Abstract
Study Objectives:
Studies of older and less active patients with obstructive sleep apnea (OSA) have reported decreased exercise capacity as measured by peak oxygen uptake (VO2 max) during cardiopulmonary exercise testing (CPET). We looked to determine whether VO2 max was decreased in younger patients with OSA who regularly exercise as would be encountered in the military.
Methods:
We evaluated military personnel who had undergone pulmonary function testing (PFT), CPET, and polysomnography (PSG) as part of the larger STAMPEDE III study for comprehensive evaluation of exertional dyspnea. For analysis, patients were classified into two groups, the OSA group with an apnea-hypopnea index (AHI) ≥ 15 events/h and a control group with an AHI < 15 events/h.
Results:
Mean AHI was 32.7 in the OSA group (n = 40) versus 5.8 in the control group (n = 58) with no significant difference in age (40.7 years versus 39.4 years) or body mass index (30.4 kg/m2 versus 29.9 kg/m2). PFT was normal in both groups including diffusing capacity (100.7% versus 96.5%) and FEV1 (89.2% versus 86.2%). VO2 max was not significantly different in the OSA group compared to the control group (101.3% versus 102.8%; P = .60) with both groups having normal exercise capacity. Exercise blood pressure response was normal and peak heart rate trended toward a blunted response in the OSA group (166.0 bpm versus 171.6 bpm, P = .09).
Conclusions:
Younger military personnel with moderate to severe OSA do not have decreased exercise capacity. The effect of OSA on exercise tolerance may be influenced by additional factors and is likely too small to be noted in this population.
Commentary:
A commentary on this article appears in this issue on page 819.
Citation:
Powell TA, Mysliwiec V, Aden JK, Morris MJ. Moderate to severe obstructive sleep apnea in military personnel is not associated with decreased exercise capacity. J Clin Sleep Med. 2019;15(6):823–829.
Keywords: cardiopulmonary exercise testing, obstructive sleep apnea, VO2 max
BRIEF SUMMARY
Current Knowledge/Study Rationale: Prior research in older and less active patients with moderate to severe obstructive sleep apnea (OSA) has shown reduced exercise capacity. In this study we looked to determine whether there was an exercise decrement as reflected by VO2 max in younger and more active patients with moderate to severe OSA as would typically be encountered in the military.
Study Impact: Younger and more active patients with OSA do not have decreased exercise tolerance. It is likely that the effect of OSA on exercise tolerance is influenced by additional factors and is too small to be appreciated in this population.
INTRODUCTION
Obstructive sleep apnea (OSA) is the most prevalent sleep-related breathing disorder and is viewed as primarily affecting obese middle-aged to older adult males.1 In this population, OSA is frequently comorbid with hypertension, ischemic heart disease, stroke, and atrial fibrillation.2–5 However, it is increasingly recognized that OSA is a chronic, insidious disorder that is underrecognized and begins at an earlier age.6 Studies in military personnel support this concept reporting that OSA is diagnosed at an earlier age and lower body mass index (BMI).7,8 Prior research has generally shown an association between OSA and reduced exercise tolerance, though it has predominantly involved an older OSA population. Understanding the effect of OSA on exercise tolerance in younger and more active patients would allow for a better determination of this disorder's effect on military service.
