Abstract
Salinity of water and soil are of the most important factors limiting the production of crops. Moreover, with the increasing population of the planet and saline fields worldwide there is no choice but to use saline soil and water in the near future. Therefore, to increase plant growth under saline stress condition, provision of sustainable and environmentally friendly management for the use of saline water and soil resources is necessary. The development of saline resistant plants is a potent approach to solve this problem. Generally, soil salinity negatively affects the plant growth through ion toxicity, oxidative stress, osmotic stress and ethylene generation. In recent years, scientists through genetic engineering techniques, which are based on molecular and physiological characteristics of plants, have made salt tolerance plants. However, the validation of the present technique is restricted to laboratory condition and it is not easily applied in the agronomy research under field environment. Another option would be to isolate and utilize salinity resistant microorganisms from the rhizosphere of halophyte plants, namely plant growth-promoting rhizobacteria (PGPR). The mechanisms of these bacteria includes; ACC-deaminase and exopolysachared production, osmolite accumulation, antioxidant system activation, ion hemostasis and etc. In this review, we will discuss mechanisms of PGPR in producing tolerate plants under salt stress and how to improve the plant–microbe interactions in future for increasing agricultural productivity to feed all of the world’s people.
Keywords: Salinity stress, Plant growth promoting rhizobacteria (PGPR), Plant–microbe interactions, Salt tolerance plants
Introduction
The world’s population is growing and food insecurity is an important issue. The global demand for food is undeniable, and this is one of the major problems of developing countries. Hence, population growth and declining land are threatening for sustainable agriculture (Shahbaz and Ashraf 2013). The world’s population is 7 billion, and it is expected to reach 10 billion in 2070 (Goswami et al. 2016). Increase in demand for food causes the increase of cultivated land to produce more for the growing population. Increasing the cultivated area to grow more crops is partially impossible due to increasing pollution/wastes, urbanization, industrialization, soil erosion and limited access to sustainable water. Therefore, increasing agricultural productivity is the only possible way for the coming years (Vaishnav et al. 2017).
To increase the yield of agricultural products, the following interventions are suggested: (1) better management of agricultural lands; (2) excessive use of chemical fertilizers; (3) use of plant growth regulators (PGRs); (4) use of effective herbicides and pesticides; (5) to mechanize the agricultural practices; (6) cultivating stress-resistant crops; (7) developing the cultivation of genetic modified (GM) crops; (8) developing the plant growth-promoting rhizobacteria (PGPR) (Glick 2014; Paul and Lade 2014; Ghorbanpour et al. 2013). Some of the above solutions are not efficient and cost-effective in the long run. Since our environment has limited resources, so any long-term effective approach to cultivate food must be sustainable and environmentally friendly.
In addition, agricultural productivity is reduced due to environmental stresses such as salinity, drought, pH and temperature (Ladeiro 2012). Up to date, more than 800 million hectares (6% of the world’s total land) is affected by salinity (FAO 2008). So, soil salinity is a very important problem and more attention should be paid to the issue because it immediately inhibits growth and yield of plants (Yan et al. 2013). Plants based on the ability to grow in saline soils are classified into two types named glycophytes and halophytes. Some of the halophytes survive through mechanisms such as excluding salts from roots and shoots, compartment of ion in organs, synthesize of the compatible solute adopted under salinity stress. However, the most of plants are grouped in glycophytes class. Thus, for overcoming this problem for a long run, plant breeding, and genetic engineering methods are applied to increase the crop yield, but they are expensive, and rarely field-practical. Moreover, engineered plants cannot be grown in different environments (Tang et al. 2014; Imam et al. 2016).
Recently, the use of plant growth-promoting rhizobacteria (PGPR) as an effective tool for agricultural operations has attracted considerable attention (Goswami et al. 2016). The rhizosphere of halophyte plants is a rich source of osmotic stress tolerant bacteria and application of these bacteria can affect the growth and yield of plants under stress positively (Jha and Saraf 2015). Rhizospheric bacteria of plants grown under ultra-saline ecosystems were investigated by Mapelli et al. (2013). The results showed that these bacteria were resistant to most environmental stresses and were capable of inducing plant growth root colonization. It means that halophyte bacteria living in saline and dry ecosystems have the potential to stimulate plant growth under stress conditions.
These bacteria can increase the growth of different plants, enhance nitrogen fixation, produce hormones such as auxin, solubilize insoluble compounds phosphorus, potassium, zinc and silicon (Vaishnav et al. 2017; Ghorbanpour et al. 2016), improve the absorption of nutrients with changes in root shape, produce Siderophores to meet the iron needs under iron deficiency conditions, and reduce the negative effects of ethylene production in stress conditions through the production of ACC-deaminase (Glick 2014; Choudhary et al. 2015), control the plant pathogenic agents through the production of HCN, chitinase, glucanase (Goswami et al. 2016), control the biotic and abiotic stress by volatile organic compounds (VOCs) production (Tyagia et al. 2018) and induce systemic resistance (ISR) in the plants (Chaudhary and Shukla 2019), produce exopolysaccharides (EPSs) under salinity conditions (Choudhary et al. 2015), which chelate excessive amount of sodium ion around roots and increase root-soli stick and regulate water movement and facilitate root growth (Kim et al. 2013; Timmusk et al. 2014). Therefore, screening and isolating PGPRs and exploiting their ability to stimulate plant growth under stress conditions is a much needed research area.
Salinity stress
Hall (2001) stated that environmental stresses in natural ecosystems are any external limiting factor, which does not allow the plant’s productivity reaches real genetic potential. Today’s, increasing demand for plant products has been coupled with decreasing cultivated land because of limited water and soil resources, soil erosion, and so on.
A soil is considered saline when the electrical conductivity (saturated extract EC) is more than 4 dS m−1 (approximately 40 mM sodium chloride). This level of salt is critical to start reducing the productivity of many crops (Jamil et al. 2011). Various types of salts, such as sodium chloride (NaCl), sodium sulfate (Na2SO4), magnesium sulfate (MgSO4), magnesium chloride (MgCl2), are present in saline soils. However, NaCl is more damaging for plants than others (Jamil et al. 2011). Salinity, indeed, is the accumulation of water-soluble salts, which included K+, Mg2+, Ca2+, Cl−, SO42−, CO32−, HCO3−, and Na+ ions. Depending on the soil, the concentration of soluble salts is different (Vaishnav et al. 2017). Mineralization is the main source of soils salinity. Human activities such as irrigation of plants with saline water or evaporation of groundwater (due to inadequate precipitation, ions are not leached from the soil profile and the result is saline soil) are some other sources of soils salinity (Vaishnav et al. 2017). It is estimated that 20% of the total cultivated land and 33% of the irrigated agricultural lands around the world are affected by salinity. If this trend continues, 50% of the cultivated land will be saline by 2050 (Vaishnav et al. 2017).
Impact of salinity on plant growth
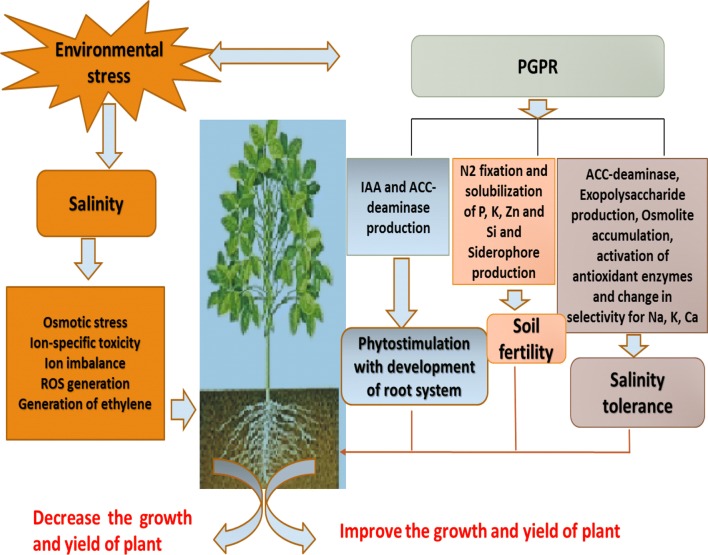
Salinity reduces growth and development by changing the biochemical and physiological processes of the plant such as leaf growth, root growth, water absorption, stomatal conductance and photosynthesis (Patil 2013). Among the above mentioned index, photosynthesis is closely related to plant growth and yield. This index includes the parts of CO2 that is assimilated, photosynthetic electron and proton transport rate (Tang et al. 2014). The presence of salts in the root zone creates osmotic (water deficiency) and ion stresses for plants (Paul and Lade 2014). Consequently, some processes such as photosynthesis and cell division are affected under stressful condition (Yan et al. 2013). Salt stress by reducing stomatal and mesophyll conductance and CO2 availability, decreases the photosynthetic activity. Moreover, salt stress indirectly can affect photosynthesis by reducing chlorophyll and total carotenoid content. The photosynthesis rate is measured by weighing gas exchange and chlorophyll a fluorescence (Yan et al. 2013; Tang et al. 2014). Some plants change the photosynthetic pathway to cope with salinity stress. For example, Mesembryanthemum crystallinum by transforming from C3 pathway to CAM adapts to water deficiency in saline condition. Atriplex lentiformis by shifting from C3 to C4 to cope with the salt stress (Tang et al. 2014). Gu et al. (2016) stated that the ratio of root biomass to shoot biomass is an indicator of plants tolerance to salinity stress. The greater value of this ratio, the higher resistance of plants to stress. Also, high concentrations of sodium and chloride, lead to ion-specific toxicity and ion imbalance (Gu et al. 2016). Under these conditions, the absorption of K+, NO3, PO42−, Ca 2+, Mg2+ and consequently, the ratios of K+/Na+, Ca2+/Na+, Mg2+/Na+, PO42−/Cl− and NO3−/Cl− decreases (Tester and Davenport 2003; Gu et al. 2016). In addition, the production of reactive oxygen species (ROS) (Yan et al. 2013; Tang et al. 2014) and the concentration of ethylene are increased under salinity stress condition (Patil 2013) (Fig. 1).
