevention and progression of adverse outcomes, sometimes there is already some degree of damage at the CNS level at the time treatment is started or the blood pressure levels are finally controlled. An ideal scenario should be the prevention of the onset of arterial hypertension.[31]
On the other hand, several studies have evidenced a strong dependence of cognitive performance on blood pressure, pointing to the fact that a low blood pressure or excessive control could lead to neural damage. The latter may be due to decreased cerebral perfusion, an effect that is greater in older persons because of related changes in autoregulatory mechanisms. [35–37 ] Further studies addressing this issue with wider and comprehensive assessments are still required, [38,39] since exploring even earlier risk factors which could exert a significant influence on risk for future cognitive impairment. [40]
On the other hand, this study also has several strengths. It is derived from a representative sample of a Latin American country, where the evidence in this particular subject is limited and there is a high prevalence of HBP. Populations with a high prevalence of HBP could be reflected in our results. As such, our findings could lead to conclusions relevant from a public health viewpoint. Our results can also be extrapolated to several regions due to limited existing information on this issue, and also due to high rates of Latin American immigrants in other regions which increases applicability elsewhere. We would like to highlight from the results that DS seems to be a predictor for the development of cognitive impairment when a vascular risk factor such as HBP is present. These results appeal for attention since DS are not usually explored in primary care settings, particularly when addressing older adults. However, the presence of DS, and psychiatric disease should be assessed in all older patients due to its implications on quality of life, as well as for its probable diagnostic value in early prediction of cognitive impairment. Also, HBP prevention and control could be crucial in preventing vascular damage and CogI progression. This study aims to disclose the importance of taking early actions given the incremental risk for development of CogI.
Thus, we conclude that even if there are no curative treatments for dementia or CogI available at the present time, prevention and delay of their installment are of paramount importance. Also, early targets for potential treatments must be identified. There are several treatable risk factors, which could be managed through counseling regarding healthy lifestyle habits, detection of prodromal symptoms and prevention of cardiovascular risk factors, including HBP which could importantly impact outcomes in older persons. [5,6,35,36] More studies are required in order to more accurately describe and confirm these findings, leading toward the creation of prevention policies for adequately treating older persons.
Footnotes
Declaration of Conflicts of Interest:
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Gutierrez-Robledo LM. Looking at the Future of Geriatric Care in Developing Countries. Journals Gerontol Ser A Biol Sci Med Sci [Internet]. 2002. March 1 [cited 2019 Jan 13];57(3):M162–7. Available from: https://academic.oup.com/biomedgerontology/article-lookup/doi/10.1093/gerona/57.3.M162 [DOI] [PubMed] [Google Scholar]
- 2.Miguel Gutiérrez-Robledo L, Arrieta-Cruz I, Luis *, Gutiérrez M, Dirección R. Demencias en México: la necesidad de un Plan de Acción GACETA MÉDICA DE MÉXICO SIMPOSIO Correspondencia [Internet]. 2015. [cited 2019 Jan 13]. Available from: www.anmm.org.mx [Google Scholar]
- 3.Mejia-Arango S, Gutierrez LM. Prevalence and Incidence Rates of Dementia and Cognitive Impairment No Dementia in the Mexican Population Whitfield K, Angel JL, Wong R, editors. J Aging Health [Internet]. 2011. October 23 [cited 2019 Jan 13];23(7): 1050–74. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21948770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rizzi L, Rosset I, Roriz-Cruz M. Global epidemiology of dementia: Alzheimer’s and vascular types. Biomed Res Int [Internet]. 2014. June 25 [cited 2019 Jan 13];2014:908915 Available from: http://www.ncbi.nlm.nih.gov/pubmed/25089278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frankish H, Horton R. Prevention and management of dementia: a priority for public health. Lancet [Internet]. 2017. December 16 [cited 2019 Jan 13];390(10113):2614–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28735854 [DOI] [PubMed] [Google Scholar]
- 6.Taragano FE, Allegri RF, Krupitzki H, Sarasola DR, Serrano CM, Loñ L, et al. Mild behavioral impairment and risk of dementia: a prospective cohort study of 358 patients. J Clin Psychiatry [Internet]. 2009. April [cited 2019 Jan 13];70(4):584–92. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19323967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balash Y, Mordechovich M, Shabtai H, Giladi N, Gurevich T, Korczyn AD. Subjective memory complaints in elders: depression, anxiety, or cognitive decline? Acta Neurol Scand [Internet]. 2013. May [cited 2019 Jan 13];127(5):344–50. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23215819 [DOI] [PubMed] [Google Scholar]