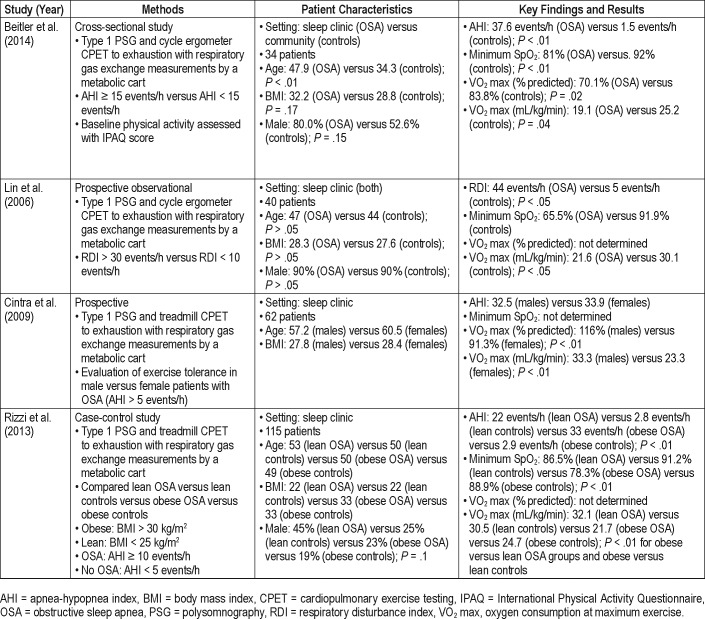
Cardiopulmonary exercise testing (CPET) provides a noninvasive assessment of cardiopulmonary fitness through measurement of various parameters to include oxygen consumption at maximum exercise (VO2 max). To date four prior studies have evaluated VO2 max in patients with moderate to severe OSA with varying results (Table 1). Studies by Beitler et al. and Lin et al.9,10 revealed significant decrements in VO2 max in patients with OSA compared to controls. Conversely, a study by Cintra et al.11 found VO2 max decrements in female but not male patients with OSA and a study by Rizzi et al.12 found no association between OSA and VO2 max. In this study we sought to determine the relationship between VO2 max and OSA in a younger and more active military population. Our hypothesis was that patients in this age category with moderate to severe OSA, as defined by an apnea-hypopnea index (AHI) ≥ 15 events/h, would have a reduced VO2 max compared to those with an AHI < 15 events/h.
Table 1.
Prior VO2 max studies in OSA.
METHODS
This study evaluated participants enrolled in the Study of Active Duty Military for Pulmonary Disease Related to Environmental Deployment Exposures (STAMPEDE) III, a prospective observational study of previously deployed military personnel with symptoms of chronic dyspnea.13 As previously described, all participants in STAMPEDE underwent pulmonary function tests, echocardiography, CPET, chest computed tomography, laryngoscopy, bronchoscopy, and if warranted by screening questionnaires (Epworth Sleepiness Scale score and STOP-BANG score), an in-laboratory polysomnography (PSG). In this study, we examined those who had undergone PSG in addition to their comprehensive pulmonary evaluation. Patients were eligible for the study if they enrolled in the STAMPEDE III study, were between 18 to 60 years of age and had complete results for pulmonary function testing (PFT), PSG, and CPET. The Army Regional Health Command-Central Institutional Review Board reviewed and approved the protocol (project approval number C.2018.007d).
For analysis purposes the patients were categorized into two groups, a moderate-to-severe OSA group with an AHI ≥ 15 events/h and a control group with an AHI < 15 events/h. Because our primary variable of interest was the patients' VO2 max, those participants in whom an underlying pulmonary disorder was diagnosed that significantly affected their CPET performance were excluded from the analysis.
Polysomnography
PSG was performed within an American Academy of Sleep Medicine (AASM) accredited laboratory (Sandman Version 9.3, Embla Systems, Broomfield, Colorado, United States) and in accordance with AASM standards. PSG was performed consisting of 16 channels, including: electrooculogram, electroencephalogram, electrocardiogram, electromyogram (submental and bilateral tibial), airflow measurements using both oronasal-thermal sensors and nasal air pressure transducers, transtracheal sounds via microphone, rib cage and abdominal movement by inductance plethysmography using thoracoabdominal belts, and continuous pulse oximetry. The studies were scored in accordance with the 2012 AASM recommended hypopnea guidelines. All PSG tests were interpreted by a board-certified sleep medicine physician.
Pulmonary Function Testing
Baseline spirometry, lung volumes, and diffusing capacity for carbon monoxide (DLCO) were performed in the Brooke Army Medical Center Pulmonary Function Laboratory using a VMax spirometer. Participants underwent a standard forced expiratory maneuver from maximal inhalation to maximal exhalation to record forced expiratory volume at 1 second (FEV1), and forced vital capacity (FVC) in accordance with American Thoracic Society standards for spirometry quality and reproducibility. The DLCO was determined using the single breath technique on the VMax spirometer and interpreted according to 1993 European Respiratory Society reference values.