Fig. 1.
The scenario of salinity stress and PGPR interactions in plant growth
Development of salinity resistant plants
Different strategies are used to develop varieties that are resistant to salinity and economically valuable. Conventional plant breeding methods, molecular methods (genetic engineering) and biological methods (biofertilizers) are some of these strategies (Vaishnav et al. 2017).
The conventional methods are money and time consuming and (Agarwal et al. 2013a, b). Also, effective conduction of these methods is hard due to the complexity of genes’ function involved in salt stress (Yan et al. 2013). Biotechnologists use genetic engineering (such as gene transfer) to increase resistant plants. This technique relied on the exploration of new genes involved in salt-tolerance halophytes. A number of genes related to salinity response have been transferred to plants to create resistant varieties. These genes are involved in various types of processes, such as the diffusion of toxic ions in vacuole, the induction of antioxidant enzymes, the synthesis of new proteins and the accumulation of compatible soluble substances under salinity stress (Ashraf and Akram 2009). However, the genetic engineering technique has not been successful because these results often were obtained under laboratory condition and not in field (Yan et al. 2013). Salinity resistant plants also can be produced through pretreatment with specific chemicals. Nitric oxide (NO), K+ and Ca2+, NaCl pretreatment, ascorbic acid, H2O2, ethylene, paraquat and glutamate, phosphorus and humic acid, silicon, sugars, hydrogen sulfide (H2S), proline, glycine betaine (GB), jasmonates (JA) and salicylic (SA) acid are some of these chemicals (Ben Rejeb et al. 2013; Yan et al. 2013). However, these are expensive and will create environmental problems in long time. Therefore, the use of chemicals as a cost-effective method in sustainable agriculture is not recommended. Other option is the use of plant growth promoters (PGRs). This is a good way to increase agricultural productivity in saline areas. Today, bacteria are used to modify stressful conditions in agriculture (Shrivastava and Kumar, 2015).
Plant growth promoting rhizobacteria (PGPR)
Various species of bacteria such as Pseudomonas, Azospirillium, Azotobacter, Klebsiella, Enterobacter, Alcaligenesis, Arthrobacter, Burkholderia, Bacillus, Sarcina, Irvinia, Flavobacterium and Rhizobium have been reported as PGPR (Egamberdiyeva 2005). These beneficial rhizobacteria are characterized by three intrinsic properties: (1) they are able to survive and tolerate soil environment; (2) they acquire the ability to compete with other micro-organisms through root colonization, growth and reproduction in root surface; (3) they are able to increase the growth rate of plants by growth promoting traits generation (Ahemad and Khan 2011). It was reported that Azopsirillum halopraeferens bacteria colonized mangrove plant roots in saline water, with the ability to tolerate 3% sodium chloride, and increased the growth of the plants irrigated with saline water (sea water) (Ramadoss et al. 2013). Similarly, Some Bacilli strains with 8% sodium chloride tolerance show growth stimulation. Mayak et al. (2004) reported that salinity resistant ACC-deaminase mediating bacteria moderated the negative effects of stress in plants through inducing plant growth stimulation. Salinity resistant bacteria not only can grow in the range of 1–33% sodium chloride but also can grow in the absence of sodium chloride (Ramadoss et al. 2013). Therefore, isolated bacteria from saline habitats can be good candidates to help ameliorate the negative effects of salinity stress in plants grown under stress conditions. It has been shown that bacteria secrete extracellular polymers, exopolysaccharide, into the environment (Tisdall 1994). These exopolysaccharides which called biofilms involved in the binding of bacteria to surfaces (Mah and O’Toole 2001). Biofilm is a complex of bacterial cells that can be linked to different living and non-living surfaces. It not only protects the microbe from the environmental stresses but also maintains moisture and protects the plants’ roots against pathogens physically and functionally (Minah and Hazarin Subair 2015). The association of rhizobacteria with the roots of plants is possible through the production of these biological polymers. Also, by growing and reproducing in the root surface micro space, they can compete with the native microflora of the soil. Exopolysaccharides have also been reported that play a role in the mobility of bacteria to compete with the native microflora of the soil (Liu et al. 2017).
PGPR mechanisms for increasing plant tolerance to salinity stress
PGPRs are involved in improving plants growth and tolerance to salinity stress through accumulating osmolytes, ion homeostasis, improvement of nutrient uptakes (N2 fixation, solubilizing of P, K, Zn and Si), producting of ACC deaminase, IAA, siderophere and exopolysaccharides, and changing in the antioxidant defense system (Fig. 1, Table 1)
Table 1.
Mechanisms of salt stress tolerance in plants inoculated by halotolerant bacteria
| PGPR | Crop | Mechanism | Results of inoculation | References |
|---|---|---|---|---|
| Bacterial strains (KS 8 and KS28) | Helianthus annus L. | ACC deaminase production | Increased growth parameters (plant height, shoot dry weight and root dry weight), phosphorus, potassium contents and K+/Na+ ratio in shoot | Kiani et al. (2015) |
| Bacillus sp. (EN1), Zhihengliuella halotolerans (EN3), Bacillus sp. (EN5), Bacillus gibsonii (EN6 and EN10), Oceanobacillus oncorhynchi (EN8), Zhihengliuella sp.(EN12), and Halomonas sp. (IA) | Triticum aestivum L. | Ammonia, IAA and ACC deaminase production, phosphate solubilizing and nitrogen fixation | Increased the root and shoot length and total fresh weight of the plants | Orhan (2016) |
| Paenibacillus xylanexedens and Enterobacter cloacae | Brassica campestris L. | IAA and ACC deaminase production | Enhanced the root elongation | Yaish et al. (2015) |
| Pseodomonas sp (S-49) and Enterobacter cloacae (R-10) | Triticum aestivum L. | potassium and zinc solubilizing | Enhanced dry matter of wheat and K concentration | Pirhadi et al. (2016) |
| Bacillus subtilis | Bassia indica L. | IAA and ACC deaminase production | Improved root and shoot growth, total lipid content, the phospholipid fraction, photosynthetic pigments | Abeer et al. (2015) |
| Halomonas variabilis (HT1) and Planococcus rifietoensis (RT4) | Cicer arietinum Var. CM-98 | Exopolysaccharide production | Enhanced the plant growth and soil aggregation | Qurashi and Sabri (2012) |
| Pseudomonas sp. strain AK-1 | Glycine max L. | Exopolysaccharide production | Rise in shoot/root length, number of lateral roots, shoot/root fresh weight and decreased Na +/K + ratio, binding of free Na from soil and makes Na unavailable to uptake | Kasotia et al. (2016) |
| Azotobacter chrococcum | Zea mays L. | Exopolysaccharide production | Increased root and shoot dry weights, chlorophyll a, b and carotenoids contents, restricted the uptake of Na and Cl and enhanced accumulation of N, P and K in shoot | Nemat et al. (2012) |
| Hallobacillus sp. SL3 | Triticum aestivum L. | IAA production and Siderophore production | Increased root elongation and dry weight | Ramadoss et al. (2013) |
| Bacillus halodenitrificans PU62 | Triticum aestivum L. | Phosphate solubilizing and SiderophoreProduction | Increased root elongation and dry weight | Ramadoss et al. (2013) |
| Bacillus megaterium | Oryza sativa L. | IAA production | Stimulated the growth of rice’s roots and dry biomass | Nguyen et al. (2017) |
|
Pseudomonas pseudoalcaligenes ST1, Bacillus pumilus ST2 |
Oryza sativa L. | Anti-oxidative activity | Enhanced the plant growth by ROS scavenging and higher accumulation of osmoprotectant | Jha et al. (2011) |
|
Bacillus pumilus STR2, Exiguobacterium oxidotolerens STR36 |
Bacopa monneri L. | Mixture of PGPs trait | High proline/lipid content peroxidation | Bharti et al. (2013) |
|
Burkholderia phytofirmans PsJN, Enterobacter sp. FD 17 |
Zea Mays L. | Mixture of PGPs trait | Decreased xylem Na concentration/maintain nutrient balance within the plant | Akhtar et al. (2015) |
| Pseudomonas putida, Pseudomonas fluorescens, Bacillus subtilis | Vicia faba L. | Mixture of PGPs trait | Modulated plant chlorophyll, protein and proline content | Metwali et al. (2015) |
|
Achromobacter xylosoxidans AUM54 |
Catharanthus Roseus L. | ACC deaminase production and nitrogen fixation | Decreased stress ethylene level, ascorbate peroxidase, superoxide dismutase, catalase | Karthikeyan et al. (2012) |
| Acinetobacter sp. ACMS25, Bacillus sp. PVMX4 | Phyllanthus Amarus L. | phosphate solubilizing | Improved anti-oxidative defense system | Joe et al. (2016) |
|
Sinorhizobium mellilote and Rhizobium legominozaroum b.v phaseoli |
Brassica napus L. | IAA, ACC deaminase production and phosphate solubilizing | Improved the growth parameters, nutrient uptake and restricted Na availability for plants | Saghafi et al. (2018) |
Accumulation of osmolytes
The accumulation of salt ions around the root of plants causes osmotic stress under salinity stress, which finally results in osmotic imbalance. However, water retention and photosynthesis integrity are vital to reduce the negative effects of stress on plant growth (Iqbal et al. 2014). Water exchange between cell and environment is regulated by aquaporins (AQPs). AQPs play important roles in various physiological processes, such as growth, development, and response to biotic and abiotic stresses. For example, aquaporins EgTIP2, TsTIP1;2 and SiTIP2;2, which were isolated from Eucalyptus grandis, Thellungiella salsuginea and Tomato plants, respectively, are induced under stress condition (Rodrigues et al. 2013; Wang et al. 2014; Xin et al. 2014). Zhang et al. (2016) isolated a novel tonoplast intrinsic protein (TIP) gene from soybean and termed GmTIP2;3. GmTIP2;3 expresses all detected tissues, such as root, stem and pod, and the accumulation of GmTIP2;3 transcripts significantly correlated with osmotic stresses, including 20% PEG6000 (polyethylene glycol) and 100 μM ABA (abscisic acid) treatments. So, GmTIP2;3 might play an important role in response to osmotic stress in plants. Also, under this condition, plants accumulate lots of metabolites, which are named as compatible (organic) solutes such as proline and glycinebetaine in the cytoplasm. These metabolites enhance plants’ resistance against salt stress through stabilizing the protein conformation, cytosolic pH, balance of cell redox condition, PSII and membrane integrity, and the activity of enzymes (Yan et al. 2013; Tang et al. 2014). In contrast to many halophyte plants, glycophyte plants cannot accumulate of enough amounts of compatible solutes. This is the major reason of these plants’ sensitivity to salt stress (Yan et al. 2013). It has been reported that PGPRs improve plants’ water relationships through the accumulation of osmolites such as proline and maintaining root hydraulic conductivity in inoculated plants (Choudhary 2012). Shukla et al. (2012), also, reported that soluble sugars besides proline, was increased in PGPR inoculated plants. Studies have shown that inoculated plants with PGPRs were more fresh and had higher photosynthetic activity and biomass in compare with plants which were not inoculated (Shukla et al. 2012; Kumari et al. 2015).