- 8.Jacinto AF, Brucki SMD, Porto CS, Arruda Martins M de, Nitrini R. Subjective memory complaints in the elderly: a sign of cognitive impairment? Clinics (Sao Paulo) [Internet]. 2014. March [cited 2019 Jan 13];69(3):194–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24626946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatr Scand [Internet]. 2014. December [cited 2019 Jan 13];130(6):439–51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25219393 [DOI] [PubMed] [Google Scholar]
- 10.Montenegro Peña M Quejas subjetivas de memoria en el envejecimiento y en adultos jóvenes: variables implicadas. 2015. [cited 2019 Jan 13];1. Available from:https://dialnet.unirioja.es/servlet/tesis?codigo=126691 [Google Scholar]
- 11.Alexopoulos GS, Kelly RE. Research advances in geriatric depression. World Psychiatry [Internet]. 2009. October [cited 2019 Jan 13];8(3):140–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19812743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geda YE, Roberts RO, Mielke MM, Knopman DS, Christianson TJH, Pankratz VS, et al. Baseline Neuropsychiatric Symptoms and the Risk of Incident Mild Cognitive Impairment: A Population-Based Study. Am J Psychiatry [Internet]. 2014. May [cited 2019 Jan 13];171(5):572–81. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24700290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skoog I, Gustafson D. Update on hypertension and Alzheimer’s disease. Neurol Res [Internet]. 2006. September 19 [cited 2019 Jan 13];28(6):605–11. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16945211 [DOI] [PubMed] [Google Scholar]
- 14.Downer B, Chen N-W, Wong R, Markides KS. Self-Reported Health and Functional Characteristics of Mexican and Mexican American Adults Aged 80 and Over. J Aging Health [Internet]. 2016. [cited 2019 Jan 13];28(7):1239–55. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27590800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong R, Michaels-Obregon A, Palloni A. Cohort Profile: The Mexican Health and Aging Study (MHAS). Int J Epidemiol [Internet]. 2017. April 1 [cited 2019 Jan 13];46(2):e2–e2. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25626437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glosser G, Wolfe N, Albert ML, Lavine L, Steele JC, Calne DB, et al. Cross-cultural cognitive examination: validation of a dementia screening instrument for neuroepidemiological research. J Am Geriatr Soc [Internet]. 1993. September [cited 2019 Jan 13];41(9):931–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8409180 [DOI] [PubMed] [Google Scholar]
- 17.Mejía-Arango S, Wong R, Michaels-Obregón A. Normative and standardized data for cognitive measures in the Mexican Health and Aging Study. Salud Publica Mex [Internet]. 2015. [cited 2019 Jan 13];57 Suppl 1(0 1):S90–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26172239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4 edition. RR Donnelley & Sons Company, editor. Washington DC: American Psychiatric Association; 2000. [Google Scholar]
- 19.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale [Internet]. [cited 2019. January 13];17(1):37–49. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7183759 [DOI] [PubMed] [Google Scholar]
- 20.Aguilar-Navarro SG, Fuentes-Cantú A, Avila-Funes JA, García-Mayo EJ. [Validity and reliability of the screening questionnaire for geriatric depression used in the Mexican Health and Age Study]. Salud Publica Mex [Internet]. [cited 2019. January 13];49(4):256–62. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17710274 [DOI] [PubMed] [Google Scholar]
- 21.Mayfield D, McLeod G, Hall P. The CAGE questionnaire: validation of a new alcoholism screening instrument. Am J Psychiatry [Internet]. 1974. October [cited 2019 Jan 13];131(10): 1121–3. Available from: http://www.ncbi.nlm.nih.gov/pubmed/4416585 [DOI] [PubMed] [Google Scholar]
- 22.Ismail Z, Malick A, Smith EE, Schweizer T, Fischer C. Depression versus dementia: is this construct still relevant? Neurodegener Dis Manag [Internet]. 2014. April 16 [cited 2019 Jan 13];4(2): 119–26. Available from: https://www.futuremedicine.com/doi/10.2217/nmt.14.5 [DOI] [PubMed] [Google Scholar]
- 23.Singh-Manoux A, Dugravot A, Fournier A, Abell J, Ebmeier K, Kivimäki M, et al. Trajectories of Depressive Symptoms Before Diagnosis of Dementia. JAMA Psychiatry [Internet]. 2017. July 1 [cited 2019 Jan 13];74(7):712 Available from: http://www.ncbi.nlm.nih.gov/pubmed/28514478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steffens DC. Late-Life Depression and the Prodromes of Dementia. JAMA Psychiatry [Internet]. 2017. July 1 [cited 2019 Jan 13];74(7):673 Available from: http://www.ncbi.nlm.nih.gov/pubmed/28514459 [DOI] [PubMed] [Google Scholar]