Cardiopulmonary Function Testing
All participants performed a graded exercise test using a Bruce incremental protocol on the Medical Graphics Platinum Series (MCG Diagnostics, St. Paul, Minnesota, United States). All patients were encouraged to exercise until exhaustion or maintenance of 85% predicted heart rate (220-age) for 10 minutes. During the test expired gas analysis was performed through the Medical Graphics Platinum Elite Series and allowed for direct measure of oxygen uptake (VO2).
Statistical Analysis
Categorical data was summarized using percentages and analyzed using chi-square tests or Fisher exact test. Means and standard deviations or medians and interquartile ranges were used as summary statistics for continuous variables and were analyzed using t test or Wilcoxon test. Significance for results was established when value of P < .05. All statistical analysis was performed using SPSS version 22.0 (IBM Corp, Armonk, New York, United States).
RESULTS
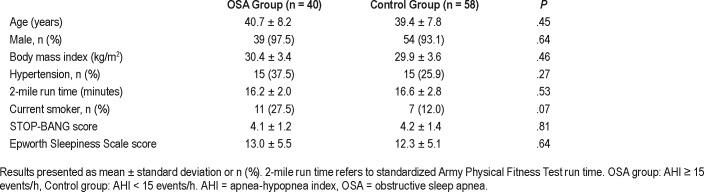
A total of 100 patients from the STAMPEDE III were evaluated in this study. Two patients from the OSA group were excluded, one for supernormal values (VO2 max > 140%) and one for subnormal exercise performance (VO2 max < 60% due to interstitial lung disease). Subsequently, our cohort included 40 patients in the OSA group (AHI ≥ 15 events/h) and 58 patients in the control group (AHI < 15 events/h). Table 2 describes the patient demographics. Overall, the two groups had similar characteristics except for their AHIs and oxygen nadir as expected for their OSA diagnoses. The patients were primarily male (97.5% versus 93.1%, P = .64) and similar in age (40.7 years versus 39.4 years; P = .45) and BMI (30.4 versus 29.9; P = .46). Regarding other comorbid disorders, the OSA group had more current smokers (27.5% versus 12.0%) near statistical significance and the rates of hypertension were similar between groups. Airway hyperreactivity or asthma was diagnosed in 7/40 (18%) in the OSA group and 10/58 (17%) during their evaluation in the STAMPEDE study. Given their normal CPET performance these patients were included in our analysis.
Table 2.
Baseline demographics.
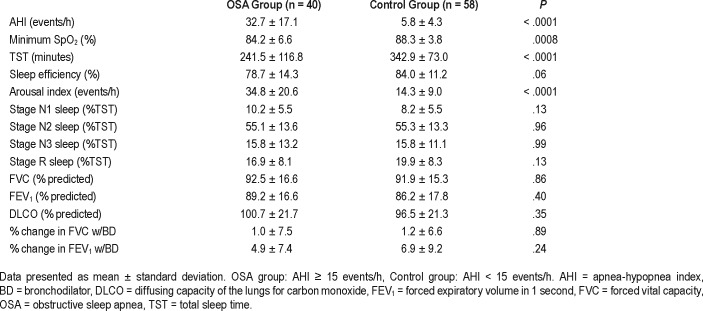
Results for PSG and PFT are displayed in Table 3. As expected, the AHI was significantly higher (32.7 versus 5.8 events/h; P < .0001) and pulse oximetry (SpO2) minimum was significantly lower (84% versus 88%; P = .0008) in the OSA group. Regarding other PSG variables, total sleep time was significantly lower (243.3 versus 324.3 minutes, P < .0001) and sleep efficiency trended lower in the OSA group though did not reach statistical significance (P = .052). Otherwise, percentages of sleep stages were similar in both groups.
Table 3.
Polysomnography and pulmonary function testing data.
Pulmonary function testing was normal in both groups to include FVC, FEV1, and DLCO (92.5% versus 91.1%, P = .86; 89.2% versus 86.2%, P = .40; and 100.7% versus 96.5%, P = .35 respectively). There was no significant change in FVC or FEV1 in either group with bronchodilator (1.0% versus 1.1%, P = .93, 4.9% versus 6.9%, P = .24).