Ion homeostasis
The increase in concentration of Na+ causes the decrease in the concentration of K+ in plant leaves under salt stress. As physicochemical properties between Na+ and K+ are similar, Na+ can compete with K+ for binding sites in process such as enzymatic reactions, protein synthesis and ribosome functions. Homeostasis retention of ions concentration is vital in plant cells under salinity stress. So, plant cells have to exclude toxic ions such as Na+ from the cytoplasm and enter them into vacuoles. This process is mediated with transporters such as the plasma membrane Na+/H+ antiporter SOS1 and the tonoplast membrane antiporters NHXs. In other way, plants preserve Na+ in roots and prevent from Na+ flux to the shoots or leaves. In some halophytes, the concentration of toxic ions such as Na+ diluted in the leaves or stems. Furthermore, the HKT family act to maintain ion homeostasis. For example, TmHKT1, OsHKT1 and OsHKT1 are correlated with Na+ exclusion and a high K+/Na+ rate in the leaves of durum wheat, rice and Arabidopsis plants, respectively (Yan et al. 2013; Tang et al. 2014). In some cases, by engineering the genes of transporters and changes of the related genes expression level in the transgenic plants, plants tolerance to salinity stress was increased (Agarwal et al. 2013a, b). Gu et al. (2016) studied the capacity of salt tolerance in cabbage (Brassica oleracea L.) seedling supplemented with sea water. The results showed that the concentration of Na+ and Cl− increased and the concentration of K+, Ca 2+, Mg2+ was decreased under saline condition. In addition, the ratios of K+/Na+, Ca2+/Na+, Mg2+/Na+ were significantly higher in the up ground biomass than underground biomass. These researchers stated that the regulation of transporters and distribution of ions among cabbage seedlings organs was the main reason of the salinity stress tolerance. Based on these findings it can be concluded that the K+/Na+ ratio is a suitable index for evaluating salt tolerance in plants. As Tang et al. (2014) showed that the K+/Na+ ratio in glycophyte plants is very low in compare to halophytes. Also, microbes can modify the absorption of nutrient elements (Cu, Zn, Mn, Fe, K, P, N) and toxic ions (Na, Cl) by plant roots through changing host plant physiology, decreasing the accumulation of toxic ions in leaves and improving the nutritional status of the plant. Zhang et al. (2008) reported that the inoculation of Arabidobis talliana with B. subtilis GB03 reduced the effect of salinity stress by regulating HKT1 potassium transporter. This result indicates that this bacterium stimulates the expression of a high-compatibility transporter for potassium ion (AtHKT1) in Arabidobis under salt stress. In a study, PGPR-inoculated plants had high potassium content and high efficiency in salt tolerance (Rojas-Tapias et al. 2012). Similarly, inoculated pea plants by Variovorax paradoxus 5C-2 bacterium increased total biomass, shoot K+/Na+ ratio, photosynthetic efficiency (Fv/Fm) and maximum electron transport rate (ETR) under salt stress (Wang et al. 2016). Ashraf et al. (2004) showed that Azospirillum inhibited sodium flow into the root. In addition, corn plants had high K+/Na+ ratios under stress condition. Yao et al. (2010) showed that PGPR increased the absorption capacity of calcium and magnesium and reduced the absorption of sodium.
The improvement of nutrient uptakes
The availability and absorption of nutrients depend on parameters such as soil pH, moisture, texture and the composition of its microorganisms. Most of the nutrients are absorbable in the 5-7 pH range. Under salinity stress, the formation of stable structures (through the bonding of cations and anions with various compounds), changes the pH of the soil so modifies the absorption of nutrients by plants. Phosphorus is present in both organic and inorganic forms in soils. It is required as an essential nutritional element for photosynthesis, energy transfer, biosynthesis of macromolecules and respiration (Fernandez et al. 2007). The availability of P is low in most agricultural soils. The concentration of phosphorus ions in these soils varies in the range of 0.1–10 μm, while the plant’s requirement of P for optimal growth is 1–5 μm (for herbaceous plants) and 5–60 μm (for plants that require high P). The phosphorus deficiency results in decreased plant yield (by 5–15%) (Zaidi et al. 2009). It has been shown that the phosphorus fertilizers that are added to the soil are deposited in large amounts (about 75–90%) as cation-metal complexes in the soil (Toro 2007). This leads farmers to add more fertilizer to soil, which has negative effects on environment. Phosphate solubilizing bacteria (PSB), belonging to the genus Bacillus, Pseudomonas, Achromobacter, Alcaligenes, Brevibacterium, Serratia, Xanthomonasand Rhizobium, have the ability to hydrolyze inaccessible phosphorus forms into absorbable form (Sindhu et al. 2010; Saghafi et al. 2018). In a study, Fluorescent Pseudomonad was screened for solubilizing of tricalcium phosphate based on the formation of a visible halo on the Pikovskaya agar (Naik et al. 2008). This bacterium solubilizes the phosphorus compounds by releasing low molecular weight organic acids, such as gluconic acid, citric acid, succinic acid, propionic acid, and lactic acid (Choudhary 2012). In addition, this bacterium solubilizes soil minerals by secretion of hydrogen ions into the rhizosphere (by reducing pH) (Khan et al. 2006). Salinity leads to depletion and sedimentation of absorbable phosphorus. In the study of Shukla et al. 2012 and Vaishnav et al. 2017, insoluble phosphate solubilizing bacteria solubilize sedimentary phosphorous in hydroponic MS medium and increased the phosphorus availability for plants under salinity stress. Also, PGPR can fix nitrogen through symbiosis and non-symbiosis mechanisms. In symbiotic fixation method, the bacteria form the node in the host roots and the fixed nitrogen is estimated to be approximately 65% of the total biological processes of the fixed nitrogen (Rajwar et al. 2013). The other group of nitrogen-fixing bacteria is not specific to the plant (Oberson et al. 2013). Azospirillum, Azotobacter, Burkholderia, Herbaspirillum, Bacillus and Paenibacillus (Goswami et al. 2016) are examples of free bacteria. It is reported that the amount of nitrogen fixed by these bacteria is about 20–30 kg h−1 year−1. Species belonging to the Azotobacter and Azospirillum are widely used in agricultural practices. Strains of these bacteria not only fix nitrogen but also produce hormones such as indole acetic acid (IAA), gibberellin and cytokinin, which all result in increased plant growth (Oberson et al. 2013). Therefore, nitrogen fixation is an important feature of PGPRs which provides nitrogen for plants. Nitrogen-fixing strains are very important in agriculture and have been traded for about 20 years as fertilizers (Goswami et al. 2015; Heulin et al. 2002).
Fe acts as a cofactor in 140 biochemical catalytic enzymes. This element is in the form of ferric (Fe3+) and forms insoluble hydroxides and oxyhydroxides which are not absorbable for plants and microorganisms (Ma et al. 2011). The accessibility of ferric iron decreases more in saline soils because solubility of the ferric form reduces with increasing pH (Thomine and Lanquar 2011). Plants have two strategies to absorb iron. The first strategy involves the release of iron chelating organic compounds which maintain iron in the form of a solution and makes it available to the plant. The solved Fe, then, is reduced and absorbed by the enzymatic system of the cell membrane of the plants. The second strategy involves absorption of the iron-organic compound by the plant so that iron is reduced and absorbed inside the plant (Goswami et al. 2016). Microorganisms use different mechanisms for accessing iron, among which Siderophores are studied more. Siderophores are iron chelating agents that have been proven in various bacteria and are important in increasing plant growth and protecting against plant pathogens (Scavino and Pedraza 2013). PGPRs produce Siderophores in the rhizosphere. Plants absorb Fe from siderophore (through destruction of chelate or direct absorption) (Rajkumar et al. 2010). Sharma and Johri (2003) reported that GRP3A and PRS siderophore generating strains of Pseudomonas spp. increased seed germination and growth of corn plants under iron deficiency stress. Pandey et al. (2005) identified P. aeruginosa GREC1 as a siderophore producing bacterium. The bacterium increased Brassica campestris growth in field condition.