- 25.Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and Risk for Alzheimer Disease. Arch Gen Psychiatry [Internet]. 2006. May 1 [cited 2019 Jan 13];63(5):530 Available from: http://www.ncbi.nlm.nih.gov/pubmed/16651510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reppermund S, Zhuang L, Wen W, Slavin MJ, Trollor JN, Brodaty H, et al. White matter integrity and late-life depression in community-dwelling individuals: diffusion tensor imaging study using tract-based spatial statistics. Br J Psychiatry [Internet]. 2014. October 2 [cited 2019 Jan 13];205(04):315–20. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25147370 [DOI] [PubMed] [Google Scholar]
- 27.Salloway S, Malloy P, Kohn R, Gillard E, Duffy J, Rogg J, et al. MRI and neuropsychological differences in early- and late-life-onset geriatric depression. Neurology [Internet]. 1996. June [cited 2019 Jan 13];46(6):1567–74. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8649550 [DOI] [PubMed] [Google Scholar]
- 28.Barnes DE, Alexopoulos GS, Lopez OL, Williamson JD, Yaffe K. Depressive Symptoms, Vascular Disease, and Mild Cognitive Impairment. Arch Gen Psychiatry [Internet]. 2006. March 1 [cited 2019 Jan 13];63(3):273 Available from: http://www.ncbi.nlm.nih.gov/pubmed/16520432 [DOI] [PubMed] [Google Scholar]
- 29.Thomas A, Kalaria RN, O’Brien JT. Depression and vascular disease: what is the relationship? J Affect Disord [Internet]. 2004. April [cited 2019 Jan 13];79(1-3):81–95. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15023483 [DOI] [PubMed] [Google Scholar]
- 30.Brommelhoff JA, Gatz M, Johansson B, McArdle JJ, Fratiglioni L, Pedersen NL. Depression as a risk factor or prodromal feature for dementia? Findings in a population-based sample of Swedish twins. Psychol Aging [Internet]. 2009. June [cited 2019 Jan 13];24(2):373–84. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19485655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Binder N, Schumacher M. Incidence of Dementia over Three Decades in the Framingham Heart Study. N Engl J Med [Internet]. 2016. July 7 [cited 2019 Jan 13];375(1):92–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27406363 [DOI] [PubMed] [Google Scholar]
- 32.Bhalla RK, Butters MA, Mulsant BH, Begley AE, Zmuda MD, Schoderbek B, et al. Persistence of Neuropsychologic Deficits in the Remitted State of Late-Life Depression. Am J Geriatr Psychiatry [Internet]. 2006. May [cited 2019 Jan 13];14(5):419–27. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16670246 [DOI] [PubMed] [Google Scholar]
- 33.Hernández-Hernández R, Silva H, Velasco M, Pellegrini F, Macchia A, Escobedo J, et al. Hypertension in seven Latin American cities: the Cardiovascular Risk Factor Multiple Evaluation in Latin America (CARMELA) study. J Hypertens [Internet]. 2010. January [cited 2019 Jan 13];28(1):24–34. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19809362 [DOI] [PubMed] [Google Scholar]
- 34.Cano-Gutierrez C, Reyes-Ortiz CA, Samper-Ternent R, Gélvez-Rueda JS, Borda MG. Prevalence and Factors Associated to Hypertension Among Older Adults in Bogotá, Colombia. J Aging Health [Internet]. 2015. September 24 [cited 2019 Jan 13];27(6):1046–65. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25804902 [DOI] [PubMed] [Google Scholar]
- 35.McLeod KJ, Jain T. Postural Hypotension and Cognitive Function in Older Adults. Gerontol Geriatr Med [Internet]. 2017. [cited 2019 Jan 21];3:2333721417733216 Available from: http://www.ncbi.nlm.nih.gov/pubmed/28979924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng S, Xanthakis V, Sullivan LM, Vasan RS. Blood Pressure Tracking Over the Adult Life Course. Hypertension [Internet]. 2012. December [cited 2019 Jan 21];60(6):1393–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23108660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cipolla MJ. The Cerebral Circulation [Internet]. The Cerebral Circulation Morgan & Claypool Life Sciences; 2009. [cited 2019 Jan 21], Available from: http://www.ncbi.nlm.nih.gov/pubmed/21452434 [PubMed] [Google Scholar]
- 38.Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet [Internet]. 2017. December 16 [cited 2019 Jan 21];390(10113):2673–734. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28735855 [DOI] [PubMed] [Google Scholar]
- 39.Fladby T, Pålhaugen L, Seines P, Waterloo K, Bråthen G, Hessen E, et al. Detecting At- Risk Alzheimer’s Disease Cases. J Alzheimer’s Dis [Internet]. 2017. August 29 [cited 2019 Jan 21];60(1):97–105. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28826181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X-J, Xu W, Li J-Q, Cao X-P, Tan L, Yu J-T. Early-Life Risk Factors for Dementia and Cognitive Impairment in Later Life: A Systematic Review and Meta-Analysis Zhu L-Q, editor. J Alzheimer’s Dis [Internet]. 2019. January 8 [cited 2019 Jan 21];67(1):221–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30636739 [DOI] [PubMed] [Google Scholar]
- 41.Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ [Internet]. 2015. March 2 [cited 2019 Jan 13];350:h369 Available from: http://www.ncbi.nlm.nih.gov/pubmed/25731881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mortby ME, Black SE, Gauthier S, Miller D, Porsteinsson A, Smith EE, et al. Dementia clinical trial implications of mild behavioral impairment. Int Psychogeriatrics [Internet]. 2018. February 16 [cited 2019 Jan 13];30(2):171–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29448970 [DOI] [PubMed] [Google Scholar]