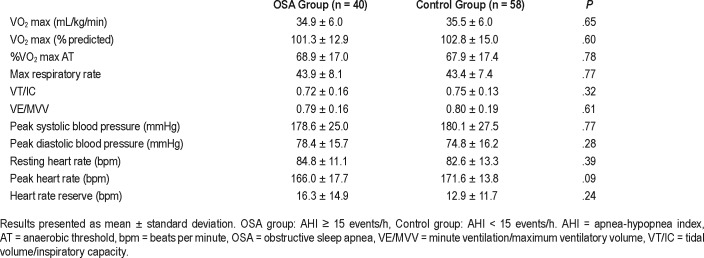
Cardiopulmonary exercise testing was not different between the OSA and control groups. There was no difference in weight-adjusted VO2 max (34.9 versus 35.5 mL/kg/min, P = .65) or percent predicted VO2 max (101.3% versus 102.8%, P = .60) between the OSA and control groups respectively. Box plots comparing percent predicted VO2 max are displayed in Figure 1. Peak heart rate with exercise trended toward a lower value in the OSA group compared to controls, but did not reach significance (166 versus 171.6 bpm, P = .09). Other exercise-related parameters to include peak systolic and diastolic blood pressure, peak heart rate, and heart rate reserve (as defined as the difference between maximum predicted and maximum observed heart rates) were also similar between groups and are displayed in Table 4. Furthermore, the reasons for CPET termination did not differ. Maximum respiratory rate with exercise > 50 breaths/min, VT/IC (tidal volume/ inspiratory capacity) > 0.80, and VE/MVV (minute ventilation/maximum ventilatory volume) > 0.84 are all indicators of pulmonary limitation to exercise.14–16 These variables were similar and below these respective thresholds in both groups (43.9 versus 43.4, P = .77; 0.72 versus 0.75, P = .32; 0.79 versus 0.80, P = .61).
Figure 1. Percent predicted VO2 max.
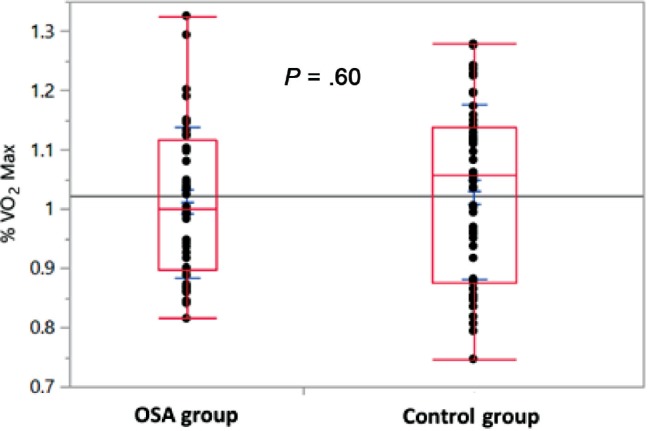
Interquartile ranges and medians (boxes), maximum and minimum observed values (whiskers), and individual values (dots) of percent predicted VO2 max for OSA and control groups. OSA group: AHI ≥ 15 events/h, Control group: AHI < 15 events/h. AHI = apnea-hypopnea index, OSA = obstructive sleep apnea.
Table 4.
Cardiopulmonary exercise testing.
To account for the effect of more pronounced oxygen desaturation, a subgroup analysis was performed on those patients with AHI ≥ 15 events/h and SpO2 nadir less than 80%. In comparing this subgroup of 9 patients (average AHI 31.2 events/h and SpO2 nadir 75%) to control patients there was no difference in weight-adjusted or percent predicted VO2 max noted (34.2 ± 5.3 versus 35.5 ± 6.0 mL/kg/min, P = .81; 101.2 ± 8.9% versus 102.8 ± 14.8%, P = .94). Peak heart rate with exercise again trended toward lower in the OSA group, but did not reach significance (160 ± 15 versus 172 ± 14 bpm, P = .07). Peak systolic and diastolic blood pressure were not significantly different (172 ± 27 versus 178 ± 27 mmHg, P = .79; 81 ± 13 versus 75 ± 16 mmHg, P = .49). Further, in reanalyzing the data using an AHI ≥ 5 events/h for the OSA group (70 patients, AHI 23.2 events/h) and AHI < 5 events/h for control patients (28 patients, AHI 2.2 events/h), similar VO2 max results were again appreciated (102.5 ± 14.5% versus 102.1 ± 13.1%, P = .92). Other exercise variables including peak systolic and diastolic blood pressure and peak heart rate with exercise were not significantly different between groups (178 ± 24 versus 180 ± 31 mmHg, P = .72; 76 ± 15 versus 77 ± 18 mmHg, P = .80; 169 ± 17 versus 171 ± 13 bpm, P = .60).