Potassium, as a non-renewable source, is an essential nutrient ingredient for plants and plays an important role in plant metabolism. In addition, potassium improves the quality of the crop production, because it plays a role in grain filling, and disease resistance which leads to increased plant resistance to stress (Sindhu et al. 2010). The key role of potassium as osmotic regulator is in cellular turgidity, regulating the opening and closure of stomata, as well as maintaining water balance in plants under stressful environments (Dubey 2005). This element exists mainly in three different forms in the soil. Usable potassium, stabilized potassium, and mineralized potassium (mica, orthoclase, and illite) are these three forms. The concentration of potassium in the soil solution is very low (1–2%), and the major part of potassium (98%) is insoluble in soil, rock and minerals. Common potassium compounds in soil are feldspar and mica (98-90%) (Sindhu et al. 2010). Although soluble and exchangeable potassium are considered as two absorbable forms for plants, studies have shown that both stabilized and structural potassium may take part in supplying plants required potassium (Rasouli Sadagiani et al. 2016). One of the strategies for using these potassium sources is the use of potassium solubilizing microorganisms (KSBs). Many researchers have studied the bio activation of non-exchangeable potassium sources of soil by KSBs and have shown that non-exchangeable potassium can contribute to the availability of potassium as a valuable resource (Vaishnav et al. 2017). KSBs solve potassium-containing minerals (mica, illite and orthoclase) by producing citric, oxalic, tartaric, succinic, and alpha-ketogluconic acids. These acids solubilize potassium ores directly or by chelating silicon ions (Parmar and Sindhu 2013). In another study, the possible reasons for the solubilization of potassium from non-exchangeable sources through production of organic and mineral acids, siderophore, and exopolysaccharides are mentioned (Ghorbanpour et al. 2014). Meena et al. (2014) stated that KSBs play an important role in increasing potassium absorption by plants and reducing the use of chemical fertilizers. In this regard, two strains of KSBs (KNP413 and KNP414) with high ability to solubilize potassium minerals, are used as potassium fertilizer in China (Hu et al. 2006). In another study, three strains of B. mucilaginosus, Azotobacter chroococcum and Rhizobium sp. were studied a mica solvent, they showed the ability to increase potassium absorption by wheat and corn plants (Singh et al. 2010).
Zn is an essential nutrient element for plants, which plays an important role in the enzymes synthesizing auxin. It also takes part in biochemical reactions, stability of biological membranes, the activity of oxidative and carbonic anhydrase enzymes (Broadley et al. 2007). The average amount of zinc in the earth’s crust is less than 89 mg/kg, which is very low compared to other minerals such as iron and manganese. Although some soils have enough amount of zinc, most of plants are not able to absorb it. Research has shown that about 30 percent of the world’s soils are zinc deficient (Kochian 2000). Due to the problem of salinity in arid and semi-arid soils, zinc deficiency is a major problem. It seems that the Zn deficiency is the most common phenomenon in calcareous soils (Rashid and Ryan 2004). Many factors, such as soil texture and calcareousity, pH, soil water content, organic matter affect Zn availability in soils (Alloway 2008). This element is found in soils in forms of sphalerite (ZnS), smithsonite (ZnCO3), zincite (ZnO), zinc oxide (ZnSO4), franklinite (ZnFe2O4) andhopeite (Zn3(PO4)2 4H2O) (Vaishnav et al. 2017). Plants absorb Zn mainly in the form of Zn2+, zinc hydrate, and organic zeolite (Alaghemand et al. 2018). The use of Zn-containing fertilizers such as zinc sulfate and zeolite, zinc-efficient cultivars, as well as microorganisms with the ability to provide Zn for the plant, is some of the most important methods to provide Zn for plants. Increasing Zn absorption by rhizobacteria is rarely studied. However, reports indicate that potential rhizobacteria have increased Zn absorption potential (Tariq et al. 2007; Biari et al. 2008; Subramanian et al. 2009). These microorganisms can increase the solubility of low soluble Zn compounds and make their Zn accessible for other organisms. They employed various mechanisms: reducing soil pH (through releasing organic acids such as gluconic acid and 2-Ketogluconic acid and proton secretion) (Koide and Kabir, 2000; Subramanian et al. 2009), chelating (through producing glutane compounds such as siderophore and EDTA) (Tariq et al. 2007), altering root system (improve root growth and absorption) (Vaishnav et al. 2017). The efficiency of Zn solubilizing bacteria has been investigated in various Zn sources (Abaid-Ullah et al. 2015). Tariq et al. (2007) found that zinc solubilizing bacteria have a positive effect on growth and grain yield in rice seedlings by increasing the absorption of this element in the plants. In another study, it has been shown that Serratia sp. has a high ability to solubilize ZnO (compared to other zinc sources) and is able to increase wheat yield under different climates (Abaid-Ullah et al. 2015).
Silicon (Si) is not an essential element but it affects the growth and health of plants. Plants have different abilities to absorb Si (1–10%) (Cherif and Belanger 1992). This element can increase the productivity and quality of crops. Also, it increases the production of certain antioxidant enzymes in plants. The effect of silicon on plant yield may be due to its sedimentation in leaf width, ability to increase leaf strength, increase chlorophyll content per leaf area, and increase the efficiency of photosystem II. Therefore, the application of soluble silicon to produce higher concentrations of the Ribulose-1,5-bisphosphate carboxylase/oxygenase (rubisco) enzyme in the leaf is required. Rubisco regulates carbon dioxide (CO2) metabolism as it plays an important role in controlling carbon dioxide stabilization in plants (Sonobe et al. 2011). Silicon is a bioactive element that helps to alleviate living and non-living stresses. Several studies have shown that the use of silicon dioxide (SiO2) increases the resistance to environmental stresses in plants such as wheat, rice, sugarcane, tomato, cucumber, citrus and barley (Bélanger et al. 2003; Ma and Yamaji 2006). Recently, Si fertilizer has been widely used to increase agricultural productivity. However, due to the high price of this fertilizer, there are many attempts to reduce the use of it (Ng et al. 2016). Although, Si is the most abundant element on the surface of the earth its presence with other elements makes it unavailable for plants root to absorb (Vasanthi et al. 2012). Si can be solubilized through the aeration of the rocks or the biological activity of microorganism and plants root. Therefore, the use of Si solubilization bacteria (SSBs) potential to solubilize Si is a good method (Ng et al. 2016). SSBs release Si from silicate minerals by producing organic acids (2-ketogluconic acid) and polysaccharides (Joseph et al. 2015). The bacteria also increase the solubilization of silicates by proton production, organic ligands, hydroxyl, anion, exopolysaccharides and enzymes (Malinovskaya et al. 1990; Hiebert and Bennett, 1992; Barker et al. 1998). Orthosilicic acid (H4SiO4) is the soluble form of Si and can be absorbed by the root of the plants (Rodrignes and Datnoff, 2005). In addition, Si sediments in the forms of silica gel in epidermal and scleroderma cells. These bacteria not only increase the fertility of the soil (through the release of phosphorus and potassium, etc.), but also enhance the defense mechanisms of the plants by solubilizing insoluble forms of silicates (Vasanthi et al. 2012). Although microorganisms are abundant in soil totally the number of SSBs in the soil is very low. Therefore, the use of effective SSBs strains is necessary to increase the fertility of soils, especially in stressful conditions.
Production of indole acetic acid (IAA)
One of the general mechanisms of plant adaptation to deal with stress is a change in root morphology. Hormones play important roles in this process. IAA is the most common type of auxin hormone in plants which plays many roles such as cell division and development, specialization of plant cells and tissues, seed germination, development of root system, control of chloriferation processes, and formation of roots. It also stimulates the lateral roots and affects photosynthesis, mediates the formation of pigments and enhances the tolerance of plants to stress conditions (Aeron et al. 2011). About 80% of rhizobacteria produce IAA. IAA producing rhizobacteria affect the root system through increasing the size, weight, branching and root surface. All of these changes lead to an increased ability to absorb food from the soil, and ultimately improved plant growth (Etesami et al. 2015; Saghafi et al. 2018). Different PGPRs have different pathways for IAA synthesis. IAA is produced by rhizobacteria through l-tryptophan dependent (use of tryptophan as a precursor to IAA synthesis) and independent pathways (Jha and Saraf 2015). The positive effect of IAA mediating bacteria on the growth of different plants under salinity and drought stresses has been proven (Paul and Lade 2014; Ghorbanpour et al. 2011). For example, the canola (Brassica napus L.) seedling inoculated by IAA producing Rhizobium Leguminosarum b.v phaseoli showed improved growth condition under salt stress (Saghafi et al. 2018). Similarity, Triticum aestivum seedling inoculated by IAA producing Azospirillum sp. and Rhizobium leguminosarum bacteria increased the uptake of nutrients and water and lateral root formation under drought stress (Hussain et al. 2018; Barnawal et al. 2019). Since phytohormone production is a general characteristic of PGPR, it is necessary to consider the selection of microbial inoculants with high potential of producing these hormones (especially IAA) to reduce negative effects of stresses (Table 1).
Production of ACC deaminase
Ethylene regulates processes such as root growth and root hairs formation, germination, seed dormancy, fruit ripening, flower senescence and leaf abscission. The hormone also plays roles in responding to environmental stresses and contributes to crop yields reduction by reducing the growth of stems and roots. Ethylene, which is produced under various environmental stresses such as high temperatures, flooding, drought, the presence of toxic metals, organic pollutants and high salinity, is called “stress ethylene” (Etesami et al. 2015; Jha and Saraf 2015). Plants respond to stress through producing 1-amino-cyclopropane-1-carboxylic acid (ACC), a precursor of ethylene production (Glick et al. 2007). ACC is secreted into the rhizosphere and is again absorbed by the roots and eventually converted to ethylene. The accumulation of ethylene prevents root growth, which consequently limits the water and nutritive elements absorption. Therefore, any factor that can modify the concentration of ethylene can regulate the growth and development of plants. PGPRs with the ability to use plant ACC as a source of nitrogen and energy in the rhizosphere, divide it into ammonia and α-ketobutyrate. They prevent the accumulation of ethylene and provides a healthy root system to cope with environmental stresses (Siddikee et al. 2010). Glick et al. (1998) presented a model to describe the role of ACC-deaminase producing bacteria in improving plant growth. When ACC is secreted from plant roots, it hydrolyzes by the ACC-deaminase producing bacteria. Therefore, ACC concentration is reduced outside the root and ACC is more secreted. Consequently, the level of ACC in plants is decreased that results in a reduced amount of ethylene. The efficiency of ACC-deaminase producing bacteria to enhance the growth of tomato and rice under salt stress was proven (Bal et al. 2013; Mayak et al. 2004) and some examples are mentioned in Table 1. Siddikee et al. (2010) reported that the salinity resistant bacteria accompanied with different ACC deaminase producing strains of Bacillus, Brevibacterium, Planococcus, Zhihengliuella, Halomonas, Exiguobacterium, Oceanimonas, Corynebacterium, Arthrobacter and Micrococcus, increase plant growth potential under salinity stress. In addition, saghafi et al. (2018) reported that the application of Sinorhizobium mellilote and Rhizobium legominozaroum b.v phaseoli with ability to ACC deaminase production, improved the growth parameters and nutrient uptake in canola plants (Brassica napus L.) under salinity stress. Furthermore, these strains restricted the uptake of Na.