DISCUSSION
The primary finding of this study is that younger individuals with untreated moderate to severe OSA as defined by an AHI ≥ 15 events/h have a similar VO2 max (34.9 versus 35.5 mL/kg/min, P = .65) compared to those with an AHI < 15 events/h. This finding may suggest that the negative effects of OSA on exercise performance have not yet developed at this younger age. The potential mechanism for the development of exercise intolerance in older, but not younger patients with OSA remains undefined, though prior research would support an abnormal cardiovascular response to exercise as the most likely etiology. This is supported by prior research showing a strong correlation between reduced VO2 max and the presence of cardiovascular dysfunction.17–19 Given the normal cardiovascular response to exercise in our cohort, it would appear that these physiologic changes had not yet developed, potentially explaining their normal exercise tolerance.
As noted previously, prior studies assessing VO2 max in patients with OSA have had variable results. Given methodological limitations in matching and comorbid medical disorders in these prior studies, we acknowledge that our finding of normal exercise tolerance in a well-matched OSA cohort without significant comorbidities may simply support that OSA does not independently affect exercise tolerance. Alternatively, the younger age of our cohort could suggest that it is the age of diagnosis that dictates how OSA affects exercise tolerance. The two prior studies showing a reduced VO2 max in OSA cohorts had mean ages of 47 and 48 years compared to 40 years in our study.9,10 It may be these additional untreated OSA years that lead to the development of adverse exercise effects, which we postulate are cardiovascular in etiology. Previous studies in older patients with OSA have reported numerous abnormal cardiovascular responses to exercise, with an exaggerated peak blood pressure and blunted peak heart rate as the most consistent findings.20–31 Peak systolic and diastolic blood pressure in our OSA cohort were not significantly different from that in the control group and although there was a trend toward a blunted heart rate response in the OSA group, this finding did not reach significance. Therefore, we suggest that it may be the development of this abnormal cardiovascular response after additional untreated years that contributes to the exercise tolerance noted in older OSA cohorts, a theory that requires further research to define. Given that prior studies in older cohorts have not shown a consistent benefit of PAP therapy on cardiovascular dysfunction, further elucidating this relationship could have therapeutic implications.32–34
Another potential explanation for our findings is that our cohort represents a less severe phenotype of OSA that would not be associated with exercise intolerance. In the study by Lin et al. showing a reduced VO2 max in patients with newly diagnosed OSA, mean SpO2 nadir was 65.5% in the OSA group compared to 84.2% in our study.10 Thus, the degree of hypoxia and not necessarily the AHI may be a greater factor regarding exercise tolerance. To account for this we performed a subgroup analysis of those with an AHI ≥ 15 events/h and SpO2 nadir less than 80%, which again revealed normal exercise capacity and cardiovascular response. Evaluating the effect of oxygen desaturation index, rather than AHI and SpO2 nadir, on VO2 max in future research would be recommended.
Last, the absence of a VO2 max decrement in our OSA cohort may also be explained by increased physical activity. Of the four previous studies of VO2 max, only the study by Beitler et al.9 assessed physical activity. In that study, exercise tolerance was poor in both groups with a VO2 max of only 83% in the control group, making regular aerobic exercise unlikely. The International Physical Activity Questionnaire used in that study requires a minimum of 3000 metabolic equivalent-min/ wk for a patient to be considered “highly active.” Mean scores were 2341 and 3936 metabolic equivalent-min/wk in the OSA group and controls respectively, suggesting the controls were more active at baseline.9,35 In the military, aerobic exercise is a standard requirement and this is substantiated by the similar and adequate 2-mile run times in our patients (which is performed biannually). It is possible that regular aerobic exercise in patients with OSA helps prevent the exercise limitations that have been previously reported. Supporting this theory are prior studies that demonstrate an aerobic exercise training regimen can improve OSA severity and decrease AHI, a finding that persists only if exercise is continued.36,37 Unfortunately, regular exercise is likely not characteristic of patients with OSA, which is supported by a prior study showing reduced physical activity in obese patients with OSA.38
In comparing our study we noted the following aspects that make it unique. First, all participants underwent a comprehensive pulmonary evaluation. Similar and normal PFTs and CPET pulmonary variables in both groups helped eliminate the possibility of significant pulmonary disease affecting the results. Second, the groups in our study were well matched for age (40.7 years versus 39.4 years, P = .45), an area that limited the study by Beitler et al (47.9 years versus 34.4 years, P < .01). Last, although significant variability exists regarding reference values for VO2 max, the results of our study are closer to expected than in prior studies. In the study by Lin et al. showing a decrement in exercise capacity in patients with OSA, VO2 max was only 30.1 mL/kg/min in controls despite having a mean age of 44 years. Similarly, control patients in the study by Beitler et al. with a mean age of 34 years had a VO2 max of only 25.2 mL/kg/min.9,10 In our study, control patients with a mean age of 39 years had a VO2 max of 35.5 mL/kg/min, which is closer to reference values for this population.39
The main limitation of our study is that all participants in the study complained of exertional dyspnea. To minimize this potential limitation, we performed comprehensive pulmonary evaluations in both groups to help eliminate confounding pulmonary disease. Normal spirometry and bronchodilator response in both groups make significant pulmonary disease unlikely. Additionally, we acknowledge that the BMI of our cohort might suggest that they were not physically fit, though performance on the 2-mile run test would support that they were exercising regularly and maintaining a fitness level above the general population. Further, it may be that percent predicted VO2 max is a poor discriminator of exercise decrements in a cohort with baseline good physical fitness. Therefore, evaluation of younger patients with OSA who do not regularly exercise may help define this in the future. Finally, our study was predominantly male and therefore we cannot reliably extrapolate these findings to female patients.
In this study we demonstrate that younger patients with moderate to severe OSA have normal exercise capacity. In the context of prior studies with variable findings, our study likely suggests that the relationship between OSA and exercise tolerance is in fact more complex. It is likely that it is not simply AHI, but rather multiple OSA-related factors including age at diagnosis, severity of desaturations, and baseline physical activity which influence how OSA effects exercise tolerance. We recommend that future research seek to better evaluate the extent to which each of these variables independently effects exercise tolerance in OSA.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. Dr. Morris is a paid speaker for Janssen Pharmaceuticals and Vyaire Medical. There are no conflicts of interest for the other authors or funding to disclose for this study. This study was presented as a poster abstract at Sleep 2018 in Baltimore, Maryland on 3 June 2018.