Production of Exopolysaccharides (EPs)
Stresses change physicochemical and biological properties of soil so affect microbial activity and yield production directly, and the soil structure indirectly. The production of exopolysaccharides by microbes keeps them from environmental fluctuating conditions. EPs are produced by soil microbes in the form of viscose materials. They can be absorbed into clay levels due to the mechanism of cationic bridges, hydrogen bond, Van der Waals forces and anionic attraction. In this way, they can form protective layer around the aggregates (Sandhya et al. 2009). EPs create a micro-environment, which maintains water and slow down the dehydration in compare with surrounding environment. Therefore, EPs protect the bacteria and roots of the plant against stress (Karimi et al. 2018). Also, the production of EPs by bacteria in saline soil can affect the plants productivity as they improve aggregate generation and physicochemical properties of soil (Minah and Hazarin Subair 2015). EPs producing bacteria can reduce damaging ion availability in saline conditions, by chelating excessive sodium ions around roots (Choudhary et al. 2015). The decrease in sodium absorption is probably due to the occupation of the root zone with soil, which prevents sodium from moving to the stem (Ashraf et al. 2004). Thus they can increase the growth of plants under salinity stress conditions (Table 1).
Change in the antioxidative defense system
Plants exposed to stressful condition produce reactive oxygen species (ROS) such as radical superoxide (O·−2), hydrogen peroxide (H2O2), radical hydroxyl (OH·), and alkaline radicals (Zhang et al. 2018). ROS react with proteins, lipids and DNA and leads to oxidative damage and impairs the general functions of plant cells (Johnson et al. 2003; Yan et al. 2013). To cope with these damages, plants use their antioxidant defense systems, which include enzymatic and non-enzymatic components. By increasing the activity of these components, the plant’s defense mechanisms try to prevent the accumulation of ROS and the oxidative stress (Miller et al. 2010). The enzymatic components include peroxidases (POX), superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), glutathione reductase (GR), dehydroascorbate reductase (DHAR), monodehydroascorbate reductase (MDAR), glutathione peroxidases (GPX), glutathione s-trasferanse (GST), etc. Non-enzymatic components include glutathione, tocopherol, anthocyanins, phenolic compounds (Such as flavonoid, lutein, tannin), ascorbic acid etc. (Yan et al. 2013; Kaushal and Wani 2015). There is a positive correlation between the level of these enzymes oxidative activity condition in plants such as cotton, citrus, foxtail millet, purslane, sugar beet, pea and plantago under stress. (Rasool et al. 2013; Tang et al. 2014). Recently, some saline resistant plants were produced through transferring and modifying the expression of the genes of some antioxidant enzymes (such as APX, GST, GPX, MnSOD, Cu/ZnSOD, DHAR, SOD, DHAR1, MDAR, AmMDAR and katE). Also, the overexpression of antioxidant enzymes such as SOD, CAT and APX in transgenic plants increased the tolerance of plants to oxidative stresses (Agarwal et al. 2013a, b; Tang et al. 2014). Using PGPR to induce the overexpression of antioxidant enzymes production is the other method. Studies have shown that the use of PGPR leads to increased activity of plant defense enzymes such as POX, SOD, CAT, APX and GR (Nautiyal et al. 2008; Chakraborty et al. 2013; Karimi et al. 2018). Hemida and Reyad (2018) inoculated the Carthamus tinctorius plants with two PGPRs namely Bacillus cereus and Bacillus aerius under salinity stress. The results showed that in inoculated plants the activity of CAT, APX and GR enzymes, also glutathione and ascorbic acid was significantly higher than control plants.
Conclusions and future prospects
The negative effects of salt stress on plants are related to osmotic stress, Na+ toxicity, nutritional imbalance and oxidative stress. Halophyte plants can ameliorate these adverse effects by accumulating compatible organic solutes such as glycinebetaine and proline, activating antioxidant system, and increasing K/Na ratio in the cytosol. As most of the agricultural crops are glycophytes, improving plant salt tolerance is important. There are three methods to do this: conventional breeding, genetic engineering and application of chemical materials. Due to the complexity of the function of salt stress responding genes the efficiency of conventional breeding method is very low. There are many studies that claimed the success of genetic engineering in producing some salt tolerant plants. Biotechnologists transferred some of genes involved in salt tolerance mechanism such as osmolite and antioxidant genes to Arabidopsis and Tobacco as model plants. However, most of these projects were successful just under controlled and abnormal laboratory condition. In addition, chemicals are expensive and will create environmental problems in long time. So, scientists should focus on the cost effective and echo-friendly approaches.
The presence of wild plants in soils affected by salinity is considered as good potentials to increase susceptible ones. Obviously, the rhizospheric soil of these plants can be a rich source for the growth-enhancing bacteria. We hope that isolation, purification, identification of these bacteria and inoculation of agricultural plants with them will increase salinity tolerance and productivity of the plants. PGPR can ameliorate the negative effects of salinity on plants through several mechanisms such as ACC-deaminase and EPs production, osmolite accumulation, antioxidant system activation, improvement of K+/Na+, Ca2+/Na+, Mg2+/Na+ (Fig. 1). However, to ensure food security of growing population of the world, it is necessary to understand the complexity of PGPR-plant interaction. Plant–microbe interaction involves various proteins and signaling pathways. The conventional biochemical and genomics methods are insufficient to study and determine the exact role of metabolites and signaling molecules. Recently, large-scale omics tools such as transcriptomics, proteomics and metabolomics are routinely used to understand the cellular processes, genetic control, and signaling networks involved in plant responses to environmental stresses (Imam et al. 2016 and 2017; Basu et al. 2018). Furthermore, through employing omics tools along with gene editing technique we can improve PGPR-plant interactions under salt stress. For example, by applying gene editing technique in PGPR bacteria, IAA and ACC-deaminase production of these bacteria improved and consequently, plants’ salinity tolerance was increased (Basu et al. 2018). Despite of the efficiency of gene editing technique, these questions must be answered: are engineered microbes safe in environmental condition? and can they survive under natural condition? However, further research is necessary to explore some new PGPRs in the future.
Compliance with ethical standards
Conflict of interest
The authors declare that he/she has no conflict of interest.
References
- Abaid-Ullah M, Hassan MN, Jamil M, Brader G, Shah MK, Sessitsch A, Hafeez FY. Plant growth promoting rhizobacteria: an alternate way to improve yield and quality of wheat (Triticum aestivum) Int J Agric Biol. 2015;17:51–60. [Google Scholar]
- Abeer H, Abdallah EF, Alqarawi AA, Alhuqail AA, Alshalawi SRM, Wirth S, Egamberdieva D. Impact of plant growth promoting bacillus subtilis on growth and physiological parameters of Bassia indica (Indian Bassia) grown under salt stress. Pak J Bot. 2015;47(5):1735–1741. [Google Scholar]
- Aeron A, Kumar S, Pandey P, Maheshwari DK. Emerging role of plant growth promoting rhizobacteriain agrobiology. In: Maheshwari DK, editor. Bacteria in agrobiology: crop ecosystems. Berlin Heidelberg: Springer; 2011. pp. 1–36. [Google Scholar]
- Agarwal DK, Billore SD, Sharma AN, Dupare BU, Srivastava SK. Soybean: introduction, improvement, and utilization in India: problems and prospects. Agric Res. 2013;2(4):293–300. [Google Scholar]
- Agarwal PK, Shukla P, Gupta K, Jha B. Bioengineering for salinity tolerance in plants: state of the art. Mol Biotechnol. 2013;54:102–123. doi: 10.1007/s12033-012-9538-3. [DOI] [PubMed] [Google Scholar]
- Ahemad M, Khan MS. Functional aspects of plant growth promoting rhizobacteria: recent advancements. Insight Microbiol. 2011;1(3):39–54. [Google Scholar]
- Ahmad MS, Zaidi E, Oves AM (2013) Functional aspect of phosphate-solubilizing bacteria: importance in crop production In: Maheshwari DK et al (ed) Bacteria in agrobiology: crop productivity
- Akhtar SS, Andersen MN, Naveed M, Zahir ZA, Liu F. Interactive effect of biochar and plant growth-promoting bacterial endophytes on ameliorating salinity stress in maize. Funct Plant Biol. 2015;42(8):770–781. doi: 10.1071/FP15054. [DOI] [PubMed] [Google Scholar]
- Alaghemand A, Khaghani S, Bihamta MR, Gomarian M, Ghorbanpour M (2018) Green synthesis of zinc oxide nanoparticles using Nigella Sativa L. extract: the effect on the height and number of branches. J Nanostruct 8(1):82–88
- Alloway BJ (2008) Zinc in soils and crop nutrition. International zinc Association and International Fertilizer Industry Association. Brussels, Belgium and Paris, France, pp 130
- Ashraf M, Akram NA. Improving salinity tolerance of plants through conventional breeding and genetic engineering: an analytical comparison. Bio technol Adv. 2009;6:744–752. doi: 10.1016/j.biotechadv.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Ashraf M, Berge SH, Mahmood OT. Inoculating wheat seedling with exopolysaccharides-producing bacteria restrict sodium uptake and stimulates plant growth under salt stress. Biol Fertil Soils. 2004;40:157–162. [Google Scholar]
- Bal HB, Nayak L, Das S, Adhya TK. Isolation of ACC deaminase producing PGPR from rice rhizosphere and evaluating their plant growth promoting activity under salt stress. Plant Soil. 2013;366(1–2):93–105. [Google Scholar]
- Barker WW, Welch SA, Chu S, Baneld JF. Experimental observations of the effects of bacteria on aluminosilicate weathering. Am Miner. 1998;83:1551–1563. [Google Scholar]
- Barnawal D, Singh R, Singh RP. Role of plant growth promoting rhizobacteria in drought tolerance: regulating growthhormones and osmolytes. In: Singh AK, Kumar A, Singh PK, editors. PGPR amelioration in sustainable agriculture. Cambridge: Woodhead Publishing; 2019. pp. 107–128. [Google Scholar]
- Basu S, Rabara RC, Negi S, Shukla P. Engineering PGPMOs through gene editing and systems biology: a solution for phytoremediation. Trends Biotechnol. 2018;1608:1–12. doi: 10.1016/j.tibtech.2018.01.011. [DOI] [PubMed] [Google Scholar]
- Bélanger RR, Benhamou N, Menzies JG. Cytological evidence of an active role of silicon in wheat resistance to powdery mildew (Blumeria graminis f. sp. tritici) Phytopathology. 2003;93:402–412. doi: 10.1094/PHYTO.2003.93.4.402. [DOI] [PubMed] [Google Scholar]
- Ben Rejeb I, Atauri Miranda L, Cordier M, Mauch-Mani B. Induced tolerance and priming for abiotic stress in plants. In: Gaur RK, Sharma P, editors. Molecular approaches in plant abiotic stress. Boca Raton: CRC Press; 2013. [Google Scholar]
- Bharti Nidhi, Yadav Deepti, Barnawal Deepti, Maji Deepamala, Kalra Alok. Exiguobacterium oxidotolerans, a halotolerant plant growth promoting rhizobacteria, improves yield and content of secondary metabolites in Bacopa monnieri (L.) Pennell under primary and secondary salt stress. World Journal of Microbiology and Biotechnology. 2012;29(2):379–387. doi: 10.1007/s11274-012-1192-1. [DOI] [PubMed] [Google Scholar]
- Biari A, Gholami A, Rahmani HA. Growth promotion and enhanced nutrient uptake of maize (Zea mays L.) by application of plant growth promoting rhizobacteria in arid region of Iran. J Biol Sci. 2008;8:1015–1020. [Google Scholar]
- Broadley MR, White PJ, Hammond JP, Zelko I, Lux A. Zinc in plants. New Phytol. 2007;173(4):677–702. doi: 10.1111/j.1469-8137.2007.01996.x. [DOI] [PubMed] [Google Scholar]
- Chakraborty N, Ghosh R, Ghosh S, Narula K, Tayal R, Datta A, Chakraborty S. Reduction of oxalate levels in tomato fruit and consequent metabolic remodeling following overexpression of a fungal oxalate decarboxylase1. Plant Physiol. 2013;162(1):364–378. doi: 10.1104/pp.112.209197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary T, Shukla P. Bioinoculants for bioremediation applications and disease resistance: innovative perspectives. Indian J Microbiol. 2019;59(2):129–136. doi: 10.1007/s12088-019-00783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherif M, Belanger RR. Use of potassium silicate amendments in recirculating nutrient solutions to suppress Pythium ultimum on long english cucumber. J Plant Dis. 1992;76(10):1008–1011. [Google Scholar]
- Choudhary DK. Microbial rescue to plant under habitat-imposed abiotic and biotic stresses. Appl Microbiol Biotechnol. 2012;96(5):1137–1155. doi: 10.1007/s00253-012-4429-x. [DOI] [PubMed] [Google Scholar]
- Choudhary DK, Kasotia A, Jain S, Vaishnav A, Kumari S, Sharma KP, Varma A. Bacterial-mediated tolerance and resistance to plants under abiotic and biotic stresses. J Plant Growth Regul. 2015;35:276–300. [Google Scholar]
- Dubey RS. Photosynthesis in plants under stressful conditions. In: Pessarakli M, editor. Handbook of photosynthesis. 2. New York: CRC Press; 2005. pp. 717–718. [Google Scholar]
- Egamberdiyeva D. Plant-growth-promoting rhizobacteria isolated from a Calcisol in a semi-arid region of Uzbekistan: biochemical characterization and effectiveness. J Plant Nutr Soil Sci. 2005;168(Suppl 1):94–99. [Google Scholar]
- Etesami H, Alikhani HA, Hosseini HM. Indole-3-acetic acid and 1-aminocyclopropane-1-carboxylate deaminase: bacterial traits required in rhizosphere, rhizoplane and/or endophytic competence by beneficial bacteria. In: Maheshwari DK, editor. Bacterial metabolites in sustainable agroecosystem. New York: Springer International; 2015. pp. 183–258. [Google Scholar]
- Fernandez LA, Zalba P, Gomez MA, Sagardoy MA. Phosphate-solubilization activity of bacterial strains in soil and their effect on soybean growth under greenhouse conditions. Biol Fertil Soil. 2007;43:805–809. [Google Scholar]
- Ghorbanpour M, Majnoun-Hoseini N, Rezazadeh SH, Omidi M, Khavazi K, Hatami M. Variations of root and shoot tropane alkaloids production of Hyoscyamus niger under two rhizobacteria strains inoculation and water deficit stress. J Med Plants. 2011;10(40):160–170. [Google Scholar]
- Ghorbanpour M, Hatami M, Khavazi K. Role of plant growth promoting rhizobacteria on antioxidant enzyme activities and tropane alkaloids production of Hyoscyamus niger under water deficit stress. Turkish J Biol. 2013;37:350–360. [Google Scholar]
- Ghorbanpour M, Hosseini N, Khodae-Motlagh M, Solgi M. The effects of inoculation with pseudomonads rhizobacteria on growth, quantity andquality of essential oils in sage (Salvia officinalis L.) plant. J Med Plants. 2014;52:89–100. [Google Scholar]
- Ghorbanpour M, Hatami M, Kariman K, Abbaszadeh Dahaji P. Phytochemical variations and enhanced efficiency of antioxidant and antimicrobial ingredients in Salvia officinalis as inoculated with different rhizobacteria. Chem Biodivers. 2016;13:319–330. doi: 10.1002/cbdv.201500082. [DOI] [PubMed] [Google Scholar]
- Glick BR. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res. 2014;169:30–39. doi: 10.1016/j.micres.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Glick BR, Penrose DM, Li J. A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J Theor Biol. 1998;190(1):63–68. doi: 10.1006/jtbi.1997.0532. [DOI] [PubMed] [Google Scholar]
- Glick BR, Cheng Z, Czarny J, Duan J. Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur J Plant Pathol. 2007;119(3):329–339. [Google Scholar]
- Goswami D, Parmar S, Vaghela H, Dhandhukia P, Thakker J. Describing paenibacillus mucilaginosus strain N3 as an efficient plant growth promoting rhizobacteria (PGPR) Cogent Food Agric. 2015;1(1):1000714. [Google Scholar]
- Goswami D, Janki N, Pinakin C. Portraying mechanics of plant growth promoting rhizobacteria (PGPR): a review. Cogent Food Agric. 2016;2:1127500. [Google Scholar]
- Gu MF, Li N, Shao TY, Long XH, Brestič M, Shao HB, Li JB, Mbarki S. Accumulation capacity of ions in cabbage (Brassica oleracea L.) supplied with sea water. Plant Soil Environ. 2016;62(7):314–320. [Google Scholar]
- Hall AE. Crop response to environment. New York: CRC Press; 2001. [Google Scholar]
- Hemida KA, Reyad AMM. Improvement salt tolerance of safflower plants by endophytic bacteria. J Hortic Plant Res. 2018;5:38–56. [Google Scholar]
- Heulin T, Achouak W, Berge O, Normand P, Guinebretière M-H. Paenibacillus graminis sp. nov. and Paenibacillus odorifer sp. nov., isolated from plant roots, soil and food. Int J Syst Evol Microbiol. 2002;52:607–616. doi: 10.1099/00207713-52-2-607. [DOI] [PubMed] [Google Scholar]
- Hiebert FK, Bennett PC. Microbial control of silicate weathering in organic-rich ground water. Science. 1992;258:278–281. doi: 10.1126/science.258.5080.278. [DOI] [PubMed] [Google Scholar]
- Hu X, Chen J, Guo J. Two phosphate-and potassium-solubilizing bacteria isolated from Tianmu Mountain, Zhejiang, China. World J Microbiol Biotechnol. 2006;22(9):983–990. [Google Scholar]
- Hussain I, Aleti G, Naidu R, Puschenreiter M, Mahmood Q, Rahman MM, Wang F, Shaheen S, Syed JH, Reichenauer TG. Microbe and plant assisted-remediation of organic xenobiotic and its enhancement by genetically modified organisms and recombinant technology: a review. Sci Total Environ. 2018;628:1582–1599. doi: 10.1016/j.scitotenv.2018.02.037. [DOI] [PubMed] [Google Scholar]
- Imam J, Singh PK, Shukla P. Plant microbe interactions in post genomic era: perspectives and applications. Front Microbiol. 2016;7:1488. doi: 10.3389/fmicb.2016.01488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam J, Singh PK, Shukla P, Mandal NP, Variar M. Microbial interactions in plants: perspectives and applications of proteomics. Curr Protein Pept Sci. 2017;18:1–10. doi: 10.2174/1389203718666161122103731. [DOI] [PubMed] [Google Scholar]