ACKNOWLEDGMENTS
Author contributions: Dr. Tyler Powell had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. Drs. Tyler Powell, Vincent Mysliwiec, and Michael Morris contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript. Dr. James Aden contributed substantially to the data analysis and interpretation. Dr. Morris is a paid speaker for Janssen Pharmaceuticals and Vyaire Medical. There are no conflicts of interest for the other authors or funding to disclose for this study.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- BMI
body mass index
- CPET
cardiopulmonary exercise testing
- DLCO
diffusing capacity for carbon monoxide
- FEV1
forced expiratory volume in one second
- FVC
forced vital capacity
- OSA
obstructive sleep apnea
- PFT
pulmonary function testing
- PSG
polysomnography
- STAMPEDE
Study of Active Duty Military for Pulmonary Disease Related to Environmental Deployment Exposures
- VO2 max
oxygen consumption at maximum exercise
REFERENCES
- 1.Senaratna C, Perret J, Lodge C, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70–81. doi: 10.1016/j.smrv.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Peppard P, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 3.Bradley T, Floras J. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373(9657):82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 4.Mehra R, Benjamin E, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173(8):910–916. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takama N, Kurabayashi M. Influence of untreated sleep-disordered breathing on the long-term prognosis of patients with cardiovascular disease. Am J Cardiol. 2009;103(5):730–734. doi: 10.1016/j.amjcard.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 6.Korson R, Guilleminault C. Obstructive sleep apnea syndrome. In: Chokroverty S, editor. Sleep Disorders Medicine. 4th ed. New York, NY: Springer; 2017. pp. 567–596. [Google Scholar]
- 7.Lettieri C, Eliasson A, Andrada T, Khramtsov A, Raphaelson M, Kristo D. Obstructive sleep apnea syndrome: are we missing an at-risk population? J Clin Sleep Med. 2005;1(4):381–385. [PubMed] [Google Scholar]
- 8.Mysliwiec V, McGraw L, Pierce R, Smith P, Trapp B, Roth B. Sleep disorders and associated medical comorbidities in active duty military personnel. Sleep. 2013;36(2):167–174. doi: 10.5665/sleep.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beitler J, Awad K, Bakker J, et al. Obstructive sleep apnea is associated with impaired exercise capacity: a cross-sectional study. J Clin Sleep Med. 2014;10(11):1199–1204. doi: 10.5664/jcsm.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin C, Hsieh W, Chou C, Liaw S. Cardiopulmonary exercise testing in obstructive sleep apnea syndrome. Respir Physiol Neurobiol. 2006;150(1):27–34. doi: 10.1016/j.resp.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Cintra F, Poyares D, Rizzi CF, et al. Cardiorespiratory response to exercise in men and women with obstructive sleep apnea. Sleep Med. 2009;10(3):368–373. doi: 10.1016/j.sleep.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Rizzi CF, Cintra F, Mello-Fujita, et al. Does obstructive sleep apnea impair the cardiopulmonary response to exercise? Sleep. 2013;36(4):547–553. doi: 10.5665/sleep.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris M, Dodson D, Lucero P, et al. Study of active duty military for pulmonary disease related to environmental deployment exposures (STAMPEDE) Am J Respir Crit Care Med. 2014;190(1):77–84. doi: 10.1164/rccm.201402-0372OC. [DOI] [PubMed] [Google Scholar]
- 14.Ross R. ATS/ ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167(2):211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 15.Weisman I, Zeballos R. An integrated approach to the interpretation of cardiopulmonary exercise testing. Clin Chest Med. 1994;15(2):421–445. [PubMed] [Google Scholar]
- 16.Johnson B, Weisman I, Zeballos R, Beck K. Emerging concepts in the evaluation of ventilatory limitation during exercise. Chest. 1998;116(2):487–503. doi: 10.1378/chest.116.2.488. [DOI] [PubMed] [Google Scholar]
- 17.Balady G, Arena R, Sietsema K, Myers J, Coke L, Milani R. Clinician's guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122(2):191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 18.Ross R, Blair S, Arena R, et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign. Circulation. 2016;134(24):e653–e699. doi: 10.1161/CIR.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 19.Guazzi M, Bandera F, Ozemek C, Systrom D, Arena R. Cardiopulmonary exercise testing: what is its value? J Am Coll Cardiol. 2017;70(13):1618–1636. doi: 10.1016/j.jacc.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Wahlin Larsson B, Kadi F, Ulfberg J, Piehl Aulin K. Skeletal muscle morphology and aerobic capacity in patients with obstructive sleep apnea syndrome. Respiration. 2008;76(1):21–27. doi: 10.1159/000126492. [DOI] [PubMed] [Google Scholar]