- Iqbal N, Umar S, Nazar R. Manipulating osmolytes for breeding salinity-tolerant plants. In: Ahmad P, Rasool S, editors. Emerging technologies and management of crop stress tolerance a sustainable approach. Amsterdam: Elsevier Inc.; 2014. [Google Scholar]
- Jamil A, Riaz S, Ashraf M, Foolad MR. Gene expression profiling of plants under salt stress. Crit Rev Plant Sci. 2011;30(5):435–458. [Google Scholar]
- Jha CK, Saraf M. Plant growth promoting rhizobacteria (PGPR): a review. J Agric Res Dev. 2015;5:108–119. [Google Scholar]
- Jha Y, Subramanian RB, Patel S. Combination of endophytic and rhizospheric plant growth promoting rhizobacteria in Oryza sativa shows higher accumulation of osmoprotectant against saline stress. Acta Physiol Plant. 2011;33(3):797–802. [Google Scholar]
- Joe MM, Devaraj S, Benson A, Sa T. Isolation of phosphate solubilizing endophytic bacteria from Phyllanthus amarus Schum & Thonn: evaluation of plant growth promotion and antioxidant activity under salt stress. J Appl Res Med Aromatic Plant. 2016;3:71–77. [Google Scholar]
- Johnson HE, Broadhurst D, Goodacre R, Smith AR. Metabolic fingerprinting of salt-stressed tomatoes. Phytochemistry. 2003;62(6):919–928. doi: 10.1016/s0031-9422(02)00722-7. [DOI] [PubMed] [Google Scholar]
- Joseph MH, Dhargave TS, Deshpande CP, Srivastava AK. Microbial Solubilisation of Phosphate: pseudomonas versus Trichoderma. Annals of Plant and Soil Research. 2015;17:227–232. [Google Scholar]
- Karimi E, Aliasgharzad N, Neyshabouri MR, Esfandiari A (2018) Isolation of biofilm formation bacteria from soil and evaluation of their effects on drought stress mediate in wheat plants. Doctoral thesis, faculty of agriculture, Tabriz University, Iran (in Persian with English abstract)
- Karthikeyan B, Joe MM, Islam MR, Sa T. ACC deaminase containing diazotrophic endophytic bacteria ameliorate salt stress in Catharanthus roseus through reduced ethylene levels and induction of antioxidative defense systems. Symbiosis. 2012;56(2):77–86. [Google Scholar]
- Kasotia A, Varma A, Tuteja N, Choudhary DK. Amelioration of soybean plant from saline-induced condition by exopolysaccharide producing Pseudomonas-mediated expression of high affinity K+-transporter (HKT1) gene. Curr Sci. 2016;111(12):25. [Google Scholar]
- Kaushal M, Wani SP. Plant-growth-promoting rhizobacteria: drought stress alleviators to ameliorate crop production in drylands. Ann Microbiol. 2015;66:1–8. [Google Scholar]
- Khan MS, Zaidi A, Wani PA. Role of phosphate-solubilizing microorganisms in sustainable agriculture – a review. Agron. Sustain. Dev. 2006;27:29–43. [Google Scholar]
- Kiani MZ, Ali A, Sultan T, Ahmad R, Hydar SI. Plant growth promoting rhizobacteria having 1-aminocyclopropane-1-carboxylic acid deaminase to induce salt tolerance in sunflower (Helianthus annus L.) Nat Res. 2015;6:391–397. [Google Scholar]
- Kim YC, Glick B, Bashan Y, Ryu CM. Enhancement of plant drought tolerance by microbes. In: Aroca R, editor. Plant responses to drought stress. Berlin: Springer; 2013. [Google Scholar]
- Kochian LV. Molecular physiology of mineral nutrient acquisition, transport, and utilization. Biochem Mol Biol Plants. 2000;20:1204–1249. [Google Scholar]
- Koide RT, Kabir Z. Extra radical hyphae of the mycorrhizal fungus Glomus intraradices can hydrolyse organic phosphate. New Phytol. 2000;148:511–517. doi: 10.1046/j.1469-8137.2000.00776.x. [DOI] [PubMed] [Google Scholar]
- Kumari S, Vaishnav A, Jain S, Varma A, Choudhary DK. Bacterial-mediated induction of systemic tolerance to salinity with expression of stress alleviating enzymes in soybean (Glycinemax L. Merrill) J Plant Growth Regul. 2015;34:558–573. [Google Scholar]
- Ladeiro Bruno. Saline Agriculture in the 21st Century: Using Salt Contaminated Resources to Cope Food Requirements. Journal of Botany. 2012;2012:1–7. [Google Scholar]
- Liu X, Luo Y, Li Z, Wang J, Wei G. Role of exopolysaccharide in salt stress resistance and cell motility of Mesorhizobium alhagi CCNWXJ12–2T. Appl Microbiol Biotechnol. 2017;101:1–12. doi: 10.1007/s00253-017-8114-y. [DOI] [PubMed] [Google Scholar]
- Ma JF, Yamaji N. Silicon uptake and accumulation in lower plants. Trends Plant Sci. 2006;11:392–397. doi: 10.1016/j.tplants.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Ma Y, Prasad MN, Rajkumar M, Freitas H. Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol Adv. 2011;29(2):248–258. doi: 10.1016/j.biotechadv.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Mah TCF, O’Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9:34–39. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- Malinovskaya IM, Kosenko LV, Votselko SK, Podgorskii VS. Role of bacillus mucilaginosus polysaccharide in degradation of silicate minerals. Mikrobiologiya. 1990;59:49–55. [Google Scholar]
- Mapelli F, Marasco R, Rolli E, Barbato M, Cherif H, Guesmi A (2013) Potential for plant growth promotion of rhizobacteria associated with Salicornia growing in Tunisian hypersaline soils. Biomed Res Int 248078 [DOI] [PMC free article] [PubMed]
- Mayak S, Tirosh T, Glick BR. Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol Biochem. 2004;42:565–572. doi: 10.1016/j.plaphy.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Meena VS, Maurya BR, Bahadur I. Potassium solubilization by bacterial strain in waste mica. Bangladesh J Bot. 2014;43(2):235–237. [Google Scholar]
- Metwali EM, Abdelmoneim TS, Bakheit MA, Kadasa NM. Alleviation of salinity stress in faba bean (Vicia faba L.) plants by inoculation with plant growth promoting rhizobacteria (PGPR) Plant Omics. 2015;8(5):449. [Google Scholar]
- Miller G, Susuki N, Ciftci-Yilmaz S, Mittler R. Reactive oxygen specieshomeostasis and signalling during drought and salinity stresses. Plant, Cell Environ. 2010;33:453–467. doi: 10.1111/j.1365-3040.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- Minah B, Hazarin Subair F. Isolation and Screening bacterial exopolysaccharide (EPS) from potato rhizosphere in highland and the potential as a producer indole acetic acid (IAA) Procedia Food Science. 2015;3:74–81. [Google Scholar]
- Naik PR, Raman G, Narayanan KB, Sakthivel N. Assessment of genetic and functional diversity of phosphate solubilizing fluorescent pseudomonads isolated from rhizospheric soil. BMC Microbiol. 2008;8(1):230. doi: 10.1186/1471-2180-8-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nautiyal CS, Govindarajan R, Lavania M, Pushpangadan P. Novel mechanism of modulating natural antioxidants in functional foods: involvement of plant growth promoting rhizobacteria NRRL B-30488. J Agric Food Chem. 2008;56(12):4474–4481. doi: 10.1021/jf073258i. [DOI] [PubMed] [Google Scholar]
- Nemat MA, Magdi TA, Magdy A. Ameliorate of environmental salt stress on the growth of Zea mays L. plants by exopolysaccharides producing bacteria. J Appl Sci Res. 2012;8(4):2033–2044. [Google Scholar]
- Ng LC, Anuar SNA, Jong JW, Elham MSH. Phytobeneficial and plant growth-promotion properties of silicon-solubilising rhizobacteria on the growth and control of rice sheath blight disease. Asian J Plant Sci. 2016;15:92–100. [Google Scholar]
- Nguyen KN, Tran TMT, Nguyen TKO, Nguyen H, Kim N. Isolation and characterization of indole acetic acid producing halophilic bacteria from salt affected soil of rice-shrimp farming system in the Mekong Delta, Vietnam. Agric, For Fish. 2017;6(3):69–77. [Google Scholar]
- Oberson A, Frossard E, Bühlmann C, Mayer J, Mader P, Luscher A. Nitrogen fixation and transfer in grass-clover leys under organic andconventional cropping systems. Plant Soil. 2013;371:237. [Google Scholar]
- Orhan F. Alleviation of salt stress by halotolerant and halophilic plant growth-promoting bacteria in wheat (Triticum aestivum) Braz J Microbiol. 2016;47:621–627. doi: 10.1016/j.bjm.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey P, Kang SC, Gupta CP, Maheshwari DK. Rhizosphere competent Pseudomonas aeruginosa GRC1 produces characteristic siderophore and enhances growth of Indian mustard (Brassica campestris) Curr Microbiol. 2005;51(5):303–309. doi: 10.1007/s00284-005-0014-1. [DOI] [PubMed] [Google Scholar]
- Parmar P, Sindhu SS. Potassium solubilization by rhizosphere bacteria: influence of nutritional and environmental conditions. J Microbiol Res. 2013;3(1):25–31. [Google Scholar]
- Patil AD (2013) Alleviating salt stress in crop plants through salt tolerant microbes. International J Sci Res (IJSR) ISSN (Online) 2319–7064
- Paul D, Lade H. Plant-growth-promoting rhizobacteria to improve crop growth in saline soils: a review. Agron Sustain Dev. 2014;34:737–752. [Google Scholar]
- Pirhadi M, Enayatizamir N, Motamedi H, Sorkheh K. Screening of salt tolerant sugarcane endophytic bacteria with potassium and zinc for their solubilizing and antifungal activity Biosci. Biotech Res Commun. 2016;9(3):530–538. [Google Scholar]
- Qurashi AW, Sabri AN. Bacterial exopolysaccharide and biofilm formation stimulate Chickpea growth and soil aggregation under salt stress. Braz J Microbiol. 2012;43:1183–1191. doi: 10.1590/S1517-838220120003000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar M, Ae N, Prasad MN, Freitas H. Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol. 2010;28(3):142–149. doi: 10.1016/j.tibtech.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Rajwar A, Sahgal M, Johri BN. Plant microbe symbiosis: fundamentals and advances. New Delhi: Springer; 2013. Legume–rhizobia symbiosis and interactions in agro ecosystems; pp. 233–265. [Google Scholar]