- 21.Sauleda J, Garcia-Palmer F, Tarraga S, Maimo A, Palou A, Agusti A. Skeletal muscle changes in patients with obstructive sleep apnea syndrome. Respir Med. 2003;97(7):804–810. doi: 10.1016/s0954-6111(03)00034-9. [DOI] [PubMed] [Google Scholar]
- 22.Aihara K, Oga T, Yoshimura C, et al. Measurement of dyspnea in patients with obstructive sleep apnea. Sleep Breath. 2013;17(2):753–761. doi: 10.1007/s11325-012-0759-2. [DOI] [PubMed] [Google Scholar]
- 23.Tryfon S, Stanopoulos I, Dascalopoulou E, Argyropoulou P, Bouros D, Mavrofridis E. Sleep apnea syndrome and diastolic blood pressure elevation during exercise. Respiration. 2004;71(5):499–504. doi: 10.1159/000080635. [DOI] [PubMed] [Google Scholar]
- 24.Kasiakogias A, Tsioufis C, Thomopoulos C, et al. A hypertensive response to exercise is prominent in patients with obstructive sleep apnea and hypertension: a controlled study. J Clin Hypertens (Greenwich) 2013;15(7):497–502. doi: 10.1111/jch.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaleth A, Chittenden T, Hawkins B, et al. Unique cardiopulmonary exercise test responses in overweight middle-aged adults with obstructive sleep apnea. Sleep Med. 2007;8(2):160–168. doi: 10.1016/j.sleep.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Hargens T, Guill S, Zedalis D, Gregg JM, Nickols-Richardson S, Herbert W. Attenuated heart rate recovery following exercise testing in overweight young men with untreated obstructive sleep apnea. Sleep. 2008;31(1):104–110. doi: 10.1093/sleep/31.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nanas S, Sakellariou D, Kapsimalakou S, et al. Heart rate recovery and oxygen kinetics after exercise in obstructive sleep apnea syndrome. Clin Cardiol. 2010;33(1):46–51. doi: 10.1002/clc.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chien M, Lee P, Tsai Y, Yang P, Wu Y. C-reactive protein and heart rate recovery in middle-aged men with severe obstructive sleep apnea. Sleep Breath. 2012;16(3):629–637. doi: 10.1007/s11325-011-0549-2. [DOI] [PubMed] [Google Scholar]
- 29.Mansukhani M, Allison T, Lopez-Jimenez F, Somers V, Caples S. Functional aerobic capacity in patients with sleep-disordered breathing. Am J Cardiol. 2013;111:1650–1654. doi: 10.1016/j.amjcard.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cholidou K, Manali E, Kapsimalis F, et al. Heart rate recovery post 6-minute walking test in obstructive sleep apnea. Clin Res Cardiol. 2014;103(10):805–815. doi: 10.1007/s00392-014-0721-3. [DOI] [PubMed] [Google Scholar]
- 31.Bonanni E, Pasquali L, Manca ML, et al. Lactate production and catecholamine profile during aerobic exercise in normotensive OSAS patients. Sleep Med. 2004;5(2):137–145. doi: 10.1016/j.sleep.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Garcia M, Capote F, Campos-Rodriguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310(22):2407–2415. doi: 10.1001/jama.2013.281250. [DOI] [PubMed] [Google Scholar]
- 33.Pengo M, Ratneswaran C, Berry M, et al. Effect of continuous positive airway pressure on blood pressure variability in patients with obstructive sleep apnea. J Clin Hypertens (Greenwich) 2016;18(11):1180–1184. doi: 10.1111/jch.12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McEvoy R, Antic N, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 35.Craig C, Marshall A, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 36.Kline C, Crowley E, Ewing G, et al. The effect of exercise training on obstructive sleep apnea and sleep quality: a randomized controlled trial. Sleep. 2011;34(12):1631–1640. doi: 10.5665/sleep.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sengul Y, Ozalevli S, Oztura I, Itil O, Baklan B. The effect of exercise on obstructive sleep apnea: a randomized and controlled trial. Sleep Breath. 2011;15(1):49–56. doi: 10.1007/s11325-009-0311-1. [DOI] [PubMed] [Google Scholar]
- 38.Vivodtzev I, Mendelson M, Croteau M, et al. Physiological correlates to spontaneous physical activity variability in obese patients with already treated sleep apnea syndrome. Sleep Breath. 2017;21(1):61–68. doi: 10.1007/s11325-016-1368-2. [DOI] [PubMed] [Google Scholar]
- 39.American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. 9th ed. Philadelphia, PA: Wolters Kluwer-Lippincott Williams & Wilkins; 2014. [Google Scholar]