- Ramadoss D, Lakkineni VK, Bose P, Ali S, Annapurna K. Mitigation of salt stress in wheat seedlings by halotolerant bacteria isolated from saline habitats. Springerplus. 2013;2:1–7. doi: 10.1186/2193-1801-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid A, Ryan J. Micronutrient constraints to crop production in soils with mediterranean type charac-teristics: a review. J Plant Nutr. 2004;27:959–975. [Google Scholar]
- Rasool S, Ahmad A, Siddiqi TO, Ahmad P. Changes in growth, lipid peroxidation and some key antioxidant enzymes in chickpea genotypes under salt stress. Acta Physiol Plant. 2013;35:1039–1050. [Google Scholar]
- Rasouli Sadagiani MH, Sadegi S, Barin M, Sepehr A, Dolati B. Effects of Suilicate solubilizing bacteria on realizing potassium from mica minerals and its uptake by Zea mays L. plants. J Soil Water Sci. 2016;78:1–14. [Google Scholar]
- Rodrignes FA, Datnoff LE. Silicon and rice disease management. Fitopatologia Brasileira. 2005;30:457–469. [Google Scholar]
- Rodrigues MI, Bravo JP, Sassaki FT, Severino FE, Maia IG. The tonoplast intrinsic aquaporin (TIP) sub family of Eucalyptus grandis: characterization of EgTIP2, a root-specific and osmotic stress-responsive gene. Plant Sci. 2013;213:106–113. doi: 10.1016/j.plantsci.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Rojas-Tapias D, Moreno-Galvan A, Pardo-Diaz S, Obando M, Rivera D, Bonilla R. Effect of inoculation with plant growth-promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays) Appl Soil Ecol. 2012;61:264–272. [Google Scholar]
- Saghafi D, Ghorbanpour M, Asgari Lajayer B. Efficiency of Rhizobium strains as plant growth promoting rhizobacteria on morpho-physiological properties of Brassica napus L. under salinity stress. J Soil Sci Plant Nutr. 2018;18(1):253–268. [Google Scholar]
- Sandhya V, Ali SKZ, Reddy Grover M, Venkateswarlu GB. Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-P45. Biol Fertil Soils. 2009;46:17–26. [Google Scholar]
- Scavino Ana Fernández, Pedraza Raúl O. Bacteria in Agrobiology: Crop Productivity. Berlin, Heidelberg: Springer Berlin Heidelberg; 2013. The Role of Siderophores in Plant Growth-Promoting Bacteria; pp. 265–285. [Google Scholar]
- Shahbaz M, Ashraf M. Improving salinity tolerance in cereals. Crit Rev Plant Sci. 2013;32:237–249. [Google Scholar]
- Sharma A, Johri BN. Growth promoting influence of siderophore-producing Pseudomonas strains GRP3A and PRS 9 in maize (Zea mays L.) under iron limiting conditions. Microbiol Res. 2003;158(3):243–248. doi: 10.1078/0944-5013-00197. [DOI] [PubMed] [Google Scholar]
- Shrivastava P, Kumar R. Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci. 2015;22:123–131. doi: 10.1016/j.sjbs.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla PS, Agarwal PK, Jha B. Improved salinity tolerance of Arachis hypogaea L. by the interaction of halotolerant plant growth promoting rhizobacteria. J Plant Growth Regul. 2012;31:195–206. [Google Scholar]
- Siddikee MA, Chauhan PS, Anandham R, Han GH, Sa T. Isolation, characterization, and use for plant growth promotion under salt stress, of ACC deaminase-producing halotolerant bacteria derived from coastal soil. J Microbiol Biotechnol. 2010;20(11):1577–1584. doi: 10.4014/jmb.1007.07011. [DOI] [PubMed] [Google Scholar]
- Sindhu Satyavir S., Dua Seema, Verma M. K., Khandelwal Aakanksha. Microbes for Legume Improvement. Vienna: Springer Vienna; 2010. Growth Promotion of Legumes by Inoculation of Rhizosphere Bacteria; pp. 195–235. [Google Scholar]
- Singh G, Biswas DR, Marwaha TS. Mobilization of potassium from waste mica by plant growth promoting rhizobacteria and its assimilation by maize (Zea mays) and wheat (Triticum aestivum L.): a hydroponics study under phytotron growth chamber. J Plant Nutr. 2010;33(8):1236–1251. [Google Scholar]
- Sonobe K, Hattori T, An P, Tsuji W, Eneji AE, Kobayashi S, Kawamura Y, Tanaka K, Inanaga S. Effect of silicon application on sorghum root responses to water stress. J Plant Nutr. 2011;34:71–82. [Google Scholar]
- Subramanian KS, Tenshia V, Jayalakshmi K, Ramachandran V. Role of arbuscular mycorrhizal fungus (Glomus intraradices)—(fungus aided) in zinc nutrition of maize. Agric Biotechnol Sust Dev. 2009;1:29–38. [Google Scholar]
- Tang X, Mu X, Shao H, Wang H, Brestic M. Global plant-responding mechanisms to salt stress: physiological and molecular levels and implications in biotechnology. Crit Rev Biotechnol. 2014;35:1–13. doi: 10.3109/07388551.2014.889080. [DOI] [PubMed] [Google Scholar]
- Tariq M, Hameed S, Malik KA, Hafeez FY. Plant root associated bacteria for zinc mobilization in rice. Pak J Bot. 2007;39(1):245. [Google Scholar]
- Tester M, Davenport R. Na+ tolerance and Na+ transport in higher plants. Ann Bot. 2003;91(5):503–527. doi: 10.1093/aob/mcg058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomine S, Lanquar V. Transporters and pumps in plant signaling. Berlin: Springer; 2011. Iron transport and signaling in plants; pp. 99–131. [Google Scholar]
- Timmusk S, Islam A Abd, El D, Lucian C, Tanilas T, Kannaste A. Drought-tolerance of wheat improved by rhizosphere bacteria from harshenvironments: enhanced biomass production and reduced emissions of stressvolatiles. PLoS One. 2014;9:1–13. doi: 10.1371/journal.pone.0096086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdall JM. Possible role of soil microorganisms in aggregation of soils. Plant Soil. 1994;159:115–121. [Google Scholar]
- Toro M. Phosphate solubilizing microorganisms in the rhizosphere of native plants from tropical savannas: an adaptive strategy to acid soils? In: Velazquez C, Rodriguez-Barrueco E, editors. Developments in plant and soil sciences. The Netherlands: Springer; 2007. pp. 249–252. [Google Scholar]
- Tyagia S, Mullaa SI, Leea K, Chaea J, Shukla P. VOCs-mediated hormonal signaling and crosstalk with plant growth promoting microbes. Crit Rev Biotechnol. 2018;38:1277–1296. doi: 10.1080/07388551.2018.1472551. [DOI] [PubMed] [Google Scholar]
- Vaishnav A, Ajit V, Narendra T, Devendra K (2017) PGPR-mediated amelioration of crops under salt stress. Block ‘E-3’, 4th Floor, Amity University Campus, Sector-125, Gautam Buddha Nagar, 201313 Noida, Uttar Pradesh, India
- Vasanthi N, Saleena LM, Raj SA. Silicon in day today life. World Appl Sci J. 2012;17:1425–1440. [Google Scholar]
- Wang LL, Chen AP, Zhong NQ, Liu N, Wu XM, Wang F. The Thellungiella salsuginea tonoplast aquaporinTsTIP1;2 functions in protection against multiple abiotic stresses. Plant Cell Physiol. 2014;55:148–161. doi: 10.1093/pcp/pct166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Dodd IC, Belimov AA, Jiang F. Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase growth and photosynthesis of pea plants under salt stress by limiting Na+ accumulation. Funct Plant Biol. 2016;43:161–172. doi: 10.1071/FP15200. [DOI] [PubMed] [Google Scholar]
- Xin S, Yu G, Sun L, Qiang X, Xu N, Cheng X. Expression of tomato SlTIP2;2 enhances the tolerance to salt stress in the transgenic Arabidopsis and interacts with target proteins. J Plant Res. 2014;127:695–708. doi: 10.1007/s10265-014-0658-7. [DOI] [PubMed] [Google Scholar]
- Yaish MW, Antony I, Glick R. Isolation and characterization of endophytic plant growth promoting bacteria from date palm tree (Phoenix dactylifera L.) and their potential role in salinity tolerance. Antonie van Leeuwenhoek. 2015;107:1519–1532. doi: 10.1007/s10482-015-0445-z. [DOI] [PubMed] [Google Scholar]
- Yan K, Shao H, Shao Ch, Chen P, Zhao Sh, Brestic M, Chen X. Physiological adaptive mechanisms of plants grown in saline soil and implications for sustainable saline agriculture in coastal zone. Acta Physiol Plant. 2013;35:2867–2878. [Google Scholar]
- Yao LX, Wu ZS, Zheng YY, Kaleem I, Li C. Growth promotion and protection against salt stress by Pseudomonas putida Rs-198 on cotton. Eur J Soil Biol. 2010;46(1):49–54. [Google Scholar]
- Zaidi A, Khan MS, Ahemad M, Oves M, Wani PA (2009) Recent advances in plant growth promotion by phosphate-solubilizing microbes. Microb Strat Crop Improve 23–50 [DOI] [PubMed]
- Zhang H, Xie X, Kim MS, Kornyeyev DA, Holaday S, Paré PW. Soil bacteria augment Arabidopsis photosynthesis by decreasing glucose sensing and abscisic acid levels in planta. Plant J. 2008;56:264–273. doi: 10.1111/j.1365-313X.2008.03593.x. [DOI] [PubMed] [Google Scholar]
- Zhang D, Tong J, He X, Xu Z, Xu L, Wei P, Huang Y, Brestic M, Ma H, Shao H. A novel soybean intrinsic protein gene, GmTIP2;3, involved in responding to osmotic stress. Front Plant Sci. 2016;6:1237. doi: 10.3389/fpls.2015.01237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Chen L, Wang J, Wang M, Yang S, Zhao C. Photosynthetic acclimation to long-term high temperature and soil drought stress in two spruce species (Picea crassifolia and P. wilsonii) used for afforestation. J For Res. 2018;29(2):363–372. [Google Scholar]